Exhibit 99.1

Corporate Presentation September 2015 MAKING BETTER HAPPEN

2 l Forward Looking Statements Any statements in this presentation about our future expectations, plans and prospects, including statements about the development of our product candidates and the timing, conduct, enrollment and outcome of our clinical studies, the availability of data from those studies and other statements containing the words "anticipate," "believe," "estimate," "expect," "intend," "goal," "may", "might," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions, constitute forward - looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 . Actual results may differ materially from those indicated by such forward - looking statements as a result of various important factors, including statements about the clinical trials of our product candidates . Such forward - looking statements involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward - looking statements . Such risks and uncertainties include, among others, those related to the timing and costs involved in developing and commercializing our products and product candidates, the timing for results, the initiation, conduct, enrollment and timing of clinical trials, delays in potential approvals by FDA of the commencement of trials, availability of data from clinical trials, positive results from such trials and timing and expectations for regulatory approvals, our scientific approach and general development progress, the composition of our management supervisory board, the availability or commercial potential of our product candidates, the sufficiency of cash resources and need for additional financing or other actions and other factors discussed in the "Risk Factors" section of our Annual Report on Form 20 - F for the year ended December 31 , 2014 , which is on file with the Securities and Exchange Commission . In addition, the forward - looking statements included in this presentation represent our views as of the date of this presentation . We anticipate that subsequent events and developments will cause our views to change . However, while we may elect to update these forward - looking statements at some point in the future, we specifically disclaim any obligation to do so . These forward - looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation .

3 l September 2015 Update COGENZIA: QIDP FDA designation – Accelerates pre - commercialization efforts COGENZIA: Phase 3 program initiated in May XARACOLL: Phase 3 program initiated in August Executive Team bolstered – Pepe Carmona, CFO – Rich Fante, CCO

4 l “What if we could consistently make medicines work better?” At Innocoll, every day we ask ourselves
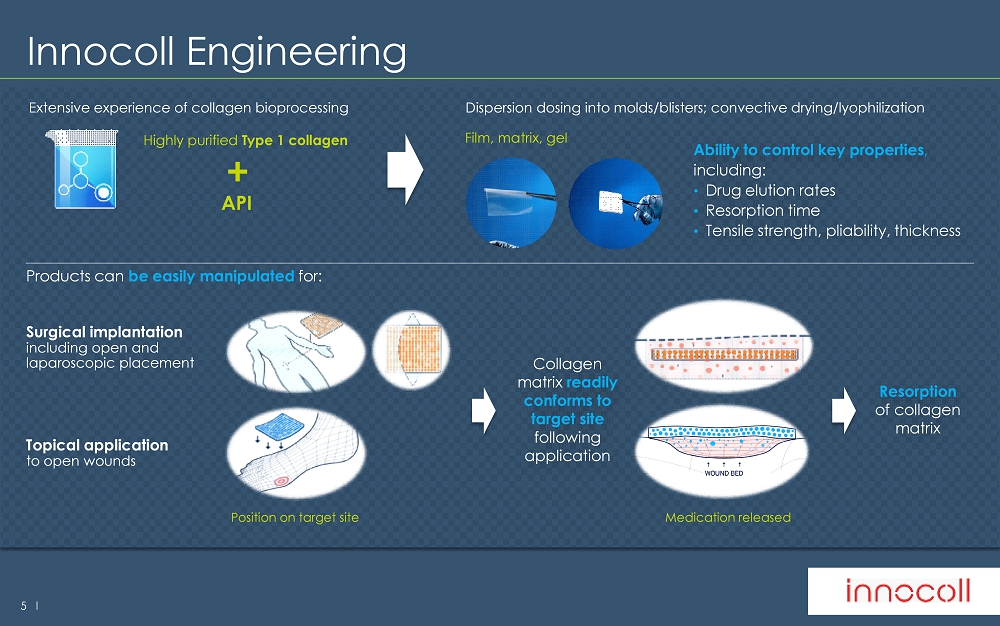
5 l Products can be easily manipulated for : Surgical implantation including open and laparoscopic placement Topical application to open wounds Innocoll Engineering H i gh l y pu rifie d T y pe 1 c oll a g e n API + Film , m atrix, g el Extensive experience of collagen bioprocessing Ability to control key properties , including: • D rug elution rates • R esorption time • Tensile strength, pliability, thickness Dispersion dosing into molds/blisters; convective drying/lyophilization Position on target site Collagen matrix readily conforms to target site following application Medication released R esorption of collagen matrix

6 l 3 Late - stage* Therapeutic Products Reduction of surgical adhesions * These products have not been approved by FDA, and therefore, the FDA has not determined their safety and effectiveness for commercial marketing and sale . Diabetic foot ulcer infection (DFI) treatment Post - operative pain

7 l “What if a new method of surgical pain management could provide more post - operative patient comfort?”
8 l XARACOLL A New Candidate to Treat Pain A resorbable, implantable collagen matrix with bupivacaine for management of post - surgical pain Engineered to provide sustained local analgesia at the surgical site Can be used in both open and laparoscopic surgery Designed to provide up to 72 hours of post - operative pain relief Targeting substantial reduction in opioid consumption post - surgery

9 l Post - operative Pain Management Opioids The standard of care for post - operative pain includes administration of systemically acting μ - opioid agonists including morphine Two areas of greatest unmet need in the treatment of postoperative pain: – Lowering postoperative opioid consumption – reducing opioid - related AEs Systemic opioids are associated with nausea, vomiting, and respiratory depression Opioid abuse and addiction are escalating at alarming rates Patients with opioid - related AEs vs. patients not experiencing opioid - related AEs have shown approximately * *In a retrospective cohort study using administrative data of patients evaluated for receipt of post - surgical opioids (N=31031). Pharmacotherapy . 2013 Apr;33(4):383 - 91 . 55% longer hospital stay 47% higher hospitalization cost 36% increased risk of readmission

10 l US Surgical Procedures Considered * *XARACOLL MATRIX - 1 and MATRIX - 2 Phase 3 studies are in open hernia repair. ** Cofactor Group March 2015 analysis on behalf of Innocoll US Surgical Procedures Approximately 70M Specific Procedures Considered • General • OB/GYN • Cosmetic • Orthopedic • Back/Spine 38% 32% 11% 10% 9% General Orthopedic OB/GYN Cosmetic Spine
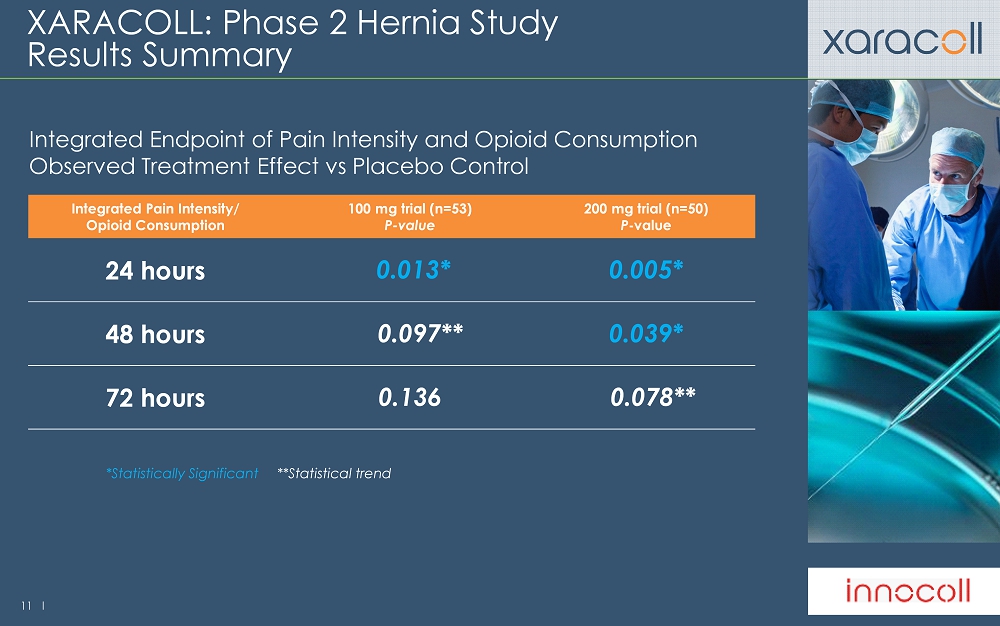
11 l XARACOLL: Phase 2 Hernia Study Results Summary Integrated Endpoint of Pain Intensity and Opioid Consumption Observed Treatment Effect vs Placebo Control Integrated Pain Intensity/ Opioid Consumption 100 mg trial (n=53) P - value 200 mg trial (n=50) P - value 24 hours 0.013* 0.005* 48 hours 0.097** 0.039* 72 hours 0.136 0.078** *Statistically Significant **Statistical trend
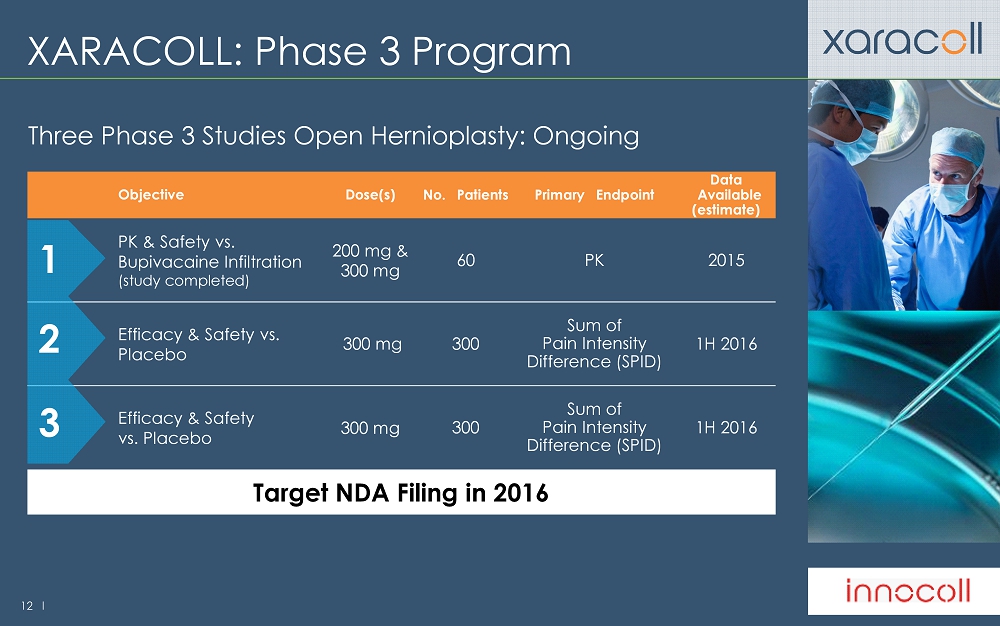
12 l XARACOLL: Phase 3 Program Three Phase 3 Studies Open Hernioplasty: Ongoing Objective Dose(s) No.
Patients Primary
Endpoint Data
Available (estimate) PK & Safety vs . Bupivacaine Infiltration (study completed) 200 mg & 300 mg 60 PK 2015 Efficacy & Safety vs . Placebo 300 mg 300 Sum of Pain Intensity Difference (SPID) 1H 2016 Efficacy & Safety vs . Placebo 300 mg 300 Sum of Pain Intensity Difference (SPID) 1H 2016 Target NDA Filing in 2016 1 2 3
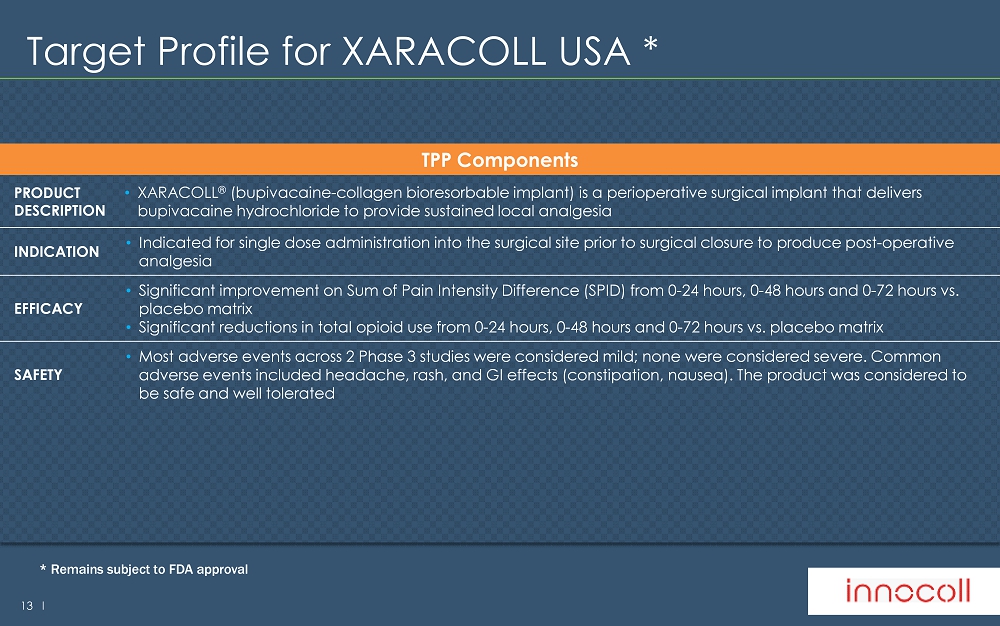
13 l Target Profile for XARACOLL USA * TPP Components PRODUCT DESCRIPTION • XARACOLL ® (bupivacaine - collagen bioresorbable implant) is a perioperative surgical implant that delivers bupivacaine hydrochloride to provide sustained local analgesia INDICATION • Indicated for single dose administration into the surgical site prior to surgical closure to produce post - operative analgesia EFFICACY • Significant improvement on Sum of Pain Intensity Difference (SPID) from 0 - 24 hours, 0 - 48 hours and 0 - 72 hours vs. placebo matrix • Significant reductions in total opioid use from 0 - 24 hours, 0 - 48 hours and 0 - 72 hours vs. placebo matrix SAFETY • Most adverse events across 2 Phase 3 studies were considered mild; none were considered severe. Common adverse events included headache, rash, and GI effects (constipation, nausea). The product was considered to be safe and well tolerated * Remains subject to FDA approval
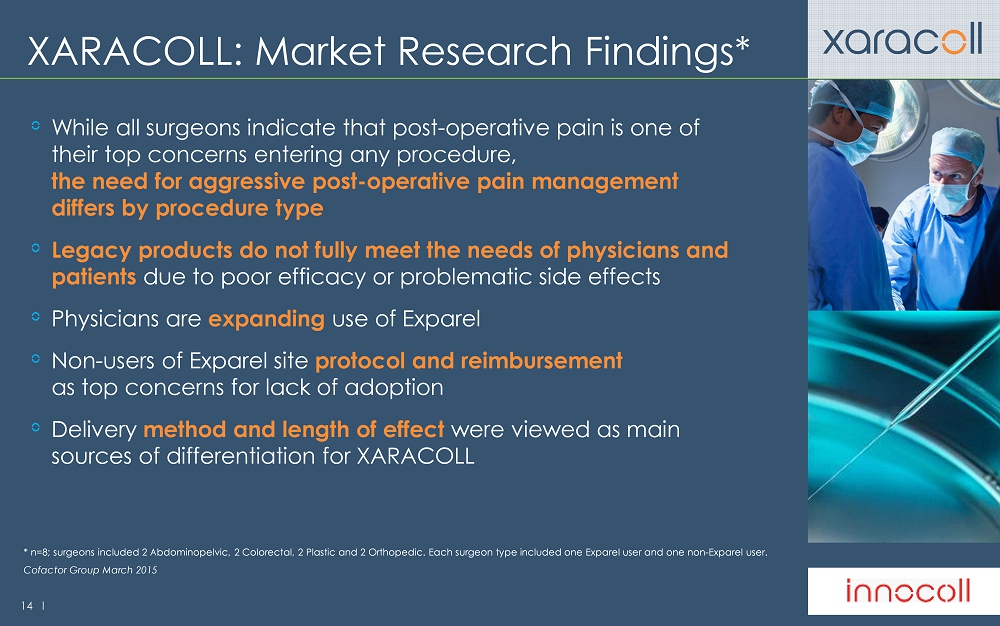
14 l XARACOLL: Market Research Findings* While all surgeons indicate that post - operative pain is one of their top concerns entering any procedure, the need for aggressive post - operative pain management differs by procedure type Legacy products do not fully meet the needs of physicians and patients due to poor efficacy or problematic side effects Physicians are expanding use of Exparel Non - users of Exparel site protocol and reimbursement as top concerns for lack of adoption Delivery method and length of effect were viewed as main sources of differentiation for XARACOLL Cofactor Group March 2015 * n=8; surgeons included 2 Abdominopelvic, 2 Colorectal, 2 Plastic and 2 Orthopedic. Each surgeon type included one Exparel u ser and one non - Exparel user.
15 l “What if diabetic foot ulcer infection rates — a major factor in amputations — could be reduced with local adjuvant therapy?”

16 l COGENZIA A resorbable, topical collagen matrix with gentamicin for treatment of DFIs ( diabetic foot infections) Engineered to provide broad antibacterial efficacy at the wound site Has potential to reduce amputations in diabetic patients Received QIDP designation Q2 2015 – Potential fast track review status – 5 year data exclusivity enhancement
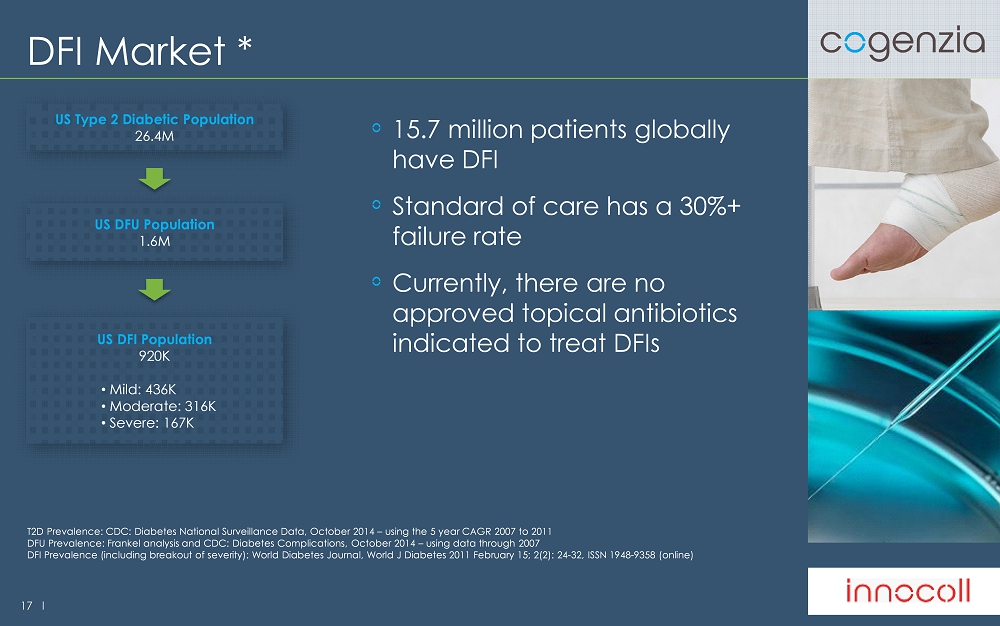
17 l DFI Market * 15.7 million patients globally have DFI S tandard of care has a 30%+ failure rate Currently, there are no approved topical antibiotics indicated to treat DFIs T2D Prevalence: CDC: Diabetes National Surveillance Data, October 2014 – using the 5 year CAGR 2007 to 2011 DFU Prevalence: Frankel analysis and CDC: Diabetes Complications, October 2014 – using data through 2007 DFI Prevalence (including breakout of severity): World Diabetes Journal, World J Diabetes 2011 February 15; 2(2): 24 - 32, ISSN 19 48 - 9358 (online) US Type 2 Diabetic Population 26.4M US DFU Population 1.6M United States 2013 2014 2015 2016 2017 2018 2019 2020 Adult Population (000s) 242,543 244,944 247,369 249,818 252,291 254,789 257,311 259,859 T2D Prevalence 10.9% 11.0% 11.1% 11.2% 11.3% 11.3% 11.4% 11.5% T2D Population (000s) 26,437 26,913 27,396 27,889 28,390 28,901 29,420 29,949 DFU Prevalence 6.0% 6.0% 5.9% 5.9% 5.9% 5.9% 5.8% 5.8% DFU Population (000s) 1,586 1,607 1,627 1,648 1,670 1,691 1,713 1,735 DFI Population (000s) 920 932 944 956 968 981 993 1,006 Mild 436 442 447 453 459 465 471 477 Moderate 316 321 325 329 333 337 342 346 Severe 167 170 172 174 176 179 181 183 US DFI Population 920K • Mild: 436K • Moderate: 316K • Severe: 167K
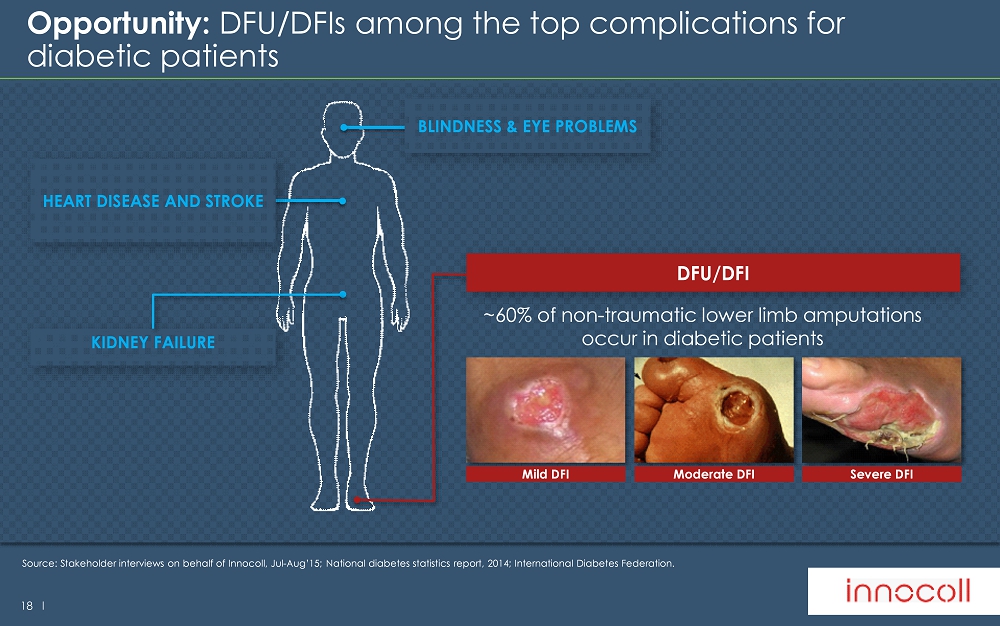
18 l HEART DISEASE AND STROKE KIDNEY FAILURE Opportunity: DFU/DFIs among the top complications for diabetic patients Source: S takeholder interviews on behalf of Innocoll , Jul - Aug’15; National diabetes statistics report, 2014; International Diabetes Federation. ~60% of non - traumatic lower limb amputations occur in diabetic patients Mild DFI Moderate DFI Severe DFI DFU/DFI BLINDNESS & EYE PROBLEMS
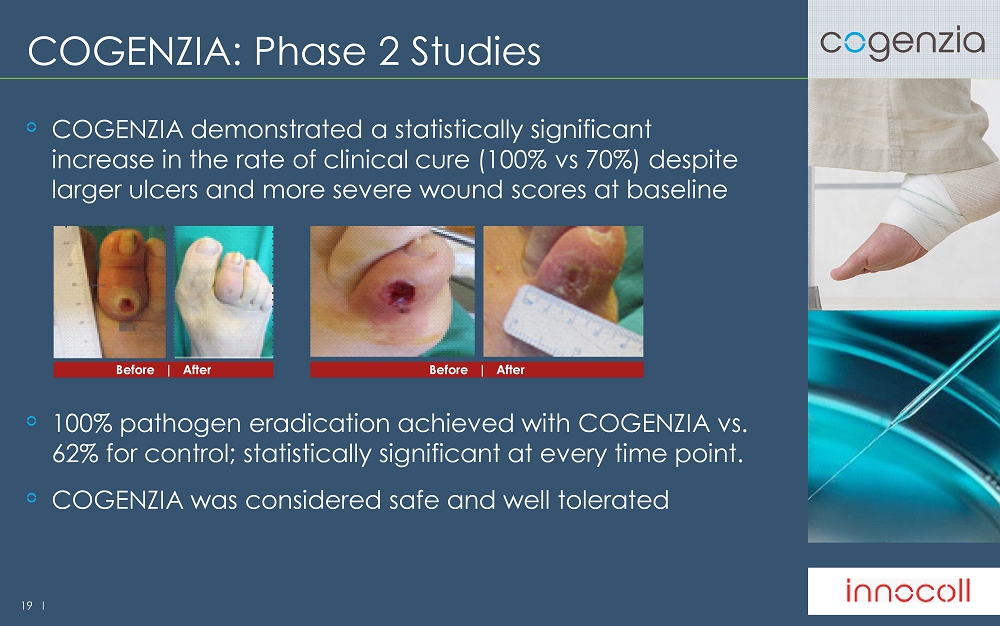
19 l COGENZIA: Phase 2 Studies COGENZIA demonstrated a statistically significant increase in the rate of clinical cure (100% vs 70%) despite larger ulcers and more severe wound scores at baseline 100% pathogen eradication achieved with COGENZIA vs. 62 % for control; statistically significant at every time point. COGENZIA was considered safe and well tolerated Before | After Before | After
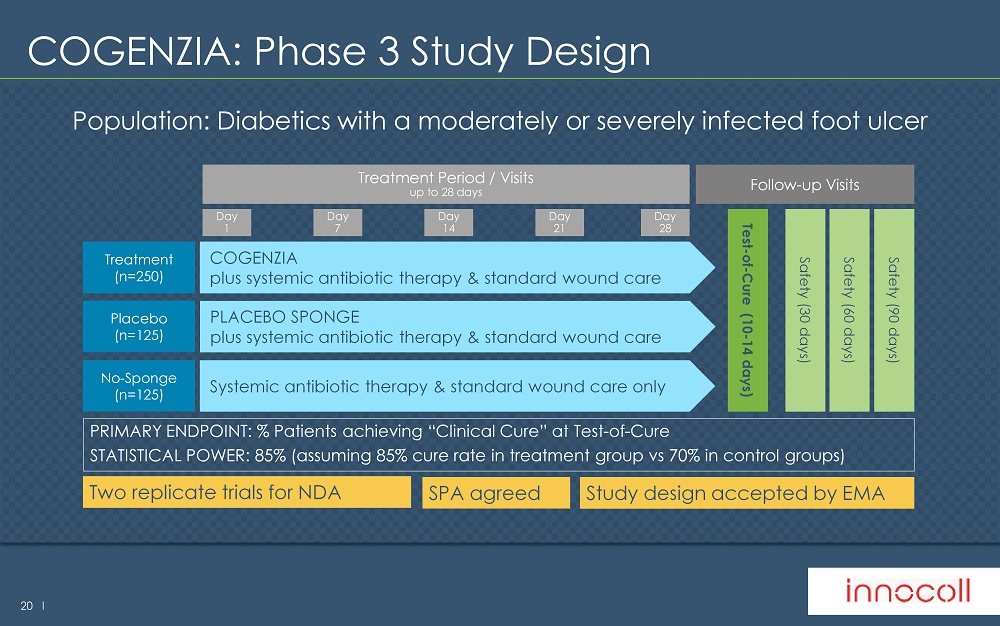
20 l COGENZIA: Phase 3 Study Design Treatment Period / Visits up to 28 days Treatment ( n=250) COGENZIA plus systemic antibiotic therapy & standard wound care Day 7 Day 14 Day 21 Day 28 Day 1 PRIMARY ENDPOINT: % Patients achieving “Clinical Cure” at Test - of - Cure STATISTICAL POWER: 85% (assuming 85% cure rate in treatment group vs 70% in control groups) Placebo ( n=125) PLACEBO SPONGE plus systemic antibiotic therapy & standard wound care Follow - up Visits Test - of - Cure (10 - 14 days) Population: Diabetics with a moderately or severely infected foot ulcer No - Sponge (n=125) Systemic antibiotic therapy & standard wound care only Safety (30 days) Safety (60 days) Safety (90 days) Two replicate trials for NDA Study design accepted by EMA SPA agreed
21 l Target Profile for COGENZIA USA * TPP Components INDICATION: US Indicated for the treatment of infected foot ulcers in diabetic patients in combination with systemic antibiotics EFFICACY When studied in large - scale Phase III clinical programs, Cogenzia, when used in combination with systemic antibiotic therapy and wound care, significantly increased the percent of patients with a clinical outcome of clinical cure (resolution of all clinical signs and symptoms of infection) when compared to placebo collagen matrix plus systemic antibiotic therapy and systemic antibiotic therapy alone . OTHER END POINTS Other endpoints studied include statistically significant improvements in: • Clinical cure and baseline pathogen eradication (approximately 10 days after end of treatment) • Percent of patients with re - infection • Percent of patients that have an amputation associated with the ulcer • Percent of patients with ulcer closure * Subject to FDA approval

22 l Profile Market Research Very positive about a topical product that delivers a high concentration of antibiotic to the site of the infection Profile concerns related to: Difficulties changing dressings due to obesity and decreased dexterity Requested information about the amount of systemic absorption of gentamicin Out - of - pocket costs to the patient Stakeholder interviews on behalf of Innocoll
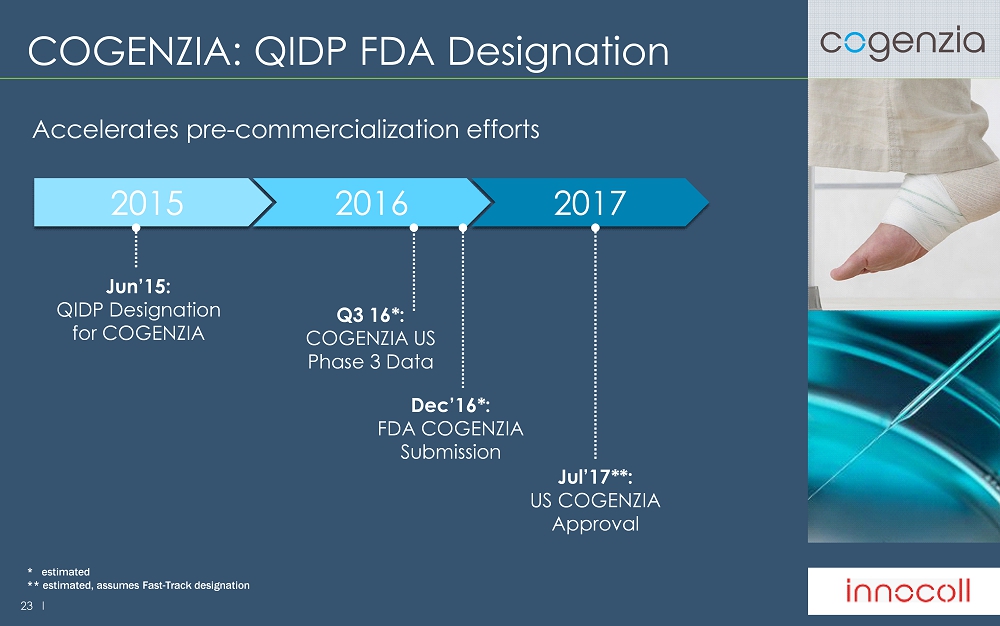
23 l COGENZIA: QIDP FDA Designation Accelerates pre - commercialization efforts 2016 2015 2017 Q3 16 * : COGENZIA US Phase 3 Data Dec’16 * : FDA COGENZIA Submission Jul’17 ** : US COGENZIA Approval Jun’15: QIDP Designation for COGENZIA * estimated ** estimated, assumes Fast - Track designation

24 l “What if one simple step could significantly reduce the incidence and extent of post - operative adhesions?”
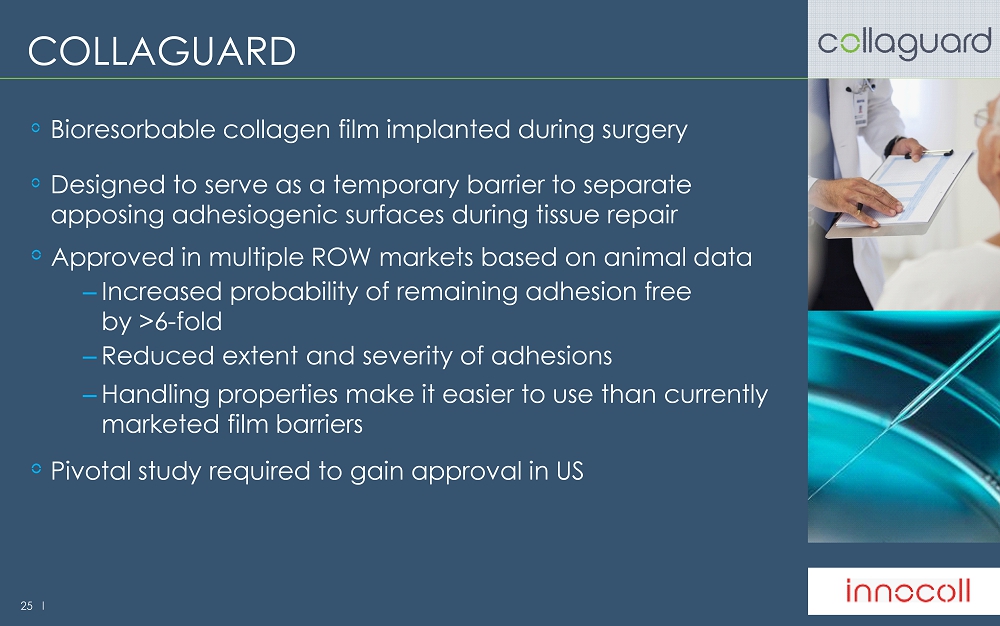
25 l COLLAGUARD Bioresorbable collagen film implanted during surgery Designed to serve as a temporary barrier to separate apposing adhesiogenic surfaces during tissue repair Approved in multiple ROW markets based on animal data – Increased probability of remaining adhesion free by >6 - fold – Reduced extent and severity of adhesions – Handling properties make it easier to use than currently marketed film barriers Pivotal study required to gain approval in US

26 l Adhesion Barrier Market Adhesion barriers are used prophylactically to reduce abnormal internal scarring and adhesions following surgery and are used for multiple procedure types Current use of adhesion barriers is low with good opportunities for growth * Cofactor Group March 2015 analysis on behalf of Innocoll US Surgical Procedures Approximately 70M US Procedures Considered • C - Section • Open OB/GYN • Open General/Abdominal • Laparoscopic OB/GYN • Laparoscopic General/Abdominal US Procedures Volume Considered* 11.5M
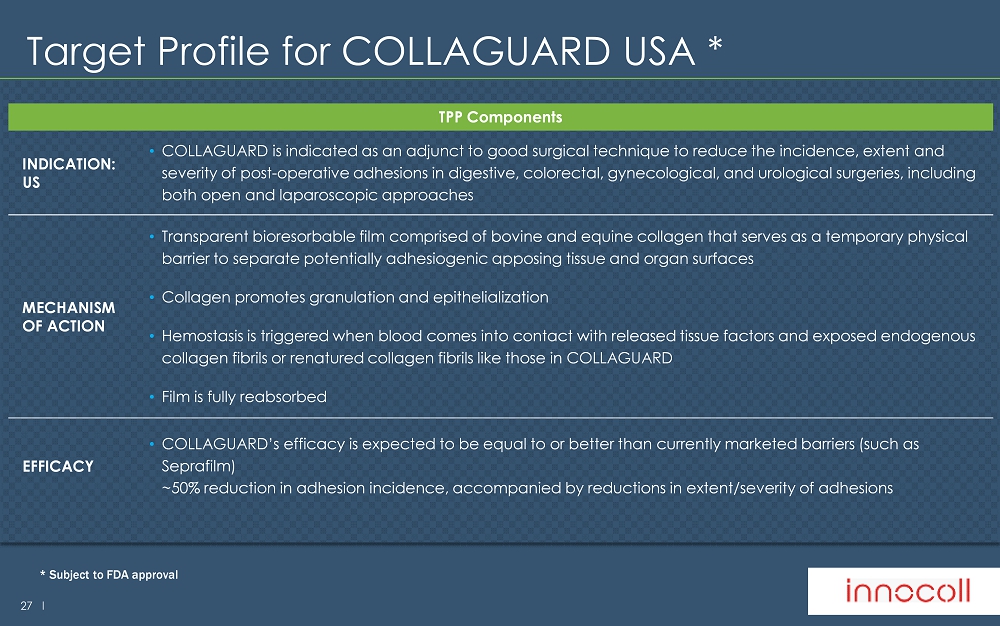
27 l Target Profile for COLLAGUARD USA * TPP Components INDICATION: US • COLLAGUARD is indicated as an adjunct to good surgical technique to reduce the incidence, extent and severity of post - operative adhesions in digestive, colorectal, gynecological, and urological surgeries, including both open and laparoscopic approaches MECHANISM OF ACTION • Transparent bioresorbable film comprised of bovine and equine collagen that serves as a temporary physical barrier to separate potentially adhesiogenic apposing tissue and organ surfaces • Collagen promotes granulation and epithelialization • Hemostasis is triggered when blood comes into contact with released tissue factors and exposed endogenous collagen fibrils or renatured collagen fibrils like those in COLLAGUARD • Film is fully reabsorbed EFFICACY • COLLAGUARD’s efficacy is expected to be equal to or better than currently marketed barriers (such as Seprafilm ) ~50% reduction in adhesion incidence, accompanied by reductions in extent/severity of adhesions * Subject to FDA approval

28 l COLLAGUARD Market Research Findings Moderate - to - high level concern for post - operative adhesion development Concern relates to future patient complications and preservation of future surgical fields Dissatisfaction with currently marketed adhesion barrier product as related to handling and placement Positive response to COLLAGUARD as related to ease of use and hemostatic characteristics

29 l Promising Clinical Studies In animal studies, COLLAGUARD increased the probability of remaining adhesion - free by more than six - fold and significantly reduced the extent and severity of adhesions COLLAGUARD is ready to enter a pivotal study once details of the study are worked out with FDA
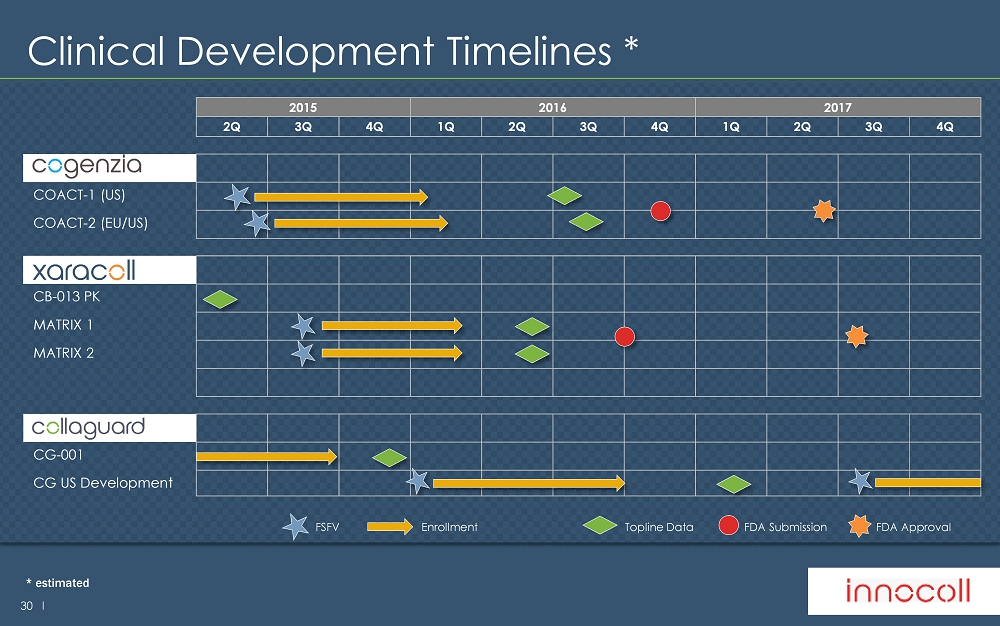
30 l Clinical Development Timelines * 2015 2016 2017 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q COGENZIA COACT - 1 (US) COACT - 2 (EU/US) XARACOLL CB - 013 PK MATRIX 1 MATRIX 2 CG - 001 CG US Development Enrollment Topline Data FSFV FDA Submission FDA Approval * estimated
31 l Our Strategy LEVERAGE EXPAND EXECUTE
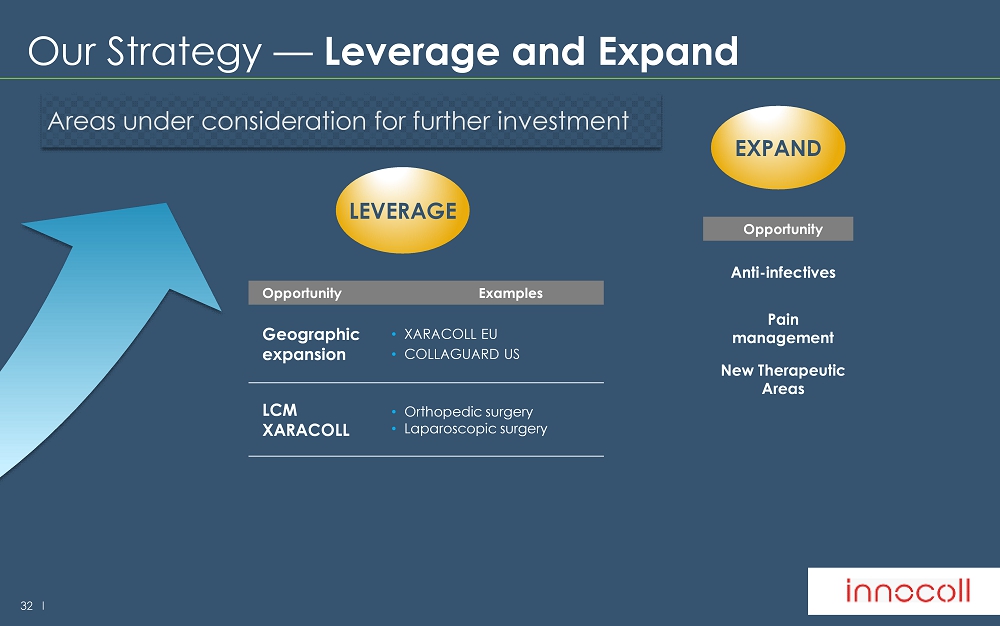
32 l Our Strategy — Leverage and Expand Areas under consideration for further investment Opportunity A nti - infectives Pain management New Therapeutic Areas Opportunity Examples G eographic expansion • XARACOLL EU • COLLAGUARD US LCM XARACOLL • Orthopedic surgery • Laparoscopic surgery LEVERAGE EXPAND

33 l Experienced Management Team Name Position Exp. Key Companies Anthony Zook President & CEO 30 yrs Jose (Pepe) Carmona Chief Financial Officer 20 yrs Michael Myers Ph.D. Head of Portfolio Operations 28 yrs Rich Fante Chief Commercial Officer & Head of Business Development 25 yrs James Croke EVP Engineering &
Technology Dev 29 yrs David Prior, Ph.D. EVP Clinical & Regulatory 30 yrs Alexandra Dietrich, Ph.D. EVP Operations 12 yrs
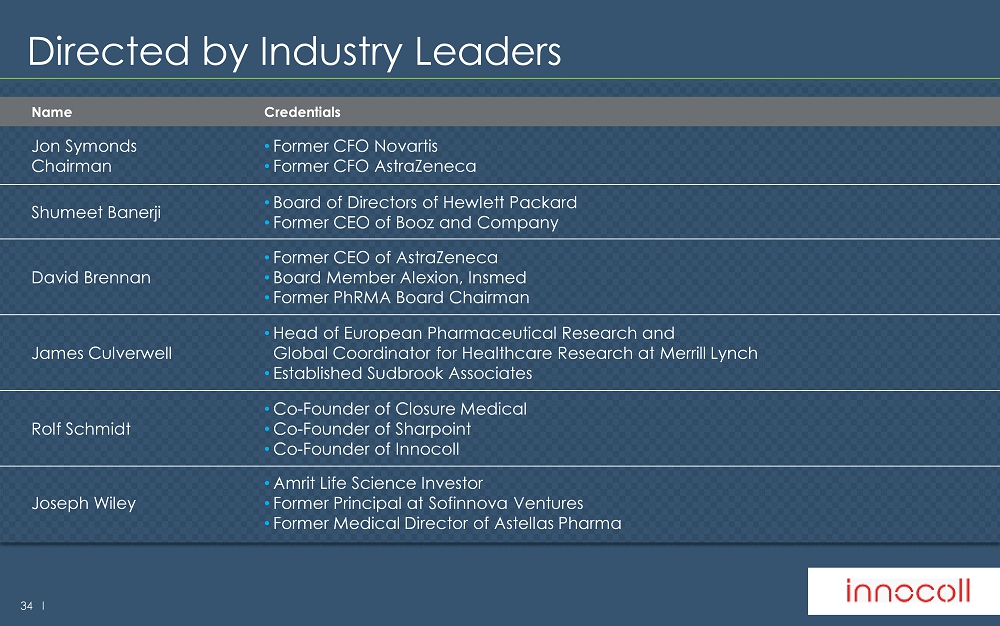
34 l Directed by Industry Leaders Name Credentials Jon Symonds Chairman • Former CFO Novartis • Former CFO AstraZeneca Shumeet Banerji • Board of Directors of Hewlett Packard • Former CEO of Booz and Company David Brennan • Former CEO of AstraZeneca • Board Member Alexion, Insmed • Former PhRMA Board Chairman James Culverwell • Head of European Pharmaceutical Research and Global Coordinator for Healthcare Research at Merrill Lynch • Established Sudbrook Associates Rolf Schmidt • Co - Founder of Closure Medical • Co - Founder of Sharpoint • Co - Founder of Innocoll Joseph Wiley • Amrit Life Science Investor • Former Principal at Sofinnova Ventures • Former Medical Director of Astellas Pharma
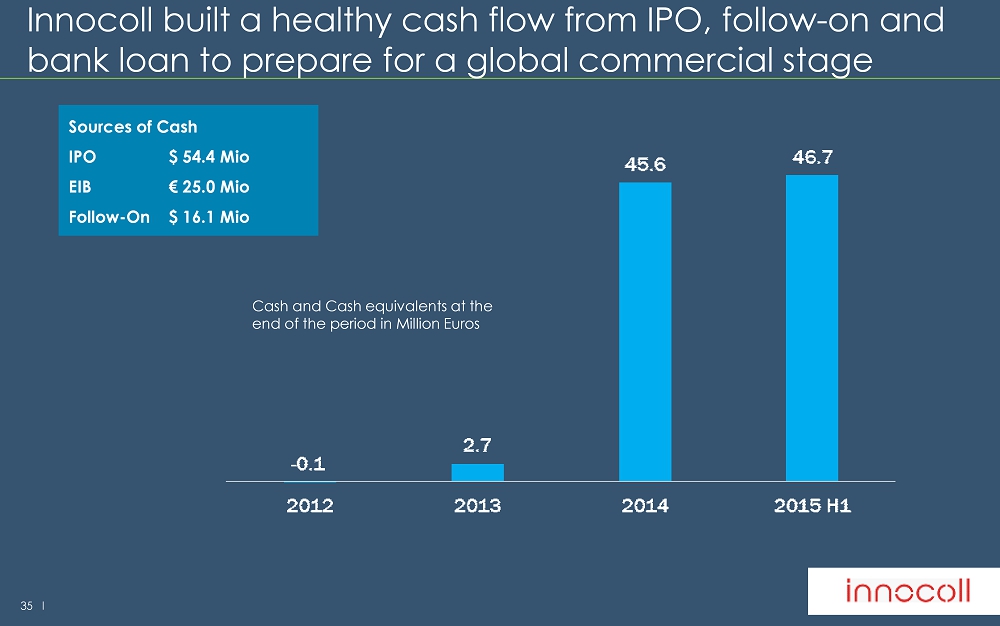
35 l Innocoll built a healthy cash flow from IPO, follow - on and bank loan to prepare for a global commercial stage Cash and Cash equivalents at the end of the period in Million Euros Sources of Cash IPO $ 54.4 Mio EIB € 25.0 Mio Follow - On $ 16.1 Mio
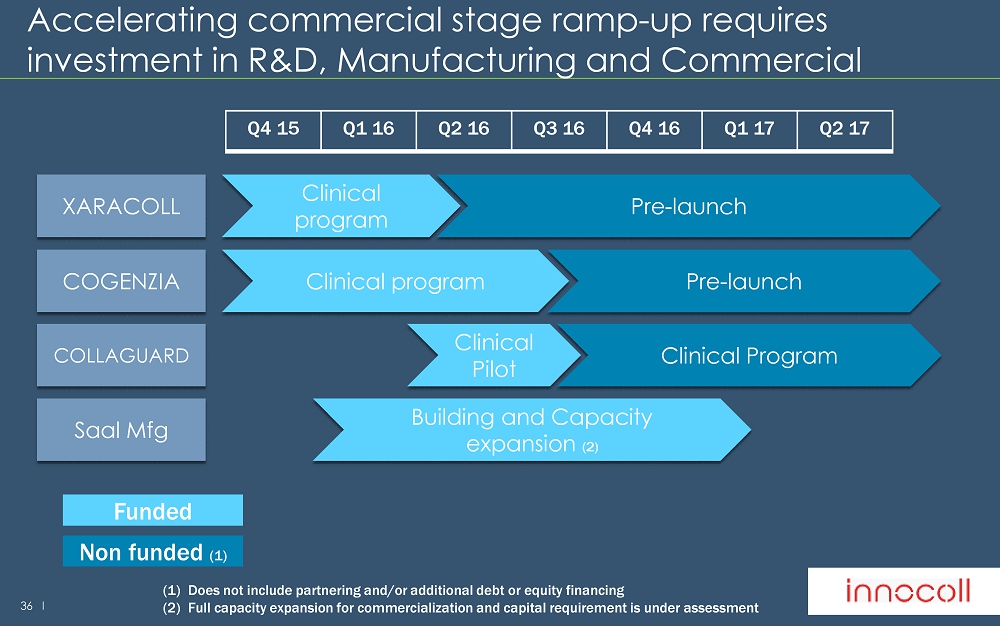
36 l Accelerating commercial stage ramp - up requires investment in R&D, Manufacturing and Commercial Clinical program XARACOLL Pre - launch Q4 15 Q1 16 Q2 16 Q3 16 Q4 16 Q1 17 Q2 17 Funded Non funded (1) Clinical program COGENZIA Pre - launch Clinical Pilot COLLAGUARD Clinical Program Building and Capacity expansion (2) Saal Mfg (1) Does not include partnering and/or additional debt or equity financing (2) Full capacity expansion for commercialization and capital requirement is under assessment

37 l September 2015 Update COGENZIA: QIDP FDA designation – Necessitates accelerated pre - commercialization efforts COGENZIA: Phase 3 program initiated in May XARACOLL: Phase 3 program initiated in August




































