|
Exhibit 99.1 
|
Dimension Therapeutics, Inc.
Quality of Science. Quality of Life.
August 2016
Forward Looking Statements
These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. For example, all statements we make regarding the initiation, timing, progress and results of our preclinical and clinical studies and our research and development programs, our ability to advance product candidates into, and successfully complete, clinical studies, the timing or likelihood of regulatory filings and approvals, our ability to develop manufacturing processes and engage third parties to manufacture our product candidates for late-stage clinical use or at commercial scale, the success of strategic partnerships, if any, and our expected cash, cash equivalents and marketable securities at year end are forward looking. All forward?looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described under the caption “Risk Factors” in Dimension Therapeutics’ Quarterly Report on Form 10-Q for the quarter ended June 30, 2016, which is on file with the Securities and Exchange Commission, as well as other risks detailed in
Dimension Therapeutics’ additional filings with the Securities and Exchange Commission. Any forward?looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
The information contained in this presentation is not an offer to sell or the solicitation of an offer to buy the
Company’s common stock or any other securities of the Company.
© 2016 Dimension Therapeutics
2
Dimension Therapeutics
A leader in Robust scientific gene therapy platform
discovering & Manufactured using mammalian process
developing new Deep expertise in liver biology
therapeutics for Unique focus on inherited metabolic diseases (IMDs)
people living with
devastating rare Highly experienced team; extensive track record in
diseases rare diseases
associated with
the liver Broad product pipeline addressing diseases with high
unmet medical need
© 2016 Dimension Therapeutics
3
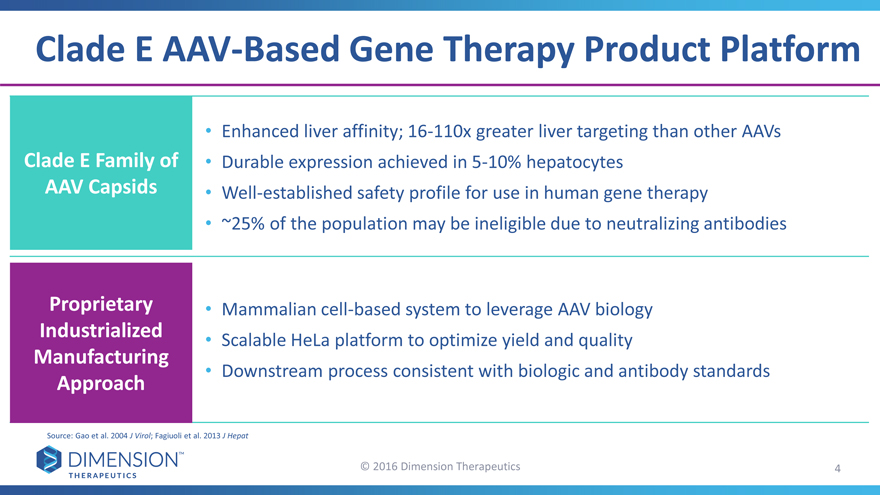
Clade E AAV-Based Gene Therapy Product Platform
Enhanced liver affinity; 16-110x greater liver targeting than other AAVs
Clade E Family of • Durable expression achieved in 5-10% hepatocytes
AAV Capsids • Well-established safety profile for use in human gene therapy
~25% of the population may be ineligible due to neutralizing antibodies
Proprietary • Mammalian cell-based system to leverage AAV biology
Industrialized • Scalable HeLa platform to optimize yield and quality
Manufacturing
Downstream process consistent with biologic and antibody standards
Approach
Source: Gao et al. 2004 J Virol; Fagiuoli et al. 2013 J Hepat
© 2016 Dimension Therapeutics
4
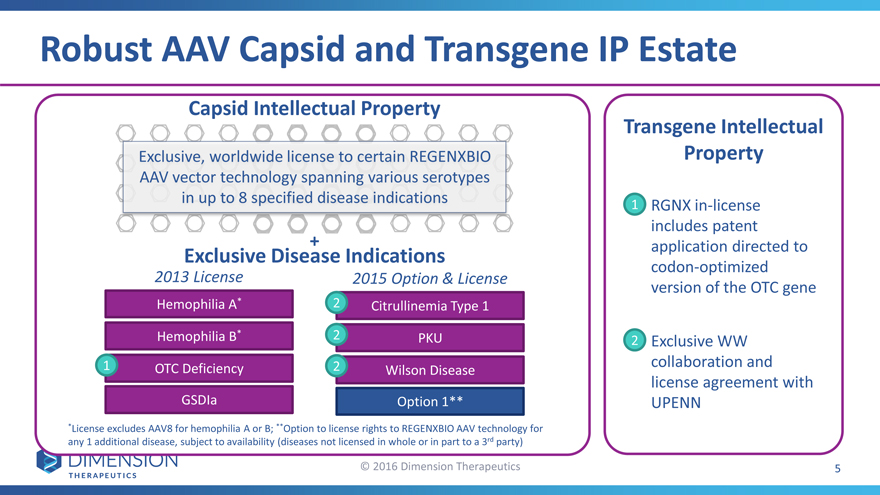
Robust AAV Capsid and Transgene IP Estate
Capsid Intellectual Property
Transgene Intellectual
Exclusive, worldwide license to certain REGENXBIO Property
AAV vector technology spanning various serotypes
in up to 8 specified disease indications 1RGNX in-license
includes patent
+application directed to
Exclusive Disease Indications codon-optimized
2013 License 2015 Option & Licenseversion of the OTC gene
Hemophilia A* 2Citrullinemia Type 1
Hemophilia B* 2PKU2Exclusive WW
1 OTC Deficiency 2Wilson Diseasecollaboration and
license agreement with
GSDIa Option 1**UPENN
*License excludes AAV8 for hemophilia A or B; **Option to license rights to REGENXBIO AAV technology for
any 1 additional disease, subject to availability (diseases not licensed in whole or in part to a 3rd party)
© 2016 Dimension Therapeutics
5
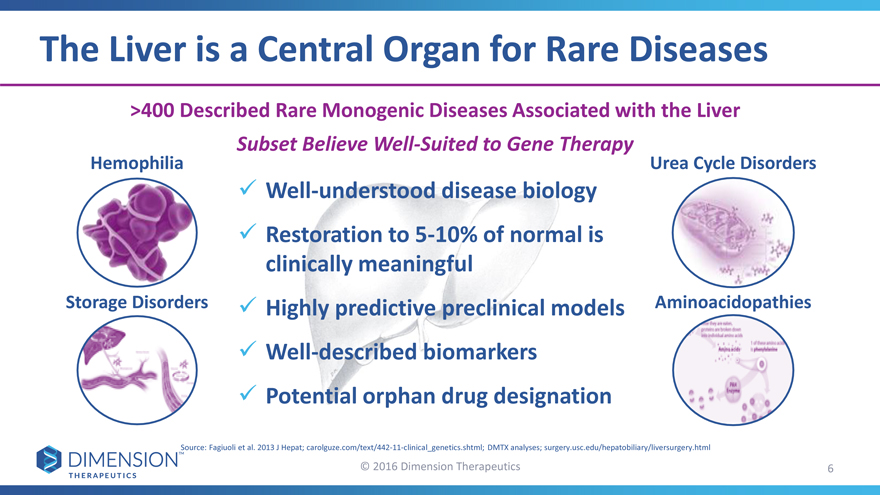
The Liver is a Central Organ for Rare Diseases
>400 Described Rare Monogenic Diseases Associated with the Liver
Subset Believe Well-Suited to Gene Therapy
Hemophilia Urea Cycle Disorders
Well-understood disease biology
Restoration to 5-10% of normal is
clinically meaningful
Storage Disorders Highly predictive preclinical modelsAminoacidopathies
Well-described biomarkers
Potential orphan drug designation
Source: Fagiuoli et al. 2013 J Hepat; carolguze.com/text/442-11-clinical_genetics.shtml; DMTX analyses; surgery.usc.edu/hepatobiliary/liversurgery.html
© 2016 Dimension Therapeutics6
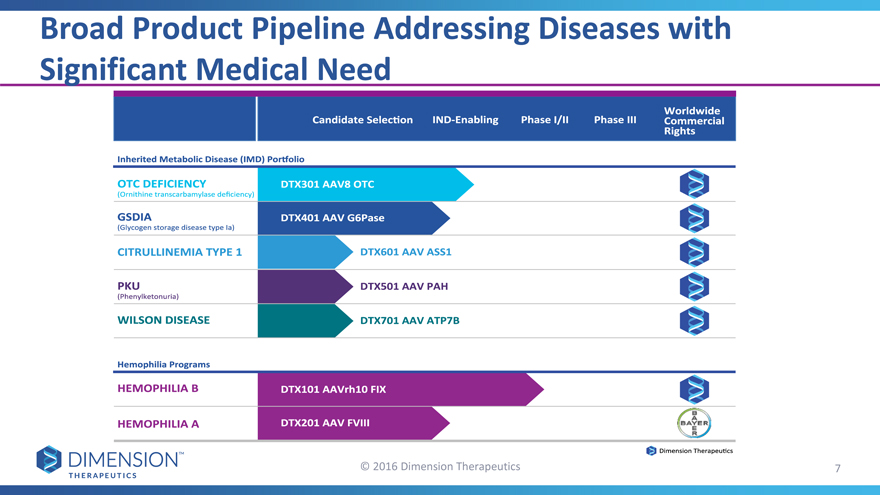
Broad Product Pipeline Addressing Diseases with Significant Medical Need
© 2016 Dimension Therapeutics 7
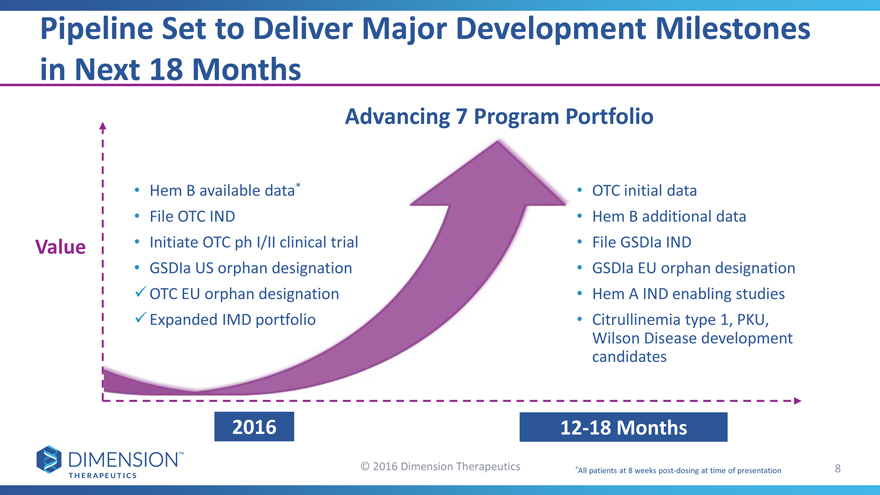
Pipeline Set to Deliver Major Development Milestones in Next 18 Months
Advancing 7 Program Portfolio
Hem B available data*•OTC initial data
File OTC IND•Hem B additional data
Value • Initiate OTC ph I/II clinical trial•File GSDIa IND
GSDIa US orphan designation•GSDIa EU orphan designation
OTC EU orphan designation •Hem A IND enabling studies
Expanded IMD portfolio •Citrullinemia type 1, PKU,
Wilson Disease development
candidates
201612-18 Months
© 2016 Dimension Therapeutics *All patients at 8 weeks post-dosing at time of presentation 8
Inherited Metabolic Disease Programs
© 2016 Dimension Therapeutics 9
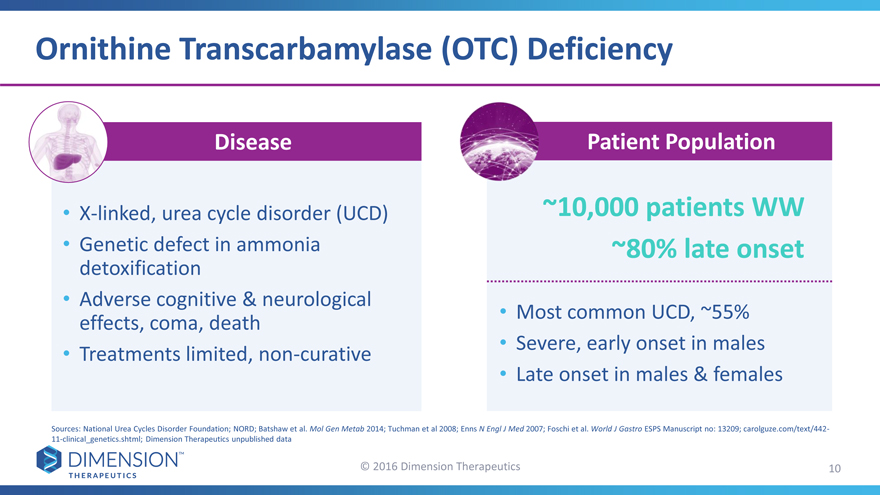
Ornithine Transcarbamylase (OTC) Deficiency
Disease Patient Population
X-linked, urea cycle disorder (UCD)~10,000 patients WW
Genetic defect in ammonia~80% late onset
detoxification
Adverse cognitive & neurological•Most common UCD, ~55%
effects, coma, death
Treatments limited, non-curative•Severe, early onset in males
•Late onset in males & females
Sources: National Urea Cycles Disorder Foundation; NORD; Batshaw et al. Mol Gen Metab 2014; Tuchman et al 2008; Enns N Engl J Med 2007; Foschi et al. World J Gastro ESPS Manuscript no: 13209; carolguze.com/text/442-11-clinical_genetics.shtml; Dimension Therapeutics unpublished data
© 2016 Dimension Therapeutics
10
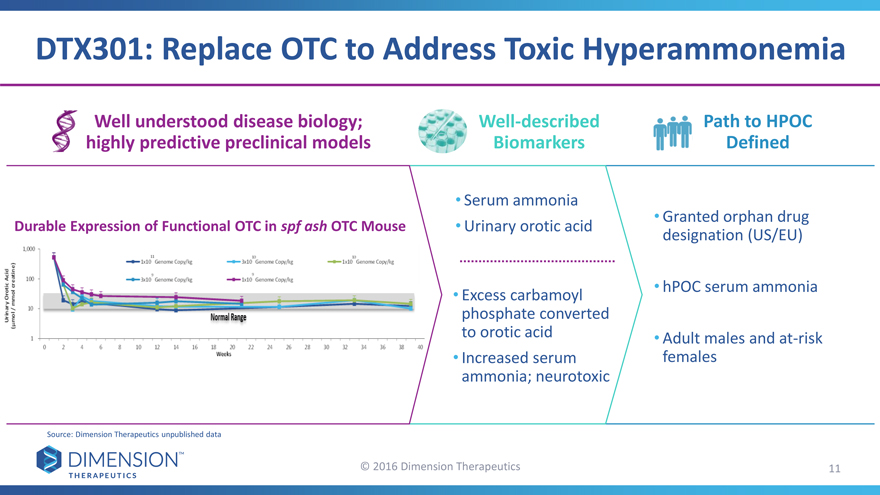
DTX301: Replace OTC to Address Toxic Hyperammonemia
Well understood disease biology; Well-described Path to HPOC
highly predictive preclinical models Biomarkers Defined
• Serum ammonia
Durable Expression of Functional OTC in spf ash OTC Mouse • Urinary orotic acid • Granted orphan drug
designation (US/EU)
• Excess carbamoyl • hPOC serum ammonia
phosphate converted
to orotic acid • Adult males and at-risk
• Increased serum females
ammonia; neurotoxic
Source: Dimension Therapeutics unpublished data
© 2016 Dimension Therapeutics
11
Glycogen Storage Disease Type Ia (GSDIa)
Disease Patient Population
Autosomal recessive, inborn error of~6,000 patients WW
glucose metabolism Dx at birth
Deficient glucose-6-phosphatase
(G6Pase)
Reduced QOL, long term risks•Most common GSD, ~25%
Strict diet and frequent feedings of•No approved drug therapies
uncooked starch or Glycosade®
Sources: Weinstein et al. Hum Gen Ther 2010; Lee et al. Hepatology 2012; Chou et al Nat Rev Endo 2010; Boers et al. Orphanet Journal of Rare Diseases 2014; www.curegsd.org; The Children’s Fund for GSD Research
© 2016 Dimension Therapeutics
12
DTX401: Replace G6Pase to Address Life-Threatening Hypoglycemia
Well understood disease biology; Well-described Path to HPOC
highly predictive preclinical models Biomarkers Defined
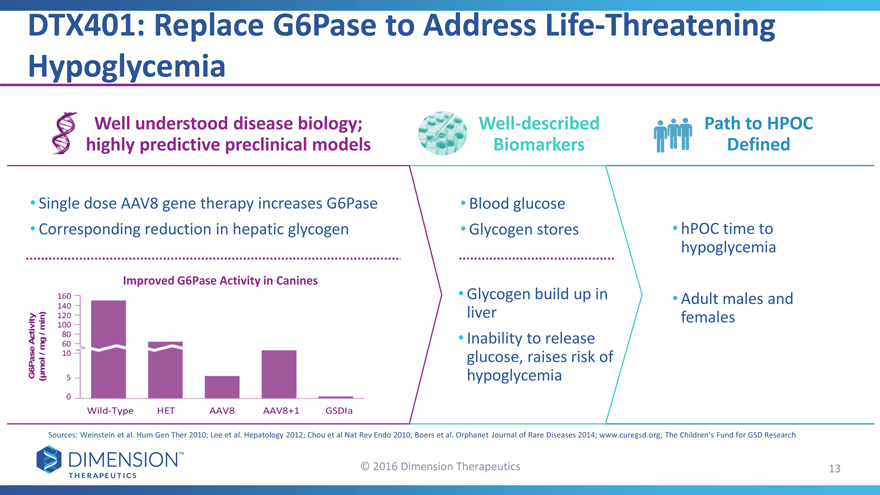
• Single dose AAV8 gene therapy increases G6Pase • Blood glucose
• Corresponding reduction in hepatic glycogen • Glycogen stores • hPOC time to
hypoglycemia
Improved G6Pase Activity in Canines
• Glycogen build up in • Adult males and
liver females
• Inability to release
glucose, raises risk of
hypoglycemia
Sources: Weinstein et al. Hum Gen Ther 2010; Lee et al. Hepatology 2012; Chou et al Nat Rev Endo 2010; Boers et al. Orphanet Journal of Rare Diseases 2014; www.curegsd.org; The Children’s Fund for GSD Research
© 2016 Dimension Therapeutics 13
Broad Inherited Metabolic Disease Portfolio
Well understood disease biology;Well-describedProduct
highly predictive preclinical modelsBiomarkersOpportunity
Wilson • Autosomal recessive copper disorder•WW prevalence >50K
Disease • Deficiency in copper transporter P-• Copperpatients
type ATPase
Autosomal recessive disorder•~50K prevalent patients
PKU • Phenylalanine
Deficiency in phenylalanine hydrolasedeveloped world
Autosomal recessive UCD
Citrullinemia •14% of all UCDs
Deficiency in argininosuccinate• Ammonia
Type 1 •WW prevalence ~2K
synthetase
Sources: Barends MGM 2014; Batshaw MGM 2014; Citrullinemia Type I NCBI Bookshelf; Engel et al Hum Mut 2009; Foschi World J Gastro 2015; Marti?n-Herna?ndez Orph J Rare Dis 2014; NORD; Ruder Ped Neuro 2014; Ruegger J Inherit Metab Dis 2014; Summar Acta Paediatr. 2008; Summar Crit Care Clin 2005; Tuchman et al Mol Genet Metab 2008; Berry Genet Med 2013; Hanley J Genet Disor Genet Rep 2013; Vockley Gen Med 2014; Zerjav Orph J Rare Dis 2015; Beinhardt Clin Gast Hepat 2014; Bull Nat Gen 1993; EASL Prac Guide; Gomes Ann Hum Bio 2015; Kaler Nat Rev Neurol 2011; Roberts & Schilsky Hepat 2008; Schilsky Biochimie 2009; WD Pathogenesis Semin Liver Dis 2000
© 2016 Dimension Therapeutics 14
Hemophilia Programs
© 2016 Dimension Therapeutics 15
~$6.2B WW Market for Hemophilia B and A Products
Disease Patient Population
Deficiency of blood coagulation~28,000 Hem B patients WW
factor IX (FIX) or factor VIII (FVIII) ~140,000 Hem A patients WW
Joint hemorrhages, potential for
severe disabilities; brain micro- •~68% Hem B, 78% Hem A
bleeds moderate to severe disease
Frequent, invasive, non-curative
infusions of factor replacement •$100,000-$300,000 annual costs
per patient
Sources: Riley et al. Haemophilia 2011; National Hemophilia Foundation; World Federation of Hemophilia; www.fiercepharmamarketing.com/story/biogens-alprolix-nod-just-first-tremor-hemophilia-market-shake/2014-04-02; Stonebraker et al. Haemophilia (2011), 1–4; Baker et al. Haemophilia (2012); Stonebraker et al. Haemophilia (2010), 16, 20–32; National Hemophilia Foundation; www.ptcommunity.com/news/2014-12-29-000000/hemophilia-market-likely-undergo-major-changes; Johnson and Zhou. ASH Annual Meeting Educational Program. 2011; Ponder. Haemophilia 2008; Manco-Johnson et al. NEJM 2007
© 2016 Dimension Therapeutics 16
DTX101 for Hemophilia B
DTX101 Phase I/II Study Ongoing
• Vector capsid Clade E AAVrh10 • Open-Label, safety, dose finding study
• Gene cassette wild type factor IX • Bayesian adaptive design
• Liver specific enhancer-promoter • Primary endpoints safety & FIX activity
• HEK293 process for hPOC • Up to 12 males with moderate/severe to
severe Hem B (?2% FIX levels)
• TPP* targeting stable FIX activity >10%
Dosing: 1.6e12-1e13 GC/kg, single
• Reduce dependency for on demand & peripheral venous infusion
prophylactic FIX therapies
Cohort 1 dosing complete
© 2016 Dimension Therapeutics * TPP: Target Product Profile 17
DTX201: Global Collaboration with Bayer
Hemophilia A
A leading gene therapy Global leader in hemophilia
company drug development and
commercialization
• $20M upfront, up to $232M in milestones
• High single-digit to low double-digit royalties not exceeding the mid-teens
• R&D expense reimbursement
• DMTX responsible for preclinical activities and Phase I/II clinical trial, funded by Bayer
• Bayer to fund subsequent trials and commercial activities at clinical POC
© 2016 Dimension Therapeutics 18
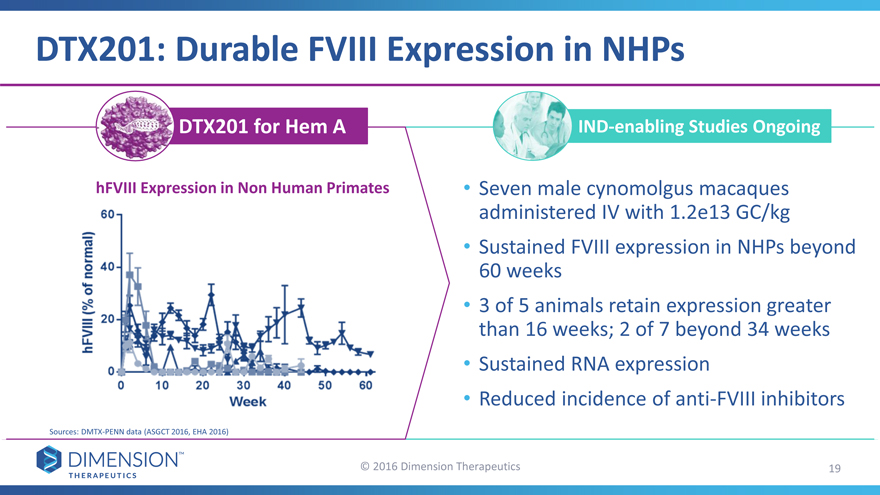
DTX201: Durable FVIII Expression in NHPs
DTX201 for Hem A IND-enabling Studies Ongoing
hFVIII Expression in Non Human Primates • Seven male cynomolgus macaques
administered IV with 1.2e13 GC/kg
Sustained FVIII expression in NHPs beyond
60 weeks
3 of 5 animals retain expression greater
than 16 weeks; 2 of 7 beyond 34 weeks
Sustained RNA expression
Reduced incidence of anti-FVIII inhibitors
Sources: DMTX-PENN data (ASGCT 2016, EHA 2016)
© 2016 Dimension Therapeutics 19
Dimension: A Leader in Liver Directed Gene Therapy
Advancing Product Portfolio Broad 7 program pipeline delivering multiple near-term
milestones
Focus on Inherited Metabolic Diseases Addressing severe, debilitating unmet medical need
Building Gene Therapy Product Platform Investing in scalable, quality HeLa manufacturing
Highly Experienced Team Strong R&D and manufacturing capabilities
Well-Capitalized Expected cash runway through 1Q 2018
© 2016 Dimension Therapeutics 20
Dimension Therapeutics, Inc.
Quality of Science. Quality of Life.
August 2016