
ROLUPERIDONE: Topline results from the Phase 3 trial: A Multicenter, Randomized, Double-blind, Parallel Group, Placebo-Controlled, Monotherapy, 12-Week Study to Evaluate the Efficacy and Safety of 2 Fixed Doses of MIN-101 in Adult Patients with Negative Symptoms of Schizophrenia, Followed by 40-Week Open-Label Extension June 5th, 2020 : NERV Exhibit 99.1

Forward-Looking Statement Safe-Harbor This presentation contains forward-looking statements which are subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this press release, and involve certain risks and uncertainties. Forward-looking statements include statements herein with respect to the timing and scope of future clinical trials and results of clinical trials with roluperidone (MIN-101); the clinical and therapeutic potential of this compound; the timing and outcomes of future interactions with U.S. and foreign regulatory bodies; our ability to successfully develop and commercialize our therapeutic products; the sufficiency of our current cash position to fund our operations; and management’s ability to successfully achieve its goals. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of factors including, without limitation, whether roluperidone will advance further in the clinical trials process and whether and when, if at all, it will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic products will be successfully marketed if approved; whether any of our therapeutic product discovery and development efforts will be successful; management’s ability to successfully achieve its goals; our ability to raise additional capital to fund our operations on terms acceptable to us; and general economic conditions. These and other potential risks and uncertainties that could cause actual results to differ from the results predicted are more fully detailed under the caption “Risk Factors” in our filings with the Securities and Exchange Commission, including our Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, filed with the Securities and Exchange Commission on May 4, 2020. Copies of reports filed with the SEC are posted on our website at www.minervaneurosciences.com. The forward-looking statements in this presentation are based on information available to us as of the date hereof, and we disclaim any obligation to update any forward-looking statements, except as required by law.

Webcast Agenda: Roluperidone phase 3 Top-Line Results (TLR) Topic Assigned Introductions William Boni, VP IR Study Results Remy Luthringer, PhD, Chairman & CEO Results Discussion by KOL’s Phil Harvey, PhD Brian Kirkpatrick, MD Concluding remarks & next steps Remy Luthringer Q&A Michael Davidson, MD, CMO Phil Harvey Brian Kirkpatrick Remy Luthringer Geoff Race, CFO & CBO Rick Russell, President Jay Saoud
Study Design Schema & Key Study Elements
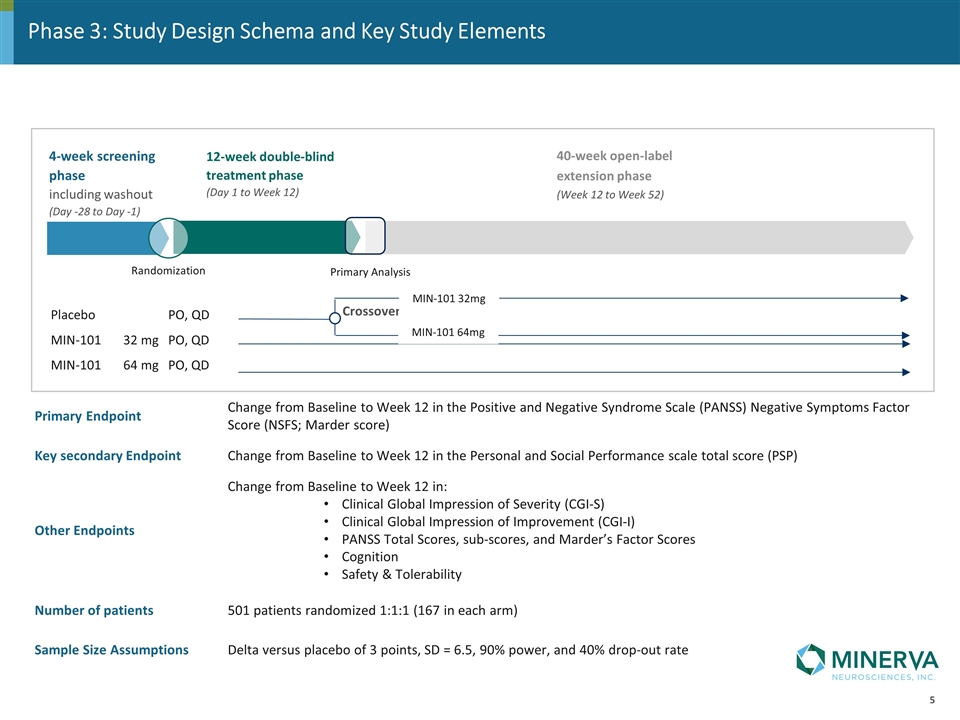
4-week screening phase including washout (Day -28 to Day -1) 12-week double-blind treatment phase (Day 1 to Week 12) 40-week open-label extension phase (Week 12 to Week 52) Placebo PO, QD MIN-101 32 mg PO, QD MIN-101 64 mg PO, QD Randomization Primary Analysis Crossover Phase 3: Study Design Schema and Key Study Elements Primary Endpoint Change from Baseline to Week 12 in the Positive and Negative Syndrome Scale (PANSS) Negative Symptoms Factor Score (NSFS; Marder score) Key secondary Endpoint Change from Baseline to Week 12 in the Personal and Social Performance scale total score (PSP) Other Endpoints Change from Baseline to Week 12 in: Clinical Global Impression of Severity (CGI-S) Clinical Global Impression of Improvement (CGI-I) PANSS Total Scores, sub-scores, and Marder’s Factor Scores Cognition Safety & Tolerability Number of patients 501 patients randomized 1:1:1 (167 in each arm) Sample Size Assumptions Delta versus placebo of 3 points, SD = 6.5, 90% power, and 40% drop-out rate MIN-101 64mg MIN-101 32mg
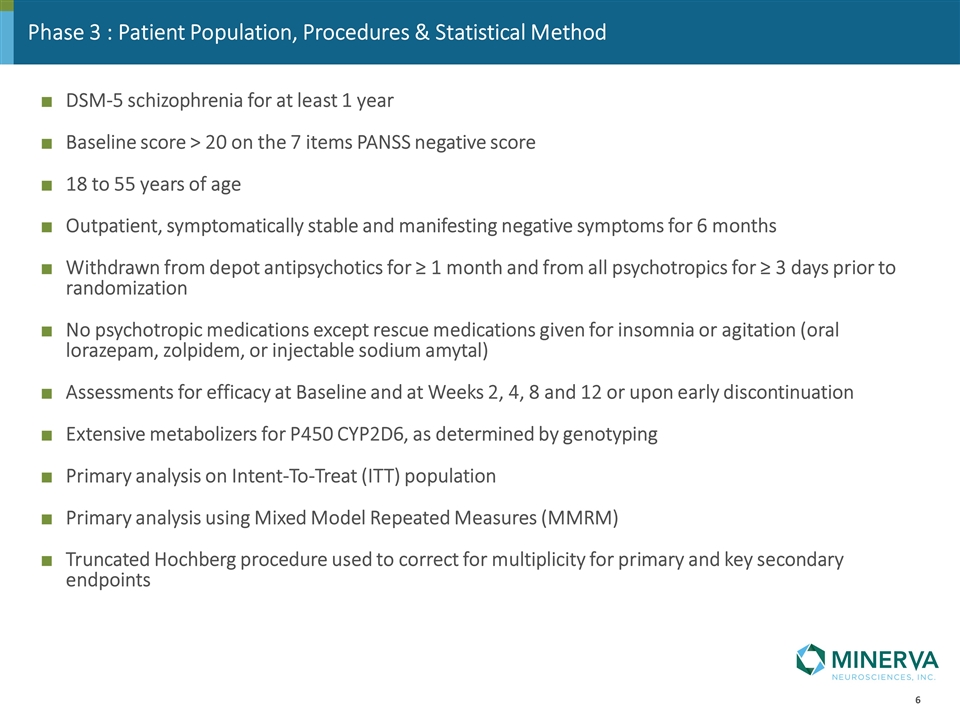
Phase 3 : Patient Population, Procedures & Statistical Method DSM-5 schizophrenia for at least 1 year Baseline score > 20 on the 7 items PANSS negative score 18 to 55 years of age Outpatient, symptomatically stable and manifesting negative symptoms for 6 months Withdrawn from depot antipsychotics for ≥ 1 month and from all psychotropics for ≥ 3 days prior to randomization No psychotropic medications except rescue medications given for insomnia or agitation (oral lorazepam, zolpidem, or injectable sodium amytal) Assessments for efficacy at Baseline and at Weeks 2, 4, 8 and 12 or upon early discontinuation Extensive metabolizers for P450 CYP2D6, as determined by genotyping Primary analysis on Intent-To-Treat (ITT) population Primary analysis using Mixed Model Repeated Measures (MMRM) Truncated Hochberg procedure used to correct for multiplicity for primary and key secondary endpoints

Disposition
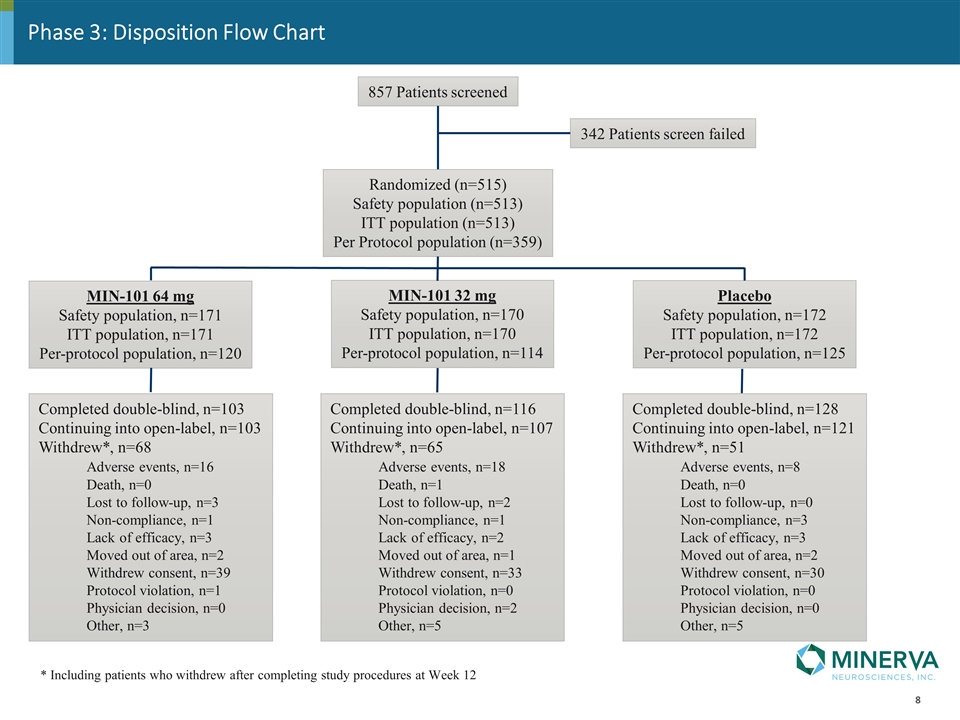
Phase 3: Disposition Flow Chart 857 Patients screened 342 Patients screen failed Randomized (n=515) Safety population (n=513) ITT population (n=513) Per Protocol population (n=359) MIN-101 64 mg Safety population, n=171 ITT population, n=171 Per-protocol population, n=120 MIN-101 32 mg Safety population, n=170 ITT population, n=170 Per-protocol population, n=114 Placebo Safety population, n=172 ITT population, n=172 Per-protocol population, n=125 Completed double-blind, n=103 Continuing into open-label, n=103 Withdrew*, n=68 Adverse events, n=16 Death, n=0 Lost to follow-up, n=3 Non-compliance, n=1 Lack of efficacy, n=3 Moved out of area, n=2 Withdrew consent, n=39 Protocol violation, n=1 Physician decision, n=0 Other, n=3 Completed double-blind, n=116 Continuing into open-label, n=107 Withdrew*, n=65 Adverse events, n=18 Death, n=1 Lost to follow-up, n=2 Non-compliance, n=1 Lack of efficacy, n=2 Moved out of area, n=1 Withdrew consent, n=33 Protocol violation, n=0 Physician decision, n=2 Other, n=5 Completed double-blind, n=128 Continuing into open-label, n=121 Withdrew*, n=51 Adverse events, n=8 Death, n=0 Lost to follow-up, n=0 Non-compliance, n=3 Lack of efficacy, n=3 Moved out of area, n=2 Withdrew consent, n=30 Protocol violation, n=0 Physician decision, n=0 Other, n=5 * Including patients who withdrew after completing study procedures at Week 12

Primary Endpoint
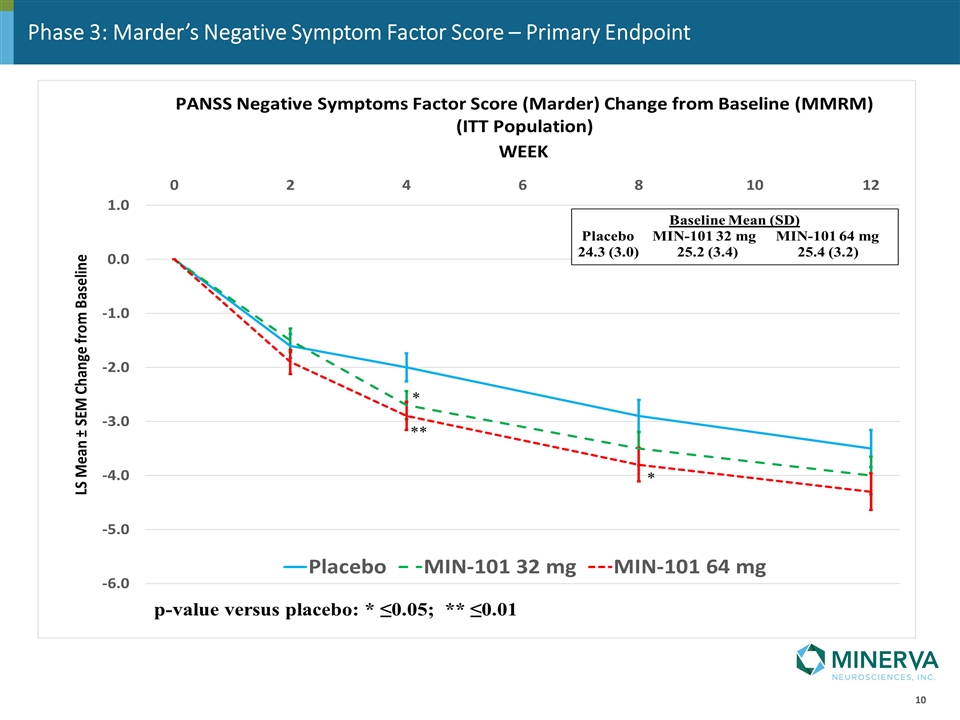
Phase 3: Marder’s Negative Symptom Factor Score – Primary Endpoint
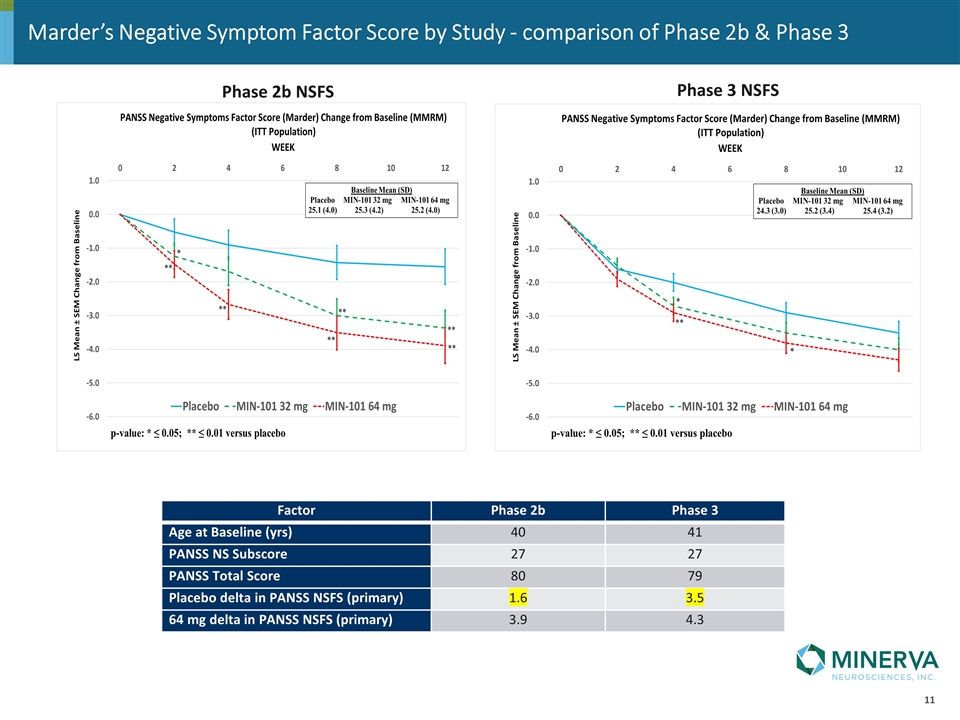
Phase 2b NSFS Phase 3 NSFS Factor Phase 2b Phase 3 Age at Baseline (yrs) 40 41 PANSS NS Subscore 27 27 PANSS Total Score 80 79 Placebo delta in PANSS NSFS (primary) 1.6 3.5 64 mg delta in PANSS NSFS (primary) 3.9 4.3 Marder’s Negative Symptom Factor Score by Study - comparison of Phase 2b & Phase 3
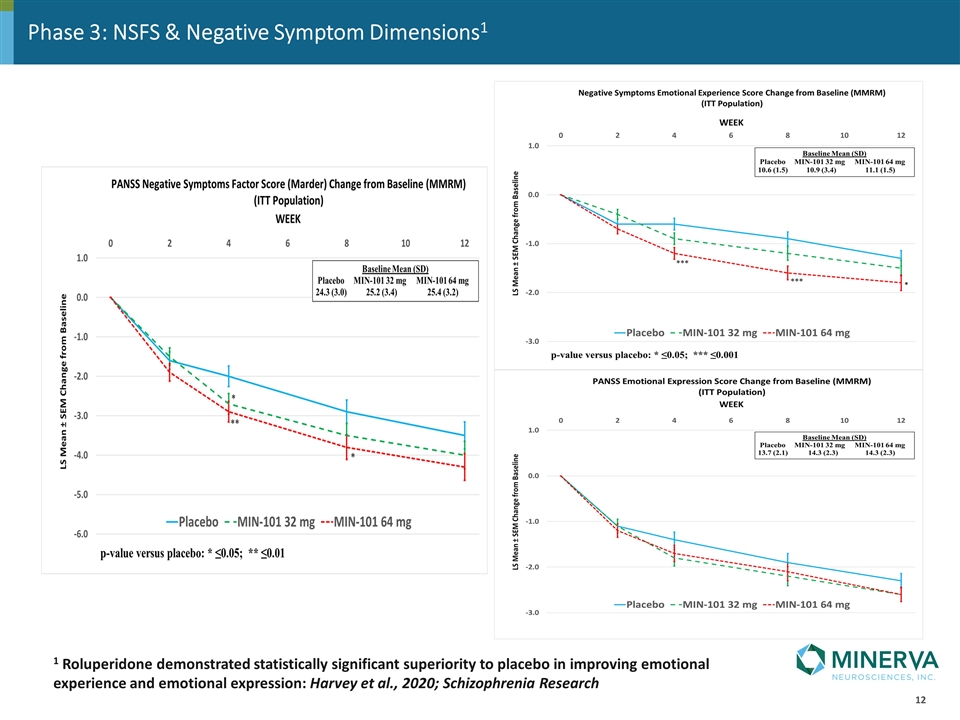
Phase 3: NSFS & Negative Symptom Dimensions1 1 Roluperidone demonstrated statistically significant superiority to placebo in improving emotional experience and emotional expression: Harvey et al., 2020; Schizophrenia Research
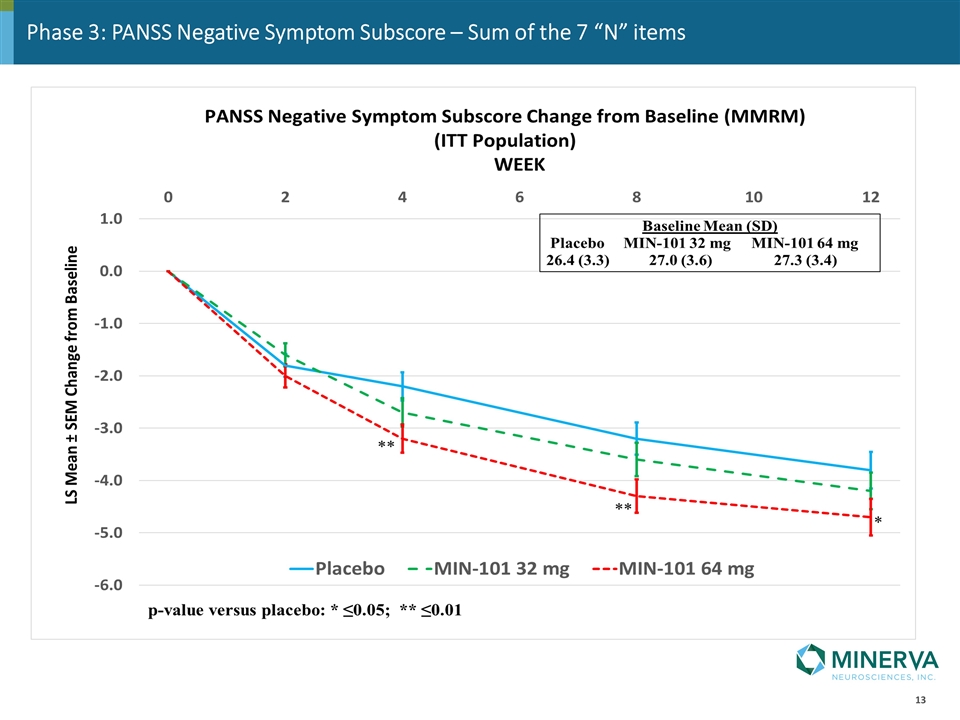
Phase 3: PANSS Negative Symptom Subscore – Sum of the 7 “N” items

Responder Analysis: PANSS Total & Marder Negative Symptoms Factor Score (NSFS)
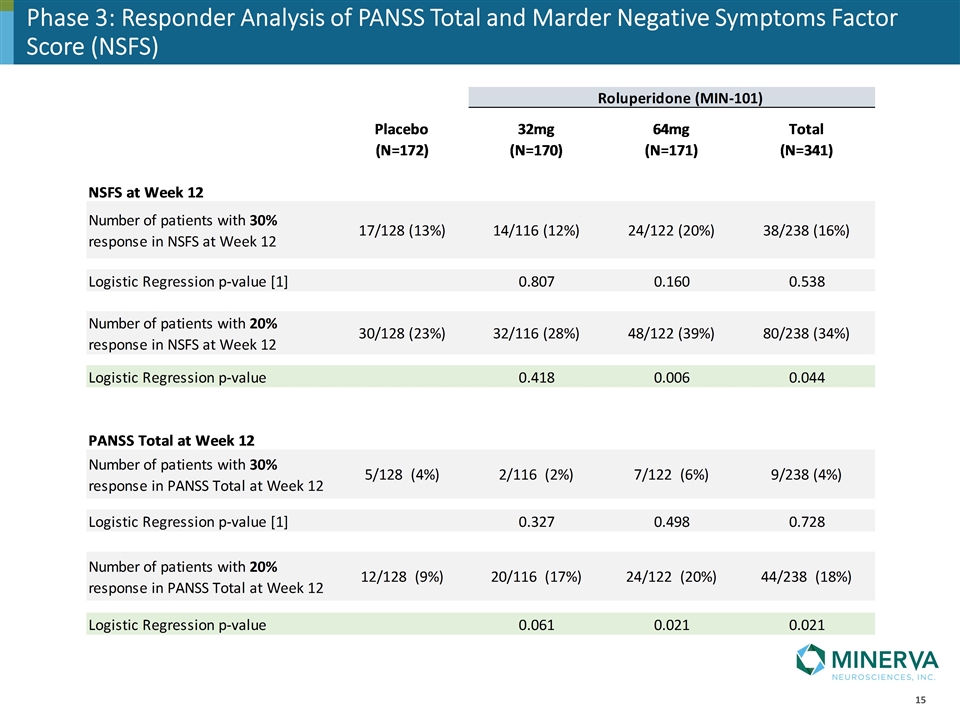
Phase 3: Responder Analysis of PANSS Total and Marder Negative Symptoms Factor Score (NSFS)

Preliminary Primary Endpoint Integrated Analysis
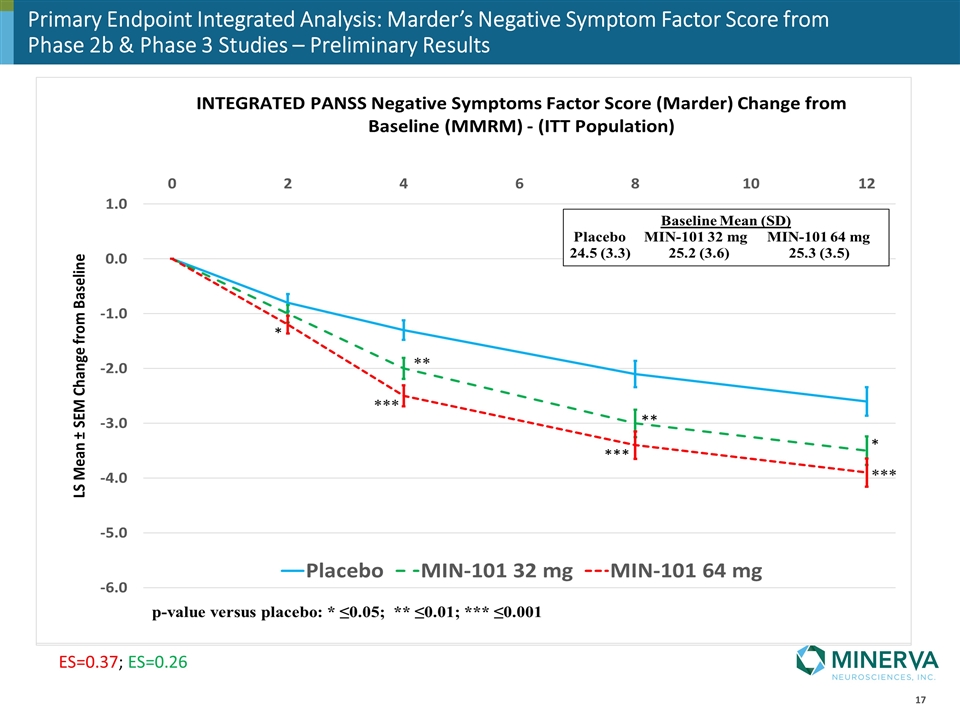
Primary Endpoint Integrated Analysis: Marder’s Negative Symptom Factor Score from Phase 2b & Phase 3 Studies – Preliminary Results ES=0.37; ES=0.26

Key Secondary Endpoint
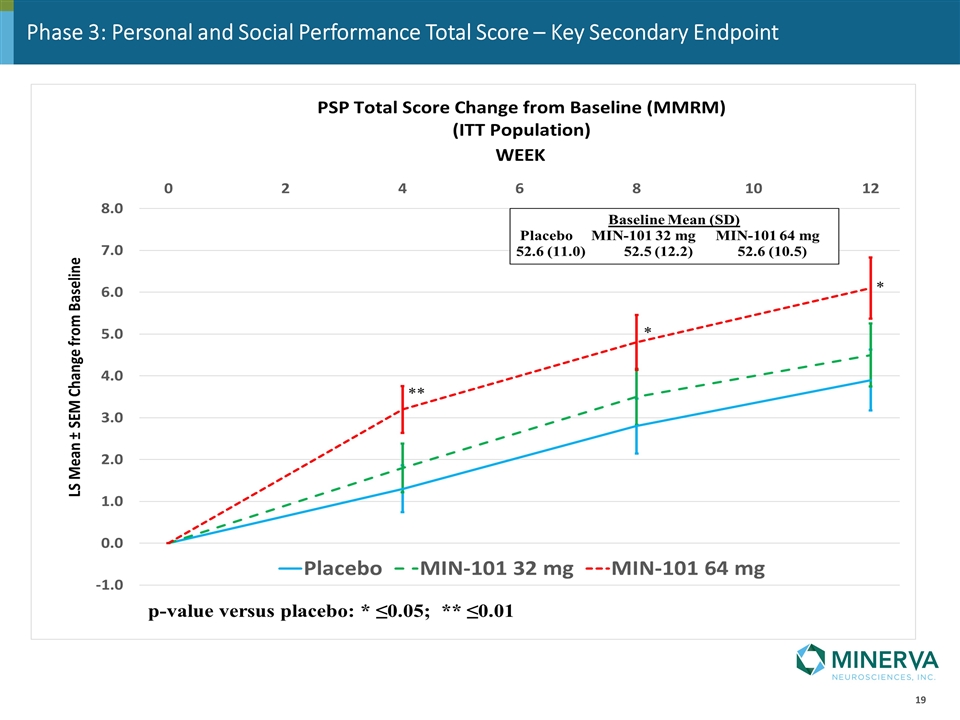
Phase 3: Personal and Social Performance Total Score – Key Secondary Endpoint

Efficacy Summary
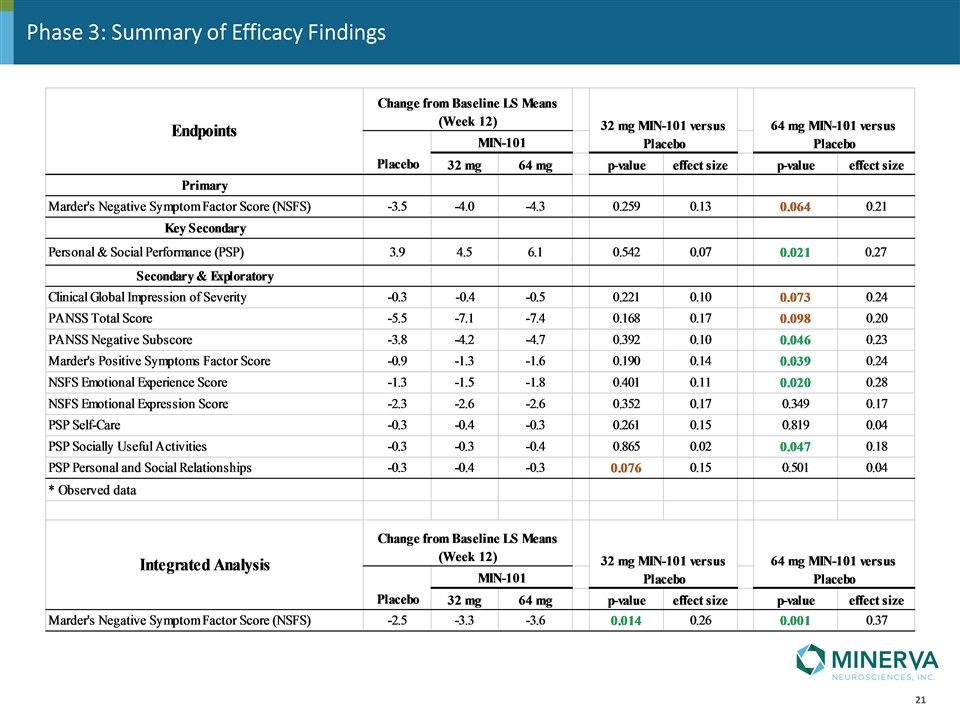
Phase 3: Summary of Efficacy Findings
Phase 3: Summary Key findings: Patient populations in the phase 2b and phase 3 are comparable Improvement of negative symptoms as measured by NSFS are similar in both studies Both doses show early separation from placebo at week 4 (both doses) and week 8 (64mg) Due to higher placebo response, roluperidone at both doses separated but did not achieve a statistically significant difference at Week 12 Higher number of responders in terms of negative symptoms and total PANSS score in the roluperidone treatment groups The reduction in negative symptoms scores in the 64 mg arm of roluperidone translated into an improvement of PSP total score and sub-scores reflective of functional improvement. Relapse rates are extremely low and confirm that a significant proportion of schizophrenic patients have stable positive symptoms for extended periods of time. The integrated analysis of the phase 3 and phase 2b study data shows a very strong statistically significant difference for both doses of roluperidone at week 12 (and earlier timepoints). Safety and efficacy of roluperidone in phase 3 are consistent with phase 2b

RESULTS DISCUSSION BY KOL’S Phil Harvey & Brian Kirkpatrick

CONCLUDING REMARKS & NEXT STEPS Remy Luthringer
Roluperidone (MIN-101) – CONCLUDING REMARKS & NEXT STEPS Further data analyses will continue over the coming weeks: Complete understanding of the data Further explore placebo group and understand the difference seen between the phase 2b and 3 study To continue our dialogue with our regulatory advisors and our KOL’s Request a meeting with the FDA to present data and obtain input and plan path forward The results also confirm the unique mechanism of action of roluperidone targeting those pathways – 5HT2A, Sigma2, Alpha1A – known to be involved in schizophrenia (1) Psychiatrists cite negative symptoms as the top unmet need in the treatment of schizophrenia (2) No product is currently approved to treat negative symptoms in the US Girgis et al., 2018. The past and future of novel, non-dopamine-2 receptor therapeutics for schizophrenia: A critical and comprehensive review. Journal of Psychiatry Research.. Schizophrenia Market Intelligence Report. Cambridge Healthcare Research. File name: Schizophrenia Intelligence 1 Dec 2017

Q&A Michael Davidson Phil Harvey Brian Kirkpatrick Remy Luthringer Geoff Race Rick Russell Jay Saoud

























