
Virtual Investor & Analyst Event Series – Volume 3 Delivering on AOCs: FSHD Exhibit 99.1

Forward Looking Statements We caution the reader that this presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, the anticipated timing, costs, design and conduct of our ongoing and planned preclinical studies and planned clinical trials, research and development plans, timing and likelihood of success, prospective products, product approvals, plans and objectives of management for future operations, and future results of anticipated product development efforts, are forward-looking statements. In some cases, the reader can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by Avidity that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: we are early in our development efforts and many of our development programs are in the preclinical or discovery stage; our approach to the discovery and development of product candidates based on our AOC platform is unproven, and we do not know whether we will be able to develop any products of commercial value; the success of our preclinical studies and clinical trials for our product candidates; the results of preclinical studies and early clinical trials are not necessarily predictive of future results; potential delays in the commencement, enrollment and completion of clinical trials; our dependence on third parties in connection with preclinical testing and product manufacturing; disruption to our operations from the COVID-19 pandemic; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval and/or commercialization, or may result in recalls or product liability claims; regulatory developments in the United States and foreign countries, including acceptance of INDs and similar foreign regulatory submissions and our proposed design of future clinical trials; our ability to obtain and maintain intellectual property protection for our product candidates and proprietary technologies; we may use our capital resources sooner than we expect; and other risks described in our filings with the SEC, including under the heading “Risk Factors” in our Form 10-K for the year ending on December 31, 2020, filed with the SEC on March 15, 2021, and any subsequent filings with the SEC. The reader is cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and the reader is cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.

Our Vision To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Luke Living with DM1
Delivering on Our Vision Progressing robust pipeline in muscle Phase 1/2 MARINATM trial of AOC 1001 ongoing Anticipated clinical trial initiations for both AOC 1044 for DMD and AOC 1020 for FSHD by the end of 2022 Leveraging expertise in clinical and commercial execution Assembling an experienced team in rare & RNA therapies Building an integrated and diverse company in service of our patients DISRUPTIVE & BROAD PLATFORM ADVANCING & EXPANDING PIPELINE AGILE & DIVERSE COMPANY Committed to delivering a new class of RNA therapies Dosed first person with an AOC – a first for the platform Broadening to other tissues & cell types through partnerships & internal discovery

PROGRAM / INDICATION TARGET DISCOVERY / LEAD OPTIMIZATION IND ENABLING PHASE 1/2 MUSCLE DISORDERS AOC 1001: Myotonic Dystrophy Type 1 (DM1) DMPK AOC 1020: Facioscapulohumeral Muscular Dystrophy (FSHD) DUX4 AOC 1044: Duchenne Muscular Dystrophy (DMD) Exon 44 Dystrophin Next AOC DMD Programs Exon 51 Dystrophin Exon 45 Dystrophin AOC Muscle Atrophy: Muscle Atrophy* MuRF1 AOC Pompe Disease: Pompe Disease GYS1 Advancing our Muscle Disease Franchise of AOCs * Opportunity for a rare disease indication Clinical trial initiations planned for 2022 Clinical trial initiations planned for 2022
Goals for the Day Demonstrate how our AOC platform expertise is leveraged across the pipeline Review the preclinical package supporting AOC 1020 Offer a broader perspective on the current treatment landscape for FSHD Answer your questions mAb OLIGO

What’s New: AOC 1001 Phase 1/2 MARINA trial ongoing: First participants dosed; marked the first time a person was dosed with an AOC Anticipate preliminary assessment of the MARINA trial in Q4 of 2022 Pipeline Progress Announcing AOC 1020 – our candidate for FSHD entering IND enabling studies Anticipate clinical trial initiations by the end of 2022 for both AOC 1020 and AOC 1044, our lead DMD program targeting Exon 44 Delivering on Progress Highlights from Today


Agenda Sarah Boyce, President & CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology University of Kansas Medical Center Sarah Boyce, President and CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology Mike MacLean, CFO (Moderator) Sarah Boyce, President & CEO Welcome & Introduction Building on our AOC Expertise Facioscapulohumeral Muscular Dystrophy (FSHD) Program FSHD: Disease Overview and Endpoints Q&A Session Closing Remarks

Building on our AOC Expertise Art Levin, Ph.D., Chief Scientific Officer
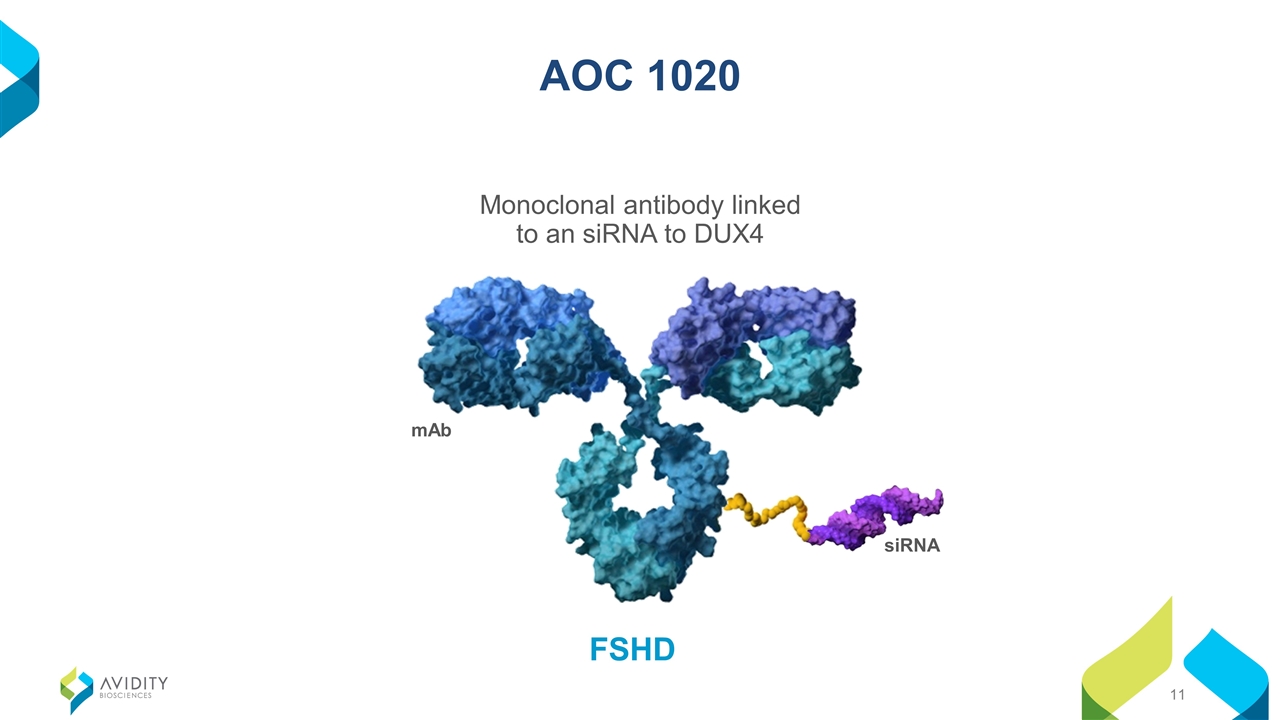
FSHD AOC 1020 Monoclonal antibody linked to an siRNA to DUX4 mAb siRNA
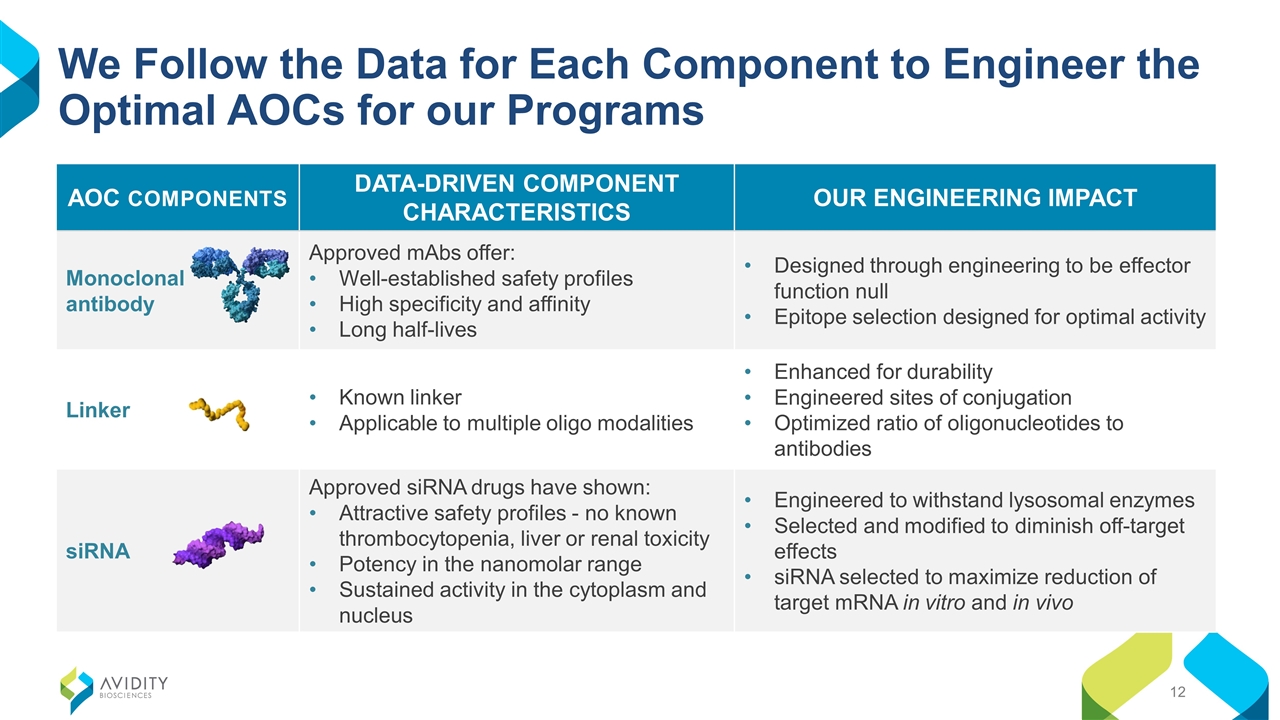
AOC COMPONENTS DATA-DRIVEN COMPONENT CHARACTERISTICS OUR ENGINEERING IMPACT Monoclonal antibody Approved mAbs offer: Well-established safety profiles High specificity and affinity Long half-lives Designed through engineering to be effector function null Epitope selection designed for optimal activity Linker Known linker Applicable to multiple oligo modalities Enhanced for durability Engineered sites of conjugation Optimized ratio of oligonucleotides to antibodies siRNA Approved siRNA drugs have shown: Attractive safety profiles - no known thrombocytopenia, liver or renal toxicity Potency in the nanomolar range Sustained activity in the cytoplasm and nucleus Engineered to withstand lysosomal enzymes Selected and modified to diminish off-target effects siRNA selected to maximize reduction of target mRNA in vitro and in vivo We Follow the Data for Each Component to Engineer the Optimal AOCs for our Programs
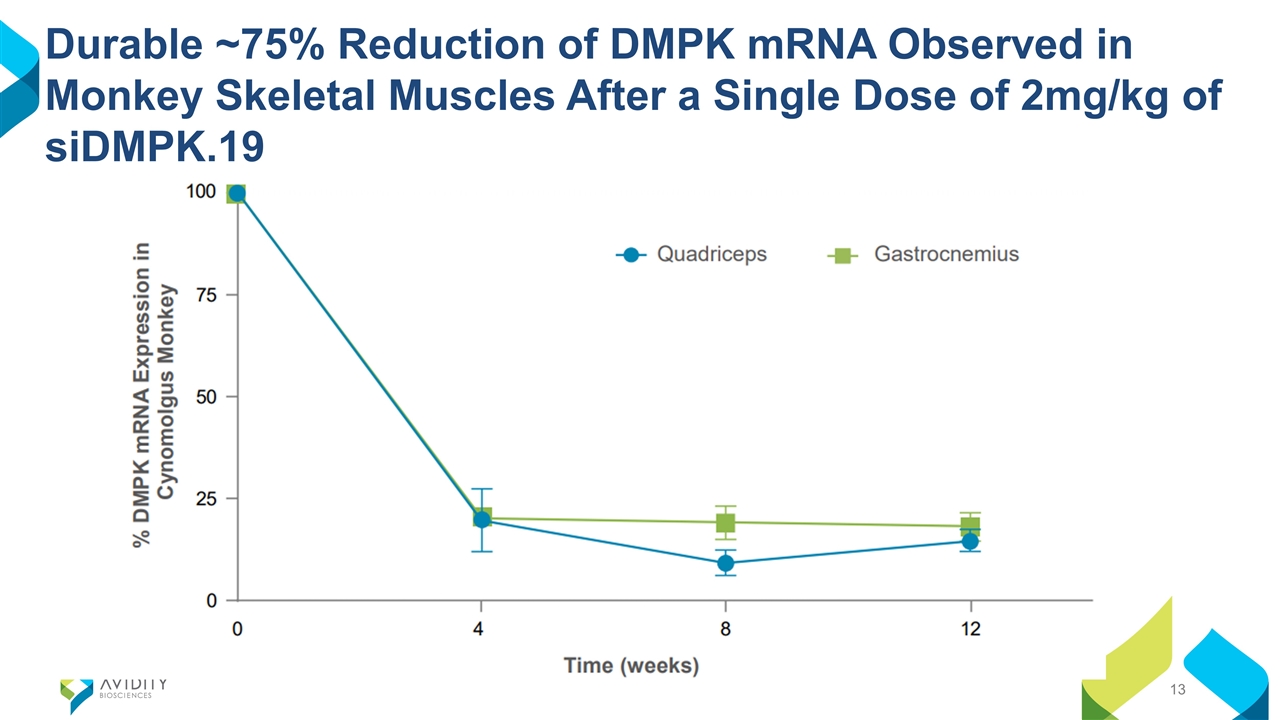
Durable ~75% Reduction of DMPK mRNA Observed in Monkey Skeletal Muscles After a Single Dose of 2mg/kg of siDMPK.19
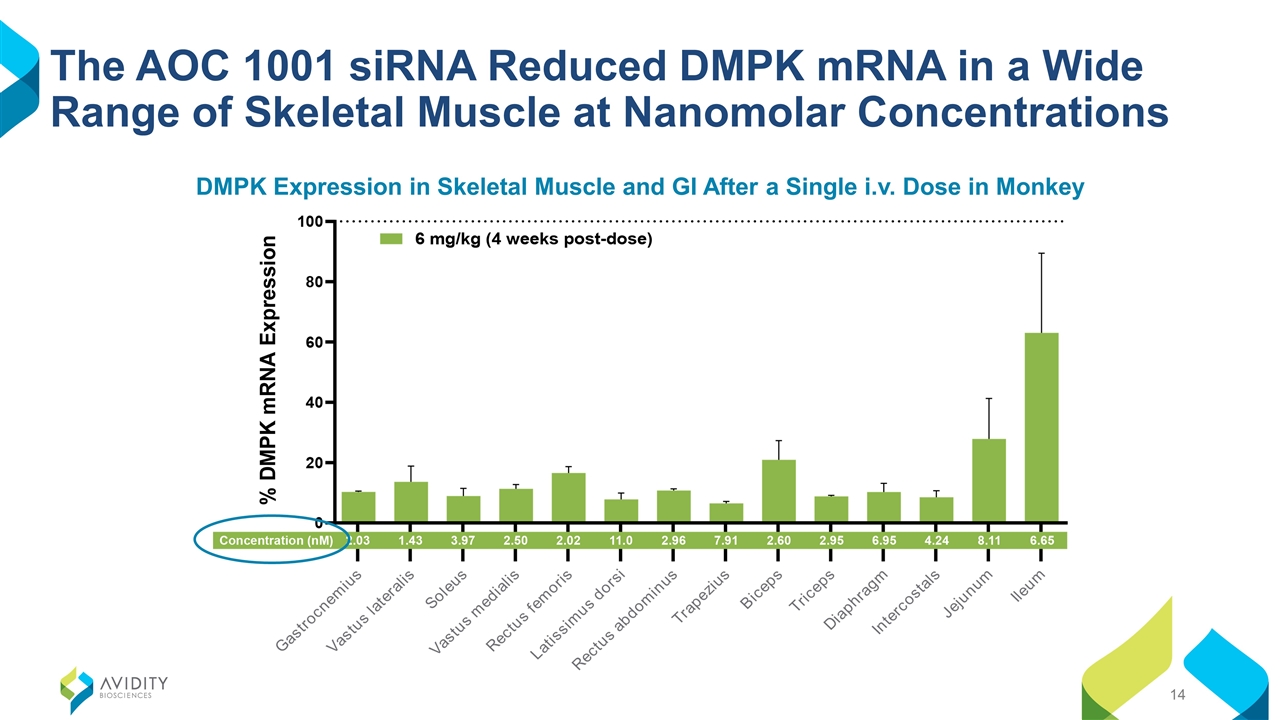
The AOC 1001 siRNA Reduced DMPK mRNA in a Wide Range of Skeletal Muscle at Nanomolar Concentrations DMPK Expression in Skeletal Muscle and GI After a Single i.v. Dose in Monkey
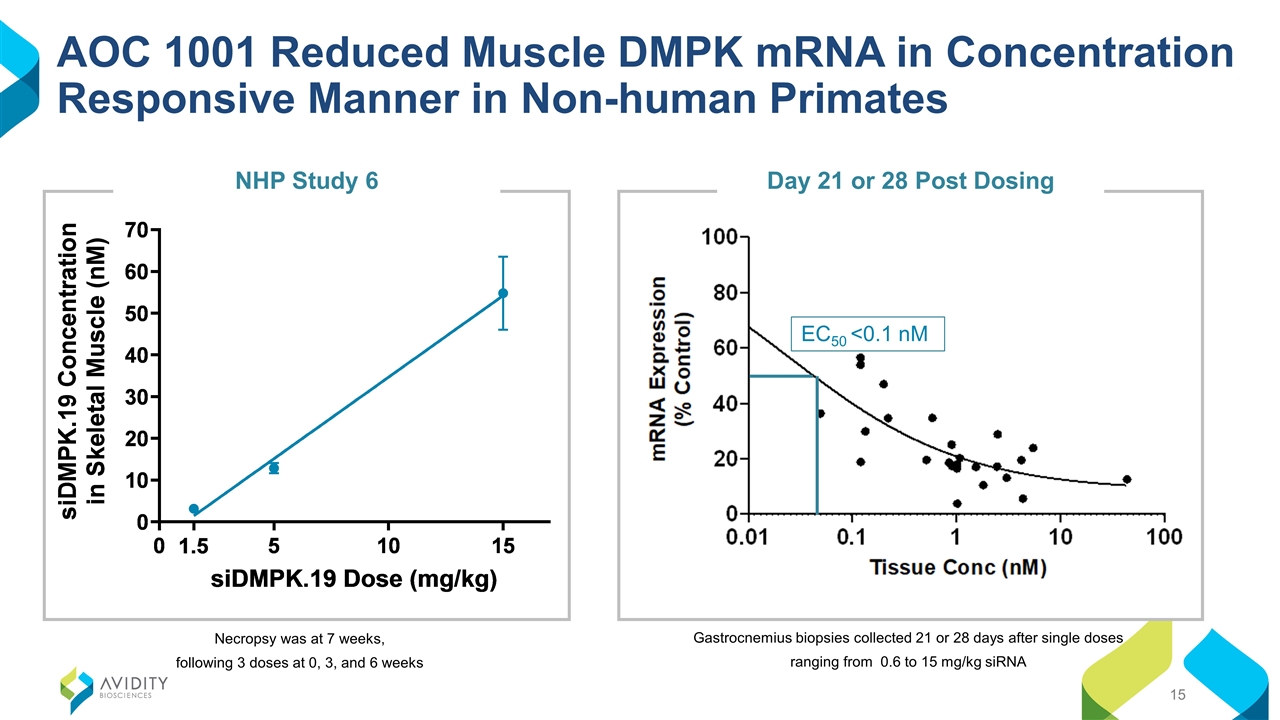
AOC 1001 Reduced Muscle DMPK mRNA in Concentration Responsive Manner in Non-human Primates Necropsy was at 7 weeks, following 3 doses at 0, 3, and 6 weeks NHP Study 6 Gastrocnemius biopsies collected 21 or 28 days after single doses ranging from 0.6 to 15 mg/kg siRNA EC50 <0.1 nM Day 21 or 28 Post Dosing
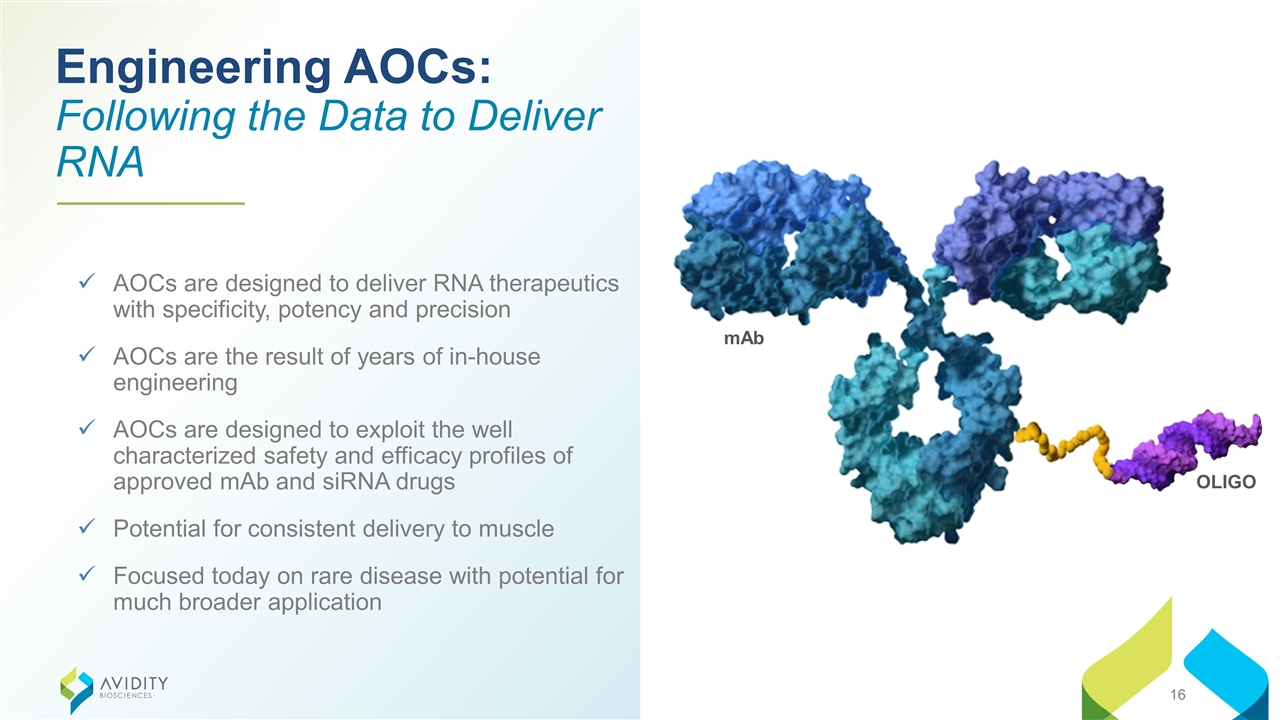
Engineering AOCs: Following the Data to Deliver RNA AOCs are designed to deliver RNA therapeutics with specificity, potency and precision AOCs are the result of years of in-house engineering AOCs are designed to exploit the well characterized safety and efficacy profiles of approved mAb and siRNA drugs Potential for consistent delivery to muscle Focused today on rare disease with potential for much broader application mAb OLIGO

Agenda Sarah Boyce, President & CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology University of Kansas Medical Center Sarah Boyce, President and CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology Mike MacLean, CFO (Moderator) Sarah Boyce, President & CEO Welcome & Introduction Building on our AOC Expertise Facioscapulohumeral Muscular Dystrophy (FSHD) Program FSHD: Disease Overview and Endpoints Q&A Session Closing Remarks

Facioscapulohumeral Muscular Dystrophy (FSHD) Program W. Michael Flanagan, Ph.D., Chief Technical Officer

Amy, Living with FSHD “Living with FSHD feels like an imprisonment in your own body.”

Delivering on AOCs: FSHD AOCs: Direct targeting of DUX4, the underlying cause of FSHD AOC 1020: Designed to be a potent DUX4 inhibitor moving into IND-enabling studies mAb OLIGO
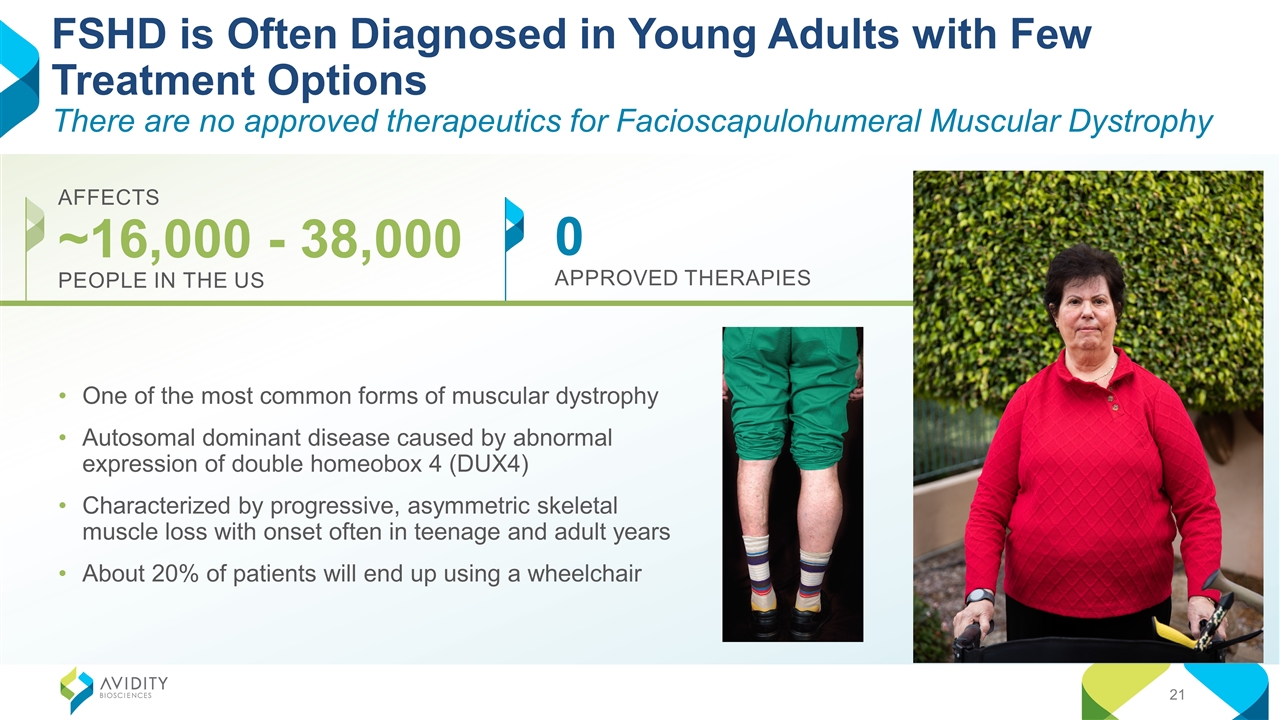
0 APPROVED THERAPIES FSHD is Often Diagnosed in Young Adults with Few Treatment Options There are no approved therapeutics for Facioscapulohumeral Muscular Dystrophy AFFECTS ~16,000 - 38,000 PEOPLE IN THE US One of the most common forms of muscular dystrophy Autosomal dominant disease caused by abnormal expression of double homeobox 4 (DUX4) Characterized by progressive, asymmetric skeletal muscle loss with onset often in teenage and adult years About 20% of patients will end up using a wheelchair
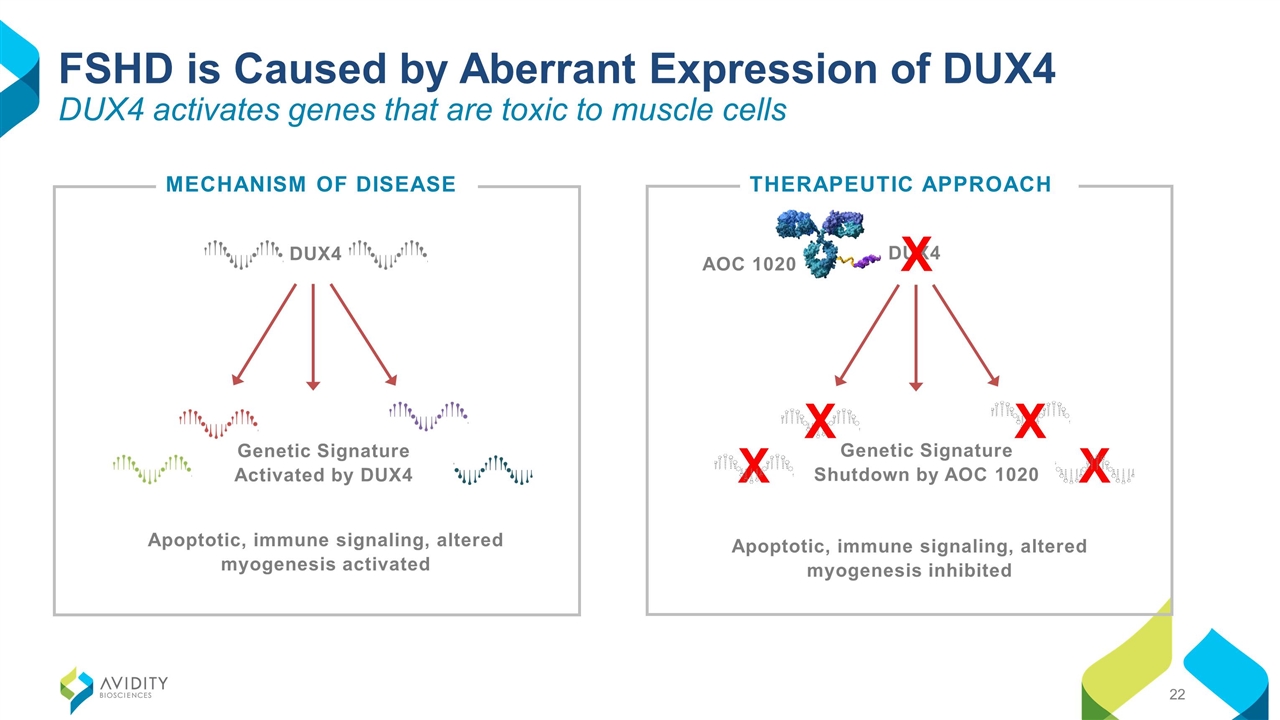
FSHD is Caused by Aberrant Expression of DUX4 DUX4 activates genes that are toxic to muscle cells MECHANISM OF DISEASE THERAPEUTIC APPROACH Apoptotic, immune signaling, altered myogenesis inhibited X X Genetic Signature Shutdown by AOC 1020 X X AOC 1020 DUX4 X Genetic Signature Activated by DUX4 Apoptotic, immune signaling, altered myogenesis activated DUX4
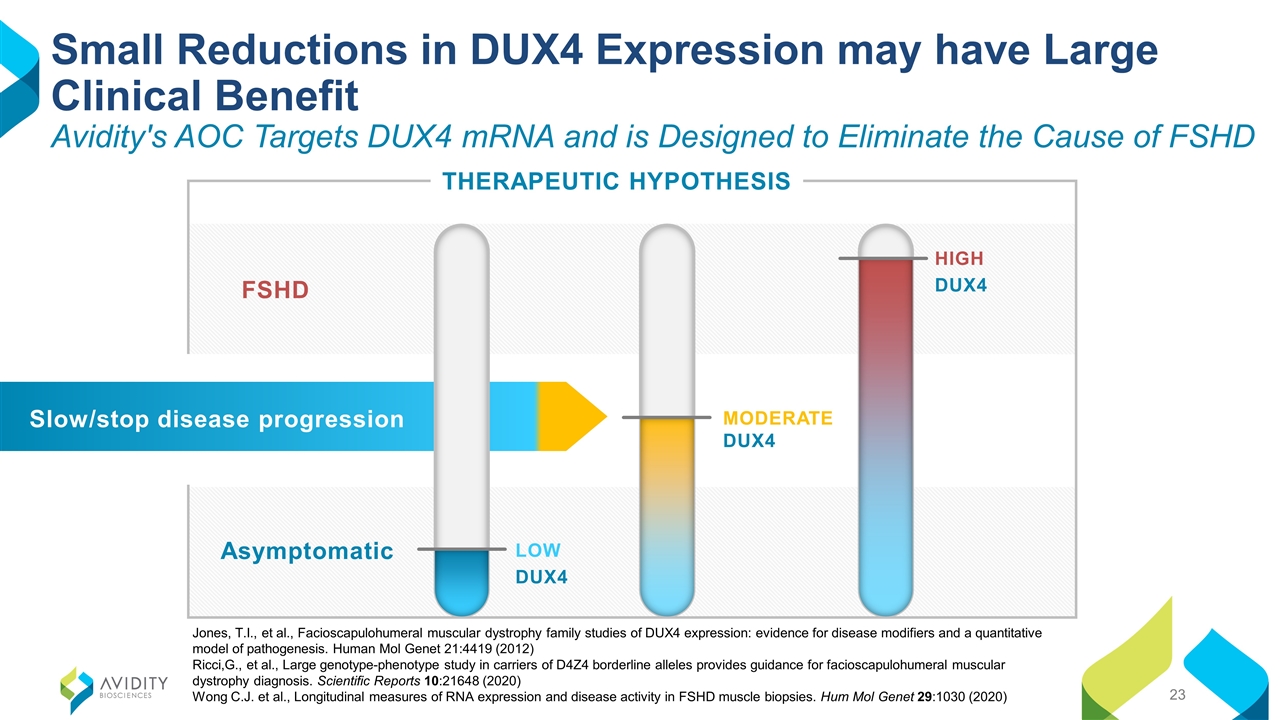
Small Reductions in DUX4 Expression may have Large Clinical Benefit Avidity's AOC Targets DUX4 mRNA and is Designed to Eliminate the Cause of FSHD THERAPEUTIC HYPOTHESIS Asymptomatic FSHD MODERATE DUX4 HIGH DUX4 LOW DUX4 Slow/stop disease progression Jones, T.I., et al., Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: evidence for disease modifiers and a quantitative model of pathogenesis. Human Mol Genet 21:4419 (2012) Ricci,G., et al., Large genotype-phenotype study in carriers of D4Z4 borderline alleles provides guidance for facioscapulohumeral muscular dystrophy diagnosis. Scientific Reports 10:21648 (2020) Wong C.J. et al., Longitudinal measures of RNA expression and disease activity in FSHD muscle biopsies. Hum Mol Genet 29:1030 (2020)

Delivering on AOCs: FSHD AOCs: Direct targeting of DUX4, the underlying cause of FSHD AOC 1020: Designed to be a potent DUX4 inhibitor moving into IND-enabling studies mAb OLIGO
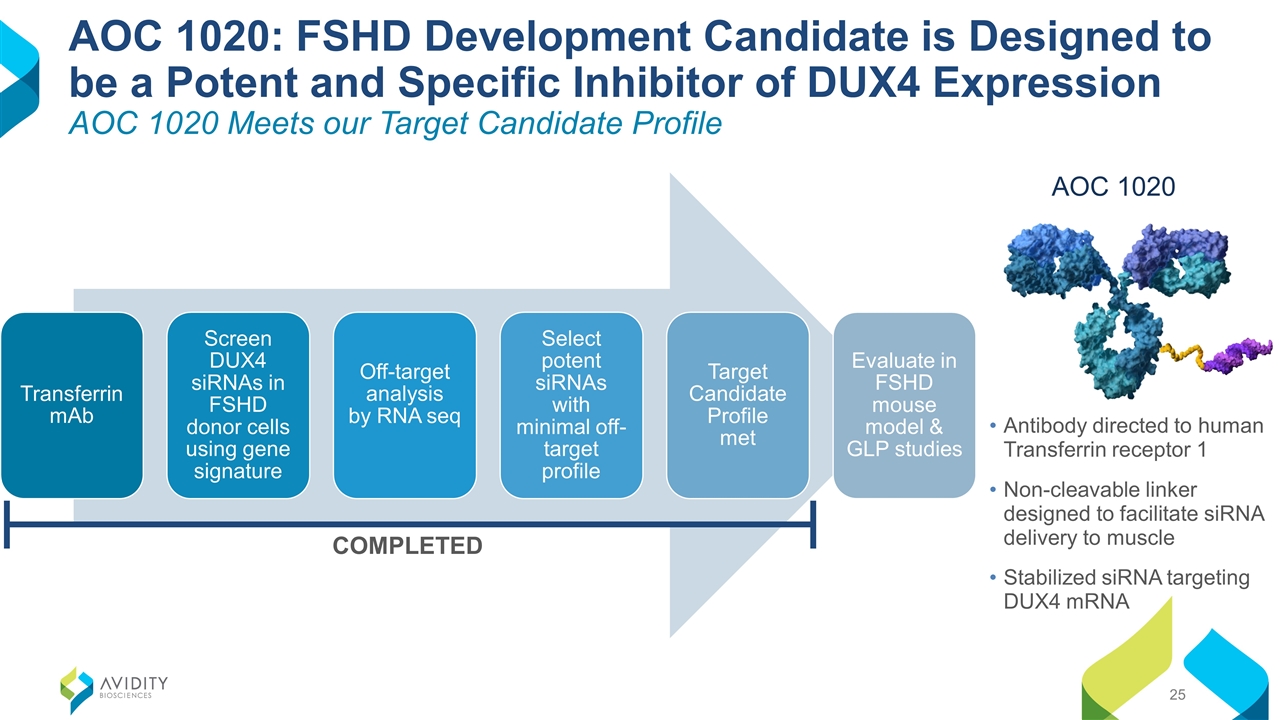
Antibody directed to human Transferrin receptor 1 Non-cleavable linker designed to facilitate siRNA delivery to muscle Stabilized siRNA targeting DUX4 mRNA AOC 1020: FSHD Development Candidate is Designed to be a Potent and Specific Inhibitor of DUX4 Expression AOC 1020 Meets our Target Candidate Profile AOC 1020 COMPLETED Transferrin mAb Screen DUX4 siRNAs in FSHD donor cells using gene signature Target Candidate Profile met Off-target analysis by RNA seq Select potent siRNAs with minimal off-target profile Evaluate in FSHD mouse model & GLP studies
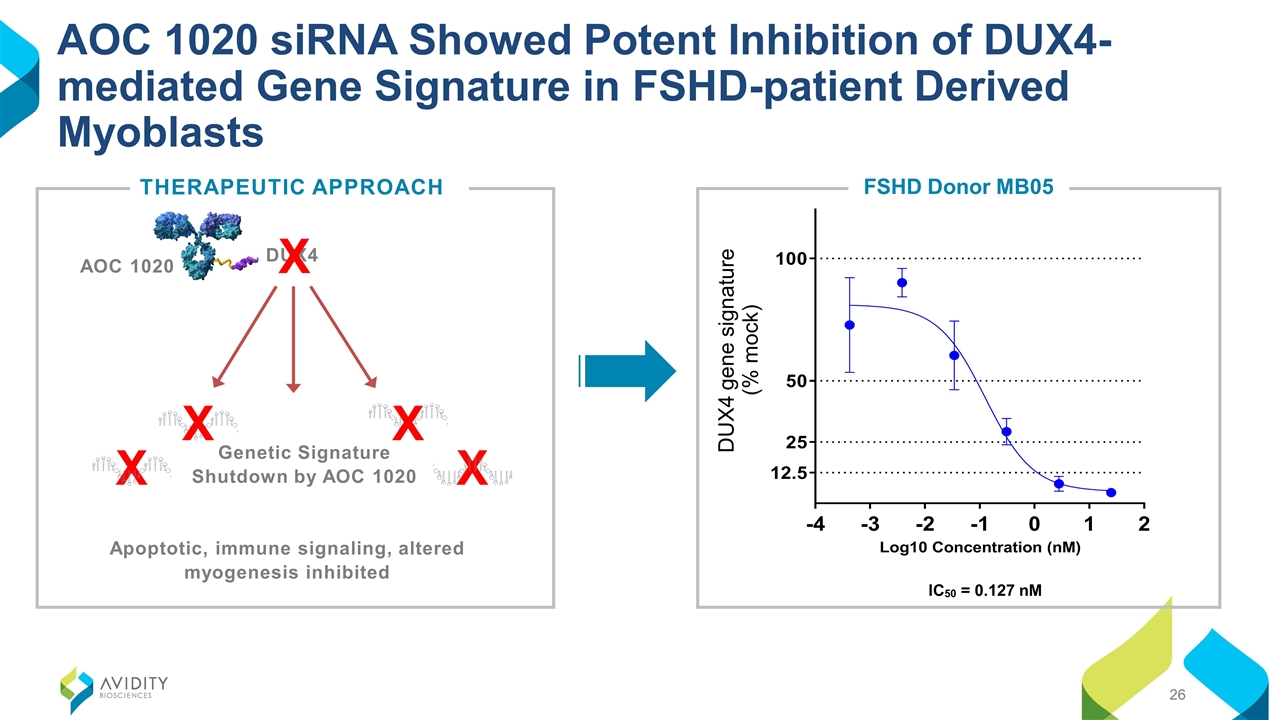
AOC 1020 siRNA Showed Potent Inhibition of DUX4-mediated Gene Signature in FSHD-patient Derived Myoblasts IC50 = 0.127 nM FSHD Donor MB05 DUX4 gene signature (% mock) THERAPEUTIC APPROACH Apoptotic, immune signaling, altered myogenesis inhibited X X Genetic Signature Shutdown by AOC 1020 X X AOC 1020 DUX4 X
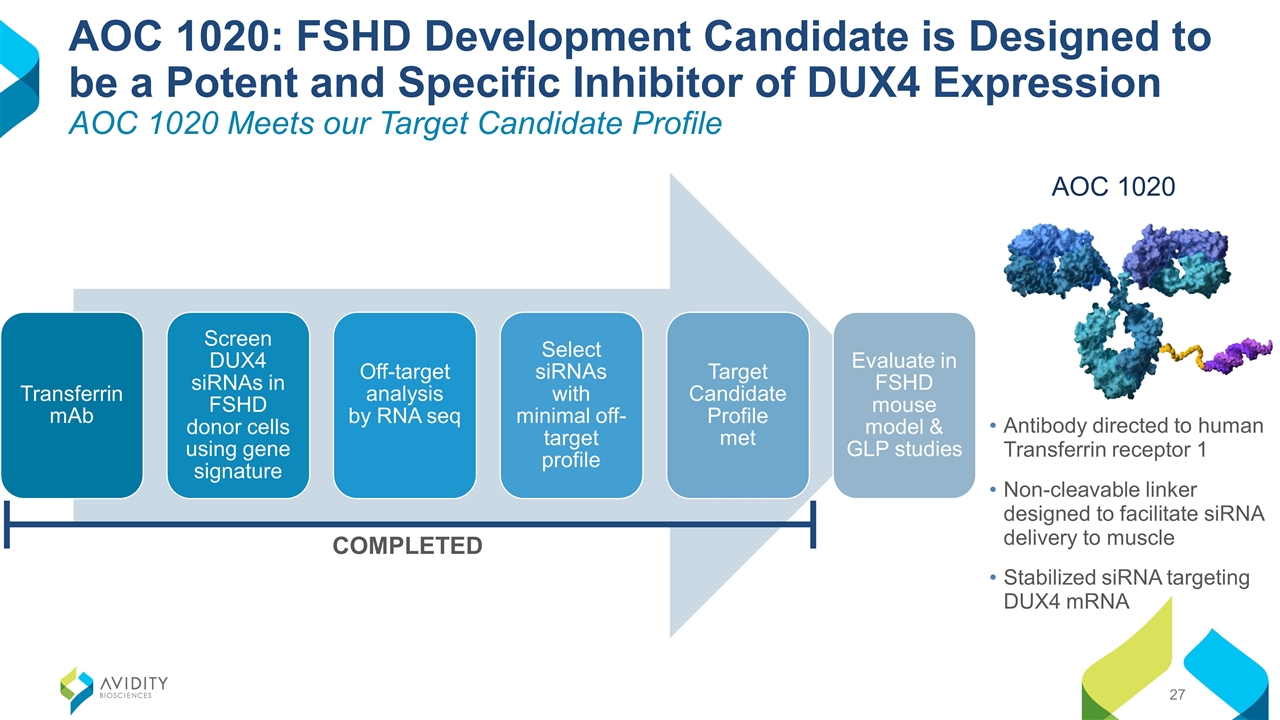
Antibody directed to human Transferrin receptor 1 Non-cleavable linker designed to facilitate siRNA delivery to muscle Stabilized siRNA targeting DUX4 mRNA AOC 1020: FSHD Development Candidate is Designed to be a Potent and Specific Inhibitor of DUX4 Expression AOC 1020 Meets our Target Candidate Profile AOC 1020 COMPLETED Transferrin mAb Screen DUX4 siRNAs in FSHD donor cells using gene signature Target Candidate Profile met Off-target analysis by RNA seq Select siRNAs with minimal off-target profile Evaluate in FSHD mouse model & GLP studies
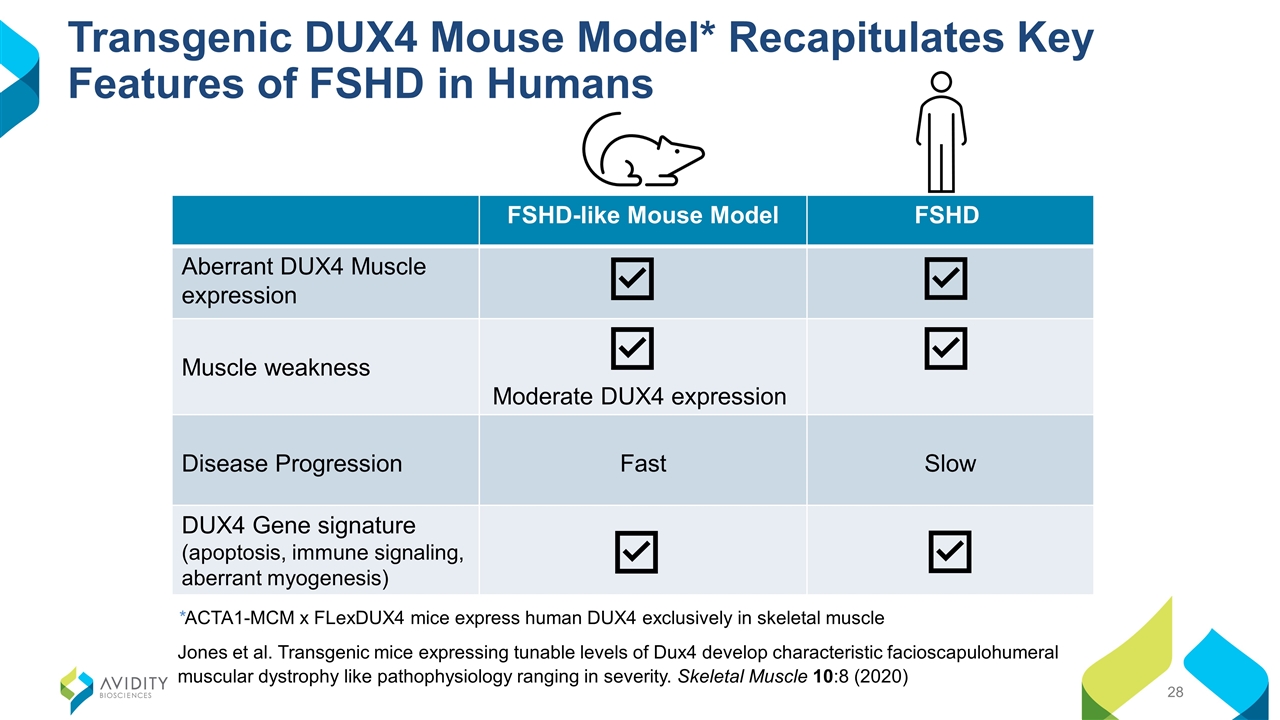
Transgenic DUX4 Mouse Model* Recapitulates Key Features of FSHD in Humans FSHD-like Mouse Model FSHD Aberrant DUX4 Muscle expression Muscle weakness Moderate DUX4 expression Disease Progression Fast Slow DUX4 Gene signature (apoptosis, immune signaling, aberrant myogenesis) *ACTA1-MCM x FLexDUX4 mice express human DUX4 exclusively in skeletal muscle Jones et al. Transgenic mice expressing tunable levels of Dux4 develop characteristic facioscapulohumeral muscular dystrophy like pathophysiology ranging in severity. Skeletal Muscle 10:8 (2020)
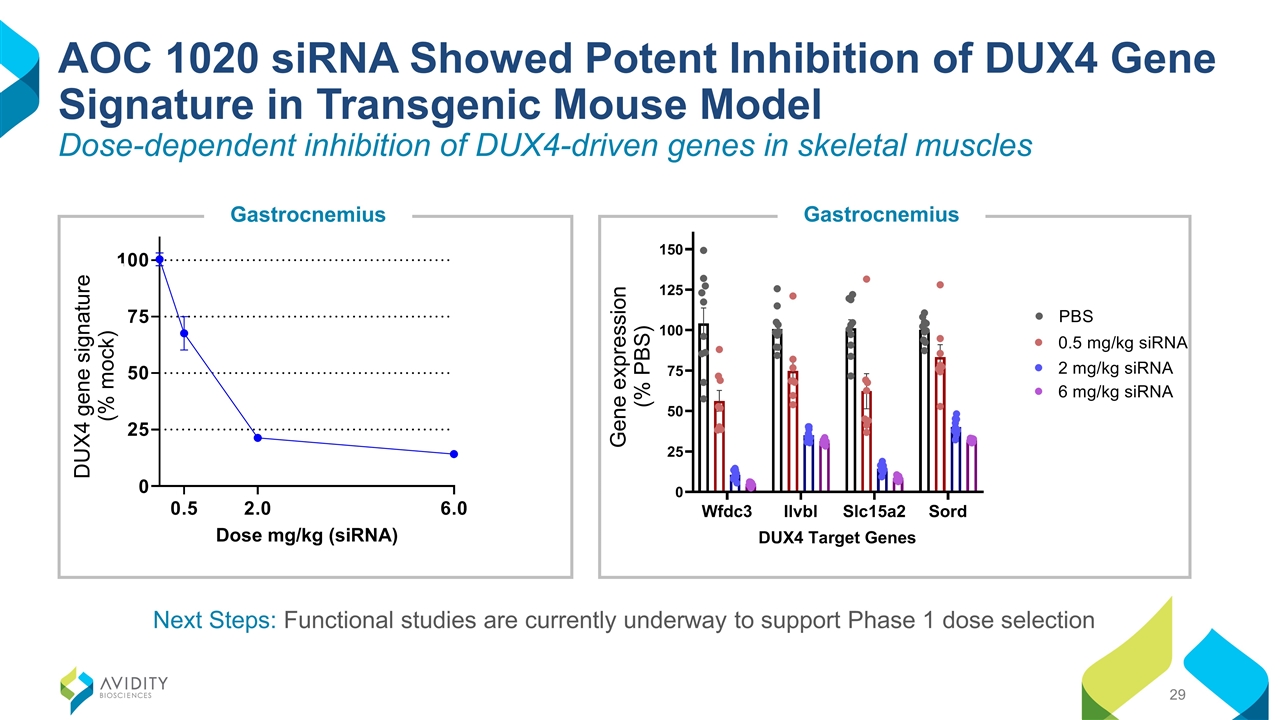
AOC 1020 siRNA Showed Potent Inhibition of DUX4 Gene Signature in Transgenic Mouse Model Dose-dependent inhibition of DUX4-driven genes in skeletal muscles Next Steps: Functional studies are currently underway to support Phase 1 dose selection Gastrocnemius Gastrocnemius DUX4 gene signature (% mock) Gene expression (% PBS)
AOC 1020 Nonclinical Activities Progressing Well to Support 2022 IND Filing No dose limiting toxicity observed in monkeys at the highest dose tested based on in-life safety assessments Supports study design for GLP toxicology studies AOC 1020 PK results consistent with anticipated exposure based on our AOC platform knowledge PK results in plasma and muscle tissue potentially support infrequent dosing AOC 1020 well tolerated in cynomolgus monkey PKPD study following single and repeat dosing Summary of non-GLP NHP studies:
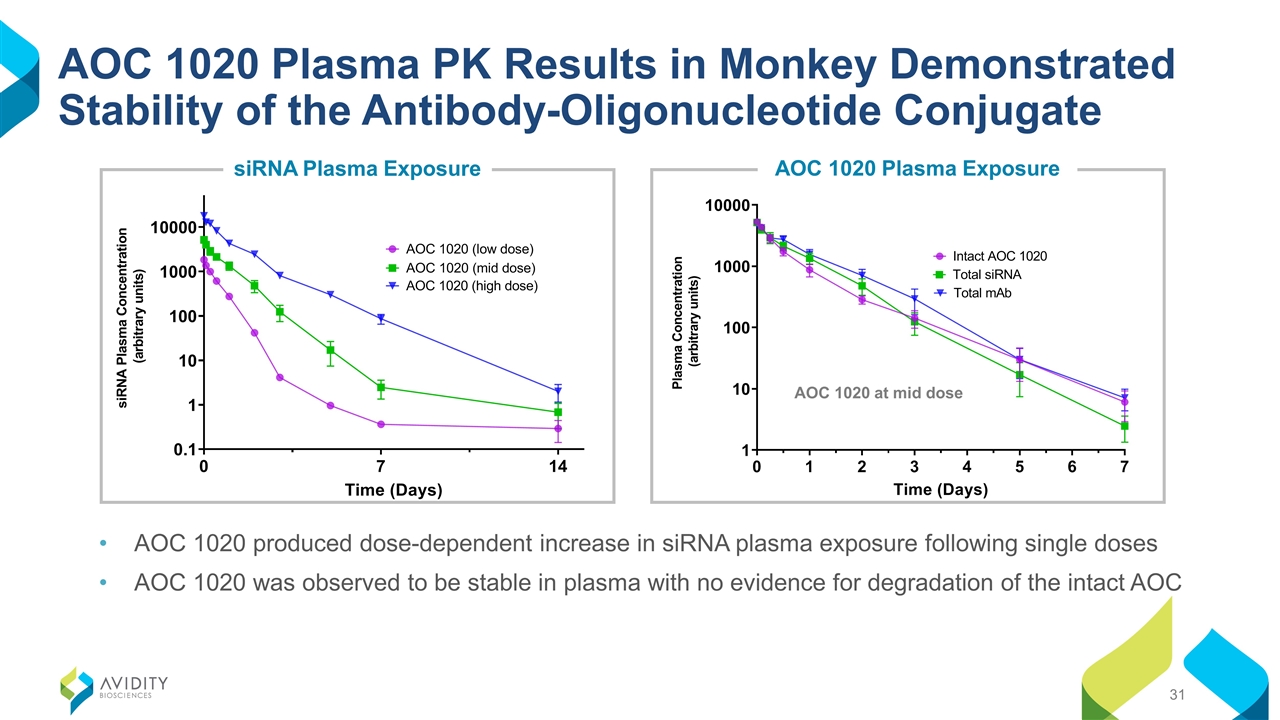
AOC 1020 Plasma PK Results in Monkey Demonstrated Stability of the Antibody-Oligonucleotide Conjugate AOC 1020 produced dose-dependent increase in siRNA plasma exposure following single doses AOC 1020 was observed to be stable in plasma with no evidence for degradation of the intact AOC AOC 1020 at mid dose siRNA Plasma Exposure AOC 1020 Plasma Exposure
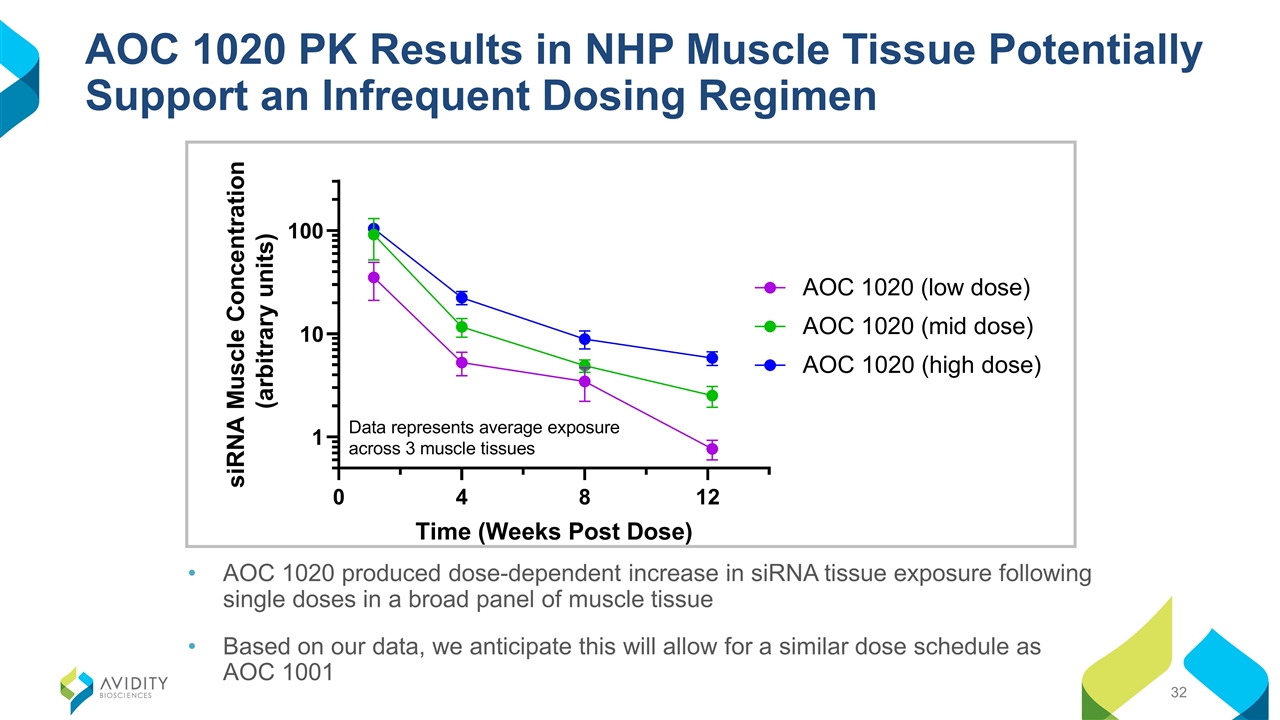
AOC 1020 PK Results in NHP Muscle Tissue Potentially Support an Infrequent Dosing Regimen AOC 1020 produced dose-dependent increase in siRNA tissue exposure following single doses in a broad panel of muscle tissue Based on our data, we anticipate this will allow for a similar dose schedule as AOC 1001

APPROACHING THE CLINIC Planned clinical trial initiation by the end of 2022 DELIVERING ON FSHD Observed reduced expression of key DUX4 gene signature in FSHD patient myotubes and in FSHD mouse disease model Well tolerated in cynomolgus monkey preclinical studies at all doses and dose frequencies tested Entering IND-enabling studies Supporting ongoing natural history study by FSHD Clinical Trial Research Network AOC 1020, Designed to be a Potent and Specific DUX4 Inhibitor Directly Targeting the Underlying Cause of FSHD

Agenda Sarah Boyce, President & CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology University of Kansas Medical Center Sarah Boyce, President and CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology Mike MacLean, CFO (Moderator) Sarah Boyce, President & CEO Welcome & Introduction Building on our AOC Expertise Facioscapulohumeral Muscular Dystrophy (FSHD) Program FSHD: Disease Overview and Endpoints Q&A Session Closing Remarks

Jeffrey M. Statland, M.D. Professor of Neurology, University of Kansas Medical Center in Kansas City, Kansas

Jeffrey M. Statland, M.D. is a Professor of Neurology at the University of Kansas Medical Center in Kansas City, Kansas. His research background has centered primarily on describing the natural history of and response to therapy for neuromuscular diseases. Dr. Statland completed a neuromuscular fellowship in Experimental Therapeutics of Neurological Diseases at the University of Rochester Medical Center and currently serves as principal investigator or co-investigator for research studies in Facioscapulohumeral Muscular Dystrophy (FSHD), Duchenne Muscular Dystrophy, Spinal Muscular Atrophy, and Myotonic Dystrophy. His specific research interest over the last 6 years has been preparing for clinical trials in FSHD. Dr. Statland has systematically analyzed the performance of strength and functional outcomes in prior FSHD clinical trials and compared to performance in a natural history study. He has worked with collaborators to develop new disease-relevant outcome measures to assess patient-reported disease burden, functional impairment, and physiological changes in muscle. Dr. Statland has obtained pilot data on the use of a number of novel outcomes for FSHD, including electrical impedance myography, a disease-specific functional rating scale, and a wireless motion analysis system in FSHD. Jeffrey M. Statland, M.D. Professor of Neurology at the University of Kansas Medical Center
FSHD: Disease Overview and Endpoints Avidity, December 2021 Jeffrey Statland, MD Professor of Neurology, University of Kansas Medical Center J Biol Chem. 2018 Jul 27;293(30):11837-11849.
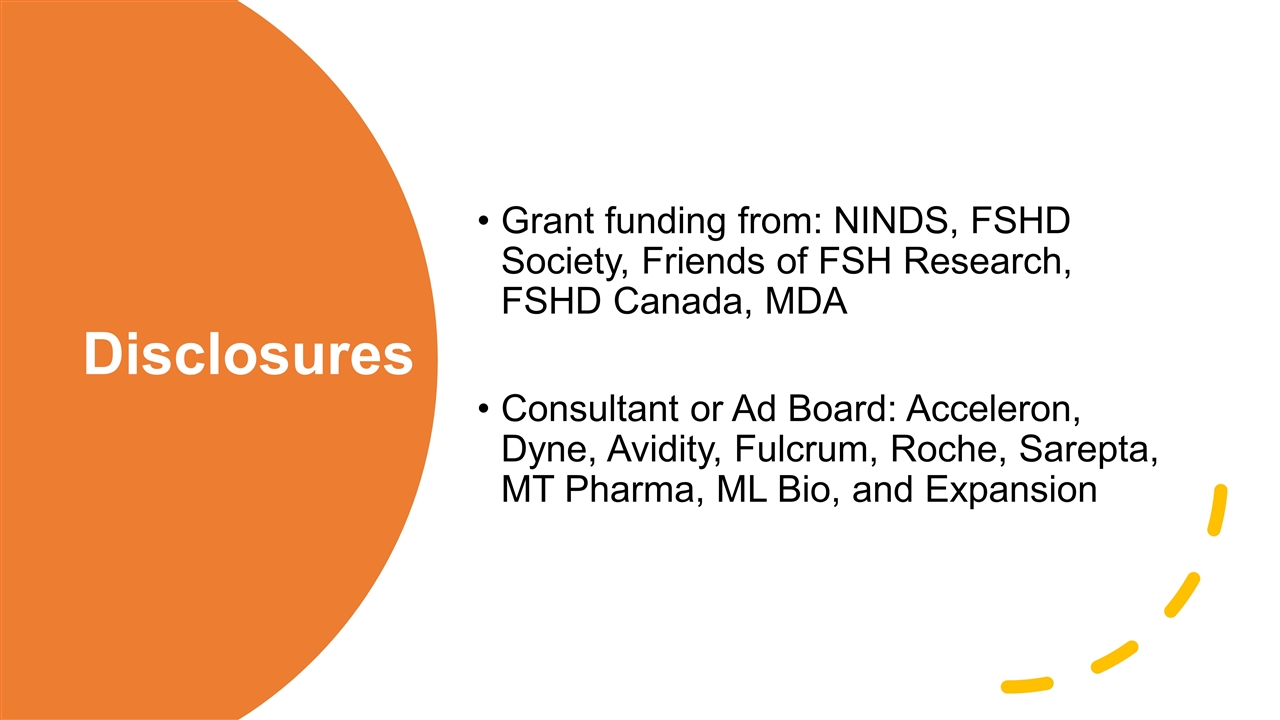
Disclosures Grant funding from: NINDS, FSHD Society, Friends of FSH Research, FSHD Canada, MDA Consultant or Ad Board: Acceleron, Dyne, Avidity, Fulcrum, Roche, Sarepta, MT Pharma, ML Bio, and Expansion
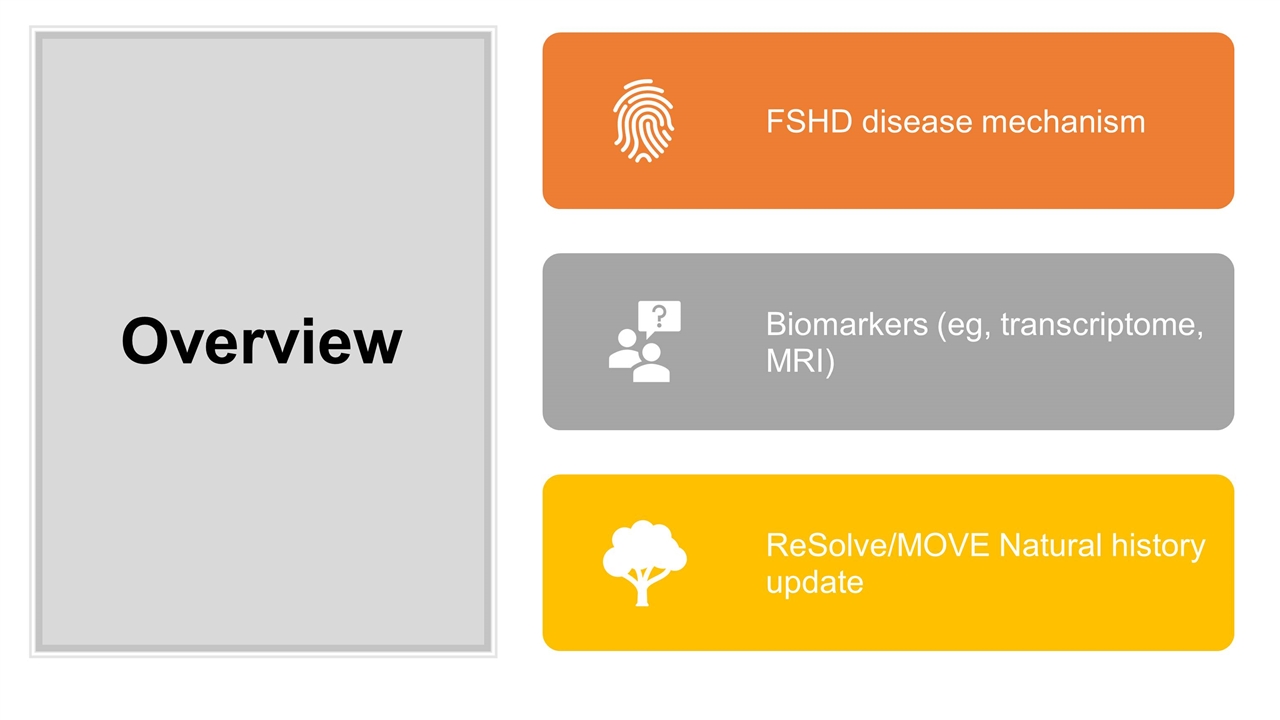
Overview FSHD disease mechanism Biomarkers ( eg , transcriptome, MRI) ReSolve /MOVE Natural history update

FSHD One of the most common muscular dystrophies Worldwide Prevalence 2-7/100,000 ~ 21,000 in US No clear racial or ethnic predispositions Can lead to significant morbidity ~20% becoming disabled or requiring full-time wheelchair use Diagnosis is based on characteristic clinical presentation and genetic testing Lemmers RJ, et al. Am J Hum Genet 2010;86:364-377 Deenen JCW, et al. Journal of Neuromuscular Diseases 2015;2:73-85.
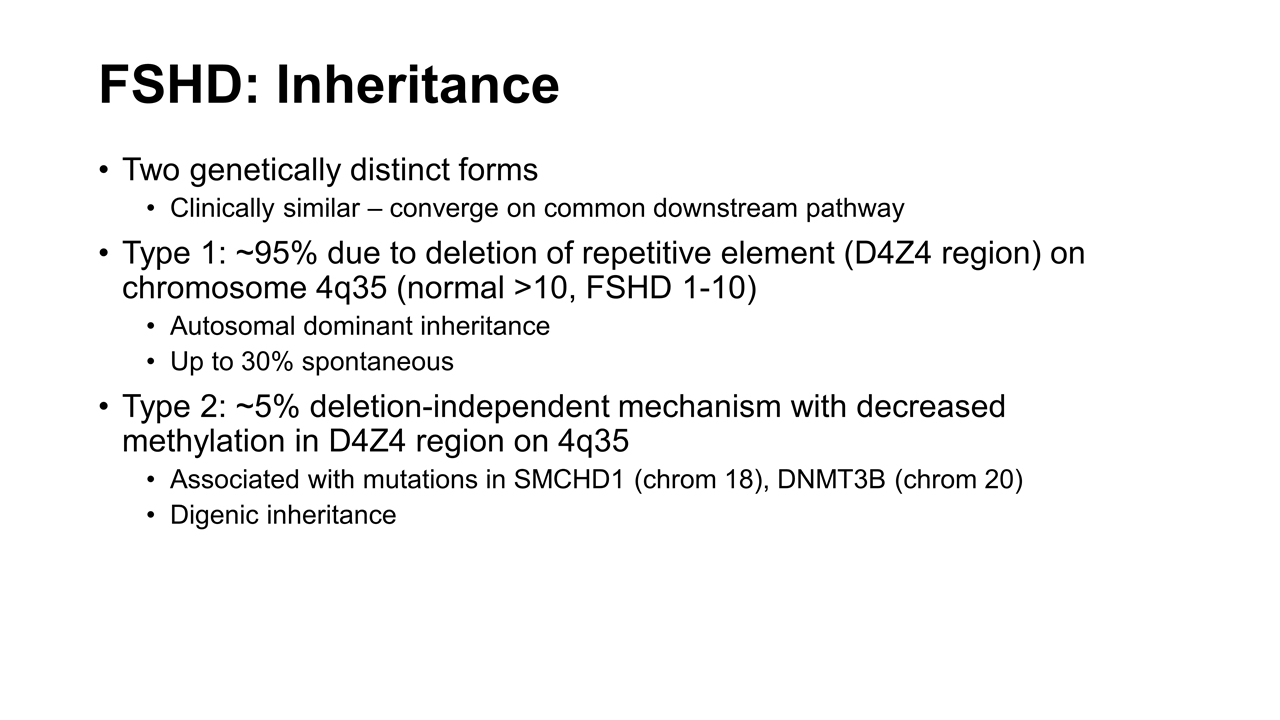
FSHD: Inheritance Two genetically distinct forms Clinically similar – converge on common downstream pathway Type 1: ~95% due to deletion of repetitive element (D4Z4 region) on chromosome 4q35 (normal >10, FSHD 1-10) Autosomal dominant inheritance Up to 30% spontaneous Type 2: ~5% deletion-independent mechanism with decreased methylation in D4Z4 region on 4q35 Associated with mutations in SMCHD1 (chrom 18), DNMT3B (chrom 20) Digenic inheritance
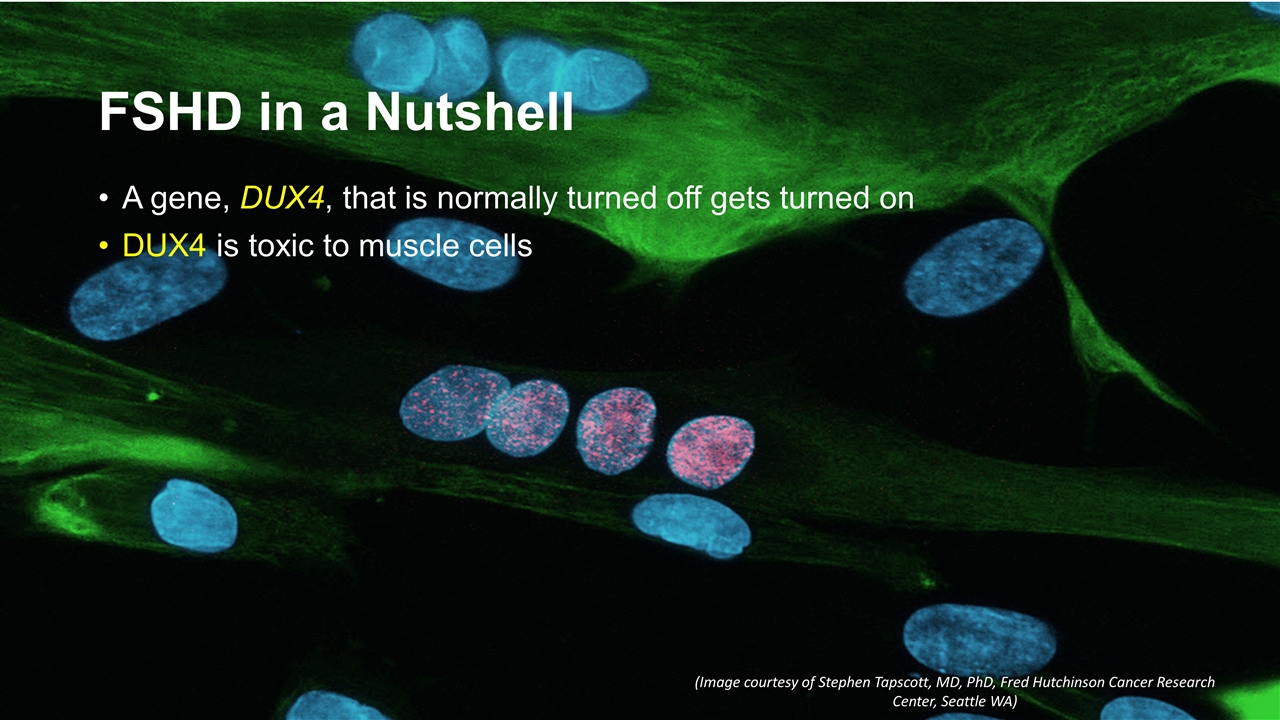
FSHD in a Nutshell A gene, DUX4, that is normally turned off gets turned on DUX4 is toxic to muscle cells (Image courtesy of Stephen Tapscott, MD, PhD, Fred Hutchinson Cancer Research Center, Seattle WA)
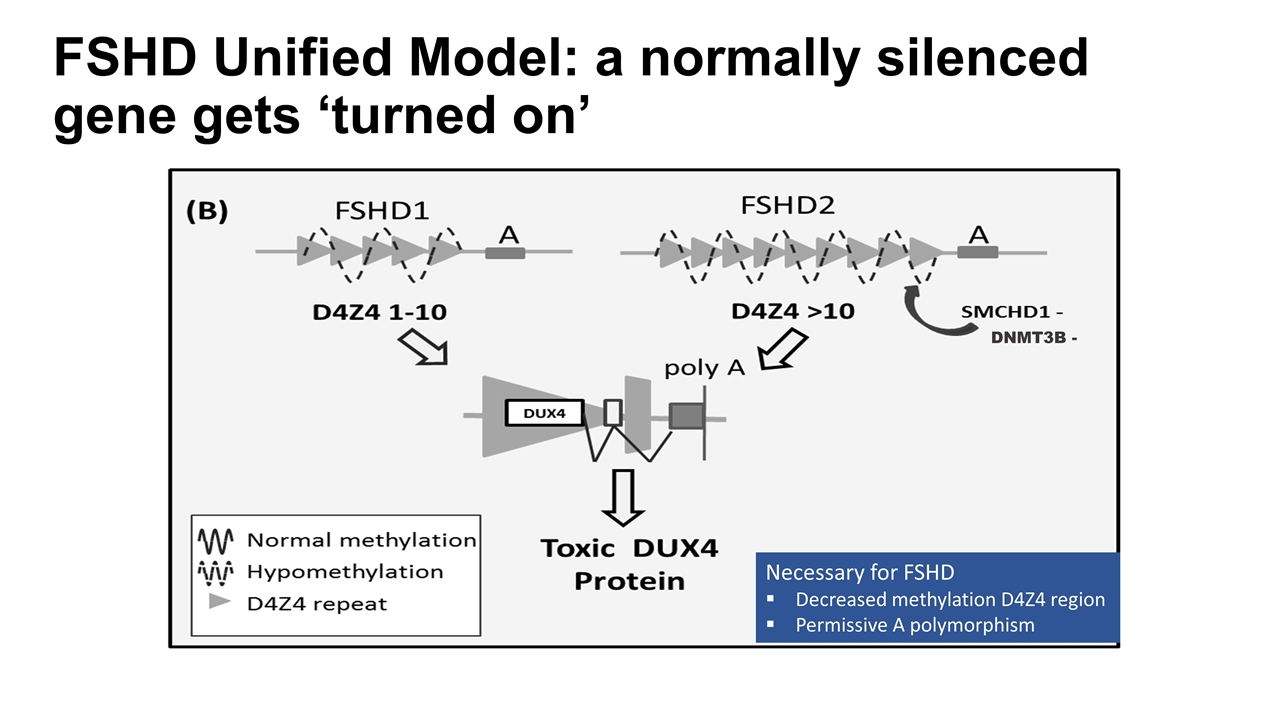
FSHD Unified Model: a normally silenced gene gets ‘turned on’ DNMT3B - Necessary for FSHD Decreased methylation D4Z4 region Permissive A polymorphism
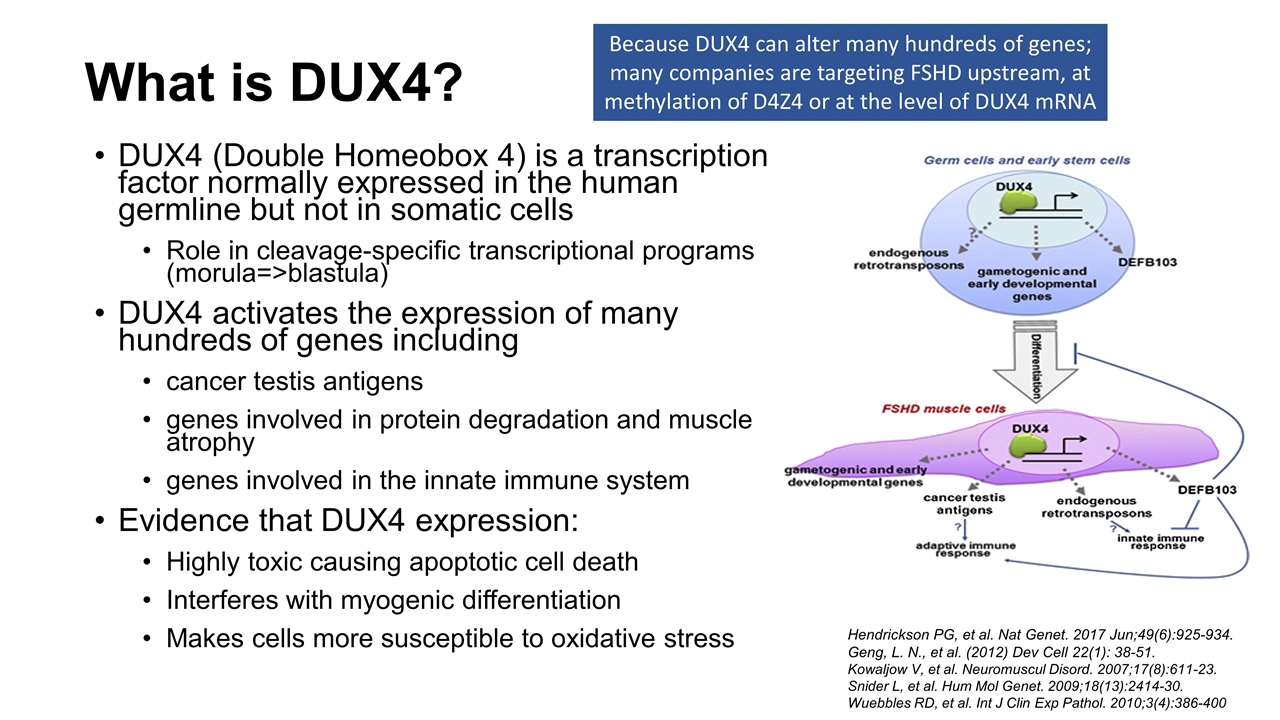
What is DUX4? DUX4 (Double Homeobox 4) is a transcription factor normally expressed in the human germline but not in somatic cells Role in cleavage-specific transcriptional programs (morula=>blastula) DUX4 activates the expression of many hundreds of genes including cancer testis antigens genes involved in protein degradation and muscle atrophy genes involved in the innate immune system Evidence that DUX4 expression: Highly toxic causing apoptotic cell death Interferes with myogenic differentiation Makes cells more susceptible to oxidative stress Hendrickson PG, et al. Nat Genet. 2017 Jun;49(6):925-934. Geng, L. N., et al. (2012) Dev Cell 22(1): 38-51. Kowaljow V, et al. Neuromuscul Disord. 2007;17(8):611-23. Snider L, et al. Hum Mol Genet. 2009;18(13):2414-30. Wuebbles RD, et al. Int J Clin Exp Pathol. 2010;3(4):386-400 Because DUX4 can alter many hundreds of genes; many companies are targeting FSHD upstream, at methylation of D4Z4 or at the level of DUX4 mRNA
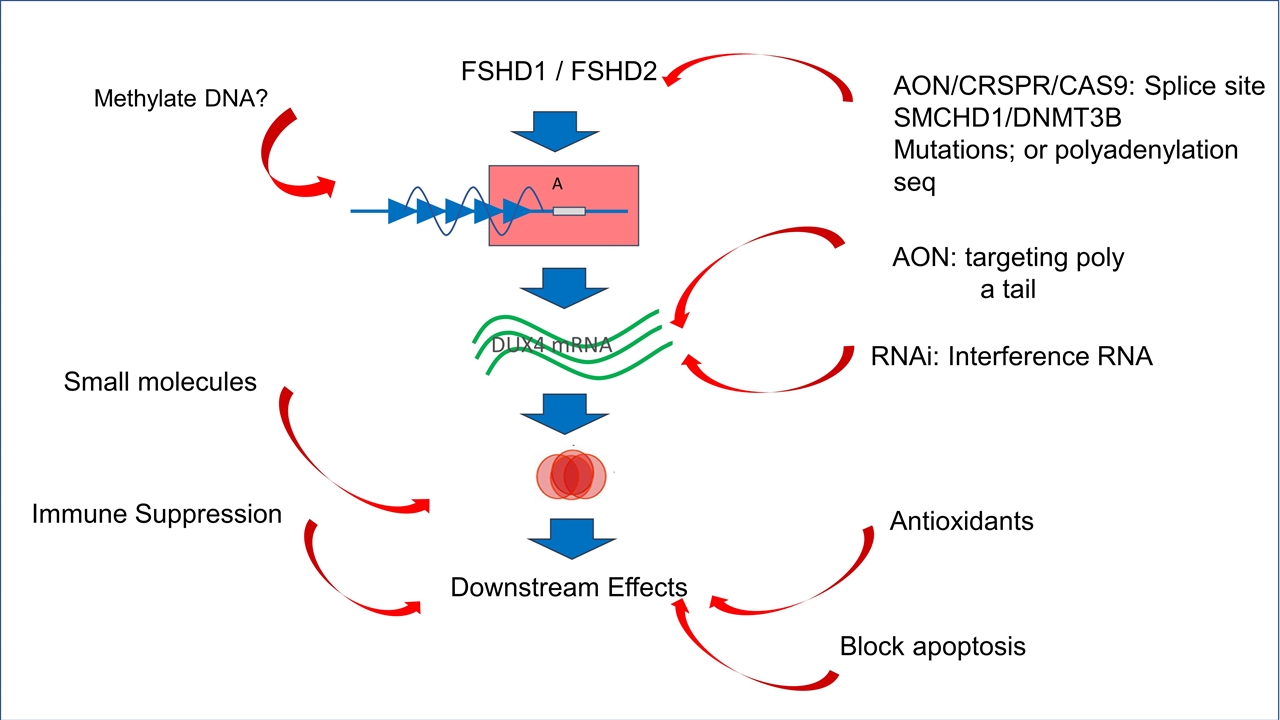
A AON/CRSPR/CAS9: Splice site SMCHD1/DNMT3B Mutations; or polyadenylation seq RNAi: Interference RNA Downstream Effects Immune Suppression Antioxidants Methylate DNA? Block apoptosis FSHD1 / FSHD2 AON: targeting poly a tail Small molecules DUX4 mRNA
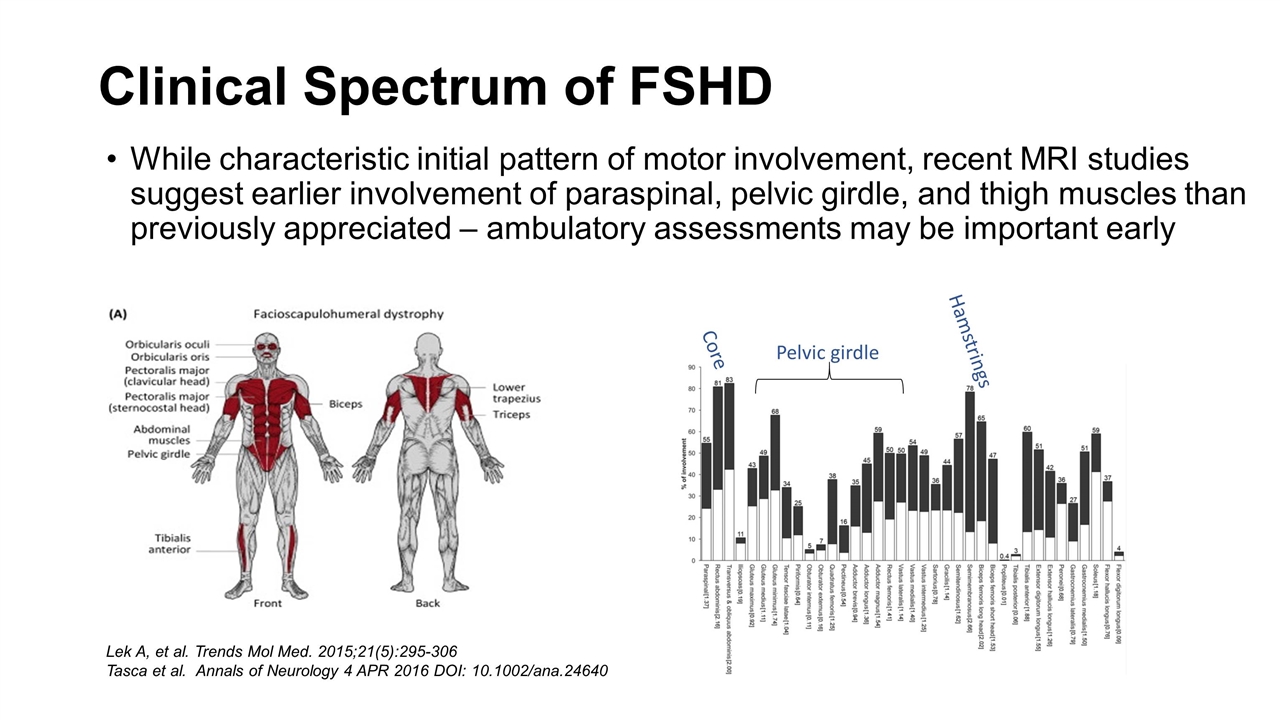
While characteristic initial pattern of motor involvement, recent MRI studies suggest earlier involvement of paraspinal, pelvic girdle, and thigh muscles than previously appreciated – ambulatory assessments may be important early Clinical Spectrum of FSHD Lek A, et al. Trends Mol Med. 2015;21(5):295-306 Tasca et al. Annals of Neurology 4 APR 2016 DOI: 10.1002/ana.24640 Hamstrings Pelvic girdle Core
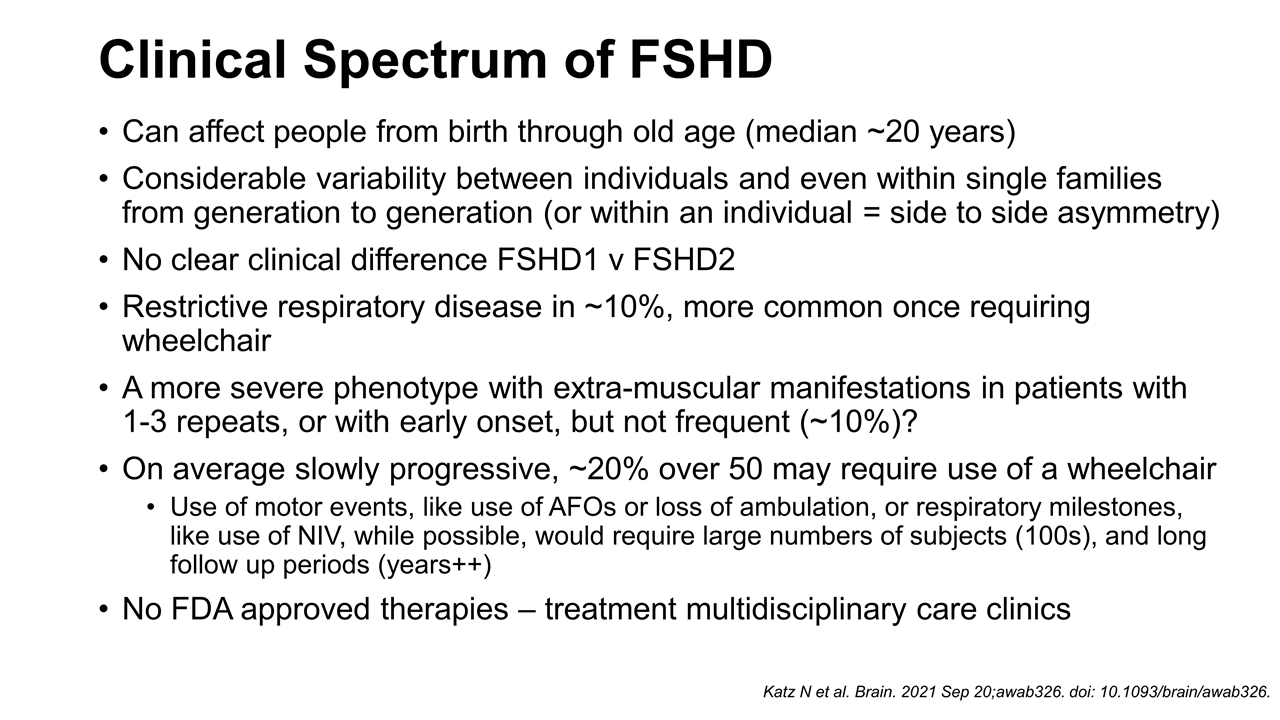
Can affect people from birth through old age (median ~20 years) Considerable variability between individuals and even within single families from generation to generation (or within an individual = side to side asymmetry) No clear clinical difference FSHD1 v FSHD2 Restrictive respiratory disease in ~10%, more common once requiring wheelchair A more severe phenotype with extra-muscular manifestations in patients with 1-3 repeats, or with early onset, but not frequent (~10%)? On average slowly progressive, ~20% over 50 may require use of a wheelchair Use of motor events, like use of AFOs or loss of ambulation, or respiratory milestones, like use of NIV, while possible, would require large numbers of subjects (100s), and long follow up periods (years++) No FDA approved therapies – treatment multidisciplinary care clinics Clinical Spectrum of FSHD Katz N et al. Brain. 2021 Sep 20;awab326. doi: 10.1093/brain/awab326.
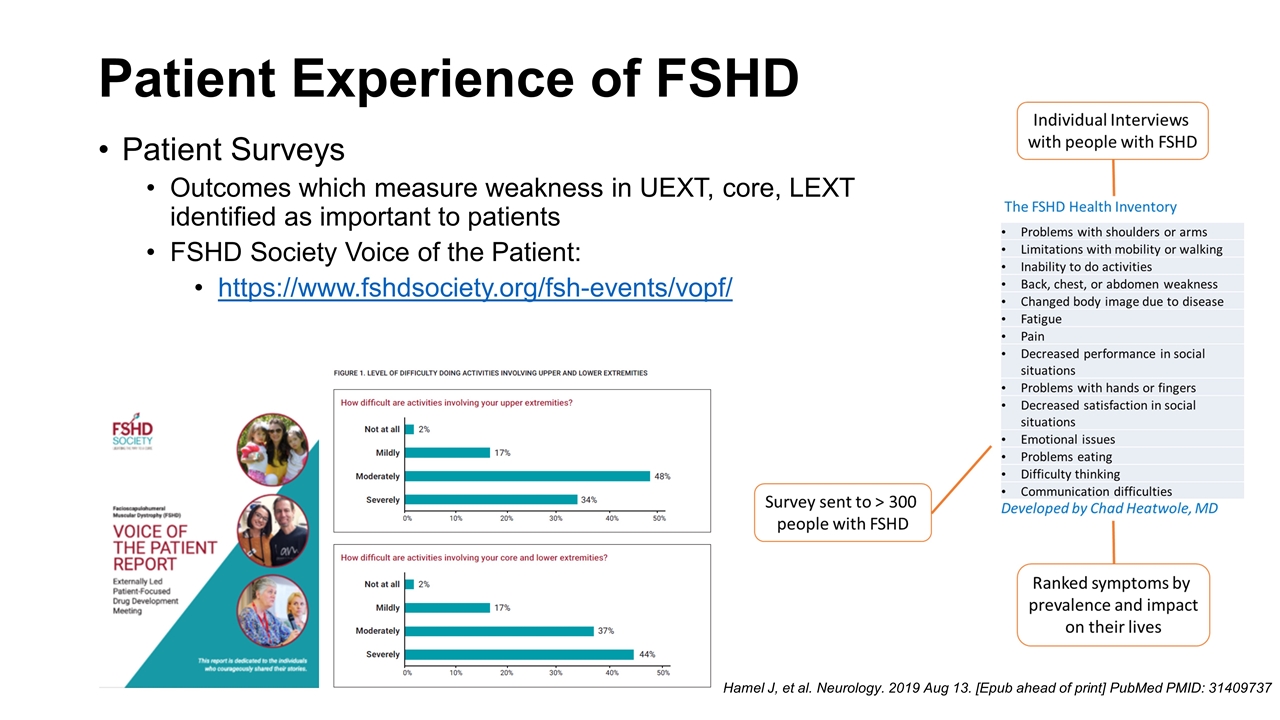
Patient Surveys Outcomes which measure weakness in UEXT, core, LEXT identified as important to patients FSHD Society Voice of the Patient: https://www.fshdsociety.org/fsh-events/vopf/ Patient Experience of FSHD Hamel J, et al. Neurology. 2019 Aug 13. [Epub ahead of print] PubMed PMID: 31409737
Overview FSHD disease mechanism Biomarkers ( eg , transcriptome, MRI) ReSolve /MOVE Natural history update
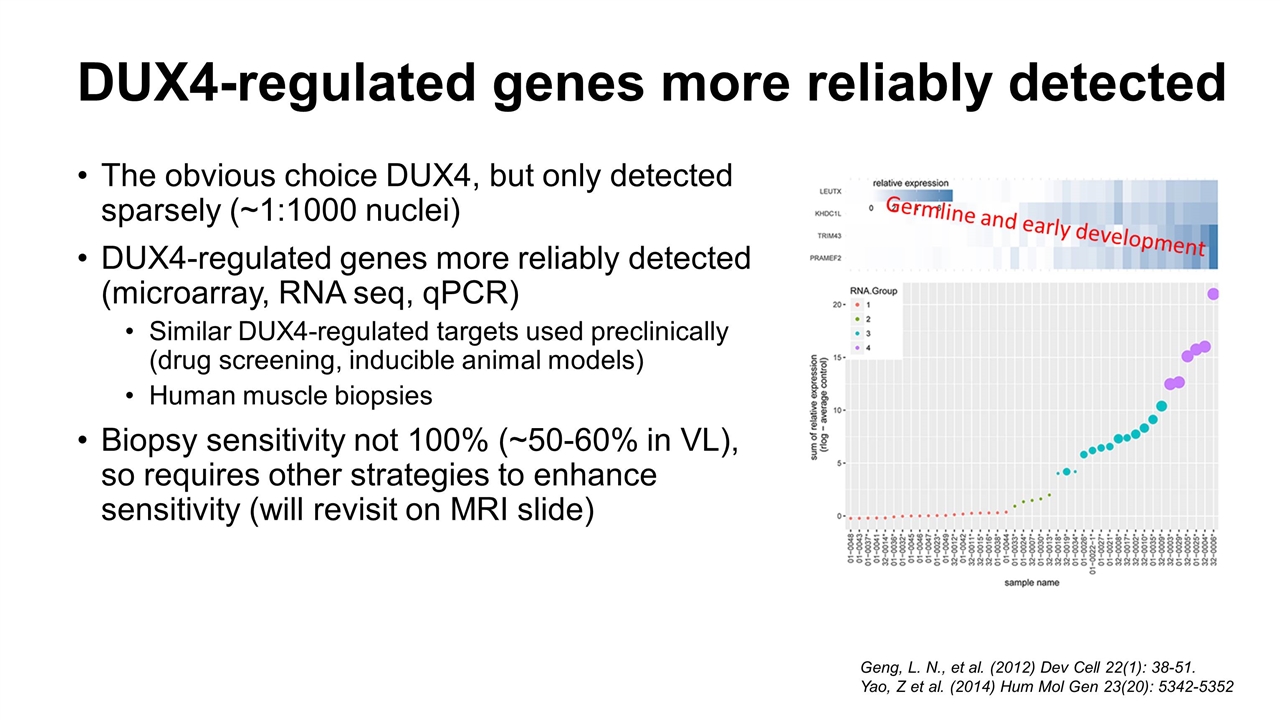
DUX4-regulated genes more reliably detected The obvious choice DUX4, but only detected sparsely (~1:1000 nuclei) DUX4-regulated genes more reliably detected (microarray, RNA seq, qPCR) Similar DUX4-regulated targets used preclinically (drug screening, inducible animal models) Human muscle biopsies Biopsy sensitivity not 100% (~50-60% in VL), so requires other strategies to enhance sensitivity (will revisit on MRI slide) Geng, L. N., et al. (2012) Dev Cell 22(1): 38-51. Yao, Z et al. (2014) Hum Mol Gen 23(20): 5342-5352
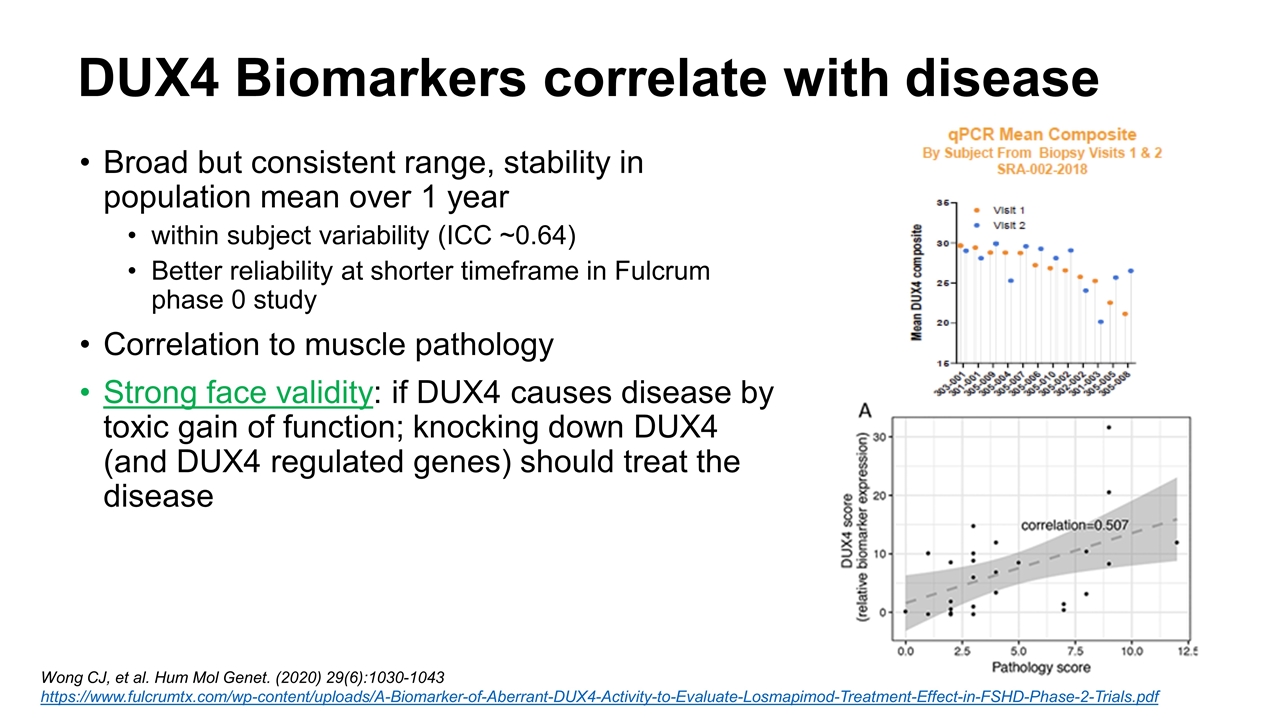
DUX4 Biomarkers correlate with disease Broad but consistent range, stability in population mean over 1 year within subject variability (ICC ~0.64) Better reliability at shorter timeframe in Fulcrum phase 0 study Correlation to muscle pathology Strong face validity: if DUX4 causes disease by toxic gain of function; knocking down DUX4 (and DUX4 regulated genes) should treat the disease Wong CJ, et al. Hum Mol Genet. (2020) 29(6):1030-1043 https://www.fulcrumtx.com/wp-content/uploads/A-Biomarker-of-Aberrant-DUX4-Activity-to-Evaluate-Losmapimod-Treatment-Effect-in-FSHD-Phase-2-Trials.pdf
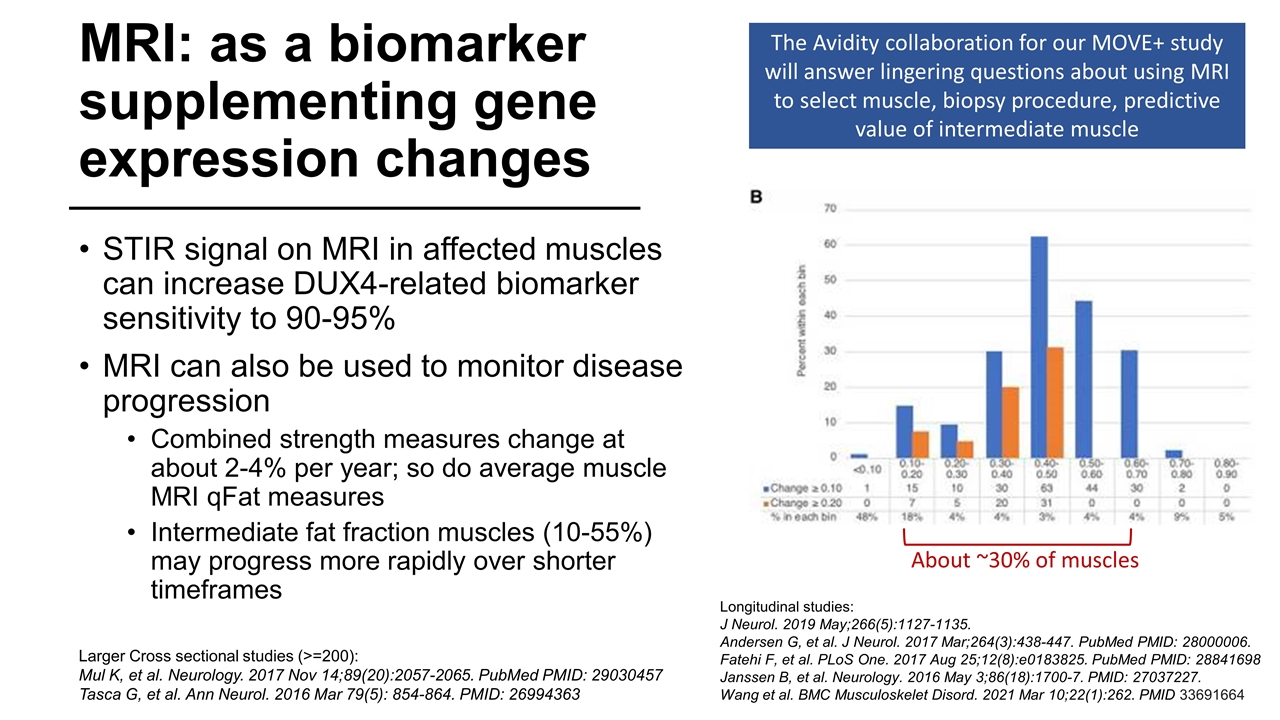
MRI: as a biomarker supplementing gene expression changes STIR signal on MRI in affected muscles can increase DUX4-related biomarker sensitivity to 90-95% MRI can also be used to monitor disease progression Combined strength measures change at about 2-4% per year; so do average muscle MRI qFat measures Intermediate fat fraction muscles (10-55%) may progress more rapidly over shorter timeframes Longitudinal studies: J Neurol. 2019 May;266(5):1127-1135. Andersen G, et al. J Neurol. 2017 Mar;264(3):438-447. PubMed PMID: 28000006. Fatehi F, et al. PLoS One. 2017 Aug 25;12(8):e0183825. PubMed PMID: 28841698 Janssen B, et al. Neurology. 2016 May 3;86(18):1700-7. PMID: 27037227. Wang et al. BMC Musculoskelet Disord. 2021 Mar 10;22(1):262. PMID 33691664 Larger Cross sectional studies (>=200): Mul K, et al. Neurology. 2017 Nov 14;89(20):2057-2065. PubMed PMID: 29030457 Tasca G, et al. Ann Neurol. 2016 Mar 79(5): 854-864. PMID: 26994363 The Avidity collaboration for our MOVE+ study will answer lingering questions about using MRI to select muscle, biopsy procedure, predictive value of intermediate muscle About ~30% of muscles
Overview FSHD disease mechanism Biomarkers ( eg , transcriptome, MRI) ReSolve /MOVE Natural history update

Prior Natural History Study (1997) Natural history study (n=81 subjects, up to 3 years) Two methods used widely in FSHD Manual Muscle Testing (MMT) can include many muscles, weak muscles Quantitative Myometry (QMT) continuous measure, can be normalized MMT used in FDA approval of deflazacort for DMD Very good to excellent inter and intra-rater (test-retest) reliability Sensitive to change at 1 year for both QMT/MMT Approximately 2-4% loss of strength per year If strength as outcome 160 per group to stop progression 40 per group for effect double that size Personius KE, et al. The FSH DY Group. Phys Ther. 1994 Mar;74(3):253-63. The FSH-DY Group. Neurology. 1997;48(1):38-46.
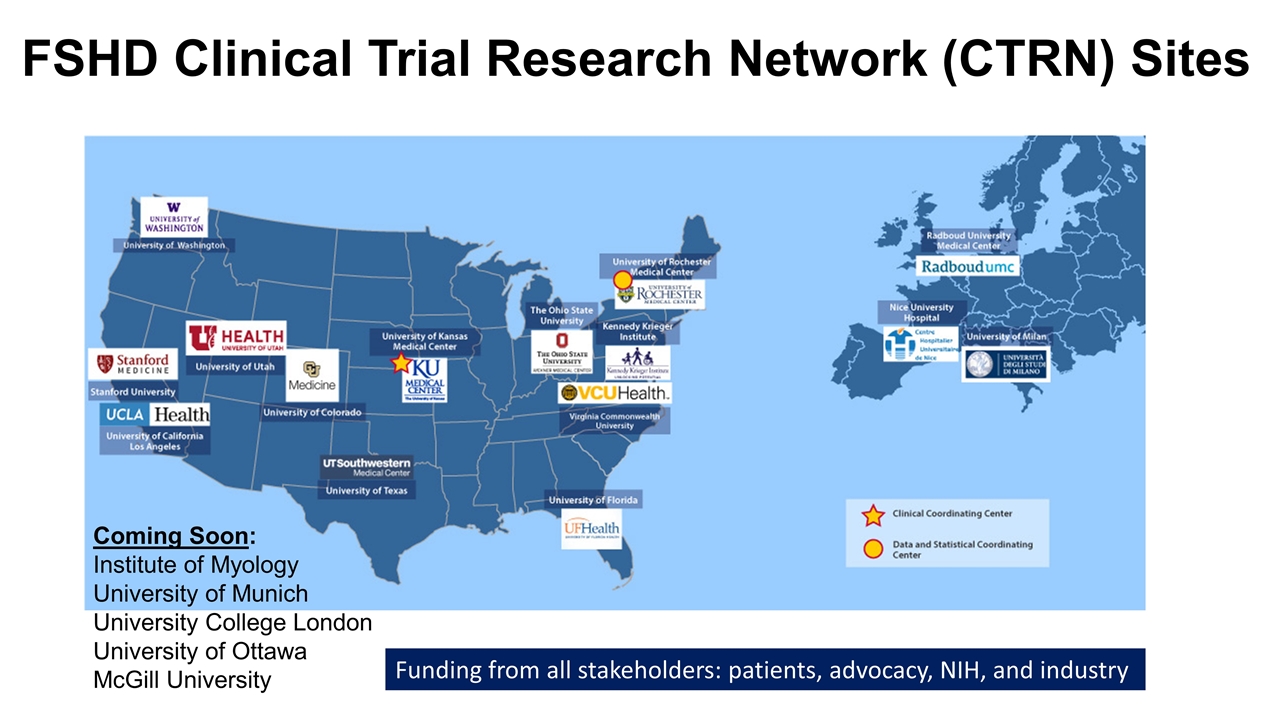
FSHD Clinical Trial Research Network (CTRN) Sites Coming Soon: Institute of Myology University of Munich University College London University of Ottawa McGill University Funding from all stakeholders: patients, advocacy, NIH, and industry
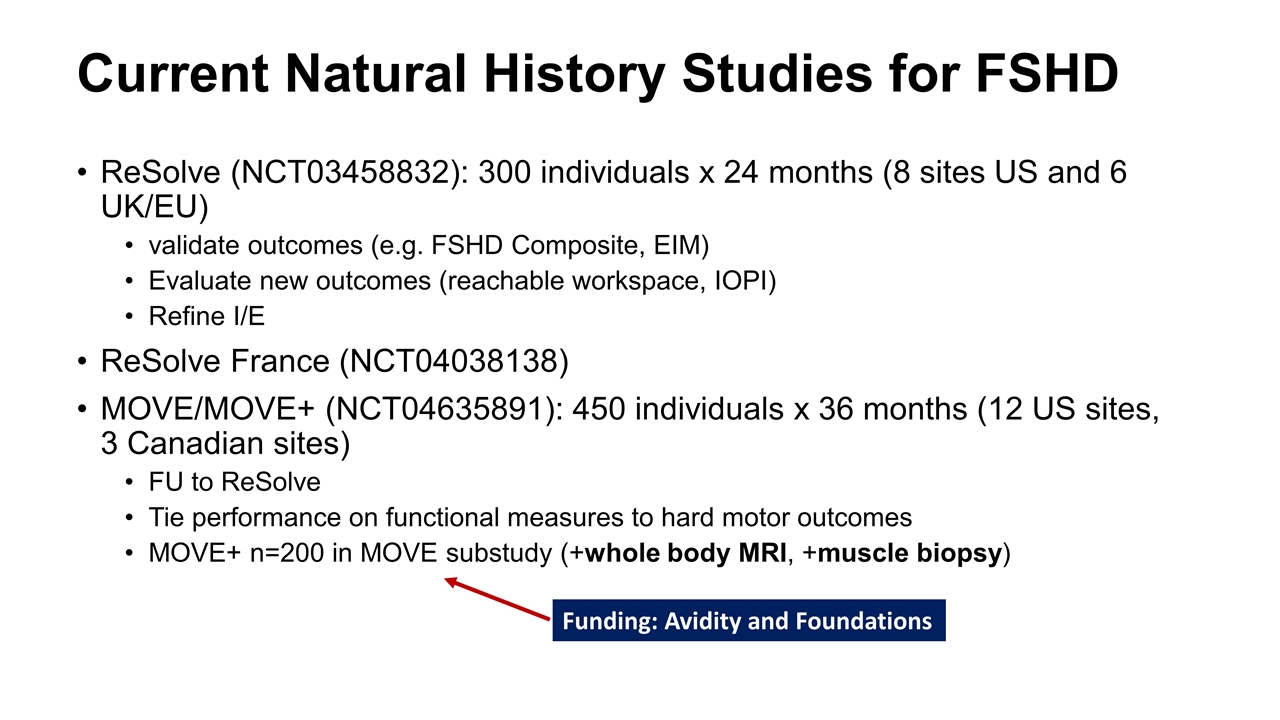
Current Natural History Studies for FSHD ReSolve (NCT03458832): 300 individuals x 24 months (8 sites US and 6 UK/EU) validate outcomes (e.g. FSHD Composite, EIM) Evaluate new outcomes (reachable workspace, IOPI) Refine I/E ReSolve France (NCT04038138) MOVE/MOVE+ (NCT04635891): 450 individuals x 36 months (12 US sites, 3 Canadian sites) FU to ReSolve Tie performance on functional measures to hard motor outcomes MOVE+ n=200 in MOVE substudy (+whole body MRI, +muscle biopsy) Funding: Avidity and Foundations
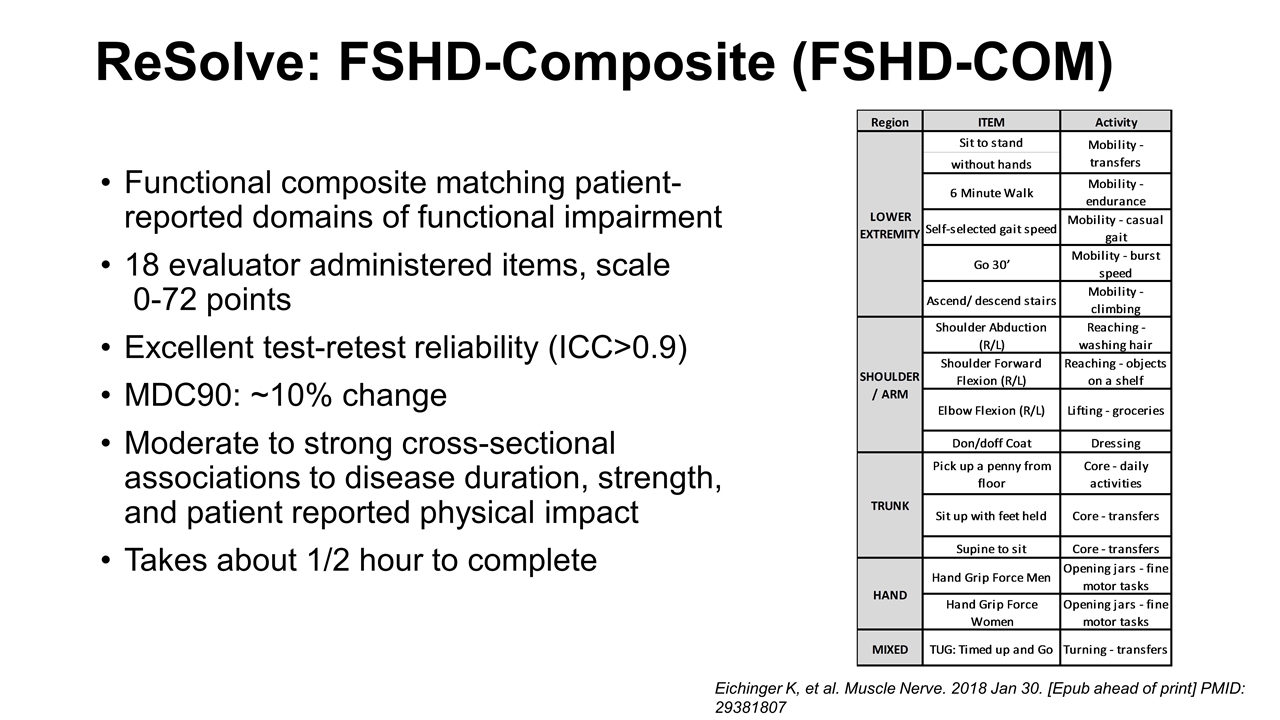
ReSolve: FSHD-Composite (FSHD-COM) Functional composite matching patient-reported domains of functional impairment 18 evaluator administered items, scale 0-72 points Excellent test-retest reliability (ICC>0.9) MDC90: ~10% change Moderate to strong cross-sectional associations to disease duration, strength, and patient reported physical impact Takes about 1/2 hour to complete Eichinger K, et al. Muscle Nerve. 2018 Jan 30. [Epub ahead of print] PMID: 29381807
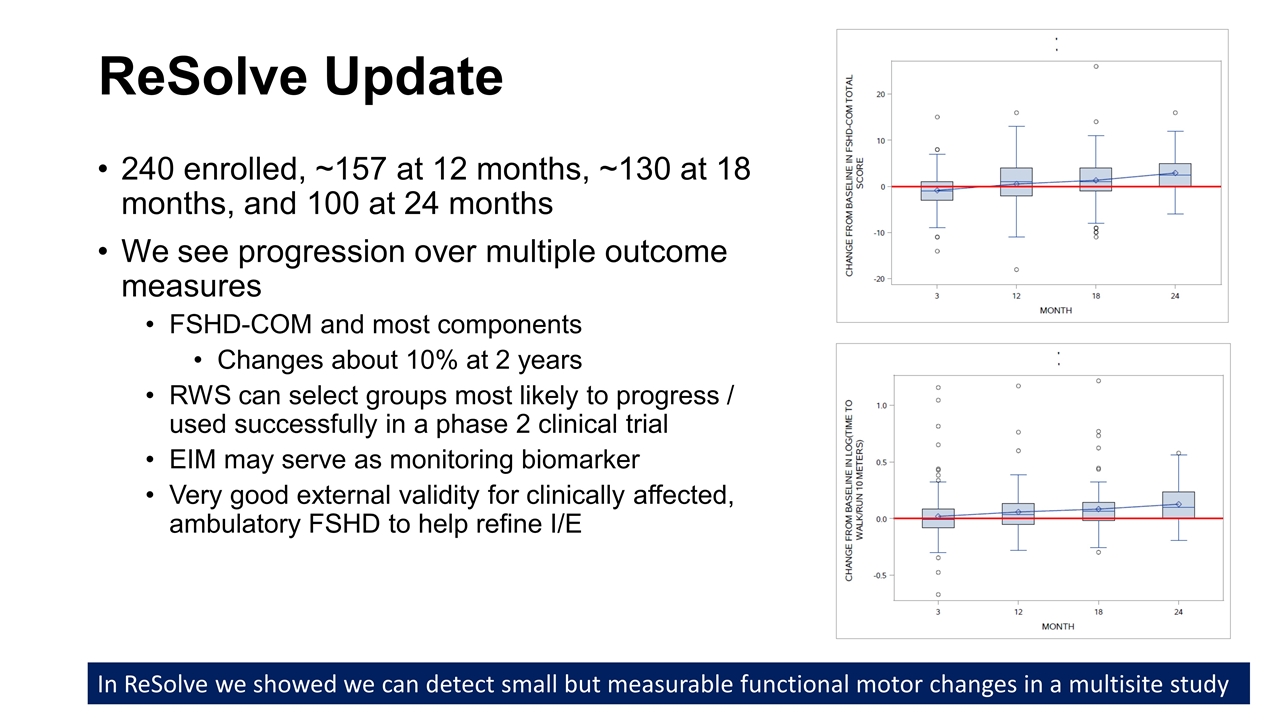
ReSolve Update 240 enrolled, ~157 at 12 months, ~130 at 18 months, and 100 at 24 months We see progression over multiple outcome measures FSHD-COM and most components Changes about 10% at 2 years RWS can select groups most likely to progress / used successfully in a phase 2 clinical trial EIM may serve as monitoring biomarker Very good external validity for clinically affected, ambulatory FSHD to help refine I/E In ReSolve we showed we can detect small but measurable functional motor changes in a multisite study
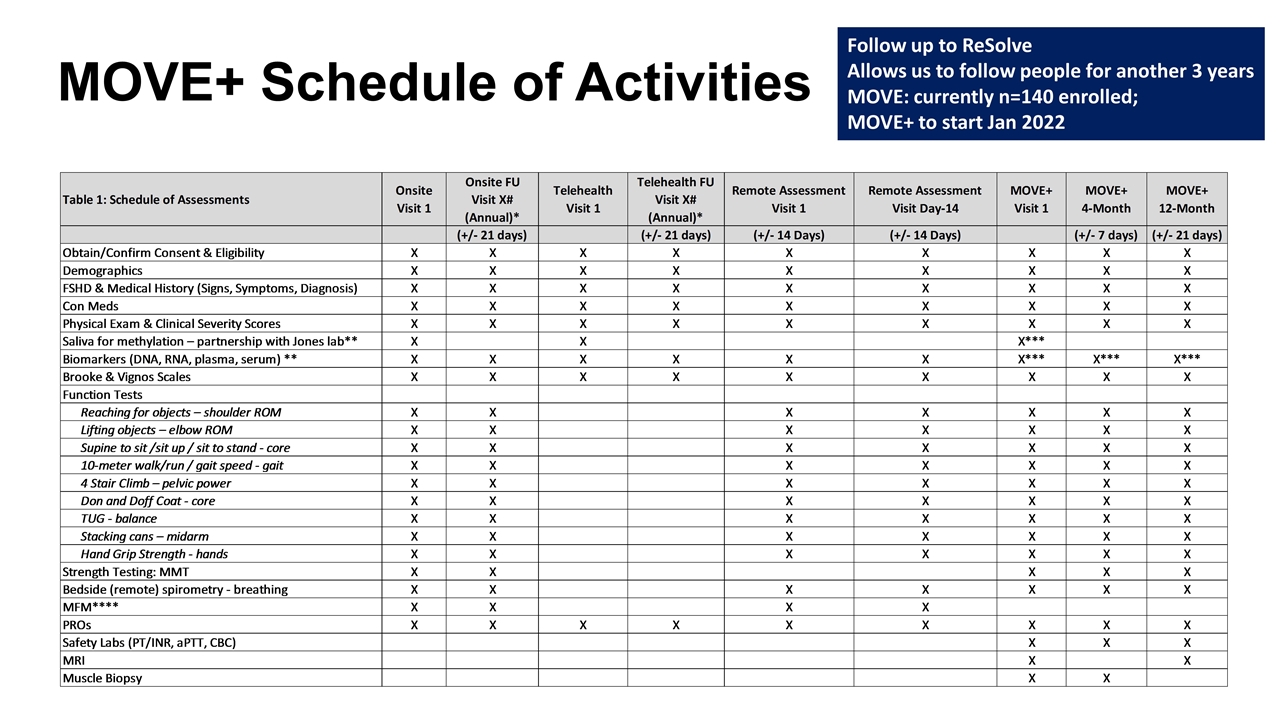
MOVE+ Schedule of Activities Follow up to ReSolve Allows us to follow people for another 3 years MOVE: currently n=140 enrolled; MOVE+ to start Jan 2022

Conclusion FSHD - the molecular basis is defined DUX4 is a clear molecular target for therapy Recently completed clinical trials to learn from Multiple candidate functional/strength outcomes for registration trial Ongoing natural history studies to help overcome barriers to trial design What will a drug do? Halt progression? Or will people improve? Will the inclusion/exclusion criteria used to select muscles for a POC study, be ideal for a definitive efficacy trial? Recent phase 2 randomized clinical trial selects moderate–severely affected individuals, who can no longer ‘reach up’ Or will we want people earlier in the disease course? Leveraging existing infrastructure and programs: collaborations between academics, advocacy, NIH, patients, and industry Highly motivated patient population!

Agenda Sarah Boyce, President & CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology University of Kansas Medical Center Sarah Boyce, President and CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology Mike MacLean, CFO (Moderator) Sarah Boyce, President & CEO Welcome & Introduction Building on our AOC Expertise Facioscapulohumeral Muscular Dystrophy (FSHD) Program FSHD: Disease Overview and Endpoints Q&A Session Closing Remarks

Q&A Participants: Avidity Management Jeffrey M. Statland, M.D. Moderator: Mike MacLean, CFO

Agenda Sarah Boyce, President & CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology University of Kansas Medical Center Sarah Boyce, President and CEO Art Levin, Ph.D., CSO Mike Flanagan, Ph.D., CTO Jeffrey M. Statland, M.D., Professor of Neurology Mike MacLean, CFO (Moderator) Sarah Boyce, President & CEO Welcome & Introduction Building on our AOC Expertise Facioscapulohumeral Muscular Dystrophy (FSHD) Program FSHD: Disease Overview and Endpoints Q&A Session Closing Remarks

Closing Remarks Sarah Boyce, President & CEO
Goals for the Day Demonstrate how our AOC platform expertise is leveraged across the pipeline Review the preclinical package supporting AOC 1020 Offer a broader perspective on the current treatment landscape for FSHD Answer your questions mAb OLIGO

Delivering on the RNA Revolution Phase 1/2 MARINA trial of AOC 1001 ongoing AOC 1020 for FSHD & AOC 1044 for DMD anticipated to enter the clinic by the end of 2022 Pursuing preclinical proof of concept in additional skeletal muscle and other tissues DELIVERING NEXT Luke Living with DM1

































































