
Summary of the Veverimer Opportunity Post-VALOR-CKD Data November 2022 Exhibit 99.1

2 Forward Looking Statements Any statements contained in this presentation that are not statements of historical facts are forward-looking statements as defined under the Federal securities laws. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, without limitation, risks associated with the Company’s business prospects, financial results and business operations. For example, the development and any potential commercialization of veverimer will require substantial additional cash. Consequently, any potential counterparty in a strategic transaction involving our Company may choose not to spend additional resources and continue development of veverimer or any alternative product candidates and may attribute little or no value, in such a transaction, to our assets. There can be no assurances that any particular course of action, business arrangement or transaction, or series of transactions, will be pursued, successfully consummated, or lead to any stockholder value. Any such forward-looking statements are based on our current expectations and assumptions, but are subject to a number of risks and uncertainties that could cause our actual future results to differ materially from our current expectations or those implied by the forward-looking statements. These risks and uncertainties are identified and described in more detail in our filings with the Securities and Exchange Commission (the “SEC”), including the Company’s most recent Annual Report filed on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. You should not place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. Except as required by applicable law, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results, later events or circumstances or to reflect the occurrence of unanticipated events.

3 Market Overview
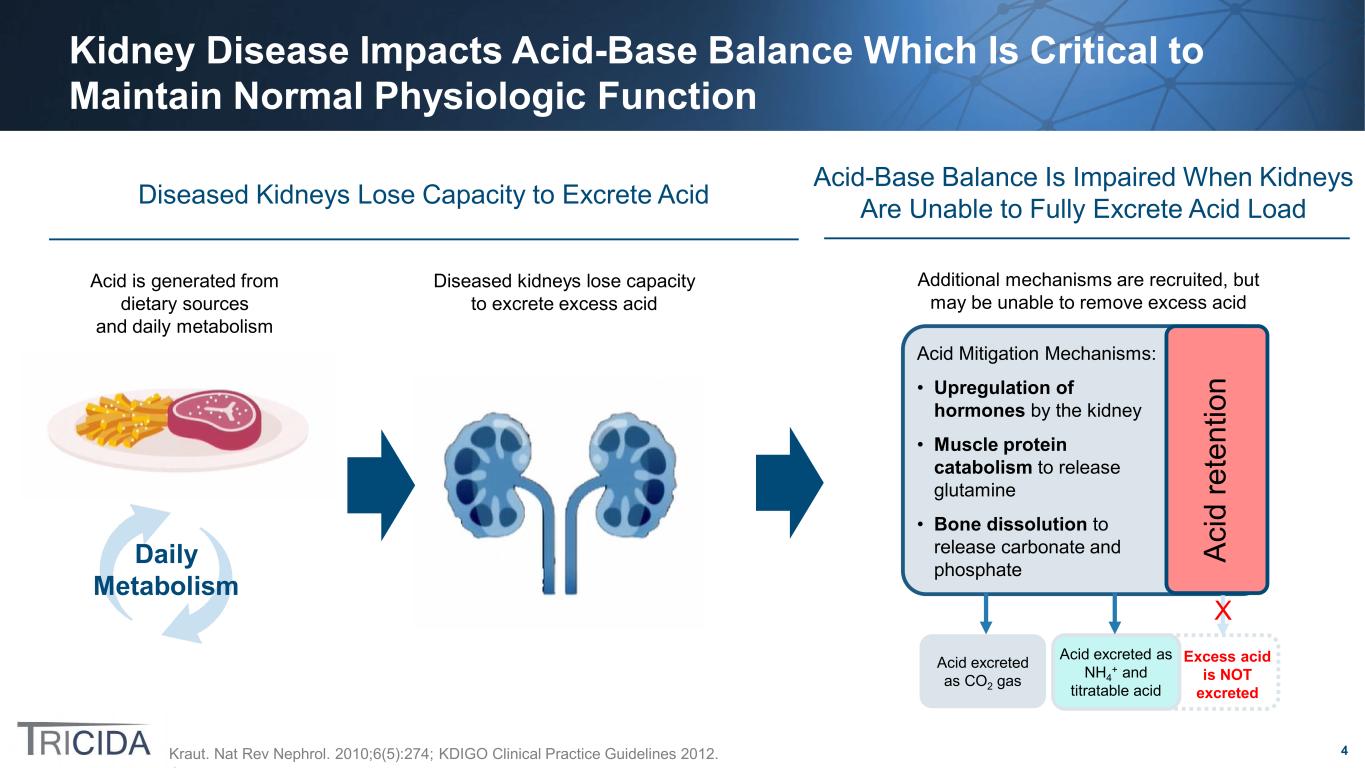
4 Daily Metabolism Kidney Disease Impacts Acid-Base Balance Which Is Critical to Maintain Normal Physiologic Function Kraut. Nat Rev Nephrol. 2010;6(5):274; KDIGO Clinical Practice Guidelines 2012. Diseased Kidneys Lose Capacity to Excrete Acid Acid is generated from dietary sources and daily metabolism Diseased kidneys lose capacity to excrete excess acid Acid Mitigation Mechanisms: • Upregulation of hormones by the kidney • Muscle protein catabolism to release glutamine • Bone dissolution to release carbonate and phosphate Acid excreted as CO2 gas Acid excreted as NH4 + and titratable acid Ac id re te nt io n Excess acid is NOT excreted Acid-Base Balance Is Impaired When Kidneys Are Unable to Fully Excrete Acid Load Additional mechanisms are recruited, but may be unable to remove excess acid X
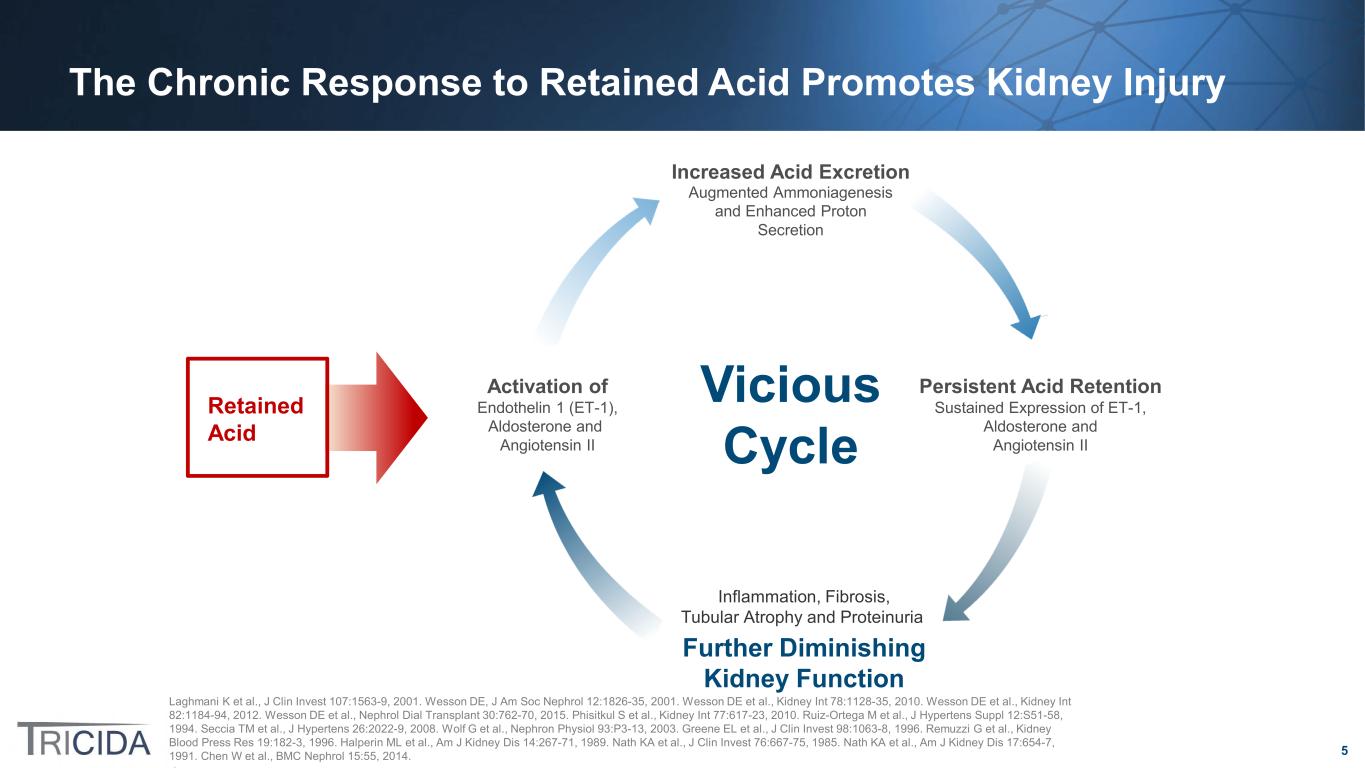
5 Laghmani K et al., J Clin Invest 107:1563-9, 2001. Wesson DE, J Am Soc Nephrol 12:1826-35, 2001. Wesson DE et al., Kidney Int 78:1128-35, 2010. Wesson DE et al., Kidney Int 82:1184-94, 2012. Wesson DE et al., Nephrol Dial Transplant 30:762-70, 2015. Phisitkul S et al., Kidney Int 77:617-23, 2010. Ruiz-Ortega M et al., J Hypertens Suppl 12:S51-58, 1994. Seccia TM et al., J Hypertens 26:2022-9, 2008. Wolf G et al., Nephron Physiol 93:P3-13, 2003. Greene EL et al., J Clin Invest 98:1063-8, 1996. Remuzzi G et al., Kidney Blood Press Res 19:182-3, 1996. Halperin ML et al., Am J Kidney Dis 14:267-71, 1989. Nath KA et al., J Clin Invest 76:667-75, 1985. Nath KA et al., Am J Kidney Dis 17:654-7, 1991. Chen W et al., BMC Nephrol 15:55, 2014. Increased Acid Excretion Augmented Ammoniagenesis and Enhanced Proton Secretion Persistent Acid Retention Sustained Expression of ET-1, Aldosterone and Angiotensin II Endothelin 1 (ET-1), Aldosterone and Angiotensin II Activation of Vicious Cycle Inflammation, Fibrosis, Tubular Atrophy and Proteinuria Further Diminishing Kidney Function Retained Acid The Chronic Response to Retained Acid Promotes Kidney Injury
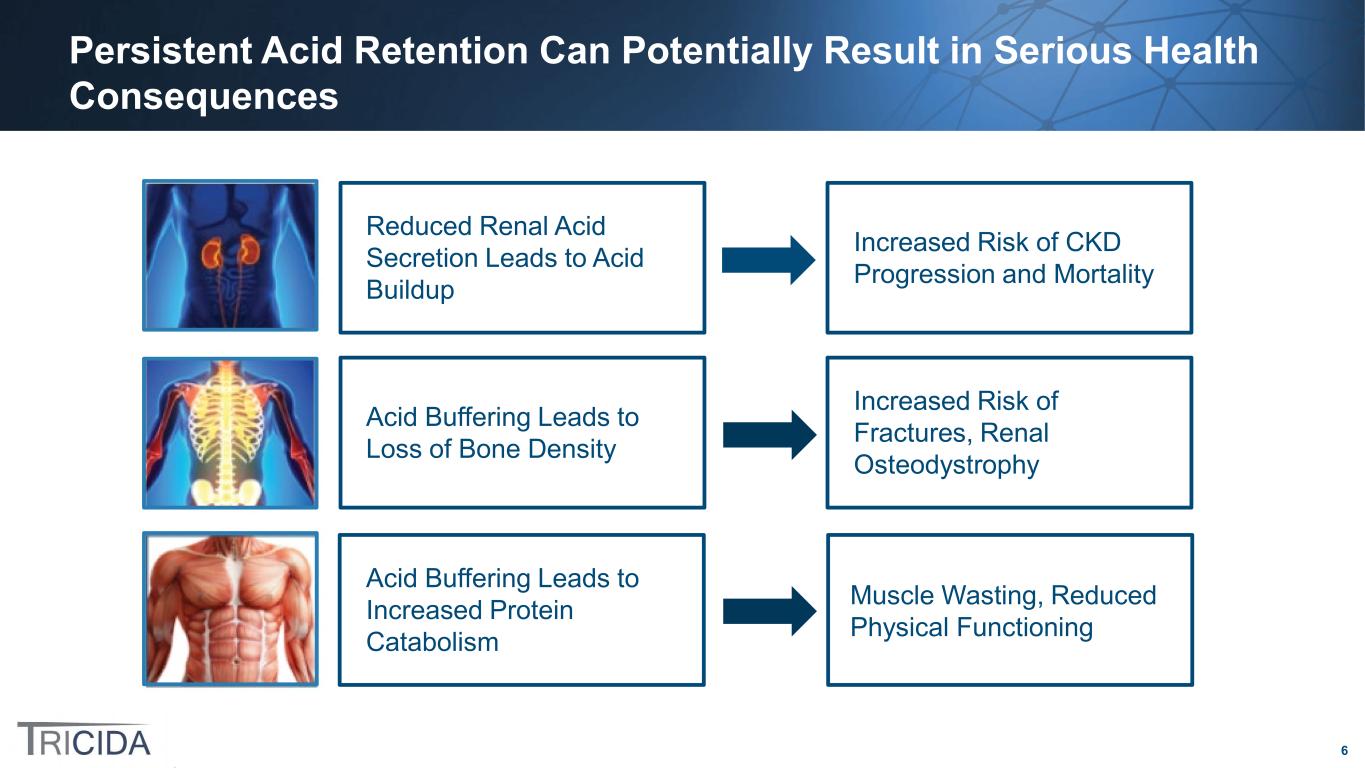
6 Persistent Acid Retention Can Potentially Result in Serious Health Consequences Acid Buffering Leads to Increased Protein Catabolism Muscle Wasting, Reduced Physical Functioning Acid Buffering Leads to Loss of Bone Density Increased Risk of Fractures, Renal Osteodystrophy Reduced Renal Acid Secretion Leads to Acid Buildup Increased Risk of CKD Progression and Mortality

7 (Data on File) Despite the High Unmet Need, There Are No FDA-Approved Therapies to Slow CKD Progression Through the Treatment of Chronic Metabolic Acidosis *Represents patients with Stages 1-5 CKD and metabolic acidosis. Data on file: 2019 US Census data, USRDS 2020 Annual Report, KDIGO 2012, Inker 2011. ** Baseline sodium bicarbonate use in the Tricida TRCA-301 clinical trial, Wesson 2019. Harmful Needs to Be Treated The Majority of Patients with Metabolic Acidosis and CKD are NOT Treated for Their Acidosis No FDA-Approved Treatments ~4,300,000 Patients 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Dobre 2013 Tangri 2022 TRCA-301** Tangri 2019 % o f P at ie nt s R ec ei vi ng O ra l A lk al i T he ra py 8.8% 15.3% 8.7%2.7% OTC Oral Alkali Treatment Rates with CKD are afflicted with metabolic acidosis*

8 Prevalence of Metabolic Acidosis in Patients with CKD and Estimated Number of Patients Treated by Physician Type Nephrologist-treated patients Patients treated by all physicians involved in CKD care Estimated US population of patients with metabolic acidosis and CKD *Represents patients with Stages 1-5 CKD and metabolic acidosis. Source: Data on file. 2019 US Census data, USRDS 2020 AR, KDIGO 2012, Inker 2011, CMS 2020, USRDS 2018 AR. Note: The market potential is based on the target product profile and could change based on actual label approved by FDA Broader Physician Specialty plus Increased Awareness Launch Nephrologist-Focused Launch ~1,700,000 ~4,300,000* ~700,000

9 Veverimer Overview
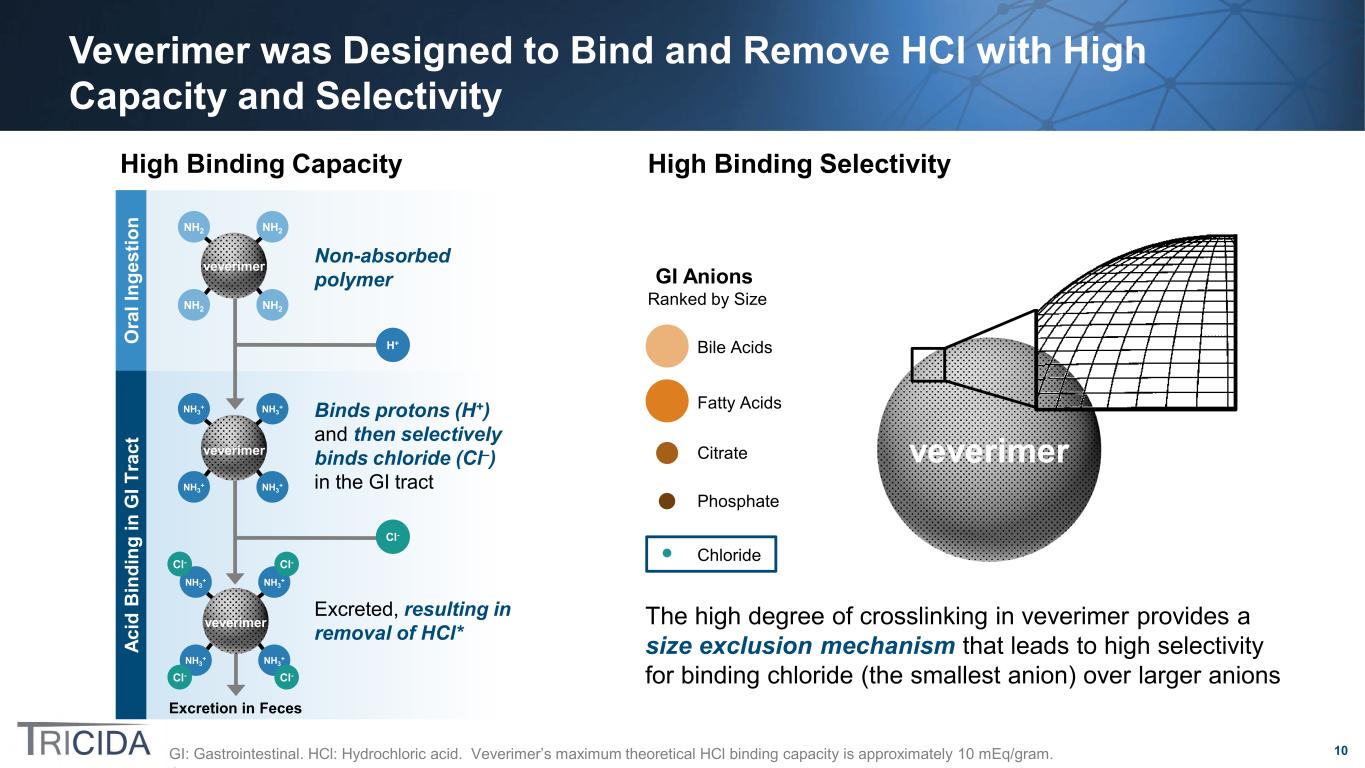
10 Veverimer was Designed to Bind and Remove HCl with High Capacity and Selectivity GI: Gastrointestinal. HCl: Hydrochloric acid. Veverimer’s maximum theoretical HCl binding capacity is approximately 10 mEq/gram. Excreted, resulting in removal of HCl* Binds protons (H+) and then selectively binds chloride (Cl–) in the GI tract Excretion in Feces O ra l I ng es tio n Ac id B in di ng in G I T ra ct NH2 NH2 NH2 NH2 veverimer NH3 + NH3 + NH3 + NH3 + Cl-Cl- Cl- Cl- veverimer Cl- H+ NH3 + NH3 + NH3 + NH3 + veverimer Non-absorbed polymer veverimerCitrate Chloride Fatty Acids Bile Acids Phosphate GI Anions Ranked by Size The high degree of crosslinking in veverimer provides a size exclusion mechanism that leads to high selectivity for binding chloride (the smallest anion) over larger anions High Binding Capacity High Binding Selectivity
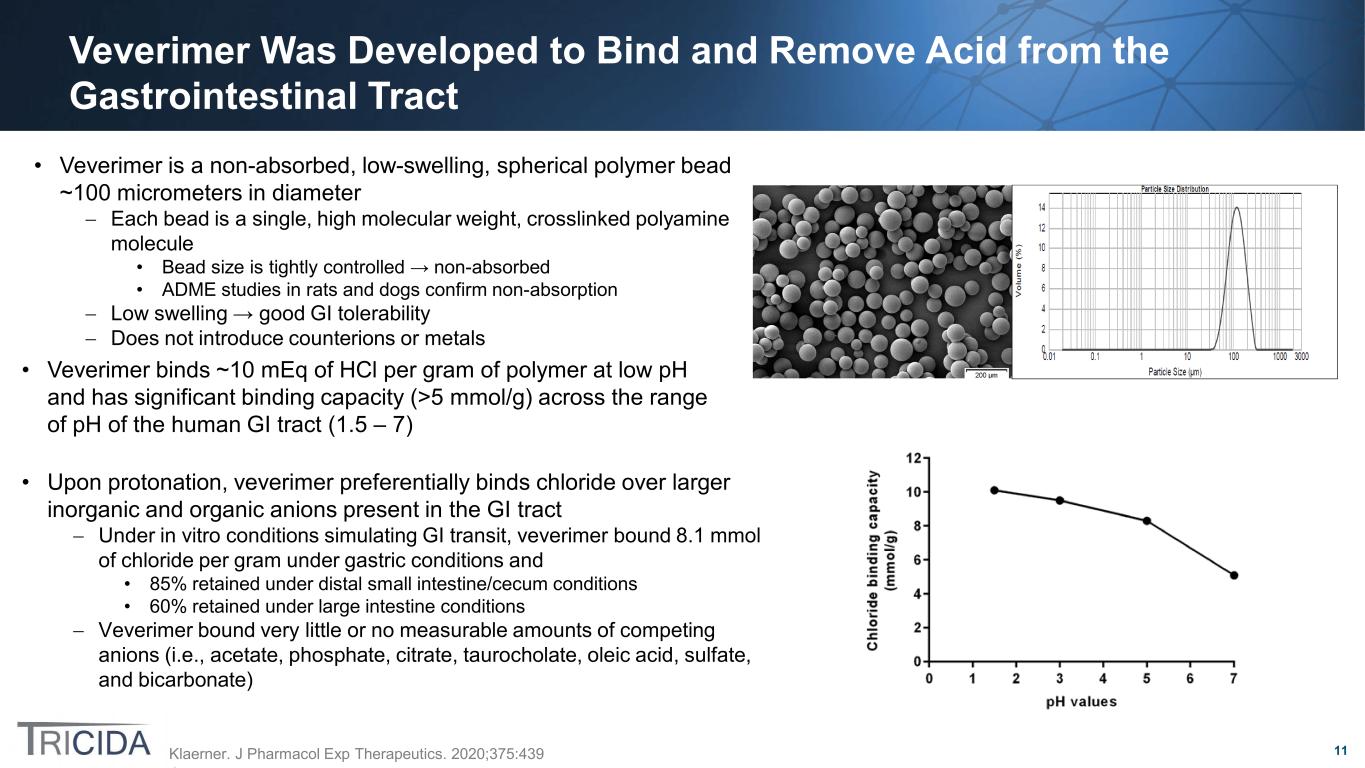
11 Veverimer Was Developed to Bind and Remove Acid from the Gastrointestinal Tract Klaerner. J Pharmacol Exp Therapeutics. 2020;375:439 • Veverimer is a non-absorbed, low-swelling, spherical polymer bead ~100 micrometers in diameter − Each bead is a single, high molecular weight, crosslinked polyamine molecule • Bead size is tightly controlled → non-absorbed • ADME studies in rats and dogs confirm non-absorption − Low swelling → good GI tolerability − Does not introduce counterions or metals • Veverimer binds ~10 mEq of HCl per gram of polymer at low pH and has significant binding capacity (>5 mmol/g) across the range of pH of the human GI tract (1.5 – 7) • Upon protonation, veverimer preferentially binds chloride over larger inorganic and organic anions present in the GI tract − Under in vitro conditions simulating GI transit, veverimer bound 8.1 mmol of chloride per gram under gastric conditions and • 85% retained under distal small intestine/cecum conditions • 60% retained under large intestine conditions − Veverimer bound very little or no measurable amounts of competing anions (i.e., acetate, phosphate, citrate, taurocholate, oleic acid, sulfate, and bicarbonate)
12 Veverimer Is Unlikely to Have Significant Drug-Drug Interactions Parsell. Drug Metab Dispos. 2021;49:490 • In vitro binding experiments using 16 test drugs, showed no positively charged, neutral, or zwitterionic drugs bound to veverimer • Three negatively charged drugs (furosemide, aspirin, ethacrynic acid) bound to veverimer – This binding was reduced or eliminated in the presence of physiologic concentrations (100–170 mM) of chloride • Human DDI studies with 2 drugs showing the most in vitro binding to veverimer (aspirin, furosemide) showed no clinically meaningful DDIs • As measured with a 24-hour gastric probe in human volunteers, veverimer increased gastric pH by 1.5–3 pH units – pH elevation peaked within 1 hour and had returned to baseline after 1.5–3 hours – Omeprazole did not alter the effect of veverimer on gastric pH • Human DDI studies with 2 drugs with pH-dependent solubility (warfarin, dabigatran) showed no clinically meaningful DDIs Effects on absorption of other drugs through binding to veverimer in the GI tract Increases in gastric pH caused by veverimer binding to HCl Veverimer is not systemically absorbed, so potential DDIs are limited to:

13 Veverimer Manufacturing • Veverimer drug substance (poly[allylamine-co-N,N′-diallyl-1,3-diaminopropane-co-1,2-diaminoethane]) is a free- flowing powder composed of low-swelling polymeric beads • Drug substance is manufactured at Patheon Austria GmbH & Co KG (Patheon) using a 2-step process at current scale of 700 kg – Step 1: preparation of crosslinked polymer beads of defined size, having a high binding capacity – Step 2: further crosslinking for low swelling and selectivity for HCl – Multiple metric tons of drug substance have been manufactured for commercial use • Drug product comprises veverimer drug substance alone (no excipients)

14 Veverimer Intellectual Property • Veverimer was discovered by Tricida utilizing its own proprietary technology • Tricida’s patent portfolio includes 233 patents in 52 different countries, including – compositions-of-matter – dosage unit forms – methods-of-treatment – medical use – methods of manufacture • Tricida’s patent protection extends through 2038
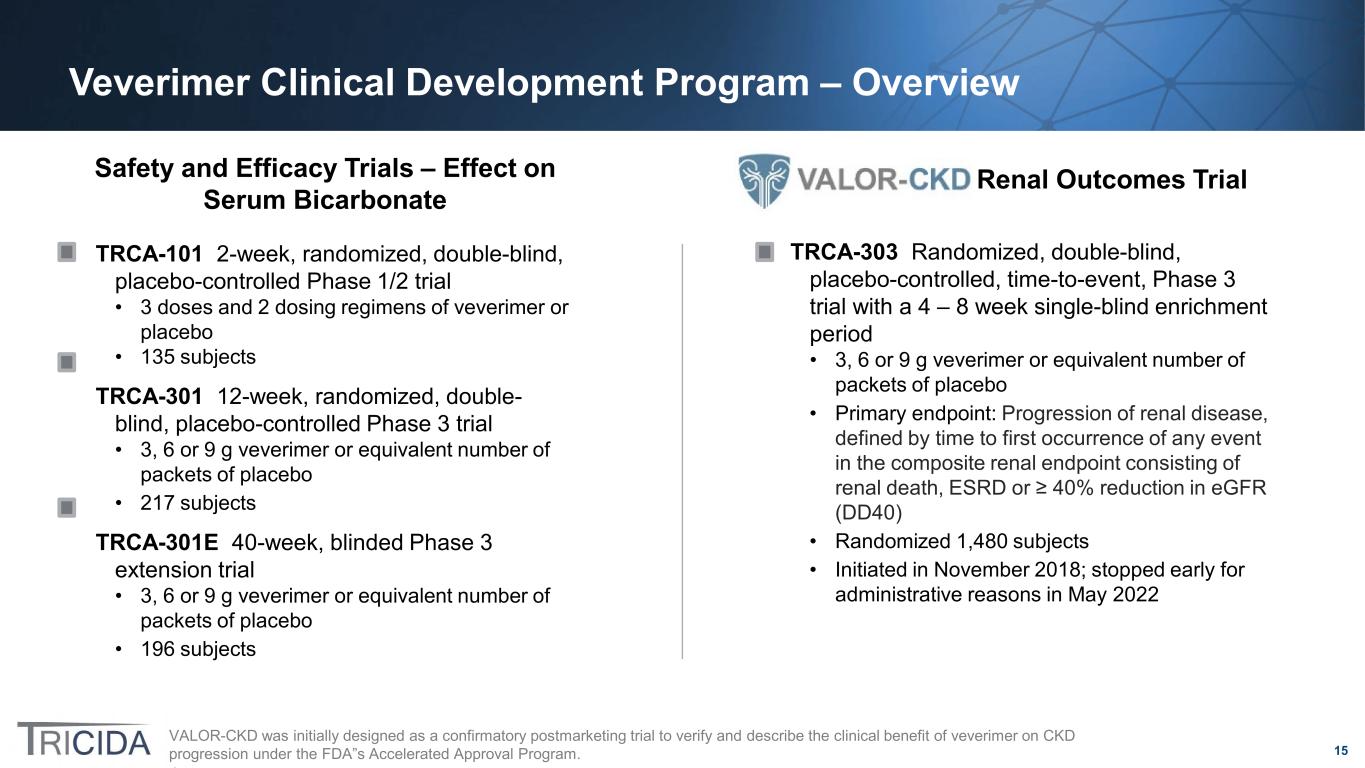
15 Veverimer Clinical Development Program – Overview VALOR-CKD was initially designed as a confirmatory postmarketing trial to verify and describe the clinical benefit of veverimer on CKD progression under the FDA”s Accelerated Approval Program. Safety and Efficacy Trials – Effect on Serum Bicarbonate TRCA-101 2-week, randomized, double-blind, placebo-controlled Phase 1/2 trial • 3 doses and 2 dosing regimens of veverimer or placebo • 135 subjects TRCA-301 12-week, randomized, double- blind, placebo-controlled Phase 3 trial • 3, 6 or 9 g veverimer or equivalent number of packets of placebo • 217 subjects TRCA-301E 40-week, blinded Phase 3 extension trial • 3, 6 or 9 g veverimer or equivalent number of packets of placebo • 196 subjects TRCA-303 Randomized, double-blind, placebo-controlled, time-to-event, Phase 3 trial with a 4 – 8 week single-blind enrichment period • 3, 6 or 9 g veverimer or equivalent number of packets of placebo • Primary endpoint: Progression of renal disease, defined by time to first occurrence of any event in the composite renal endpoint consisting of renal death, ESRD or ≥ 40% reduction in eGFR (DD40) • Randomized 1,480 subjects • Initiated in November 2018; stopped early for administrative reasons in May 2022 Renal Outcomes Trial

16 VALOR-CKD Pivotal Trial Summary

17 The VALOR-CKD Trial Design Overview • Time-to-event, multicenter, randomized, double-blind, placebo-controlled trial • Primary endpoint: DD40, defined as time to first occurrence of renal death, ESRD or a confirmed ≥ 40% reduction in eGFR Veverimer QD (N = 741) Placebo QD (N = 739)Tr ea tm en t Trial terminated early for administrative reasons in May 2022 Subjects with serum bicarbonate of 12 – 20 mEq/L, eGFR of 20 – 40 ml/min/1.73m2 Screening VALOR-CKD Renal Outcomes Trial Single-Blind Active Treatment Period: 4 to 8 Weeks All on Veverimer R Part A Part B – Randomized Withdrawal Patients who experienced a ≥4 mEq/L increase in serum bicarbonate and those whose serum bicarbonate increased into the normal range of ≥22 mEq/L were randomized to veverimer or to placebo treatment To enter Part A, patients were required to have three serum bicarbonate values at least 2 weeks apart, each within the range 12 – 20 mEq/L, within a 6-week period
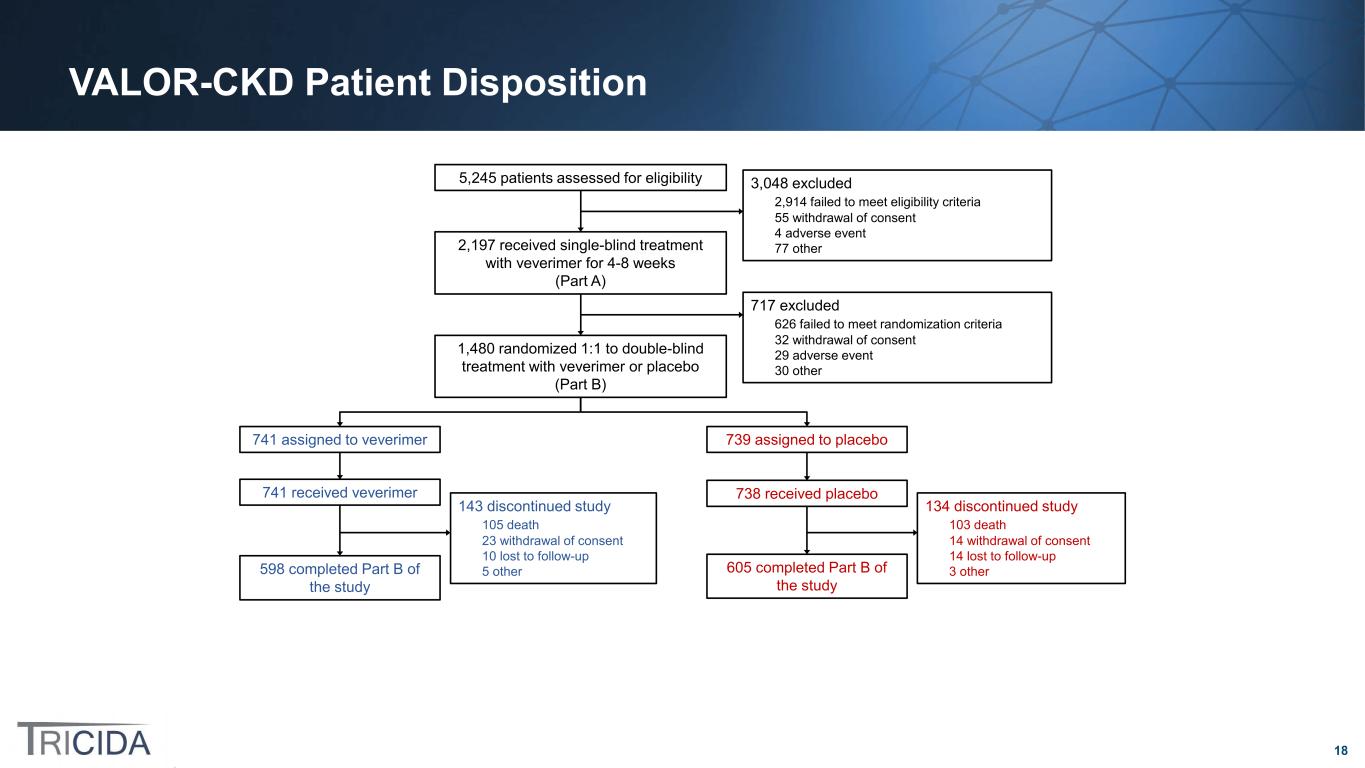
18 VALOR-CKD Patient Disposition 741 assigned to veverimer 739 assigned to placebo 143 discontinued study 105 death 23 withdrawal of consent 10 lost to follow-up 5 other598 completed Part B of the study 741 received veverimer 738 received placebo 5,245 patients assessed for eligibility 3,048 excluded 2,914 failed to meet eligibility criteria 55 withdrawal of consent 4 adverse event 77 other2,197 received single-blind treatment with veverimer for 4-8 weeks (Part A) 717 excluded 626 failed to meet randomization criteria 32 withdrawal of consent 29 adverse event 30 other 1,480 randomized 1:1 to double-blind treatment with veverimer or placebo (Part B) 605 completed Part B of the study 134 discontinued study 103 death 14 withdrawal of consent 14 lost to follow-up 3 other
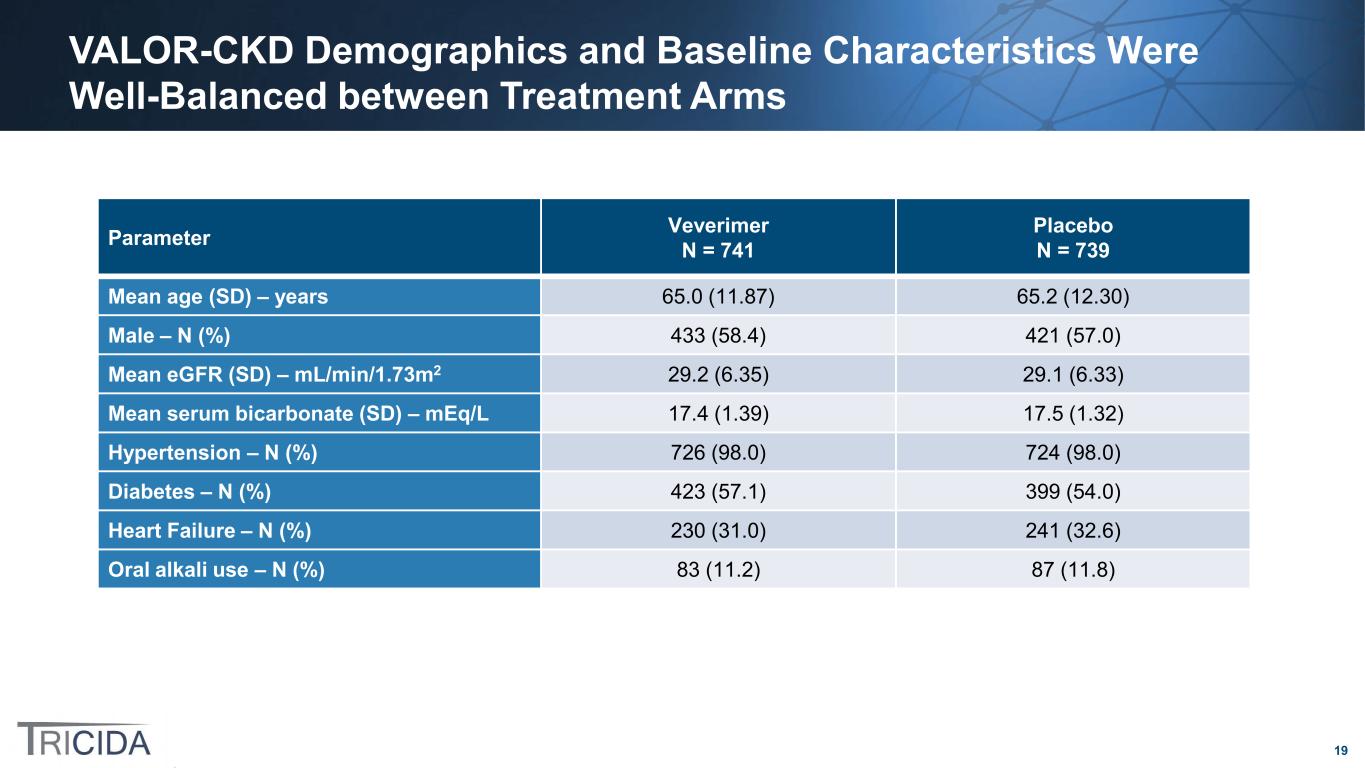
19 VALOR-CKD Demographics and Baseline Characteristics Were Well-Balanced between Treatment Arms Parameter Veverimer N = 741 Placebo N = 739 Mean age (SD) – years 65.0 (11.87) 65.2 (12.30) Male – N (%) 433 (58.4) 421 (57.0) Mean eGFR (SD) – mL/min/1.73m2 29.2 (6.35) 29.1 (6.33) Mean serum bicarbonate (SD) – mEq/L 17.4 (1.39) 17.5 (1.32) Hypertension – N (%) 726 (98.0) 724 (98.0) Diabetes – N (%) 423 (57.1) 399 (54.0) Heart Failure – N (%) 230 (31.0) 241 (32.6) Oral alkali use – N (%) 83 (11.2) 87 (11.8)
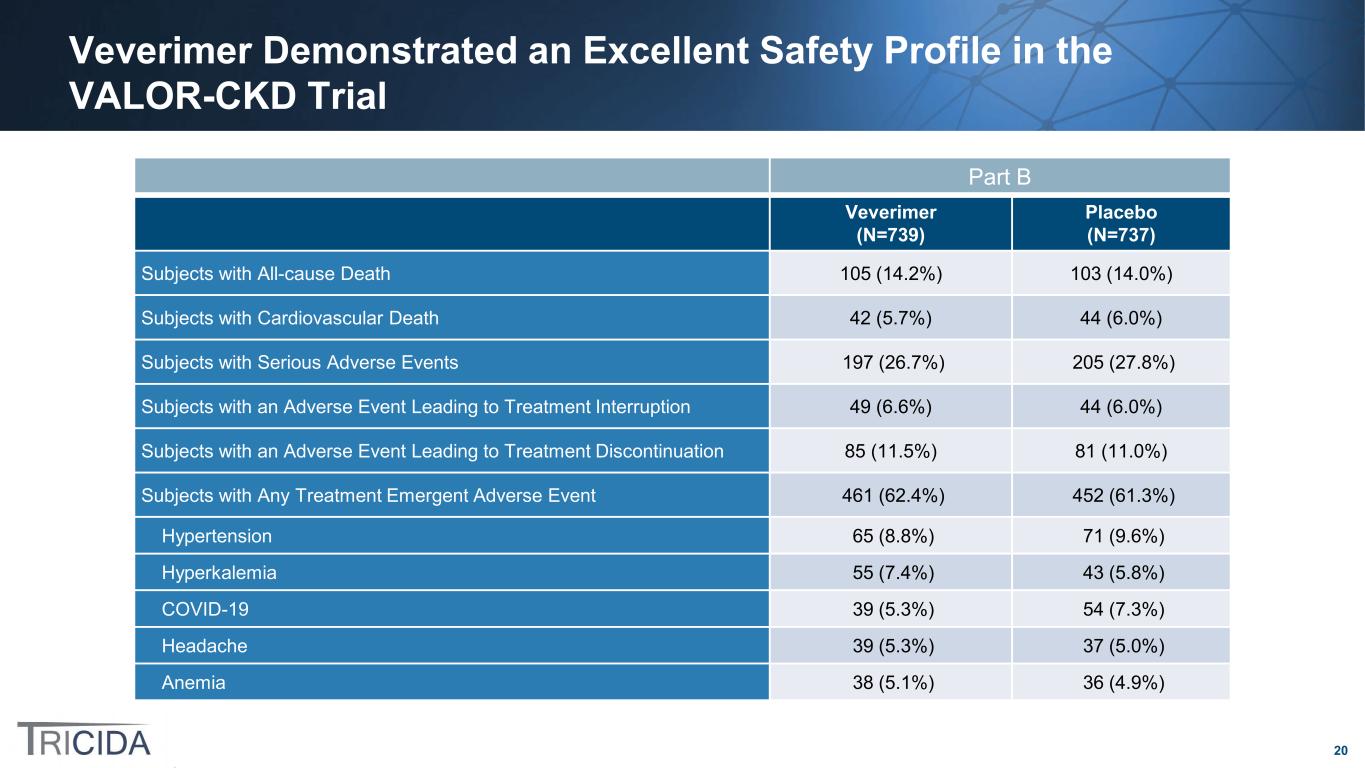
20 Veverimer Demonstrated an Excellent Safety Profile in the VALOR-CKD Trial Part B Veverimer (N=739) Placebo (N=737) Subjects with All-cause Death 105 (14.2%) 103 (14.0%) Subjects with Cardiovascular Death 42 (5.7%) 44 (6.0%) Subjects with Serious Adverse Events 197 (26.7%) 205 (27.8%) Subjects with an Adverse Event Leading to Treatment Interruption 49 (6.6%) 44 (6.0%) Subjects with an Adverse Event Leading to Treatment Discontinuation 85 (11.5%) 81 (11.0%) Subjects with Any Treatment Emergent Adverse Event 461 (62.4%) 452 (61.3%) Hypertension 65 (8.8%) 71 (9.6%) Hyperkalemia 55 (7.4%) 43 (5.8%) COVID-19 39 (5.3%) 54 (7.3%) Headache 39 (5.3%) 37 (5.0%) Anemia 38 (5.1%) 36 (4.9%)
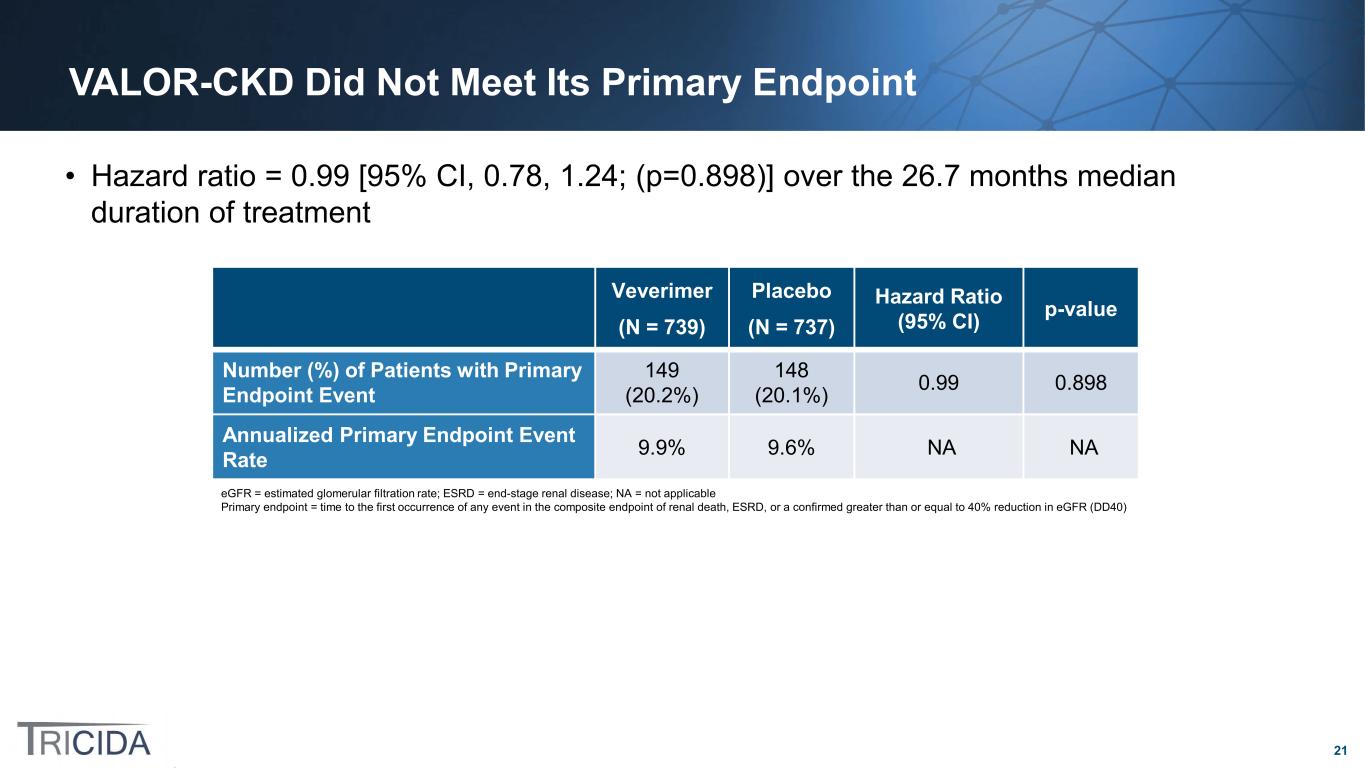
21 VALOR-CKD Did Not Meet Its Primary Endpoint • Hazard ratio = 0.99 [95% CI, 0.78, 1.24; (p=0.898)] over the 26.7 months median duration of treatment Veverimer (N = 739) Placebo (N = 737) Hazard Ratio (95% CI) p-value Number (%) of Patients with Primary Endpoint Event 149 (20.2%) 148 (20.1%) 0.99 0.898 Annualized Primary Endpoint Event Rate 9.9% 9.6% NA NA eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; NA = not applicable Primary endpoint = time to the first occurrence of any event in the composite endpoint of renal death, ESRD, or a confirmed greater than or equal to 40% reduction in eGFR (DD40)
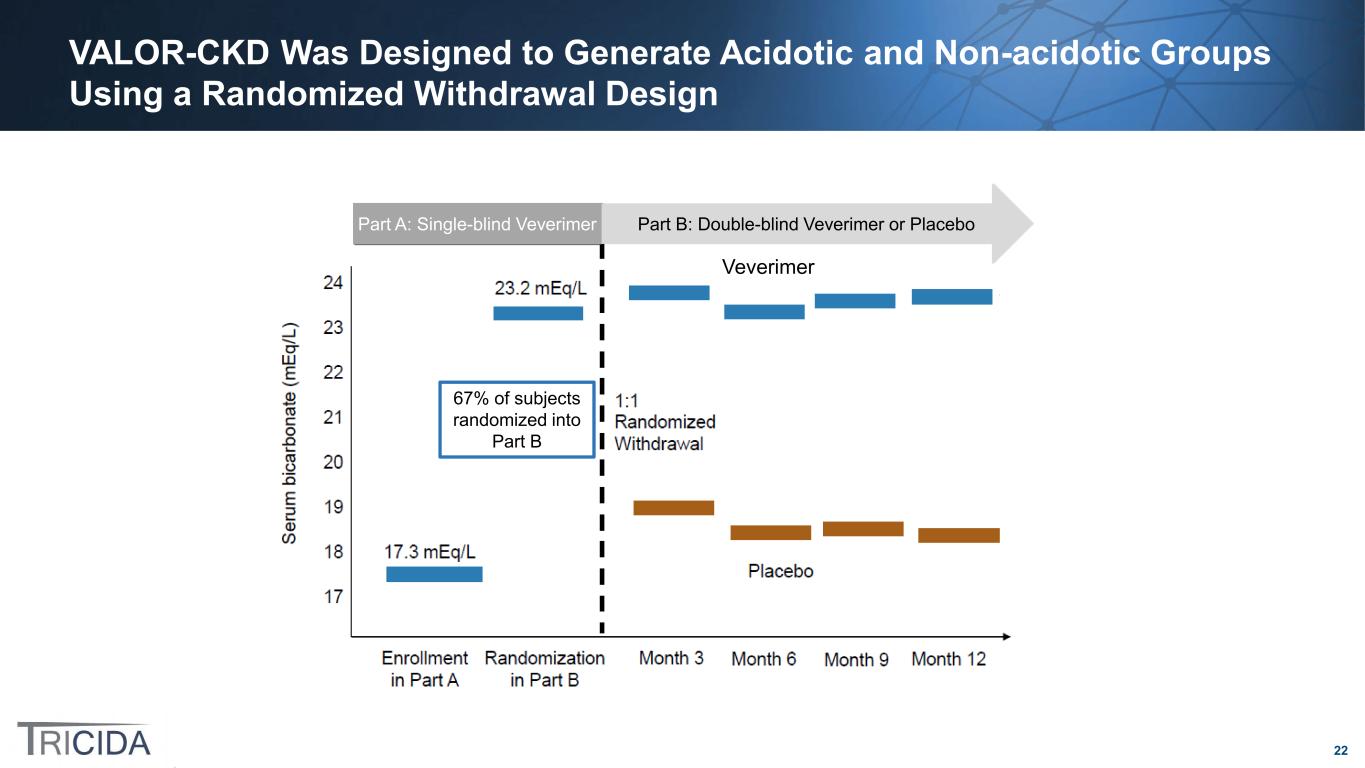
22 VALOR-CKD Was Designed to Generate Acidotic and Non-acidotic Groups Using a Randomized Withdrawal Design Part B: Double-blind Veverimer or PlaceboPart A: Single-blind Veverimer Veverimer 67% of subjects randomized into Part B

23 In the VALOR-CKD Trial, the Veverimer and Placebo Groups Demonstrated Unexpected Serum Bicarbonate Results • In Part A, the average increase in serum bicarbonate was 5.9 mEq/L, increasing from 17.5 mEq/L to 23.4 mEq/L • In Part B: ‒ The average serum bicarbonate over months 3 to 12 was: Veverimer = 21.8 mEq/L Placebo = 20.9 mEq/L ‒ The veverimer group unexpectedly dropped by 1.41 mEq/L after randomization ‒ The placebo group only dropped by 2.55 mEq/L and did not decrease to baseline after randomization Serum Bicarbonate Measurements In the VALOR-CKD Trial Part A Part B
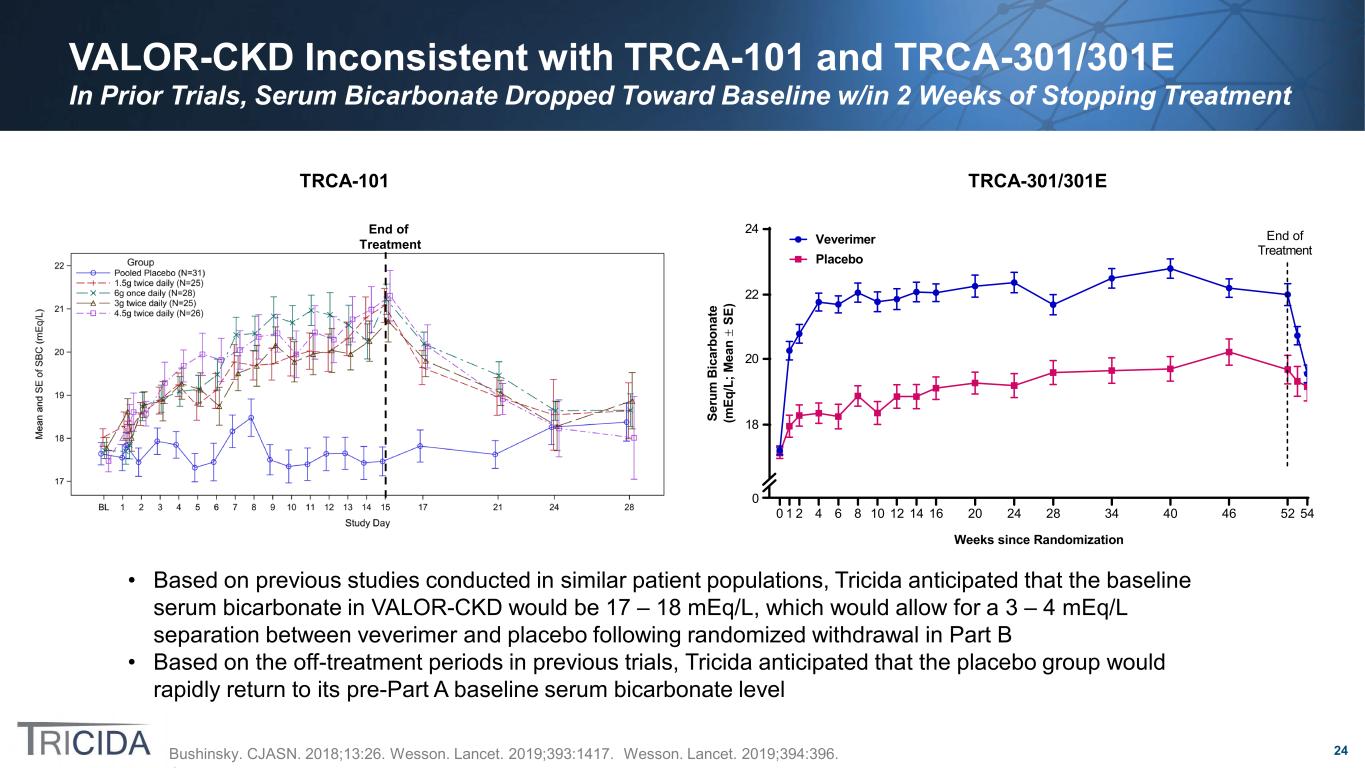
24 VALOR-CKD Inconsistent with TRCA-101 and TRCA-301/301E In Prior Trials, Serum Bicarbonate Dropped Toward Baseline w/in 2 Weeks of Stopping Treatment 0 1 2 4 6 8 10 12 14 16 20 24 28 34 40 46 52 54 Weeks since Randomization Se ru m B ic ar bo na te (m Eq /L ; M ea n ± SE ) Veverimer Placebo End of Treatment 0 18 20 22 24End of Treatment TRCA-101 TRCA-301/301E Bushinsky. CJASN. 2018;13:26. Wesson. Lancet. 2019;393:1417. Wesson. Lancet. 2019;394:396. • Based on previous studies conducted in similar patient populations, Tricida anticipated that the baseline serum bicarbonate in VALOR-CKD would be 17 – 18 mEq/L, which would allow for a 3 – 4 mEq/L separation between veverimer and placebo following randomized withdrawal in Part B • Based on the off-treatment periods in previous trials, Tricida anticipated that the placebo group would rapidly return to its pre-Part A baseline serum bicarbonate level
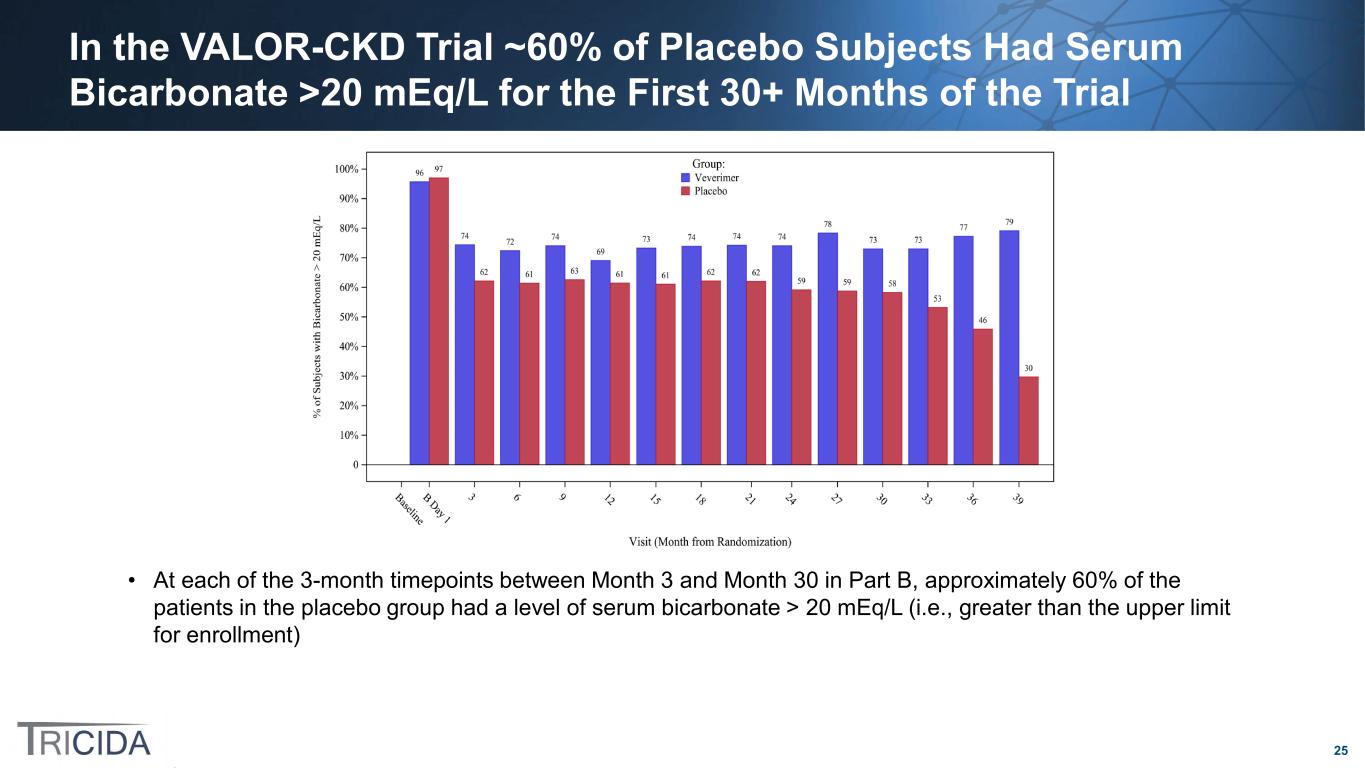
25 In the VALOR-CKD Trial ~60% of Placebo Subjects Had Serum Bicarbonate >20 mEq/L for the First 30+ Months of the Trial • At each of the 3-month timepoints between Month 3 and Month 30 in Part B, approximately 60% of the patients in the placebo group had a level of serum bicarbonate > 20 mEq/L (i.e., greater than the upper limit for enrollment)

26 Potential Alternatives for Future Development of Veverimer for the Treatment of Metabolic Acidosis Post-VALOR-CKD
27 Interpretation of the Negative VALOR-CKD Primary Endpoint Results to Facilitate Design of a Potential New Outcomes Trial in Chronic Metabolic Acidosis • The difference in serum bicarbonate levels between the active and placebo treatment groups was insufficient to evaluate the effect of veverimer on slowing CKD progression by treating metabolic acidosis in the VALOR-CKD trial • More stringent entry requirements for a stable acidotic baseline and a more robust response to veverimer could result in sufficient separation in serum bicarbonate to assess the effect of veverimer on CKD progression
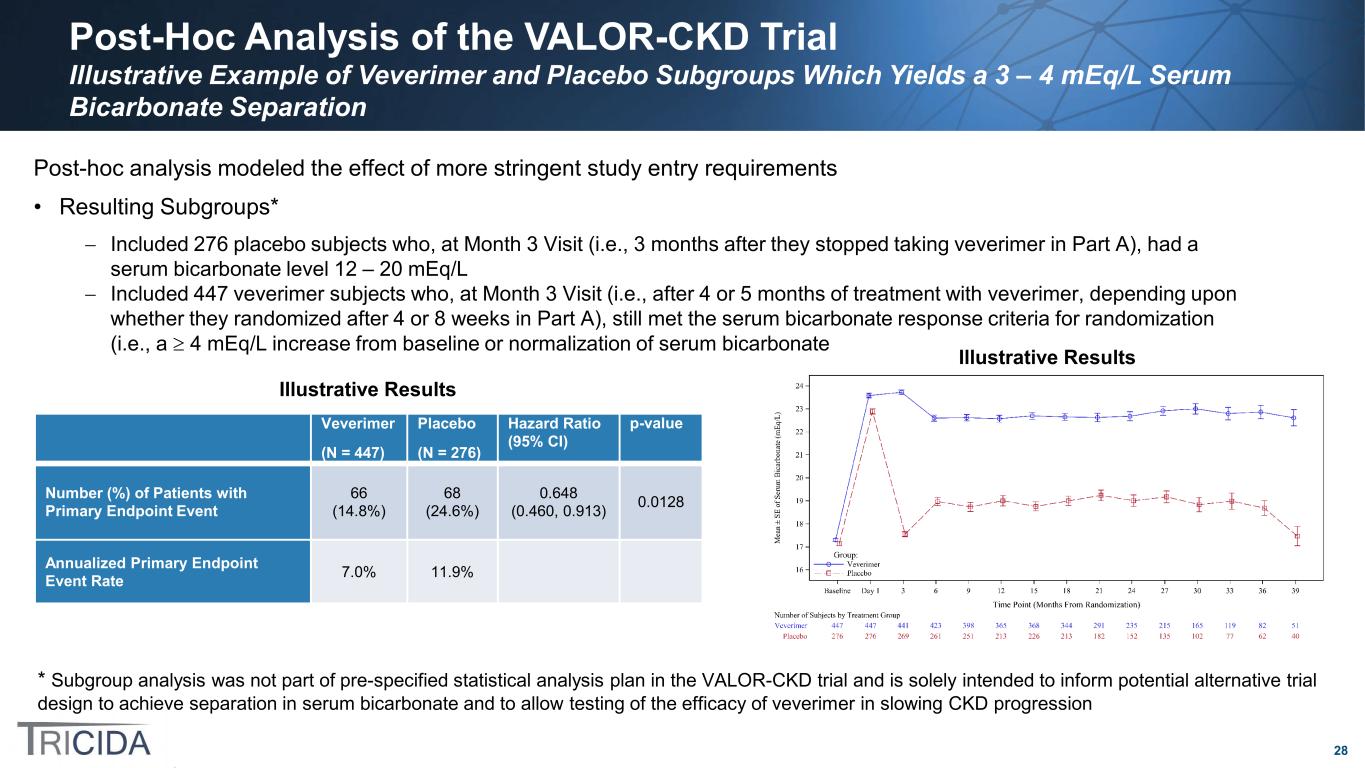
28 Post-Hoc Analysis of the VALOR-CKD Trial Illustrative Example of Veverimer and Placebo Subgroups Which Yields a 3 – 4 mEq/L Serum Bicarbonate Separation * Subgroup analysis was not part of pre-specified statistical analysis plan in the VALOR-CKD trial and is solely intended to inform potential alternative trial design to achieve separation in serum bicarbonate and to allow testing of the efficacy of veverimer in slowing CKD progression Veverimer (N = 447) Placebo (N = 276) Hazard Ratio (95% CI) p-value Number (%) of Patients with Primary Endpoint Event 66 (14.8%) 68 (24.6%) 0.648 (0.460, 0.913) 0.0128 Annualized Primary Endpoint Event Rate 7.0% 11.9% Post-hoc analysis modeled the effect of more stringent study entry requirements • Resulting Subgroups* − Included 276 placebo subjects who, at Month 3 Visit (i.e., 3 months after they stopped taking veverimer in Part A), had a serum bicarbonate level 12 – 20 mEq/L − Included 447 veverimer subjects who, at Month 3 Visit (i.e., after 4 or 5 months of treatment with veverimer, depending upon whether they randomized after 4 or 8 weeks in Part A), still met the serum bicarbonate response criteria for randomization (i.e., a ≥ 4 mEq/L increase from baseline or normalization of serum bicarbonate Illustrative Results Illustrative Results
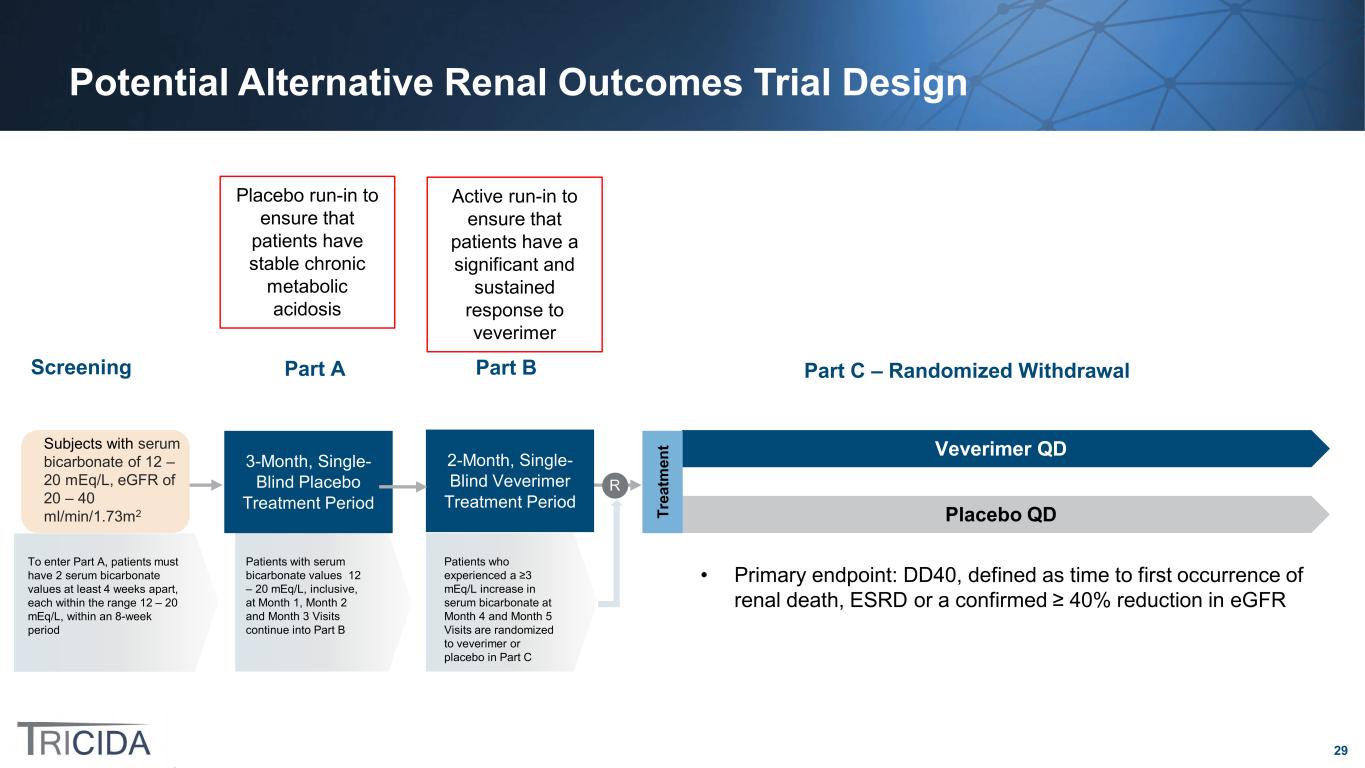
29 Potential Alternative Renal Outcomes Trial Design Placebo run-in to ensure that patients have stable chronic metabolic acidosis Veverimer QD Placebo QDTr ea tm en tSubjects with serum bicarbonate of 12 – 20 mEq/L, eGFR of 20 – 40 ml/min/1.73m2 Screening 3-Month, Single- Blind Placebo Treatment Period R Part A Part C – Randomized Withdrawal Patients who experienced a ≥3 mEq/L increase in serum bicarbonate at Month 4 and Month 5 Visits are randomized to veverimer or placebo in Part C To enter Part A, patients must have 2 serum bicarbonate values at least 4 weeks apart, each within the range 12 – 20 mEq/L, within an 8-week period Patients with serum bicarbonate values 12 – 20 mEq/L, inclusive, at Month 1, Month 2 and Month 3 Visits continue into Part B 2-Month, Single- Blind Veverimer Treatment Period Part B • Primary endpoint: DD40, defined as time to first occurrence of renal death, ESRD or a confirmed ≥ 40% reduction in eGFR Active run-in to ensure that patients have a significant and sustained response to veverimer
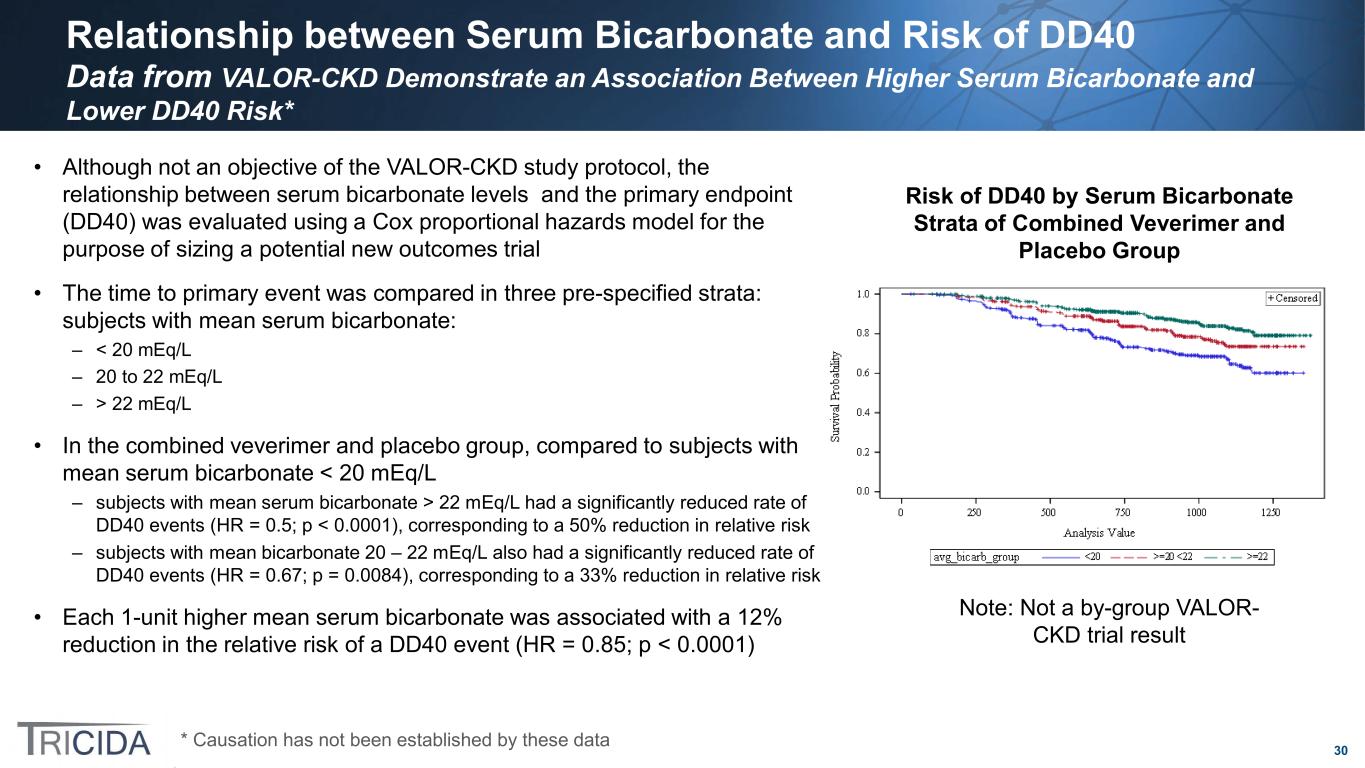
30 Relationship between Serum Bicarbonate and Risk of DD40 Data from VALOR-CKD Demonstrate an Association Between Higher Serum Bicarbonate and Lower DD40 Risk* • Although not an objective of the VALOR-CKD study protocol, the relationship between serum bicarbonate levels and the primary endpoint (DD40) was evaluated using a Cox proportional hazards model for the purpose of sizing a potential new outcomes trial • The time to primary event was compared in three pre-specified strata: subjects with mean serum bicarbonate: – < 20 mEq/L – 20 to 22 mEq/L – > 22 mEq/L • In the combined veverimer and placebo group, compared to subjects with mean serum bicarbonate < 20 mEq/L – subjects with mean serum bicarbonate > 22 mEq/L had a significantly reduced rate of DD40 events (HR = 0.5; p < 0.0001), corresponding to a 50% reduction in relative risk – subjects with mean bicarbonate 20 – 22 mEq/L also had a significantly reduced rate of DD40 events (HR = 0.67; p = 0.0084), corresponding to a 33% reduction in relative risk • Each 1-unit higher mean serum bicarbonate was associated with a 12% reduction in the relative risk of a DD40 event (HR = 0.85; p < 0.0001) Risk of DD40 by Serum Bicarbonate Strata of Combined Veverimer and Placebo Group Note: Not a by-group VALOR- CKD trial result * Causation has not been established by these data
31 Illustrative Example of Sample Size Calculation Based on VALOR-CKD Data and the Observed Relationship between Serum Bicarbonate and DD40 • Illustrative sample size assumptions – Placebo event rate = 9.6% per year – Hazard ratio = 0.723 (derived from relationship between serum bicarbonate and DD40 in VALOR- CKD; VALOR-CKD showed a hazard ratio for the DD40 endpoint of 0.85 per 1 mEq/L higher serum bicarbonate; a HR of 0.723 would be expected for a 2 mEq/L difference in serum bicarbonate) – Enrollment = 3 years – Maximum study duration = 5 years – Study discontinuation rate = 8.5% per year – 2-sided alpha = 0.05 – 90% power: 1824 subjects total; 400 primary endpoint events
32 VALOR-CKD Data Related to Physical Function • No significant effects of veverimer were observed on the two secondary endpoints that assessed physical function (in contrast to results from the TRCA-301E study) – Change from baseline to Month 12 in the total score of the KDQOL physical function domain (p = NS) – Change from baseline to Month 12 in the time to complete a 5-times repeated chair stand test (p = NS) • As an exploratory endpoint, DXA scans were performed at a subset of sites in VALOR-CKD to evaluate potential effects of veverimer on bone (i.e., bone mineral density, trabecular bone score) and muscle (i.e., total lean mass and appendicular muscle mass – No significant differences between the veverimer and placebo treatment groups for measures of bone mineral density or trabecular bone score – In the small group of patients who participated at study centers with the capability to conduct DXA scans that measured muscle mass (veverimer = 14 subjects; placebo = 13 subjects) a significant difference was observed between groups in the percent change from baseline to end of treatment in total lean mass (4.82%), favoring veverimer (p = 0.0289) • A trend favoring veverimer (p = 0.0584) was also observed in the between-group difference in percentage change in appendicular muscle mass (5.02%) Note: The very small group of patients in this analysis is a limitation

33 Alternative Development Opportunities
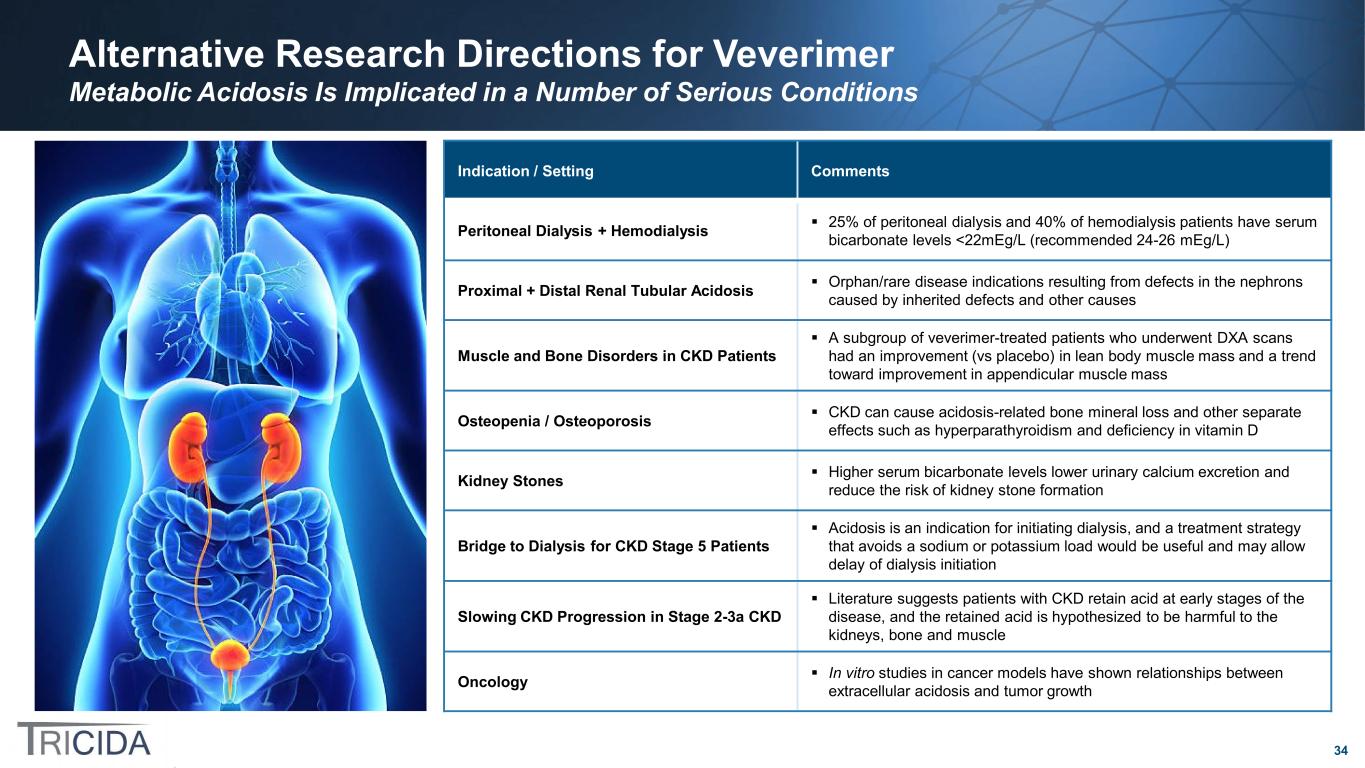
34 Alternative Research Directions for Veverimer Metabolic Acidosis Is Implicated in a Number of Serious Conditions Indication / Setting Comments Peritoneal Dialysis + Hemodialysis 25% of peritoneal dialysis and 40% of hemodialysis patients have serum bicarbonate levels <22mEg/L (recommended 24-26 mEg/L) Proximal + Distal Renal Tubular Acidosis Orphan/rare disease indications resulting from defects in the nephrons caused by inherited defects and other causes Muscle and Bone Disorders in CKD Patients A subgroup of veverimer-treated patients who underwent DXA scans had an improvement (vs placebo) in lean body muscle mass and a trend toward improvement in appendicular muscle mass Osteopenia / Osteoporosis CKD can cause acidosis-related bone mineral loss and other separate effects such as hyperparathyroidism and deficiency in vitamin D Kidney Stones Higher serum bicarbonate levels lower urinary calcium excretion and reduce the risk of kidney stone formation Bridge to Dialysis for CKD Stage 5 Patients Acidosis is an indication for initiating dialysis, and a treatment strategy that avoids a sodium or potassium load would be useful and may allow delay of dialysis initiation Slowing CKD Progression in Stage 2-3a CKD Literature suggests patients with CKD retain acid at early stages of the disease, and the retained acid is hypothesized to be harmful to the kidneys, bone and muscle Oncology In vitro studies in cancer models have shown relationships between extracellular acidosis and tumor growth
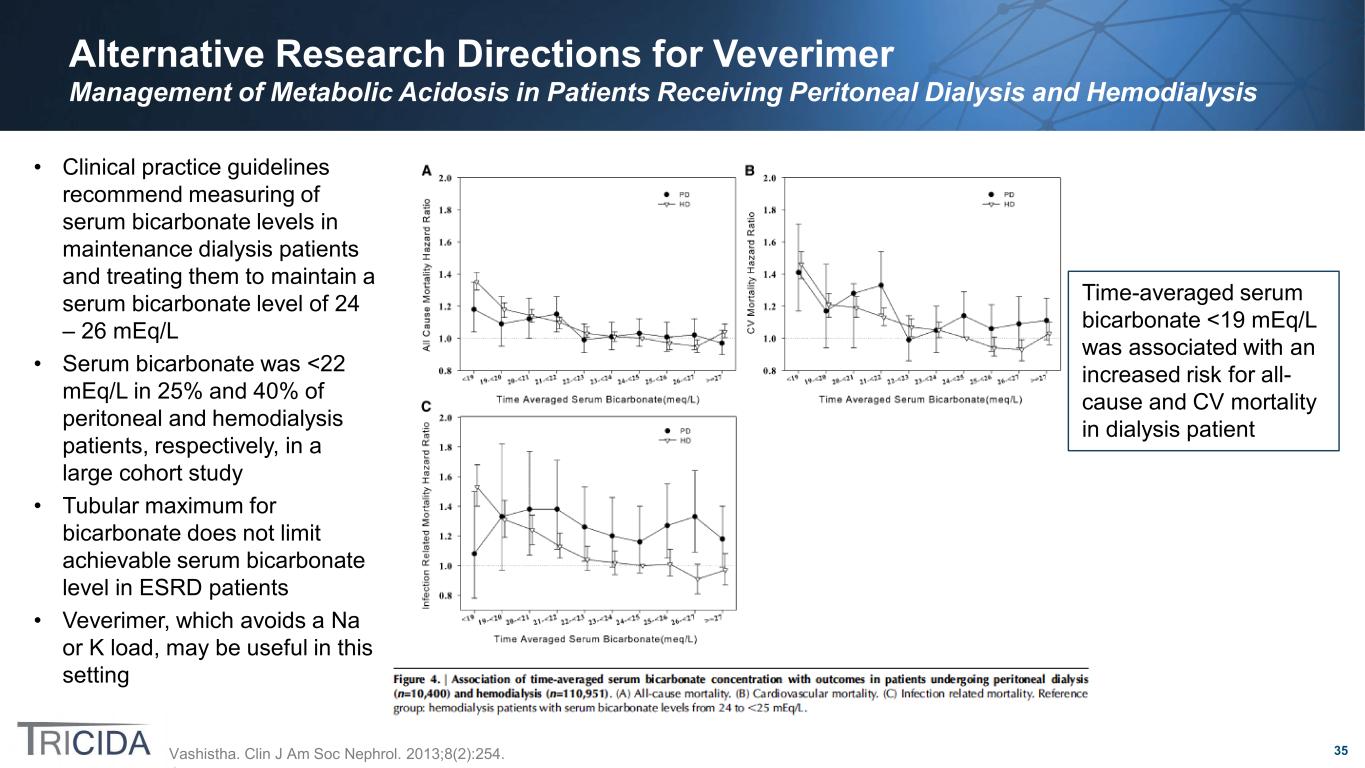
35 Alternative Research Directions for Veverimer Management of Metabolic Acidosis in Patients Receiving Peritoneal Dialysis and Hemodialysis • Clinical practice guidelines recommend measuring of serum bicarbonate levels in maintenance dialysis patients and treating them to maintain a serum bicarbonate level of 24 – 26 mEq/L • Serum bicarbonate was <22 mEq/L in 25% and 40% of peritoneal and hemodialysis patients, respectively, in a large cohort study • Tubular maximum for bicarbonate does not limit achievable serum bicarbonate level in ESRD patients • Veverimer, which avoids a Na or K load, may be useful in this setting Vashistha. Clin J Am Soc Nephrol. 2013;8(2):254. Time-averaged serum bicarbonate <19 mEq/L was associated with an increased risk for all- cause and CV mortality in dialysis patient
36 Alternative Research Directions for Veverimer Management of Acidosis in Patients with Proximal and Distal Renal Tubular Acidosis (RTA) • Renal tubular acidosis is caused by a defect in the nephrons that either causes bicarbonate to be wasted at a lower- than-normal tubular maximum for bicarbonate (proximal RTA [Type 1]) or that prevents normal excretion of acid by the kidneys (distal RTA [Type 2]), independent of kidney function (GFR) • RTA can be due to – Inherited defects – Disease (e.g., diabetes, Sjogren’s syndrome, rheumatoid arthritis, sarcoidosis) – Medications (e.g., lithium) – Toxins (e.g., lead) – Kidney diseases (e.g., obstructive uropathy, kidney transplant rejection) • Current RTA treatment (i.e., alkali supplements) aims to correct or manage acid-base and electrolyte disturbances and prevent or treat consequences of these, including nephrocalcinosis, kidney stones, osteopenia, growth retardation in children and CKD – Sodium containing alkali supplements (e.g., sodium bicarbonate) can worsen hypercalciuria and increase risk of stone formation – Type 2 RTA typically cannot be corrected completely with oral alkali – Tolerability of oral alkali is low – Infants and children require very large doses of alkali • RTA indications may meet criteria for orphan and/or rare diseases

37 Alternative Research Directions for Veverimer Treatment of Muscle and Bone Disorders in Patients with CKD, Low Bicarbonate Levels and Osteoporosis/Sarcopenia • Metabolic acidosis in CKD is associated with abnormalities in bone and muscle function in animal models and clinical studies • TRCA-301E showed clinically meaningful improvement in muscle function and physical function related quality of life, and while this was not replicated in the VALOR-CKD study, a subgroup of veverimer-treated patients who underwent DXA scans had an improvement (vs placebo) in lean body muscle mass and a trend toward improvement in appendicular muscle mass • Patients with metabolic acidosis and osteoporosis or sarcopenia, as verified on DXA scanning, may benefit from treatment with veverimer – In these populations, there may be an additional benefit on quality of life and/or objectively measured physical function

38 Alternative Research Directions for Veverimer Treatment of Osteopenia/Osteoporosis in Post-menopausal Women and Men with Osteopenia • Treatment with potassium citrate (an alkali salt) has been shown to improve bone mineral density and bone architecture and to reduce fracture risk scores in older men and women and in post-menopausal women • The effects of CKD on bone metabolism are complex. In addition to acidosis-related bone mineral loss, there are separate effects of hyperparathyroidism and deficiency in vitamin D • While bone mineral density was not improved in the VALOR-CKD study among CKD patients with metabolic acidosis, the possibility of benefit on bone in other populations remains an open question • Regulatory pathways for development of drugs for osteoporosis are known Jehle. J Am Soc Nephrol. 2006;17(11):3213. Jehle. J Clin Endocrinol Metab. 2013;98(1):207.

39 Alternative Research Directions for Veverimer Management of Patients with Kidney Stones • Increasing serum bicarbonate lowers urinary calcium excretion and reduces the risk of recurrent calcium oxalate kidney stone formation – Potassium citrate is a current treatment • Alkalinization of urine is a cornerstone of treatment for uric acid kidney stones – In TRCA-301, while the patient population was not selected based on serum or urine uric acid levels, an increase in urine pH was observed in the veverimer group and the mean 24-hour uric acid excretion decreased more in the veverimer group than in the placebo group

40 Alternative Research Directions for Veverimer Management of Metabolic Acidosis in Patients with Stage 5 CKD as a “Bridge to Dialysis” • Uncontrolled acidosis is an indication for initiating dialysis. Acidosis often becomes markedly worse as patients approach eGFRs at which dialysis is typically initiated (8 – 10 mL/min/1.73m2) • Patients with very low eGFR are precarious in terms of volume status and blood pressure because their ability to excrete sodium and water is limited – Use of sodium containing alkali may contribute to severe volume overload/heart failure and/or uncontrollable hypertension – Potassium-containing alkali supplements are contraindicated because of the risk of hyperkalemia • A strategy for treating metabolic acidosis that avoids a sodium or potassium load would be useful in this setting and may allow dialysis initiation to be delayed

41 Alternative Research Directions for Veverimer Slowing CKD Progression in Patients with Stage 2 – 3a CKD and Normal Serum Bicarbonate • Published literature suggests that patients with CKD retain acid, even at early stages of the disease at a time when compensatory mechanisms have been able to maintain serum bicarbonate in the normal range. Retained acid is hypothesized to be injurious to the kidneys, bone, and muscle – “eubicarbonatemic metabolic acidosis” – urine citrate is an emerging marker of acid retention, and it can be affected by alkali treatment • Slowing CKD by treating eubicarbonatemic metabolic acidosis in these earlier stages of CKD would require the use of eGFR slope as an endpoint because outcome events (e.g., dialysis, ≥ 40% eGFR decline) would occur infrequently • Citrate as a marker and predictor of CKD progression would need further study Goraya. Am J Physiol Renal Physiol. 2019;317(2):F502. Goraya. Kidney Int. 2019;95(5):1190.

42 Alternative Research Directions for Veverimer Oncology indications • Acidosis may be a target in cancer treatment • In vitro studies in various cancer models have shown relationships between extracellular acidosis and tumor growth – In pancreatic adenocarcinomas, a metastatic phenotype is associated with induction of localized extracelluar acidification – Increasing extracellular alkalinity in cultured breast cancer stem cells is affected by metabolic parameters including HIF1α regulation, glucose consumption, extracellular lactate production, and LDH activity which seem to promote programmed cell death and suppress the proliferation of stem cells – Based on these types of basic science experiments, it is plausible that there might be utility of alkalinization as an adjunct to cancer treatment • Alternative uses in patients with malignancy include urinary alkalinization in patients on high dose methotrexate therapy and those at risk for tumor lysis syndrome due to high rates of cell lysis from chemotherapy or intrinsic tumor burden Gillies. Biochim Biophys Acta Rev Cancer. 2019;1871(2):273. Olszewski. Mol Oncol. 2009;3(3):204. Wanandi. Brax J Med Biol Res. 2017;50(8):e6538. Wang. Chinese Chemical Letters. 2021.









































