Exhibit 99.1
Corporate Presentation
Juno Therapeutics Proprietary Materials September 2015
Forward-Looking Statements
This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties, and assumptions. If
the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by
such forward-looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited
to, any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest
trends for our business; any statements of the plans, strategies, and objectives of management for future operations, including our manufacturing
and process development; any statements of expectation or belief regarding future events, potential markets or market size, technology
developments, our product pipeline, clinical data, enforceability of our intellectual property rights, competitive strengths or our position within the
industry; any statements regarding the anticipated benefits of our Celgene collaboration or other strategic transactions; and any statements of
assumptions underlying any of the items mentioned.
These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future
performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited
to, risks associated with: the success, cost, and timing of our product development activities and clinical trials; our ability to obtain regulatory
approval for and to commercialize our product candidates; our ability to establish a commercially-viable manufacturing process and manufacturing
infrastructure; regulatory requirements and regulatory developments; the effects of competition and technological advances; our dependence on
third-party collaborators and other contractors in our research and development activities, including for the conduct of clinical trials and the
manufacture of our product candidates; our dependence on Celgene for the development and commercialization outside of North America of product
candidates for which Celgene exercises an option; our ability to obtain, maintain, or protect intellectual property rights related to our product
candidates; among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in
these forward-looking statements, as well as risks relating to our business in general, see our Quarterly Report on Form 10-Q filed with the
Securities and Exchange Commission on August 14, 2015 and our other periodic reports filed from time to time with the Securities and Exchange
Commission. Except as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these
statements to actual results or to changes in our expectations.
Juno Therapeutics Proprietary Materials
Re-engaging the immune system to revolutionize medicine
Founded on the premise that engineered T cells will revolutionize cancer treatments and medicine
Investing in pipeline and platform that will extend reach to a broader array of cancers
Enrolling first pivotal trial with potential FDA filing in late 2016/early 2017
Ten therapeutic candidates against six targets in human testing by early 2016 using either CAR or TCR technologies
Celgene collaboration drives global footprint, expands capabilities, and offers new pipeline growth opportunities
Deep experience and a significant investment in process development and manufacturing
Juno Therapeutics Proprietary Materials
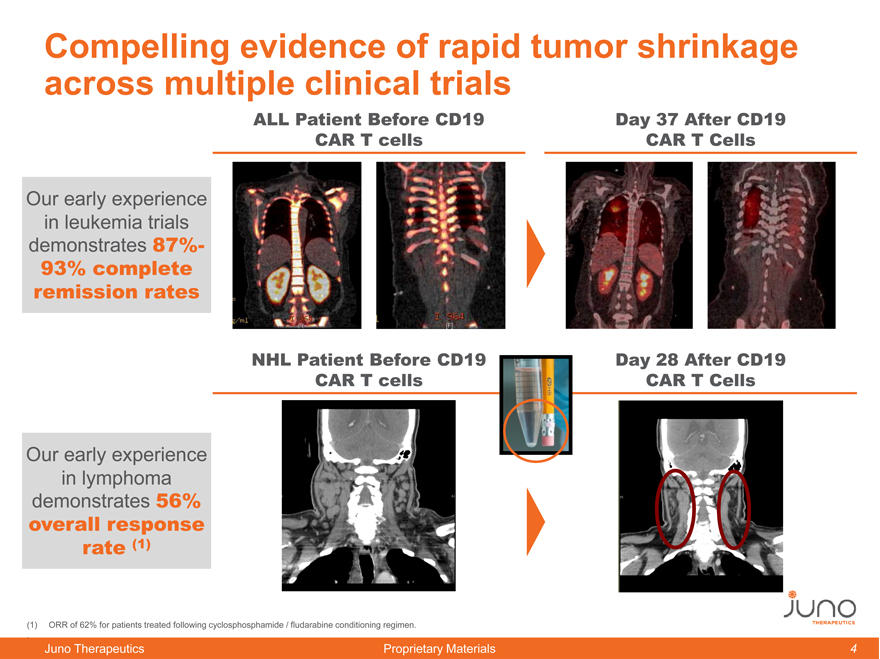
Compelling evidence of rapid tumor shrinkage across multiple clinical trials
ALL Patient Before CD19 Day 37 After CD19 CAR T cells CAR T Cells
Our early experience in leukemia trials demonstrates 87%-93% complete remission rates
Our early experience in lymphoma demonstrates 56% overall response rate (1)
NHL Patient Before CD19 CAR T cells ymphoma)w
Day 28 After CD19
nced)Lymphoma)with)CD19) CAR T Cells AR)T) ells))
(1) | | ORR of 62% for patients treated following cyclosphosphamide / fludarabine conditioning regimen. |
Juno Therapeutics Proprietary Materials
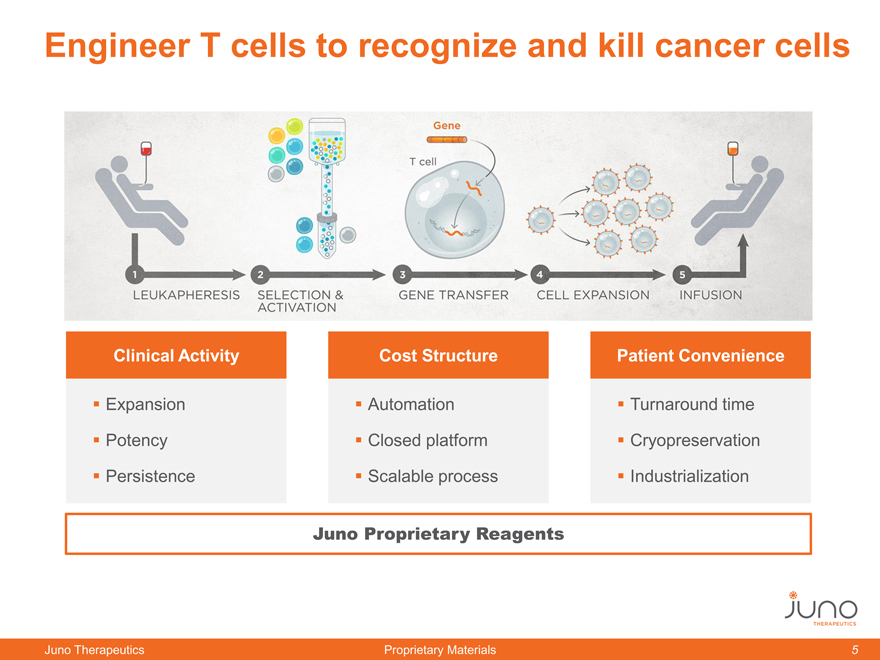
Engineer T cells to recognize and kill cancer cells
Clinical Activity Cost Structure Patient Convenience
Expansion Potency Persistence
Automation Closed platform Scalable process
Turnaround time Cryopreservation Industrialization
Juno Proprietary Reagents
Juno Therapeutics Proprietary Materials
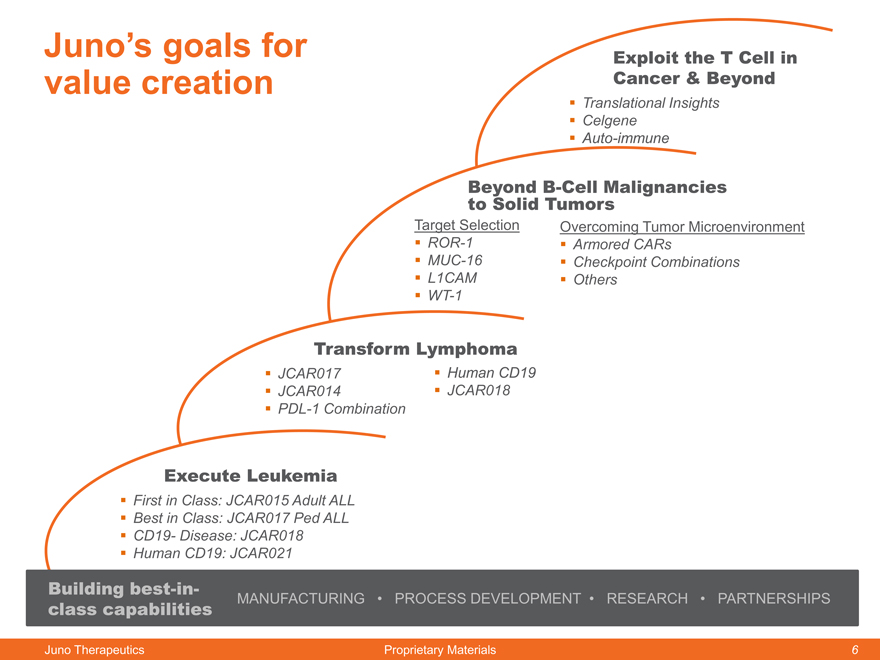
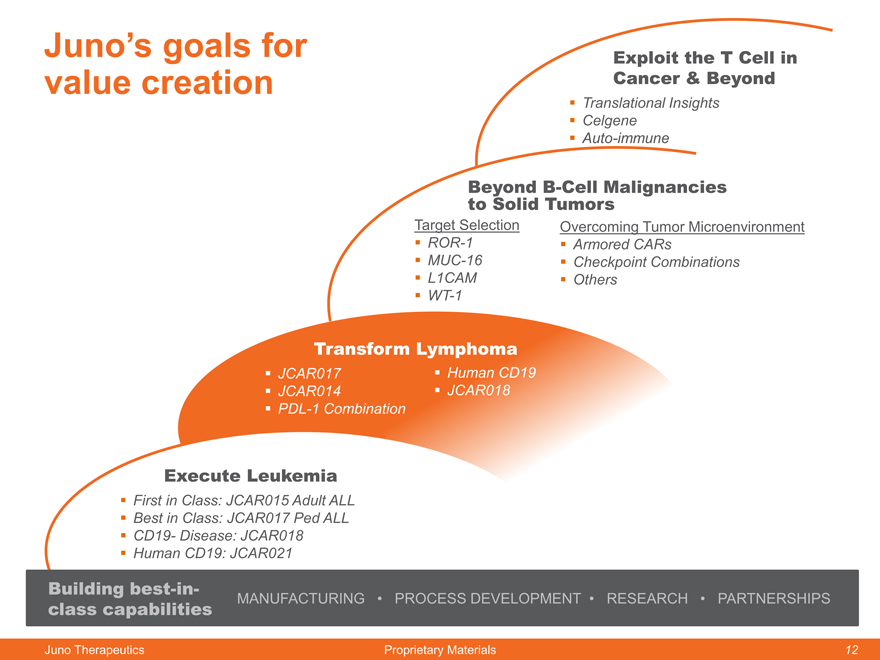
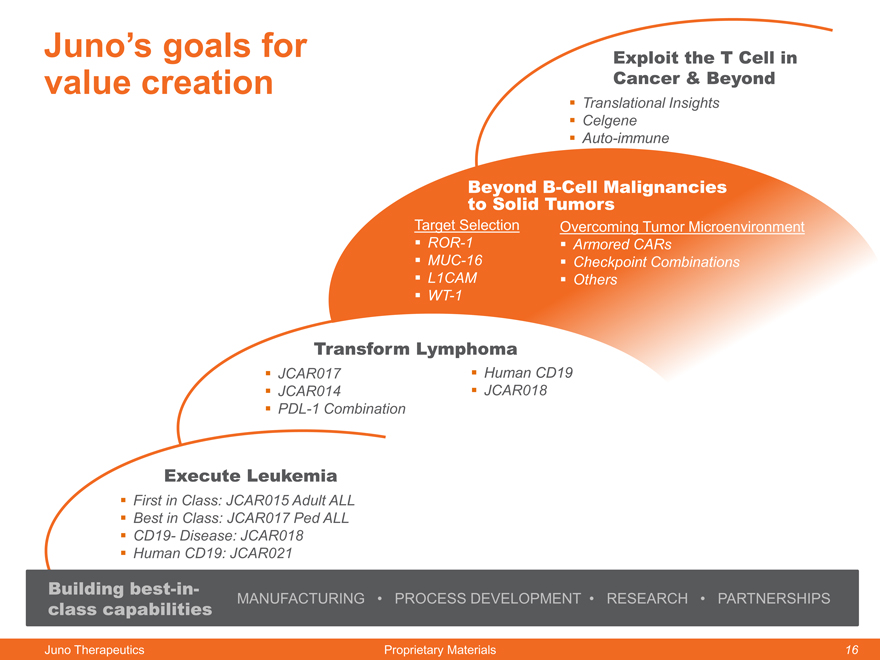
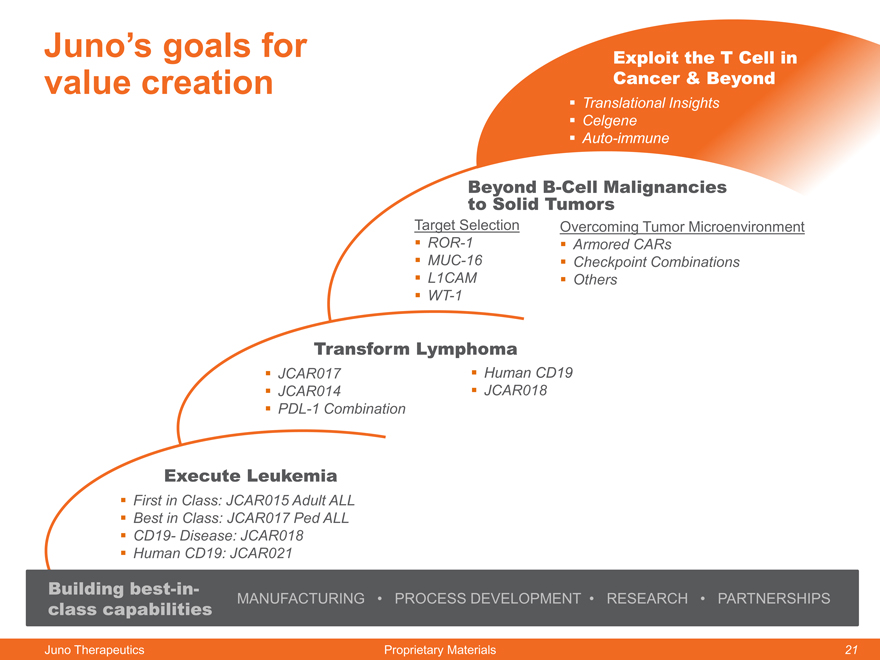
Juno’s goals for value creation
Exploit the T Cell in Cancer & Beyond
Juno’s goals for Exploit the T Cell in
value creation Cancer & Beyond
Translational Insights
Celgene
Auto-immune
Beyond B-Cell Malignancies
to Solid Tumors
Target Selection Overcoming Tumor Microenvironment
ROR-1 Armored CARs
MUC-16 Checkpoint Combinations
L1CAM Others
WT-1
Transform Lymphoma
JCAR017 Human CD19
JCAR014 JCAR018
PDL-1 Combination
Execute Leukemia
First in Class: JCAR015 Adult ALL
Best in Class: JCAR017 Ped ALL
CD19- Disease: JCAR018
Human CD19: JCAR021
Building best-in-
class capabilities MANUFACTURING PROCESS DEVELOPMENT RESEARCH PARTNERSHIPS
Juno Therapeutics Proprietary Materials
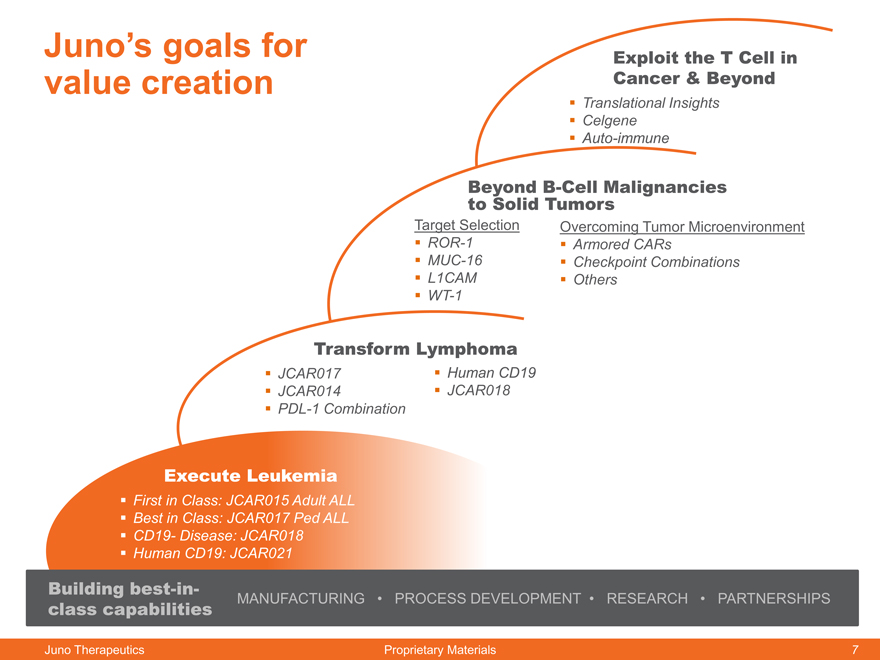
Juno’s goals for Exploit the T Cell in
value creation Cancer & Beyond
Translational Insights
Celgene
Auto-immune
Beyond B-Cell Malignancies
to Solid Tumors
Target Selection Overcoming Tumor Microenvironment
ROR-1 Armored CARs
MUC-16 Checkpoint Combinations
L1CAM Others
WT-1
Transform Lymphoma
JCAR017 Human CD19
JCAR014 JCAR018
PDL-1 Combination
Execute Leukemia
First in Class: JCAR015 Adult ALL
Best in Class: JCAR017 Ped ALL
CD19- Disease: JCAR018
Human CD19: JCAR021
Building best-in-
class capabilities MANUFACTURING PROCESS DEVELOPMENT RESEARCH PARTNERSHIPS
Juno Therapeutics Proprietary Materials
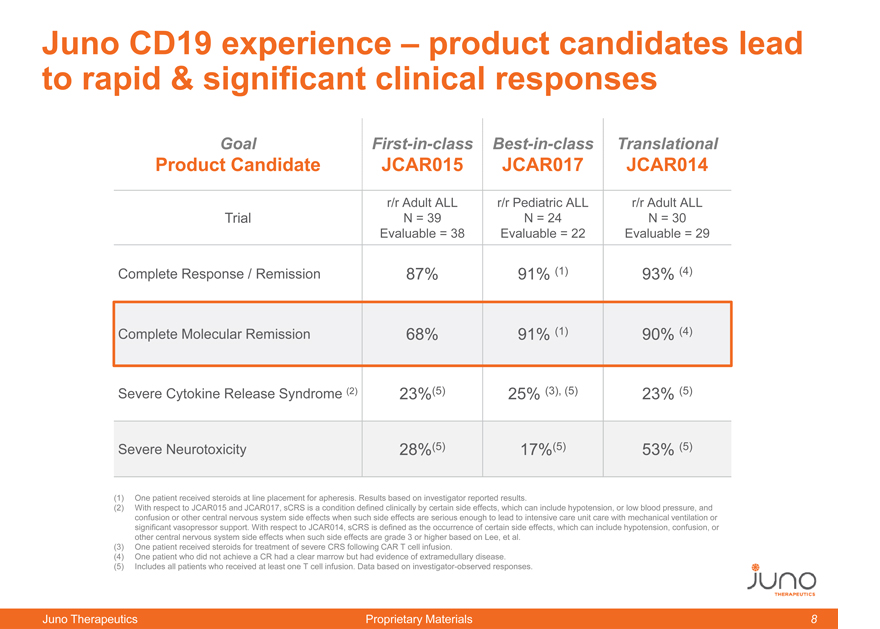
Juno CD19 experience – product candidates lead
to rapid & significant clinical responses
Goal First-in-class Best-in-class Translational
Product Candidate JCAR015 JCAR017 JCAR014
r/r Adult ALL r/r Pediatric ALL r/r Adult ALL
Trial N = 39 N = 24 N = 30
Evaluable = 38 Evaluable = 22 Evaluable = 29
Complete Response / Remission 87% 91% (1) 93% (4)
Complete Molecular Remission 68% 91% (1) 90% (4)
Severe Cytokine Release Syndrome (2) 23%(5) 25% (3)(5) 23% (5)
Severe Neurotoxicity 28%(5) 17%(5) 53% (5)
(1) | | One patient received steroids at line placement for apheresis. Results based on investigator reported results. |
(2) With respect to JCAR015 and JCAR017, sCRS is a condition defined clinically by certain side effects, which can include hypotension, or low blood pressure, and
confusion or other central nervous system side effects when such side effects are serious enough to lead to intensive care unit care with mechanical ventilation or
significant vasopressor support. With respect to JCAR014, sCRS is defined as the occurrence of certain side effects, which can include hypotension, confusion, or
other central nervous system side effects when such side effects are grade 3 or higher based on Lee, et al.
(3) | | One patient received steroids for treatment of severe CRS following CAR T cell infusion. |
(4) | | One patient who did not achieve a CR had a clear marrow but had evidence of extramedullary disease. |
(5) | | Includes all patients who received at least one T cell infusion. Data based on investigator-observed responses. |
Juno Therapeutics Proprietary Materials
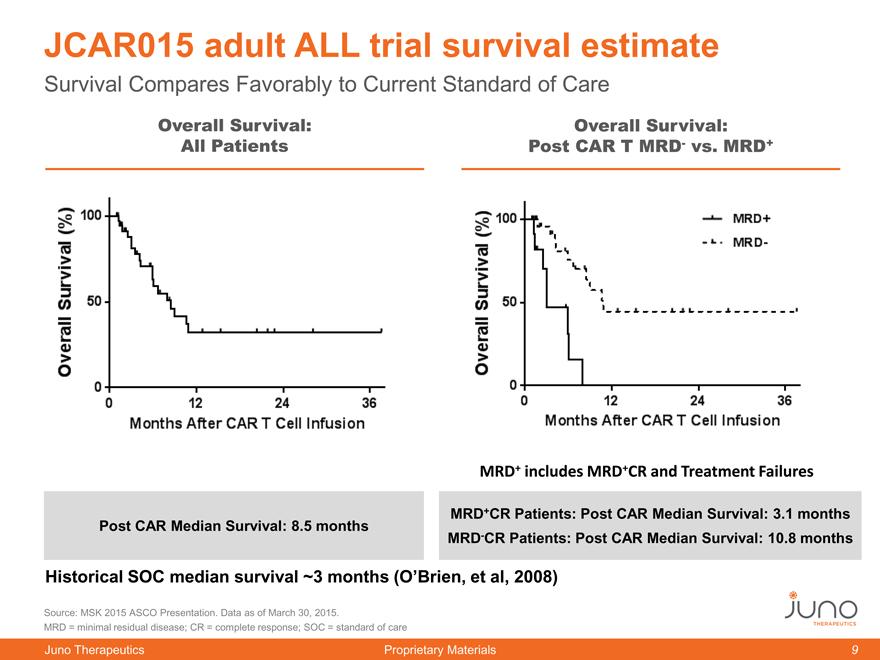
JCAR015 adult ALL trial survival estimate
Survival Compares Favorably to Current Standard of Care
Overall Survival: Overall Survival:
All Patients Post CAR T MRD- vs. MRD+
MRD+ includes MRD+CR and Treatment Failures
MRD+CR Patients: Post CAR Median Survival: 3.1 months
Post CAR Median Survival: 8.5 months
MRD-CR Patients: Post CAR Median Survival: 10.8 months
Historical SOC median survival ~3 months (O’Brien, et al, 2008)
Source: MSK 2015 ASCO Presentation. Data as of March 30, 2015.
MRD = minimal residual disease; CR = complete response; SOC = standard of care
Juno Therapeutics Proprietary Materials
9
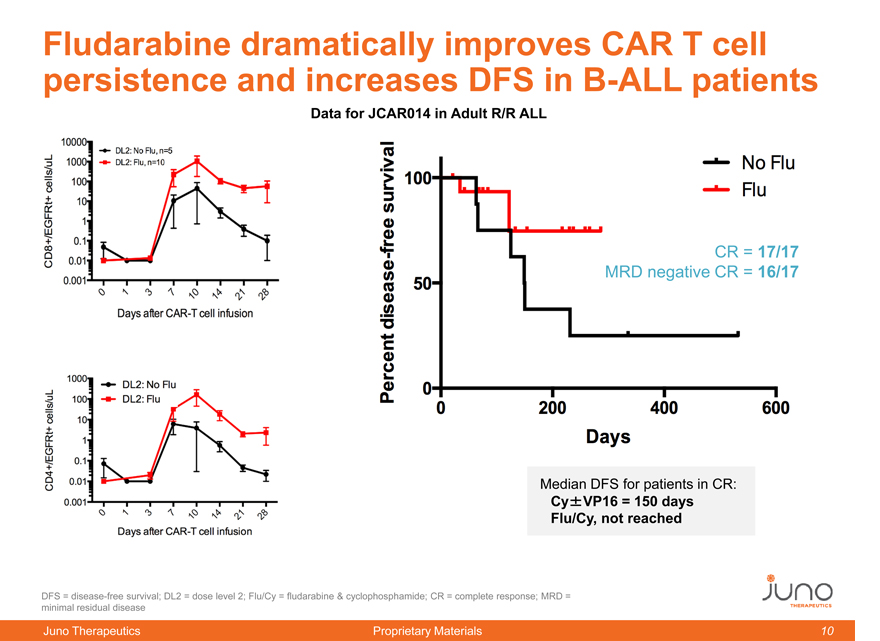
Fludarabine dramatically improves CAR T cell
persistence and increases DFS in B-ALL patients
Data for JCAR014 in Adult R/R ALL
CR = 17/17
MRD negative CR = 16/17
Median DFS for patients in CR:
Cy±VP16 = 150 days
Flu/Cy, not reached
DFS = disease-free survival; DL2 = dose level 2;
Flu/Cy = fludarabine & cyclophosphamide; CR = complete response; MRD = minimal residual disease
Juno Therapeutics Proprietary Materials
10
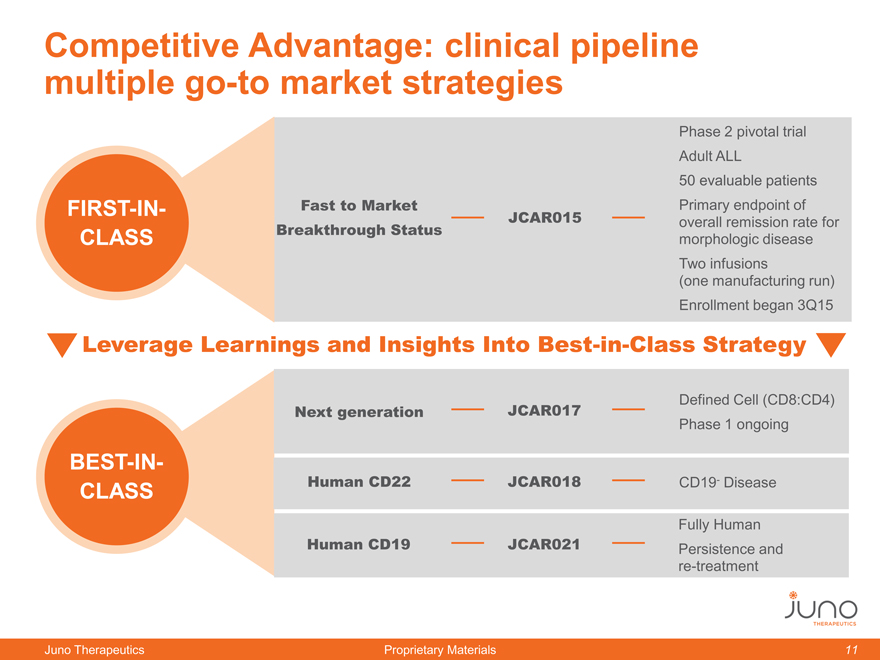
Competitive Advantage: clinical pipeline
multiple go-to market strategies
Phase 2 pivotal trial
Adult ALL
50 evaluable patients
FIRST-IN- Fast to Market Primary endpoint of
Breakthrough Status JCAR015 overall remission rate for
CLASS morphologic disease
Two infusions
(one manufacturing run)
Enrollment began 3Q15
Leverage Learnings and Insights Into Best-in-Class Strategy
Next generation JCAR017 Defined Cell (CD8:CD4)
Phase 1 ongoing
BEST-IN-
CLASS Human CD22 JCAR018 CD19- Disease
Fully Human
Human CD19 JCAR021 Persistence and
re-treatment
Juno Therapeutics Proprietary Materials
11
Juno’s goals for Exploit the T Cell in
value creation Cancer & Beyond
Translational Insights
Celgene
Auto-immune
Beyond B-Cell Malignancies
to Solid Tumors
Target Selection Overcoming Tumor Microenvironment
ROR-1 Armored CARs
MUC-16 Checkpoint Combinations
L1CAM Others
WT-1
Transform Lymphoma
JCAR017 Human CD19
JCAR014 JCAR018
PDL-1 Combination
Execute Leukemia
First in Class: JCAR015 Adult ALL
Best in Class: JCAR017 Ped ALL
CD19- Disease: JCAR018
Human CD19: JCAR021
Building best-in-
class capabilities MANUFACTURING PROCESS DEVELOPMENT RESEARCH PARTNERSHIPS
Juno Therapeutics Proprietary Materials
12
Non-Hodgkin Lymphoma – The Next Significant Frontier
Despite significant progress, NHL remains a large unmet need with over 19,000 deaths in the U.S. annually.
NHL is a CD19 positive disease. We, and others, have shown that CD19 directed CAR T cells can rapidly shrink NHL tumors.
Keys to durable, long-term remissions appear to be improving cell expansion, improving cell persistence, and overcoming the tumor micro-environment.
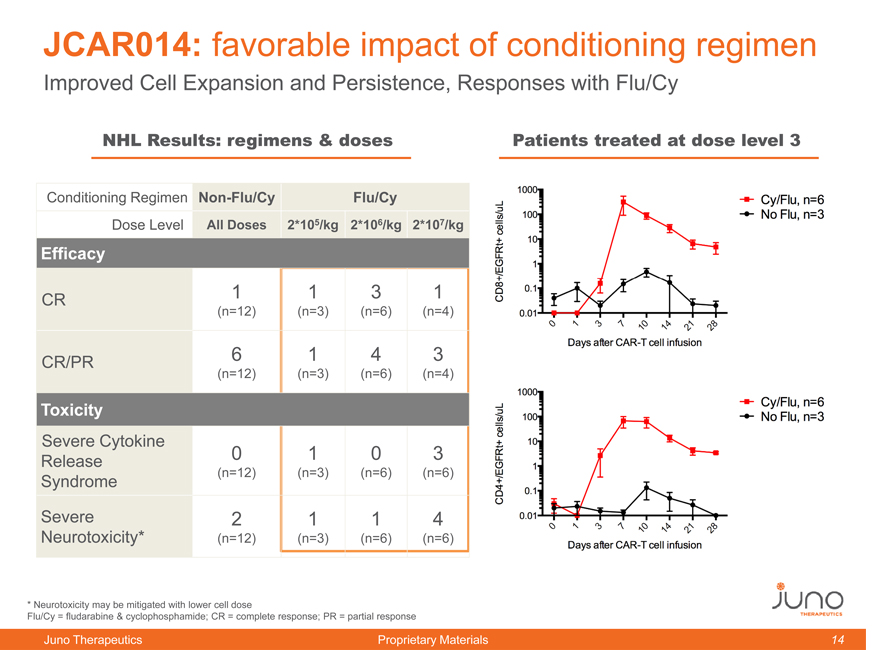
JCAR014 with improved conditioning regimen leads to a marked improvement in cell expansion and cell persistence, translating into early improvement in response rate.
Multi-pronged strategy to create a best-in-class NHL product.
Biology and progress suggest NHL efficacy has the potential to improve dramatically over time.
Juno Therapeutics Proprietary Materials
13
JCAR014: favorable impact of conditioning regimen
Improved Cell Expansion and Persistence, Responses with Flu/Cy
NHL Results: regimens & doses Patients treated at dose level 3
Conditioning Regimen Non-Flu/Cy Flu/Cy
Dose Level All Doses 2*105/kg 2*106/kg 2*107/kg
Efficacy
CR 1 1 3 1
(n=12) (n=3) (n=6) (n=4)
CR/PR 6 1 4 3
(n=12) (n=3) (n=6) (n=4)
Toxicity
Severe Cytokine Release 0 1 0 3
Syndrome (n=12) (n=3) (n=6) (n=6)
Severe 2 1 1 4
Neurotoxicity* (n=12) (n=3) (n=6) (n=6)
* | | Neurotoxicity may be mitigated with lower cell dose |
Flu/Cy = fludarabine & cyclophosphamide; CR = complete response; PR = partial response
Juno Therapeutics Proprietary Materials
14
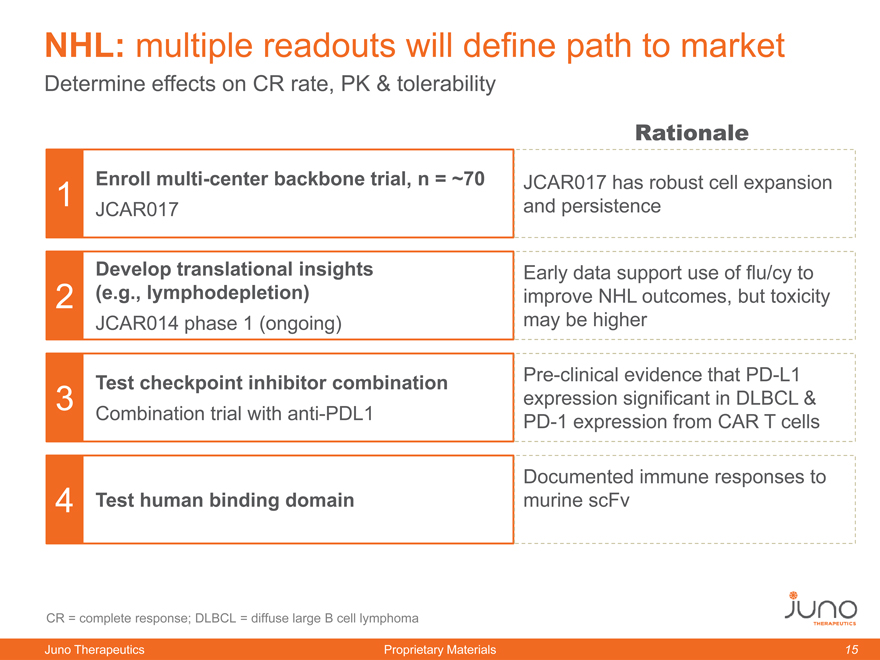
NHL: multiple readouts will define path to market
Determine effects on CR rate, PK & tolerability
Rationale
1 | | Enroll multi-center backbone trial, n =70 JCAR017 has robust cell expansion |
JCAR017 and persistence
Develop translational insights Early data support use of flu/cy to
2 | | (e.g., lymphodepletion) improve NHL outcomes, but toxicity |
JCAR014 phase 1 (ongoing) may be higher
Test checkpoint inhibitor combination Pre-clinical evidence that PD-L1
3 | | expression significant in DLBCL & |
Combination trial with anti-PDL1 PD-1 expression from CAR T cells
Documented immune responses to
4 | | Test human binding domain murine scFv |
CR = complete response; DLBCL = diffuse large B cell lymphoma
Juno Therapeutics Proprietary Materials
15
Juno’s goals for Exploit the T Cell in
value creation Cancer & Beyond
Translational Insights
Celgene
Auto-immune
Beyond B-Cell Malignancies
to Solid Tumors
Target Selection Overcoming Tumor Microenvironment
ROR-1 Armored CARs
MUC-16 Checkpoint Combinations
L1CAM Others
WT-1
Transform Lymphoma
JCAR017 Human CD19
JCAR014 JCAR018
PDL-1 Combination
Execute Leukemia
First in Class: JCAR015 Adult ALL
Best in Class: JCAR017 Ped ALL
CD19- Disease: JCAR018
Human CD19: JCAR021
Building best-in-
class capabilities MANUFACTURING PROCESS DEVELOPMENT RESEARCH PARTNERSHIPS
Juno Therapeutics Proprietary Materials
16
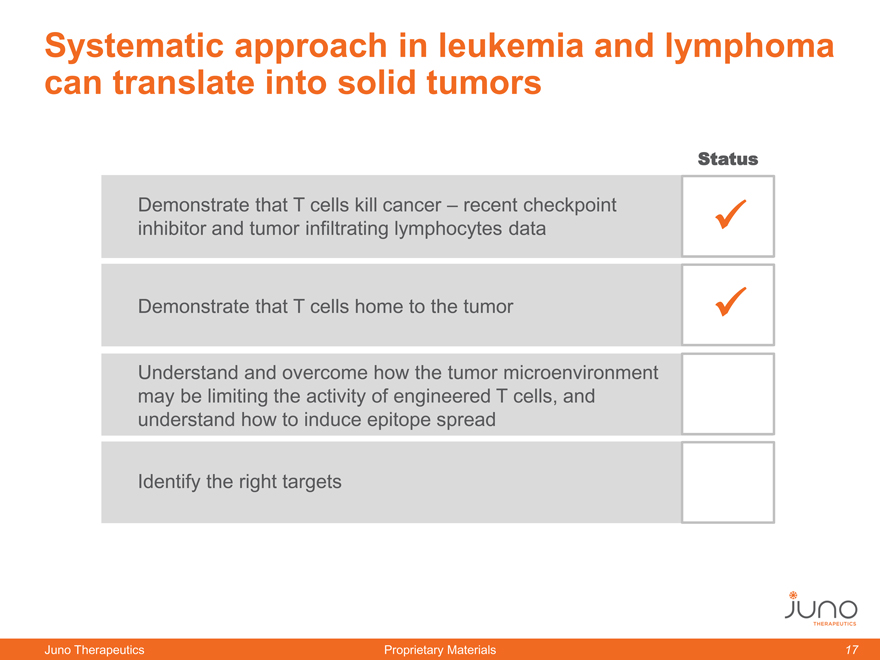
Systematic approach in leukemia and lymphoma
can translate into solid tumors
Status
Demonstrate that T cells kill cancer – recent checkpoint
inhibitor and tumor infiltrating lymphocytes data
Demonstrate that T cells home to the tumor
Understand and overcome how the tumor microenvironment
may be limiting the activity of engineered T cells, and
understand how to induce epitope spread
Identify the right targets
Juno Therapeutics Proprietary Materials
17
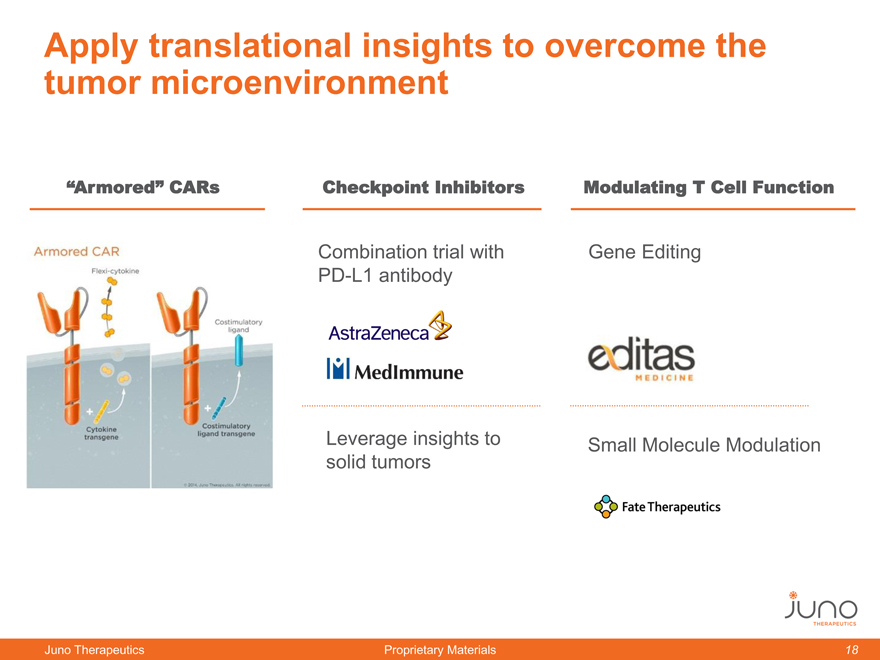
Apply translational insights to overcome the
tumor microenvironment
“Armored” CARs Checkpoint Inhibitors Modulating T Cell Function
Combination trial with Gene Editing
PD-L1 antibody
Leverage insights to Small Molecule Modulation
solid tumors
Juno Therapeutics Proprietary Materials
18
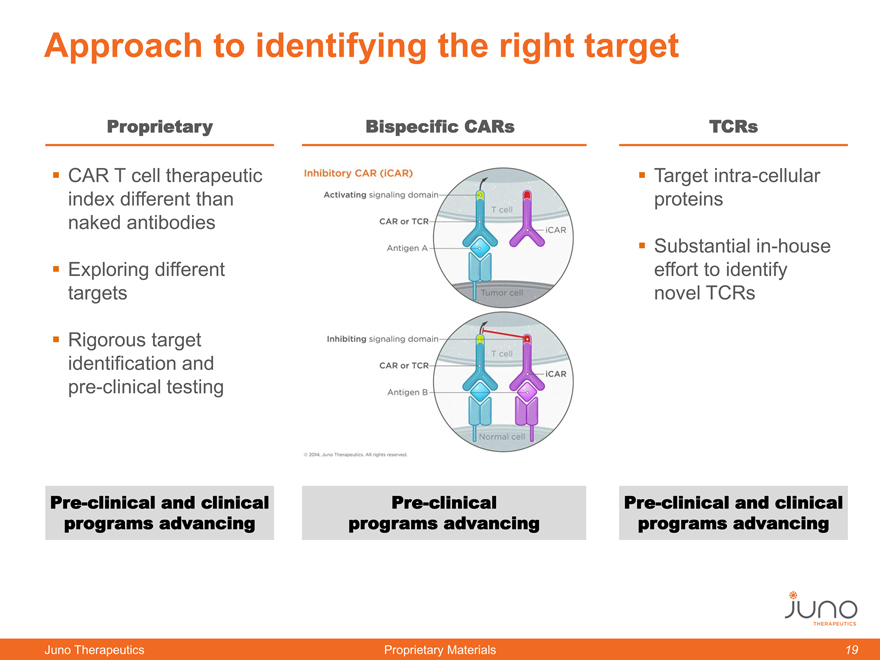
Approach to identifying the right target
Proprietary Bispecific CARs TCRs
CAR T cell therapeutic Target intra-cellular
index different than proteins
naked antibodies
Substantial in-house
Exploring different effort to identify
targets novel TCRs
Rigorous target
identification and
pre-clinical testing
Pre-clinical and clinical Pre-clinical Pre-clinical and clinical
programs advancing programs advancing programs advancing
Juno Therapeutics Proprietary Materials
19
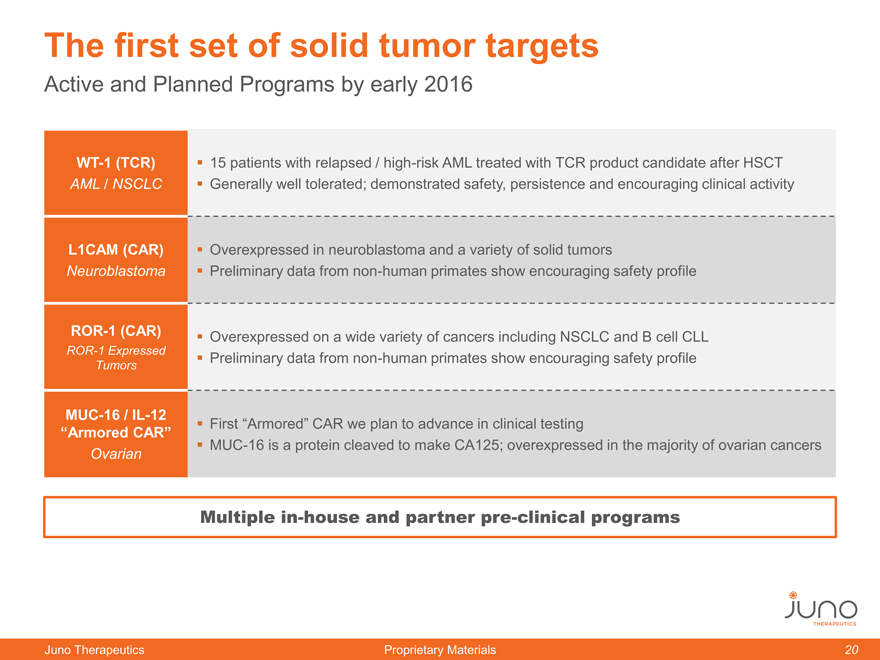
The first set of solid tumor targets
Active and Planned Programs by early 2016
WT-1 (TCR) 15 patients with relapsed / high-risk AML treated with TCR product candidate after HSCT
AML / NSCLC Generally well tolerated; demonstrated safety, persistence and encouraging clinical activity
L1CAM (CAR) Overexpressed in neuroblastoma and a variety of solid tumors
Neuroblastoma Preliminary data from non-human primates show encouraging safety profile
ROR-1 (CAR) Overexpressed on a wide variety of cancers including NSCLC and B cell CLL
ROR-1 Expressed Preliminary data from non-human primates show encouraging safety profile
Tumors
MUC-16 / IL-12 First “Armored” CAR we plan to advance in clinical testing
“Armored CAR”
Ovarian MUC-16 is a protein cleaved to make CA125; overexpressed in the majority of ovarian cancers
Multiple in-house and partner pre-clinical programs
Juno Therapeutics Proprietary Materials
20
Juno’s goals for Exploit the T Cell in
value creation Cancer & Beyond
Translational Insights
Celgene
Auto-immune
Beyond B-Cell Malignancies
to Solid Tumors
Target Selection Overcoming Tumor Microenvironment
ROR-1 Armored CARs
MUC-16 Checkpoint Combinations
L1CAM Others
WT-1
Transform Lymphoma
JCAR017 Human CD19
JCAR014 JCAR018
PDL-1 Combination
Execute Leukemia
First in Class: JCAR015 Adult ALL
Best in Class: JCAR017 Ped ALL
CD19- Disease: JCAR018
Human CD19: JCAR021
Building best-in-
class capabilities MANUFACTURING PROCESS DEVELOPMENT RESEARCH PARTNERSHIPS
Juno Therapeutics Proprietary Materials
21
Our consolidation of multiple technologies is a
competitive advantage in this evolving field
FOUNDING ACADEMIC STRATEGIC TECHNOLOGY & ACQUISITIONS
INSTITUTIONS INSTITUTIONS PARTNER DEVELOPMENT
PARTNERS
Opus Bio
Juno Therapeutics Proprietary Materials
22
Solidifying leadership in the development of
transformative immunotherapies
EXPANDED CAPABILITIES ACCELERATED PIPELINE
Cell therapies, small molecules, and proteins Deep translational capabilities
Immuno-oncology combinations Global commercialization
BUSINESS DEVELOPMENT PIPELINE ENHANCEMENT
COOPERATION
A partner of choice for cellular immunotherapies Access to an array of Celgene pipeline assets
Enhanced capabilities to identify and add value to over time
immuno-oncology assets more broadly Potential to exploit T cell biology more broadly
Juno Therapeutics Proprietary Materials
23
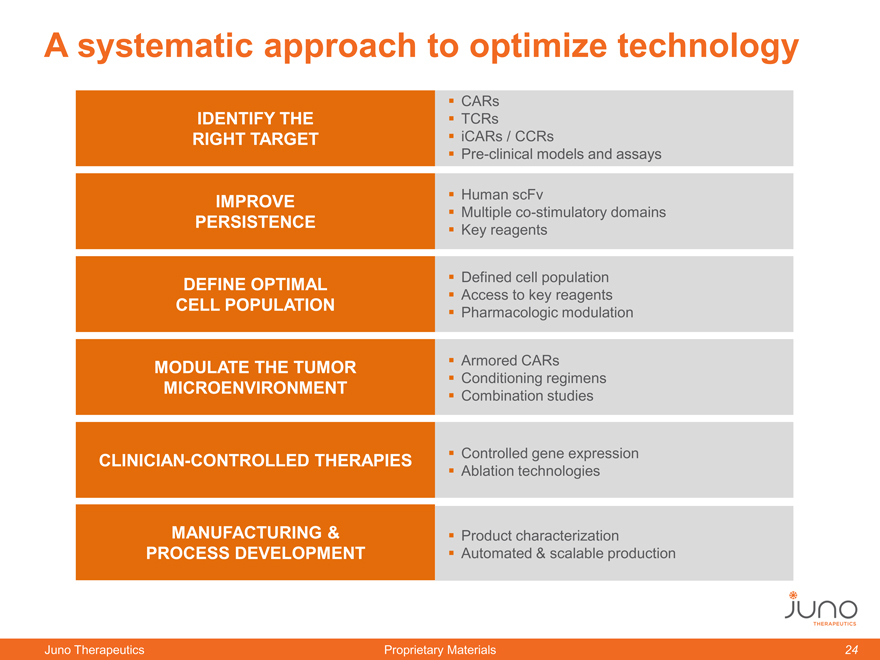
A systematic approach to optimize technology
CARs
IDENTIFY THE TCRs
RIGHT TARGET iCARs / CCRs
Pre-clinical models and assays
IMPROVE Human scFv
Multiple co-stimulatory domains
PERSISTENCE Key reagents
DEFINE OPTIMAL Defined cell population
Access to key reagents
CELL POPULATION Pharmacologic modulation
MODULATE THE TUMOR Armored CARs
Conditioning regimens
MICROENVIRONMENT Combination studies
CLINICIAN-CONTROLLED THERAPIES Controlled gene expression
Ablation technologies
MANUFACTURING & Product characterization
PROCESS DEVELOPMENT Automated & scalable production
Juno Therapeutics Proprietary Materials
24
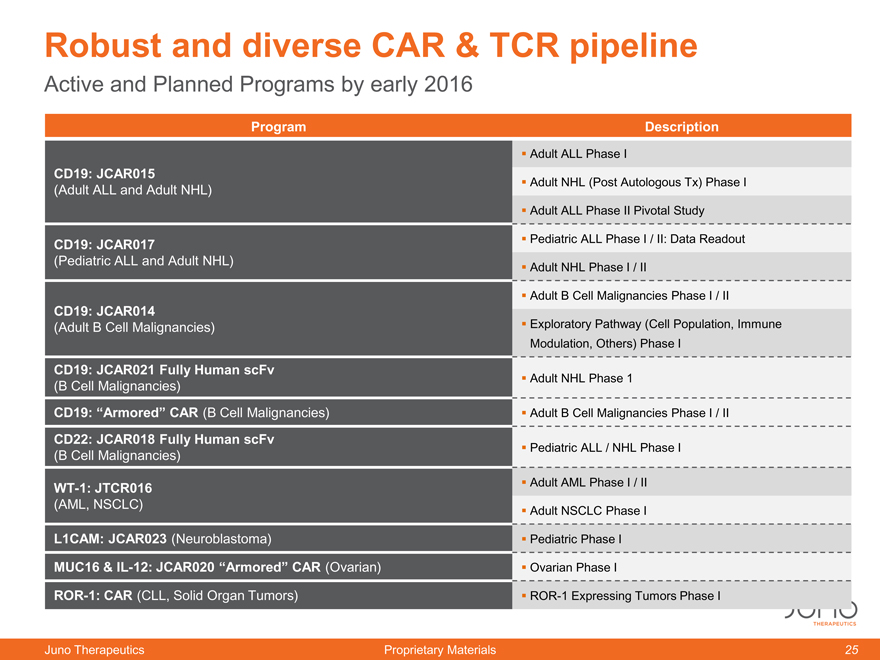
Robust and diverse CAR & TCR pipeline
Active and Planned Programs by early 2016
Program Description
Adult ALL Phase I
CD19: JCAR015
(Adult ALL and Adult NHL) Adult NHL (Post Autologous Tx) Phase I
Adult ALL Phase II Pivotal Study
CD19: JCAR017 Pediatric ALL Phase I / II: Data Readout
(Pediatric ALL and Adult NHL) Adult NHL Phase I / II
Adult B Cell Malignancies Phase I / II
CD19: JCAR014
(Adult B Cell Malignancies) Exploratory Pathway (Cell Population, Immune
Modulation, Others) Phase I
CD19: JCAR021 Fully Human scFv
(B Cell Malignancies) Adult NHL Phase 1
CD19: “Armored” CAR (B Cell Malignancies) Adult B Cell Malignancies Phase I / II
CD22: JCAR018 Fully Human scFv
(B Cell Malignancies) Pediatric ALL / NHL Phase I
WT-1: JTCR016 Adult AML Phase I / II
(AML, NSCLC) Adult NSCLC Phase I
L1CAM: JCAR023 (Neuroblastoma) Pediatric Phase I
MUC16 & IL-12: JCAR020 “Armored” CAR (Ovarian) Ovarian Phase I
ROR-1: CAR (CLL, Solid Organ Tumors) ROR-1 Expressing Tumors Phase I
Juno Therapeutics Proprietary Materials
25