UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________
FORM 10-Q
_________________
| | | | | |
| (Mark One) |
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
For the quarterly period ended March 31, 2024
or
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from _________ to _________ |
Commission File Number: 001-36866
_______________________________
Summit Therapeutics Inc.
(Exact name of registrant as specified in its charter)
_____________________
| | | | | | | | |
| Delaware | | 37-1979717 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
| | |
| | |
601 Brickell Key Drive, Suite 1000,
Miami, FL
(Address of principal executive offices)
650-460-8308
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address and former fiscal year, if changed since last report)
_________________
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, $0.01 par value per share | SMMT | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | |
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| | | |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | |
| Emerging growth company | ☐ | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of April 22, 2024, there were 701,979,596 shares of common stock, par value $0.01 per share, outstanding.
| | | | | | | | |
| | Page |
| |
| PART I | |
| Item 1. | | |
| | |
| | |
| | |
| | |
| | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| PART II | |
| Item 1. | | |
| Item 1A. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| Item 5 | | |
| Item 6. | | |
| | |
| | |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 regarding the future financial performance, business prospects and growth of Summit Therapeutics Inc., that involve substantial risks and uncertainties. All statements contained in this Quarterly Report on Form 10-Q, other than statements of historical fact, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements in this Quarterly Report on Form 10-Q include, among other things, statements about:
•the ability to develop a successful product candidate under the License Agreement (as defined below);
•our ability to raise sufficient additional funds to make payments under the License Agreement;
•the timing of and the ability to effectively execute clinical development of ivonescimab;
•the timing, costs, conduct and outcomes of clinical trials for any product candidates;
•our plans with respect to possible future collaborations and partnering arrangements;
•the potential benefits of possible future acquisitions or investments in other businesses, products or technologies;
•our plans to pursue research and development of other future product candidates;
•our estimates regarding the potential market opportunity and patient population for commercializing our product candidates, if approved for commercial use;
•our sales, marketing and distribution capabilities and strategy;
•our ability to establish and maintain arrangements with third parties, such as contract research organizations, contract manufacturing organizations, suppliers, and distributors;
•the costs and timing of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against any intellectual property-related claims;
•our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
•the impact of government laws and regulations in the United States and in foreign countries;
•the timing and likelihood of regulatory filings and approvals for our product candidates;
•whether regulatory authorities determine that additional trials or data are necessary in order to accept a new drug application for review and/or approval;
•our competitive position;
•the need to raise additional capital to fund ongoing operations and capital needs;
•our ability to attract and retain key scientific or management personnel;
•the impact of public health epidemics, such as the novel coronavirus pandemic (“COVID-19”), the response to such epidemics and the potential effects of such epidemics on our clinical trials, business, financial results, supply chain and market; and
•other risks and uncertainties, including those described under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K for the year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (SEC) on February 20, 2024.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this Report, particularly in the “Risk Factors” section in this Report, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this Report and the documents that we have filed as exhibits to this Report completely and with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking statements.
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements.
Summit Therapeutics Inc.
Condensed Consolidated Balance Sheets
(in thousands, except share and per share data)
(Unaudited)
| | | | | | | | | | | | | | |
| | March 31, 2024 | | December 31, 2023 |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 61,294 | | | $ | 71,425 | |
| Restricted cash | | 317 | | | — | |
| Short-term investments | | 95,386 | | | 114,817 | |
| | | | |
| Prepaid expenses and other current assets | | 5,323 | | | 2,622 | |
| | | | |
| Research and development tax credit receivable | | 945 | | | 848 | |
| Total current assets | | 163,265 | | | 189,712 | |
| | | | |
| Non-current assets: | | | | |
| Property and equipment, net | | 181 | | | 204 | |
| Right-of-use assets | | 9,373 | | | 5,859 | |
| Goodwill | | 1,875 | | | 1,893 | |
| | | | |
| Research and development tax credit receivable | | 212 | | | 959 | |
| Other assets | | 1,875 | | | 4,322 | |
| Total assets | | $ | 176,781 | | | $ | 202,949 | |
| | | | |
| Liabilities and stockholders' equity | | | | |
| Current liabilities: | | | | |
| Accounts payable | | $ | 6,371 | | | $ | 2,667 | |
| Accrued liabilities | | 6,547 | | | 8,783 | |
| Accrued compensation | | 6,033 | | | 5,429 | |
| Lease liabilities | | 3,699 | | | 2,809 | |
| | | | |
| Other current liabilities | | 788 | | | 717 | |
| | | | |
| Total current liabilities | | 23,438 | | | 20,405 | |
| | | | |
| Non-current liabilities: | | | | |
| Lease liabilities, net of current portion | | 5,823 | | | 3,290 | |
| Other non-current liabilities | | 3,310 | | | 1,562 | |
| Promissory note payable to a related party | | 100,000 | | | 100,000 | |
| Total liabilities | | 132,571 | | | 125,257 | |
| | | | |
| Commitments and contingencies (Note 17) | | | | |
| | | | |
| Stockholders' equity: | | | | |
| Preferred stock, $0.01 par value, 20,000,000 shares authorized; none issued and outstanding at March 31, 2024 and December 31, 2023, respectively | | — | | | — | |
| Common stock, $0.01 par value: 1,000,000,000 shares authorized; 701,974,596 and 701,660,053 shares issued and outstanding at March 31, 2024 and December 31, 2023, respectively | | 7,020 | | | 7,017 | |
| Additional paid-in capital | | 1,076,370 | | | 1,066,381 | |
| Accumulated other comprehensive loss | | (2,449) | | | (2,448) | |
| Accumulated deficit | | (1,036,731) | | | (993,258) | |
| Total stockholders' equity | | 44,210 | | | 77,692 | |
| Total liabilities and stockholders' equity | | $ | 176,781 | | | $ | 202,949 | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Summit Therapeutics Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
(Unaudited)
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, | | |
| | 2024 | | 2023 | | | | |
| | | | | | | | |
| Operating expenses: | | | | | | | | |
| Research and development | | $ | 30,873 | | | $ | 9,883 | | | | | |
| In-process research and development | | — | | | 520,915 | | | | | |
| General and administrative | | 11,729 | | | 6,940 | | | | | |
| | | | | | | | |
| Total operating expenses | | 42,602 | | | 537,738 | | | | | |
| Other operating income, net | | 213 | | | 584 | | | | | |
| Operating loss | | (42,389) | | | (537,154) | | | | | |
| Other expense, net | | (1,084) | | | (5,222) | | | | | |
| | | | | | | | |
| | | | | | | | |
| Net loss | | $ | (43,473) | | | $ | (542,376) | | | | | |
| | | | | | | | |
| Net loss per share: | | | | | | | | |
| Basic and diluted | | $ | (0.06) | | | $ | (1.43) | | | | | |
| Weighted-average shares used to compute net loss per share: | | | | | | | | |
| Basic and diluted | | 701,785,250 | | | 378,163,980 | | | | | |
| | | | | | | | |
| Comprehensive loss: | | | | | | | | |
| Net loss | | $ | (43,473) | | | $ | (542,376) | | | | | |
| | | | | | | | |
| Other comprehensive (loss) income: | | | | | | | | |
| Foreign currency translation adjustments | | (12) | | | (51) | | | | | |
| Reclassification of cumulative currency translation gain to other expense, net | | — | | | (419) | | | | | |
| Net changes related to short-term investments | | 11 | | | 968 | | | | | |
| Comprehensive loss | | $ | (43,474) | | | $ | (541,878) | | | | | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Summit Therapeutics Inc.
Condensed Consolidated Statements of Stockholders' Equity
(in thousands, except share data)
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| | | | | | | | | | |
| | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | Three Months Ended March 31, 2024 |
| | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Loss | | Accumulated Deficit | | Total Stockholders' Equity |
| | Shares | | Amount | | | | |
| Balance at December 31, 2023 | | 701,660,053 | | | $ | 7,017 | | | $ | 1,066,381 | | | $ | (2,448) | | | $ | (993,258) | | | $ | 77,692 | |
| Issuance of common stock under stock purchase plans and exercise of stock options | | 314,543 | | | 3 | | | 482 | | | — | | | — | | | 485 | |
| Stock-based compensation | | — | | | — | | | 9,507 | | | — | | | — | | | 9,507 | |
| | | | | | | | | | | | |
| Net other comprehensive loss | | — | | | — | | | — | | | (1) | | | — | | | (1) | |
| Net loss | | — | | | — | | | — | | | — | | | (43,473) | | | (43,473) | |
| Balance at March 31, 2024 | | 701,974,596 | | | $ | 7,020 | | | $ | 1,076,370 | | | $ | (2,449) | | | $ | (1,036,731) | | | $ | 44,210 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, 2023 |
| | Common Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Loss | | Accumulated Deficit | | Total Stockholders' Equity |
| | Shares | | Amount | | | | |
| Balance at December 31, 2022 | | 211,091,425 | | | $ | 2,110 | | | $ | 504,767 | | | $ | (1,893) | | | $ | (378,330) | | | $ | 126,654 | |
| Rights offering of common stock, net of offering costs of $619 | | 476,190,471 | | | 4,762 | | | 494,619 | | | — | | | — | | | 499,381 | |
| Issuance of common stock under stock purchase plans and exercise of stock options | | 403,469 | | | 4 | | | 647 | | | — | | | — | | | 651 | |
| Issuance of common stock in lieu of cash for Akeso upfront payment | | 10,000,000 | | | 100 | | | 45,800 | | | — | | | — | | | 45,900 | |
| Stock-based compensation | | — | | | — | | | 2,775 | | | — | | | — | | | 2,775 | |
| Net other comprehensive income | | — | | | — | | | — | | | 497 | | | — | | | 497 | |
| Net loss | | — | | | — | | | — | | | — | | | (542,376) | | | (542,376) | |
| Balance at March 31, 2023 | | 697,685,365 | | | $ | 6,976 | | | $ | 1,048,608 | | | $ | (1,396) | | | $ | (920,706) | | | $ | 133,482 | |
| | | | | | | | | | | | |
| | |
| | | | | | | | | | |
| | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Summit Therapeutics Inc.
Condensed Consolidated Statements of Cash Flows
(in thousands)
(Unaudited)
| | | | | | | | | | | |
| Three Months Ended
March 31, |
| 2024 | | 2023 |
| Cash flows from operating activities: | | | |
| Net loss | $ | (43,473) | | | (542,376) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Non-cash interest expense | — | | | 6,063 | |
| Amortization of discount on short-term investments | (402) | | | — | |
| Unrealized foreign exchange loss (gain) | 9 | | | (470) | |
| Reclassification of currency translation gain | — | | | (419) | |
| Impairment of fixed assets | — | | | 474 | |
| | | |
| Depreciation | 27 | | | 90 | |
| | | |
| | | |
| Stock-based compensation | 9,507 | | | 2,775 | |
| In-process research and development expense | — | | | 520,915 | |
| Change in operating assets and liabilities: | | | |
| Accounts receivable | — | | | 350 | |
| Prepaid expenses | (2,792) | | | 331 | |
| Other current and long-term assets | 2,531 | | | 508 | |
| Research and development tax credit receivable | 632 | | | 850 | |
| | | |
| Accounts payable | 3,704 | | | 308 | |
| Accrued liabilities | (2,154) | | | 730 | |
| Other long- term liabilities | 1,763 | | | — | |
| Accrued compensation | 606 | | | (3,238) | |
| Operating lease right-of-use assets and lease liabilities, net | (92) | | | (22) | |
| Net cash used in operating activities | (30,134) | | | (13,131) | |
| | | |
| Cash flows from investing activities: | | | |
| Purchases of property and equipment | (4) | | | (53) | |
| | | |
| Purchase of short-term investments | (92,982) | | | (169,995) | |
| Maturities and sales of short-term investments | 112,857 | | | — | |
Payments to Akeso for upfront milestone payments and associated
direct transaction costs | — | | | (475,015) | |
| Net cash provided by (used in) investing activities | 19,871 | | | (645,063) | |
| | | |
| Cash flows from financing activities: | | | |
| Proceeds from the issuance of common stock for rights offering | — | | | 104,686 | |
| Transaction costs related to the issuance of common stock for rights offering | — | | | (539) | |
| Repayment of related party promissory notes | — | | | (24,686) | |
| | | |
| | | |
| | | |
| Proceeds received related to employee stock awards | 485 | | | 651 | |
| Net cash provided by financing activities | 485 | | | 80,112 | |
| Effect of exchange rate changes on cash | (36) | | | 444 | |
| Decrease in cash and cash equivalents | (9,814) | | | (577,638) | |
| Cash, cash equivalents and restricted cash at beginning of period | 71,425 | | | 648,607 | |
| Cash, cash equivalents and restricted cash at end of period | $ | 61,611 | | | $ | 70,969 | |
| | | |
| |
| | | |
| | | |
| Supplemental Disclosure of Cash Flow Information: | | | |
| Cash paid for interest on related party promissory notes | $ | 1,501 | | | $ | 1,306 | |
| | | |
| Supplemental Disclosure of Non-Cash Investing and Financing Activities: | | | |
| Consideration for the issuance of common stock for rights offering used to satisfy a portion of a related party promissory note (Note 14) | $ | — | | | $ | 395,314 | |
| Issuance of common stock pursuant to the Akeso License Agreement (Note 7) | $ | — | | | $ | 45,900 | |
| Transaction costs included in accrued expenses | $ | — | | | $ | 80 | |
| Lease assets obtained in exchange for operating lease liabilities | $ | 4,216 | | | $ | — | |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
1. Nature of Business and Operations
Nature of Business and Operations
Summit Therapeutics Inc. (“we”, “Summit” or the “Company”) is a biopharmaceutical company focused on the discovery, development, and commercialization of patient-, physician-, caregiver- and societal-friendly medicinal therapies intended to improve quality of life, increase potential duration of life, and resolve serious unmet medical needs.
The Company’s current lead development candidate is ivonescimab, a novel, potential first-in-class bispecific antibody intending to combine the effects of immunotherapy via a blockade of PD-1 with the anti-angiogenesis effects of an anti-VEGF compound into a single molecule. On December 5, 2022, the Company entered into a Collaboration and License Agreement (the “License Agreement”) with Akeso, Inc. and its affiliates (“Akeso”) pursuant to which the Company has in-licensed ivonescimab as further described in Note 7. Through the License Agreement, the Company obtained the rights to develop and commercialize ivonescimab in the United States, Canada, Europe, and Japan (the “Licensed Territory”). The License Agreement and transaction closed in January 2023 following customary waiting periods. The Company’s operations will be focused on the development of ivonescimab and other future activities, as the Company determines.
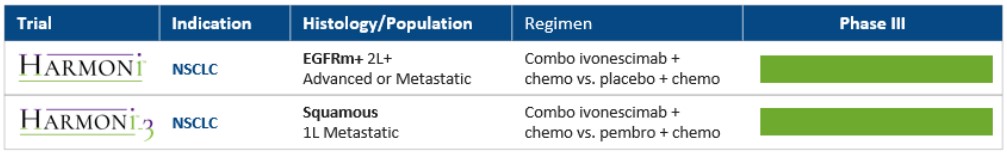
The Company has begun its development for ivonescimab in non-small cell lung cancer (“NSCLC”), specifically launching Phase III clinical trials in the following indications:
a) ivonescimab combined with chemotherapy in patients with epidermal growth factor receptor (“EGFR”)-mutated, locally advanced or metastatic non-squamous NSCLC who have progressed after treatment with a third-generation EGFR tyrosine kinase inhibitor (“TKI”) (“HARMONi”); and
b) ivonescimab combined with chemotherapy in first-line metastatic squamous NSCLC patients (“HARMONi-3”)
As of the date of these financial statements, both studies are enrolling patients.
The entry into the License Agreement with Akeso represents a significant change in the Company’s strategy and its future operations will be focused on the development of ivonescimab and other future activities as the Company determines. The Company’s portfolio also includes ridinilazole, a product candidate for treating patients suffering from Clostridioides difficile infection, also known as C. difficile infection, or CDI, and SMT-738, the first of a novel class of precision antibiotics for combating multidrug resistant infections, specifically carbapenem-resistant Enterobacteriaceae (“CRE”) infections. All prior development activities related to ridinilazole and SMT-738 have been terminated; the Company will continue to pursue partnerships for both assets.
2. Basis of Presentation and Use of Estimates
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP") and pursuant to the rules and regulations of the U.S. Securities and Exchange Commission. Accordingly, certain information and disclosures required by U.S. GAAP for complete consolidated financial statements are not included herein. All intercompany accounts and transactions have been eliminated in consolidation. The interim financial data as of March 31, 2024 and for the three months ended March 31, 2024 are unaudited; however, in the opinion of management, the interim data includes all adjustments, consisting of normal recurring adjustments, necessary for a fair statement of the results for the interim periods. The condensed consolidated balance sheet presented as of December 31, 2023 has been derived from the consolidated audited financial statement as of that date. The results of the period are not necessarily indicative of full year results or any other interim period. These unaudited interim condensed consolidated financial statements should be read in conjunction with the audited financial statements and notes thereto of the Company which are included in the Company's Annual Report on Form 10-K for the year ended December 31, 2023 filed with the Securities and Exchange Commission on February 20, 2024. The financial results of the Company's activities are reported in United States Dollars
Our accounting policies are consistent with the Notes to the Consolidated Financial Statement in the Company's Annual Report on Form 10-K filed with the Securities and Exchange Commission, except as updated below:
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
Cash Equivalents
Cash equivalents consist of non-interest-bearing deposits denominated in the U.S. dollar and British pound, and the Company invested cash in money market funds and U.S. treasury securities. All highly liquid investments with a maturity date of 90 days or less at the date of purchase are considered to be cash equivalents. The appropriate classification of investments in securities as to whether it is classified as a cash equivalent is determined by the Company at the time of purchase.
Marketable Securities
Marketable securities consist of investments with original maturities greater than ninety days from the date of acquisition. The Company classifies investments with maturities of greater than 90 days as short-term, based on the liquid nature of the securities and because such marketable securities represent the investment of cash that is available for current operations. The Company considers its investment portfolio of investments as available-for-sale. Accordingly, these investments are recorded at fair value, which is based on quoted market prices or other observable inputs. Unrealized gains and losses are recorded as a component of other comprehensive income (loss). Realized gains and losses are determined on a specific identification basis and are included in other (expense) income. Amortization and accretion of discounts and premiums is also recorded in other (expense) income.
When the fair value is below the amortized cost of the asset, an estimate of expected credit losses is made, this estimate is limited to the amount by which fair value is less than amortized cost. The credit-related impairment amount is recognized in the condensed consolidated statements of operations and comprehensive loss and the remaining impairment amount and unrealized gains are reported as a component of accumulated other comprehensive income (loss) in shareholders’ equity. Credit losses are recognized through the use of an allowance for credit losses account and subsequent improvements in expected credit losses are recognized as a reversal of the allowance account. If the Company has the intent to sell the security or it is more likely than not that the Company will be required to sell the security prior to recovery of its amortized cost basis the allowance for credit loss is written off and the excess of the amortized cost basis of the asset over its fair value is recorded in the condensed consolidated statements of operations and comprehensive loss..
Use of Estimates
The preparation of these unaudited condensed consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent liabilities at the date of the unaudited condensed consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an on-going basis, management evaluates its estimates and judgments, including those related to accrued research and development expenses, stock-based compensation, goodwill, other long-lived assets and income taxes. Management bases its estimates and judgments on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
3. Summary of Significant Accounting Policies and Recently Issued or Adopted Accounting Pronouncements
Summary of Accounting Policies
The significant accounting policies used in the preparation of these condensed consolidated financial statements for the three months ended March 31, 2024 are consistent with those discussed in Note 4 to the consolidated financial statements in the Company's Annual Report on Form 10-K for the year ended December 31, 2023.
Recently Issued or Adopted Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standard setting bodies that the Company adopts as of the specified effective date. The Company does not believe that the adoption of recently issued standards have or may have a material impact on the accompanying unaudited condensed consolidated financial statements.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
4. Liquidity and Capital Resources
During the three months ended March 31, 2024, the Company incurred a net loss of $43,473 and cash flows used in operating activities for the three months ended March 31, 2024 was $30,134. As of March 31, 2024, the Company had an accumulated deficit of $1,036,731, cash and cash equivalents of $61,294, short-term investments in U.S. treasury securities of $95,386 and current and long-term U.K. research and development tax credits receivable of $1,157. The Company expects to continue to generate operating losses for the foreseeable future.
The Company has evaluated whether its cash, cash equivalents and U.K. research and development tax credits provide sufficient cash to fund its operating cash needs for the next twelve months from the date of issuance of these quarterly financials. The Company is investing in the clinical development of ivonescimab, including its ongoing clinical trials. In addition, the Company has a $100,000 promissory note and interest payable to a related party (refer to Note 14 for further details) that matures on April 1, 2025. In order to repay this promissory note, the Company intends to raise additional capital. As of the date of issuance of these condensed consolidated financial statements, additional capital has not yet been secured. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for at least one year from the date these condensed consolidated financial statements are issued.
Until the Company can generate substantial revenue and achieve profitability, the Company will need to raise additional capital to fund its ongoing operations and capital needs. The Company continues to evaluate options to further finance its operating cash needs for its product candidates through a combination of some, or all, of the following: equity and debt offerings, collaborations, strategic alliances, grants and clinical trial support from government entities, philanthropic, non-government and not-for-profit organizations, and marketing, distribution or licensing arrangements. There is no assurance, however, that additional financing will be available when needed or that management of the Company will be able to obtain financing on terms acceptable to the Company. If the Company is unable to obtain funding when required in the future, the Company could be required to delay, reduce, or eliminate research and development programs, product portfolio expansion, or future commercialization efforts, which could adversely affect its business prospects.
The accompanying condensed consolidated financial statements are prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of the business. The condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classifications of liabilities that might result from the outcome of this uncertainty.
5. Segment Reporting
The Company's chief operating decision makers (the "CODM function"), which are the Company's CEOs, Mr. Duggan and Dr. Zanganeh, utilize consolidated financial information to make decisions about allocating resources and assessing performance for the entire Company. The CODM function approves of key operating and strategic decisions, including key decisions in clinical development and clinical operating activities, entering into significant contracts, such as revenue contracts and collaboration agreements and approves the Company's consolidated operating budget. The CODM function views the Company's operations and manages its business as a single reportable operating segment. The Company's single operating segment covers the Company’s research and development activities, primarily comprising of oncology product research activities (including ivonescimab). As the Company operates as one operating segment, all required financial segment information can be found in these condensed consolidated financial statements.
The Company operates in two geographic regions: the U.K. and the U.S. The following table summarizes the Company's long-lived assets, which include the Company's property and equipment, net and right-of-use assets by geography:
| | | | | | | | | | | | | | |
| | March 31, 2024 | | December 31, 2023 |
| United Kingdom | | $ | 742 | | | $ | 808 | |
United States(1) | | 8,812 | | | 5,254 | |
| | $ | 9,554 | | | $ | 6,062 | |
(1) The increase in long-lived assets as of March 31, 2024 as compared to December 31, 2023, is primarily due to $4,106 of net right-of use assets recorded as a result of the Company entering into a new lease agreement for their Miami, FL headquarters, partially offset by $534 of amortization expense for right-of-use assets relating to lease agreements for its office space in Menlo Park, CA.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
6. Other Operating Income, net
The following table sets forth the components of other operating income, net by category:
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| Other operating income, net by category: | | 2024 | | 2023 | | | | |
| | | | | | | | |
| Research and development tax credits | | 213 | | | 542 | | | | | |
| Grant income from CARB-X (as defined below) | | — | | | 34 | | | | | |
| Other income | | — | | | 8 | | | | | |
| | $ | 213 | | | $ | 584 | | | | | |
Research and development tax credits
Income from tax credits, consist of R&D tax credits received in the U.K. The Company benefits from the Small and Medium Enterprise Program ("SME Program") U.K. research and development tax credit cash rebate regime, and The Research and Development Expenditure Credit ("The RDEC scheme"), a UK government tax incentive that promotes innovation amongst UK's larger businesses. Qualifying expenditures largely comprise of employment costs for research staff, consumables, a proportion of relevant, permitted sub-contract costs and certain internal overhead costs incurred as part of research projects for which the Company does not receive income. Tax credits related to the SME Program and The RDEC scheme are recorded as other operating income in the consolidated statements of operations and other comprehensive loss. Under these scheme, the Company receives cash payments that are not dependent on the Company’s pre-tax net income levels.
Based on criteria established by His Majesty’s Revenue and Customs ("HMRC"), a portion of expenditures being carried out in relation to the Company's pipeline research and development, clinical trials management and third-party manufacturing development activities are eligible for the SME regime and the Company expects such elements of research and development expenditure incurred in its UK entities will also continue to be eligible for the SME regime for future periods.
As of March 31, 2024, the current and non-current research and development tax credit receivable was $945 and $212, respectively. As of December 31, 2023, the current and non-current research and development tax credit receivable was $848 and $959, respectively.
CARB-X (as defined below)
In May 2021, the Company announced the selection of a new preclinical candidate, SMT-738, from the DDS-04 series for development in the fight against multi-drug resistant infections, specifically Carbapenem-resistant Enterobacteriaceae ("CRE") infections. Simultaneously, the Company announced it had received an award from the Trustees of Boston University under the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator program ("CARB-X") to progress this candidate through preclinical development and Phase Ia clinical trials. The award commits initial funding of up to $4,100, with the possibility of up to another $3,700 based on the achievement of future milestones. As of March 31, 2024, based on translation of historical foreign currency amounts in the period, the Company has recognized $2,920 of cumulative income since contract inception.
7. Akeso Collaboration and License Agreement
On December 5, 2022, the Company entered into a Collaboration and License Agreement (the “License Agreement”) with Akeso, Inc. and its affiliates (“Akeso”) pursuant to which the Company is in-licensing its breakthrough bispecific antibody, ivonescimab. The License Agreement and transaction closed in January 2023 following customary waiting periods.
Ivonescimab, known as AK112 in China and Australia, and also as SMT112 in the United States, Canada, Europe, and Japan, is a novel, potential first-in-class bispecific antibody intending to combine the effects of immunotherapy via a blockade of PD-1 with the anti-angiogenesis effects of an anti-VEGF into a single molecule. Ivonescimab was engineered to bring two well established oncology targeted mechanisms together. Ivonescimab is currently in clinical development and, pursuant to the terms of the License Agreement, Summit will design and conduct the clinical trial activities to support regulatory filings in the Licensed Territory that Summit will submit.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
Pursuant to the terms of the License Agreement, Summit will have final decision-making authority with respect to clinical development strategy and execution in the Licensed Territory. For co-joined studies in which both Summit and Akeso participate, mutual agreement is required for material decisions; Summit retains the exclusive decision making with respect to participating in, and continuing its participation in, co-joined studies. Pursuant to the terms of the License Agreement, Summit will have final decision making authority with respect to commercial strategy, pricing and reimbursement and other commercialization matters in the Licensed Territory. In connection with the License Agreement, the Company has also entered into a Supply Agreement with Akeso, pursuant to which Summit agrees to purchase a certain portion of drug substance for clinical and commercial supply. Summit is not assuming any liabilities (including contingent liabilities), acquiring any physical assets or trade names, or hiring or acquiring any employees from Akeso in connection with the License Agreement. Through the License Agreement, the Company obtained the rights to develop and commercialize ivonescimab in the United States, Canada, Europe, and Japan (the “Licensee Territory”).
In exchange for the rights obtained, an upfront payment of $500,000 was made to Akeso, of which $274,900 was paid in cash and, pursuant to the License Agreement and Issuance Agreement, Akeso elected to receive 10,000,000 shares of the Company's common stock in lieu of $25,100 cash. The remaining $200,000 amount of the upfront payment was paid on March 6, 2023.
The Company has accounted for the License Agreement to acquire the rights to develop and commercialize ivonescimab as the acquisition of an asset. All of the consideration relates to ivonescimab and technological feasibility of the asset has not yet been established since ivonescimab is in clinical development. As such, the Company has expensed the consideration as in-process research and development upon closing of the transaction in the condensed consolidated statement of comprehensive loss. In-process research and development expense for the three months ended March 31, 2023 was $520,915, which is comprised of the $474,900 paid in cash, the fair value of the 10,000,000 shares of common stock on the date of closing the transaction of $45,900, and $115 of direct transactions costs incurred.
In addition to the payments already made to Akeso, under the License Agreement, there are additional potential milestone payments of up to $4,500,000, as Akeso will be eligible to receive regulatory milestones of up to $1,050,000 and commercial milestones of up to $3,450,000. In addition, Akeso will be eligible to receive low double-digit royalties on net sales.
8. Other Expense, net
The following table sets forth the components of other (expense) income:
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, | | |
| | 2024 | | 2023 | | | | |
| Foreign currency gains | | $ | 208 | | | $ | 508 | | | | | |
| Interest expense on promissory notes payable to related parties | | (3,121) | | | (8,327) | | | | | |
| Investment income | | 1,829 | | | 1,761 | | | | | |
Reclassification of cumulative currency translation gain(1) | | — | | | 419 | | | | | |
| Other expense, net | | — | | | 417 | | | | | |
| | $ | (1,084) | | | $ | (5,222) | | | | | |
(1) During the three months ended March 31, 2023, the Company dissolved certain dormant entities and as a result, $419 of cumulative foreign currency translation adjustments were re-classified from accumulated other comprehensive loss relating to these entities.
For the three months ended March 31, 2024, other expense, net primarily consisted of loan interest expense incurred related to the $100,000 promissory note and for the three months ended March 31, 2023, other expense, net primarily consisted of loan interest expense incurred related to the $520,000 promissory notes, as described in Note 14. These amounts for both periods are partially offset by investment income related to the Company's money market funds and short-term investments in U.S. treasury securities.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
9. Net Loss per Share
The following table sets forth the computation of basic and diluted net loss per share:
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| | 2024 | | 2023 | | | | |
| Net loss | | $ | (43,473) | | | $ | (542,376) | | | | | |
| | | | | | | | |
| Basic weighted average number of shares of common stock outstanding | | 701,785,250 | | | 378,163,980 | | | | | |
| Diluted weighted average number of shares of common stock outstanding | | 701,785,250 | | | 378,163,980 | | | | | |
| | | | | | | | |
| Basic net loss per share | | $ | (0.06) | | | $ | (1.43) | | | | | |
| Diluted net loss per share | | $ | (0.06) | | | $ | (1.43) | | | | | |
Basic net loss per share is computed by dividing the net loss by the weighted-average number of common shares outstanding for the period. Diluted net loss per share is computed by dividing the diluted net loss by the weighted-average number of common shares outstanding for the period, including potentially dilutive common shares. Since the Company was in a loss position for all periods presented, basic net loss per share is the same as diluted net loss per share for all periods, as the inclusion of all potential common share equivalents outstanding would have been anti-dilutive.
The following potentially dilutive securities were excluded from the computation of the diluted net loss per share of common stock for the periods presented because their effect would have been anti-dilutive:
| | | | | | | | | | | | | | |
| | March 31, |
| | 2024 | | 2023 |
| Options to purchase common stock | | 54,851,855 | | 20,179,822 |
| Warrants | | 5,015,642 | | 5,821,137 |
| Shares expected to be purchased under employee stock purchase plan | | 127,978 | | | 206,772 | |
| | 59,995,475 | | 26,207,731 |
Stock options that are outstanding and contain performance-based or market-based vesting criteria for which the performance or market conditions have not been met are excluded from the presentation of common stock equivalents outstanding in the chart above
10. Fair Value Measurements and Short-Term Investments
In accordance with the provisions of fair value accounting, a fair value measurement assumes that the transaction to sell an asset or transfer a liability occurs in the principal market for the asset or liability or, in the absence of a principal market, the most advantageous market for the asset or liability and defines fair value based on the exit price model.
The fair value measurement guidance establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The guidance describes three levels of inputs that may be used to measure fair value:
Level 1
Quoted prices in active markets for identical assets or liabilities as of the reporting date. Active markets are those in which transactions for the asset or liability occur in sufficient frequency and volume to provide pricing information on an ongoing basis.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
Level 2
Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Level 2 assets and liabilities include debt securities with quoted prices that are traded less frequently than exchange-traded instruments or securities or derivative contracts that are valued using a pricing model with inputs that are observable in the market or can be derived principally from or corroborated by observable market data.
Level 3
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Level 3 assets and liabilities include financial instruments whose value is determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant management judgment or estimation.
In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the Company categorizes such assets and liabilities based on the lowest level input that is significant to the fair value measurement in its entirety. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset.
The following tables sets forth the Company’s fair value hierarchy for its assets and liabilities that are measured at fair value on a recurring basis as of March 31, 2024 and December 31, 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements as of March 31, 2024 using: |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Cash equivalents: | | | | | | | |
| Money market funds | $ | 16,946 | | | $ | — | | | $ | — | | | $ | 16,946 | |
| U.S. Government treasury bills | — | | | 40,157 | | | — | | | 40,157 | |
| Short-term investments: | | | . | | | | |
| U.S. Government treasury bills | — | | | 95,386 | | | — | | | 95,386 | |
| Total financial assets | $ | 16,946 | | | $ | 135,543 | | | $ | — | | | $ | 152,489 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements as of December 31, 2023 using: |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Cash equivalents: | | | | | | | |
| Money market funds | $ | 21,016 | | | $ | — | | | $ | — | | | $ | 21,016 | |
| U.S. Government treasury bills | — | | | 39,341 | | | — | | | 39,341 | |
| Short-term investments | | | | | | | |
| U.S. Government treasury bills | — | | | 114,817 | | | — | | | 114,817 | |
| Total financial assets | $ | 21,016 | | | $ | 154,158 | | | $ | — | | | $ | 175,174 | |
The tables above do not include cash at March 31, 2024 and December 31, 2023 of $4,191 and $11,068, respectively.
The Company believes that the carrying amounts of prepaid expenses, other current assets, accounts payable, and accrued expenses approximates their fair values due to the short-term nature of those instruments. The carrying value of the Company’s promissory note approximates its fair value and the current interest rate of the note outstanding when compared to market interest rates (which represents a Level 2 measurement). Refer to Note 14 for further details.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
The following table sets forth the Company’s short-term investments as of March 31, 2024 and December 31, 2023, which have a contractual maturity of less than one year:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | March 31, 2024 |
| | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized (Losses) | | Credit (Loss) | | Fair Value |
| Assets | | | | | | | | | | |
| U.S. Government treasury bills | | $ | 95,375 | | | $ | 11 | | | | | $ | — | | | $ | 95,386 | |
| Total | | $ | 95,375 | | | $ | 11 | | | $ | — | | | $ | — | | | $ | 95,386 | |
| | | | | | | | | | |
| | December 31, 2023 |
| | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized (Losses) | | Credit (Loss) | | Fair Value |
| Assets | | | | | | | | | | |
| U.S. Government treasury bills | | $ | 114,781 | | | $ | 36 | | | $ | — | | | $ | — | | | $ | 114,817 | |
| Total | | $ | 114,781 | | | $ | 36 | | | $ | — | | | $ | — | | | $ | 114,817 | |
11. Goodwill
Goodwill
As of March 31, 2024 and December 31, 2023, goodwill was $1,875 and $1,893, respectively. Changes in the gross carrying amount of goodwill during the three months ended March 31, 2024 as compared to December 31, 2023, are the result of changes in foreign currency. As of December 31, 2023, the Company performed its annual impairment assessment of goodwill and determined that it is more likely than not that the fair value of the reporting unit exceeds its carrying amount. There have been no cumulative goodwill impairments recognized during the three months ended March 31, 2024.
12. Leases
The Company has operating leases for real estate. The Company does not have any finance leases.
During the three months ended March 31, 2024, the Company recorded $4,216 of additional right-of-use assets related to a new lease for office space that commenced during the period for its Miami, Florida headquarter location. Total future lease payments as of March 31, 2024, which include base rent and sales tax are approximately $4,814 on an undiscounted basis. This lease commenced on February 1, 2024 and has a term of 64 months. As of March 31, 2024 the Company has $317 of restricted cash associated with an irrevocable letter of credit required by the landlord to enter into this lease. There were no new right-of-use assets recorded during the three month period ended March 31, 2023. The carrying value of the right-of-use assets as of March 31, 2024 and December 31, 2023 was $9,373 and $5,859, respectively.
13. Research and Development Prepaid Expenses and Accrued Liabilities
Included within prepaid expenses and other current assets at March 31, 2024 and December 31, 2023 is $4,351 and $1,466, respectively, of prepayments relating to research and development expenditures. Included within accrued liabilities at March 31, 2024 and December 31, 2023 is $6,074 and $7,289, respectively, relating to research and development expenditures.
These amounts are determined based on the estimated costs to complete each study or activity, the estimation of the current stage of completion and the invoices received, as well as predetermined milestones which are not reflective of the current stage of development for prepaid expenses. However, prepaid expenses decrease and accrued liabilities increase as the activities progress, and if actual costs incurred exceed the prepaid expense, an accrual will be recorded for the liability. The
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
key sensitivity is the estimated current stage of completion of each study or activity, which is based on information received from the supplier and the Company’s operational knowledge of the work completed under those contracts.
14. Promissory Note Payable to Related Parties
Non-current promissory note payable to related parties was $100,000 as of March 31, 2024 and December 31, 2023.
December 2022 Promissory Note
On December 6, 2022, the Company entered into a Note Purchase Agreement (the "Note Purchase Agreement"), with Mr. Duggan and Dr. Zanganeh, pursuant to which the Company agreed to sell to each of Mr. Duggan and Dr. Zanganeh unsecured promissory notes in the aggregate amount of $520,000. Pursuant to the Note Purchase Agreement, the Company issued to Mr. Duggan and Dr. Zanganeh unsecured promissory notes in the amount of $400,000 (the "Duggan February Note") and $20,000 (the "Zanganeh Note"), respectively, which would mature and become due on February 15, 2023 and an unsecured promissory note to Mr. Duggan in the amount of $100,000 (the “Duggan September Note” and together with the Duggan February Note and the Zanganeh Note, the “December 2022 Notes”), which was originally due on September 15, 2023. The maturity dates of the December 2022 Notes could be extended one or more times at the Company’s election, but in no event to a date later than September 6, 2024. In addition, if the Company consummates a public offering, then upon the later to occur of (i) five business days after the Company receives the net cash proceeds therefrom or (ii) May 15, 2023, the Duggan February Note and the Zanganeh Note shall be prepaid by an amount equal to the lesser of (a) 100% of the amount of the net proceeds of such offering and (b) the outstanding principal amount on such Notes.
On January 19, 2023, the Company provided notice to extend the term of the Duggan February Note and Duggan September Note to a maturity date of September 6, 2024. Furthermore, on January 19, 2023, the Company and Mr. Duggan rectified the Duggan February Note and Duggan September Note in order to correctly reflect the parties’ intent that the Company may only prepay (i) the Duggan February Note following the completion of a public rights offering to be conducted by Summit in the approximate amount of $500,000, or a similar capital raise, in an amount equal to the lesser of (x) the net proceeds of the Rights Offering or such capital raise or (y) the full amount outstanding of the Duggan February Note, and (ii) Duggan September Note following the completion of a capital raising transaction subsequent to the 2023 Rights Offering in an amount equal to the lesser of (A) the net proceeds of such capital raise or (B) the full amount outstanding of the Duggan September Note. Following the issuance of the two new Promissory Notes (the “Duggan Promissory Notes”), the Duggan February Note and Duggan September Note were marked as “cancelled” on their face and replaced in their entirety by the Duggan Promissory Notes (together with the Zanganeh Note, the "Notes").
On February 15, 2023, the $20,000 Zanganeh Note matured and the Company repaid the outstanding principal balance. In connection with the closing of the 2023 Rights Offering, the $400,000 Duggan Promissory Note matured and became due, and the Company satisfied all principal and accrued interest thereunder using a combination of a portion of the cash proceeds from the 2023 Rights Offering and the extinguishment of a portion of the amount due equal to the subscription price for shares subscribed by Mr. Duggan in the 2023 Rights Offering.
The Notes accrued interest at an initial rate of 7.5%. All interest on the Notes was paid on the date of signing for the period through February 15, 2023. Such prepaid interest was paid in a number of shares of the Company’s common stock, par value $0.01 (“Common Stock”) equal to the dollar amount of such prepaid interest, divided by $0.7913 (the consolidated closing bid price immediately preceding the time the Company entered into the Note Purchase Agreement, plus $0.01), which was 9,720,291 shares. For all applicable periods following February 15, 2023, interest shall accrue on the outstanding principal balance of the Notes at the US prime interest rate, as reported in the Wall Street Journal, plus 50 basis points, as adjusted monthly, for three months immediately following February 15, 2023, and thereafter at the US prime rate plus 300 basis points, as adjusted monthly. Such accrued interest shall be paid in cash, quarterly in arrears, on each of March 31, June 30, September 30 and December 31.
Debt issuance costs associated with the Notes were $44 and were capitalized as part of the carrying value of the promissory notes payable to related parties.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
On February 17, 2024, the Duggan February Note was amended and restated to extend the maturity date from September 6, 2024 to April 1, 2025. For all applicable periods commencing February 17, 2024, interest shall accrue on the outstanding principal balance at the greater of 12% or the US prime interest rate, as reported in the Wall Street Journal plus 350 basis points, as adjusted monthly, compounded quarterly. Interest shall be paid upon maturity of the loan.
The debt discount is amortized to interest expense using an effective interest rate method. The effective interest rate of the Duggan February Note and Zanganeh Note was 8.9% and the effective interest rate of the Duggan September Note is 12.4%.
During the three months ended March 31, 2024 and 2023, the Company incurred interest expense of $3,121 and $8,327, respectively. Interest expense incurred during the three months ended March 31, 2023 included amortized imputed interest of $761. As of March 31, 2024, accrued interest was $1,740 and was recorded in other long-term liabilities. As of December 31, 2023, accrued interest was $120 and was recorded in accrued liabilities.
The estimated future principal payments are $0 and $100,000 for 2024 and 2025, respectively, as the note matures on April 1, 2025.
15. Stock-Based Compensation and Warrants
The Company currently grants stock options to employees and directors under the 2020 Stock Incentive Plan (the "2020 Plan") and formerly, the Company granted stock options under the 2016 Long Term Incentive Plan (the "2016 Plan"). The 2020 Plan is administered by the Compensation Committee of the Company's Board of Directors. The 2020 Plan is intended to attract and retain employees and directors and provide an incentive for these individuals to assist the Company to achieve long-range performance goals and to enable these individuals to participate in the long-term growth of the Company.
On October 12, 2023, the Company's stockholders approved an increase in the number of shares of the Company's common stock issuable under the Plan by 70,000,000 shares.
The following table summarizes the Company's time-based stock option activity for the three months ended March 31, 2024:
| | | | | | | | | | | | | | |
| | Number of Options | | Weighted average exercise price |
| Outstanding at December 31, 2023 | | 54,209,289 | | | $ | 2.28 | |
| Granted | | 1,503,876 | | 3.49 | |
| | | | |
| Forfeited | | (780,500) | | | 2.51 | |
| Exercised | | (80,810) | | | 1.99 | |
| Outstanding at March 31, 2024 | | 54,851,855 | | | $ | 2.31 | |
| Exercisable at March 31, 2024 | | 6,002,166 | | | $ | 4.79 | |
The total intrinsic value of all outstanding time-based stock options and exercisable stock options at March 31, 2024 was $113,727 and $3,146, respectively The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s common stock for those stock options that had exercise prices lower than the fair value of the Company’s common stock.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
The following table summarizes the Company's performance-based stock option activity for the three months ended March 31, 2024:
| | | | | | | | | | | | | | |
| | Number of Options | | Weighted average exercise price |
| Outstanding at December 31, 2023 | | 46,654,220 | | $ | 1.62 |
| Granted | | 150,000 | | | $ | 3.77 | |
| | | | |
| Forfeited | | (275,000) | | | $ | 1.76 | |
| Exercised | | — | | | $ | — | |
| Outstanding at March 31, 2024 | | 46,529,220 | | | $ | 1.63 | |
| Exercisable at March 31, 2024 | | — | | | $ | — | |
The total intrinsic value of all performance-based stock options at March 31, 2024 was $117,019.
As of March 31, 2024, total unrecognized compensation expense related to performance-based stock options that were deemed probable of vesting was approximately $11,313 which excludes 37,223,376 of unvested performance-based stock options that were deemed not-probable of vesting totaling unrecognized stock-based compensation expense of $44,816.
The total stock-based compensation expense included in the Company's condensed consolidated statements of operations and comprehensive loss was as follows:
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, | | |
| | 2024 | | 2023 | | | | |
| Research and development | | $ | 2,414 | | | $ | 1,086 | | | | | |
| General and administrative | | 7,093 | | | $ | 1,689 | | | | | |
| Total stock-based compensation expense | | $ | 9,507 | | $ | 2,775 | | | | |
The following summarizes share-based compensation expense associated with each of the Company's stock-based compensation arrangements:
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, | | |
| | 2024 | | 2023 | | | | |
| Time-based stock options | | $ | 8,115 | | | $ | 2,671 | | | | | |
| Performance-based stock options | | 1,321 | | | 48 | | | | | |
| Employee stock purchase plan | | 71 | | | 56 | | | | | |
| Total stock-based compensation expense | | $ | 9,507 | | $ | 2,775 | | | | |
Warrants
The Company had outstanding and exercisable 5,015,642 warrants with a weighted average exercise price of $1.57 as of March 31, 2024 and December 31, 2023.
16. Related Party Transactions
July 25, 2022 First Amendment to Sublease Agreement with Maky Zanganeh and Associates, Inc.
Summit Therapeutics Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
(in thousands, except share and per share data)
On July 25, 2022 the Company entered into a first amendment, dated July 19, 2022, to its existing sublease agreement with Maky Zanganeh and Associates, Inc. ("MZA"), consisting of 4,500 square feet of office space at 2882 Sand Hill Road, Menlo Park, California. The existing sublease term, which was set to expire on September 30, 2022, was extended for a period of thirty-nine months from October 1, 2022 through December 31, 2025. The rent payable under the terms of the sublease is equivalent to the proportionate share of the net payable by MZA to the third-party landlord, based on the square footage of office space sublet by the Company, and no mark-up has been applied. During the three months ended March 31, 2024 and 2023, payments of $195 and $189, respectively, were made pursuant to the first amendment to the Sublease Agreement.
July 29, 2022 Second Amendment to Sublease Agreement with Maky Zanganeh and Associates, Inc.
On July 29, 2022, the Company entered into a second amendment, dated August 1, 2022, to its existing sublease agreement with MZA, described above. The second amendment was effective as of August 1, 2022 and expires on December 31, 2025. The second amendment includes an additional 1,277 square feet (the "Expansion Premises") of office space at 2882 Sand Hill Road, Menlo Park, California. The rent payable under the terms of the sublease is equivalent to the proportionate share of the net payable by MZA to the third-party landlord, based on the square footage of office space sublet by the Company, and no mark-up has been applied. During the three months ended March 31, 2024 and 2023, payments of $55 and $54 respectively, were made pursuant to the second amendment to the Sublease Agreement.
December 6, 2022 Note Purchase Agreement
Refer to Note 14 for a discussion of the promissory note payable to a related party.
Akeso License Agreement
Upon the closing of the License Agreement, the Board of Directors (the “Board”) of the Company appointed Dr. Yu (Michelle) Xia to serve as a member of the Board pursuant to the terms of the License Agreement. Dr. Xia is the founder of Akeso, Inc. ("Akeso"), and has been the chairwoman, president and CEO of Akeso since its inception in 2012. For details on the License Agreement, see Note 7. Furthermore, in connection with the License Agreement, the Company also entered into a Supply Agreement with Akeso, pursuant to which Summit agreed to purchase a certain portion of drug substance for clinical and commercial supply (the “Supply Agreement”).
2023 Rights Offering
On December 6, 2022, the Company announced a rights offering for its existing shareholders to participate in the purchase of additional shares of its Common Stock for $1.05 per share. The 2023 Rights Offering commenced on February 7, 2023 and the associated subscription rights expired on March 1, 2023. Aggregate gross proceeds from the 2023 Rights Offering were $500,000 from the sale of 476,190,471 shares of the Company's common stock and issuance costs were $619. Mr. Duggan and Dr. Zanganeh fully subscribed to their respective basic subscription rights at a price of $1.05 per share. To satisfy the $395,314 subscription price for the shares subscribed by Mr. Duggan in the 2023 Rights Offering, Mr. Duggan agreed with the Company to extinguish a portion of the amount due and payable to him by the Company at the closing of the 2023 Rights Offering pursuant to the $400,000 Duggan Promissory Note in an amount equal to the subscription price.
Private Placement
On October 16, 2023, the Company announced the appointment of Mr. Manmeet Soni as its Chief Operating Officer, effective immediately. Mr. Soni has been a part of the Company's Board of Directors since 2019. He will remain a member of the Board of Directors. In conjunction with his appointment, Mr. Soni entered into a share purchase agreement with the Company to invest $5,000 in shares of common stock via a private placement. The transaction was effective October 13, 2023 with a closing price of $1.68, resulting in the purchase of 2,976,190 shares of the Company's common stock.
17. Commitments and Contingencies
Lease Commitments
There were no material changes to the Company's lease commitments that were disclosed in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the Securities and Exchange Commission on February 20, 2024, other than the new lease for the Miami, FL headquarter location as described in Note 12.
Debt Commitments
Refer to Note 14 for a discussion of the promissory note payable to a related party.
Other Commitments
The Company enters into contracts in the normal course of business with various third parties for clinical trials, preclinical research studies and testing, manufacturing and other services and products for operating purposes. Most contracts provide for termination upon notice, and therefore are cancellable contracts. The majority of these commitments are due within one year. There have been no material changes to the Company's other contractual commitments that were disclosed in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
Indemnifications
The Company's certificate of incorporation provides that it will indemnify the directors and officers to the fullest extent permitted by Delaware law. In addition, the Company has entered into indemnification agreements with all of the directors and executive officers. These indemnification agreements may require the Company, among other things, to indemnify each such director or executive officer for some expenses, including attorneys’ fees, judgments, fines, and settlement amounts incurred by him or her in any action or proceeding arising out of his or her service as one of the Company's directors or executive officers. The Company believes the fair value for these indemnification obligations is minimal. Accordingly, the Company has not recognized any liabilities relating to these obligations as of March 31, 2024 and December 31, 2023.
Legal Proceedings
The Company is not currently subject to any material legal proceedings.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited condensed consolidated financial statements and related notes included herein and our audited consolidated financial statements and related notes for the year ended December 31, 2023 included in our Form 10-K, filed on February 20, 2024. Some of the information contained in this discussion and analysis or set forth elsewhere in this filing, including information with respect to our plans and strategy for our business, includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks and uncertainties. All statements other than statements relating to historical matters including statements to the effect that we “believe,” “expect,” “anticipate,” “plan,” “target,” “intend” and similar expressions should be considered forward-looking statements. As a result of many factors, including those factors set forth in the risks identified the “Risk Factors’’ section of our other filings with the Securities and Exchange Commission, or the SEC, our actual results could differ materially from the results, performance or achievements expressed in or implied by these forward-looking statements.
Company Overview
Summit Therapeutics Inc. (“we”, “Summit” or the “Company”) is a biopharmaceutical company focused on the discovery, development, and commercialization of patient-, physician-, caregiver- and societal-friendly medicinal therapies intended to improve quality of life, increase potential duration of life, and resolve serious unmet medical needs. The Company’s pipeline of product candidates is designed with the goal to become the patient-friendly, new-era standard-of-care medicines, in the therapeutic area of oncology.
The Company’s current lead development candidate is ivonescimab, a novel, potential first-in-class bispecific antibody intending to combine the effects of immunotherapy via a blockade of PD-1 with the anti-angiogenesis effects of an anti-VEGF compound into a single molecule. On December 5, 2022, the Company entered into a Collaboration and License Agreement (the “License Agreement”) with Akeso, Inc. and its affiliates (“Akeso”) pursuant to which the Company has in-licensed ivonescimab. Through the License Agreement, the Company obtained the rights to develop and commercialize ivonescimab in the United States, Canada, Europe, and Japan (the “Licensed Territory”). The License Agreement and transaction closed in January 2023 following customary waiting periods. The Company’s operations will be focused on the development of ivonescimab and other future activities, as the Company determines.
The Company has begun its development for ivonescimab in non-small cell lung cancer (“NSCLC”), specifically launching Phase III clinical trials in the following indications:
a) ivonescimab combined with chemotherapy in patients with epidermal growth factor receptor (“EGFR”)-mutated, locally advanced or metastatic non-squamous NSCLC who have progressed after treatment with a third-generation EGFR tyrosine kinase inhibitor (“TKI”) (“HARMONi”); and
b) ivonescimab combined with chemotherapy in first-line metastatic squamous NSCLC patients (“HARMONi-3”)
As of the date of this filing, both studies are enrolling patients.
The entry into the License Agreement with Akeso represents a significant change in the Company’s strategy and its future operations will be focused on the development of ivonescimab and other future activities as the Company determines. The Company’s portfolio also includes ridinilazole, a product candidate for treating patients suffering from Clostridioides difficile infection, also known as C. difficile infection, or CDI, and SMT-738, the first of a novel class of precision antibiotics for combating multidrug resistant infections, specifically carbapenem-resistant Enterobacteriaceae (“CRE”) infections. All prior development activities related to ridinilazole and SMT-738 have been terminated; the Company will continue to pursue partnerships for both assets.
Akeso Collaboration and License Agreement
Pursuant to the License Agreement with Akeso, the Company received the rights to develop and commercialize ivonescimab in the Licensed Territory. Akeso will retain development and commercialization rights for the rest of the world excluding the
Licensed Territory. In exchange for these rights, Summit made an upfront payment during the first quarter of 2023 comprised of $474.9 million cash and the issuance of 10 million shares of Company common stock in lieu of $25.1 million cash pursuant to a share transfer agreement. In addition, the Company will potentially owe Akeso (a) milestone payments tied to achievement of regulatory approval of ivonescimab with various regulatory authorities in the Licensed Territory (b) milestone payments tied to achievement of annual revenue from ivonescimab in the Licensed Territory and (c) Royalty payments equal to low-double-digit percentage of annual revenues from ivonescimab in the Licensed Territory.
Pursuant to the terms of the License Agreement, Summit will have final decision-making authority with respect to clinical development strategy and execution in the Licensed Territory. For co-joined studies in which both Summit and Akeso participate, mutual agreement is required for material decisions; Summit retains the exclusive decision making with respect to participating in, and continuing its participation in, co-joined studies. In connection with the License Agreement, the Company has also agreed to enter into a Supply Agreement with Akeso (the "Supply Agreement"). Pursuant to the Supply Agreement, Summit agreed to purchase a certain portion of drug substance for clinical and commercial supply. Pursuant to the terms of the License Agreement, Summit will have final decision-making authority with respect to commercial strategy, pricing and reimbursement and other commercialization matters in the Licensed Territory.
Summit has not assumed any liabilities (including contingent liabilities), nor acquired any physical assets or trade names, or hired or acquired any employees from Akeso in connection with the License Agreement.
Ivonescimab
Ivonescimab is a novel potential first-in-class PD-1 / VEGF bispecific antibody, believed to be the most advanced in clinical development in the Licensed Territory; there are no known PD-1 / VEGF bispecific antibodies approved in our Licensed Territory. Engineered with Akeso’s unique Tetrabody technology, ivonescimab, as a single molecule, blocks programmed cell death protein 1 (“PD-1”) from binding to PD-L1 and PD-L2, and blocks vascular endothelial growth factor (“VEGF”) from binding to VEGF receptors. Ivonescimab is designed to potentially allow cooperative binding of the intended targets, such that the binding of PD-1 increases the binding affinity of VEGF and the binding of VEGF increases the affinity towards PD-1. In view of the co-expression of VEGF and PD-1 in the tumor micro-environment (“TME”), ivonescimab may block these two pathways more effectively and enhance the antitumor activity, as compared to combination therapy through what is believed to be a differentiated cooperative binding mechanism.
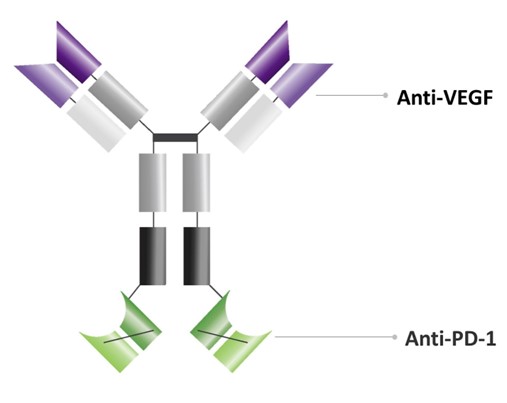
This could differentiate ivonescimab as there is potentially higher expression (presence) of both PD-1 and VEGF in tumor tissue and the TME as compared to normal tissue in the body. As shown in Akeso’s in-vitro studies, ivonescimab’s tetravalent structure (four binding sites) enables higher avidity (accumulated strength of multiple binding interactions) in the tumor microenvironment with over 18 fold increased binding affinity to PD-1 in the presence of VEGF in vitro, and over 4 times increased binding affinity to VEGF in the presence of PD-1 in vitro. This tetravalent structure, the intentional novel design of the molecule, and bringing these two targets into a single bispecific antibody with cooperative binding qualities have the potential to direct ivonescimab to the tumor tissue versus healthy tissue. The intent of this design, together with a half-life of six to seven days, is to improve upon previously established efficacy thresholds, in addition to side effects and safety profiles associated with these targets.
In addition to the two Phase III clinical trials sponsored by the Company, ivonescimab is also being developed in China and Australia by Akeso in multiple solid tumors and has been dosed in more than 1,600 patients globally.
Based on data published by Akeso at the 2024 European Lung Cancer Conference with a cut-off date of October 2023 after a median follow-up time of approximately 25.8 months, ivonescimab treatment, was associated with an overall response rate (ORR) in a Phase II study in patients with NSCLC (n=19) who have failed an EGFR-TKI of 68.4%, a median Progression-Free Survival (“mPFS”) time period of 8.5 months, and a median Overall Survival (“mOS”) of 22.5 months when combined with doublet chemotherapy (pemetrexed and carboplatin). The Phase II study, AK112-201, Cohort 2, was considered to have demonstrated a tolerable safety profile and a low discontinuation rate for adverse events.
In a separate group in the same Phase II study, AK112-201 (Cohort 1) for first-line advanced or metastatic NSCLC patients with squamous histology, (n=63), ivonescimab, combined with carboplatin and paclitaxel, in demonstrated a mPFS of 11.1 months. Median overall survival was not reached after a median follow-up period of 22.1 months. Phase II study was considered to have demonstrated a tolerable safety profile and a low discontinuation rate for adverse events.
Summit plans to conduct its current clinical trials, as well as design and conduct additional clinical trial activities for ivonescimab in its Licensed Territory, to support and submit relevant regulatory filings. Summit also plans to support additional study activities through its investigator initiated study program.
Product Pipeline
Summit Sponsored Ivonescimab Trials: Ivonescimab is currently being investigated in global Phase III clinical trials. Phase I and II trials were completed by our partner Akeso. This pipeline reflects clinical trials that have been initiated by Summit in its Licensed Territory.
HARMONi study is a multi-regional, registration-enabling clinical trial that we joined with Akeso, and for which we started initiating and activating sites in North America and Europe during 2023. The first patient in our Licensed Territory was enrolled during the second quarter of 2023. We plan to initiate additional sites in North America and Europe by the end of the second quarter of 2024. We expect to complete enrollment during the second half of 2024. The co-primary endpoints for this study are progression free survival (PFS) and overall survival (OS).
HARMONi-3 study is a phase III, is multi-regional, registration-enabling clinical trial for which we initiated activating sites in North Americas and China during fourth quarter of 2023. We plan to initiate additional sites in North America, China, European countries, Japan and several other countries through early 2025. Our plan to initiate in new countries and sites is dependent upon the timing and requirements for getting the regulatory approval of the clinical trial applications with the respective regulatory authorities and approvals from central or local independent review boards. We may decide to modify our plans to go in certain regions or countries based on the timelines and requirements from the respective regulatory regions. We commenced patient enrollment in the HARMONi-3 study during fourth quarter of 2023. The primary endpoint for this study is overall survival (OS).
In Q4 2023, we began collaborating with multiple institutions globally and opened our investigator initiated study program across several disease areas.
In addition, our partners at Akeso are sponsoring multiple, ongoing Phase II and III clinical trials in NSCLC and other cancers outside of our Licensed Territory. We plan to review the data generated from these clinical trials as a part of our consideration for advancing our clinical development pipeline for ivonescimab in our Licensed Territory.
Status of Anti-Infectives Pipeline
Discuva Platform
In December 2017, we acquired Discuva Limited, a privately held United Kingdom-based company. Through this acquisition, we obtained a bacterial genetics platform and a suite of software-based technologies (collectively termed our “Discuva Platform”), which facilitate the discovery and development of new mechanism antibiotics. Our Discuva Platform can be used to identify new bacterial targets for drug discovery, understand the mechanism of action of small molecules targeting varying types of bacteria and select the most optimal preclinical candidates, including those with the least propensity to develop bacterial resistance.
On January 20, 2023, we announced that, given the License Agreement that we entered into in December 2022 and the shift in Company’s focus to oncology, we will cease further investment in the Discuva platform and evaluate further options for the use of the Discuva Platform.
Results of Operations
Amounts reported in millions within this Quarterly Report are computed based on the amounts in thousands, and therefore, the sum of components may not equal the total amount reported in millions due to rounding.
The following table sets forth our results of operations for the three month periods ended March 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| (in millions) | | 2024 | | 2023 | | | | |
| | | | | | | | |
| Operating expenses: | | | | | | | | |
| Research and development | | $ | 30.9 | | | $ | 9.9 | | | | | |
| In-process research and development | | — | | | 520.9 | | | | | |
| General and administrative | | 11.7 | | | 6.9 | | | | | |
| | | | | | | | |
| Total operating expenses | | 42.6 | | | 537.7 | | | | | |
| Other operating income | | 0.2 | | | 0.6 | | | | | |
| Operating loss | | (42.4) | | | (537.1) | | | | | |
| Other expense, net | | (1.1) | | | (5.2) | | | | | |
| | | | | | | | |
| | | | | | | | |
| Net loss | | $ | (43.5) | | | $ | (542.3) | | | | | |
Operating Expenses
Research and Development Expenses
The table below summarizes our research and development expenses by category for the three month periods ended March 31, 2024 and 2023, respectively.
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| (in millions) | | 2024 | | 2023 | | | | |
| Oncology | | $ | 20.7 | | | $ | 2.4 | | | | | |
| Anti-infectives | | 0.1 | | | 0.4 | | | | | |
| Compensation related costs, excluding stock-based compensation | | 6.5 | | | 4.8 | | | | | |
| Stock-based compensation | | 2.4 | | | 1.1 | | | | | |
| Other research and development costs | | 1.2 | | | 1.2 | | | | | |
| Total | | $ | 30.9 | | | $ | 9.9 | | | | | |
The entry into the License Agreement with Akeso, Inc., effective in January 2023, represents a significant change in our strategy from anti-infectives to the therapeutic area of oncology.
Oncology expenses represent our investment in the clinical development of ivonescimab, known as SMT112 in the United States, Canada, Europe, and Japan. During the second quarter of 2023, we announced plans to initiate Phase III clinical studies for ivonescimab in NSCLC and we announced that the first United States-based patient had been enrolled in the Phase III HARMONi study. We have recently commenced enrollment in our second Phase III HARMONi-3 clinical trial. We are continuing to focus our efforts on patient enrollment in both the Phase III HARMONi and Phase III HARMONi-3 studies.
With this shift in focus from anti-infectives to oncology, research and development expenses increased by $21 million during the three months ended March 31, 2024, compared to the same period in the prior year, primarily due to our investment in oncology expenses of $18.3 million, and an increase in compensation related expenses of $1.7 million to support the clinical development of ivonescimab as we hire experts in the oncology field, partially offset by a decrease of $0.3 million related to discontinuing the development activities related to ridinilazole and SMT-738. We expect oncology-related research and development costs to continue to increase as we progress with the development of ivonescimab.
In-process research and development
The table below summarizes our in-process research and development expenses by category for the three month periods ended March 31, 2024 and 2023, respectively.
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| (in millions) | | 2024 | | 2023 | | | | |
| Upfront milestone payments | | $ | — | | | $ | 520.8 | | | | | |
| Direct transaction costs | | — | | | 0.1 | | | | | |
| Total | | $ | — | | | $ | 520.9 | | | | | |
Our investment in ivonescimab totaled $520.9 million for the three months ended March 31, 2023 and primarily related to our upfront milestone payments pursuant to the License Agreement with Akeso. The License Agreement closed in January 2023, and both Akeso and Summit entered into the Common Stock Issuance Agreement (“Issuance Agreement”). Pursuant to the License Agreement and Issuance Agreement, Akeso elected to receive 10 million shares of our common stock in lieu of $25.1 million cash and was paid $274.9 million in cash as the initial upfront payment. The remaining $200.0 million upfront payment was paid on March 6, 2023. In-process research and development expense comprised of the $474.9 million paid in cash, the fair value of the 10 million shares of common stock on the date of closing the transaction of $45.9 million, and $0.1 million of direct transactions costs incurred.
General and Administrative Expenses
The table below summarizes our general and administrative expenses by category for the three month periods ended March 31, 2024 and 2023, respectively.
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| (in millions) | | 2024 | | 2023 | | | | |
| Compensation related costs, excluding stock-based compensation | | $ | 2.5 | | | $ | 2.5 | | | | | |
| Stock-based compensation | | 7.0 | | | 1.7 | | | | | |
| Legal fees and professional services | | 1.0 | | | 1.7 | | | | |
| Other general and administrative expenses | | 1.2 | | | 1.0 | | | | | |
| Total | | $ | 11.7 | | | $ | 6.9 | | | | | |
General and administrative expenses increased by $4.8 million for the three months ended March 31, 2024, compared to the same period in the prior year, primarily due to an increase of $5.3 million in stock-based compensation as the Company is focused on building its executive management team to continue supporting the growth of the Company, partially offset by a decrease of $0.7 million in legal and professional services related to corporate projects and transactions. We expect general and administrative expenses to continue to increase as we scale our infrastructure and management to support development of ivonescimab.
Other Operating Income
The table below summarizes our other operating income for the three month periods ended March 31, 2024 and 2023, respectively. | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| (in millions) | | 2024 | | 2023 | | | | |
| Research and development tax credits | | $ | 0.2 | | | $ | 0.6 | | | | | |
| | | | | | | | |
| | | | | | | | |
| | $ | 0.2 | | | $ | 0.6 | | | | | |
U.K. research and development tax credits decreased by $0.4 million for the three month period ended March 31, 2024, compared to the same periods in the prior year, respectively, due to a decrease in clinical and manufacturing activity spend, which resulted in a decrease in tax credits claimed, coupled with a decrease in eligible expenses claimed due to changes in U.K. tax legislation.
Other Expense, net
The table below summarizes our other expense, net by category for the three month periods ended March 31, 2024 and 2023, respectively.
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | | |
| (in millions) | | 2024 | | 2023 | | | | |
| Foreign currency gains | | $ | 0.2 | | | $ | 0.5 | | | | | |
| Interest expense on promissory notes payable to related parties | | (3.1) | | | (8.3) | | | | | |
| Investment income | | 1.8 | | | 1.8 | | | | | |
| Reclassification of cumulative currency translation gain | | — | | | 0.4 | | | | | |
| Other expense, net | | — | | | 0.4 | | | | | |
| | $ | (1.1) | | | $ | (5.2) | | | | | |
For the three months ended March 31, 2024, other expense, net primarily consisted of loan interest expense incurred related to the $100 million promissory note and for the three months ended March 31, 2023, other expense, net primarily consisted of loan interest expense incurred related to the $520 promissory notes, as described in Note 14. These amounts for both periods are partially offset by investment income related to the Company's money market funds and short-term investments in U.S. treasury securities.
Liquidity and Capital Resources
Sources of Liquidity
To date, we have financed our operations primarily through issuances of our common stock, issuance of debt, receipt of payments to us under license, collaboration, and commercialization arrangements, for example, our license and commercialization agreement with Eurofarma Laboratórios SA, or Eurofarma, development funding and other assistance from government entities, philanthropic, non-government and not-for-profit organizations for our product candidates. In particular, we have received funding from BARDA, CARB-X, Innovate UK, Wellcome Trust and a number of not-for-profit organizations.
We have devoted substantially all of our efforts to research and development, including clinical trials. We have not completed the development of any drugs. We expect to continue to incur significant expenses and increasing operating losses for at least the next few years. The net losses we incur may fluctuate significantly from quarter to quarter and year to year, due to the nature and timing of our research and development activities. We expect that our research and development and general and administrative expenses will continue to be significant in connection with our ongoing research and development efforts. In addition, if we obtain marketing approval for any of our product candidates in the United States or other jurisdictions where we retain commercial rights, and if we choose to retain those rights, we would expect to incur significant sales, marketing, distribution and outsourced manufacturing expenses, as well as ongoing research and development expenses. In addition, our expenses will increase if and as we:
•invest in clinical development of invonescimab in our Licensed Territory;
•conduct research and continue development of additional product candidates;
•maintain and augment our intellectual property portfolio and opportunistically acquire complimentary intellectual property;
•seek further regulatory advancement for ivonescimab;
•invest in our manufacturing capabilities for ivonescimab and any other products for which we may obtain regulatory approval;
•seek marketing approvals for any product candidates that successfully complete clinical development;
•ultimately establish a sales, marketing and distribution infrastructure in jurisdictions where we have retained commercialization rights and scale up external manufacturing capabilities to commercialize any product candidates for which we receive marketing approval;
•perform our obligations under our collaboration agreements;
•pursue business development opportunities, including investing in other businesses, products and technologies;
•experience any delays or encounter any issues with any of the above, including but not limited to failed studies, complex results, safety issues or other regulatory challenges
•hire additional clinical, regulatory, scientific and administrative personnel;
•expand our physical presence;
•add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts; and
•borrow capital to fund our resources and have to pay interest expenses on such borrowings.
During the three months ended March 31, 2024, we incurred a net loss of $43.5 million, and cash flows used in operating activities for the three months ended March 31, 2024 was $30.1 million. As of March 31, 2024, we had an accumulated deficit of $1,036.7 million, cash and cash equivalents of $61.3 million, short-term investments in U.S. treasury securities of $95.4 million. We expect to continue to generate operating losses for the foreseeable future.
We have evaluated whether our cash, cash equivalents and U.K. research and development tax credits provide sufficient cash to fund our operating cash needs for the next twelve months from the date of issuance of these quarterly financials. We are investing in the clinical development of ivonescimab, including its ongoing clinical trials. In addition, we have a $100 million promissory note and interest payable to a related party (refer to Note 14 for further details) that matures on April 1, 2025. Based upon our cash and cash equivalents and short-term investments as of March 31, 2024, we expect to be able to operate through the first quarter of 2025. In order to further fund our operating cash needs and repay this promissory note, we intend to raise additional capital. As of the date of this filing, the additional capital has not been secured. As a result, these conditions raise substantial doubt about our ability to continue as a going concern.
In addition to the payments already made to Akeso, under the License Agreement there are additional potential milestone payments of $4.5 billion, as Akeso will be eligible to receive regulatory milestones of up to $1.05 billion and commercial milestones of up to $3.45 billion. In addition, Akeso will be eligible to receive low double-digit royalties on net sales. Until we can generate substantial revenue and achieve profitability, we will need to raise additional capital to fund ongoing operations and capital needs, including the payment of the milestone payments referenced above.
We have based the foregoing estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. This estimate assumes, among other things, that we do not obtain any additional funding through grants and clinical trial support or through new collaboration arrangements. Our future capital requirements will depend on many factors, including:
•the costs, timing and outcome of clinical trials required for clinical development of ivonescimab;
•the number and development requirements of other future product candidates that we pursue;
•the costs, timing and outcome of regulatory review of ivonescimab and/or our other product candidates we develop;
•the costs and timing of commercialization activities, including product sales, marketing, distribution and manufacturing, for any of our product candidates that receive marketing approval;
•the extent to which we become liable for milestone payments under our Licensing Agreement for ivonescimab;
•subject to receipt of marketing approval, revenue received from commercial sales of any product candidates;
•the costs and timing of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against any intellectual property-related claims;
•our ability to establish and maintain collaborations, licensing or other arrangements and the financial terms of such arrangements;
•the extent to which we acquire or invest in other businesses, products and technologies;
•the rate of the expansion of or the extent to which we change our physical presence.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of some, or all, of the following: equity and debt offerings, collaborations, strategic alliances, grants and clinical trial support from government entities, philanthropic, non-government and not-for-profit organizations, and marketing, distribution or licensing arrangements.
We will need to seek additional funding in the future to fund operations. Additional capital, when needed, may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing stockholders may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing stockholders. Additional debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific
actions, such as incurring additional debt, making capital expenditures or declaring dividends or other distributions. If we raise additional funds through collaborations, strategic alliances or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves, which could materially adversely affect our business prospects or our ability to continue operations.
Cash Flows
The following table summarizes the results of our cash flows for the three months ended March 31, 2024 and 2023:
| | | | | | | | | | | | | | | | | |
| | Three Months Ended
March 31, | |
| (in millions) | | 2024 | | 2023 | |
| Net cash used in operating activities | | $ | (30.1) | | | $ | (13.1) | | |
| Net cash provided by (used in) investing activities | | $ | 19.8 | | | $ | (645.1) | | |
| Net cash provided by financing activities | | $ | 0.5 | | | $ | 80.1 | | |
Operating Activities
Net cash used in operating activities for the three months ended March 31, 2024 was $30.1 million and was due to a net loss of $43.5 million, which included non-cash charges of $9.2 million and a net change in working capital of $4.2 million. The non-cash charges primarily consisted of $9.5 million of stock-based compensation, partially offset by $0.4 million for amortization of the discount on short-term investments. The net change in working capital was primarily due to an increase of $3.7 million of accounts payable, a $1.8 million increase in other long-term liabilities, which represents the interest on the promissory notes payable and an increase in accrued compensation of $0.6 million, offset by decrease of $2.2 million in accrued liabilities.
Net cash used in operating activities for the three months ended March 31, 2023 was $13.1 million and resulted from a net loss of $542.4 million, which included an adjustment of $475.0 million cash payments to investing activities for the purchase of in-process research and development from Akeso under the terms of the License Agreement and the associated direct transaction costs, non-cash charges of $54.8 million and a net decrease in working capital of $0.5 million. The non-cash charges primarily comprised of $45.9 million issuance of shares in lieu of cash for Akeso upfront payment, $6.1 million non-cash interest expense and $2.8 million of non-cash charges related to stock-based compensation. The net increase in working capital was primarily due to a decrease of $3.2 million in accrued compensation, partially offset by a decrease of $0.9 million in the research and development tax credit receivable, and a decrease of $0.4 million in other non-current assets.
Investing Activities
Net cash provided by investing activities for the three months ended March 31, 2024 was $19.8 million and was primarily due to $112.9 million received from the maturity and redemption of short-term investments in U.S. treasury securities, offset by $93.0 million related to the purchase of short-term investments.
Net cash used in investing activities for the three months ended March 31, 2023 was $645.1 million and was primarily due to $475.0 million cash payments made to Akeso for the upfront payment pursuant to the License Agreement and $170.0 million for the purchase of short-term investments in U.S. treasury securities.
Financing Activities
Net cash provided by financing activities was $0.5 million for the three months ended March 31, 2024, and was due to proceeds received of $0.5 million related to employee stock awards.
Net cash provided by financing activities was $80.1 million for the three months ended March 31, 2023 and was due to net proceeds received of $104.1 million (net of paid issuance costs) related to the issuance of common stock from the 2023 Rights Offering and net of the extinguishment of $395.3 million of principal and accrued interest due and payable by us under the $400 million Duggan Promissory Note in satisfaction of the subscription price for the shares subscribed by Mr. Duggan in the 2023 Rights Offering, proceeds received of $0.7 million related to employee stock awards, offset by the repayment of $24.7 million related to promissory notes from related parties.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our condensed consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, research and development costs, intangible assets, stock-based compensation and income taxes. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations, and in Critical Accounting Policies and Significant Judgments and Estimates in our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission on February 20, 2024. There have been no material changes to our critical accounting policies and estimates that were disclosed in the Annual Report on Form 10-K.
Contractual obligations and commitments
Lease Commitments
We lease office space in Menlo Park, California, Miami, Florida, United States and in Oxfordshire, United Kingdom. In addition to our lease commitments as of December 31, 2023 which were disclosed in our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission on February 20, 2024, we entered into a new lease agreement for our Miami, Florida headquarters during the three months ended March 31, 2024. Total future lease payments as of March 31, 2024, which include base rent and sales tax are approximately $4.8 million on an undiscounted basis. This lease commenced on February 1, 2024 and has a term of 64 months. As of March 31, 2024 we have $0.3 million of restricted cash associated with an irrevocable letter of credit required by the landlord to enter into this lease.
Debt Commitments
Refer to Note 14 for a discussion of the promissory note payable to a related party.
Other Commitments
We enter into contracts in the normal course of business with various third parties for clinical trials, preclinical research studies and testing, manufacturing and other services and products for operating purposes. Most contracts provide for termination upon notice, and therefore are cancellable contracts. The majority of these commitments are due within one year.
There have been no material changes to the Company's other contractual commitments that were disclosed in the our Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
We have certain commitments under our agreements with Akeso, Wellcome Trust, the University College London and certain employees, former employees and former directors of Discuva, pursuant to which we will be required to pay royalties or make milestone payments. The License Agreement with Akeso also contains certain manufacturing and purchase commitments. As of March 31, 2024, we are unable to estimate the amount, timing or likelihood of achieving the milestones, making future product sales or assessing estimated forecasts for manufacturing and supplied materials which these contingent payment obligations relate to.
Indemnifications
Our certificate of incorporation provides that it will indemnify the directors and officers to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with all of the directors and executive officers. These indemnification agreements may require us, among other things, to indemnify each such director or executive officer for some expenses, including attorneys’ fees, judgments, fines, and settlement amounts incurred by him or her in any action or proceeding arising out of his or her service as one of our directors or executive officers. We believe the fair value for these
indemnification obligations is minimal. Accordingly, we have not recognized any liabilities relating to these obligations as of March 31, 2024 and December 31, 2023.
Legal Proceedings
We are not currently subject to any material legal proceedings.
Off-Balance Sheet Arrangements
Other than the contractual obligations and commitments described above, we did not have during the periods presented, and we do not currently have, any off‑balance sheet arrangements, as defined in the rules and regulations of the Securities and Exchange Commission.
Recently Issued Accounting Pronouncements
For a discussion of recently issued accounting pronouncements, refer to Note 3, Recently Issued or Adopted Accounting Pronouncements, to our condensed consolidated financial statements included in this report.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Pursuant to Item 305(e) of Regulation S-K (§ 229.305(e)), the Company is not required to provide the information required by this Item as it is a “smaller reporting company,” as defined by Rule 229.10(f)(1).
Item 4. Controls and Procedures.
We have carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) under the supervision and the participation of the Company’s management, which is responsible for the management of the internal controls, and which includes our Chief Executive Officers and our Chief Financial Officer. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Securities Exchange Act of 1934 is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based upon our evaluation of our disclosure controls and procedures as of March 31, 2024, our Chief Executive Officers and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at a reasonable level of assurance.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended March 31, 2024, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
We are not currently subject to any material legal proceedings.
Item 1A. Risk Factors.
An investment in our common stock or other securities involves a number of risks. You should carefully consider each of the risks described in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (the "Annual Report") filed with the Securities and Exchange Commission on February 20, 2024, which Annual Report includes a detailed discussion of the Company’s risk factors. If any of the risks develop into actual events, our business, financial condition, or results of operations could be negatively affected, the market price of our common stock or other securities could decline, and you may lose all or part of your investment.
There have been no material changes to the risk factors disclosed in Item 1A of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
None.
Item 5. Other Information.
None.
Item 6. Exhibits.
Exhibit Index
| | | | | | | |
| Exhibit No. | Description | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document | | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document | | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | | |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document | | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document | | |
| 104* | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | | |
| | | | | | | | |
| * | | Filed herewith. |
| ** | | Furnished herewith. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| Date: May 1, 2024 | | SUMMIT THERAPEUTICS INC. |
| | | |
| | By: | /s/ Manmeet Soni |
| | Name: | Manmeet Soni |
| | Title | Chief Operating Officer and Chief Financial Officer |
| | | (Principal Financial Officer) |
| | | |
| | | |