

This presentation and any accompanying discussion and documents contain information that includes or is based upon "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995. These forward-looking statements are based on our current expectations, estimates and projections about our industry and our company, management's beliefs and certain assumptions we have made. The words “plan,” “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. Forward-looking statements made in this presentation include the occurrence or realization of any near- or medium-term potential milestones; the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical and clinical studies, including timelines for enrollment in studies, data readouts, and progression toward IND-enabling studies; outcomes and benefits from licenses, partnerships and collaborations, including option exercises by partners, additional partnerships, and the ability to house tools on the BioNeMo Marketplace; outcomes and benefits expected from the Tempus partnership, including our ability to leverage the datasets acquired through the license agreement into increased machine learning capabilities and accelerate clinical trial enrollment; outcomes and benefits expected from the Helix partnership, including the development of causal AI models and biomarker and patient stratification strategies; the potential size of the market opportunity for our drug candidates; outcomes and benefits expected from the Enamine partnership, including the generating and co-branding of new chemical libraries; expected BioHive supercomputer capabilities; the potential for additional partnerships and making data and tools available to third parties; advancements of our Recursion OS, including augmentation of our dataset; outcomes and benefits expected from the Large Language Model-Orchestrated Workflow Engine (LOWE); our ability to identify viable new drug candidates for clinical development and the accelerating rate at which we expect to identify such candidates; our expectation that the assets that will drive the most value for us are those that we will identify in the future using our datasets and tools, and many others. Forward-looking statements made in this presentation are neither historical facts nor assurances of future performance, are subject to significant risks and uncertainties, and may not occur as actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. For a discussion of factors that could affect our business, please refer to the "Risk Factors" sections in our filings with the U.S. Securities and Exchange Commission, including our most recent Quarterly Report on Form 10-Q and our Annual Report on Form 10-K for the Fiscal Year ended December 31, 2023. This presentation does not purport to contain all the information that may be required to make a full analysis of the subject matter. We undertake no obligation to correct or update any forward-looking statements, whether as a result of new information, future events or otherwise. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the company’s own internal estimates and research. While the company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the company believes its own internal research is reliable, such research has not been verified by any independent source. Information contained in, or that can be accessed through our website (including the company’s ESG report referenced herein) is not a part of and is not incorporated into this presentation. Cross-trial or cross-candidate comparisons against other clinical trials and other drug candidates are not based on head-to-head studies and are presented for informational purposes; comparisons are based on publicly available information for other clinical trials and other drug candidates. Any non-Recursion logos or trademarks included herein are the property of the owners thereof and are used for reference purposes only. 2

Recursion Poised to Hit TechBio Escape Velocity Pipeline, Platform, and People 3

Pipeline 4

5 More than a dozen discovery and research programs in oncology or with our partners – first program optioned by Roche-Genentech in GI-oncology All populations defined above are US and EU5 incidence unless otherwise noted. EU5 is defined as France, Germany, Italy, Spain, and UK. (1) Prevalence for hereditary and sporadic symptomatic population. (2) Annual US and EU5 incidence for all NF2-driven meningiomas. (3) Prevalence for adult and pediatric population. (4) Our program has the potential to address several indications. (5) We have not finalized a target product profile for a specific indication. (6) Incidence for US only. (7) 2L+ drug-treatable population. (8) 2L+ drug-treatable population comprising ovarian, prostate, breast, and pancreatic cancers. Program Indication Target Patient Population Preclinical Phase 1 Phase 2 Phase 3 Near-Term Milestones REC-994 Cerebral Cavernous Malformation Superoxide ~ 360K1 • Topline readout in Q3 2024 REC-2282 Neurofibromatosis Type 2 HDAC ~ 33K2 • Preliminary data readout in Q4 2024 REC-4881 Familial Adenomatous Polyposis MEK ~ 50K3 • Preliminary data readout in H1 2025 REC-3964 Clostridioides difficile Infection TcdB ~730K • Phase 2 initiation Epsilon Fibrotic Diseases Undisclosed ~ 50K4,5,6 • IND submission REC-4881 Advanced AXIN1/APC-Mutant Cancers MEK ~ 104K7 • Preliminary data readout in H1 2025 RBM39 Advanced HR-Proficient Cancers RBM39 ~ 220K8 • IND submission • Phase 1 initiation Ra re & O th er O nc ol og y LILAC SYCAMORE POPLAR TUPELO

Platform 6

7 Access to hundreds of thousands of de-identified records, including Helix’s Exome+(R) genomics & longitudinal health data, to train causal AI models and design biomarker & patient stratification strategies across broad disease areas

We just announced that we have sequenced our 1 MILLIONTH transcriptome In the process of creating a full genome transcriptomic map to explore novel biology and complement our phenotypic maps 8
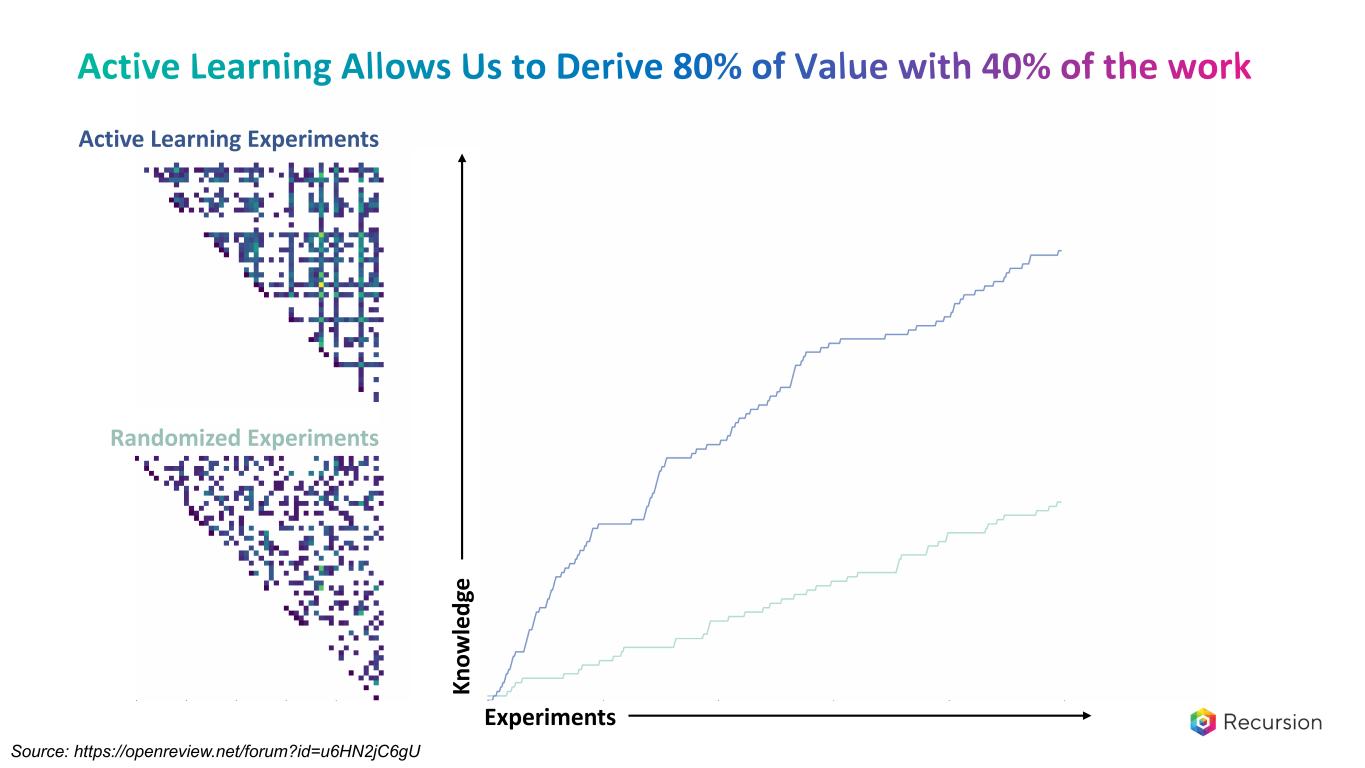
Kn ow le dg e Experiments Source: https://openreview.net/forum?id=u6HN2jC6gU Active Learning Experiments Randomized Experiments
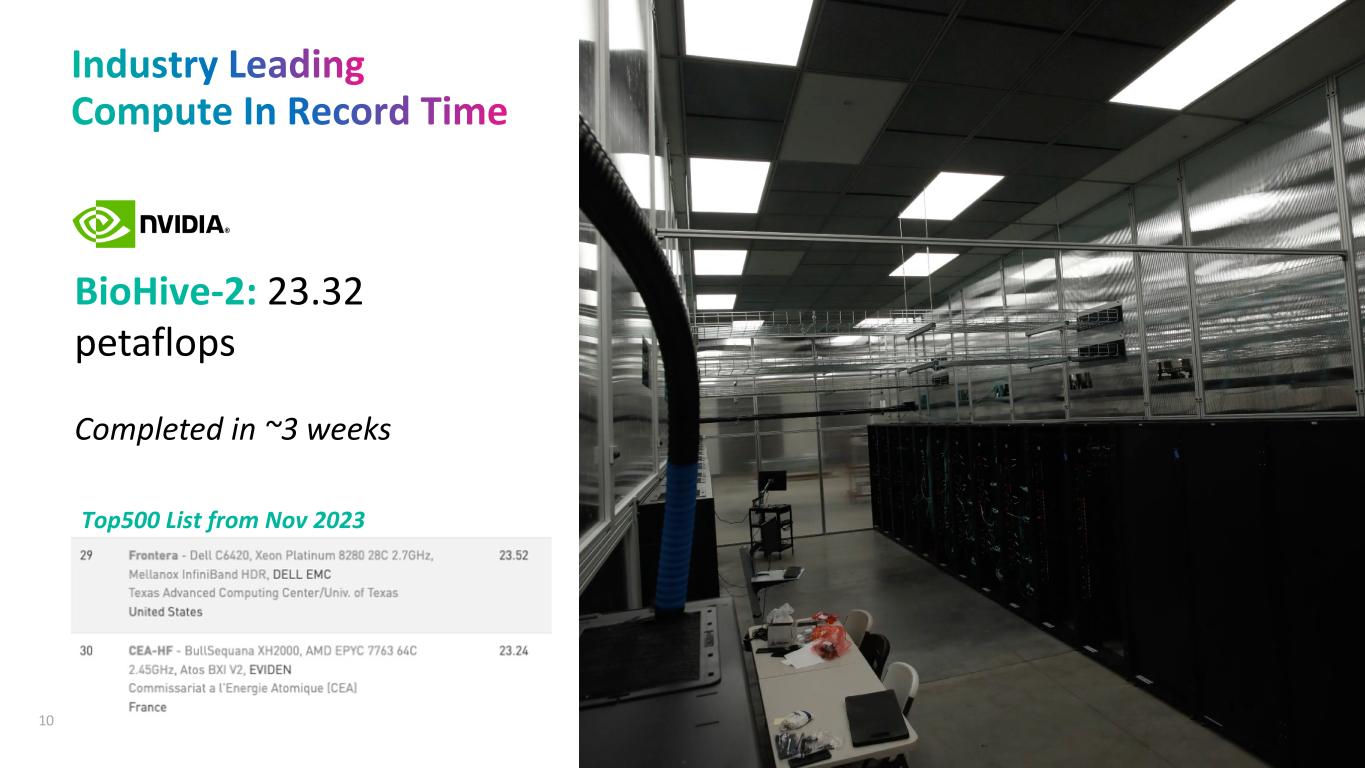
BioHive-2: 23.32 petaflops Completed in ~3 weeks 10 Top500 List from Nov 2023

People 11

Najat Khan, PhD, Appointed Chief R&D Officer & Chief Commercial Officer and Board Member • Former Chief Data Science Officer and Co- Chair of Data Science Council at Johnson & Johnson Michael Bronstein, DeepMind Professor of Artificial Intelligence, Oxford • Appointed as Recursion/Valence advisor Recursion plans to open first European location in London in June 12

Guidance 13

Pipeline: • Five expected Ph2 readouts in the next 18 months • Additional Ph2 trial starts in 2024 • Potential for additional INDs Partners: • Roche and Genentech: Pioneering collaboration, potential near-term program & map options • Bayer: Significant deal value, focused on undruggable oncology, potential near-term program options • Tempus: Potential near term novel NSCLC targets and large-scale causal AI models to generate target hypotheses across cancer • Potential for additional partnership(s) in large, intractable areas of biology (CV/Met) • Potential to make some data and tools available to biopharma and commercial users Platforms: • Recursion OS moves towards autonomous discovery • Active learning and exploration of proteomics, organoids, spheroids, and automated synthesis Strong Financial Position ~$296M in cash Q1 2024 Cash refers to cash and cash equivalents at the end of Q1 2024 14

Questions?














