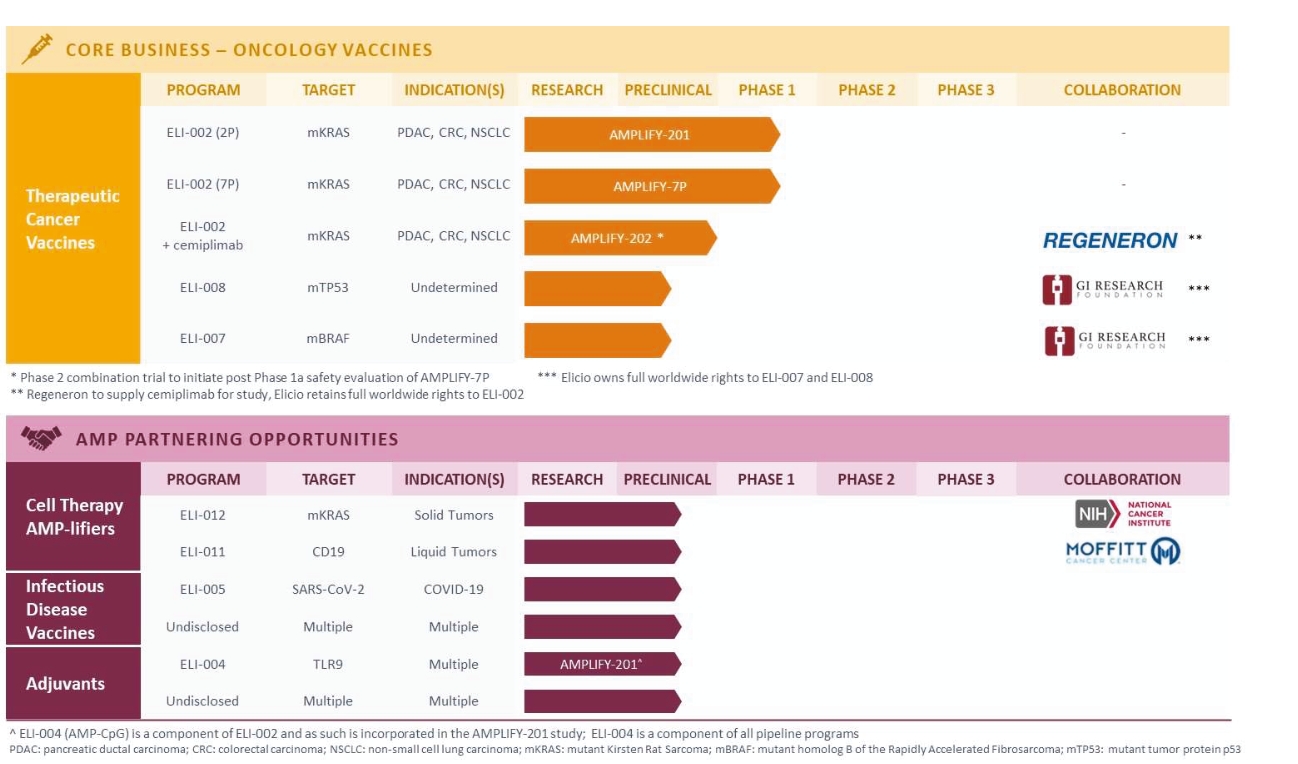
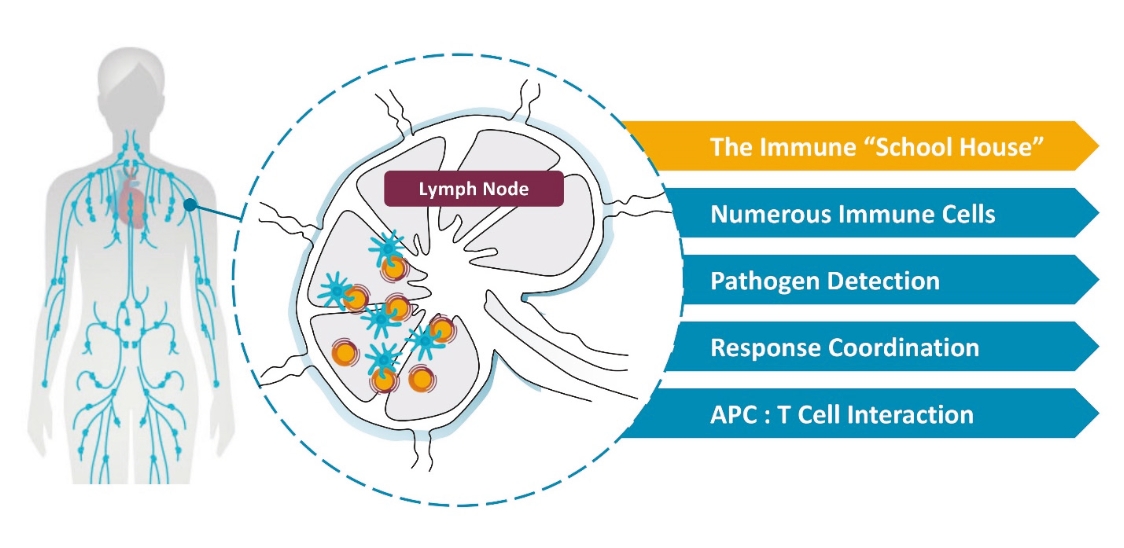
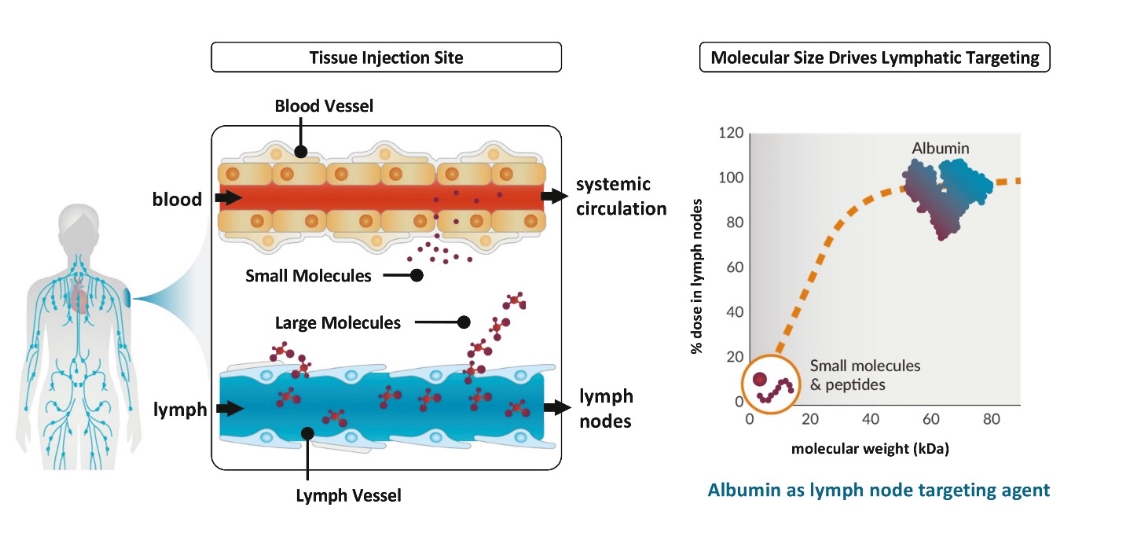
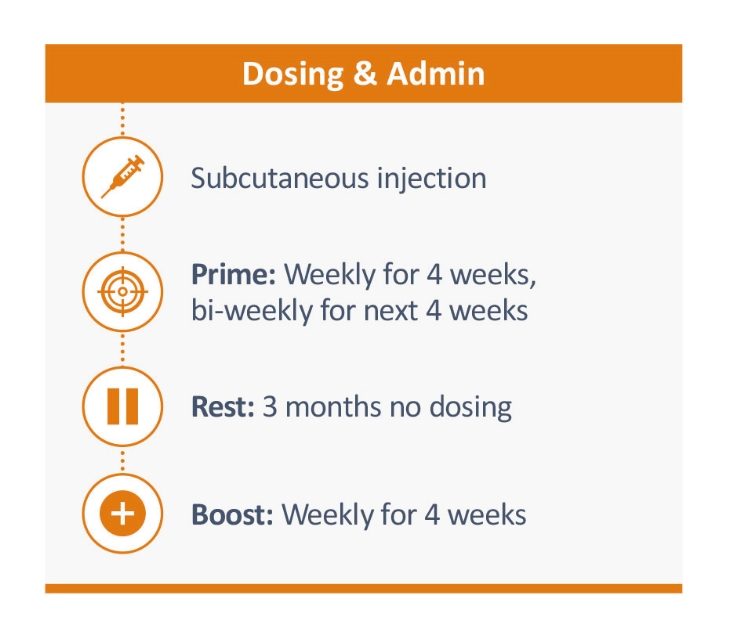
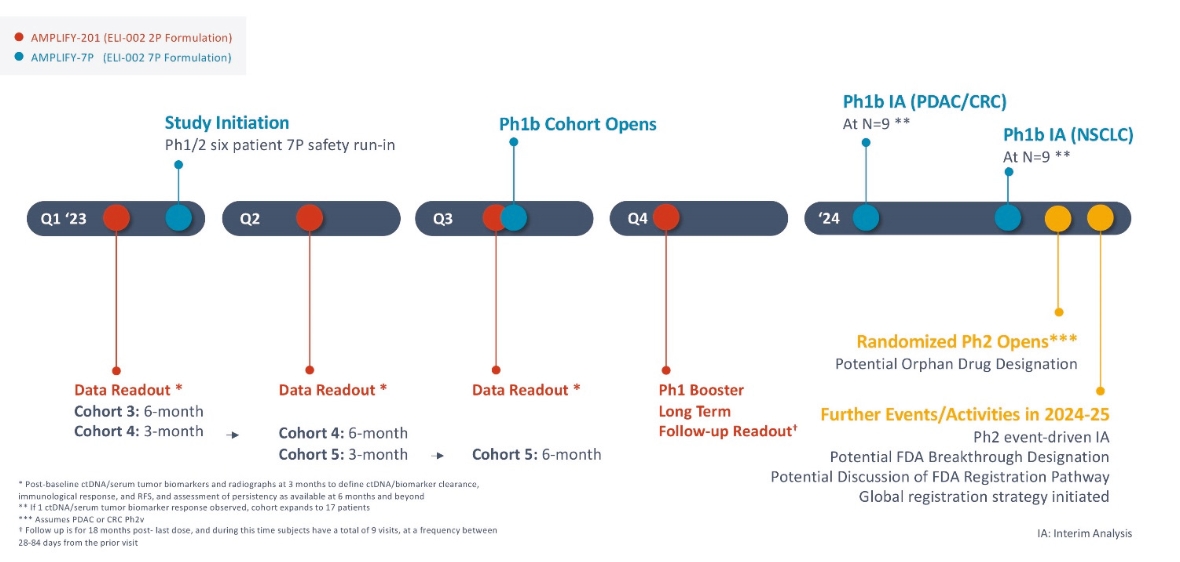
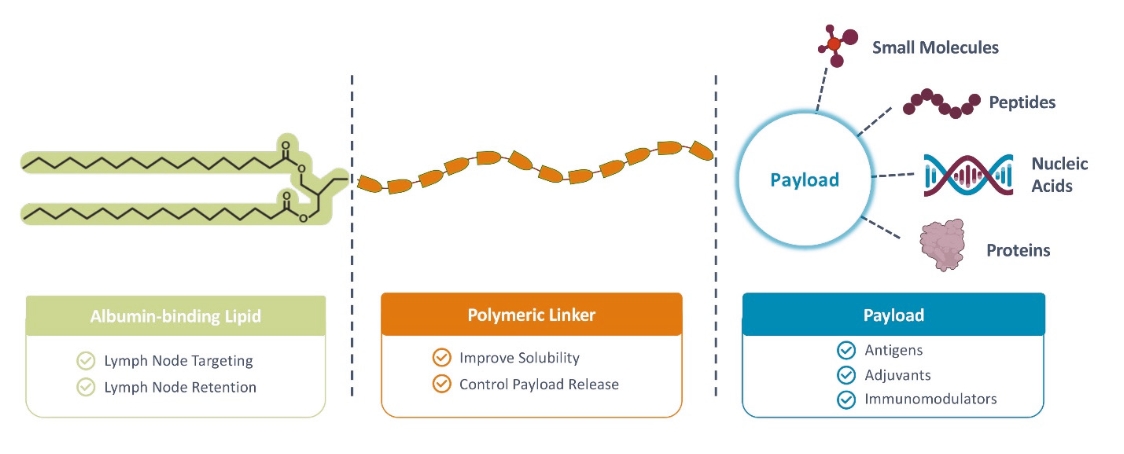
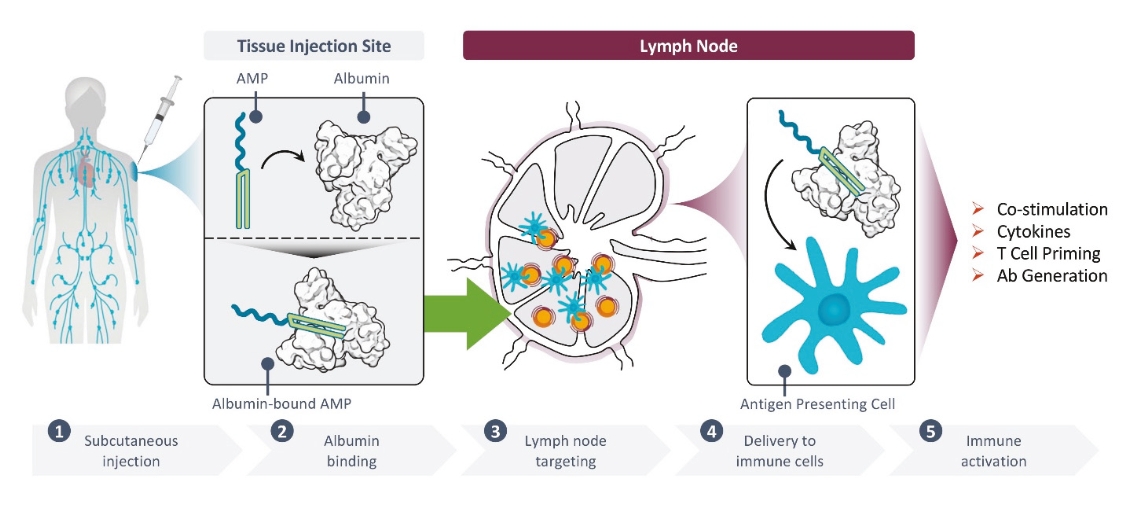
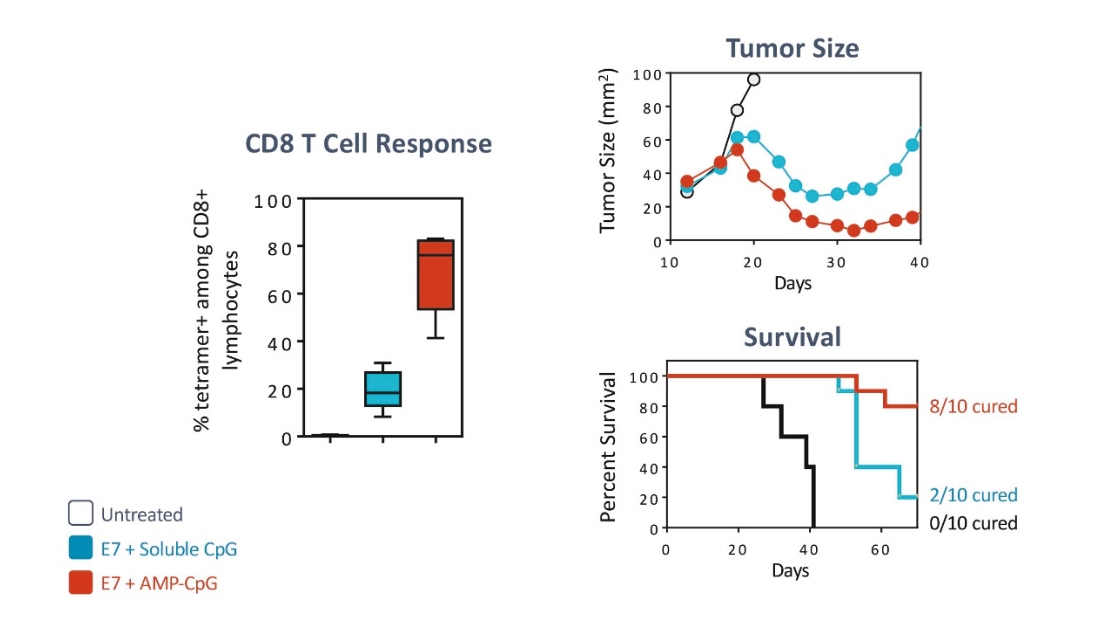
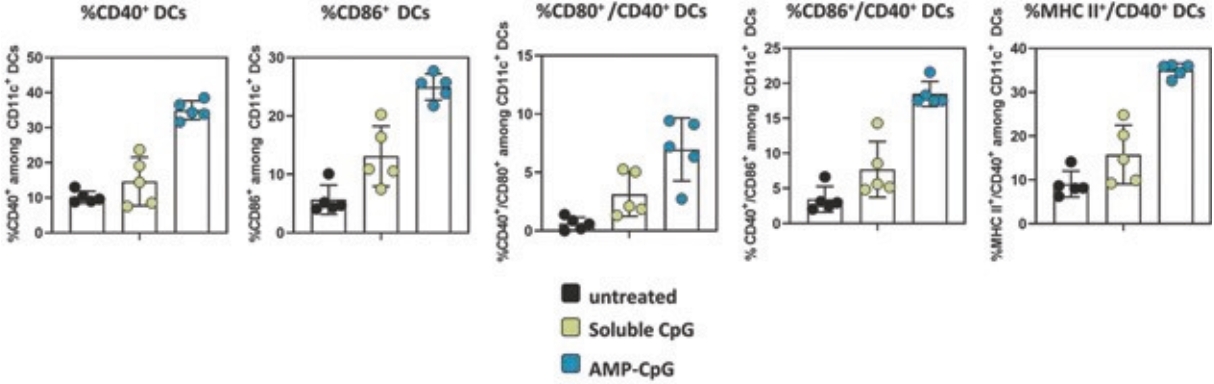
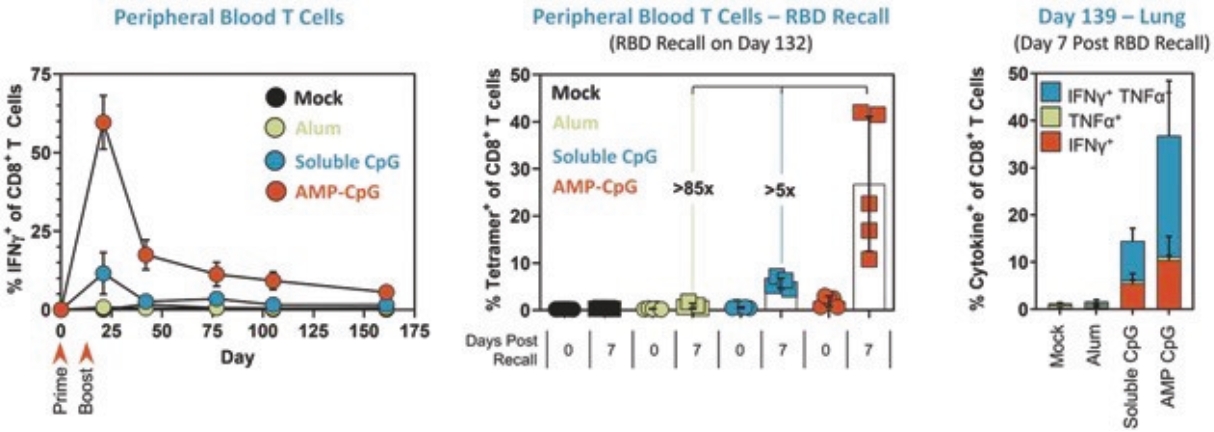
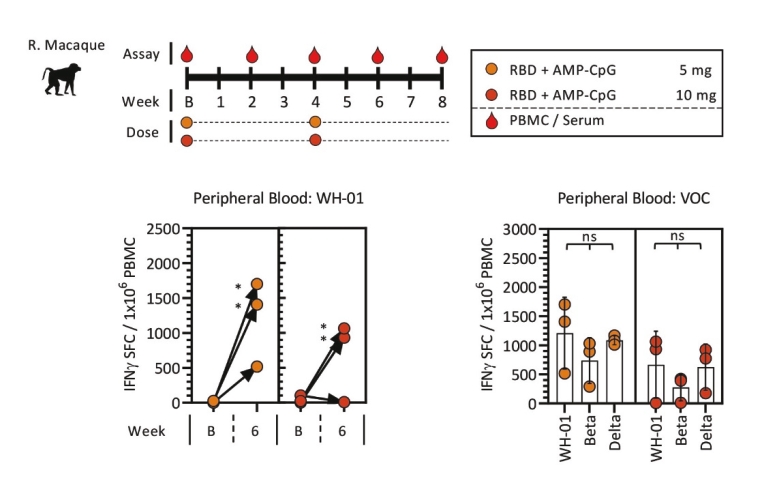
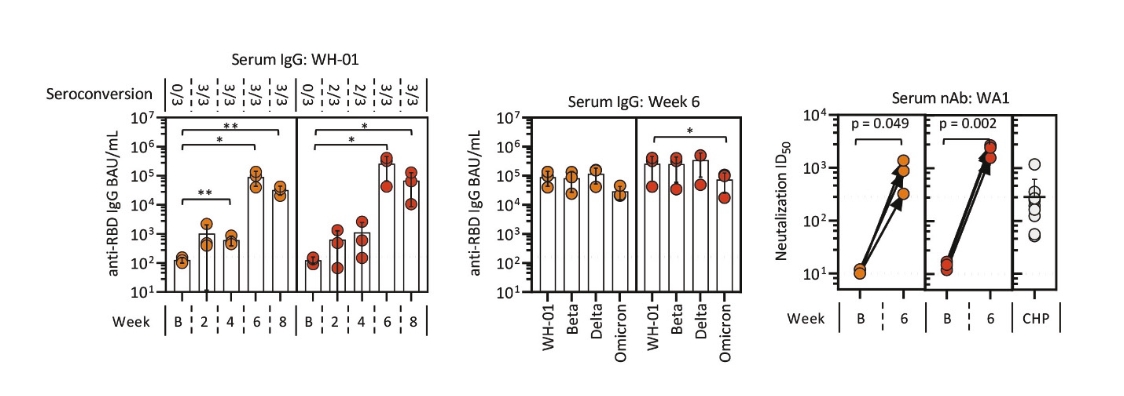
rights as required in order to obtain, maintain, and/or enforce them. The expiration dates of the patents discussed below assume in all cases that the appropriate maintenance, renewal, annuity, or other governmental fees are paid to maintain the patent(s) in force for the full extent of their term and any extension(s) thereof.
Angion’s ANG-3070 Tyrosine Kinase Inhibitor Program
As of March 17, 2023, compound, pharmaceutical composition and methods of use claims to Angion's kinase inhibitors are covered in patents issued in the United States. Angion also owned issued patents in Australia, Canada, China, Europe, Hong Kong, Israel, India, and Japan. The European patent was validated in Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Luxembourg, Monaco, Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland/Liechtenstein, Turkey, and the United Kingdom. A continuation application is pending in the United States. These patents, and patents that may issue from the pending applications, provide patent protection until 2033, assuming payment of all appropriate annuities and/or maintenance fees.
As of March 17, 2023, Angion had filed applications directed to the use of ANG-3070 in the treatment of irritable bowel disease (IBD) in Australia, Canada, China, Europe, Israel, Japan, and the United States. Patents issuing from corresponding national applications will expire in 2040.
As of March 17, 2023, Angion had filed applications in the United States and Europe relating to solid forms of ANG-3070. Patents issuing from corresponding national applications will expire in 2041.
As of March 17, 2023, Angion had filed a PCT patent application relating to gene expression levels associated with treatment comprising ANG-3070. Patents issuing from corresponding national applications, if granted, will expire in 2042.
Under the Hatch-Waxman Act, a single patent term restoration of up to five years in the United States may be available. Angion also may be eligible for similar restoration of term in Europe under supplementary protection certificate rights, and similar extensions in certain other countries.
Angion’s ROCK2 Inhibitor Program
As of March 17, 2023, the patent portfolio for the ROCK2 inhibitor program consists of three composition of matter patents. The first family includes granted patents in the United States and India, and pending applications in the United States, Australia, Canada, China, Europe, Hong Kong, Israel, and Japan, each of which would have presumed twenty-year terms expiring in 2038. A second family includes pending applications in the United States, Australia, Canada, China, Europe, Israel, India, and Japan, each of which would have presumed twenty-year terms expiring in 2040. A third family includes or will include pending applications in the United States, Canada, China, Europe, Israel, and Japan, each of which would have presumed twenty-year terms expiring in 2041.
Angion’s CYP11B2 Inhibitor Program
As of March 17, 2023, the patent portfolio for the CYP11B2 inhibitor program includes pending applications in the United States, Canada, Europe and Israel, each of which would have presumed twenty-year terms expiring in 2038. As of March 17, 2023, Angion owned issued patents in the United States that claim, among other things, ANG-3598 composition of matter, pharmaceutical compositions comprising ANG-3598, and methods of treating renal fibrosis. Angion also owned issued patents in Australia, China, Israel, India and Japan. Each of the patents expire in 2035.
Angion’s ANG-3777
As of March 17, 2023, the patent portfolio for ANG-3777 includes patents and patent applications that describe and/or specifically claim pharmaceutical compositions whose active agent is ANG-3777 and uses thereof, as well as compounds structurally related to ANG-3777, pharmaceutical compositions and uses thereof. As of March 17, 2023, Angion owned issued patents in the United States that claim, among other things, pharmaceutical compositions comprising ANG-3777. Angion also owned issued patents in Australia, Canada, China, Europe, Hong Kong, Israel, and Japan. Granted European patents have been validated in the following European countries: Denmark, France, Germany, Hungary, Ireland, Italy, Luxembourg, Monaco, Netherlands, Sweden, Switzerland/Liechtenstein, and the United Kingdom. These patents should remain in force, if the appropriate maintenance, renewal, annuity or other governmental fees are paid, in the United States until 2024, and in other jurisdictions until 2023.