
Corporate Presentation January 2022 Exhibit 99.1

Forward-Looking Statement Safe-Harbor Forward-Looking Safe Harbor Statement This presentation contains forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this presentation, and involve certain risks and uncertainties. Forward-looking statements include, but are not limited to, statements herein with respect to the timing and scope of clinical trials and regulatory review and results and outcomes of such clinical trials and regulatory review with roluperidone (MIN-101); the clinical and therapeutic potential of this compound; the likelihood of successful clinical trials, regulatory review, commercialization, and future sales of and potential royalty stream from seltorexant; the timing and outcomes of future interactions with U.S. and foreign regulatory bodies, including the U.S. Food and Drug Administration; our ability to successfully develop and commercialize our therapeutic products; the sufficiency of our current cash position to fund our operations; and management’s ability to successfully achieve its goals. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of factors including, without limitation, whether roluperidone will advance further in the clinical trials process and whether and when, if at all, it will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic products or seltorexant will be successfully marketed if approved; whether any of our therapeutic product discovery and development efforts will be successful; management’s ability to successfully achieve its goals; our ability to raise additional capital to fund our operations on terms acceptable to us; changes in expected or existing competition; unexpected litigation or other disputes; the impacts of the COVID-19 pandemic on our business; and general economic conditions. These and other potential risks and uncertainties that could cause actual results to differ from the results predicted are more fully detailed under the caption “Risk Factors” in our filings with the Securities and Exchange Commission, including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2021, filed with the Securities and Exchange Commission on November 8th, 2021. Copies of reports filed with the SEC are posted on our website at www.minervaneurosciences.com. The forward-looking statements in this presentation are based on information available to us as of the date hereof, and we disclaim any obligation to update any forward-looking statements, except as required by law. 5th January 2022
Founded in 2014 Our goal is to transform the lives of patients suffering from CNS disease including schizophrenia, depression, insomnia and Parkinson’s disease. Roluperidone is our lead program for the treatment of negative symptoms in patients diagnosed with schizophrenia. Minerva Neurosciences (NASDAQ: NERV)

Roluperidone Treatment of negative symptoms in patients diagnosed with schizophrenia
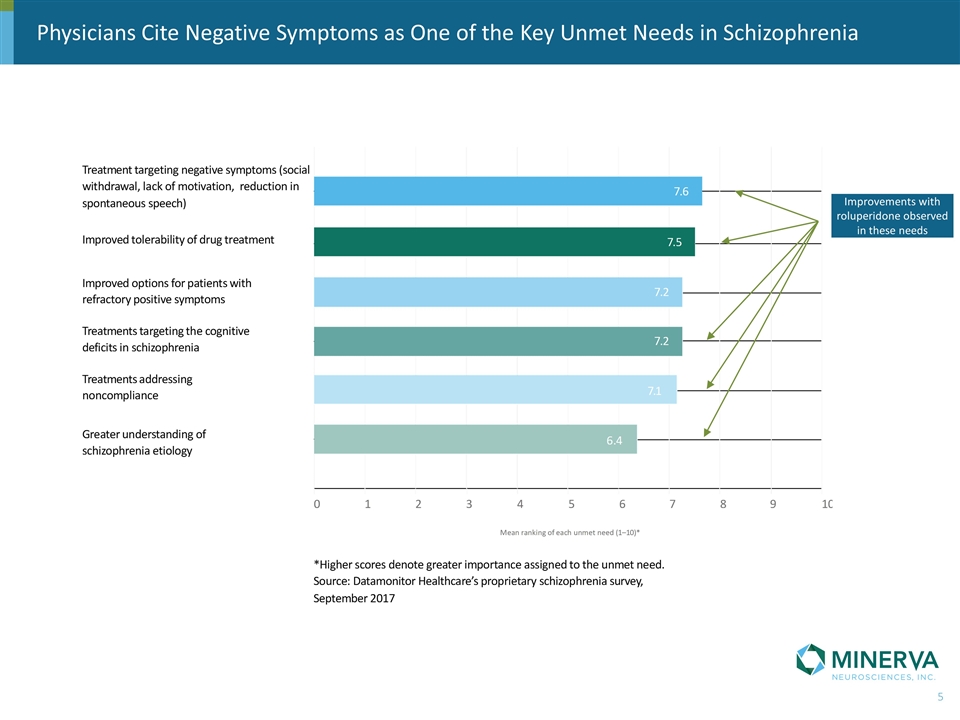
Physicians Cite Negative Symptoms as One of the Key Unmet Needs in Schizophrenia Improvements with roluperidone observed in these needs
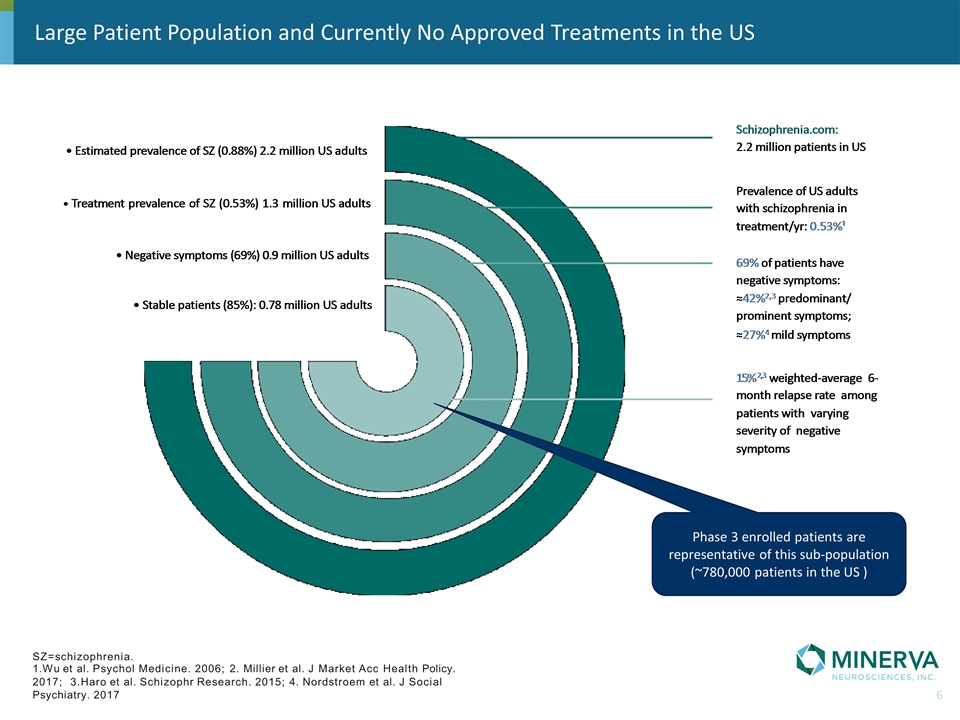
Large Patient Population and Currently No Approved Treatments in the US SZ=schizophrenia. 1.Wu et al. Psychol Medicine. 2006; 2. Millier et al. J Market Acc Health Policy. 2017; 3.Haro et al. Schizophr Research. 2015; 4. Nordstroem et al. J Social Psychiatry. 2017 Phase 3 enrolled patients are representative of this sub-population (~780,000 patients in the US )
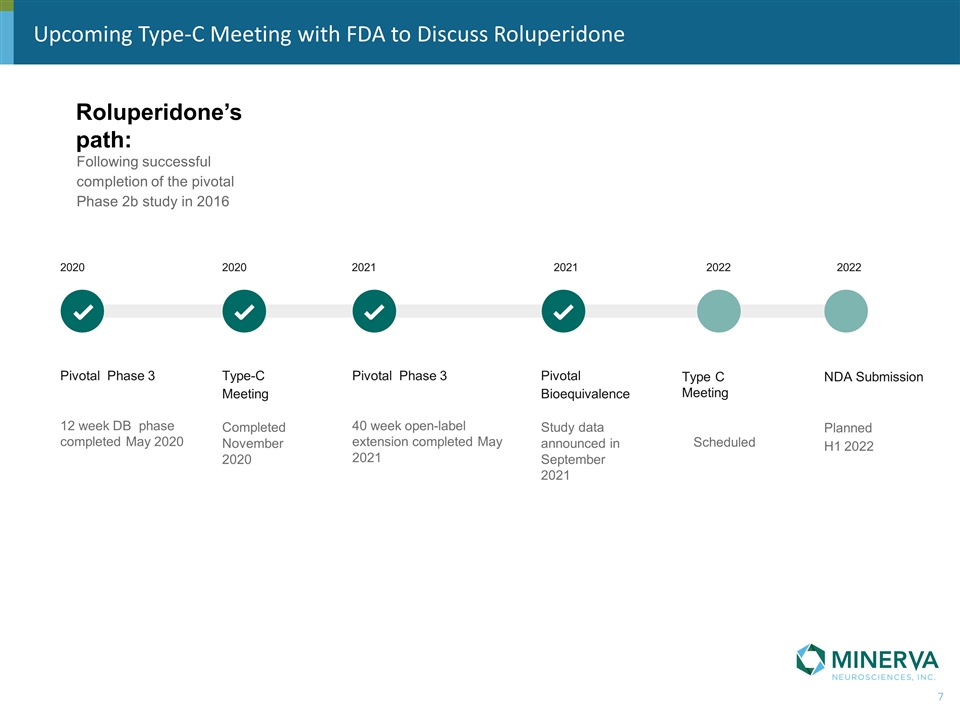
Roluperidone’s path: Summary of Progress Following successful completion of the pivotal Phase 2b study in 2016 Pivotal Phase 3 12 week DB phase completed May 2020 Type-C Meeting Completed November 2020 Pivotal Phase 3 40 week open-label extension completed May 2021 2020 2020 2021 2021 Pivotal Bioequivalence Study data announced in September 2021 Type C Meeting Scheduled 2022 NDA Submission Planned H1 2022 2022 Upcoming Type-C Meeting with FDA to Discuss Roluperidone

Phase 2b Recap
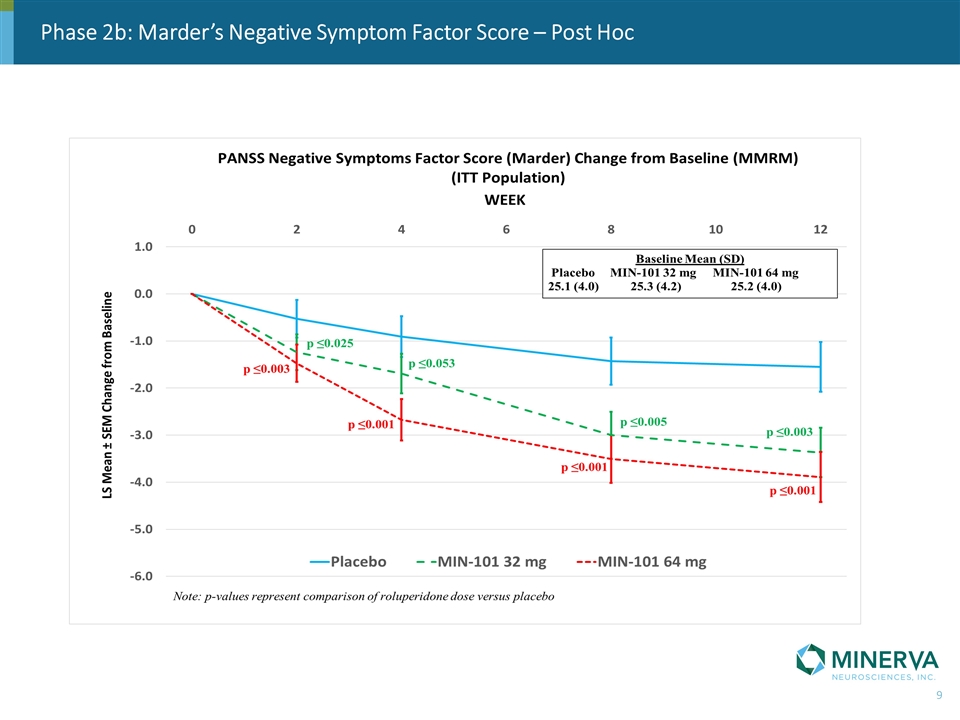
Phase 2b: Marder’s Negative Symptom Factor Score – Post Hoc

Phase 3: Recap
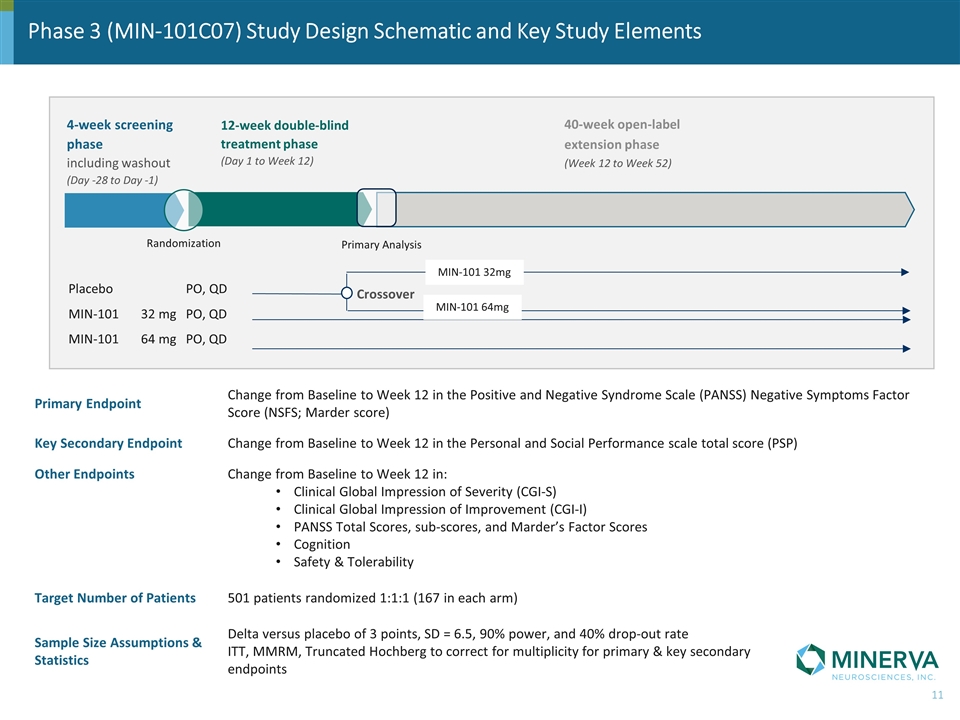
Phase 3 (MIN-101C07) Study Design Schematic and Key Study Elements Primary Endpoint Change from Baseline to Week 12 in the Positive and Negative Syndrome Scale (PANSS) Negative Symptoms Factor Score (NSFS; Marder score) Key Secondary Endpoint Change from Baseline to Week 12 in the Personal and Social Performance scale total score (PSP) Other Endpoints Change from Baseline to Week 12 in: Clinical Global Impression of Severity (CGI-S) Clinical Global Impression of Improvement (CGI-I) PANSS Total Scores, sub-scores, and Marder’s Factor Scores Cognition Safety & Tolerability Target Number of Patients 501 patients randomized 1:1:1 (167 in each arm) Sample Size Assumptions & Statistics Delta versus placebo of 3 points, SD = 6.5, 90% power, and 40% drop-out rate ITT, MMRM, Truncated Hochberg to correct for multiplicity for primary & key secondary endpoints 4-week screening phase including washout (Day -28 to Day -1) 12-week double-blind treatment phase (Day 1 to Week 12) 40-week open-label extension phase (Week 12 to Week 52) Placebo PO, QD MIN-101 32 mg PO, QD MIN-101 64 mg PO, QD Randomization Primary Analysis Crossover MIN-101 64mg MIN-101 32mg
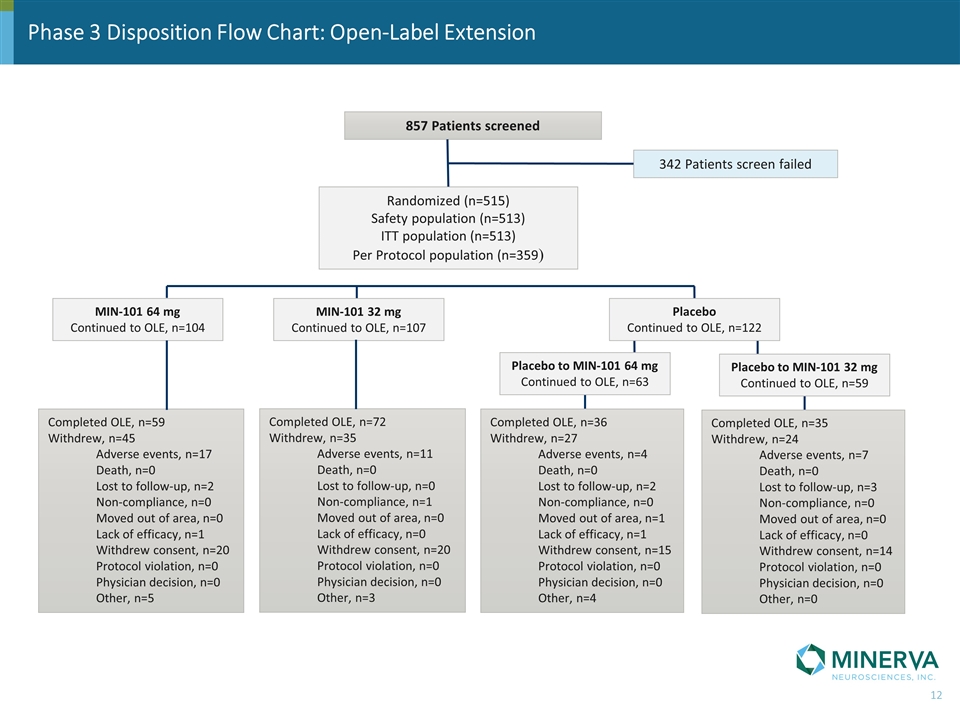
Phase 3 Disposition Flow Chart: Open-Label Extension 857 Patients screened 342 Patients screen failed MIN-101 64 mg Continued to OLE, n=104 Completed OLE, n=59 Withdrew, n=45 Adverse events, n=17 Death, n=0 Lost to follow-up, n=2 Non-compliance, n=0 Moved out of area, n=0 Lack of efficacy, n=1 Withdrew consent, n=20 Protocol violation, n=0 Physician decision, n=0 Other, n=5 Completed OLE, n=72 Withdrew, n=35 Adverse events, n=11 Death, n=0 Lost to follow-up, n=0 Non-compliance, n=1 Moved out of area, n=0 Lack of efficacy, n=0 Withdrew consent, n=20 Protocol violation, n=0 Physician decision, n=0 Other, n=3 Completed OLE, n=35 Withdrew, n=24 Adverse events, n=7 Death, n=0 Lost to follow-up, n=3 Non-compliance, n=0 Moved out of area, n=0 Lack of efficacy, n=0 Withdrew consent, n=14 Protocol violation, n=0 Physician decision, n=0 Other, n=0 Randomized (n=515) Safety population (n=513) ITT population (n=513) Per Protocol population (n=359) MIN-101 32 mg Continued to OLE, n=107 Placebo to MIN-101 64 mg Continued to OLE, n=63 Completed OLE, n=36 Withdrew, n=27 Adverse events, n=4 Death, n=0 Lost to follow-up, n=2 Non-compliance, n=0 Moved out of area, n=1 Lack of efficacy, n=1 Withdrew consent, n=15 Protocol violation, n=0 Physician decision, n=0 Other, n=4 Placebo to MIN-101 32 mg Continued to OLE, n=59 Placebo Continued to OLE, n=122
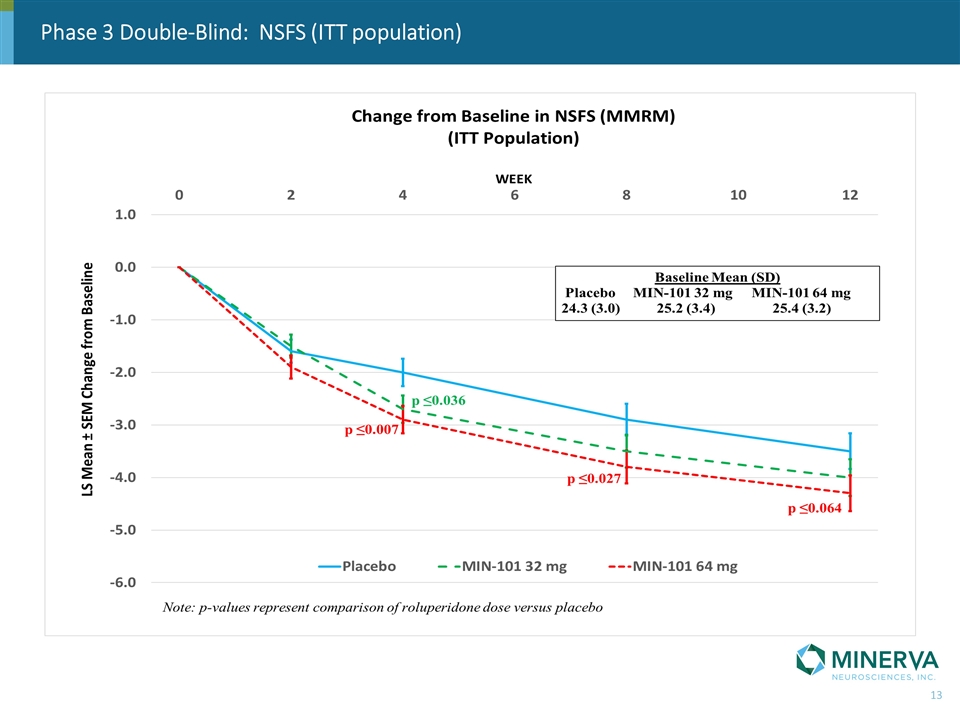
Phase 3 Double-Blind: NSFS (ITT population)
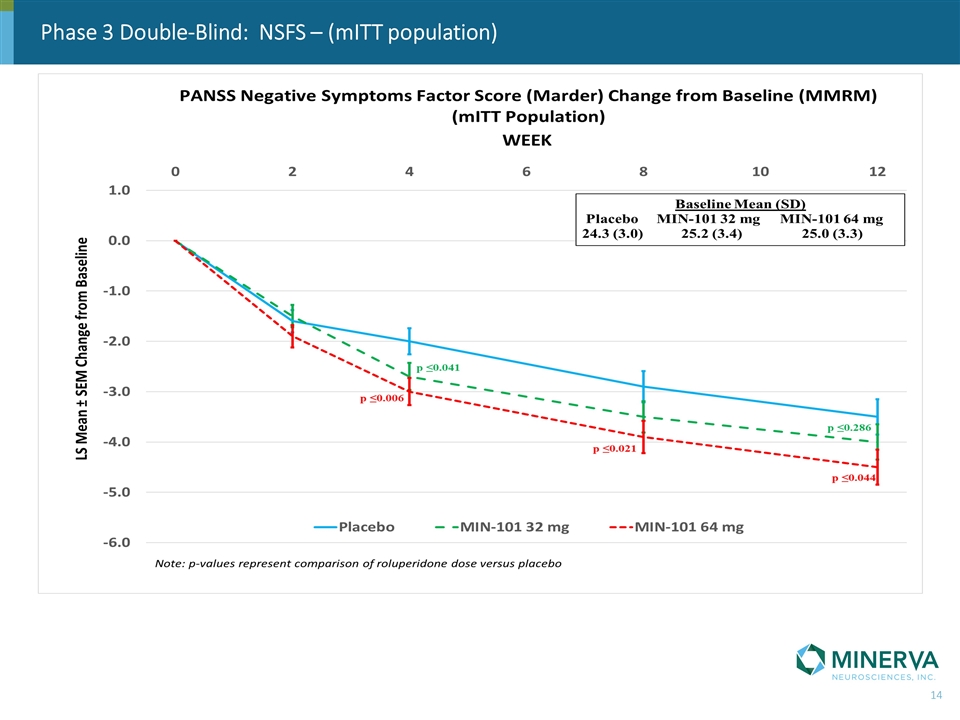
Phase 3 Double-Blind: NSFS – (mITT population)
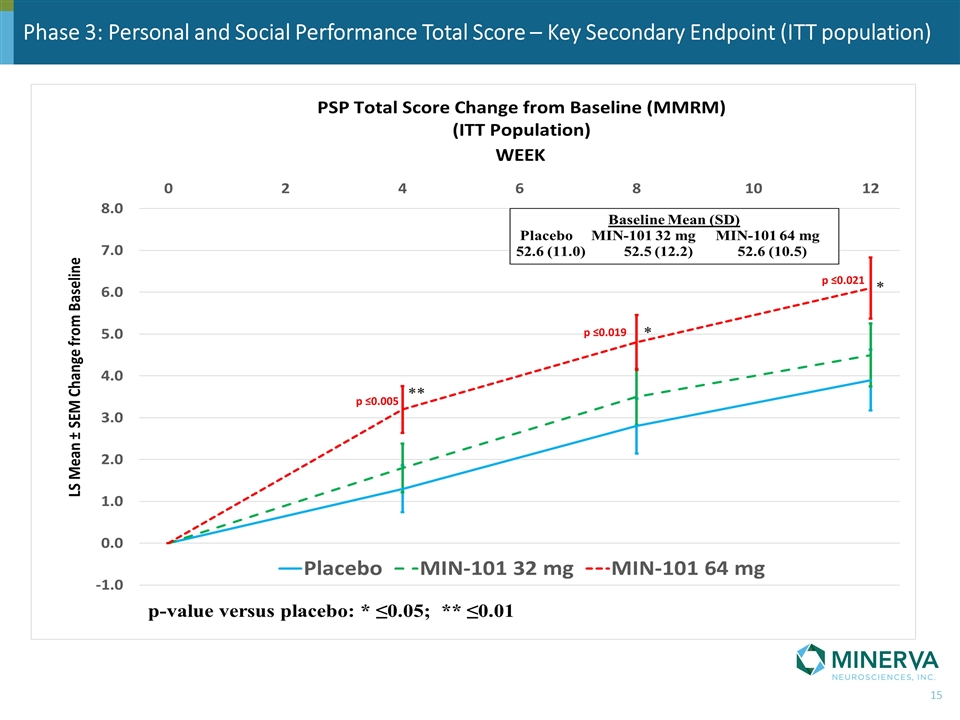
Phase 3: Personal and Social Performance Total Score – Key Secondary Endpoint (ITT population) p ≤0.005 p ≤0.019 p ≤0.021

Phase 3 Extension: Recap

Key Objectives of the open-Label Extension (OLE) Long term safety Long term efficacy: Negative Symptoms Marder Negative Symptom Factor Score (NSFS) (Primary Endpoint) Functioning Personal and Social Performance Total score (PSP) (Key Secondary Endpoint) Overall Psychopathology Other dimensions of the disease, including CGI-S and other PANSS scale items Relapse rate of Schizophrenia Cognition
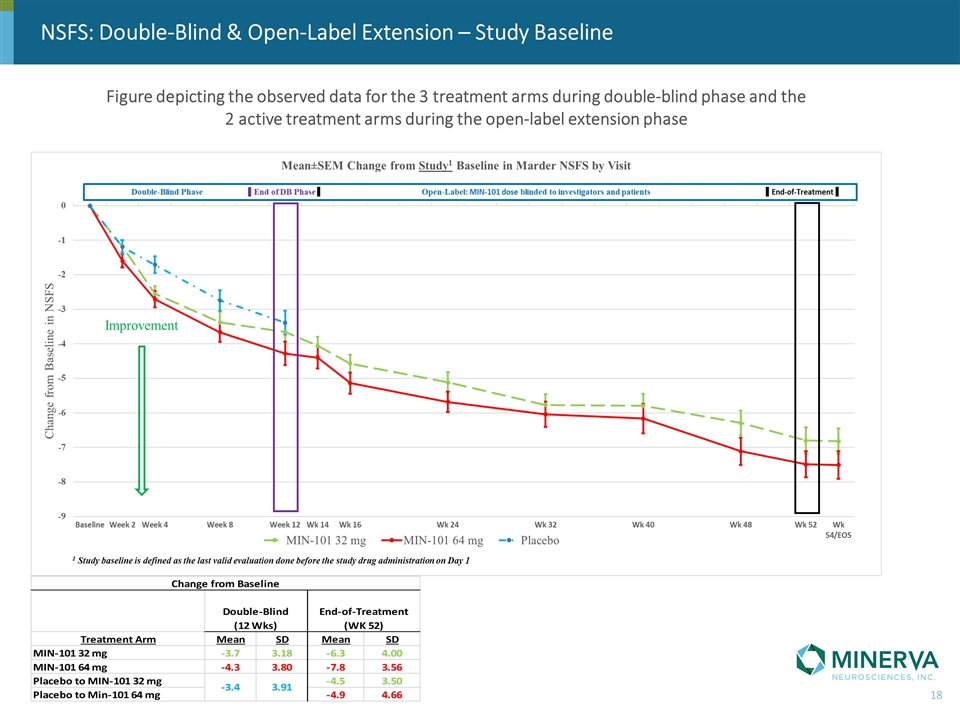
NSFS: Double-Blind & Open-Label Extension – Study Baseline Figure depicting the observed data for the 3 treatment arms during double-blind phase and the 2 active treatment arms during the open-label extension phase
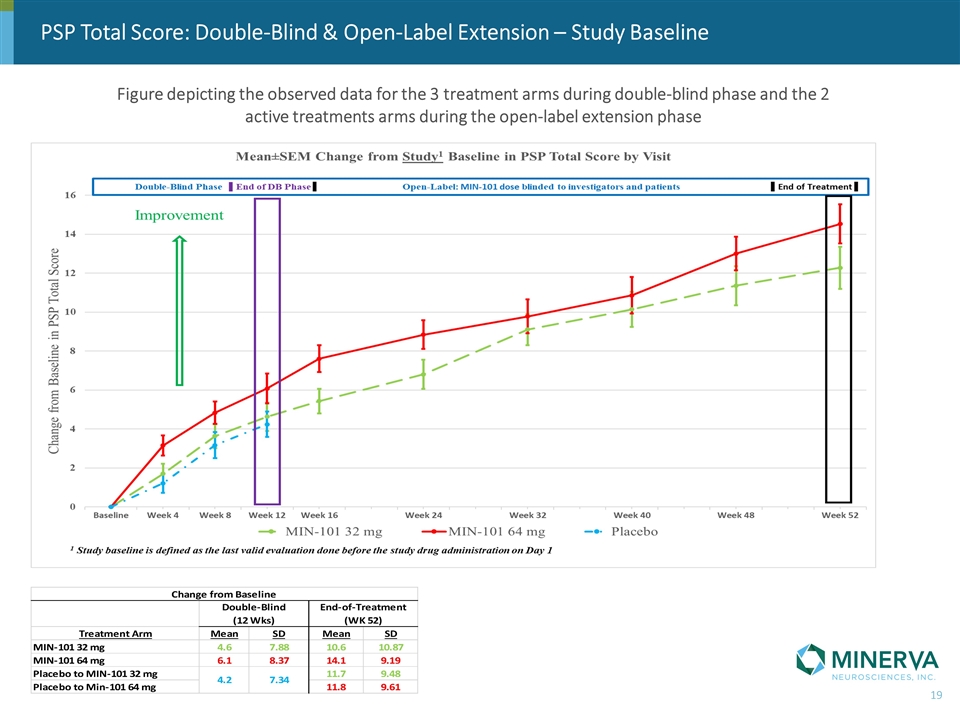
PSP Total Score: Double-Blind & Open-Label Extension – Study Baseline Figure depicting the observed data for the 3 treatment arms during double-blind phase and the 2 active treatments arms during the open-label extension phase
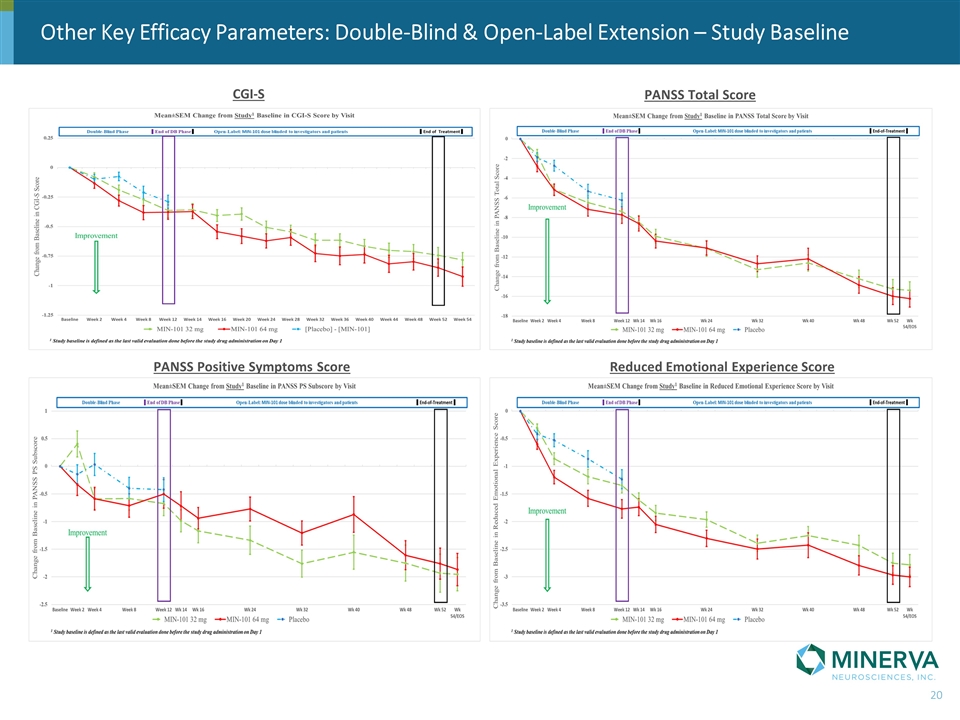
Other Key Efficacy Parameters: Double-Blind & Open-Label Extension – Study Baseline CGI-S PANSS Total Score PANSS Positive Symptoms Score Reduced Emotional Experience Score
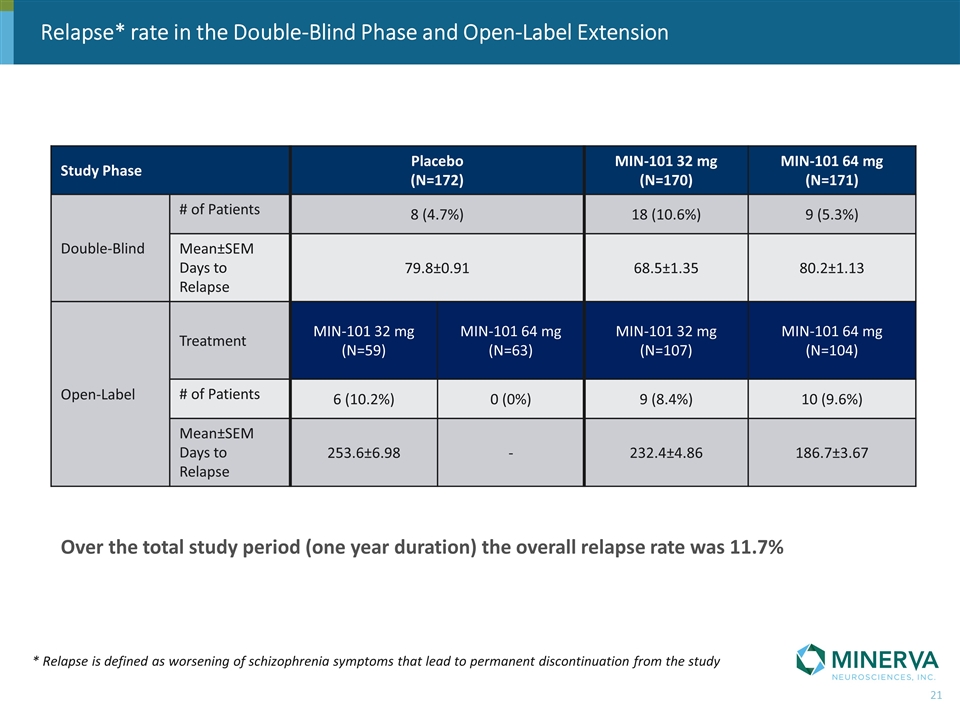
Relapse* rate in the Double-Blind Phase and Open-Label Extension * Relapse is defined as worsening of schizophrenia symptoms that lead to permanent discontinuation from the study Study Phase Placebo (N=172) MIN-101 32 mg (N=170) MIN-101 64 mg (N=171) Double-Blind # of Patients 8 (4.7%) 18 (10.6%) 9 (5.3%) Double-Blind Mean±SEM Days to Relapse 79.8±0.91 68.5±1.35 80.2±1.13 Open-Label Treatment MIN-101 32 mg (N=59) MIN-101 64 mg (N=63) MIN-101 32 mg (N=107) MIN-101 64 mg (N=104) Open-Label # of Patients 6 (10.2%) 0 (0%) 9 (8.4%) 10 (9.6%) Mean±SEM Days to Relapse 253.6±6.98 - 232.4±4.86 186.7±3.67 Over the total study period (one year duration) the overall relapse rate was 11.7%

Pivotal BE study: Recap
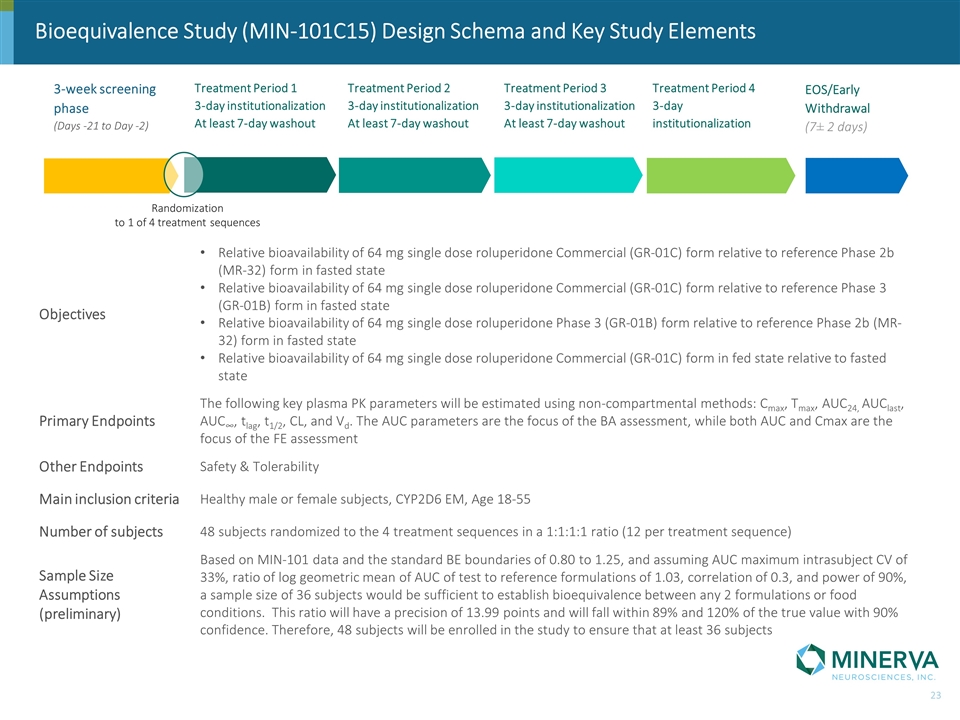
Bioequivalence Study (MIN-101C15) Design Schema and Key Study Elements Objectives Relative bioavailability of 64 mg single dose roluperidone Commercial (GR-01C) form relative to reference Phase 2b (MR-32) form in fasted state Relative bioavailability of 64 mg single dose roluperidone Commercial (GR-01C) form relative to reference Phase 3 (GR-01B) form in fasted state Relative bioavailability of 64 mg single dose roluperidone Phase 3 (GR-01B) form relative to reference Phase 2b (MR-32) form in fasted state Relative bioavailability of 64 mg single dose roluperidone Commercial (GR-01C) form in fed state relative to fasted state Primary Endpoints The following key plasma PK parameters will be estimated using non-compartmental methods: Cmax, Tmax, AUC24, AUClast, AUC∞, tlag, t1/2, CL, and Vd. The AUC parameters are the focus of the BA assessment, while both AUC and Cmax are the focus of the FE assessment Other Endpoints Safety & Tolerability Main inclusion criteria Healthy male or female subjects, CYP2D6 EM, Age 18-55 Number of subjects 48 subjects randomized to the 4 treatment sequences in a 1:1:1:1 ratio (12 per treatment sequence) Sample Size Assumptions (preliminary) Based on MIN-101 data and the standard BE boundaries of 0.80 to 1.25, and assuming AUC maximum intrasubject CV of 33%, ratio of log geometric mean of AUC of test to reference formulations of 1.03, correlation of 0.3, and power of 90%, a sample size of 36 subjects would be sufficient to establish bioequivalence between any 2 formulations or food conditions. This ratio will have a precision of 13.99 points and will fall within 89% and 120% of the true value with 90% confidence. Therefore, 48 subjects will be enrolled in the study to ensure that at least 36 subjects 3-week screening phase (Days -21 to Day -2) Treatment Period 1 3-day institutionalization At least 7-day washout Randomization to 1 of 4 treatment sequences EOS/Early Withdrawal (7± 2 days) Treatment Period 2 3-day institutionalization At least 7-day washout Treatment Period 3 3-day institutionalization At least 7-day washout Treatment Period 4 3-day institutionalization
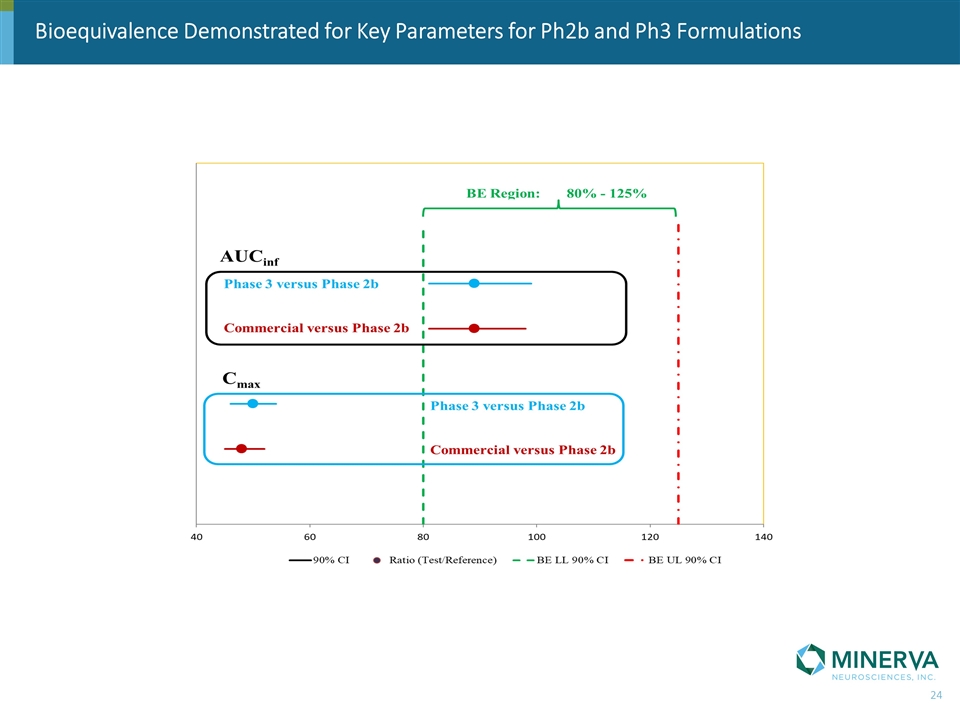
Bioequivalence Demonstrated for Key Parameters for Ph2b and Ph3 Formulations

Summary
Summary Roluperidone Negative Symptoms in Schizophrenia Regulatory Process Phase 3 Double-Blind TLR data announced in May 2020 Phase 3 40 week OLE data announced in May 2021 Bioequivalence Study data announced in September 2021 Type C meeting scheduled NDA filing targeted H1 2022 (Subject to FDA feedback) Seltorexant MDD & Insomnia Symptoms Phase 3 studies initiated by Janssen in 2020 Royalty rights sold to Royalty Pharma for $155m in Jan 2021 ($60m up-front) $95m future revenue dependent on clinical, regulatory & sales milestones Cash $65.7m cash & cash equivalents at September 30th, 2021

Thank you


























