
June 2016 Innovative CNS therapies to address unmet medical needs Exhibit 99.1

This presentation contains certain forward-looking statements about Minerva Neurosciences that are intended to be covered by the safe harbor for “forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “expect(s),” “feel(s),” “believe(s),” “will,” “may,” “anticipate(s)” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to: the benefits, efficacy and safety of our new formulations; whether studies performed on analogs or backups of our compounds are a good predictor of the clinical efficacy of our compounds; the timing and results of future clinical milestones; the timing of future clinical trials and results of such clinical trials; statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; our expectations regarding approval for our products by the U.S. Food and Drug Administration or equivalent foreign regulatory agencies; estimates regarding the market potential for our products; financial projections and estimates and their underlying assumptions; and future performance. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking statements. These risks and uncertainties include, but are not limited to: the benefits, efficacy and safety of our new formulations; whether analogs or backups of our compounds are a good predictor of the clinical efficacy of our compounds; the timing and results of future clinical milestones; the timing of future clinical trials and results of such clinical trials; whether any of our therapeutic candidates will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic candidates will be successfully marketed if approved; whether our therapeutic product discovery and development efforts will be successful; our ability to achieve the results contemplated by our collaboration agreements; the strength and enforceability of our intellectual property rights; competition from pharmaceutical and biotechnology companies; the development of and our ability to take advantage of the market for our therapeutic products; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; and the other risk factors contained in our periodic and interim reports filed with the Securities and Exchange Commission which are available on the SEC website at www.sec.gov. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. All trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. Forward-Looking Statement Safe-Harbor
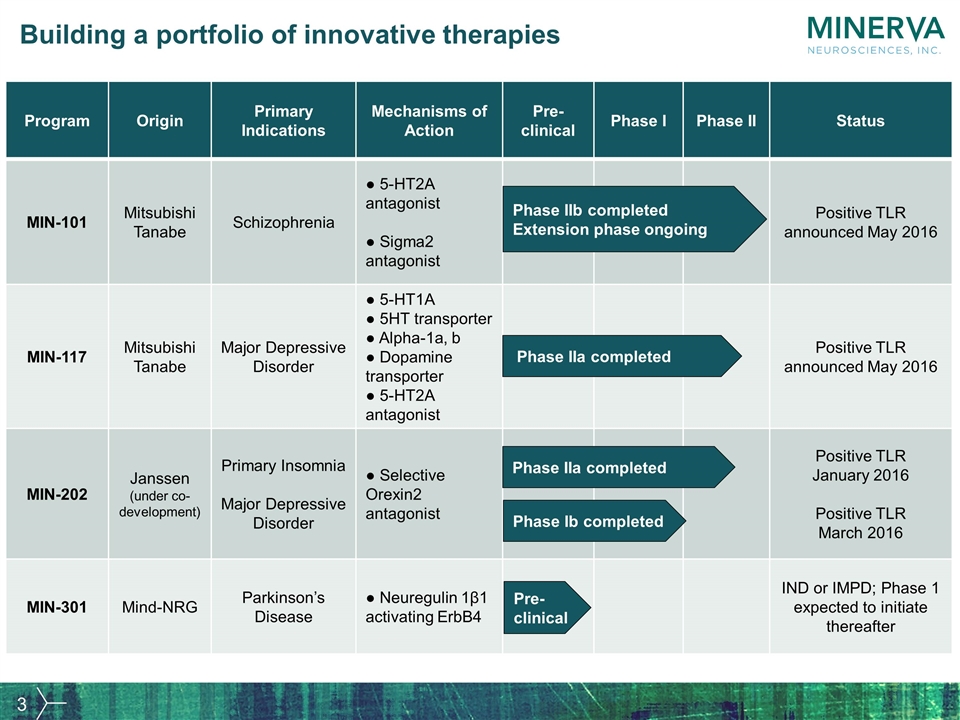
Building a portfolio of innovative therapies Program Origin Primary Indications Mechanisms of Action Pre- clinical Phase I Phase II Status MIN-101 Mitsubishi Tanabe Schizophrenia ● 5-HT2A antagonist ● Sigma2 antagonist Positive TLR announced May 2016 MIN-117 Mitsubishi Tanabe Major Depressive Disorder ● 5-HT1A ● 5HT transporter ● Alpha-1a, b ● Dopamine transporter ● 5-HT2A antagonist Positive TLR announced May 2016 MIN-202 Janssen (under co-development) Primary Insomnia Major Depressive Disorder ● Selective Orexin2 antagonist Positive TLR January 2016 Positive TLR March 2016 MIN-301 Mind-NRG Parkinson’s Disease ● Neuregulin 1β1 activating ErbB4 IND or IMPD; Phase 1 expected to initiate thereafter Phase IIb completed Extension phase ongoing Phase IIa completed Phase Ib completed Pre-clinical Phase IIa completed

MIN-101 A new drug with the potential to address unmet needs in schizophrenia
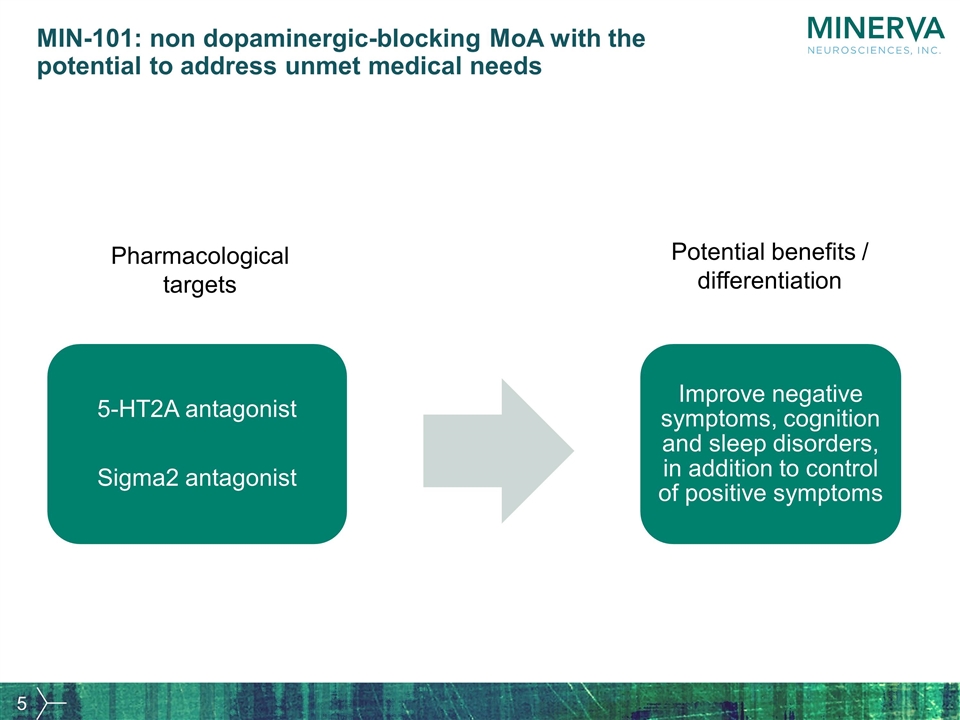
MIN-101: non dopaminergic-blocking MoA with the potential to address unmet medical needs Pharmacological targets Potential benefits / differentiation 5-HT2A antagonist Sigma2 antagonist Improve negative symptoms, cognition and sleep disorders, in addition to control of positive symptoms
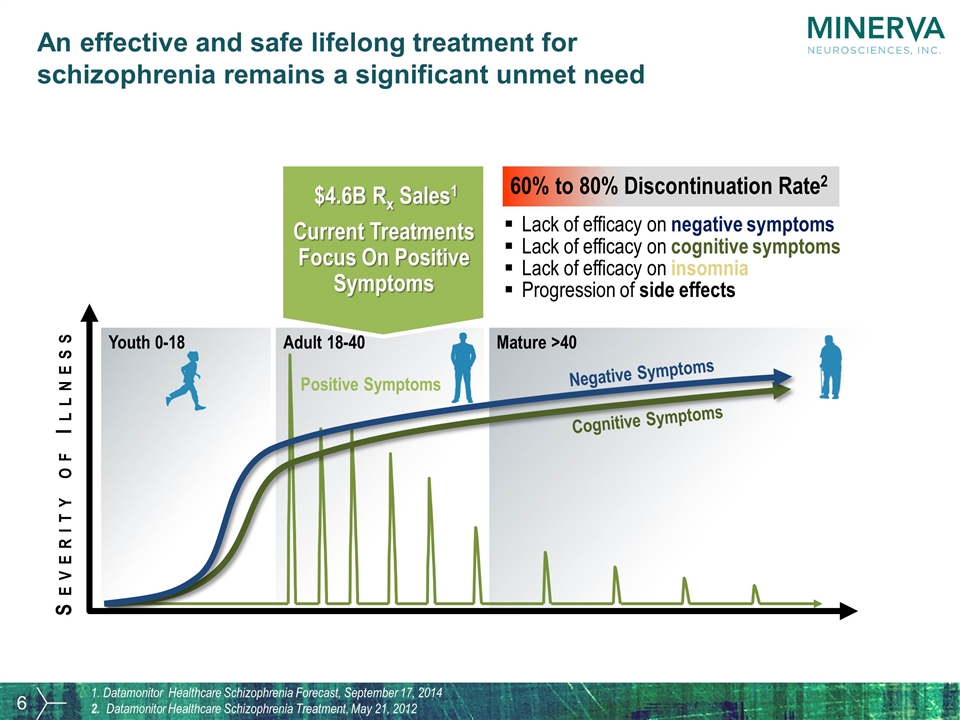
Severity of Illness Chronic Progression of Negative Symptoms & Cognitive Impairment Youth 0-18 Adult 18-40 Mature >40 Cognitive Symptoms Negative Symptoms Positive Symptoms Current Treatments Focus On Positive Symptoms $4.6B Rx Sales1 60% to 80% Discontinuation Rate2 Lack of efficacy on negative symptoms Lack of efficacy on cognitive symptoms Lack of efficacy on insomnia Progression of side effects 1. Datamonitor Healthcare Schizophrenia Forecast, September 17, 2014 2. Datamonitor Healthcare Schizophrenia Treatment, May 21, 2012 An effective and safe lifelong treatment for schizophrenia remains a significant unmet need
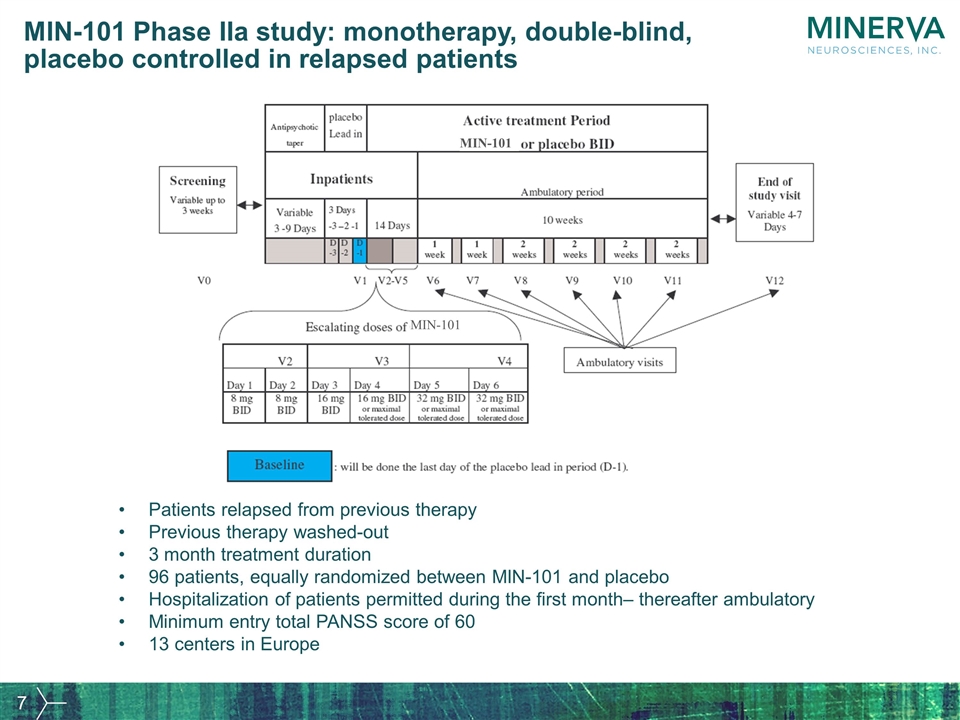
MIN-101 Phase IIa study: monotherapy, double-blind, placebo controlled in relapsed patients Patients relapsed from previous therapy Previous therapy washed-out 3 month treatment duration 96 patients, equally randomized between MIN-101 and placebo Hospitalization of patients permitted during the first month– thereafter ambulatory Minimum entry total PANSS score of 60 13 centers in Europe MIN-101 MIN-101
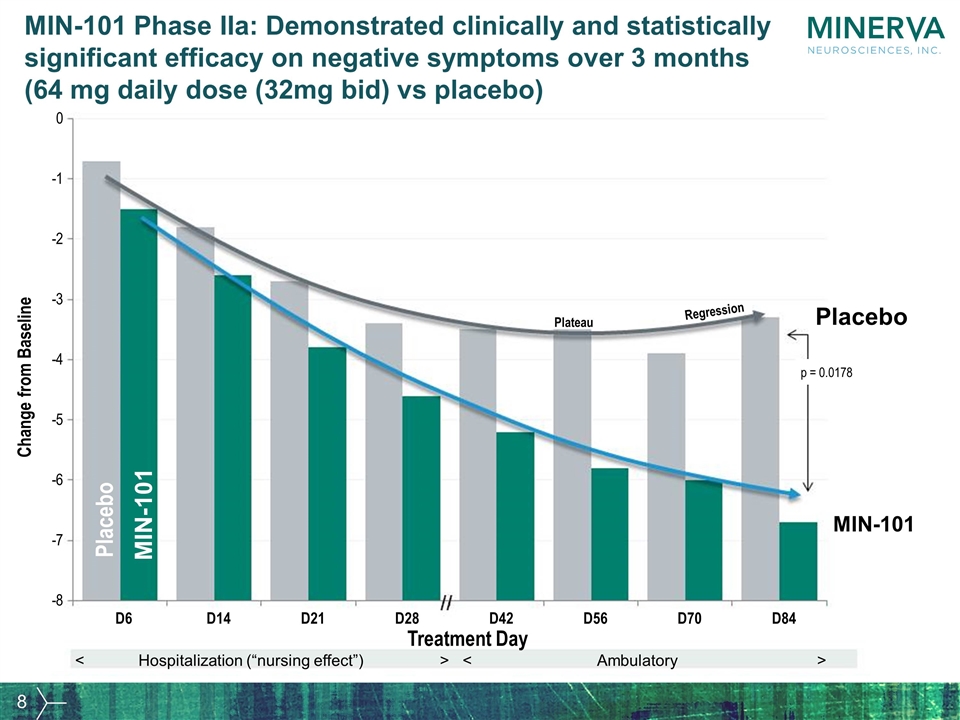
MIN-101 Phase IIa: Demonstrated clinically and statistically significant efficacy on negative symptoms over 3 months (64 mg daily dose (32mg bid) vs placebo) Change from Baseline Placebo Plateau p = 0.0178 Regression Placebo MIN-101 MIN-101 - 8 - 7 - 6 - 5 - 4 - 3 - 2 - 1 0 D6 D14 D21 D28 D42 D56 D70 D84 Treatment Day > < Hospitalization (“nursing effect”) > < Ambulatory
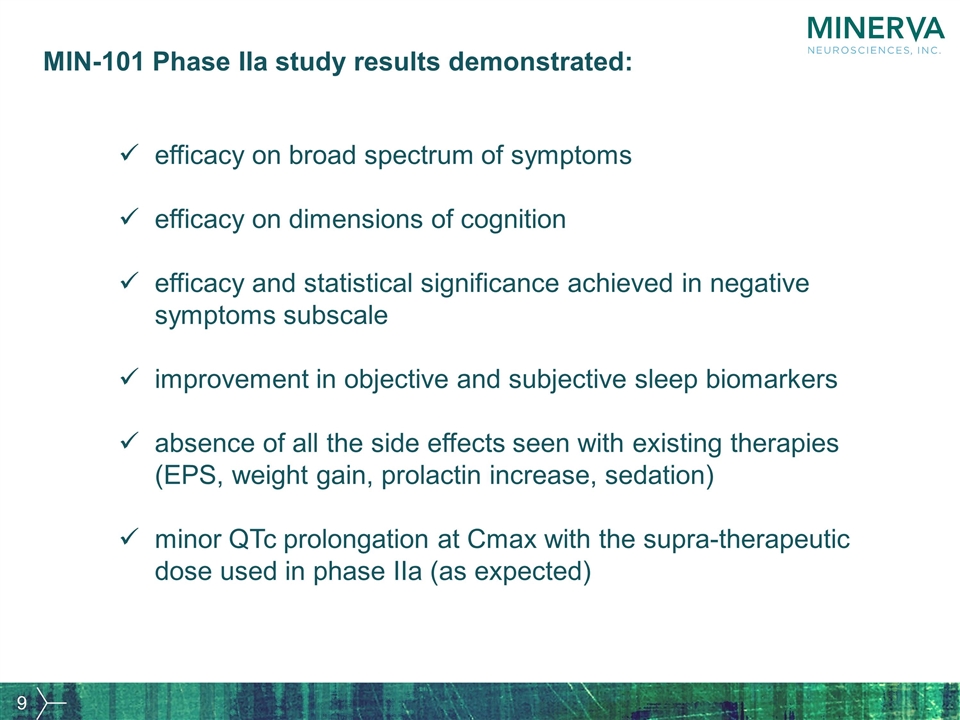
MIN-101 Phase IIa study results demonstrated: efficacy on broad spectrum of symptoms efficacy on dimensions of cognition efficacy and statistical significance achieved in negative symptoms subscale improvement in objective and subjective sleep biomarkers absence of all the side effects seen with existing therapies (EPS, weight gain, prolactin increase, sedation) minor QTc prolongation at Cmax with the supra-therapeutic dose used in phase IIa (as expected)

MIN-101 Phase IIb A multi-center, randomized, double-blind, parallel-group, placebo-controlled 12 week study to evaluate the efficacy, tolerability and safety of MIN-101 in patients with negative symptoms of schizophrenia followed by a 24-week, open-label extension
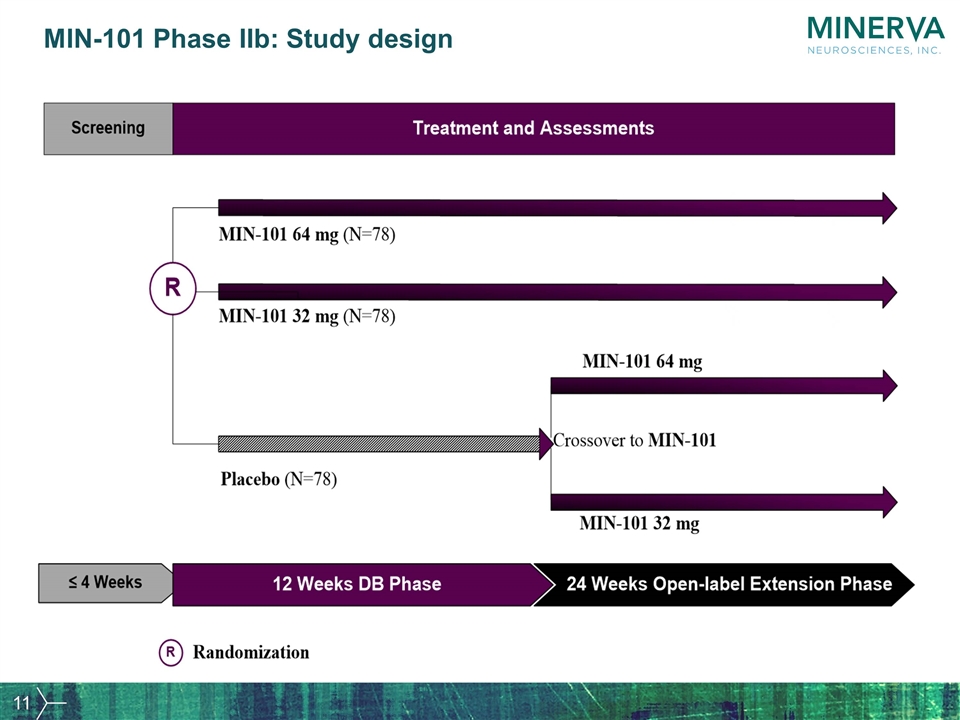
MIN-101 Phase IIb: Study design
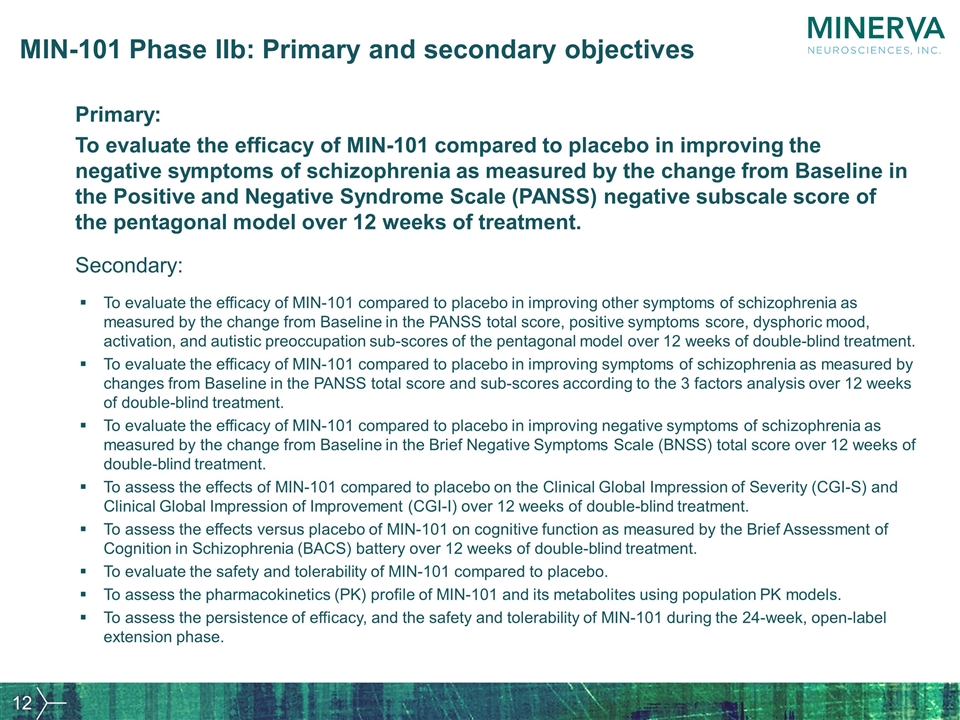
MIN-101 Phase IIb: Primary and secondary objectives Primary: To evaluate the efficacy of MIN-101 compared to placebo in improving the negative symptoms of schizophrenia as measured by the change from Baseline in the Positive and Negative Syndrome Scale (PANSS) negative subscale score of the pentagonal model over 12 weeks of treatment. To evaluate the efficacy of MIN-101 compared to placebo in improving other symptoms of schizophrenia as measured by the change from Baseline in the PANSS total score, positive symptoms score, dysphoric mood, activation, and autistic preoccupation sub-scores of the pentagonal model over 12 weeks of double-blind treatment. To evaluate the efficacy of MIN-101 compared to placebo in improving symptoms of schizophrenia as measured by changes from Baseline in the PANSS total score and sub-scores according to the 3 factors analysis over 12 weeks of double-blind treatment. To evaluate the efficacy of MIN-101 compared to placebo in improving negative symptoms of schizophrenia as measured by the change from Baseline in the Brief Negative Symptoms Scale (BNSS) total score over 12 weeks of double-blind treatment. To assess the effects of MIN-101 compared to placebo on the Clinical Global Impression of Severity (CGI-S) and Clinical Global Impression of Improvement (CGI-I) over 12 weeks of double-blind treatment. To assess the effects versus placebo of MIN-101 on cognitive function as measured by the Brief Assessment of Cognition in Schizophrenia (BACS) battery over 12 weeks of double-blind treatment. To evaluate the safety and tolerability of MIN-101 compared to placebo. To assess the pharmacokinetics (PK) profile of MIN-101 and its metabolites using population PK models. To assess the persistence of efficacy, and the safety and tolerability of MIN-101 during the 24-week, open-label extension phase. Secondary:
MIN-101 Phase IIb: Statistical methods Primary endpoint analysis: Efficacy analyses are based on the Intent to Treat (ITT) population Mixed-effects model for repeated measures (MMRM) is applied Changes from baseline to week 12 in PANSS negative subscale score using the pentagonal structured model is the primary endpoint The Hochberg procedure is applied in order to maintain the Type I error rate due to multiple comparisons of the primary endpoint results at or below 0.050%
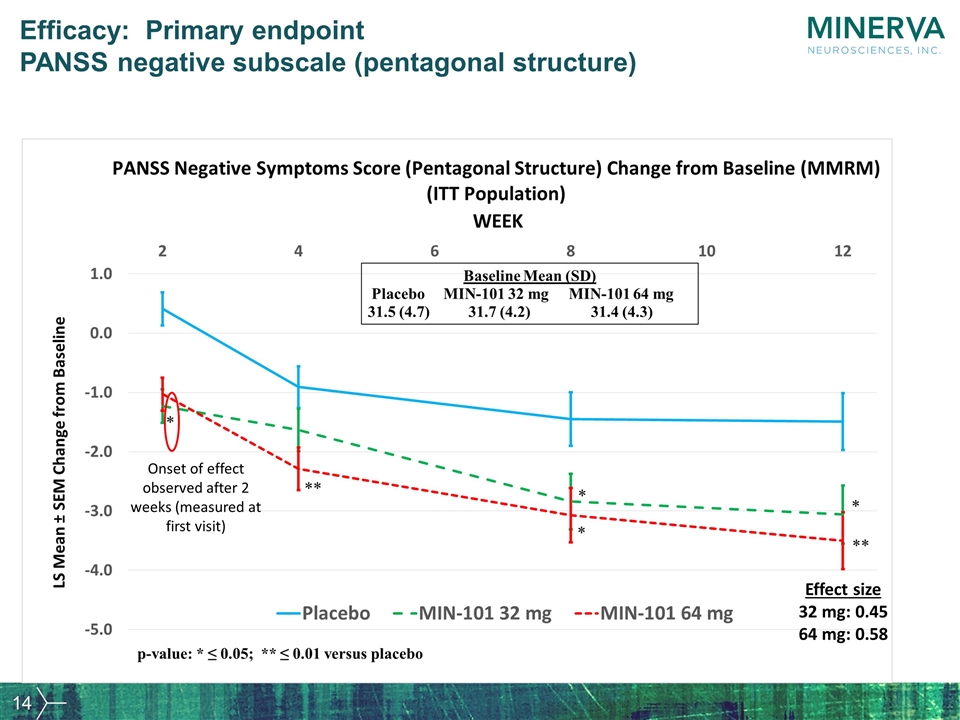
Effect size 32 mg: 0.45 64 mg: 0.58 Onset of effect observed after 2 weeks (measured at first visit) Efficacy: Primary endpoint PANSS negative subscale (pentagonal structure)
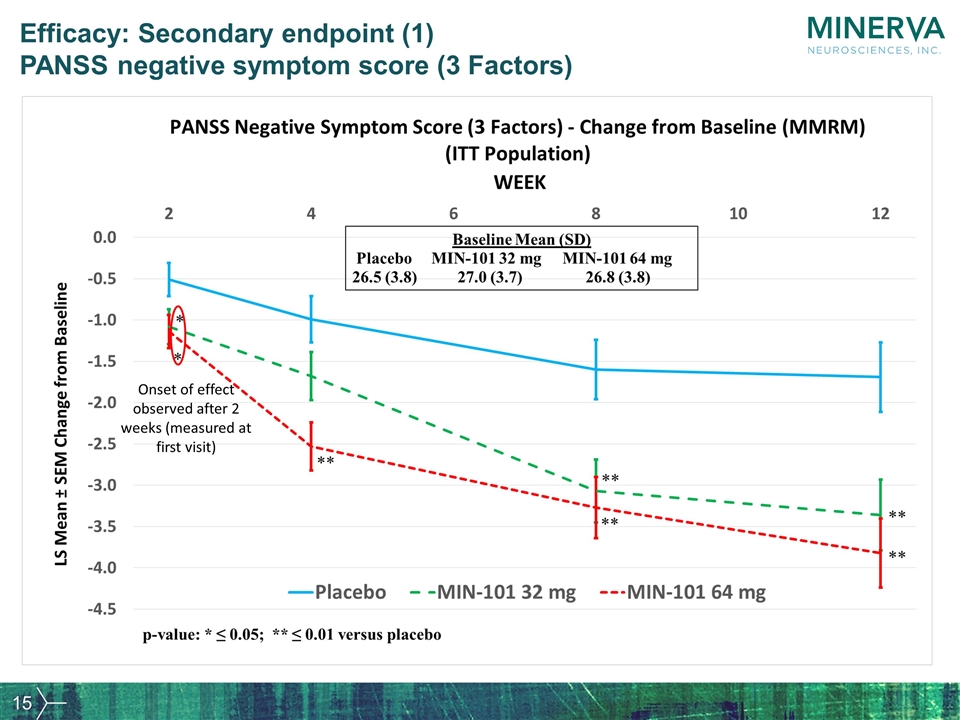
Efficacy: Secondary endpoint (1) PANSS negative symptom score (3 Factors) Onset of effect observed after 2 weeks (measured at first visit)
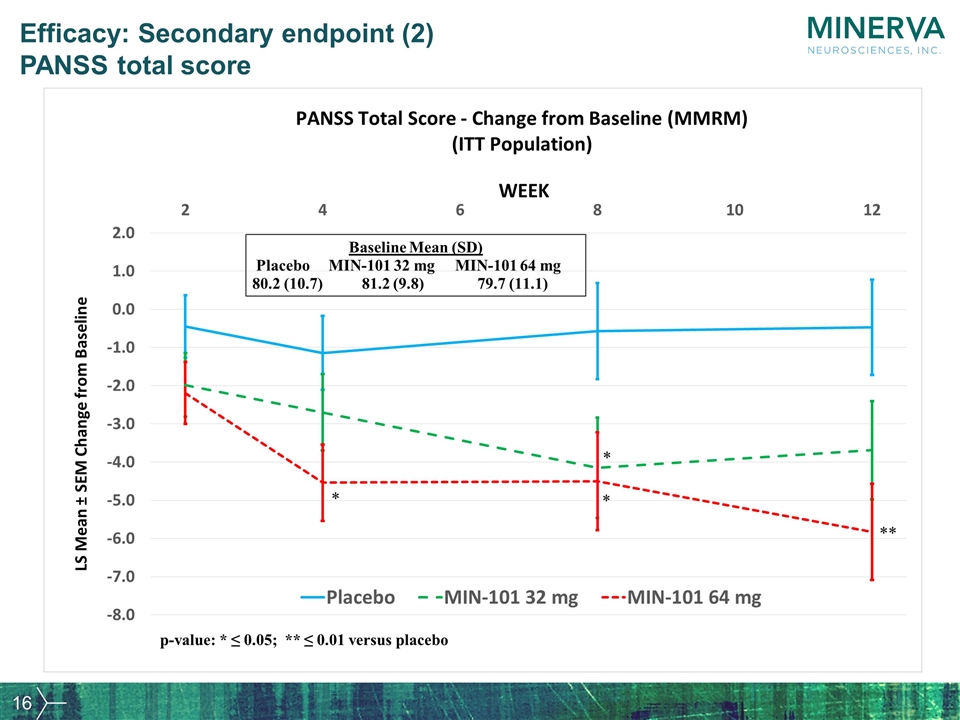
Efficacy: Secondary endpoint (2) PANSS total score
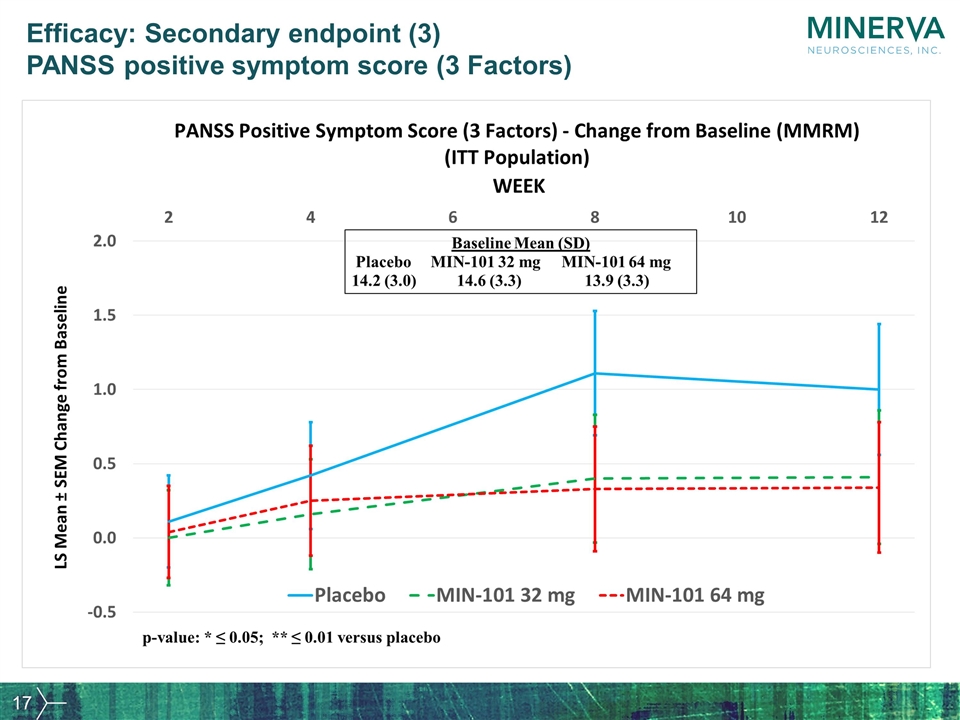
Efficacy: Secondary endpoint (3) PANSS positive symptom score (3 Factors)
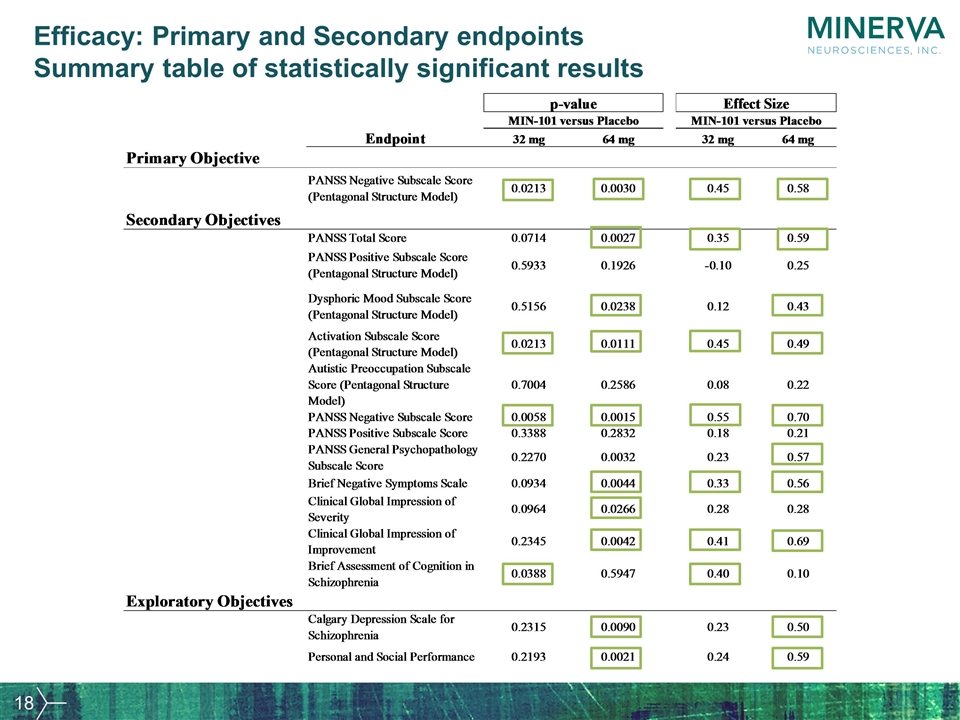
Efficacy: Primary and Secondary endpoints Summary table of statistically significant results
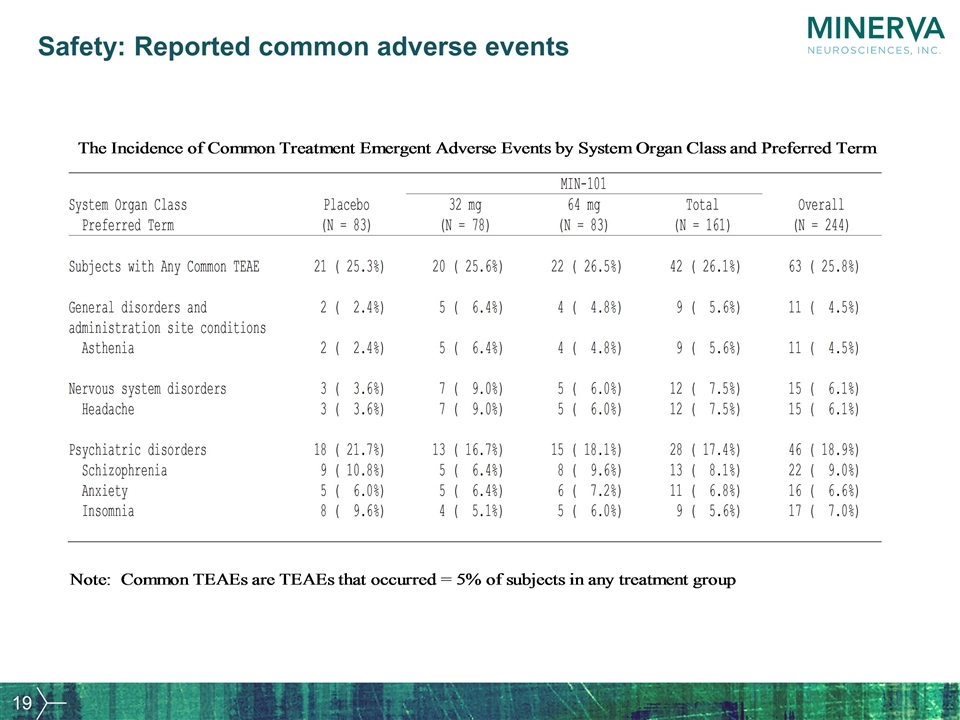
Safety: Reported common adverse events
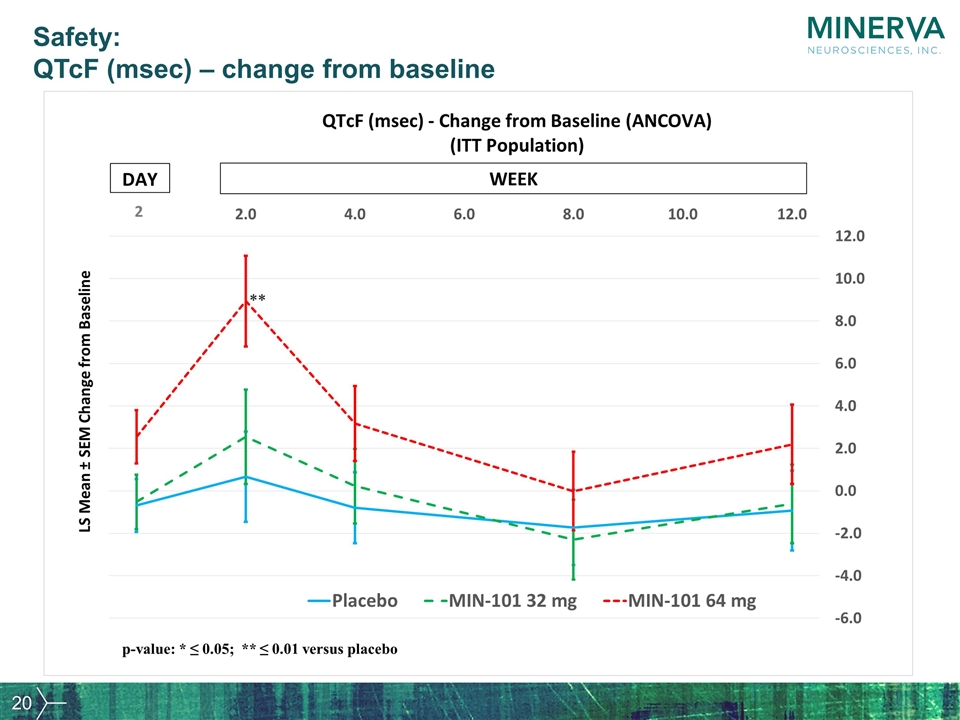
Safety: QTcF (msec) – change from baseline
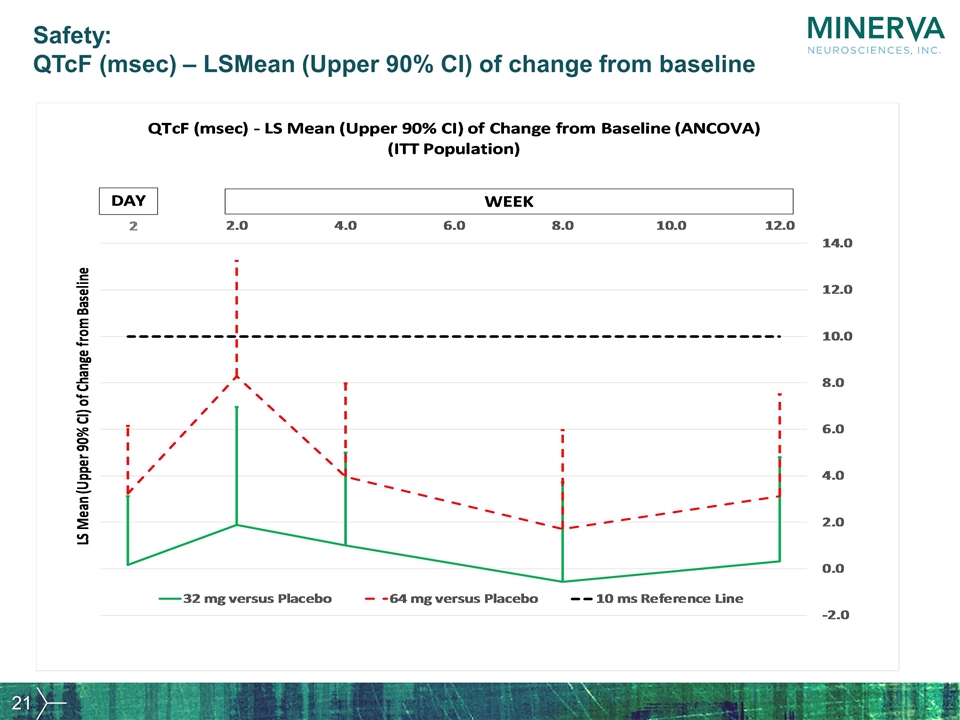
Safety: QTcF (msec) – LSMean (Upper 90% CI) of change from baseline
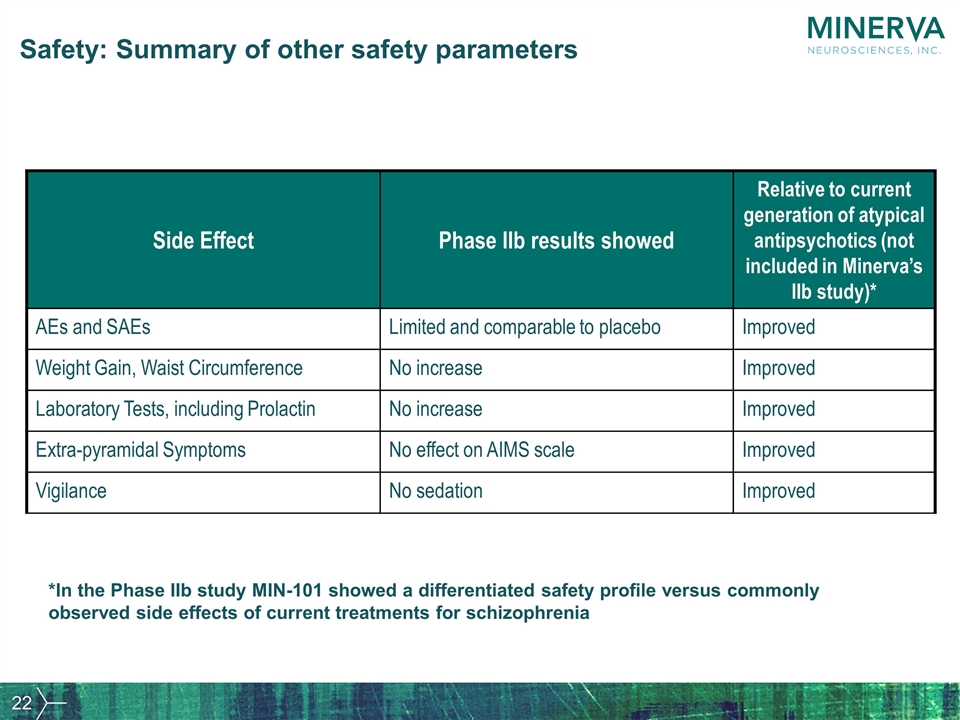
Safety: Summary of other safety parameters Side Effect Phase IIb results showed Relative to current generation of atypical antipsychotics (not included in Minerva’s IIb study)* AEs and SAEs Limited and comparable to placebo Improved Weight Gain, Waist Circumference No increase Improved Laboratory Tests, including Prolactin No increase Improved Extra-pyramidal Symptoms No effect on AIMS scale Improved Vigilance No sedation Improved *In the Phase IIb study MIN-101 showed a differentiated safety profile versus commonly observed side effects of current treatments for schizophrenia

U.S. Investigational New Drug Application (IND) accepted by FDA in December 2015 Advanced-stage U.S. clinical program to be defined following results of Phase IIb trial in Europe Under consideration Multiple potential clinical late-stage pathways / routes to registration Trials to be conducted independently or with a partner MIN-101 US development plan
MIN-101: In-licensed from Mitsubishi Tanabe Pharmaceutical Company (MTPC) Minerva: exclusive worldwide license includes rights to develop, commercialize and sub-license MIN-101 (and back-ups) outside of certain Asian countries MTPC: retains rights to commercialize and sell MIN-101 in certain Asian countries including China, Japan, India and South Korea (MTPC Territory) Milestones upon launch and commercialization goals could total up to $47.5 million payable Royalties payable on net sales range from high single digits to low teens

MIN-117 Our objective is to develop a new drug to address unmet needs in major depressive disorder (MDD)
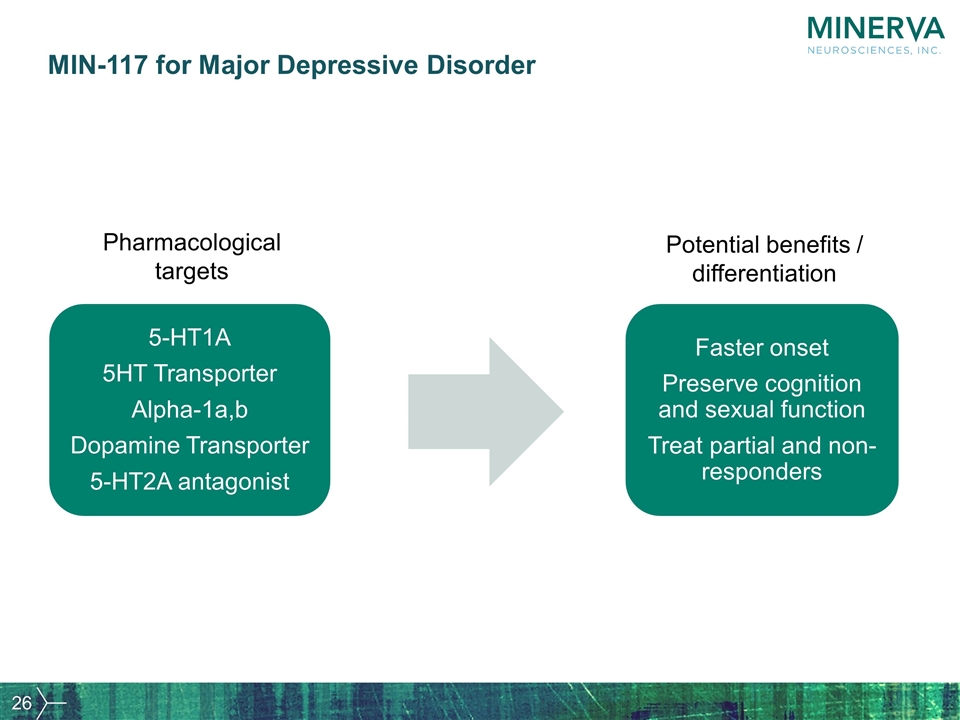
MIN-117 for Major Depressive Disorder 5-HT1A 5HT Transporter Alpha-1a,b Dopamine Transporter 5-HT2A antagonist Faster onset Preserve cognition and sexual function Treat partial and non-responders Potential benefits / differentiation Pharmacological targets

MIN117: Phase IIa A Randomized, Double-Blind, Parallel-Group, Placebo- and Active-Controlled Study to Evaluate the Efficacy and Safety of 2 Doses of MIN-117 in Adult Subjects with Major Depressive Disorder
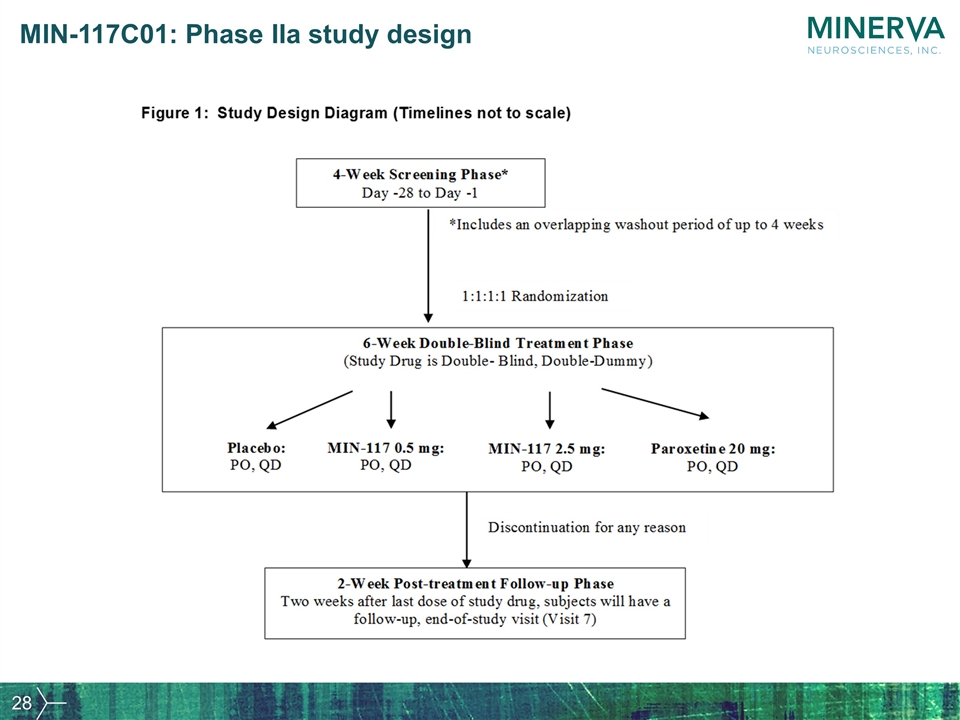
MIN-117C01: Phase IIa study design
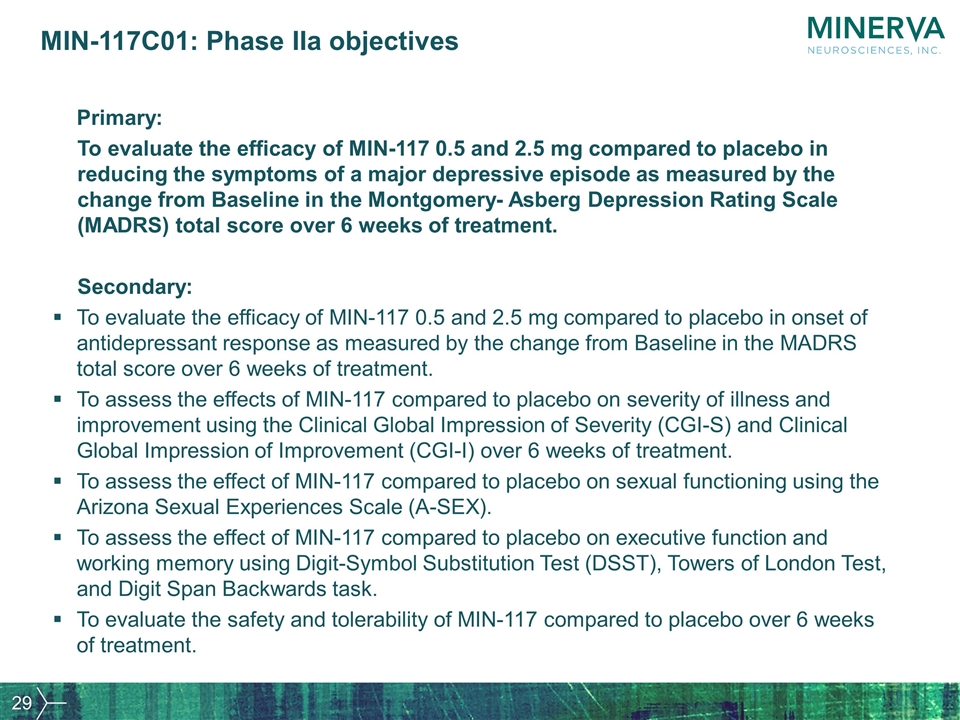
MIN-117C01: Phase IIa objectives Primary: To evaluate the efficacy of MIN-117 0.5 and 2.5 mg compared to placebo in reducing the symptoms of a major depressive episode as measured by the change from Baseline in the Montgomery- Asberg Depression Rating Scale (MADRS) total score over 6 weeks of treatment. Secondary: To evaluate the efficacy of MIN-117 0.5 and 2.5 mg compared to placebo in onset of antidepressant response as measured by the change from Baseline in the MADRS total score over 6 weeks of treatment. To assess the effects of MIN-117 compared to placebo on severity of illness and improvement using the Clinical Global Impression of Severity (CGI-S) and Clinical Global Impression of Improvement (CGI-I) over 6 weeks of treatment. To assess the effect of MIN-117 compared to placebo on sexual functioning using the Arizona Sexual Experiences Scale (A-SEX). To assess the effect of MIN-117 compared to placebo on executive function and working memory using Digit-Symbol Substitution Test (DSST), Towers of London Test, and Digit Span Backwards task. To evaluate the safety and tolerability of MIN-117 compared to placebo over 6 weeks of treatment.
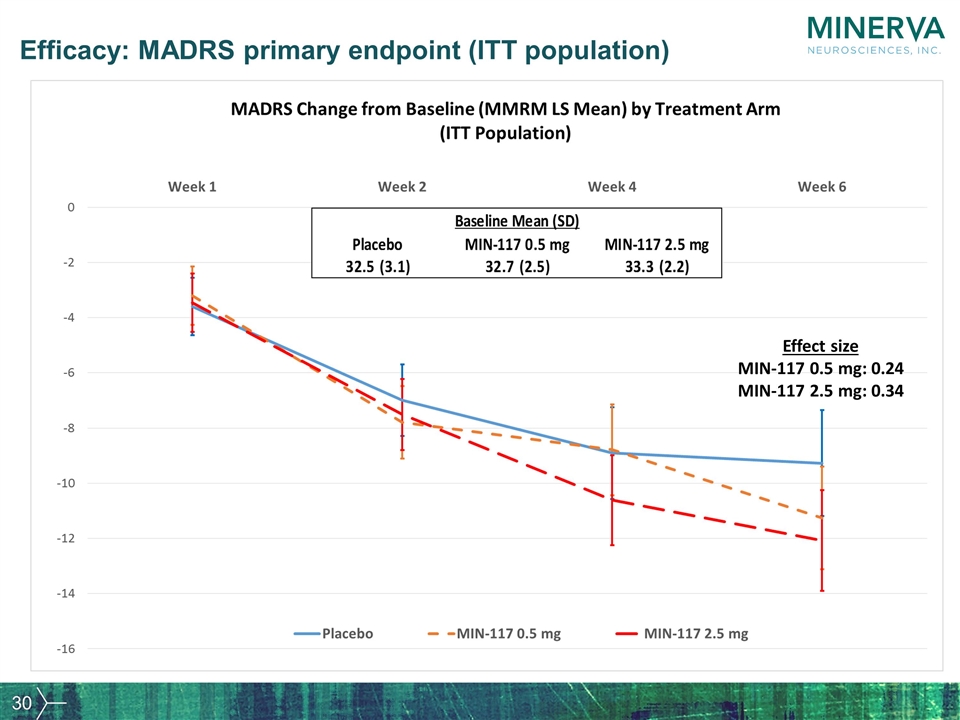
Efficacy: MADRS primary endpoint (ITT population) Effect size MIN-117 0.5 mg: 0.24 MIN-117 2.5 mg: 0.34
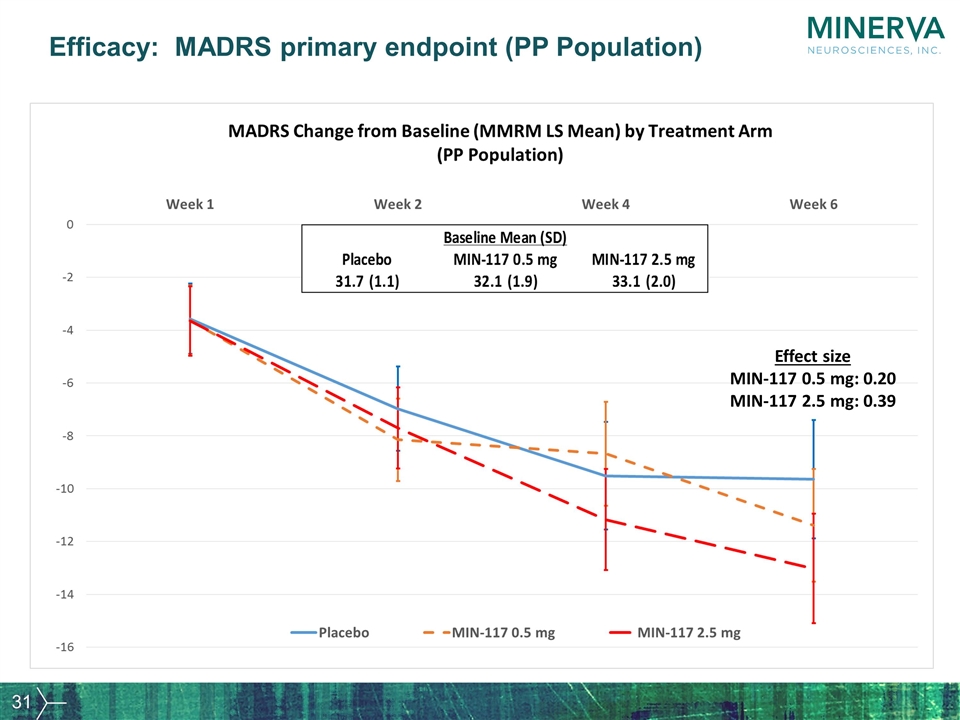
Efficacy: MADRS primary endpoint (PP Population) Effect size MIN-117 0.5 mg: 0.20 MIN-117 2.5 mg: 0.39
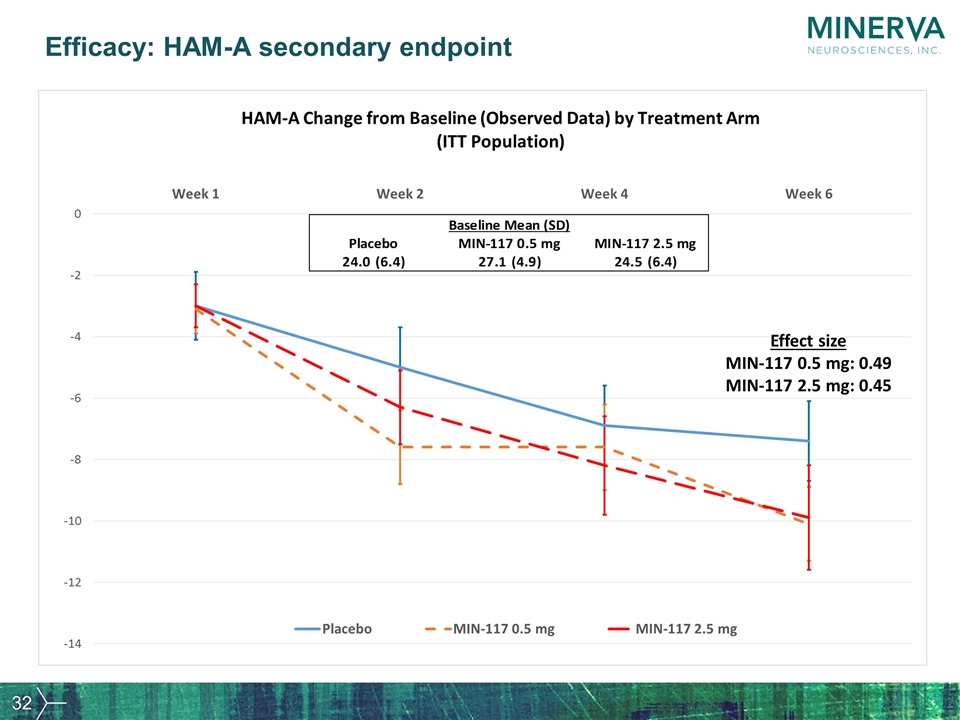
Efficacy: HAM-A secondary endpoint Effect size MIN-117 0.5 mg: 0.49 MIN-117 2.5 mg: 0.45
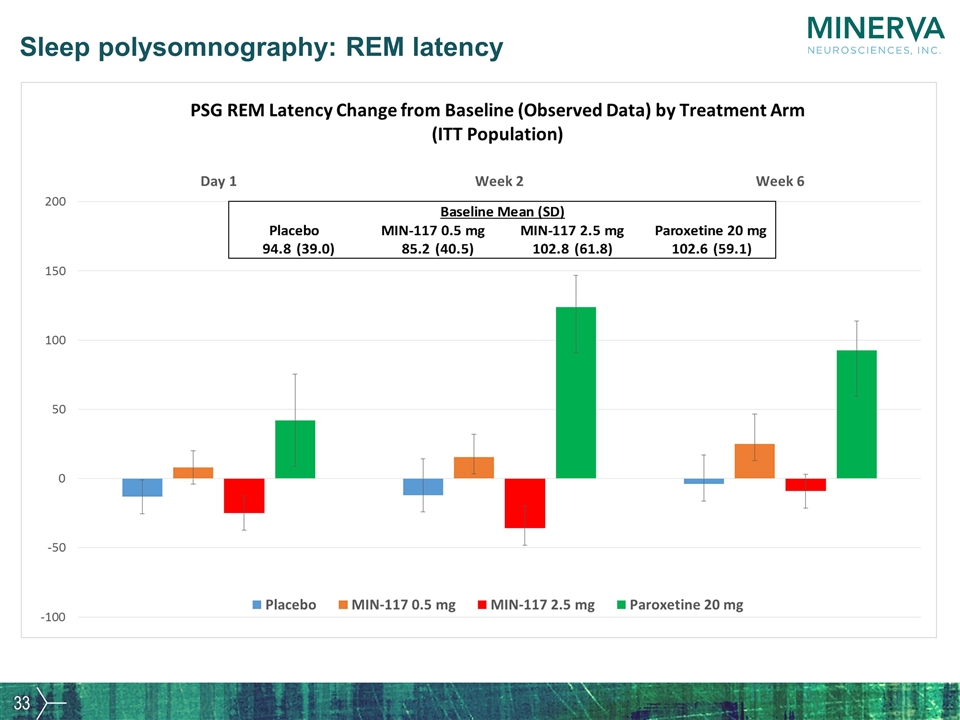
Sleep polysomnography: REM latency
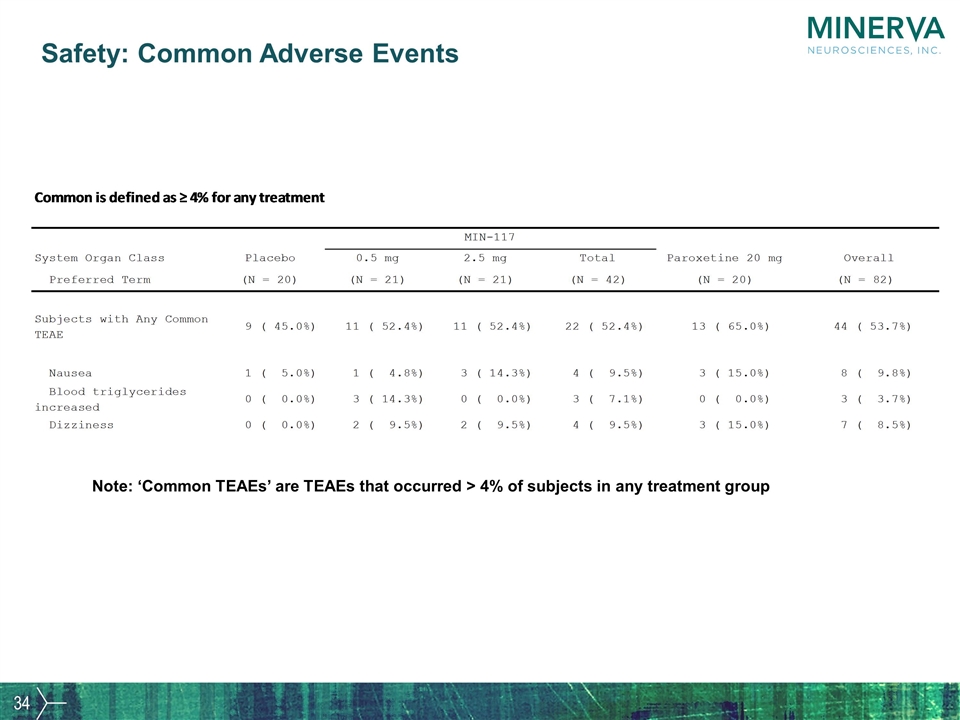
Safety: Common Adverse Events Note: ‘Common TEAEs’ are TEAEs that occurred > 4% of subjects in any treatment group
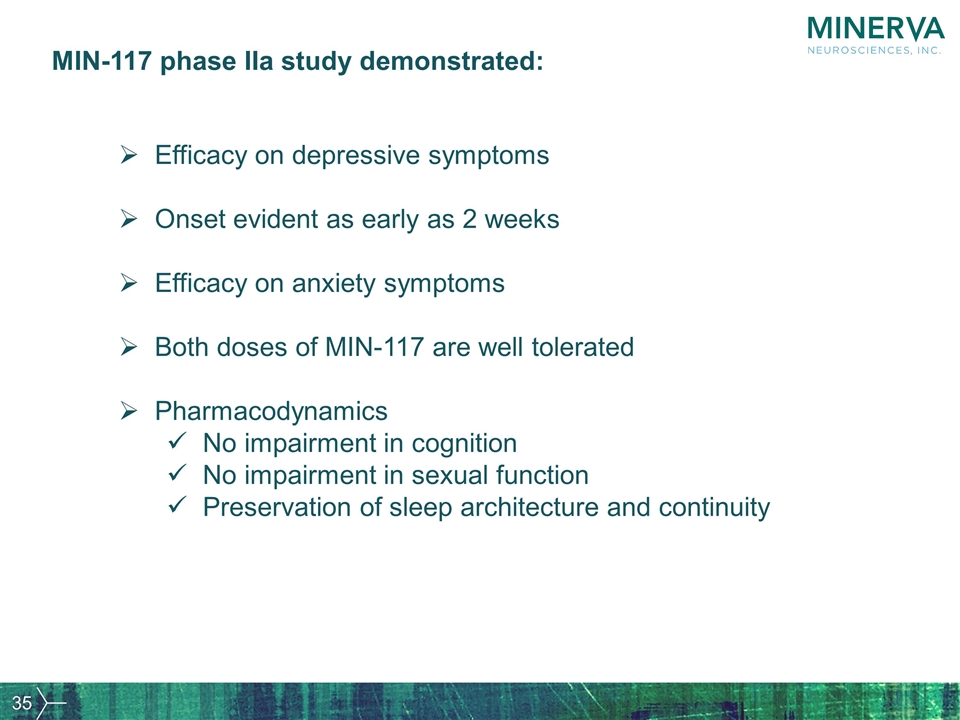
MIN-117 phase IIa study demonstrated: Efficacy on depressive symptoms Onset evident as early as 2 weeks Efficacy on anxiety symptoms Both doses of MIN-117 are well tolerated Pharmacodynamics No impairment in cognition No impairment in sexual function Preservation of sleep architecture and continuity

Minerva: exclusive worldwide license includes rights to develop, commercialize and sub-license MIN-117 outside of certain Asian countries MTPC: retains rights to commercialize and sell MIN-117 in certain Asian countries including China, Japan, India and South Korea (MTPC Territory) Milestones upon launch and commercialization goals could total up to $47.5 million payable Royalties payable on net sales range from high single digits to low teens MIN-117 in-licensed from Mitsubishi Tanabe Pharmaceutical Company (MTPC)

MIN-202 (JNJ-42847922) Insomnia Disorder and Major Depressive Disorder (MDD) A co-development & commercialization program with Janssen
MIN-202: A specific Orexin-2 antagonist for insomnia and mood disorders Selective Orexin2 antagonist Physiological regulation of biological rhythms and control of wake drive Potential benefits / differentiation Pharmacological target
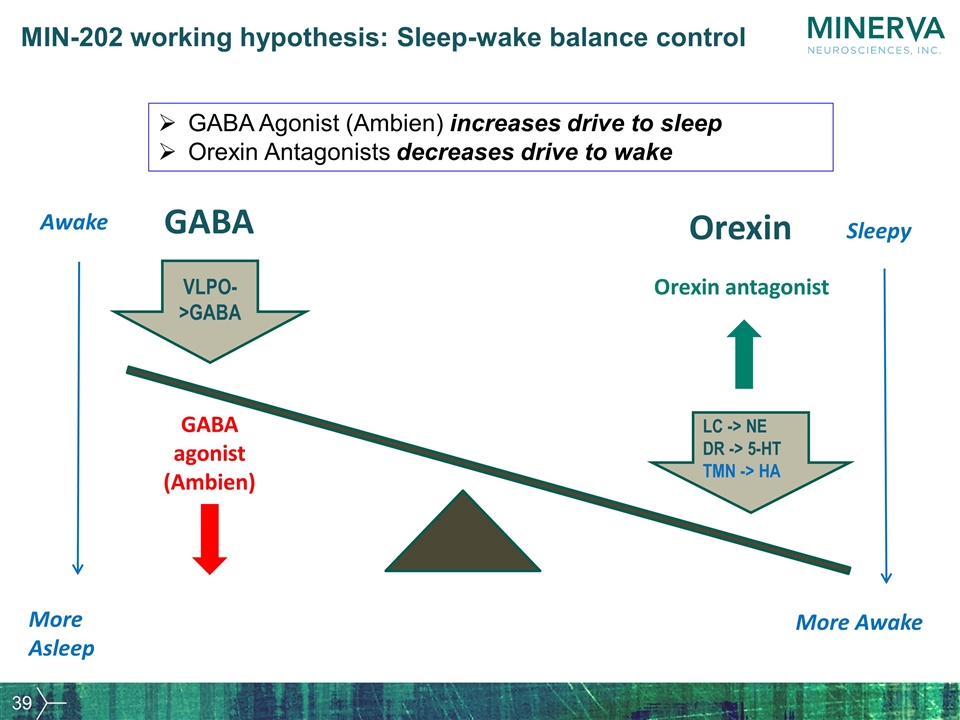
More Awake More Asleep Orexin LC -> NE DR -> 5-HT TMN -> HA GABA VLPO->GABA GABA agonist (Ambien) Orexin antagonist Sleepy Awake GABA Agonist (Ambien) increases drive to sleep Orexin Antagonists decreases drive to wake MIN-202 working hypothesis: Sleep-wake balance control
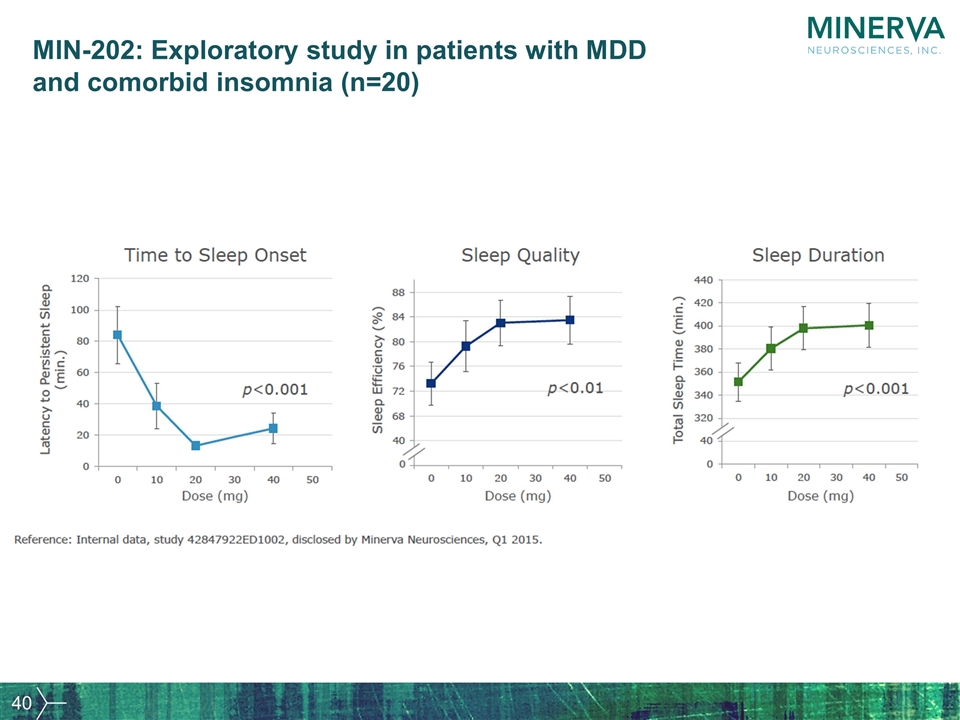
MIN-202: Exploratory study in patients with MDD and comorbid insomnia (n=20)
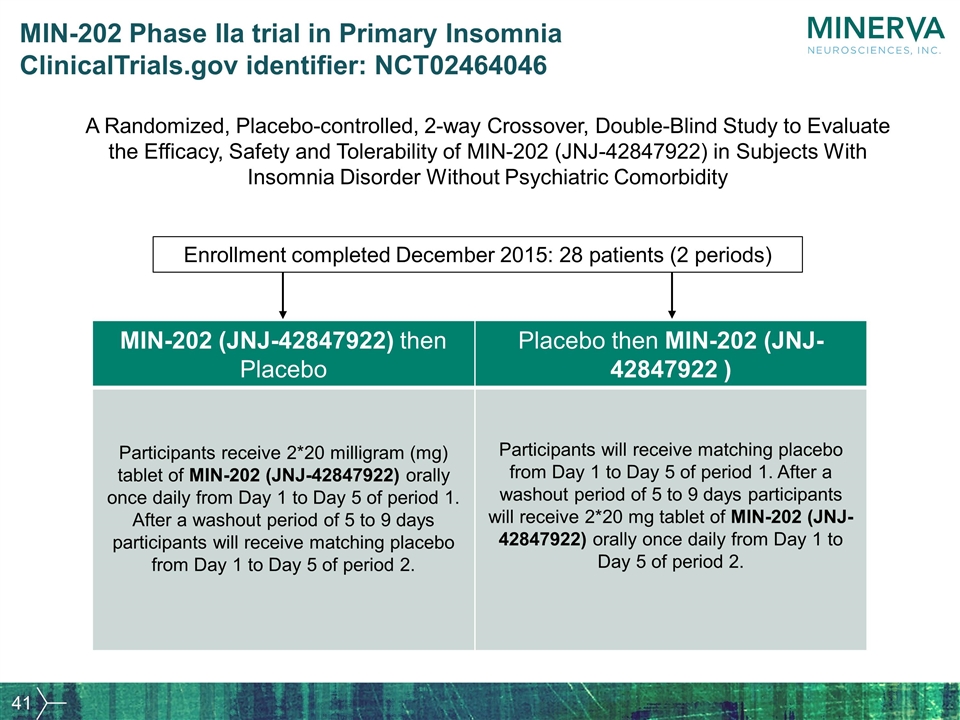
MIN-202 Phase IIa trial in Primary Insomnia ClinicalTrials.gov identifier: NCT02464046 A Randomized, Placebo-controlled, 2-way Crossover, Double-Blind Study to Evaluate the Efficacy, Safety and Tolerability of MIN-202 (JNJ-42847922) in Subjects With Insomnia Disorder Without Psychiatric Comorbidity MIN-202 (JNJ-42847922) then Placebo Placebo then MIN-202 (JNJ-42847922 ) Participants receive 2*20 milligram (mg) tablet of MIN-202 (JNJ-42847922) orally once daily from Day 1 to Day 5 of period 1. After a washout period of 5 to 9 days participants will receive matching placebo from Day 1 to Day 5 of period 2. Participants will receive matching placebo from Day 1 to Day 5 of period 1. After a washout period of 5 to 9 days participants will receive 2*20 mg tablet of MIN-202 (JNJ-42847922) orally once daily from Day 1 to Day 5 of period 2. Enrollment completed December 2015: 28 patients (2 periods)
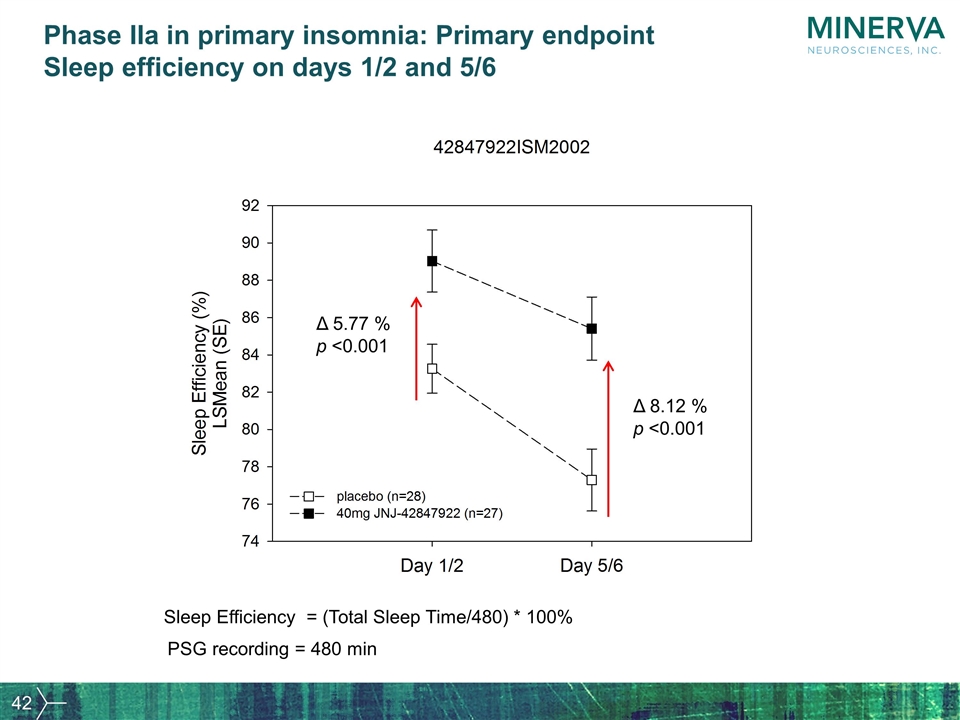
Phase IIa in primary insomnia: Primary endpoint Sleep efficiency on days 1/2 and 5/6 Δ 8.12 % p <0.001 Δ 5.77 % p <0.001 Sleep Efficiency = (Total Sleep Time/480) * 100% PSG recording = 480 min
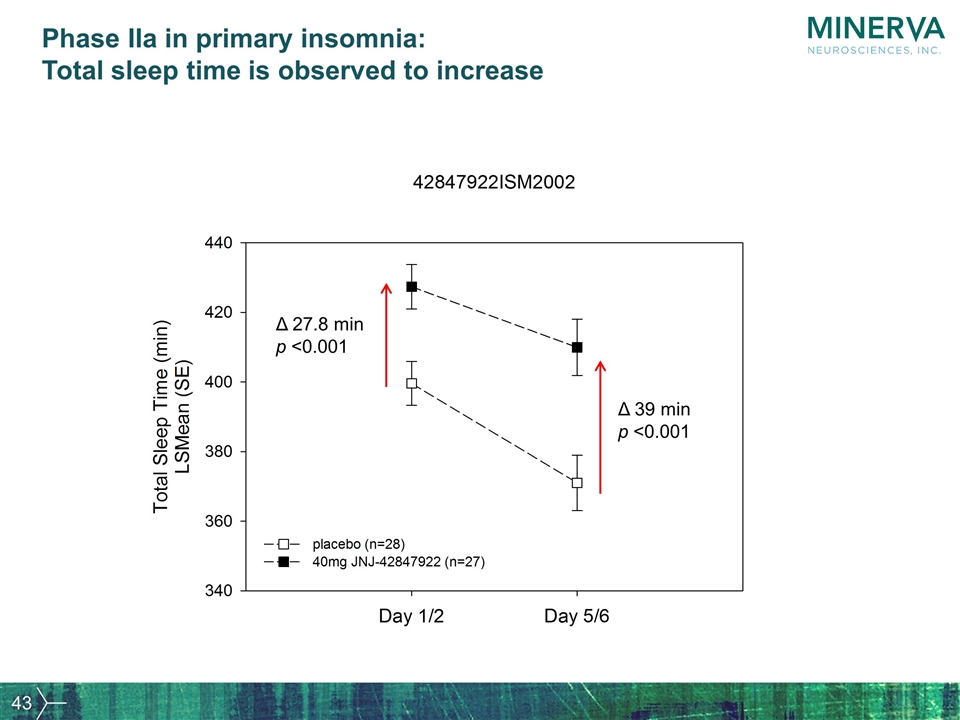
Phase IIa in primary insomnia: Total sleep time is observed to increase Δ 39 min p <0.001 Δ 27.8 min p <0.001
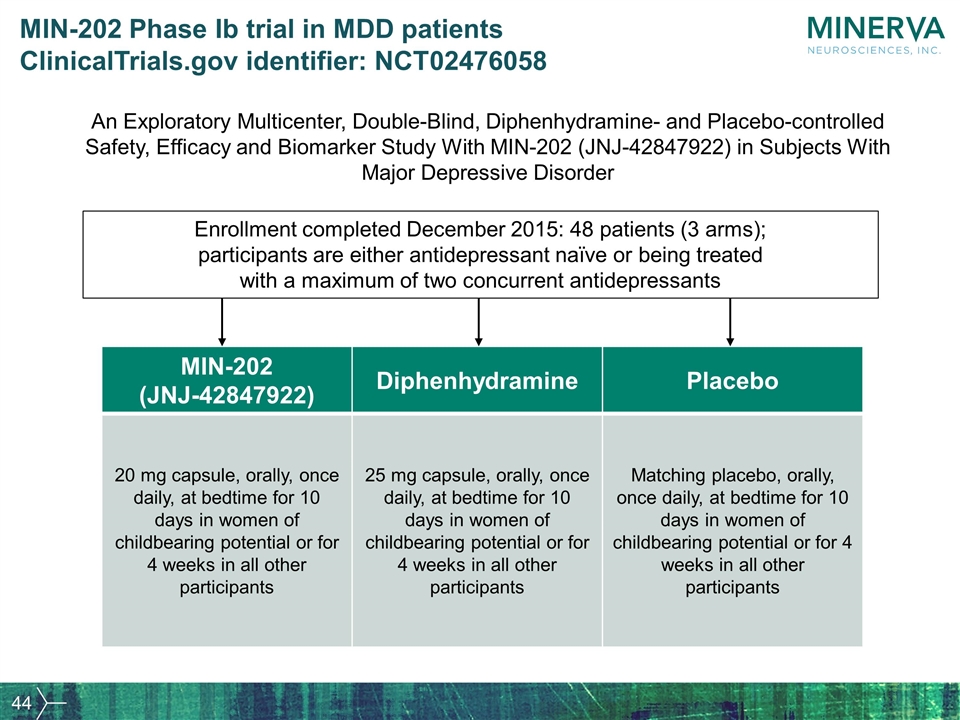
MIN-202 Phase Ib trial in MDD patients ClinicalTrials.gov identifier: NCT02476058 An Exploratory Multicenter, Double-Blind, Diphenhydramine- and Placebo-controlled Safety, Efficacy and Biomarker Study With MIN-202 (JNJ-42847922) in Subjects With Major Depressive Disorder MIN-202 (JNJ-42847922) Diphenhydramine Placebo 20 mg capsule, orally, once daily, at bedtime for 10 days in women of childbearing potential or for 4 weeks in all other participants 25 mg capsule, orally, once daily, at bedtime for 10 days in women of childbearing potential or for 4 weeks in all other participants Matching placebo, orally, once daily, at bedtime for 10 days in women of childbearing potential or for 4 weeks in all other participants Enrollment completed December 2015: 48 patients (3 arms); participants are either antidepressant naïve or being treated with a maximum of two concurrent antidepressants
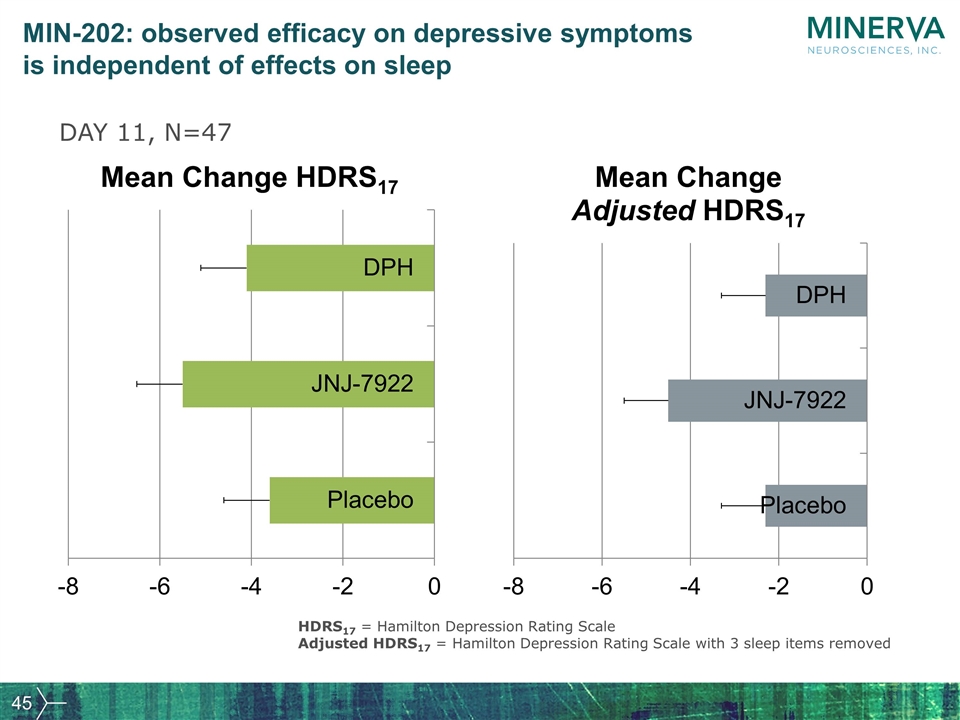
MIN-202: observed efficacy on depressive symptoms is independent of effects on sleep HDRS17 = Hamilton Depression Rating Scale Adjusted HDRS17 = Hamilton Depression Rating Scale with 3 sleep items removed DAY 11, N=47

MIN-202: Co-development and license agreement with Janssen Pharmaceutica NV (Janssen) Minerva has commercialization rights for EU, Switzerland, Liechtenstein, Iceland & Norway (Minerva Territory) with rights to sub-license Minerva pays quarterly high single digit % royalty to Janssen on Minerva Territory net sales Janssen has commercialization rights in all territories outside of the Minerva Territory Janssen pays high single digit % royalty to Minerva on all sales outside of Minerva Territory Minerva contributes 40% of development cost subject to ceiling caps at various points in the clinical development plan

MIN-301: Parkinson’s Disease
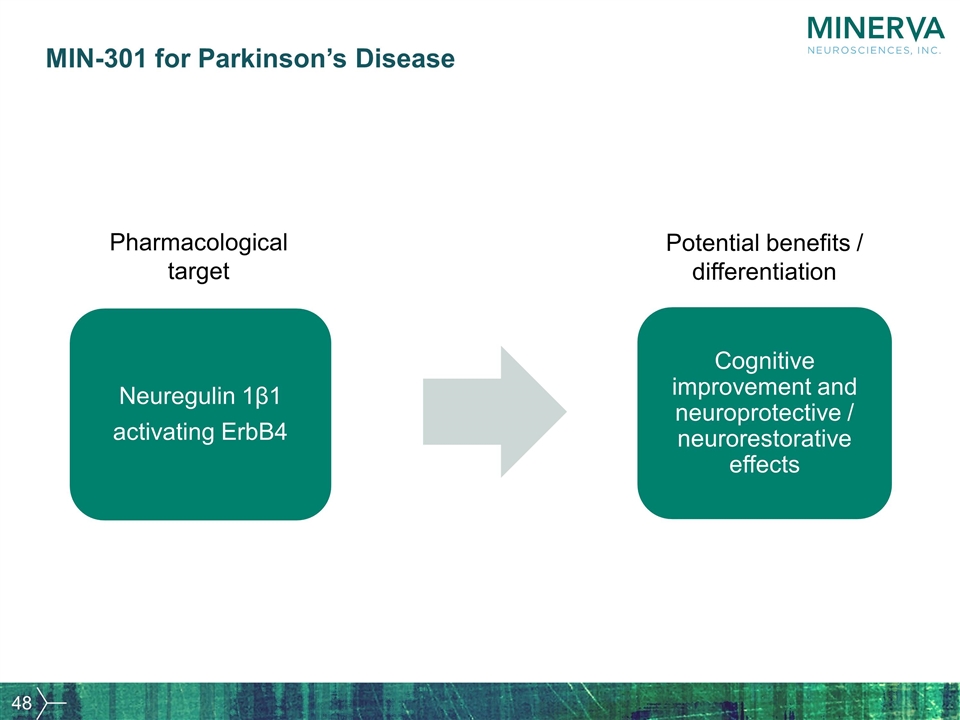
MIN-301 for Parkinson’s Disease Neuregulin 1β1 activating ErbB4 Cognitive improvement and neuroprotective / neurorestorative effects Potential benefits / differentiation Pharmacological target
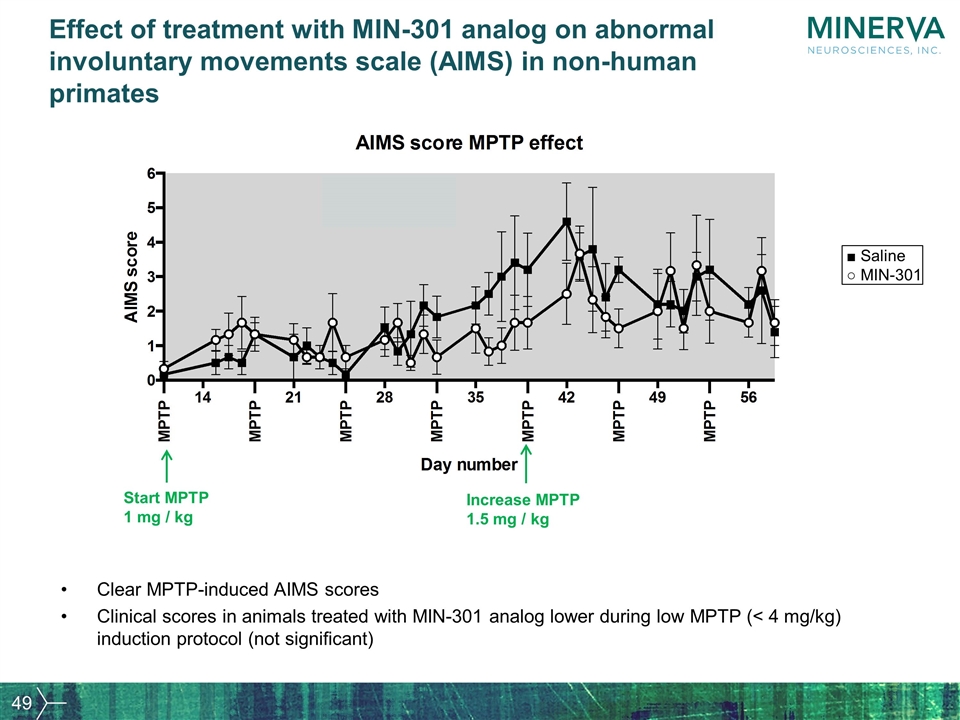
Effect of treatment with MIN-301 analog on abnormal involuntary movements scale (AIMS) in non-human primates Clear MPTP-induced AIMS scores Clinical scores in animals treated with MIN-301 analog lower during low MPTP (< 4 mg/kg) induction protocol (not significant) Start MPTP 1 mg / kg Increase MPTP 1.5 mg / kg ■ Saline ○ MIN-301
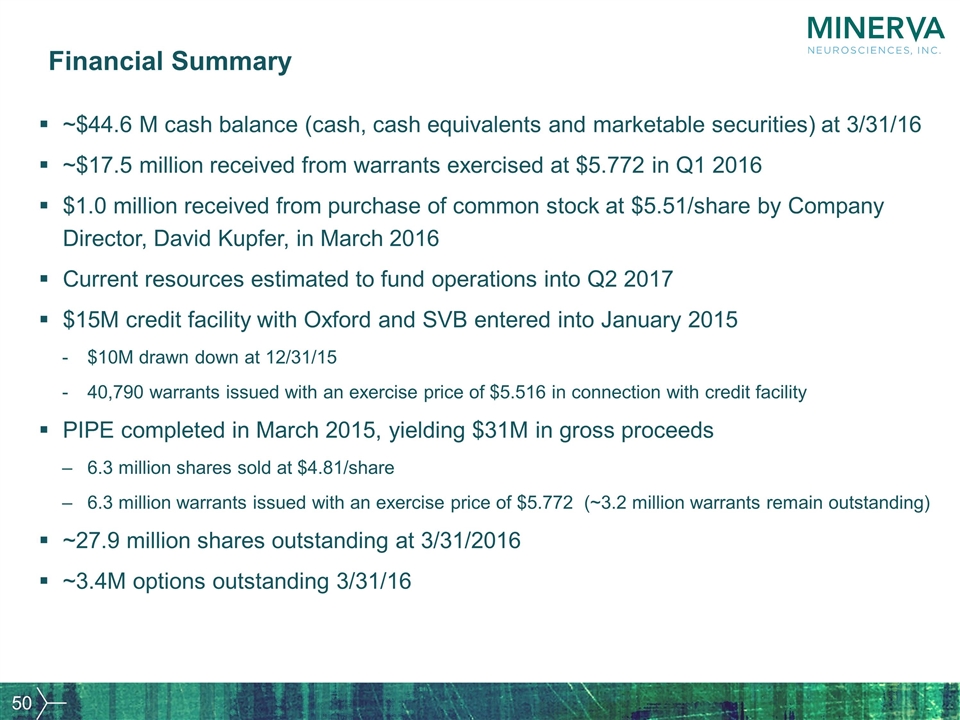
Financial Summary ~$44.6 M cash balance (cash, cash equivalents and marketable securities) at 3/31/16 ~$17.5 million received from warrants exercised at $5.772 in Q1 2016 $1.0 million received from purchase of common stock at $5.51/share by Company Director, David Kupfer, in March 2016 Current resources estimated to fund operations into Q2 2017 $15M credit facility with Oxford and SVB entered into January 2015 $10M drawn down at 12/31/15 40,790 warrants issued with an exercise price of $5.516 in connection with credit facility PIPE completed in March 2015, yielding $31M in gross proceeds 6.3 million shares sold at $4.81/share 6.3 million warrants issued with an exercise price of $5.772 (~3.2 million warrants remain outstanding) ~27.9 million shares outstanding at 3/31/2016 ~3.4M options outstanding 3/31/16
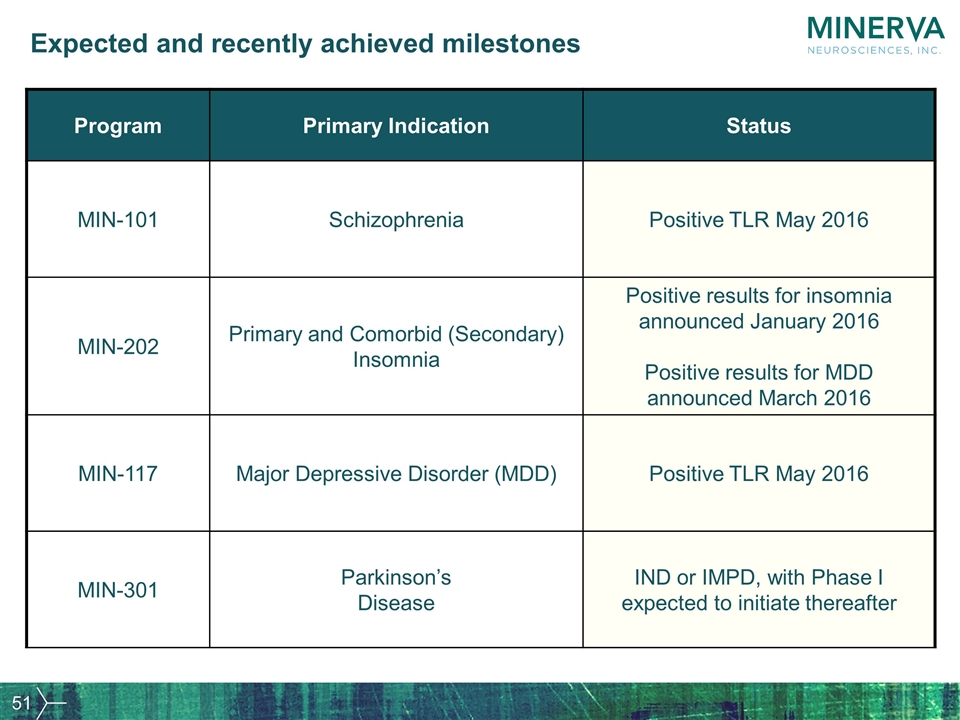
Expected and recently achieved milestones Program Primary Indication Status MIN-101 Schizophrenia Positive TLR May 2016 MIN-202 Primary and Comorbid (Secondary) Insomnia Positive results for insomnia announced January 2016 Positive results for MDD announced March 2016 MIN-117 Major Depressive Disorder (MDD) Positive TLR May 2016 MIN-301 Parkinson’s Disease IND or IMPD, with Phase I expected to initiate thereafter

Thank You Minerva Neurosciences, Inc. 1601 Trapelo Road, Suite 284, Waltham, MA 02451



















































