
TARGETING THE IMMUNE SYSTEM IN RARE DISEASES NASDAQ:CRBP | CORBUSPHARMA.COM EXHIBIT 99.1

This presentation contains certain forward-looking statements, including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities and other statements that are predictive in nature. Additional written and oral forward-looking statements may be made by the Company from time to time in filings with the Securities and Exchange Commission (SEC) or otherwise. The Private Securities Litigation Reform Act of 1995 provides a safe-harbor for forward-looking statements. These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential,” “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the SEC. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this presentation. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. FORWARD-LOOKING STATEMENTS
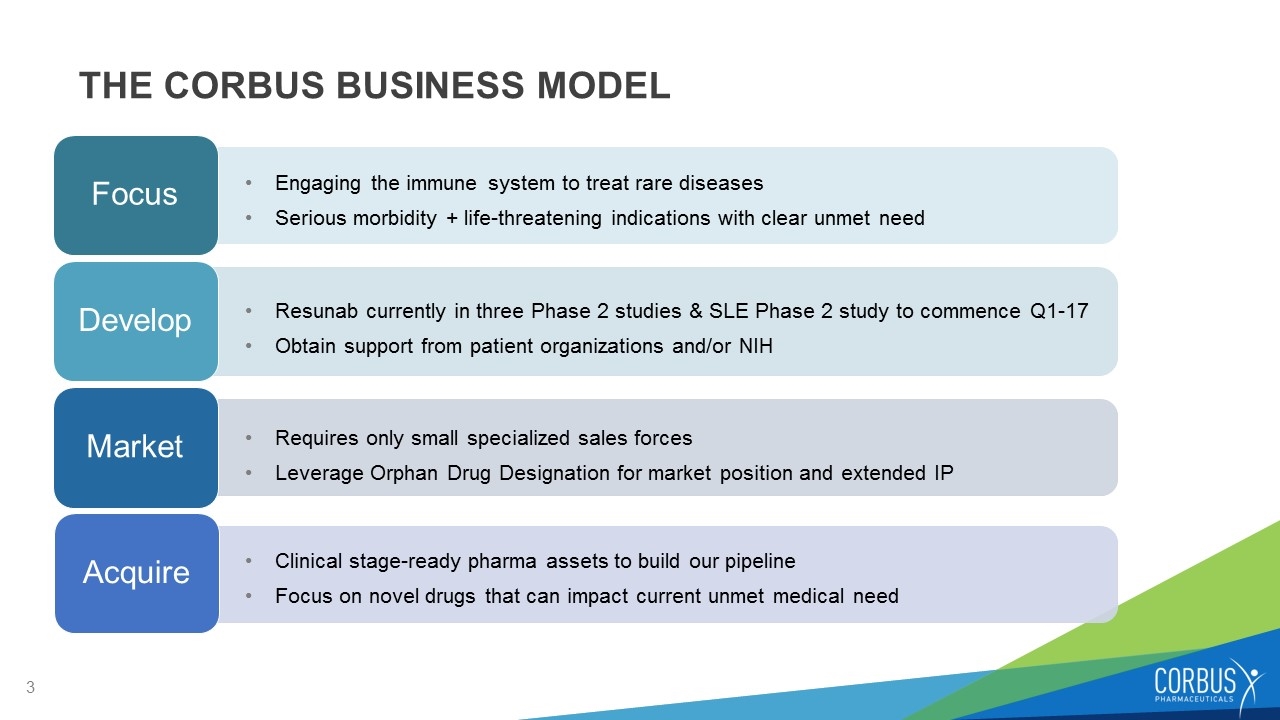
THE CORBUS BUSINESS MODEL Focus Develop Market Acquire Engaging the immune system to treat rare diseases Serious morbidity + life-threatening indications with clear unmet need Resunab currently in three Phase 2 studies & SLE Phase 2 study to commence Q1-17 Obtain support from patient organizations and/or NIH Requires only small specialized sales forces Leverage Orphan Drug Designation for market position and extended IP Clinical stage-ready pharma assets to build our pipeline Focus on novel drugs that can impact current unmet medical need

YUVAL COHEN PH.D. CHIEF EXECUTIVE OFFICER Co-founder and former President of Celsus Therapeutics (CLTX). Expertise in developing anti-inflammatory drugs including for CF MANAGEMENT TEAM BARBARA WHITE M.D. CHIEF MEDICAL OFFICER Board-certified Rheumatologist and clinical immunologist. Previously SVP and Head, R&D Stiefel, VP and Head of Inflammation Clin. Dev. for UCB & MedImmune, and Director, Medical Affairs, Amgen SEAN MORAN C.P.A. M.B.A. CHIEF FINANCIAL OFFICER Former CFO: InVivo (NVIV), Celsion (CLSN), Transport Pharma, Echo Therapeutics (ECTE) & Anika Therapeutics (ANIK) SCOTT CONSTANTINE M.S. DIRECTOR, CLINICAL OPERATIONS Expertise in CF and Pulmonary diseases trials. Former Director, Clinical Research & Operations of Insmed and Clinical Program Scientist at PTC Therapeutics (PTCT) MARK TEPPER PH.D. PRESIDENT & CHIEF SCIENTIFIC OFFICER Former VP U.S. Research & Operations, EMD Serono; Sr. Investigator, Bristol-Myers Squibb

YUVAL COHEN, PH.D. CHIEF EXECUTIVE OFFICER AMB. ALAN HOLMER CHAIRMAN OF THE BOARD Former CEO of PhRMA (1996-2005) Over two decades of public service in Washington, D.C. including Special Envoy to China (2007-2009) Former board member Inspire Pharma Chairman of the Board of the Metropolitan Washington, D.C. Chapter of the Cystic Fibrosis Foundation AVERY W. (CHIP) CAITLIN CFO Celldex Therapeutics (CLDX) since 2000 Raised over $600MM financing Over 20 years experience in industry: Repligen (CFO) and Endogen (CFO) BOARD OF DIRECTORS DAVID HOCHMAN Managing Partner of Orchestra Medical Ventures Over 17 years of venture capital and investment banking experience Former Managing Director of Spencer Trask Ventures, Inc. securing over $420 million in equity capital RENU GUPTA, M.D. Over 25 years of development, regulatory and senior management experience in the biopharm industry Former CMO of Insmed, a specialty CF company and current advisor to the CEO Former VP and Head of U.S. Clinical Research and Devp Novartis (2003-2006)

RESUNAB: OUR FIRST ASSET As of 3/31/16, Corbus has received $2.5MM of the $5.MM CFF award. NIH grants fund Ph. 2 trials of Resunab in dermatomyositis and systemic lupus erythematosus; Corbus retains all rights to the product and owns the IND’data. Novel synthetic oral endocannabinoid-mimetic with unique MOA First-in-class therapeutic currently targeting four indications Acquired pharma asset with extensive Phase 1 safety data IP portfolio à 2033 Cystic Fibrosis (“CF”) Orphan Designation + Fast Track Status granted by FDA Diffuse Cutaneous Systemic Sclerosis (“SSc or Scleroderma”) Orphan Designation + Fast Track Status granted by FDA + Open Label Extension Dermatomyositis (“DM”) Systemic Lupus Erythematosus (“SLE”) $5MM Award from CFF (1) NIH Grants (2)
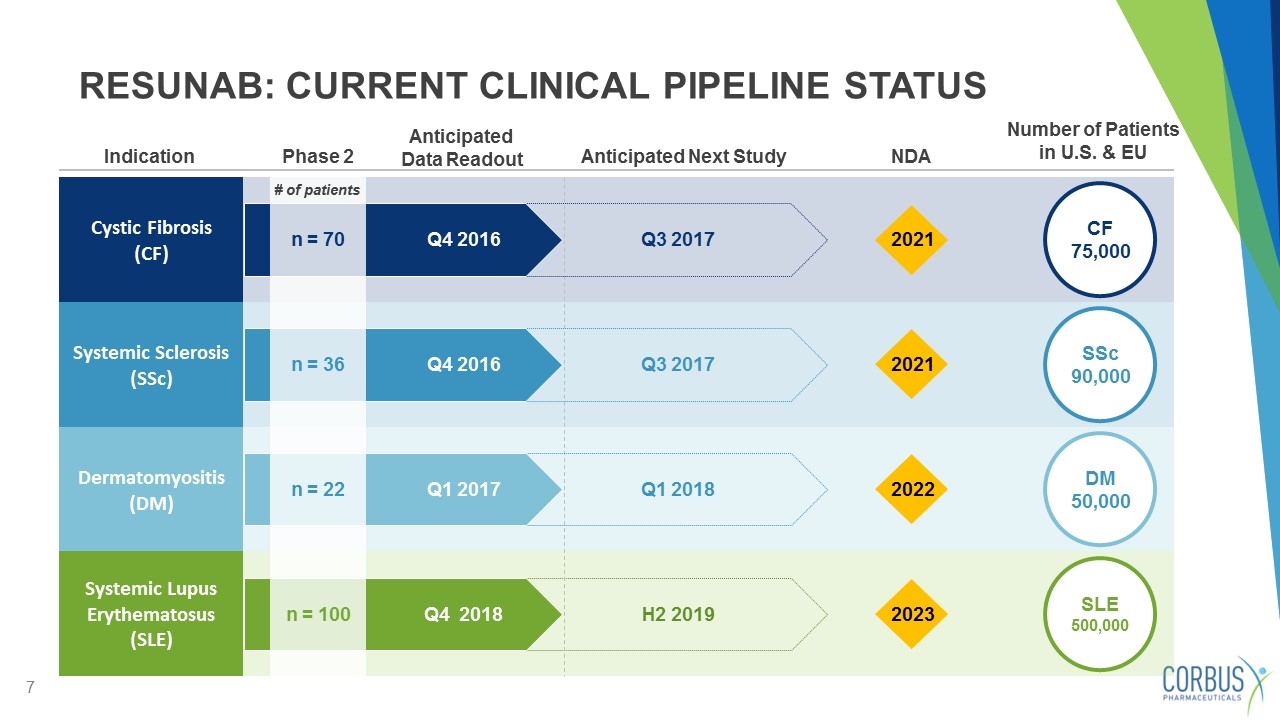
RESUNAB: CURRENT CLINICAL PIPELINE STATUS CF 75,000 SSc 90,000 DM 50,000 Cystic Fibrosis (CF) Systemic Sclerosis (SSc) Dermatomyositis (DM) Indication Number of Patients in U.S. & EU NDA Anticipated Next Study Phase 2 2021 2021 2022 Q3 2017 Q3 2017 Q1 2018 Anticipated Data Readout n = 70 n = 36 n = 22 # of patients Q4 2016 Q4 2016 Q1 2017 SLE 500,000 Systemic Lupus Erythematosus (SLE) 2023 n = 100 Q4 2018 H2 2019
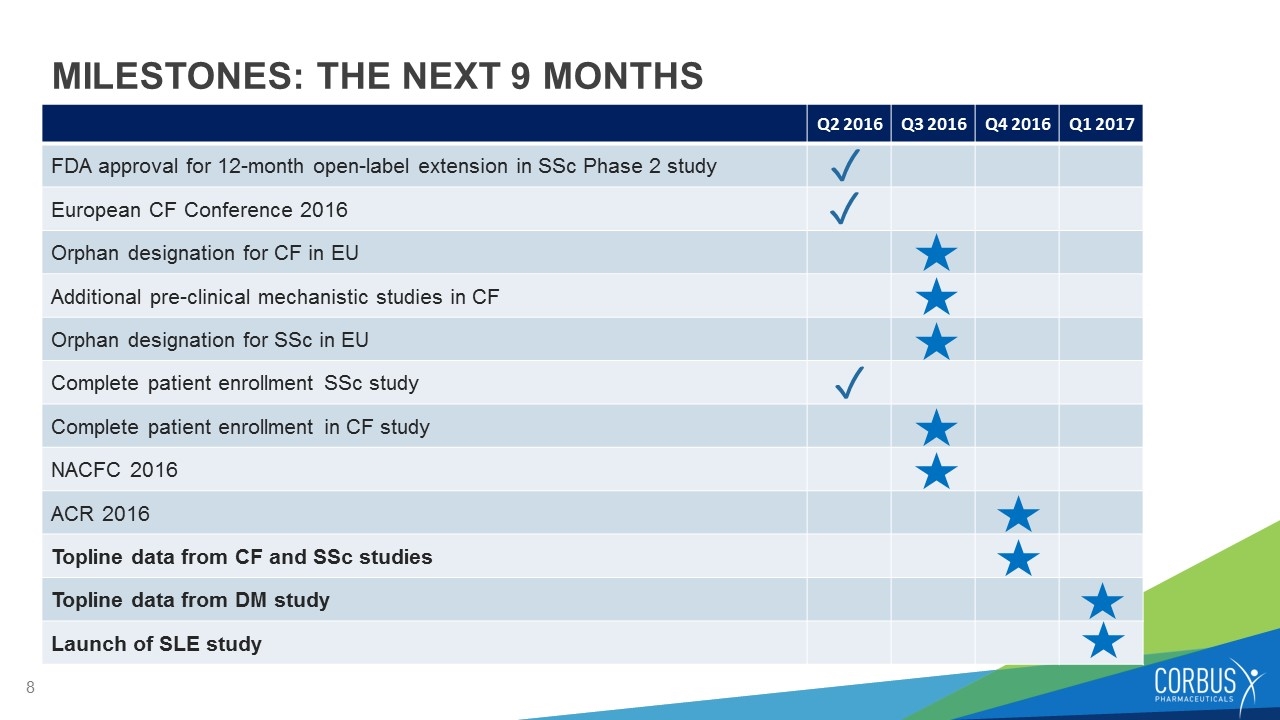
MILESTONES: THE NEXT 9 MONTHS Q2 2016 Q3 2016 Q4 2016 Q1 2017 FDA approval for 12-month open-label extension in SSc Phase 2 study European CF Conference 2016 Orphan designation for CF in EU Additional pre-clinical mechanistic studies in CF Orphan designation for SSc in EU Complete patient enrollment SSc study Complete patient enrollment in CF study NACFC 2016 ACR 2016 Topline data from CF and SSc studies Topline data from DM study Launch of SLE study ✓ ✓ ✓
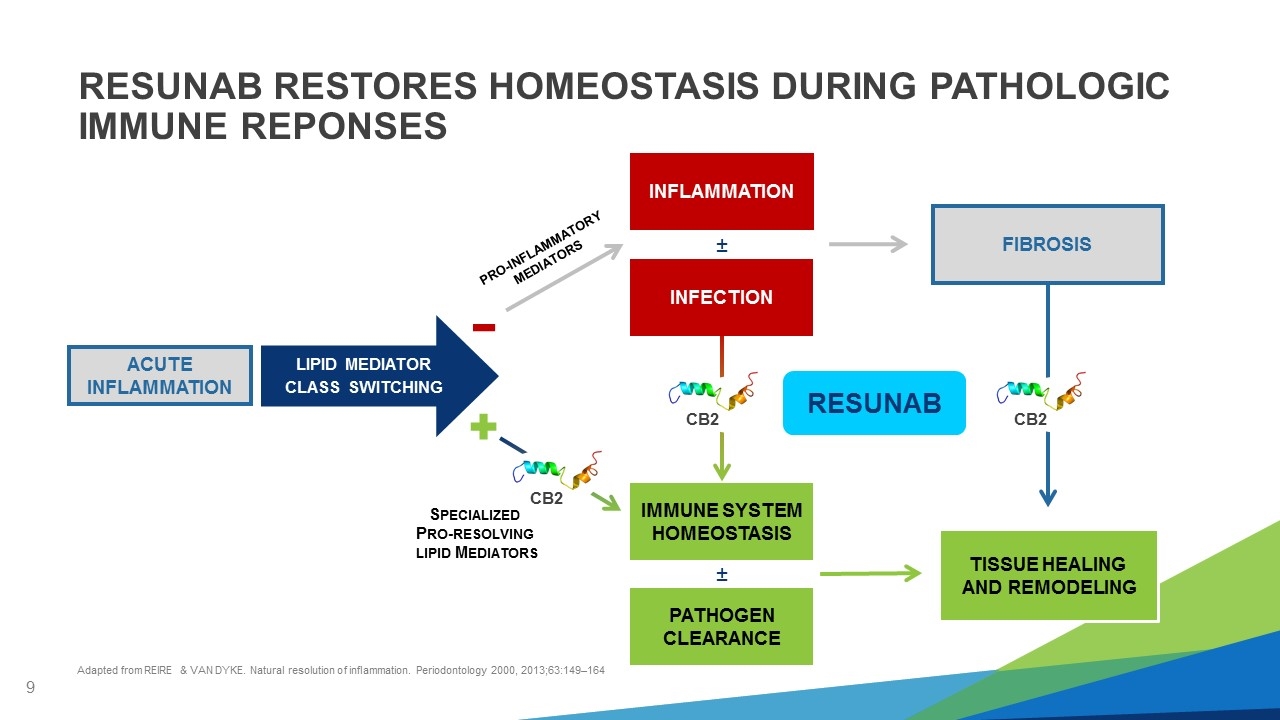
IMMUNE SYSTEM HOMEOSTASIS PRO-INFLAMMATORY MEDIATORS SPECIALIZED PRO-RESOLVING LIPID MEDIATORS LIPID MEDIATOR CLASS SWITCHING Adapted from REIRE & VAN DYKE. Natural resolution of inflammation. Periodontology 2000, 2013;63:149–164 RESUNAB RESTORES HOMEOSTASIS DURING PATHOLOGIC IMMUNE REPONSES INFLAMMATION RESUNAB INFECTION PATHOGEN CLEARANCE ± ± CB2 CB2 CB2 FIBROSIS TISSUE HEALING AND REMODELING ACUTE INFLAMMATION
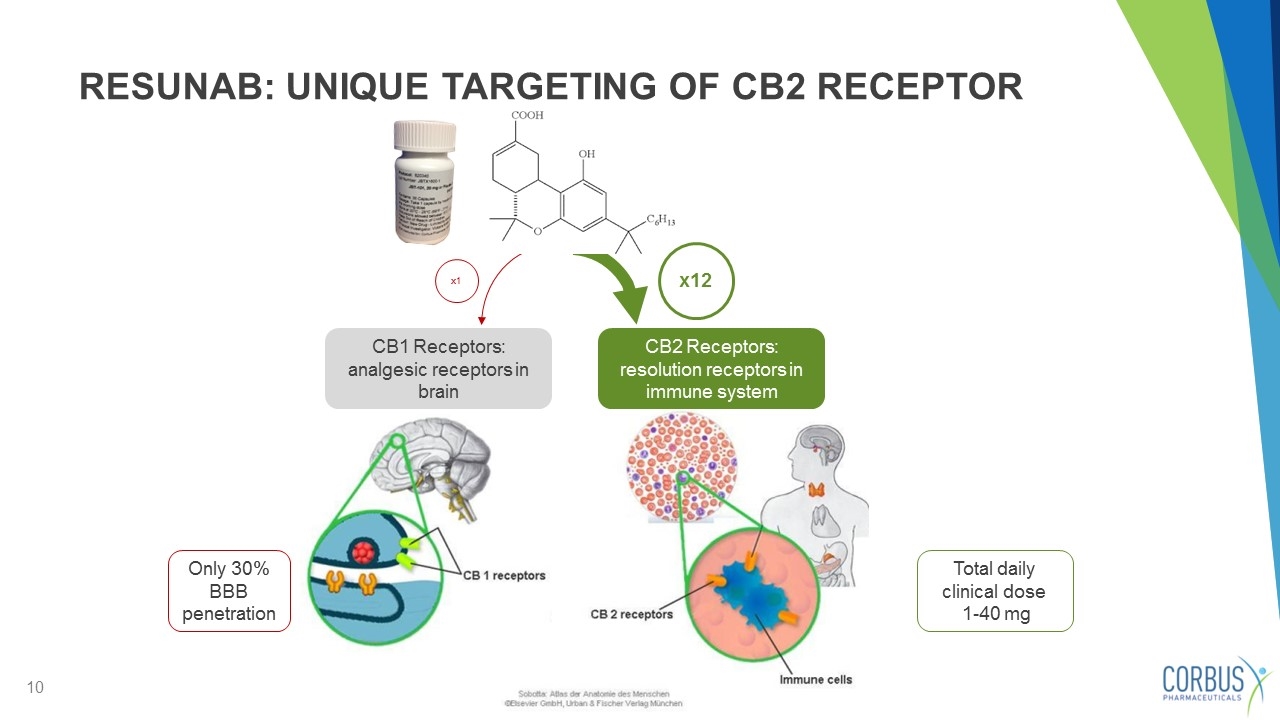
RESUNAB: UNIQUE TARGETING OF CB2 RECEPTOR x1 CB1 Receptors: analgesic receptors in brain CB2 Receptors: resolution receptors in immune system Total daily clinical dose 1-40 mg x12 Only 30% BBB penetration
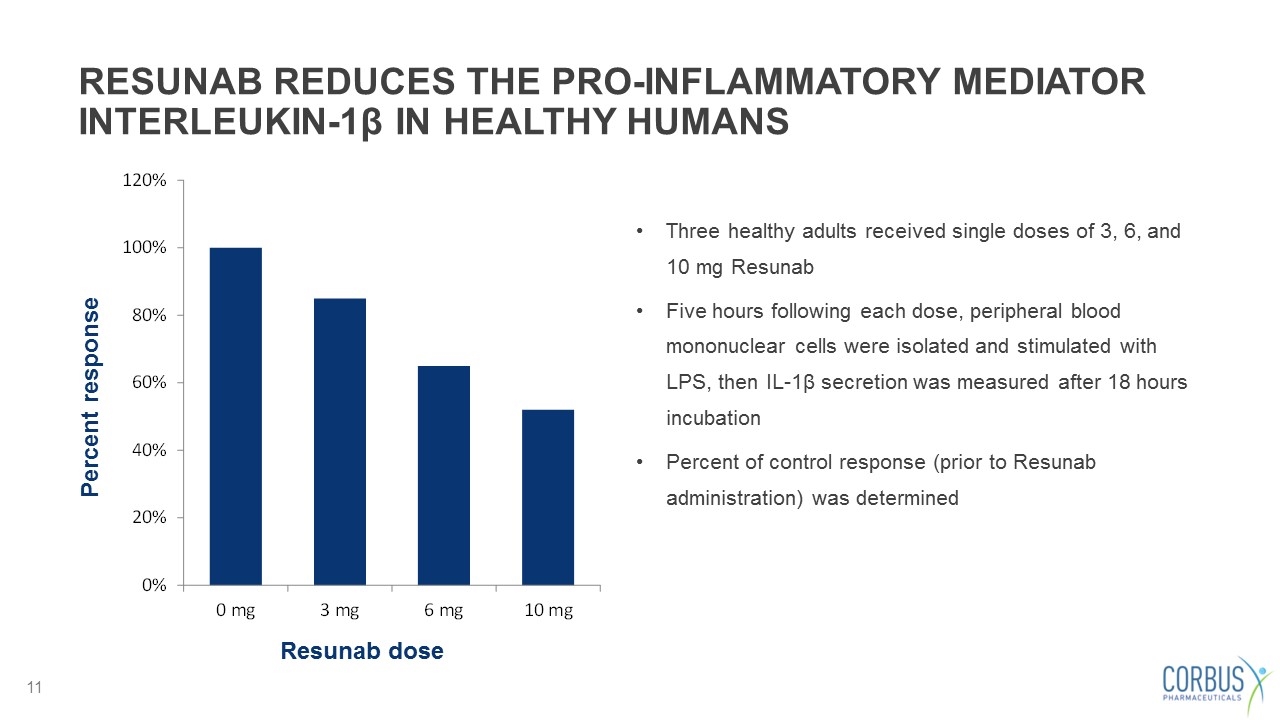
RESUNAB REDUCES THE PRO-INFLAMMATORY MEDIATOR INTERLEUKIN-1β IN HEALTHY HUMANS Resunab dose Percent response Three healthy adults received single doses of 3, 6, and 10 mg Resunab Five hours following each dose, peripheral blood mononuclear cells were isolated and stimulated with LPS, then IL-1β secretion was measured after 18 hours incubation Percent of control response (prior to Resunab administration) was determined
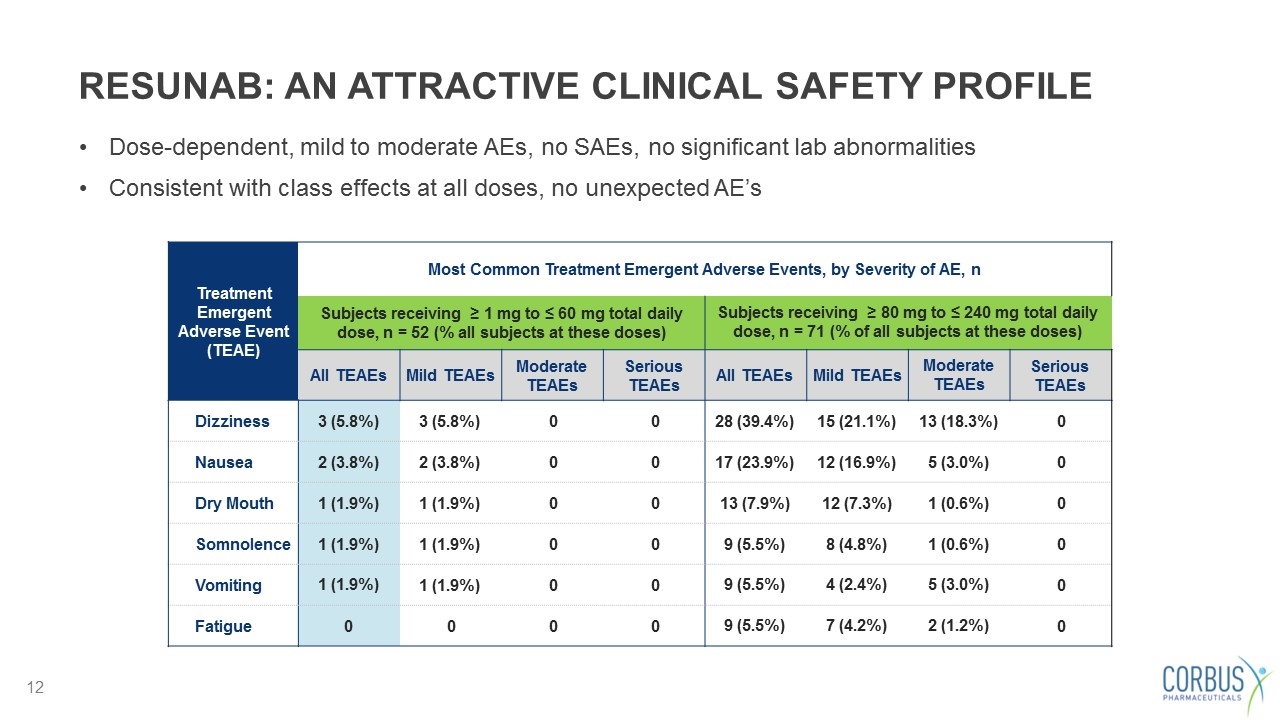
RESUNAB: AN ATTRACTIVE CLINICAL SAFETY PROFILE Treatment Emergent Adverse Event (TEAE) Most Common Treatment Emergent Adverse Events, by Severity of AE, n Subjects receiving ≥ 1 mg to ≤ 60 mg total daily dose, n = 52 (% all subjects at these doses) Subjects receiving ≥ 80 mg to ≤ 240 mg total daily dose, n = 71 (% of all subjects at these doses) All TEAEs Mild TEAEs Moderate TEAEs Serious TEAEs All TEAEs Mild TEAEs Moderate TEAEs Serious TEAEs Dizziness 3 (5.8%) 3 (5.8%) 0 0 28 (39.4%) 15 (21.1%) 13 (18.3%) 0 Nausea 2 (3.8%) 2 (3.8%) 0 0 17 (23.9%) 12 (16.9%) 5 (3.0%) 0 Dry Mouth 1 (1.9%) 1 (1.9%) 0 0 13 (7.9%) 12 (7.3%) 1 (0.6%) 0 Somnolence 1 (1.9%) 1 (1.9%) 0 0 9 (5.5%) 8 (4.8%) 1 (0.6%) 0 Vomiting 1 (1.9%) 1 (1.9%) 0 0 9 (5.5%) 4 (2.4%) 5 (3.0%) 0 Fatigue 0 0 0 0 9 (5.5%) 7 (4.2%) 2 (1.2%) 0 Dose-dependent, mild to moderate AEs, no SAEs, no significant lab abnormalities Consistent with class effects at all doses, no unexpected AE’s

CYSTIC FIBROSIS: FOCUSING ON INFLAMMATION & FIBROSIS
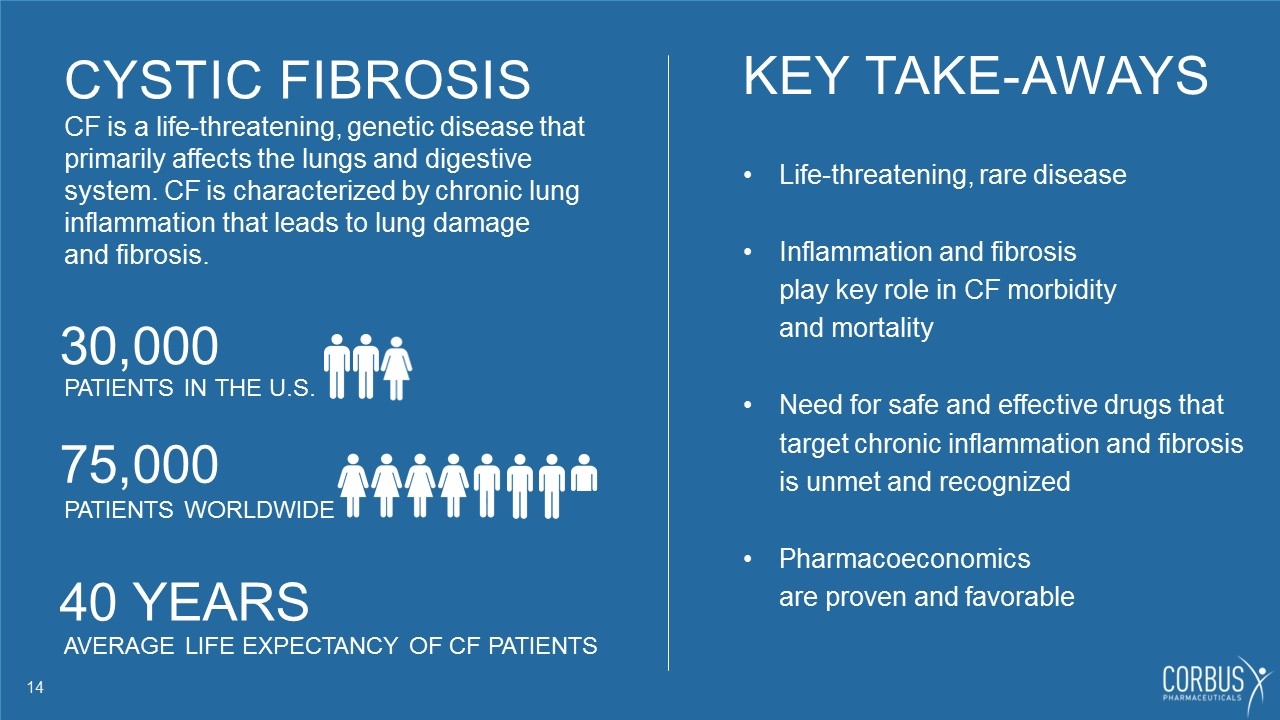
CYSTIC FIBROSIS CF is a life-threatening, genetic disease that primarily affects the lungs and digestive system. CF is characterized by chronic lung inflammation that leads to lung damage and fibrosis. 30,000 PATIENTS IN THE U.S. 75,000 PATIENTS WORLDWIDE 40 YEARS AVERAGE LIFE EXPECTANCY OF CF PATIENTS KEY TAKE-AWAYS Life-threatening, rare disease Inflammation and fibrosis play key role in CF morbidity and mortality Need for safe and effective drugs that target chronic inflammation and fibrosis is unmet and recognized Pharmacoeconomics are proven and favorable
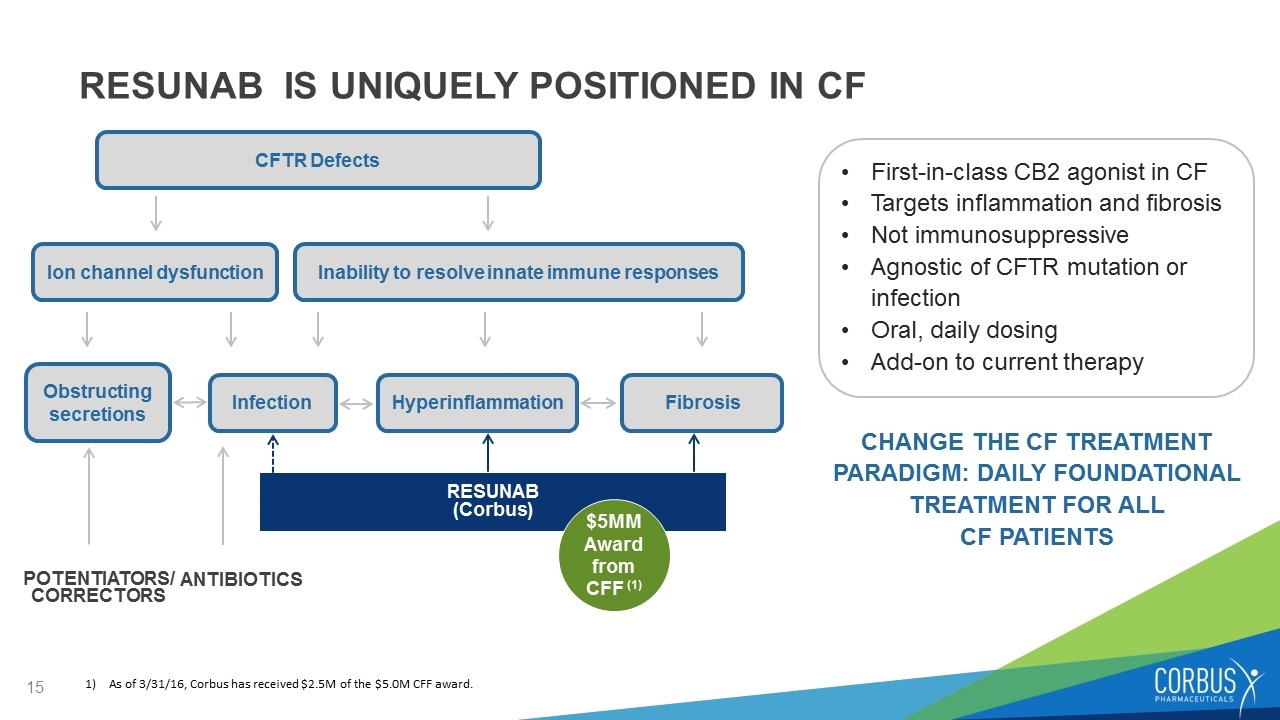
RESUNAB IS UNIQUELY POSITIONED IN CF First-in-class CB2 agonist in CF Targets inflammation and fibrosis Not immunosuppressive Agnostic of CFTR mutation or infection Oral, daily dosing Add-on to current therapy CHANGE THE CF TREATMENT PARADIGM: DAILY FOUNDATIONAL TREATMENT FOR ALL CF PATIENTS CFTR Defects Hyperinflammation Infection Fibrosis POTENTIATORS/ CORRECTORS RESUNAB (Corbus) $5MM Award from CFF (1) As of 3/31/16, Corbus has received $2.5M of the $5.0M CFF award. Obstructing secretions Ion channel dysfunction Inability to resolve innate immune responses ANTIBIOTICS
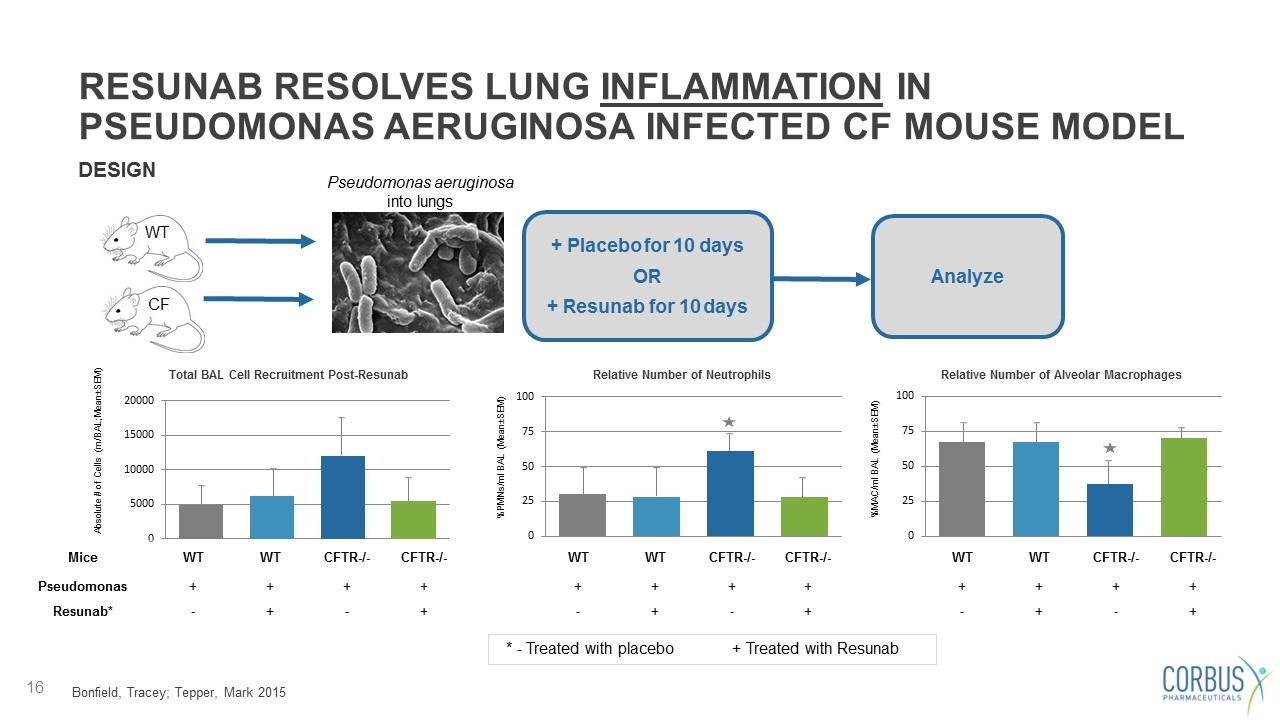
RESUNAB RESOLVES LUNG INFLAMMATION IN PSEUDOMONAS AERUGINOSA INFECTED CF MOUSE MODEL DESIGN WT CF Pseudomonas aeruginosa into lungs + Placebo for 10 days OR + Resunab for 10 days Analyze Total BAL Cell Recruitment Post-Resunab Absolute # of Cells (m/BAL; Mean±SEM) %MAC/ml BAL (Mean±SEM) Relative Number of Alveolar Macrophages Relative Number of Neutrophils %PMNs/ml BAL (Mean±SEM) Mice WT WT CFTR-/- CFTR-/- WT WT CFTR-/- CFTR-/- WT WT CFTR-/- CFTR-/- Pseudomonas + + + + + + + + + + + + Resunab* - + - + - + - + - + - + Bonfield, Tracey; Tepper, Mark 2015 * - Treated with placebo + Treated with Resunab
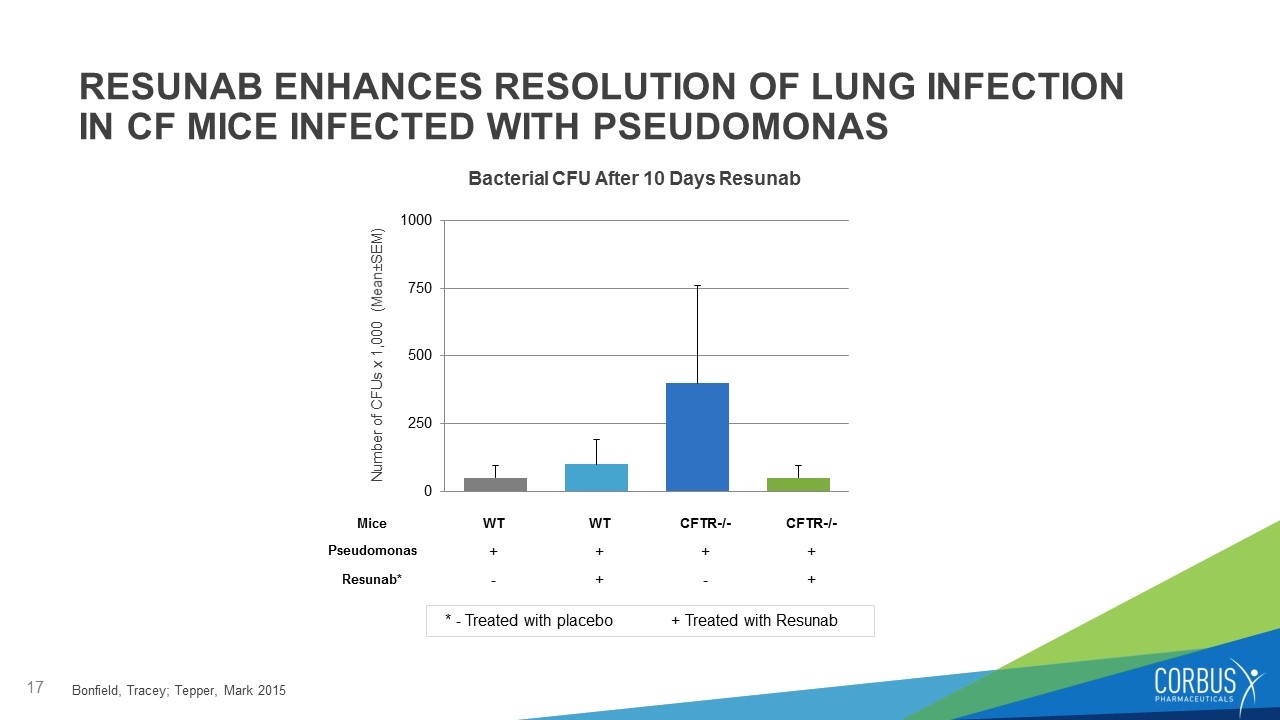
RESUNAB ENHANCES RESOLUTION OF LUNG INFECTION IN CF MICE INFECTED WITH PSEUDOMONAS - Associated with worsening symptoms Bacterial CFU After 10 Days Resunab Number of CFUs x 1,000 (Mean±SEM) Mice WT WT CFTR-/- CFTR-/- Pseudomonas + + + + Resunab* - + - + Bonfield, Tracey; Tepper, Mark 2015 * - Treated with placebo + Treated with Resunab
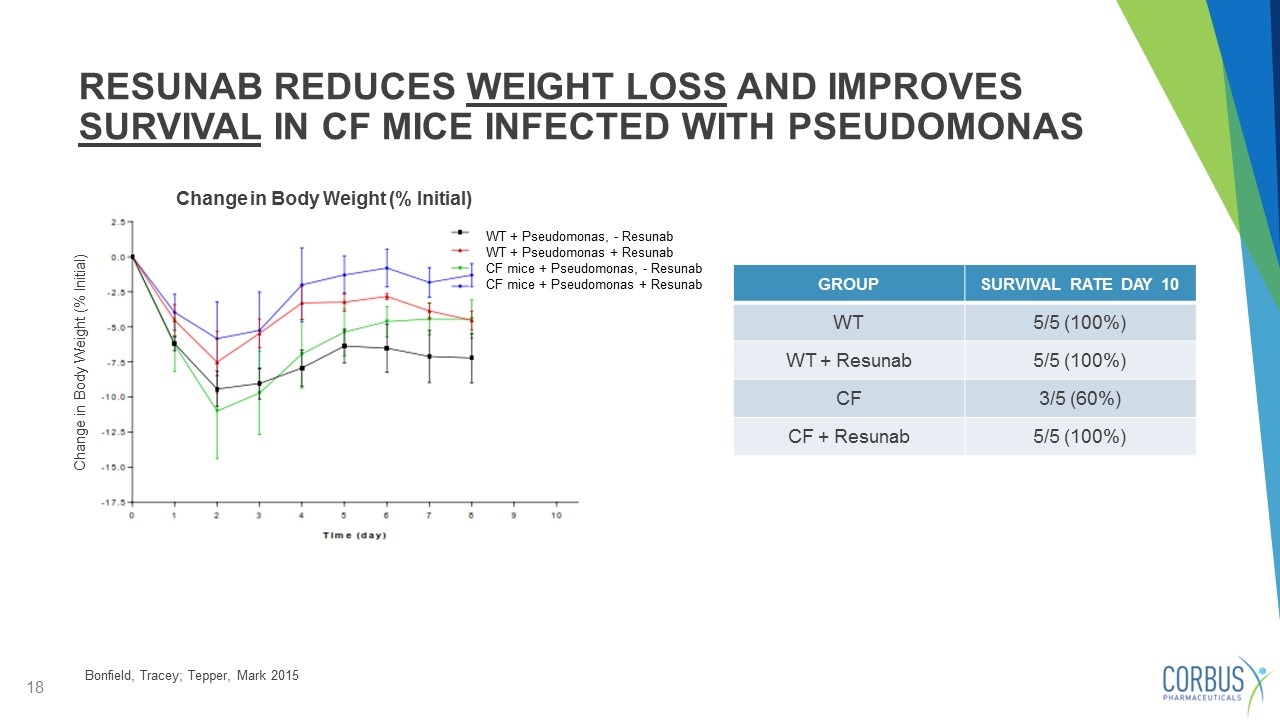
RESUNAB REDUCES WEIGHT LOSS AND IMPROVES SURVIVAL IN CF MICE INFECTED WITH PSEUDOMONAS - Associated with worsening symptoms WT + Pseudomonas, - Resunab WT + Pseudomonas + Resunab CF mice + Pseudomonas, - Resunab CF mice + Pseudomonas + Resunab Bonfield, Tracey; Tepper, Mark 2015 GROUP SURVIVAL RATE DAY 10 WT 5/5 (100%) WT + Resunab 5/5 (100%) CF 3/5 (60%) CF + Resunab 5/5 (100%) Change in Body Weight (% Initial) Change in Body Weight (% Initial)
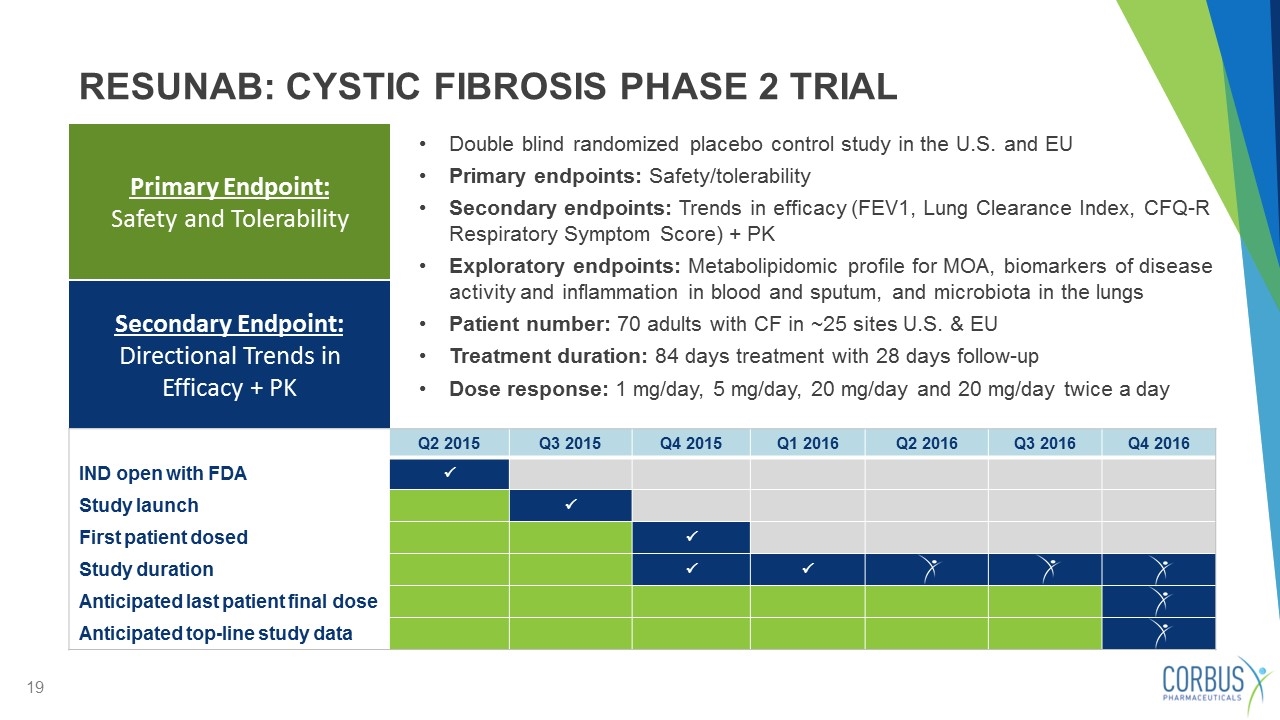
Double blind randomized placebo control study in the U.S. and EU Primary endpoints: Safety/tolerability Secondary endpoints: Trends in efficacy (FEV1, Lung Clearance Index, CFQ-R Respiratory Symptom Score) + PK Exploratory endpoints: Metabolipidomic profile for MOA, biomarkers of disease activity and inflammation in blood and sputum, and microbiota in the lungs Patient number: 70 adults with CF in ~25 sites U.S. & EU Treatment duration: 84 days treatment with 28 days follow-up Dose response: 1 mg/day, 5 mg/day, 20 mg/day and 20 mg/day twice a day Primary Endpoint: Safety and Tolerability RESUNAB: CYSTIC FIBROSIS PHASE 2 TRIAL Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 Q4 2016 IND open with FDA P Study launch P First patient dosed P Study duration P P Anticipated last patient final dose Anticipated top-line study data Secondary Endpoint: Directional Trends in Efficacy + PK
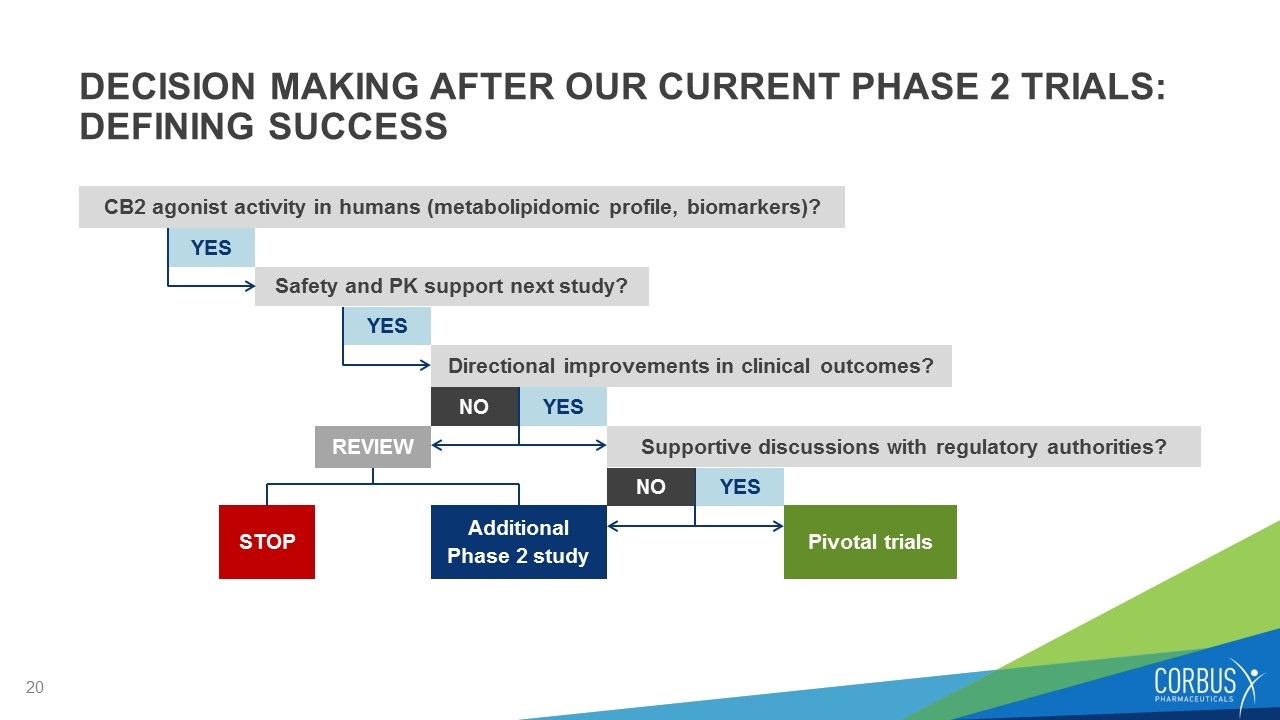
DECISION MAKING AFTER OUR CURRENT PHASE 2 TRIALS: DEFINING SUCCESS CB2 agonist activity in humans (metabolipidomic profile, biomarkers)? YES Safety and PK support next study? Additional Phase 2 study STOP Pivotal trials YES Directional improvements in clinical outcomes? YES Supportive discussions with regulatory authorities? NO REVIEW YES NO

DIFFUSE CUTANEOUS SYSTEMIC SCLEROSIS: RELIEF FOR A DISEASE WITH NO APPROVED TARGETED THERAPY
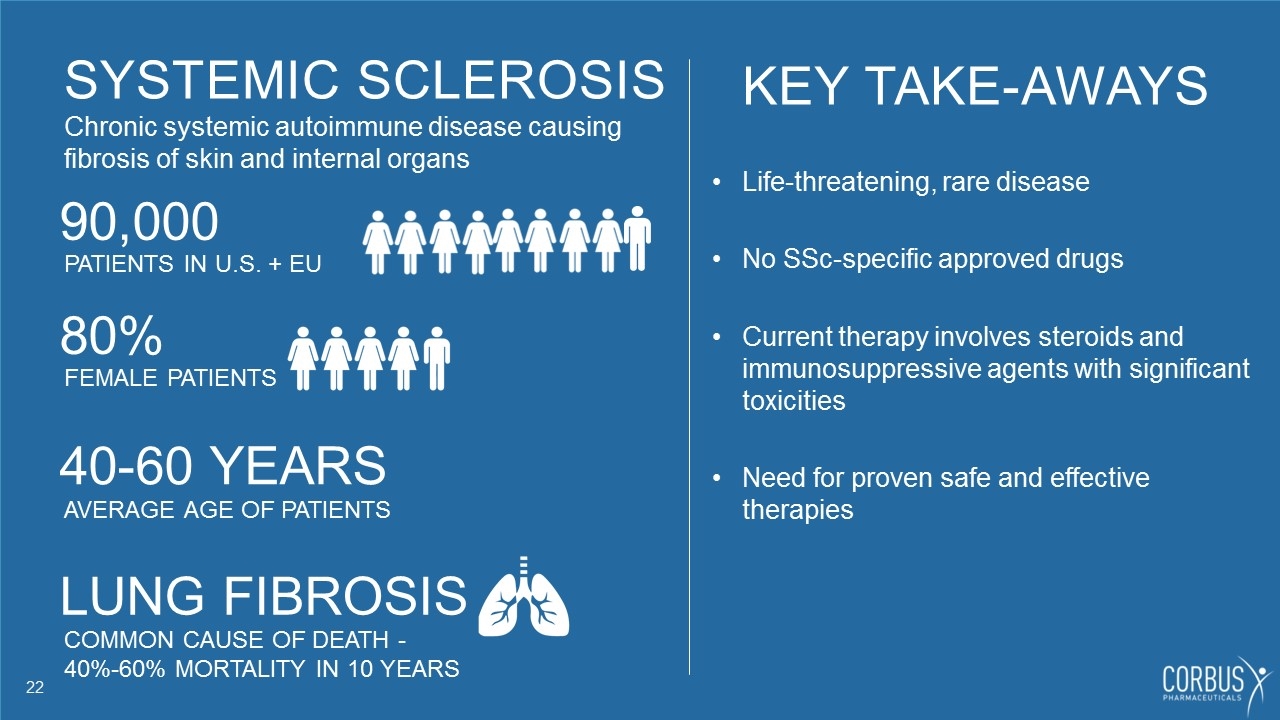
SYSTEMIC SCLEROSIS Chronic systemic autoimmune disease causing fibrosis of skin and internal organs 80% FEMALE PATIENTS 40-60 YEARS AVERAGE AGE OF PATIENTS KEY TAKE-AWAYS Life-threatening, rare disease No SSc-specific approved drugs Current therapy involves steroids and immunosuppressive agents with significant toxicities Need for proven safe and effective therapies 90,000 PATIENTS IN U.S. + EU LUNG FIBROSIS COMMON CAUSE OF DEATH - 40%-60% MORTALITY IN 10 YEARS
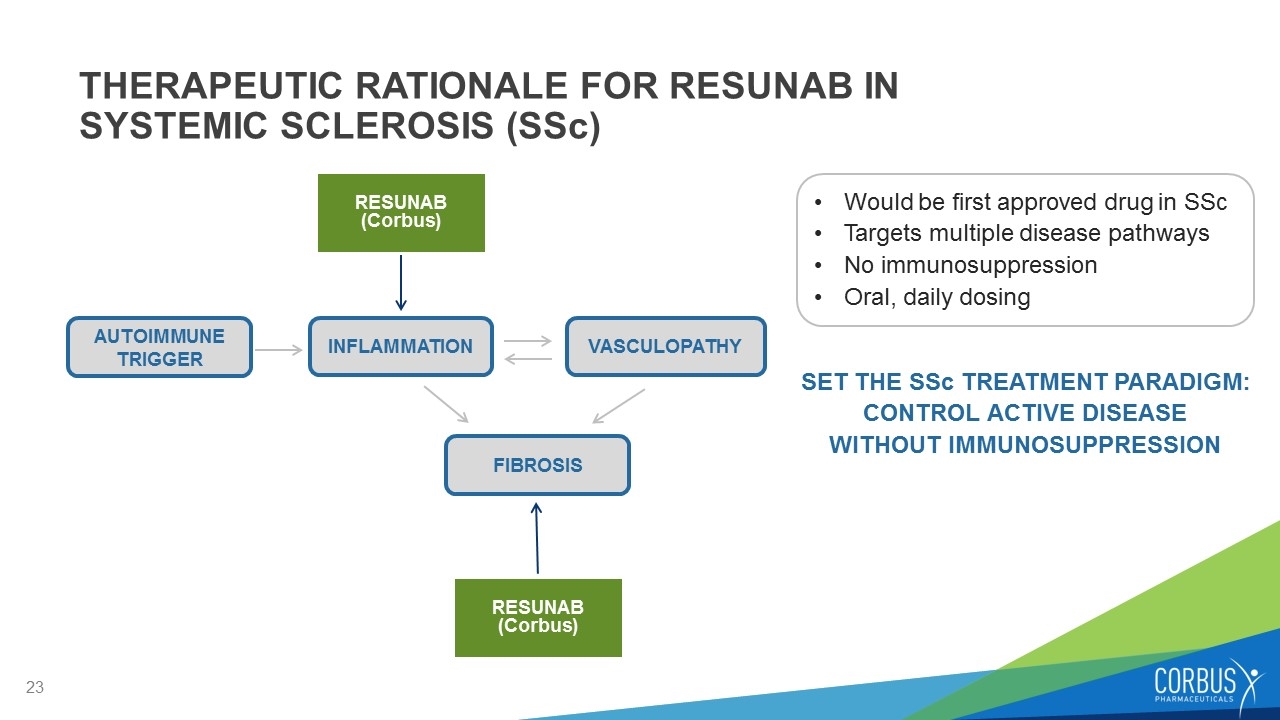
THERAPEUTIC RATIONALE FOR RESUNAB IN SYSTEMIC SCLEROSIS (SSc) Would be first approved drug in SSc Targets multiple disease pathways No immunosuppression Oral, daily dosing SET THE SSc TREATMENT PARADIGM: CONTROL ACTIVE DISEASE WITHOUT IMMUNOSUPPRESSION AUTOIMMUNE TRIGGER INFLAMMATION VASCULOPATHY FIBROSIS RESUNAB (Corbus) RESUNAB (Corbus)
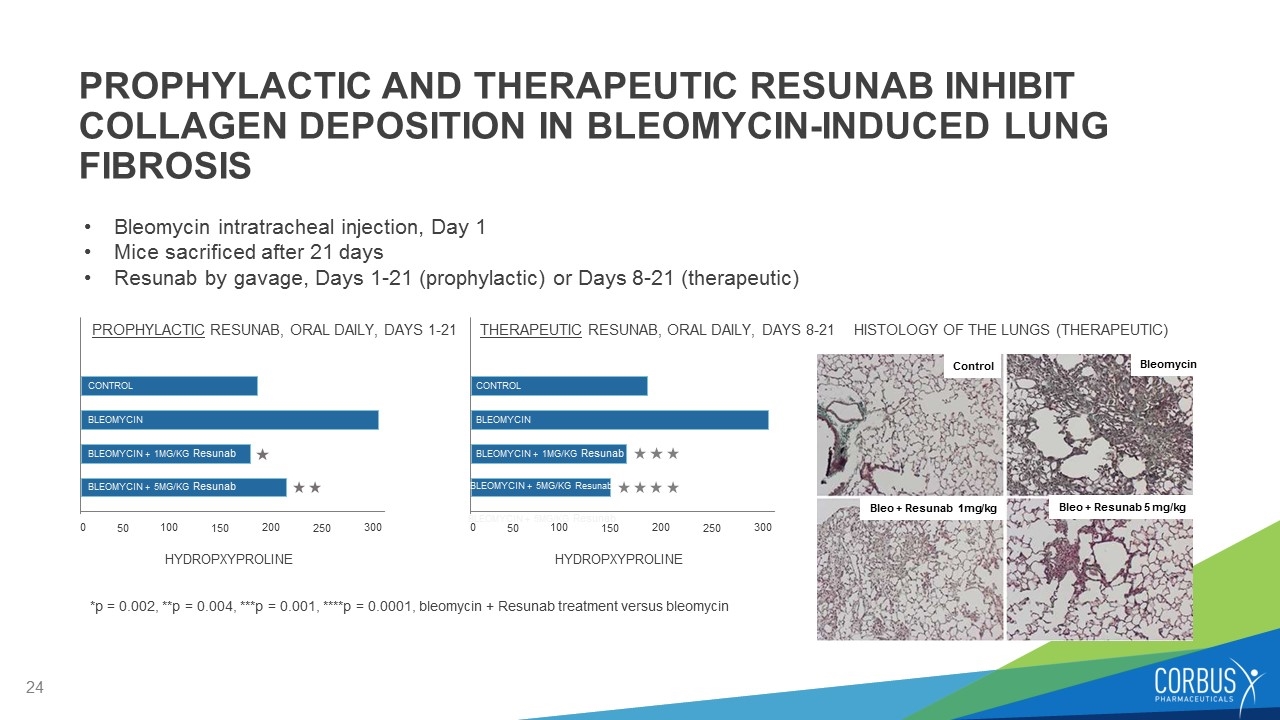
Bleomycin intratracheal injection, Day 1 Mice sacrificed after 21 days Resunab by gavage, Days 1-21 (prophylactic) or Days 8-21 (therapeutic) *p = 0.002, **p = 0.004, ***p = 0.001, ****p = 0.0001, bleomycin + Resunab treatment versus bleomycin PROPHYLACTIC AND THERAPEUTIC RESUNAB INHIBIT COLLAGEN DEPOSITION IN BLEOMYCIN-INDUCED LUNG FIBROSIS HYDROPXYPROLINE 0 50 100 150 200 250 300 BLEOMYCIN + 5MG/KG Resunab BLEOMYCIN CONTROL BLEOMYCIN + 1MG/KG Resunab PROPHYLACTIC RESUNAB, ORAL DAILY, DAYS 1-21 HYDROPXYPROLINE 0 50 100 150 200 250 300 BLEOMYCIN CONTROL THERAPEUTIC RESUNAB, ORAL DAILY, DAYS 8-21 BLEOMYCIN + 1MG/KG Resunab BLEOMYCIN + 5MG/KG Resunab HISTOLOGY OF THE LUNGS (THERAPEUTIC) Control Bleomycin Bleo + Resunab 1mg/kg Bleo + Resunab 5 mg/kg BLEOMYCIN + 5MG/KG Resunab
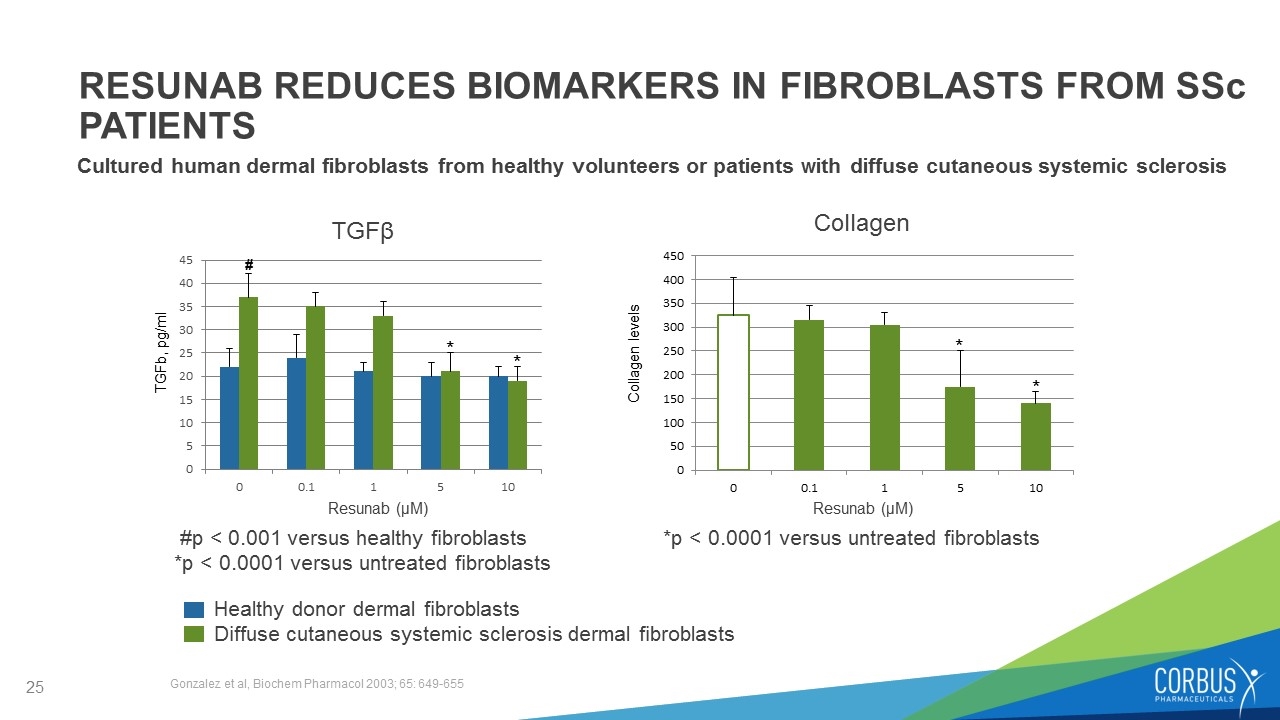
RESUNAB REDUCES BIOMARKERS IN FIBROBLASTS FROM SSc PATIENTS Cultured human dermal fibroblasts from healthy volunteers or patients with diffuse cutaneous systemic sclerosis Resunab (μM) Resunab (μM) Healthy donor dermal fibroblasts Diffuse cutaneous systemic sclerosis dermal fibroblasts TGFβ *p < 0.0001 versus untreated fibroblasts * #p < 0.001 versus healthy fibroblasts *p < 0.0001 versus untreated fibroblasts TGFb, pg/ml * * * Collagen Gonzalez et al, Biochem Pharmacol 2003; 65: 649-655 # Collagen levels
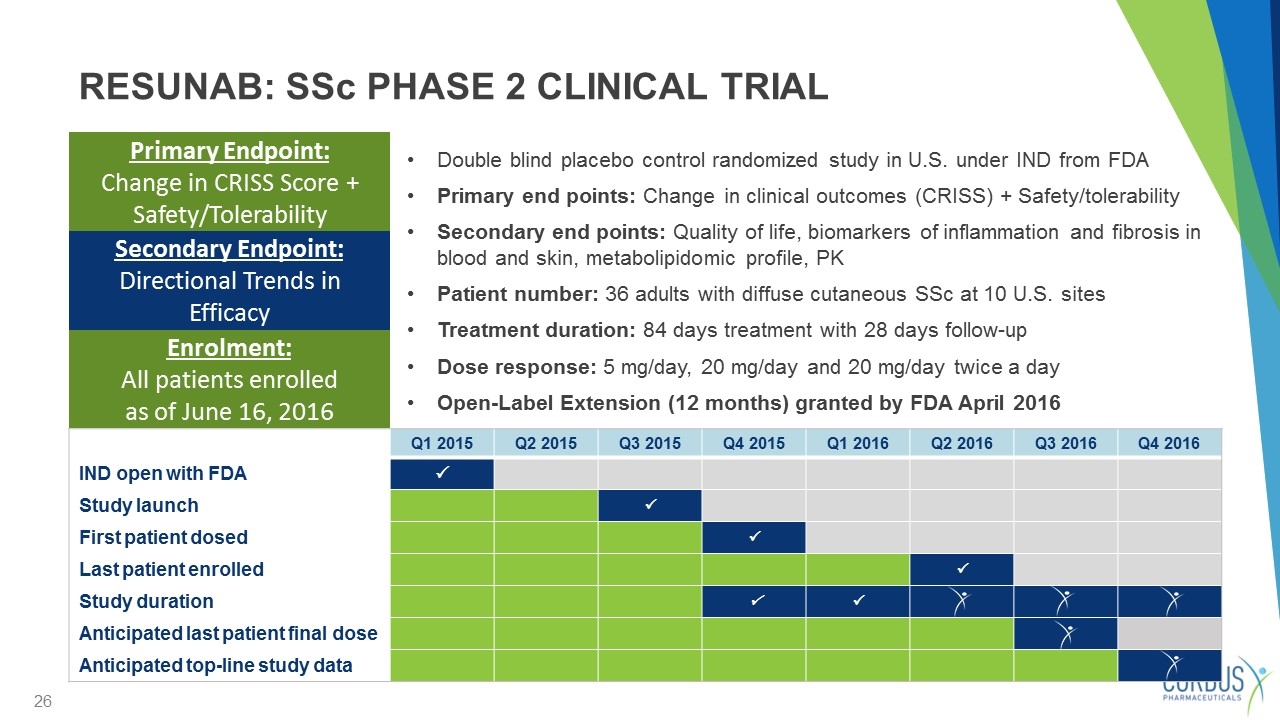
Double blind placebo control randomized study in U.S. under IND from FDA Primary end points: Change in clinical outcomes (CRISS) + Safety/tolerability Secondary end points: Quality of life, biomarkers of inflammation and fibrosis in blood and skin, metabolipidomic profile, PK Patient number: 36 adults with diffuse cutaneous SSc at 10 U.S. sites Treatment duration: 84 days treatment with 28 days follow-up Dose response: 5 mg/day, 20 mg/day and 20 mg/day twice a day Open-Label Extension (12 months) granted by FDA April 2016 Primary Endpoint: Change in CRISS Score + Safety/Tolerability RESUNAB: SSc PHASE 2 CLINICAL TRIAL Q1 2015 Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 Q4 2016 IND open with FDA P Study launch P First patient dosed P Last patient enrolled P Study duration P P Anticipated last patient final dose Anticipated top-line study data Secondary Endpoint: Directional Trends in Efficacy Enrolment: All patients enrolled as of June 16, 2016

RESUNAB: SSc PHASE 2 CLINICAL TRIAL OPEN-LABEL EXTENSION 12-month open-label extension study of ongoing Phase 2 clinical trial of Resunab granted by U.S. FDA Goal of the open-label extension is to collect long term safety and efficacy data on Resunab All subjects from ongoing double-blind placebo-controlled study provided with option to continue treatment for an additional 12 months following the completion of the 84-day treatment period All subjects in the extension study will receive Resunab, including those who received placebo in the current study Same clinical endpoint used in ongoing double-blinded placebo-controlled portion of the trial will be monitored throughout the extension

DERMATOMYOSITIS & LUPUS (SLE): WORKING WITH THE NIH ON RARE AUTOIMMUNE DISEASES
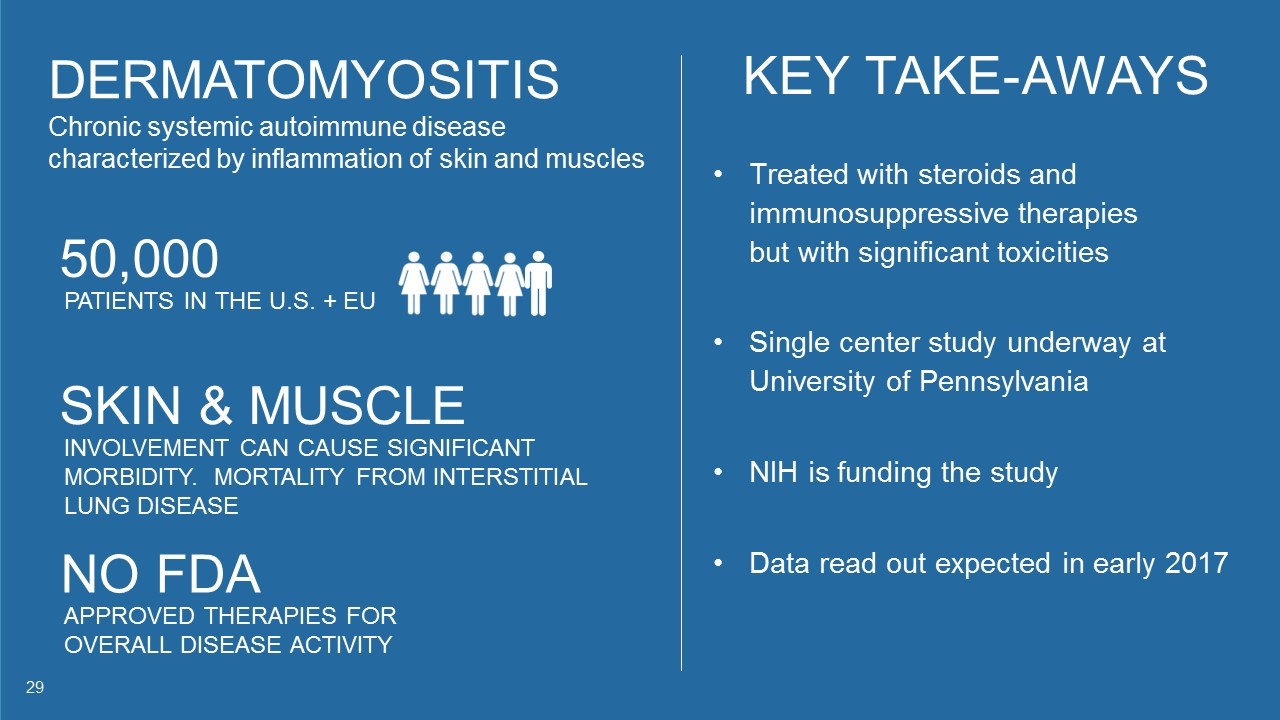
DERMATOMYOSITIS Chronic systemic autoimmune disease characterized by inflammation of skin and muscles 50,000 PATIENTS IN THE U.S. + EU KEY TAKE-AWAYS Treated with steroids and immunosuppressive therapies but with significant toxicities Single center study underway at University of Pennsylvania NIH is funding the study Data read out expected in early 2017 NO FDA APPROVED THERAPIES FOR OVERALL DISEASE ACTIVITY SKIN & MUSCLE INVOLVEMENT CAN CAUSE SIGNIFICANT MORBIDITY. MORTALITY FROM INTERSTITIAL LUNG DISEASE
SYSTEMIC LUPUS ERYTHEMATOSUS Chronic systemic autoimmune disease characterized by arthritis, skin rashes, kidney disease, and involvement of the nervous system and other organs KEY TAKE-AWAYS Treated with steroids and immunosuppressive therapies Multi-center study planned (n=100) NIH is funding the study Data read out expected in Q4 2018 NON-IMMUNOSUPRESSIVE TREATMENTS NEEDED 500,000 – 600,000 PATIENTS IN THE U.S. + EU 10-12:1 WOMEN TO MEN HIGHER INCIDENCE AND MORE SEVERE IN BLACKS AND ASIANS
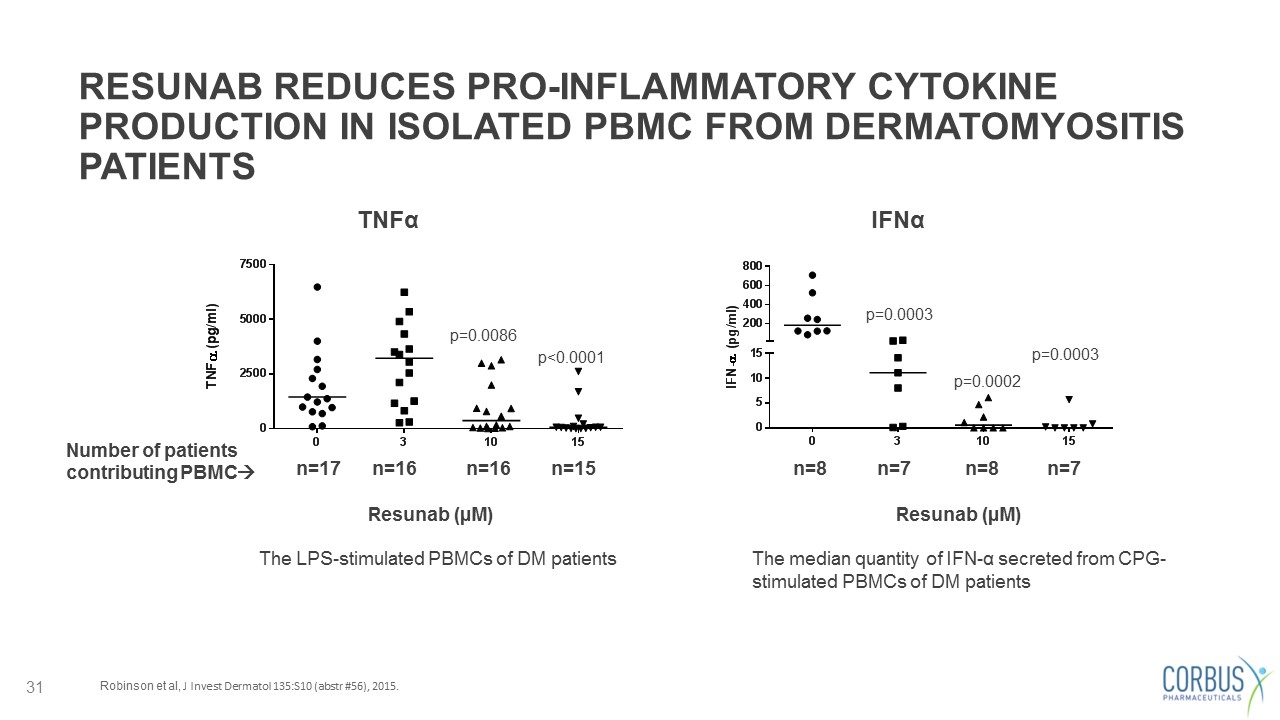
RESUNAB REDUCES PRO-INFLAMMATORY CYTOKINE PRODUCTION IN ISOLATED PBMC FROM DERMATOMYOSITIS PATIENTS Robinson et al, J Invest Dermatol 135:S10 (abstr #56), 2015. The LPS-stimulated PBMCs of DM patients The median quantity of IFN-α secreted from CPG-stimulated PBMCs of DM patients Resunab (µM) Resunab (µM) TNFα p=0.0086 p<0.0001 n=17 n=16 n=16 n=15 p=0.0003 p=0.0002 p=0.0003 n=8 n=7 n=8 n=7 Number of patients contributing PBMCà IFNα
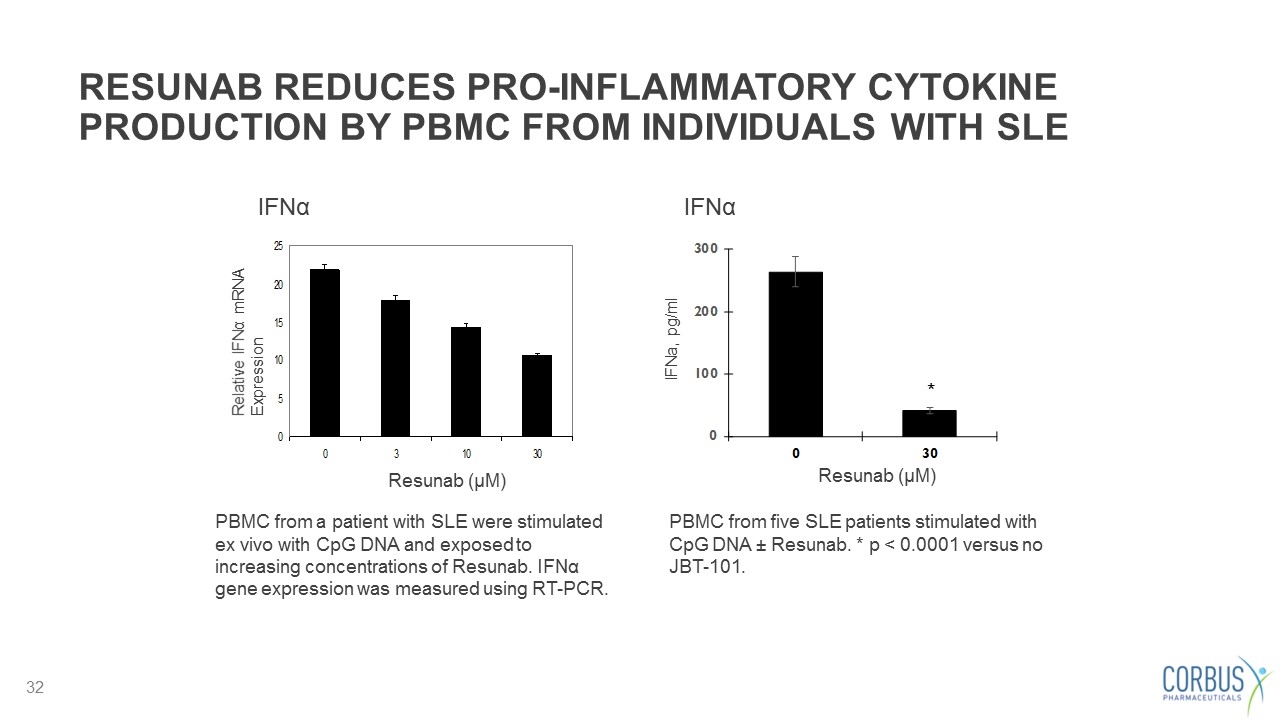
RESUNAB REDUCES PRO-INFLAMMATORY CYTOKINE PRODUCTION BY PBMC FROM INDIVIDUALS WITH SLE PBMC from a patient with SLE were stimulated ex vivo with CpG DNA and exposed to increasing concentrations of Resunab. IFNα gene expression was measured using RT-PCR. PBMC from five SLE patients stimulated with CpG DNA ± Resunab. * p < 0.0001 versus no JBT-101. Relative IFNα mRNA Expression * IFNa, pg/ml Resunab (µM) Resunab (µM) IFNα IFNα
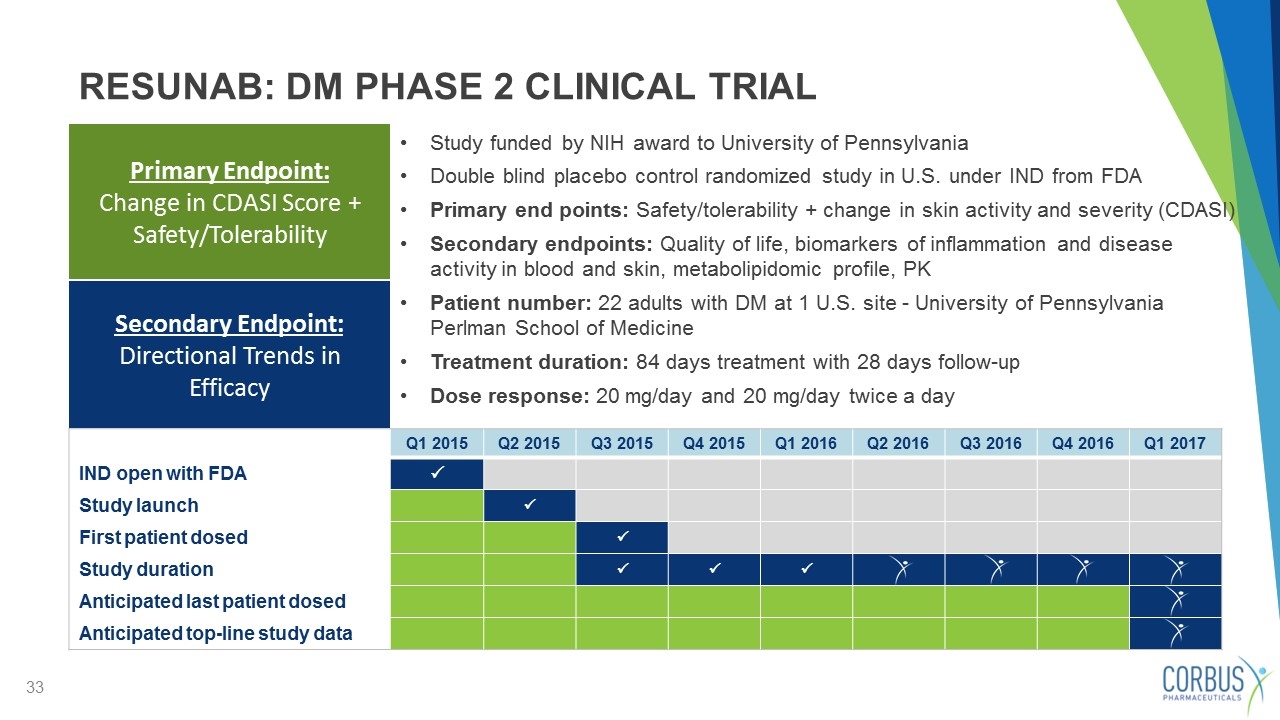
Study funded by NIH award to University of Pennsylvania Double blind placebo control randomized study in U.S. under IND from FDA Primary end points: Safety/tolerability + change in skin activity and severity (CDASI) Secondary endpoints: Quality of life, biomarkers of inflammation and disease activity in blood and skin, metabolipidomic profile, PK Patient number: 22 adults with DM at 1 U.S. site - University of Pennsylvania Perlman School of Medicine Treatment duration: 84 days treatment with 28 days follow-up Dose response: 20 mg/day and 20 mg/day twice a day Primary Endpoint: Change in CDASI Score + Safety/Tolerability RESUNAB: DM PHASE 2 CLINICAL TRIAL Q1 2015 Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 Q4 2016 Q1 2017 IND open with FDA P Study launch P First patient dosed P Study duration P P P Anticipated last patient dosed Anticipated top-line study data Secondary Endpoint: Directional Trends in Efficacy

Study funded by NIH award to Feinstein Institute for Medical Research Double blind placebo control randomized study in U.S. under IND from FDA Primary end points: Efficacy in inflammatory pain in subjects with active musculoskeletal disease Secondary endpoints: Efficacy in overall disease activity, musculoskeletal disease, and quality of life, safety and tolerability, biomarkers of inflammation, metabolipidomic profile, PK Patient number: 100 adults with SLE at 10 U.S. sites Treatment duration: 84 days treatment with 28 days follow-up Dose response: 5 mg/day, 20 mg/day and 20 mg/day twice a day Primary Endpoint: Efficacy in inflammatory pain in active musculoskeletal disease RESUNAB: SLE PHASE 2 CLINICAL TRIAL Secondary Endpoints: Efficacy in overall disease activity, quality of life, safety

JAMES CHMIEL, M.D. CASE WESTERN RESERVE MEDICAL SCHOOL Professor of Pediatrics, National PI on largest ever anti-inflammatory CF study U.S. PI for Cystic Fibrosis ROBERT SPIERA, M.D. HOSPITAL FOR SPECIAL SURGERY Director of the Vasculitis and Scleroderma Program U.S. PI for Scleroderma VICTORIA WERTH, M.D. UNIVERSITY OF PENNSYLVANIA Professor of Dermatology U.S. PI for Dermatomyositis STUART ELBORN, M.D. FRCP QUEEN’S UNIVERSITY, BELFAST Dean of School of Medicine, Dentistry and Biomedical Sciences EU PI for Cystic Fibrosis MEGGAN MACKAY, M.D. HOFSTRA NORTHWELL SCHOOL OF MEDICINE Associate Professor, Molecular Medicine and Medicine Investigator, The Feinstein Institute SCIENTIFIC ADVISORS AND PRINCIPAL INVESTIGATORS CHARLES N. SERHAN, PH.D. BRIGHAM AND WOMEN’S HOSPITAL; HARVARD MEDICAL SCHOOL Director of CET&RI; Professor of Anesthesia, Perioperative and Pain Medicine, Infection and Immunity MICHAEL KNOWLES, M.D., PH.D. UNC CHAPEL HILL Professor of Pulmonary and Critical Care Medicine DANIEL FURST, M.D. UCLA SCHOOL OF MEDICINE Director of UCLA Scleroderma Program ROBERT ZURIER, M.D. UMASS MEDICAL SCHOOL Ex-Chair of Rheumatology Scientific Advisors Principal Investigators
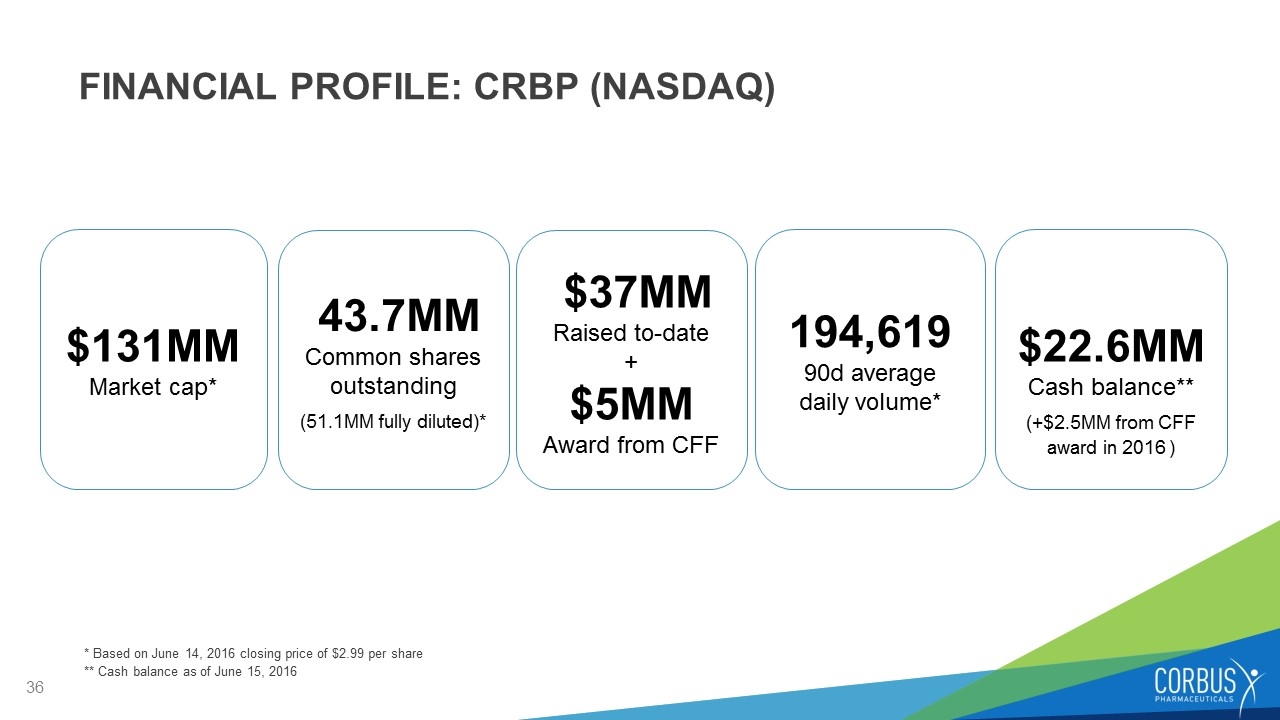
FINANCIAL PROFILE: CRBP (NASDAQ) $131MM Market cap* 43.7MM Common shares outstanding (51.1MM fully diluted)* $37MM Raised to-date + $5MM Award from CFF 194,619 90d average daily volume* $22.6MM Cash balance** (+$2.5MM from CFF award in 2016 ) * Based on June 14, 2016 closing price of $2.99 per share ** Cash balance as of June 15, 2016

CONTACT US Corbus Pharmaceuticals Holdings, Inc. 617.963.0100 info@corbuspharma.com www.corbuspharma.com 100 River Ridge Drive Norwood, MA 02062




































