
Summit Therapeutics Q4 & Full Year 2023 Earnings Call February 20, 2024 9:00am ET

Forward Looking Statement Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. 2 Summit Proprietary Information - Do Not Copy, Photograph or Distribute Oppenheimer 34th Annual Healthcare Life Sciences Conference, February 2024 Any statements in this presentation about the Company’s future expectations, plans and prospects, including but not limited to, statements about the clinical and preclinical development of the Company’s product candidates, entry into and actions related to the Company’s partnership with Akeso Inc., the therapeutic potential of the Company’s product candidates, the potential commercialization of the Company’s product candidates, the timing of initiation, completion and availability of data from clinical trials, the potential submission of applications for marketing approvals, potential acquisitions and other statements containing the words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "would," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the results of our evaluation of the underlying data in connection with the development and commercialization activities for ivonescimab, the outcome of discussions with regulatory authorities, including the Food and Drug Administration, the uncertainties inherent in the initiation of future clinical trials, availability and timing of data from ongoing and future clinical trials, the results of such trials, and their success, and global public health crises that may affect timing and status of our clinical trials and operations, whether preliminary results from a clinical trial will be predictive of the final results of that trial or whether results of early clinical trials or preclinical studies will be indicative of the results of later clinical trials, whether business development opportunities to expand the Company’s pipeline of drug candidates, including without limitation, through potential acquisitions of, and/or collaborations with, other entities occur, expectations for regulatory approvals, laws and regulations affecting government contracts and funding awards, availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements and other factors discussed in the "Risk Factors" section of filings that the Company makes with the Securities and Exchange Commission. Any change to our ongoing trials could cause delays, affect our future expenses, and add uncertainty to our commercialization efforts, as well as to affect the likelihood of the successful completion of clinical development of ivonescimab. Accordingly, the audience should not place undue reliance on forward-looking statements or information. In addition, any forward-looking statements included in this presentation represent the Company’s views only as of the date of this presentation and should not be relied upon as representing the Company’s views as of any subsequent date. The Company specifically disclaims any obligation to update any forward-looking statements included in this presentation.
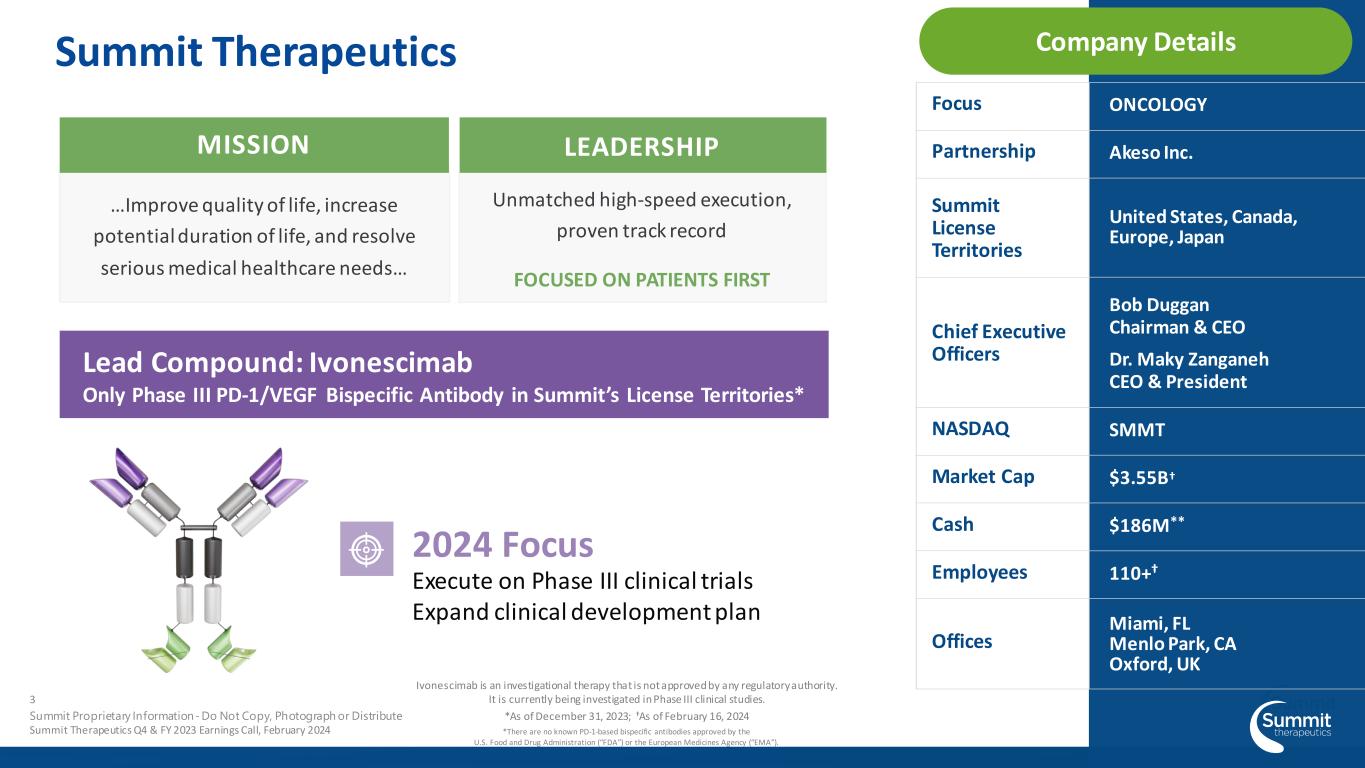
Company Details Focus ONCOLOGY Partnership Akeso Inc. Summit License Territories United States, Canada, Europe, Japan Chief Executive Officers Bob Duggan Chairman & CEO Dr. Maky Zanganeh CEO & President NASDAQ SMMT Market Cap $3.55B† Cash $186M** Employees 110+† Offices Miami, FL Menlo Park, CA Oxford, UK …Improve quality of life, increase potential duration of life, and resolve serious medical healthcare needs… MISSION Unmatched high-speed execution, proven track record FOCUSED ON PATIENTS FIRST LEADERSHIP Lead Compound: Ivonescimab Only Phase III PD-1/VEGF Bispecific Antibody in Summit’s License Territories* 2024 Focus Execute on Phase III clinical trials Expand clinical development plan Summit Therapeutics 3 Summit Proprietary Information - Do Not Copy, Photograph or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. *There are no known PD-1-based bispecific antibodies approved by the U.S. Food and Drug Administration (“FDA”) or the European Medicines Agency (“EMA”). *As of December 31, 2023; †As of February 16, 2024
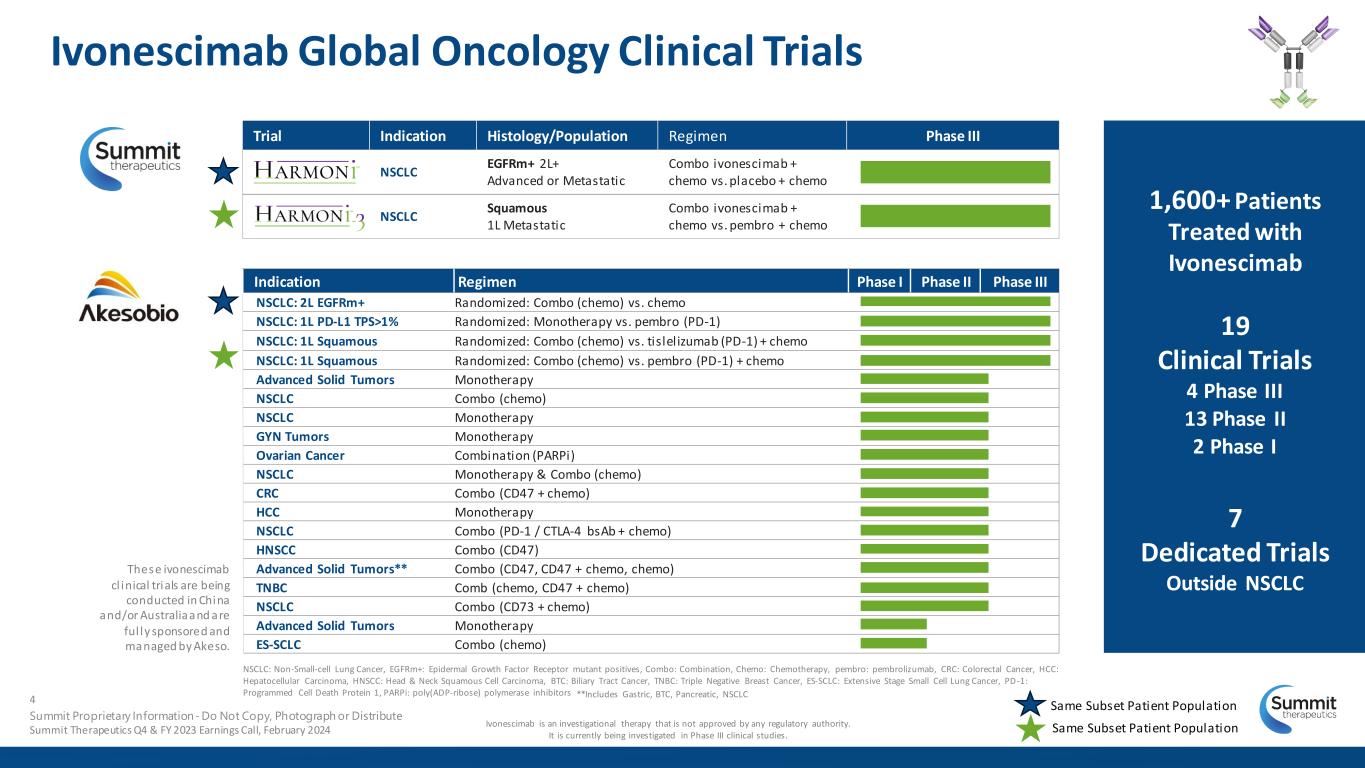
Trial Indication Histology/Population Regimen Phase III NSCLC EGFRm+ 2L+ Advanced or Metastatic Combo ivonescimab + chemo vs. placebo + chemo NSCLC Squamous 1L Metastatic Combo ivonescimab + chemo vs. pembro + chemo Indication Regimen Phase I Phase II Phase III NSCLC: 2L EGFRm+ Randomized: Combo (chemo) vs. chemo NSCLC: 1L PD-L1 TPS>1% Randomized: Monotherapy vs. pembro (PD-1) NSCLC: 1L Squamous Randomized: Combo (chemo) vs. tislelizumab (PD-1) + chemo NSCLC: 1L Squamous Randomized: Combo (chemo) vs. pembro (PD-1) + chemo Advanced Solid Tumors Monotherapy NSCLC Combo (chemo) NSCLC Monotherapy GYN Tumors Monotherapy Ovarian Cancer Combination (PARPi) NSCLC Monotherapy & Combo (chemo) CRC Combo (CD47 + chemo) HCC Monotherapy NSCLC Combo (PD-1 / CTLA-4 bsAb + chemo) HNSCC Combo (CD47) Advanced Solid Tumors** Combo (CD47, CD47 + chemo, chemo) TNBC Comb (chemo, CD47 + chemo) NSCLC Combo (CD73 + chemo) Advanced Solid Tumors Monotherapy ES-SCLC Combo (chemo) Ivonescimab Global Oncology Clinical Trials These ivonescimab cl inical trials are being conducted in China and/or Australia and are ful ly sponsored and managed by Akeso. NSCLC: Non-Small-cell Lung Cancer, EGFRm+: Epidermal Growth Factor Receptor mutant positives, Combo: Combination, Chemo: Chemotherapy, pembro: pembrolizumab, CRC: Colorectal Cancer, HCC: Hepatocellular Carcinoma, HNSCC: Head & Neck Squamous Cell Carcinoma, BTC: Biliary Tract Cancer, TNBC: Triple Negative Breast Cancer, ES-SCLC: Extensive Stage Small Cell Lung Cancer, PD-1: Programmed Cell Death Protein 1, PARPi: poly(ADP-ribose) polymerase inhibitors Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. **Includes Gastric, BTC, Pancreatic, NSCLC Same Subset Patient Population 1,600+ Patients Treated with Ivonescimab 19 Clinical Trials 4 Phase III 13 Phase II 2 Phase I 7 Dedicated Trials Outside NSCLC 4 Summit Proprietary Information - Do Not Copy, Photograph or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 Same Subset Patient Population
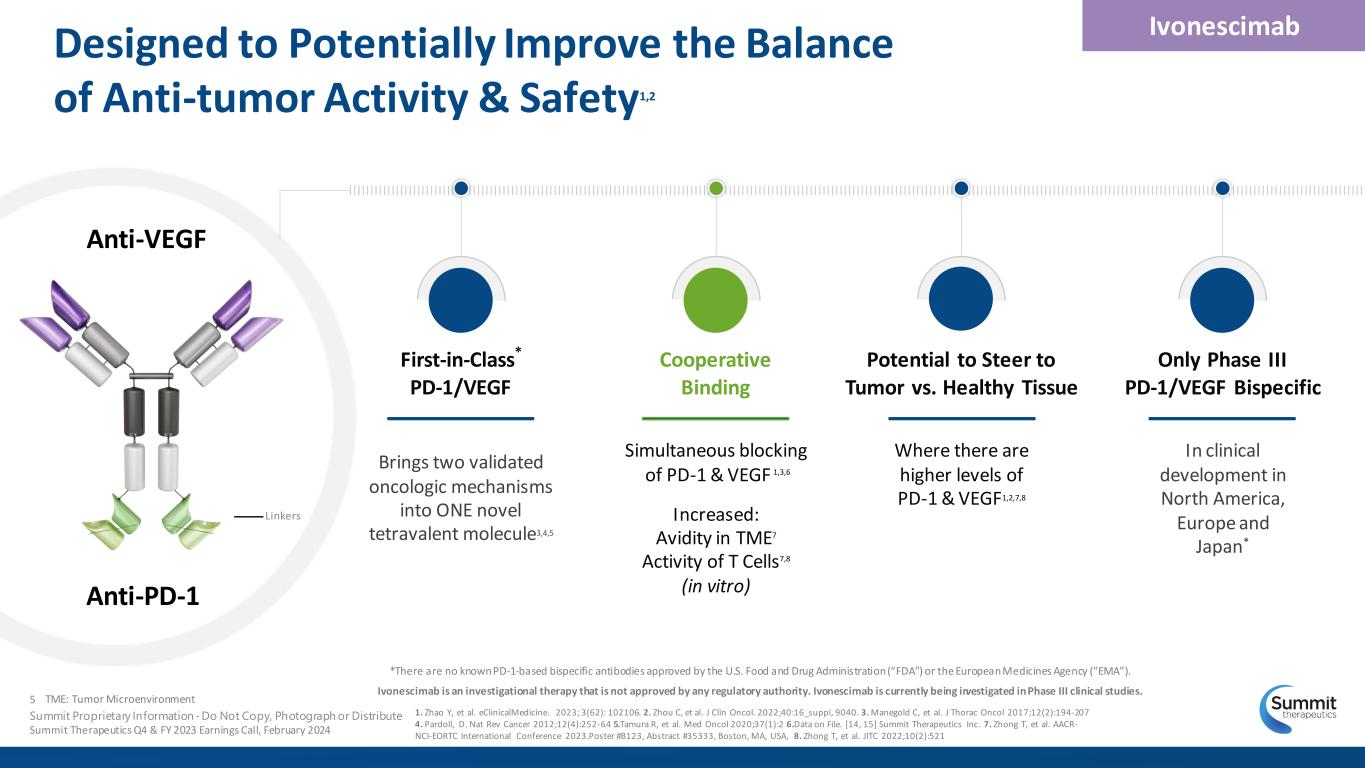
Designed to Potentially Improve the Balance of Anti-tumor Activity & Safety1,2 *There are no known PD-1-based bispecific antibodies approved by the U.S. Food and Drug Administration (“FDA”) or the European Medicines Agency (“EMA”). Ivonescimab is an investigational therapy that is not approved by any regulatory authority. Ivonescimab is currently being investigated in Phase III clinical studies. Ivonescimab First-in-Class* PD-1/VEGF Brings two validated oncologic mechanisms into ONE novel tetravalent molecule3,4,5 Potential to Steer to Tumor vs. Healthy Tissue Where there are higher levels of PD-1 & VEGF1,2,7,8 Only Phase III PD-1/VEGF Bispecific In clinical development in North America, Europe and Japan* Cooperative Binding Simultaneous blocking of PD-1 & VEGF 1,3,6 Increased: Avidity in TME7 Activity of T Cells7,8 (in vitro) 5 Summit Proprietary Information - Do Not Copy, Photograph or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 1. Zhao Y, et al. eClinicalMedicine. 2023; 3(62): 102106. 2. Zhou C, et al. J Clin Oncol. 2022;40:16_suppl, 9040. 3. Manegold C, et al. J Thorac Oncol 2017;12(2):194-207 4. Pardoll, D. Nat Rev Cancer 2012;12(4):252-64 5.Tamura R, et al. Med Oncol 2020;37(1):2 6.Data on File. [14, 15] Summit Therapeutics Inc. 7. Zhong T, et al. AACR- NCI-EORTC International Conference 2023.Poster #B123, Abstract #35333, Boston, MA, USA, 8. Zhong T, et al. JITC 2022;10(2):521 TME: Tumor Microenvironment Anti-VEGF Anti-PD-1 Linkers
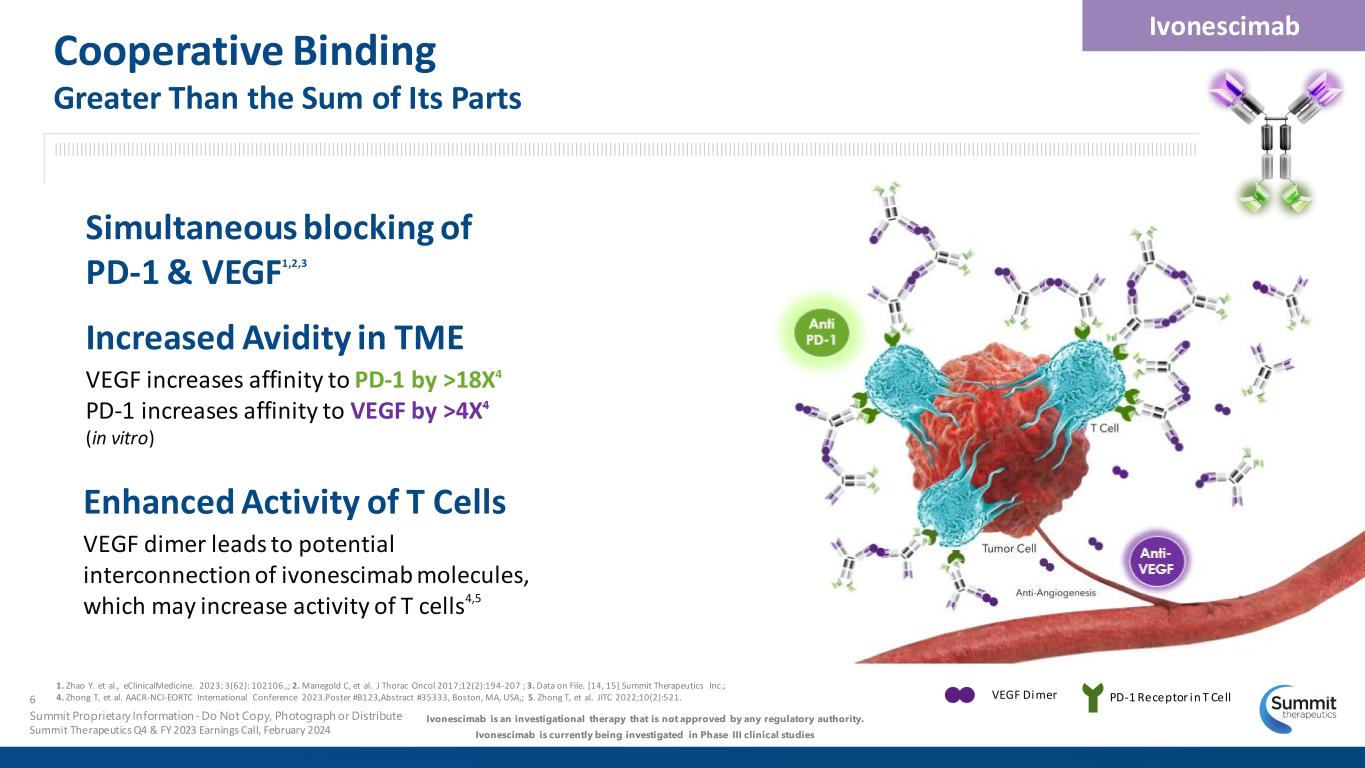
VEGF Dimer PD-1 Receptor in T Cell Increased Avidity in TME VEGF increases affinity to PD-1 by >18X4 PD-1 increases affinity to VEGF by >4X4 (in vitro) Enhanced Activity of T Cells VEGF dimer leads to potential interconnection of ivonescimab molecules, which may increase activity of T cells4,5 Ivonescimab is an investigational therapy that is not approved by any regulatory authority. Ivonescimab is currently being investigated in Phase III clinical studies Simultaneous blocking of PD-1 & VEGF1,2,3 Ivonescimab 6 Summit Proprietary Information - Do Not Copy, Photograph or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 Cooperative Binding Greater Than the Sum of Its Parts 1. Zhao Y. et al., eClinicalMedicine. 2023; 3(62): 102106.,; 2. Manegold C, et al. J Thorac Oncol 2017;12(2):194-207 ; 3. Data on File. [14, 15] Summit Therapeutics Inc.; 4. Zhong T, et al. AACR-NCI-EORTC International Conference 2023.Poster #B123,Abstract #35333, Boston, MA, USA,; 5. Zhong T, et al. JITC 2022;10(2):521.
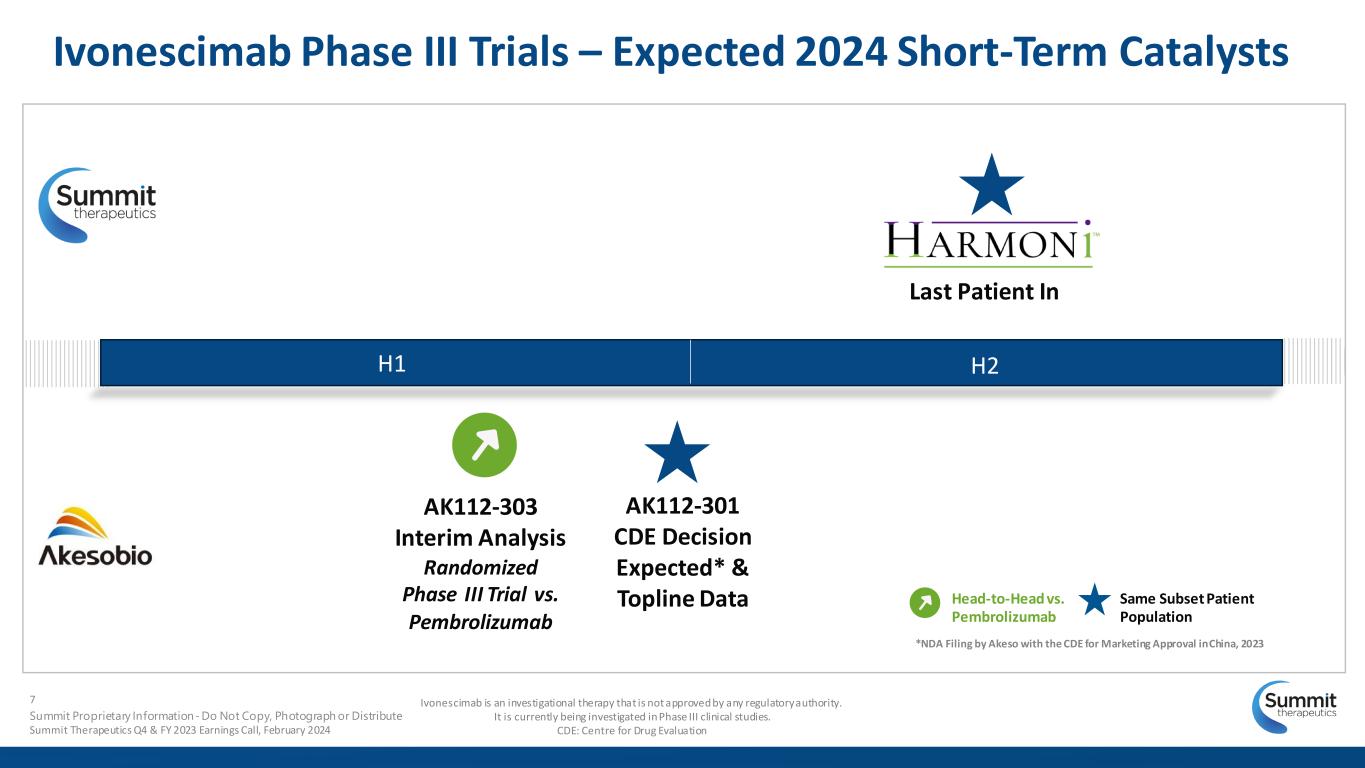
Ivonescimab Phase III Trials – Expected 2024 Short-Term Catalysts Ivonescimab is an investigational therapy that is not approved by any regulatory authority. It is currently being investigated in Phase III clinical studies. CDE: Centre for Drug Evaluation *NDA Filing by Akeso with the CDE for Marketing Approval in China, 2023 Head-to-Head vs. Pembrolizumab Same Subset Patient Population 7 Summit Proprietary Information - Do Not Copy, Photograph or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 AK112-301 CDE Decision Expected* & Topline Data AK112-303 Interim Analysis Randomized Phase III Trial vs. Pembrolizumab Last Patient In H1 H2
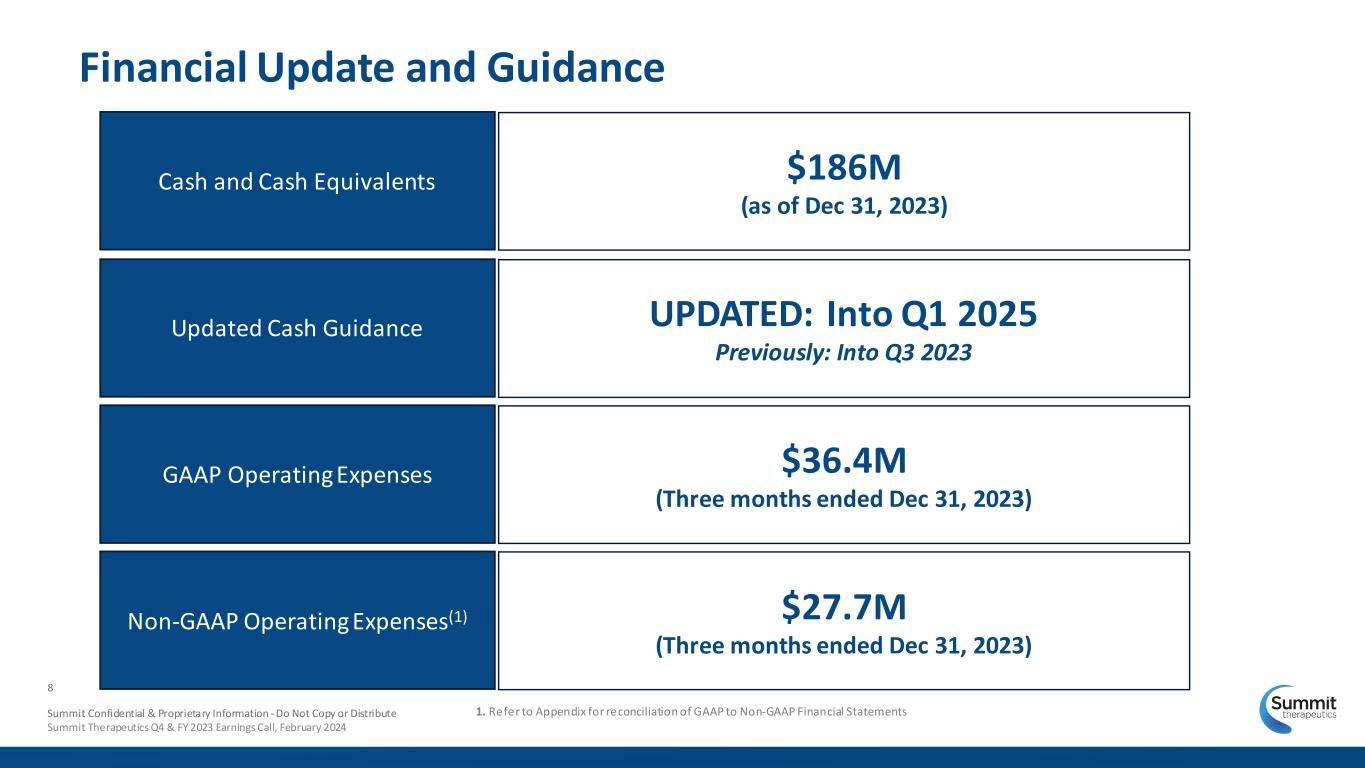
Summit Confidential & Proprietary Information - Do Not Copy or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 8 Financial Update and Guidance Cash and Cash Equivalents Updated Cash Guidance GAAP Operating Expenses $186M (as of Dec 31, 2023) UPDATED: Into Q1 2025 Previously: Into Q3 2023 $36.4M (Three months ended Dec 31, 2023) Non-GAAP Operating Expenses(1) $27.7M (Three months ended Dec 31, 2023) 1. Refer to Appendix for reconciliation of GAAP to Non-GAAP Financial Statements

APPENDIX

Summit Confidential & Proprietary Information - Do Not Copy or Distribute 10 Statement Regarding Use of Non-GAAP Financial Measures Non-GAAP financial measures adjust GAAP financial measures for the items listed below. These Non-GAAP measures should be viewed in addition to, and not as a substitute for Summit's reported GAAP results, and may be different from Non-GAAP measures used by other companies. In addition, these Non-GAAP measures are not based on any comprehensive set of accounting rules or principles. Summit management uses these non-GAAP measures for internal budgeting and forecasting purposes and to evaluate Summit’s financial performance. Summit management believes the presentation of these Non-GAAP measures is useful to investors for comparing prior periods and analyzing ongoing business trends and operating results. Each of non-GAAP Research and Development Expense, non-GAAP General and Administrative Expenses, non-GAAP Operating Expenses, Non-GAAP Net Loss and Non- GAAP EPS differ from GAAP in that such measures exclude the non-cash charges and costs associated with stock-based compensation. In addition, (i) non-GAAP Operating Expenses, non-GAAP Net Loss and non-GAAP EPS each exclude certain one-time charges associated with in-process research and development and impairment of intangible assets, (ii) non-GAAP In-Process Research and Development Expenses excludes certain in-process research and development charges and (iii) non-GAAP Impairment of Intangible Assets excludes certain one-time impairment charges, in each case as described further in the notes below and as expressed in the tabular reconciliation presented in following slides. Note 1: Stock-based compensation is a non-cash charge and costs calculated for this expense can vary year-over-year depending on the stock price of awards on the date of grant as well as the timing of compensation award arrangements. Note 2: In-process research and development represents a one-time charge associated with the Company's in-licensing of ivonescimab from Akeso. Note 3: The Company determined that it would cease investment in the Discuva Platform in 2022 and focus on the therapeutic area of oncology and as such recognized an impairment charge for the carrying value.
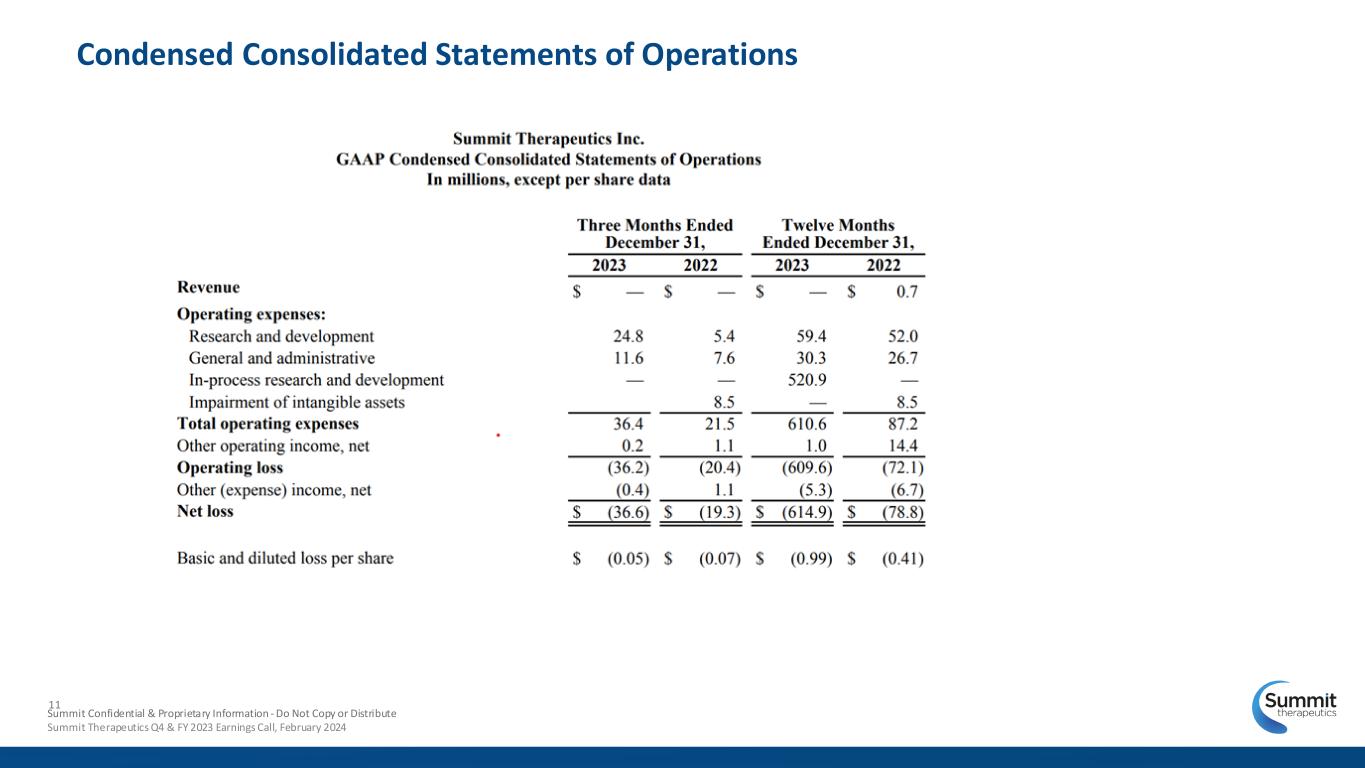
Condensed Consolidated Statements of Operations 11 Summit Confidential & Proprietary Information - Do Not Copy or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024
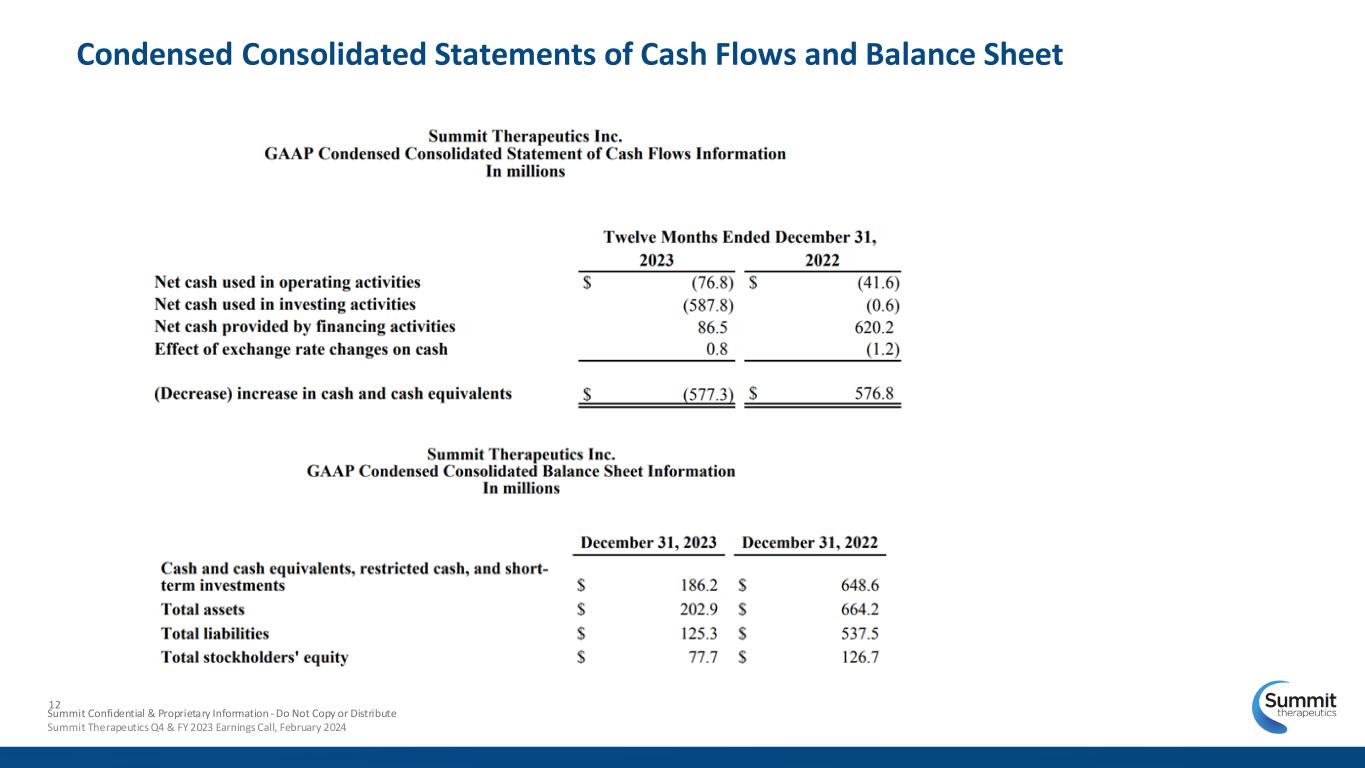
12 Condensed Consolidated Statements of Cash Flows and Balance Sheet Summit Confidential & Proprietary Information - Do Not Copy or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024
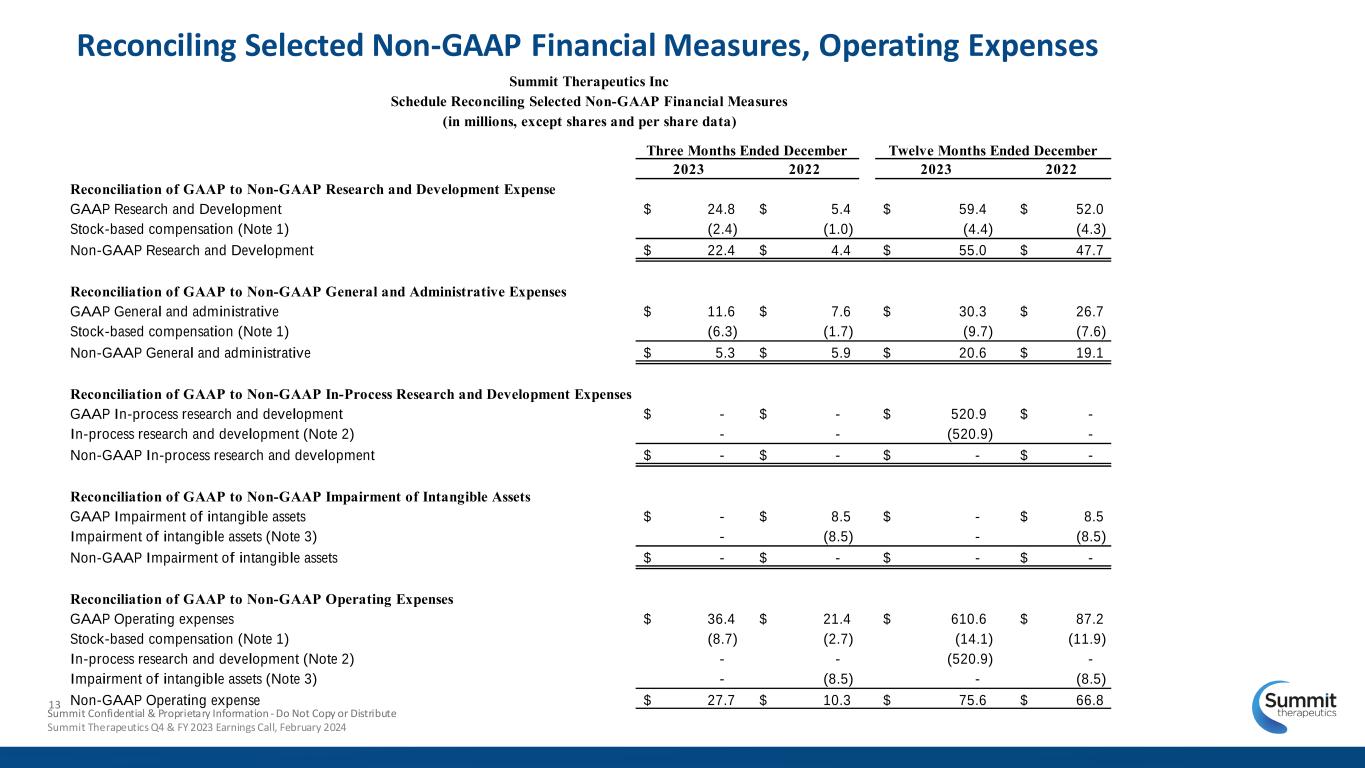
13 Reconciling Selected Non-GAAP Financial Measures, Operating Expenses Summit Confidential & Proprietary Information - Do Not Copy or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024 2023 2022 2023 2022 Reconciliation of GAAP to Non-GAAP Research and Development Expense GAAP Research and Development $ 24.8 $ 5.4 $ 59.4 $ 52.0 Stock-based compensation (Note 1) (2.4) (1.0) (4.4) (4.3) Non-GAAP Research and Development $ 22.4 $ 4.4 $ 55.0 $ 47.7 Reconciliation of GAAP to Non-GAAP General and Administrative Expenses GAAP General and administrative $ 11.6 $ 7.6 $ 30.3 $ 26.7 Stock-based compensation (Note 1) (6.3) (1.7) (9.7) (7.6) Non-GAAP General and administrative $ 5.3 $ 5.9 $ 20.6 $ 19.1 Reconciliation of GAAP to Non-GAAP In-Process Research and Development Expenses GAAP In-process research and development $ - $ - $ 520.9 $ - In-process research and development (Note 2) - - (520.9) - Non-GAAP In-process research and development $ - $ - $ - $ - Reconciliation of GAAP to Non-GAAP Impairment of Intangible Assets GAAP Impairment of intangible assets $ - $ 8.5 $ - $ 8.5 Impairment of intangible assets (Note 3) - (8.5) - (8.5) Non-GAAP Impairment of intangible assets $ - $ - $ - $ - Reconciliation of GAAP to Non-GAAP Operating Expenses GAAP Operating expenses $ 36.4 $ 21.4 $ 610.6 $ 87.2 Stock-based compensation (Note 1) (8.7) (2.7) (14.1) (11.9) In-process research and development (Note 2) - - (520.9) - Impairment of intangible assets (Note 3) - (8.5) - (8.5) Non-GAAP Operating expense $ 27.7 $ 10.3 $ 75.6 $ 66.8 Three Months Ended December Twelve Months Ended December Summit Therapeutics Inc Schedule Reconciling Selected Non-GAAP Financial Measures (in millions, except shares and per share data)
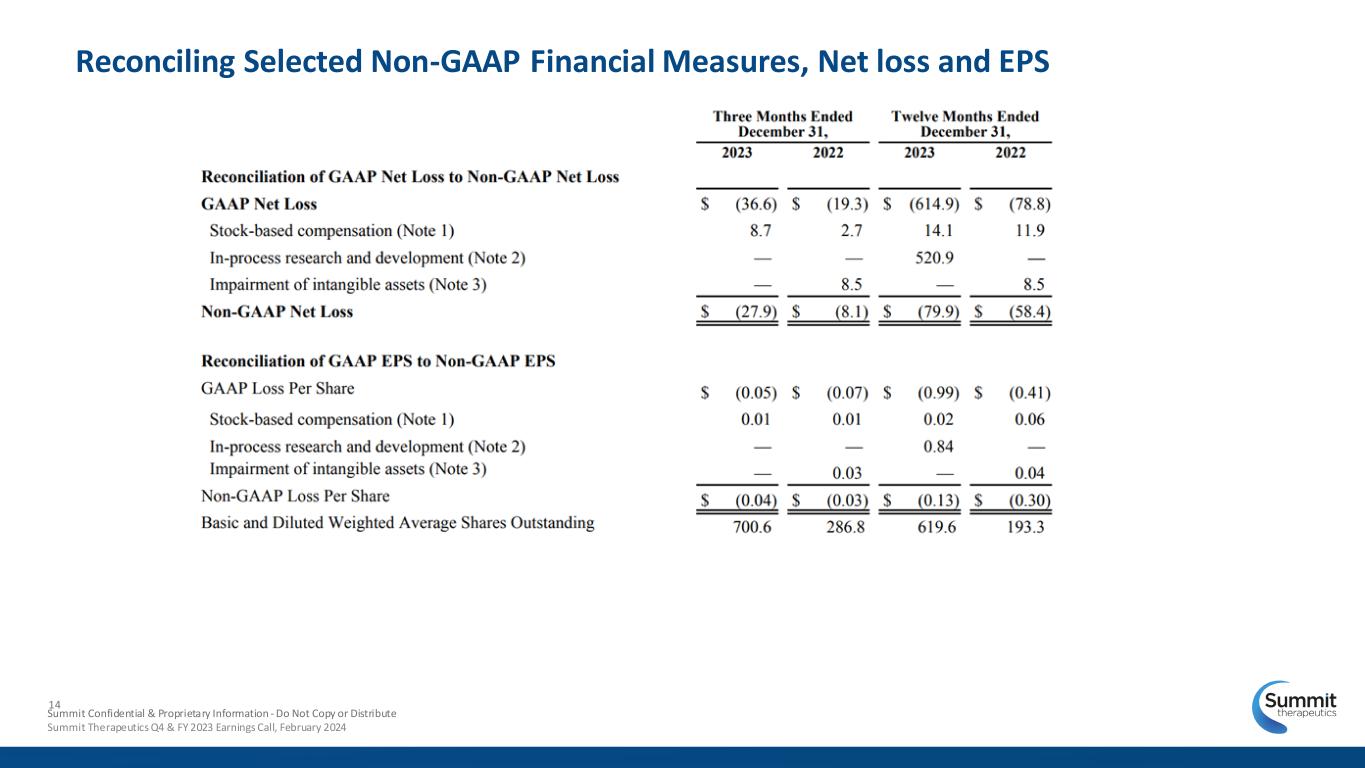
14 Reconciling Selected Non-GAAP Financial Measures, Net loss and EPS Summit Confidential & Proprietary Information - Do Not Copy or Distribute Summit Therapeutics Q4 & FY 2023 Earnings Call, February 2024

Summit Therapeutics Q4 & Full Year 2023 Earnings Call February 20, 2024 9:00am ET














