Exhibit 99.1

Precision that Moves Diane L. Patient with EGFR - driven lung cancer
Blueprint Medicines call participants 2 Introduction and portfolio overview Kate Haviland Chief Executive Officer Initial data from the SYMPHONY trial of BLU - 945 presented at the AACR Annual Meeting 2022 David Spigel, M.D. Chief Scientific Officer Sarah Cannon Research Institute Portfolio strategy and next steps Fouad Namouni, M.D. President, Research and Development Q&A All PREPARED REMARKS AACR, American Association of Cancer Research Not for promotional use.

Forward - looking statements 3 This presentation contains forward - looking statements as defined in the Private Securities Litigation Reform Act of 1995 , as amended . The words "aim," "may," "will," "could," "would," "should," "expect," "plan," "anticipate," "intend," "believe," "estimate," "predict," "project," "potential," "continue," "target" and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . In this presentation, forward - looking statements include, without limitation, express or implied statements regarding plans, strategies, timelines and expectations for the current or future approved drugs and drug candidates of Blueprint Medicines Corporation (the “Company”), including timelines for the initiation of clinical trials and trial cohorts, the results of ongoing and planned clinical trials, data publications, marketing applications and approvals ; the anticipated benefits of the preclinical profiles of the Company's drug candidates ; the Company’s plans, strategies and timelines for the development of the Company’s drug candidates as monotherapies and combination with other agents ; anticipated indications for the Company's drug candidates ; plans and timelines for additional marketing applications for avapritinib and pralsetinib and, if approved, commercialization of avapritinib and pralsetinib in additional indications or in additional geographies ; the potential benefits of any of the Company’s current or future approved drugs or drug candidates in treating patients ; the Company’s scientific platform expansion plans and plans to announce new research programs ; and ௗ the Company’s ௗ financial performance, strategy, goals and anticipated milestones, business plans and focus . The Company has based these forward - looking statements on management's current expectations, assumptions, estimates and projections . If such expectations, assumptions, estimates and projections do not fully materialize or prove incorrect, the events or circumstances referred to in the forward - looking statements may not occur . While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward - looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond the Company's control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward - looking statements . These risks and uncertainties include, without limitation, risks and uncertainties related to the impact of the COVID - 19 pandemic to the Company's business, operations, strategy, goals and anticipated milestones, including the Company's ongoing and planned research and discovery activities, ability to conduct ongoing and planned clinical trials, clinical supply of current or future drug candidates, commercial supply of current or future approved drugs, and launching, marketing and selling current or future approved drugs ; the Company’s ability and plans in establishing a commercial infrastructure, and successfully launching, marketing and selling current or future approved products ; the Company’s ability to successfully expand the approved indications for AYVAKIT® /AYVAKYT® (avapritinib) and GAVRETO® (pralsetinib) or obtain marketing approval for AYVAKIT/AYVAKYT in additional geographies in the future ; the delay of any current or planned clinical trials or the development of the Company's drug candidates or the licensed drug candidate ; the Company's advancement of multiple early - stage efforts ; the Company's ability to successfully demonstrate the efficacy and safety of its drug candidates and gain approval of its drug candidates on a timely basis, if at all ; the timing and results of preclinical and clinical studies for the Company's drug candidates, which may not support further development of such drug candidates either as monotherapies or in combination with other agents or may impact the anticipated timing of data or regulatory submissions ; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of clinical trials or marketing applications ; the Company's ability to obtain, maintain and enforce patent and other intellectual property protection for AYVAKIT/AYVAKYT, GAVRETO or any drug candidates it is developing ; the Company's ability to develop and commercialize companion diagnostic tests for any of the Company's current or future approved drugs or drug candidates ; the Company’s ability to successfully expand its operations and scientific platform and the costs thereof ; the Company's ability to realize the benefits of its executive leadership transition plan ; and the success of the Company's current and future collaborations, partnerships and licenses . These and other risks and uncertainties are described in greater detail under "Risk Factors" in the Company's filings with the Securities and Exchange Commission ("SEC"), including its most recent Annual Report on Form 10 - K, as supplemented by its most recent Quarterly Report on Form 10 - Q, and any other filings it has made or may make with the SEC in the future . The Company cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that its expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished . The forward - looking statements in this presentation are made only as of the date hereof, and except as required by law, the Company undertakes no obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or otherwise . Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements . This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company's industry . These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions and estimates of the Company's future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk . Blueprint Medicines, AYVAKIT, AYVAKYT, GAVRETO and associated logos are trademarks of Blueprint Medicines Corporation. Not for promotional use.

Blueprint Medicines is poised for growth in 2022 and beyond * Cash, cash equivalents and investments as of December 31, 2021. 4 STRONG FINANCIAL POSITION WITH ~$1B CASH AT YEAR - END 2021* Advance our clinical - stage pipeline targeting difficult - to - treat and prevalent cancer drivers Transform the lives of patients with systemic mastocytosis by improving treatment options across the spectrum disease Harness our research engine to bring forward new precision oncology and hematology programs Not for promotional use. KEY GOALS
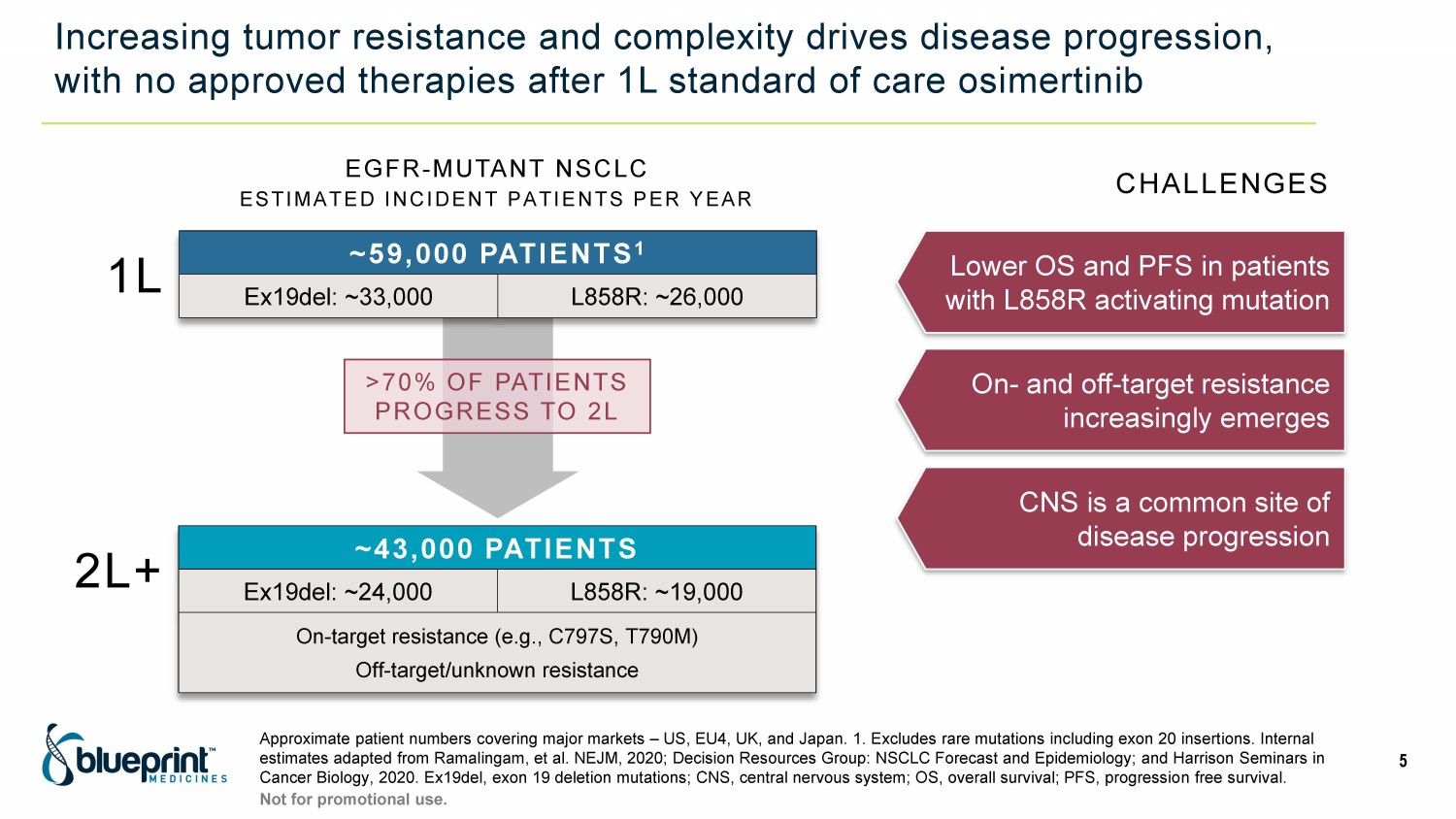
Increasing tumor resistance and complexity drives disease progression, with no approved therapies after 1L standard of care osimertinib Approximate patient numbers covering major markets – US, EU4, UK, and Japan. 1. Excludes rare mutations including exon 20 insert ions. Internal estimates adapted from Ramalingam, et al. NEJM, 2020; Decision Resources Group: NSCLC Forecast and Epidemiology; and Harrison Se minars in Cancer Biology, 2020. Ex19del, exon 19 deletion mutations; CNS, central nervous system; OS, overall survival; PFS, progressio n f ree survival. 5 Not for promotional use. EGFR - MUTANT NSCLC ESTIMATED INCIDENT PATIENTS PER YEAR >70% OF PATIENTS PROGRESS TO 2L ~59,000 PATIENTS 1 Ex19del: ~33,000 L858R: ~26,000 1L 2L+ ~43,000 PATIENTS Ex19del: ~24,000 L858R: ~19,000 On - target resistance (e.g., C797S, T790M) Off - target/unknown resistance Lower OS and PFS in patients with L858R activating mutation On - and off - target resistance increasingly emerges CNS is a common site of disease progression CHALLENGES
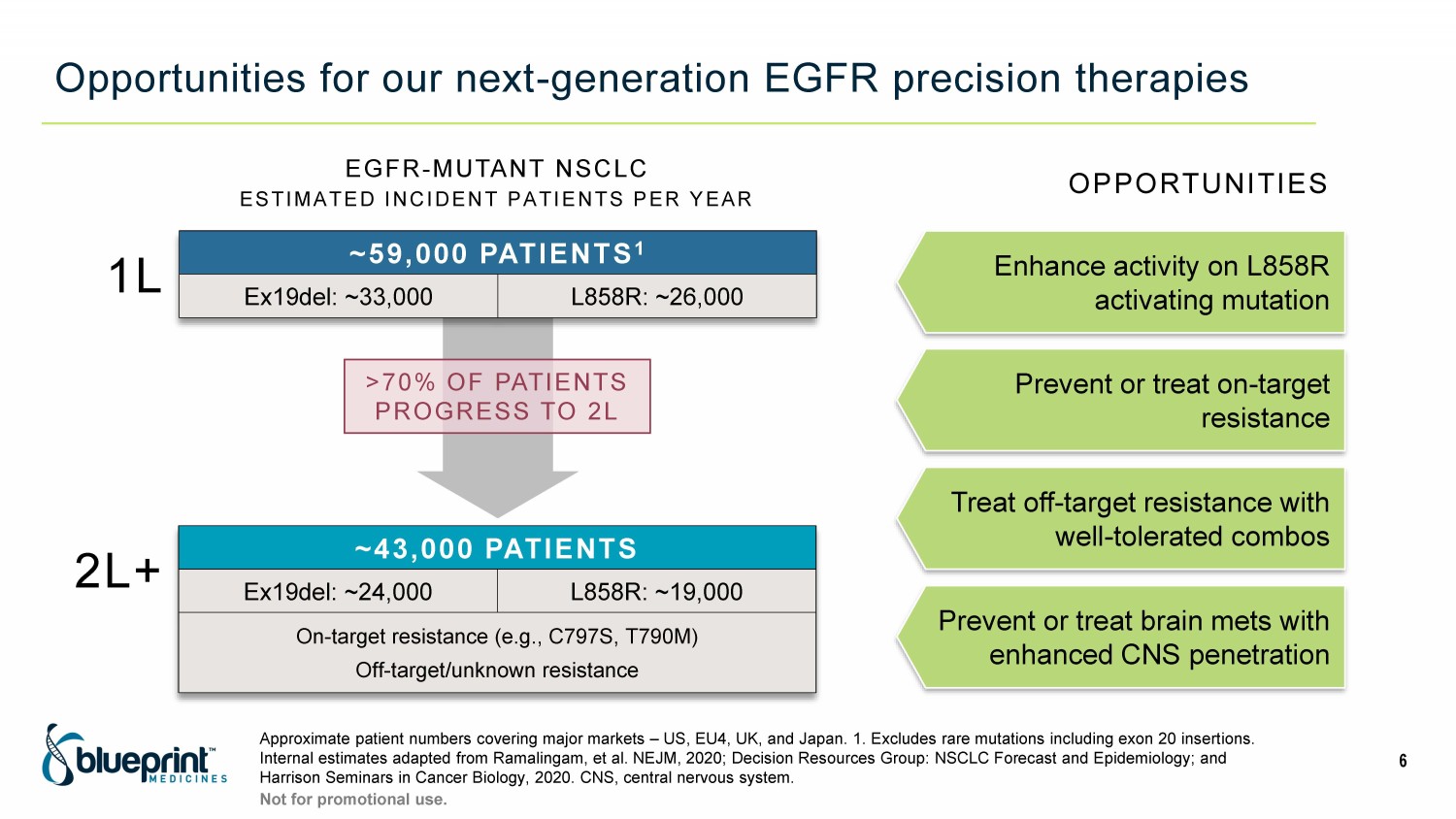
Opportunities for our next - generation EGFR precision therapies Approximate patient numbers covering major markets – US, EU4, UK, and Japan. 1. Excludes rare mutations including exon 20 insert ions. Internal estimates adapted from Ramalingam, et al. NEJM, 2020; Decision Resources Group: NSCLC Forecast and Epidemiology; and Harrison Seminars in Cancer Biology, 2020. CNS, central nervous system. 6 Not for promotional use. EGFR - MUTANT NSCLC ESTIMATED INCIDENT PATIENTS PER YEAR >70% OF PATIENTS PROGRESS TO 2L ~59,000 PATIENTS 1 Ex19del: ~33,000 L858R: ~26,000 1L 2L+ ~43,000 PATIENTS Ex19del: ~24,000 L858R: ~19,000 On - target resistance (e.g., C797S, T790M) Off - target/unknown resistance Enhance activity on L858R activating mutation Prevent or treat on - target resistance Treat off - target resistance with well - tolerated combos OPPORTUNITIES Prevent or treat brain mets with enhanced CNS penetration

Our portfolio of EGFR therapies are purpose - built to address medical needs CS, C797S resistance mutation; Ex20in, activating exon 20 insertion mutations; LR, L858R activating mutation; TM, T790M resis tan ce mutation. 7 Not for promotional use. Effectively block the EGFR pathway Establish 2L+ SOC with combinations that treat on - and off - target resistance TREATMENT GOALS • Potent EGFR mutation coverage: o LR and LR/CS o TM and TM/CS regardless of activating mutation o Potential for broader coverage at higher exposures • Highly selective over wild - type EGFR • Potent EGFR mutation coverage: o Ex19del and LR o CS regardless of activating mutation • Highly CNS penetrant BLU - 451 • Potent inhibitor of all common Ex20ins • Highly selective over wild - type EGFR • CNS penetrant BLU - 701 BLU - 945 BLUEPRINT MEDICINES EGFR PORTFOLIO

Emerging evidence of activity of BLU - 945 in patients with advanced EGFR - mutant NSCLC utilizing circulating tumor DNA in the Phase 1/2 SYMPHONY study American Association for Cancer Research Annual Meeting 2022 April 8, 2022 Elaine Shum, Yasir Elamin, Karen L Reckamp, Zofia Piotrowska, Julie Rotow, Daniel SW Tan, Koichi Goto, Jagan Parepally, Faris Albayya, Melinda Louie - Gao, Renata Sawtell, Alena Zalutskaya, David Spigel

Disclosures Dr. David Spigel, MD, Chief Scientific Officer, Sarah Cannon Research Institute • Research funding : Aeglea Biotherapeutics, Agios, Apollomics, Arcus, Arrys Therapeutics, Astellas, AstraZeneca, Bayer, BeiGene, BIND Therapeutics, BioNTech RNA Pharmaceuticals, Blueprint Medicines, Boehringer - Ingelheim, Bristol - Myers Squibb, Calithera, Celgene, Celldex, Clovis, Cyteir Therapeutics, Daiichi Sankyo, Denovo Biopharma, Eisai, Elevation Oncology, EMD Serono, Evelo Biosciences, GI Therapeutics, Roche/Genentech, GlaxoSmithKline, GRAIL, Hutchison MediPharma, ImClone Systems, Incyte, ImmunoGen, Ipsen, Janssen, Kronos Bio, Eli Lilly, Loxo Oncology, MacroGenics, MedImmune, Merck, Molecular Partners, Molecular Template, Nektar, Neon Therapeutics, Novartis, Novocure, Oncologie, Pfizer, PTC Therapeutics, PureTech Health, Razor Genomics, Repare Therapeutics, Rgenix, Takeda, Tesaro, Tizona Therapeutics, Transgene, UT Southwestern, Verstem • Consulting/advisory : Amgen, AstraZeneca, Bristol - Myers Squibb, Curio Science, EMD Serono, Evidera, Exelixis, GlaxoSmithKline, Intellisphere, Ipsen, Janssen, Jazz, Lilly, Mirati Therapeutics, Molecular Templates, Novartis, Novocure, Pfizer, Puma Biotechnology, Regeneron Pharmaceuticals, Roche/Genentech, Sanofi - Aventis *All payments made to Sarah Cannon Research Institute
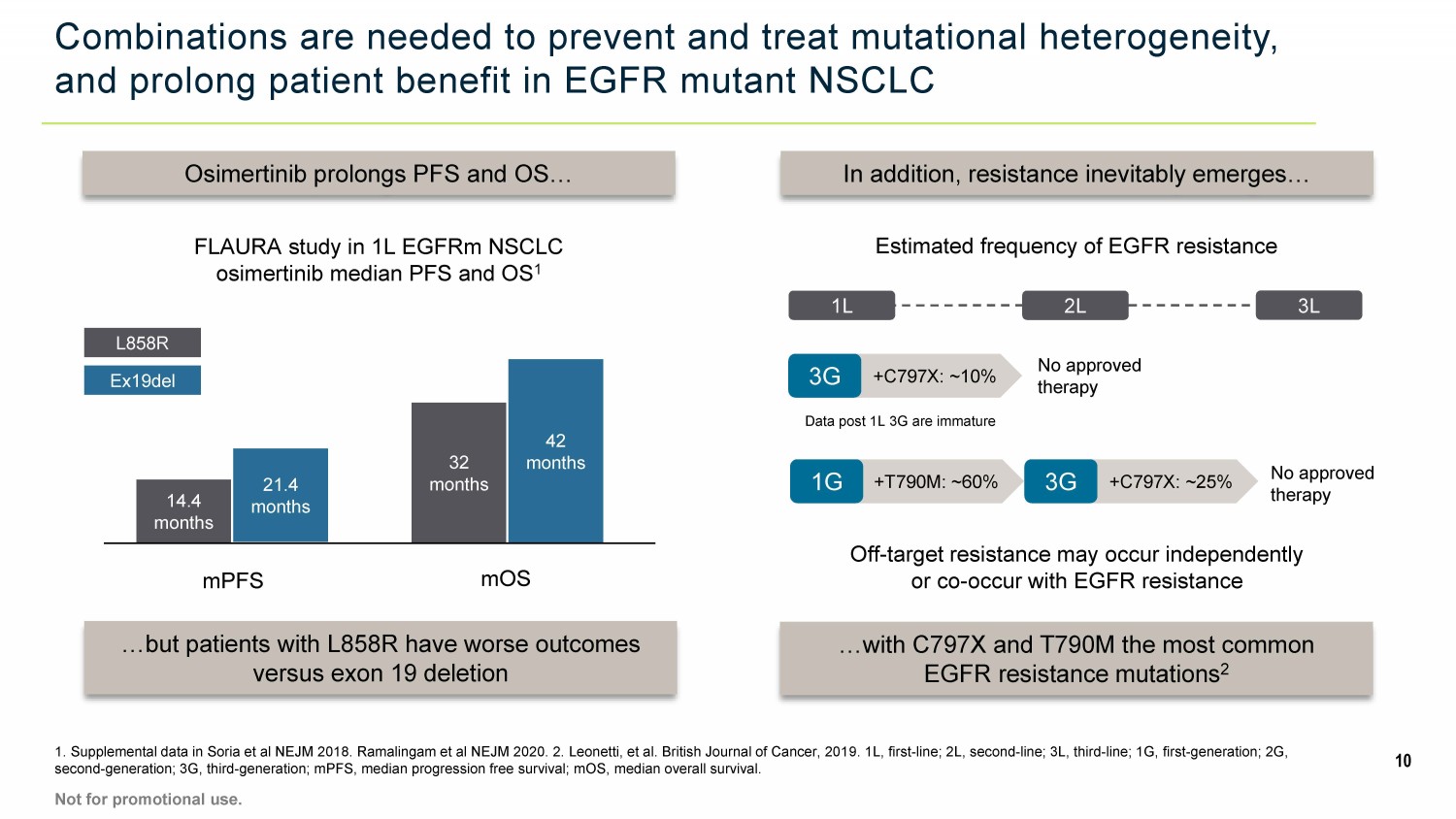
Combinations are needed to prevent and treat mutational heterogeneity, and prolong patient benefit in EGFR mutant NSCLC 1. Supplemental data in Soria et al NEJM 2018. Ramalingam et al NEJM 2020. 2. Leonetti, et al. British Journal of Cancer, 201 9. 1L, first - line; 2L, second - line; 3L, third - line; 1G, first - generation; 2G, second - generation; 3G, third - generation; mPFS, median progression free survival; mOS, median overall survival. 10 Osimertinib prolongs PFS and OS… …but patients with L858R have worse outcomes versus exon 19 deletion FLAURA study in 1L EGFRm NSCLC osimertinib median PFS and OS 1 In addition, resistance inevitably emerges… …with C797X and T790M the most common EGFR resistance mutations 2 Off - target resistance may occur independently or co - occur with EGFR resistance Estimated frequency of EGFR resistance mPFS mOS 14.4 months 21.4 months 32 months 42 months L858R Ex19del Not for promotional use. 1L 2L +T790M: ~60% 1G +C797X: ~25% 3G No approved therapy 3L +C797X: ~10% 3G Data post 1L 3G are immature No approved therapy

BLU - 945 potency and selectivity enable wide therapeutic index and broad EGFR coverage, including activating L858R mutation BLU - 945’s therapeutic index enables potent inhibition of EGFR mutants compared to SOC therapies 1 BLU - 945 is active alone in combination with BLU - 701 in an osimertinib - resistant L858R/C797S CDX model 2 11 1. Company data on file. IC 50 measured by pEGFR in cells. 2. Data presented at AACR annual meeting 2022. Abstract #3328. Not for promotional use. L 8 5 8 R L 8 5 8 R / C 7 9 7 S L 8 5 8 R / T 7 9 0 M L 8 5 8 R / T 7 9 0 M / C 7 9 7 S E x 1 9 d e l / T 7 9 0 M / C 7 9 7 S 1 10 100 1000 R a t i o : W T I C 5 0 / M u t a n t I C 5 0 BLU-945 osimertinib gefitinib
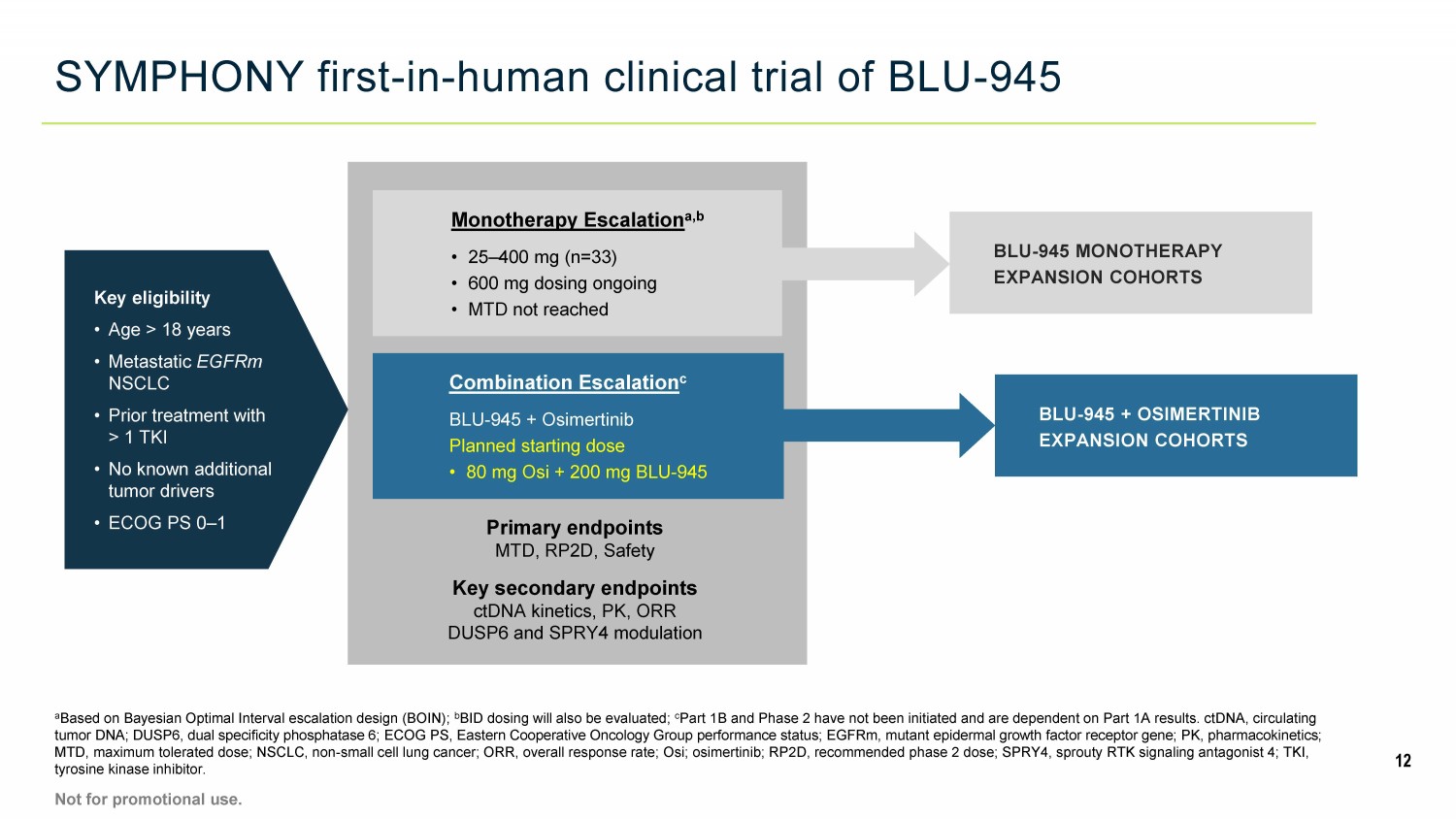
SYMPHONY first - in - human clinical trial of BLU - 945 a Based on Bayesian Optimal Interval escalation design (BOIN); b BID dosing will also be evaluated; c Part 1B and Phase 2 have not been initiated and are dependent on Part 1A results. ctDNA, circulating tumor DNA; DUSP6, dual specificity phosphatase 6; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFRm, muta nt epidermal growth factor receptor gene; PK, pharmacokinetics; MTD, maximum tolerated dose; NSCLC, non - small cell lung cancer; ORR, overall response rate; Osi; osimertinib; RP2D, recommended phase 2 dose; SPRY4, sprouty RTK signaling antagonist 4; TKI, tyrosine kinase inhibitor. 12 Not for promotional use. Key eligibility • Age > 18 years • Metastatic EGFRm NSCLC • Prior treatment with > 1 TKI • No known additional tumor drivers • ECOG PS 0 – 1 Monotherapy Escalation a,b • 25 – 400 mg (n=33) • 600 mg dosing ongoing • MTD not reached Combination Escalation c BLU - 945 + Osimertinib Planned starting dose • 80 mg Osi + 200 mg BLU - 945 Primary endpoints MTD, RP2D, Safety Key secondary endpoints ctDNA kinetics, PK, ORR DUSP6 and SPRY4 modulation BLU - 945 + OSIMERTINIB EXPANSION COHORTS BLU - 945 MONOTHERAPY EXPANSION COHORTS
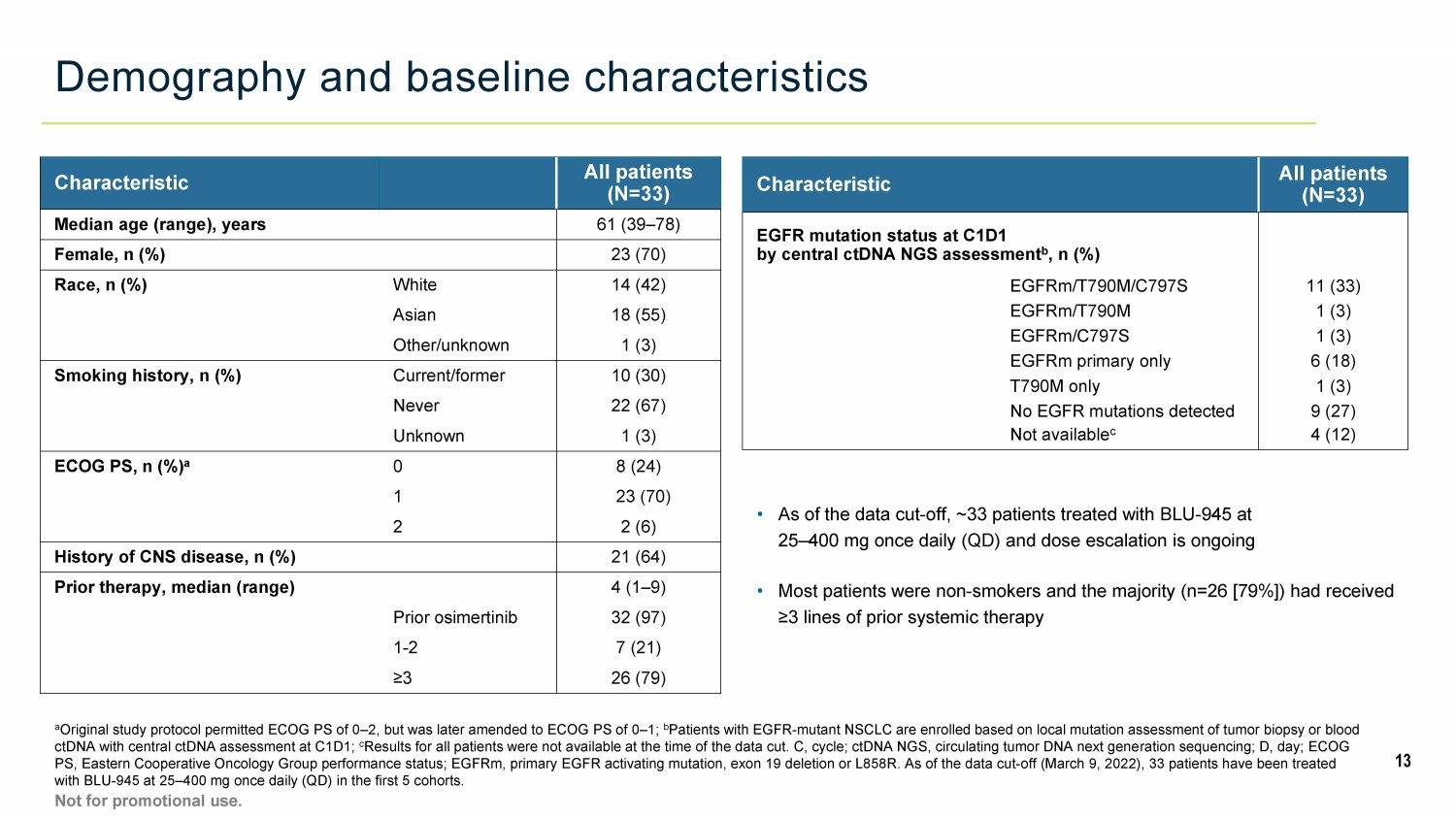
Demography and baseline characteristics a Original study protocol permitted ECOG PS of 0 – 2, but was later amended to ECOG PS of 0 – 1; b Patients with EGFR - mutant NSCLC are enrolled based on local mutation assessment of tumor biopsy or blood ctDNA with central ctDNA assessment at C1D1; c Results for all patients were not available at the time of the data cut. C, cycle; ctDNA NGS, circulating tumor DNA next gene rat ion sequencing; D, day; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFRm, primary EGFR activating mutation, exon 19 deletion or L858R . A s of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 cohorts. 13 Not for promotional use. • As of the data cut - off, ~33 patients treated with BLU - 945 at 25 – 400 mg once daily (QD) and dose escalation is ongoing • Most patients were non - smokers and the majority (n=26 [79%]) had received ≥3 lines of prior systemic therapy Characteristic All patients (N=33) Median age (range), years 61 (39 – 78) Female, n (%) 23 (70) Race, n (%) White 14 (42) Asian 18 (55) Other/unknown 1 (3) Smoking history, n (%) Current/former 10 (30) Never 22 (67) Unknown 1 (3) ECOG PS, n (%) a 0 8 (24) 1 23 (70) 2 2 (6) History of CNS disease, n (%) 21 (64) Prior therapy, median (range) 4 (1 – 9) Prior osimertinib 32 (97) 1 - 2 7 (21) ≥ 3 26 (79) Characteristic All patients (N=33) EGFR mutation status at C1D1 by central ctDNA NGS assessment b , n (%) EGFRm/T790M/C797S 11 (33) EGFRm/T790M 1 (3) EGFRm/C797S 1 (3) EGFRm primary only 6 (18) T790M only 1 (3) No EGFR mutations detected 9 (27) Not available c 4 (12)
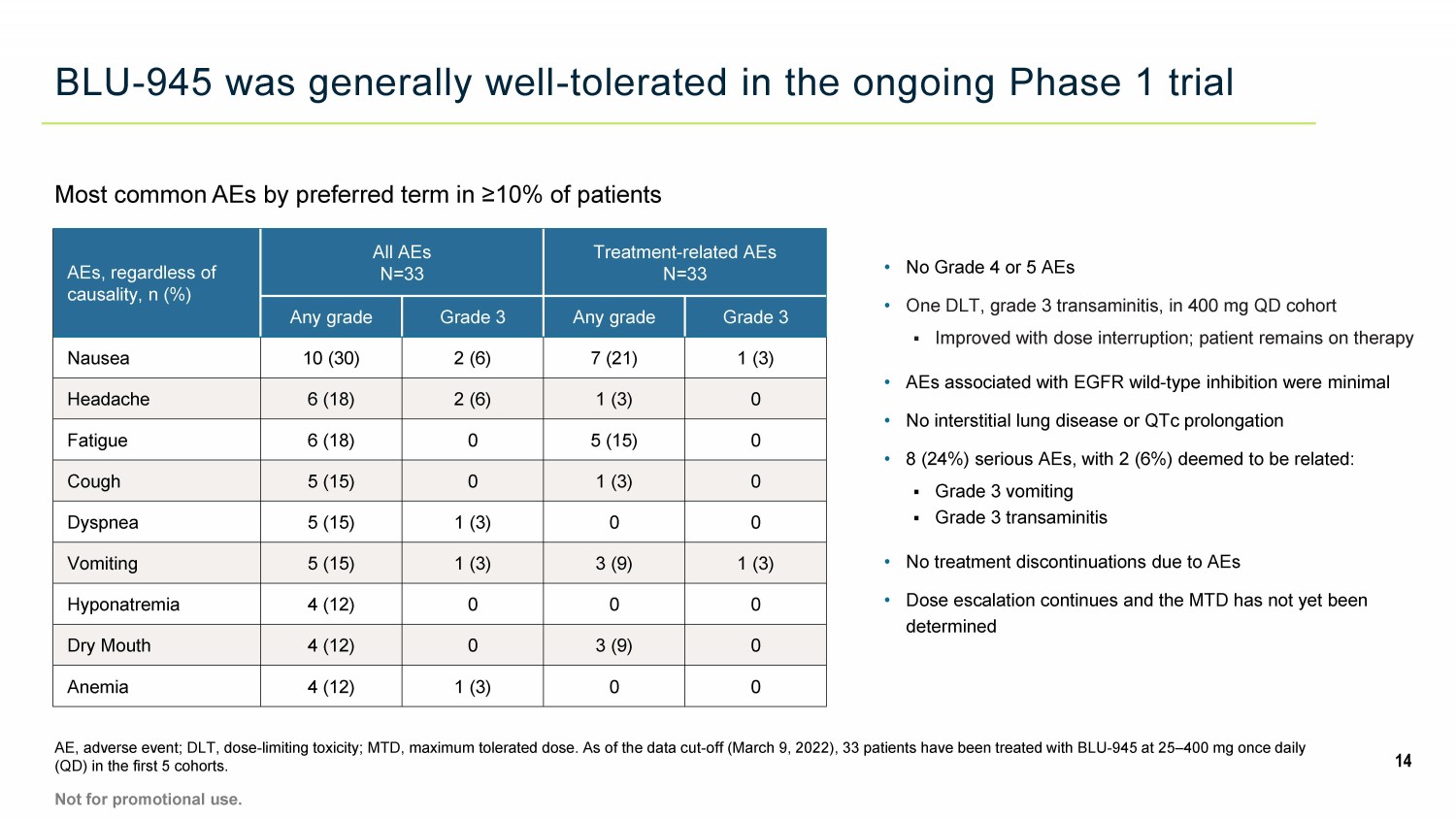
BLU - 945 was generally well - tolerated in the ongoing Phase 1 trial 14 AE, adverse event; DLT, dose - limiting toxicity; MTD, maximum tolerated dose. As of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 cohorts. Not for promotional use. AEs, regardless of causality, n (%) All AEs N=33 Treatment - related AEs N=33 Any grade Grade 3 Any grade Grade 3 Nausea 10 (30) 2 (6) 7 (21) 1 (3) Headache 6 (18) 2 (6) 1 (3) 0 Fatigue 6 (18) 0 5 (15) 0 Cough 5 (15) 0 1 (3) 0 Dyspnea 5 (15) 1 (3) 0 0 Vomiting 5 (15) 1 (3) 3 (9) 1 (3) Hyponatremia 4 (12) 0 0 0 Dry Mouth 4 (12) 0 3 (9) 0 Anemia 4 (12) 1 (3) 0 0 • No Grade 4 or 5 AEs • One DLT, grade 3 transaminitis, in 400 mg QD cohort ▪ Improved with dose interruption; patient remains on therapy • AEs associated with EGFR wild - type inhibition were minimal • No interstitial lung disease or QTc prolongation • 8 (24%) serious AEs, with 2 (6%) deemed to be related: ▪ Grade 3 vomiting ▪ Grade 3 transaminitis • No treatment discontinuations due to AEs • Dose escalation continues and the MTD has not yet been determined Most common AEs by preferred term in ≥10% of patients
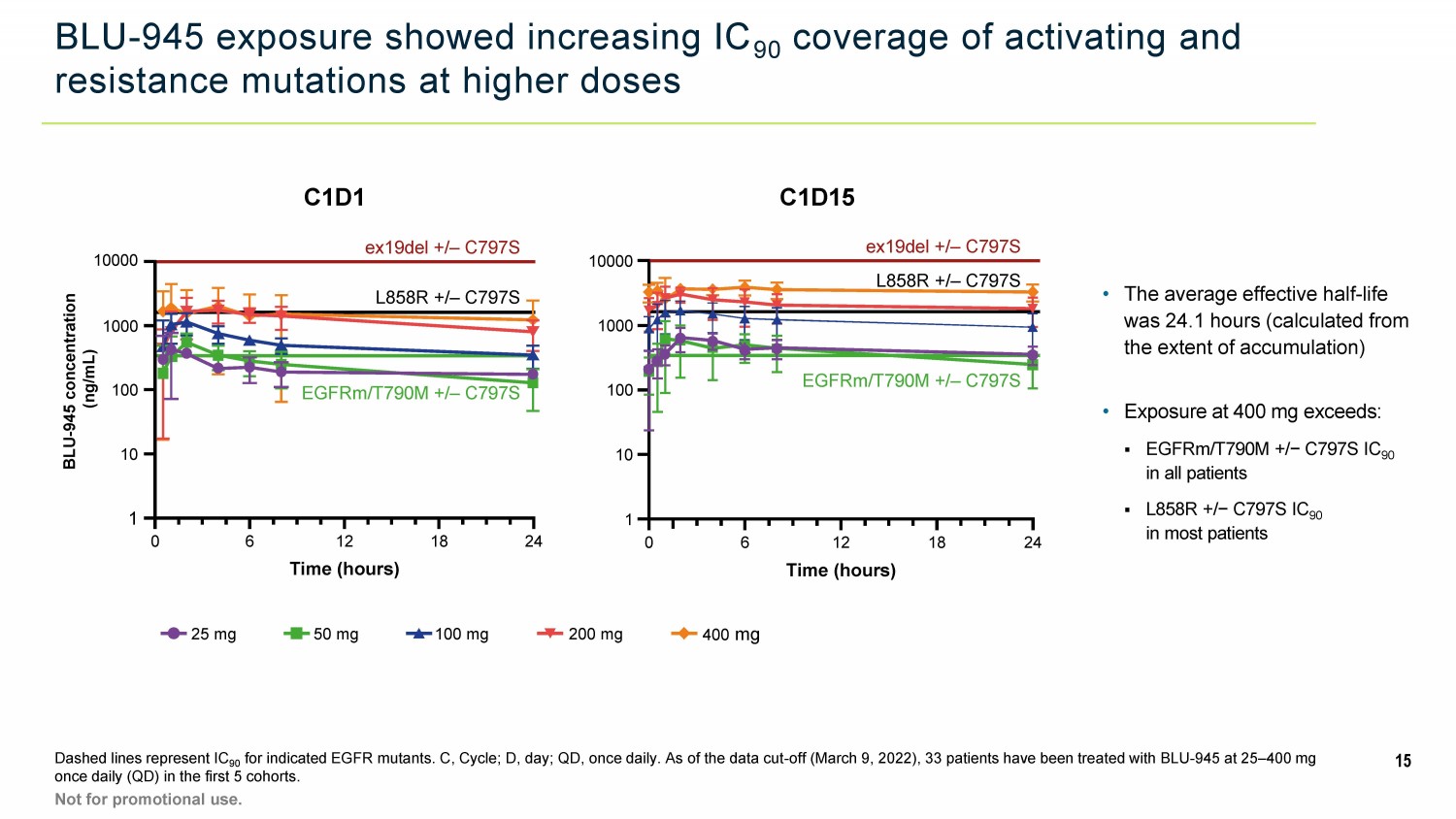
BLU - 945 exposure showed increasing IC 90 coverage of activating and resistance mutations at higher doses Dashed lines represent IC 90 for indicated EGFR mutants. C, Cycle; D, day; QD, once daily. As of the data cut - off (March 9, 2022), 33 patients have been tre ated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 cohorts. 15 Not for promotional use. C1D1 C1D15 25 mg 50 mg 100 mg 200 mg 400 mg ex19del +/ – C797S EGFRm/T790M +/ – C797S 1 10 100 1000 10000 0 6 12 18 24 Time (hours) L858R +/ – C797S BLU - 945 concentration (ng/mL) 10000 1 10 100 1000 ex19del +/ – C797S L858R +/ – C797S EGFRm/T790M +/ – C797S 0 6 12 18 24 Time (hours) • Exposure at 400 mg exceeds: ▪ EGFRm/T790M +/− C797S IC 90 in all patients ▪ L858R +/− C797S IC 90 in most patients • The average effective half - life was 24.1 hours (calculated from the extent of accumulation)

BLU - 945 treatment led to dose - dependent reductions in ctDNA a P atient had two different DNA mutations in C797S. Note: reductions in individual variant allele fractions as shown; therefore, pa tients with multiple mutations may be represented on both plots. All T790M and C797S allele fractions with available baseline and C1D15 data are shown. Increases of greater than 100% were truncated at 10 0%. C, Cycle; ctDNA, circulating tumor DNA; D, day; F1LCDx, FoundationOne Liquid CDx assay; QD, once daily.1. Ku BM et al. Oncology. 2022; Epub ahead of print. PMID: 35196661; 2 Ma L et al . Front Oncol. 2021;11:643199; 3. Fernandes MGO et al. Cells. 2021;10:1912. As of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 c ohorts. Not for promotional use. 16 83% OF EGFR - T790M VARIANT ALLELES REDUCED WITH TREATMENT 81% OF EGFR - C797S VARIANT ALLELES REDUCED WITH TREATMENT EGFR - T790M ctDNA levels EGFR - C797S ctDNA levels • In the 400 - mg cohort, all detectable T790M and C797S alleles showed reduction, including three that fell below the limit of dete ction (clearance)
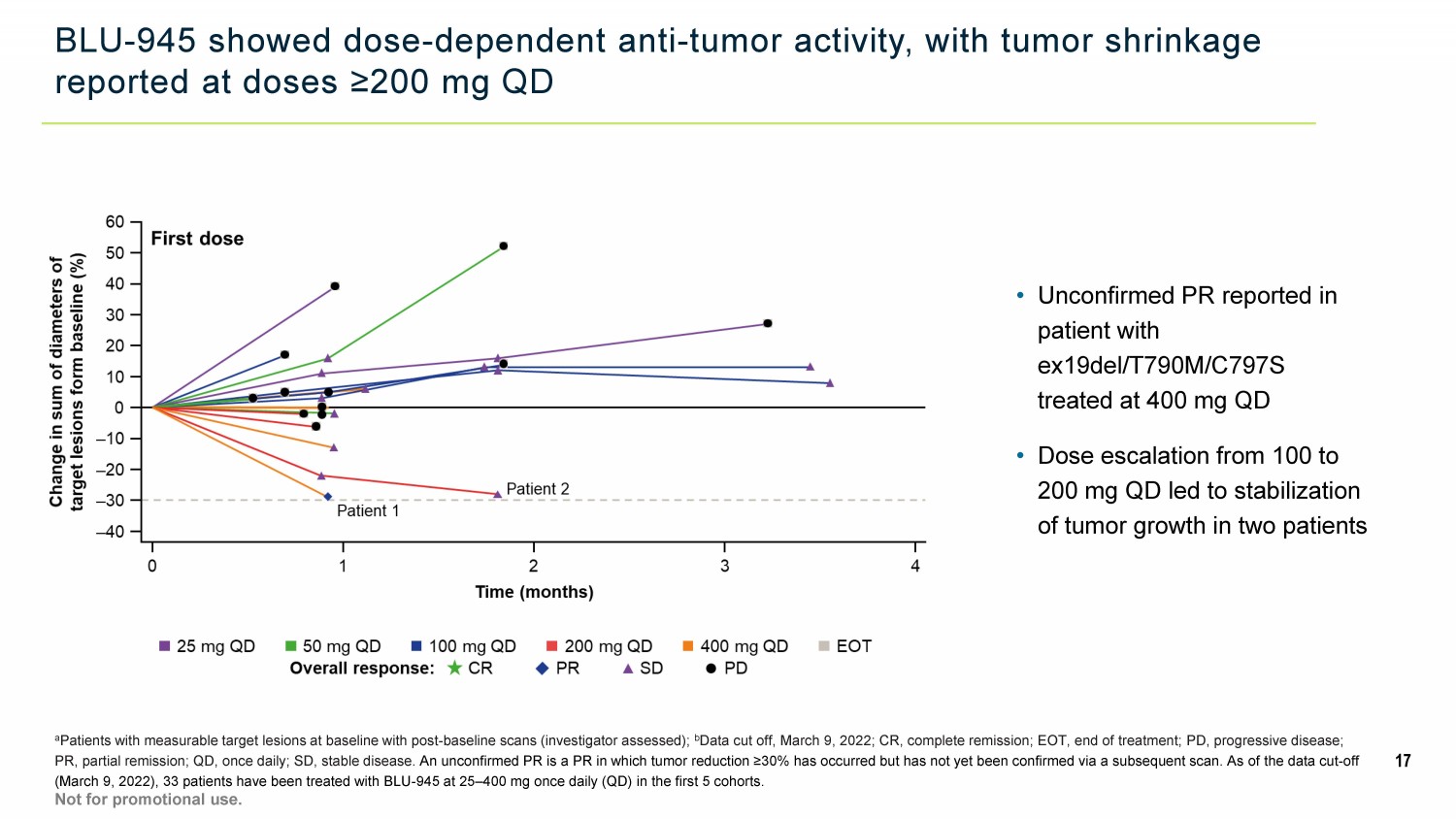
BLU - 945 showed dose - dependent anti - tumor activity, with tumor shrinkage reported at doses ≥200 mg QD a Patients with measurable target lesions at baseline with post - baseline scans (investigator assessed); b Data cut off, March 9, 2022; CR, complete remission; EOT, end of treatment; PD, progressive disease; PR, partial remission; QD, once daily; SD, stable disease. An unconfirmed PR is a PR in which tumor reduction ≥30% has occurred but has not yet been confirmed via a subsequent scan. As of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 cohorts . 17 Not for promotional use. • Unconfirmed PR reported in patient with ex19del/T790M/C797S treated at 400 mg QD • Dose escalation from 100 to 200 mg QD led to stabilization of tumor growth in two patients
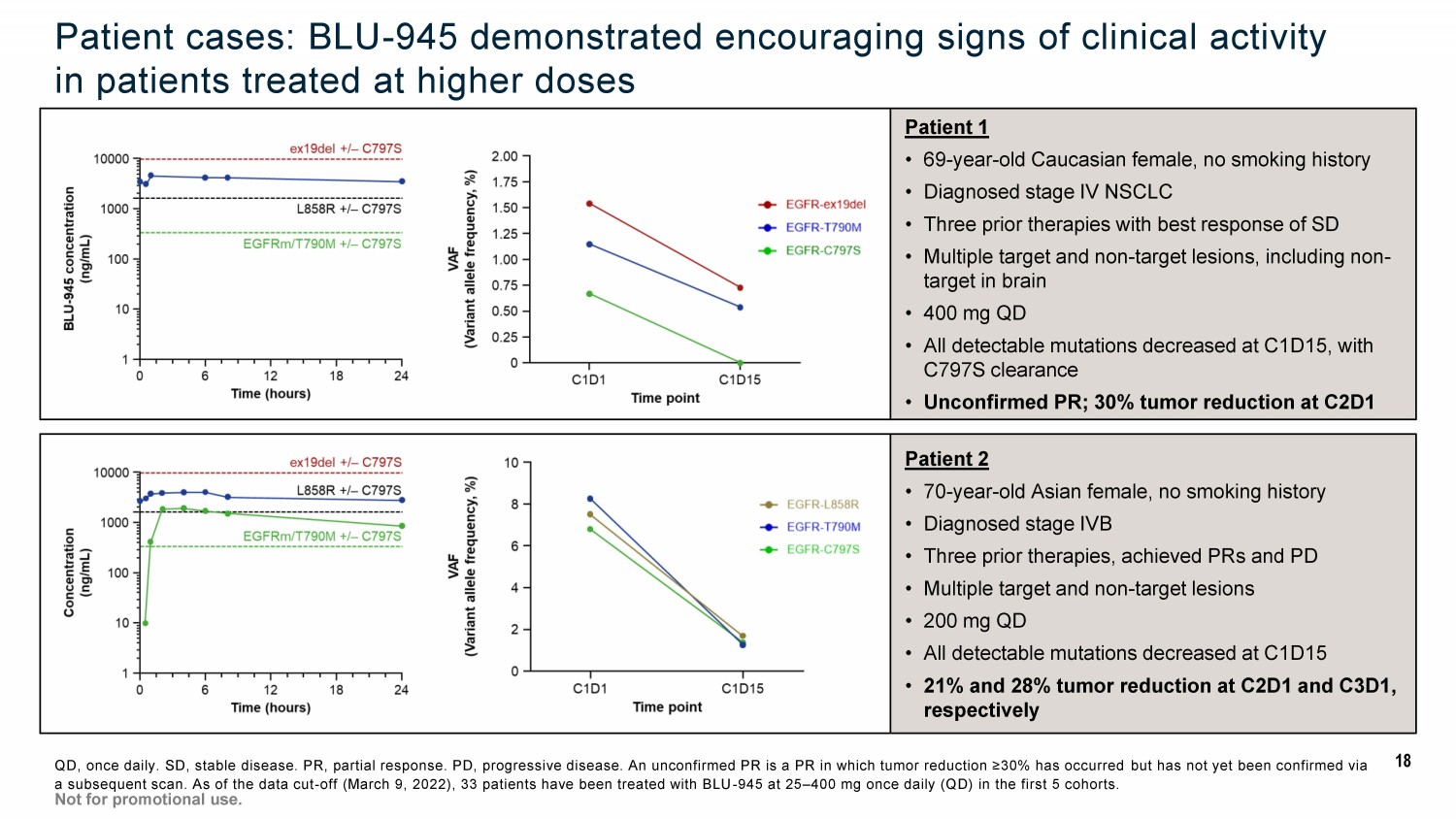
Patient 2 • 70 - year - old Asian female, no smoking history • Diagnosed stage IVB • Three prior therapies, achieved PRs and PD • Multiple target and non - target lesions • 200 mg QD • All detectable mutations decreased at C1D15 • 21% and 28% tumor reduction at C2D1 and C3D1, respectively Patient cases: BLU - 945 demonstrated encouraging signs of clinical activity in patients treated at higher doses QD, once daily. SD, stable disease. PR, partial response. PD, progressive disease. An unconfirmed PR is a PR in which tumor reduction ≥30% has occurred but has not yet been confirmed via a subsequent scan. As of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (Q D) in the first 5 cohorts. Not for promotional use. Patient 1 • 69 - year - old Caucasian female, no smoking history • Diagnosed stage IV NSCLC • Three prior therapies with best response of SD • Multiple target and non - target lesions, including non - target in brain • 400 mg QD • All detectable mutations decreased at C1D15, with C797S clearance • Unconfirmed PR; 30% tumor reduction at C2D1 18
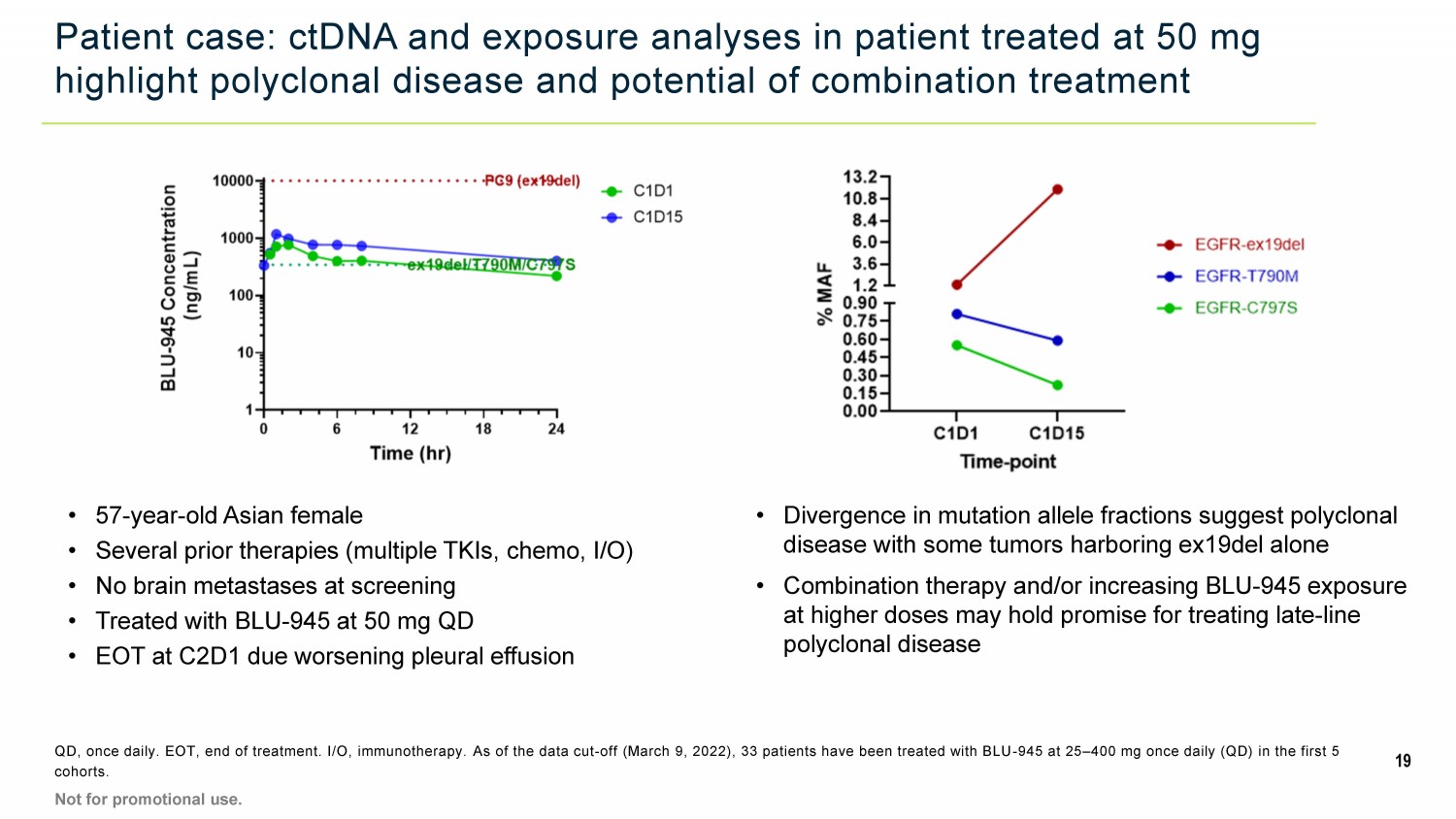
Patient case: ctDNA and exposure analyses in patient treated at 50 mg highlight polyclonal disease and potential of combination treatment QD, once daily. EOT, end of treatment. I/O, immunotherapy. As of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 cohorts. • 57 - year - old Asian female • Several prior therapies (multiple TKIs, chemo, I/O) • No brain metastases at screening • Treated with BLU - 945 at 50 mg QD • EOT at C2D1 due worsening pleural effusion • Divergence in mutation allele fractions suggest polyclonal disease with some tumors harboring ex19del alone • Combination therapy and/or increasing BLU - 945 exposure at higher doses may hold promise for treating late - line polyclonal disease 19 Not for promotional use.

Conclusions • In the ongoing Phase 1 trial, BLU - 945, a highly potent and selective oral EGFR inhibitor, was generally well tolerated at clinically active doses in heavily pre - treated patients with EGFRm NSCLC • Few AEs characteristic of wild - type EGFR toxicity observed at doses up to 400 mg QD • Despite presence of EGFR mutations conferring resistance to osimertinib, treatment with BLU - 945 resulted in rapid dose - dependent reductions in ctDNA, consistent with preclinical data • Increasing BLU - 945 doses were associated with increasing antitumor activity, with tumor shrinkage noted at doses of 200 mg QD and above, including an unconfirmed partial response at 400 mg QD • The clonal evolution and resulting mutational complexity of EGFR - driven NSCLC tumor cells demonstrates the need for precision medicine combinations to improve clinical outcomes • Initial safety and clinical activity results support expanded clinical development of BLU - 945 in combination with osimertinib and other complementary agents 20 Not for promotional use. AE, adverse event. QD, once daily. ctDNA, circulating tumor DNA. An unconfirmed PR is a PR in which tumor reduction ≥30% has occ urred but has not yet been confirmed via a subsequent scan. As of the data cut - off (March 9, 2022), 33 patients have been treated with BLU - 945 at 25 – 400 mg once daily (QD) in the first 5 cohorts.

Acknowledgements 21 • Participating patients and families • SYMPHONY trial investigators and research coordinators • Clinical trial sites: • Colleagues at Blueprint Medicines Corporation Cedars - Sinai Medical Center, Los Angeles, CA Samsung Medical Center, Seoul, Korea Dana - Farber Cancer Institute, Boston, MA Sarah Cannon Research Institute, Nashville, TN Kanagawa Cancer Center, Yokohama - shi, Kanagawa, Japan Seoul National University, Department of Internal Medicine, Seoul, Korea Massachusetts General Hospital, Boston, MA The Royal Marsden NHS Foundation Trust, Sutton, Surrey, UK National Cancer Centre Singapore, Singapore The University of Texas MD Anderson Cancer Center, Houston, TX National Cancer Center Hospital, Chuo Ku, Tokyo, Japan UC Irvine Health, Chao Family Comprehensive Cancer Center, Orange, CA National Cancer Center Hospital East, Kashiwa, Chiba, Japan University of Colorado Hospital - Anschutz Cancer Pavilion (ACP), Aurora, CA National Taiwan University Hospital, Taipei, Taiwan Vall d'Hebron University Hospital, Oncology Department, Barcelona, Spain NYU Langone Health, Laura and Isaac Perlmutter Cancer Center, New York, NY Yonsei Cancer Center, Severance Hospital, Yonsei University, Seoul, Korea Not for promotional use.

Portfolio strategy and next steps Fouad Namouni, MD, President, R&D
Early BLU - 945 dose escalation data achieve clinical proof - of - concept 23 Dose - dependent reductions in ctDNA allele fractions for EGFR resistance mutations targeted by BLU - 945 Increasing coverage of EGFR activating and resistance mutations at higher doses, based on pharmacokinetic data Generally well - tolerated, with no significant adverse events associated with wild - type EGFR inhibition Dose - dependent antitumor activity, with reductions in target lesions observed at 200 mg QD and higher DATA SUPPORT INITIATION OF BROAD COMBINATION DEVELOPMENT STRATEG Y Not for promotional use.
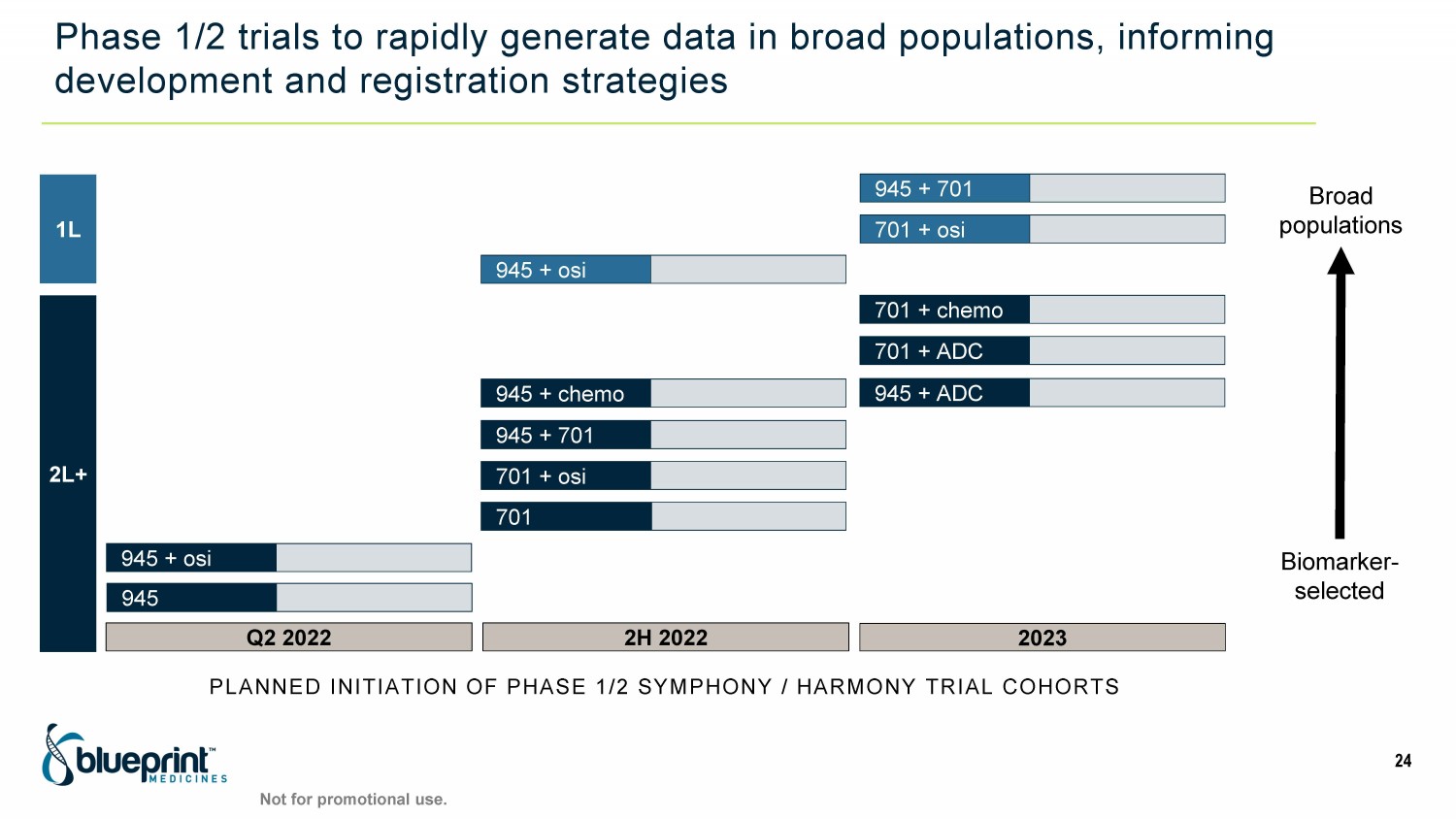
Phase 1/2 trials to rapidly generate data in broad populations, informing development and registration strategies PLANNED INITIATION OF PHASE 1/2 SYMPHONY / HARMONY TRIAL COHORTS 1L 2L+ 2023 701 + ADC 945 + osi 701 + chemo 701 + osi Q2 2022 945 + osi 945 2H 2022 945 + ADC 701 945 + 701 945 + chemo 701 + osi Biomarker - selected Broad populations 24 945 + 701 Not for promotional use.
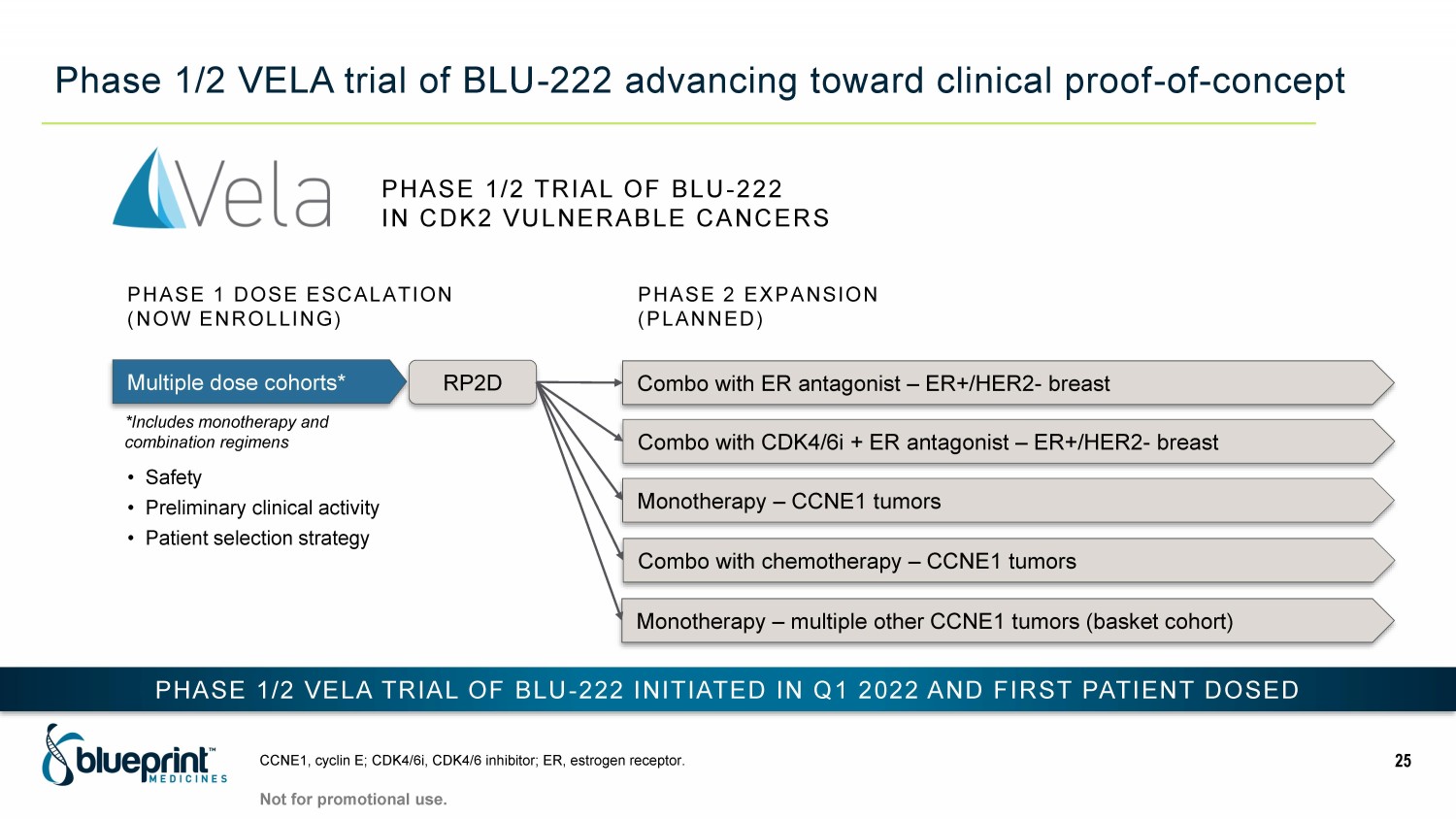
Phase 1/2 VELA trial of BLU - 222 advancing toward clinical proof - of - concept CCNE1, cyclin E; CDK4/6i, CDK4/6 inhibitor; ER, estrogen receptor. 25 Multiple dose cohorts* RP2D Combo with ER antagonist – ER+/HER2 - breast Combo with CDK4/6i + ER antagonist – ER+/HER2 - breast Monotherapy – CCNE1 tumors PHASE 2 EXPANSION (PLANNED) PHASE 1 DOSE ESCALATION (NOW ENROLLING) Combo with chemotherapy – CCNE1 tumors PHASE 1/2 VELA TRIAL OF BLU - 222 INITIATED IN Q1 2022 AND FIRST P ATIENT DOSED • Safety • Preliminary clinical activity • Patient selection strategy PHASE 1/2 TRIAL OF BLU - 222 IN CDK2 VULNERABLE CANCERS Not for promotional use. *Includes monotherapy and combination regimens Monotherapy – multiple other CCNE1 tumors (basket cohort)
Significant progress across our portfolio will drive near - term news flow 26 Top - line PIONEER trial data in non - advanced SM with potential to significantly expand AYVAKIT label Multiple anticipated datasets for EGFR and CDK2 programs with potential to unlock broad patient opportunities Plan to unveil new research programs and vision for scientific platform expansion at R&D Day Not for promotional use. MID - 2022 2H 2022 THRU 2023 2H 2022

Thank You


























