The patent positions of therapeutics companies like us are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and patent scope can be reinterpreted by the courts after issuance. Moreover, many jurisdictions permit third parties to challenge issued patents in administrative proceedings which may result in further narrowing or even cancellation of patent claims. Consequently, we do not know whether any of our product candidates will be protectable or remain protected by enforceable patents. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors. Any patents that we own or license may be challenged, narrowed, circumvented or invalidated by third parties.
Because patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months or potentially even longer, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office, or USPTO, to determine priority of inventions for any patent applications filed with the USPTO on or before March 15, 2013. Likewise, derivation proceedings may also be declared for any patent filings filed after March 15, 2013.
The patents and patent applications that relate to our programs are described below.
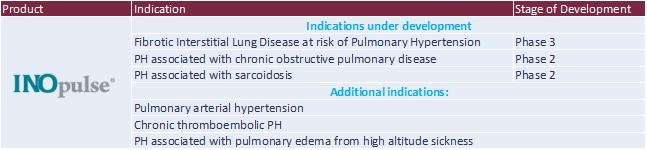
INOpulse
As of December 31, 2021, we hold exclusive licenses from Ikaria to at least 100 patents and pending patent applications in both the United States and foreign countries including Australia, Brazil, Canada, China, Eurasia, Europe, Hong Kong, India, Indonesia, Israel, Japan, Korea, Mexico, the Philippines, Russia, Singapore and South Africa. Certain of these issued patents and patent applications, if issued, will expire as late as 2033. These patent rights have been exclusively licensed for the treatment of patients with Bellerophon indications and cover methods of delivery and the drug delivery device, as well as important safety features and the ornamental design of the drug delivery device.
A primary basis for patent exclusivity is based on pending and issued in-licensed patents directed to proprietary methods of administering pulsed inhaled nitric oxide, as well as a device for delivering the same. At least one patent has been issued in the United States as well as Australia, Brazil, Canada, China, Europe, Hong Kong, Japan and Mexico. Patent applications are pending in Australia, Hong Kong, Mexico and the United States. This patent family expires as late as 2027 in the United States and in 2026 in the other countries.
Another important basis for patent exclusivity is based on an in-licensed portfolio of patents, directed to novel nasal cannula features that we believe are necessary for the accurate, safe and efficacious administration of pulsed nitric oxide. The patent family consists of seven issued U.S. patents and issued patents in Australia, Brazil, China, Eurasia, Europe, Hong Kong, Israel, Japan, Korea, Mexico and South Africa, as well as pending applications in the United States as well as Australia, Canada, China, Eurasia, Europe, Israel, India, Japan, Korea, Mexico and South Africa. Each of these patents and patent applications, if issued, will expire in 2033 in the United States and abroad.
Another in-licensed patent family relates to features of the drug delivery canister necessary for providing drug product for use with our proprietary pulsing drug delivery device. This patent family includes at least one issued patent in each of the United States, Australia, Canada, China, Europe, Hong Kong, Indonesia, Israel, Japan, Korea, Mexico, the Philippines, Russia and Singapore, as well as pending patent applications in the United States, Brazil, India, Mexico and Singapore. These pending applications, if issued, as well as the non-U.S. issued patents will expire in 2029. Two issued U.S. patents will expire in 2030.
Several other patent families directed to device and safety features are issued and pending. One U.S. issued patent directed to the valve configuration of our proprietary drug delivery device and the shape of the nitric oxide pulses will expire in 2039. Furthermore, design patents covering the ornamental designs of the intended commercial device and clinical device have been granted.