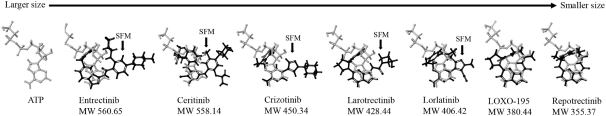
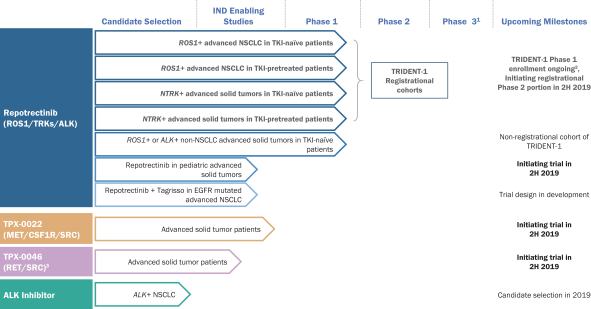
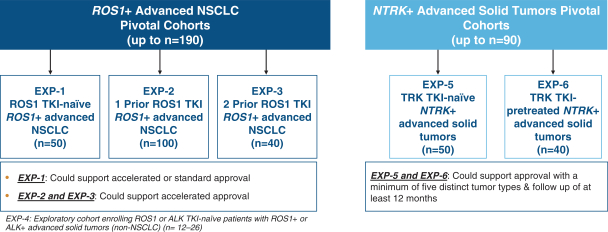
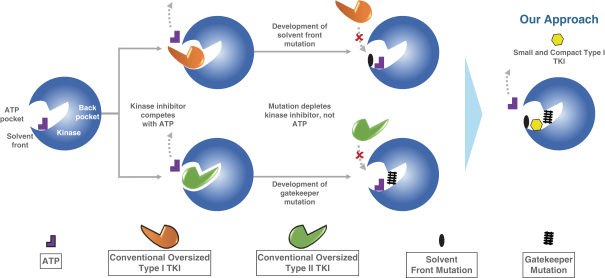
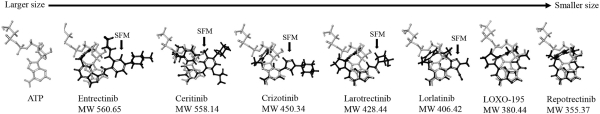
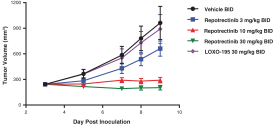
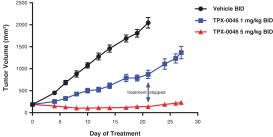
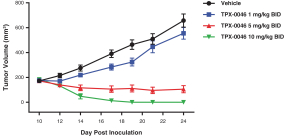
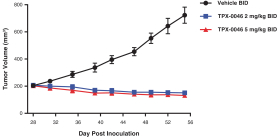
patents are expected to expire in January 2035, without taking into account any possible patent term extension, where applicable. As of December 31, 2018, we also had 78 pending patent applications directed to repotrectinib and its use in the United States, North America, Europe, Asia and other global regions which, if issued, are expected to expire at dates ranging between January 2035 and January 2038, without taking potential patent term extensions into account.
With regard to our preclinical drug candidates, we do not currently have any issued patents, but we intend to pursue patent protection for these candidates relating to their composition of matter, their methods of use and related technologies that we consider important to our business.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filinga non-provisional patent application.
In the United States, the term of a patent coveringan FDA-approved drug may, in certain cases, be eligible for a patent term extension under the Hatch-Waxman Act as compensation for the loss of patent term during the FDA regulatory review process. The period of extension may be up to five years, but cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent applicable to an approved drug is eligible for extension and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar provisions are available in Europe and in certain other jurisdictions to extend the term of a patent that covers an approved drug. It is possible that issued U.S. patents covering repotrectinib, and patents forTPX-0046,TPX-0022 and novel ALK inhibitors, if issued, may or will be entitled to patent term extensions. If our drug candidates receive FDA approval, we intend to apply for patent term extensions, if available, to extend the term of patents that cover the approved drug candidates. We also intend to seek patent term extensions in any jurisdictions where they are available; however, there is no guarantee that the applicable authorities, including the FDA, will agree with our assessment of whether such extensions should be granted, and even if granted, the length of such extensions.
In addition to patent protection, we also rely on trade secret protection for our proprietary information that is not amenable to, or that we do not consider appropriate for, patent protection, including certain aspect of our manufacturing processes. However, trade secrets can be difficult to protect. Although we take steps to protect our proprietary information, including restricting access to our confidential information, as well as entering intonon-disclosure and confidentiality agreements with our employees, consultants, independent contractors, advisors, contract manufacturers, CROs, hospitals, independent treatment centers, suppliers, collaborators and other third parties, such parties may breach such agreements and disclose our proprietary information including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. In addition, third parties may independently develop the same or similar proprietary information or may otherwise gain access to our proprietary information. As a result, we may be unable to meaningfully protect our trade secrets and proprietary information. For more information regarding the risks related to our intellectual property please see “Risk Factors—Risks Related to Our Intellectual Property.”
Government Regulation
The FDA and other regulatory authorities at federal, state, and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring, and post-approval reporting of drug products such as those we are developing. We, along with third-party contractors, will
126