
Exhibit 99.1 Next-Generation Precision Oncology Medicines Company Overview January 2021Exhibit 99.1 Next-Generation Precision Oncology Medicines Company Overview January 2021

Forward-Looking Statements Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development activities, plans and projected timelines, business strategy and plans, regulatory matters, objectives of management for future operations, market size and opportunity, our ability to complete certain milestones and our expectations regarding the relative benefits of our drug candidates versus competitive therapies. Words such as “believe,” “can”, “continue,” “anticipate,” “could,” “estimate,” “plan,” “predict,” “expect,” “intend,” “will,” “may,” “goal,” “upcoming,” “near term”, “milestone”, “potential,” “target” or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation: our preclinical studies and clinical trials may not be successful; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; the COVID-19 pandemic may disrupt our business and that of third parties on which we depend, including delaying or otherwise disrupting our research and development activities; and we may not be able to obtain additional financing. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these drug candidates for the use for which such drug candidates are being studied. 2Forward-Looking Statements Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development activities, plans and projected timelines, business strategy and plans, regulatory matters, objectives of management for future operations, market size and opportunity, our ability to complete certain milestones and our expectations regarding the relative benefits of our drug candidates versus competitive therapies. Words such as “believe,” “can”, “continue,” “anticipate,” “could,” “estimate,” “plan,” “predict,” “expect,” “intend,” “will,” “may,” “goal,” “upcoming,” “near term”, “milestone”, “potential,” “target” or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation: our preclinical studies and clinical trials may not be successful; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; the COVID-19 pandemic may disrupt our business and that of third parties on which we depend, including delaying or otherwise disrupting our research and development activities; and we may not be able to obtain additional financing. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these drug candidates for the use for which such drug candidates are being studied. 2

Significant Pipeline Progress in 2020 ü Breakthrough Therapy Designation and Third FDA Fast Track Designation Granted ü Reported Early, Interim Data from TRIDENT-1 Phase 2 Registrational Study ü Continued Site Activation, ~90 Sites in 14 Countries in TRIDENT-1 Study Repotrectinib ü Received FDA Feedback to Potentially Accelerate Development Timelines ü Advanced Combination Strategy in KRAS Tumors with Strong Preclinical Evidence ü Partnership with Zai Lab in Greater China¹ ü Reported Early, Interim Data from SHIELD-1 Phase 1 Study TPX-0022 ü Partnership with Zai Lab in Greater China announced Jan. 2021² TPX-0046ü Continued in Dose Finding Portion of Phase 1 Study with Early, Interim Data Anticipated in 1H 2021 TPX-0131ü Advanced IND-enabling Studies Toward Anticipated IND Submission in Q1 2021 Discoveryü Continued Investment in Multiple Oncology Discovery Programs ¹ Announced 07-Jul-2020. Economics include $25mm upfront, up to $151mm in milestones and mid-to-high teen royalties. ² Economics include $25mm upfront, up to approximately $336mm in potential milestones and mid-teen to low-twenty-percent royalties. 3Significant Pipeline Progress in 2020 ü Breakthrough Therapy Designation and Third FDA Fast Track Designation Granted ü Reported Early, Interim Data from TRIDENT-1 Phase 2 Registrational Study ü Continued Site Activation, ~90 Sites in 14 Countries in TRIDENT-1 Study Repotrectinib ü Received FDA Feedback to Potentially Accelerate Development Timelines ü Advanced Combination Strategy in KRAS Tumors with Strong Preclinical Evidence ü Partnership with Zai Lab in Greater China¹ ü Reported Early, Interim Data from SHIELD-1 Phase 1 Study TPX-0022 ü Partnership with Zai Lab in Greater China announced Jan. 2021² TPX-0046ü Continued in Dose Finding Portion of Phase 1 Study with Early, Interim Data Anticipated in 1H 2021 TPX-0131ü Advanced IND-enabling Studies Toward Anticipated IND Submission in Q1 2021 Discoveryü Continued Investment in Multiple Oncology Discovery Programs ¹ Announced 07-Jul-2020. Economics include $25mm upfront, up to $151mm in milestones and mid-to-high teen royalties. ² Economics include $25mm upfront, up to approximately $336mm in potential milestones and mid-teen to low-twenty-percent royalties. 3
Our Vision Drives Our Strategy to be a Leader in Precision Oncology Clinical Research Commercial Development • Growing commercial team with • Deep expertise in tumor biology, and • Rapid clinical proof-of-concept data proven track record in oncology structure-based drug design • Oncology development • Preparing for potential launch of • Existing macrocyclic scaffolds, and new and regulatory expertise lead drug candidate, repotrectinib discovery against novel or validated targets • Strategies that utilize the most rapid • Potential best-in-class products to address paths to market unmet needs in TKI-naive and resistance settings • Pipeline expansion through synergistic combinations 4Our Vision Drives Our Strategy to be a Leader in Precision Oncology Clinical Research Commercial Development • Growing commercial team with • Deep expertise in tumor biology, and • Rapid clinical proof-of-concept data proven track record in oncology structure-based drug design • Oncology development • Preparing for potential launch of • Existing macrocyclic scaffolds, and new and regulatory expertise lead drug candidate, repotrectinib discovery against novel or validated targets • Strategies that utilize the most rapid • Potential best-in-class products to address paths to market unmet needs in TKI-naive and resistance settings • Pipeline expansion through synergistic combinations 4
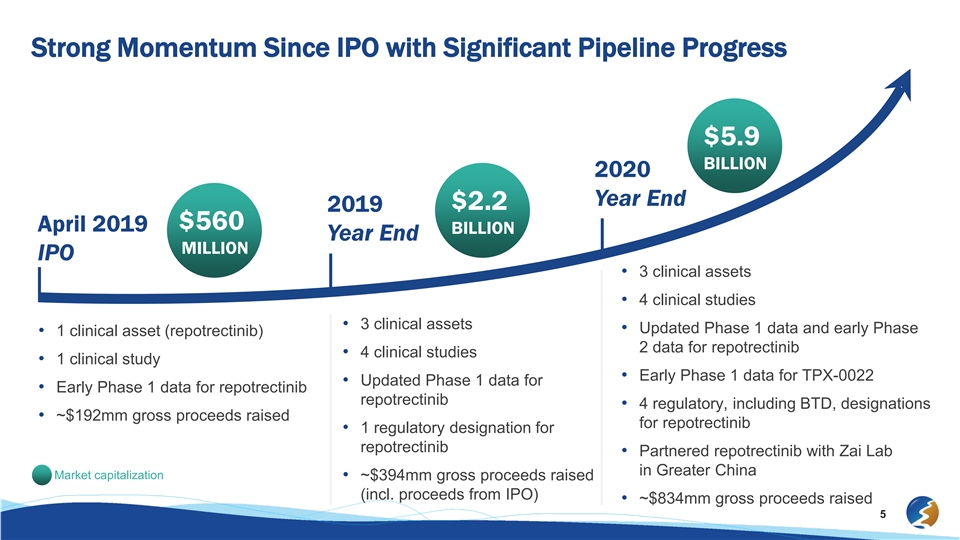
Strong Momentum Since IPO with Significant Pipeline Progress $5.9 BILLION 2020 Year End $2.2 2019 $560 April 2019 BILLION Year End MILLION IPO • 3 clinical assets • 4 clinical studies • 3 clinical assets • Updated Phase 1 data and early Phase • 1 clinical asset (repotrectinib) 2 data for repotrectinib • 4 clinical studies • 1 clinical study • Early Phase 1 data for TPX-0022 • Updated Phase 1 data for • Early Phase 1 data for repotrectinib repotrectinib • 4 regulatory, including BTD, designations • ~$192mm gross proceeds raised for repotrectinib • 1 regulatory designation for repotrectinib • Partnered repotrectinib with Zai Lab in Greater China Market capitalization • ~$394mm gross proceeds raised (incl. proceeds from IPO) • ~$834mm gross proceeds raised 5Strong Momentum Since IPO with Significant Pipeline Progress $5.9 BILLION 2020 Year End $2.2 2019 $560 April 2019 BILLION Year End MILLION IPO • 3 clinical assets • 4 clinical studies • 3 clinical assets • Updated Phase 1 data and early Phase • 1 clinical asset (repotrectinib) 2 data for repotrectinib • 4 clinical studies • 1 clinical study • Early Phase 1 data for TPX-0022 • Updated Phase 1 data for • Early Phase 1 data for repotrectinib repotrectinib • 4 regulatory, including BTD, designations • ~$192mm gross proceeds raised for repotrectinib • 1 regulatory designation for repotrectinib • Partnered repotrectinib with Zai Lab in Greater China Market capitalization • ~$394mm gross proceeds raised (incl. proceeds from IPO) • ~$834mm gross proceeds raised 5
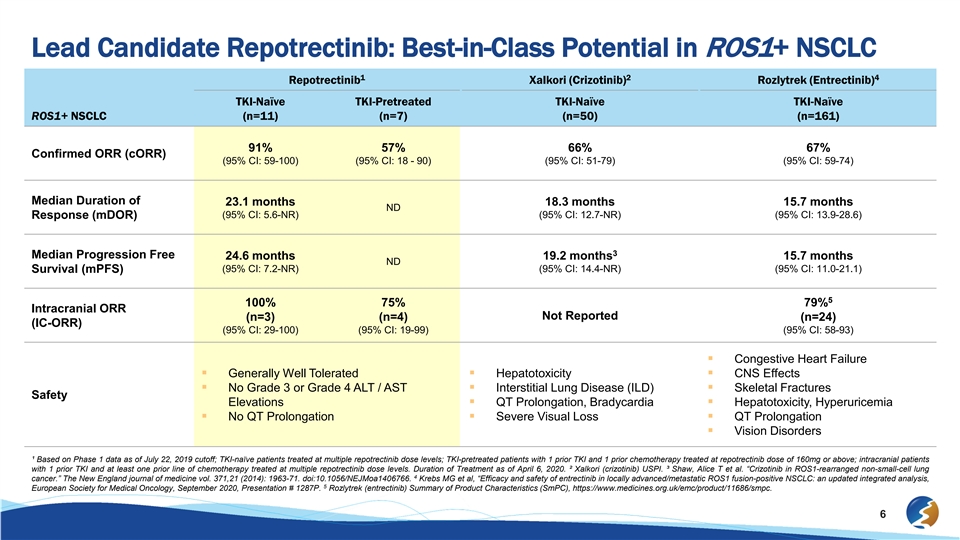
Lead Candidate Repotrectinib: Best-in-Class Potential in ROS1+ NSCLC 1 2 4 Repotrectinib Xalkori (Crizotinib) Rozlytrek (Entrectinib) TKI-Naïve TKI-Pretreated TKI-Naïve TKI-Naïve ROS1+ NSCLC (n=11) (n=7) (n=50) (n=161) 91% 57% 66% 67% Confirmed ORR (cORR) (95% CI: 59-100) (95% CI: 18 - 90) (95% CI: 51-79) (95% CI: 59-74) Median Duration of 23.1 months 18.3 months 15.7 months ND (95% CI: 5.6-NR) (95% CI: 12.7-NR) (95% CI: 13.9-28.6) Response (mDOR) 3 Median Progression Free 24.6 months 19.2 months 15.7 months ND (95% CI: 7.2-NR) (95% CI: 14.4-NR) (95% CI: 11.0-21.1) Survival (mPFS) 5 100% 75% 79% Intracranial ORR Not Reported (n=3) (n=4) (n=24) (IC-ORR) (95% CI: 29-100) (95% CI: 19-99) (95% CI: 58-93) § Congestive Heart Failure § Generally Well Tolerated§ Hepatotoxicity§ CNS Effects § No Grade 3 or Grade 4 ALT / AST § Interstitial Lung Disease (ILD)§ Skeletal Fractures Safety Elevations§ QT Prolongation, Bradycardia§ Hepatotoxicity, Hyperuricemia § No QT Prolongation§ Severe Visual Loss§ QT Prolongation § Vision Disorders ¹ Based on Phase 1 data as of July 22, 2019 cutoff; TKI-naïve patients treated at multiple repotrectinib dose levels; TKI-pretreated patients with 1 prior TKI and 1 prior chemotherapy treated at repotrectinib dose of 160mg or above; intracranial patients with 1 prior TKI and at least one prior line of chemotherapy treated at multiple repotrectinib dose levels. Duration of Treatment as of April 6, 2020. ² Xalkori (crizotinib) USPI. ³ Shaw, Alice T et al. “Crizotinib in ROS1-rearranged non-small-cell lung 4 cancer.” The New England journal of medicine vol. 371,21 (2014): 1963-71. doi:10.1056/NEJMoa1406766. Krebs MG et al, “Efficacy and safety of entrectinib in locally advanced/metastatic ROS1 fusion-positive NSCLC: an updated integrated analysis, 5 European Society for Medical Oncology, September 2020, Presentation # 1287P. Rozlytrek (entrectinib) Summary of Product Characteristics (SmPC), https://www.medicines.org.uk/emc/product/11686/smpc. 6Lead Candidate Repotrectinib: Best-in-Class Potential in ROS1+ NSCLC 1 2 4 Repotrectinib Xalkori (Crizotinib) Rozlytrek (Entrectinib) TKI-Naïve TKI-Pretreated TKI-Naïve TKI-Naïve ROS1+ NSCLC (n=11) (n=7) (n=50) (n=161) 91% 57% 66% 67% Confirmed ORR (cORR) (95% CI: 59-100) (95% CI: 18 - 90) (95% CI: 51-79) (95% CI: 59-74) Median Duration of 23.1 months 18.3 months 15.7 months ND (95% CI: 5.6-NR) (95% CI: 12.7-NR) (95% CI: 13.9-28.6) Response (mDOR) 3 Median Progression Free 24.6 months 19.2 months 15.7 months ND (95% CI: 7.2-NR) (95% CI: 14.4-NR) (95% CI: 11.0-21.1) Survival (mPFS) 5 100% 75% 79% Intracranial ORR Not Reported (n=3) (n=4) (n=24) (IC-ORR) (95% CI: 29-100) (95% CI: 19-99) (95% CI: 58-93) § Congestive Heart Failure § Generally Well Tolerated§ Hepatotoxicity§ CNS Effects § No Grade 3 or Grade 4 ALT / AST § Interstitial Lung Disease (ILD)§ Skeletal Fractures Safety Elevations§ QT Prolongation, Bradycardia§ Hepatotoxicity, Hyperuricemia § No QT Prolongation§ Severe Visual Loss§ QT Prolongation § Vision Disorders ¹ Based on Phase 1 data as of July 22, 2019 cutoff; TKI-naïve patients treated at multiple repotrectinib dose levels; TKI-pretreated patients with 1 prior TKI and 1 prior chemotherapy treated at repotrectinib dose of 160mg or above; intracranial patients with 1 prior TKI and at least one prior line of chemotherapy treated at multiple repotrectinib dose levels. Duration of Treatment as of April 6, 2020. ² Xalkori (crizotinib) USPI. ³ Shaw, Alice T et al. “Crizotinib in ROS1-rearranged non-small-cell lung 4 cancer.” The New England journal of medicine vol. 371,21 (2014): 1963-71. doi:10.1056/NEJMoa1406766. Krebs MG et al, “Efficacy and safety of entrectinib in locally advanced/metastatic ROS1 fusion-positive NSCLC: an updated integrated analysis, 5 European Society for Medical Oncology, September 2020, Presentation # 1287P. Rozlytrek (entrectinib) Summary of Product Characteristics (SmPC), https://www.medicines.org.uk/emc/product/11686/smpc. 6

Four Ongoing Clinical Studies Against Well Validated Oncogenic Targets Multiple Additional Studies Anticipated in 2021 Early Stage Late Stage Clinical Clinical Discovery Preclinical Development Development Repotrectinib (ROS1/TRK) § TRIDENT-1: Advanced NSCLC (ROS1) and solid tumors (NTRK) § Pediatric advanced solid tumors § TRIDENT-2: KRAS-targeting combination TPX-0022 (MET) § SHIELD-1: Advanced solid tumors § SHIELD-2: EGFR combination TPX-0046 (RET) § Advanced solid tumors TPX-0131 (ALK) § Advanced NSCLC Discovery Programs § Multiple Oncology Targets Note: Turning Point retains worldwide rights for all pipeline assets, except for repotrectinib and TPX-0022 in Greater China (partnered with Zai Lab). 7Four Ongoing Clinical Studies Against Well Validated Oncogenic Targets Multiple Additional Studies Anticipated in 2021 Early Stage Late Stage Clinical Clinical Discovery Preclinical Development Development Repotrectinib (ROS1/TRK) § TRIDENT-1: Advanced NSCLC (ROS1) and solid tumors (NTRK) § Pediatric advanced solid tumors § TRIDENT-2: KRAS-targeting combination TPX-0022 (MET) § SHIELD-1: Advanced solid tumors § SHIELD-2: EGFR combination TPX-0046 (RET) § Advanced solid tumors TPX-0131 (ALK) § Advanced NSCLC Discovery Programs § Multiple Oncology Targets Note: Turning Point retains worldwide rights for all pipeline assets, except for repotrectinib and TPX-0022 in Greater China (partnered with Zai Lab). 7

Single Agent and Combination Opportunities To Treat Patients with Biomarker Driven Cancers Patient Populations: US+EU5 Incidence³ 4,5 ROS1+ NSCLC 6k 3.4 6 NTRK+ Solid Tumors 4k MILLION 4,7-8 MET Altered NSCLC 13k estimated patients diagnosed 9-12 MET Amplified Gastric Cancer 2k with solid tumors annually in the US and EU5¹ 4,5 RET+ NSCLC 4k 13-15 RET+ Thyroid Cancer 4k 4,16 ALK+ NSCLC 11k 57% 4,17-18 EGFRm / MET Amplified NSCLC 6k 19-20 KRASm NSCLC 92k of tumors harbor potentially actionable targets² Repotrectinib TPX-0022 TPX-0046 TPX-0131 ¹ American Cancer Society 2020. ² Bailey MH, et al: Cell 2018. ³ Estimates rounded and include locally advanced and metastatic populations per SEER, based on incidence reported by American Cancer Society Cancer Facts & Figures 2020 (US) and GLOBOCAN 2018 4 5 6 7 8 (EU5). Zappa, et al: Transl Lung Cancer Res 2016. Hirsch FR, et al: Lancet 2017. Rosen EY, et al: Clin Cancer Res 2020. Drilon A, et al: J Thorac Oncol. 2017. Overbeck TR, et al: Translational lung cancer research 2020; based on gene copy number of 10 or 9 10 11 12 13 greater. Loberg RD et al: Journal of Clinical Oncology 2014. Yang Y, et al: Gastric Cancer 2016. Inokuchi M, et al: World J Gastrointest Oncol. 2015. Lennerz JK, et al: J Clin Oncol. 2011. Includes MTC and PTC. For MTC, based on weighted average of 14 15 sporadic (75%) and familial (25%) per American Cancer Society, with biomarker frequencies of 60% (Taccaliti A, et al: Curr Genomics 2011) and 98% (Elisei R, et al: Genes (Basel) 2019), respectively. Lee MY, et al: Cancer Res Treat. 2017. Prescott JD, et al: Cancer 16 17 18 2015. Garber K.: J Natl Cancer Inst. 2010. Assumes 15% of US and EU5 NSCLC patients are EGFR+ (Hirsch FR, et al: Lancet 2017). Does not account for proportion of patients who will not progress to a 2L therapy. Ramalingam SS, et al: Annals of Oncology 19 20 2018. Based on repotrectinib combination data presented at AACR 2020 showing synergy in certain mutant KRAS models. Based on overall mutation rate in the KRAS gene in NSCLC based on Biernacka A, et al: Cancer Genet 2016. 8 Combos Single AgentSingle Agent and Combination Opportunities To Treat Patients with Biomarker Driven Cancers Patient Populations: US+EU5 Incidence³ 4,5 ROS1+ NSCLC 6k 3.4 6 NTRK+ Solid Tumors 4k MILLION 4,7-8 MET Altered NSCLC 13k estimated patients diagnosed 9-12 MET Amplified Gastric Cancer 2k with solid tumors annually in the US and EU5¹ 4,5 RET+ NSCLC 4k 13-15 RET+ Thyroid Cancer 4k 4,16 ALK+ NSCLC 11k 57% 4,17-18 EGFRm / MET Amplified NSCLC 6k 19-20 KRASm NSCLC 92k of tumors harbor potentially actionable targets² Repotrectinib TPX-0022 TPX-0046 TPX-0131 ¹ American Cancer Society 2020. ² Bailey MH, et al: Cell 2018. ³ Estimates rounded and include locally advanced and metastatic populations per SEER, based on incidence reported by American Cancer Society Cancer Facts & Figures 2020 (US) and GLOBOCAN 2018 4 5 6 7 8 (EU5). Zappa, et al: Transl Lung Cancer Res 2016. Hirsch FR, et al: Lancet 2017. Rosen EY, et al: Clin Cancer Res 2020. Drilon A, et al: J Thorac Oncol. 2017. Overbeck TR, et al: Translational lung cancer research 2020; based on gene copy number of 10 or 9 10 11 12 13 greater. Loberg RD et al: Journal of Clinical Oncology 2014. Yang Y, et al: Gastric Cancer 2016. Inokuchi M, et al: World J Gastrointest Oncol. 2015. Lennerz JK, et al: J Clin Oncol. 2011. Includes MTC and PTC. For MTC, based on weighted average of 14 15 sporadic (75%) and familial (25%) per American Cancer Society, with biomarker frequencies of 60% (Taccaliti A, et al: Curr Genomics 2011) and 98% (Elisei R, et al: Genes (Basel) 2019), respectively. Lee MY, et al: Cancer Res Treat. 2017. Prescott JD, et al: Cancer 16 17 18 2015. Garber K.: J Natl Cancer Inst. 2010. Assumes 15% of US and EU5 NSCLC patients are EGFR+ (Hirsch FR, et al: Lancet 2017). Does not account for proportion of patients who will not progress to a 2L therapy. Ramalingam SS, et al: Annals of Oncology 19 20 2018. Based on repotrectinib combination data presented at AACR 2020 showing synergy in certain mutant KRAS models. Based on overall mutation rate in the KRAS gene in NSCLC based on Biernacka A, et al: Cancer Genet 2016. 8 Combos Single Agent

Repotrectinib: A Highly Selective ROS1/TRK InhibitorRepotrectinib: A Highly Selective ROS1/TRK Inhibitor

Repotrectinib: Potential Best-in-Class ROS1 and NTRK Targeted Therapy in TKI-Naïve and Treatment Resistance Settings § Highly potent, structurally differentiated: small (low Our Approach molecular weight), compact, with a rigid 3D Repotrectinib macrocycle ATP § Designed to bind completely inside the ATP pocket even in the presence of solvent front or gatekeeper Kinase mutations § Potential to address resistance from prior lines of TKI therapy § May also prevent or delay the emergence of new Solvent-Front Gatekeeper resistant mutations mutation mutation 10Repotrectinib: Potential Best-in-Class ROS1 and NTRK Targeted Therapy in TKI-Naïve and Treatment Resistance Settings § Highly potent, structurally differentiated: small (low Our Approach molecular weight), compact, with a rigid 3D Repotrectinib macrocycle ATP § Designed to bind completely inside the ATP pocket even in the presence of solvent front or gatekeeper Kinase mutations § Potential to address resistance from prior lines of TKI therapy § May also prevent or delay the emergence of new Solvent-Front Gatekeeper resistant mutations mutation mutation 10
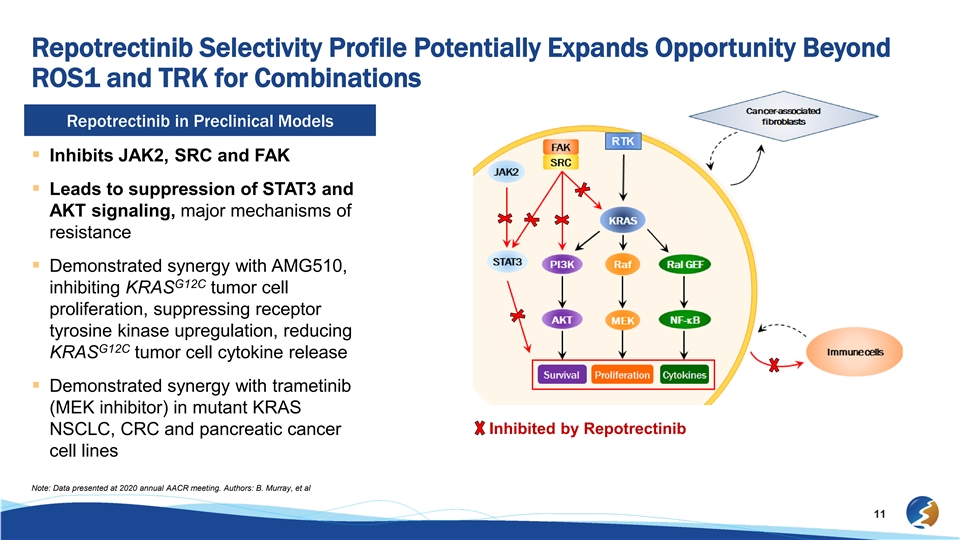
Repotrectinib Selectivity Profile Potentially Expands Opportunity Beyond ROS1 and TRK for Combinations Repotrectinib in Preclinical Models § Inhibits JAK2, SRC and FAK § Leads to suppression of STAT3 and AKT signaling, major mechanisms of resistance § Demonstrated synergy with AMG510, G12C inhibiting KRAS tumor cell proliferation, suppressing receptor tyrosine kinase upregulation, reducing G12C KRAS tumor cell cytokine release § Demonstrated synergy with trametinib (MEK inhibitor) in mutant KRAS Inhibited by Repotrectinib NSCLC, CRC and pancreatic cancer cell lines Note: Data presented at 2020 annual AACR meeting. Authors: B. Murray, et al 11Repotrectinib Selectivity Profile Potentially Expands Opportunity Beyond ROS1 and TRK for Combinations Repotrectinib in Preclinical Models § Inhibits JAK2, SRC and FAK § Leads to suppression of STAT3 and AKT signaling, major mechanisms of resistance § Demonstrated synergy with AMG510, G12C inhibiting KRAS tumor cell proliferation, suppressing receptor tyrosine kinase upregulation, reducing G12C KRAS tumor cell cytokine release § Demonstrated synergy with trametinib (MEK inhibitor) in mutant KRAS Inhibited by Repotrectinib NSCLC, CRC and pancreatic cancer cell lines Note: Data presented at 2020 annual AACR meeting. Authors: B. Murray, et al 11
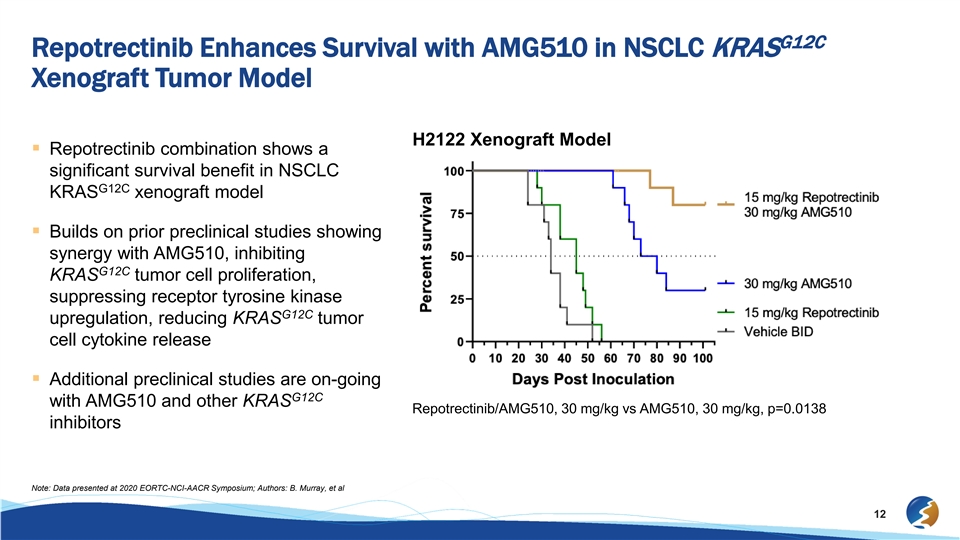
G12C Repotrectinib Enhances Survival with AMG510 in NSCLC KRAS Xenograft Tumor Model H2122 Xenograft Model § Repotrectinib combination shows a significant survival benefit in NSCLC G12C KRAS xenograft model § Builds on prior preclinical studies showing synergy with AMG510, inhibiting G12C KRAS tumor cell proliferation, suppressing receptor tyrosine kinase G12C upregulation, reducing KRAS tumor cell cytokine release § Additional preclinical studies are on-going G12C with AMG510 and other KRAS Repotrectinib/AMG510, 30 mg/kg vs AMG510, 30 mg/kg, p=0.0138 inhibitors Note: Data presented at 2020 EORTC-NCI-AACR Symposium; Authors: B. Murray, et al 12G12C Repotrectinib Enhances Survival with AMG510 in NSCLC KRAS Xenograft Tumor Model H2122 Xenograft Model § Repotrectinib combination shows a significant survival benefit in NSCLC G12C KRAS xenograft model § Builds on prior preclinical studies showing synergy with AMG510, inhibiting G12C KRAS tumor cell proliferation, suppressing receptor tyrosine kinase G12C upregulation, reducing KRAS tumor cell cytokine release § Additional preclinical studies are on-going G12C with AMG510 and other KRAS Repotrectinib/AMG510, 30 mg/kg vs AMG510, 30 mg/kg, p=0.0138 inhibitors Note: Data presented at 2020 EORTC-NCI-AACR Symposium; Authors: B. Murray, et al 12

TRIDENT-1: An Ongoing Phase 1/2 Study of Repotrectinib in Patients with Advanced Solid Tumors Harboring ROS1, NTRK1-3 Rearrangements Phase 2 Early Interim Update Based on Data Cutoff of July 10, 2020TRIDENT-1: An Ongoing Phase 1/2 Study of Repotrectinib in Patients with Advanced Solid Tumors Harboring ROS1, NTRK1-3 Rearrangements Phase 2 Early Interim Update Based on Data Cutoff of July 10, 2020

TRIDENT-1 Efficacy Overview – Early Phase 2 Data Compared to Phase 1 1 Phase 2 (N=39 ) Efficacy by Physician ROS1+ TKI-Naïve ROS1+ TKI Pretreated ROS1+ TKI Pretreated ROS1+ TKI Pretreated NTRK+ TKI-Pretreated Assessment (EXP-1: N=7) 1 Prior TKI with prior 1 Prior TKI without prior 2 Prior TKI without prior (EXP-6: N=6) platinum-based platinum-based chemotherapy platinum-based chemotherapy 3 chemotherapy (EXP-4: N=6) (EXP-Other : N=5) (EXP-2: N=5) 2 Overall Response 6 (86% ) 2 (40%) 4 (67%) 2 (40%) 3 (50%) Rate (42 – 100) (5 – 85) (22 – 96) (5 – 85) (12 – 88) (95% CI) Duration of Response 0.9+ - 2.0+ 4.5 - 5.6+ 1.0+ – 5.7+ 1.9+ – 1.9+ 1.7+ – 3.6+ (Range) (months) 4 Phase 1 (N=33 ): All Dose Levels Studied Efficacy by BICR ROS1+ TKI-Naïve ROS1+ TKI Pretreated ROS1+ TKI Pretreated ROS1+ TKI Pretreated NTRK+ TKI-Pretreated (N=11) 1 Prior TKI with prior 1 Prior TKI without prior 2 Prior TKI without prior (N=3) platinum-based platinum-based chemotherapy platinum-based chemotherapy chemotherapy (N=4) (N=1) (N=14) Overall Response Rate 10 (91%) 5 (36%) 2 (50%) 0 1 (33%) (95% CI) (59 - 100) (13 - 65) (7 – 93) (1 – 91) Duration of Response 3.7+ - 23.3+ 2.7+ - 13.0 0.8+ - 5.5+ NA 9.8 (Range) (months) ¹ Represents the first 39 patients treated. In EXP-3 (Two prior TKIs with prior chemotherapy; N=10): No objective responses observed. EXP-3 to be modified to exclude patients treated with prior chemotherapy ² The seventh patient achieved an unconfirmed PR (uPR) after the data cutoff date and remained on treatment awaiting a confirmatory scan as of the early interim update on Aug. 19, 2020. uPR is not counted in ORR calculations ³ Represents the planned modified EXP-3 cohort of patients previously treated with 2 prior TKIs without prior chemotherapy 4 Phase 1 dataset previously reported 4 patients treated with 3 prior TKIs and 6 patients who had 2 TKIs and prior chemotherapy Phase 2 Data cutoff of 10 July 2020, Phase 1 Data cutoff of 22 July 2019 14 ConfidentialTRIDENT-1 Efficacy Overview – Early Phase 2 Data Compared to Phase 1 1 Phase 2 (N=39 ) Efficacy by Physician ROS1+ TKI-Naïve ROS1+ TKI Pretreated ROS1+ TKI Pretreated ROS1+ TKI Pretreated NTRK+ TKI-Pretreated Assessment (EXP-1: N=7) 1 Prior TKI with prior 1 Prior TKI without prior 2 Prior TKI without prior (EXP-6: N=6) platinum-based platinum-based chemotherapy platinum-based chemotherapy 3 chemotherapy (EXP-4: N=6) (EXP-Other : N=5) (EXP-2: N=5) 2 Overall Response 6 (86% ) 2 (40%) 4 (67%) 2 (40%) 3 (50%) Rate (42 – 100) (5 – 85) (22 – 96) (5 – 85) (12 – 88) (95% CI) Duration of Response 0.9+ - 2.0+ 4.5 - 5.6+ 1.0+ – 5.7+ 1.9+ – 1.9+ 1.7+ – 3.6+ (Range) (months) 4 Phase 1 (N=33 ): All Dose Levels Studied Efficacy by BICR ROS1+ TKI-Naïve ROS1+ TKI Pretreated ROS1+ TKI Pretreated ROS1+ TKI Pretreated NTRK+ TKI-Pretreated (N=11) 1 Prior TKI with prior 1 Prior TKI without prior 2 Prior TKI without prior (N=3) platinum-based platinum-based chemotherapy platinum-based chemotherapy chemotherapy (N=4) (N=1) (N=14) Overall Response Rate 10 (91%) 5 (36%) 2 (50%) 0 1 (33%) (95% CI) (59 - 100) (13 - 65) (7 – 93) (1 – 91) Duration of Response 3.7+ - 23.3+ 2.7+ - 13.0 0.8+ - 5.5+ NA 9.8 (Range) (months) ¹ Represents the first 39 patients treated. In EXP-3 (Two prior TKIs with prior chemotherapy; N=10): No objective responses observed. EXP-3 to be modified to exclude patients treated with prior chemotherapy ² The seventh patient achieved an unconfirmed PR (uPR) after the data cutoff date and remained on treatment awaiting a confirmatory scan as of the early interim update on Aug. 19, 2020. uPR is not counted in ORR calculations ³ Represents the planned modified EXP-3 cohort of patients previously treated with 2 prior TKIs without prior chemotherapy 4 Phase 1 dataset previously reported 4 patients treated with 3 prior TKIs and 6 patients who had 2 TKIs and prior chemotherapy Phase 2 Data cutoff of 10 July 2020, Phase 1 Data cutoff of 22 July 2019 14 Confidential

ROS1+ TKI Naïve Advanced NSCLC Patients EXP-1 + Phase 1 Phase 2 Phase 1+2 (n=7) (n=14) Confirmed ORR 86% 86% (42 – 100) (57 – 98) (95% CI) 0.9+ –2.0+ 0.9+ – 17.6+ months months Duration of Response (range) (range) n=6 n=12 ^Patient achieved an unconfirmed partial response after the data cutoff date and remained on treatment awaiting a confirmatory scan as of the early interim update on Aug. 19, 2020. uPR is not counted in cORR calculation. Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib 15ROS1+ TKI Naïve Advanced NSCLC Patients EXP-1 + Phase 1 Phase 2 Phase 1+2 (n=7) (n=14) Confirmed ORR 86% 86% (42 – 100) (57 – 98) (95% CI) 0.9+ –2.0+ 0.9+ – 17.6+ months months Duration of Response (range) (range) n=6 n=12 ^Patient achieved an unconfirmed partial response after the data cutoff date and remained on treatment awaiting a confirmatory scan as of the early interim update on Aug. 19, 2020. uPR is not counted in cORR calculation. Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib 15
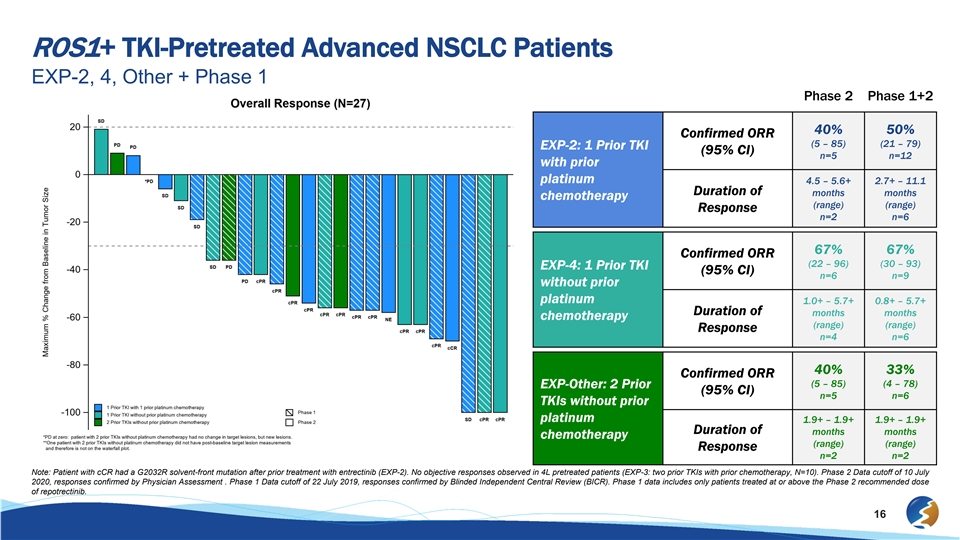
ROS1+ TKI-Pretreated Advanced NSCLC Patients EXP-2, 4, Other + Phase 1 Phase 2 Phase 1+2 40% 50% Confirmed ORR (5 – 85) (21 – 79) EXP-2: 1 Prior TKI (95% CI) n=5 n=12 with prior platinum 4.5 – 5.6+ 2.7+ – 11.1 Duration of months months chemotherapy (range) (range) Response n=2 n=6 67% 67% Confirmed ORR (22 – 96) (30 – 93) EXP-4: 1 Prior TKI (95% CI) n=6 n=9 without prior platinum 1.0+ – 5.7+ 0.8+ – 5.7+ Duration of months months chemotherapy (range) (range) Response n=4 n=6 40% 33% Confirmed ORR (5 – 85) (4 – 78) EXP-Other: 2 Prior (95% CI) n=5 n=6 TKIs without prior platinum 1.9+ – 1.9+ 1.9+ – 1.9+ Duration of months months chemotherapy (range) (range) Response n=2 n=2 Note: Patient with cCR had a G2032R solvent-front mutation after prior treatment with entrectinib (EXP-2). No objective responses observed in 4L pretreated patients (EXP-3: two prior TKIs with prior chemotherapy, N=10). Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment . Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR). Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib. 16ROS1+ TKI-Pretreated Advanced NSCLC Patients EXP-2, 4, Other + Phase 1 Phase 2 Phase 1+2 40% 50% Confirmed ORR (5 – 85) (21 – 79) EXP-2: 1 Prior TKI (95% CI) n=5 n=12 with prior platinum 4.5 – 5.6+ 2.7+ – 11.1 Duration of months months chemotherapy (range) (range) Response n=2 n=6 67% 67% Confirmed ORR (22 – 96) (30 – 93) EXP-4: 1 Prior TKI (95% CI) n=6 n=9 without prior platinum 1.0+ – 5.7+ 0.8+ – 5.7+ Duration of months months chemotherapy (range) (range) Response n=4 n=6 40% 33% Confirmed ORR (5 – 85) (4 – 78) EXP-Other: 2 Prior (95% CI) n=5 n=6 TKIs without prior platinum 1.9+ – 1.9+ 1.9+ – 1.9+ Duration of months months chemotherapy (range) (range) Response n=2 n=2 Note: Patient with cCR had a G2032R solvent-front mutation after prior treatment with entrectinib (EXP-2). No objective responses observed in 4L pretreated patients (EXP-3: two prior TKIs with prior chemotherapy, N=10). Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment . Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR). Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib. 16
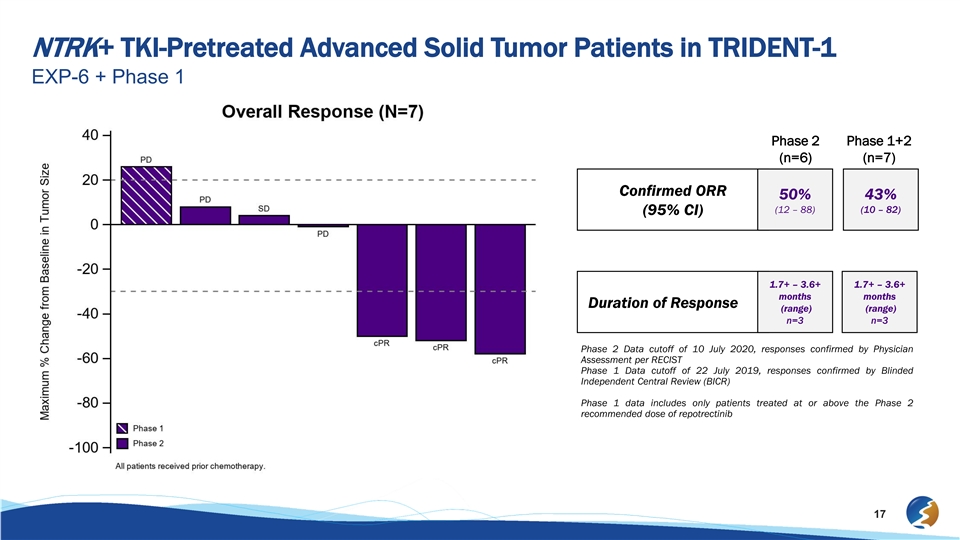
NTRK+ TKI-Pretreated Advanced Solid Tumor Patients in TRIDENT-1 EXP-6 + Phase 1 Phase 2 Phase 1+2 (n=6) (n=7) Confirmed ORR 50% 43% (12 – 88) (10 – 82) (95% CI) 1.7+ – 3.6+ 1.7+ – 3.6+ months months Duration of Response (range) (range) n=3 n=3 Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment per RECIST Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib 17NTRK+ TKI-Pretreated Advanced Solid Tumor Patients in TRIDENT-1 EXP-6 + Phase 1 Phase 2 Phase 1+2 (n=6) (n=7) Confirmed ORR 50% 43% (12 – 88) (10 – 82) (95% CI) 1.7+ – 3.6+ 1.7+ – 3.6+ months months Duration of Response (range) (range) n=3 n=3 Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment per RECIST Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib 17

Phase 2 Safety Summary: Treatment-Emergent and Treatment-Related AEs All Treated Patients (n=39) § Generally well-tolerated TEAEs (≥10% of patients) TRAEs § Most treatment emergent adverse events All Grades Grade 3 Grade 4 Grade 3¹ Grade 4 (TEAEs) and treatment-related adverse Adverse Event n (%) n (%) n (%) n (%) n (%) events (TRAEs) were Grade 1 or 2 Dizziness 24 (61.5) --- – – – § No Grade 4 or Grade 5 TRAEs Fatigue 15 (38.5) 1 (2.6) – 1 (2.6) – Constipation 13 (33.3) – – – – § The most commonly reported TEAE was Dysgeusia 13 (33.3) – – – – low grade dizziness Dyspnea 11 (28.2) 1 (2.6) – – – – 83% (20/24) were Grade 1 Paresthesia 8 (20.5) – – – – – No cases of dizziness have led Muscular weakness 7 (17.9) 1 (2.6) – – – to treatment discontinuation Nausea 7 (17.9) – – – – § No Grade 3 or Grade 4 treatment-related Pain 5 (12.8) – – – – ALT/AST elevations reported Anemia 4 (10.3) 2 (5.1) – 2 (5.1) – Aspartate aminotransferase increased 4 (10.3) – – – – Hypoesthesia oral 4 (10.3) – – – – Sensitive skin 4 (10.3) – – – – Data cut-off date of July 10, 2020. ¹ Additional Grade 3 TRAEs:: depressed level of consciousness (n=1), neutrophil count decreased (n=2), and lymphopenia (n=1). 18Phase 2 Safety Summary: Treatment-Emergent and Treatment-Related AEs All Treated Patients (n=39) § Generally well-tolerated TEAEs (≥10% of patients) TRAEs § Most treatment emergent adverse events All Grades Grade 3 Grade 4 Grade 3¹ Grade 4 (TEAEs) and treatment-related adverse Adverse Event n (%) n (%) n (%) n (%) n (%) events (TRAEs) were Grade 1 or 2 Dizziness 24 (61.5) --- – – – § No Grade 4 or Grade 5 TRAEs Fatigue 15 (38.5) 1 (2.6) – 1 (2.6) – Constipation 13 (33.3) – – – – § The most commonly reported TEAE was Dysgeusia 13 (33.3) – – – – low grade dizziness Dyspnea 11 (28.2) 1 (2.6) – – – – 83% (20/24) were Grade 1 Paresthesia 8 (20.5) – – – – – No cases of dizziness have led Muscular weakness 7 (17.9) 1 (2.6) – – – to treatment discontinuation Nausea 7 (17.9) – – – – § No Grade 3 or Grade 4 treatment-related Pain 5 (12.8) – – – – ALT/AST elevations reported Anemia 4 (10.3) 2 (5.1) – 2 (5.1) – Aspartate aminotransferase increased 4 (10.3) – – – – Hypoesthesia oral 4 (10.3) – – – – Sensitive skin 4 (10.3) – – – – Data cut-off date of July 10, 2020. ¹ Additional Grade 3 TRAEs:: depressed level of consciousness (n=1), neutrophil count decreased (n=2), and lymphopenia (n=1). 18
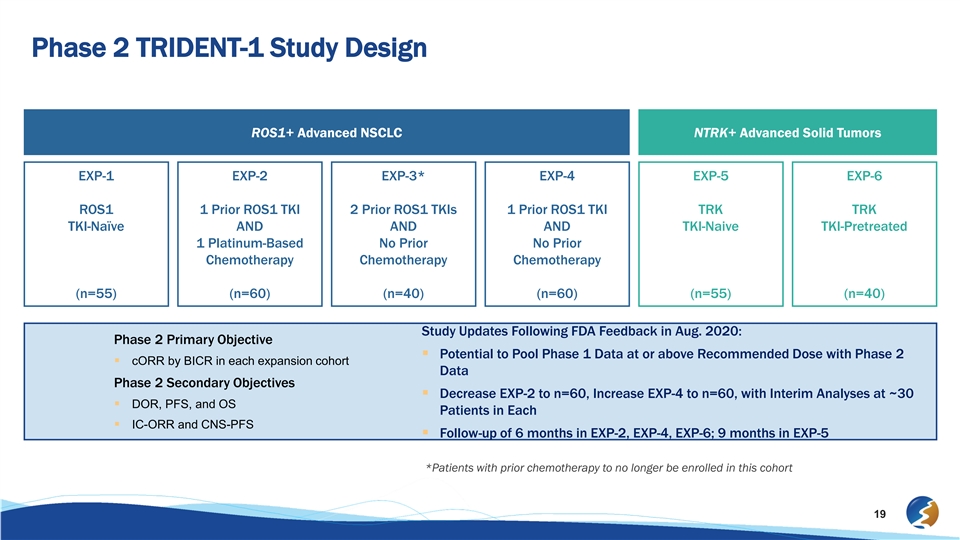
Phase 2 TRIDENT-1 Study Design ROS1+ Advanced NSCLC NTRK+ Advanced Solid Tumors EXP-1 EXP-2 EXP-3* EXP-4 EXP-5 EXP-6 ROS1 1 Prior ROS1 TKI 2 Prior ROS1 TKIs 1 Prior ROS1 TKI TRK TRK TKI-Naïve AND AND AND TKI-Naive TKI-Pretreated 1 Platinum-Based No Prior No Prior Chemotherapy Chemotherapy Chemotherapy (n=55) (n=60) (n=40) (n=60) (n=55) (n=40) Study Updates Following FDA Feedback in Aug. 2020: Phase 2 Primary Objective § Potential to Pool Phase 1 Data at or above Recommended Dose with Phase 2 § cORR by BICR in each expansion cohort Data Phase 2 Secondary Objectives § Decrease EXP-2 to n=60, Increase EXP-4 to n=60, with Interim Analyses at ~30 § DOR, PFS, and OS Patients in Each § IC-ORR and CNS-PFS § Follow-up of 6 months in EXP-2, EXP-4, EXP-6; 9 months in EXP-5 *Patients with prior chemotherapy to no longer be enrolled in this cohort 19Phase 2 TRIDENT-1 Study Design ROS1+ Advanced NSCLC NTRK+ Advanced Solid Tumors EXP-1 EXP-2 EXP-3* EXP-4 EXP-5 EXP-6 ROS1 1 Prior ROS1 TKI 2 Prior ROS1 TKIs 1 Prior ROS1 TKI TRK TRK TKI-Naïve AND AND AND TKI-Naive TKI-Pretreated 1 Platinum-Based No Prior No Prior Chemotherapy Chemotherapy Chemotherapy (n=55) (n=60) (n=40) (n=60) (n=55) (n=40) Study Updates Following FDA Feedback in Aug. 2020: Phase 2 Primary Objective § Potential to Pool Phase 1 Data at or above Recommended Dose with Phase 2 § cORR by BICR in each expansion cohort Data Phase 2 Secondary Objectives § Decrease EXP-2 to n=60, Increase EXP-4 to n=60, with Interim Analyses at ~30 § DOR, PFS, and OS Patients in Each § IC-ORR and CNS-PFS § Follow-up of 6 months in EXP-2, EXP-4, EXP-6; 9 months in EXP-5 *Patients with prior chemotherapy to no longer be enrolled in this cohort 19

Advancing Three Additional Next-Generation Kinase InhibitorsAdvancing Three Additional Next-Generation Kinase Inhibitors
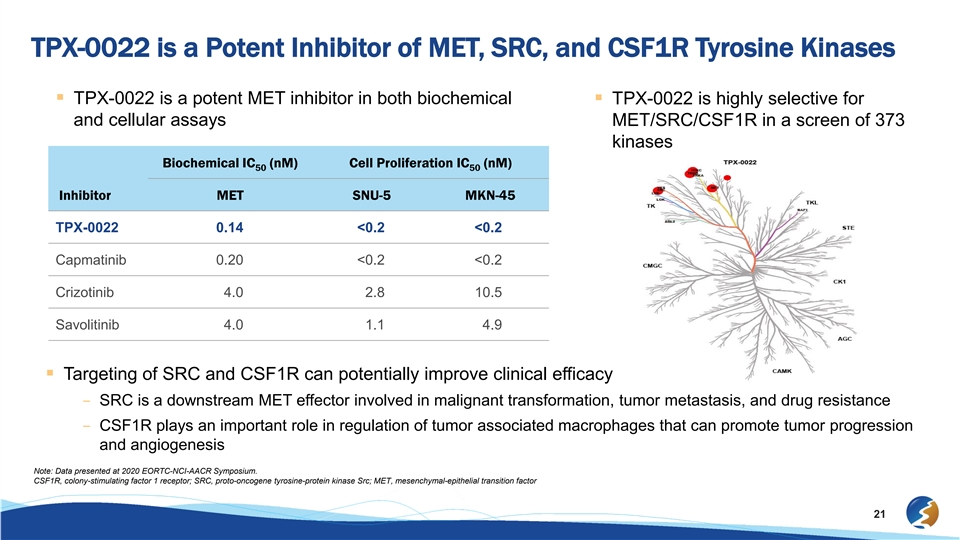
TPX-0022 is a Potent Inhibitor of MET, SRC, and CSF1R Tyrosine Kinases § TPX-0022 is a potent MET inhibitor in both biochemical § TPX-0022 is highly selective for and cellular assays MET/SRC/CSF1R in a screen of 373 kinases Biochemical IC (nM) Cell Proliferation IC (nM) 50 50 Inhibitor MET SNU-5 MKN-45 TPX-0022 0.14 <0.2 <0.2 Capmatinib 0.20 <0.2 <0.2 Crizotinib 4.0 2.8 10.5 Savolitinib 4.0 1.1 4.9 § Targeting of SRC and CSF1R can potentially improve clinical efficacy – SRC is a downstream MET effector involved in malignant transformation, tumor metastasis, and drug resistance – CSF1R plays an important role in regulation of tumor associated macrophages that can promote tumor progression and angiogenesis Note: Data presented at 2020 EORTC-NCI-AACR Symposium. CSF1R, colony-stimulating factor 1 receptor; SRC, proto-oncogene tyrosine-protein kinase Src; MET, mesenchymal-epithelial transition factor 21TPX-0022 is a Potent Inhibitor of MET, SRC, and CSF1R Tyrosine Kinases § TPX-0022 is a potent MET inhibitor in both biochemical § TPX-0022 is highly selective for and cellular assays MET/SRC/CSF1R in a screen of 373 kinases Biochemical IC (nM) Cell Proliferation IC (nM) 50 50 Inhibitor MET SNU-5 MKN-45 TPX-0022 0.14 <0.2 <0.2 Capmatinib 0.20 <0.2 <0.2 Crizotinib 4.0 2.8 10.5 Savolitinib 4.0 1.1 4.9 § Targeting of SRC and CSF1R can potentially improve clinical efficacy – SRC is a downstream MET effector involved in malignant transformation, tumor metastasis, and drug resistance – CSF1R plays an important role in regulation of tumor associated macrophages that can promote tumor progression and angiogenesis Note: Data presented at 2020 EORTC-NCI-AACR Symposium. CSF1R, colony-stimulating factor 1 receptor; SRC, proto-oncogene tyrosine-protein kinase Src; MET, mesenchymal-epithelial transition factor 21
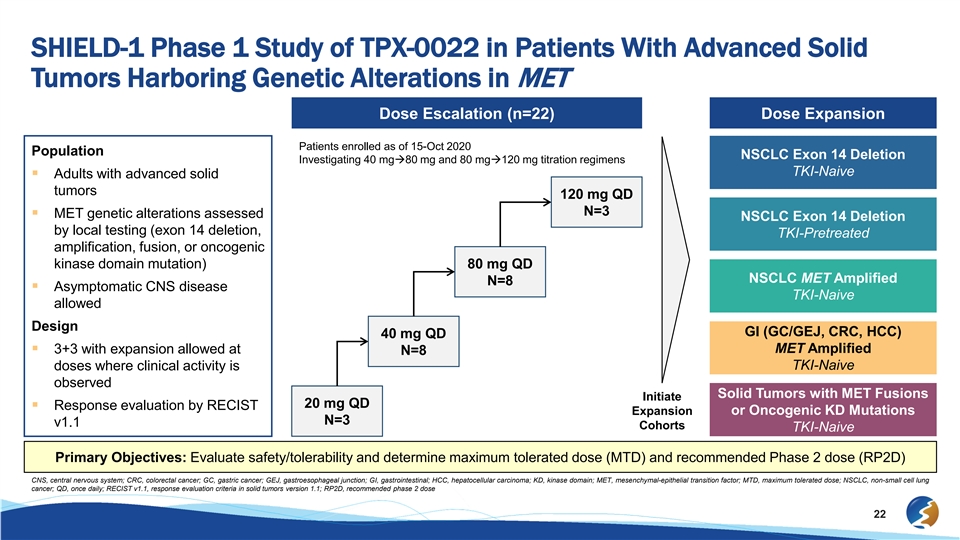
SHIELD-1 Phase 1 Study of TPX-0022 in Patients With Advanced Solid Tumors Harboring Genetic Alterations in MET Dose Escalation (n=22) Dose Expansion Patients enrolled as of 15-Oct 2020 Population NSCLC Exon 14 Deletion Investigating 40 mgà80 mg and 80 mgà120 mg titration regimens TKI-Naive § Adults with advanced solid tumors 120 mg QD N=3 § MET genetic alterations assessed NSCLC Exon 14 Deletion by local testing (exon 14 deletion, TKI-Pretreated amplification, fusion, or oncogenic kinase domain mutation) 80 mg QD NSCLC MET Amplified N=8 § Asymptomatic CNS disease TKI-Naive allowed Design GI (GC/GEJ, CRC, HCC) 40 mg QD MET Amplified § 3+3 with expansion allowed at N=8 doses where clinical activity is TKI-Naive observed Solid Tumors with MET Fusions Initiate 20 mg QD § Response evaluation by RECIST Expansion or Oncogenic KD Mutations N=3 v1.1 Cohorts TKI-Naive Primary Objectives: Evaluate safety/tolerability and determine maximum tolerated dose (MTD) and recommended Phase 2 dose (RP2D) CNS, central nervous system; CRC, colorectal cancer; GC, gastric cancer; GEJ, gastroesophageal junction; GI, gastrointestinal; HCC, hepatocellular carcinoma; KD, kinase domain; MET, mesenchymal-epithelial transition factor; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; QD, once daily; RECIST v1.1, response evaluation criteria in solid tumors version 1.1; RP2D, recommended phase 2 dose 22SHIELD-1 Phase 1 Study of TPX-0022 in Patients With Advanced Solid Tumors Harboring Genetic Alterations in MET Dose Escalation (n=22) Dose Expansion Patients enrolled as of 15-Oct 2020 Population NSCLC Exon 14 Deletion Investigating 40 mgà80 mg and 80 mgà120 mg titration regimens TKI-Naive § Adults with advanced solid tumors 120 mg QD N=3 § MET genetic alterations assessed NSCLC Exon 14 Deletion by local testing (exon 14 deletion, TKI-Pretreated amplification, fusion, or oncogenic kinase domain mutation) 80 mg QD NSCLC MET Amplified N=8 § Asymptomatic CNS disease TKI-Naive allowed Design GI (GC/GEJ, CRC, HCC) 40 mg QD MET Amplified § 3+3 with expansion allowed at N=8 doses where clinical activity is TKI-Naive observed Solid Tumors with MET Fusions Initiate 20 mg QD § Response evaluation by RECIST Expansion or Oncogenic KD Mutations N=3 v1.1 Cohorts TKI-Naive Primary Objectives: Evaluate safety/tolerability and determine maximum tolerated dose (MTD) and recommended Phase 2 dose (RP2D) CNS, central nervous system; CRC, colorectal cancer; GC, gastric cancer; GEJ, gastroesophageal junction; GI, gastrointestinal; HCC, hepatocellular carcinoma; KD, kinase domain; MET, mesenchymal-epithelial transition factor; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; QD, once daily; RECIST v1.1, response evaluation criteria in solid tumors version 1.1; RP2D, recommended phase 2 dose 22
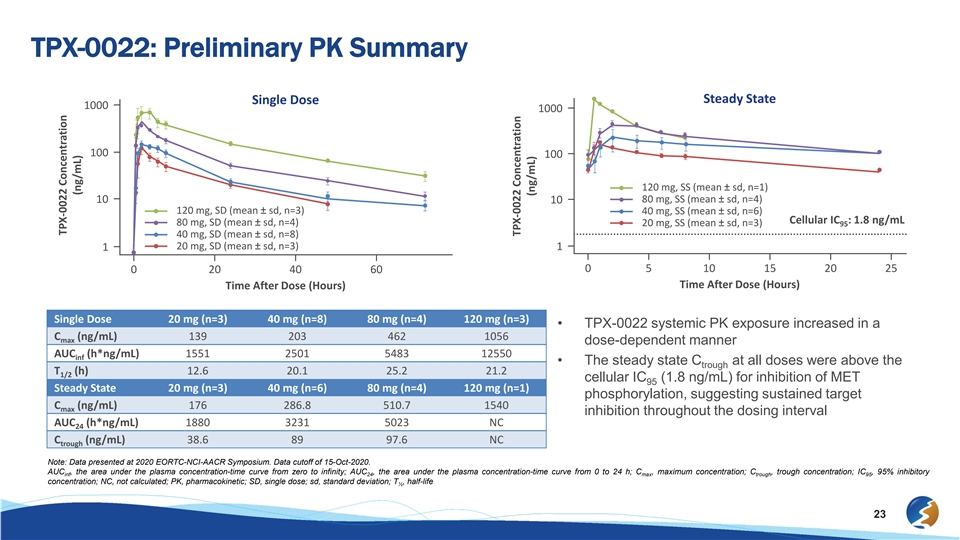
TPX-0022: Preliminary PK Summary Steady State Single Dose 1000 1000 100 100 120 mg, SS (mean ± sd, n=1) 10 80 mg, SS (mean ± sd, n=4) 10 120 mg, SD (mean ± sd, n=3) 40 mg, SS (mean ± sd, n=6) Cellular IC : 1.8 ng/mL 80 mg, SD (mean ± sd, n=4) 20 mg, SS (mean ± sd, n=3) 95 40 mg, SD (mean ± sd, n=8) 20 mg, SD (mean ± sd, n=3) 1 1 0 5 10 15 20 25 0 20 40 60 Time After Dose (Hours) Time After Dose (Hours) Single Dose 20 mg (n=3) 40 mg (n=8) 80 mg (n=4) 120 mg (n=3) • TPX-0022 systemic PK exposure increased in a C (ng/mL) 139 203 462 1056 max dose-dependent manner AUC (h*ng/mL) 1551 2501 5483 12550 inf • The steady state C at all doses were above the trough T (h) 12.6 20.1 25.2 21.2 1/2 cellular IC (1.8 ng/mL) for inhibition of MET 95 Steady State 20 mg (n=3) 40 mg (n=6) 80 mg (n=4) 120 mg (n=1) phosphorylation, suggesting sustained target C (ng/mL) 176 286.8 510.7 1540 max inhibition throughout the dosing interval AUC (h*ng/mL) 1880 3231 5023 NC 24 C (ng/mL) 38.6 89 97.6 NC trough Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. AUC , the area under the plasma concentration-time curve from zero to infinity; AUC , the area under the plasma concentration-time curve from 0 to 24 h; C , maximum concentration; C , trough concentration; IC , 95% inhibitory inf 24 max trough 95 concentration; NC, not calculated; PK, pharmacokinetic; SD, single dose; sd, standard deviation; T , half-life ½ 23 TPX-0022 Concentration (ng/mL) TPX-0022 Concentration (ng/mL)TPX-0022: Preliminary PK Summary Steady State Single Dose 1000 1000 100 100 120 mg, SS (mean ± sd, n=1) 10 80 mg, SS (mean ± sd, n=4) 10 120 mg, SD (mean ± sd, n=3) 40 mg, SS (mean ± sd, n=6) Cellular IC : 1.8 ng/mL 80 mg, SD (mean ± sd, n=4) 20 mg, SS (mean ± sd, n=3) 95 40 mg, SD (mean ± sd, n=8) 20 mg, SD (mean ± sd, n=3) 1 1 0 5 10 15 20 25 0 20 40 60 Time After Dose (Hours) Time After Dose (Hours) Single Dose 20 mg (n=3) 40 mg (n=8) 80 mg (n=4) 120 mg (n=3) • TPX-0022 systemic PK exposure increased in a C (ng/mL) 139 203 462 1056 max dose-dependent manner AUC (h*ng/mL) 1551 2501 5483 12550 inf • The steady state C at all doses were above the trough T (h) 12.6 20.1 25.2 21.2 1/2 cellular IC (1.8 ng/mL) for inhibition of MET 95 Steady State 20 mg (n=3) 40 mg (n=6) 80 mg (n=4) 120 mg (n=1) phosphorylation, suggesting sustained target C (ng/mL) 176 286.8 510.7 1540 max inhibition throughout the dosing interval AUC (h*ng/mL) 1880 3231 5023 NC 24 C (ng/mL) 38.6 89 97.6 NC trough Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. AUC , the area under the plasma concentration-time curve from zero to infinity; AUC , the area under the plasma concentration-time curve from 0 to 24 h; C , maximum concentration; C , trough concentration; IC , 95% inhibitory inf 24 max trough 95 concentration; NC, not calculated; PK, pharmacokinetic; SD, single dose; sd, standard deviation; T , half-life ½ 23 TPX-0022 Concentration (ng/mL) TPX-0022 Concentration (ng/mL)
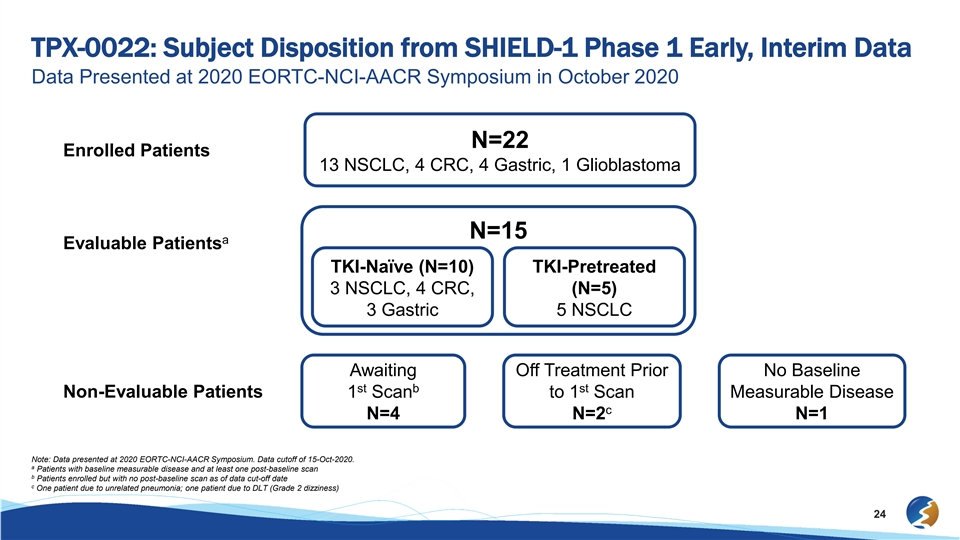
TPX-0022: Subject Disposition from SHIELD-1 Phase 1 Early, Interim Data Data Presented at 2020 EORTC-NCI-AACR Symposium in October 2020 N=22 Enrolled Patients 13 NSCLC, 4 CRC, 4 Gastric, 1 Glioblastoma N=15 a Evaluable Patients TKI-Naïve (N=10) TKI-Pretreated 3 NSCLC, 4 CRC, (N=5) 3 Gastric 5 NSCLC Awaiting Off Treatment Prior No Baseline st b st Non-Evaluable Patients 1 Scan to 1 Scan Measurable Disease c N=4 N=2 N=1 Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. a Patients with baseline measurable disease and at least one post-baseline scan b Patients enrolled but with no post-baseline scan as of data cut-off date c One patient due to unrelated pneumonia; one patient due to DLT (Grade 2 dizziness) 24TPX-0022: Subject Disposition from SHIELD-1 Phase 1 Early, Interim Data Data Presented at 2020 EORTC-NCI-AACR Symposium in October 2020 N=22 Enrolled Patients 13 NSCLC, 4 CRC, 4 Gastric, 1 Glioblastoma N=15 a Evaluable Patients TKI-Naïve (N=10) TKI-Pretreated 3 NSCLC, 4 CRC, (N=5) 3 Gastric 5 NSCLC Awaiting Off Treatment Prior No Baseline st b st Non-Evaluable Patients 1 Scan to 1 Scan Measurable Disease c N=4 N=2 N=1 Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. a Patients with baseline measurable disease and at least one post-baseline scan b Patients enrolled but with no post-baseline scan as of data cut-off date c One patient due to unrelated pneumonia; one patient due to DLT (Grade 2 dizziness) 24
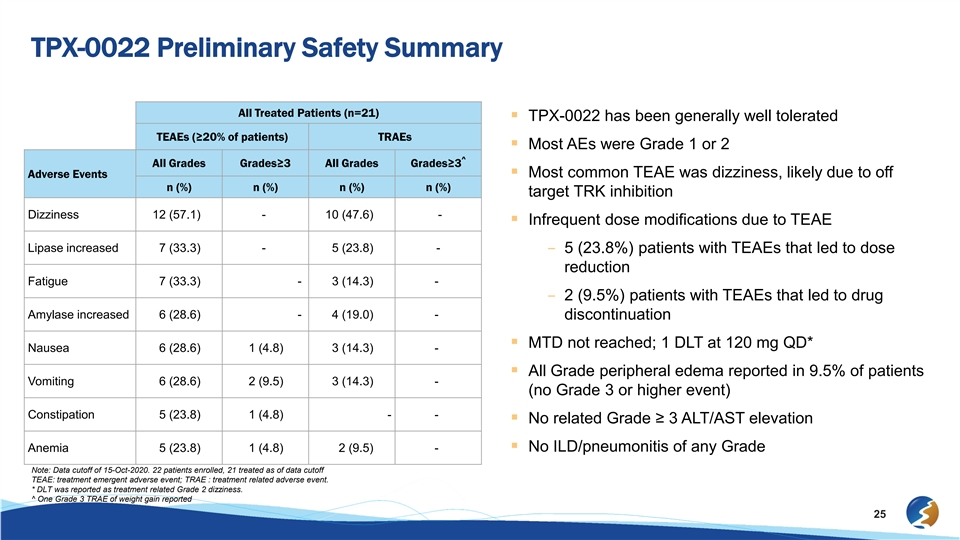
TPX-0022 Preliminary Safety Summary All Treated Patients (n=21) § TPX-0022 has been generally well tolerated TEAEs (≥20% of patients) TRAEs § Most AEs were Grade 1 or 2 ^ All Grades Grades≥3 All Grades Grades≥3 Adverse Events§ Most common TEAE was dizziness, likely due to off n (%) n (%) n (%) n (%) target TRK inhibition Dizziness 12 (57.1) - 10 (47.6) - § Infrequent dose modifications due to TEAE Lipase increased 7 (33.3) - 5 (23.8) - – 5 (23.8%) patients with TEAEs that led to dose reduction Fatigue 7 (33.3) - 3 (14.3) - – 2 (9.5%) patients with TEAEs that led to drug Amylase increased 6 (28.6) - 4 (19.0) - discontinuation § MTD not reached; 1 DLT at 120 mg QD* Nausea 6 (28.6) 1 (4.8) 3 (14.3) - § All Grade peripheral edema reported in 9.5% of patients Vomiting 6 (28.6) 2 (9.5) 3 (14.3) - (no Grade 3 or higher event) Constipation 5 (23.8) 1 (4.8) - - § No related Grade ≥ 3 ALT/AST elevation Anemia 5 (23.8) 1 (4.8) 2 (9.5) -§ No ILD/pneumonitis of any Grade Note: Data cutoff of 15-Oct-2020. 22 patients enrolled, 21 treated as of data cutoff TEAE: treatment emergent adverse event; TRAE : treatment related adverse event. * DLT was reported as treatment related Grade 2 dizziness. ^ One Grade 3 TRAE of weight gain reported 25TPX-0022 Preliminary Safety Summary All Treated Patients (n=21) § TPX-0022 has been generally well tolerated TEAEs (≥20% of patients) TRAEs § Most AEs were Grade 1 or 2 ^ All Grades Grades≥3 All Grades Grades≥3 Adverse Events§ Most common TEAE was dizziness, likely due to off n (%) n (%) n (%) n (%) target TRK inhibition Dizziness 12 (57.1) - 10 (47.6) - § Infrequent dose modifications due to TEAE Lipase increased 7 (33.3) - 5 (23.8) - – 5 (23.8%) patients with TEAEs that led to dose reduction Fatigue 7 (33.3) - 3 (14.3) - – 2 (9.5%) patients with TEAEs that led to drug Amylase increased 6 (28.6) - 4 (19.0) - discontinuation § MTD not reached; 1 DLT at 120 mg QD* Nausea 6 (28.6) 1 (4.8) 3 (14.3) - § All Grade peripheral edema reported in 9.5% of patients Vomiting 6 (28.6) 2 (9.5) 3 (14.3) - (no Grade 3 or higher event) Constipation 5 (23.8) 1 (4.8) - - § No related Grade ≥ 3 ALT/AST elevation Anemia 5 (23.8) 1 (4.8) 2 (9.5) -§ No ILD/pneumonitis of any Grade Note: Data cutoff of 15-Oct-2020. 22 patients enrolled, 21 treated as of data cutoff TEAE: treatment emergent adverse event; TRAE : treatment related adverse event. * DLT was reported as treatment related Grade 2 dizziness. ^ One Grade 3 TRAE of weight gain reported 25

TPX-0022: Preliminary Efficacy by Investigator Assessment from Dose Finding Study Tumor Assessment 50 PD 40 PD 30§ Of 10 MET TKI-Naïve patients, 5 have PD achieved PRs SD 20 PD PD – 3 gastric/GE junction, 1 CRC, and 1 10 NSCLC 40 80 40 120 80 80 40 120 40 0 • 3 cPRs are ongoing with DOR 40 20 40 20 80 40 SD# ^ -10 range 1.0+ to 3.9+ months • 2 uPR on treatment pending -20 PD confirmation -30 SD § Of the 5 MET TKI-Pretreated NSCLC -40 patients, 3 had stable disease -50 uPR# PR# PR# – All 5 patients were treated at 20 or 40 PR# -60 mg QD dose levels -70 uPR# § Clinical benefit rate for all patients is 9/15 MET ex14 NSCLC METex14 NSCLC MET AMP NSCLC MET AMP NSCLC -80 SD* MET ex14 CRC METex14 CRC MM ET F E uT Fu sion CRs Cion CRC -90 TKI Pretreated ME M T A ET MP AM CRC P CRC No change in tumor measurement from baseline ^ MET AMP GC/GEJ # Patient remains on treatment MET AMP GC/GEJ TKI Naive MET Fusion GC/GEJ Dose: mg QD Patient had PR on C3D1 but PD on C4D1 MET Fusion GC/GEJ -100 * Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. uPR: unconfirmed PR patient remains on treatment and awaits confirmatory scan; clinical benefit rate: confirmed or unconfirmed PR or SD. 26 Maximum change(%) from baseline in SLDTPX-0022: Preliminary Efficacy by Investigator Assessment from Dose Finding Study Tumor Assessment 50 PD 40 PD 30§ Of 10 MET TKI-Naïve patients, 5 have PD achieved PRs SD 20 PD PD – 3 gastric/GE junction, 1 CRC, and 1 10 NSCLC 40 80 40 120 80 80 40 120 40 0 • 3 cPRs are ongoing with DOR 40 20 40 20 80 40 SD# ^ -10 range 1.0+ to 3.9+ months • 2 uPR on treatment pending -20 PD confirmation -30 SD § Of the 5 MET TKI-Pretreated NSCLC -40 patients, 3 had stable disease -50 uPR# PR# PR# – All 5 patients were treated at 20 or 40 PR# -60 mg QD dose levels -70 uPR# § Clinical benefit rate for all patients is 9/15 MET ex14 NSCLC METex14 NSCLC MET AMP NSCLC MET AMP NSCLC -80 SD* MET ex14 CRC METex14 CRC MM ET F E uT Fu sion CRs Cion CRC -90 TKI Pretreated ME M T A ET MP AM CRC P CRC No change in tumor measurement from baseline ^ MET AMP GC/GEJ # Patient remains on treatment MET AMP GC/GEJ TKI Naive MET Fusion GC/GEJ Dose: mg QD Patient had PR on C3D1 but PD on C4D1 MET Fusion GC/GEJ -100 * Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. uPR: unconfirmed PR patient remains on treatment and awaits confirmatory scan; clinical benefit rate: confirmed or unconfirmed PR or SD. 26 Maximum change(%) from baseline in SLD

TPX-0022 Duration of Treatment 40 u 40 80 u 20 80 u 40 u 40 Duration of Treatment 120 u NSCLC (N=8): 3.4 to 34+ weeks 20 CRC (N=4): 5.1 to 17 weeks 40 GC/GEJ (N=3): 9.4+ to 23.6+ weeks 40 MET ex14 NSCLC MET ex14 NSCLC 80 MET AMP NSCLC MET AMP NSCLC MET ex14 CRC 120 Legend u MET Fusion CRC MET FusionCRC Ongoing on Treatment 80 MET AMP CRC MET AMP CRC TKI Pretreated Time to Response MET AMP GC/GEJu MET AMP GC/GEJ 40 TKI Naive MET Fusion GC/GEJ Progressive Disease MET FusionGC/GEJ 0 5 10 15 20 25 30 35 40 Weeks Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. 27 Starting Dose (mg QD)TPX-0022 Duration of Treatment 40 u 40 80 u 20 80 u 40 u 40 Duration of Treatment 120 u NSCLC (N=8): 3.4 to 34+ weeks 20 CRC (N=4): 5.1 to 17 weeks 40 GC/GEJ (N=3): 9.4+ to 23.6+ weeks 40 MET ex14 NSCLC MET ex14 NSCLC 80 MET AMP NSCLC MET AMP NSCLC MET ex14 CRC 120 Legend u MET Fusion CRC MET FusionCRC Ongoing on Treatment 80 MET AMP CRC MET AMP CRC TKI Pretreated Time to Response MET AMP GC/GEJu MET AMP GC/GEJ 40 TKI Naive MET Fusion GC/GEJ Progressive Disease MET FusionGC/GEJ 0 5 10 15 20 25 30 35 40 Weeks Note: Data presented at 2020 EORTC-NCI-AACR Symposium. Data cutoff of 15-Oct-2020. 27 Starting Dose (mg QD)
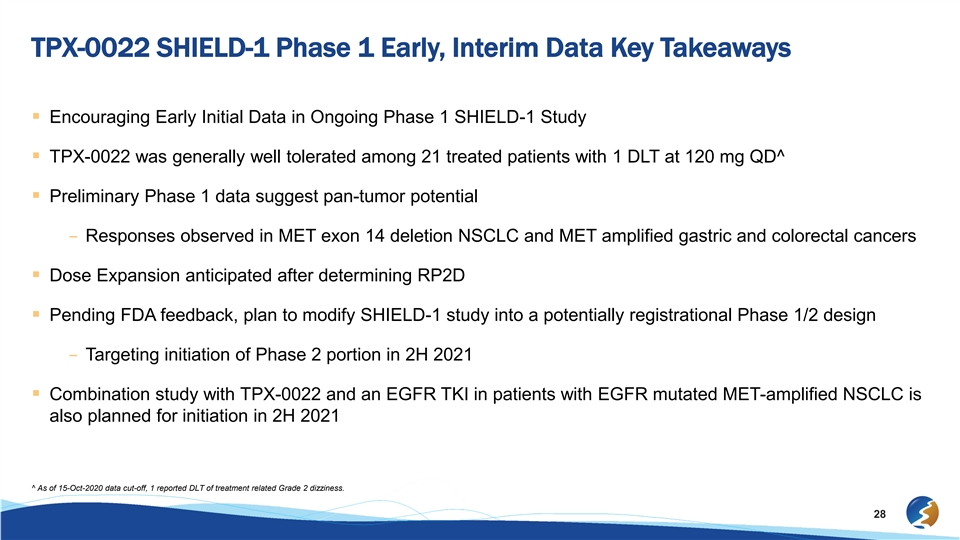
TPX-0022 SHIELD-1 Phase 1 Early, Interim Data Key Takeaways § Encouraging Early Initial Data in Ongoing Phase 1 SHIELD-1 Study § TPX-0022 was generally well tolerated among 21 treated patients with 1 DLT at 120 mg QD^ § Preliminary Phase 1 data suggest pan-tumor potential – Responses observed in MET exon 14 deletion NSCLC and MET amplified gastric and colorectal cancers § Dose Expansion anticipated after determining RP2D § Pending FDA feedback, plan to modify SHIELD-1 study into a potentially registrational Phase 1/2 design – Targeting initiation of Phase 2 portion in 2H 2021 § Combination study with TPX-0022 and an EGFR TKI in patients with EGFR mutated MET-amplified NSCLC is also planned for initiation in 2H 2021 ^ As of 15-Oct-2020 data cut-off, 1 reported DLT of treatment related Grade 2 dizziness. 28TPX-0022 SHIELD-1 Phase 1 Early, Interim Data Key Takeaways § Encouraging Early Initial Data in Ongoing Phase 1 SHIELD-1 Study § TPX-0022 was generally well tolerated among 21 treated patients with 1 DLT at 120 mg QD^ § Preliminary Phase 1 data suggest pan-tumor potential – Responses observed in MET exon 14 deletion NSCLC and MET amplified gastric and colorectal cancers § Dose Expansion anticipated after determining RP2D § Pending FDA feedback, plan to modify SHIELD-1 study into a potentially registrational Phase 1/2 design – Targeting initiation of Phase 2 portion in 2H 2021 § Combination study with TPX-0022 and an EGFR TKI in patients with EGFR mutated MET-amplified NSCLC is also planned for initiation in 2H 2021 ^ As of 15-Oct-2020 data cut-off, 1 reported DLT of treatment related Grade 2 dizziness. 28
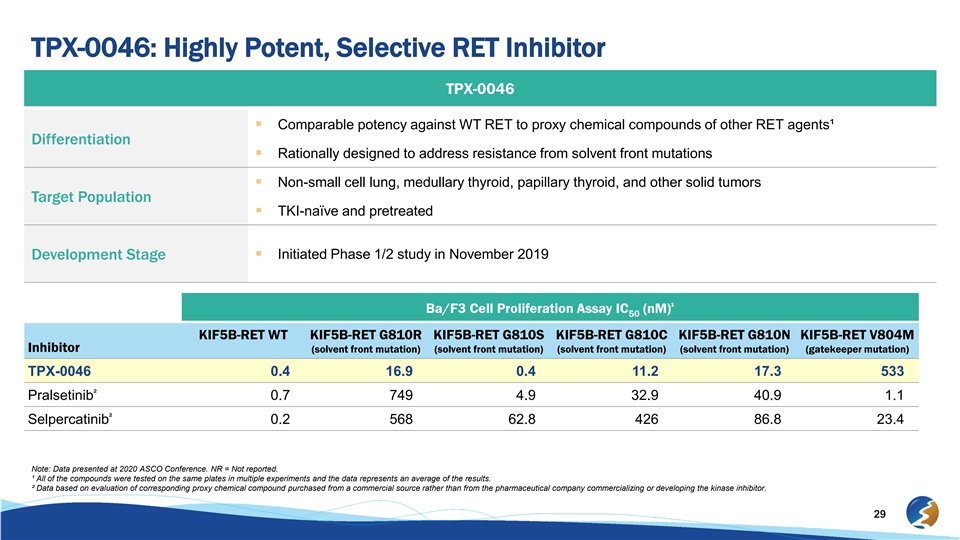
TPX-0046: Highly Potent, Selective RET Inhibitor TPX-0046 § Comparable potency against WT RET to proxy chemical compounds of other RET agents¹ Differentiation § Rationally designed to address resistance from solvent front mutations § Non-small cell lung, medullary thyroid, papillary thyroid, and other solid tumors Target Population § TKI-naïve and pretreated § Initiated Phase 1/2 study in November 2019 Development Stage ¹ Ba/F3 Cell Proliferation Assay IC (nM) 50 KIF5B-RET WT KIF5B-RET G810R KIF5B-RET G810S KIF5B-RET G810C KIF5B-RET G810N KIF5B-RET V804M Inhibitor (solvent front mutation) (solvent front mutation) (solvent front mutation) (solvent front mutation) (gatekeeper mutation) TPX-0046 0.4 16.9 0.4 11.2 17.3 533 ² Pralsetinib 0.7 749 4.9 32.9 40.9 1.1 ² Selpercatinib 0.2 568 62.8 426 86.8 23.4 Note: Data presented at 2020 ASCO Conference. NR = Not reported. ¹ All of the compounds were tested on the same plates in multiple experiments and the data represents an average of the results. ² Data based on evaluation of corresponding proxy chemical compound purchased from a commercial source rather than from the pharmaceutical company commercializing or developing the kinase inhibitor. 29TPX-0046: Highly Potent, Selective RET Inhibitor TPX-0046 § Comparable potency against WT RET to proxy chemical compounds of other RET agents¹ Differentiation § Rationally designed to address resistance from solvent front mutations § Non-small cell lung, medullary thyroid, papillary thyroid, and other solid tumors Target Population § TKI-naïve and pretreated § Initiated Phase 1/2 study in November 2019 Development Stage ¹ Ba/F3 Cell Proliferation Assay IC (nM) 50 KIF5B-RET WT KIF5B-RET G810R KIF5B-RET G810S KIF5B-RET G810C KIF5B-RET G810N KIF5B-RET V804M Inhibitor (solvent front mutation) (solvent front mutation) (solvent front mutation) (solvent front mutation) (gatekeeper mutation) TPX-0046 0.4 16.9 0.4 11.2 17.3 533 ² Pralsetinib 0.7 749 4.9 32.9 40.9 1.1 ² Selpercatinib 0.2 568 62.8 426 86.8 23.4 Note: Data presented at 2020 ASCO Conference. NR = Not reported. ¹ All of the compounds were tested on the same plates in multiple experiments and the data represents an average of the results. ² Data based on evaluation of corresponding proxy chemical compound purchased from a commercial source rather than from the pharmaceutical company commercializing or developing the kinase inhibitor. 29
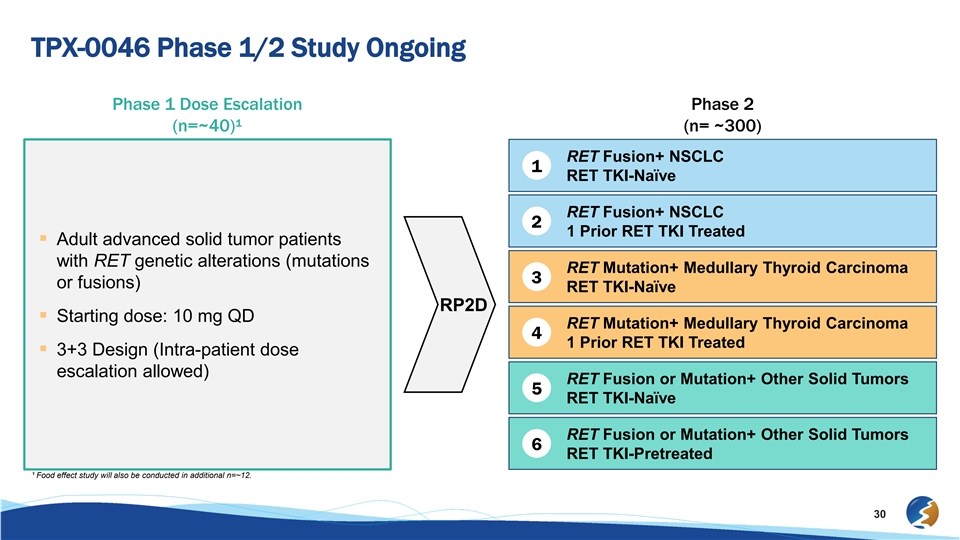
TPX-0046 Phase 1/2 Study Ongoing Phase 1 Dose Escalation Phase 2 (n=~40)¹ (n= ~300) RET Fusion+ NSCLC 1 RET TKI-Naïve RET Fusion+ NSCLC 2 1 Prior RET TKI Treated § Adult advanced solid tumor patients with RET genetic alterations (mutations RET Mutation+ Medullary Thyroid Carcinoma 3 or fusions) RET TKI-Naïve RP2D § Starting dose: 10 mg QD RET Mutation+ Medullary Thyroid Carcinoma 4 1 Prior RET TKI Treated § 3+3 Design (Intra-patient dose escalation allowed) RET Fusion or Mutation+ Other Solid Tumors 5 RET TKI-Naïve RET Fusion or Mutation+ Other Solid Tumors 6 RET TKI-Pretreated ¹ Food effect study will also be conducted in additional n=~12. 30TPX-0046 Phase 1/2 Study Ongoing Phase 1 Dose Escalation Phase 2 (n=~40)¹ (n= ~300) RET Fusion+ NSCLC 1 RET TKI-Naïve RET Fusion+ NSCLC 2 1 Prior RET TKI Treated § Adult advanced solid tumor patients with RET genetic alterations (mutations RET Mutation+ Medullary Thyroid Carcinoma 3 or fusions) RET TKI-Naïve RP2D § Starting dose: 10 mg QD RET Mutation+ Medullary Thyroid Carcinoma 4 1 Prior RET TKI Treated § 3+3 Design (Intra-patient dose escalation allowed) RET Fusion or Mutation+ Other Solid Tumors 5 RET TKI-Naïve RET Fusion or Mutation+ Other Solid Tumors 6 RET TKI-Pretreated ¹ Food effect study will also be conducted in additional n=~12. 30
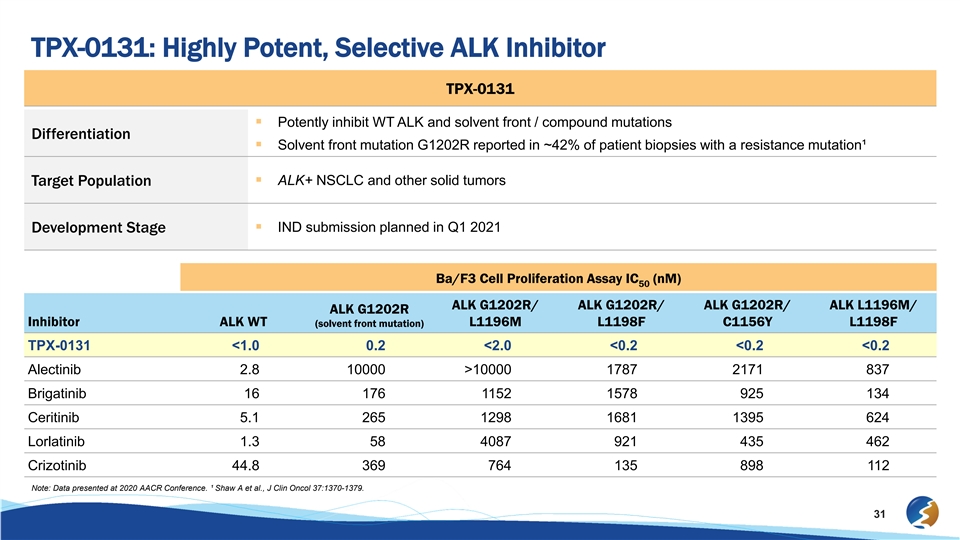
TPX-0131: Highly Potent, Selective ALK Inhibitor TPX-0131 § Potently inhibit WT ALK and solvent front / compound mutations Differentiation § Solvent front mutation G1202R reported in ~42% of patient biopsies with a resistance mutation¹ § ALK+ NSCLC and other solid tumors Target Population § IND submission planned in Q1 2021 Development Stage Ba/F3 Cell Proliferation Assay IC (nM) 50 ALK G1202R/ ALK G1202R/ ALK G1202R/ ALK L1196M/ ALK G1202R Inhibitor ALK WT L1196M L1198F C1156Y L1198F (solvent front mutation) TPX-0131 <1.0 0.2 <2.0 <0.2 <0.2 <0.2 Alectinib 2.8 10000 >10000 1787 2171 837 Brigatinib 16 176 1152 1578 925 134 Ceritinib 5.1 265 1298 1681 1395 624 Lorlatinib 1.3 58 4087 921 435 462 Crizotinib 44.8 369 764 135 898 112 Note: Data presented at 2020 AACR Conference. ¹ Shaw A et al., J Clin Oncol 37:1370-1379. 31TPX-0131: Highly Potent, Selective ALK Inhibitor TPX-0131 § Potently inhibit WT ALK and solvent front / compound mutations Differentiation § Solvent front mutation G1202R reported in ~42% of patient biopsies with a resistance mutation¹ § ALK+ NSCLC and other solid tumors Target Population § IND submission planned in Q1 2021 Development Stage Ba/F3 Cell Proliferation Assay IC (nM) 50 ALK G1202R/ ALK G1202R/ ALK G1202R/ ALK L1196M/ ALK G1202R Inhibitor ALK WT L1196M L1198F C1156Y L1198F (solvent front mutation) TPX-0131 <1.0 0.2 <2.0 <0.2 <0.2 <0.2 Alectinib 2.8 10000 >10000 1787 2171 837 Brigatinib 16 176 1152 1578 925 134 Ceritinib 5.1 265 1298 1681 1395 624 Lorlatinib 1.3 58 4087 921 435 462 Crizotinib 44.8 369 764 135 898 112 Note: Data presented at 2020 AACR Conference. ¹ Shaw A et al., J Clin Oncol 37:1370-1379. 31

§ Report updated Phase 2 TRIDENT-1 data in TKI-Naïve ROS1+ Jan. 2021 NSCLC patients at the World Conference on Lung Cancer¹ § Provide update on overall Phase 2 TRIDENT-1 study timeline Q1 2021 Repotrectinib § Initiate Phase 2 TRIDENT-2 combination study in KRAS Mid 2021 Anticipated mutant NSCLC § Provide clinical data updates from certain cohorts of the Phase 2H 2021 Milestones 2 TRIDENT-1 study § Report updated Phase 1 SHIELD-1 data 2H 2021 § Initiate Phase 2 portion of SHIELD-1 study, pending FDA 2H 2021 TPX-0022 feedback § Initiate Phase 2 SHIELD-2 combination study with EGFR TKI 2H 2021 TPX-0046§ Report early interim data from initial patients in Phase 1 study 1H 2021 § Submit IND Q1 2021 TPX-0131 § Initiate Phase 1 clinical study 1H 2021 § Outline research strategy and milestones for future pipeline 2H 2021 Discovery programs 32 ¹ Abstract accepted for mini-oral presentation at 20th IASLC World Conference on Lung Cancer.§ Report updated Phase 2 TRIDENT-1 data in TKI-Naïve ROS1+ Jan. 2021 NSCLC patients at the World Conference on Lung Cancer¹ § Provide update on overall Phase 2 TRIDENT-1 study timeline Q1 2021 Repotrectinib § Initiate Phase 2 TRIDENT-2 combination study in KRAS Mid 2021 Anticipated mutant NSCLC § Provide clinical data updates from certain cohorts of the Phase 2H 2021 Milestones 2 TRIDENT-1 study § Report updated Phase 1 SHIELD-1 data 2H 2021 § Initiate Phase 2 portion of SHIELD-1 study, pending FDA 2H 2021 TPX-0022 feedback § Initiate Phase 2 SHIELD-2 combination study with EGFR TKI 2H 2021 TPX-0046§ Report early interim data from initial patients in Phase 1 study 1H 2021 § Submit IND Q1 2021 TPX-0131 § Initiate Phase 1 clinical study 1H 2021 § Outline research strategy and milestones for future pipeline 2H 2021 Discovery programs 32 ¹ Abstract accepted for mini-oral presentation at 20th IASLC World Conference on Lung Cancer.

Next-Generation Precision Oncology Medicines Company Overview January 2021Next-Generation Precision Oncology Medicines Company Overview January 2021
































