
Needham & Co. 15th Annual Healthcare Conference New York April 2016 Investor Presentation Exhibit 99.1

Disclosure To the extent statements contained in this presentation are not descriptions of historical facts regarding Presbia PLC and its subsidiaries (collectively “Presbia,” “we,” “us,” or “our”), they are forward-looking statements reflecting management’s current beliefs and expectations. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “intends,” or “continue,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding: (i) the initiation, timing, progress and results of our clinical trials, our regulatory submissions and our research and development programs; (ii) our ability to advance our products into, and successfully complete, clinical trials; (iii) our ability to obtain pre-market approvals; (iv) the commercialization of our products; (v) the implementation of our business model, strategic plans for our business, products and technology; (vi) the scope of protection we are able to establish and maintain for intellectual property rights covering our products and technology; (vii) estimates of our expenses, future revenues, growth of operations, capital requirements and our needs for additional financing; (viii) the timing or likelihood of regulatory filings and approvals; (ix) our financial performance; (x) developments relating to our competitors and our industry; and (xi) statements regarding our markets, including the estimated size and anticipated growth in those markets. Various factors may cause differences between our expectations and actual results, including those risks discussed under “Risk Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 28, 2016 and those risks discussed under “Risk Factors” in other reports we may file with the Securities and Exchange Commission. Except as required by law, we assume no obligation to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
Business Highlights Large, Underserved Presbyopia Opportunity Best-in-Class Near Vision Microlens Refractive corneal inlay restores reading vision, multiple lines of improvement Wide range of lens refractive powers to offer patients a customized therapy Compatible with other refractive surgical procedures - LASIK and cataract surgery CE-marked with over 1000 lenses safely implanted globally Clear FDA Pathway Second and final FDA two-stage clinical trial enrollment completed: 421 U.S. patients received Presbia Flexivue Microlens™ 18 months into 36-month follow-up; submission of final PMA module in Q4 2017 expected to lead to FDA approval Q4 2018 + 5 lines of uncorrected near visual acuity in treated eyes (90% of 6 month US study cohort) 113 million presbyopes in the U.S.; 1.8 billion worldwide (2014)* 4,000+ ophthalmic surgery centers with no effective treatment of presbyopia Refractive surgeons are highly motivated to develop this market to replace lost LASIK volumes and utilize installed base of expensive femtosecond lasers Compelling surgery center economics: 100% private pay, ~10 minute procedure Two beach-heads Now: Asia Pacific – South Korea Next: Europe – Germany Ongoing: Centers of Excellence – Ireland, Netherlands, Canada, Australia Commercial Strategy *Source: 2013 Market Scope.
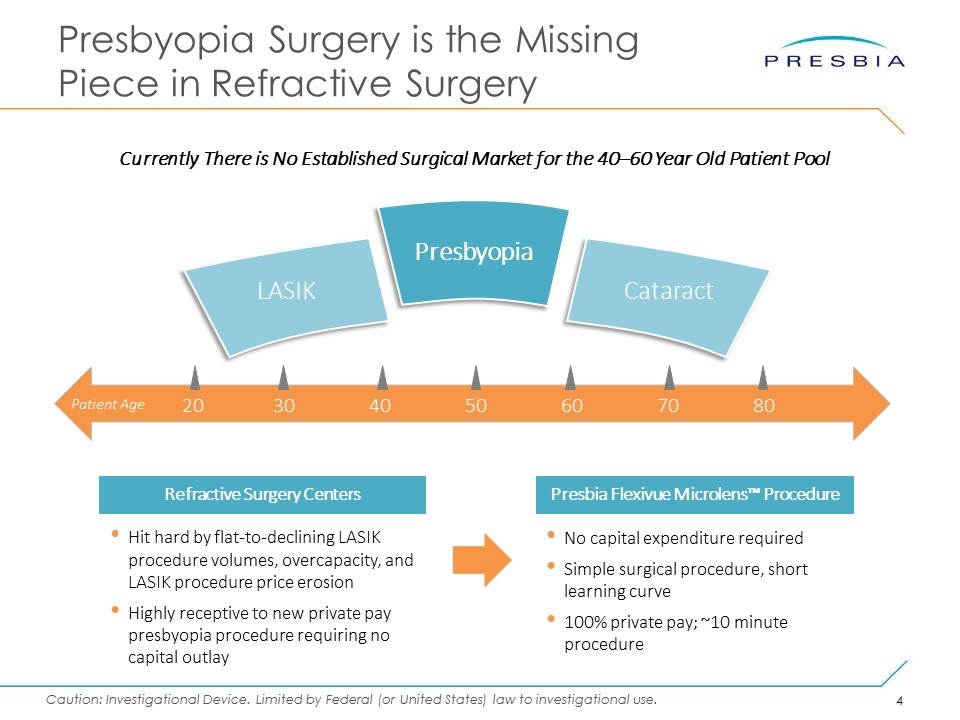
Presbyopia Surgery is the Missing Piece in Refractive Surgery Hit hard by flat-to-declining LASIK procedure volumes, overcapacity, and LASIK procedure price erosion Highly receptive to new private pay presbyopia procedure requiring no capital outlay Refractive Surgery Centers No capital expenditure required Simple surgical procedure, short learning curve 100% private pay; ~10 minute procedure Presbia Flexivue Microlens™ Procedure 20304050607080 Patient Age LASIK Cataract Presbyopia Currently There is No Established Surgical Market for the 40–60 Year Old Patient Pool

A Loss of Near Vision Affecting the Majority of People over the Age of 40 Presbia and related research shows: The Inconvenience of Presbyopia “I can never find my glasses”* “Can’t read using my iPhone”* “The hassle of my glasses on and off all day”* Freedom and confidence Reading glasses are one of the most ubiquitous signs of aging Recent Bausch & Lomb survey found “almost half of women over the age of 40 admit to feeling embarrassed, frumpy, or annoyed when reaching for reading glasses”* “I feel younger”* “My friends notice I don’t have reading glasses”* “I feel free now that I don’t need them”* * Focus Groups, February, 2015, Dublin, Ireland
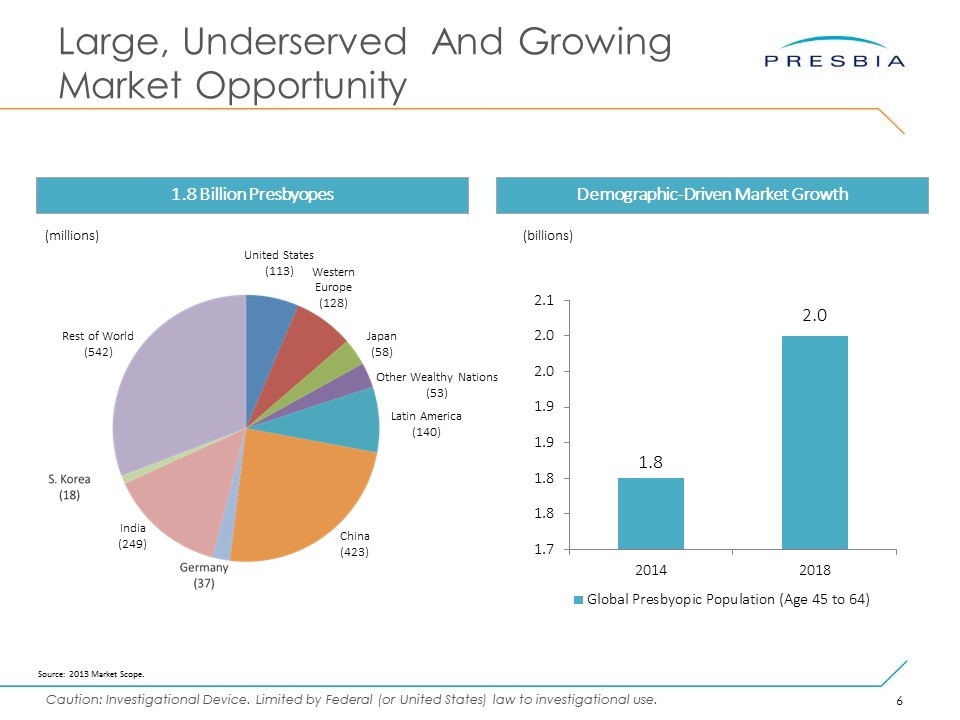
Large, Underserved And Growing Market Opportunity Demographic-Driven Market Growth 1.8 Billion Presbyopes (billions) Source: 2013 Market Scope. United States (113) Western Europe (128) Latin America (140) China (423) India (249) Rest of World (542) Other Wealthy Nations (53) Japan (58) (millions)
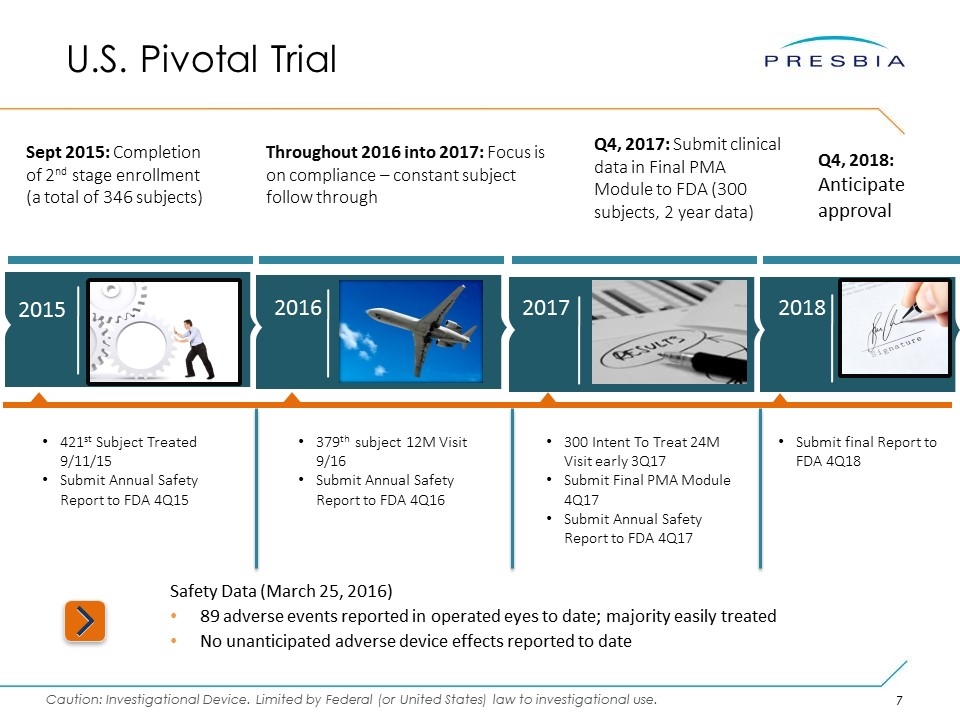
Safety Data (March 25, 2016) 89 adverse events reported in operated eyes to date; majority easily treated No unanticipated adverse device effects reported to date Sept 2015: Completion of 2nd stage enrollment (a total of 346 subjects) Throughout 2016 into 2017: Focus is on compliance – constant subject follow through Q4, 2017: Submit clinical data in Final PMA Module to FDA (300 subjects, 2 year data) U.S. Pivotal Trial 2017 2015 2016 2018 Q4, 2018: Anticipate approval 421st Subject Treated 9/11/15 Submit Annual Safety Report to FDA 4Q15 379th subject 12M Visit 9/16 Submit Annual Safety Report to FDA 4Q16 300 Intent To Treat 24M Visit early 3Q17 Submit Final PMA Module 4Q17 Submit Annual Safety Report to FDA 4Q17 Submit final Report to FDA 4Q18
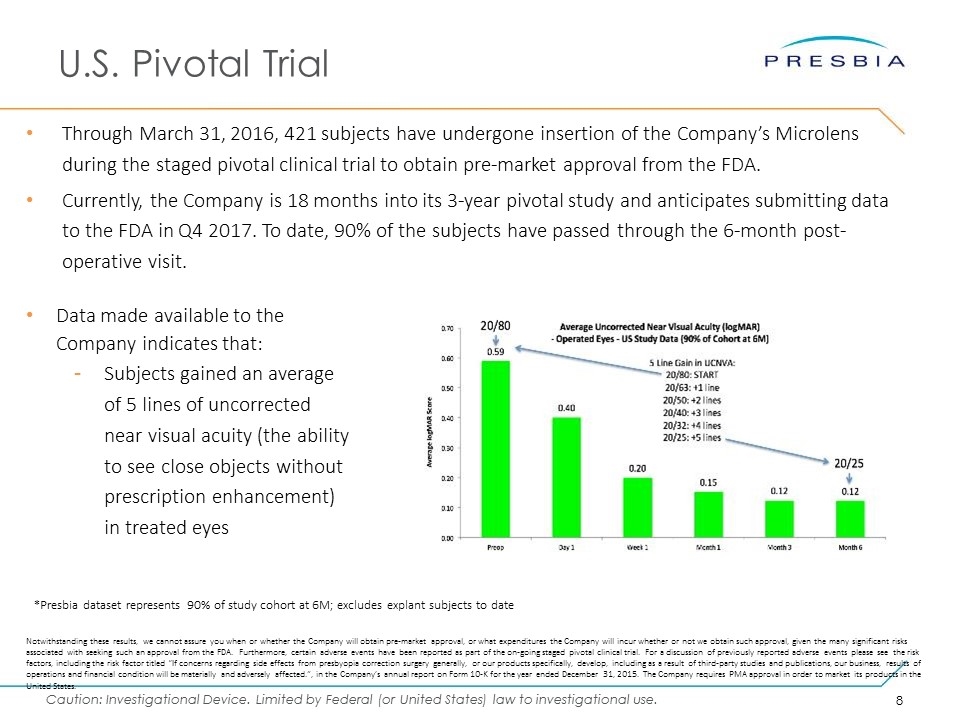
U.S. Pivotal Trial Through March 31, 2016, 421 subjects have undergone insertion of the Company’s Microlens during the staged pivotal clinical trial to obtain pre-market approval from the FDA. Currently, the Company is 18 months into its 3-year pivotal study and anticipates submitting data to the FDA in Q4 2017. To date, 90% of the subjects have passed through the 6-month post-operative visit. *Presbia dataset represents 90% of study cohort at 6M; excludes explant subjects to date Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States. Data made available to the Company indicates that: Subjects gained an average of 5 lines of uncorrected near visual acuity (the ability to see close objects without prescription enhancement) in treated eyes
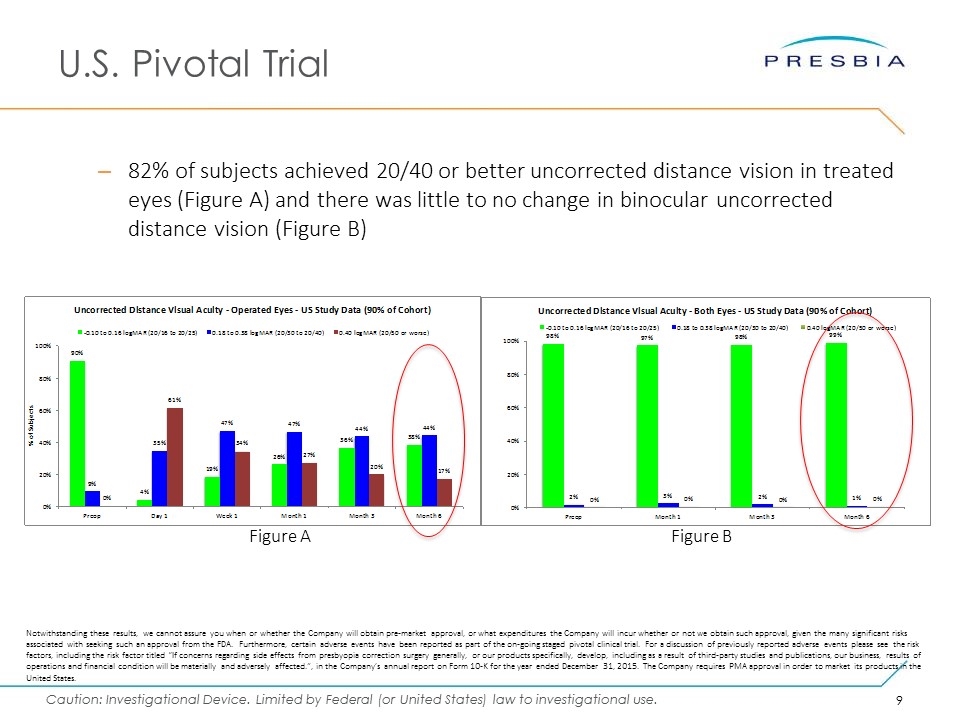
U.S. Pivotal Trial 82% of subjects achieved 20/40 or better uncorrected distance vision in treated eyes (Figure A) and there was little to no change in binocular uncorrected distance vision (Figure B) Figure A Figure B Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States.
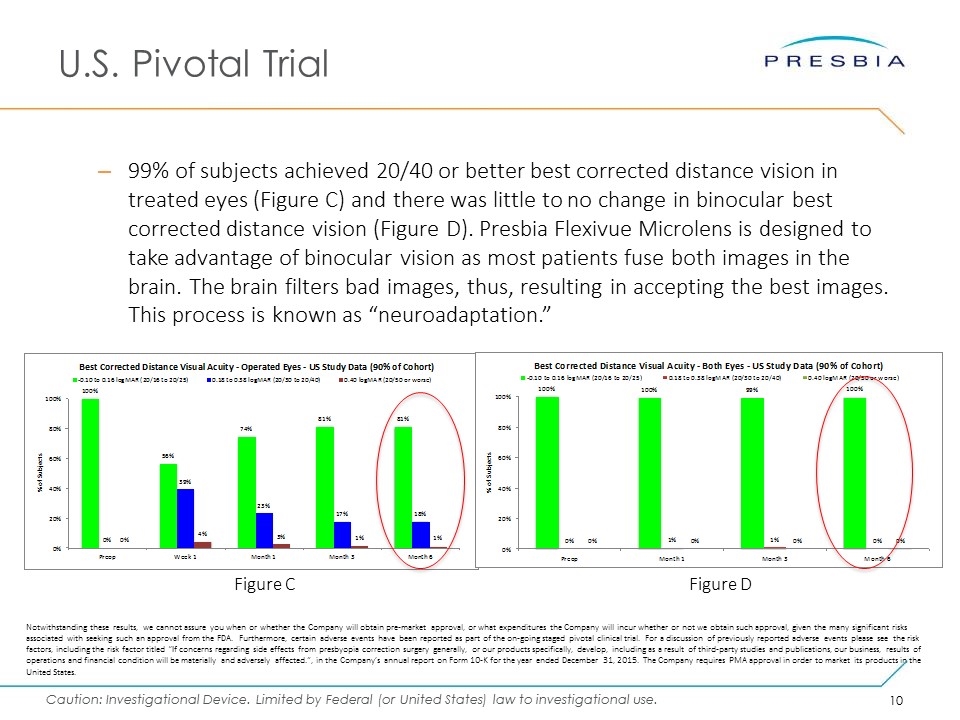
U.S. Pivotal Trial Figure C Figure D 99% of subjects achieved 20/40 or better best corrected distance vision in treated eyes (Figure C) and there was little to no change in binocular best corrected distance vision (Figure D). Presbia Flexivue Microlens is designed to take advantage of binocular vision as most patients fuse both images in the brain. The brain filters bad images, thus, resulting in accepting the best images. This process is known as “neuroadaptation.” Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015. The Company requires PMA approval in order to market its products in the United States.
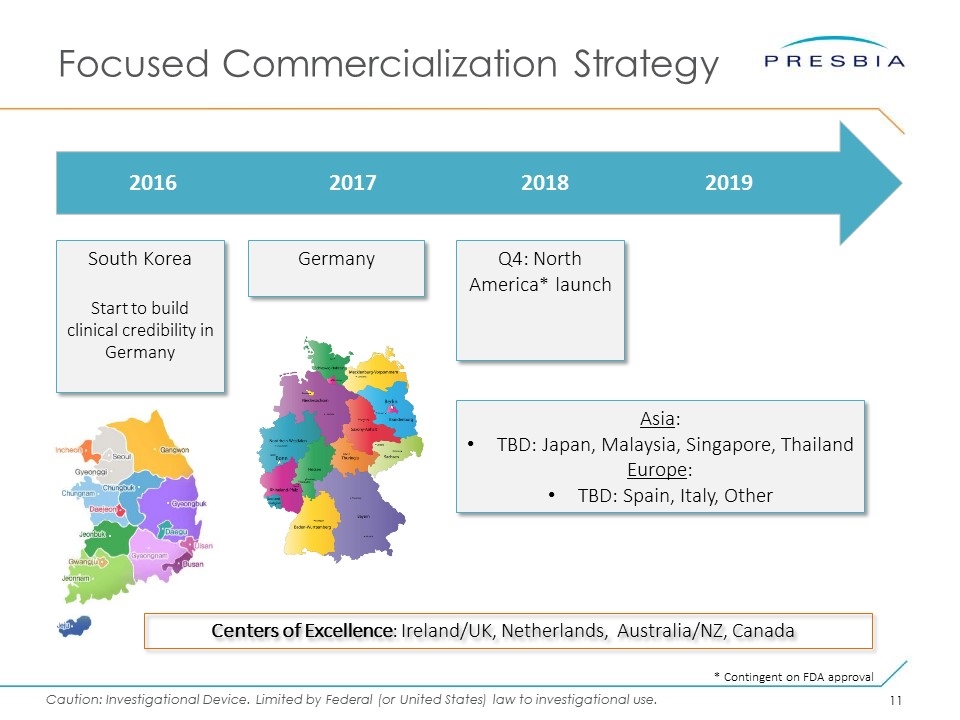
Focused Commercialization Strategy 2016 2017 2018 2019 South Korea Start to build clinical credibility in Germany Centers of Excellence: Ireland/UK, Netherlands, Australia/NZ, Canada Q4: North America* launch Asia: TBD: Japan, Malaysia, Singapore, Thailand Europe: TBD: Spain, Italy, Other Germany * Contingent on FDA approval
Two Pronged Approach in Country * Contingent FDA approval Clinical Local data Local key opinion leader (KOL) Increase global data Local reference site (peer-to-peer education) PR driven by clinical data (positive clinical outcomes result in a believable and newsworthy campaign) Commercial Local experience Acceptance Competition Increased awareness drives more patients
Focus Market South Korea Launch Symposium in Q4 2015 Wet labs completed Currently engaging top hospitals to participate in local Korean/Asian clinical studies Initial KOLs targeted to increase surgeon confidence and expedite adoption Lead generation marketing and PR in each metro area Go deep – hands-on with clinic Target largest metro areas and saturate each city: surgical trainer, clinical applications specialist business development director, sales account manager & marketing Sustained Resource Concentration – multiple refractive practices per metro area to create competition
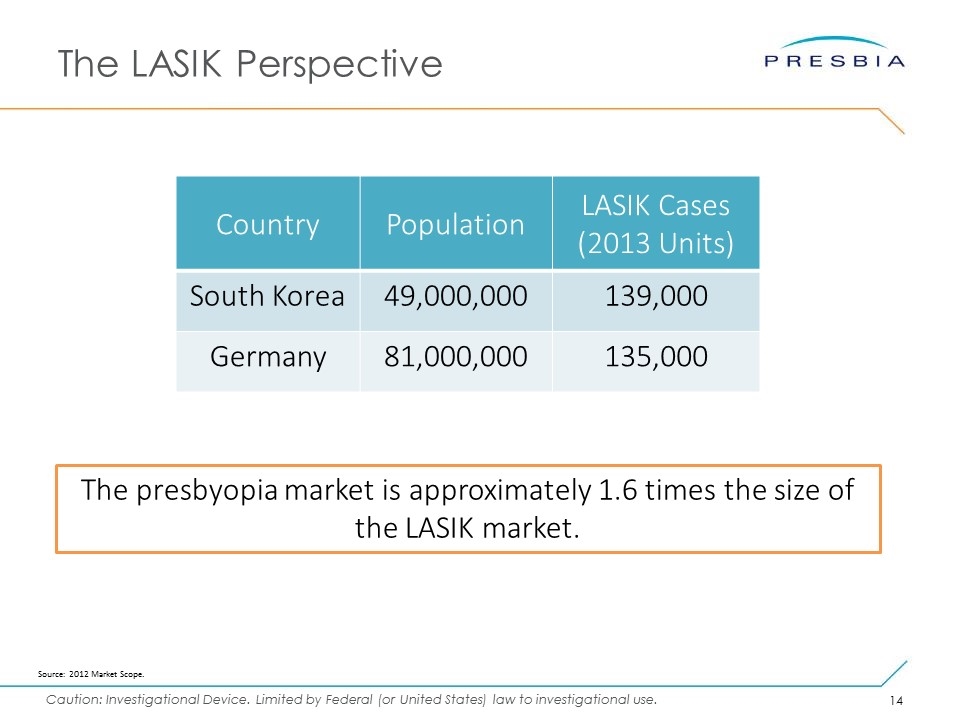
The LASIK Perspective Country Population LASIK Cases (2013 Units) South Korea 49,000,000 139,000 Germany 81,000,000 135,000 The presbyopia market is approximately 1.6 times the size of the LASIK market. Source: 2012 Market Scope.

Clearly Differentiated Near Vision Solution
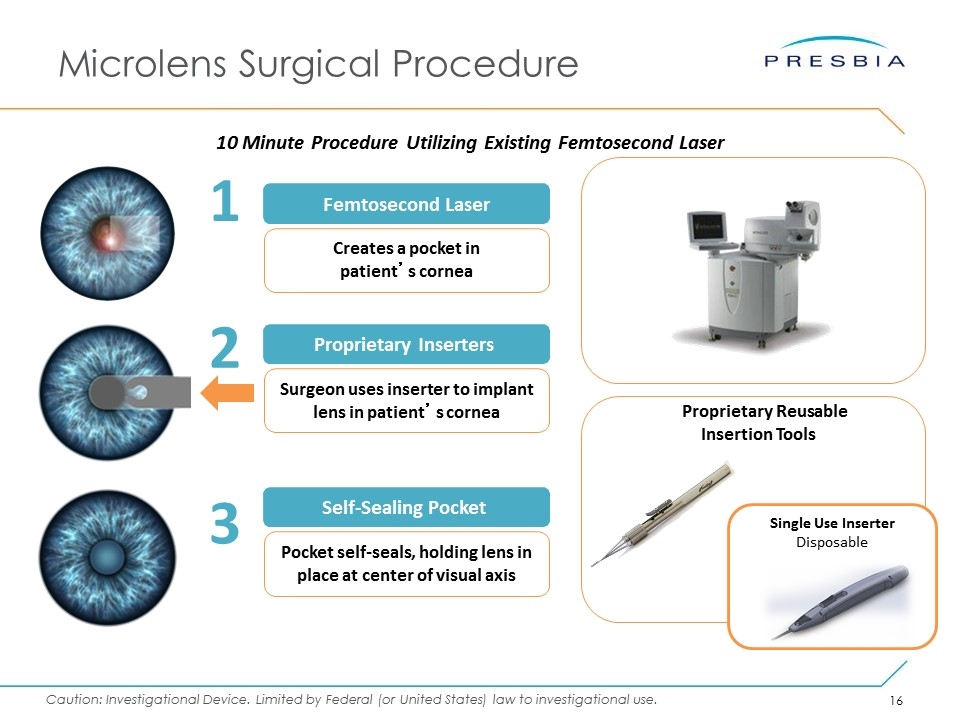
Microlens Surgical Procedure 10 Minute Procedure Utilizing Existing Femtosecond Laser Femtosecond Laser 1 Creates a pocket in patient’s cornea 3 2 Proprietary Inserters Surgeon uses inserter to implant lens in patient’s cornea Self-Sealing Pocket Pocket self-seals, holding lens in place at center of visual axis Proprietary Reusable Insertion Tools Single Use Inserter Disposable
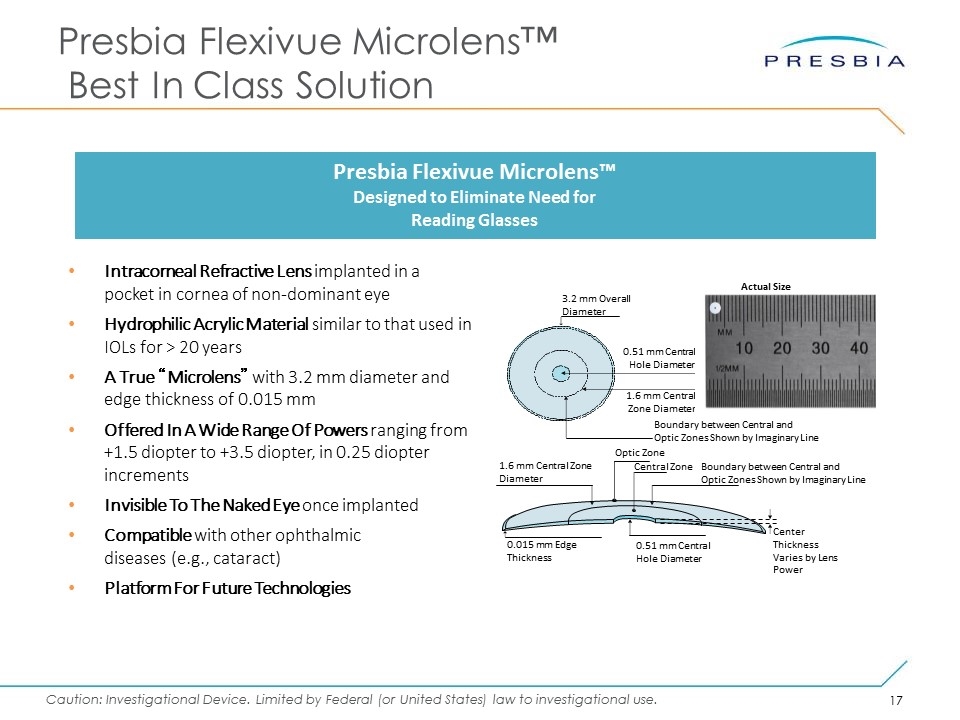
Intracorneal Refractive Lens implanted in a pocket in cornea of non-dominant eye Hydrophilic Acrylic Material similar to that used in IOLs for > 20 years A True “Microlens” with 3.2 mm diameter and edge thickness of 0.015 mm Offered In A Wide Range Of Powers ranging from +1.5 diopter to +3.5 diopter, in 0.25 diopter increments Invisible To The Naked Eye once implanted Compatible with other ophthalmic diseases (e.g., cataract) Platform For Future Technologies Presbia Flexivue Microlens™ Best In Class Solution Presbia Flexivue Microlens™ Designed to Eliminate Need for Reading Glasses 1.6 mm Central Zone Diameter Boundary between Central and Optic Zones Shown by Imaginary Line 0.51 mm Central Hole Diameter 3.2 mm Overall Diameter Central Zone Boundary between Central and Optic Zones Shown by Imaginary Line Optic Zone Center Thickness Varies by Lens Power 1.6 mm Central Zone Diameter 0.51 mm Central Hole Diameter 0.015 mm Edge Thickness Actual Size
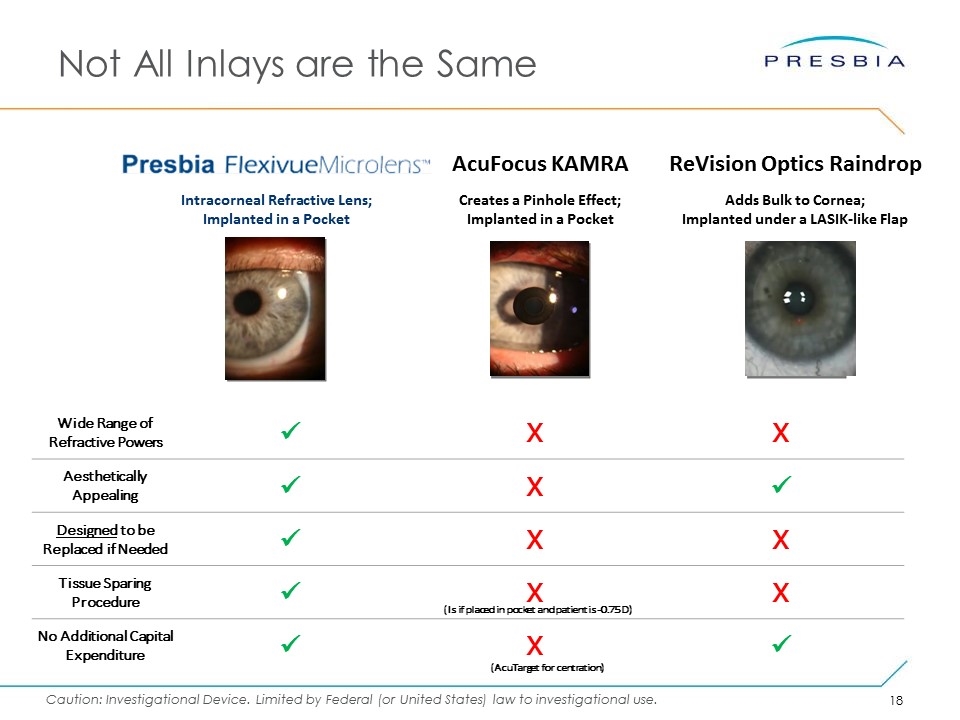
Not All Inlays are the Same Intracorneal Refractive Lens; Implanted in a Pocket Creates a Pinhole Effect; Implanted in a Pocket AcuFocus KAMRA Adds Bulk to Cornea; Implanted under a LASIK-like Flap ReVision Optics Raindrop Wide Range of Refractive Powers ü X X Aesthetically Appealing ü X ü Designed to be Replaced if Needed ü X X Tissue Sparing Procedure ü X X No Additional Capital Expenditure ü X ü (Is if placed in pocket and patient is -0.75 D) (AcuTarget for centration)
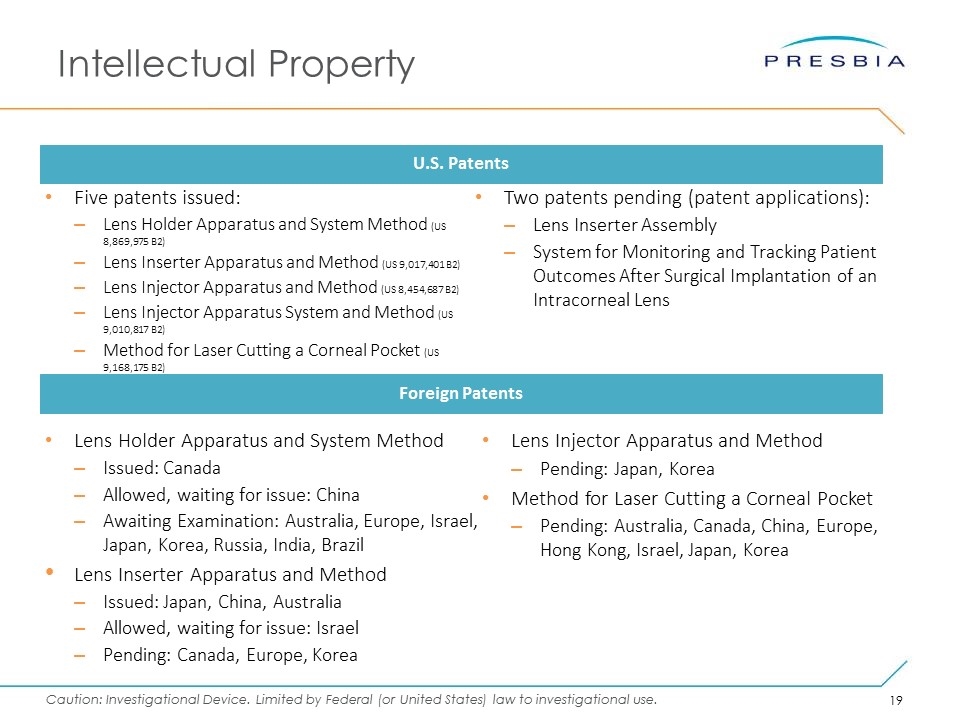
Intellectual Property Five patents issued: Lens Holder Apparatus and System Method (US 8,869,975 B2) Lens Inserter Apparatus and Method (US 9,017,401 B2) Lens Injector Apparatus and Method (US 8,454,687 B2) Lens Injector Apparatus System and Method (US 9,010,817 B2) Method for Laser Cutting a Corneal Pocket (US 9,168,175 B2) U.S. Patents Foreign Patents Lens Holder Apparatus and System Method Issued: Canada Allowed, waiting for issue: China Awaiting Examination: Australia, Europe, Israel, Japan, Korea, Russia, India, Brazil Lens Inserter Apparatus and Method Issued: Japan, China, Australia Allowed, waiting for issue: Israel Pending: Canada, Europe, Korea Two patents pending (patent applications): Lens Inserter Assembly System for Monitoring and Tracking Patient Outcomes After Surgical Implantation of an Intracorneal Lens Lens Injector Apparatus and Method Pending: Japan, Korea Method for Laser Cutting a Corneal Pocket Pending: Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Korea

Manufacturing 4,000 square-foot, two-part (wet/dry) manufacturing facility Approved to manufacture devices for U.S. IDE by State of California FDA in 2013 Sufficient capacity to handle projected Presbia Flexivue Microlens™ volume through U.S. launch Approved to manufacture devices for OUS sale by Intertek (ISO 13485:2012 certified) Additional third-party manufacturing facility in Israel supplies product for all current OUS requirements Distribution facilities in Ireland and the Netherlands Irvine, CA Manufacturing Facility

Experienced Leadership Team & Board Vanessa Tasso Vice President of Clinical Affairs John Strobel Vice President of Sales Vlad Feingold Chief Technology Officer & Director Todd Cooper President, Chief Executive Officer & Director Randy Thurman Executive Chairman Richard Ressler Director Bob Cresci Director Gerd Auffarth Director Zohar Loshitzer Director EXECUTIVE BOARD Gerald Farrell Director
Business Highlights Large, Underserved Presbyopia Opportunity Best-in-Class Near Vision Microlens Refractive corneal inlay restores reading vision, multiple lines of improvement Wide range of lens refractive powers to offer patients a customized therapy Compatible with other refractive surgical procedures - LASIK and cataract surgery CE-marked with over 1000 lenses safely implanted globally Clear FDA Pathway Second and final FDA two-stage clinical trial enrollment completed: 421 U.S. patients received Presbia Flexivue Microlens™ 18 months into 36-month follow-up; submission of final PMA module in Q4 2017 expected to lead to FDA approval Q4 2018 + 5 lines of uncorrected near visual acuity in treated eyes (90% of 6 month US study cohort) 113 million presbyopes in the U.S.; 1.8 billion worldwide (2014)* 4,000+ ophthalmic surgery centers with no effective treatment of presbyopia Refractive surgeons are highly motivated to develop this market to replace lost LASIK volumes and utilize installed base of expensive femtosecond lasers Compelling surgery center economics: 100% private pay, ~10 minute procedure Two beach-heads Now: Asia Pacific – South Korea Next: Europe – Germany Ongoing: Centers of Excellence – Ireland, Netherlands, Canada, Australia Commercial Strategy *Source: 2013 Market Scope.

Words From Our Surgeons "Thank you for enabling us to implant an invisible product into a transparent organ, with dogma defying outcomes and joyous recipients. It is these inspiring experiences that make medicine fun". Kerry Assil, MD (USA) “It (Presbia Microlens) marks a new era in the treatment of this natural part of the ageing process, and in a sense it allows you to turn back the clock and regain the vision you enjoyed when younger.” Wayne Crewe-Brown, MD (Ireland/UK) “Presbia offers a unique technology that provides the patient with correction for nearsightedness while allowing the dominant eye to continue to provide effective distance vision. Most importantly, this is a very simple and reversible procedure. Long-time research and follow-up has proven the highest level of bio-compatibility of this miniature intracorneal lens.” Prof. Ioannis Pallikaris (Greece) “Corneal inlays are an exciting new approach to the treatment of presbyopia. In my opnion, the Presbia Microlens represents the most robust corneal inlay platform to date!” Dean Smith, MD (Canada) “We’re looking for a solution that’s safe, that’s simple, that’s effective, that’s customizable to the patient, that’s reversible and I think the Presbia Flexivue Microlens™ is the solution for the future.” Mickey Gordon, MD (USA)

Presbia PLC (Headquarters) 120-121 Lower Baggot St. Dublin 2, Ireland tel: +353-1-659-9446 Presbia Coöperatief U.A. Pieter Pauwstraat 2A – I · 1017 ZJ Amsterdam, Netherlands tel: +31-20-7084-556 · fax: +31-20-7084-556 PresbiBio LLC 8845 Irvine Center Dr., Suite 100 · Irvine, CA 92618 tel: +1-949‐502‐7010 · fax: +1-323-832-8447 info@presbia.com • www.presbia.com























