J.P. Morgan Healthcare Conference January 2025 Exhibit 99.1

Safe Harbor Statement The slides presented today and the accompanying oral presentations contain forward-looking statements, which may be identified by the use of words such as “may,” “might,” “will,” “should,” “can,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “opportunity”, “goal”, “mission”, "vision", “potential,” “target”, or “continue,” and other similar expressions. Forward-looking statements in this presentation include statements regarding: plans, expectations, strategy and goals for commercialization of ZURZUVAE as a treatment for women with PPD, including our goal for ZURZUVAE to become first line therapy and standard of care in this indication, plans to scale and accelerate growth of ZURZUVAE in PPD, our plans to increase investment in ZURZUVAE to help accelerate market and topline revenue growth in 2025 and our overall expectations on the impact of such increased investment, including expectations regarding salesforce expansion, future media campaigns, and disease state awareness efforts and related impacts, expectations on reimbursement and access, and plans and goals related to other aspects of commercialization; our belief in the potential benefit and profile of ZURZUVAE for the treatment of women with PPD; the potential for success of our commercialization of ZURZUVAE for the treatment of women with PPD and our belief in the size of the potential market opportunity in PPD and the role of ZURZUVAE in unlocking such potential; our clinical development plans and expectations, including expected timelines for data read-outs and other activities, such as the expected timing of readout of the multiple ascending dose study for SAGE-319; our plans to apply learnings and advance a recalibrated and focused R&D approach; our plans to evaluate potential indications for our product candidates, including SAGE-324, and our expected announcement of next steps regarding the SAGE-324 program; our plans to explore targeted early discovery work within our NMDA NAMs platform; our belief in the potential profile and benefit of our product candidates, potential indications for our product candidates, the potential for success of our programs, and the opportunity to help patients in various indications; our estimates as to the number of patients with disorders and diseases of interest; the potential drivers of value for our business; the opportunity, mission, goals, core priorities, and vision for our business; and our expectations with respect to our cash runway and our anticipated reduction in operating expenses in 2025 relative to 2024, including the impact of the 2024 strategic reorganization, and maintaining a strong financial focus. These forward-looking statements are neither promises nor guarantees of future performance, and are subject to a variety of risks and uncertainties, many of which are beyond our control, which could cause actual results to differ materially from those contemplated in these forward-looking statements, including the risk that: We may not be successful in our commercialization efforts with respect to ZURZUVAE for the treatment of women with PPD; the market size and market acceptance for ZURZUVAE in PPD by healthcare professionals, patients and payors may be significantly smaller than we expect; we may encounter reimbursement, market access, process-related or other issues in the course of our commercialization activities, including competition in the market; early positive signs, including ZURZUVAE results in 2024, may not be a signal of future success; ZURZUVAE may not achieve the clinical benefit for the treatment of PPD that we expect; we may be unsuccessful in driving ZURZUVAE growth in 2025, including as a result of our plans for increased investment; we may not generate revenue from sales of ZURZUVAE at the levels or on the timing we expect, or meet our other goals for market access, sales and marketing, customer support, or distribution strategies. Our clinical trials may not meet their primary endpoints or key secondary endpoints. Success in nonclinical studies or in prior clinical trials of our product candidates may not be repeated or observed in ongoing, planned or future studies involving the same compound or other product candidates. Non-clinical and clinical results from ongoing or future trials may not support further development of the product candidate, our planned regulatory pathway, or filing for or obtaining regulatory approval on the timelines we expect or at all and we may be required to conduct additional clinical trials or nonclinical studies which may not be feasible or successful. We may encounter delays in initiation, conduct, completion of enrollment or completion and reporting of data with respect to any of our ongoing clinical trials, such as the completion of the multiple ascending dose study for SAGE-319, including as a result of slower than expected site initiation, slower than expected enrollment, the need or decision to expand the trials or other changes, that may impact our ability to meet our expected timelines and may increase our costs. We may encounter unexpected safety or tolerability issues with respect to any of our product candidates or marketed products; we may encounter different or more severe adverse events at higher doses, different frequency or length of dosing or in new indications. At any stage, regulatory authorities may ask for additional clinical trials, nonclinical studies or other data in order for us to proceed further in development or to file for or obtain regulatory approval. Other decisions or actions of the FDA or other regulatory authorities may affect the initiation, timing, design, size, progress and cost of clinical trials or development efforts and our ability to proceed with further development or gain regulatory approval of products beyond ZURZUVAE and ZULRESSO. Even if our other product candidates are successfully developed and approved, the number of patients with the diseases or disorders our products treat or the subset of such patients we believe will use our products, the need for new treatment options, and the actual market for such products may be smaller than our current estimates. The anticipated benefits of our collaborations, including our collaboration with Biogen, may never be achieved. The need to align with our collaborators may hamper or delay our development and commercialization efforts or increase our costs; our business may be adversely affected, and our costs may increase if any of our key collaborators fails to perform its obligations or terminates our collaboration. We may not be able to obtain and maintain adequate intellectual property protection or other forms of data and marketing exclusivity for our products, or to defend our patent portfolio against challenges from third parties. We may face competition from others developing products or with approved products for similar uses as those for which our product candidates are being developed. Our operating expenses may be higher than forecasted and we may face unexpected expenses which could cause us to use our cash faster or change our plans or both. Also, we may not achieve anticipated cost savings from our October 2024 reorganization and pipeline prioritization efforts at the levels we expect. Our revenues may be lower than we expect, including if we do not achieve market acceptance of ZURZUVAE for the treatment of women with PPD or if we do not achieve our access/reimbursement goals in this indication, or if our launch for other reasons is not as successful as we expect which may cause us to not achieve our cash runway expectations. We may not achieve expected milestones that trigger cash payments on the timing we expect, or at all. We may be opportunistic in our future financing plans even if available cash is sufficient or additional funding may not be available on acceptable terms, or at all. For these and other reasons, our expectations with respect to cash, expenses and financial strength may not prove to be accurate. We may not be able to establish and maintain key business relationships with third parties on acceptable terms or we may encounter problems with the performance of such third parties. We may encounter technical and other unexpected hurdles in the manufacture, development or commercialization of our products. Any of the foregoing or other factors may negatively impact our ability to achieve our goals, mission, vision, opportunities, plans or expectations for our business and the potential for value creation. For additional disclosure regarding these and other risks Sage faces, see the disclosure contained in the "Risk Factors" section of our most recent report, and in our other public filings, with the Securities and Exchange Commission, available on the SEC's website at http://www.sec.gov. Any forward-looking statement represents our views only as of today and should not be relied upon as representing our views as of any subsequent date. We undertake no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise.

person can thrive OUR VISION Fearlessly lead the way to create a world with better brain health. OUR MISSION Pioneer solutions to deliver life-changing brain health medicines, so every person can thrive.
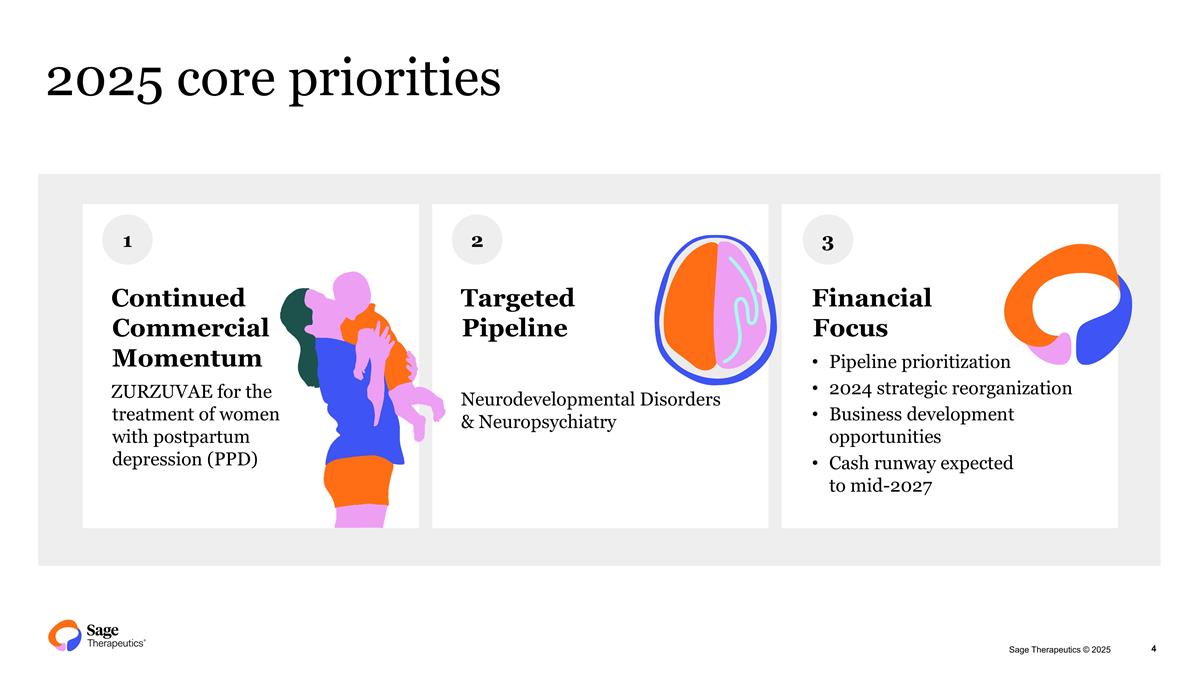
2025 core priorities Continued Commercial Momentum ZURZUVAE for the treatment of women with postpartum depression (PPD) 1 2 3 Targeted Pipeline Neurodevelopmental Disorders & Neuropsychiatry Financial Focus Pipeline prioritization 2024 strategic reorganization Business development opportunities Cash runway expected to mid-2027
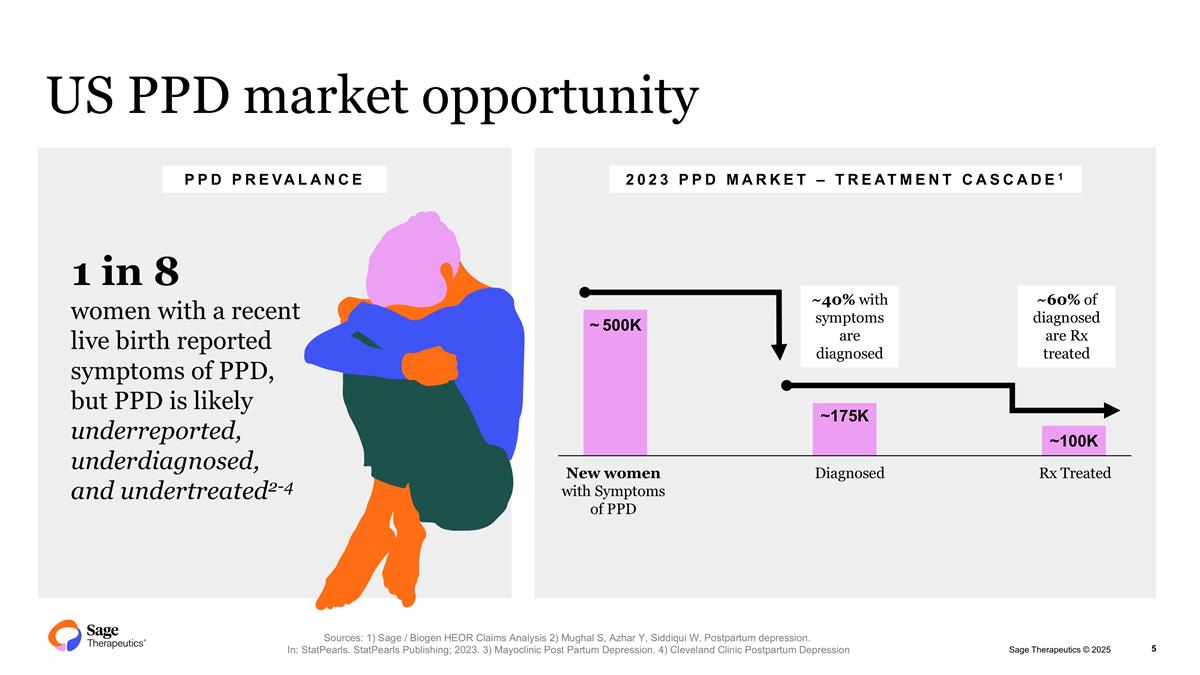
US PPD market opportunity women with a recent live birth reported symptoms of PPD, but PPD is likely underreported, underdiagnosed, and undertreated2-4 1 in 8 PPD PREVALANCE 2023 PPD MARKET – TREATMENT CASCADE1 ~500K ~175K ~100K New women with Symptoms of PPD Diagnosed Rx Treated Sources: 1) Sage / Biogen HEOR Claims Analysis 2) Mughal S, Azhar Y, Siddiqui W. Postpartum depression. In: StatPearls. StatPearls Publishing; 2023. 3) Mayoclinic Post Partum Depression. 4) Cleveland Clinic Postpartum Depression ~40% with symptoms are diagnosed ~60% of diagnosed are Rx treated

ZURZUVAE – Think big, start with focus, scale with success Please refer to the U.S. Prescribing Information for ZURZUVAE (https://documents.sage-biogen.com/us/zurzuvae/pi.pdf) women with PPD treated with ZURZUVAE as of Q3 2024 Majority of ZURZUVAE patients are receiving ZURZUVAE as their first line treatment for PPD 90% brand awareness among OBGYNs and psychiatrists >90% Commercial and Medicaid lives are favorably covered >70% of prescriptions are written by OBGYNs >4,100 Significant growth in new and repeat prescribers

Scaling with Success in 2025 Joint salesforce expansion to cover a wider range of HCPs who treat PPD 1 Build on ZURZUVAE branded media 2 Expand social media influencer campaigns/DTC 3 Increase investment in disease state awareness to support improved PPD screening and diagnosis 4 HCP WEBSITE PATIENT WEBSITE
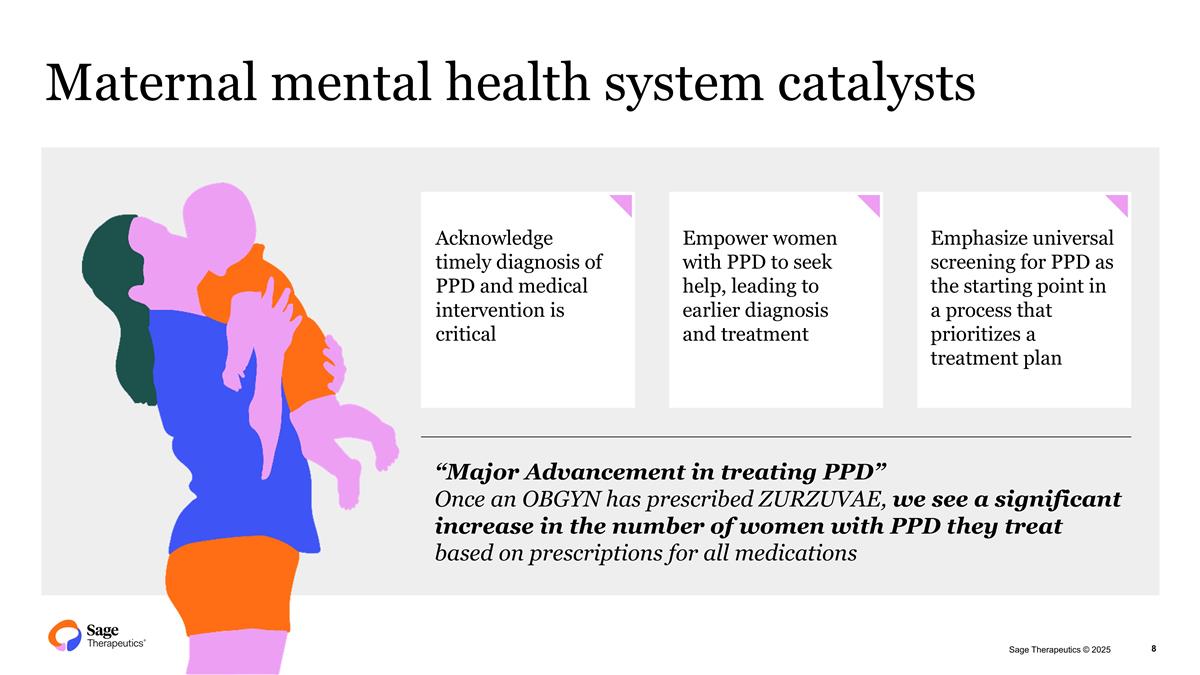
Maternal mental health system catalysts XS Acknowledge timely diagnosis of PPD and medical intervention is critical Empower women with PPD to seek help, leading to earlier diagnosis and treatment Emphasize universal screening for PPD as the starting point in a process that prioritizes a treatment plan “Major Advancement in treating PPD” Once an OBGYN has prescribed ZURZUVAE, we see a significant increase in the number of women with PPD they treat based on prescriptions for all medications
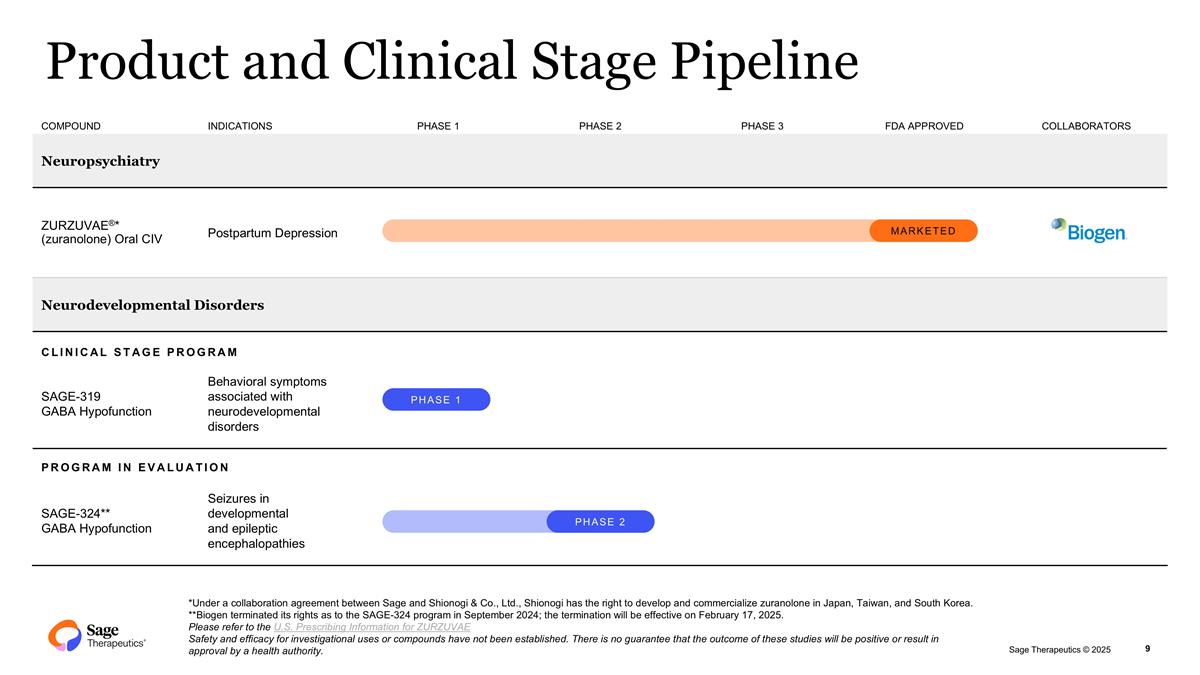
COMPOUND INDICATIONS PHASE 1 PHASE 2 PHASE 3 FDA APPROVED COLLABORATORS Neuropsychiatry ZURZUVAE®* (zuranolone) Oral CIV Postpartum Depression Neurodevelopmental Disorders CLINICAL STAGE PROGRAM SAGE-319 GABA Hypofunction Behavioral symptoms associated with neurodevelopmental disorders PROGRAM IN EVALUATION SAGE-324** GABA Hypofunction Seizures in developmental and epileptic encephalopathies Product and Clinical Stage Pipeline 9 *Under a collaboration agreement between Sage and Shionogi & Co., Ltd., Shionogi has the right to develop and commercialize zuranolone in Japan, Taiwan, and South Korea. **Biogen terminated its rights as to the SAGE-324 program in September 2024; the termination will be effective on February 17, 2025. Please refer to the U.S. Prescribing Information for ZURZUVAE Safety and efficacy for investigational uses or compounds have not been established. There is no guarantee that the outcome of these studies will be positive or result in approval by a health authority. MARKETED PHASE 2 PHASE 1
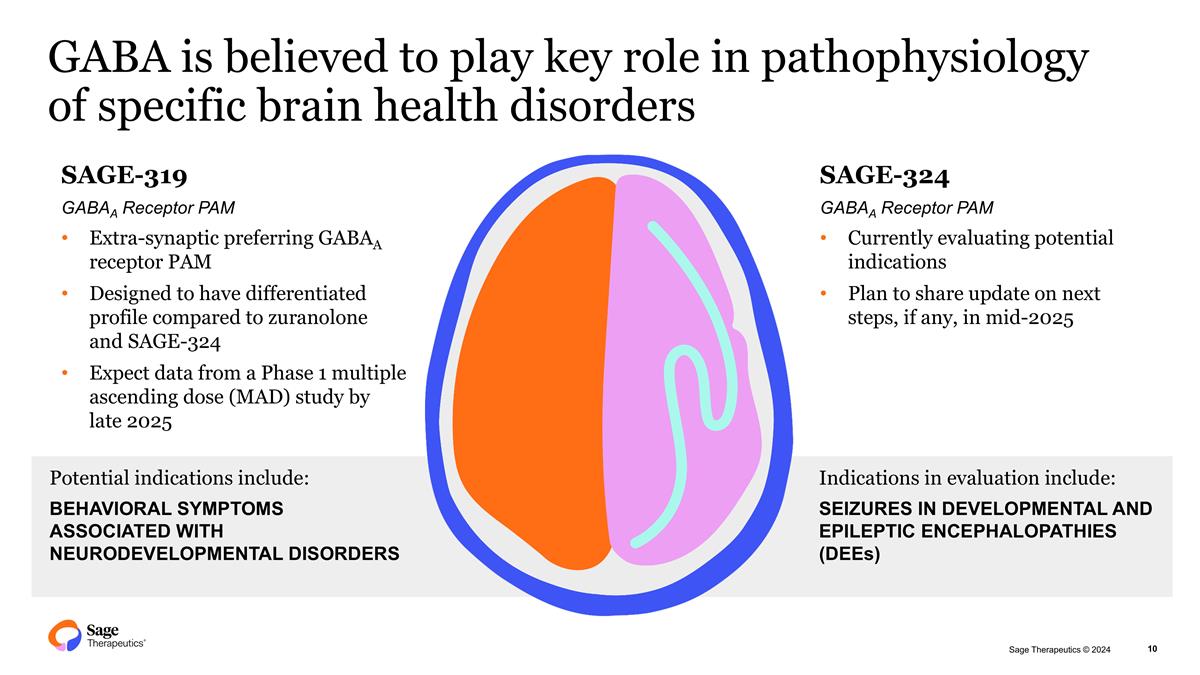
Indications in evaluation include: SEIZURES IN DEVELOPMENTAL AND EPILEPTIC ENCEPHALOPATHIES (DEEs) GABA is believed to play key role in pathophysiology of specific brain health disorders Potential indications include: BEHAVIORAL SYMPTOMS ASSOCIATED WITH NEURODEVELOPMENTAL DISORDERS SAGE-319 GABAA Receptor PAM Extra-synaptic preferring GABAA receptor PAM Designed to have differentiated profile compared to zuranolone and SAGE-324 Expect data from a Phase 1 multiple ascending dose (MAD) study by late 2025 SAGE-324 GABAA Receptor PAM Currently evaluating potential indications Plan to share update on next steps, if any, in mid-2025
Advancing commitment to brain health Patient inspired, patient led, patient first ZURZUVAE® First and only oral product specifically for adults with postpartum depression Focused approach to drug development in neuropsychiatry and neurodevelopmental disorders Development programs based on our neurosteroid platform Value-driven culture focused on doing what's right for patients

Our purpose is personal. Sahar, Ashley, Katlyn: Experienced PPD











