
End of Q4 2021

This presentation and any accompanying discussion or documents may contain information that includes or is based upon "forward- looking statements" within the meaning of the Securities Litigation Reform Act of 1995. These forward-looking statements are based on our current expectations, estimates and projections about our industry, management's beliefs and certain assumptions we have made. They are neither historical facts nor assurances of future performance, are subject to significant risks and uncertainties, and may turn out to be wrong. For a discussion of factors that could affect our business, please refer to the "Risk Factors" sections in our Prospectus filed with the SEC on April 16, 2021 and in our periodic filings with the SEC. This presentation does not purport to contain all the information that may be required to make a full analysis of the subject matter. We undertake no obligation to correct or update any forward-looking statements. 2

Recursion is a clinical stage Pharmatech company Mapping and Navigating biology designed to bring better medicines to patients faster and at lower cost via an Internal Pipeline and Partnerships 3 The Leading Pharmatech >150+ Biologists, chemists and drug developers >150+ Data scientists, software programmers, and engineers Mapping & Navigating 13 Petabytes of proprietary biological and chemical data generated in-house >200B inferred biological relationships to mine using our maps of biology Internal Pipeline 3 Programs entering Ph2 or Ph2/3 and 1 program entering Ph1 in 2022 >10 programs in late discovery or preclinical Dozens of programs in early discovery Partnerships >$230M in upfront payments and investment to date from partners >$500M in performance/data-sharing milestones possible in intermediate term >$13B in project milestones across up to 50+ programs possible Royalties on all partnered programs 3

4 Political sentiment the world over, and increasingly in the U.S., against high drug prices will create additional pressure 1Deloitte, "Measuring the Return from Pharmaceutical innovation" (2020 and 2015 editions). 2Mullard, A. Nature Reviews Drug Discovery 2021. 3Biopharma Dive, 2018 (leerink) and Brown, D and Wobst H J Med Chem 2021 (FIC) 60% of sales growth of the top selling drugs is accounted for by price increases3 The number of new drug approvals is up only 47% over the last 25 years and first in class drug approvals have fallen 17% over the past decade2 $2.4B of R&D per new drug is 2.1 times more than a decade ago1

Well-known primary relationships found by the Recursion OS between key members of five pathways: JAK/STAT | SWI/SNF | TGFβ | RAS | Proteasome All primary relationships found by the Recursion OS between key members of five pathways: JAK/STAT | SWI/SNF | TGFβ | RAS | Proteasome 5

6

1 3 Real (atoms) Digital (bits) 1 Profile real systems Capture high-dimensional data to create a digital record of reality (things, places, behaviors, preferences, etc) Aggregate and analyze Aggregate data to create digital maps of reality Algorithmic Inference Navigate digital maps to predict or suggest novel relationships and routes - then try them in reality 3 2 2 7

8

How we build maps of biology 9 Enables quality, reliability, repeatability and scale Novel Efficacy and Insights At Scale Human cell types Small Molecules Arrayed CRISPR guides Cytokines & soluble factors Biological and Chemical Diversity Automated Execution at Scale Experiments per week Up to Digitization of Complex Biology Proprietary High-Dimensional Biological Data ML/AI-Based Analysis Supercomputer on Earth (TOP500 List - Nov 2021) ML-enabled hypotheses ML/AI-Based ExplorationHigh-Dimensional Validation Near whole exomes per week Proteomics panels in 2021 Up to

10

To the left is a whole-genome arrayed CRISPR KO Map generated in primary human endothelial cells Recursion visualizes its Maps in different ways. Below is a Map of thousands of new chemical entities, clustered by chemical similarity and colored by potency, which demonstrated a strong anti-inflammatory response on the Recursion OS 11

Many known mitochondrial-related genes cluster together along with a few less well-known genes 12

JAK1 Many known JAK / STAT genes cluster together along with a few less well-known genes 13

JAK1 JAK1 The JAK / STAT pathway now clustered by strength of interaction, including both similar genes (red) and ‘opposite’ genes (blue) – note that both expected and less well-known genes can serve as novel hypotheses 14
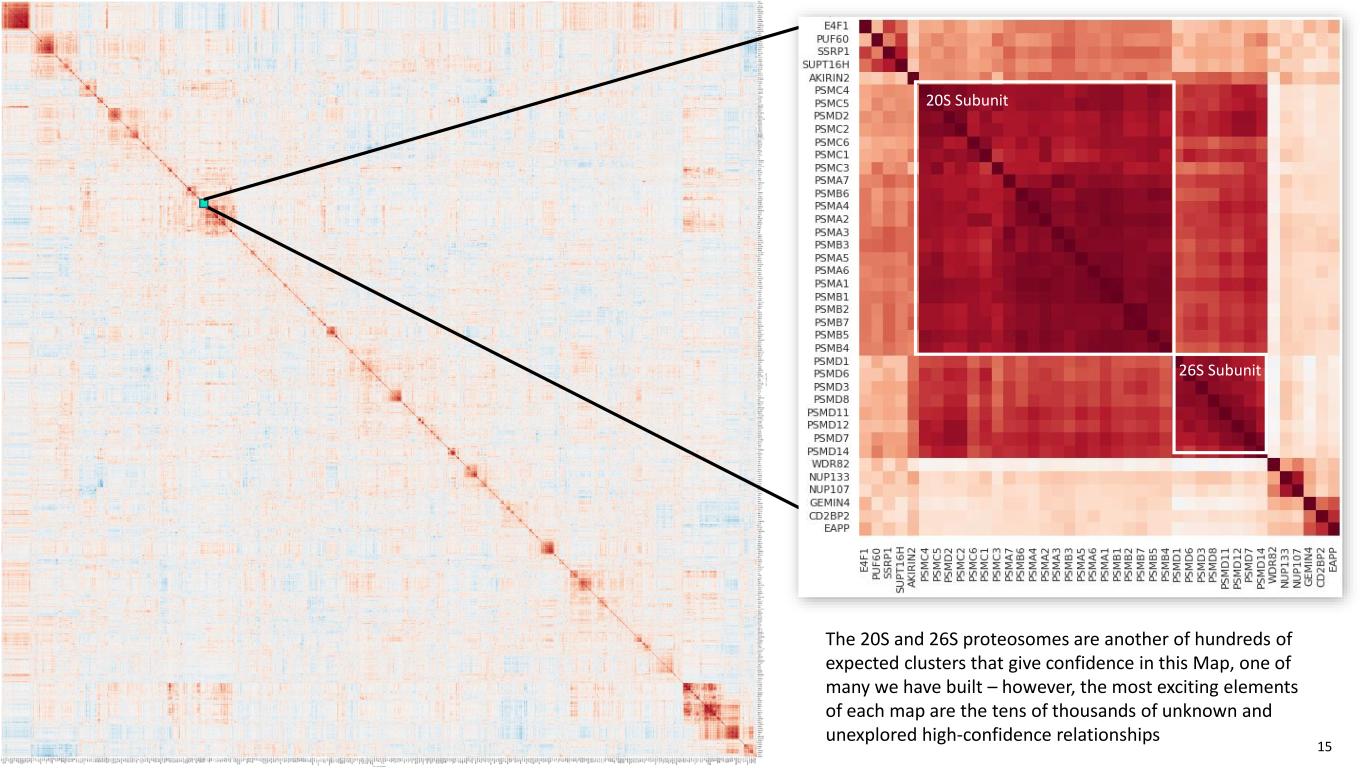
20S Subunit 26S Subunit The 20S and 26S proteosomes are another of hundreds of expected clusters that give confidence in this Map, one of many we have built – however, the most exciting elements of each map are the tens of thousands of unknown and unexplored high-confidence relationships 15

World’s literature reviewed People generate hypotheses Hypotheses validated in low dimensional assays 1. Millions of disparate journal articles and publications 2. Many data cannot be independently replicated 3. Human-selected low dimensional assays prone to confirmation bias 4. Humans prone to confirmation bias MAP OF BIOLOGY >200B predicted relationships from >100M highly reproducible experiments People navigate machine-generated hypotheses with unmet need and scientific intuition in mind Chosen hypotheses automatically validated using orthogonal high- dimensional assays 1. Massive, relatable proprietary map of searchable biology 2. Data highly replicable and scalable 3. High-dimensional orthogonal validation minimizes ‘leak’ of poor hypotheses to later stages 4. Minimization of human bias 5. Maximization of biological systems relevance This approach is designed to achieve higher clinical success rates Traditional Approach LIMITATIONS BENEFITS <10% clinical success rate Increased efficiency of translation: more scale, more speed, less costTranslation Recursion Approach 16

Data shown are the averages of all our programs from 2017 through 2021. All industry data adapted from Paul, et al. Nature Reviews Drug Discovery. (2010) 9, 203–214 17

18 All populations are US and EU5 incidence unless otherwise noted. EU5 is defined as France, Germany, Italy, Spain and UK. (1) Prevalence for hereditary and sporadic symptomatic population. (2) Annual US and EU5 incidence for all NF2-driven meningiomas. (3) Worldwide prevalence; conducting dose optimization study in animal model with a potential trial start in 2024 (4) US and EU5 prevalence (5) Our program has the potential to address a number of indications with systemic or neural inflammatory components We have not finalized a target product profile for a specific indication. (6) Our program has the potential to address a number of indications driven by MYC alterations, totalling 54,000 patients in the US and EU5 annually. We have not finalized a target product profile for a specific indication. (7) Our program has the potential to address a number of indications in this space.

Internal Pipeline - Rare Genetic Diseases Asset Sales / Licenses / Commercialization Partnership strategy • Partner in large, difficult and/or competitive therapeutic areas • Add knowledge to and improve our growing atlas of biology Internal Pipeline Discovery Partnerships Commercialization, licensing and asset sales Fibrosis Other large, intractable areas of biology Neuroscience *and a single oncology indication 19 • Exclusive multi-year discovery deal signed in ‘20 and expanded in ‘21 • $30M upfront and $50M equity investment • Up to or exceeding $1.2B in milestones for up to or exceeding 12 programs • Mid single-digit royalties on net sales • Exclusive multi-year discovery deal signed in ‘21 • $150M upfront and up to or exceeding $500M in performance-based research milestones and data usage options • Up to or exceeding $300M in possible milestones per program for up to 40 programs • Mid to high single-digit tiered royalties on net sales Pipeline strategy • Advance programs where we have a data arbitrage • Focus on capital efficient therapeutic areas Oncology Inflammation & Immunology Rare Diseases

The earliest iterations of the Recursion OS leveraged brute-force search (where small molecules were tested directly in the context of each disease model we built) and used a small molecule library restricted primarily to known chemical entities. Programs arising from this iteration of the Recursion OS are deemed First Generation Programs. As we developed our chemistry capabilities and new chemical entity library at Recursion, Second Generation Programs arose, though the throughput needed to screen large libraries of new chemical entities presents a powerful but relatively inefficient solution. Today, most of our new programs, as well as new partnerships or expansions of prior partnerships, are Next Generation Programs, whereby we use our maps of biology to navigate to novel or unexpected relationships between molecules (known or new chemical entities) and then validate those predictions in our wet labs 20

US + EU5 PREVALENCE CAUSE LOF mutations in genes CCM1, CCM2 & CCM3, key for maintaining the structural integrity of the vasculature due to unknown mechanisms PATHOPHYSIOLOGY Vascular malformations of the CNS leading to focal neurological deficits, hemorrhage and other symptoms OUR REASON TO BELIEVE Efficacy in Recursion OS as well as functional validation via scavenging of massive superoxide accumulation in cellular models; reduction in lesion number with chronic administration in mice KEY ELEMENTS • Targeting sporadic and familial symptomatic CCM • Patients with CCM1, CCM2, and CCM3 mutations • Once daily oral dosing • US and EU Orphan Drug Designation granted Julia – living with CCM High Dose Low Dose Placebo 1:1:1 52-Week Treatment Open Label Extension 1° - Safety and Tolerability 2° - Imaging, assessments and PROs CCM Confirmed with MRI 21

US + EU5 INCIDENCE CAUSE LOF mutations in NF2 tumor suppressor gene PATHOPHYSIOLOGY OUR REASON TO BELIEVE Efficacy in Recursion OS, cellular, & animal models; suppression of aberrant ERK, AKT, and S6 pathway activation in a Phase 1 PD Study in NF2 patient tumors KEY ELEMENTS • Targeting familial and sporadic NF2 meningioma patients • Adult and adolescent patient populations • Oral bioavailability and CNS exposure together are unique among clinical-stage HDAC inhibitors • Potentially reduced cardiac toxicity compared to class Inherited rare CNS tumor syndrome leading to loss of hearing and mobility, other focal neurologic deficits Ricki – living with NF2 REC-2282 40mg REC-2282 60mg 1:1 6 x 1 Month Cycles 1° - PFS 2° - TTR, DOR & ORR Interim Analysis 20 x 1 Month Cycles Phase 2 REC-2282 Placebo 2:1 26 x 1M Cycles Phase 3 Progressive NF2-mutated meningioma Progressive NF2-mutated meningioma 22

US + EU5 PREVALENCE CAUSE Inactivating mutations in the tumor suppressor gene APC PATHOPHYSIOLOGY Polyps throughout the GI tract with extremely high risk of malignant transformation OUR REASON TO BELIEVE Efficacy in the Recursion OS shows that specific MEK 1/2 inhibitors had a specific effect in context of APC LOF. Subsequent mouse model APCmin showed potent reduction in polyps and dysplastic adenomas KEY ELEMENTS • Targeting Classical FAP patients (w/ APC mutation) • Benign polyps and dysplastic adenomas • Oral Dosing & Gut-Biased • US Orphan Drug Designation granted High Dose Low Dose Placebo 2:2:1 Food Effect Open Label Extension 1° -Δ Polyp burden at 12M 2° - PL, Safety, polyp # and histological grade FAP with APC mutation confirmed 48 Week Treatment 23

US + EU5 INCIDENCE CAUSE Release of C. Difficile toxins by colonizing bacterium causes degradation of colon cell junction, toxin transit to bloodstream, and morbidity to host PATHOPHYSIOLOGY Highly recurrent infectious disease with severe diarrhea, colitis, and risk of toxic megacolon, sepsis, and death OUR REASON TO BELIEVE Efficacy on the Recursion OS identified a new chemical entity for prophylaxis and recurrent C. difficile infection via glycosyl transferase inhibition with potential to be both orally active and gut-biased KEY TPP ELEMENTS • Orally active small molecule toxin inhibitor • Non-antibiotic approach with potential for combination with SOC and other therapies for recurrent disease • Designed for gut-biased pharmacology to target infection in the GI tract while reducing systemic exposure and potential systemic effects • Not expected to negatively impact the gut microbiome 24 Colleen – overcame recurrent C diff.

Efficacy demonstrated in CT26 checkpoint resistance (left) mouse model; complete response (CR) mice show minimal tumor growth when rechallenged (middle left). Peripheral IL-6 remain unchanged (middle right) while intertumoral IFNg increases EMT6 Gene B Gene B REC- 648918 REC-648918 BIRC2 Gene A Gene A BIRC2 • Goal: Identify novel compounds capable of re-sensitizing tumors with tumor- intrinsic resistance factors to checkpoint therapy • Phenomap insight: Novel compound (REC-648918) identified with similarity to knockout of potential immunotherapy resistance gene targets (Gene A, Gene B) • Result: Reduction in tumor growth vs. anti-PD-1 alone in both CT26 checkpoint resistance and EMT6 models (including 40% and 80% complete response in combination in each model respectively) Efficacy demonstrated in EMT6 mouse model days CT26: mouse colon carcinoma. REC-648918 was dosed PO, QD for 5 weeks at 100mg/kg. Anti-PD-1 was dosed IP, BIW for 5 weeks at 10mg/kg. 10 mice per group, dosing initiated when tumors reached ~ 80 mm3; * p<0.05 ** p<0.01 **** p<0.0001; ^ Combination treatment in EMT6 resulted in 8 CR and 8 rejections on re-challenge 25 CT26 REC-648918 REC-648918 + aPD1 Vehicle Anti-PD-1 Tumor-intrinsic immune resistance STK11 Target Alpha Target Beta More... Similar Opposite 25 80% CR in combo CT26 rechallenge 40% CR in combo

Gene A CDK12 2.5μM 1.0μM REC-65029 0.1μM 0.25μM REC-65029 • Goal: Identify novel compounds capable of sensitizing HRD-negative ovarian cancer and other tumors to PARP inhibition • Phenomap insight: Inhibition of target Gene A (for example, with REC-65029) may mimic inhibition of CDK12 while mitigating toxicity due to CDK13 inhibition • Result: Single agent and combo activity with olaparib in an HRD-negative ovarian cancer CDX and PDX models with durable response Single agent activity in olaparib-resistant CDX mouse model Single agent and combination (with olaparib) in an HRD-negative ovarian cancer PDX model with durable response 26 OVCAR-3 CDX – animals dosed with REC-65029 for 5 days at 100 mg/kg BID, a holiday from days 6-9 (due to body weight loss) and dosing resumed at 85 mg/kg BID; OV0273 PDX – REC-65029 dosed at 85 mg/kg PO, BID, olalparib dosed at 90mg/kg PO QD; ** p<0.01 **** p<0.0001 Treatment stopped on Day 28 OV0273 PDX OV0273 PDX (Survival)OVCAR-3 CDX 80% CR by day 14 in combo 100% CR Tumor-targeted precision therapeutics Hepatocellular Carcinoma MYC CDK12 More... Similar Opposite 26

Life Sciences - biology, chemistry, development, etc. Tech - data science, software engineering, automation, etc. Strategic Operations Advanced degrees Employees today Team Credentials 400+ 44% All employees VP and above Gender: % Women 41% 41% 27

28 Enables quality, reliability, repeatability and scale Recursion OS Continued digital chemistry and predictive ADMET OS build Clinical Predictions Near-TermToday Intermediate-Term

29 Near-Term Milestones • Rec-994 for CCM Ph2a clinical start in Q1 • Rec-2282 for NF2 Ph2/3 clinical start in Q2 • Rec-4881 for FAP Ph2 clinical start in Q2 or Q3 • Rec-3964 for C diff. IND and Phase 1 start in 2H • Additional INDs and Clinical Starts • Option Exercises for Partnership Programs • $517M in cash, equivalents & investments with no substantial debt at end of Q4, 2021 • Does not include $150M upfront from Roche/Genentech collaboration Strong Financials C lin ic al P ro gr am s Medium-term milestones • Multiple POC readout(s) for AI-discovered programs • Potential additional partnership in large, intractable area of biology • Additional option exercises for partnership programs • Recursion OS Begins to move to Autonomous Map Building and Navigation with Automated Chemical Synthesis, Digital Chemistry and Predictive ADMET Tools • In-House Small Molecule Manufacturing Capabilities come On-Line

IMPACT

Board of Directors TINA LARSON President & COO MASON VICTORS Chief Product Officer Executive Team CHRIS GIBSON, PHD Co-Founder & CEO DEAN LI, MD/PHD Recursion Co-founder, President of Merck Research Labs ZAVAIN DAR Partner, Lux Capital ZACHARY BOGUE, JD Partner, Data Collective ROBERT HERSHBERG, MD/PHD Former EVP CSO & BD, Celgene BLAKE BORGESON, PHD Recursion Co-founder, Board member Machine Intelligence Research Institute SHAFIQUE VIRANI, MD FRCS Chief Corp Dev Officer MICHAEL SECORA, PHD Chief Financial Officer HEATHER KIRKBY Chief People Officer BEN MABEY Chief Technology Officer CHRIS GIBSON, PHD Co-founder & CEO TERRY-ANN BURRELL, MBA CFO & Treasurer Beam Therapeutics R. MARTIN CHAVEZ Vice-Chair of 6th Street Financial. Former CFO/CIO at GS RAMONA DOYLE, MD Chief Medical Officer LOUISA DANIELS, JD Chief Legal Officer & General Counsel Trademarks are the property of their respective owners and used for informational purposes only. 31

• Growth in capabilities, proprietary data, programs, and partnerships • Increasing business opportunities • Reducing binary risks We are a biotechnology company scaling more like a technology company, as demonstrated by our growth in inputs (experiments) and growth in outputs (data, biological and chemical relationships, programs, and partnerships). (1) Includes approximately 500,000 compounds from Bayer’s proprietary library. (2) ‘Predicted Relationships’ refers to the number of Unique Perturbations that have been predicted using our maps. (3) Announced a collaboration with Roche and Genentech in December 2021 and received an upfront payment of $150 million in January 2022. Year 2018 2019 2020 2021 Total Phenomic Experiments (Millions) 8 24 56 115 Data (PB) 1.8 4.3 6.8 12.9 Cell Types 12 25 36 38 Total Chemical Library1 (Thousands) 24 106 706 978 In Silico Chemistry Library (Billions) 0 0.02 3 12 Predicted Biological and Chemical Relationships2 (Billions) NA NA 13 203 IND-Enabling and Clinical Stage Programs 1 2 4 5 Cumulative Upfront and Investment Payments Committed by Partners3 $0 $0 $80M $230M Cumulative Potential Payments from Partners Excluding Royalties $0 $0 >$1B >$13B 32
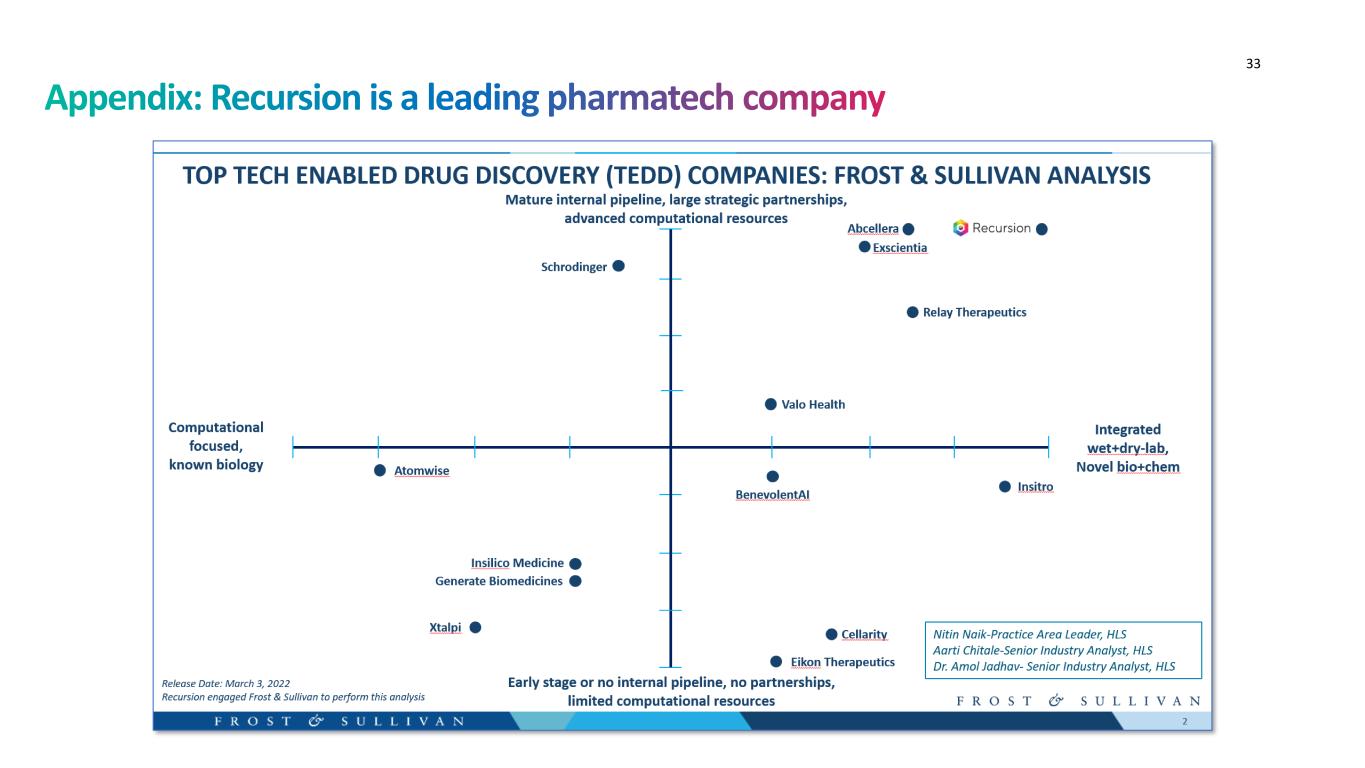
33

34

































