
© 2020 Innate Pharma. All rights reserved. PARIS: IPH.PA NASDAQ: IPHA Company Overview April 2022

This document has been prepared by Innate Pharma S.A. (the “Company”) solely for the purposes of a presentation to investors concerning the Company. This document is not to be reproduced by any person, nor to be distributed. This document contains forward-looking statements. The use of certain words, including “believe,” “potential,” “expect” and “will” and similar expressions, is intended to identify forward- looking statements. Although the Company believes its expectations are based on reasonable assumptions, these forward-looking statements are subject to various risks and uncertainties, which could cause the Company’s actual results or financial condition to differ materially from those anticipated. These risks and uncertainties include, among other things, the uncertainties inherent in research and development, including related to safety, progression of and results from its ongoing and planned clinical trials and preclinical studies, review and approvals by regulatory authorities of its product candidates, the Company’s commercialization efforts and the Company’s continued ability to raise capital to fund its development. For an additional discussion of risks and uncertainties which could cause the Company's actual results, financial condition, performance or achievements to differ from those contained in the forward-looking statements, please refer to the Risk Factors (“Facteurs de Risque”) section of the Universal Registration Document filed with the Autorité des Marchés Financiers (“AMF”), available on the AMF website (www.amf-france.org) or on the Company’s website (www.innate-pharma.com), and public filings and reports filed with the U.S. Securities and Exchange Commission (“SEC”), including the Company’s Annual Report on Form 20-F for the year ended December 31, 2021, and subsequent filings and reports filed with the AMF or SEC, or otherwise made public, by the Company. Such documents may not be necessarily up to date. This document contains data pertaining to the Company's potential markets and the industry and environment in which it operates. Some of this data comes from external sources that are recognized in the field or from Company’s estimates based on such sources. This presentation discusses product candidates that are under clinical development and which have not yet been approved for marketing by the U.S. Food and Drug Administration or the European Medicines Agency. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. The information contained herein has not been independently verified. No representation, warranty or undertaking, express or implied, is made as to, and no reliance should be placed on, the fairness, accuracy, completeness or correctness of the information or opinions contained herein. The Company is under no obligation to keep current the information contained in this presentation and any opinion expressed is subject to change without notice. The Company shall not bear any liability whatsoever for any loss arising from any use of this document or its contents or otherwise arising in connection therewith. This document and the information contained herein do not constitute an offer to sell or a solicitation of an offer to buy or subscribe to shares of the Company in any country. Disclaimer on Forward-Looking Information and Risk Factors 2

A Leading Company in the Field of Innate Immunity M A R S E I L L E F R A N C E R O C K V I L L E M A R Y L A N D Founded: 1999 Paris Euronext listing: 2006 Nasdaq listing: 2019 3 • Global, clinical-stage oncology-focused biotech company. • Scientific excellence in the field of innate immunity with expertise in natural killer cell biology and antibody engineering. • Focused pipeline of antibodies, including several potentially first-in-class clinical and preclinical candidates in cancers with high unmet medical need.

Drive near-term value with Lacutamab Advance our innovative R&D pipeline Build a sustainable business through partnerships 4 Scientific Innovation Drives Our Strategy We aim to harness our scientific know-how in innate immunity and antibody engineering to develop oncology products that improve the lives of patients

5 Strong Science + Strong Partnerships = Robust Pipeline Validated Science in high-impact publications Strong Track Record of Collaborations with industry and academia Robust Pipeline with innovative pre-clinical and clinical assets
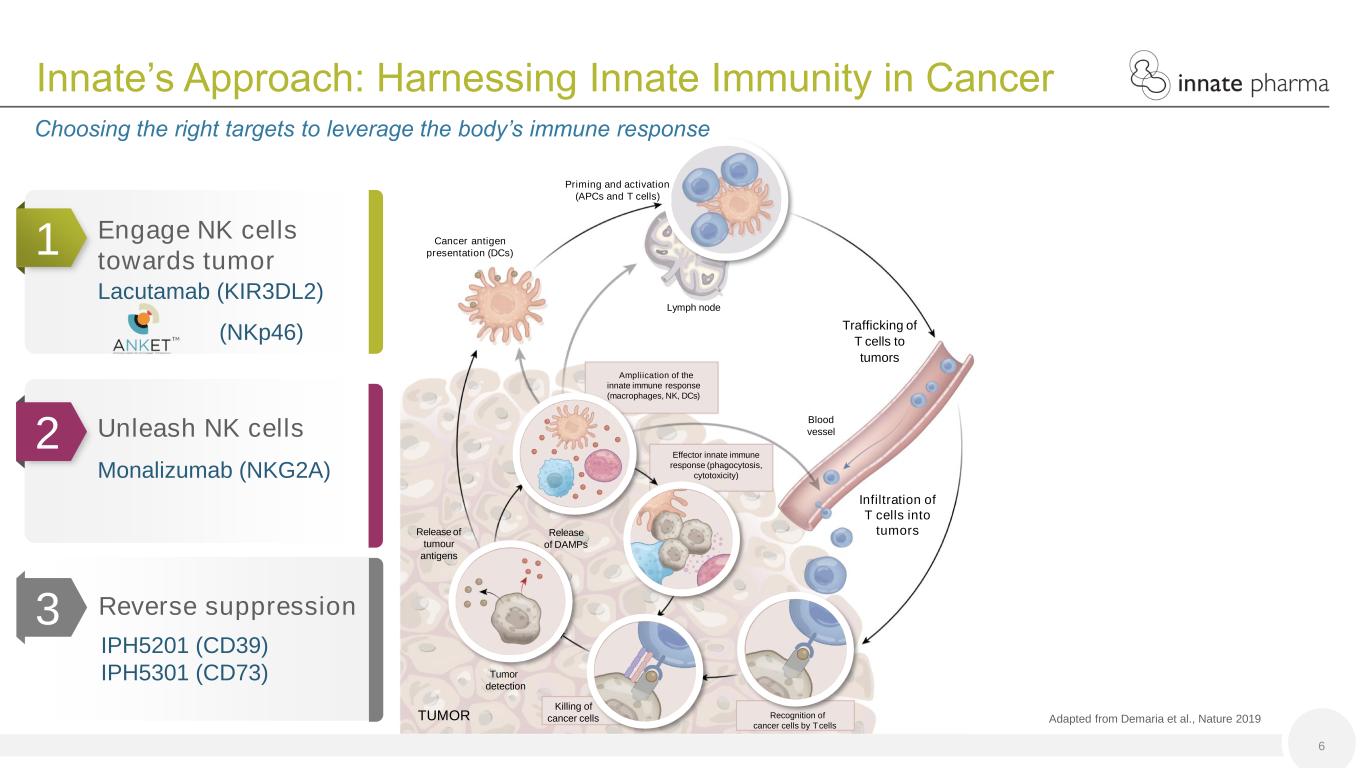
Lymph node Blood vessel TUMOR Priming and activation (APCs and T cells) Trafficking of T cells to tumors Infiltration of T cells into tumors Effector innate immune response (phagocytosis, cytotoxicity) Cancer antigen presentation (DCs) Ampliication of the innate immune response (macrophages, NK, DCs) Release of DAMPs Release of tumour antigens Killing of cancer cells Recognition of cancer cells by Tcells Tumor detection 1 Engage NK cells towards tumor Lacutamab (KIR3DL2) (NKp46) Adapted from Demaria et al., Nature 2019 6 Innate’s Approach: Harnessing Innate Immunity in Cancer Choosing the right targets to leverage the body’s immune response Reverse suppression IPH5201 (CD39) IPH5301 (CD73) 2 3 Unleash NK cells Monalizumab (NKG2A)

Program Target Indication Pre-Clinical Phase 1 Phase 2 Phase 3 Status Lacutamab KIR3DL2 Sézary Syndrome Preliminary Phase 2 data expected 2022 Mycosis Fungoides Early data presented Preliminary Phase 2 data expected 2022 PTCL (combo with GemOx) Phase 2 start H2 2021 PTCL (mono) Phase 1 start H2 2021 Monalizumab* NKG2A Post-PDx HNSCC Phase 3 data expected 2022+* Unresectable Stg III NSCLC Phase 3 starting Neoadjuvant NSCLC, other Phase 2 study in progress Avdoralimab C5aR BP Data expected 2024 IPH5201* CD39 Cancer (solid tumors) Data expected 2023 IPH5301 CD73 Cancer (solid tumors) Phase 1 study in progress IPH6101** (CD123 tri-specific) IPH62* (tri-specific) IPH64** (tri-specific) IPH65 (tetra-specific) IPH6101 Phase 1 study in progress Progress towards IND-enabling studies Other preclinical IPH25*, IPH26* (Siglec-9), IPH43* (MICA/B ADC), IPH45 7 Our Robust Pipeline of Proprietary & Partnered Assets INTERLINK-1 PHASE 3 TELLOMAK PHASE 2 (FDA FAST TRACK/EMA PRIME DESIGNATION) TELLOMAK PHASE 2 NeoCOAST-2 PHASE 2 PHASE 2 PHASE 1 PHASE 1 Pre-clinical Pre-clinical PHASE 2 PHASE 1b * ** PACIFIC-9 PHASE 3 GemOx: gemcitabine and oxaliplatin; PDX: anti-PD-1/L1; HNSCC: Squamous Cell Carcinoma of the Head and Neck; BP: Bullous Penphigoid; PTCL: Peripheral T Cell Lymphoma; IND: Investigational new drug PHASE 1

Driving Our Proprietary Portfolio
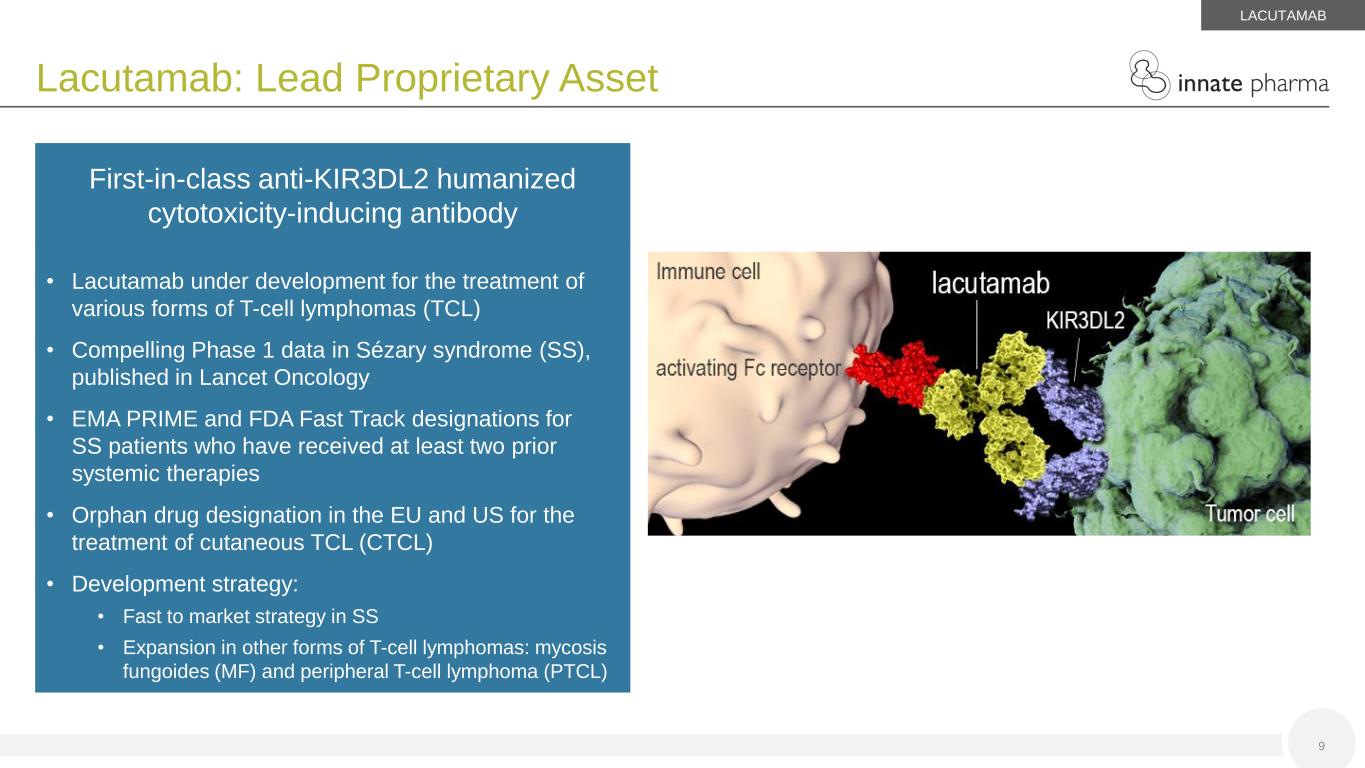
9 Lacutamab: Lead Proprietary Asset • Lacutamab under development for the treatment of various forms of T-cell lymphomas (TCL) • Compelling Phase 1 data in Sézary syndrome (SS), published in Lancet Oncology • EMA PRIME and FDA Fast Track designations for SS patients who have received at least two prior systemic therapies • Orphan drug designation in the EU and US for the treatment of cutaneous TCL (CTCL) • Development strategy: • Fast to market strategy in SS • Expansion in other forms of T-cell lymphomas: mycosis fungoides (MF) and peripheral T-cell lymphoma (PTCL) First-in-class anti-KIR3DL2 humanized cytotoxicity-inducing antibody Tumor cell lacutamab KIR3DL2 LACUTAMAB
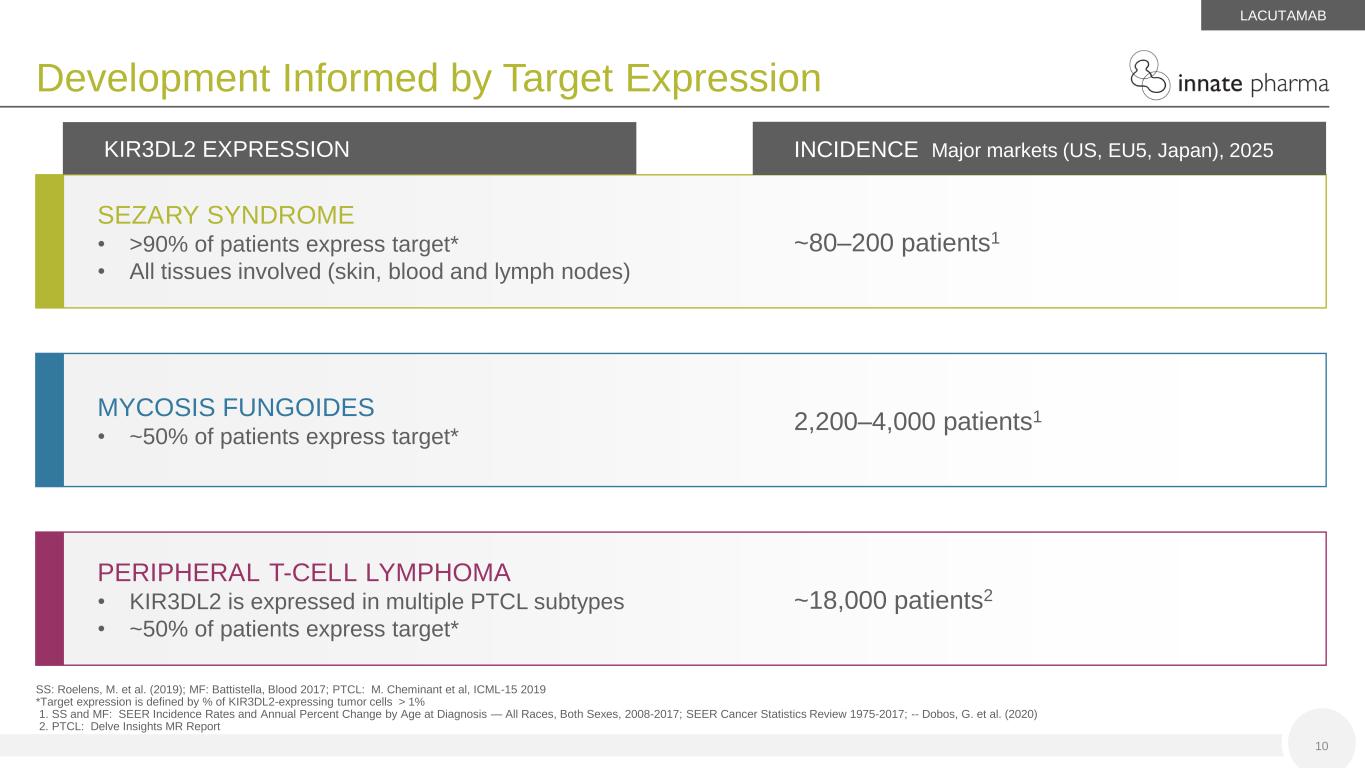
SEZARY SYNDROME • >90% of patients express target* • All tissues involved (skin, blood and lymph nodes) MYCOSIS FUNGOIDES • ~50% of patients express target* PERIPHERAL T-CELL LYMPHOMA • KIR3DL2 is expressed in multiple PTCL subtypes • ~50% of patients express target* ~80–200 patients1 2,200–4,000 patients1 ~18,000 patients2 KIR3DL2 EXPRESSION INCIDENCE Major markets (US, EU5, Japan), 2025 10 SS: Roelens, M. et al. (2019); MF: Battistella, Blood 2017; PTCL: M. Cheminant et al, ICML-15 2019 *Target expression is defined by % of KIR3DL2-expressing tumor cells > 1% 1. SS and MF: SEER Incidence Rates and Annual Percent Change by Age at Diagnosis — All Races, Both Sexes, 2008-2017; SEER Cancer Statistics Review 1975-2017; -- Dobos, G. et al. (2020) 2. PTCL: Delve Insights MR Report Development Informed by Target Expression LACUTAMAB

11 Developing New Standard of Care in KIR3DL2-Expressing T-Cell Lymphomas LACUTAMAB Mycosis Fungoides Expand potential beyond SS Explore impact of KIR3DL2 expression on clinical outcome Multiple trials to support PTCL Detecting early signal in relapsed population then expanding in earlier lines or in combinations Cutaneous T-Cell Lymphoma (CTCL) Peripheral T-Cell Lymphoma (PTCL) Sezary Syndrome Fast to market approach Niche indication with high unmet need
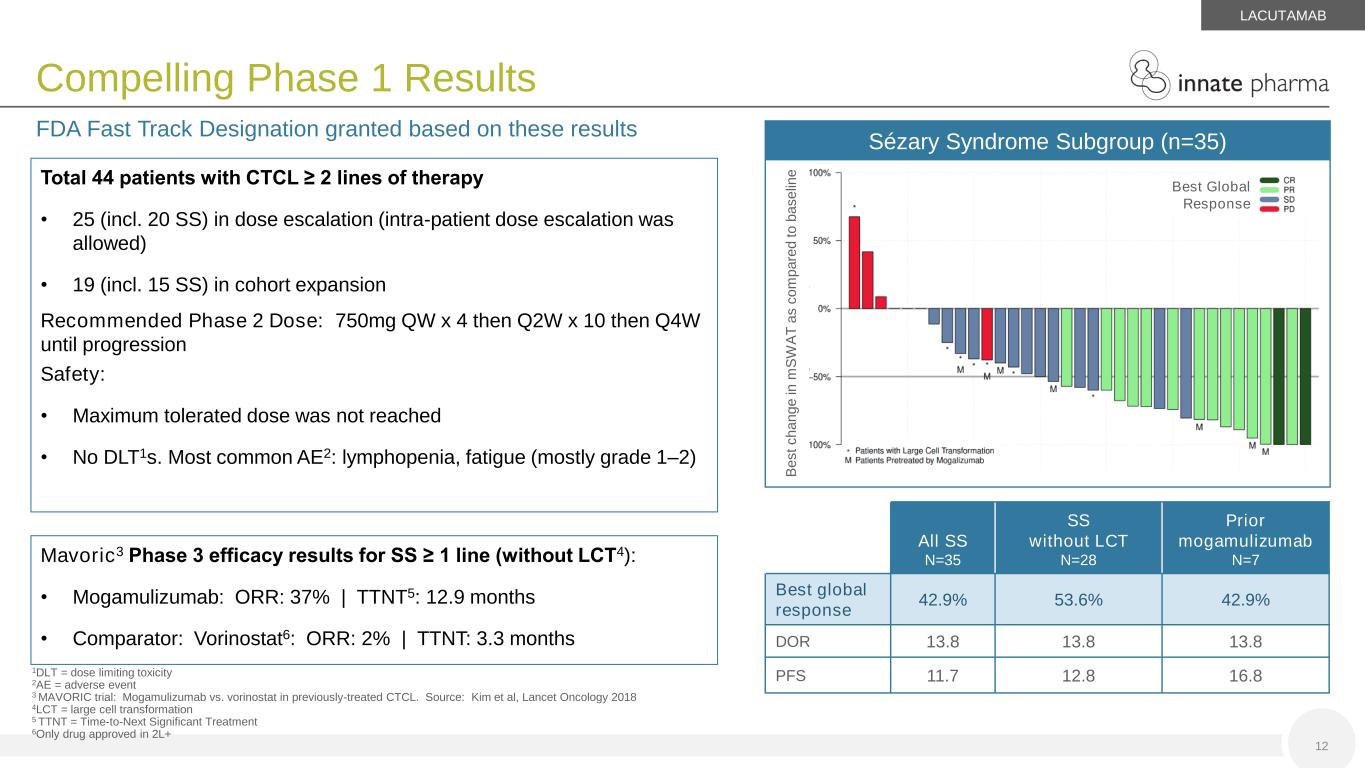
Compelling Phase 1 Results 12 FDA Fast Track Designation granted based on these results 1DLT = dose limiting toxicity 2AE = adverse event 3 MAVORIC trial: Mogamulizumab vs. vorinostat in previously-treated CTCL. Source: Kim et al, Lancet Oncology 2018 4LCT = large cell transformation 5 TTNT = Time-to-Next Significant Treatment 6Only drug approved in 2L+ LACUTAMAB Sézary Syndrome Subgroup (n=35) Best Global Response B e s t c h a n g e in m S W A T a s c o m p a re d t o b a s e lin e All SS N=35 SS without LCT N=28 Prior mogamulizumab N=7 Best global response 42.9% 53.6% 42.9% DOR 13.8 13.8 13.8 PFS 11.7 12.8 16.8 Total 44 patients with CTCL ≥ 2 lines of therapy • 25 (incl. 20 SS) in dose escalation (intra-patient dose escalation was allowed) • 19 (incl. 15 SS) in cohort expansion Recommended Phase 2 Dose: 750mg QW x 4 then Q2W x 10 then Q4W until progression Safety: • Maximum tolerated dose was not reached • No DLT1s. Most common AE2: lymphopenia, fatigue (mostly grade 1–2) Mavoric3 Phase 3 efficacy results for SS ≥ 1 line (without LCT4): • Mogamulizumab: ORR: 37% | TTNT5: 12.9 months • Comparator: Vorinostat6: ORR: 2% | TTNT: 3.3 months
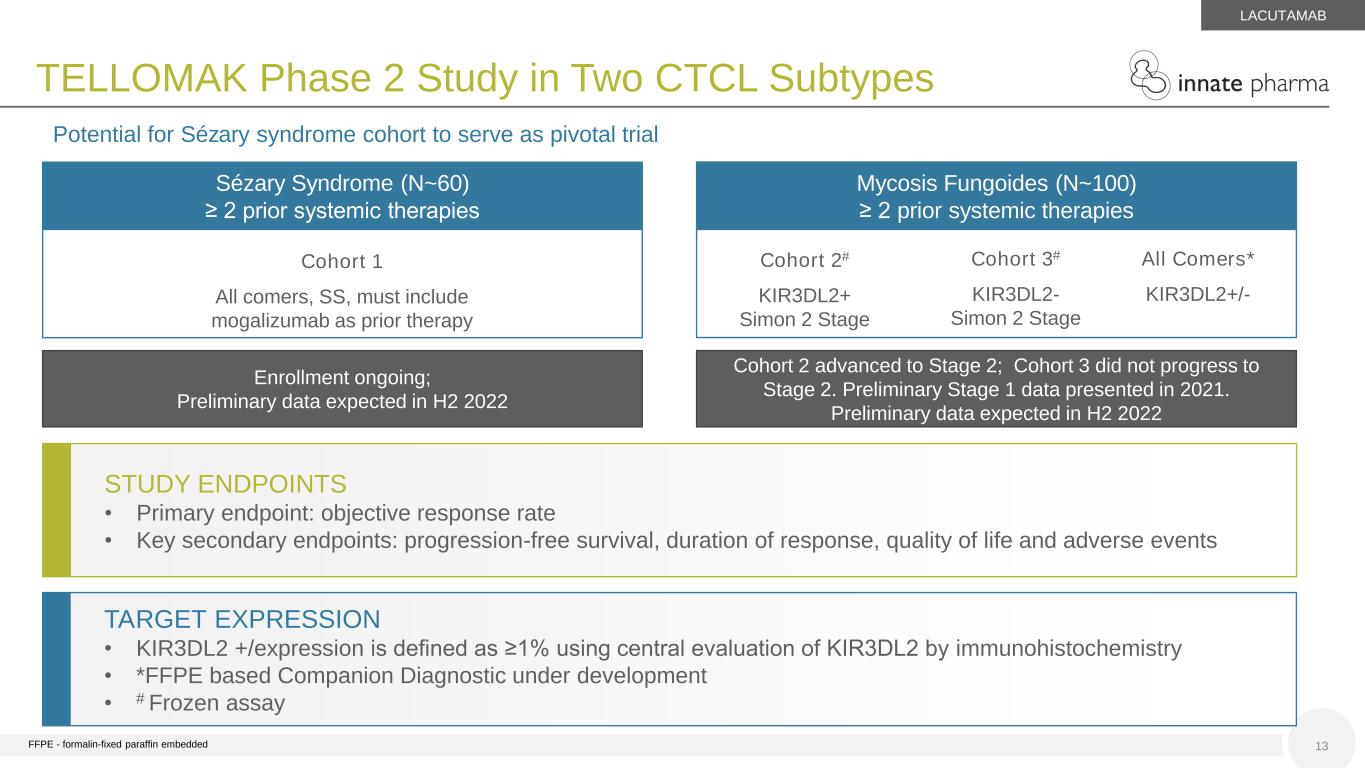
13 TELLOMAK Phase 2 Study in Two CTCL Subtypes Mycosis Fungoides (N~100) ≥ 2 prior systemic therapies Cohort 1 All comers, SS, must include mogalizumab as prior therapy Sézary Syndrome (N~60) ≥ 2 prior systemic therapies Cohort 2# KIR3DL2+ Simon 2 Stage All Comers* KIR3DL2+/- STUDY ENDPOINTS • Primary endpoint: objective response rate • Key secondary endpoints: progression-free survival, duration of response, quality of life and adverse events TARGET EXPRESSION • KIR3DL2 +/expression is defined as ≥1% using central evaluation of KIR3DL2 by immunohistochemistry • *FFPE based Companion Diagnostic under development • # Frozen assay LACUTAMAB Potential for Sézary syndrome cohort to serve as pivotal trial Cohort 2 advanced to Stage 2; Cohort 3 did not progress to Stage 2. Preliminary Stage 1 data presented in 2021. Preliminary data expected in H2 2022 Enrollment ongoing; Preliminary data expected in H2 2022 Cohort 3# KIR3DL2- Simon 2 Stage FFPE - formalin-fixed paraffin embedded

14 Encouraging Preliminary Results in KIR3DL2-Expressing MF Cohort of TELLOMAK Trial LACUTAMAB * reported after data cut off (DCO) date, May 10, 2021. Bagot et al. ICML June 2021. Response rate reported after data cut off. CR: complete response, PR: partial response, uPR: unconfirmed partial response Cohort 2 Overall response rate of ~35%* in advanced patient population with no current standard of care Cohort 3 Overall response rate of ~11%* KIR3DL2 non-expressors cohort did not progress to Stage 2 Skin (N = 17) Skin (N = 19) Cohort 2 KIR3DL2 expressors Cohort 3 KIR3DL2 non expressors 2 confirmed global responses (2PR) 11 / 19 patients still ongoing therapy 6 confirmed (1CR, 5PR) global responses 9 / 17 patients still ongoing therapy

15 Initiating Data-Driven Strategy in PTCL 03 02 01 RELAPSED SETTING Highest unmet medical need; two-pronged approach: • Monotherapy (Phase 1b study in H2 2021) • Combination with: • GemOx (LYSA-sponsored Phase 2 KILT study in H2 2021); and • other SOC N O W FRONTLINE Driven by data in the relapsed setting to advance into earlier lines • Combination with CHOP N E X T S T E P S LACUTAMAB CHOP: Cyclophosphamide, Hydroxydaunorubicin, Oncovin and PrednisoneSOC: Standard of care

16 Developing a New Standard of Care Across KIR3DL2- Expressing T-Cell Lymphomas Sezary Syndome Mycosis Fungoides • Trial expanded (pivotal potential) • Fast Track & PRIME Designation • Preliminary data expected in 2022 (H2) • Advanced Cohort 2 to Stage 2 with earlier-than-expected efficacy signal • 2 active cohorts – KIR3DL2 expressing and all comers • Reported preliminary Cohort 2, Stage 1 data at ICML – 35% ORR • Preliminary data expected in 2022 (H2) Multi-trial Strategy From Relapsed to Frontline PTCL 80-200 patients >90% KIR3DL2 expression 2,200-4,400 patients ~50% KIR3DL2 expression • Monotherapy + combination with GemOX (LYSA) & SOC in relapsed setting • Follow data into earlier lines (in combination with CHOP) ~18,000 patients ~50% KIR3DL2 expression Cutaneous T-Cell Lymphoma (CTCL) Peripheral T-Cell Lymphoma (PTCL) Phase 2 TELLOMAK Trial GemOX: gemcitabine and oxaliplatin SOC: Standard of care, CHOP: cyclophosphamide, hydroxydaunomycin, oncovin, prednisolone LACUTAMAB
Next-Generation Assets: NK Cell Engagers

18 Tumor-infiltrating Natural Killer cells. Cózar et al., Cancer Discovery 2021 Multifunctional Natural Killer Cell Engagers targeting NKp46 trigger protective tumor immunity. Gauthier et al., Cell 2019 Harnessing innate immunity in cancer therapy. Demaria et al., Nature 2019 Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Walzer et al., PNAS 2007 p46, a novel natural killer cell-specific surface molecule that mediates cell activation. Sivori et al, J Exp Med. 1997 NK Cells Offer Unique Cancer-Fighting Abilities NK cells in solid tumors NK cells in periphery • NK cells are not expected to produce a cytokine storm • NKp46 is the most specific activating receptor of NK cells N K S P E C I F I C I TY • Unlike many other activating receptors, NKp46 is conserved on NK cells infiltrating solid tumors N K p 4 6 E X P R E S S I O N S TA B I L I TY ANKETTM NKp46

19 ANKET™: Innate’s Proprietary NK Cell Engager Platform is a versatile, fit-for- purpose technology that is creating an entirely new class of tri- and tetra-specific molecules to induce synthetic immunity against cancer ANKET™ Harnesses NK cell effector functions against cancer cells, through the most conserved activating receptor on NK cells: NKp46 Provides proliferation and activation signals targeted to NK cells Demonstrates better anti-tumor efficacy than approved benchmark antibodies in preclinical tumor models ANKETTM TAg: Tumor antigen

New Data from IPH6101/SAR443579, Innate’s Lead ANKET™ Asset 20 Research collaboration with partner Sanofi; Phase 1/2 clinical trial underway First NKp46/CD16-based NK cell engager that targets CD123 on AML cells Uses Innate’s proprietary multi-specific antibody format Announced €7M milestone payment to Innate to date ANKETTM • Companies are collaborating on the generation and evaluation of up to two NK cell engagers. • Sanofi is responsible for the development, manufacturing and commercialization of products resulting from the research collaboration. • Innate is eligible for up to €400m in development and commercial milestone payments and royalties on net sales.

21 CD123 tri-specific targeted SAR443579/IPH6101 Demonstrates Potent Antitumor Activity Against AML and Favorable Safety Profile ANKETTM AML: Acute Myeloid Leukemia, HR-MDS: Higher-Risk Myelodysplastic Syndromes, ADCC: antibody drug conjugate, NHP: non-human primates The first NKp46/CD16-based NK Cell engager using ANKET Phase 1 trial in AML and HR-MDS underway SAR443579/IPH6101 kills AML cells resistant to ADCC SAR443579/IPH6101 induce CD123-expressing cell depletion in NHP with minor cytokine release ADCC-sensitive AML cells ADCC-resistant AML cells

22 Optimal Efficacy Requires All Arms in Tetra-specific ANKET™ Demonstrate Superiority in Preclinical Model of Tumors ANKETTM *ADCC-enhanced mAbTAg: Tumor antigen Progressing towards IND enabling studies Disseminated tumor modelsSolid tumor models

23 Adapted from Lanier, Nature Immunol 2008; Vivier et al., Science, 2004 Different Receptors have Different Effects on NK cells NKp46 CD3ζ, FcRγ #DAP10 NKG2D • NK cell activation + •cytotoxicity • NK cell activation +++ •cytotoxicity •cytokines and chemokines CD16 IL-2Rb IL-2Rg • Proliferation +++ ANKETTM

Building a Sustainable Business
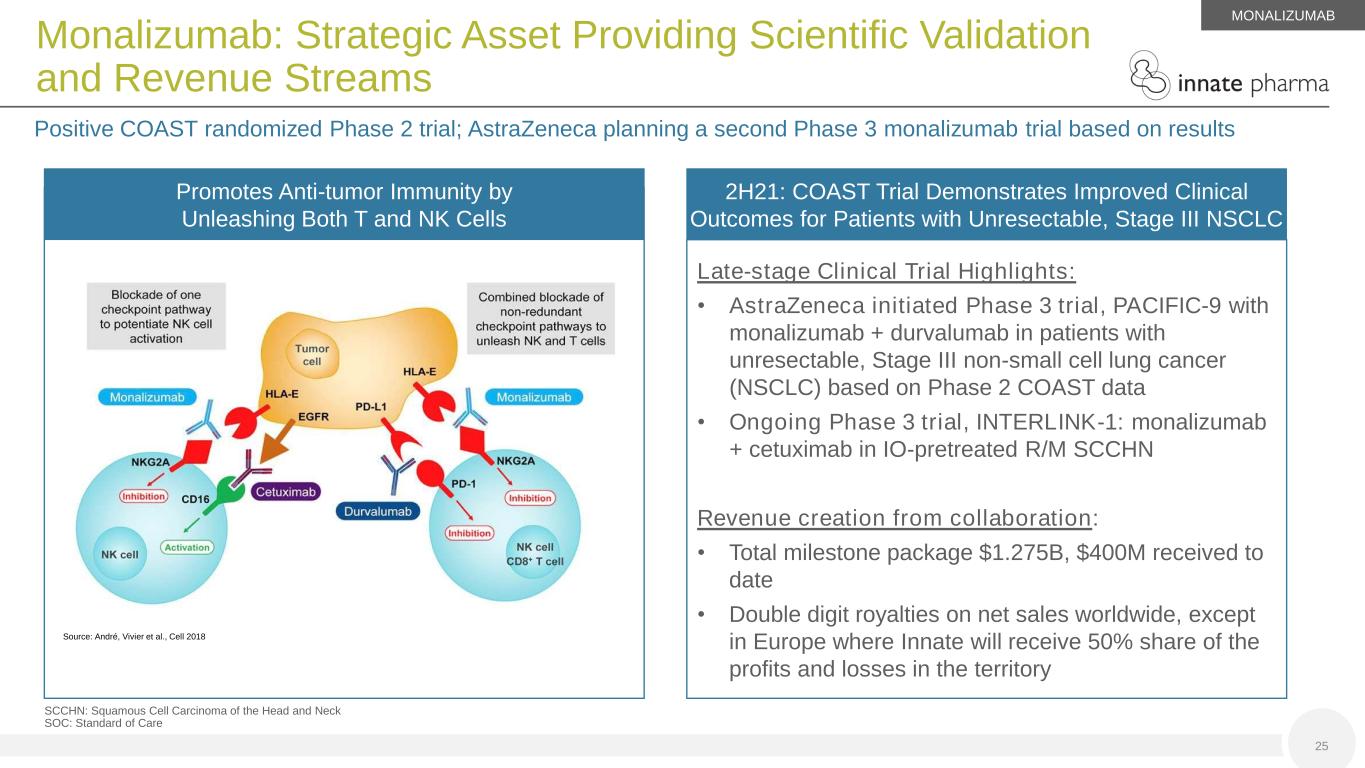
Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells SCCHN: Squamous Cell Carcinoma of the Head and Neck SOC: Standard of Care 25 Monalizumab: Strategic Asset Providing Scientific Validation and Revenue Streams Positive COAST randomized Phase 2 trial; AstraZeneca planning a second Phase 3 monalizumab trial based on results Late-stage Clinical Trial Highlights: • AstraZeneca initiated Phase 3 trial, PACIFIC-9 with monalizumab + durvalumab in patients with unresectable, Stage III non-small cell lung cancer (NSCLC) based on Phase 2 COAST data • Ongoing Phase 3 trial, INTERLINK-1: monalizumab + cetuximab in IO-pretreated R/M SCCHN Revenue creation from collaboration: • Total milestone package $1.275B, $400M received to date • Double digit royalties on net sales worldwide, except in Europe where Innate will receive 50% share of the profits and losses in the territory 2H21: COAST Trial Demonstrates Improved Clinical Outcomes for Patients with Unresectable, Stage III NSCLC MONALIZUMAB Source: André, Vivier et al., Cell 2018
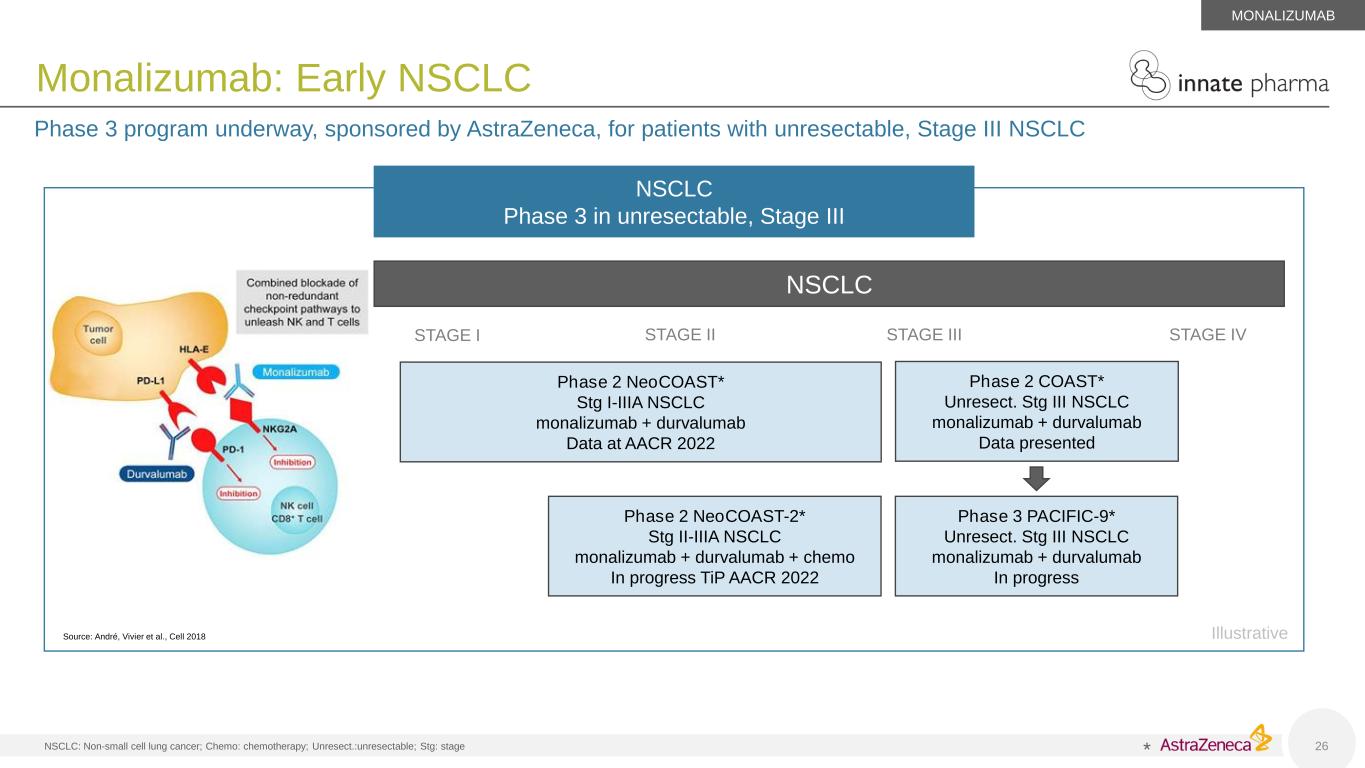
NSCLC: Non-small cell lung cancer; Chemo: chemotherapy; Unresect.:unresectable; Stg: stage 26 Monalizumab: Early NSCLC Phase 3 program underway, sponsored by AstraZeneca, for patients with unresectable, Stage III NSCLC MONALIZUMAB Source: André, Vivier et al., Cell 2018 NSCLC Phase 3 in unresectable, Stage III * NSCLC STAGE I STAGE II STAGE III STAGE IV Phase 2 COAST* Unresect. Stg III NSCLC monalizumab + durvalumab Data presented Phase 3 PACIFIC-9* Unresect. Stg III NSCLC monalizumab + durvalumab In progress Phase 2 NeoCOAST* Stg I-IIIA NSCLC monalizumab + durvalumab Data at AACR 2022 Phase 2 NeoCOAST-2* Stg II-IIIA NSCLC monalizumab + durvalumab + chemo In progress TiP AACR 2022 Illustrative
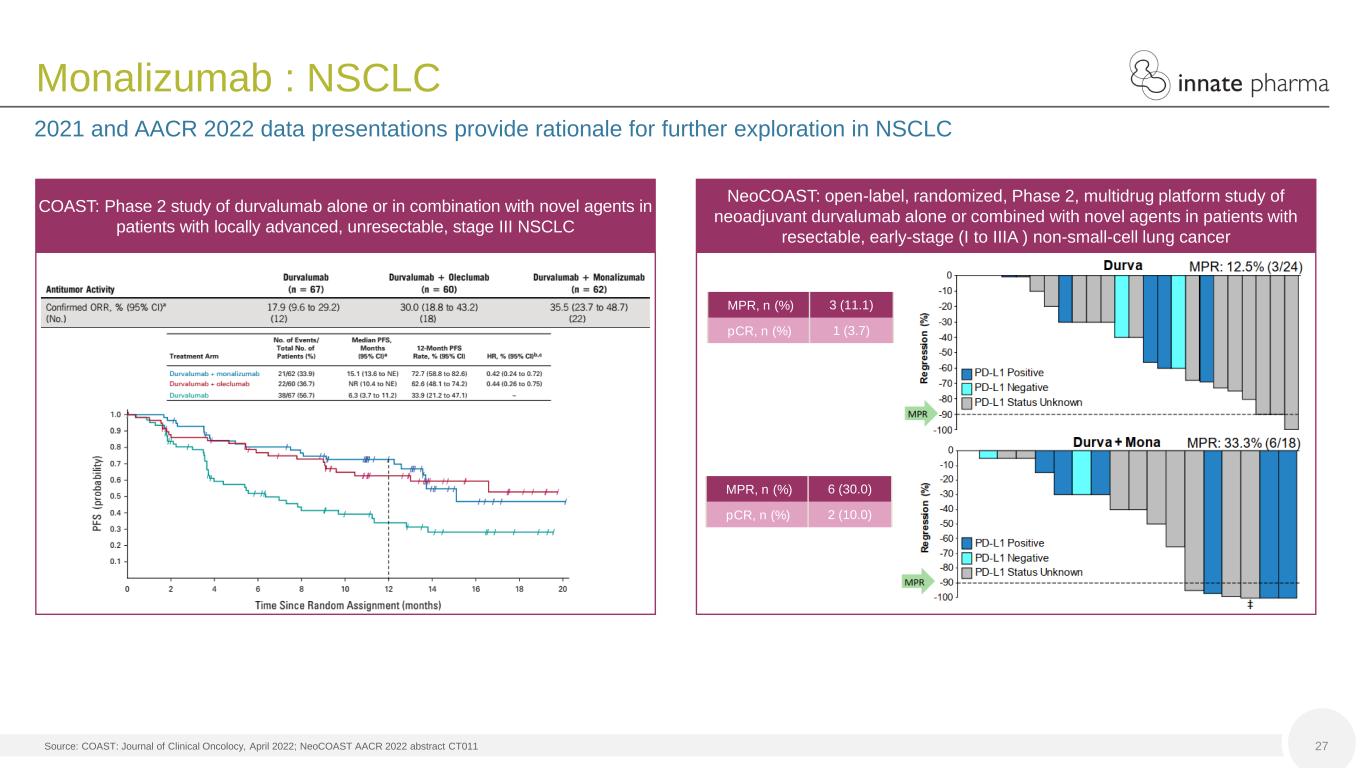
27 Monalizumab : NSCLC COAST: Phase 2 study of durvalumab alone or in combination with novel agents in patients with locally advanced, unresectable, stage III NSCLC NeoCOAST: open-label, randomized, Phase 2, multidrug platform study of neoadjuvant durvalumab alone or combined with novel agents in patients with resectable, early-stage (I to IIIA ) non-small-cell lung cancer MPR, n (%) 3 (11.1) pCR, n (%) 1 (3.7) MPR, n (%) 6 (30.0) pCR, n (%) 2 (10.0) 2021 and AACR 2022 data presentations provide rationale for further exploration in NSCLC Source: COAST: Journal of Clinical Oncolocy, April 2022; NeoCOAST AACR 2022 abstract CT011
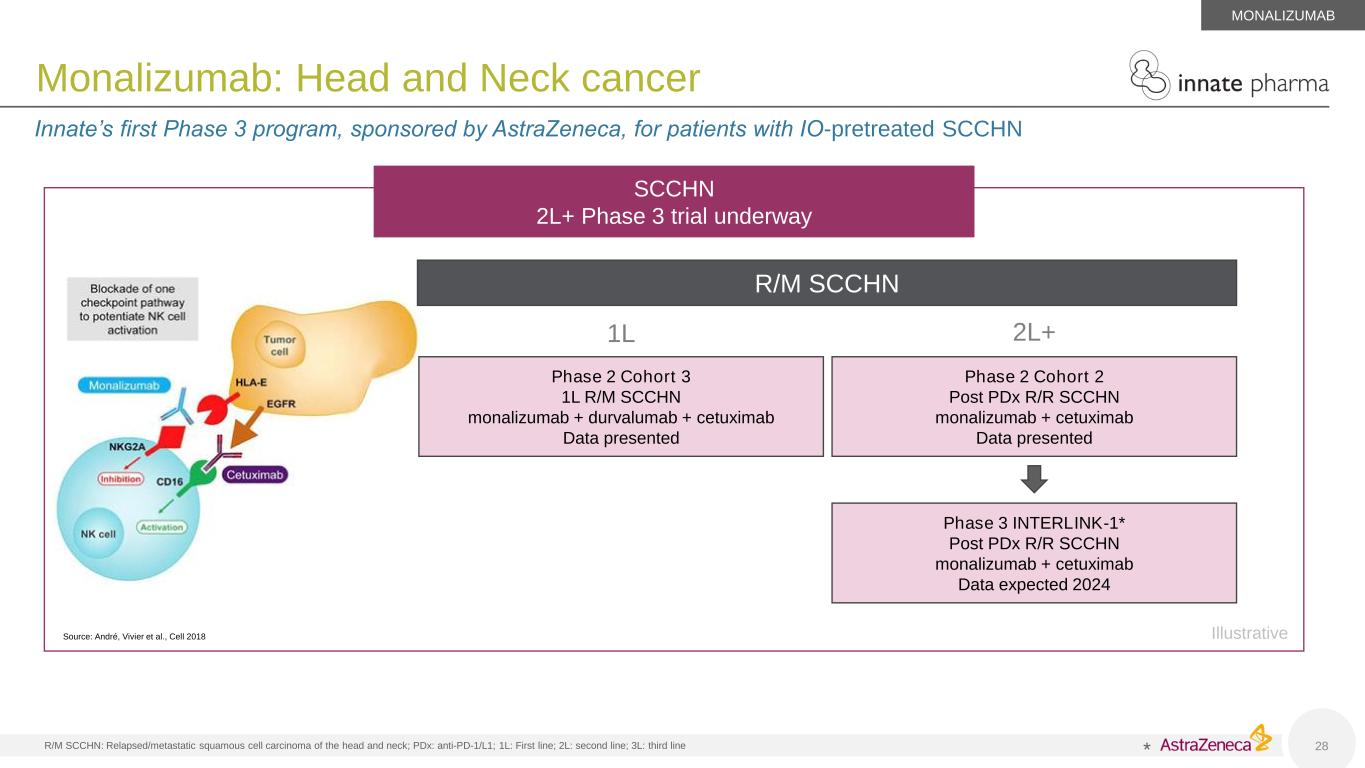
R/M SCCHN: Relapsed/metastatic squamous cell carcinoma of the head and neck; PDx: anti-PD-1/L1; 1L: First line; 2L: second line; 3L: third line 28 Monalizumab: Head and Neck cancer Innate’s first Phase 3 program, sponsored by AstraZeneca, for patients with IO-pretreated SCCHN MONALIZUMAB Source: André, Vivier et al., Cell 2018 SCCHN 2L+ Phase 3 trial underway * R/M SCCHN 1L 2L+ Phase 2 Cohort 2 Post PDx R/R SCCHN monalizumab + cetuximab Data presented Phase 2 Cohort 3 1L R/M SCCHN monalizumab + durvalumab + cetuximab Data presented Phase 3 INTERLINK-1* Post PDx R/R SCCHN monalizumab + cetuximab Data expected 2024 Illustrative

Catalysts and Summary

30 Key Newsflow Over the Next 2 Years IST: Investigator Sponsored Trial; HNSCC: Squamous Cell Carcinoma of the Head and Neck; BP: Bullous Penphigoid; MF: Mycosis Fungoides; SS: Sezary Syndrome; PTCL: Peripheral T Cell Lymphoma; IND: Investigational New Drug 2 0 2 2 2 0 2 3 Data Readouts Clinical Progress • Monalizumab NeoCOAST Ph2 NSCLC (AstraZeneca) AACR • Lacutamab Phase 2 MF data (preliminary) H2 • Lacutamab Phase 2 SS data (preliminary) H2 • Monalizumab PACIFIC-9 NSCLC Phase 3 start (AstraZeneca) • Monalizumab NeoCOAST-2 Phase 2 start (AstraZeneca) • Lacutamab r/r PTCL mono Phase 1b starting • Lacutamab r/r PTCL combo Phase 2 starting (IST) • IPH5201 (CD39) next step planning (AstraZeneca) • IPH5301 (CD73) Phase 1 start (IST) • Proprietary tetra-specific IPH65 ANKETTM progress towards IND Data Readouts Clinical Progress • Lacutamab Phase 2 MF data (final) • Lacutamab Phase 2 SS data (final) • Lacutamab PTCL data (preliminary) • IPH5201 (CD39) Phase 1 data (AstraZeneca) • Lacutamab PTCL next steps • Proprietary tetra-specific IPH65 ANKETTM IND filing

31 Early R&D focus to drive value through later stage partnerships Cash position of €159.7 million* as of December 31, 2021 with runway into mid 2023 Lacutamab R&D pipeline Partnerships • TELLOMAK read-out with MF in 2021 and preliminary data for MF and SS in 2022 • Initiating monotherapy and combination PTCL studies • Advancing proprietary tetra- specific NK cell-targeted platform and portfolio • Advancing our adenosine franchise • Strong Phase 3 monalizumab collaboration with AstraZeneca • Sanofi CD123 tri-specific Phase 1 clinical trial underway *Including short term investments (€16.1m) and non-current financial instruments (€39.9m). Cash position as of December 31, 2021 includes proceeds (€28.7m) relating to State-Guaranteed Loans (Prêts Garantis “PGE”) received in December 2021.
PARIS: IPH.PA NASDAQ: IPHA © 2020 Innate Pharma. All rights reserved. THANK YOU www.innate-pharma.com

Appendix

34 2021 Financial Highlights Licensing and collaborations: €12.1m • €7.4m for monalizumab (AZ) • €1.6m R&D costs sharing – IPH5201/avdoralimab (AZ) • €3.0m for IPH6101/SARSAR443579 (Sanofi) Government funding for research expenditures: • €10.3m 65% expenses related to R&D R&D expenses €47m: decrease in depreciation and amortization expenses G&A expenses €25.5m: increase mainly due to increase in staff costs, non-scientific advisory, consulting and insurance Revenue/other income from continuing operations**: €24.7m Operating expenses from continuing operations** : €72.5m Cash, cash equivalents and financial assets: €159.7m* as of December 31, 2021 • Sufficient to fund operations until mid 2023 * Including short term investments (€16.1m) and non-current financial instruments (€39.9m). Cash position as of December 31, 2021 includes proceeds (€28.7m) relating to State-Guaranteed Loans (Prêts Garantis par l'Etat “PGE”) received in December 2021. ** Operations related to Lumoxiti are presented as discontinued operations from the notification made to the FDA relating to the transfer of the U.S marketing authorization (October 1, 2021)
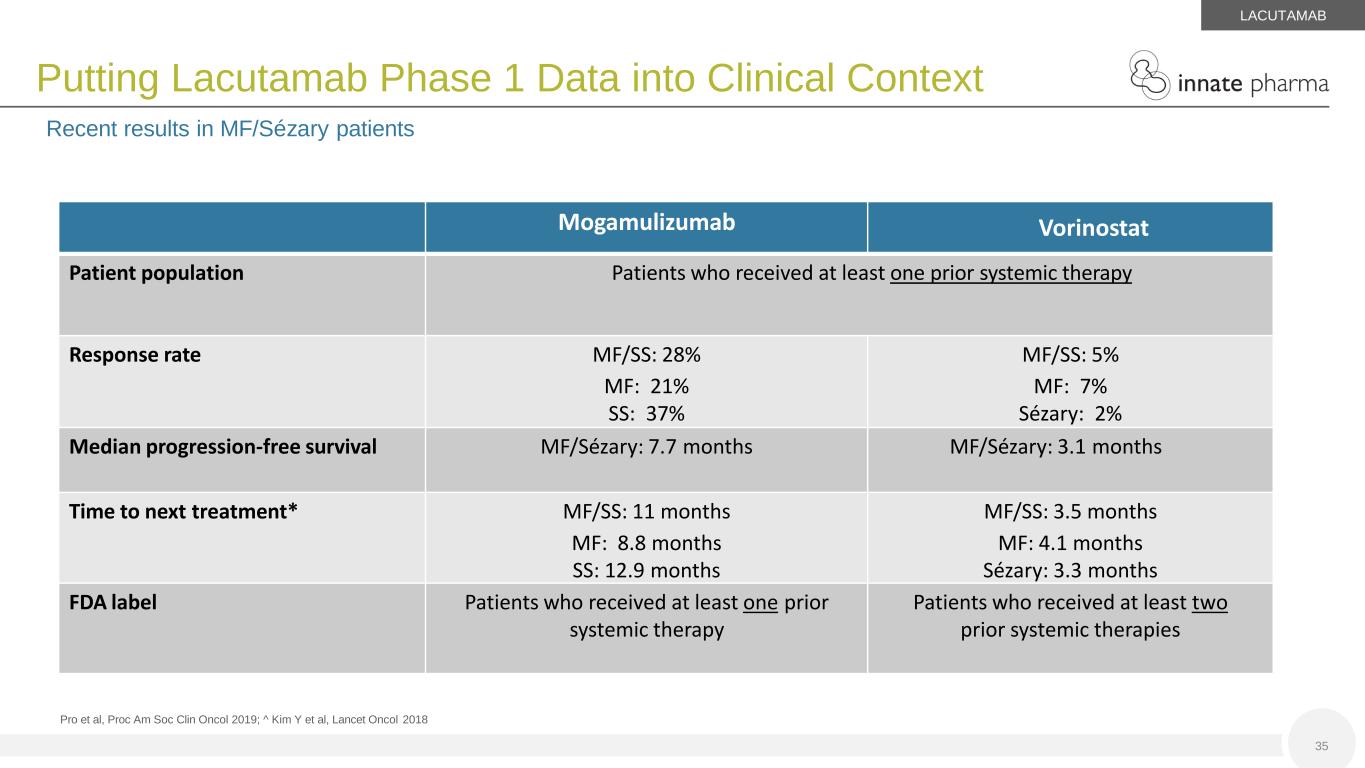
35 Putting Lacutamab Phase 1 Data into Clinical Context Recent results in MF/Sézary patients LACUTAMAB Mogamulizumab Vorinostat Patient population Patients who received at least one prior systemic therapy Response rate MF/SS: 28% MF: 21% SS: 37% MF/SS: 5% MF: 7% Sézary: 2% Median progression-free survival MF/Sézary: 7.7 months MF/Sézary: 3.1 months Time to next treatment* MF/SS: 11 months MF: 8.8 months SS: 12.9 months MF/SS: 3.5 months MF: 4.1 months Sézary: 3.3 months FDA label Patients who received at least one prior systemic therapy Patients who received at least two prior systemic therapies Pro et al, Proc Am Soc Clin Oncol 2019; ^ Kim Y et al, Lancet Oncol 2018

LACUTAMAB IN PATIENTS (PTS) WITH MYCOSIS FUNGOIDES (MF) ACCORDING TO KIR3DL2 EXPRESSION: EARLY RESULTS FROM THE TELLOMAK PHASE 2 TRIAL Presented at ICML (Jun2021) and EORTC (Oct2021) Clinicaltrials.gov: NCT03902184 LACUTAMAB

Tellomak : T-cell lymphoma anti-KIr3dl2 therapy A Multi-cohort International Open-label Phase 2 Trial Mycosis Fungoides ≥ 2 prior systemic therapies including biological agents Stage 1 (N=21) Stage 1 (N=18) Cohort # 2 KIR3DL2 ≥ 1% Cohort # 3 KIR3DL2 < 1% If N ≥3 responses If N ≥3 responses Lacutamab monotherapy 750mg (1 hour IV infusion) • weekly x 5, every 2 weeks x 10, every 4 weeks until progression or unacceptable toxicity. Clinicaltrials.gov: NCT03902184 Sponsor: Innate Pharma Stage 2 (N=29) Stage 2 (N=20) LACUTAMAB

PRELIMINARY RESULTS FROM STAGE 1 OF THE MF COHORTS Data cut-off (DCO): 10 May 2021 LACUTAMAB

Patient characteristics Cohort 2 KIR3DL2 ≥ 1% (N=17) Cohort 3 KIR3DL2 < 1% (N=19) Age in years, Median (range) 59 (33 – 76) 60 (19 – 81) - Female - Male 6 (35%) 11 (65%) 3 (16%) 16 (84%) - Stage IB / II - Stage III / IV 12 (70%) 5 (30%) 17 (89%) 2 (11%) Blood involvement at baseline (B1) 7 (41%) 2 (11%) Nodal involvement at baseline 8 (47%) 6 (32%) Months since initial diagnosis, Median (range) 55 (12 – 213) 64 (7 – 218) N prior lines of systemic therapy, Median (range) 4 (2 – 7) 4 (1* – 10) Data cut-off (DCO): 10 May 2021 • Cohort 2: 17 / 21 recruited in Stage 1 (recruitment ongoing) • Cohort 3: 19 / 18 recruited in Stage 1 (recruitment completed) * 1 patient had protocol deviation having received only 1 prior systemic therapy Clinicaltrials.gov: NCT03902184 LACUTAMAB
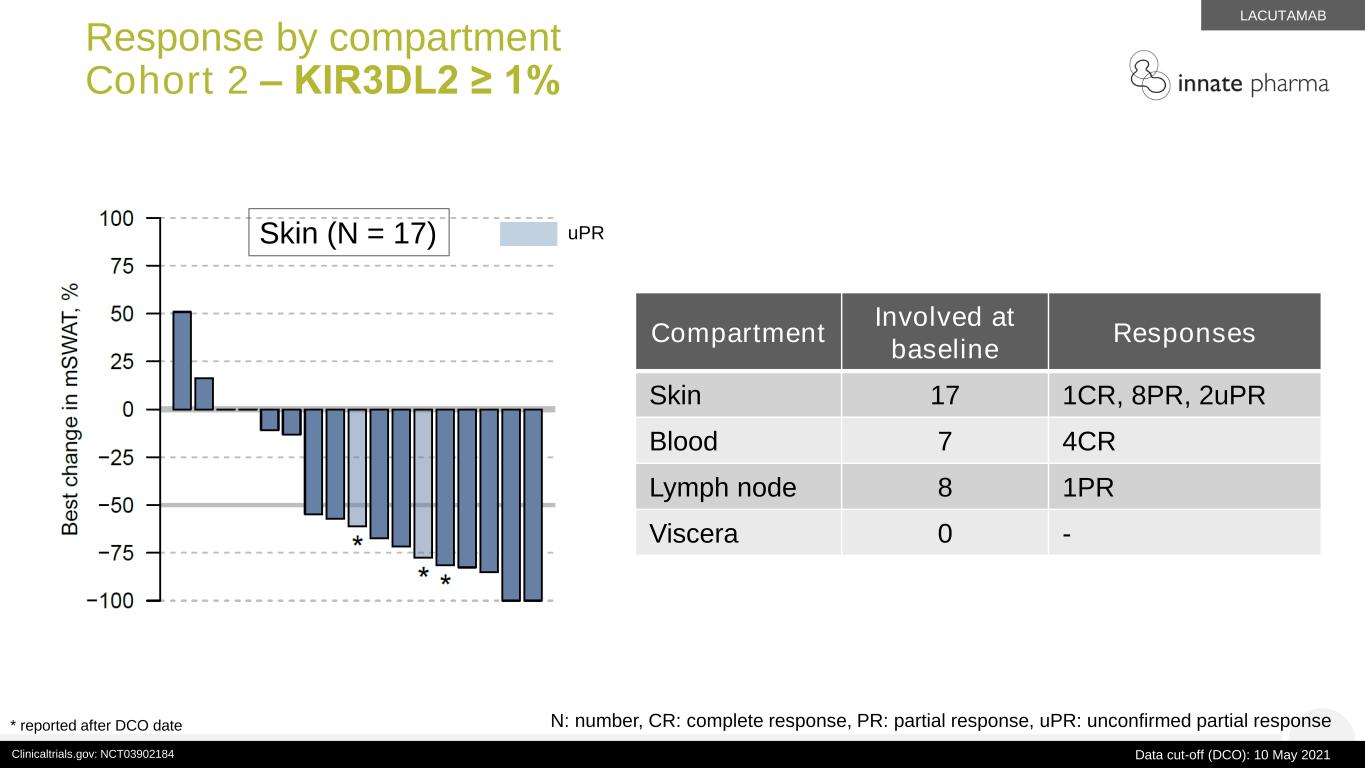
Response by compartment Cohort 2 – KIR3DL2 ≥ 1% Compartment Involved at baseline Responses Skin 17 1CR, 8PR, 2uPR Blood 7 4CR Lymph node 8 1PR Viscera 0 - N: number, CR: complete response, PR: partial response, uPR: unconfirmed partial response Skin (N = 17) uPR * reported after DCO date Data cut-off (DCO): 10 May 2021Clinicaltrials.gov: NCT03902184 LACUTAMAB

Response by compartment Cohort 3 – KIR3DL2 < 1% Compartmen t Involved at baseline Responses Skin 19 2 PR Blood 2 - Lymph node 6 - Viscera 0 - N: number, CR: complete response, PR: partial response Skin (N = 19) * reported after DCO date Data cut-off (DCO): 10 May 2021Clinicaltrials.gov: NCT03902184 LACUTAMAB

Treatment related adverse events* (at least 5%) Cohort 2 + 3 (N = 36) Any grade Grade 3 / 4 Asthenia 4 (11%) 0 Nausea 4 (11%) 0 Arthralgia 2 (6%) 0 Diarrhea 2 (6%) 0 Fatigue 2 (6%) 0 AEs leading to treatment discontinuation 1 (3%) 1 (3%)^ Serious adverse events 1 (3%) 1 (3%) ^ Fatal adverse events 0 0 * as defined by the treating investigator ^ Interstitial lung disease grade 3 • Updated in Apr2022: The following patient developed interstitial lung disease on 24Nov2020 which was probably related to lacutamab. The patient recovered without sequelae on 17Jun2021 and discontinued from study treatment on 11Nov2020. The patient went on to be treated with subsequent treatments (May2021 to Dec2021) for Mycosis Fungoides, had infections and died on 29Mar2022 which we consider unrelated to lacutamab. The TELLOMAK trial is continuing as planned. Data cut-off (DCO): 10 May 2021Clinicaltrials.gov: NCT03902184 LACUTAMAB

ESMO-IO 2020 Abstract 235 Poster 81P 43 Monalizumab R/M SCCHN: Updated Results from Phase 2 Trial Cohort 2 post platinum and post anti-PD-(L)1 (n=40) • Population with high medical need • R/M SCCHN post platinum and post anti-PD-(L)1 where no treatment options are currently approved globally. • Promising activity • Response rate of 20% and 6- and 12-month OS of 80% and 33% • Manageable safety profile Based on these results, a randomized phase 3 trial is underway to evaluate the combination of monalizumab + cetuximab versus cetuximab + placebo in R/M SCCHN post platinum and post anti-PD-(L)1 patients. Best Change of Tumor Size From Baseline Overall Survival MONALIZUMAB
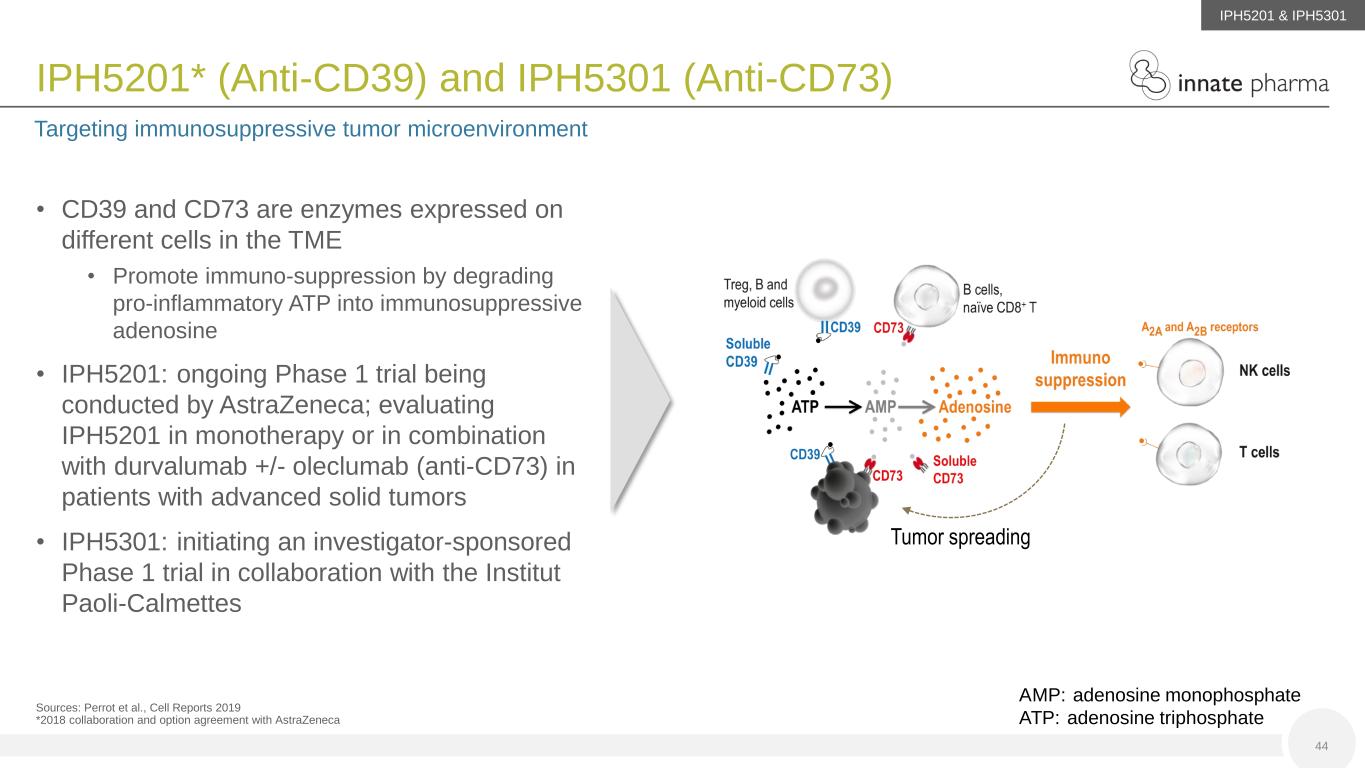
• CD39 and CD73 are enzymes expressed on different cells in the TME • Promote immuno-suppression by degrading pro-inflammatory ATP into immunosuppressive adenosine • IPH5201: ongoing Phase 1 trial being conducted by AstraZeneca; evaluating IPH5201 in monotherapy or in combination with durvalumab +/- oleclumab (anti-CD73) in patients with advanced solid tumors • IPH5301: initiating an investigator-sponsored Phase 1 trial in collaboration with the Institut Paoli-Calmettes IPH5201* (Anti-CD39) and IPH5301 (Anti-CD73) Targeting immunosuppressive tumor microenvironment Sources: Perrot et al., Cell Reports 2019 *2018 collaboration and option agreement with AstraZeneca Tumor spreading AMP: adenosine monophosphate ATP: adenosine triphosphate 44 IPH5201 & IPH5301

Sources: Perrot et al., Cell Reports 2019 *2018 collaboration and option agreement with AstraZeneca IPH5201*: Preclinical Data Significant tumor responses observed in response to treatment with PD-1 inhibitors and ADCC-inducing antibodies, as well as with immunogenic chemotherapy, compared to responses to these agents in wild-type mice 45 IPH5201

I P H 5 3 M E D I 9 4 4 7 C D 7 3 . 4 0 2 0 4 0 6 0 8 0 1 0 0 C D 4 + T c e l l s P r o li f e r a t io n r a t e ( n o r m a li z e d t o c o n t r o l) * * * * * * IPH5301: Preclinical Data IPH5301 more potent in restoring CD4+ and CD8+ T-cell proliferation in an ATP-suppression assay, than the most advanced clinical candidates CD4+ T cells I P H 5 3 M E D I 9 4 4 7 C D 7 3 . 4 0 2 0 4 0 6 0 8 0 1 0 0 C D 8 + T c e l l s * * * * CD8+ T cells IPH53 MEDI a-CD73 Ab BMS a-CD73 Ab 46 IPH5301













































