Exhibit 99.2

H.C. WAINWRIGHT BIOCONNECT CONFERENCE JANUARY 10 - 13, 2022, VIRTUAL CONFERENCE Dr Faz Chowdhury, CEO Nasdaq : NMRD

Forward - Looking Statements This presentation includes forward - looking statements that are subject to many risks and uncertainties. These forward - looking statements, such as statements about Nemaura’s short - term and long - term growth strategies, can sometimes be identified by use of terms such as “intend,” “expect,” “plan,” “estimate,” “future,” “strive,” and similar words. These statements involve many risks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements. These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including the risks identified under the section captioned “Risk Factors” in Nemaura’s Annual Report on Form 10 - K filed with the Commission on June 29, 2021 as the same may be updated from time to time. Nemaura disclaims any obligation to update information contained in these forward - looking statements whether as a result of new information, future events, or otherwise.

Clinical Need… There are over 463 million people living with diabetes worldwide, and over $ 760 Billion was spent in the U . S . alone in 2019 for diabetes related healthcare expenditure 1 . The total addressable market exceeds $ 150 Billion 2 , 3 , 4 . Obesity and Diabetes are two of the major drivers of the chronic disease epidemic .
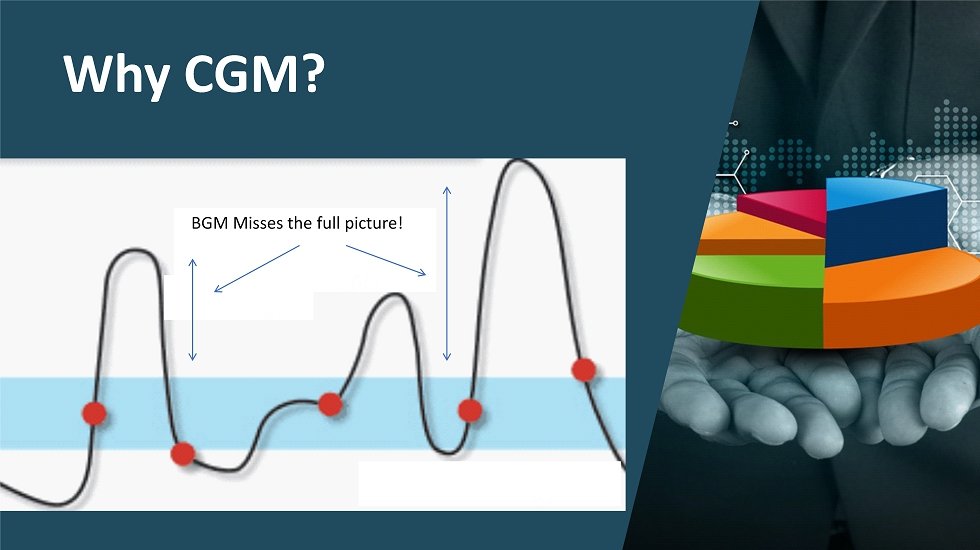
Why CGM? BGM Misses the full picture!
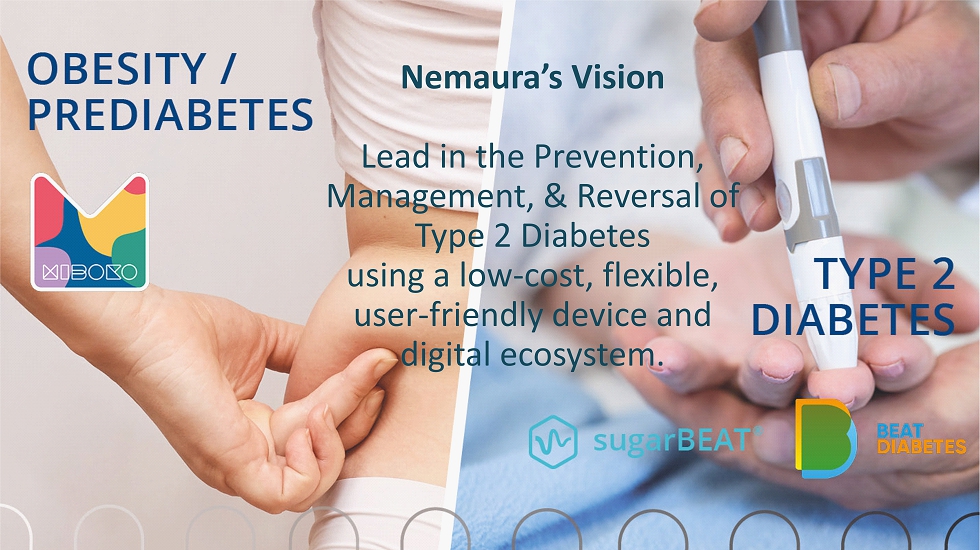
Nemaura’s Vision Lead in the Prevention, Management, & Reversal of Type 2 Diabetes using a low - cost, flexible, user - friendly device and digital ecosystem.

Our Approach ▪ Skin mounted (disposable) sensor & (non - disposable) transmitter. Glucose extracted to the surface of the skin ▪ Sensors use well established glucose oxidase enzyme to convert glucose to an electric current signal ▪ Low energy bluetooth sends this to the phone app every 5 minutes where it’s converted to a glucose value and viewed by the user. The information is used in various forms to educate and empower the user to improve health outcomes (and not to make the user become ‘dependent’ on the technology).

Product Positioning sugarBEAT® CGM – Direct sales to consumers that wish to purchase sensors to measure glucose profiles on days they choose. www.sugarBEAT.com BEAT®diabetes Program: a subscription - based diabetes management program. Currently in pilot and plan is to sell to employers and insurers. www.BEATdiabetes.Life MiBoKo ® Consumer Metabolic Health Program – Direct to consumer sales to commence after Beta Phase (Beta was launched in October 2021). Plan is also to sell to employers and insurers globally, driven by the low cost approach. www.Miboko.com

sugarBEAT ® Regulatory Status ▪ CE Approved Class 2b Medical Device in Europe ▪ FDA PMA submitted and review still in progress: • BIMO Audit conducted in December 2021 at Nemaura’s UK facility. A single 483 observation was issued. Company has committed to a full and complete response this quarter. • BIMO Audit of Clinical Investigator site scheduled for this quarter . • Premarket inspection covering FDA's Quality System/Current Good Manufacturing Practice regulations for medical devices (21 CFR Part 820) scheduled for this quarter .

sugarBEAT ® Sales status UK ▪ UK: 200,000 sensors ordered by licensee following soft launch success ▪ Delivered first batch of transmitter devices in December 2021, with rolling bi - weekly/monthly delivery planned. ▪ Purchase order forecast for (approximately) a further 100,000 per month for the next 2 years, totaling over 2 million sensors. ▪ Licensee selling these based on a diabetes management subscription service.
sugarBEAT ® Sales Status - Outside - UK Planning direct to consumer launch of sugarBEAT outside of UK where CE approval is accepted, such as Europe and Middle East.
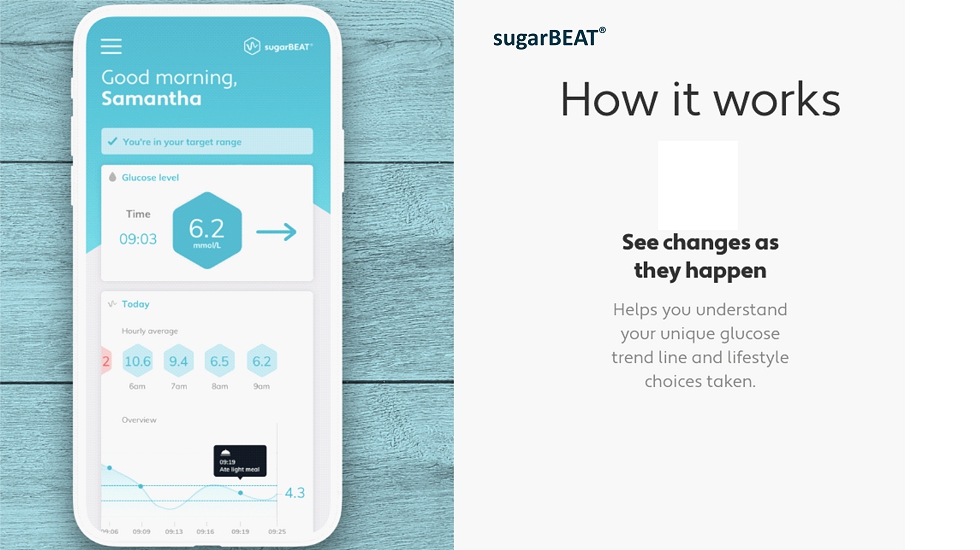
sugarBEAT ®

sugarBEAT ® Testimonials* MySugarWatch offered me a needle - free blood glucose monitoring solution that’s non - invasive and easy to use. I didn’t even realize I had the My Sugar watch device on my arm as it is so lightweight. It gives me the assurance that my blood sugar reading is accurate, and I have access to my levels on my phone at all times. I was diagnosed with gestational diabetes, and I was informed by my healthcare professional that this may lead to a diagnosis of Type 2 diabetes in the future. Unfortunately, I was diagnosed with Type 2 diabetes after this and I have to manage this diagnosis all by myself and learn to control my blood glucose levels. Using MySugarWatch has alerted me to changes in my blood glucose levels and helped me understand how these changes make an impact on my body and how I am feeling. To have this information at my fingertips gives me so much control to manage my diabetes. I have been a Type 2 diabetic for 10 years. I sporadically manage my blood sugar with a blood glucose monitoring device. I know that if not controlled or managed effectively I can have real highs and lows and not know when this will happen. I was wearing the MySugarWatch device and it alerted me to the fact I was about to have a hypo before it happened. This alert enabled me to quickly balance my medication . * MySugarWatch ® is the UK Licensee brand for sugarBEAT®
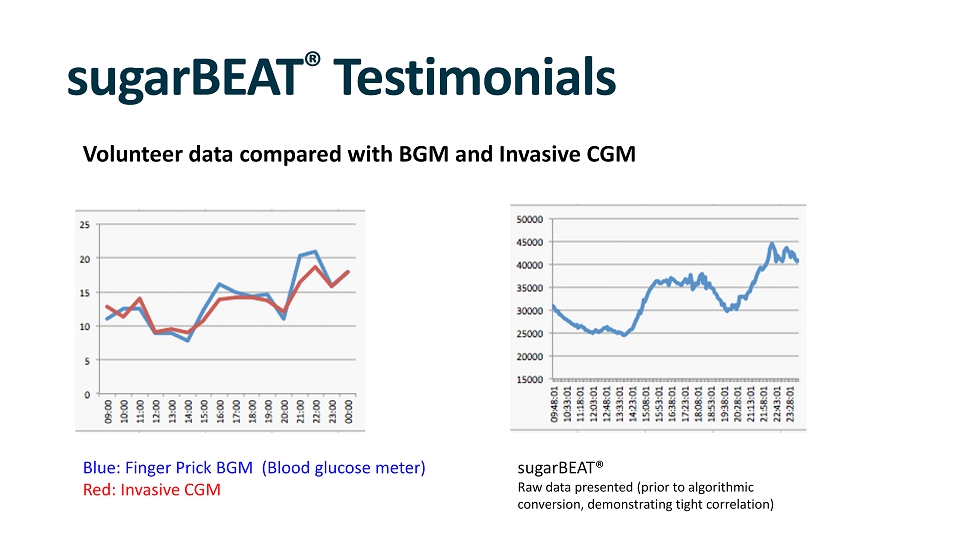
sugarBEAT ® Testimonials Volunteer data compared with BGM and Invasive CGM Blue: Finger Prick BGM (Blood glucose meter) Red: Invasive CGM sugarBEAT® Raw data presented (prior to algorithmic conversion, demonstrating tight correlation)

Type 2 Diabetes prevention and management program launched in the U.S.

BEAT ® diabetes – 3 Components ▪ Weight loss program originally developed at the Joslin Diabetes Centre – over 12 years of clinical evidence (based on an in - clinic program, subsequently replicated using a virtual program). Sustained long term weight loss achieved without loss of muscle mass ▪ proBEAT Ρ Intermittent glucose profiling – using world’s first daily - wearable glucose sensor, developed in - house ▪ Coaching: digital 24/7 using app, and specialist 1 to 1 coaching

Miboko ‘MIND BODY CONNECT’ A metabolic health program comprising an app and integrated glucose sensor
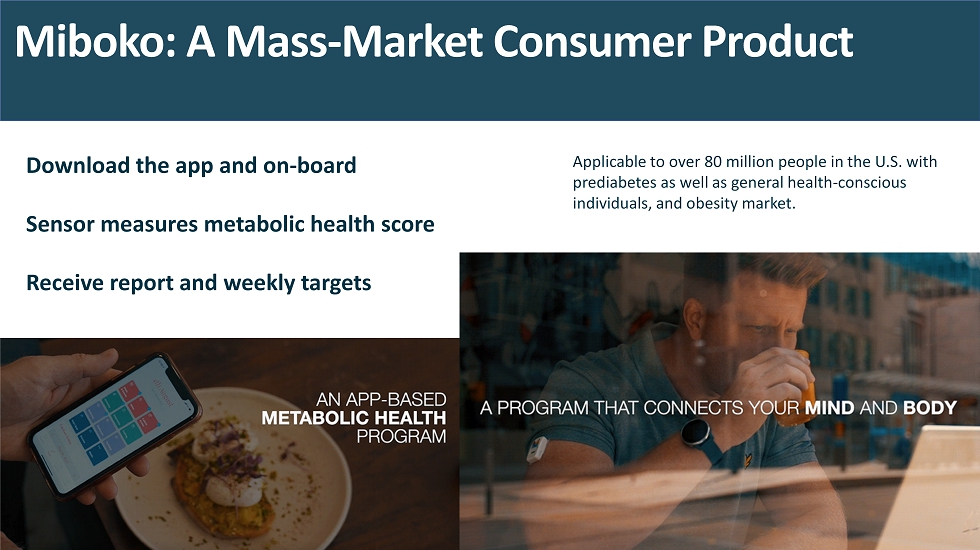
Miboko : A Mass - Market Consumer Product Download the app and on - board Sensor measures metabolic health score Receive report and weekly targets Applicable to over 80 million people in the U.S. with prediabetes as well as general health - conscious individuals, and obesity market.

Miboko : Key Competition Noom: Over 50 million subscribers, >$400m revenues in 2020 5 Miboko USP: A holistic metabolic health approach to weight loss and better health supported using our proprietary wearable sensor that gives an insight into what’s really happening inside the body

Miboko : Launch Update Marketing activities thus far: restricted mainly to low - key organic social media campaign. Over 5000 registrations during the last few weeks of BETA launch, with more than 60% conversion for on - boarding Large scale marketing campaign planned for 2022
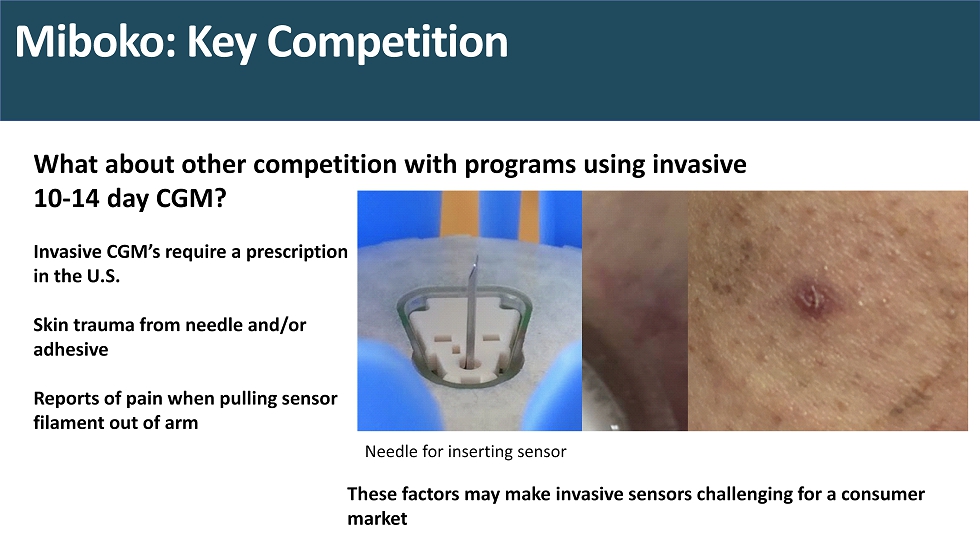
Miboko : Key Competition What about other competition with programs using invasive 10 - 14 day CGM? Invasive CGM’s require a prescription in the U.S. Skin trauma from needle and/or adhesive Reports of pain when pulling sensor filament out of arm These factors may make invasive sensors challenging for a consumer market Needle for inserting sensor

CONTINUOUS LACTATE MONITORING Assists in threshold maximization in performance athletes Early identification of tissue hypoperfusion or shock for aggressive early resuscitation of critically ill patients to improve the their chances of survival BODY TEMPERATURE MONITORING Gives a more accurate and large data set. For monitoring viral infections and lower limb blood circulation tracking the effectiveness of drugs Wearable temperature sensors market is expected to register a CAGR of 8.3% during the forecast period 2021 - 2026 6 Future Product Opportunities Leveraging the BEAT ® Technology A rich portfolio of additional products to complement existing offering and contribute to increased revenues

ALCOHOL MONITORING Support personal health goals and provide warnings prior to driving. Provide physicians with individual’s drinking habits . Prevention of progression - to - alcohol - related disease DRUG MONITORING Monitoring the impact of drugs and personalized treatment plan for patients. Global therapeutic drug monitoring device market to reach $3.37B by 2024 7 Future Product Opportunities Leveraging the BEAT ® Technology

The Team ▪ We are building a world class team ▪ So far includes senior level appointments, with experience from companies including: Dexcom, Lifescan , Abbvie , & Eli Lilly ▪ Hiring for Chief Marketing and Sales Officers – U.S. and EU

The Team – Most recent addition Chief Operating Officer, COO Dr Arash Ghadar : Over 20 years as technical and operational experience, last 10 years as COO of an electronics development and manufacturing c ompany. Dr Ghadar obtained a PhD in Bio - sensors from Warwick University and holds a Bachelors and Masters in Electronics and Control Systems Engineering, respectively, for which he achieved First C lass degrees, and was the highest ranking student on both occasions. Dr Ghadar brings with him manufacturing, operational, and management expertise that strongly complement Nemaura’s growth plans.
Intellectual Property ▪ IP consists of several filed p atents and substantial know - how ▪ Two key sensor - related patents are not yet published, relating to novel device and composition

Summary ▪ CE Class 2b Medical Device Approval – and subsequent user testimonials and order for 200,000 sensors (from UK Licensee) and first batch delivery made in D ecember 2021. ▪ FDA PMA review is in progress; audits in relation to PMA, conducted and in - progress ▪ Simple revenue generation models: Direct to consumer subscription services, direct sales to employers and insurers. ▪ Highly Scalable business model with potential for rapid growth ▪ Cash balance over $26m as of September 30, 2021; last quarterly burn rate approx. $2m/ qtr (plus debt repayment).

References 1. https://www.idf.org/aboutdiabetes/what - is - diabetes/facts - figures.html 2. https://drug - dev.com/global - type - 2 - diabetes - market - set - to - almost - double - to - 58 - 7 - billion/ 3. https ://www.prnewswire.com/news - releases/global - digital - diabetes - market - outlook - to - 2026 - a - 16 - billion - industry - opportunity - 300980794. html 4. https://www.absolutemarketsinsights.com/reports/global - Noninsulin - Therapies - for - Diabetes - Market - 2019 - 2027 - 259 5. https ://www.bloomberg.com/news/articles/2021 - 05 - 25/weight - loss - app - noom - gets - 540 - million - in - silver - lake - led - round 6. https://www.mordorintelligence.com/industry - reports/global - wearable - temperature - sensors - market - industry 7. https://www.grandviewresearch.com/press - release/global - therapeutic - drug - monitoring - market


























