
Earnings Call – Q3 2021 November 4, 2021

This presentation has been prepared solely for use at this meeting and is intended for investors and analysts only. The material is given in conjunction with an oral presentation and should not be taken out of context. Unless the context requires otherwise, references to “ViewRay,” “the company,” “we,” “us” and “our,” refer to ViewRay, Inc. Except for historical information, ViewRay’s written and accompanying oral presentation may contain forward-looking statements, including statements about the overall industry, including but not limited to: our financial guidance; current expectations of the market; growth drivers; future trends; demand for radiation oncology products and features; treatment results; and innovation and growth opportunities. Forward-looking statements also include, but are not limited to, statements about ViewRay’s: future orders; backlog or earnings growth; future financial results; and market acceptance of ViewRay’s existing products, future products, or technology. Words such as “could,” “anticipates,” “expects,” “outlook,” “intends,” “plans,” “believes,” “seeks,” “vision,” “estimates,” “may,” “will,” “future,” “horizon,” “aiming,” “driving,” “target” (or variations of them,) and similar statements, are forward-looking statements. These types of statements express management’s beliefs based on the information available to us as of the date of this presentation, are subject to change, and are not guarantees of future performance. Forward-looking statements involve risks, uncertainties, and assumptions that are difficult to predict and could cause ViewRay’s results to differ materially from those presented. These risks, uncertainties, and assumptions include, but are not limited to, changes in: the regulatory environment; global economics; trade compliance requirements, duties or tariffs; third-party reimbursement levels; currency exchange rates; taxation, healthcare law, and product clearance requirements, as well as those related to: the effect of COVID-19 on our business operations and financial condition; adverse publicity about ViewRay and our products; our reliance on sole or limited source suppliers; our ability to commercialize our products successfully; the impact of competitive products and pricing, and all other risks listed from time to time in the company’s filings with the Securities and Exchange Commission, which are incorporated into this Forward-Looking Statements disclosure by this reference. We do not assume any obligation to update or revise the forward-looking statements in ViewRay’s written or oral presentation, whether based on future events, new or additional information or otherwise. ViewRay’s written and oral presentation does not constitute an offer to sell, or the solicitation of an offer to buy, securities. The opinions and clinical experiences presented herein are specific to the featured physicians and/or the featured patients and are for information purposes only. Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations. Individual customer and patient results are illustrative only and are not predictive of future results. The opinions and clinical experiences presented herein are specific to the featured physicians and the featured patients and are for information purposes only. Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations. ViewRay issued a press release and presentation for today’s call. The presentation can be viewed live on the webcast or downloaded from the “financial events and webinars” portion of our website at www.investors.viewray.com. The call is being broadcast and webcast live, and a replay will be available for 14 days. Listeners are cautioned that comments made by management during this call may include forward-looking statements within the meaning of federal securities laws. These statements involve material risks and uncertainties, and actual results could differ from those projected in any forward-looking statement due to numerous factors. For a description of these risks and uncertainties, please see ViewRay's Annual Report on Form 10-K for the fiscal year ended December 31, 2020, and its Quarterly Reports on Form 10-Q, as updated periodically with the company's other SEC filings. Furthermore, the content of this conference call contains time-sensitive information accurate only as of today, November 4, 2021. ViewRay undertakes no obligation to revise or otherwise update any statements to reflect events or circumstances after the date of this call. Financial Disclosure: Drs. Chuong and Nagar, respectively, have received consulting fees and research grants from ViewRay, Inc. and each serves on the Medical Advisory Board of ViewRay, Inc. Medical Advice Disclaimer: ViewRay is a medical device manufacturer and cannot and does not recommend specific treatment approaches. Individual results may vary. Forward-Looking Statements & Disclaimer
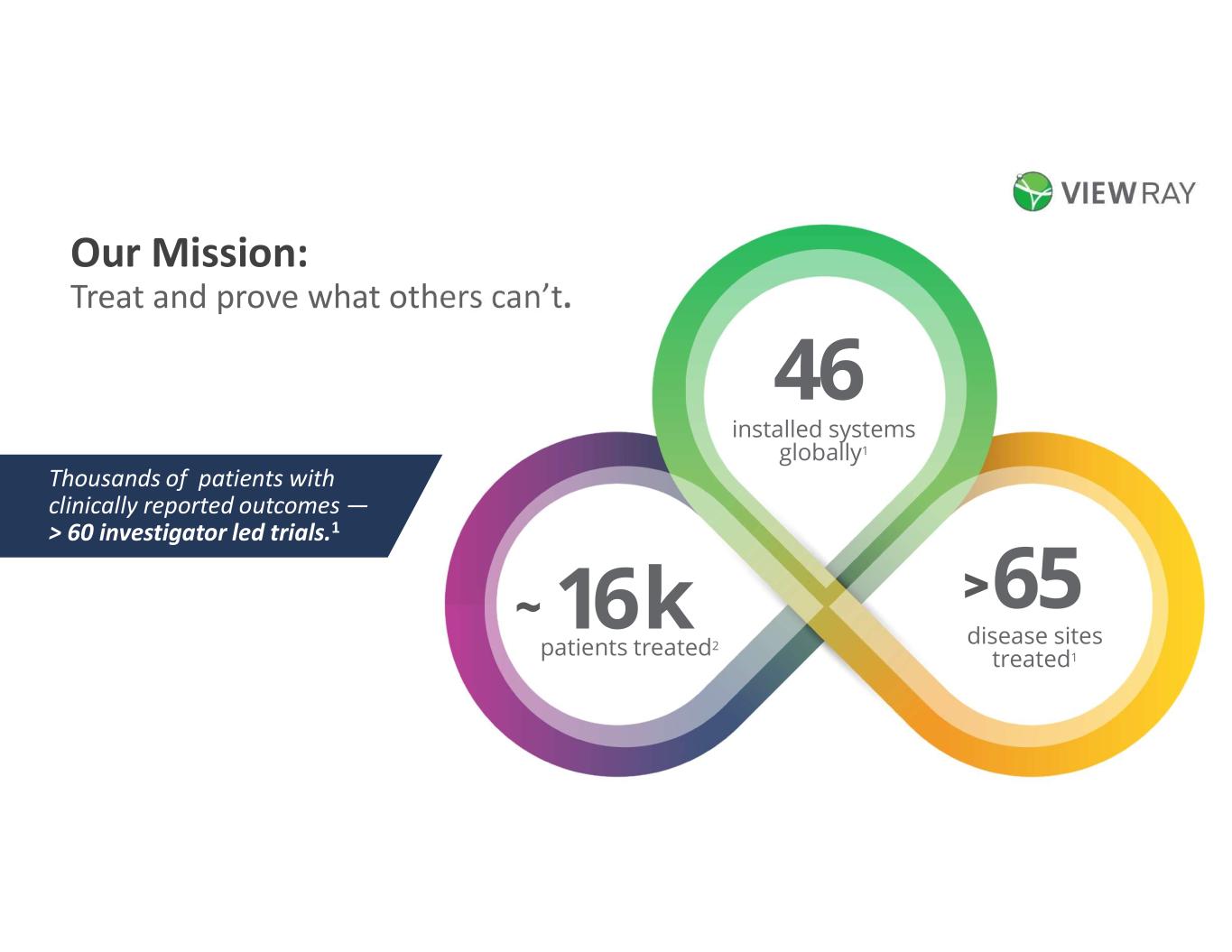
Our Mission: Treat and prove what others can’t. installed systems globally1 46 16 k patients treated2 ~ disease sites treated1 65> Thousands of patients with clinically reported outcomes ― > 60 investigator led trials.1
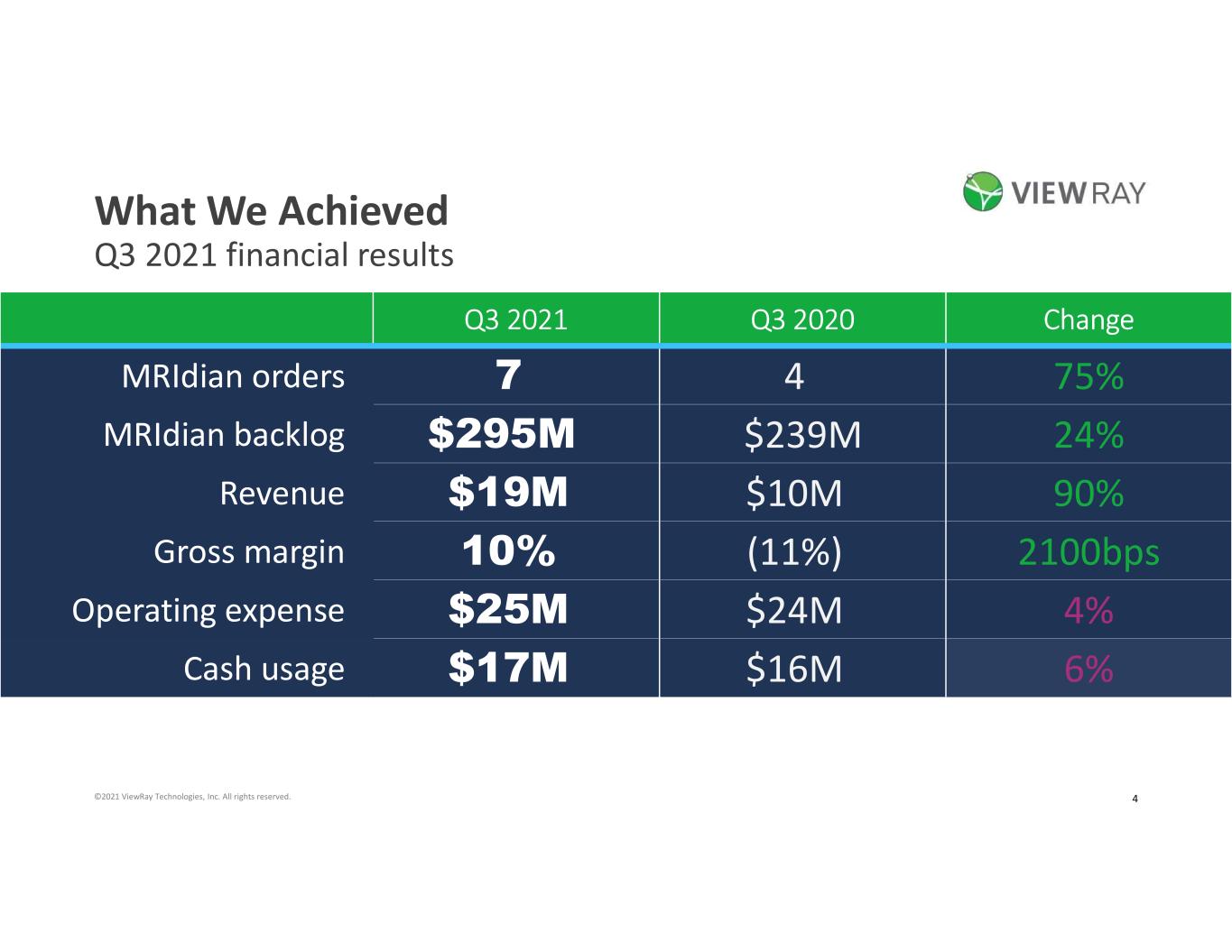
4©2021 ViewRay Technologies, Inc. All rights reserved. What We Achieved Q3 2021 financial results Q3 2021 Q3 2020 Change MRIdian orders 7 4 75% MRIdian backlog $295M $239M 24% Revenue $19M $10M 90% Gross margin 10% (11%) 2100bps Operating expense $25M $24M 4% Cash usage $17M $16M 6%
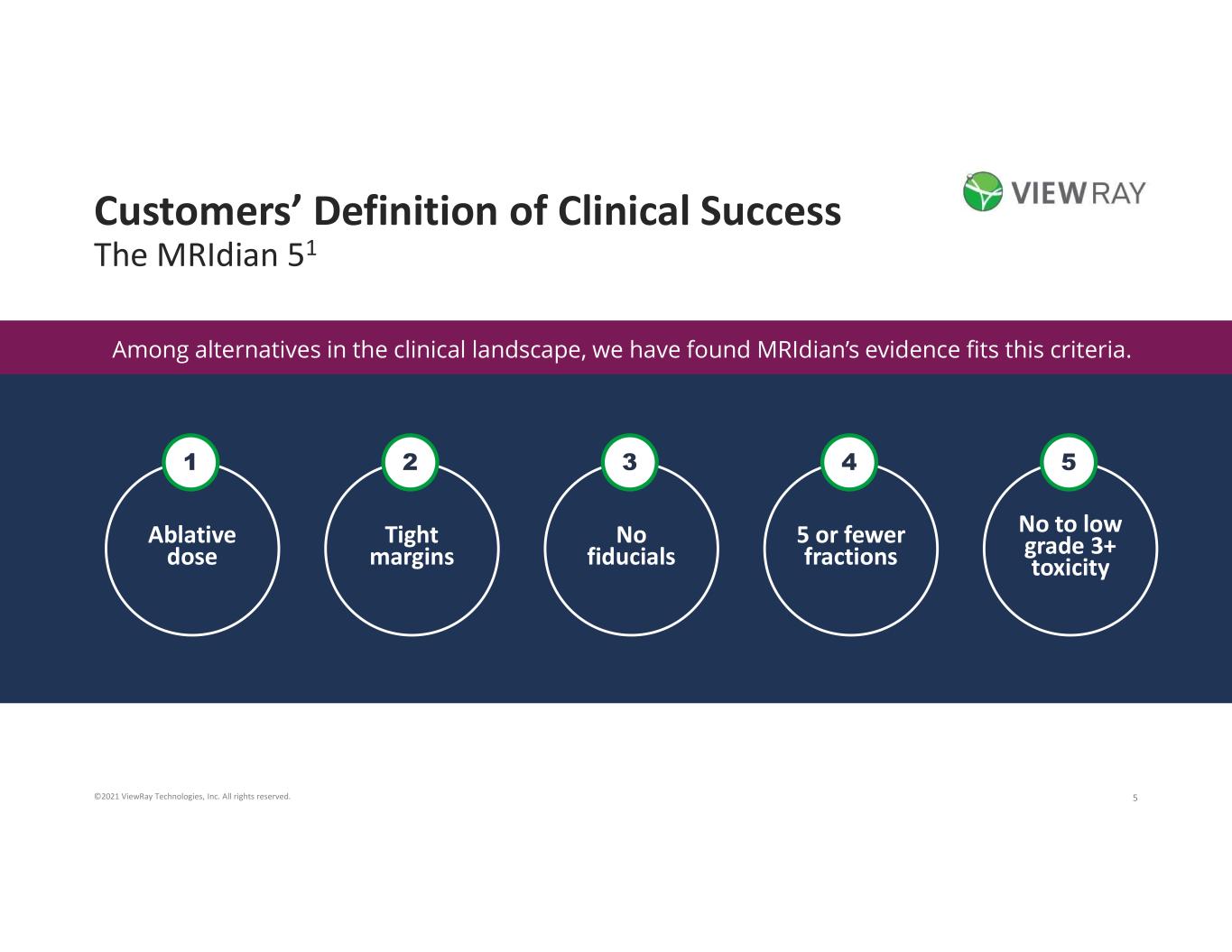
5©2021 ViewRay Technologies, Inc. All rights reserved. Customers’ Definition of Clinical Success The MRIdian 51 Ablative dose 1 Tight margins 2 No fiducials 3 5 or fewer fractions 4 No to low grade 3+ toxicity 5 Among alternatives in the clinical landscape, we have found MRIdian’s evidence fits this criteria.

Michael Chuong, MD, FACRO Vice Chair, Medical Director, Clinical Research Director, Lead GI Radiation Oncologist - Dept of Radiation Oncology at Miami Cancer Institute Recent Clinical Data Event 2-year overall survival among patients with inoperable pancreatic cancer1 ©2021 ViewRay Technologies, Inc. All rights reserved. • 148 patients from 3 institutions − All treated on MRIdian Linac from 2018-2020 − Median dose 50 Gy in 5 fractions (BED10 = 100 Gy) − No fiducial markers • 26-month median survival • 52.7% 2-year OS
Himanshu Nagar, MD Assistant Professor, Department of Radiation Oncology at Weill Cornell Medicine NewYork-Presbyterian Recent Clinical Data Event Evolution of prostate treatment1 ©2021 ViewRay Technologies, Inc. All rights reserved. • X-ray to MRI • Real-time MRI is future • SHORTER – Post-op Prostate − 20fx vs 5fx − Complimentary to surgery • FORT – Intact Prostate − 5fx vs 2fx • 4X increase in prostate cancer patient volumes

MRIdian Target Demonstrate safety of MRIdian SMART to treat what others can’t. Prostate • MIRAGE – Demonstrate the safety profile of 5fx SBRT vs CBCT • SCIMITAR & SHORTER – Safety of 5fx SBRT in post-op prostate • FORT - Redefine standard prostate treatment from 5fx to 2fx Mission: Improve patient quality of life Pancreas Extend to all pancreatic cancer patients and become an alternative to surgery (SMART Pancreas Trial) Mission: Safely extend survival rate Lung Mission: Safe treatment—curative intent Prove safe MRIdian SMART in tough to treat tumors in patients with few options (LUNG STAAR) Meaningful Clinical Data

©2021 ViewRay Technologies, Inc. All rights reserved.
VISIBLY BETTER Newest Generation of Innovations Suite of workflow and clinical features •Focused on treatment time improvement •Remote Access •Brain Treatment Package • Focused on enhancing MRI Imaging IMPORTANT NOTE This technology is FDA 510(k) pending and not available for sale anywhere.

Clinical ASTRO Pancreas Data Innovation 510(k) Pending Status on newest generation Commercial Customers purchasing multiple systems: TJU, Baptist Health South Florida and more in the pipeline + Recent Highlights Innovation and clinical pipelines accrue customer traction

Financial Results Q3 2021
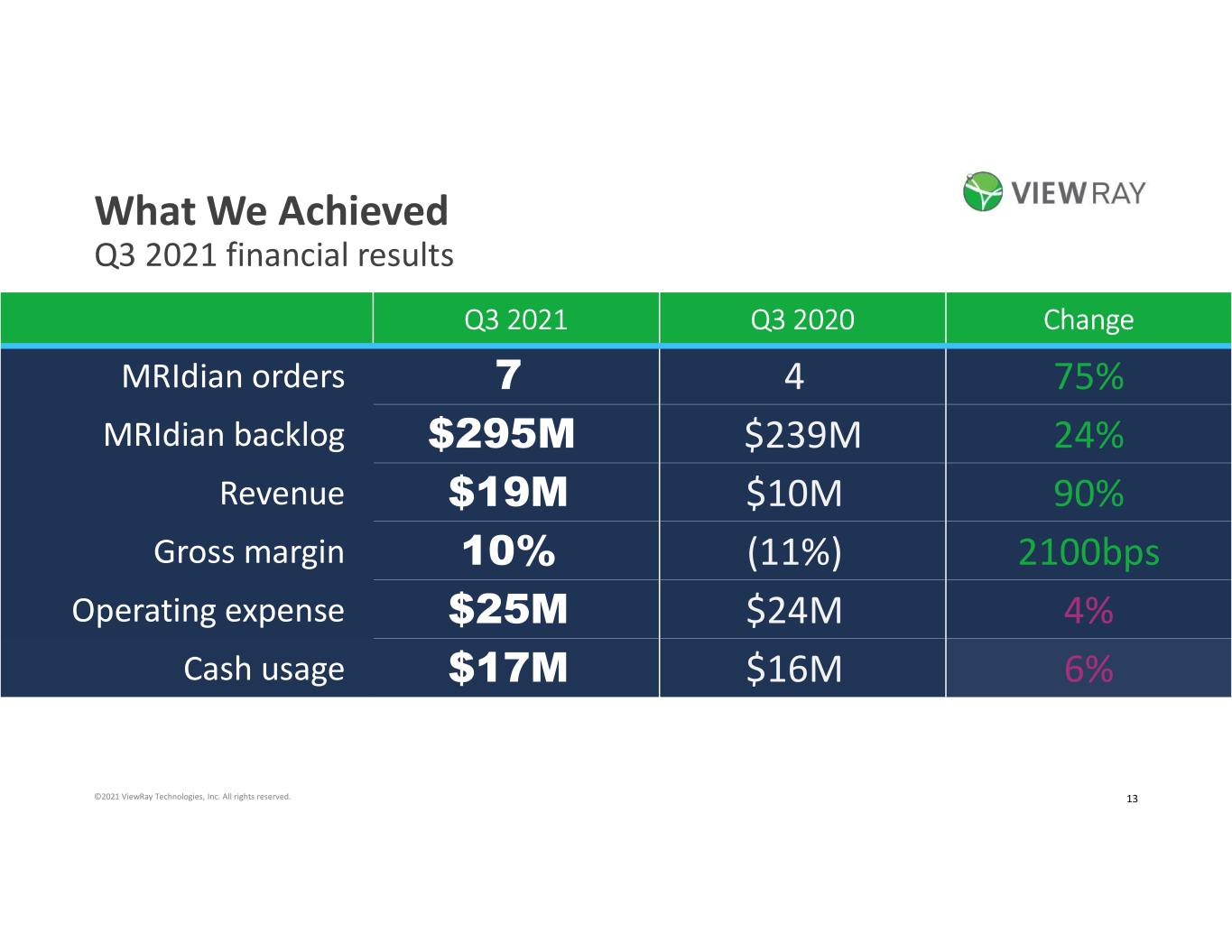
13©2021 ViewRay Technologies, Inc. All rights reserved. What We Achieved Q3 2021 financial results Q3 2021 Q3 2020 Change MRIdian orders 7 4 75% MRIdian backlog $295M $239M 24% Revenue $19M $10M 90% Gross margin 10% (11%) 2100bps Operating expense $25M $24M 4% Cash usage $17M $16M 6%
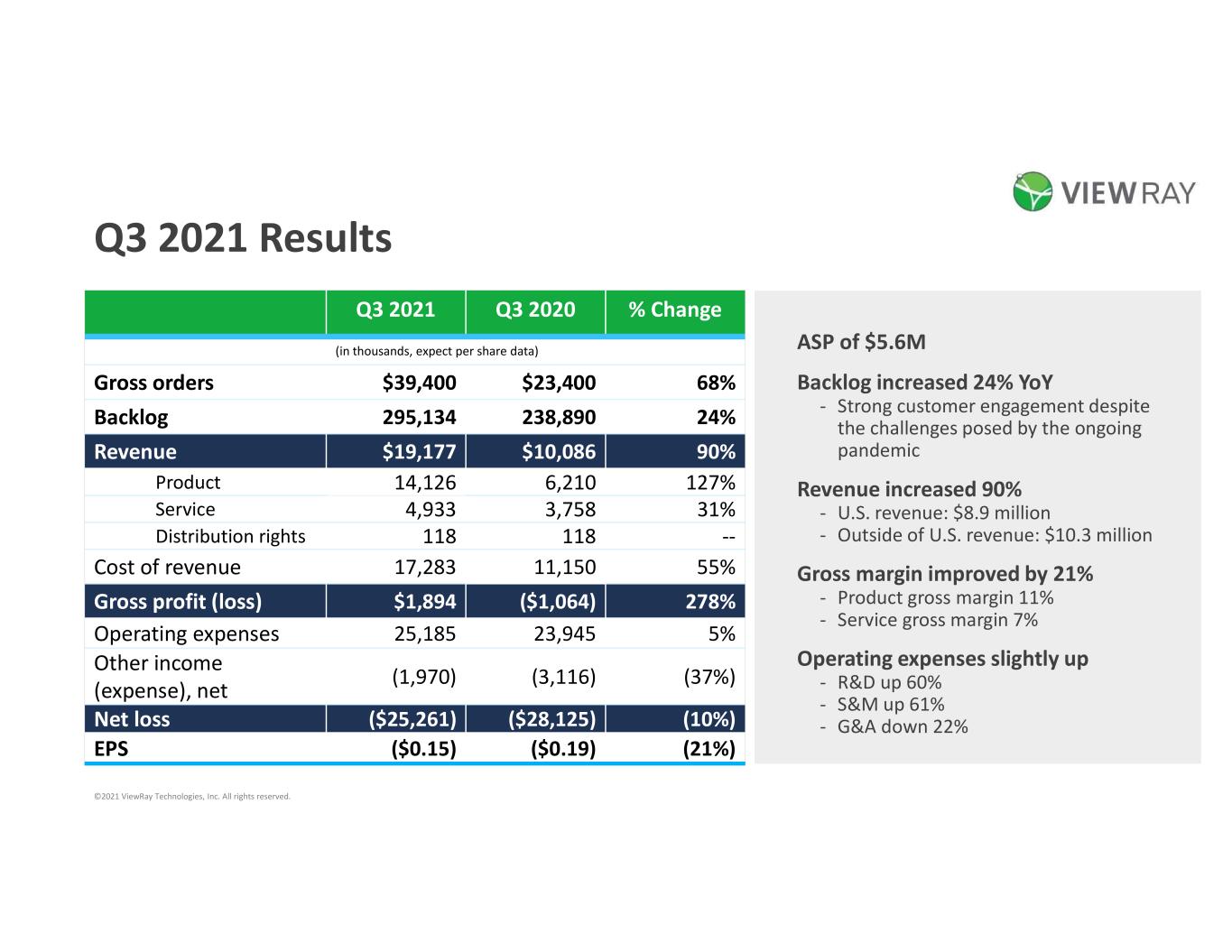
ASP of $5.6M Backlog increased 24% YoY - Strong customer engagement despite the challenges posed by the ongoing pandemic Revenue increased 90% - U.S. revenue: $8.9 million - Outside of U.S. revenue: $10.3 million Gross margin improved by 21% - Product gross margin 11% - Service gross margin 7% Operating expenses slightly up - R&D up 60% - S&M up 61% - G&A down 22% Q3 2021 Results Q3 2021 Q3 2020 % Change (in thousands, expect per share data) Gross orders $39,400 $23,400 68% Backlog 295,134 238,890 24% Revenue $19,177 $10,086 90% Product 14,126 6,210 127% Service 4,933 3,758 31% Distribution rights 118 118 -- Cost of revenue 17,283 11,150 55% Gross profit (loss) $1,894 ($1,064) 278% Operating expenses 25,185 23,945 5% Other income (expense), net (1,970) (3,116) (37%) Net loss ($25,261) ($28,125) (10%) EPS ($0.15) ($0.19) (21%) ©2021 ViewRay Technologies, Inc. All rights reserved.
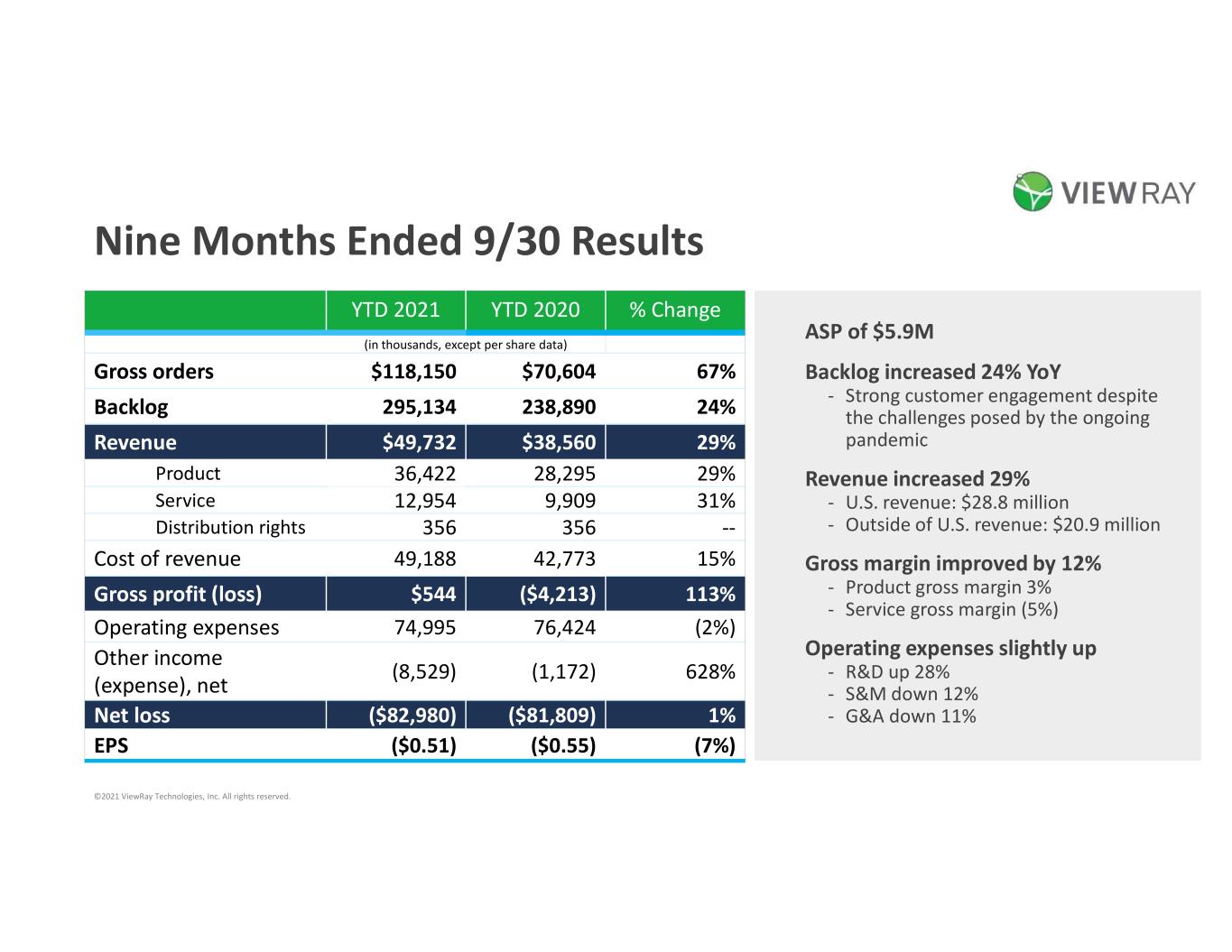
Nine Months Ended 9/30 Results YTD 2021 YTD 2020 % Change (in thousands, except per share data) Gross orders $118,150 $70,604 67% Backlog 295,134 238,890 24% Revenue $49,732 $38,560 29% Product 36,422 28,295 29% Service 12,954 9,909 31% Distribution rights 356 356 -- Cost of revenue 49,188 42,773 15% Gross profit (loss) $544 ($4,213) 113% Operating expenses 74,995 76,424 (2%) Other income (expense), net (8,529) (1,172) 628% Net loss ($82,980) ($81,809) 1% EPS ($0.51) ($0.55) (7%) ©2021 ViewRay Technologies, Inc. All rights reserved. ASP of $5.9M Backlog increased 24% YoY - Strong customer engagement despite the challenges posed by the ongoing pandemic Revenue increased 29% - U.S. revenue: $28.8 million - Outside of U.S. revenue: $20.9 million Gross margin improved by 12% - Product gross margin 3% - Service gross margin (5%) Operating expenses slightly up - R&D up 28% - S&M down 12% - G&A down 11%
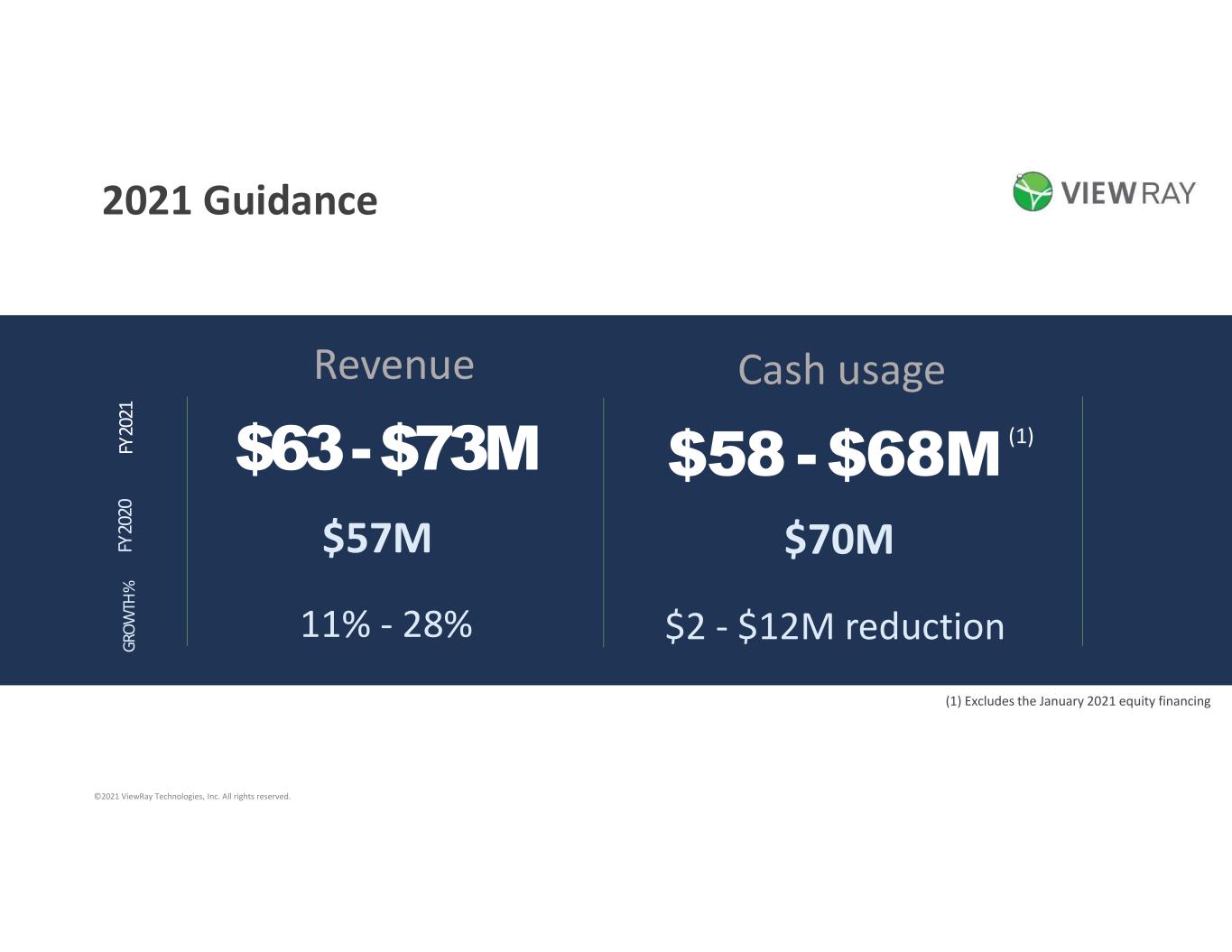
FY 20 20 FY 20 21 GR OW TH % Revenue $63-$73M $57M 11% - 28% 2021 Guidance (1) Excludes the January 2021 equity financing ©2021 ViewRay Technologies, Inc. All rights reserved. Cash usage $58 -$68M $70M $2 - $12M reduction (1)

VISIBLY BETTER

Presentation Citations Slide 3 1. Internal and historical company data 2. 09/30/2021 MRIdian log data Slide 5 1. Ablative dose, Tight margins, No fiducials: Chuong MD, Bryant J, Mittauer KE, Hall M, Kotecha R, Alvarez D, Romaguera T, Rubens M, Adamson S, Godley A, Mishra V, Luciani G, Gutierrez AN, Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer, Practical Radiation Oncology (2020) Five or fewer factions, No or low grade 3 toxicity: Finazzi T, Haasbeek CJA, Spoelstra FOB, Palacios MA, Admiraal MA, Bruynzeel AME, Slotman BJ, Lagerwaard FJ, Senan S, Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors International Journal of Radiation Oncology • Biology• Physics (2020); Rosenberg S. A. et al. (2018). A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Advances in Radiation Oncology, 4(1), 142-149; Rudra S. et al. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123-2132; Henke, L., et al. (2018). Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiotherapy and Oncology, 126(3), 519-526. Henke LE, et al. Stereotactic mr-guided online adaptive radiation therapy (smart) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol 2019;4:201-209; Rosenberg SA, et al. A multi- institutional experience of mr-guided liver stereotactic body radiation therapy. Adv Radiat Oncol 2019;4:142-149; Kennedy WR, Thomas MA, Stanley JA, Luo J, Ochoa LL, Clifton KK, Cyr AE, Margenthaler JA, DeWees TA, Price A, Kashani R, Green O, Zoberi I, Single Institution Phase I/II Prospective Clinical Trial of Single Fraction High Gradient Adjuvant Partial Breast Irradiation for Hormone Sensitive Stage 0-I Breast Cancer, International Journal of Radiation Oncology • Biology • Physics (2020); Bruynzeel AME, Tetar SU, Oei SS, Senan S, Haasbeek CJA, Spoelstra FOB, Piet AHM, Meijnen P, Bakker van der Jagt MAB, Fraikin T, Slotman BJ, van Moorselaar RJA, Lagerwaard FJ, A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results, International Journal of Radiation Oncology • Biology • Physics (2019) Slide 6 1. Choung, M.D., et al. Long-Term Multi-Institutional Outcomes of 5-Fraction Ablative Stereotactic MR-Guided Adaptive Radiation Therapy (SMART) for Inoperable Pancreas Cancer With Median Prescribed Biologically Effective Dose of 100 Gy10 [abstract]. In: Proceedings of the 2021 Annual Meeting of the American Society for Radiation Oncology.; 2021 Oct 24-27; Chicago, IL, USA. Arlington, VA: ASTRO; 2021. Abstract nr 1106. Abstract retrieved from https://plan.core-apps.com/myastroapp2021/abstract/16c0367a-e1bf-415d-b026-66bca5cf7b14. Slide 7 1. Nagar, Himanshu. Weill Medical College of Cornell University (U.S.). (2020, Sept 24 - ). Randomized Phase II Trial of Salvage Radiotherapy for Prostate Cancer In 4 Weeks v. 2 Weeks. Identifier NCT04422132. https://clinicaltrials.gov/ct2/show/NCT04422132. Nagar, Himanshu. Weill Medical College of Cornell University (U.S.). (2021, Nov 1 - ). Randomized Phase II Trial of Five or Two MRI-Guided Adaptive Radiotherapy Treatments for Prostate Cancer. Identifier NCT04984343. https://clinicaltrials.gov/ct2/show/NCT04984343. Kishan, Amar. UCLA / Jonsson Comprehensive Cancer Center (U.S.). (2020, May 12 - ). Magnetic Resonance Imaging-Guided Stereotactic Body Radiotherapy for Prostate Cancer (Mirage): A Phase III Randomized Trial. Identifier NCT04384770. https://clinicaltrials.gov/ct2/show/NCT04384770. Kishan, Amar. UCLA / Jonsson Comprehensive Cancer Center (U.S.). (2019, Jan 29 - ). Prospective Study of Stereotactic Body Radiotherapy (SBRT) Following Radical Prostatectomy. Identifier NCT03541850. https://clinicaltrials.gov/ct2/show/NCT03541850.


















