
J.P. Morgan Healthcare Conference January 2022 Exhibit 99.1

2©2022 ViewRay Technologies, Inc. All rights reserved. This presentation has been prepared solely for use at this meeting and is intended for investors and analysts only. The material is given in conjunction with an oral presentation and should not be taken out of context. Unless the context requires otherwise, references to “ViewRay,” “the company,” “we,” “us” and “our,” refer to ViewRay, Inc. Except for historical information, ViewRay’s written and accompanying oral presentation may contain forward-looking statements, including statements about the overall industry, including but not limited to: our financial guidance; current expectations of the market; growth drivers; future trends; demand for radiation oncology products and features; treatment results; and innovation and growth opportunities. Forward-looking statements also include, but are not limited to, statements about ViewRay’s: future orders; backlog or earnings growth; future financial results; and market acceptance of ViewRay’s existing products, future products, or technology. Words such as “could,” “anticipates,” “expects,” “outlook,” “intends,” “plans,” “believes,” “seeks,” “vision,” “estimates,” “may,” “will,” “future,” “horizon,” “aiming,” “driving,” “target” (or variations of them,) and similar statements, are forward-looking statements. These types of statements express management’s beliefs based on the information available to us as of the date of this presentation, are subject to change, and are not guarantees of future performance. Forward-looking statements involve risks, uncertainties, and assumptions that are difficult to predict and could cause ViewRay’s results to differ materially from those presented. These risks, uncertainties, and assumptions include, but are not limited to, changes in: the regulatory environment; global economics; trade compliance requirements, duties or tariffs; third-party reimbursement levels; currency exchange rates; taxation, healthcare law, and product clearance requirements, as well as those related to: the effect of COVID-19 on our business operations and financial condition; adverse publicity about ViewRay and our products; our reliance on sole or limited source suppliers; our ability to commercialize our products successfully; the impact of competitive products and pricing, and all other risks listed from time to time in the company’s filings with the Securities and Exchange Commission, which are incorporated into this Forward-Looking Statements disclosure by this reference. We do not assume any obligation to update or revise the forward-looking statements in ViewRay’s written or oral presentation, whether based on future events, new or additional information or otherwise. ViewRay’s written and oral presentation does not constitute an offer to sell, or the solicitation of an offer to buy, securities. The opinions and clinical experiences presented herein are specific to the featured physicians and/or the featured patients and are for information purposes only. Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations. Individual customer and patient results are illustrative only and are not predictive of future results. The opinions and clinical experiences presented herein are specific to the featured physicians and the featured patients and are for information purposes only. Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations. ViewRay issued presentation for today’s meeting. The presentation can be viewed live on the webcast or downloaded from the “financial events and webinars” portion of our website at www.investors.viewray.com. The presentation is being broadcast and webcast live, and a replay will be available for 14 days. Listeners are cautioned that comments made by management during this presentation may include forward-looking statements within the meaning of federal securities laws. These statements involve material risks and uncertainties, and actual results could differ from those projected in any forward-looking statement due to numerous factors. For a description of these risks and uncertainties, please see ViewRay's Annual Report on Form 10-K for the fiscal year ended December 31, 2020, and its Quarterly Reports on Form 10-Q, as updated periodically with the company's other SEC filings. Furthermore, the content of this conference presentation contains time-sensitive information accurate only as of today, January 13, 2022. ViewRay undertakes no obligation to revise or otherwise update any statements to reflect events or circumstances after the date of this presentation. Financial Disclosure: Drs. Chuong and Nagar, respectively, have received consulting fees and research grants from ViewRay, Inc. and each serves on the Medical Advisory Board of ViewRay, Inc. Medical Advice Disclaimer: ViewRay is a medical device manufacturer and cannot and does not recommend specific treatment approaches. Individual results may vary. Forward-Looking Statements & Disclaimer

3 Agenda •Preliminary Q4 & FY 2021 Results •Trends in Cancer Care •Value of MRIdian Clinical, Innovation, and Commercial Pipeline •Driving Therapy Adoption and Shareholder Value

4©2022 ViewRay Technologies, Inc. All rights reserved. What We Achieved Q4 2021 preliminary financial results (unaudited) Q4 2021 Q4 2020 Change MRIdian orders 7 5 40% MRIdian backlog $313M $241M 30% Revenue $20M $18M 10% Cash usage* $7M $7M 0% (*) Excluding proceeds from equity offerings
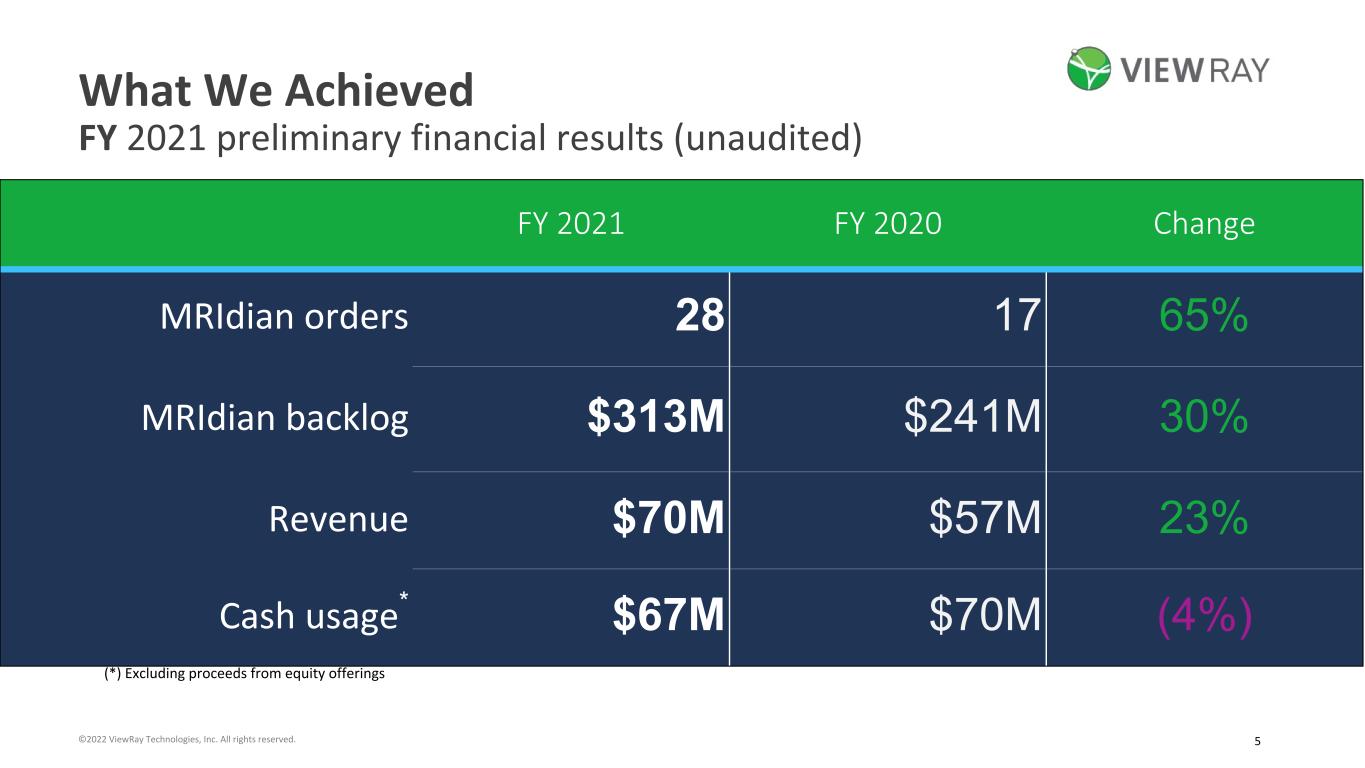
5©2022 ViewRay Technologies, Inc. All rights reserved. What We Achieved FY 2021 preliminary financial results (unaudited) FY 2021 FY 2020 Change MRIdian orders 28 17 65% MRIdian backlog $313M $241M 30% Revenue $70M $57M 23% Cash usage* $67M $70M (4%) (*) Excluding proceeds from equity offerings
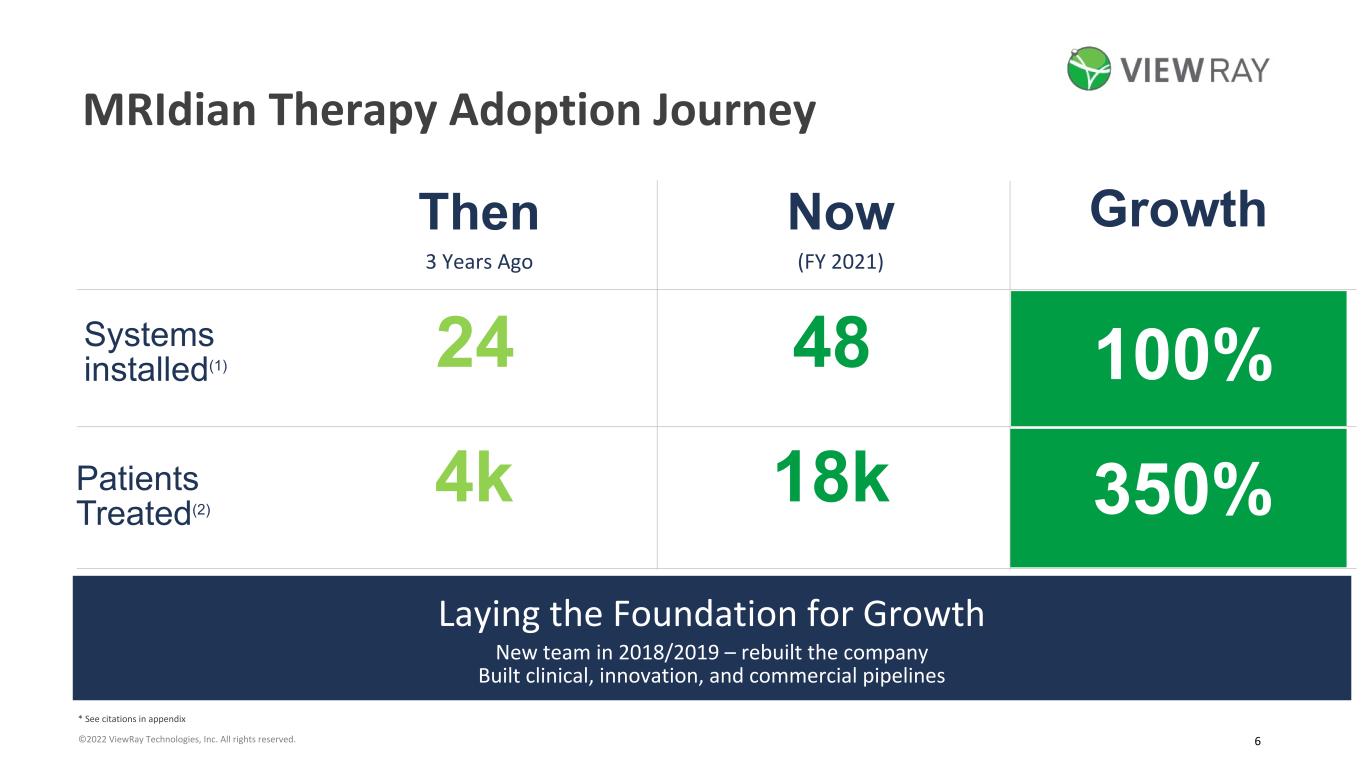
6©2022 ViewRay Technologies, Inc. All rights reserved. Thousands of patients with clinically reported outcomes. MRIdian Therapy Adoption Journey Systems installed(1) Patients Treated(2) Then 3 Years Ago Now (FY 2021) Growth 18k 48 4k 24 350% 100% Laying the Foundation for Growth New team in 2018/2019 – rebuilt the company Built clinical, innovation, and commercial pipelines * See citations in appendix

7©2022 ViewRay Technologies, Inc. All rights reserved. Trends in Cancer Care
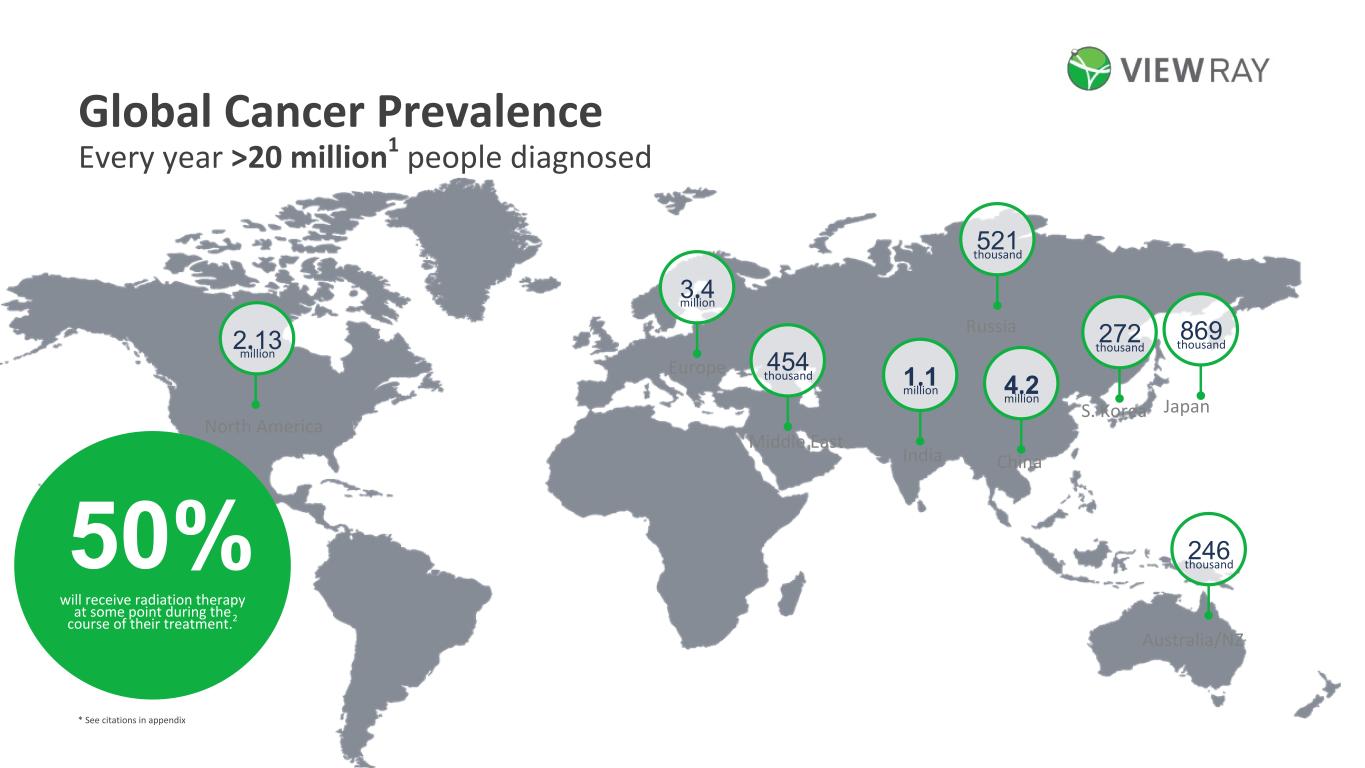
8©2022 ViewRay Technologies, Inc. All rights reserved. China Japan India Russia Middle East Europe S. Korea Australia/NZ North America 2.13 million 3.4 million 454 thousand 521 thousand 246 thousand 272 thousand 869 thousand 4.2 million 1.1 million 50% will receive radiation therapy at some point during the course of their treatment.2 Every year >20 million1 people diagnosed Global Cancer Prevalence * See citations in appendix

9©2022 ViewRay Technologies, Inc. All rights reserved. Radiation Oncology Market Opportunity ~$6.5B Global radiotherapy market in 2023 (projected)1 Installed base2 ~14,000 Replacment2 800/year De novo units 2 200/year ~1,000 – 1,250 LINAC units sold / year2 * See citations in appendix

10©2022 ViewRay Technologies, Inc. All rights reserved. MRIdian Paradigm Change is Clear * See citations in appendix
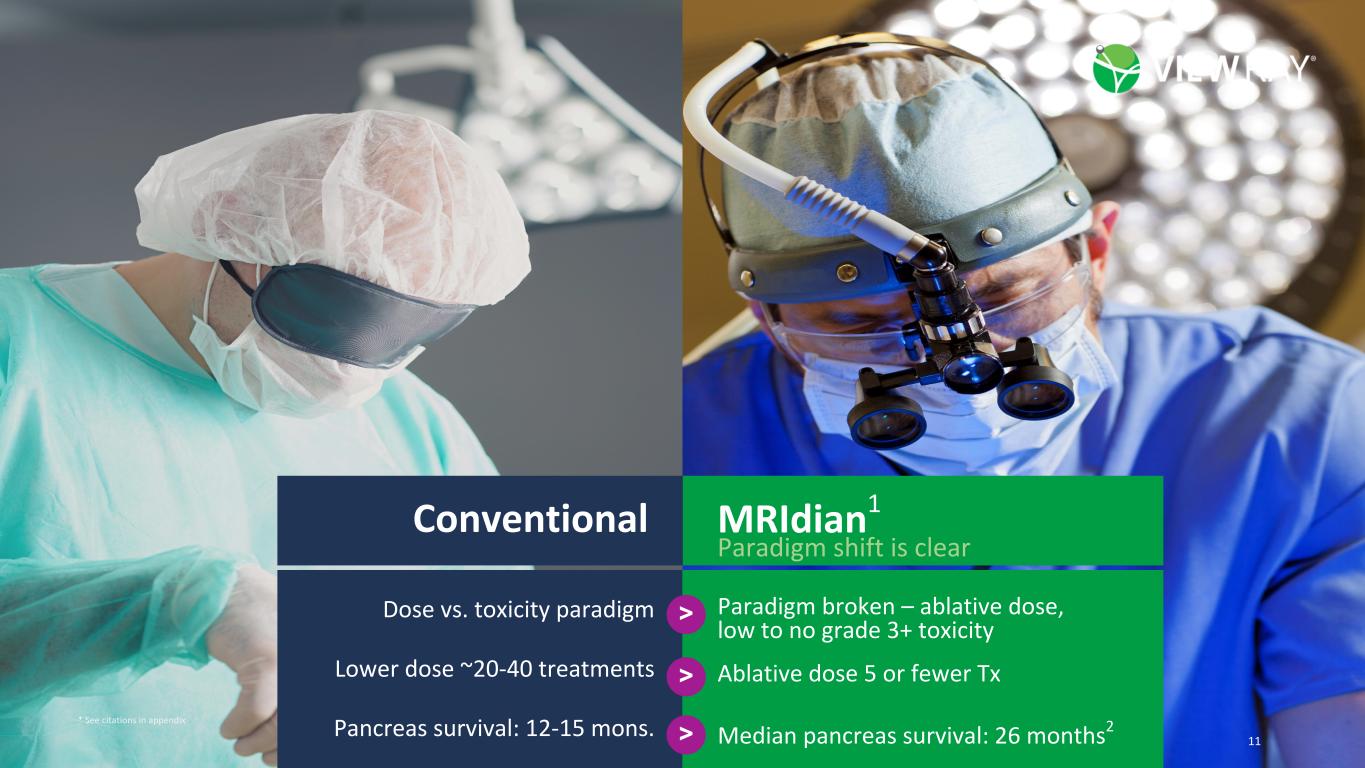
11©2022 ViewRay Technologies, Inc. All rights reserved. Dose vs. toxicity paradigm Lower dose ~20-40 treatments Pancreas survival: 12-15 mons. Paradigm broken – ablative dose, low to no grade 3+ toxicity Conventional MRIdian1 Paradigm shift is clear > > > Ablative dose 5 or fewer Tx Median pancreas survival: 26 months2 * See citations in appendix

12©2022 ViewRay Technologies, Inc. All rights reserved. Customers’ Definition of Clinical Success The MRIdian 51 Ablative dose 1 Tight margins 2 No fiducials 3 5 or fewer fractions 4 No to low grade 3+ toxicity 5 Among alternatives in the clinical landscape, we have found MRIdian’s evidence fits this criteria. * See citations in appendix
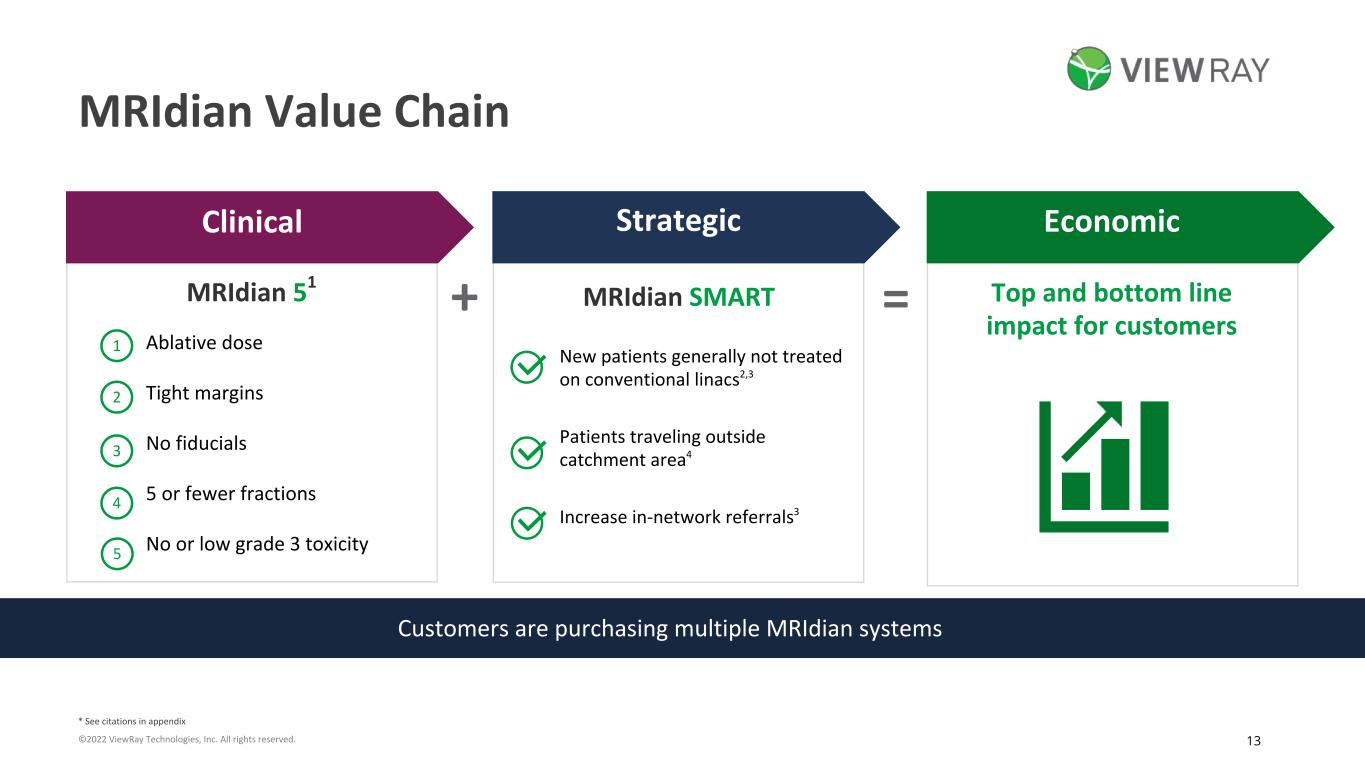
13©2022 ViewRay Technologies, Inc. All rights reserved. MRIdian Value Chain Customers are purchasing multiple MRIdian systems = Top and bottom line impact for customers +MRIdian 51 1 2 3 5 4 Ablative dose Tight margins No fiducials 5 or fewer fractions No or low grade 3 toxicity Clinical Strategic Economic New patients generally not treated on conventional linacs2,3 Patients traveling outside catchment area4 Increase in-network referrals3 MRIdian SMART * See citations in appendix
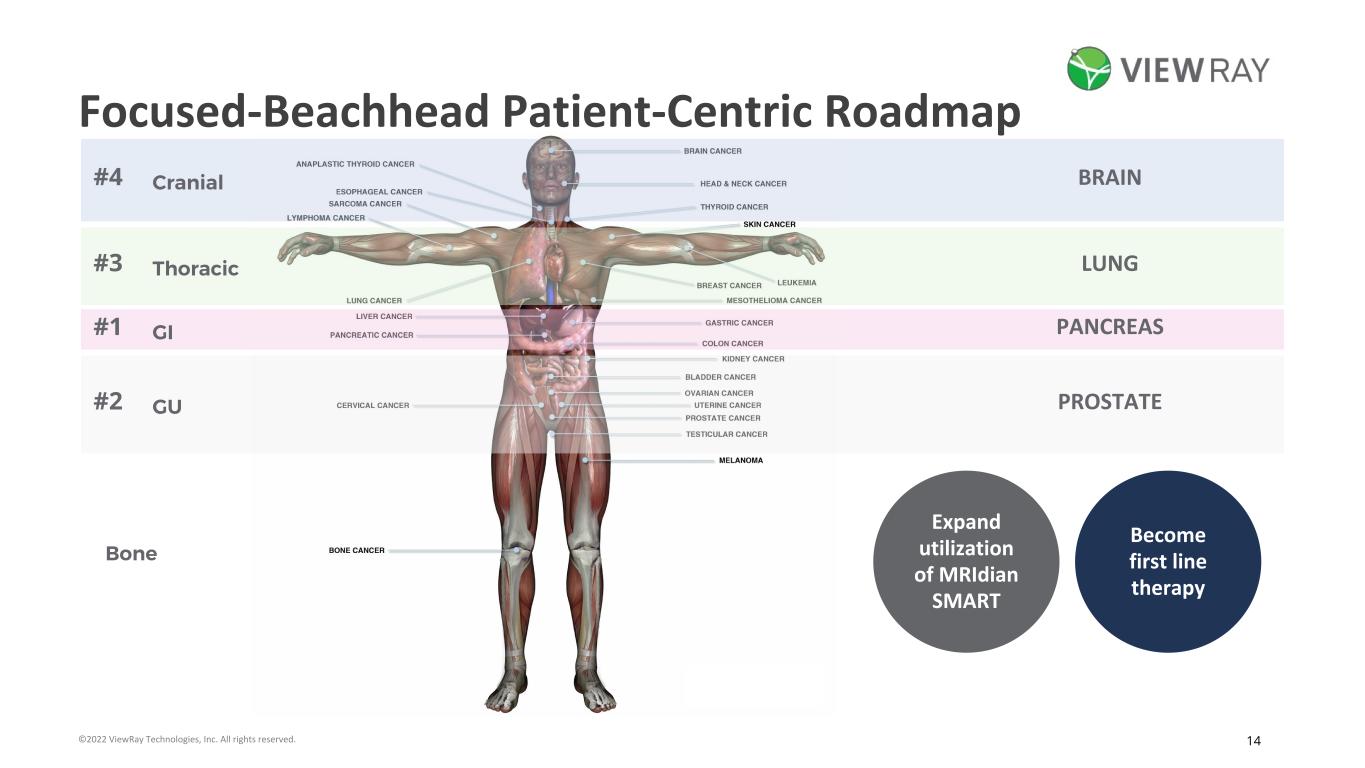
14©2022 ViewRay Technologies, Inc. All rights reserved. Focused-Beachhead Patient-Centric Roadmap Bone #1 #2 #3 #4 Expand utilization of MRIdian SMART Become first line therapy PANCREAS PROSTATE LUNG BRAIN GI GU Thoracic Cranial

15©2022 ViewRay Technologies, Inc. All rights reserved. MRIdian Clinical Pipeline Constant flow of proof to drive adoption • Prospective studies. Larger ‘N’ • Confirm signals in key tumor sites • Safety and Efficacy endpoints • Randomized controlled trials • Comparative • Safety and efficacy endpoints • 80+ investigator-initiated studies • Feasibility • Explore new indications/expand SMART • Reduce fx and improve workflow MIRAGE (CT vs. MRIdian prostate) PANCOSAR (SMART in medically inoperable pancreas) SMART Pancreas SHORTER (20 vs 5 fx post-op prostate) SCIMITAR (post-op SMART) Immunotherapy + SMART Lung STAAR (central/ultra central SMART) Pre-op Gastric SMILE (prostate SBRT German multi-cent) MARTHA (head and neck) MASPAC (late-stage pancreas multi-cent) Compassionate Access Program (pancreas) Radiomics Synthetic CT Workflow efficiency Prone Breast APBI Oligomets AI dose calculation DWI SMART ONE Sarcoma Lymphoma Cervical Rectal Liver mets SMART Master CONFIRM Phase 3 (Definitive) 3 Phase 2 (Confirmatory) 2 Phase 1 (Exploratory)1 * See citations in appendix

16©2022 ViewRay Technologies, Inc. All rights reserved. Recent Clinical Data Event Michael Chuong, MD, FACRO Vice Chair, Medical Director, Clinical Research Director, Lead GI Radiation Oncologist - Dept of Radiation Oncology at Miami Cancer Institute • 148 patients from 3 institutions ◦ All treated on MRIdian Linac from 2018-2020 ◦ Median dose 50 Gy in 5 fractions (BED10 = 100 Gy) ◦ No fiducial markers • 26-month median survival • 52.7% 2-year OS 2-year overall survival among patients with inoperable pancreatic cancer1,2 * See citations in appendix
17©2022 ViewRay Technologies, Inc. All rights reserved. Recent Clinical Data Event Evolution of prostate treatment1 Himanshu Nagar, MD Assistant Professor, Department of Radiation Oncology at Weill Cornell Medicine NewYork-Presbyterian • X-ray to MRI • Real-time MRI is future • SHORTER – Post-op Prostate ◦ 20fx vs 5fx ◦ Complimentary to surgery • FORT – Intact Prostate ◦ 5fx vs 2fx • 4X increase in prostate cancer patient volumes * See citations in appendix
MRIdian Target Demonstrate safety of MRIdian SMART to treat what others can’t. Prostate • MIRAGE – Released at ASCO GU in February 2022 • SCIMITAR & SHORTER – Demonstrate the safety of 5fx SBRT in post-op prostate • FORT - Redefine standard prostate treatment from 5fx to 2fx Mission: Improve patient quality of life Pancreas Extend to all pancreatic cancer patients and become an alternative to surgery (SMART Pancreas Trial) Mission: Safely extend survival rate Lung Mission: Safe treatment—curative intent Prove safety of MRIdian SMART in tough to treat tumors in patients with few options (LUNG STAAR) Meaningful Clinical Data1-3 18 * See citations in appendix

Newest Generation of Innovations – MRIdian A3i System •Workflow efficiency ◦ Streamline adaptive workflow ◦ Integrate auto-contouring ◦ Remote, parallel workflow ◦ Enable increased patient throughput • Clinical utility ◦ Brain treatment package ◦ New imaging and planning scans ◦ 3D multiplanar tracking and automated beam gating ◦ Improved patient experience • Compatibility ◦ Expanded OIS connectivity ◦ Enhanced cybersecurity *ViewRay stock images and videos Note: Not available for sale outside the United States. 19
20©2022 ViewRay Technologies, Inc. All rights reserved. Recent Highlights Innovation and clinical pipelines accrue customer traction Clinical ASTRO Pancreas Data Innovation 510(k) Clearance on MRIdian A3i System Commercial Customers purchasing multiple systems: TJU, Baptist Health South Florida and more in the pipeline +

21©2022 ViewRay Technologies, Inc. All rights reserved. Thousands of patients with clinically reported outcomes. MRIdian Therapy Adoption Journey Now (FY 2021) Future (2-4 years) 18k 48 2x (again) 4x (again) Positioned for Growth & Therapy Adoption • Innovation pipeline accelerating • Clinical data releases to build into future (MIRAGE, Lung STAAR, SCIMITAR, SHORTER, FORT, SMART Pancreas, SMART ONE, etc.) • Team in place to scale 4k 24 Then (3 years ago) * See citations in appendix Systems installed(1) Patients Treated(2) NOTE: Individual customer and patient results are illustrative only and are not predictive of future results

22

23 Appendix

24©2022 ViewRay Technologies, Inc. All rights reserved. Slide 6: 1. Internal and historical company data 2. 12/31/2021 MRIdian log data Slide 8: 3. https://jamanetwork.com/journals/jamaoncology/fullarticle/2787350 4. https://www.medsci.org/v09p0193.htm 5. https://pubmed.ncbi.nlm.nih.gov/16080176/ Slide 9: 6. Infoholic Research, 2018 Radiation Oncology Market, Drivers, Opportunities, Trends, and Forecasts: 2018-2024 7. IAEA/DIRAC Directory of Radiation Centers 2019, Internal reports and survey data Slide 10: 8. Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. 9. Aluwini, S., Pos, F., Schimmel, E., et al. (2015). Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Acute toxicity results from a randomised non inferiority phase 3 trial. Lancet Oncology, 16(3), 274–283. Dearnaley, D., Syndikus, I., Mossop, H., et al. (2016). Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the andomised, non-nferiority, phase 3 CHHiP trial. Lancet Oncology, 17(8), 1047–1060. Published correction appears in Lancet Oncology, 17(8), e321. Schneider, B. J., Daly, M. E., Kennedy, E. B., et al. (2018). Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: American Society of Clinical Oncology endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol, 36(7), 710–719. Toesca, D. A., Osmundson, E.C., Eyben, R. V., et al. (2017). Central liver toxicity after SBRT: An expanded analysis and predictive nomogram. Radiother Oncol, 122(1), 130–136. Herman, J.M., Chang, D.T., Goodman, K.A., et al. (2015). Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer, 121(7), 1128–1137. Palma, D. A., Olson, R., Harrow, S., et al. (2019). Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet, 393(10185):2051–2058. 10. Chuong MD, Bryant J, Mittauer KE, Hall M, Kotecha R, Alvarez D, Romaguera T, Rubens M, Adamson S, Godley A, Mishra V, Luciani G, Gutierrez AN, Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer, Practical Radiation Oncology (2020). 11. Finazzi T, Haasbeek CJA, Spoelstra FOB, Palacios MA, Admiraal MA, Bruynzeel AME, Slotman BJ, Lagerwaard FJ, Senan S, Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors International Journal of Radiation Oncology • Biology• Physics (2020); Rosenberg S. A. et al. (2018). A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Advances in Radiation Oncology, 4(1), 142-149; Rudra S. et al. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123-2132; Henke, L., et al. (2018). Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiotherapy and Oncology, 126(3), 519-526. Henke LE, et al. Stereotactic mr-guided online adaptive radiation therapy (smart) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol 2019;4:201-209; Rosenberg SA, et al. A multi- institutional experience of mr-guided liver stereotactic body radiation therapy. Adv Radiat Oncol 2019;4:142-149; Kennedy WR, Thomas MA, Stanley JA, Luo J, Ochoa LL, Clifton KK, Cyr AE, Margenthaler JA, DeWees TA, Price A, Kashani R, Green O, Zoberi I, Single Institution Phase I/II Prospective Clinical Trial of Single Fraction High Gradient Adjuvant Partial Breast Irradiation for Hormone Sensitive Stage 0-I Breast Cancer, International Journal of Radiation Oncology • Biology • Physics (2020); Bruynzeel AME, Tetar SU, Oei SS, Senan S, Haasbeek CJA, Spoelstra FOB, Piet AHM, Meijnen P, Bakker van der Jagt MAB, Fraikin T, Slotman BJ, van Moorselaar RJA, Lagerwaard FJ, A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results, International Journal of Radiation Oncology • Biology • Physics (2019). Slide 11 12. Ablative dose, Tight margins, No fiducials: Chuong MD, Bryant J, Mittauer KE, Hall M, Kotecha R, Alvarez D, Romaguera T, Rubens M, Adamson S, Godley A, Mishra V, Luciani G, Gutierrez AN, Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer, Practical Radiation Oncology (2020) Five or fewer factions, No or low grade 3 toxicity: Finazzi T, Haasbeek CJA, Spoelstra FOB, Palacios MA, Admiraal MA, Bruynzeel AME, Slotman BJ, Lagerwaard FJ, Senan S, Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors International Journal of Radiation Oncology • Biology• Physics (2020); Rosenberg S. A. et al. (2018). A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Advances in Radiation Oncology, 4(1), 142-149; Rudra S. et al. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123-2132; Henke, L., et al. (2018). Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiotherapy and Oncology, 126(3), 519-526. Henke LE, et al. Stereotactic mr-guided online adaptive radiation therapy (smart) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol 2019;4:201-209; Rosenberg SA, et al. A multi-institutional experience of mr-guided liver stereotactic body radiation therapy. Adv Radiat Oncol 2019;4:142-149; Kennedy WR, Thomas MA, Stanley JA, Luo J, Ochoa LL, Clifton KK, Cyr AE, Margenthaler JA, DeWees TA, Price A, Kashani R, Green O, Zoberi I, Single Institution Phase I/II Prospective Clinical Trial of Single Fraction High Gradient Adjuvant Partial Breast Irradiation for Hormone Sensitive Stage 0-I Breast Cancer, International Journal of Radiation Oncology • Biology • Physics (2020); Bruynzeel AME, Tetar SU, Oei SS, Senan S, Haasbeek CJA, Spoelstra FOB, Piet AHM, Meijnen P, Bakker van der Jagt MAB, Fraikin T, Slotman BJ, van Moorselaar RJA, Lagerwaard FJ, A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results, International Journal of Radiation Oncology • Biology • Physics (2019) 13. Choung, M.D., et al. Long-Term Multi-Institutional Outcomes of 5-Fraction Ablative Stereotactic MR-Guided Adaptive Radiation Therapy (SMART) for Inoperable Pancreas Cancer With Median Prescribed Biologically Effective Dose of 100 Gy10 [abstract]. In: Proceedings of the 2021 Annual Meeting of the American Society for Radiation Oncology.; 2021 Oct 24-27; Chicago, IL, USA. Arlington, VA: ASTRO; 2021. Abstract nr 1106. Abstract retrieved from https://plan.core-apps.com/myastroapp2021/abstract/16c0367a-e1bf-415d- b026-66bca5cf7b14. NOTE: Individual customer and patient results are illustrative only and are not predictive of future results Presentation Citations

25©2022 ViewRay Technologies, Inc. All rights reserved. Slide 12 1. Ablative dose, Tight margins, No fiducials: Chuong MD, Bryant J, Mittauer KE, Hall M, Kotecha R, Alvarez D, Romaguera T, Rubens M, Adamson S, Godley A, Mishra V, Luciani G, Gutierrez AN, Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer, Practical Radiation Oncology (2020) Five or fewer factions, No or low grade 3 toxicity: Finazzi T, Haasbeek CJA, Spoelstra FOB, Palacios MA, Admiraal MA, Bruynzeel AME, Slotman BJ, Lagerwaard FJ, Senan S, Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors International Journal of Radiation Oncology • Biology• Physics (2020); Rosenberg S. A. et al. (2018). A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Advances in Radiation Oncology, 4(1), 142-149; Rudra S. et al. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123-2132; Henke, L., et al. (2018). Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiotherapy and Oncology, 126(3), 519-526. Henke LE, et al. Stereotactic mr-guided online adaptive radiation therapy (smart) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol 2019;4:201-209; Rosenberg SA, et al. A multi-institutional experience of mr-guided liver stereotactic body radiation therapy. Adv Radiat Oncol 2019;4:142-149; Kennedy WR, Thomas MA, Stanley JA, Luo J, Ochoa LL, Clifton KK, Cyr AE, Margenthaler JA, DeWees TA, Price A, Kashani R, Green O, Zoberi I, Single Institution Phase I/II Prospective Clinical Trial of Single Fraction High Gradient Adjuvant Partial Breast Irradiation for Hormone Sensitive Stage 0-I Breast Cancer, International Journal of Radiation Oncology • Biology • Physics (2020); Bruynzeel AME, Tetar SU, Oei SS, Senan S, Haasbeek CJA, Spoelstra FOB, Piet AHM, Meijnen P, Bakker van der Jagt MAB, Fraikin T, Slotman BJ, van Moorselaar RJA, Lagerwaard FJ, A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results, International Journal of Radiation Oncology • Biology • Physics (2019) Slide 13 1. - Henke, L., et al. (2018). Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiotherapy and Oncology, 126(3), 519-526; Henke LE, et al. Stereotactic mr-guided online adaptive radiation therapy (smart) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol 2019;4:201-209; - Rosenberg SA, et al. A multi-institutional experience of mr-guided liver stereotactic body radiation therapy. Adv Radiat Oncol 2019;4:142-149; - Finazzi T, Haasbeek CJA, Spoelstra FOB, Palacios MA, Admiraal MA, Bruynzeel AME, Slotman BJ, Lagerwaard FJ, Senan S, Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors International Journal of Radiation Oncology • Biology• Physics (2020); - Rudra S. et al. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123-2132; Finazzi, et al. Role of on-table plan adaptation in MR-guided ablative radiation therapy for central lung tumors. Int J Radiat Oncol Biol Phys. 2019 Jul 15;104(4):933-941. doi: 10.1016/j.ijrobp.2019.03.035. Epub 2019 Mar 28; - Chuong, M.D., Bryant, J., Mittauer, K.E., Hall, M., Kotecha, R., Alvarez, D., et al. (2020). Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas - Hassanzadeh, C., Rudra, S., Bommireddy, A., Hawkins, W.G., Wang-Gillam, A., Fields, R.C., et al. (2020) Ablative Five-Fraction Stereotactic Body Radiotherapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Advances in Radiation Oncology, Advance online publication. - Kennedy WR, Thomas MA, Stanley JA, Luo J, Ochoa LL, Clifton KK, Cyr AE, Margenthaler JA, DeWees TA, Price A, Kashani R, Green O, Zoberi I, Single Institution Phase I/II Prospective Clinical Trial of Single Fraction High Gradient Adjuvant Partial Breast Irradiation for Hormone Sensitive Stage 0-I Breast Cancer, International Journal of Radiation Oncology • Biology • Physics (2020); - Henke, L., et al. (2018). Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiotherapy and Oncology, 126(3), 519-526; - Bruynzeel AME, Tetar SU, Oei SS, Senan S, Haasbeek CJA, Spoelstra FOB, Piet AHM, Meijnen P, Bakker van der Jagt MAB, Fraikin T, Slotman BJ, van Moorselaar RJA, Lagerwaard FJ, A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results, International Journal of Radiation Oncology • Biology • Physics (2019); - Tetar, S., Bruynzeel, A., Oei, S., Senan, S., Fraikin T., Slotman, B. et al. (2020) Magnetic Resonance-guided stereotactic radiotherapy for localized prostate cancer: final results on patient-reported outcomes of a prospective phase 2 study. Eu Urology Oncology epub June 12, 2020. - Witt, J., et al. (2020). MRI-guided adaptive radiotherapy for liver tumours: visualizing the future. Lancet Oncol 2020; 21:e74-82. - Finazzi, T., van Sörnsen de Koste, J.R., Palacios, M.A., Spoelstra, F.O.B., Slotman, B.J., Haasbeek, C.J.A., Senan, S. (2020). Delivery of magnetic resonance-guided single-fraction stereotactic lung radiotherapy. Physics and Imaging in Radiation Oncology, 14, P17-23. 2. “Day One, All-Day Adaptive: Dana-Farber/Brigham and Women's Cancer Center Maximizes Clinical Value of MRIdian,” 7/23/20 3.Dr. Nagar, Cornell University, October 2020. 4.Dr. Michael Choung, Miami Cancer Institute. “Physician-led Webinar for Investors and Analysts at 2020 ASTRO Annual Meeting”, 10/27/2020. https://investors.viewray.com/events/event-details/physician-led-webinar-investors-and- analysts-2020-astro-annual-meeting. Presentation Citations

26©2022 ViewRay Technologies, Inc. All rights reserved. Presentation Citations Slide 15 and Slide 18 1. Phase 1 (Exploratory) - SMART ONE: Miami Cancer Institute, PI: Dr. Michael Chuong, “Stereotactic MRI-Guided Adaptive Radiation Therapy Delivered in One Fraction for Inoperable Primary or Metastatic Carcinoma (SMART One)” - SMART Master: https://clinicaltrials.gov/ct2/show/NCT04115254 - CONFIRM: https://clinicaltrials.gov/ct2/show/NCT04368702 2. Phase 2 (Confirmatory) - SMART Pancreas: https://clinicaltrials.gov/ct2/show/NCT03621644 - SHORTER: https://clinicaltrials.gov/ct2/show/NCT04422132 - SCIMITAR: https://clinicaltrials.gov/ct2/show/NCT03541850 - Immunotherapy + SMART: https://clinicaltrials.gov/ct2/show/NCT04376502 - LUNG STAAR: LUNG STAAR: Miami Cancer Institute, PI: Dr. Rupesh Kotecha, “Phase II Study of Stereotactic MR Guided Adaptive Radiotherapy for Central and Ultra-central Lung Tumors” - Pre-op Gastric: https://clinicaltrials.gov/ct2/show/NCT04162665 - SMILE: Heidelberg University, PI: Dr. Stefan Korber, “Stereotactic MRI-guided radiation therapy for Localized prostate cancer (SMILE)” - MARTHA: https://clinicaltrials.gov/ct2/show/NCT03972072 - MASPAC: University of Zurich, PI: Dr. Matea Pavic, “MR-guided Adaptive Stereotactic Body Radiotherapy (SBRT) of primary tumor for pain control in metastatic Pancreatic ductal adenocarcinoma (mPDAC) – a multicenter randomized, controlled, open-label, phase IIb trial (MASPAC Study)” - Compassionate Access Program: https://www.genesiscare.com/uk/compassionate-access-programme/ 3. Phase 3 (Definitive) - MIRAGE: https://clinicaltrials.gov/ct2/show/NCT04384770 - PANCOSAR: https://www.oncologie.nu/nieuws/stereotactische-radiotherapie-voor-kwetsbare-pati%C3%ABnten-met-lokaal-pancreascarcinoom/ Slide 16 1. Choung, M.D., et al. Long-Term Multi-Institutional Outcomes of 5-Fraction Ablative Stereotactic MR-Guided Adaptive Radiation Therapy (SMART) for Inoperable Pancreas Cancer With Median Prescribed Biologically Effective Dose of 100 Gy10 [abstract]. In: Proceedings of the 2021 Annual Meeting of the American Society for Radiation Oncology.; 2021 Oct 24-27; Chicago, IL, USA. Arlington, VA: ASTRO; 2021. Abstract nr 1106. Abstract retrieved from https://plan.core- apps.com/myastroapp2021/abstract/16c0367a-e1bf-415d-b026-66bca5cf7b14. NOTE: Individual customer and patient results are illustrative only and are not predictive of future results 2. Rudra, S., Jiang, N., Rosenberg, S. A., Olsen, J. R., Roach, M. C., Wan, L., … Lee, P. P. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123– 2132. doi: 10.1002/cam4.2100 Slide 17 3. Nagar, Himanshu. Weill Medical College of Cornell University (U.S.). (2020, Sept 24 - ). Randomized Phase II Trial of Salvage Radiotherapy for Prostate Cancer In 4 Weeks v. 2 Weeks. Identifier NCT04422132. https://clinicaltrials.gov/ct2/ show/NCT04422132. Nagar, Himanshu. Weill Medical College of Cornell University (U.S.). (2021, Nov 1 - ). Randomized Phase II Trial of Five or Two MRI-Guided Adaptive Radiotherapy Treatments for Prostate Cancer. Identifier NCT04984343. https://clinicaltrials.gov/ct2/show/NCT04984343. Kishan, Amar. UCLA / Jonsson Comprehensive Cancer Center (U.S.). (2020, May 12 - ). Magnetic Resonance Imaging-Guided Stereotactic Body Radiotherapy for Prostate Cancer (Mirage): A Phase III Randomized Trial. Identifier NCT04384770. https://clinicaltrials.gov/ct2/show/NCT04384770. Kishan, Amar. UCLA / Jonsson Comprehensive Cancer Center (U.S.). (2019, Jan 29 - ). Prospective Study of Stereotactic Body Radiotherapy (SBRT) Following Radical Prostatectomy. Identifier NCT03541850. https://clinicaltrials.gov/ct2/show/NCT03541850. Slide 21 4. Internal and historical company data 5. 12/31/2021 MRIdian log data 6. NOTE: Individual customer and patient results are illustrative only and are not predictive of future results

























