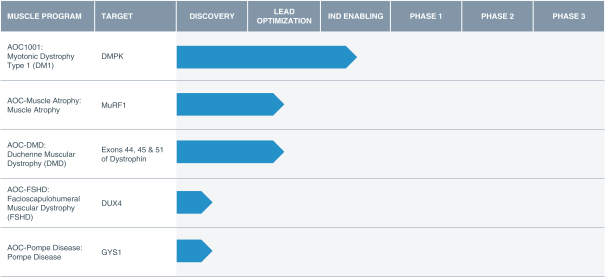
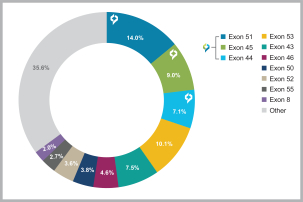
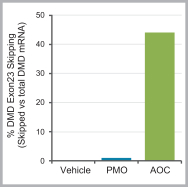
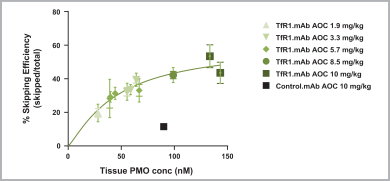
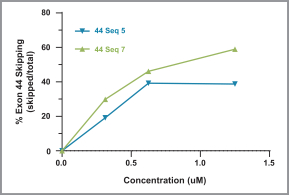
Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Mexico, Singapore, and South Korea. These patent rights relate to the DMD AOCs composition of matter, formulations containing the DMD AOCs, methods of manufacturing, and methods of treating diseases, using our DMD AOCs. Any patents issued from these applications are expected to expire in 2038; however, a patent term extension may be available.
Intellectual Property Relating to Our Muscle Atrophy AOC Product Candidate
With regard to our muscle atrophy AOC product candidate, as of May 31, 2020 we owned one pending U.S. patent application and one pending PCT patent application. These patent rights relate to the muscle atrophy AOC composition of matter, formulations containing the muscle atrophy AOC, methods of manufacturing, and methods of treating diseases, using our muscle atrophy AOC. Any patents issued from these applications are expected to expire in 2038; however, a patent term extension may be available.
Intellectual Property Relating to Our FSHD AOC Product Candidate
With regard to our FSHD AOC product candidate, as of May 31, 2020 we owned one pending U.S. patent application. These patent rights relate to the FSHD AOC composition of matter, formulations containing the FSHD AOC, methods of manufacturing, and methods of treating diseases, using our FSHD AOC. Any patents issued from these applications are expected to expire in 2041; however, a patent term extension may be available.
Intellectual Property Relating to Our Pompe AOC Product Candidate
With regard to our Pompe AOC product candidate, as of May 31, 2020, we owned one pending U.S. patent application. This application relates to the Pompe disease AOC composition of matter, formulations containing the Pompe disease AOC, methods of manufacturing, and method of treating diseases, using our Pompe disease AOC. Any patents issued from this application are expected to expire in 2041; however, a patent term extension may be available.
Intellectual Property Relating to Our AOC Product Platform
As of May 31, 2020, we owned 14 families of U.S. and foreign patents and patent applications generally covering our AOC product platform. These families include two issued U.S. patents, 14 pending U.S. patent applications, three PCT patent applications and 24 foreign patent applications in the European Patent Office, Australia, Canada, China, Israel, Hong Kong, Japan, South Korea, Mexico, Singapore, and Taiwan, relating to key aspects and components of our AOC product platform systems. Our patent applications contain claims covering (i) proprietary antibodies; (ii) proprietary oligonucleotide chemical structures; (iii) proprietary oligonucleotide sequences; (iv) proprietary AOC structures; and (v) methods for manufacturing and using our AOC technologies. Some of these AOC platform cases generically cover our various product candidates. The issued U.S. patents and any U.S. patents issuing from our pending U.S. patent applications are expected to expire between 2037 and 2040.
The term of individual patents depends upon the laws of the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing of anon-provisional patent application. However, the term of United States patents may be extended for delays incurred due to compliance with the FDA requirements or by delays encountered during prosecution that are caused by the USPTO. For example, for drugs that are regulated by the FDA under the Hatch-Waxman Act, it is permitted to extend the term of a patent that covers such drug for up to five years beyond the normal expiration date of the patent. In the future, if and when our biopharmaceutical product candidates receive FDA approval, we expect to apply for patent term extensions on patents covering those product candidates. We intend to seek patent term extensions to any of our issued patents in any jurisdiction where these are available; however, there is no guarantee that the applicable authorities, including the USPTO and FDA, will agree with our assessment of whether such extensions
123