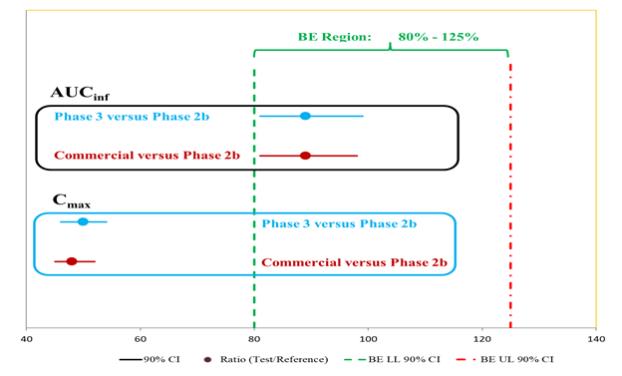
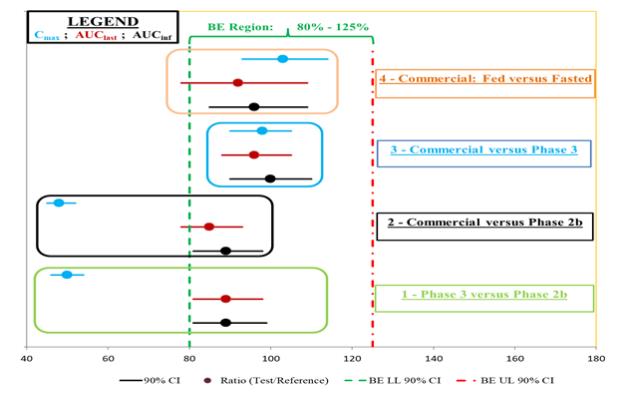
About Schizophrenia and Negative Symptoms
Schizophrenia is a chronic, severe and debilitating type of mental illness characterized by distortions in thinking, perception, emotions, language, sense of self and behavior. Schizophrenia affects 20 million people worldwide. (World Health Organization).
Negative symptoms can cause individuals with schizophrenia to withdraw from society, become disinterested or unable to complete tasks or feel pleasure. Negative symptoms are characterized by five constructs: blunted affect, alogia, avolition, anhedonia, and asociality (Marder and Galderisi, 2017).
Negative symptoms are the main cause of the poor functional outcome of patients suffering from schizophrenia (Harvey et al., 2020) and may also be one of the main reasons ultrahigh risk adolescents may develop full blown schizophrenia (Gomes and Grace, 2017). There are currently no treatments approved for negative symptoms of schizophrenia.
About Minerva Neurosciences
Minerva Neurosciences, Inc. (Nasdaq: NERV) is a clinical-stage biopharmaceutical company focused on developing product candidates to treat central nervous system (CNS) diseases. Our goal is to transform the lives of patients with improved therapeutic options. Minerva’s portfolio of compounds includes roluperidone (MIN-101), in clinical development for negative symptoms of schizophrenia, and MIN-301, in pre-clinical development for Parkinson’s disease. For more information, please visit our website.
Forward-Looking Safe Harbor Statement
This press release contains forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this press release, and involve certain risks and uncertainties. Forward-looking statements include, but are not limited to, statements herein with respect to the timing and scope of clinical trials and regulatory review and results and outcomes of such clinical trials, including the clinical development of roluperidone (MIN-101) for the treatment of negative symptoms of schizophrenia; the clinical and therapeutic potential of this compound, including its potential benefits in the treatment of negative symptoms of schizophrenia or any other indication; the timing and outcomes of future interactions with U.S. and foreign regulatory bodies, including the U.S. Food and Drug Administration; our ability to successfully develop and commercialize our therapeutic products; and management’s ability to successfully achieve its goals. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of factors including, without limitation, the risk that trials and studies may be delayed and may not have satisfactory outcomes, the risk that initial or interim results from a clinical trial may not be predictive of the final results of the trial or the results of future trials, whether roluperidone will advance further in the clinical trials process and whether and when, if at all, it will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic products or seltorexant will be successfully marketed if approved; whether any of our therapeutic product discovery and development efforts will be successful; management’s ability to successfully achieve its goals; our ability to raise additional capital to