
1 Investor & Analyst Event Series – Volume 7 AOC 1001 MARINATM Phase 1/2 Topline Data Kristl, Zen & Loraine living with DM1 Nathan living with DMD Josh living with FSHD

2 Forward-Looking Statements We caution the reader that this presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical fact contained in this presentation are forward-looking statements, including, but not limited to, statements regarding: the characterization and implications of the Phase 1/2 MARINA topline safety, biomarker and functional data; expectations related to Avidity's discussions with, and data to be provided to, the FDA and any change of status in the existing partial clinical hold related to the Phase 1/2 MARINA trial; the safety, tolerability and benefits of AOC 1001; the severity of adverse events related to AOC 1001; our future results of operations and financial position; our business strategy; the anticipated timing, costs, design and conduct of our ongoing and planned preclinical studies and clinical trials; research and development plans; plans and projected timelines for AOC 1001, AOC 1020 and AOC 1044; timing and likelihood of success; prospective products; product approvals; the potential of the AOC platform; plans and objectives of management for future operations; and future results of anticipated product development efforts. In some cases, the reader can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by Avidity that any of these items will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: we may not be able to resolve the partial clinical hold, and the analysis of the underlying cause of the related serious adverse event may result in delays in the MARINA study or an inability to compete the study; the Phase 1/2 MARINA trial data as of the date hereof may differ materially from the final results of the trial; additional participant data related to AOC 1001 that continues to become available may be inconsistent with the data produced as of the date hereof and the conclusions drawn therefrom; unexpected adverse side effects or inadequate efficacy of our product candidates may delay or limit their development, regulatory approval and/or commercialization, or may result in clinical holds, recalls or product liability claims; we are early in our development efforts and many of our development programs are in the preclinical or discovery stage; our approach to the discovery and development of product candidates based on our AOC platform is unproven, and we do not know whether we will be able to develop any products of commercial value; the success of our preclinical studies and clinical trials for our product candidates; the results of preclinical studies and early clinical trials are not necessarily predictive of future results; potential delays in the commencement, enrollment and completion of clinical trials; our dependence on third parties in connection with preclinical and clinical testing and product manufacturing; regulatory developments in the United States and foreign countries, including acceptance of INDs and similar foreign regulatory submissions and our proposed design of future clinical trials; our ability to obtain and maintain intellectual property protection for our AOC platform, product candidates and proprietary technologies; we may exhaust our capital resources sooner than we expect and fail to raise additional needed funds; and other risks described in our filings with the SEC, including under the heading “Risk Factors” in our Form 10-K for the year ending on December 31, 2022, filed with the SEC on February 28, 2023, and any subsequent filings with the SEC. The reader is cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and the reader is cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.

3 Our Vision To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Luke Living with DM1

4 Advancing a New Class of RNA Therapeutics • Progressing robust pipeline in muscle; three programs in clinical development in approximately one year • Leveraging expertise in rare diseases and RNA therapies to execute clinical programs and commercial operations • Building an integrated and diverse company in service of our patients • Strong financials with cash runway into mid-2025 BROAD & DISRUPTIVE P L A T F O R M ADVANCING & EXPANDING P I P E L I N E AGILE & DIVERSE C O M P A N Y • Committed to delivering a new class of RNA therapies • Advancing three AOCs in clinical development; two siRNAs and first PMO • Broadening beyond muscle to other indications like cardiology and immunology through internal discovery & partnerships *Sept. 2022, FDA placed a partial clinical hold on new participant enrollment. All current participants may continue in their current dosing cohort. All participants in MARINA may roll over into the MARINA-OLE where they will receive AOC 1001 as planned. Avidity is working to resolve the partial clinical hold as quickly as possible.

5 Proof of AOC Platform AOC 1001 Topline Data: Disease Modification, Functional Improvement, and Dose Range Identified in DM1 Impact on Disease Mechanism Functional Improvement Safety & Tolerability Completed Successful MARINA Trial *Sept. 2022, FDA placed a partial clinical hold on new participant enrollment. All current participants may continue in their current dosing cohort. Avidity is working to resolve the partial clinical hold as quickly as possible. AOC 1001 demonstrates directional improvements in myotonia, strength and early signs of mobility in 6-month period Functional improvements follow DMPK knockdown and splicing changes consistent with our understanding of the mechanism of disease Identified Phase 3 dose range: 2-4mg/kg with 2mg/kg to be dosed more frequently Favorable safety & tolerability profile* Next step: align with health authorities on a path forward

6 • Share AOC 1001 MARINA topline safety, functional and biomarker data • Discuss Avidity’s disruptive and broad AOC Platform • Answer your questions Goals for the Day mAb OLIGO AOC 1001

7 7 AOC 1001 MARINATM Phase 1/2 Trial Topline Data Assessment Avidity Management Team Nicholas E. Johnson, M.D. M.Sci., FAAN Virginia Commonwealth University GUEST SPEAKER Art Levin, Ph.D. Distinguished Scientist & Strategic Leader Steve Hughes, M.D. Chief Medical Officer Sarah Boyce President & CEO W. Michael Flanagan, Ph.D. Chief Scientific & Technical Officer Mike MacLean Chief Financial & Business Officer Kath Gallagher SVP Corporate Communications & Investor Relations Elizabeth Ackermann SVP Clinical Development

8 AGENDA Sarah Boyce, President & CEO Steve Hughes, M.D., CMO Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University Michael Flanagan, Ph.D., CSO & CTO Art Levin, Ph.D., Avidity Board Member & Distinguished Scientist & Strategic Leader Sarah Boyce, President & CEO Avidity Management & Dr. Nicholas Johnson, VCU Kath Gallagher, SVP, Communications & IR (Moderator) • Welcome & Introduction • AOC 1001 MARINATM Topline Data Topline Data & Clinical Overview Biomarker Overview & Assessment • Expanding the AOC platform • Closing Remarks • Q&A Session

MARINA: A Phase 1/2 Clinical Trial to Evaluate AOC 1001 in Adult Patients with DM1 Steve Hughes, M.D., Chief Medical Officer

10 0 APPROVED THERAPIES DM1 Burden and Significant Unmet Need >40,000 PEOPLE WITH DM1 IN THE US • Complex disease with symptoms that present with high variability from patient to patient • Monogenic, autosomal dominant, progressive disease that primarily affects muscle: skeletal, cardiac & smooth • Increases in severity from generation to generation • Significant impact on quality of life • Shortened life-expectancy • Caused by triplet-repeat in DMPK gene, resulting in a toxic gain of function mRNA Loraine, Kristl & Zen Living with DM1

11 Update on Clinical Trials • Discussions with FDA on the AOC 1001 partial clinical hold for new participant enrollment remain ongoing • The partial hold is in response to a serious adverse event (SAE) reported in a single participant in the 4mg/kg cohort comprising of bilateral ischemia in the region of the lateral geniculate nuclei in the thalamus with subsequent hemorrhagic transformation. This was reported as thalamic hemorrhage • No plausible biological link to any component of AOC 1001, the AOC platform, the transferrin receptor delivery mechanism or reduction of DMPK was identified • Will share information on next steps once agreed plan is in place with FDA • MARINA trial delivered solid data package to inform pivotal studies • MARINA trial concluded with 38 participants • Topline data was presented in oral presentation American Academy of Neurology (AAN) Annual Meeting • First look at data from the MARINA-OLE study at the end of 2023

12 *Sept. 2022, FDA placed a partial clinical hold on new participant enrollment. All current participants may continue in their current dosing cohort. Avidity is working to resolve the partial clinical hold as quickly as possible. **Upon entry into MARINA OLE, biopsies were only taken for participants in Cohort A. ‡Booster dose was only given to participants who were in Cohort A1 and placebo B1/B2 † As of early March 2023 A ll Pa rt ic ip an ts R ec ei ve AO C 10 01 MARINATM Trial Designed to Evaluate Safety and Tolerability of AOC 1001* 2mg/kg 4mg/kg3: 1 Ra nd om iz at io n (A O C 10 01 :P la ce bo ) A 1 mg/kg B1 2 mg/kg B2 4 mg/kg (N=8) (N=12) (N=18) AOC 1001, n=6 AOC 1001, n=9 AOC 1001, n=13 Placebo, n=2 Placebo, n=3 Placebo, n=5 • With the 38 participants treated with AOC 1001 in MARINA and/or MARINA-OLE, we've administered 132 doses and accumulated ~27 total patient years of exposure† • One participant receiving 4 mg/kg AOC 1001 discontinued treatment due to SAE • As of April 20, 36 participants have enrolled in the MARINA-OLE ‡ Dose Booster Biopsy ** ‡ Dose listed is siRNA.

13 Preliminary Assessment in December 2022: DM1 Cascade to Functional Benefit Early responders demonstrated myotonia improvement • 16% splicing improvement across 22 gene panel • 31% improvement in key muscle-specific panel • 100% of treated participants had a DMPK reduction • 45% mean DMPK reduction in treated participants • Delivered RNA to muscle: reinforcing disruptive and broad potential of the AOC platform DELIVERY TO MUSCLE DMPK REDUCTION SPLICING IMPROVEMENT FUNCTIONAL BENEFIT

14 • Directional improvement in multiple scores on a patient level • Improvements in myotonia & muscle strength and early signs in mobility • Splicing changes followed by functional improvement in 2- 4mg/kg doses • Broad splicing changes in over a thousand genes confirm activity in the nucleus • Delivered RNA to muscle • DMPK knockdown demonstrating target engagement (42% mean) Proof of AOC Platform Impact on Disease Mechanism Functional Improvement FUNCTIONAL BENEFIT Successfully Completed MARINA Trial • Selected dose range of 2-4mg/kg; with increased frequency expected at 2mg/kg • Next step: align with health authorities on a path forward AOC 1001 Positive Topline Data Demonstrates Disease Modification, Functional Improvement and Identifies Pivotal Dose Range

15 EARLY SIGNS OF IMPROVEMENT IN MOBILITY MEASURES IMPROVEMENT IN MYOTONIA IMPROVEMENT IN STRENGTH MARINA Topline Functional Assessments Timed Up and Go (TUG) Quantitative Muscle Testing (QMT) Total Score* Hand Grip Elbow Extension & Elbow Flexion Knee Extension & Knee Flexion Ankle Dorsiflexion 10 Meter Walk Run Test (10mWRT) Video Hand Opening Time (vHOT) Independently adjudicated Timed 10mWRT 1m Slow Down

16 AGENDA Sarah Boyce, President & CEO Steve Hughes, M.D., CMO Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University Michael Flanagan, Ph.D., CSO & CTO Art Levin, Ph.D., Avidity Board Member & Distinguished Scientist & Strategic Leader Sarah Boyce, President & CEO Avidity Management & Dr. Nicholas Johnson, VCU Kath Gallagher, SVP, Communications & IR (Moderator) • Welcome & Introduction • AOC 1001 MARINATM Topline Data Topline Data & Clinical Overview Biomarker Overview & Assessment • Expanding the AOC platform • Closing Remarks • Q&A Session

17 Topline Data from the AOC 1001 MARINA Phase 1/2 Trial Nicholas E. Johnson, M.D., M.Sci., FAAN Associate Professor & Vice Chair of Research, Department of Neurology, Virginia Commonwealth University Dr. Johnson is an associate professor and vice chair of research in the department of neurology at Virginia Commonwealth University with a focus in inherited neuromuscular disorders. He received his undergraduate degree in molecular and cellular biology and psychology at the University of Arizona. He then obtained his medical degree at the University of Arizona. He completed his neurology residency and combined fellowship in neuromuscular medicine and experimental therapeutics at the University of Rochester. His laboratory is focused on identifying the pathogenesis of myotonic dystrophy, the limb girdle muscular dystrophies, and facioscapulohumeral muscular dystrophy and identifying appropriate clinical endpoints for these conditions. Dr. Johnson conducts therapeutic trials in many other inherited nerve and muscle disorders.

Topline Data from the AOC 1001 MARINA Phase 1/2 Trial Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University
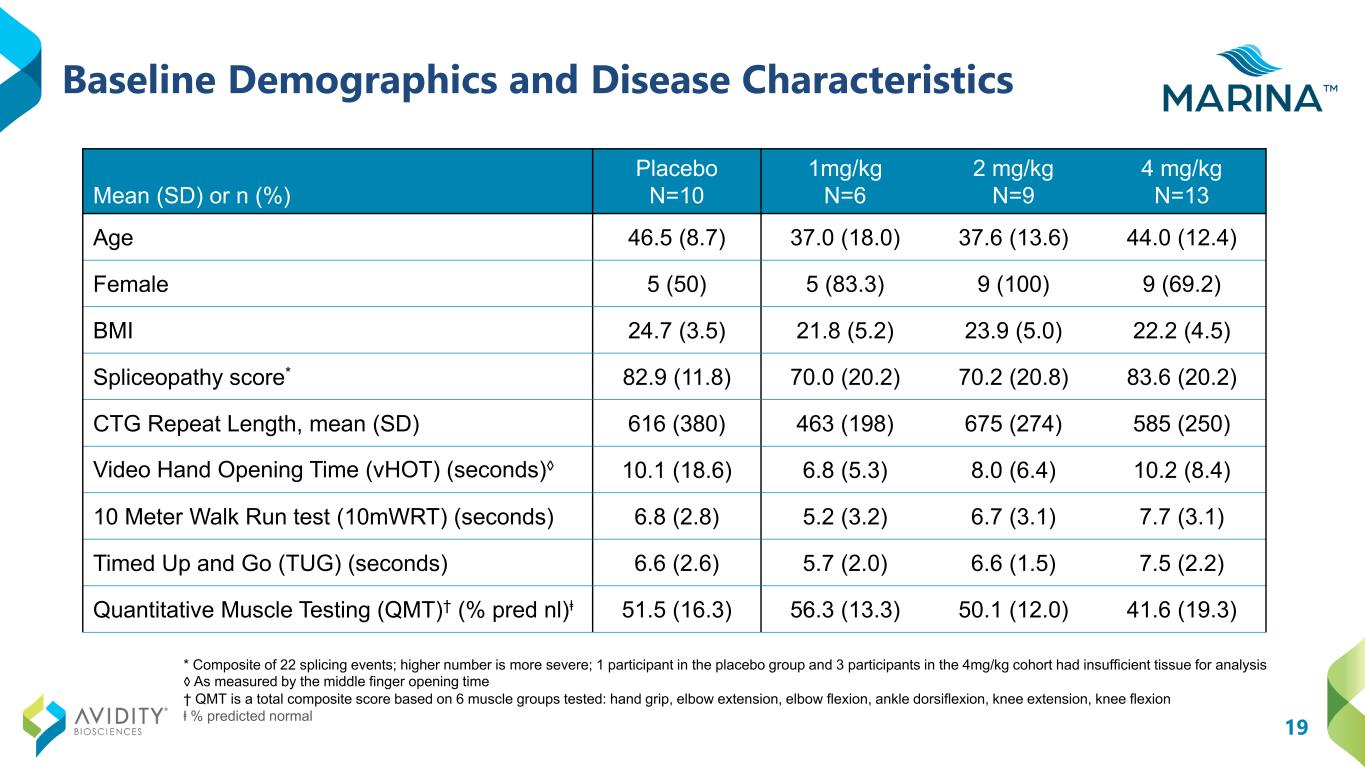
19 Baseline Demographics and Disease Characteristics * Composite of 22 splicing events; higher number is more severe; 1 participant in the placebo group and 3 participants in the 4mg/kg cohort had insufficient tissue for analysis ◊ As measured by the middle finger opening time † QMT is a total composite score based on 6 muscle groups tested: hand grip, elbow extension, elbow flexion, ankle dorsiflexion, knee extension, knee flexion ⱡ % predicted normal Mean (SD) or n (%) Placebo N=10 1mg/kg N=6 2 mg/kg N=9 4 mg/kg N=13 Age 46.5 (8.7) 37.0 (18.0) 37.6 (13.6) 44.0 (12.4) Female 5 (50) 5 (83.3) 9 (100) 9 (69.2) BMI 24.7 (3.5) 21.8 (5.2) 23.9 (5.0) 22.2 (4.5) Spliceopathy score* 82.9 (11.8) 70.0 (20.2) 70.2 (20.8) 83.6 (20.2) CTG Repeat Length, mean (SD) 616 (380) 463 (198) 675 (274) 585 (250) Video Hand Opening Time (vHOT) (seconds)◊ 10.1 (18.6) 6.8 (5.3) 8.0 (6.4) 10.2 (8.4) 10 Meter Walk Run test (10mWRT) (seconds) 6.8 (2.8) 5.2 (3.2) 6.7 (3.1) 7.7 (3.1) Timed Up and Go (TUG) (seconds) 6.6 (2.6) 5.7 (2.0) 6.6 (1.5) 7.5 (2.2) Quantitative Muscle Testing (QMT)† (% pred nl)ⱡ 51.5 (16.3) 56.3 (13.3) 50.1 (12.0) 41.6 (19.3)

20 Generally Favorable Safety and Tolerability Subjects with ≥ 1 AE n (%) Placebo N=10 1 mg/kg N=6 2 mg/kg N=9 4 mg/kg N=13 Any AE 8 (80%) 6 (100%) 9 (100%) 13 (100%) Related to study drug 2 (20%) 1 (17%) 3 (33%) 10 (77%) Serious AE (SAE)* 0 0 1 (11%) 1 (8%) AE leading to study discontinuation** 0 0 0 1 (8%) AE leading to death 0 0 0 0 *1 SAE considered related to AOC 1001 4 mg/kg: resulted in a partial clinical hold; 1 SAE considered unrelated to treatment: reaction to opioid pain medication after an elective surgery **Patient discontinued from the study due to the SAE † Most common AEs are defined as those above 20% in combined 2 and 4 mg/kg treated participants Summary of Treatment emergent Adverse Events Most treatment emergent adverse events (AEs) were mild or moderate • Most common AEs† • procedural pain (36%) • anemia (32%) • COVID-19 (23%) • headache (23%) • nausea (23%) • 1 discontinuation due to an SAE • Anemia events were monitorable and reversible • Liver enzyme increases were observed (18%); interpretation is complicated by underlying disease & elevated baseline values (up to ~2.5x greater than upper limit of normal) • No impact on bilirubin

21 AOC 1001 Demonstrates Myotonia Reduction in Early Responder from 2mg/kg Cohort Improvement visible 3 months following the third dose at 2 mg/kg Participant from 2mg/kg Multidose Baseline vHOT Day 183 vHOT 3 months after third dose vHOT, video hand opening time.

22 Participants Treated with AOC 1001 Demonstrated Improvements in Myotonia, a Hallmark of DM1 at 2 mg/kg and 4 mg/kg *Measurements for vHOT are based on middle finger opening time. Placebo group combined from Cohort B1 (2mg/kg) and Cohort B2 (4mg/kg) AOC 1001 achieved statistical significance at 4mg/kg in a post-hoc analysis at all time points Video Hand Opening Time (vHOT)* Im pr ov em en t -4 -2 0 2 middle finger time SEM Days C ha ng e fr om B as el in e (s ec ) Placebo 2 mg/kg 4 mg/kg Dosing D43 D92D1 D183 Video Hand Opening Time (vHOT) Independently adjudicated IMPROVEMENT IN MYOTONIA

23 Participants Treated with AOC 1001 had Improvements in Total Muscle Strength at 2 mg/kg and 4 mg/kg Placebo group combined from Cohort B1 (2mg/kg) and Cohort B2 (4mg/kg) Error bars = standard error of the mean (SEM) †QMT Total Score is based on 6 muscle groups from both upper and lower body ⱡ % predicted normal AOC 1001 achieved statistical significance at 4mg/kg in a post-hoc analysis at day 183 -4 -2 0 2 4 6 Days Ch an ge fr om B as el in e (P PN ) Placebo 2 mg/kg 4 mg/kg Dosing D92D1 D43 ⱡ ⱡ Quantitative Muscle Testing (QMT) Total Score† Im pr ov em en t D183 IMPROVEMENT IN STRENGTH Quantitative Muscle Testing (QMT) Total Score* Hand Grip Elbow Extension & Elbow Flexion Knee Extension & Knee Flexion Ankle Dorsiflexion

24 AOC 1001 Showed Early Signs of Improvement in Mobility Measures -1.0 -0.5 0.0 0.5 Days Ch an ge fr om B as el in e (s ec ) Placebo 2 mg/kg 4 mg/kg Dosing D43 D92D1 10 Meter Walk Run Test (10mWRT) Im pr ov em en t D183 EARLY SIGNS OF IMPROVEMENT IN MOBILITY MEASURES 10 Meter Walk Run Test (10mWRT) Timed 10mWRT 1m Slow Down

25 EARLY SIGNS OF IMPROVEMENT IN MOBILITY MEASURES -0.5 0.0 0.5 Days Ch an ge fr om B as el in e (s ec ) Placebo 2 mg/kg 4 mg/kg Dosing D92D1 D43 AOC 1001 Showed Early Signs of Improvement in Mobility Measures Timed Up and Go (TUG) Im pr ov em en t D183 Timed Up and Go (TUG)

26 AOC 1001 Demonstrates Myotonia Reduction in 4 mg/kg Cohort Baseline vHOT Day 183 vHOT Improvement visible 3 months following the third dose at 4 mg/kg vHOT, video hand opening time. ` Participant from 4 mg/kg Multidose

27 MARINA Phase 1/2 Trial Demonstrates AOC 1001 Impacts Disease Mechanism and Achieves Functional Improvement • DM1 is an underrecognized, progressive, and often fatal neuromuscular disease with a high unmet need and no approved therapies • AOC 1001 is an investigational antibody oligonucleotide conjugate that successfully delivered siRNA to muscle resulting in DMPK reductions and splicing improvements leading to functional improvements • Top line data from MARINA demonstrate directional improvement in multiple clinical endpoints in the dose range of 2-4mg/kg of AOC 1001 including: o Improvements in myotonia (vHOT) as early as 6 weeks after dosing with a sustained effect at month 6 o Improvement in QMT total strength measure observed at month 6 o Early signs of mobility improvements in the 10mWRT and the TUG • AOC 1001 had a generally favorable safety and tolerability profile • Data support advancement of AOC 1001 into Phase 3 study

28 AGENDA Sarah Boyce, President & CEO Steve Hughes, M.D., CMO Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University Michael Flanagan, Ph.D., CSO & CTO Art Levin, Ph.D., Avidity Board Member & Distinguished Scientist & Strategic Leader Sarah Boyce, President & CEO Avidity Management & Dr. Nicholas Johnson, VCU Kath Gallagher, SVP, Communications & IR (Moderator) • Welcome & Introduction • AOC 1001 MARINATM Topline Data Topline Data & Clinical Overview Biomarker Overview & Assessment • Expanding the AOC platform • Closing Remarks • Q&A Session

AOC 1001: Impact on Disease Mechanism W. Michael Flanagan Chief Scientific and Technical Officer

30 • Directional improvement in multiple scores on a patient level • Improvements in myotonia & muscle strength and early signs in mobility • Splicing changes followed by functional improvement in 2- 4mg/kg doses • Broad splicing changes in over a thousand genes confirm activity in the nucleus • Delivered RNA to muscle • DMPK knockdown demonstrating target engagement (42% mean) Proof of AOC Platform Impact on Disease Mechanism Functional Improvement FUNCTIONAL BENEFIT Successfully Completed MARINA Trial • Selected dose range of 2-4mg/kg; with increased frequency expected at 2mg/kg • Next step: align with health authorities on a path forward AOC 1001 Positive Topline Data Demonstrates Disease Modification, Functional Improvement and Identifies Pivotal Dose Range

31 MARINA™ Topline Data at 6 Weeks Post-Dose Dose BoosterBiopsy - 1 participant in 2 mg/kg group had a muscle biopsy soon after the third dose and was removed from the PK analysis - 1 participant in the placebo group, 1 from the 1mg/kg and 3 participants in the 4mg/kg cohort had insufficient tissue for analysis - The participant who experienced the SAE only received a baseline biopsy NOTE: Day 92 biopsy in 2mg/kg & 4mg/kg cohorts taken prior to third dose of AOC 1001 3: 1 Ra nd om iz at io n (A O C 10 01 :P la ce bo ) A 1 mg/kg B1 2 mg/kg B2 4 mg/kg (N=8) (N=12) (N=18) D43 D92BL D92 D183BL D92 D183BL Cohort Number of Participants Enrolled Number of Biopsies Available for PK/PD Analysis PK PD Placebo 10 N/A 9 1mg/kg 6 6 5 2mg/kg 9 8 9 4mg/kg 13 10 9

32 AOC 1001 Delivered siRNA to Muscle Expanding siRNA Therapeutics Beyond Liver Dose Proportional Increase in Muscle siRNA Concentrations 1 2 4 Dose (mg/kg) si R N A in M us cl e (n M ) 6 w ee ks a fte r d os e 1 dose q6W x 2 q6W x 2 PROOF OF AOC PLATFORM FUNCTIONAL IMPROVEMENT IMPACT ON DISEASE MECHANISM IDENTIFIED PIVOTAL DOSE RANGE

33 AOC 1001 Produced 42% mean DMPK Knockdown Demonstrating Target Engagement DMPK Reduction Across all Cohorts Placebo group combined from both cohorts and shown as standard error of mean Data shown at 6 weeks post a single dose of 1mg/kg or two doses of 2 or 4 mg/kg 1mg/kg (n=5) 2mg/kg (n=9) Placebo (n=9) Group 4mg/kg (n=9) PROOF OF AOC PLATFORM FUNCTIONAL IMPROVEMENT IMPACT ON DISEASE MECHANISM IDENTIFIED PIVOTAL DOSE RANGE

34 Sp lic e In de x (c ha ng e fro m b as el in e) Splicing Improvements in 22 Gene Panel Demonstrates AOC 1001 is Impacting DM1 Disease Mechanism Splicing measured by targeted RNA sequencing and calculated using published formula (Tanner et. al 2021) Splicing Index for each participant is calculated as absolute change from baseline (22 gene panel) Placebo group combined from all cohorts (standard error of the mean) mean absolute change from baseline score across all matched samples in a cohort 22 Gene Splicing Panel for Individual Patients and Cohorts Data shown at 6 weeks post a single dose of the 1mg/kg and 6 weeks post two doses of 2mg/kg or 4 mg/kg Sp lic in g im pr ov em en t Sp lic e in de x m ea n ch an ge fr om b as el in e 1mg/kg (n=5) 2mg/kg (n=9) Placebo (n=9) Group 4mg/kg (n=9) Cohorts PROOF OF AOC PLATFORM FUNCTIONAL IMPROVEMENT IMPACT ON DISEASE MECHANISM IDENTIFIED PIVOTAL DOSE RANGE

35 Muscle-Specific Biomarkers Show Splicing Improvement 4-Gene Muscle Splicing Panel for Individual Patients and Cohorts Data shown at 6 weeks post a single dose of the 1mg/kg and 6 weeks post two doses of 2mg/kg or 4 mg/kg Sp lic in g im pr ov em en t Muscle panel: CLCN1, CACNA1S, ATP2A1, BIN1 1mg/kg (n=5) 2mg/kg (n=9) Placebo (n=9) Group 4mg/kg (n=9) M us cl e- sp ec ifi c sp lic in g in de x m ea n ch an ge fr om b as el in e M us cl e- sp ec ifi c sp lic in g in de x ch an ge fr om b as el in e Splicing measured by targeted RNA sequencing and calculated using published formula (Tanner et. al 2021) Splicing Index for each participant is calculated as absolute change from baseline (22 gene panel) Placebo group combined from all cohorts (standard error of the mean) Mean change from baseline is the mean absolute change from baseline score across all matched samples in a cohort Cohorts PROOF OF AOC PLATFORM FUNCTIONAL IMPROVEMENT IMPACT ON DISEASE MECHANISM IDENTIFIED PIVOTAL DOSE RANGE

AOC 1001 (2 mg/kg) Broadly Impacts DM1 Disease Mechanism in Over 1,000 Splicing Events Responsive to mutant DMPK mRNA reduction Specific to muscle Biological relevance to disease RNA sequencing utilized to identify mis-spliced mRNA transcripts changed (p <0.05, mean PSI difference > 5) at 6 weeks post 2nd AOC 1001 (2 mg/kg) dosed participants relative to baseline. 4 mg/kg cohort RNA sequencing analysis not completed. Baseline (before dosing) 6 Weeks Post 2nd Dose Ex on s pl ic ed in ~7 00 Ex on s pl ic ed o ut ~4 50 Each column represents 1 participant’s splicing at baseline compared to 6 weeks post dose Same Participant 36

37 Delivering on the AOC Platform and Impacting Disease Mechanism Platform Achievements: • AOC technology delivered siRNA to muscle – a first for the RNA field • AOC 1001 achieved DMPK knockdown demonstrating target engagement • 42% mean DMPK reduction in treated participants DM1 Advancements: • Demonstrated splicing improvement leading to functional benefit • Broad splicing changes in over a thousand genes confirm activity in the nucleus Next Steps: • Analyze & interpret additional biomarker data from MARINA including: • Analysis of third biopsies in MARINA • Continue to analyze RNA sequencing data from all cohorts • Analyze day 183 biopsies from MARINA and MARINA-OLE PROOF OF AOC PLATFORM FUNCTIONAL IMPROVEMENT IMPACT ON DISEASE MECHANISM IDENTIFIED PIVOTAL DOSE RANGE

38 AGENDA Sarah Boyce, President & CEO Steve Hughes, M.D., CMO Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University Michael Flanagan, Ph.D., CSO & CTO Art Levin, Ph.D., Avidity Board Member & Distinguished Scientist & Strategic Leader Sarah Boyce, President & CEO Avidity Management & Dr. Nicholas Johnson, VCU Kath Gallagher, SVP, Communications & IR (Moderator) • Welcome & Introduction • AOC 1001 MARINATM Topline Data Topline Data & Clinical Overview Biomarker Overview & Assessment • Expanding the AOC platform • Closing Remarks • Q&A Session

Delivering on the AOC Platform - Broad Utility & Power of the Platform Art Levin, Avidity Board Member and Distinguished Scientist & Strategic Leader

40 AOC™ Platform: Building on the Power of Oligonucleotides AOC platform advantages include: MONOCLONAL ANTIBODIES mAb OLIGO Designed to combine the specificity of mAbs with the precision of oligonucleotide therapies OLIGONUCLEOTIDE THERAPIES OLIGOmAb ANTIBODY OLIGONUCLEOTIDE CONJUGATE (AOC) Ability to target new tissue and cell types beyond the liver Flexibility to select and deploy the most potent oligonucleotides (e.g., siRNAs, PMOs) Maximizes therapeutic durability, enabling infrequent dosing Readily reproducible and scalable

41 Diverse and Expanding AOC Pipeline PROGRAM / INDICATION TARGET LEAD OPTIMIZATION IND ENABLING PHASE 1/2 PHASE 3 AOC 1001 Myotonic Dystrophy Type 1 (DM1) DMPK AOC 1044 Duchenne Muscular Dystrophy (DMD) Exon 44 AOC 1020 Facioscapulohumeral Muscular Dystrophy (FSHD) DUX4 Additional DMD Programs Exon 45 & Undisclosed Rare Skeletal Muscle Program Undisclosed Rare Cardiac Program Undisclosed *Sept. 2022, FDA placed a partial clinical hold on new participant enrollment. All current participants may continue in their current dosing cohort. All participants in MARINA may roll over into the MARINA-OLE where they will receive AOC 1001 as planned. Avidity is working to resolve the partial clinical hold as quickly as possible.

42 A New Paradigm of RNA Therapeutics with AOCs AOC mAb OLIGO Past 30 Years 2021 First AOC Dosed in Humans Today AOCs deliver RNA into Skeletal Muscle CARDIAC IMMUNOLOGY OTHER INDICATIONS 2023 and Beyond RNA Therapeutics Focused on delivery to the liver or local delivery Targeting muscle and other tissues beyond the liver

43 AGENDA Sarah Boyce, President & CEO Steve Hughes, M.D., CMO Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University Michael Flanagan, Ph.D., CSO & CTO Art Levin, Ph.D., Avidity Board Member & Distinguished Scientist & Strategic Leader Sarah Boyce, President & CEO Avidity Management & Dr. Nicholas Johnson, VCU Kath Gallagher, SVP, Communications & IR (Moderator) • Welcome & Introduction • AOC 1001 MARINATM Topline Data Topline Data & Clinical Overview Biomarker Overview & Assessment • Expanding the AOC platform • Closing Remarks • Q&A Session

44 Our Vision To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Luke Living with DM1

45 AGENDA Sarah Boyce, President & CEO Steve Hughes, M.D., CMO Nicholas E. Johnson, M.D., M.Sci., FAAN Virginia Commonwealth University Michael Flanagan, Ph.D., CSO & CTO Art Levin, Ph.D., Avidity Board Member & Distinguished Scientist & Strategic Leader Sarah Boyce, President & CEO Avidity Management & Dr. Nicholas Johnson, VCU Kath Gallagher, SVP, Communications & IR (Moderator) • Welcome & Introduction • AOC 1001 MARINATM Topline Data Topline Data & Clinical Overview Biomarker Overview & Assessment • Expanding the AOC platform • Closing Remarks • Q&A Session












































