Exhibit 99.3
DESCRIPTION OF ELICIO’S BUSINESS
BUSINESS
Overview
Elicio is a clinical-stage biotechnology company pioneering the development of therapeutic cancer vaccines for patients with limited treatment options and poor outcomes. Through its AMP platform technology, Elicio’s goal is to re-engineer the body’s immune response to defeat diseases using potent lymph node targeted vaccines and immunotherapies.
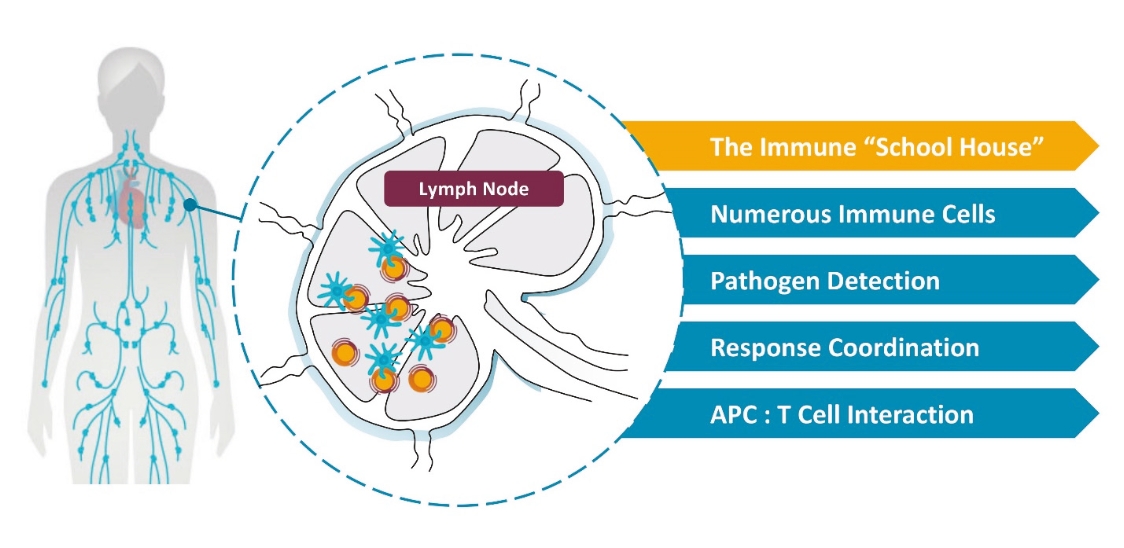
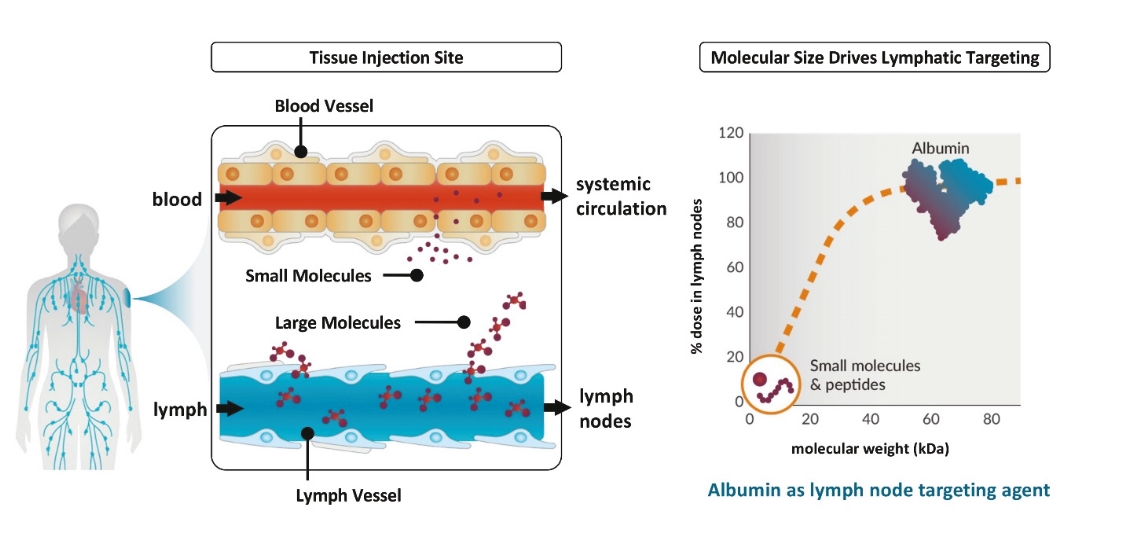
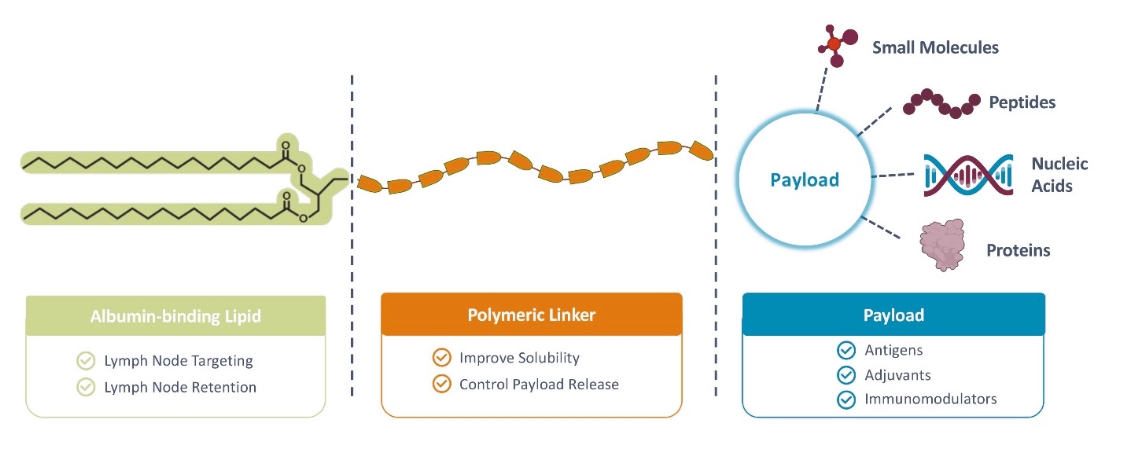
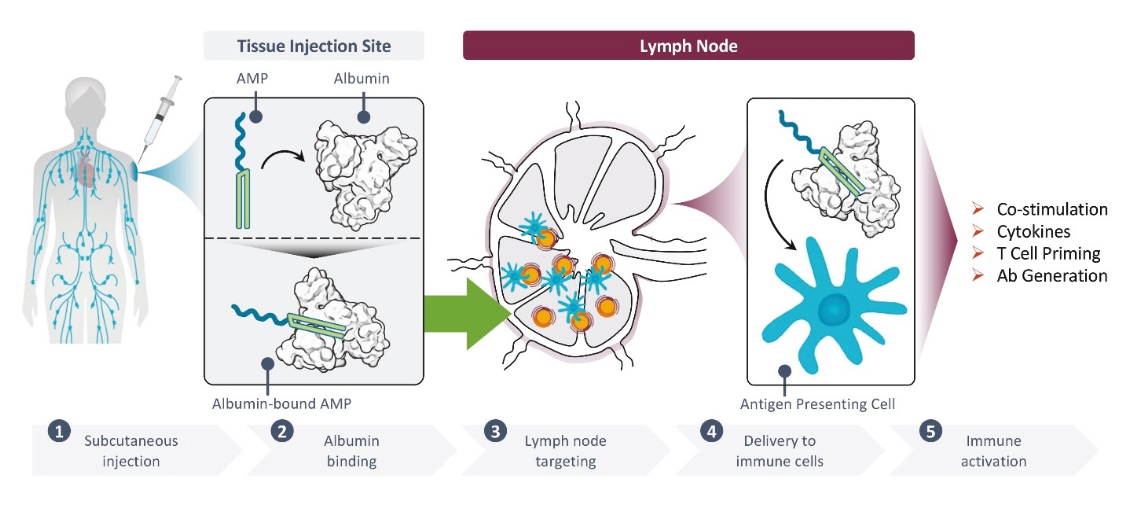
Elicio’s proprietary AMP technology precisely traffics immuno-modulatory molecules to the lymph nodes, the “schoolhouse” of the immune system, enhancing the magnitude, potency, functionality, and durability of the immune response. The lymph nodes are a primary site in the body where most immune cells are located. The lymph nodes are where the immune system naturally collects information about health and disease in order to orchestrate the mechanisms of immunity which protect us from pathogens and tumors. By efficiently targeting these sites within the body we are taking advantage of the power and the unique biology of the lymph nodes to improve responses across a broad range of diseases. Elicio’s is utilizing its lymph-node targeting technology to build a pipeline of therapeutic cancer vaccines, which will be the focus of Elicio. Other applications of the AMP technology, such as infectious disease vaccines and immune cell therapies, will be developed through partnerships.
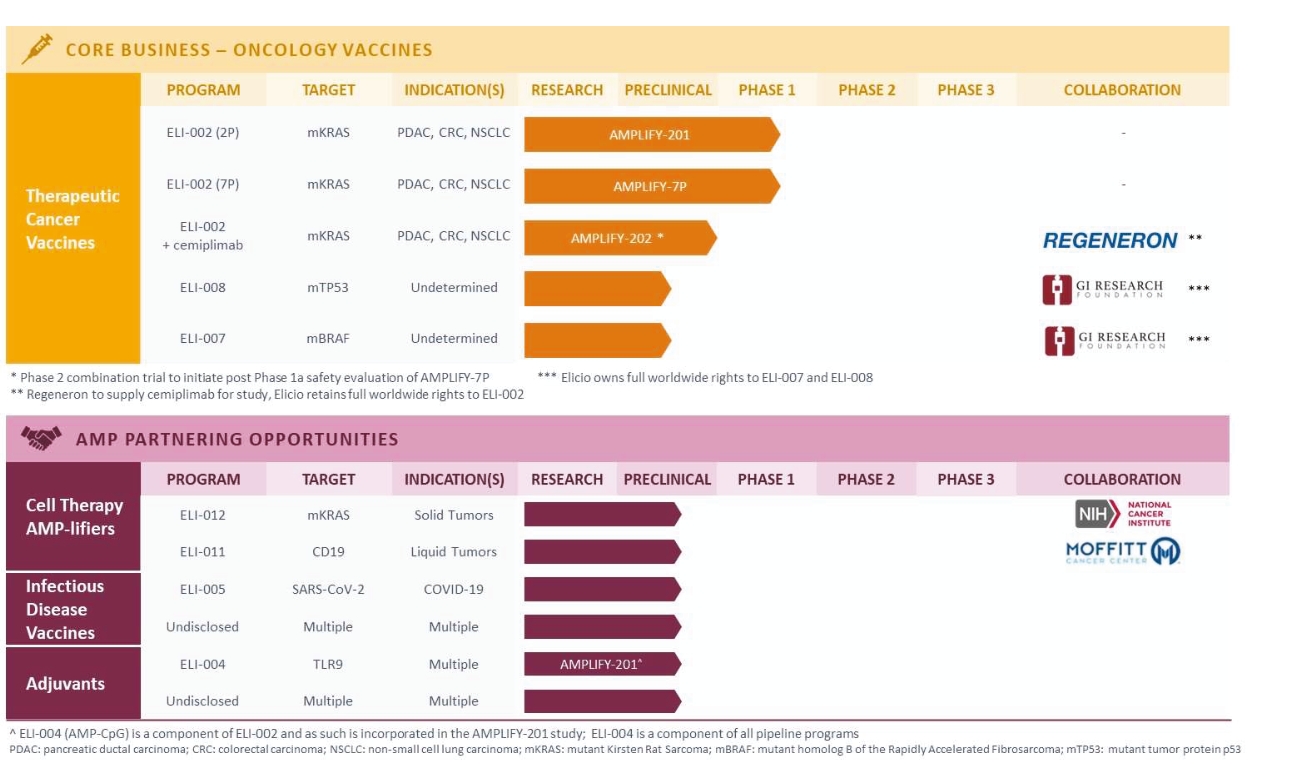
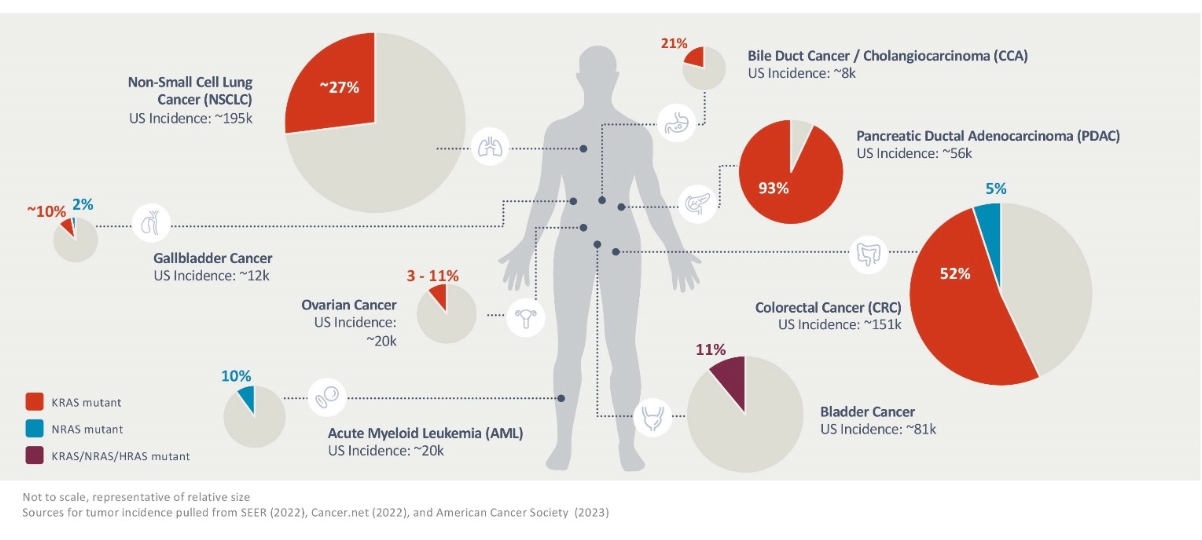
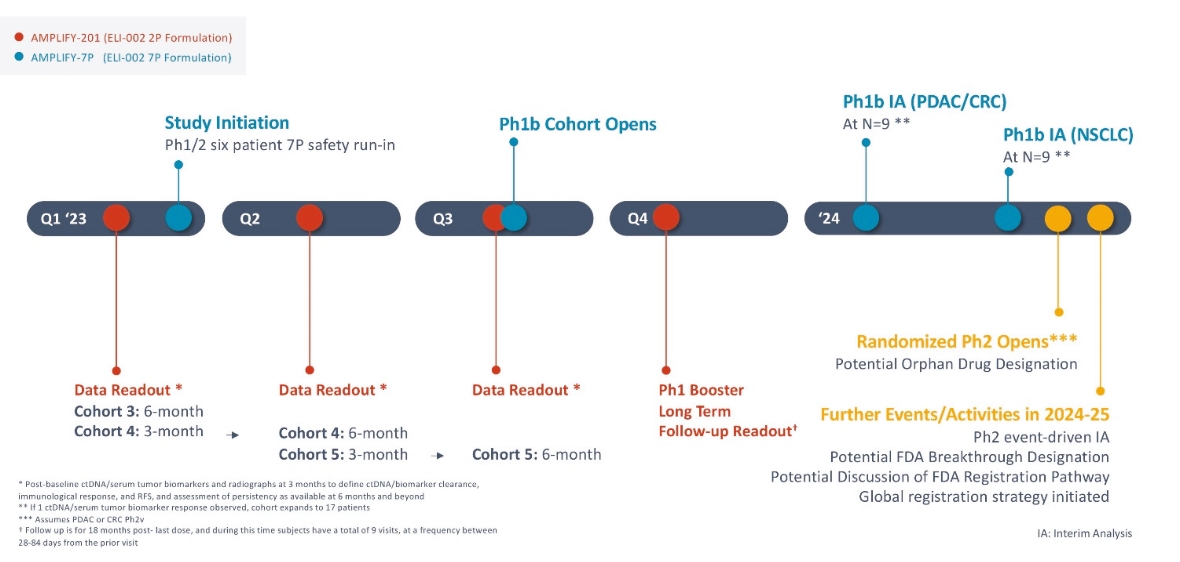
Elicio’s core business is the development of therapeutic cancer vaccines. ELI-002, its lead clinical program, is designed to stimulate an immune response against the KRAS mutations driving 25% of solid tumors. ELI-002 is currently AMPLIFY-201 in patients with mutant (m)KRAS-driven PDAC and CRC.
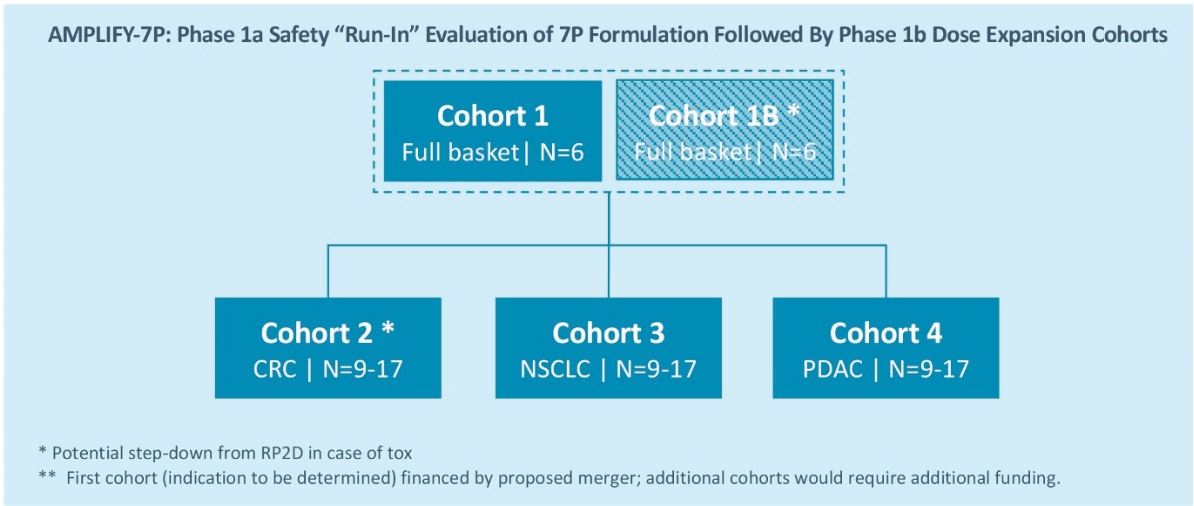
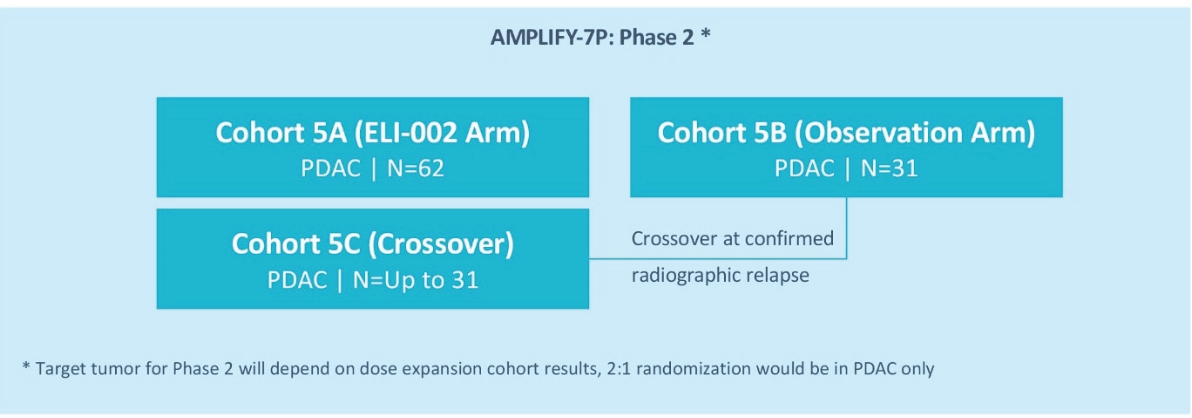
The ELI-002 program is planned to provide multiple potential pathways to success, including enrolling a cohort of patients with PDAC or CRC in a planned Phase 1/2 study called “AMPLIFY-7P”. Also, subject to receipt of additional funding following the Merger, a clinical trial to study the combination of ELI-002 + LIBTAYO® (cemiplimab, Regeneron’s FDA-approved anti-PD-1 therapy) is planned. Studies are also being considered in other solid tumors and where ELI-002 could target additional RAS isoforms, such as neuroblastoma ras (NRAS) and Harvey rat sarcoma (HRAS), to further expand the addressable population for ELI-002. ELI-002 has the potential to be a universal, all-stage immunotherapeutic for treating and preventing mKRAS-driven cancers.
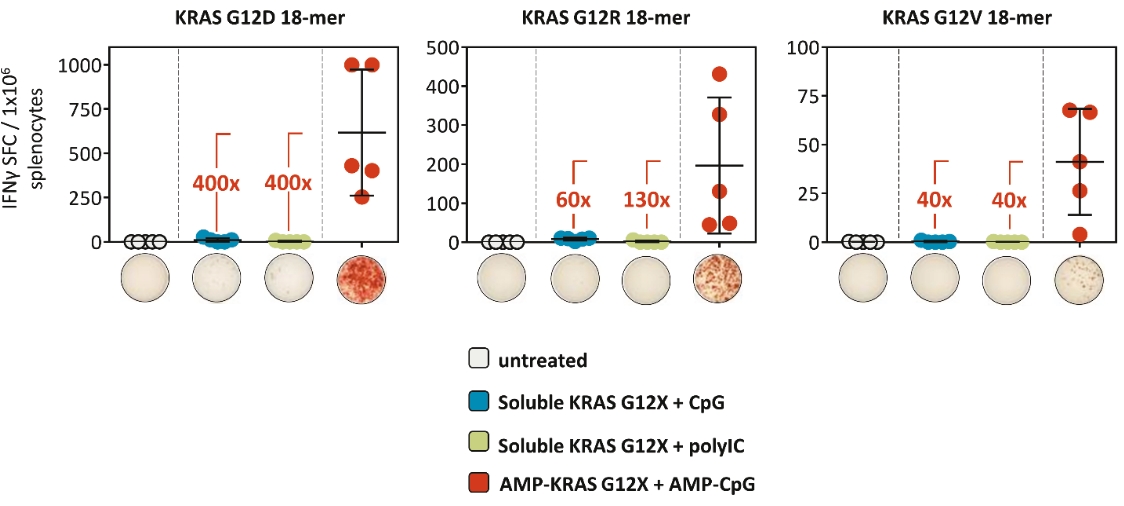
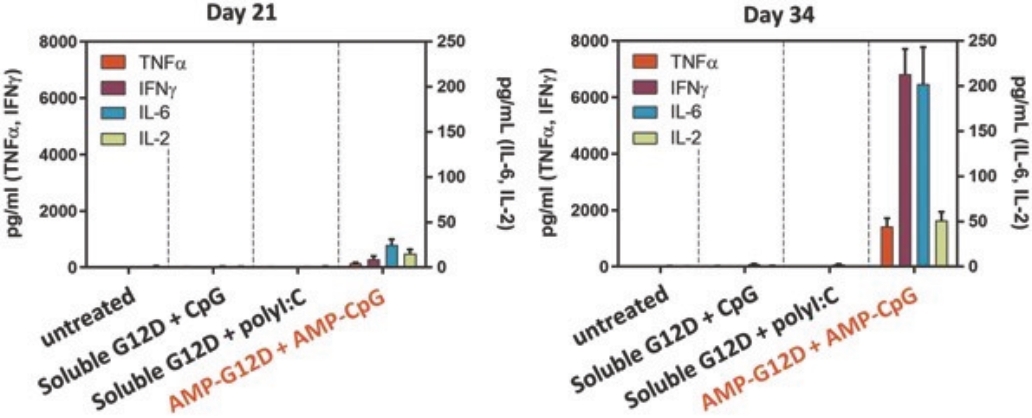
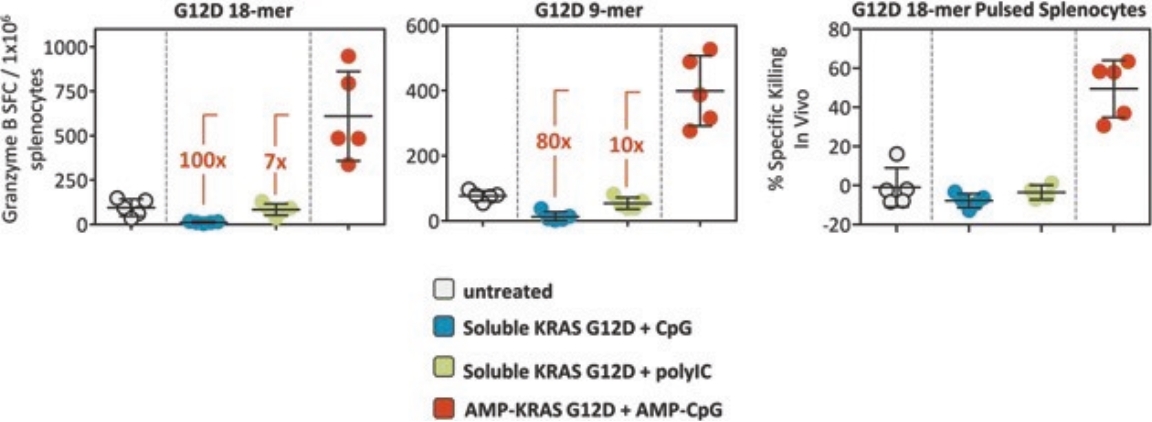
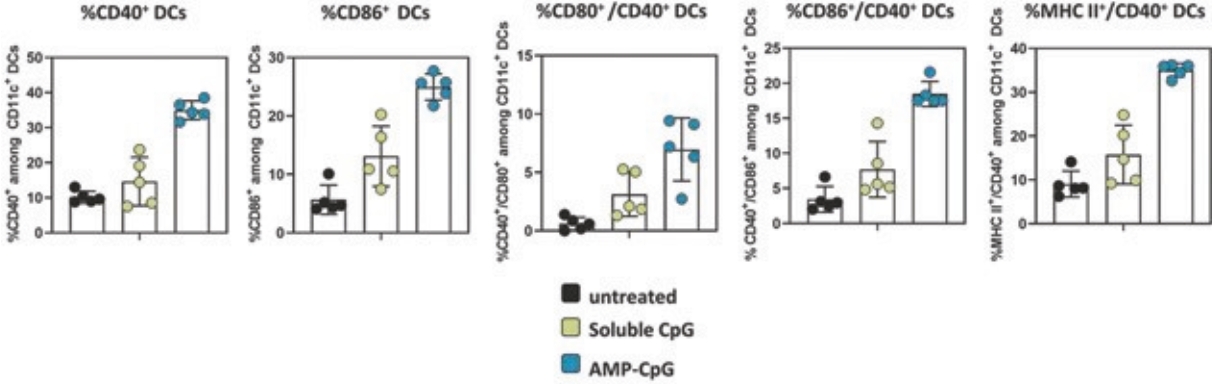
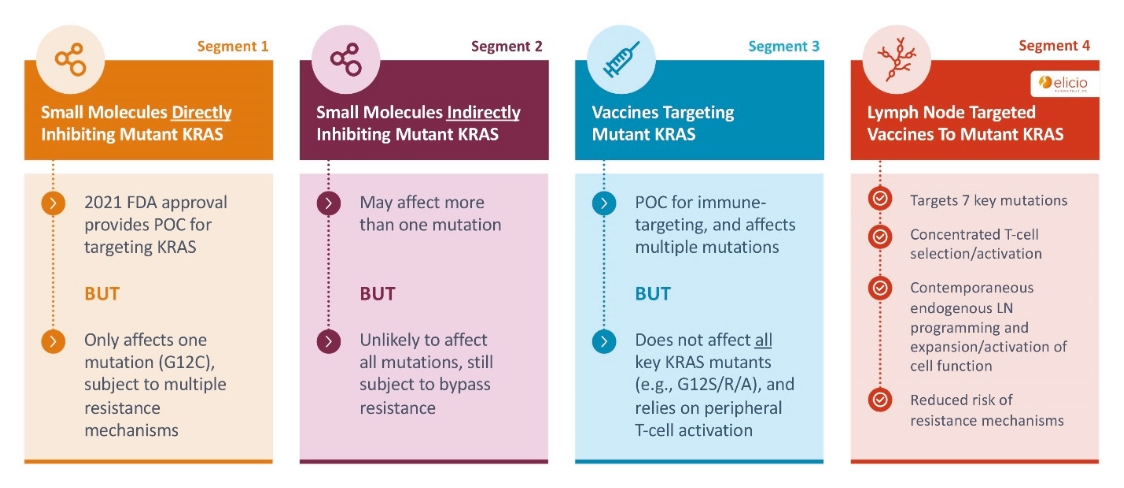
While past cancer vaccine efforts have struggled to induce a sufficient immune response to drive meaningful clinical benefit to patients, ELI-002 is poised to overcome these historical challenges through three advances intended to induce potent antitumor immune activity:
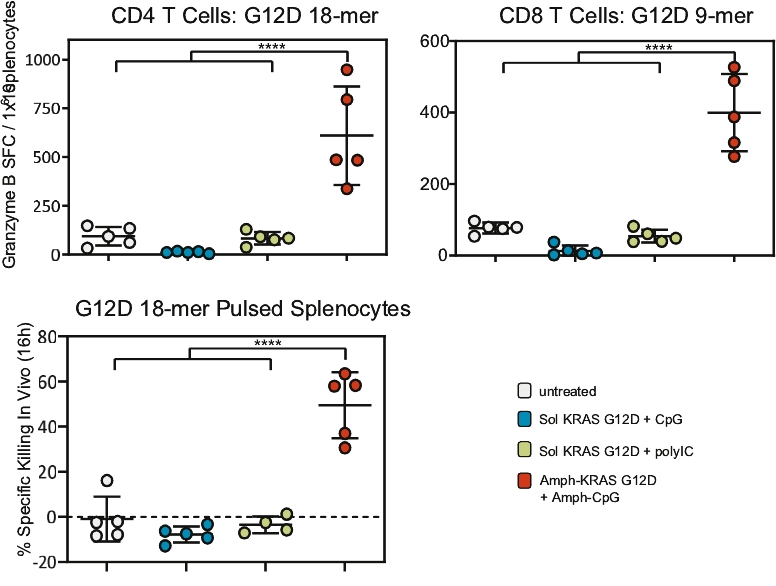
| • | The first major advance is the smart trafficking of ELI-002 to the lymph nodes after subcutaneous administration, generating tumor-targeted immune responses of increased magnitude, function, and durability. |
| • | The second advance is the clinical innovation of administering ELI-002 in the adjuvant setting, where ELI-002 is given after the patient has completed surgery and initial standard of care treatments to reduce the size and quantity of existing tumors and micrometastases. Using ELI-002 in the adjuvant setting is intended to maximize the number of mKRAS-targeting immune cells relative to the number of tumor cells. This strategy provides the potential for ELI-002 to eliminate any remaining residual disease in order to give the patient a longer period of disease control. |
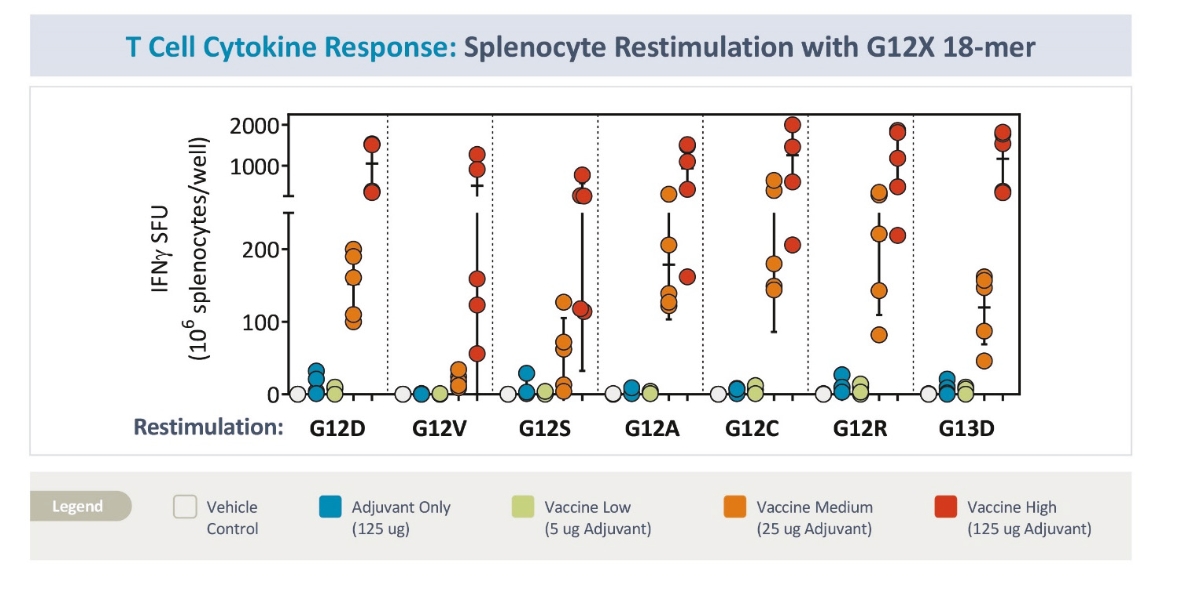
| • | The third advance is the inclusion of seven mKRAS peptides allowing ELI-002 to be used in additional patient populations, as well as offering the potential to target potential tumor-acquired escape mutations, thereby increasing the durability of responses to treatment. |
Through ELI-002, Elicio is exploring whether its lymph node targeting technology can create effective therapeutics out of biologically validated targets that have previously failed to induce clinically meaningful immune responses. This can be done quickly and efficiently, minimizing the time and cost for new target validation. Elicio is developing additional lymph node targeted therapeutic cancer vaccines using a similar approach to ELI-002, including ELI-007 for use in the treatment of mutant (v-raf murine sarcoma viral oncogene homolog B1) BRAF-driven cancers and ELI-008 for use in the treatment of mutated Tumor protein p53 (TP53) expressing cancers. Examples of mutant BRAF-driven and TP53-expressing cancers include melanoma, CRC, and NSCLC.
Elicio is also committed to maximizing the potential value of the AMP platform in non-core applications via collaboration and partnership opportunities. These non-core applications include immune cell therapy ‘AMP-lifiers’ which