
3 Authors: Michael Davidson1,2 MD, Jay Saoud1 PhD, Corinne Staner3 MD, Nadine Noel3 PhD, Sandra Werner3 PhD, Elisabeth Luthringer3 RN, Philip Harvey PhD, Gregory Strauss PhD, Mark Weiser4MD, Remy Luthringer1 PhD Efficacy and safety of roluperidone for the treatment of negative symptoms of schizophrenia Study registration: Eudra-CT: 2017-003333-29; NCT03397134 Affiliations: (1) Minerva Neurosciences; (2) Nicosia University Medical School; (3) PPRS, (4) University of Tel Aviv School of Medicine (6) University of Miami Miller School of Medicine (7) University of Georgia Minerva Neurosciences is the sponsor of the trials Exhibit 99.1
Abstract Objective: In a previous large multi-national trial, roluperidone (MIN-101) a compound with antagonistic properties for 5‑HT2A, sigma2 and α1A-adrenergic receptors demonstrated statistically significant efficacy in reducing negative symptoms and good tolerability in stable schizophrenia patients. The objective of the current study was to confirm roluperidone’s efficacy, safety, and tolerability in similar schizophrenia patients. Methods: Roluperidone 32 mg/day, roluperidone 64 mg/day, or placebo was administered for 12 weeks to 513 schizophrenia patients with moderate to severe negative symptoms. The primary endpoint was the PANSS-derived Negative Symptoms Factor Score (NSFS) (Marder negative symptoms subscore) and the key secondary endpoint was Personal and Social Performance scale (PSP) total score. Results. NSFS scores were lower (improved) for roluperidone 64 mg compared to placebo and marginally missing statistical significance for the intent-to-treat (ITT) analysis set (p ≤0.064) but reaching nominal significance (p ≤0.044) for the modified-ITT (mITT)* set. Change in PSP total score was significantly higher (better) on roluperidone 64 mg compared to placebo for ITT and mITT (p≥ 0.021 and p≥ 0.017, respectively). Conclusions: Results of this trial confirm roluperidone’s potential for the treatment of negative symptoms of schizophrenia leading to better daily functioning. * Modified ITT excluded 17/513 patients before the data lock and unblinding because of behavioural and physiologic implausible data
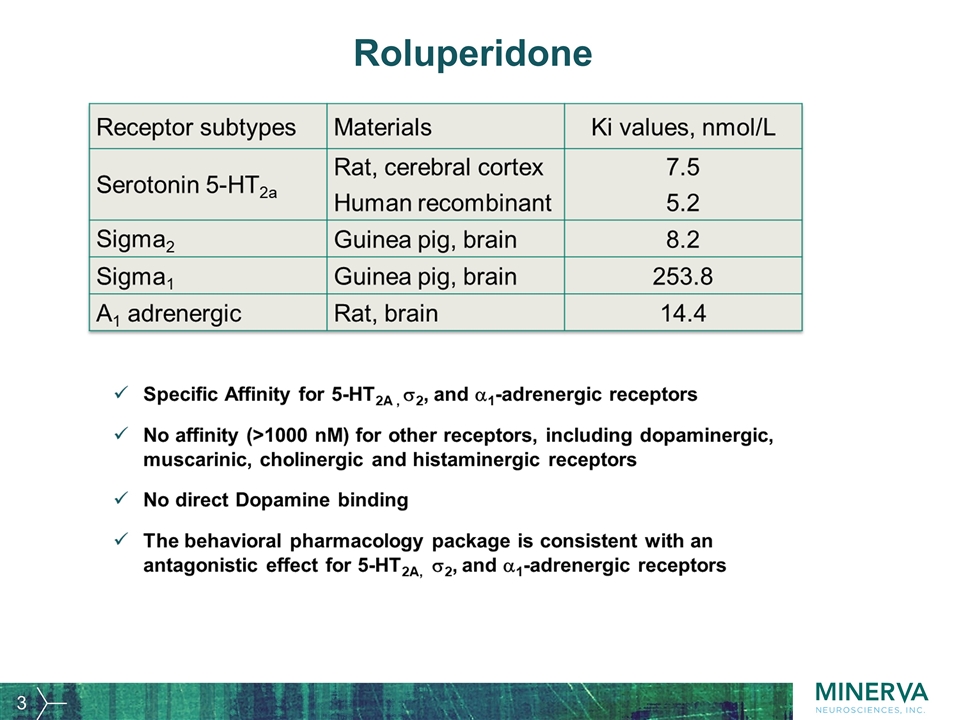
Roluperidone
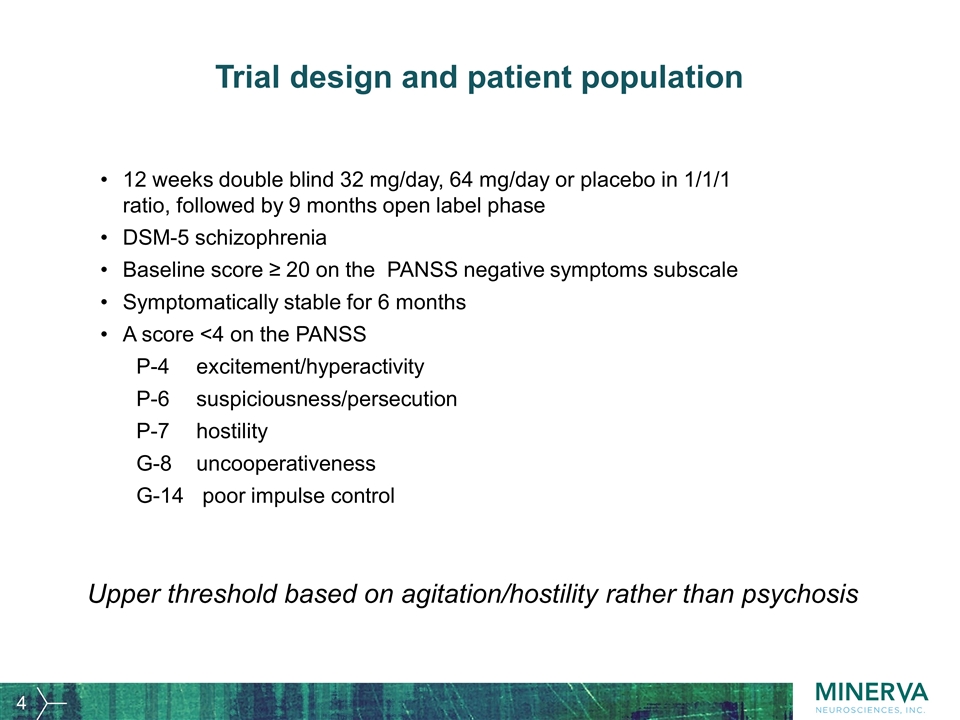
Trial design and patient population 12 weeks double blind 32 mg/day, 64 mg/day or placebo in 1/1/1 ratio, followed by 9 months open label phase DSM-5 schizophrenia Baseline score ≥ 20 on the PANSS negative symptoms subscale Symptomatically stable for 6 months A score <4 on the PANSS P-4excitement/hyperactivity P-6suspiciousness/persecution P-7hostility G-8uncooperativeness G-14 poor impulse control Upper threshold based on agitation/hostility rather than psychosis
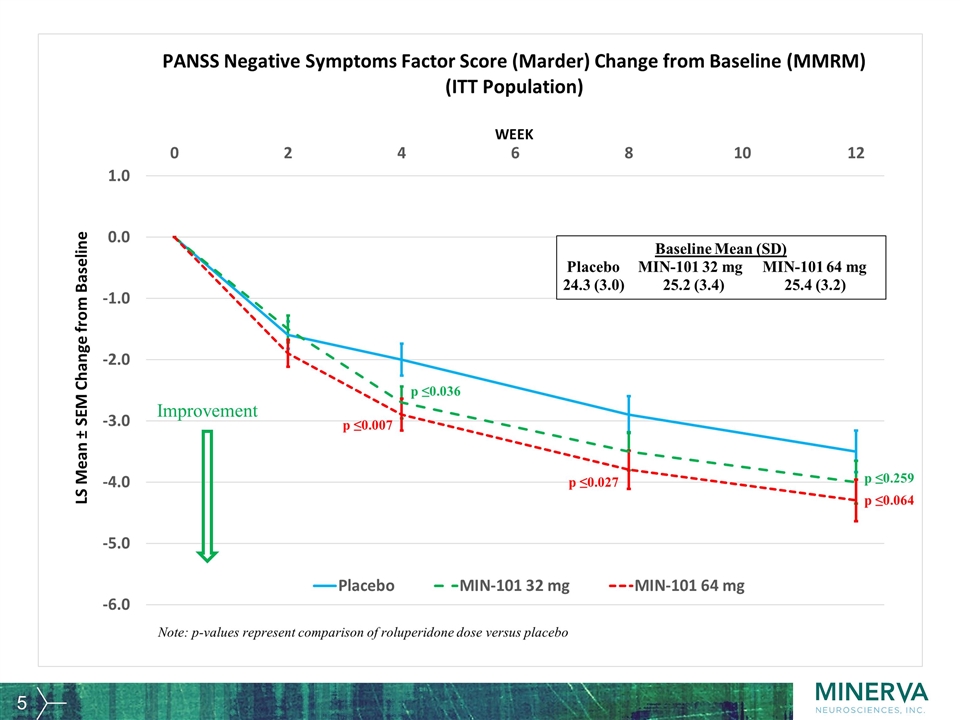
Improvement
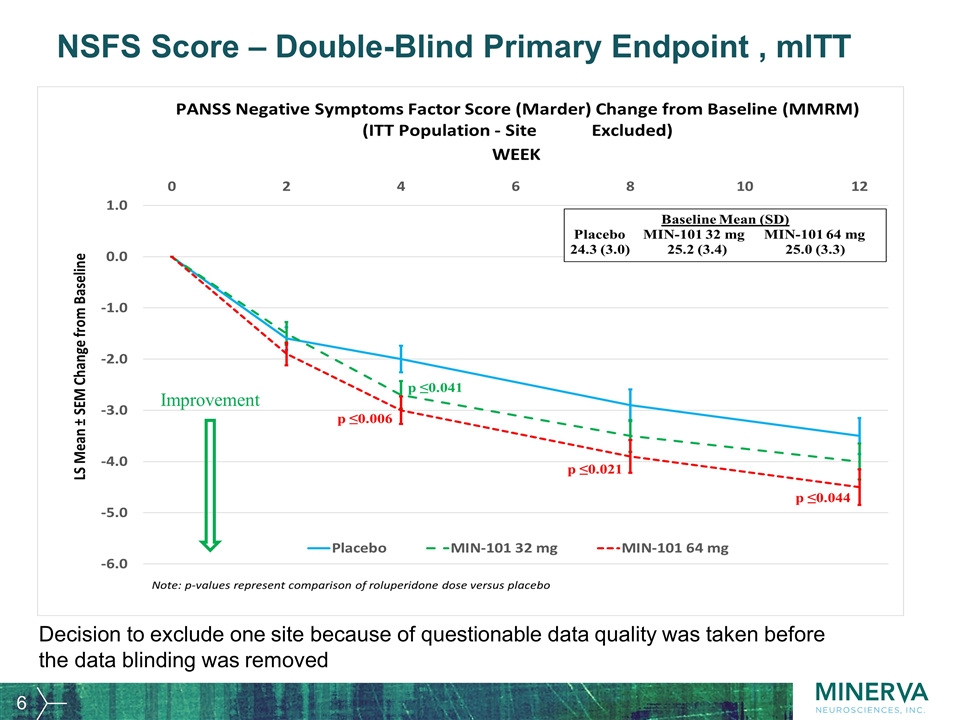
NSFS Score – Double-Blind Primary Endpoint , mITT Improvement Decision to exclude one site because of questionable data quality was taken before the data blinding was removed
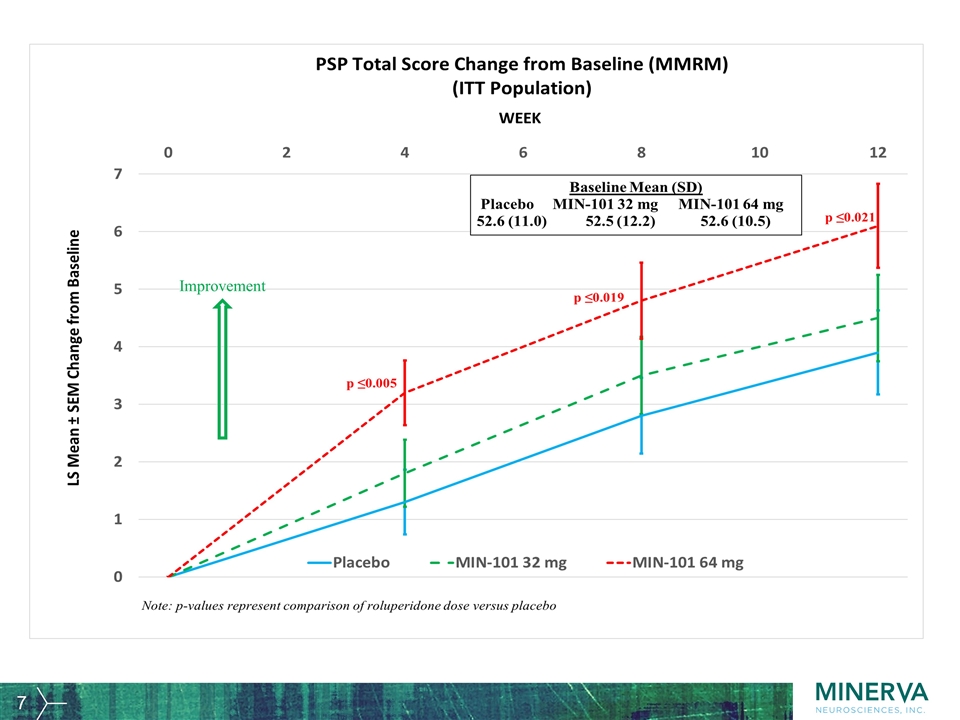
Improvement
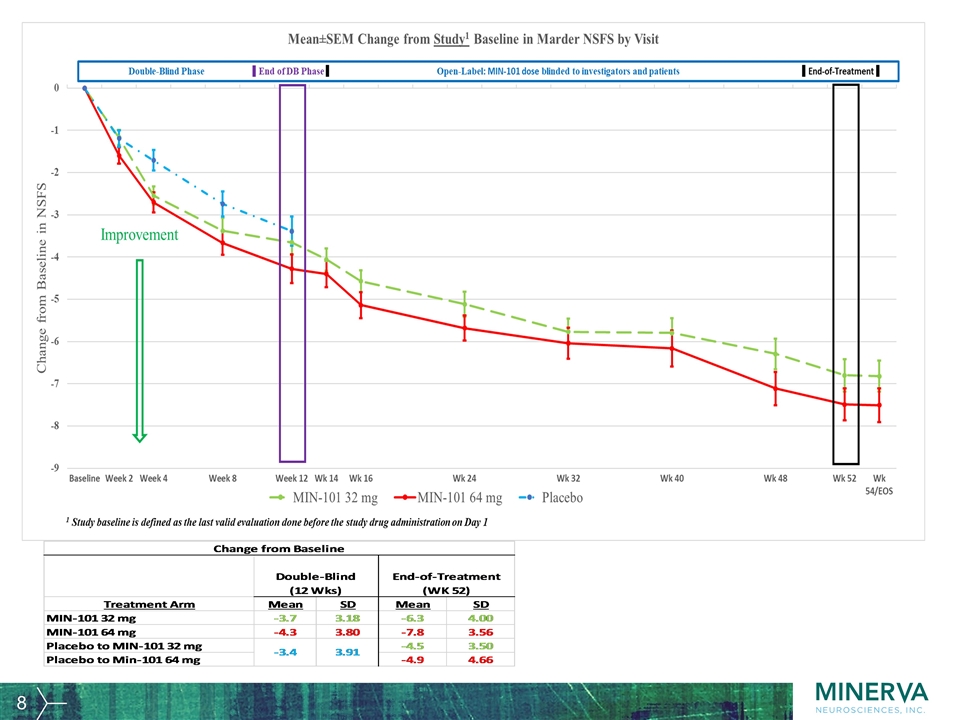
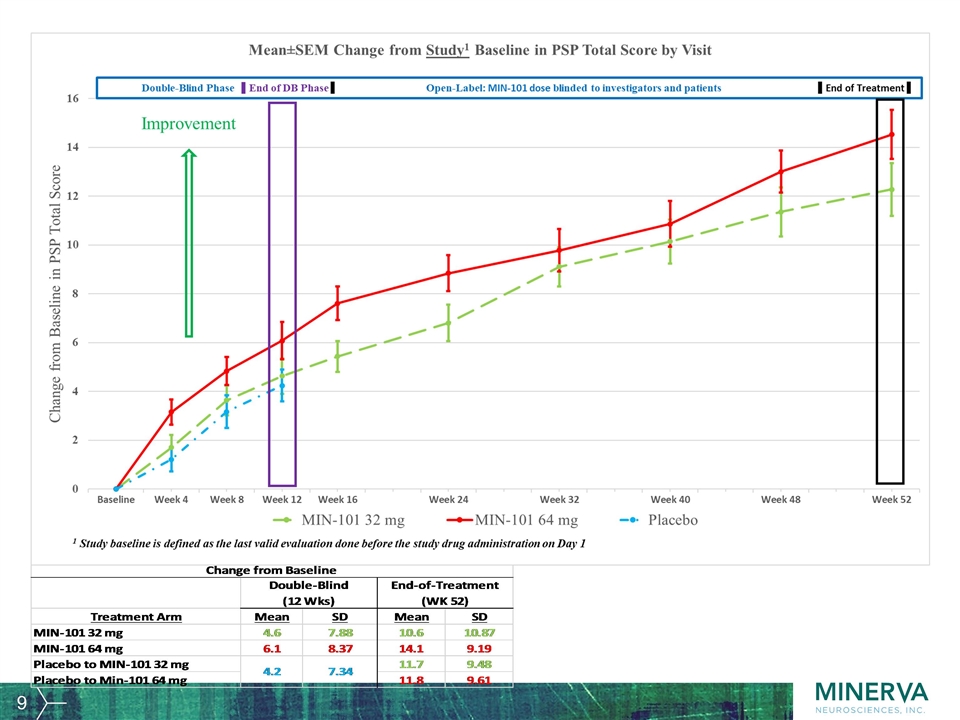
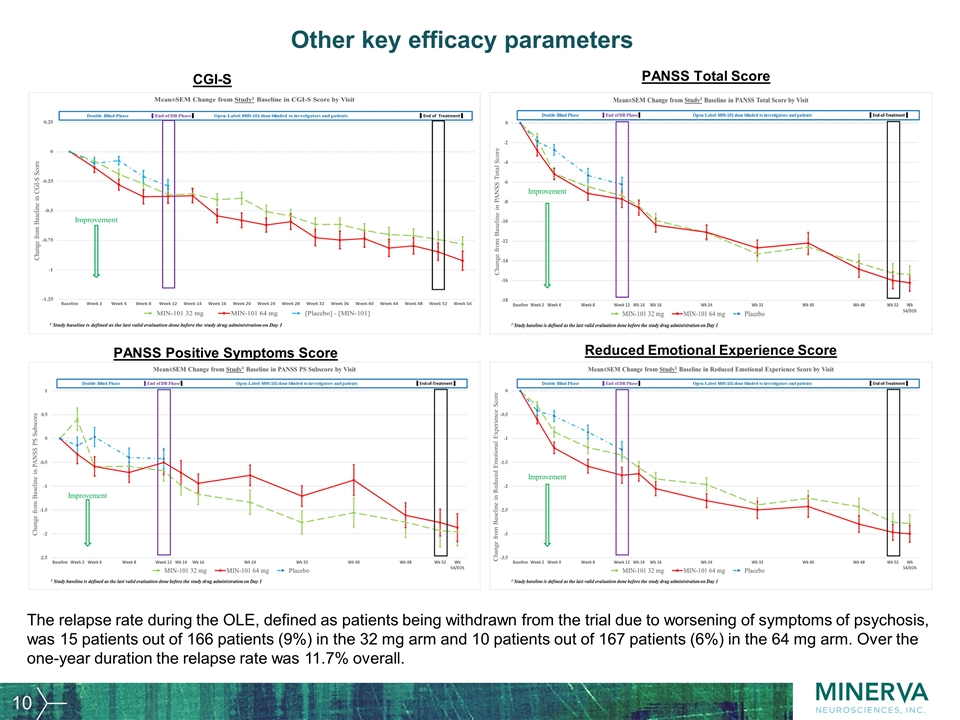
Other key efficacy parameters The relapse rate during the OLE, defined as patients being withdrawn from the trial due to worsening of symptoms of psychosis, was 15 patients out of 166 patients (9%) in the 32 mg arm and 10 patients out of 167 patients (6%) in the 64 mg arm. Over the one-year duration the relapse rate was 11.7% overall. CGI-S PANSS Total Score PANSS Positive Symptoms Score Reduced Emotional Experience Score

Phase 2b study showed improvements in NS over 12 weeks and 36 weeks in both doses and stable positive symptoms Core 12-week phase: Statistically significant improvements in the primary endpoint 12-Week DB & 24-week extension phase Core Phase Extension Phase Source: Davidson et al., 2017
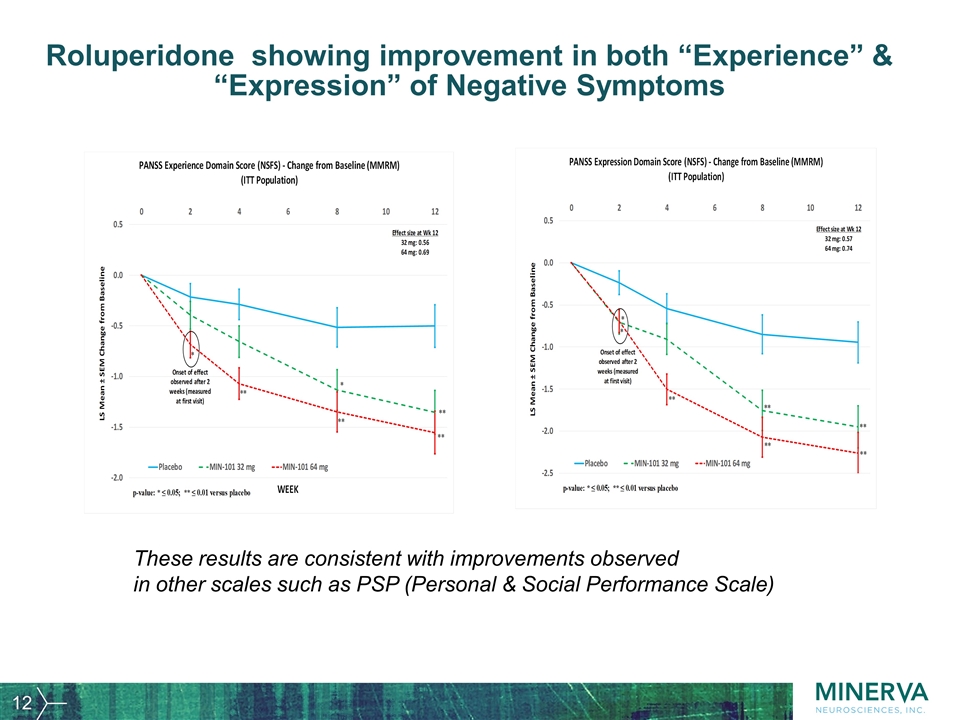
Roluperidone showing improvement in both “Experience” & “Expression” of Negative Symptoms These results are consistent with improvements observed in other scales such as PSP (Personal & Social Performance Scale)
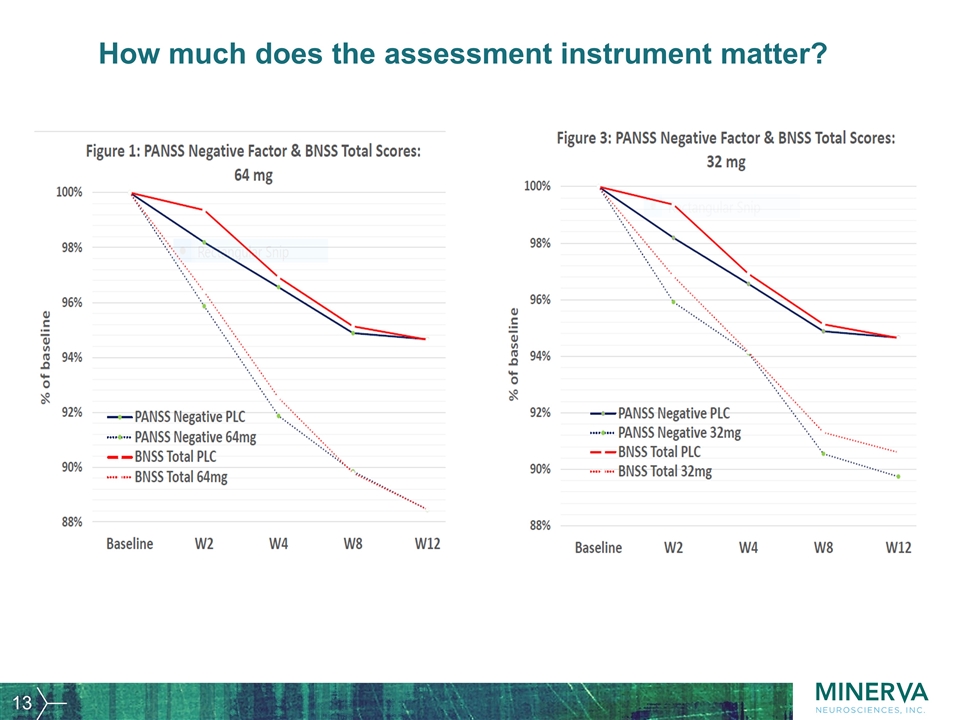
How much does the assessment instrument matter?

Discussion Roluperidone administered as monotherapy may improve negative symptoms and social functioning in patients suffering from schizophrenia and moderate to severe negative symptoms. Monotherapy, rather than add-on to antipsychotics, design was selected for two trials because: antipsychotics may produce secondary negative symptoms such as rigidity, akathisia and sedation hence confounding the effects of the experimental compound antipsychotics by blocking DA receptors and interfering with brain reward circuits, might enhance negative symptoms It is possible that a subgroup of patients with stable positive symptoms, low level of symptoms related to agitation and moderate to severe negative symptoms can maintain symptomatic stability in absence of maintenance treatment with antipsychotic drugs













