
October 7, 2021 Next-Generation Precision Oncology Medicines��AACR-NCI-EORTC Investor Meeting Exhibit 99.1

Forward-Looking Statements Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development activities, plans and projected timelines, business strategy and plans, regulatory matters, objectives of management for future operations, market size and opportunity, our ability to complete certain milestones and our expectations regarding the relative benefits of our drug candidates versus competitive therapies. Words such as “believe,” “can”, “continue,” “anticipate,” “could,” “estimate,” “plan,” “predict,” “expect,” “intend,” “will,” “may,” “goal,” “upcoming,” “near term”, “milestone”, “potential,” “target” or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation: our preclinical studies and clinical trials may not be successful; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; the COVID-19 pandemic may disrupt our business and that of third parties on which we depend, including delaying or otherwise disrupting our research and development activities; and we may not be able to obtain additional financing. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these drug candidates for the use for which such drug candidates are being studied.

Agenda Introduction Athena Countouriotis, M.D., President & CEO Repotrectinib TRIDENT-1 Presented Data�ROS1+ TKI-pretreated NSCLC Mohammad Hirmand, M.D., Chief Medical Officer Elzovantinib (TPX-0022) SHIELD-1 Presented Data Solid Tumors with MET Genetic Alterations Mohammad Hirmand, M.D., Chief Medical Officer Q&A Panel Athena Countouriotis, M.D., President & CEO Mohammad Hirmand, M.D., Chief Medical Officer Alexander Drilon, M.D.�Chief, Early Drug Development Service, Memorial Sloan Kettering Cancer Center David S. Hong, M.D. Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center Clinical data for repotrectinib from the NTRK-positive TKI-naïve and TKI-pretreated advanced solid tumor cohorts of TRIDENT-1 will be presented at a late breaker plenary presentation on October 8 at 10:05 am ET at the AACR-NCI-EORTC conference.

Introduction Athena Countouriotis, M.D.�President & Chief Executive Officer
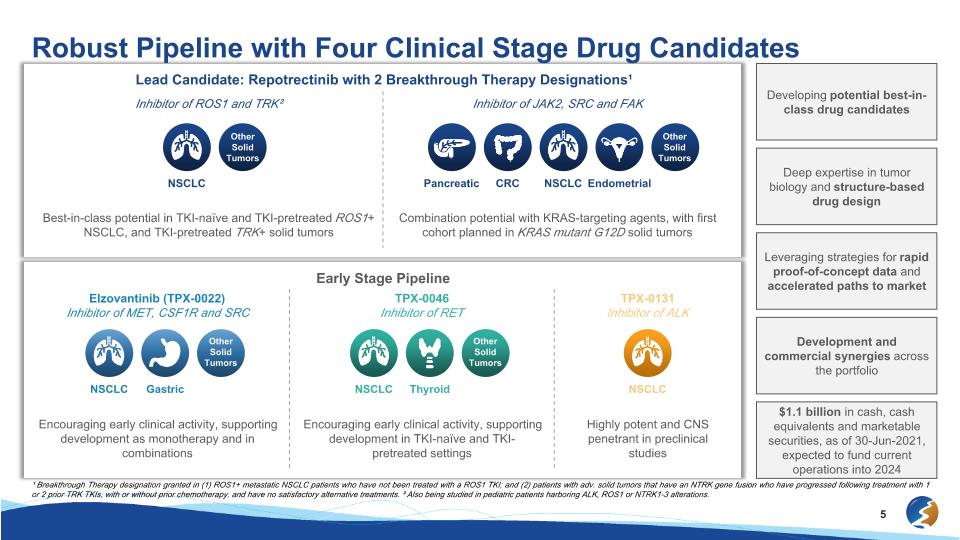
Lead Candidate: Repotrectinib with 2 Breakthrough Therapy Designations¹ NSCLC Other Solid Tumors Robust Pipeline with Four Clinical Stage Drug Candidates Developing potential best-in-class drug candidates Inhibitor of JAK2, SRC and FAK Combination potential with KRAS-targeting agents, with first cohort planned in KRAS mutant G12D solid tumors Inhibitor of ROS1 and TRK² Best-in-class potential in TKI-naïve and TKI-pretreated ROS1+ NSCLC, and TKI-pretreated TRK+ solid tumors Early Stage Pipeline Elzovantinib (TPX-0022) Inhibitor of MET, CSF1R and SRC Encouraging early clinical activity, supporting development as monotherapy and in combinations TPX-0046 Inhibitor of RET Encouraging early clinical activity, supporting development in TKI-naïve and TKI-pretreated settings TPX-0131 Inhibitor of ALK Highly potent and CNS penetrant in preclinical studies Leveraging strategies for rapid proof-of-concept data and accelerated paths to market Development and commercial synergies across the portfolio $1.1 billion in cash, cash equivalents and marketable securities, as of 30-Jun-2021, expected to fund current operations into 2024 ¹ Breakthrough Therapy designation granted in (1) ROS1+ metastatic NSCLC patients who have not been treated with a ROS1 TKI; and (2) patients with adv. solid tumors that have an NTRK gene fusion who have progressed following treatment with 1 or 2 prior TRK TKIs, with or without prior chemotherapy, and have no satisfactory alternative treatments. ² Also being studied in pediatric patients harboring ALK, ROS1 or NTRK1-3 alterations. Pancreatic NSCLC CRC Endometrial Other Solid Tumors NSCLC NSCLC Gastric Other Solid Tumors NSCLC Thyroid Other Solid Tumors Deep expertise in tumor biology and structure-based drug design
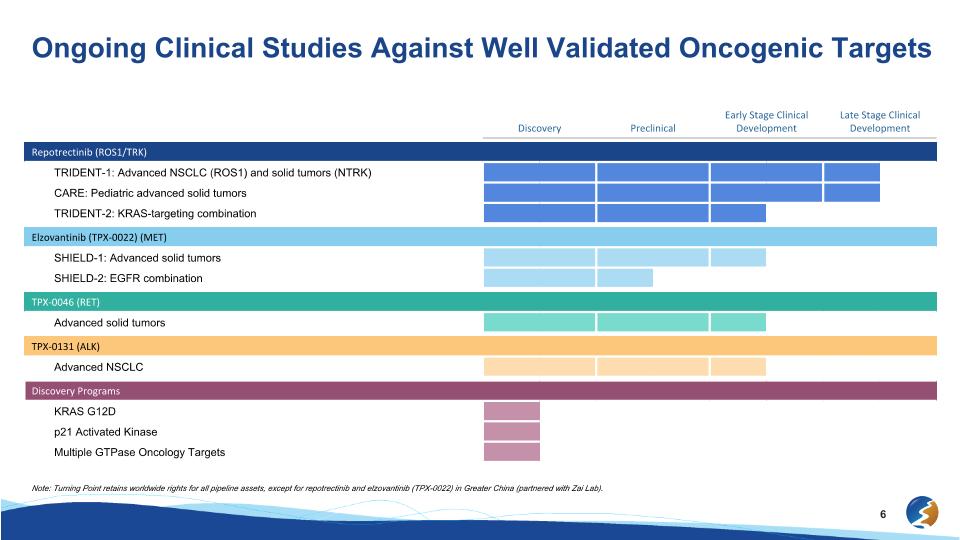
Discovery Preclinical Early Stage Clinical Development Late Stage Clinical Development Repotrectinib (ROS1/TRK) TRIDENT-1: Advanced NSCLC (ROS1) and solid tumors (NTRK) CARE: Pediatric advanced solid tumors TRIDENT-2: KRAS-targeting combination Elzovantinib (TPX-0022) (MET) SHIELD-1: Advanced solid tumors SHIELD-2: EGFR combination TPX-0046 (RET) Advanced solid tumors TPX-0131 (ALK) Advanced NSCLC Discovery Programs KRAS G12D p21 Activated Kinase Multiple GTPase Oncology Targets Ongoing Clinical Studies Against Well Validated Oncogenic Targets Note: Turning Point retains worldwide rights for all pipeline assets, except for repotrectinib and elzovantinib (TPX-0022) in Greater China (partnered with Zai Lab).

Highlights at AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics Poster Presentations Update from the Phase 2 registrational trial of repotrectinib in TKI-pretreated patients with ROS1+ advanced non-small cell lung cancer and with NTRK+ advanced solid tumors (TRIDENT-1)¹ Preliminary interim data of elzovantinib (TPX-0022), a novel inhibitor of MET/SRC/CSF1R, in patients with advanced solid tumors harboring genetic alterations in MET: Update from the Phase 1 SHIELD-1 trial² Plenary Presentation, Session 2: New Drugs on the Horizon I Repotrectinib in patients with NTRK fusion-positive advanced solid tumors: update from the registrational phase 2 TRIDENT-1 trial³�Friday, October 8 at 10:05 am ET ¹ P224; Authors: J. Lin, et al. ² P225; Authors: David S. Hong, et al. ³ LB #6545; Authors: Benjamin Besse, et al.

Repotrectinib: Data from TRIDENT-1�ROS1+ TKI-pretreated NSCLC Mohammad Hirmand, M.D.�Chief Medical Officer

Update from the Phase 2 registrational trial of repotrectinib in TKI-pretreated patients with ROS1+ advanced non-small cell lung cancer and with NTRK+ advanced solid tumors (TRIDENT-1) Jessica J. Lin,1 Byoung Chul Cho,2 Christoph Springfeld,3 D. Ross Camidge,4 Benjamin Solomon,5 Christina Baik,6 Vamsidhar Velcheti,7 Young-Chul Kim,8 Victor Moreno,9 Anthonie J. van der Wekken,10 Enriqueta Felip,11 Dipesh Uprety,12 Denise Trone,13 Shanna Stopatschinskaja,13 Alexander Drilon14 1Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 2Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea; 3Heidelberg University Hospital, National Center for Tumor Diseases, Department of Medical Oncology, Heidelberg, Germany; 4University of Colorado Denver, Anschutz Medical Campus, Aurora, CO, USA; 5Peter MacCallum Cancer Center, Melbourne, Australia; 6University of Washington School of Medicine, Seattle Cancer Care Alliance, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; 7NYU Perlmutter Cancer Center, New York, NY, USA; 8Chonnam National University Medical School, and CNU Hwasun Hospital, Hwasun-gun, Republic of Korea; 9Fundación Jiménez Díaz - START Madrid, Madrid, Spain; 10University of Groningen, University Medical Centre Groningen, Groningen, Netherlands; 11Vall d’Hebron University Hospital, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain; 12Karmanos Cancer Institute, Detroit, MI, USA; 13Turning Point Therapeutics Inc, San Diego, CA, USA; 14Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, NY, USA. Poster #: P224 9
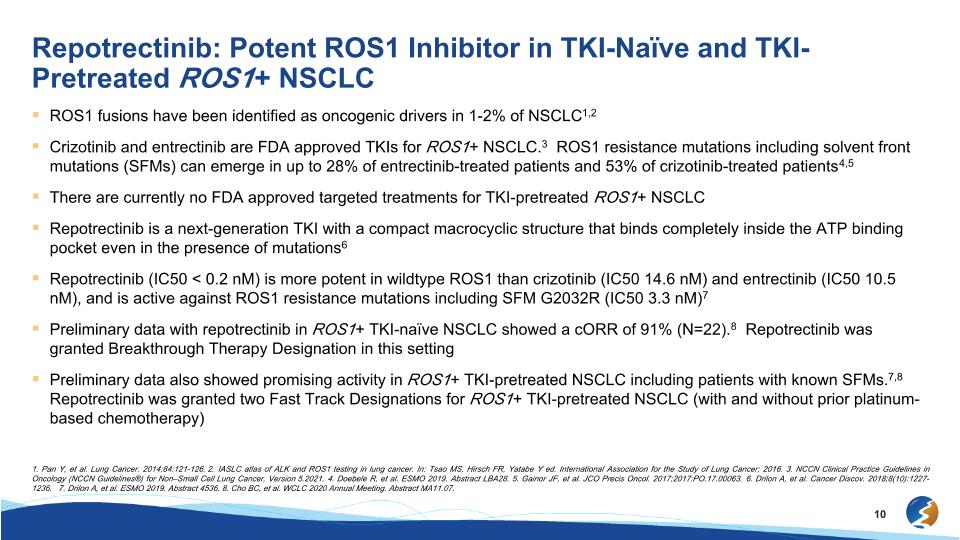
Repotrectinib: Potent ROS1 Inhibitor in TKI-Naïve and TKI-Pretreated ROS1+ NSCLC ROS1 fusions have been identified as oncogenic drivers in 1-2% of NSCLC1,2 Crizotinib and entrectinib are FDA approved TKIs for ROS1+ NSCLC.3 ROS1 resistance mutations including solvent front mutations (SFMs) can emerge in up to 28% of entrectinib-treated patients and 53% of crizotinib-treated patients4,5 There are currently no FDA approved targeted treatments for TKI-pretreated ROS1+ NSCLC Repotrectinib is a next-generation TKI with a compact macrocyclic structure that binds completely inside the ATP binding pocket even in the presence of mutations6 Repotrectinib (IC50 < 0.2 nM) is more potent in wildtype ROS1 than crizotinib (IC50 14.6 nM) and entrectinib (IC50 10.5 nM), and is active against ROS1 resistance mutations including SFM G2032R (IC50 3.3 nM)7 Preliminary data with repotrectinib in ROS1+ TKI-naïve NSCLC showed a cORR of 91% (N=22).8 Repotrectinib was granted Breakthrough Therapy Designation in this setting Preliminary data also showed promising activity in ROS1+ TKI-pretreated NSCLC including patients with known SFMs.7,8 Repotrectinib was granted two Fast Track Designations for ROS1+ TKI-pretreated NSCLC (with and without prior platinum-based chemotherapy) 1. Pan Y, et al. Lung Cancer. 2014;84:121-126. 2. IASLC atlas of ALK and ROS1 testing in lung cancer. In: Tsao MS, Hirsch FR, Yatabe Y ed. International Association for the Study of Lung Cancer; 2016. 3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non–Small Cell Lung Cancer, Version 5.2021. 4. Doebele R, et al. ESMO 2019. Abstract LBA28. 5. Gainor JF, et al. JCO Precis Oncol. 2017;2017:PO.17.00063. 6. Drilon A, et al. Cancer Discov. 2018;8(10):1227-1236. 7. Drilon A, et al. ESMO 2019. Abstract 4536. 8. Cho BC, et al. WCLC 2020 Annual Meeting. Abstract MA11.07.
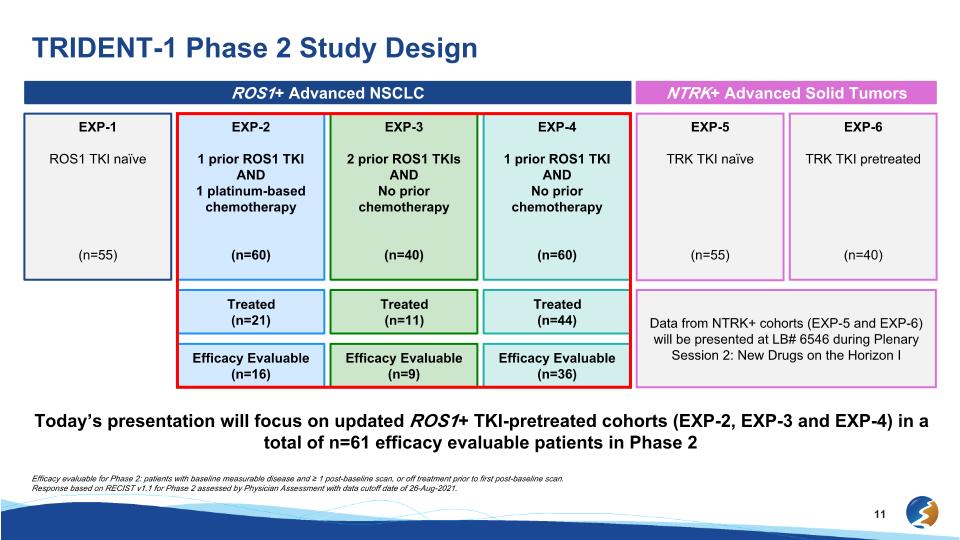
TRIDENT-1 Phase 2 Study Design ROS1+ Advanced NSCLC NTRK+ Advanced Solid Tumors EXP-1 ROS1 TKI naïve (n=55) EXP-2 1 prior ROS1 TKI AND 1 platinum-based chemotherapy (n=60) EXP-3 2 prior ROS1 TKIs AND No prior chemotherapy (n=40) EXP-4 1 prior ROS1 TKI AND No prior chemotherapy (n=60) EXP-5 TRK TKI naïve (n=55) EXP-6 TRK TKI pretreated (n=40) Treated (n=21) Treated (n=11) Treated (n=44) Data from NTRK+ cohorts (EXP-5 and EXP-6) will be presented at LB# 6546 during Plenary Session 2: New Drugs on the Horizon I Efficacy Evaluable (n=16) Efficacy Evaluable (n=9) Efficacy Evaluable (n=36) Efficacy evaluable for Phase 2: patients with baseline measurable disease and ≥ 1 post-baseline scan, or off treatment prior to first post-baseline scan. Response based on RECIST v1.1 for Phase 2 assessed by Physician Assessment with data cutoff date of 26-Aug-2021. Today’s presentation will focus on updated ROS1+ TKI-pretreated cohorts (EXP-2, EXP-3 and EXP-4) in a total of n=61 efficacy evaluable patients in Phase 2
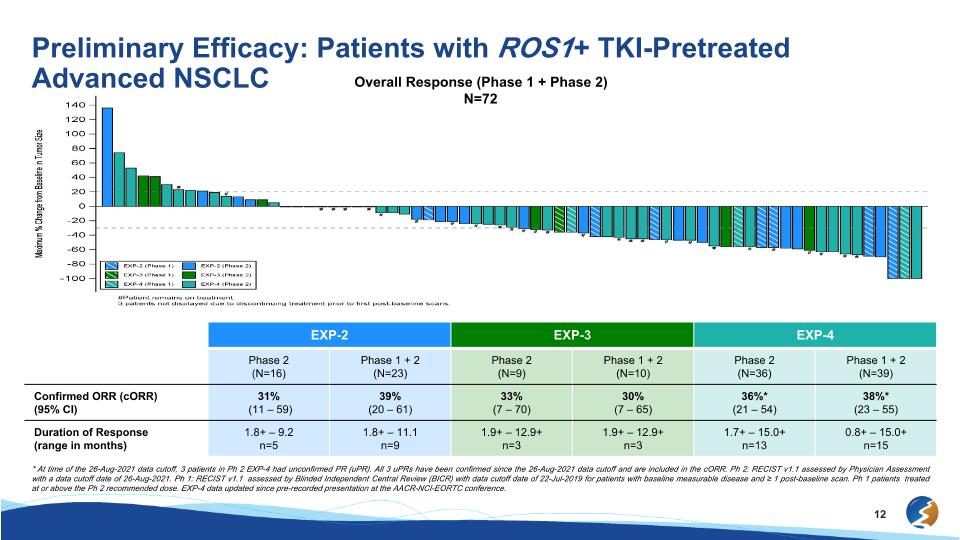
Preliminary Efficacy: Patients with ROS1+ TKI-Pretreated Advanced NSCLC Overall Response (Phase 1 + Phase 2) N=72 EXP-2 EXP-3 EXP-4 Phase 2 (N=16) Phase 1 + 2 (N=23) Phase 2 (N=9) Phase 1 + 2 (N=10) Phase 2 (N=36) Phase 1 + 2 (N=39) Confirmed ORR (cORR) (95% CI) 31% (11 – 59) 39%�(20 – 61) 33%�(7 – 70) 30%�(7 – 65) 36%*�(21 – 54) 38%*�(23 – 55) Duration of Response (range in months) 1.8+ – 9.2 n=5 1.8+ – 11.1 n=9 1.9+ – 12.9+ n=3 1.9+ – 12.9+ n=3 1.7+ – 15.0+ n=13 0.8+ – 15.0+ n=15 * At time of the 26-Aug-2021 data cutoff, 3 patients in Ph 2 EXP-4 had unconfirmed PR (uPR). All 3 uPRs have been confirmed since the 26-Aug-2021 data cutoff and are included in the cORR. Ph 2: RECIST v1.1 assessed by Physician Assessment with a data cutoff date of 26-Aug-2021. Ph 1: RECIST v1.1 assessed by Blinded Independent Central Review (BICR) with data cutoff date of 22-Jul-2019 for patients with baseline measurable disease and ≥ 1 post-baseline scan. Ph 1 patients treated at or above the Ph 2 recommended dose. EXP-4 data updated since pre-recorded presentation at the AACR-NCI-EORTC conference.
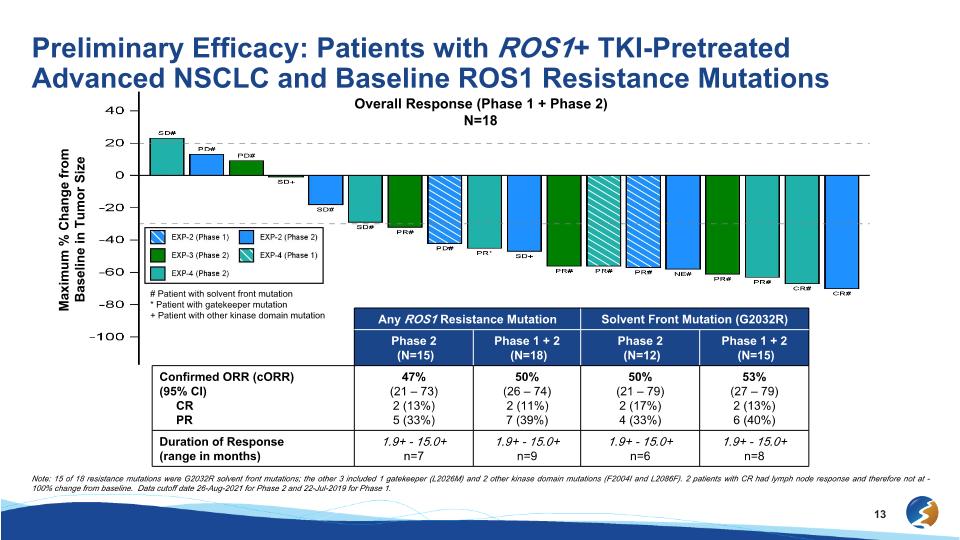
Preliminary Efficacy: Patients with ROS1+ TKI-Pretreated Advanced NSCLC and Baseline ROS1 Resistance Mutations Overall Response (Phase 1 + Phase 2) N=18 # Patient with solvent front mutation * Patient with gatekeeper mutation + Patient with other kinase domain mutation Maximum % Change from Baseline in Tumor Size Any ROS1 Resistance Mutation Solvent Front Mutation (G2032R) Phase 2 (N=15) Phase 1 + 2 (N=18) Phase 2 (N=12) Phase 1 + 2 (N=15) Confirmed ORR (cORR) (95% CI) CR PR 47% (21 – 73) 2 (13%) 5 (33%) 50% (26 – 74) 2 (11%) 7 (39%) 50% (21 – 79) 2 (17%) 4 (33%) 53% (27 – 79) 2 (13%) 6 (40%) Duration of Response (range in months) 1.9+ - 15.0+ n=7 1.9+ - 15.0+ n=9 1.9+ - 15.0+ n=6 1.9+ - 15.0+ n=8 Note: 15 of 18 resistance mutations were G2032R solvent front mutations; the other 3 included 1 gatekeeper (L2026M) and 2 other kinase domain mutations (F2004I and L2086F). 2 patients with CR had lymph node response and therefore not at -100% change from baseline. Data cutoff date 26-Aug-2021 for Phase 2 and 22-Jul-2019 for Phase 1.
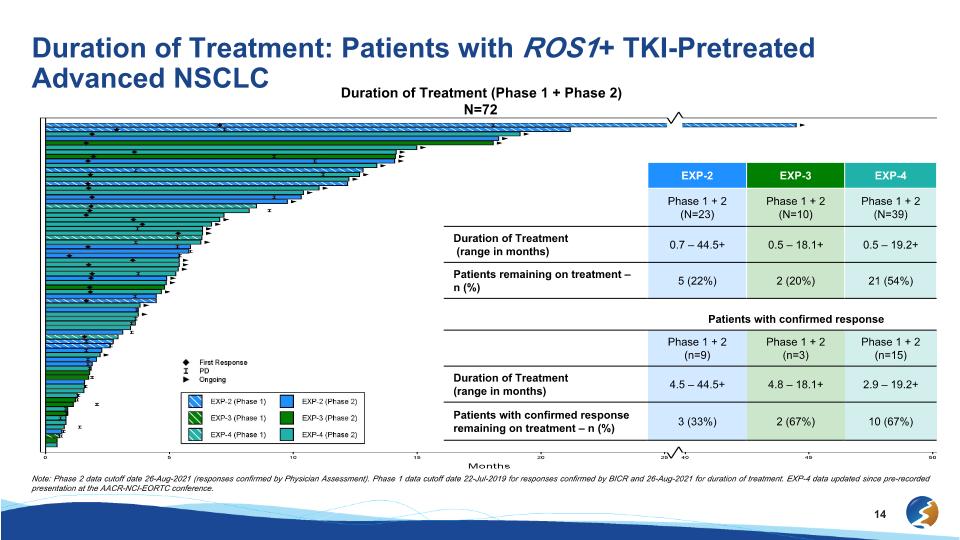
Duration of Treatment: Patients with ROS1+ TKI-Pretreated Advanced NSCLC Duration of Treatment (Phase 1 + Phase 2) N=72 EXP-2 EXP-3 EXP-4 Phase 1 + 2 (N=23) Phase 1 + 2 (N=10) Phase 1 + 2 (N=39) Duration of Treatment (range in months) 0.7 – 44.5+ 0.5 – 18.1+ 0.5 – 19.2+ Patients remaining on treatment – n (%) 5 (22%) 2 (20%) 21 (54%) Patients with confirmed response Phase 1 + 2 (n=9) Phase 1 + 2 (n=3) Phase 1 + 2 (n=15) Duration of Treatment (range in months) 4.5 – 44.5+ 4.8 – 18.1+ 2.9 – 19.2+ Patients with confirmed response remaining on treatment – n (%) 3 (33%) 2 (67%) 10 (67%) Note: Phase 2 data cutoff date 26-Aug-2021 (responses confirmed by Physician Assessment). Phase 1 data cutoff date 22-Jul-2019 for responses confirmed by BICR and 26-Aug-2021 for duration of treatment. EXP-4 data updated since pre-recorded presentation at the AACR-NCI-EORTC conference.
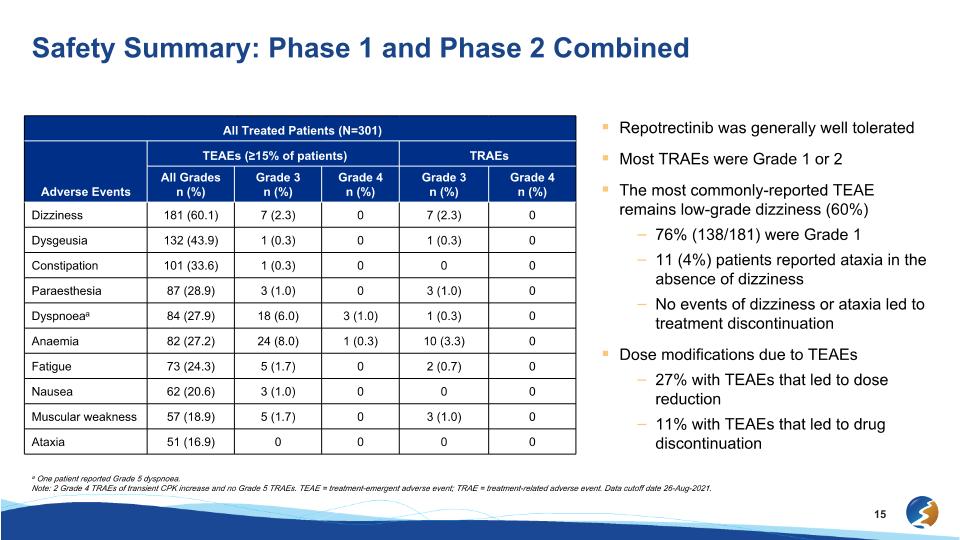
Safety Summary: Phase 1 and Phase 2 Combined All Treated Patients (N=301) All Treated Patients (N=301) TEAEs (≥15% of patients) TRAEs Adverse Events All Grades�n (%) Grade 3�n (%) Grade 4�n (%) Grade 3�n (%) Grade 4�n (%) Dizziness 181 (60.1) 7 (2.3) 0 7 (2.3) 0 Dysgeusia 132 (43.9) 1 (0.3) 0 1 (0.3) 0 Constipation 101 (33.6) 1 (0.3) 0 0 0 Paraesthesia 87 (28.9) 3 (1.0) 0 3 (1.0) 0 Dyspnoeaa 84 (27.9) 18 (6.0) 3 (1.0) 1 (0.3) 0 Anaemia 82 (27.2) 24 (8.0) 1 (0.3) 10 (3.3) 0 Fatigue 73 (24.3) 5 (1.7) 0 2 (0.7) 0 Nausea 62 (20.6) 3 (1.0) 0 0 0 Muscular weakness 57 (18.9) 5 (1.7) 0 3 (1.0) 0 Ataxia 51 (16.9) 0 0 0 0 Repotrectinib was generally well tolerated Most TRAEs were Grade 1 or 2 The most commonly-reported TEAE remains low-grade dizziness (60%) 76% (138/181) were Grade 1 11 (4%) patients reported ataxia in the absence of dizziness No events of dizziness or ataxia led to treatment discontinuation Dose modifications due to TEAEs 27% with TEAEs that led to dose reduction 11% with TEAEs that led to drug discontinuation a One patient reported Grade 5 dyspnoea. Note: 2 Grade 4 TRAEs of transient CPK increase and no Grade 5 TRAEs. TEAE = treatment-emergent adverse event; TRAE = treatment-related adverse event. Data cutoff date 26-Aug-2021.

Conclusions Repotrectinib is a next-generation ROS1/TRK inhibitor Repotrectinib was generally well-tolerated In the setting of ROS1+ TKI pretreated advanced NSCLC with limited treatment options, repotrectinib demonstrated clinical activity in Phase 1 and Phase 2 EXP-2: N=23, cORR 39% (95% CI: 20 – 61) EXP-3: N=10, cORR 30% (95% CI: 7 – 65) EXP-4: N=39, cORR 38% (95% CI: 23 – 55) In TKI-pretreated NSCLC and ROS1 G2032R SFM (N=15): cORR 53% (95% CI: 27 – 79) Enrollment is ongoing in the registrational Phase 2 TRIDENT-1 study evaluating repotrectinib for the treatment of TKI-naive and TKI-pretreated patients with ROS1+ advanced NSCLC and NTRK+ advanced solid tumors Data for NTRK+ cohorts (EXP-5 and EXP-6) will be presented at LB# 6546 during Plenary Session 2: New Drugs on the Horizon I

Elzovantinib (TPX-0022): Data from SHIELD-1�Solid Tumors with MET Genetic Alterations Mohammad Hirmand, M.D.�Chief Medical Officer

Preliminary interim data of elzovantinib (TPX-0022), a novel inhibitor of MET/SRC/CSF1R, in patients with advanced solid tumors harboring genetic alterations in MET: Update from the Phase 1 SHIELD-1 trial David S. Hong1, Daniel Catenacci2, Lyudmila Bazhenova3, Byoung Chul Cho4, Mariano Ponz-Sarvise5, Rebecca Heist6, Victor Moreno7, Gerald Falchook8, Viola W. Zhu9, Aurélie Swalduz10, Benjamin Besse11, Dong-Wan Kim12, Shinkyo Yoon13, Xiuning Le1, Tingting Zhao14, Justine Lam14, Zachary Zimmerman14, Jeeyun Lee15 1The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 2University of Chicago Medical Center, Chicago, IL, USA; 3UC San Diego Moores Cancer Center, San Diego, CA, USA; 4Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea; 5Clinica Universidad de Navarra, Pamplona, Spain; 6Massachusetts General Hospital, Boston, MA, USA; 7Fundación Jiménez Díaz - START Madrid, Madrid, Spain; 8Sarah Cannon Research Institute at HealthONE, Denver, CO, USA; 9University of California Irvine, Orange, CA, USA; 10Centre de Lutte Contre le Cancer - Centre Leon Berard, Lyon, France; 11Institut Gustave Roussy, Villejuif Cedex, France; 12Seoul National University Hospital, Seoul, Republic of Korea; 13Asan Medical Center, Seoul, Republic of Korea; 14Turning Point Therapeutics, Inc., San Diego, CA, USA; 15Samsung Medical Center, Seoul, Republic of Korea Poster #: P225 18
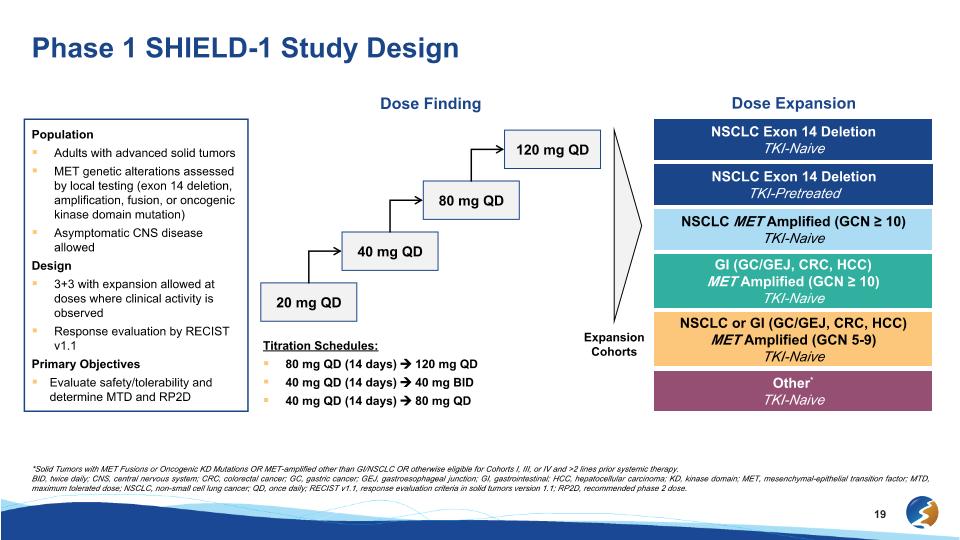
Phase 1 SHIELD-1 Study Design *Solid Tumors with MET Fusions or Oncogenic KD Mutations OR MET-amplified other than GI/NSCLC OR otherwise eligible for Cohorts I, III, or IV and >2 lines prior systemic therapy. BID, twice daily; CNS, central nervous system; CRC, colorectal cancer; GC, gastric cancer; GEJ, gastroesophageal junction; GI, gastrointestinal; HCC, hepatocellular carcinoma; KD, kinase domain; MET, mesenchymal-epithelial transition factor; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; QD, once daily; RECIST v1.1, response evaluation criteria in solid tumors version 1.1; RP2D, recommended phase 2 dose. Population Adults with advanced solid tumors MET genetic alterations assessed by local testing (exon 14 deletion, amplification, fusion, or oncogenic kinase domain mutation) Asymptomatic CNS disease allowed Design 3+3 with expansion allowed at doses where clinical activity is observed Response evaluation by RECIST v1.1 Primary Objectives Evaluate safety/tolerability and determine MTD and RP2D Dose Finding Expansion Cohorts Dose Expansion NSCLC Exon 14 Deletion TKI-Naive GI (GC/GEJ, CRC, HCC) MET Amplified (GCN ≥ 10) TKI-Naive NSCLC Exon 14 Deletion TKI-Pretreated NSCLC MET Amplified (GCN ≥ 10) TKI-Naive NSCLC or GI (GC/GEJ, CRC, HCC)�MET Amplified (GCN 5-9) TKI-Naive Other* TKI-Naive 40 mg QD 80 mg QD 120 mg QD 20 mg QD Titration Schedules: 80 mg QD (14 days) 120 mg QD 40 mg QD (14 days) 40 mg BID 40 mg QD (14 days) 80 mg QD
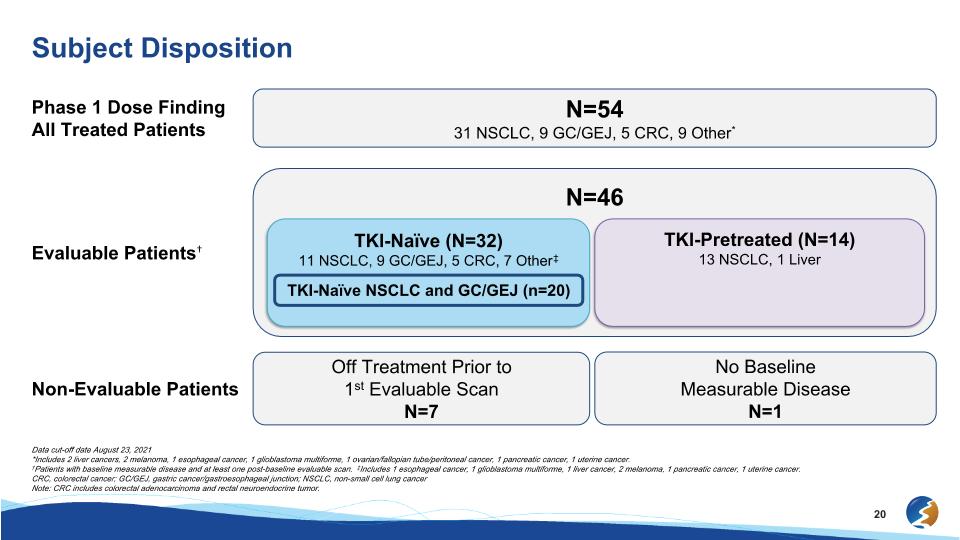
Subject Disposition Data cut-off date August 23, 2021 *Includes 2 liver cancers, 2 melanoma, 1 esophageal cancer, 1 glioblastoma multiforme, 1 ovarian/fallopian tube/peritoneal cancer, 1 pancreatic cancer, 1 uterine cancer. †Patients with baseline measurable disease and at least one post-baseline evaluable scan. ‡Includes 1 esophageal cancer, 1 glioblastoma multiforme, 1 liver cancer, 2 melanoma, 1 pancreatic cancer, 1 uterine cancer. CRC, colorectal cancer; GC/GEJ, gastric cancer/gastroesophageal junction; NSCLC, non-small cell lung cancer Note: CRC includes colorectal adenocarcinoma and rectal neuroendocrine tumor. Phase 1 Dose Finding All Treated Patients N=54 31 NSCLC, 9 GC/GEJ, 5 CRC, 9 Other* Non-Evaluable Patients Off Treatment Prior to 1st Evaluable Scan N=7 No Baseline Measurable Disease N=1 N=46 Evaluable Patients† TKI-Naïve (N=32) 11 NSCLC, 9 GC/GEJ, 5 CRC, 7 Other‡ TKI-Pretreated (N=14) 13 NSCLC, 1 Liver TKI-Naïve NSCLC and GC/GEJ (n=20)
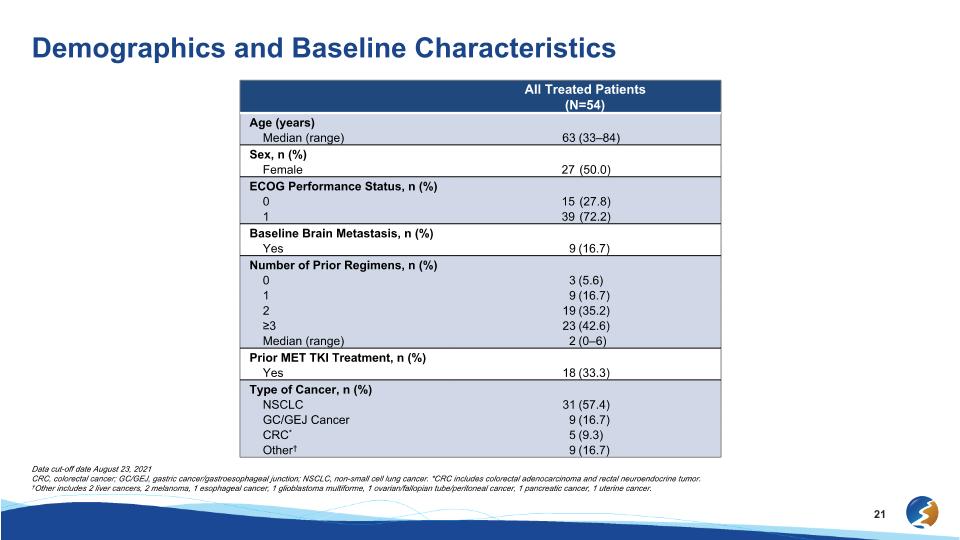
Demographics and Baseline Characteristics Data cut-off date August 23, 2021 CRC, colorectal cancer; GC/GEJ, gastric cancer/gastroesophageal junction; NSCLC, non-small cell lung cancer. *CRC includes colorectal adenocarcinoma and rectal neuroendocrine tumor. †Other includes 2 liver cancers, 2 melanoma, 1 esophageal cancer, 1 glioblastoma multiforme, 1 ovarian/fallopian tube/peritoneal cancer, 1 pancreatic cancer, 1 uterine cancer. All Treated Patients �(N=54) Age (years) Median (range) 63 (33–84) Sex, n (%) Female 27 (50.0) ECOG Performance Status, n (%) 0 15 (27.8) 1 39 (72.2) Baseline Brain Metastasis, n (%) Yes 9 (16.7) Number of Prior Regimens, n (%) 0 3 (5.6) 1 9 (16.7) 2 19 (35.2) ≥3 23 (42.6) Median (range) 2 (0–6) Prior MET TKI Treatment, n (%) Yes 18 (33.3) Type of Cancer, n (%) NSCLC 31 (57.4) GC/GEJ Cancer 9 (16.7) CRC* 5 (9.3) Other† 9 (16.7)
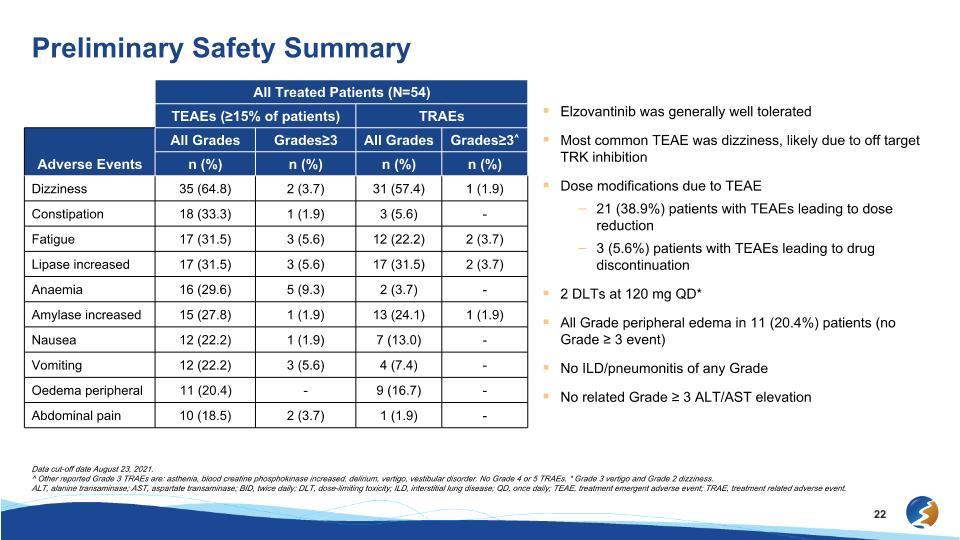
Elzovantinib was generally well tolerated Most common TEAE was dizziness, likely due to off target TRK inhibition Dose modifications due to TEAE 21 (38.9%) patients with TEAEs leading to dose reduction 3 (5.6%) patients with TEAEs leading to drug discontinuation 2 DLTs at 120 mg QD* All Grade peripheral edema in 11 (20.4%) patients (no Grade ≥ 3 event) No ILD/pneumonitis of any Grade No related Grade ≥ 3 ALT/AST elevation Preliminary Safety Summary All Treated Patients (N=54) TEAEs (≥15% of patients) TRAEs Adverse Events All Grades Grades≥3 All Grades Grades≥3^ n (%) n (%) n (%) n (%) Dizziness 35 (64.8) 2 (3.7) 31 (57.4) 1 (1.9) Constipation 18 (33.3) 1 (1.9) 3 (5.6) - Fatigue 17 (31.5) 3 (5.6) 12 (22.2) 2 (3.7) Lipase increased 17 (31.5) 3 (5.6) 17 (31.5) 2 (3.7) Anaemia 16 (29.6) 5 (9.3) 2 (3.7) - Amylase increased 15 (27.8) 1 (1.9) 13 (24.1) 1 (1.9) Nausea 12 (22.2) 1 (1.9) 7 (13.0) - Vomiting 12 (22.2) 3 (5.6) 4 (7.4) - Oedema peripheral 11 (20.4) - 9 (16.7) - Abdominal pain 10 (18.5) 2 (3.7) 1 (1.9) - Data cut-off date August 23, 2021. ^ Other reported Grade 3 TRAEs are: asthenia, blood creatine phosphokinase increased, delirium, vertigo, vestibular disorder. No Grade 4 or 5 TRAEs. * Grade 3 vertigo and Grade 2 dizziness. ALT, alanine transaminase; AST, aspartate transaminase; BID, twice daily; DLT, dose-limiting toxicity; ILD, interstitial lung disease; QD, once daily; TEAE, treatment emergent adverse event; TRAE, treatment related adverse event.
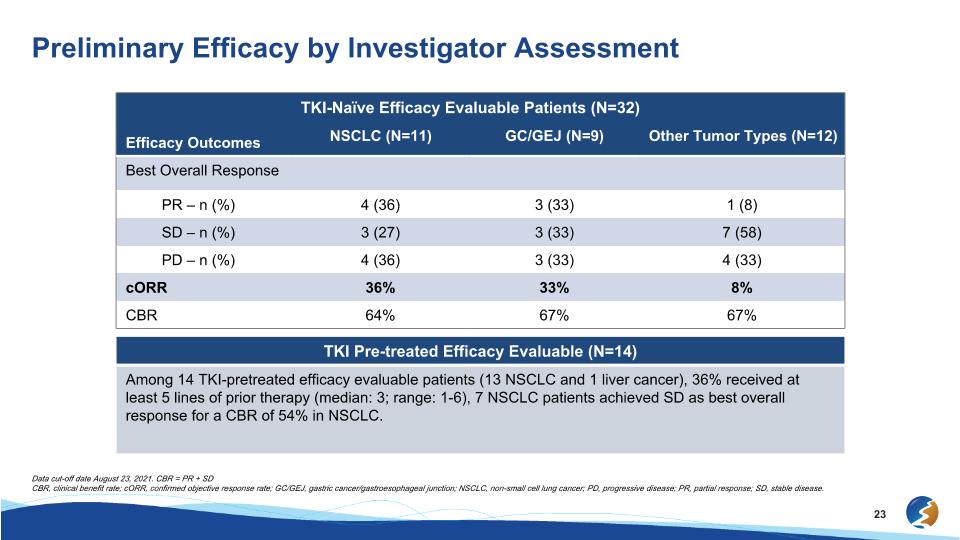
Preliminary Efficacy by Investigator Assessment Efficacy Outcomes TKI-Naïve Efficacy Evaluable Patients (N=32) Efficacy Outcomes NSCLC (N=11) GC/GEJ (N=9) Other Tumor Types (N=12) Best Overall Response PR – n (%) 4 (36) 3 (33) 1 (8) SD – n (%) 3 (27) 3 (33) 7 (58) PD – n (%) 4 (36) 3 (33) 4 (33) cORR 36% 33% 8% CBR 64% 67% 67% TKI Pre-treated Efficacy Evaluable (N=14) Among 14 TKI-pretreated efficacy evaluable patients (13 NSCLC and 1 liver cancer), 36% received at least 5 lines of prior therapy (median: 3; range: 1-6), 7 NSCLC patients achieved SD as best overall response for a CBR of 54% in NSCLC. Data cut-off date August 23, 2021. CBR = PR + SD CBR, clinical benefit rate; cORR, confirmed objective response rate; GC/GEJ, gastric cancer/gastroesophageal junction; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease.
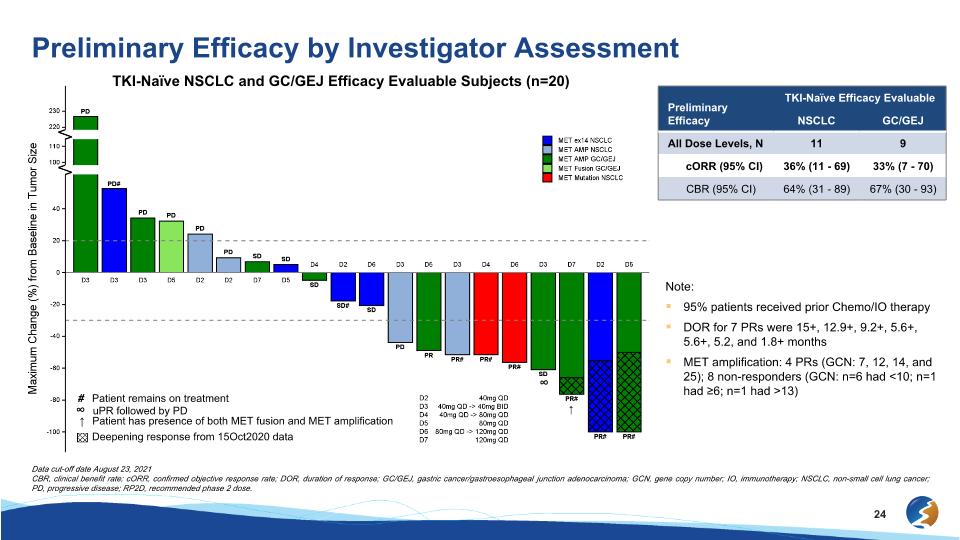
Preliminary Efficacy by Investigator Assessment TKI-Naïve NSCLC and GC/GEJ Efficacy Evaluable Subjects (n=20) ↑ ∞ # Patient remains on treatment ↑ Patient has presence of both MET fusion and MET amplification ∞ uPR followed by PD Deepening response from 15Oct2020 data Preliminary Efficacy TKI-Naïve Efficacy Evaluable NSCLC GC/GEJ All Dose Levels, N 11 9 cORR (95% CI) 36% (11 - 69) 33% (7 - 70) CBR (95% CI) 64% (31 - 89) 67% (30 - 93) Note: 95% patients received prior Chemo/IO therapy DOR for 7 PRs were 15+, 12.9+, 9.2+, 5.6+, 5.6+, 5.2, and 1.8+ months MET amplification: 4 PRs (GCN: 7, 12, 14, and 25); 8 non-responders (GCN: n=6 had <10; n=1 had ≥6; n=1 had >13) Data cut-off date August 23, 2021 CBR, clinical benefit rate; cORR, confirmed objective response rate; DOR, duration of response; GC/GEJ, gastric cancer/gastroesophageal junction adenocarcinoma; GCN, gene copy number; IO, immunotherapy; NSCLC, non-small cell lung cancer; PD, progressive disease; RP2D, recommended phase 2 dose.
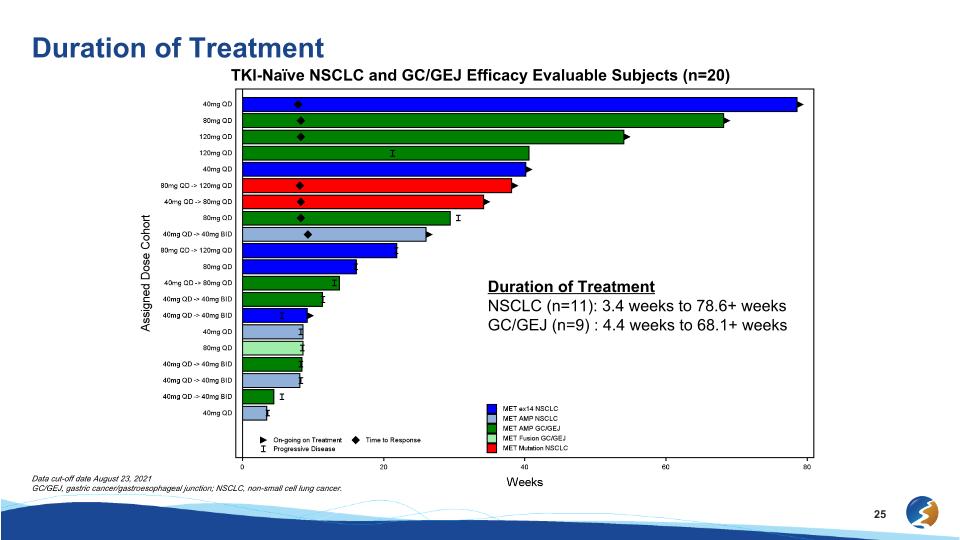
Duration of Treatment TKI-Naïve NSCLC and GC/GEJ Efficacy Evaluable Subjects (n=20) Duration of Treatment NSCLC (n=11): 3.4 weeks to 78.6+ weeks GC/GEJ (n=9) : 4.4 weeks to 68.1+ weeks Data cut-off date August 23, 2021 GC/GEJ, gastric cancer/gastroesophageal junction; NSCLC, non-small cell lung cancer.
Conclusions Elzovantinib was generally well tolerated Responses in MET TKI-naïve NSCLC and GC/GEJ cancers 95% patients received prior Chemo/IO therapy NSCLC: cORR 36% (all dose levels) GC/GEJ Cancer: cORR 33% (all dose levels) Limited activity in heavily pretreated MET TKI-pretreated patients (36% with ≥5 lines of prior therapy) with a CBR of 54% in NSCLC patients
Regulatory Updates Repotrectinib At the anticipated meeting with the FDA in 1H 2022 to discuss the topline BICR results from EXP-1 of the TRIDENT-1 study, plan to also discuss available BICR data in at least 50 patients from the ROS1-positive TKI-pretreated NSCLC cohorts of the study (EXP-2, EXP-3, EXP-4), with at least 6 months of follow-up for the majority of responders Elzovantinib�(TPX-0022) Recent End of Phase 1 meeting with the FDA focused on next steps in patients with NSCLC In initial feedback, FDA recommended exploring an intermediate dose level using the QD titration to BID dosing strategy in at least 6 to 10 patients prior to starting the Phase 2 portion of the study Plan to enroll at least 6 to 10 patients at 60 mg QD (14 days) 60 mg BID in Phase 1 Plan to revise SHIELD-1 into a potentially registrational Phase 1/2 study and initiate the Phase 2 portion in 2022, pending FDA feedback on data from the intermediate dose level FDA feedback focused on gastric/gastroesophageal junction cancer (GEJ) is pending Plan to initiate the SHIELD-2 combination study with an EGFR targeted therapy in 2022, pending filing of an IND application by the FDA

Thank you to the patients and investigators for participating in the TRIDENT-1 and SHIELD-1 studies

October 7, 2021 Next-Generation Precision Oncology Medicines��AACR-NCI-EORTC Investor Meeting




























