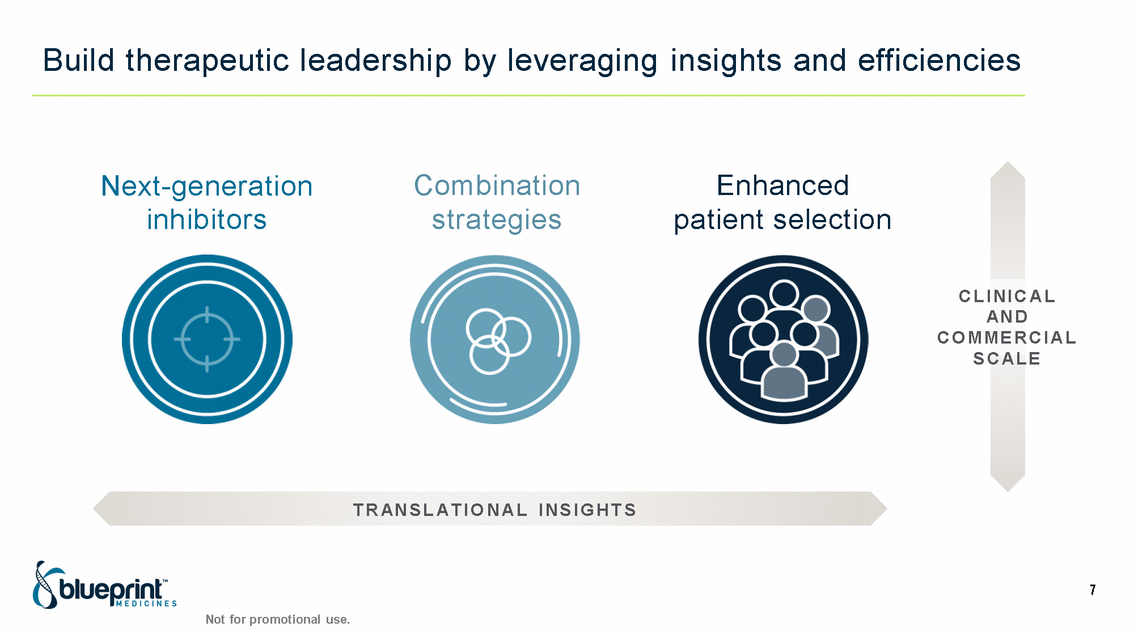
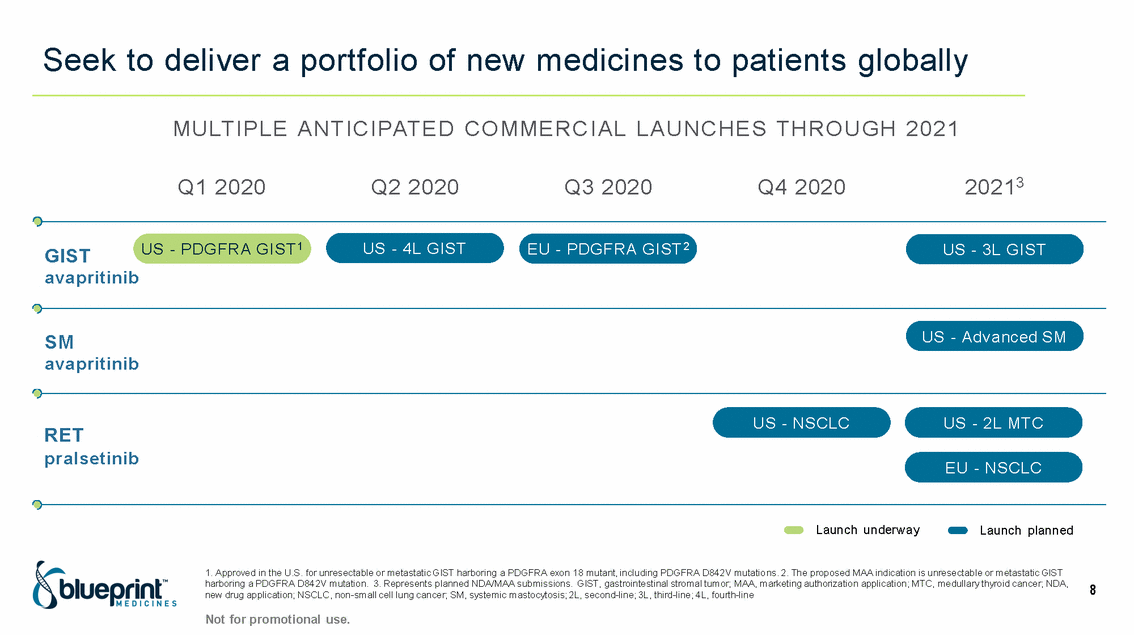
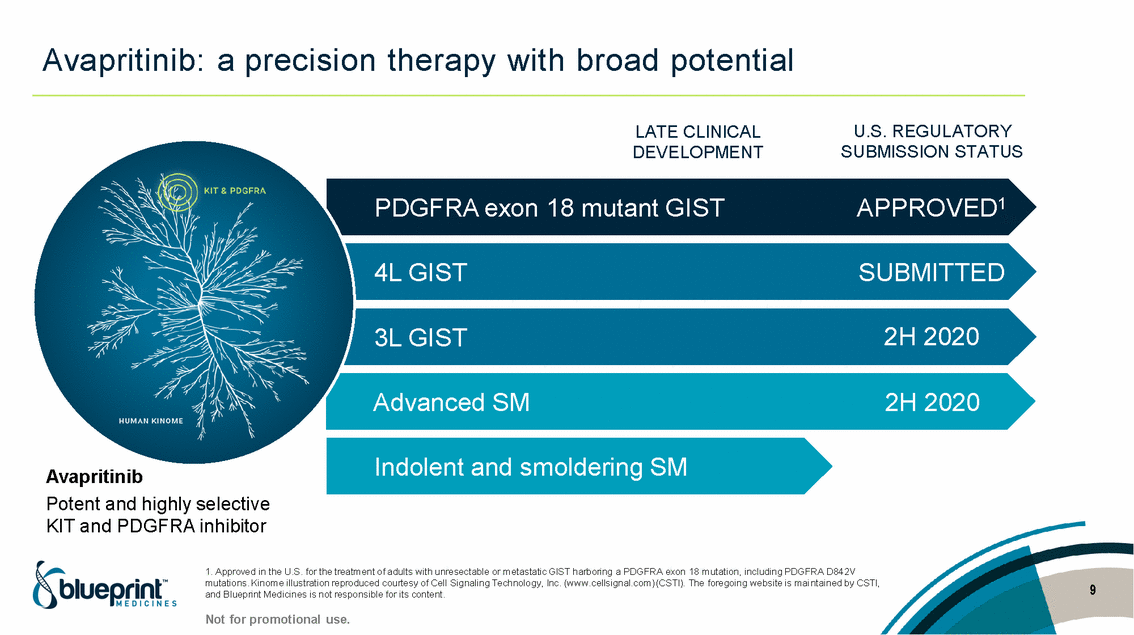
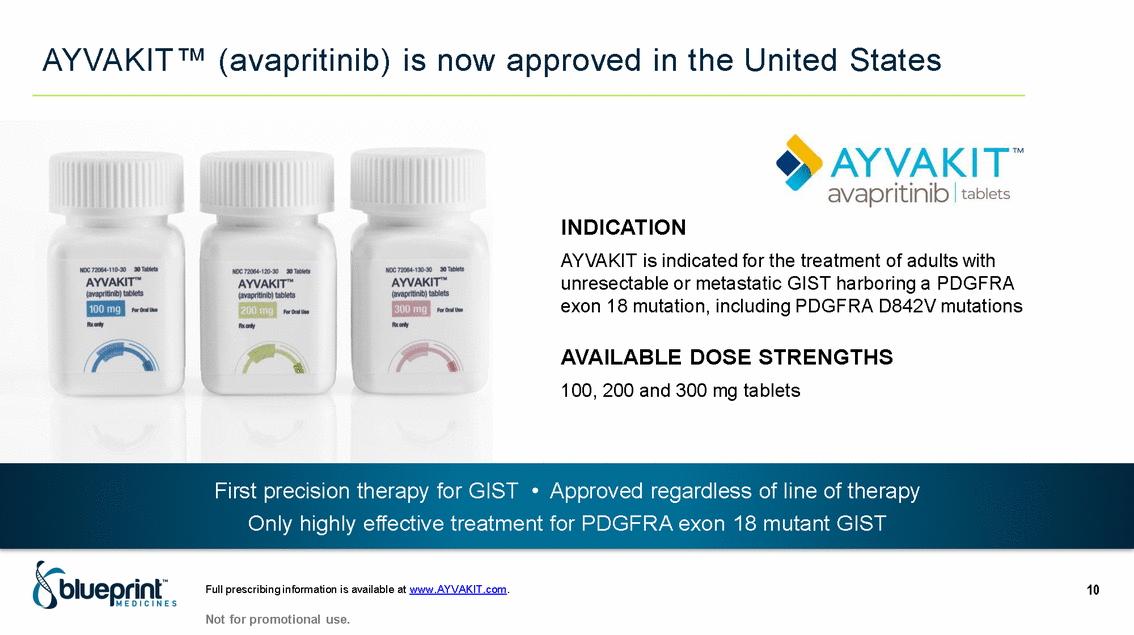
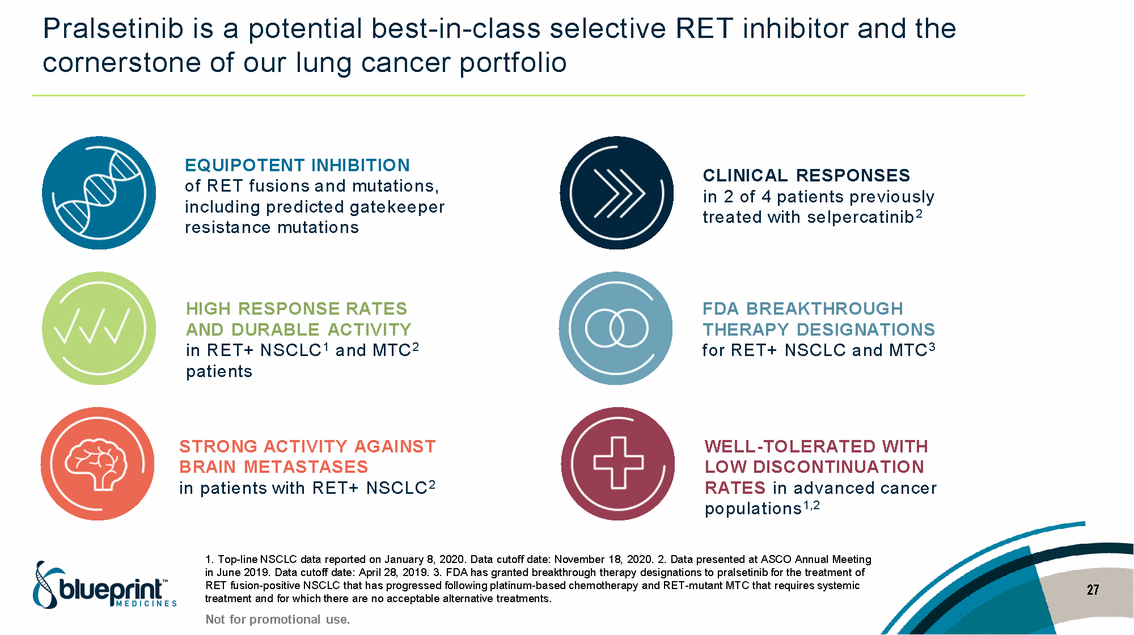
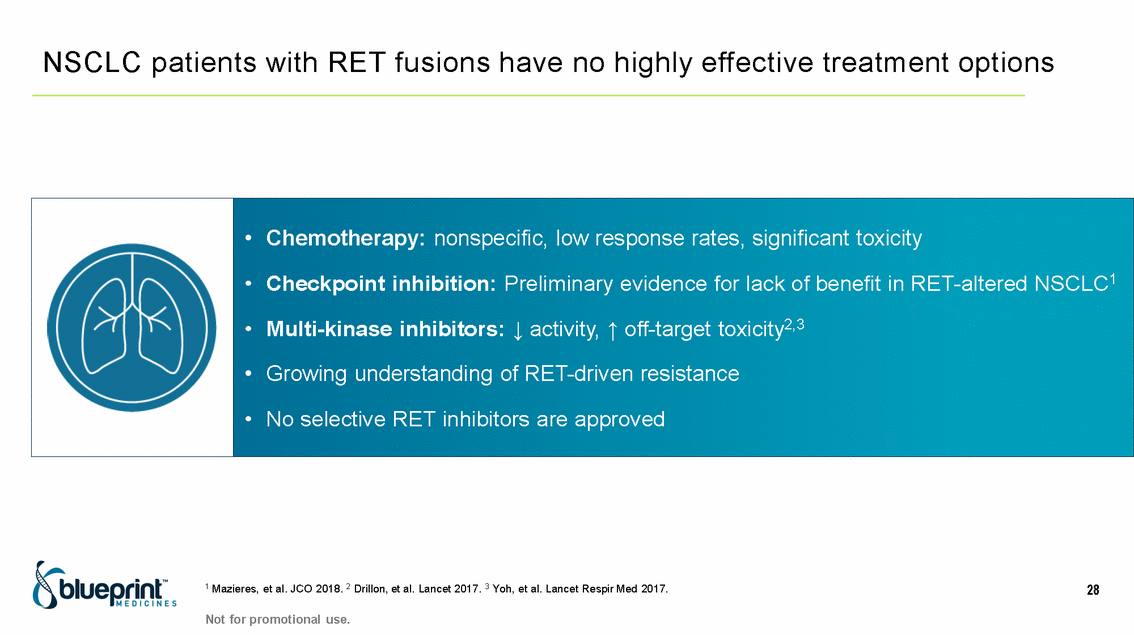
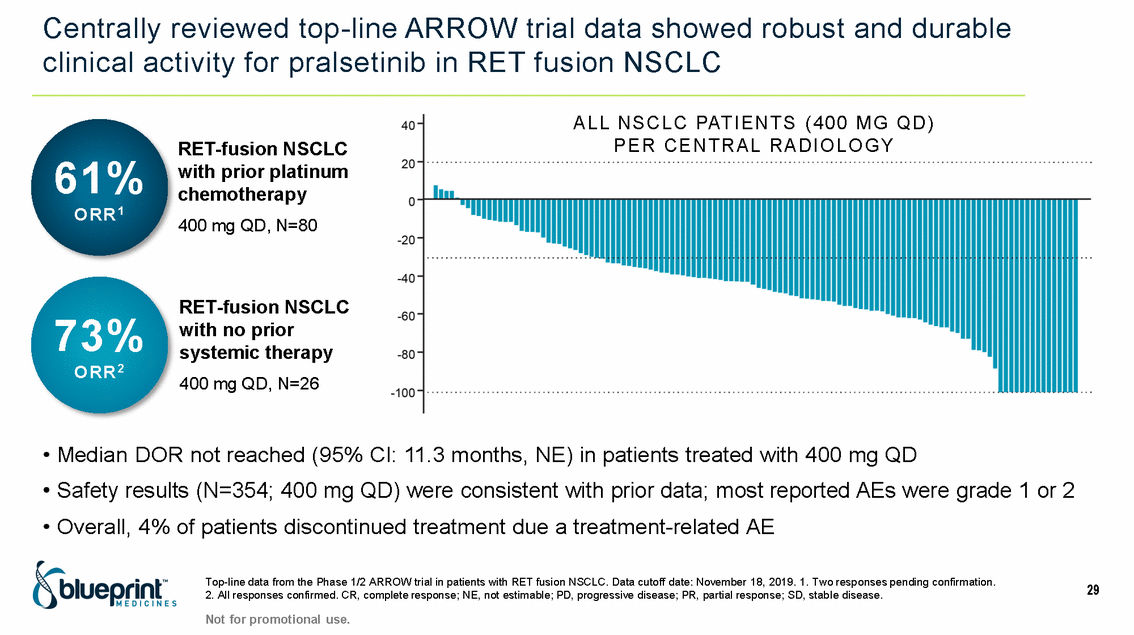
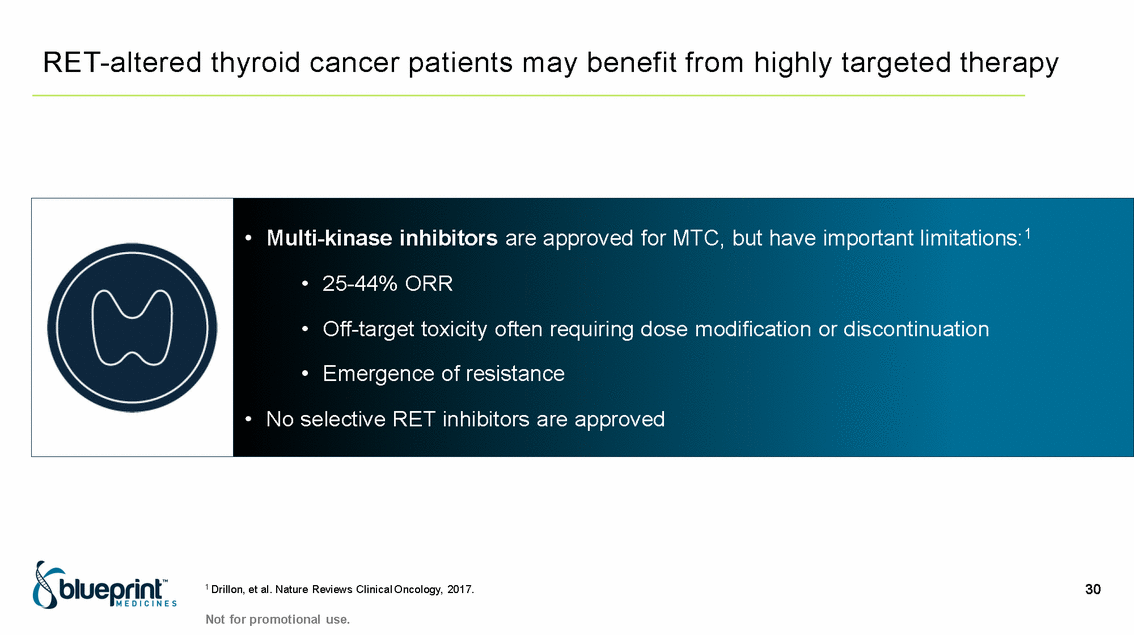
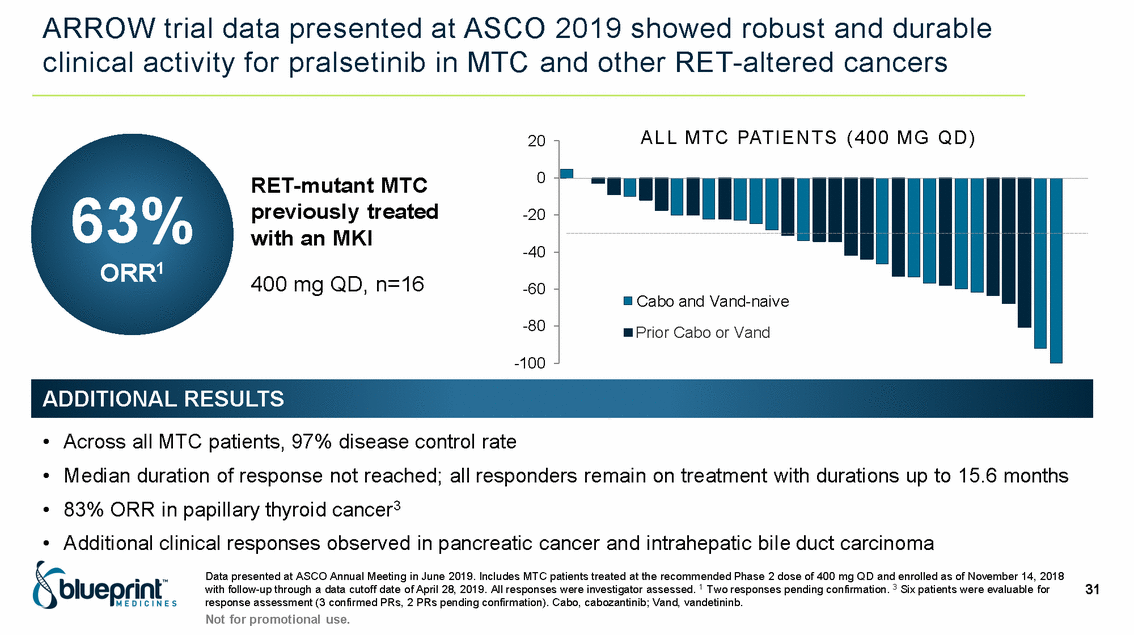
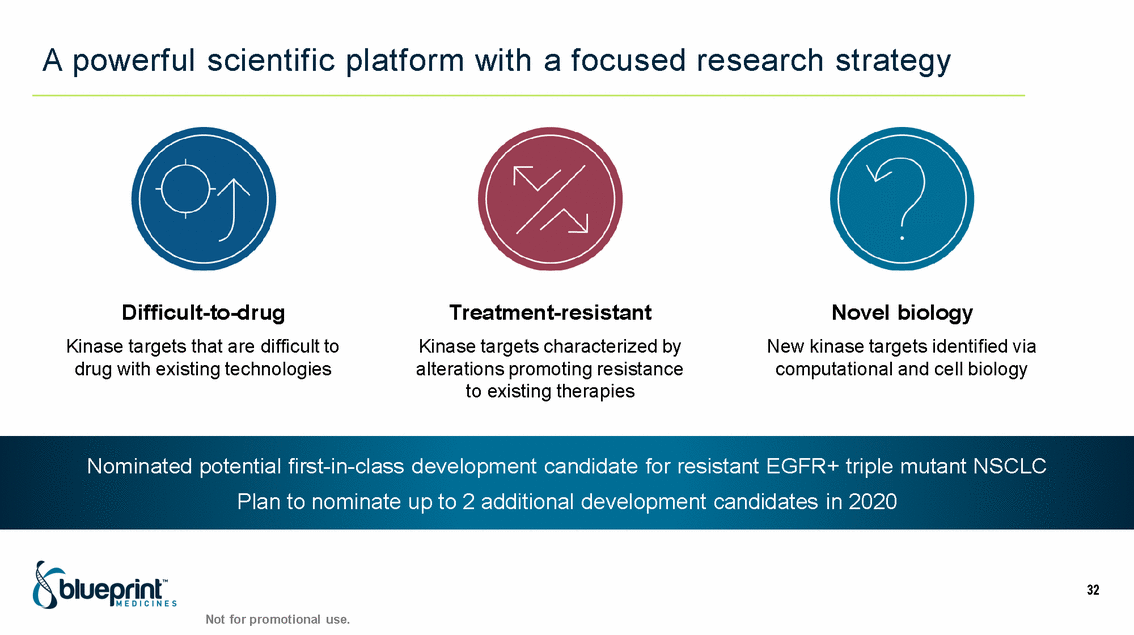
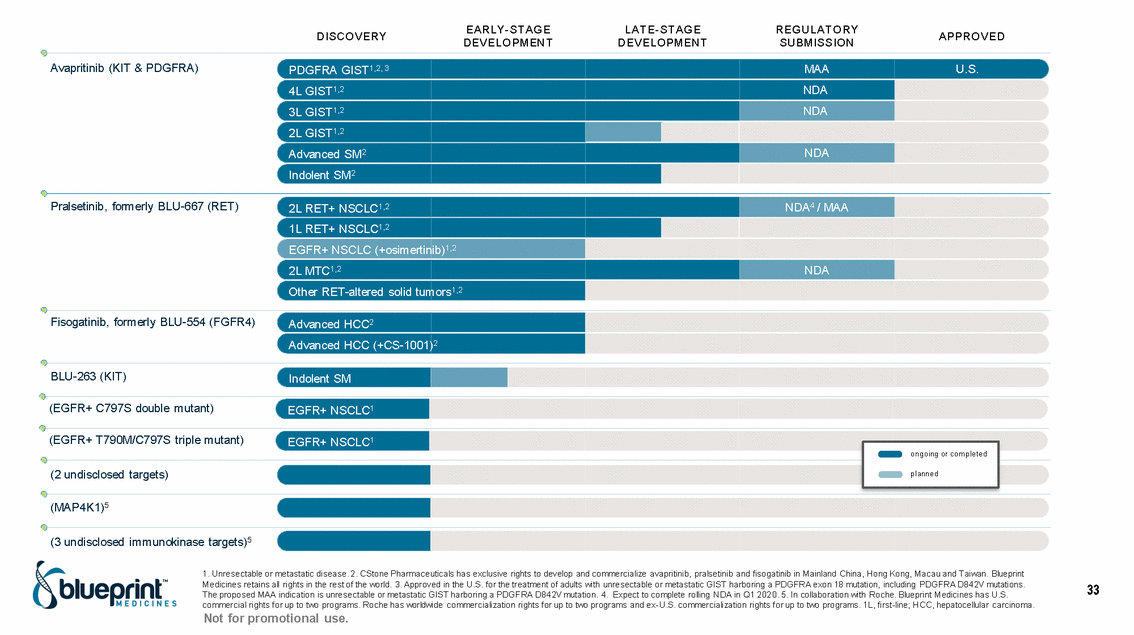
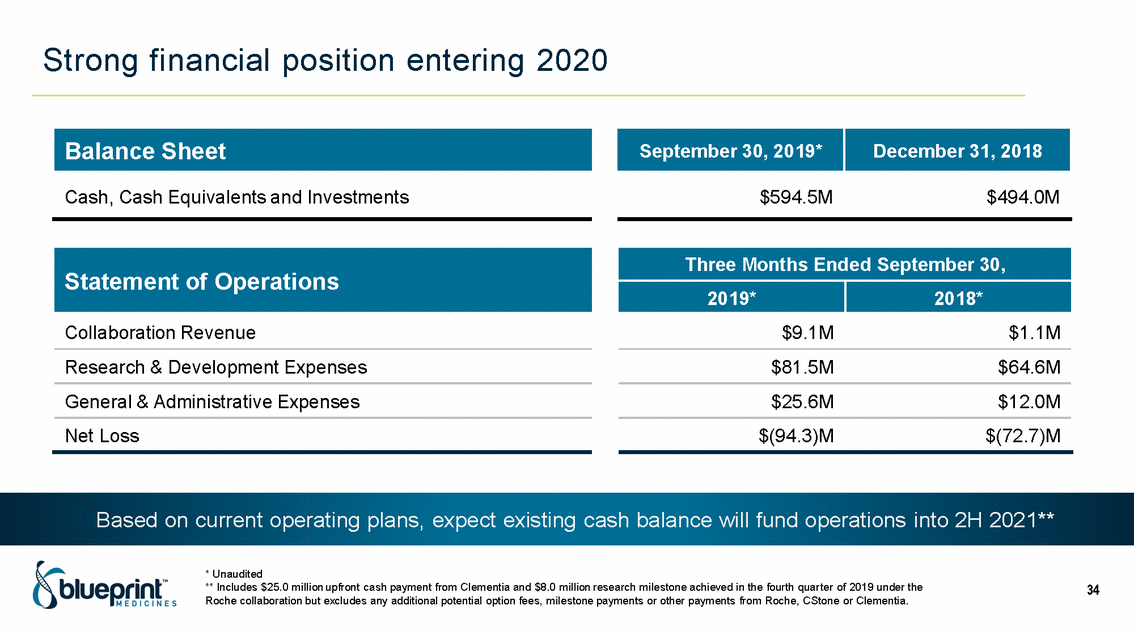
| Forward-looking statements T his presentation contains forward -looking statements as defined in the Private Securities Litigation Reform Act of 1995, as ame nded. T he words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar ex pressions are intended to identify forward -looking statements, although not all forward-looking statements contain these identifying words. In this presentation, forward -looking statements include, without li mitation, statements regarding Blueprint Medicines’ 2020 key milestones; Blueprint Medicines’ plans, strategies, timelines and expectations for the preclinical and clinical development a nd commercialization of AYVAKIT ™ (avapritinib), pralsetinib, fisogatinib, and BLU-263; plans and timelines for submitting marketing applications for avapritinib and pralsetinib and, if approved, commercializing avapritinib for additional indications or pralsetinib; the potential benefits of Blueprint Medicines’ current and future drug candidates in treating patients; expectations regarding Bl ueprint Medicines’ existing cash, cash equivalents and investments; and Blueprint Medicines’ strategy, goals and anticipated milestones, business plans and focus. Blueprint Medicines has based thes e forward-looking statements on management’s current expectations, assumptions, estimates and projections. W hile Blueprint Medicines believes these expectations, assumptions, estimates and pro jections are reasonable, such forward -looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond Blueprin t Medicines’ control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward -looking statements. T hese risks and uncertainti es include, without limitation, risks and uncertainties related to the delay of any current or planned clinical trials or the development of Blueprint Medicines’ drug candidates, including avaprit inib, pralsetinib, fisogatinib and BLU-263, or the licensed products, including BLU-782; Blueprint Medicines’ advancement of multiple early-stage efforts; Blueprint Medicines’ ability to successfully demonstr ate the efficacy and safety of its drug candidates and gain approval of its drug candidates on a timely basis, if at all; the preclinical and clinical results for Blueprint Medicines’ drug candidat es, which may not support further development of such drug candidates; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of clinical trials o r marketing applications; Blueprint Medicines’ ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing; Blueprint Medicines’ abil ity and plans for establishing a commercial infrastructure, and successfully launching, marketing and selling its current or future approved products; Blueprint Medicines’ ability to successfully expand the indications for AYVAKIT in the future; Blueprint Medicines’ ability to develop and commercialize companion diagnostic tests for its current and future drug candidates; and the success of Blueprint Medicines’ current and future collaborations, partnerships, and license, including its collaborations with F. Hoffmann -La Roche Ltd and Hoffmann-La Roche Inc. (collectively, “Roche”) and CStone Pharmaceuticals (“CStone”) and its license agreement with Clementia Pharmaceuticals Inc. (“ Clementia”). T hese and other risks and uncertainties are described in greater detail under “Risk Factors” in Blueprint Medicines’ filings with the Securities and Exchange Commission (“SEC”), including Blueprint Medicines’ most recent Quarterly Report on Form 10 -Q and any other filings Blueprint Medicines has made or may make with the SEC in the future. Blueprint Medicines cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that Bluepri nt Medicines’ expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished. T he forward -looking statements in this presentation are made only as of the date hereof, and except as required by law, Blueprint Medicines undertakes no obligation to update any forward -looking statements contained in this presentation as a result of new information, future events or otherwise. T his presentation also contains estimates, projections and other statistical data made by independent parties and by Blueprin t Medicines relating to market size and growth and other data about Blueprint Medicines’ industry. T hese data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of Blueprint Medicines’ future performance and the future performance of the markets in which Blueprint Medicin es operates are necessarily subject to a high degree of uncertainty and risk. 2 |