Exhibit 99.1

Corporate Presentation October 2019 NASDAQ: NMRD

Forward - Looking Statements • This presentation includes forward - looking statements that are subject to many risks and uncertainties. These forward - looking statements, such as statements about Nemaura’s short - term and long - term growth strategies, can sometimes be identified by use of terms such as “intend,” “expect,” “plan,” “estimate,” “future,” “strive,” and similar words. These statements involve many risks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements. • These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including the risks identified under the section captioned “Risk Factors” in Nemaura’s Annual Report on Form 10 - K filed with the Commission in June 2019 as the same may be updated from time to time. • Nemaura disclaims any obligation to update information contained in these forward - looking statements whether as a result of new information, future events, or otherwise. 2 www.nemauramedical.com

Our Mission • By utilizing the world’s first CE Mark approved Non - invasive CGM (continuous glucose monitoring) product, SugarBEAT ®, we aspire to combine our SELF MONITORING/WEARABLE platform, with one - on - one COACHING (independently clinically proven to enhance the impact of diabetes management) to disrupt the $80B+ Diabetes Market. • Diabetes is a global pandemic affecting over 450 million people worldwide. • Within 5 years Nemaura aims to lead in the wearables market and self - management of medical conditions with our pipeline products of sensors and digital healthcare platforms using AI. • Employers, healthcare providers and insurers are already paying substantial fees to support patients for the long term, using apps and coaching, so SugarBEAT ® , together with the planned digital and on - demand coaching services, is poised to disrupt this space. • https://sugarbeat.com/introducing - sugarbeat/ 3 NASDAQ: NMRD

Investment Highlights x World’s first non - invasive CGM (continuous glucose monitor) SugarBEAT ® , targeting $179B+ Global opportunity: • Digital health sector comprising $69B+ type II diabetic market 1 • $50B+ pre - diabetic market 2 • Wearable health - tech sector comprising $60B+ weight loss & wellness markets 3 x US FDA approval and launch anticipated by end of 2020. x CE Mark Approved; UK & Ireland commercial launch expected in Q1 2020, and Germany in Q2 2020. x Lowest priced, high recurring margin model, with lowest COGS per CGM patch in the industry x Growing IP portfolio with over 30 issued & pending patents x Platform BEAT™ diagnostic technology with robust product pipeline: • Launching continuous lactate monitoring (CLM) near term, targeting $60B+ wearable tech market • Pipeline of four other products including non - invasive continuous alcohol monitoring (CAM) x Proven management team with successful track records x Clean capital structure with no long - term debt 1 PiperJaffray Company Note DXCM Sep 5 2018 2 Assuming 50% usage as compared to type II non insulin market 3 Juniper Research Digital Health Report Jan 14 2019 NASDAQ: NMRD

Our Offering 5 • We are poised to disrupt the multi - billon dollar glucose trending and diabetes management space • SugarBEAT ® is the World’s first non - invasive CGM (Continuous Glucose Monitor) where the sensor sits on top of the skin. Does not require needles and does not puncture the skin to insert a sensor • SugarBEAT ® is a flexible CGM which can be worn for a single day at a time, with no commitment to wear the device continuously for 10 - 14 days as is the case with other CGM, making it unlikely that the daily cost - of - use can be matched by our competitors • CE approved Class IIB Medical Device • US FDA approval and launch anticipated by end of 2020 • EU commercial Launch first, with U.S. and others to follow. • Empowering Glucose Trend data over the course of the day, with measurements recorded every 5 minutes • Replacing point in time finger - stick measurements which provide very little and often misleading information as the previous and subsequent readings are not known NASDAQ: NMRD
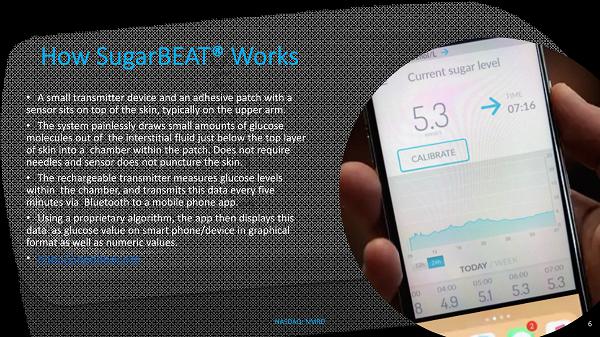
How SugarBEAT ® Works • A small transmitter device and an adhesive patch with a sensor sits on top of the skin, typically on the upper arm. • The system painlessly draws small amounts of glucose molecules out of the interstitial fluid just below the top layer of skin into a chamber within the patch. Does not require needles and sensor does not puncture the skin. • The rechargeable transmitter measures glucose levels within the chamber, and transmits this data every five minutes via Bluetooth to a mobile phone app. • Using a proprietary algorithm, the app then displays this data as glucose value on smart phone/device in graphical format as well as numeric values. • https://sugarbeat.com 6 NASDAQ: NMRD
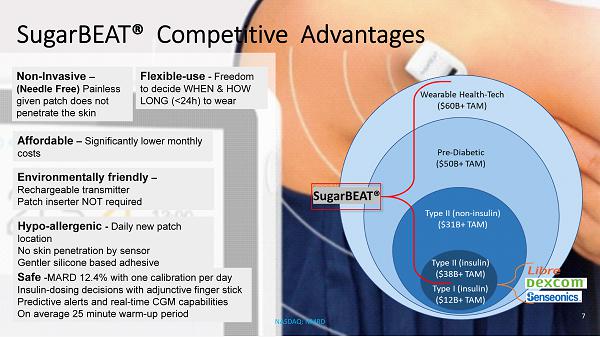
SugarBEAT ® Competitive Advantages Safe - MARD 12.4% with one calibration per day Insulin - dosing decisions with adjunctive finger stick Predictive alerts and real - time CGM capabilities On average 25 minute warm - up period Hypo - allergenic - Daily new patch location No skin penetration by sensor Gentler silicone based adhesive Environmentally friendly – Rechargeable transmitter Patch inserter NOT required Affordable – Significantly lower monthly costs Flexible - use - Freedom to decide WHEN & HOW LONG (<24h) to wear Non - Invasive – (Needle Free) Painless given patch does not penetrate the skin 7 NASDAQ: NMRD SugarBEAT ®

Potentially game - changing moment for big data in the health industry The Value of Medical Data Realtime Mobile technology has been one of the largest contributors to big data for the past several years. Proliferation of mobile phones, the implementation of app - enabled smart phones, and now the growth of the wearable device market are all creating massive new data flows that can be put to use for health and other purposes. Wearable medical devices are not new, but they have long been expensive and not always viable for every patient or subject of a medical study to wear. That’s why the growth of the wearable personal electronic device market – and the lower prices that come with a higher volume of devices being sold – is such a potentially game - changing moment for big data in the health industry Extracting personal data from wearable Medical devices: • Predictive analytics is based on logic that is drawn from the wearable Medical devices uses an algorithm to seek patterns and structure in data and cluster them into groups or insights. • Improving efficiencies per patient’s management of health care • Accuracy of diagnosis and treatment in personal medicine • Increased insights to enhance lifestyle, diabetes, drug management and cohort treatment Development of Artificial Intelligence and intermittent testing using SugarBEAT® has the potential to disrupt diabetes management from the following perspectives: • Empowering users with interpretations of SugarBEAT ® data. Empowering Industry such as Big Pharma to enhance drug treatment regimens and develop personalized therapy. • Seeking to capitalize on this groundbreaking approach to making large datasets more accessible, the U.S. National Institutes of Health — the preeminent U.S. government medical research organization, which oversees an annual $31 billion budget — is now working with IBM to connect a very wide variety of clinical and research datasets to the IBM Watson system. SugarBEAT®

Nemaura vs Livongo ® The technical difference! Starts with a supporting approved medical device . The Trends In Mobile Healthcare • What we are seeing is a total shift towards digital medical care. The records are more often kept electronically, there are more opportunities for remote patient monitoring, and more tools for individual health monitoring are emerging. Digital health deals are booming: the value of investments reached $2 billion in the first quarter of 2019 alone. • Nemaura is the latest breakthrough in mobile/wearable healthcare technology regarding functionality, technology, interconnectivity and all supported with real medical diagnostics from their CE approved medical device. Livongo ® Limitations: • Livongo ® for Diabetes: includes a cellular - connected interactive glucometer, unlimited blood glucose test strips, real - time coaching, and 24 - hour monitoring • Livongo for Pre - Diabetes and Weight Management: offers a cellular - connected weight scale, health educational content, personalized coaching service, and group classes Nemaura Limitless: • SugarBEAT ® CGM provides very powerful data that will allow long term therapy and lifestyle adjustments that finger prick testing cannot achieve • Clinically proven, evidence based health education on diabetes that has been demonstrated to lead to behavioral changes in diabetics . On - demand coaching service for personal fitness, motivation, and dietary advice, (through in - licensing and collaboration) 9 NASDAQ: NMRD 1 1 Livongo ® is a registered trademark of Livongo Health, Inc.

Commercial Strategy: Priority 1: Subscription based service • A monthly subscription to receive a set number of sensors each month and the app which will allow the user to share their data with family and care givers, and be able to monitor their glucose profile relative to lifestyle interventions and habits. Priority 2: Digital Coaching • Subscription based digital coaching supporting diabetes / health / nutrition / exercise/ behavioral changes and modification etc. Priority 3: AI Platform • Creation of AI platform to enhance user experience. This will be developed as we gather data from users. Priority 4: One - to - one digital coaching • Subscription based high value, low volume service, with coaches recruited in all local territories. 10 NASDAQ: NMRD

N EMAURA M EDICAL L AUNCH S TRATEGY • G ERMANY : M ARKET O PPORTUNITY OF $3 - 5B • UK: M ARKET O PPORTUNITY OF $2 - 4B • I RELAND : M ARKET O PPORTUNITY OF $1B • Nemaura's product offering is being led by their Vice President of Strategy and Strategic Alliances, Dr Fred Shaebsdau . • From September 2016 until January 2019, he was the General Manager of Dexcom Germany, a key global player in the CGM market, which during his leadership became the fastest growing organization in Dexcom’s history achieving triple digit revenue, and substantial growth of users . • There are currently around 2m diabetics and 5m pre - diabetics in the UK. There are 2m employees in the largest 10 UK companies alone. In the UK, Nemaura forecast having the potential of achieving over 200,000 UK subscribers. 11 NASDAQ: NMRD

The number of people that could benefit from SugarBEAT® is significant but even in a scenario where this was only rolled out ac ross staff considered to be high value assets to the 100 biggest companies in the UK, we forecast this having the potential of achieving over 200,000 su bscribers in the UK alone . NHS (UK) Diabetes Prevention Program (DPP) Digital Stream • DPP to support 200,000 people per year to transform their lifestyles • Five digital behavior change providers • Based on continuous engagement with patients to make new habits and behaviors • Help patients to improve their conditions by personalized health lifestyle plans and coaching NHS (UK) Type 2 behavior change at scale • Partnership with NHS England offering free digital support for people diagnosed with Type 2 diabetes • Online platform will deliver this behavior change service nationally • Evidence based education with innovative technology to provide personalization at scale SugarBEAT ® $179B+ Global Market opportunity consists of three target markets: 1 • $69B+ TAM Type 2 diabetics (90% of all diabetics) • $50B+ TAM Pre - diabetics — approximately 3x population • $60B+ TAM Wearable Health - Tech market: low carb / weight loss / fitness U.S. CGM Market Overview 2 • U.S. has the largest number of CGM users globally (630k in 2018) Only 2.6% of 25M US diagnosed diabetics used CGM in 2018 • U.S. annual CGM usage increased by 117% in 2018 30% of U.S. Type I diabetics use CGM • 3% of U.S. Type 2 insulin users use CGM CGM usage amongst non - insulin diabetics negligible • U.S. has 84M pre - diabetics Continuous glucose monitoring (CGM): TAM Understanding the opportunity 1&2 PiperJaffray Company Note DXCM Sep 5 2018 12 NASDAQ: NMRD

SugarBEAT ® Key Milestones 13 1Q 2016: SugarBEAT ® received CE Mark in Europe on Predecessor Product (wristwatch) 4Q 2017: Successful 525 patient trial completed in Europe for SugarBEAT ® 1Q 2018: NMRD Lists on Nasdaq 2Q 2018: SugarBEAT ® began FDA clinical program 3Q 2018: Published interim FDA clinical data 2Q 2019: Submitted 510K De - Novo application 1Q 2020: Commercial launch expected in Europe: UK and Ireland first followed by Germany End of 2020: target FDA approval for SugarBEAT ® 4Q 2018: Completed trials for FDA submission 2Q 2019: CE approval The worlds first non - invasive CGM SugarBEAT ® 4Q 2020: Launch of second line of products & US launch of SugarBEAT ® NASDAQ: NMRD

Current Product Pipeline NASDAQ: NMRD 14 Product Key Features Market SugarBEAT ® Gen II • Include pediatric cover • Improved accuracy (MARD) • Longer patch wear time • Include Gestational use • Type II Diabetics • Pre - Diabetics • Wearable Health - Tech • Pregnancy Continuous Lactate Monitoring • World’s o nly non - invasive skin patch for continuous lactate monitoring • Determines appropriate training intensity levels and monitors progression • Athletes • Fitness • Wearable Health - Tech market expected to be worth $60B+ by 2023 1 1 Juniper Research Digital Health Report Jan 14 2019

Future Product Pipeline 15 Product Uses Diagnostics Alcohol Monitoring Support personal health goals, and provide warnings prior to driving, and provide physicians with individual drinking habits Prevention of progression to alcohol related diseases. Prostaglandin Monitoring Screening for inflammatory irritants in formulations during drug and cosmetic development Inflammation Lactate Monitoring Lactate monitoring in intensive care Anaerobic Metabolism Drug Monitoring Monitoring the impact of drug treatment for treatment - regimen calibration and pharmacokinetics Treatment Regimen Optimization Phenylalanine Monitoring Phenylalanine monitoring to ensure that the level is sufficiently suppressed Phenylketonuria (PKU) NASDAQ: NMRD

THE MANAGEMENT TEAM Dr. Faz Chowdhury Chief Executive Officer Dr. Chowdhury has served as CEO and chair of the board of Nemaura Medical since formation in December 2013. He is sole inventor on more than 100 granted and pending patents across over 20 technology platforms within the medical device and pharmaceutical sectors. He has 20 years experience and track record taking products from concept to approval, and has been pivotal in the company’s technical and strategic development. He has authored Textbook Chapters on Nano - biosciences for Wiley and Elsevier, and serves on the Board of Medilink East Midlands, UK. Dr. Chowdhury holds a Masters in Microsystems and Nanotechnology from Cranfield University, UK, and Doctorate from the University of Oxford on nano - medicine and drug delivery. Chris Avery Vice President - Global Business Operations Mr . Avery has 35 years’ experience in diabetes, gaining vast experience in glucose monitoring and insulin delivery markets. He co - founded a UK diabetes distributorship in 2000 and served as UK Managing Director and European Director later acquired by Nipro. To date he has successfully launched over 20 glucose systems either direct or with distributors and negotiated partnerships and distribution deals with pharmaceutical & medtech companies across Europe and other international markets. In 2016 he joined Nemaura Medical’s European JV partner, Dallas Burston Ethitronix as SVP Global Business Development, and worked closely with Dr. Chowdury until joining Nemaura Medical in June 2019. Dr. Fred Schaebsdau VP Strategy and Strategic Alliances Dr. Schaebsdau has over 15 years of executive level experience in the CGM, Blood Glucose Monitoring (BGM) and insulin delivery industries, which started in 2004 during his tenure with Abboq Diabetes Care, where he was a member of the M&A and post - merger integration teams responsible for the acquisition of TheraSense and its FreeStyle Navigator CGM. From September 2016 until January 2019, he was the General Manager of Dexcom Germany, which during his leadership became the fastest growing organization in Dexcom’s history achieving triple digit revenue and new patient growth every year. Kathryn Farrar Financial Controller Kathryn received a Degree and Masters in Chemistry from the University of Oxford in 1998, and qualified as a Chartered Accountant in 2001. Ms. Farrar worked within the audit department at KPMG for 9 years, prior to joining Nemaura in 2010, where she is currently the Financial Controller. Kathryn has extensive experience managing Nemaura’s compliance with US GAAP and SOX regulations, and provides strategic direction to the board. 16 NASDAQ: NMRD
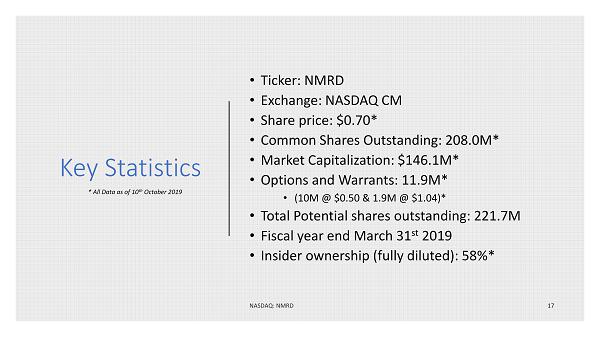
Key Statistics • Ticker: NMRD • Exchange: NASDAQ CM • Share price: $0.70* • Common Shares Outstanding: 208.0M* • Market Capitalization: $146.1M* • Options and Warrants: 11.9M* • (10M @ $0.50 & 1.9M @ $1.04)* • Total Potential shares outstanding: 221.7M • Fiscal year end March 31 st 2019 • Insider ownership (fully diluted): 58%* * All Data as of 10 th October 2019

Investment Opportunity Summary Nemaura Medical aims to dominate the global diabetes markets for putting Type 2 diabetes into remission, using the SugarBEAT ® device and digital healthcare platform. • Within 5 years the company aims to lead in the wearables market and self - management of medical conditions with its pipeline products of sensors and digital healthcare platform using AI. • The company has the potential to disrupt the multi - Billion dollar glucose trending and diabetes management space targeting $179B+ Global opportunity: • Planned Launch of SugarBEAT® in key global territories by 2020 and aiming for 1 million users by 2021, and 3 million users by 2023 Digital health sector comprising $69B+ type II diabetic market & $50B+ pre - diabetic market • Aiming to build on the Livongo model, but using the company’s proprietary unique device platform, hence a more substantial investment proposition. • Wearable health - tech sector comprising $60B+ weight loss & wellness markets • US approval and launch anticipated by end of 2020. • CE Mark Approved; UK & Ireland commercial launch expected in Q1 2020, followed by Germany • Lowest priced, high recurring margin model, with lowest COGS per CGM patch in the industry Growing IP portfolio with over 30 issued & pending patents • Launching continuous lactate monitoring (CLM) near term, targeting $60B+ wearable tech market • Pipeline of four other products including non - invasive continuous alcohol monitoring (CAM) Proven management team with successful track records Clean capital structure with no long - term debt 18 NASDAQ: NMRD

Appendices 19 NASDAQ: NMRD
Manufacturing The Device And Sensors • Transmitter device and charging station • All manufactured in the UK and can be readily scaled to by adding multiple lines to what is a linear operation. • Disposable skin adhesive and ancillary disposable parts • All manufactured in the UK and can be readily scaled to millions of units per month. • Disposable daily sensor • All manufactured in the UK and can be readily scaled to millions of units per month, by adding multiple lines to what is a linear operation. • Manufacturing scale • Current scale will allow us to launch in the UK, Ireland and Germany. • Future manufacturing will be outsourced to various low cost regions for the non - specialized parts, but sensor production to remain in UK. 20 NASDAQ: NMRD

SugarBEAT ® Clinical Data • Completed clinical studies to support FDA submission • The clinical studies used were split between Type I and Type II diabetics • Consisted of 75 patients over 225 patient days • Generated over 12,000 paired data points, with blood samples taken via catheter every 15 minutes over a 12 - hour period for three non - consecutive days for each patient • Study design was based on two previous pre - sub meetings Nemaura held with the FDA • The clinical study results indicated a MARD (Mean Absolute Relative Deviation) of 12.4% (with a lower figure denoting greater accuracy), using a single point finger stick calibration • No device - related adverse events were noted NASDAQ: NMRD
SugarBEAT® Regulatory Pathway • U.S. FDA De - Novo 510(k) x Completed clinical studies to support FDA De - Novo 510(k) submission x FDA Approval: target by end of 2020. • European CE Mark x Approval Received at the end of Q2 2019. De - Novo 510(k) • Lower risk (Class II graded medium risk) • Quicker review process compared with PMA which is Class III graded (higher risk) • The recent De - Novo and subsequent 510(k) by Dexcom shows current FDA thinking on invasive CGM devices • SugarBEAT® is non - invasive and adjunctive, and therefore lower risk NASDAQ: NMRD

Intellectual Property Building an extensive intellectual property portfolio to position the Company to become a leader in the non - invasive CGM space The Company has over thirty patents (~70% approved and ~30% pending) spanning the following patent families: 1. Sensor related 2. Algorithm and methods of using the CGM data 3. Devices & methods to enhance glucose sensing 4. Methods to enhance glucose sensing 5. Devices and methods to extract glucose The Company anticipates filing multiple additional patents over the course of the next 18 months based on ongoing findings and improvements. 23 NASDAQ: NMRD






















