
Earnings Call – Q3 2022 November 1, 2022 Exhibit 99.2

2©2022 ViewRay Technologies, Inc. All rights reserved. Forward-looking statement This presentation contains forward-looking statements within the meaning of Section 27A of the Private Securities Litigation Reform Act. Statements in this presentation that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, ViewRay's financial guidance for the full year 2022, anticipated future orders, anticipated future operating and financial performance, treatment results, therapy adoption, innovation, and the performance of the MRIdian systems. Actual results could differ from those projected in any forward-looking statements due to numerous factors. Such factors include, among others, the ability to commercialize the MRIdian Linac System, demand for ViewRay's products, the ability to convert backlog into revenue, the timing of delivery of ViewRay's products, the timing, length, and severity of the COVID-19 pandemic, including its impacts across our businesses on demand, our operations and global supply chains, disruptions in the supply or changes in costs of raw materials, labor, product components or transportation services as a result of inflation, the results and other uncertainties associated with clinical trials, the ability to raise the additional funding needed to continue to pursue ViewRay's business and product development plans, the inherent uncertainties associated with developing new products or technologies, competition in the industry in which ViewRay operates, and overall market conditions. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to ViewRay's business in general, see ViewRay's current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2021 and its Quarterly Reports on Form 10-Q, as updated periodically with the Company's other filings with the SEC. These forward-looking statements are made as of the date of this presentation, and ViewRay assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Individual customer and patient results are illustrative only and are not predictive of future results. The opinions and clinical experiences presented herein are specific to the featured physicians and the featured patients and are for information purposes only. Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations. ViewRay issued a press release and presentation for today’s call. The presentation can be viewed live on the webcast or downloaded from the “financial events and webinars” portion of our website at www.investors.viewray.com. The call is being broadcast and webcast live, and a replay will be available for 14 days. Listeners are cautioned that comments made by management during this presentation may include forward-looking statements within the meaning of federal securities laws. These statements involve material risks and uncertainties, and actual results could differ from those projected in any forward-looking statement due to numerous factors. For a description of these risks and uncertainties, please see ViewRay's Annual Report on Form 10-K for the fiscal year ended December 31, 2021, and its Quarterly Reports on Form 10-Q, as updated periodically with the company's other SEC filings. Furthermore, the content of this conference call contains time-sensitive information accurate only as of today, November 1, 2022. ViewRay undertakes no obligation to revise or otherwise update any statements to reflect events or circumstances after the date of this presentation. Medical advice disclaimer: ViewRay is a medical device manufacturer and cannot and does not recommend specific treatment approaches. Individual results may vary. Financial disclosure: Dr. Chuong has received consulting fees and research grants from ViewRay, Inc. and serves on the Medical Advisory Board of ViewRay, Inc. Forward-looking statements and disclaimer
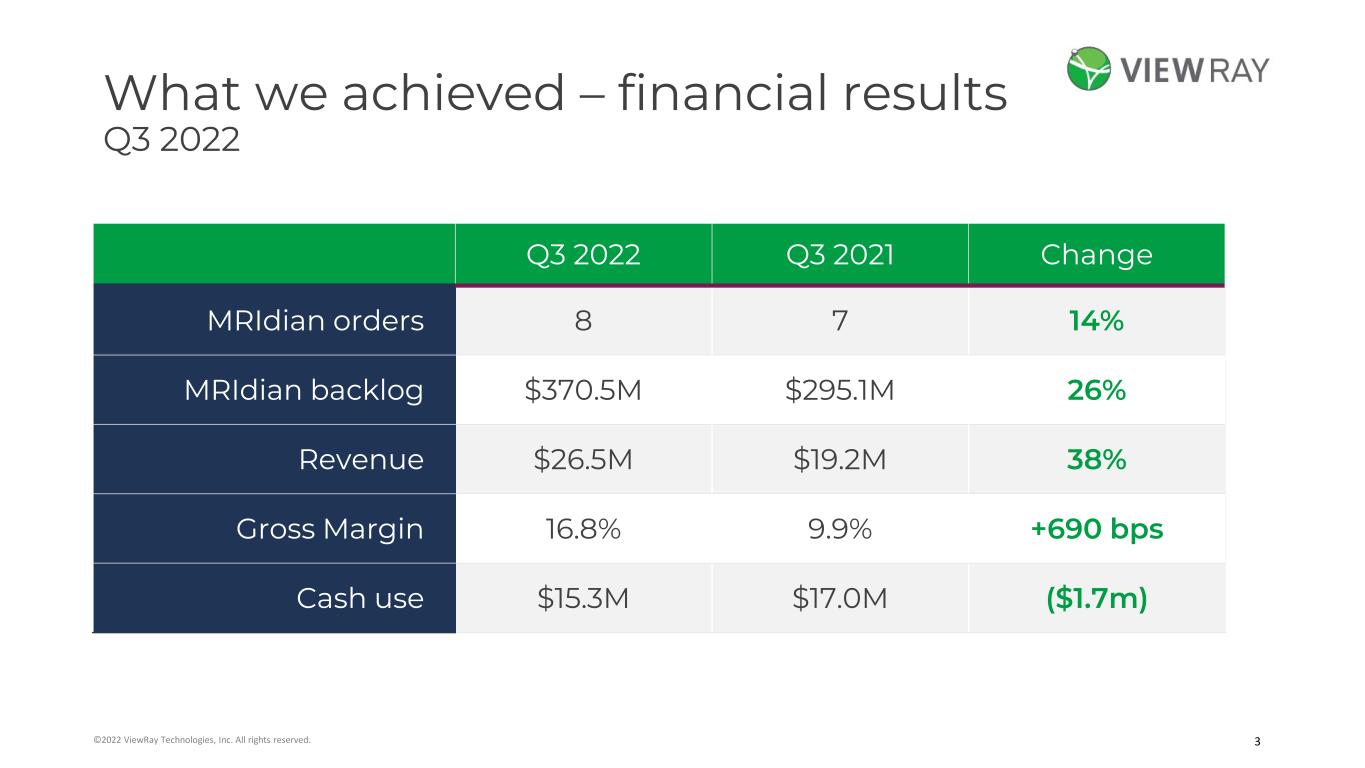
3©2022 ViewRay Technologies, Inc. All rights reserved. What we achieved – financial results Q3 2022 Q3 2022 Q3 2021 Change MRIdian orders 8 7 14% MRIdian backlog $370.5M $295.1M 26% Revenue $26.5M $19.2M 38% Gross Margin 16.8% 9.9% +690 bps Cash use $15.3M $17.0M ($1.7m)
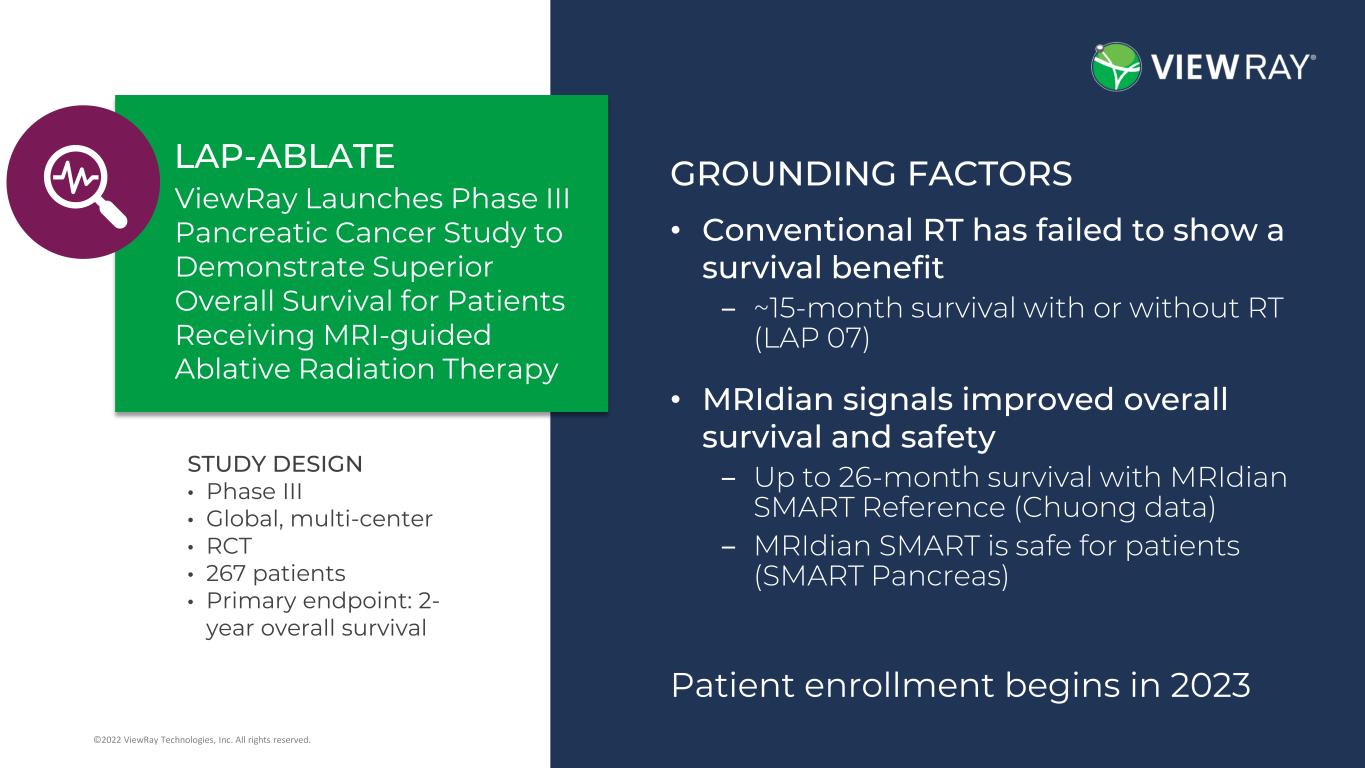
4©2022 ViewRay Technologies, Inc. All rights reserved. • Phase 3 • Global • Multi-center • RCT • 267 patients • 2 year Overall survival primary endpoint Enrollment to begin in 2023 Study design Patient enrollment begins in 2023 GROUNDING FACTORS • Conventional RT has failed to show a survival benefit ‒ ~15-month survival with or without RT (LAP 07) • MRIdian signals improved overall survival and safety ‒ Up to 26-month survival with MRIdian SMART Reference (Chuong data) ‒ MRIdian SMART is safe for patients (SMART Pancreas) STUDY DESIGN • Phase III • Global, multi-center • RCT • 267 patients • Primary endpoint: 2- year overall survival ViewRay Launches Phase III Pancreatic Cancer Study to Demonstrate Superior Overall Survival for Patients Receiving MRI-guided Ablative Radiation Therapy LAP-ABLATE
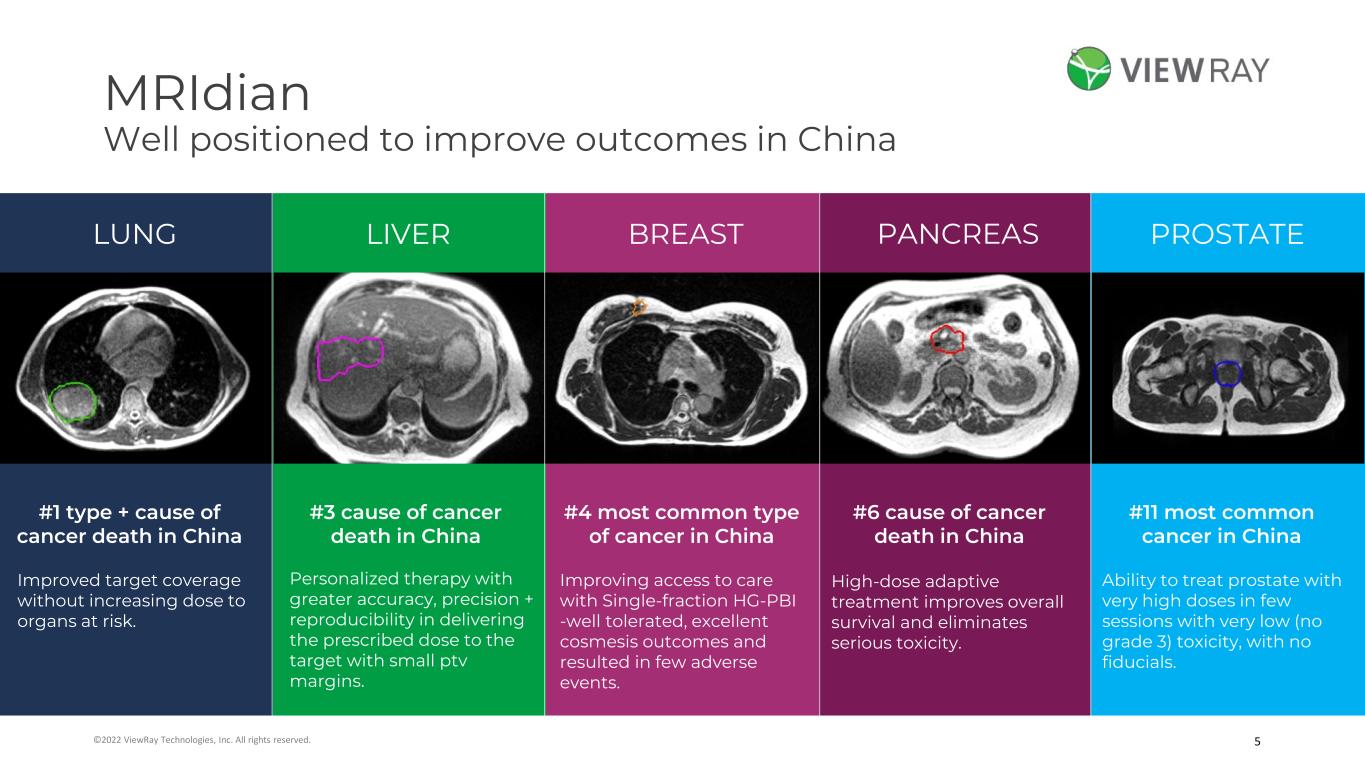
5©2022 ViewRay Technologies, Inc. All rights reserved. MRIdian Well positioned to improve outcomes in China LUNG LIVER PROSTATEPANCREASBREAST #1 type + cause of cancer death in China #3 cause of cancer death in China #4 most common type of cancer in China #6 cause of cancer death in China #11 most common cancer in China High-dose adaptive treatment improves overall survival and eliminates serious toxicity. Ability to treat prostate with very high doses in few sessions with very low (no grade 3) toxicity, with no fiducials. Improving access to care with Single-fraction HG-PBI -well tolerated, excellent cosmesis outcomes and resulted in few adverse events. Personalized therapy with greater accuracy, precision + reproducibility in delivering the prescribed dose to the target with small ptv margins. Improved target coverage without increasing dose to organs at risk.

Financial results Q3 2022
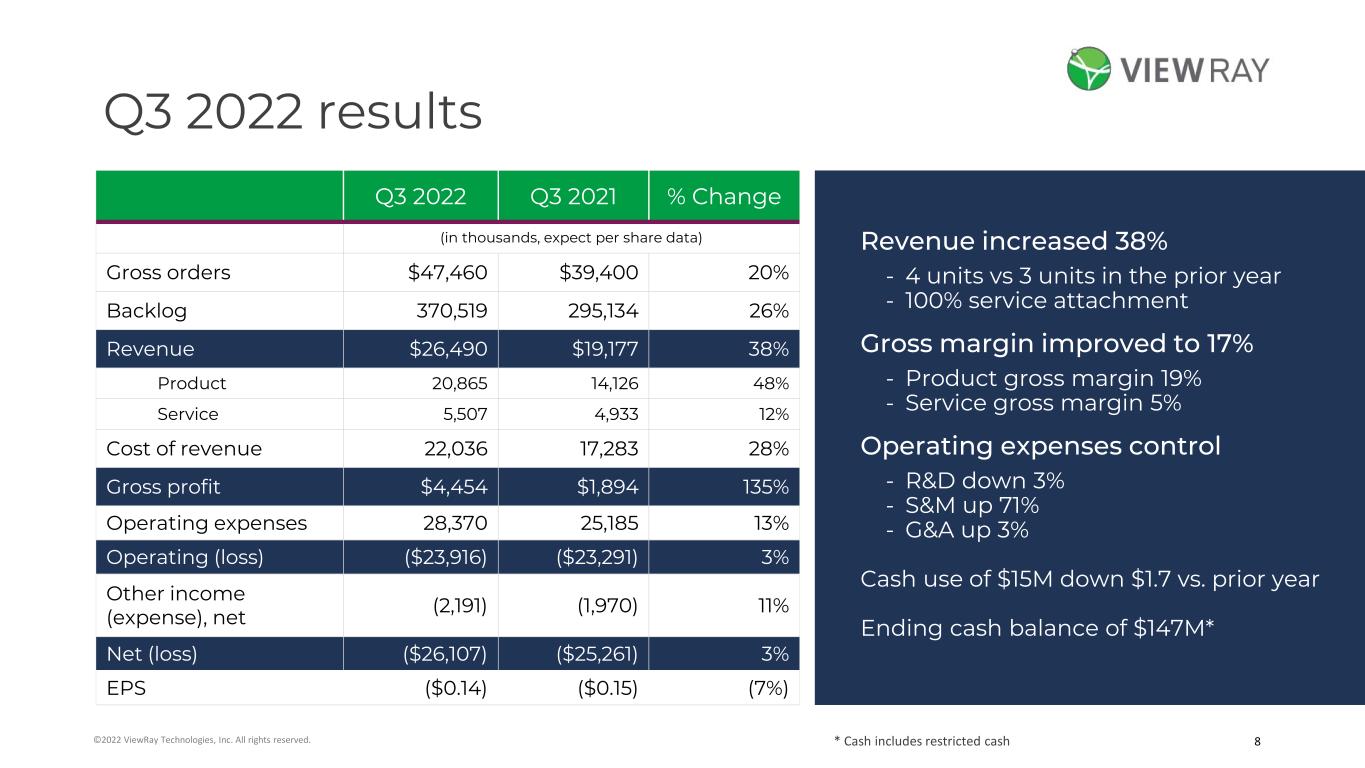
8©2022 ViewRay Technologies, Inc. All rights reserved. Revenue increased 38% - 4 units vs 3 units in the prior year - 100% service attachment Gross margin improved to 17% - Product gross margin 19% - Service gross margin 5% Operating expenses control - R&D down 3% - S&M up 71% - G&A up 3% Cash use of $15M down $1.7 vs. prior year Ending cash balance of $147M* Q3 2022 Q3 2021 % Change (in thousands, expect per share data) Gross orders $47,460 $39,400 20% Backlog 370,519 295,134 26% Revenue $26,490 $19,177 38% Product 20,865 14,126 48% Service 5,507 4,933 12% Cost of revenue 22,036 17,283 28% Gross profit $4,454 $1,894 135% Operating expenses 28,370 25,185 13% Operating (loss) ($23,916) ($23,291) 3% Other income (expense), net (2,191) (1,970) 11% Net (loss) ($26,107) ($25,261) 3% EPS ($0.14) ($0.15) (7%) * Cash includes restricted cash Q3 2022 results
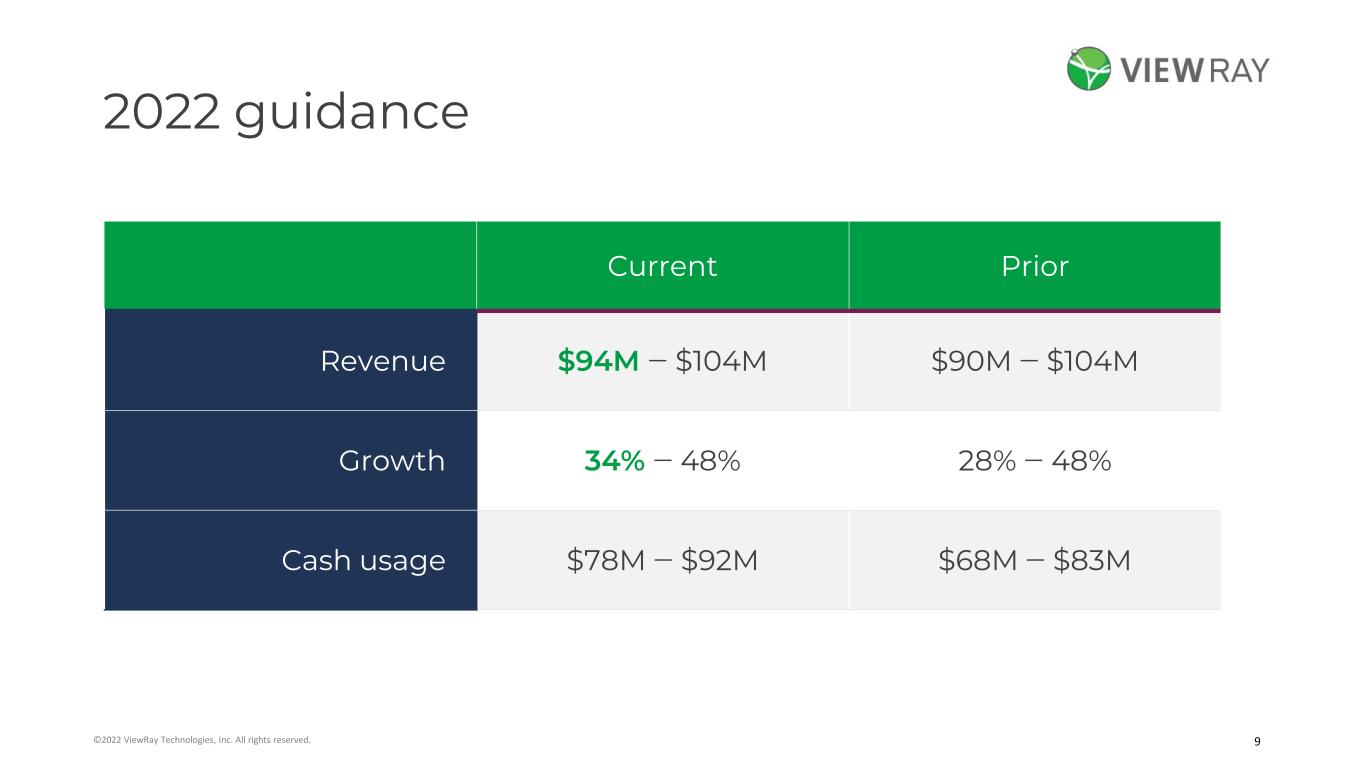
9©2022 ViewRay Technologies, Inc. All rights reserved. 2022 guidance Current Prior Revenue $94M ‒ $104M $90M ‒ $104M Growth 34% ‒ 48% 28% ‒ 48% Cash usage $78M ‒ $92M $68M ‒ $83M

10©2022 ViewRay Technologies, Inc. All rights reserved. Delivering P&L leverage ‘22 and beyond Meaningful operating expense leverage… 2.3x YTD On track to deliver ~40% revenue growth… 36% YTD Well capitalized: sufficiently capitalized to drive to cash flow breakeven In 2022… Placements expected to drive 750 - 1,000 bps of gross margin expansion… +725 bps YTD

11©2022 ViewRay Technologies, Inc. All rights reserved. ViewRay Investor Day Intercontinental Hotel New York, NY November 17, 2022

VISIBLY BETTER 12

13©2022 ViewRay Technologies, Inc. All rights reserved. References 1Rudra S. et al. (2019). Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Medicine, 8(5), 2123-2132. 2Chen A. M. et al. (2018). MRI-guided radiotherapy for head and neck cancer: initial clinical experience. Clinical Translational Oncology, 20(2), 160-168. 3Henke L, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (smart) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol 2018;126:519-526. 4Rosenberg, S. A., et al. (2019). A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Advances in Radiation Oncology, 4(1), 142-149. 5Bruynzeel A. M. E. et al. (2019). A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results. International Journal of Radiation Oncology*Biology*Physics, in press. 6Kennedy W. R., et al. (2018). Postoperative single-fraction partial breast irradiation for low-risk stage 0 and I breast carcinomas: results of a prospective clinical trial. Radiation Oncology, 102(3), S227-S228. 7Global Cancer Observatory. (2018, August). Cancer today. Retrieved from https://gco.iarc.fr/today 8China Med Device. (2018, May). Every minute, 4 people die of cancer in China. Retrieved from https://chinameddevice.com/seven-cancer-patient- diagnosed-every-minute-in-china/ 9Dargan, R. (2017, November). Opportunities abound for radiation oncology in the era of personalized medicine, RSNA: Daily Bulletin, https://rsna2017.rsna.org/dailybulletin/index.cfm?pg=17thu01 10Finazzi, T., et al. (2019). Role of on-table plan adaptation in MR-guided ablative radiation therapy for central lung tumors. International Journal of Radiation Oncology*Biology*Physics, 104(4), 933-941.












