United States
Securities and Exchange Commission
Washington, D.C. 20549
FORM 20-F
| ¨ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(B) OR (G) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED MARCH 31, 2015
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| ¨ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number -
MEDIFOCUS INC.
(Exact name of Registrant as specified in its charter)
MEDIFOCUS INC.
(Translation of Registrant’s name into English)
Province of Ontario, Canada
(Jurisdiction of incorporation or organization)
10240 Old Columbia Road, Suite G
Columbia, Maryland 21046
(Address of principal executive offices)
Dr. Augustine Cheung
410-290-5734
acheung@medifocusinc.com
10240 Old Columbia Road, Suite G
Columbia, Maryland 21046
(Name, Telephone, E-Mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
None
| | | | |
Title of each class | | | | Name of each exchange on which registered |
| | | | |
| | | | |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
Common Shares
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common shares as of the close of the period covered by the annual report.
127,542,120
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ¨ Yes x No
If this report is an annual or transition report, indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ¨ Yes ¨ No
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes x No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes x No
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one)
| | | | |
| Large accelerated filer ¨ | | Accelerated filer ¨ | | Non-accelerated filer x |
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| | | | |
| U.S. GAAP x | | International Financial Reporting Standards as issued By the International Accounting Standards Board ¨ | | Other ¨ |
If “Other” has been check in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
¨ Item 17 ¨ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes x No
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. ¨ Yes ¨ No
TABLE OF CONTENTS
PART I
In this Registration Statement on Form 20-F, the “Company,” “we,” “us” and “our” refers to Medifocus Inc. and its subsidiaries.
Unless otherwise indicated, all dollar amounts in this registration statement are expressed in United States dollars.
Unless we indicate otherwise, all information in this Report is stated as of March 31, 2015.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements contained in or incorporated by reference in this annual report are “forward-look statements.” Except for the statements of historical fact contained herein, the information presented constitutes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 (“PSLRA”). Often, but not always, forward-looking statements can be identified by the use of words such as “plans”, “expects”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, “believes”, or variation of such words and phrases that refer to certain actions, events or results to be taken, occur or achieved. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of the Company to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements.
Such factors include, among others, the actual results of the Prolieve business, requirements for additional capital, delays in obtaining governmental approvals, as well as those factors discussed in “Item 3. Key Information” and “Item 4. Information on the Company” of this annual report. Although the Company has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results not to be as anticipated, estimated or intended. There can be no assurance that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements.
In addition, forward-looking statements represent our estimates and assumptions only as of the date of this annual report. You should carefully review this annual report and the documents that the Company references in this annual report, or that are incorporated by reference into this annual report, with the understanding that the Company’s actual future results may differ materially from what is presented in this annual report.
Except as required by law, the Company assumes no obligation to update any forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in any forward-looking statements, even if new information becomes available in the future.
Notwithstanding the above, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, expressly state that the safe harbor for forward looking statements does not apply to companies that issue penny stocks. Accordingly, the safe harbor for forward looking statements under the PSLRA is not available to the Company because we are considered to be an issuer of penny stock.
1
| Item 1. | Identity of Directors, Senior Management and Advisers. |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
| Item 2. | Offer Statistics and Expected Timetable. |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
| A. | Selected financial data. |
The following selected financial and other data summarize our historical financial information. We derived the selected balance sheet information as of March 31, 2015, 2014, 2013 and 2012, and the selected statement of operations information for the years ended March 31, 2015, 2014, 2013 and 2012 from our audited financial statements as of those dates, prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”). The information herein should be read in conjunction with our historical financial statements and the notes thereto included elsewhere in this annual report.See “Item 5. Operating and Financial Review and Prospects,” “Item 8. Financial Information” and “Item 18. Financial Statements.”
We have not provided selected balance sheet data as of March 31, 2011 because our audited financial statements as of that date were prepared in accordance with International Financial Reporting Standards or generally accepted accounting principles in Canada, which differ in certain significant respects from U.S. GAAP. We believe we would have to incur unreasonable expenses to provide this information.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended March 31, | |
Statement of Operations Data | | 2015 | | | 2014 | | | 2013 | | | 2012 | | | 2011 | |
Total Sales | | $ | 4,219,459 | | | $ | 5,116,506 | | | $ | 1,800,371 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (4,087,985 | ) | | | (4,886,807 | ) | | | (5,333,515 | ) | | | (1,484,302 | ) | | | (2,339,835 | ) |
Net loss | | | (5,971,470 | ) | | | (5,992,897 | ) | | | (5,846,523 | ) | | | (1,665,402 | ) | | | (2,388,252 | ) |
Net loss per common share | | $ | (0.05 | ) | | $ | (0.05 | ) | | $ | (0.07 | ) | | $ | (0.05 | ) | | $ | (0.09 | ) |
Dividends declared | | | — | | | | — | | | | — | | | | — | | | | — | |
Weighted average common shares outstanding - basic and diluted | | | 122,809,928 | | | | 117,260,870 | | | | 84,042,487 | | | | 31,565,402 | | | | 26,002,635 | |
| | | | | |
Balance Sheet Data | | | | | | | | | | | | | | | |
Total assets | | $ | 5,418,487 | | | $ | 7,328,130 | | | $ | 6,268,583 | | | $ | 438,925 | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Common stock (no par value) | | | 12,782,563 | | | | 12,372,498 | | | | 12,524,735 | | | | 4,774,837 | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total stockholders’ equity (deficit) | | | (2,897,012 | ) | | | (134,369 | ) | | | 478,084 | | | | (2,553,772 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | |
All amounts are presented in U.S. dollars and in accordance with U.S. GAAP.
2
| B. | Capitalization and indebtedness. |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
| C. | Reasons for the offer and use of proceeds. |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
An investment in shares of our common stock (which we refer to as the “Shares”) involves a high degree of risk. You should carefully consider the risks described below and the risks described elsewhere in this annual report under the sections entitled “Item 4. Information on the Company” before deciding whether to invest in our shares. The following is a summary of the risk factors that we believe are most relevant to our business. These are factors that, individually or in the aggregate, could cause our actual results to differ significantly from anticipated or historical results. The occurrence of any of the risks could harm our business and cause the price of our common stock to decline, and investors may lose all or part of their investment. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. The risks and uncertainties described below and in the incorporated documents are not the only risks and uncertainties that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of these risks actually occurs, our business, financial condition and results of operations would suffer. The risks discussed below also include forward-looking statements, and our actual results may differ substantially from those discussed in these forward-looking statements. See “Special Note Regarding Forward-Looking Statements” at the beginning of Part I of this annual report. Except as required by law, we undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events, or otherwise.
We have a history of significant losses and expect to continue such losses for the foreseeable future.
Since our inception in 2005, our expenses have substantially exceeded our revenues, resulting in continuing losses and an accumulated deficit of $26,900,315 at March 31, 2015. In addition, our net loss for the year ended March 31, 2015 was $5,971,470. Such operating losses are the result of our commitment to continuing our product research, development and commercialization programs, which is only partially offset by limited revenues from the sale of our Prolieve system and related disposables. We expect to continue to experience significant operating losses unless and until we generate significant revenue from Prolieve, as well as the development of other new products and these products have been clinically tested, approved by the FDA or other regulatory authorities, and successfully commercialized.
We may not be able to generate significant revenue for the foreseeable future.
Since 2005, we have devoted our resources to developing the APA 1000, but we will not be able to market the APA 1000 until we have completed clinical testing and obtained all necessary governmental approvals. On July 26, 2012, we acquired from Boston Scientific Corporation the Prolieve Thermodilatation system business for the treatment of BPH and, since that time, we have assembled a sales and service team to market the Prolieve system. All of our current revenue is derived from sales of our Prolieve control units and more importantly, our single-use treatment catheters and treatments
3
delivered through our mobile service. There can be no assurance as to how much revenue will be generated by Prolieve sales. Our lack of product diversification means that we may be negatively affected by changes in market conditions and in regulation (including regulation affecting reimbursement for our products). In addition, at the present time our APA 1000 system is still in clinical testing stage and cannot be marketed until we have completed clinical testing and obtained necessary governmental approval. Accordingly, our revenue sources are, and will remain extremely limited until and unless our Prolieve system is marketed successfully and/or until our other new products are clinically tested, approved by the FDA or other regulatory authorities, and successfully commercialized. We cannot guarantee that our products will be successfully tested, approved by the FDA or other regulatory authorities, or commercialized, successfully or otherwise, at any time in the foreseeable future, if at all.
Our future is dependent upon our ability to obtain additional financing. If we do not obtain such financing, we may have to cease our operations and investors could lose their entire investment.
We have yet to operate profitably or generate positive cash flows from operations, and there is no assurance that we will operate profitably or will generate positive cash flow in the future. As a result, we have very limited funds, and such funds may not be adequate to take advantage of current, planned and unanticipated business opportunities. Even if our funds prove to be sufficient to pursue current, planned and unanticipated business opportunities, we may not have enough capital to fully develop such opportunities.
Further, our capital requirements relating to the manufacturing and marketing of our products have been, and will continue to be, significant. We are dependent on the proceeds of future financing in order to continue in business and to develop and commercialize proposed products. There can be no assurance that we will be able to raise the additional capital resources necessary to permit us to pursue our business plan. Finally, the continued growth of our business may require additional funding from time to time to be used by us for general corporate purposes, such as acquisitions, investments, repayment of debt, capital expenditures, repurchase of capital stock and additional purposes identified by the Company.
Accordingly, our ultimate success may depend upon our ability to raise additional capital. There can be no assurance that any additional financing will be available to us. As additional capital is needed, we may not be able to obtain additional equity or debt financing. Even if financing is available, it may not be available on terms that are favorable or acceptable to us, or in sufficient amounts to satisfy our requirements. Any inability to obtain additional financing will likely have a material adverse effect on our business operations, and could result in the loss of your entire investment.
Our independent registered public accountants have expressed substantial doubt regarding our ability to continue as a going concern.
Our auditors have expressed their opinion that there is substantial doubt about the Company’s ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of these uncertainties. Our ability to continue as a going concern is dependent upon our ability to successfully raise adequate additional financing and our ability to successfully develop our sales and marketing programs and commence our planned operations. We cannot assure you that we will be able to obtain additional financing or achieve profitability in our operations. Our failure to obtain additional financing or achieve profitability in our operations could require the Company to liquidate our business interests, and could result in the loss of your entire investment.
4
Our failure to have a full-time Chief Financial Officer may negatively affect our business and operations.
Our Chief Financial Officer (or “CFO”) serves only on a part-time basis and may be subject to conflicts of interest. The CFO devotes a portion of his working time to other business endeavors, which may lead to conflicts of interest, including deciding how much time to devote to our affairs. The CFO position is critical to our operations, and our failure to fill this position on a full-time basis may negatively impact our business and operations. It may also lead to the late filing of financial reports and other required disclosures, or the filing of noncompliant financial reports and other required disclosures, which could have numerous consequences, including administrative proceedings by the Securities and Exchange Commission (the “SEC”), claims under Section 10 of the Exchange Act and, if our Shares become listed on a national exchange, cease trade orders or the de-listing of the Shares on such exchange. Further, having a part-time CFO has, and may continue to, negatively impact the effectiveness of our disclosure controls and our internal controls over financial reporting. No assurances can be given that our CFO will transition to a full-time basis, or that we will be able to identify or afford a full-time qualified candidate for this position.
We operate with de-centralized management, and may be unable to hire additional personnel to support, manage and control our operations.
Our CFO performs his functions for us on a part-time, non-exclusive basis, and resides in Toronto, Canada. Further, we believe that we are understaffed and need to hire additional personnel to support, manage and control our operations in order to operate optimally. In the past, the combination of not having our CFO at our headquarters and being understaffed have contributed to the late filing of financial reports in Canada. These late filings resulted in temporary cease trade orders being issued, and a multi-month suspension of trading of our shares on the Toronto Venture Exchange (the “TSXV”). Although the Company has recently hired a qualified outside accounting consultant to minimize this risk. until we become profitable or obtain additional financing, these factors will persist, raising the risk in the future of us making late filings of financial reports in Canada and in the U.S., and having our Shares suspended from trading. Moreover, until we can afford to hire additional staff, we will continue to operate with less than optimal support, management and control of our operations. The Company recently hired a qualified outside accounting consultant to provide support to our management and CFO, but we cannot assure you that such efforts will effectively minimize such risks. If we continue to operate with de-centralized management and insufficient staffing, it may have a material adverse effect on our business.
We identified a material weakness in our internal control over financial reporting that could affect the reliability of our financial statements and have other adverse consequences.
We identified a material weakness in our internal control over financial reporting as of March 31, 2015. We determined that a material weakness existed because we did not employ a sufficient number of qualified accounting personnel to ensure all required adjustments are made to the Company’s books, which would allow for the preparation and presentation of the Company’s financial statements in conformity with accounting principles generally accepted in the United States of America.
Failure to have effective internal control over financial reporting could impair our ability to produce accurate financial statements on a timely basis and could lead to a restatement of our financial statements. If, as a result of deficiencies in our internal control over financial reporting, we cannot provide reliable financial statements, our business decision processes may be adversely affected, our business and results of operations could be harmed, investors could lose confidence in our reported financial information and our ability to obtain additional financing, or additional financing on favorable terms, could be adversely affected. In addition, failure to maintain effective internal control over financial reporting could result in investigations or sanctions by regulatory authorities.
5
In an effort to remediate this material weakness, we retained and have continued to work closely with qualified consultants. Further, we continue to search for qualified personnel and consultants to help ensure proper and timely accounting disclosures. Thus far, retaining outside consultants has not yet remediated this material weakness. Further, we are in the process of updating all internal controls, including financial reporting, and properly documenting all such controls. There can be no assurance that the material weakness identified or that any additional material weaknesses will not arise in the future due to our failure to implement and maintain adequate internal control over financial reporting.
The loss of certain of our key personnel, or any inability to attract and retain additional personnel, could negatively affect our business.
Our future success depends to a significant extent on the continued service of Dr. Augustine Cheung, our President, and John Mon, our Chief Operating Officer. Both of these individuals have been intimately involved with, and primarily responsible for, the invention, development and commercialization efforts for both of our products. The loss of services of either individual would adversely affect our business and our ability to implement our business plan.
Our future success will also depend on our ability to attract, retain and motivate highly skilled personnel to assist us with product development, commercialization and other facets of our business plan. If we fail to hire and retain a sufficient number of qualified individuals to fully meet the needs of the business of the Company, it may have an adverse effect on our business and results of operations.
One of our shareholders owns a significant percentage of our Shares and could exert significant influence over matters requiring shareholder approval.
Mr. Tak Cheung Yam, a former director of the Company, through Integrated Assets Management (Asia) Ltd, currently owns 26,023,106 Shares, or 14.97% of the Company’s outstanding common stock. Together with Integrated Assets Management (Asia) Ltd, Mr. Yam also owns exercisable warrants and options to purchase an additional 18,595,833 Shares. If Mr. Yam chooses to exercise all these warrants and options, he will control 23.18% of our Common Stock. In addition, Mr. Yam, through Integrated Assets Management (Asia) Ltd, owns a convertible note that can be converted into 7,400,000 Shares. If Mr. Yam chooses to convert the note to Shares, and to exercise all his warrants and options, he will effectively control 26.03% of our outstanding shares. As a result, Mr. Yam may have significant influence over our management, our decision-making process, our business strategy and affairs and matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as mergers, consolidations or the sale of substantially all of our assets. Mr. Yam’s interests may differ from those of other shareholders of the Company, and, Mr. Yam will have the ability to exercise influence over our business and may take actions that are not in our or our public shareholders’ best interests. Furthermore, this concentration of ownership may have the effect of delaying or preventing a change in control, including a merger, consolidation or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control, even if such a change in control would benefit our other stockholders.
Our internal sales and marketing capability is limited and we may need to enter into alliances with others possessing such capabilities to commercialize our products internationally.
Currently our primary source of revenue is through the sales of disposable catheter treatment kits and mobile services in the U.S. Consequently, we are dependent upon our limited sales and marketing capability for the successful marketing of our Prolieve system. There can be no assurance that we will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our Prolieve system.
6
We intend to market our other products, if and when such products are approved for commercialization by the FDA or other regulatory authorities, either directly or through other strategic alliances and distribution arrangements with third parties. There can be no assurance that we will be able to enter into third-party marketing or distribution arrangements on advantageous terms or at all. To the extent that we do enter into such arrangements, we will be dependent on our marketing and distribution partners. In entering into third-party marketing or distribution arrangements, we expect to incur significant additional expense. There can be no assurance that, to the extent that we sell products directly or we enter into any commercialization arrangements with third parties, such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our products and services.
We do not manufacture the Prolieve system ourselves, and rely on a third-party supplier to supply us with the proprietary disposable catheters used with our Prolieve system.
The Prolieve systems we currently have in inventory were manufactured by Sanmina Corporation for Boston Scientific Corporation prior to our acquisition of the Prolieve assets, and we do not currently have an agreement with Sanmina for the production of additional Prolieve systems. Accordingly, if our current inventory becomes insufficient to meet the business growth in both the U.S. and international markets, we will have to engage Sanmina Corporation, or another manufacturer, to produce such additional systems. Further, the proprietary disposable catheter kits used with the Prolieve system are manufactured by Lake Regional Medical Center (formerly Accellent Inc.) in its facility in Mexico. Due to the complexity of these catheter kits, as well as FDA standards applicable to manufacturers of such kits, the Company has not identified an alternative supplier for these catheter kits. If, for any reason, we are unable to obtain new Prolieve systems manufactured by Sanmina Corporation, or we are no longer able to purchase the catheter kits from Lake Regional Medical Center in sufficient amounts, on an as-needed basis and on acceptable terms, or if either manufacturer becomes unable or unwilling to continue to supply us with new Prolieve systems and disposable catheter kits, it would have a material adverse effect on our business and operations. There can be no assurance that we could find new manufacturers to fulfill our needs, that any such manufacturer would be FDA approved, or that such manufacturers would be willing to provide us with the required products under commercially acceptable terms. If we are unable to find additional manufacturers and suppliers and it results in a disruption to our business, there would be a material adverse effect on our business and results of operations.
The slow pace of our APA 1000 Breast Cancer System’s Phase III clinical trials could result in additional delays and increased costs of completing the trials in the future.
Our main focus at this time is attaining profitability for our Prolieve business. Accordingly, we have allocated most of our resources to this goal, compounding this with the lack of funding the progress of the pivotal Phase III clinical trials of our APA 1000 breast cancer treatment system has been very slow. We estimate that the Phase III clinical trials will cost approximately $7,500,000. We currently do not have the financing in place to accelerate and complete these trials. There can be no assurance that such financings will be available at all, or on terms favorable to us. Further, there can be no assurance as to when, or even if, we will succeed in making Prolieve profitable. Our inability to do so may make it more difficult for us to raise funds for the pivotal Phase III clinical trial of the APA 1000. In the event that we are able achieve profitable Prolieve operations, there can be no assurance that we will be able to generate enough funds from the Prolieve business to finance the pivotal Phase III clinical trial. Furthermore, we cannot predict the effect of the slow pace of the pivotal Phase III trial could have on the costs and other critical aspects of the Phase III clinical trial. There is the risk that this uncertainty could negatively impact our business plans, and our ability to raise additional funds for further development of our APA 1000 business.
7
We may not receive regulatory approval from the U.S. Food and Drug Administration (“FDA”) to market the APA 1000.
Drugs and medical devices in the United States are regulated by the FDA, which requires that new medicines and medical devices be demonstrated to be both safe and effective. This is accomplished by conducting staged clinical trials that are subject to the FDA’s review, analysis and approval. While the Phase I and Phase II clinical trials for APA 1000 have been completed, and we received approval from the FDA and Health Canada to begin the pivotal Phase III clinical trials, as of today, a very limited number of patients out of a planned 238 person trial in the pivotal Phase III clinical trial, have been treated with APA 1000. There can be no assurance that our Phase III clinical trial will be completed, and if it is completed, that it will demonstrate APA 1000’s safety and efficacy, and that we will subsequently receive the FDA’s approval for us to commence marketing. In the event that we complete the pivotal Phase III clinical trial and receive FDA approval to market APA 1000, there can be no assurance that APA 1000 will be adopted for use by the healthcare industry, and that this business will be profitable. If the APA 1000 is not adopted for use by the healthcare industry, or we are not able to become profitable, it would have a material adverse effect on our business and results of operations.
We may not succeed in developing a meaningful market share of the benign prostatic hyperplasia (“BPH”) treatment markets with Prolieve, and our Prolieve business may not become profitable.
The BPH market is highly competitive, and is presently dominated by large, international pharmaceutical companies that promote the use of proprietary drugs to treat this condition. These companies, which include, Eli Lilly, Glaxo Smith Kline, Merck & Co., and others, aggressively market their drugs to primary care physicians, and to consumers through television, print, digital and other media. Because the market for BPH treatment is large and growing, and the manufacturers of these medications have made substantial investments in their development and marketing, we expect them to vigorously defend their market positions. In addition, we face strong competition from surgical and other minimally invasive treatment modalities. Although we have received a PMA from the FDA for our Prolieve system for the treatment of BPH, we can offer no assurance that the Prolieve system will be accepted by the medical community widely. Because our financial, marketing and sales resources are much smaller than those of the pharmaceutical companies, we are at significant competitive disadvantage, which will make it difficult for us to substantially expand our Prolieve business. Our inability to expand our Prolieve business achieve profitability and capture significant market share of the BPH treatment market will adversely affect us.
Recent health care reform laws in the U.S. could have a negative impact on our business.
Our business, financial condition, results of operations and cash flows could be significantly and adversely affected by recent healthcare reform legislation, including, most immediately, by the medical device excise tax that became effective on January 1, 2013. The Patient Protection and Affordable Care Act and Health Care and Education Reconciliation Act of 2010 (the “Healthcare Reform Acts”) were enacted into law in March 2010. As a company that operated in the United States, the Healthcare Reform Acts may materially impact our business and operations. Certain provisions of the Healthcare Reform Acts will not be effective for a number of years and there are many programs and requirements for which the details have not yet been fully established. Accordingly, it is unclear what the full impact will be from the Healthcare Reform Acts.
However, beginning in January 2013, the Healthcare Reform Acts impose a 2.3% excise tax on sales of our Prolieve products in the United States. We expect the new tax will materially and adversely affect our business, cash flows and results of operations. The Healthcare Reform Acts also contain a number of Medicare provisions aimed at improving quality and decreasing costs. The Medicare provisions include value-based payment programs, increased funding of comparative effectiveness research, reduced hospital payments for avoidable readmissions and hospital acquired conditions, and pilot programs to evaluate alternative payment methodologies that promote care coordination (such as bundled physician and hospital payments). Additionally, the Healthcare Reform Acts include a reduction in the annual rate of
8
inflation for Medicare payments to hospitals that began in 2011 and the establishment of an independent payment advisory board to recommend ways of reducing the rate of growth in Medicare spending beginning in 2014. We cannot predict what healthcare programs and regulations will ultimately be implemented at the federal or state level, or the effect of any future legislation or regulation. However, any changes that lower reimbursement for our products or reduce medical procedure volumes could have a material adverse effect our business and results of operations.
Our APA 1000 system and future products utilizing the adaptive phased array technology depend on license agreements with MIT to permit us to use patented technologies.
Our success depends, in substantial part, on our ability to maintain our rights under license agreements that grant us the rights to use patented technologies. We have entered into a license agreement with MIT under which we have exclusive rights to commercialize medical treatment products and procedures based on MIT’s Adaptive Phased Array technology. The MIT license agreement contains license fee, royalty and/or research support provisions, testing and regulatory milestones, and other performance requirements that we must meet by certain deadlines. If we were to breach these or other provisions of the license agreement, we could lose our ability to use the subject technology, as well as compensation for our efforts in developing or exploiting the technology. Any such loss of rights and access to technology could have a material adverse effect on our business.
Further, we cannot guarantee that any patent or other technology rights licensed to us by others will not be challenged or circumvented successfully by third parties, or that the rights granted will provide adequate protection. We are aware of published patent applications and issued patents belonging to others, and it is not clear whether any of these patents or applications, or other patent applications of which we may not have any knowledge, will require us to alter any of our potential products or processes, pay licensing fees to others or cease certain activities. Litigation, which could result in substantial costs, may also be necessary to enforce any patents issued to or licensed by us or to determine the scope and validity of others’ claimed proprietary rights. We also rely on trade secrets and confidential information that we seek to protect, in part, by confidentiality agreements with our corporate partners, collaborators, employees, and consultants. We cannot guarantee that these agreements will not be breached, that, even if not breached, that they are adequate to protect our trade secrets, that we will have adequate remedies for any breach or that our trade secrets will not otherwise become known to, or will not be discovered independently by, competitors.
We may not be able to protect the intellectual property that is integral to our business, or we may be subject to claims of intellectual property infringement by third parties, either of which could have a material adverse effect on our business.
Much of our potential success and value lies in our ownership and use of intellectual property. Our inability or failure to protect our intellectual property may negatively affect our business and value. Our ability to compete effectively is dependent in large part upon the maintenance and protection of the intellectual property we own and licenses from MIT. We will rely on patents, trademarks, trade secret and copyright law, as well as confidentiality procedures to establish and protect our intellectual property rights. It may be possible for a third party to copy or otherwise obtain and use the proprietary technology presently owned by or licensed to us without authorization. Policing unauthorized use of our intellectual property is difficult. The steps we take may not prevent misappropriation of our intellectual property, and the agreements we enter into may not be enforceable. In addition, effective intellectual property protection may be unavailable or limited in some jurisdictions outside the United States. Litigation may be necessary in the future to enforce or protect our intellectual property rights or to determine the validity and scope of the proprietary rights of others. Such litigation could cause us to incur substantial costs and divert resources away from our business, which in turn could have a material adverse effect on our business, results of operations, financial condition and profitability.
9
We may be subject to damaging and disruptive intellectual property litigation.
Although we are not currently aware that our products or services infringe any published patents or registered trademarks, we may be subject to infringement claims in the future. Because patent applications are kept confidential for a period of time after filing, applications may have been filed that, if issued as patents, could relate to our business.
Parties making claims of infringement may be able to obtain injunctive or other equitable relief that could effectively block us from providing its products and services in the United States and other jurisdictions and could cause us to pay substantial damages. In the event of a successful claim of infringement, we may need to obtain one or more licenses from third parties, which may not be available at a reasonable cost, if at all. The defense of any lawsuit could result in time-consuming and expensive litigation, regardless of the merits of such claims, as well as resulting damages, license fees, royalty payments and restrictions on our ability to provide products or services, any of which could harm our business.
Intellectual property rights are difficult to enforce in China, which could harm our business.
Chinese commercial law is relatively undeveloped compared with the commercial law in many of our other major markets and limited protection of intellectual property is available in China as a practical matter. We have formed a joint venture with Ideal Concepts Inc. to commercialize our products in the “Asia Pacific,” including China. Accordingly, any local design, manufacture, distribution or marketing of products that we undertake in China could subject us to an increased risk that unauthorized parties will be able to copy or otherwise obtain or use our intellectual property, which could harm our business. We may also have limited legal recourse in the event we encounter patent or trademark infringers, which could have a material adverse effect on our business and results of operations.
Our business is subject to numerous and evolving state, federal and foreign regulations and we may not be able to secure the government approvals needed to develop and market our products.
Our research and development activities, pre-clinical tests and clinical trials, and ultimately the manufacturing, marketing and labeling of our products, are all subject to extensive regulation by the FDA and foreign regulatory agencies. Pre-clinical testing and clinical trial requirements and the regulatory approval process typically take years and require the expenditure of substantial resources. Further, additional government regulation may be established that could prevent or delay regulatory approval of our product candidates. Delays or rejections in obtaining regulatory approvals would adversely affect our ability to commercialize any product candidates and our ability to generate product revenues or royalties.
The FDA and foreign regulatory agencies require that the safety and efficacy of product candidates be supported through adequate and well-controlled clinical trials. If the results of pivotal clinical trials do not establish the safety and efficacy of our product candidates to the satisfaction of the FDA and other foreign regulatory agencies, we will not receive the approvals necessary to market such product candidates.
Even if regulatory approval of a product candidate is granted, the approval may include significant limitations on the indicated uses for which the product may be marketed. In addition, we are subject to inspections and regulations by the FDA. Medical devices must also continue to comply with the FDA’s Quality System Regulation, or QSR. Compliance with such regulations requires significant expenditures of time and effort to ensure full technical compliance. The FDA stringently applies regulatory standards for manufacturing.
10
We are subject to the periodic inspection of our clinical trials, facilities, procedures and operations and/or the testing of our products by the FDA to determine whether our systems and processes are in compliance with FDA regulations. Following such inspections, the FDA may issue notices on Form 483 and warning letters that could cause us to modify certain activities identified during the inspection. A Form 483 notice is generally issued at the conclusion of an FDA inspection and lists conditions the FDA inspectors believe may violate FDA regulations. FDA guidelines specify that a warning letter is issued only for violations of “regulatory significance” for which the failure to adequately and promptly achieve correction may be expected to result in an enforcement action.
Failure to comply with FDA and other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production and/or distribution, suspension of the FDA’s review of product applications, enforcement actions, injunctions and criminal prosecution. Under certain circumstances, the FDA also has the authority to revoke previously granted product approvals. Although we have internal compliance programs, if these programs do not meet regulatory agency standards or if our compliance is deemed deficient in any significant way, it could have a material adverse effect on the Company.
We are also subject to record keeping and reporting regulations, including FDA’s mandatory Medical Device Reporting, or MDR, regulation. Labeling and promotional activities are regulated by the FDA and, in certain instances, by the Federal Trade Commission.
Many states in which we do or in the future may do business or in which our products may be sold impose licensing, labeling or certification requirements that are in addition to those imposed by the FDA. There can be no assurance that one or more states will not impose regulations or requirements that have a material adverse effect on our ability to sell our products.
In many of the foreign countries in which we may do business or in which our products may be sold, we will be subject to regulation by national governments and supranational agencies as well as by local agencies affecting, among other things, product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. There can be no assurance that one or more countries or agencies will not impose regulations or requirements that could have a material adverse effect on our ability to sell our products.
Failure to comply with applicable regulatory requirements, can result in, among other things, warning letters, fines, injunctions and other equitable remedies, civil penalties, recall or seizure of products, total or partial suspension of production, refusal of the government to grant approvals, pre-market clearance or pre-market approval, withdrawal of approvals and criminal prosecution of the Company and its employees, all of which would have a material adverse effect on our business.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act.
Our business operations in countries outside the United States, for example through our Chinese joint venture, may be subject to anti-corruption laws and regulations, including restrictions imposed by the Foreign Corrupt Practices Act (the “FCPA”). The FCPA and similar anti-corruption laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. We cannot provide assurance that our internal controls and procedures will always protect us from criminal acts committed by our employees or third parties with whom we work. If we are found to be liable for violations of the FCPA or similar anti-corruption laws in international jurisdictions, either due to our own acts or out of inadvertence, or due to the acts or inadvertence of others, we could suffer from criminal or civil penalties which could have a material and adverse effect on our results of operations, financial condition and cash flows.
11
The success of our products may be harmed if the government, private health insurers and other third-party payors do not provide sufficient coverage or reimbursement.
Our current and future revenues are subject to uncertainties regarding health care reimbursement and reform. Our ability to commercialize our new cancer treatment system successfully will depend in part on the extent to which reimbursement for the costs of such products and related treatments will be available from government health administration authorities, private health insurers and other third-party payors. The reimbursement status of newly approved medical products is subject to significant uncertainty. We cannot guarantee that adequate third-party insurance coverage will be available for us to establish and maintain price levels sufficient for us to realize an appropriate return on our investment in developing new therapies. Government, private health insurers, and other third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products approved for marketing by the FDA. Accordingly, even if coverage and reimbursement are provided by government, private health insurers, and third-party payors for uses of our products, market acceptance of these products would be adversely affected if the reimbursement available proves to be unprofitable for health care providers. We may be unable to sell our products on a profitable basis if third-party payers deny coverage, or provide low reimbursement rates.
Our products may not achieve sufficient acceptance by the medical community to sustain our business.
Although we have received a PMA from the FDA for our Prolieve system for the treatment of BPH, we can offer no assurance that the Prolieve system will be accepted by the medical community widely. Our breast cancer treatment development project using the APA technology is currently in Phase III clinical trials. It may prove not to be effective in practice. If testing and clinical practice do not confirm the safety and efficacy of our systems or, even if further testing and practice produce positive results but the medical community does not view these new forms of treatment as effective and desirable, our efforts to market our new products may fail, with material adverse consequences to our business.
We face intense competition and the failure to compete effectively could adversely affect our ability to develop and market our products.
There are many companies and other institutions engaged in research and development of various technologies, both for prostate disease and cancer treatment products that seek treatment outcomes similar to those that we are pursuing. We believe that the level of interest by others in investigating the potential of possible competitive treatments and alternative technologies will continue and may increase. Potential competitors engaged in all areas of BPH and cancer treatment research in the United States and other countries include, among others, major pharmaceutical, specialized technology companies, and universities and other research institutions. Most of our competitors and potential competitors have substantially greater financial, technical, human and other resources, and may also have far greater experience, than do we, both in pre-clinical testing and human clinical trials of new products and in obtaining FDA and other regulatory approvals. One or more of these companies or institutions could succeed in developing products or other technologies that are more effective than the products and technologies that we have been or are developing, or which would render our technology and products obsolete and non-competitive. Furthermore, if we are permitted to commence commercial sales of any of our products, we will also be competing, with respect to manufacturing efficiency and marketing, with companies having substantially greater resources and experience in these areas.
12
If we become subject to product liability claims, we may be required to pay damages that exceed our insurance coverage and the value of our assets.
We currently carry product liability insurance in the amount of $5,000,000 per occurrence, which may be inadequate to satisfy liabilities we may incur. Any claim brought against us, regardless of its merit, could result in the increase of our product liability insurance rates or our inability to obtain future coverage on acceptable terms, or at all. In addition, if our product liability coverage is inadequate to pay a damage award, we would have to pay any shortfall out of our assets, which may be insufficient, or by securing additional funds, of which there can be no assurance. Even a meritless or unsuccessful product liability claim made against us could harm our reputation, cause us to incur significant legal fees and result in the diversion of management’s attention from managing our business. Any of these occurrences or events would have a material adverse effect on our business.
Our newly formed joint venture with Ideal Concepts Inc. could cause us to effectively transfer rights to our technology in major markets in Asia, and to lose rights to sell and market our products in Asia.
We recently formed a joint venture with Ideal Concept Inc. As a new business, this joint venture is subject to a variety of risks including, without limitation, obtaining adequate financing to operate the business, recruiting management with expertise to market, promote, and produce products and having the capability of obtaining required regulatory approvals from various foreign governments in order sell products. Pursuant to the terms of our joint venture, our equity ownership in the joint venture can be reduced, and eventually eliminated, if we are unable to contribute financing to it in the future. This is a distinct possibility because of our current financial condition, and also because we will be borrowing funds from Ideal Concept Inc. to enable us to contribute a portion of the funds required to be invested by us in the joint venture. In addition, our right to receive royalties from the sale of products by the joint venture will prove to be worthless if there are no sales.
We could have disagreements with Ideal Concepts Inc. over the territory covered by the joint venture, and over other key aspects of the joint venture.
The territory covered by the joint venture is described as “Asia Pacific”, which is not defined in the agreement. In addition, other important aspects, terms and conditions of the joint venture are absent or unclear in the agreement establishing the joint venture. Accordingly, we could have disagreements with Ideal Concepts Inc. over rights and responsibilities of Ideal Concepts Inc. and us, as well as on other issues. If not resolved, these issues could have adverse consequences on the joint venture, make it difficult or impossible to sell products, result in litigation and cause us to incur substantial liabilities.
Damage to our reputation, for whatever reason, could have a material adverse effect on our business.
Our ability to market and sell Prolieve, APA 1000 and new products in major world markets, including the United States, could be adversely affected in the future by negative publicity resulting from, among others, the joint venture, adverse regulatory decisions by international bodies related to our products, controversy surrounding our products and the businesses activities of the joint venture, litigation arising from the joint venture and use of products, over which we will have very little, if any, control.
We have elected to use the extended transition period for complying with new or revised accounting standards.
Pursuant to Section 107(b) of the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), we have elected to use the extended transition period for complying with new or revised accounting standards for an “emerging growth company.” This election will permit, but not require, us to delay the adoption of new or revised accounting standards that will have different effective dates for public and private companies until those standards apply to private companies. Consequently, our financial statements may not be comparable to companies that comply with public company effective dates.
13
Our Shares are deemed to be “Penny Stocks,” which means that there are significant restrictions on stockbrokers and dealers recommending our Shares for purchase.
Our common stock is considered to be a “penny stock” pursuant to the rules promulgated under Section 15(g) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As a result, our securities are subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in the sale of shares of penny stock to persons other than established customers or “accredited investors” (as such term is defined in Rule 501 of Regulation D promulgated under the Securities Act of 1933, as amended (the “Securities Act”)). Under such rules, a broker-dealer must, prior to a transaction in a penny stock not otherwise exempt from those rules, deliver a standardized risk disclosure document prepared by the SEC, which specifies information about penny stocks and the nature and significance of risks of the penny stock market. A broker-dealer must also provide the customer with bid and offer quotations for the penny stock, the compensation of the broker-dealer, and sales person in the transaction, and monthly account statements indicating the market value of each penny stock held in the customer’s account. In addition, the penny stock rules require that, prior to a transaction in a penny stock not otherwise exempt from the penny stock rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for stock that is subject to the penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules may discourage investor interest in and limit the marketability of our securities, and limit the current investors’ ability to sell their shares of our common stock.
We may never pay dividends.
We have never declared or paid any dividends on our Shares since our inception. We do not intend to pay cash dividends on our Shares for the foreseeable future, and currently intend to retain any future earnings to fund the development and growth of our business. The payment of cash dividends, if any, on the Shares will rest solely within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors. We currently intend to use any revenues, as well as proceeds from any financings, to assist us in obtaining our business objectives, and not for the payment of any dividends upon our Shares.
Shareholders may suffer dilution of the value of their Shares by our issuance of additional Shares in the future.
As of March 31, 2015, we have outstanding debt, warrants and options that are convertible or exchangeable into 137,043,719 Shares. Additionally, we have plans to sell and issue additional Shares, or other securities that are convertible into Shares, in the future, in order to raise funds and for other purposes. The issuance of additional Shares, whether through the conversion of convertible notes, the exercise of warrants or options, or an issuance of Shares in connection with a financing, will dilute our current shareholders’ ownership in the Company, and will reduce shareholders’ voting power proportionally.
Future sales of Shares, securities convertible into Shares, and other securities may negatively affect our stock price.
Future sales of Shares and/or other securities that are convertible into Shares could have a significant negative effect on the market price of our Shares, and the number of Shares outstanding could increase substantially. This increase, in turn, could dilute future earnings per share. Dilution and the availability of a large amount of securities for sale, and the possibility of additional issuances and sales of Shares or other classes of securities may negatively affect both the trading price and liquidity of our Shares.
14
The market for our Shares is, and may continue to be, limited and highly volatile, which may generally affect any future price of our Shares.
The lack of an orderly market for our common stock may negatively affect the volume of trading and market price for our common stock.
Historically, the volume of trades for our Shares has been limited. Moreover, the prices at which our Shares have traded have fluctuated widely on a percentage basis. There can be no assurance as to the prices at which our Shares will trade in the future, although they may continue to fluctuate significantly. Prices for our Shares will be determined in the marketplace and may be influenced by many factors, including, without limitation, the following:
| | • | | the depth and liquidity of the markets for our Shares; |
| | • | | investor perception of the Company and the industry in which we participate; |
| | • | | general economic and market conditions; |
| | • | | statements or changes in opinions, ratings or earnings estimates made by brokerage firms or industry analysts relating to the market in which we do business or relating to us specifically, as has occurred in the past; |
| | • | | quarterly variations in our results of operations; |
| | • | | general market conditions or market conditions specific to technology industries; and |
| | • | | domestic and international macroeconomic factors. |
An active trading market for the Shares may not exist in the future. Even if a market for our Shares continues to exist, investors may not be able to resell their Shares at or above the purchase price for which such investors purchased such Shares.
In addition, the stock market has recently experienced extreme price and volume fluctuations. These fluctuations are often unrelated to the operating performance of the specific companies. As a result of the factors identified above, a stockholder (due to personal circumstances) may be required to sell its Shares at a time when our stock price is depressed due to random fluctuations, possibly based on factors beyond our control.
| Item 4. | Information on the Company. |
Emerging Growth Company Status
We are an “emerging growth company” as defined in section 3(a) of the Exchange Act, as amended by the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), and will continue to qualify as an “emerging growth company” until the earliest to occur of: (a) the last day of the fiscal year during which we have total annual gross revenues of $1,000,000,000 (as such amount is indexed for inflation every 5 years by the SEC) or more; (b) the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (c) the date on which we have, during the previous 3-year period, issued more than $1,000,000,000 in non-convertible debt; or (d) the date on which we are deemed to be a ‘large accelerated filer’, as defined in Exchange Act Rule 12b–2.
15
Generally, a company that registers any class of its securities under section 12 of the Exchange Act is required to include in the second and all subsequent annual reports filed by it under the Exchange Act, a management report on internal controls over financial reporting and, subject to an exemption available to companies that meet the definition of a “smaller reporting company” in Exchange Act Rule 12b-2, an auditor attestation report on management’s assessment of internal controls over financial reporting. However, for so long as we continue to qualify as an emerging growth company, we will be exempt from the requirement to include an auditor attestation report in our annual reports filed under the Exchange Act, even if we do not qualify as a “smaller reporting company”. In addition, section 103(a)(3) of the Sarbanes-Oxley Act of 2002 has been amended by the JOBS Act to provide that, among other things, auditors of an emerging growth company are exempt from any rules of the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the company.
Any U.S. domestic issuer that is an emerging growth company is able to avail itself to the reduced disclosure obligations regarding executive compensation in periodic reports and proxy statements, and to not present to its shareholders a nonbinding advisory vote on executive compensation, obtain approval of any golden parachute payments not previously approved, or present the relationship between executive compensation actually paid and our financial performance. As a foreign private issuer, we are not subject to such requirements, and will not become subject to such requirements even if we were to cease to be an emerging growth company.
As a reporting issuer under the securities legislation of the Canadian provinces of Ontario, British Columbia, and Alberta, we are required to comply with all new or revised accounting standards that apply to Canadian public companies. Pursuant to Section 107(b) of the JOBS Act, an emerging growth company may elect to utilize an extended transition period for complying with new or revised accounting standards for public companies until such standards apply to private companies. We have elected to utilize this extended transition period. However, while we have elected to utilize this extended transition period, our audited consolidated financial statements as of March 31, 2015 reflect the adoption of all required accounting standards for public companies.
| | A. | History and development of the company. |
General
We are in the business of developing and selling medical device systems that deliver precisely focused, microwave-generated heat to diseased tissue, thereby destroying or shrinking it. We have developed two thermotherapy platforms for delivering this heat. The first platform delivers heat via a catheter that is inserted through a body opening directly to the diseased tissue. The catheter is attached to a modular, free-standing unit that generates controls and monitors the heat delivery. We refer to this platform as our “Endo-thermotherapy platform.” Our Prolieve Thermodilation System (“Prolieve”), utilized for the treatment of BPH, discussed below, uses this method. The Prolieve system has been commercialized and we are currently generating limited revenues from it.
Our second thermotherapy platform delivers heat to the diseased tissue via microwave beams delivered from outside of the body. The beams are precisely focused on the diseased tissue by utilizing sophisticated identification and targeting technology which we have licensed from MIT. This technology was originally developed at MIT as part of the United States’ “Star Wars” missile shield defense system, but we have adapted this technology for use in our products. With this method of heat delivery, a fine needle probe is inserted into the targeted tissue using conventional radio frequency positioning technology. This probe acts as a receptor for the microwave generated heat beams that are delivered to the targeted tissue from a module incorporating the MIT technology. We refer to this technology of heat delivery as our Adaptive Phased Array or “APA platform”. Our APA 1000 system for the treatment of
16
breast cancer, discussed below, uses this technology. APA 1000 has not been approved for use by the FDA to treat locally advanced tumors in breast cancer patients. We have completed Phase I and Phase II clinical trials for APA 1000. We have begun conducting pivotal Phase III clinical trial, but the progress of the clinical trials has been slow due to insufficient funding. Subject to the availability of funds, we plan to fully resume the pivotal Phase III clinical trial during the current fiscal year.
We believe that our two focused heat technology platforms can provide the design basis for the future development of additional cancer treatment systems for surface, subsurface and deep internal localized and regional cancers. We also believe that our technology platforms could form the basis for us to develop new therapeutic systems in the future that may (i) prevent breast cancer, and (ii) have cosmetic applications in treating cellulite and minimally invasive liposuction.
History
Our business was started by Dr. Augustine Cheung, our President and Chief Executive Officer, as an outgrowth of his academic interest and work in the field of microwave technology and the thermotherapy treatment of disease while he was a professor at the University of Maryland and George Washington University. In 1982, he founded A.Y. Cheung Associates Inc. to pursue this work. A.Y. Cheung Associates Inc. changed its name to Cheung Laboratories, Inc. in 1984, and Cheung Laboratories Inc. subsequently changed its name to Celsion Corporation (“Celsion”) in 1998.
At Celsion, Dr. Cheung began developing technologies for the treatment of BPH and breast cancer using thermotherapy technology, leading to the development and commercialization of the Prolieve system for the treatment of BPH. In 2007, Celsion sold the Prolieve system and technology to Boston Scientific Corporation (“Boston Scientific”) for $60 million. Dr. Cheung also began developing the APA 1000 system for the treatment of breast cancer. The rights to key elements of APA 1000 were licensed from MIT pursuant to an Exclusive Patent License Agreement (“Patent License Agreement”) dated October 24, 1997.
In 2005 Celsion transferred all its interest in this license and other rights to APA 1000 to its wholly-owned subsidiary, Celsion (Canada) Limited (“Celsion Canada”). On January 16, 2006, Dr. Cheung resigned from Celsion’s board of directors and his position as Celsion’s Chief Scientific Officer, and purchased Celsion Canada for $20,000,000 (Canadian dollars). The purchase price was paid by issuing: (a) a personal $1.5 million promissory note; and (b) an $ 18.5 million royalty payable at the rate of 5% of the net sales on sales of products developed using APA technology, once such products become commercialized. The $1.5 million promissory note was secured by 1,508,050 shares of Celsion’s common stock. After Dr. Cheung’s default on payment of the promissory note, Celsion agreed in 2009 with Dr. Cheung to retain the 1,508,000 shares of Celsion’s common stock that it held as security in full satisfaction of the $1.5 million promissory note.
Medifocus Inc. was incorporated on April 25, 2005 under the Business Corporations Act (Ontario) as a CPC. Under Canadian law, a CPC is a newly created Canadian company having no assets, other than cash, which is permitted to conduct an initial public offering of its securities (“IPO”) and obtain a listing of its shares on the TSXV. A CPC may then uses the funds raised in the IPO to identify and evaluate assets or businesses which, when acquired, qualify the CPC for listing as a regular issuer on the TSXV.
On June 29, 2006 Medifocus Inc., completed its IPO on the TSXV of 4,600,000 shares at a price of $0.20 (Canadian dollars) per share receiving gross proceeds of $920,000 (Canadian dollars). In order to gain improved access to funding, Medifocus Inc. engaged in a share exchange offer with Celsion Canada in 2008 pursuant to which Celsion Canada became a wholly-owned subsidiary of Medifocus.
17
Concurrently with the exchange offer, Medifocus completed a private placement of units, receiving gross proceeds of $2 million (Canadian dollars). In addition, Medifocus issued 903,112 shares to Celsion at a deemed value of $0.50 (Canadian dollars) per share, in partial satisfaction of an approximate $600,000 (Canadian dollars) liability that was owed to Celsion. After the completion of the share exchange transaction, we continued our development of the APA 1000 technology for the treatment of breast cancer. Phase I and Phase II clinical trials were originally completed by Celsion. Subsequently, the Company received approvals from both the FDA and the Canadian Bureau of Medical Devices to conduct a pivotal Phase III breast cancer treatment study. We have begun the pivotal Phase III clinical trials but, such trials have been proceeding at a slow pace due to lack of funding. We plan to complete the pivotal Phase III trial when funding is available.
The Patent License Agreement with MIT was amended on June 16, 2007. The amended agreement requires us to pay MIT a 5% royalty on the net sales of any products derived from APA 1000, and an annual maintenance fee of $50,000. MIT is entitled to receive royalties for so long as the patents relating to the APA technology are valid or the Patent License Agreement is terminated.
On July 24, 2012 we acquired the Prolieve technology and related assets from Boston Scientific pursuant to an Asset Purchase Agreement dated June 25, 2012, amended on July 24, 2012 (the “Asset Purchase Agreement”). The purchase price was $3,662,115, of which $2,535,610 was paid on the closing of the transaction. Additionally, we entered into a contingent consideration arrangement under which we will pay Boston Scientific up to $2,500,000, to be paid in quarterly installments at a rate of 10% of the sales of Prolieve products. Sales are defined as the gross amount invoiced for sales, distributions, licenses, leases, transfers, and other dispositions. At March 31, 2015, approximately 2,190,867 remains payable to Boston Scientific under the contingent consideration arrangement, $728,632 of which is past due.
See the information contained in the subsection titled “Our Products” of the section titled “B. Business Overview,” below.
As a medical technology company, all of our products marketed in the United States are regulated by the FDA. The FDA has established extensive rules, policies and procedures regarding the approval of new products and technologies for use in the United States. Generally, the FDA requires that a new technology undergo controlled human studies to determine safety and efficacy before the technology can be marketed and sold. Typically, such studies are conducted in three separate clinical trials, Phase I and Phase II to establish safety and efficacy on a modest sized sample, leading to a larger pivotal Phase III trial. We operate in a highly competitive environment, our business is speculative in nature, and we face substantial risks and challenges. Please refer to “Risk Factors” in “Item 3. Key Information.”
Our Products
Prolieve
Our first commercial heat-based therapy system, Prolieve, is used to treat benign prostatic hyperplasia or “BPH.” BPH is a condition in which the prostate gland becomes enlarged and restricts the flow of urine through the urethra. Our clinical studies have shown that the treatment of this condition with the Prolieve system improves urine flow by decreasing the enlarged prostate’s pressure on the urethra through the heating, dilation and shrinking of the prostate tissue surrounding it. The BPH drug therapy market is estimated to be about $4 billion in major developed countries according to Decision Resources Group.
18
This number does not include non-drug treatments and the patients who are on “Watchful Waiting” due to the side effects of some of the treatment options. While the market for minimally invasive BPH treatment is approximately $150 million according to Medtech Insight, we believe that Prolieve can be a viable alternative to drug therapy due to its safety and efficacy profiles and thus has the potential to increase the market for minimally invasive BPH treatment.
What Is Benign Prostatic Hyperplasia?
Millions of aging men experience symptoms resulting from BPH, a non-cancerous urological disease in which the prostate enlarges and constricts the urethra. The prostate is a walnut-sized gland surrounding the male urethra that produces seminal fluid and plays a key role in sperm preservation and transportation. The prostate frequently enlarges with age. As the prostate expands, it compresses or constricts the urethra, thereby restricting the normal passage of urine. This restriction may require a patient to exert excessive bladder pressure to urinate. Because urination is one of the body’s primary means of cleansing impurities, the inability to urinate adequately increases the possibility of infection and bladder and kidney damage.
BPH Symptoms
The symptoms of BPH usually involve problems with emptying the bladder or storing urine in the bladder. However, the severity of the symptoms can vary widely, from mild and barely noticeable to serious and disruptive. Common BPH symptoms include:
| | • | | Pushing or straining to begin urination; |
| | • | | Dribbling after urination; |
| | • | | A frequent need to urinate, sometimes every 2 hours or less; |
| | • | | A recurrent, sudden, or uncontrollable urge to urinate; |
| | • | | Feeling the bladder has not completely emptied after urination; |
| | • | | Pain during urination; and |
| | • | | Waking at night to urinate. |
In extreme cases, a man may be completely unable to urinate. In such situations, emergency medical attention is required.
An enlarged prostate does not cause prostate cancer or directly affect sexual function. However, many men experience sexual dysfunction and BPH symptoms at the same time. This is due to aging and the common medical conditions older men often encounter, including vascular disease and diabetes. Because all of these conditions take place with aging, sexual dysfunction tends to be more pronounced in men with BPH.
BPH Complications
BPH is not a form of prostate cancer and does not lead to prostate cancer. Accordingly, BPH is not life-threatening. However, as many men know, BPH may be lifestyle-threatening and can cause great discomfort, inconvenience, and awkwardness and complications such as:
| | • | | Acute urinary retention, which is a condition that results in a complete inability to urinate. A tube called a catheter may be needed to drain urine from the bladder. |
| | • | | Chronic urinary retention, which is a partial blockage of urine flow that causes urine to remain in the bladder. In rare cases, this may lead to kidney damage if it goes undiagnosed for too long. |
19
| | • | | Urinary tract infection, which can cause pain or burning during urination, foul-smelling urine, or fever and chills. |
| | • | | Other complications from BPH may include bladder stones or bladder infections. |
| | • | | Having BPH does not directly affect one’s sexual function. However, it is common for the symptoms of BPH and sexual dysfunction to occur at the same time. |
Prevalence of BPH and Market Opportunity
BPH is an age-related disorder the incidence of which increases with maturation of the population. According to urologyhealth.org, by age 60, more than half of men have BPH. By age 85, about 90 percent of men have BPH. As the population continues to age and life expectancy increases, the prevalence of BPH can be expected to continue to increase.
Treatment Alternatives for BPH
Several types of treatments are available for enlarged prostate. They include medications, surgery and minimally invasive surgery. The best treatment choice for patients depends on several factors, including how much the symptoms bother them, the size of their prostate, other health conditions the patients may have, their age and preference. If symptoms are not severe, a patient may decide not to have treatment and wait to see whether their symptoms become more bothersome over time.
Watchful Waiting
When a patient first develops symptoms caused by BPH, physicians generally prescribe drugs as the first treatment option, but usually leave the decision to their patients. Due to the low success rate, high costs, side effects and complications associated with BPH drug therapies, some patients diagnosed with BPH prefer to be regularly monitored by their doctors, but choose not to begin a drug therapy. The patients who opt out of therapy fall into a group referred to as “watchful waiting.” Often, BPH symptom persistence and worsening or an acute urinary event may force the patient to move on to some other form of therapy.
Drug Therapy
Medications are the most common treatment for moderate symptoms of prostate enlargement but if a patient stops taking medicine, the symptoms will usually return. Medications used to relieve symptoms of enlarged prostate include several different types of drugs, such as Alpha-Blockers (such as Flomax®) and Alpha Reductase Inhibitors (such as Proscar®). Drug therapy costs approximately $1,000 per year or more in the United States, must be maintained for life, and does not offer consistent relief to a large number of BPH patients. Many of the currently available BPH drugs also have appreciable side effects, such as: headache, fatigue, impotence, dizziness, and low blood pressure.
Surgical Intervention
Two of the primary surgical procedures to treat BPH are transurethral resection of the prostate (“TURP”) and laser procedures. TURP has traditionally been a common procedure for enlarged prostate for many years. It is a procedure in which the prostatic urethra and surrounding diseased tissue in the prostate are trimmed with a telescopic knife, thereby widening the urethral channel for urine flow. While the TURP procedure generally has been considered the most effective treatment available for the relief of BPH symptoms, the procedure has its shortcomings. In the first instance, TURP generally requires from one to three days of post-operative hospitalization. In addition, a substantial percentage, approximately 5-10%, of patients who undergo TURP encounter significant complications, which can include painful
20
urination, infection, impotence, incontinence, and excessive bleeding. Further, retrograde ejaculation, a condition in which semen released during ejaculation enters the bladder rather than exiting the penis, occurs in up to 90% of patients who undergo a TURP procedure, with a long-term side effect in up to 75% of such patients.
Laser surgeries (also called laser therapies) use high-energy lasers to destroy or remove overgrown prostate tissue. Options for laser therapy depend on prostate size, the location of the overgrown areas. During prostate laser surgery, a combined visual scope and laser is inserted through the tip of the patient’s penis into the urethra, which is surrounded by the prostate. Using the laser, doctors remove prostate tissue that are squeezing the urethra and blocking urine flow, thus making a new larger tube for urine to pass through. Lasers use concentrated light to generate precise and intense heat. Risks of laser surgery include: temporary difficulty urinating and post treatment catheterization, urinary tract infection, narrowing of the urethra as scars form, retrograde ejaculation, and erection problems. Accordingly, neither drug therapies nor the surgical alternatives appear to provide fully satisfactory, cost-effective treatment solutions for BPH sufferers.
Our Approach: The Prolieve Thermodilatation System
The Prolieve Thermodilatation System was originally and primarily developed and commercialized by our current management, product development, clinical and regulatory teams. Such development occurred while such teams were employed at Celsion Corporation from 1997 to 2004, at an estimated cost of $20,000,000. Further, the development and commercialization occurred under the leadership of Dr. Augustine Cheung, who was Celsion’s president at the time. Dr. Cheung is currently our chief executive officer. As discussed above, Celsion sold the Prolieve system, technology and related assets to Boston Scientific Corporation in 2007 for $60 million.
Prolieve is an in-office procedure that minimizes patient discomfort and the need for post-treatment catheterization. In a randomized one-year clinical trial, conducted at 14 centers across the United States, patients undergoing treatment with Prolieve achieved measurably greater improvement in symptoms after three months compared to a control group using a drug, Proscar, which is commonly prescribed to treat BPH condition. In June 2012, Medifocus reached an agreement with Boston Scientific for the purchase of all of the assets of its Prolieve business, including all Prolieve inventory, the mobile service distribution assets, as well as the intellectual property associated with the Prolieve technology.
The purpose of the Prolieve system is to provide a relatively painless and effective alternative to drug therapy and certain types of surgical procedures to treat the symptoms of BPH. Prolieve is a minimally invasive treatment option for BPH. Unlike other microwave-based BPH treatments, Prolieve utilizes both microwave heat, delivered via a catheter, and proprietary balloon compression to both heat the prostate and dilate the prostatic urethra, and to shrink enlarged prostate tissue. The 45-minute Prolieve treatment is administered on an outpatient basis in a physician’s office and can be done with topical anesthesia only. We estimate that approximately 95,000 patients have been treated since the FDA PMA was granted. Many patients treated with Prolieve experience immediate symptom relief. Based upon a study conducted by Boston Scientific (the “Prolieve Study”), patients treated with the Prolieve system experienced a symptom reduction of 22% three days following treatment. Furthermore, most patients that undergo the Prolieve treatment do not require post-treatment catheterization. Based upon the Prolieve Study, 94% of patients that underwent the Prolieve treatment were catheter free immediately following the treatment, and 100% of such patients were catheter free after three days. Accordingly, we believe that patients that undergo the Prolieve treatment should be able to resume their normal activities shortly after the treatment.
21
The Prolieve system is comprised of two components. The first component is a freestanding module that contains a microwave generator and computerized controls that regulates and monitors the delivery of heat to the enlarged prostate tissue. The second component is our proprietary disposable catheter that is attached to the module. This component contains an internal balloon that is inflated after it is inserted through the urethra to the point of constriction. Upon inflation of the balloon, the tissue is heated by microwaves delivered via the catheter, resulting in dilation of the urethra. Our computer system in the module monitors and regulates the heat being applied to ensure maximum safety and efficiency. The Prolieve system is covered by 55 core patents, which were acquired as part of the acquisition of the Prolieve assets from Boston Scientific Corporation in 2012.
The combined effect of this “heat plus compression” therapy is twofold: first, the heat denatures the proteins in the wall of the urethra, causing a stiffening of the opening created by the inflated balloon, forming a biological stent. Second, the heat serves effectively to kill off prostate cells outside the wall of the urethra, thereby creating sufficient space for the enlarged natural opening. In addition, the Prolieve system’s temperature (46º C to 54º C) is sufficient to kill prostatic cells surrounding the urethra wall, thereby creating space for the enlargement of the urethra opening. However, the relatively low temperature is not sufficient to cause swelling in the urethra.
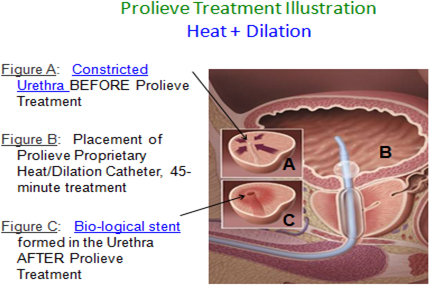
The Prolieve system is designed with patients’ needs and comfort in mind. In general, it does not require sedation or post-operative catheterization and provides rapid symptomatic relief from BPH. BPH patients can be treated using Prolieve in urologic offices throughout the United States. In addition, the Prolieve treatment is also made available to physicians utilizing our nationwide mobile service.
The Prolieve system is currently in use in the United States. Although it is generating revenue, (gross revenues of $4,219,459 for the year ended March 31, 2015) our Prolieve operations are not, and have never been profitable, and there can be no assurance that they will ever become profitable.
22
Since acquiring the Prolieve assets from Boston Scientific Corporation in July 2012, we have been concentrating our corporate development efforts on developing these assets into a business. We are focusing on increasing sales from our installed base of systems, and from our mobile systems, described below. We have been increasing the number of persons directly supporting our Prolieve operations from eight in July 2012 to 21 at March 31, 2015. The Prolieve operations are currently supported by three sales professionals, 11 mobile technicians, one schedule coordinator, three persons responsible for regulatory compliance matters, and three engineering and support staff.
Boston Scientific Corporation had sold approximately 250 Prolieve systems and approximately 80,000 disposable catheter kits in the United States prior to Boston Scientific Corporation’s sale of the Prolieve assets to us in 2012. Our current business strategy is to increase revenues from these installed systems. In the U.S. market, we do not intend to actively market the Prolieve system itself but, rather, our strategy is to grow revenue through the direct sale of disposable catheter kits to physicians with Prolieve systems installed and, increasingly, through our mobile service, which eliminates physicians’ need to purchase, and learn how to operate, the Prolieve system. However, if U.S. or international customers choose to purchase the Prolieve system itself, we will accommodate such costumers’ needs to the best of our ability.
We currently have approximately 120 systems that were acquired as part of the Prolieve asset purchase from Boston Scientific Corporation. We do not currently have an agreement with a manufacturer for the production of additional Prolieve systems, although we believe that there are several qualified medical device contract manufacturers, including Sanmina, that are capable of manufacturing the system if our current inventory is depleted. At this time, 100% of our revenues come from the sales of our disposable catheters used in each treatment or the provision of mobile services that provide therapy using our disposable catheters. The disposable catheters are manufactured in Mexico by Lake Region Medical Center, formerly known as Accelent Corporation. We currently have an agreement with Lake Region Medical Center to supply these catheters, pursuant to which we order the number of catheters we estimate we will need for a 12-month period. We have no other source of catheters at the present time. Due to the complicated nature of these kits, as well as FDA manufacturing standards imposed on suppliers, the Company does not believe that an alternate supplier of catheters is readily available.
In addition to the Prolieve technology, the installed base of Prolieve systems and related patents acquired from Boston Scientific Corporation, we also acquired a fleet of 15 vans, each equipped with two Prolieve systems. This mobile fleet allows us to provide Prolieve therapy to patients whose health care providers do not have access to one of our permanently installed systems. The mobile Prolieve system is identical to the permanently installed systems.
Our mobile Prolieve systems are deployed by our dispatcher and scheduler upon the request of a physician. Our scheduler then coordinates the timing of the requested appointment with one of our medical technicians. On the day of the appointment, our medical technician arrives at the physician’s office and the Prolieve module is brought into the physician’s office. Under the physician’s supervision, a catheter is inserted into the urethra to the point of constriction, and the Prolieve treatment is administered by our medical technician under the physician’s supervision. In most cases, the patient’s symptoms are eliminated immediately and normal urination bladder function is restored.
Competition
There are several treatment options for BPH. The first is traditional surgery, known as trans-urethral resection procedure, or “TURP.” This surgery, requires a hospital stay, sedation, and a post-operative recovery period. Further, we are aware of two other minimally invasive, microwave-based, treatments with which we compete. The first such treatment is offered by Urologix. The second is offered
23
by Thermatrix. Unlike these two treatments, which solely utilize heat, our Prolieve therapy combines heat and compression (via the inflated balloon). According to Medtech Insight, the surgical and minimally invasive treatment market for BPH is approximately $150 million in the U.S.
However, the majority of BPH patients undergoing treatment today choose medical therapy instead of surgery. Pursuant to such medical therapy, patients take daily doses of medicine to shrink the prostate in order to improve function. These medicines are known to cause side effects, and must be taken daily to be effective. We believe our Prolieve treatment can be a viable alternative to drug therapy due the demonstrated efficacy and side effect profile. We intend to explore the possibility of delivering the Prolieve treatment using our mobile service at general practitioners’ offices to provide a treatment option for BPH patients who are in drug therapy and patients on “Watchful Waiting”.
Prescribed medicines for BPH treatment in major industrialized countries is currently believed to be approximately $4 billion annually. These medicines are manufactured and sold by some of the world’s largest pharmaceutical companies, including Eli Lilly, Glaxo Smith Kline and Merck & Co. These companies market their drugs to physicians and directly to the public through television, radio, the internet and conventional print media. With the substantial investment made by these companies in developing, commercializing and marketing these drugs, and the size of the BPH treatment market, these companies represent a significant competitive threat to our Prolieve therapy, and to our company. We are also aware that non-prescription herbal supplements promoted to relieve BPH symptoms are being aggressively marketed to the public; these products also compete with Prolieve.
APA 1000
Our second product, APA 1000, which is a minimally invasive breast cancer treatment, is developed, but has not been cleared by the FDA for commercial use. Both Phase I and Phase II clinical trials were completed by Celsion, establishing the system’s safety and efficacy on a limited scale. We have begun pivotal Phase III clinical trials, but have proceeded slowly in such trials because of insufficient funds. We are planning to complete the pivotal Phase III clinical trial of APA 1000 when we obtain adequate funding to do so. The Phase III clinical trial is designed to demonstrate that the combination of focused heat and neo-adjuvant chemotherapy could shrink the size of the tumor 40% more over using chemotherapy alone. In the Phase II clinical trial, a 50% increase in tumor size reduction using focused heat and neo-adjuvant chemotherapy was observed over using chemotherapy alone. In the Phase II trial, two heat treatments were applied while in the Phase III trial, three heat treatments are applied. We believe that, if the Phase III trial is successful, it will show that the combination of focused heat and neo-adjuvant chemotherapy could downsize a cancer tumor enough to allow a surgeon to perform a lumpectomy rather than a mastectomy, thereby preserving the affected breast.
The APA 1000 system delivers heat precisely to breast tumors. While using heat to kill cancerous tumors has been considered effective for many years, heat therapy has not become a part of standard treatment for cancer because of the inability to safely apply it to tumors without damaging healthy tissue. When treating cancer, physicians seek to minimize damage to healthy tissue. It is our belief that the APA 1000 system precisely focuses microwave heat on diseased tissue, sparing adjacent tissue. Precision is achieved through the utilization of “Star Wars” technology that we have exclusively licensed from MIT and have adapted for medical use in our APA 1000 system.
Adaptive Phased Array Technology Illustration
Our current management team has been working with researchers at Massachusetts Institute of Technology (“MIT”) who had developed, originally for the U.S. Department of Defense, a microwave control technology known as “Adaptive Phased Array,” or “APA.” This technology permits properly
24
designed microwave devices to focus and concentrate energy targeted at diseased tissue areas deep within the body and to heat them selectively, without adverse impact on surrounding healthy tissue. Since licensing the APA technology from MIT, our management team has been working together with Dr. Alan J. Fenn, the inventor of the patented technology. This collaboration has included technology transfer and technical/engineering assistance to develop and design our current APA Breast Cancer treatment device. In addition, Dr. Fenn has collaborated and advised the Company on the design of the clinical protocol, clinical study support, and device usage training of the current FDA breast cancer study. as well as assisting the Company in developing new clinical protocol and new treatment devices using the APA technology licensed from MIT.
In the treatment of breast cancer, the APA technology applies the same principal used in MIT’s “Star Wars” program of detecting missiles.
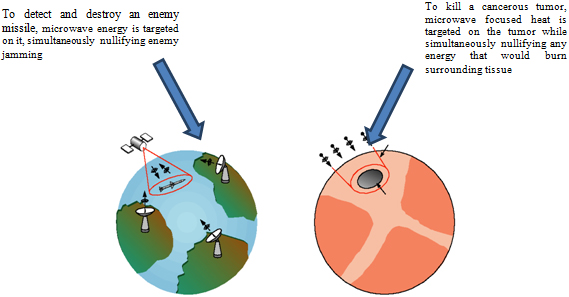
APA 1000 Breast Cancer Treatment Illustration
| | • | | An RF needle probe inserted at tumor center provides feedback signal to focus microwave energy at tumor center to induce shrinkage without harming surrounding tissue. |
| | • | | Focused microwave energy (43-44°C) combined with chemotherapy achieves an average of 88% tumor size reduction in Phase II clinical trials. |
25

Treatment with APA 1000 may accomplish several objectives. First, we believe that it destroys many cancer cells, and substantially shrinks cancerous cells that are not destroyed. If tumors are shrunk small enough, a patient may not need to have the entire breast removed. Second, we believe that the application of APA 1000 heat therapy boosts the effectiveness of subsequent chemotherapy and radiation therapy.
There can be no assurance that we will complete the pivotal Phase III clinical trial, or that the FDA will approve of the APA 1000 for sale in the United States. Even if the APA 1000 successfully completes the pivotal Phase III clinical trial and the FDA permits us to sell this system, there can be no assurance that it will be adopted by health care industry.
26
As stated earlier, we are progressing at a slow pace through Phase III clinical trials due to lack of funding, and are currently focusing our corporate activities and resources on expanding our Prolieve operations. We estimate that the cost of completing Phase III clinical trials will be approximately $7,500,000. We currently plan, subject to obtaining financing, to fully resume of the pivotal Phase III trial during the present calendar year, although there can be no assurance we will resume clinical trials.
Further, in anticipation of commencing the pivotal Phase III clinical trial, we previously negotiated arrangements with physicians and medical centers in the United States and Canada to conduct this trial. Because the pace of the trial has been slow, there can be no assurance that the persons and institutions with which we have previously made arrangements will be available to proceed on the same terms, or at all, when we are ready. In such event, we would then need to make alternative arrangements, of which there can be no assurance.
Our Intellectual Property
We have 55 core patents that cover our Prolieve system. These patents expire over several years, commencing in September 2021 and continuing until February 2029. We have 41 core patents that cover our APA 1000 system. We have also licensed 15 patents from MIT covering our APA 1000 system. Of the 15 patents licensed from MIT, five patents in foreign countries expired in November 2014. Our MIT and APA 1000 patents expire over a multi-year period, commencing June 2017, when one patent in the U.S. will expire, and ending when the final patents expire in January 2023. In total, we have over 100 patents and patents pending in the United States and in foreign jurisdictions.
Medifocus Holding Joint Venture
On November 8, 2013, we entered into an agreement with Ideal Concept Group Limited (“Ideal Concept”) to develop our Prolieve business and APA technology in a geographic area referred to as “Asia Pacific” in the agreement (the “JV Agreement”). The countries comprising of Asia Pacific are not specified in the JV Agreement. Pursuant to the JV Agreement, Medifocus and Ideal Concept agreed to capitalize a company, Medifocus Holding Limited (“Medifocus Holding”), to develop this business. Medifocus Holding was incorporated in the British Virgin Islands on June 28, 2012.
The JV Agreement states that, at the outset, Ideal Concept will own 60% of Medifocus Holding and we will own 40%. Through March 31, 2015, Medifocus Inc. has made total contributions to Medifocus Holding of approximately $214,735 in cash and Prolieve equipment. In addition to capital contributions, the shareholders are obligated to provide loans to the JV of up to HKD 4,000,000 (or approximately $520,000). Ideal Concept previously agreed, through November 8, 2014, to loan us the funds necessary to satisfy our portion of the required shareholder contributions to Medifocus Holding. Such loan would bear interest at 6% per year and be secured by our ownership interest in Medifocus Holding. No such loans were made to us by Ideal Concept and, based on Medifocus Holdings’ current business plan, we do not expect to make any further investments or loans in the joint venture.
Pursuant to the terms of the JV Agreement and a License and Distribution Agreement dated as of November 8, 2013, Medifocus Holding will engage in clinical testing, and obtaining approval from China Food and Drug Administration of the People’s Republic of China (“CFDA”) for all products relating to Prolieve and the APA technology. Medifocus Holding has been in communication with the CFDA and is currently in the process of preparing the required documentation with the CFDA for commercialization of Prolieve in China. There is no assurance that the CFDA will approve Prolieve for commercialization in China. Additionally, Medifocus Holding has been in discussions with several hospitals in China regarding conducting clinical testing. As of the date of this annual report, no clinical testing has begun in China.
27
The JV Agreement outlines the respective obligations of Ideal Concept and ourselves. We will be responsible for: (i) providing Medifocus Holding with an exclusive license to our rights in the Prolieve business and APA technology; (ii) applying for and maintaining the patents and other intellectual property rights throughout the world; (iii) directing and managing all research and development activities in Asia Pacific; and (iv) providing on-site and off-site technical support and training to Medifocus Holding’s personnel. Ideal Concept’s responsibilities will include: (i) formulating a business plan to evaluate opportunities in Asia Pacific; (ii) assisting in the performance of clinical trials relating to CFDA approval; (iii) providing assistance in establishing manufacturing arrangements for products; and (iv) managing the financial affairs of Medifocus Holding, including the cash flow, arranging funding, and assisting in developing markets in Asia Pacific.
Medifocus Holding is required to pay us a royalty of 5% of the first $10,000,000 in sales of the catheter kits and control units utilized in the Prolieve business. After $10,000,000 in sales has been reached, the royalty decreases to 3%. For all other products we develop, Medifocus Holding is required to pay us a royalty of 7.5% on net sales of such products.
| C. | Organizational structure. |
Our only subsidiary is Celsion (Canada) Limited, a corporation organized under the laws of the Province of Ontario, Canada.
| D. | Property, plant and equipment. |
Our main offices are located at 10240 Old Columbia Road, Suite G, Columbia, Maryland, 21040. We lease these premises, which comprise of 10,833 square feet of office and storage space. Our current annual rental is approximately $ 140,000 per year, which increases incrementally each year of the lease to approximately $150,000 in the final year of the lease. The lease expires on February 28, 2018.
| Item 4A. | Unresolved Staff Comments. |
Not applicable.
| Item 5. | Operating and Financial Review and Prospects. |
The following discussions should be read in conjunction with our consolidated financial statements and related notes thereto included in this Annual Report on Form 20-F. The following discussion contains “forward-looking statements” made pursuant to the safe harbor provisions of Section 21E of the Securities Exchange Act and the Private Securities Litigation Reform Act of 1995. These statements are based on our beliefs and expectations about future outcomes and are subject to risks and uncertainties that could cause actual results to differ materially from anticipated results. Forward-looking statements are typically identified by the use of terms such as “may,” “will,” “should,” “potential,” “predicts,” “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” or the negative of such terms and variations of these words and similar expressions. These statements are not guarantees of future performance and are subject to risks, uncertainties and other factors, some of which are beyond our control, are difficult to predict and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements.
Factors that could cause or contribute to such differences include, without limitation, those described under Part I, “Item 3.D. Risk Factors” appearing in this Annual Report on Form 20-F and factors described in other cautionary statements, cautionary language and risk factors set forth in other documents that we file with the Securities and Exchange Commission. We undertake no obligation to publicly update, except as required by law, any forward-looking statements, whether as a result of new information, future events or otherwise.
28
Notwithstanding the above, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, expressly state that the safe harbor for forward looking statements does not apply to companies that issue penny stocks. Accordingly, the safe harbor for forward looking statements under the PSLRA is not available to the Company because we are considered to be an issuer of penny stock.
Unless otherwise noted, all references to “$” or “dollar” refer to the United States dollar, the Company’s reporting and functional currency. Prior to fiscal 2015, the functional currency of the Company and its subsidiaries was the Canadian dollar.
Overview and Background
The Company was incorporated under theBusiness Corporations Act (Ontario) on April 25, 2005. The Company is listed in Canada on the TSX Venture Exchange Inc. (the “Exchange”) under the symbol “MFS” and in the United States on the OTC QX market under the symbol “MDFZF”.
On January 16, 2006, the Company’s wholly-owned subsidiary Celsion Canada Inc. purchased from Celsion Corporation(USA) all of the assets relating to the Microfocus APA 1000 Breast Cancer Treatment System (“APA 1000”), consisting of the microwave machine, the adaptive phased array (“APA”) technology licensed from the Massachusetts Institute of Technology (“MIT”), and all related intellectual and regulatory property (collectively, the “Business”). The Company has a commitment to pay a 5% royalty on the net sales of products sold by and patent royalties received by the Company and its successors and assignees, the royalty not to exceed $18,500,000. Royalties will not be payable until the APA 1000 can be commercialized following successful completion of the pivotal clinical trial and receipt of marketing approval in the United States from the United States Food and Drug Administration (the “FDA”) and in Canada from Health Canada. The Company will expense the royalties as paid.
The Company currently owns two technology platforms with over 100 U.S. and international patents:
| | 1. | The Endo-thermotherapy Platform is a catheter-based focused heat technology platform that utilizes natural body openings to deliver precise microwave thermotherapy to the diseased sites. The Prolieve Thermodilatation System for the treatment of BPH was developed based on the Endo-thermotherapy Platform. The same platform can potentially be used to treat cancers in prostate, rectum, cervix and esophagus. |
| | 2. | The Adaptive Phased Array Microwave Focusing Platform-invented by MIT and licensed to Medifocus, directs precisely focused microwave energy at tumor center to induce shrinkage or eradication of tumors without undue harm to surrounding tissue. The APA technology was originally developed by MIT for military applications in the U.S. Department of Defense’ “Star Wars Program” to focus microwave energy on missiles, in order to detect and destroy them. The APA technology has been licensed exclusively to Medifocus for medical applications. The Company’s APA 1000 Breast Cancer Treatment System, developed from the APA technology platform, has received approval from the U.S. FDA and Health Canada to conduct the pivotal Phase III clinical trials. The APA Microwave Focusing Platform can provide the design basis for future focused heat cancer treatment systems for surface, subsurface and deep seated localized and regional cancers, such as lung and liver cancers. Limited pivotal Phase III clinical trials are being conducted and Medifocus expects to accelerate such trials when sufficient financial resources become available. |
29
On July 24, 2012, the Company acquired the Prolieve Thermodilatation System technology (“Prolieve”) and related business assets from Boston Scientific Corporation (“BSC”) through an asset purchase agreement. Prolieve is a U.S. FDA approved device for the treatment of enlarged prostate, medically known as Benign Prostatic Hyperplasia (“BPH”). The total purchase price for the transaction was approximately $3.7 million of which $2.5 million was paid on the closing of the transaction. The balance consists of up to $2.5 million in contingent consideration that will be paid in quarterly installments at a rate of 10% of Medifocus’ Prolieve sales.
As a result of this acquisition, the Company acquired a revenue-generating heat technology that was successfully engineered and developed by the same management team that now operates Medifocus. The Company believes that with management’s extensive knowledge and past success with this product, they are in the best position to maximize Prolieve’s potential within the marketplace.
Financial Condition
Our future capital requirements will depend upon numerous unpredictable factors, including, without limitation,
| | • | | the revenue generated by Prolieve, |
| | • | | the cost, timing and outcomes of clinical studies and regulatory reviews of our products, |
| | • | | our efforts to implement new collaborations, licenses and strategic transactions, and |
| | • | | our ability to manage general and administrative expenses, capital expenditures and other uses of cash. |
Our operations are subject to certain risks and uncertainties including, among others, current and potential competitors with greater resources, dependence on significant customers, lack of operating history and uncertainty of future profitability and possible fluctuations in financial results. Since inception, the Company has incurred substantial operating losses, principally from expenses associated with the Company’s research and development programs. The Company believes these expenditures are essential for the commercialization of its technologies. The Company expects its operating losses to continue for the foreseeable future as it continues its product development efforts, and when it undertakes sales and marketing activities. The Company’s ability to achieve profitability is dependent upon its ability to obtain governmental approvals, produce, and market and sell its new product candidates. There can be no assurance that the Company will be able to commercialize its technology successfully or that profitability will ever be achieved. The operating results of the Company have fluctuated significantly in the past. The Company expects that its operating results will fluctuate significantly in the future and will depend on a number of factors, many of which are outside the Company’s control.
In 2012 and 2013 we raised significant capital from equity and debt financings. During the fiscal year ended March 31, 2012, we received approximately $0.3 million of gross proceeds from the sale of 1,000,000 shares of our common stock.
During the fiscal year ended March 31, 2013, we received approximately $11.2 million of gross proceeds from the sale of 75,821,055 shares of our common stock and warrants to purchase 75,821,055 shares of our common stock. The net proceeds from the offering were primarily used to fund the purchase of the Prolieve assets from BSC and the subsequent costs for Prolieve operations and for general corporate purposes, including research and development activities, capital expenditures, repayment of debt and working capital.
30
During the fiscal year ended March 31, 2014, the Company raised gross proceeds of $5.6 million from the sale of convertible redeemable promissory notes and warrants (the “Units”). Each Unit consists of (i) a $10,000 face value convertible redeemable promissory note, bearing 8% annual interest and due in three years (“Note”), which is convertible into shares of common stock beginning six months after the Closing Date of the offering at a conversion price of $0.25 per share, and (ii) three-year warrants to purchase 20,000 shares of common stock at a price of $0.30 per Share. The net proceeds from the offering is to be used for Prolieve operations and for general corporate purposes, including research and development activities, capital expenditures, repayment of debt and working capital.
Our $0.6 million promissory note made to a lender in July 2012 and the accrued but unpaid interest thereon, was originally due October 23, 2103. The lender previously extended the due date of this promissory note to June 30, 2014 and we are currently in discussion with the lender to negotiate an extension of the maturity date.
For the fiscal year ending March 31, 2015, the Company raised approximately $1.6 million from the sale of common stock and warrants (the “Units”). Each Unit was priced at $0.16 and consists of one Share, and a detachable stock purchase warrant to purchase one Share at $0.25 per share. The Company received funds prior to March 31, 2015 for common shares in the amount of $1,705,000 for future shares to be issued pursuant to a private placement offering. In May 2015, the Company issued 38,750,000 shares of common stock at the price of $0.044 per share for the $1,705,000 received.
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Management is continuing its efforts to obtain additional funds so that the Company can meet its obligations and sustain operations.
We do not have any committed sources of financing and cannot give assurance that alternate funding will be available in a timely manner, on acceptable terms or at all. We may need to pursue dilutive equity financings, such as the issuance of shares of common stock, convertible debt or other equity-linked securities, which financings could dilute the percentage ownership of our current common stockholders and could significantly lower the market value of our common stock. For example, in September 2014, we raised gross proceeds of approximately $1.6 million from the sale of our common stock and detachable stock purchase warrants. We previously entered into arrangements and agreements with an investment banker to help us raise capital. While no such arrangements or agreements are effective, we intend to continue to raise funds, likely with the assistance of such investment banker, when we determine that such capital raises are in the company’s best interests.
Our cash and cash equivalents of approximately $1,312,000 on hand at March 31, 2015 are not sufficient to fund operations through 2015. We will need to raise substantial additional capital in the near future to fund our planned future operations beyond our fiscal year ending March 31, 2016, and we anticipate that such financing transactions will likely be dilutive to our current shareholders. If we are not
31
able to raise additional capital, we will need to take certain measures to reduce our operating costs, including reducing our staff, curtailing our research and development efforts and our clinical trials, and reducing the costs we plan spend to grow our Prolieve business. As such, we would not be able to maintain the growth of the Prolieve business, complete the development, testing and commercialization of our product candidates.
Critical Accounting Policies
A “critical accounting policy” is one that is both important to the portrayal of our financial condition and results of operations and that requires management’s most difficult, subjective or complex judgments. Such judgments are often the result of a need to make estimates about the effect of matters that are inherently uncertain. The preparation of our financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
A summary of our critical accounting policies, including those that require the use of significant estimates and judgment, follows. A more comprehensive description of all of our significant accounting policies is contained in Note 1 to our consolidated financial statements.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. In the accompanying consolidated financial statements, estimates are used for, but not limited to, stock-based compensation, allowance for doubtful accounts, accruals for estimated product returns, allowance for inventory obsolescence, allowances for contingencies, value of contingent consideration, deferred taxes and valuation allowance, and the depreciable lives of tangible and intangible assets. Actual results could differ from those estimates.
Revenue Recognition
The Company sells products that are used in the treatment of Benign Prostate Hyperplasia. The Company recognizes revenue, net of sales taxes, from the sale of Prolieve consoles and catheters upon shipment delivery to the customer. Revenue from the sale of products is measured at the fair value of the consideration received or receivable, net of estimated returns. Revenue from the mobile service is recognized upon completion of the services, which is generally upon treatment of the patient.
The Company does not have a return policy that allows customers to return product, however the company has allowed returns on a limited customer by customer basis. The Company’s estimate for returns is based upon its historical experience with actual returns. While such experience has allowed for reasonable estimation in the past, history may not always be an accurate indicator of future returns. The Company continually monitors its estimates for returns and makes adjustments when it believes that actual product returns may differ from the established accruals. We record a provision for estimated returns in the same period as the related revenue is recorded. The provision for estimated sales returns is based on historical sales returns, analysis of credit memo data and specific customer-based circumstances.
32
Inventory
Inventory consists primarily of console units and single-use treatment catheters. Inventory consists of the direct costs of acquiring the inventory from vendors. Inventory of console units are considered non-current since the sales period is usually in excess of one year.
Inventory is valued at the lower of cost or net realizable value. Net realizable value represents the estimated selling price for inventories less costs necessary to make the sale. In determining net realizable value, we consider, at a minimum, selling prices, reimbursements charges, and changes in demand for products due to competitive conditions or market acceptance. A provision is recognized to reduce the cost of inventories to the estimated net realizable values, if required. We also analyze the level of inventory on hand on a periodic basis, in relation to estimated customer requirements to determine whether write-downs for excess, obsolete, or slow-moving inventory are required. Any significant or unanticipated change in the factors noted above could have a significant impact on the value of inventories and on reported operating results.
Inventory is relieved using the first-in, first-out method.
Stock-Based Compensation
Compensation costs for all stock-based awards is measured at fair value on the date of the grant using an option pricing model and is recognized over the service period for awards expected to vest. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the current estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Research and Development Expenses
Research and development costs are expensed as incurred.
Contingent Consideration
In accordance with ASC 805, upon the purchase of Prolieve, the Company recognized a contingent consideration obligation as part of the consideration transferred in exchange for the acquired business. The initial measurement of the contingent consideration obligation was based on its estimated fair value. The contingent consideration obligation has been remeasured to fair value at each reporting date and will continue to be remeasured until the contingency is resolved. The changes in fair value are recognized in earnings. The obligation outstanding totaled $1,031,179 and $1,172,654 as of March 31, 2015 and 2014.
Intangible Assets
Intangible assets consist of intellectual property and customer relationships for our Prolieve business acquired in July 2012. These intangible assets were originally recorded at fair value and are amortized on a straight line basis over their estimated useful lives of 10 years. The Company reviews its intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, in a manner similar to that for property and equipment.
Recent Accounting Pronouncements
None of the accounting pronouncements we were required to or otherwise adopted in any of the periods contained in this report had a material impact on our results of operations, financial condition or cash flows. Additionally, we have evaluated all issued and unadopted Accounting Standards Updates and believe the adoption of these standards also will not have a material impact on our results of operations, financial position, or cash flows.
33
Comparison of Fiscal Years Ended March 31, 2015 and 2014
The table below summarizes our results of operations for fiscal year ended March 31, 2015 and fiscal year ended March 31, 2014:
| | | | | | | | |
| | | Year ended March 31, | |
| | | 2015 | | | 2014 | |
Sales | | | | | | | | |
Products | | $ | 1,212,224 | | | $ | 2,704,593 | |
Services | | | 3,007,235 | | | | 2,411,913 | |
| | | | | | | | |
Total Sales | | | 4,219,459 | | | | 5,116,506 | |
| | | | | | | | |
Costs of Sales | | | | | | | | |
Products | | | 665,382 | | | | 1,167,493 | |
Services | | | 2,990,089 | | | | 2,381,605 | |
| | | | | | | | |
Total Costs of Sales | | | 3,655,471 | | | | 3,549,098 | |
| | | | | | | | |
Gross Profit (Deficit) | | | 563,988 | | | | 1,567,408 | |
| | | | | | | | |
| | |
Operating Expenses | | | | | | | | |
Research and development | | | 399,212 | | | | 472,810 | |
Sales and marketing | | | 1,549,460 | | | | 2,122,203 | |
General and administrative | | | 2,703,301 | | | | 3,859,202 | |
| | | | | | | | |
Total Operating Expenses | | | 4,651,973 | | | | 6,454,215 | |
| | | | | | | | |
Loss from Operations | | | (4,087,985 | ) | | | (4,886,807 | ) |
| | | | | | | | |
| | |
Total Other Income (Expense) | | | (1,883,485 | ) | | | (1,106,090 | ) |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Net Loss | | | (5,971,470 | ) | | | (5,992,897 | ) |
Sales
The Company’s revenue from the sale of its Prolieve system products and services decreased from $5,116,506 in fiscal year 2014 to $4,219,459 in fiscal year 2015. Product sales in 2015 and 2014 consisted solely of single-use catheters. The decrease of 18% was due to a significant decrease in product revenue offset by increased services revenue, as a result of our restructuring of the Prolieve operation initiated in August 2014.
We anticipate that sales of Prolieve products and service will increase in fiscal year 2016 as a result of our continuing efforts to sell our single-use catheters product and services across the U.S. While we expect single-use catheter product sales to increase in 2016 over the 2015 levels, we expect sales of our mobile services to continue to increase at a somewhat higher rate.
34
Costs of Good Sold, Costs of Services and Gross Profit
The costs of sales for products primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our Prolieve intellectual property, warranty costs, warehousing costs, freight and handling charges. Warehousing costs include payroll and benefit costs, including related stock-based compensation expense. Costs of sales for services consist primarily of the costs to provide mobile services to our patients, including depreciation of our mobile consoles and vehicle fleet, and payroll and benefit costs, including related stock-based compensation expense.
Costs of goods sold as a percentage of product sales was 55% in 2015 as compared to 43% in 2014, and costs of services as a percentage of services sales was 99% in 2015 as compared to 99% in 2014. As a result, total gross profit decreased from $1,567,408 in fiscal year 2014 to $563,988 in fiscal year 2015. The gross profit related to our sales decreased as fixed amortization expense of our intangible assets had a greater impact. We anticipate that as our sales continue to grow, margins on both product sales and services will become positive as the effect of fixed charges have a relative lesser impact on total margin.
Net Loss
Our net loss of $5,971,470 decreased slightly from our net loss of $5,992,897 in 2014. The company had significant decreases in gross profit due to increased service revenue, which has a lower gross profit percentage, offset by a significant decrease in our operating expenses from $6,454,215 in fiscal year 2014 to $4,651,973 for fiscal year 2015 which was mainly due to decreases in general administrative and sales and marketing expenses.
Research and Development Expenses
For the fiscal year ended March 31, 2015, the Company incurred research and development expenses of $399,212, a 16% decrease from the $472,810 for the same period in 2014. Research and development expenses for both periods consisted primarily of costs incurred with respect to the Phase III clinical study for our APA 1000 Breast Cancer System, as well as costs related to the Prolieve post-marketing study.
Sales and Marketing Expenses
Sales and marketing expenses include the costs of our sales force (including labor, travel, stock-based compensation, and other direct marketing expenses) for Prolieve.
Sales and marketing expenses in fiscal year 2015 were $1,549,460, a decrease of 27% from 2014 expenses of $2,122,203. The decrease is primarily the result of the Company’s efforts to reduce costs due to the limited cash resources available during the year by eliminating certain sales positions and scaling back marketing activities and travel expenses.
General and Administrative Expenses
General and administrative expenses in fiscal year 2015 decreased 30% to $2,703,301, from 2014 expenses of $3,859,202, as there was a decrease in legal and accounting fees, corporate salaries and related benefits and consultant fees as the company focused on operating more efficiently. Further, our expenses associated with legal and accounting fees and consulting fees were abnormally high in fiscal year 2014 because the company was going through the process of initial registration under the Exchange Act. The company continues to monitor operating costs and our efforts to the Prolieve business more efficiently.
35
Other Income (Expenses)
Other income (expenses) consists of interest expense, losses from our equity method investments, foreign exchange loss and changes in the fair value of contingent consideration related to our Prolieve acquisition. Total other income (expense) of ($1,883,485) in 2015 reflects a significant increase from our total other income (expense) of ($1,160,090) in 2014 primarily as a result of an increase in interest and accretion costs related to our convertible debt offset by a decrease in the loss from the change in the fair value of the contingent consideration of $425,059.
Comparison of Fiscal Years Ended March 31, 2014 and 2013
The table below summarizes our results of operations for fiscal year ended March 31, 2014 and fiscal year ended March 31, 2013. Comparative numbers have been restated to conform to the presentation adopted in the current year.
| | | | | | | | |
| | | Year ended March 31, | |
| | | 2014 | | | 2013 | |
Sales | | | | | | | | |
Products | | $ | 2,704,593 | | | $ | 1,188,288 | |
Services | | | 2,411,913 | | | | 612,083 | |
| | | | | | | | |
Total Sales | | | 5,116,506 | | | | 1,800,371 | |
| | | | | | | | |
| | |
Costs of Sales | | | | | | | | |
Products | | | 1,167,493 | | | | 1,057,171 | |
Services | | | 2,381,605 | | | | 855,737 | |
| | | | | | | | |
Total Costs of Sales | | | 3,549,098 | | | | 1,912,908 | |
| | | | | | | | |
Gross Profit (Deficit) | | | 1,567,408 | | | | (112,537 | ) |
| | | | | | | | |
| | |
Operating Expenses | | | | | | | | |
Research and development | | | 472,810 | | | | 411,045 | |
Sales and marketing | | | 2,122,203 | | | | 2,407,723 | |
General and administrative | | | 3,859,202 | | | | 2,402,210 | |
| | | | | | | | |
Total Operating Expenses | | | 6,454,215 | | | | 5,220,978 | |
| | | | | | | | |
Loss from Operations | | | (4,886,807 | ) | | | (5,333,515 | ) |
| | | | | | | | |
| | |
Other Income (Expense) | | | | | | | | |
Net loss from equity method investment | | | (159,000 | ) | | | — | |
Loss on elimination of translation adjustment | | | — | | | | — | |
Foreign exchange gain (loss) | | | (24,398 | ) | | | (135,044 | ) |
Loss from change in fair value of contingent consideration | | | (705,355 | ) | | | — | |
Interest and discount accretion | | | (217,337 | ) | | | (377,964 | ) |
| | | | | | | | |
Total Other Income (Expense) | | | (1,106,090 | ) | | | (513,008 | ) |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Net Loss | | | (5,992,897 | ) | | | (5,846,523 | ) |
36
Sales
The Company’s revenue from the sale of its Prolieve system products and services increased from $1,800,371 in fiscal year 2013 to $5,116,506 in fiscal year 2014; product sales in 2014 consisted solely of single-use catheters. The increase of over 184% reflects a full fiscal year of sales in 2014 (the Prolieve business was acquired in July 2012) as well an increase in individual product sales in our mobile services.
Costs of Good Sold, Costs of Services and Gross Profit
The costs of sales for products primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our Prolieve intellectual property, warranty costs, warehousing costs, freight and handling charges. Warehousing costs include payroll and benefit costs, including related stock-based compensation expense. Costs of sales for services consist primarily of the costs to provide mobile services to our patients, including depreciation of our mobile consoles and vehicle fleet, and payroll and benefit costs, including related stock-based compensation expense.
Costs of goods sold as a percentage of product sales was 43% in 2014 as compared to 89% in 2013, and costs of services as a percentage of services sales was 99% in 2014 as compared to 140% in 2013. As a result, total gross profit increased, from a loss of $112,537 in fiscal year 2013 to a gross profit of $1,567,408 in fiscal year 2014. The gross profit related to our services increased as fixed depreciation expense of our mobile consoles and vehicle fleet had a lesser impact. We anticipate that as our sales continue to grow, margins on both product sales and services will become positive as the effect of fixed charges have a relative lesser impact on total margin.
Net Loss
Our net loss in 2014 of $5,992,897 increased slightly (2.5%) from our net loss of $5,846,523 in 2013. The increase is primarily the result of our increased gross profit offset by increased operating expenses (namely general and administrative expenses), as discussed below.
Research and Development Expenses
For the fiscal year ended March 31, 2014, the Company incurred research and development expenses of $472,810, a 15% increase from the $411,045 for the same period in 2013. Research and development expenses for both periods consisted primarily of costs incurred with respect to the Phase III clinical study for our APA 1000 Breast Cancer System, as well as costs related to the Prolieve post-marketing study.
Sales and Marketing Expenses
Sales and marketing expenses include the costs of our sales force (including labor, travel, stock-based compensation, and other direct marketing expenses) for Prolieve.
Sales and marketing expenses in fiscal year 2014 of $2,122,203, a decrease of 12% from 2013 expenses of $2,407,723. While we had expected sales and marketing expense to increase in 2014 as compared to 2013, those expenses actually decreased primarily the result of the Company’s efforts to reduce costs due to the limited cash resources available during the year. We anticipate sales and marketing expenses to increase in 2015 as compared to 2014 as a result of adding additional sales employees as well as additional administrative and mobile service team employees.
37
General and Administrative Expenses
General and administrative expenses in fiscal year 2014 increased 61% to $3,859,202, from the 2013 expenses of $2,402,210, as the Company significantly increased its corporate activities and expansion as a result of commercializing Prolieve and the related increase in corporate staff headcounts, professional fees, director compensations, as well as increase in costs in investor relations and business development activities.
Other Income (Expenses)
Other income (expenses) consists of interest expense, losses from our equity method investments, and changes in the fair value of contingent consideration related to our Prolieve acquisition. Total other income (expense) of ($1,106,090) in 2014 reflects a significant increase from our total other income (expense) of ($513,008) in 2013 primarily as a result of our share of losses of $159,000 in 2014 from our joint venture and the loss from the change in the fair value of the contingent consideration of $703,355. Interest and accretion expense was $217,337 in 2014 as compared to $ 377,964 in 2013, while net foreign currency transaction losses decreased to $24,298 in 2014 as compared to net losses of $135,044 in 2013. The decrease in accretion and interest expense is due to the timing of payments and proceeds on notes payable and the decrease in foreign transaction loss is due to the fluctuation of US and Canadian foreign exchange rates.
| B. | Liquidity and Capital Resources |
The Company’s primary cash requirements are to fund operations, including research and development programs and collaborations, to support general and administrative activities, and to fund acquisitions of products or businesses. The Company’s future capital requirements will depend on many factors, including, but not limited to:
| | • | | sales of the Company’s Prolieve products and services; |
| | • | | pricing and payment terms with customers; |
| | • | | costs of raw materials and payment terms with suppliers; |
| | • | | capital expenditures and equipment purchases to support product launches; and |
| | • | | business and product acquisitions. |
In December 2013, the Company raised gross proceeds of $3.6 million from the sale of convertible redeemable promissory notes and warrants (the “Units”). Each Unit consists of (i) a $10,000 face value convertible redeemable promissory note, bearing 8% annual interest and due in three years (“Note”), which is convertible into shares of common stock beginning six months after the Closing Date of the offering at a conversion price of $0.25 per share, and (ii) three-year warrants to purchase 20,000 shares of common stock at a price of $0.30 per Share. The net proceeds from the offering is to be used for Prolieve operations and for general corporate purposes, including research and development activities, capital expenditures, repayment of debt and working capital.
In a second closing in March 2014, the Company issued 200 additional Units to the investors, receiving gross proceeds of $2,000,000. The additional notes are convertible into 8,000,000 shares of common stock. Each warrant entitles the holder to acquire 20,000 common shares (for a total of 4,000,000 common shares) at an exercise price of $0.30 per share and expire on December 18, 2016. The warrants were classified as equity and were recorded as additional paid in capital at their estimated fair value of $572,999.
38
Our $0.6 million unsecured promissory note made to a lender in July 2012 (included in our contractual obligations table on page 42) and the accrued but unpaid interest of $0.2 million as of December 31, 2013, was originally due October 23, 2013. The lender has extended the due date of this promissory note to June 30, 2014 and we are currently in discussion with the lender to negotiate a payment plan for this note. Subsequent to March 31, 2015 we have made no principal and interest payments to the lender and are currently in negotiations with the lender regarding the extension of the due date. If such negotiations fail, the lender may declare all amounts due and payable immediately. The lender would not have a right to seize any of the Company’s assets because the promissory note is unsecured. Further, if the lender were to retain counsel or initiate litigation to enforce its rights and interests under the promissory note, the Company would be required to pay all reasonable costs and expenses of the lender.
In the fiscal year ending March 31, 2015, the Company raised approximately $1.6 million from the sale of Units. Each Unit was priced at $0.16 and consists of one Share, and a detachable stock purchase warrant to purchase one Share at $0.25 per share.
The company also received funds prior to March 31, 2015 for common shares in the amount of $1,705,000 for future shares to be issued. On May 12, 2015, the company issued 38,750,000 common shares at a price of $0.044 per common share for gross proceeds of $ 1,705,000 as part of this transaction.
The Company extends credit to customers on an unsecured basis and payment terms are typical 30 days from delivery or service. The Company’s receivables have increased significantly since its acquisition of Prolieve in July 2012 as a result of increasing sales of Prolieve products and services. Management assesses the collectability of its receivables based on a periodic customer-by-customer analysis, considering historical collection experience as well as customer-specific conditions; when a specific customer account is determined to be uncollectible the Company provides an allowance equal to the estimated uncollectible amounts. Receivables are written off when it is determined that amounts are uncollectible. The Company established an allowance for doubtful accounts of approximately $74,000 and $34,000 as of March 31, 2015 and 2014, respectively.
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Management is continuing its efforts to obtain additional funds so that the Company can meet its obligations and sustain operations.
We do not have any committed sources of financing and cannot give assurance that alternate funding will be available in a timely manner, on acceptable terms or at all. We may need to pursue dilutive equity financings, such as the issuance of shares of common stock, convertible debt or other equity-linked securities, which financings could dilute the percentage ownership of our current common stockholders and could significantly lower the market value of our common stock. For example, in August 2014, we raised gross proceeds of approximately $1.6 million from the sale of our common stock and detachable stock purchase warrants.
39
Our cash and cash equivalents of approximately $1,312,000 on hand at March 31, 2015 is not sufficient to fund operations through our fiscal year ending March 31, 2016. We estimate that the external funding requirement for the next 12 months will be approximately $8 million to grow the Prolieve business in the U.S. and internationally, to accelerate the APA 1000’s Phase III clinical trials, and to start the research and development activities in the heat-activated immunotherapy business that the Company intends to pursue. We will need to raise substantial additional capital in the near future to fund our planned future operations beyond 2015, and we anticipate that such financing transactions will likely be dilutive to our current shareholders. If we are not able to raise additional capital, we will need to take certain measures to reduce our operating costs, including reducing our staff, curtailing our research and development efforts and our clinical trials, and reducing the costs we plan spend to grow our Prolieve business. As such, we would not be able to maintain the growth of the Prolieve business, complete the development, testing and commercialization of our product candidates.
Net Cash Used In/Provided By Operations
Net cash used in operating activities was $2,821,240 for the year ended March 31, 2015 compared to $5,461,991 during the same period in 2014. The reduction in the use of cash of approximately $2,640,751 is primarily due to the cash received from our accounts receivable, a reduction in our inventory levels and an increase in our non-cash expenses offset by a decrease in our accounts payable balances.
Net cash used in operating activities was $5,461,991 for the year ended March 31, 2014 compared to $7,481,836 during the same period in 2013, a reduction in the use of cash of $2,019,845. The primary reason for the decrease in the use of cash was the significant increase in accounts receivable ($1,334,881) as our sales from Prolieve products and services continued to ramp up. The total decrease in the use of cash was specifically due to an increase in our net loss of $146,374 and a decrease in changes in our operating assets and liabilities of $2,161,480, offset by increases in non-cash expenses such as depreciation and amortization, loss on fair value of contingent consideration, losses in equity method investments and non-cash interest expense.
Net Cash Provided by/Used in Investing Activities
Net cash used in investing activities for the year ended March 31, 2015 was limited to purchases of property and equipment in the amount of approximately $45,000.
Net cash used in investing activities for the year ended March 31, 2014 was $484,192, consisting primarily of cash payments for contingent consideration on our Prolieve acquisition and our investment in our joint venture; we made no such payments in 2013. In 2013, the Company purchased from Boston Scientific Corporation in a taxable transaction, all of the assets, relating to the Prolieve Thermodilatation System a FDA approved device for the treatment of Benign Prostatic Hyperplasia for a total consideration of $3,662,115.
Net Cash Used in/Provided by Financing Activities
Net cash provided by financing activities was approximately $2,826,786 for the year ended March 31, 2015, compared to $5,540,000 in 2014.
Net cash provided by financing activities was $5,540,000 for the year ended March 31, 2014, compared to $11,626,852 in 2013. The higher level in 2013 was the result of our raising significantly more capital from the issuance of equity, equity-linked and debt capital in 2013 as compared to 2014.
40
| C. | Research, Development, Patents and Licenses, etc. |
For the fiscal years ended March 31, 2015, 2014 and 2013, the Company incurred research and development expenses of $399,212, $472,810, and $411,045, respectively. Research and development expenses include pre-market approval fees and other fees payable to the U.S. Food and Drug Administration (FDA), the costs of implementing a Quality Management System (“QMS”) system which has passed FDA audit, the costs of our technology transfer and post-marketing efforts related to our Prolieve product, as well as the costs incurred with respect to the Phase III clinical study for our APA 1000 Breast Cancer System.
As a result of our acquisition of the Prolieve business in July 2012, for the first time in 2013 we had revenues and costs of sales and those sales increased significantly in 2014. We anticipate that sales of Prolieve products and service will increase over the next few years as a result of our continuing efforts to sell our products and services across the U.S. While we expect product sales to increase in 2015 over 2014 levels, we expect sales of our mobile services to increase at a somewhat higher rate.
We face significant competition from competitors with different approaches to treating BPH, some of which have been highly effective and are generally seen to be safe and affordable. In order to continue to grow our business and sales, our potential customers (primarily individual doctors and group medical practices) we will need to embrace our technology and in some cases switch from competitors’ products and services. There can be no assurance that our potential customers will be able or willing to embrace our products and services and that we will be able to continue or grow our business and sales.
We anticipate that as our sales continue to grow, margins on both product sales and services will become positive as the effect of fixed charges (such as amortization and depreciation expenses and to some extent warehousing costs) have a relative lesser impact on total margin. However, if we are unable to grow our business and sales, our gross margins will not increase as expected and we may continue to generate gross losses on products sales and/or sour mobile service.
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Management is continuing its efforts to obtain additional funds so that the Company can meet its obligations and sustain operations.
| E. | Off-Balance Sheet Arrangements |
As of each of March 31, 2015 and 2014, the Company did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K.
41
Tabular Disclosure of Contractual Obligations
The following are contractual commitments at March 31, 2015:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Total | | | Year 1 | | | Year 2 | | | Year 3 | | | Year 4 | | | Thereafter | |
Office lease obligation | | $ | 423,423 | | | $ | 150,062 | | | $ | 145,372 | | | $ | 136,913 | | | | | | | | | |
Vehicle lease obligation | | | 163,424 | | | | 45,430 | | | | 45,430 | | | | 45,430 | | | | 27,135 | | | | | |
Long-term and short-term debt obligations (1) | | | 6,834,422 | | | | 6,834,422 | | | | | | | | | | | | | | | | | |
Other contractual obligation (2) | | | 250,000 | | | | 50,000 | | | | 50,000 | | | | 50,000 | | | | 50,000 | | | | 50,000 | |
Total | | $ | 7,671,269 | | | $ | 7,079,914 | | | $ | 251,545 | | | $ | 219,419 | | | $ | 77,135 | | | $ | 50,000 | |
| (1) | Excluding $2,190,867 remaining balance on contingent consideration payable to Boston Scientific Corporation pursuant to the Prolieve Assets Purchase Agreement. The Company shall make quarterly sales royalty payment equal to ten percent (10%) of the amount of Prolieve sales. As of March 31, 2015, approximately $310,000 of such payments had been made to Boston Scientific Corporation. |
| (2) | Excluding potential royalty arrangements with Massachusetts Institute of Technology and Celsion Corporation for various products once commercialized. |
| Item 6. | Directors, Senior Management and Employees. |
| | A. | Directors and senior management. |
The following table sets forth important information regarding our directors and senior management. Our directors serve one-year terms or until their successors are elected and accept their positions. Except as disclosed in the biographies contained below, there are no family relationships or understandings between any of the directors and executive officers. In addition, there was no arrangement or understanding between any executive officer and any other person pursuant to which any person was selected as an executive officer.
| | | | |
| Name | | Position with the Company | | Date Position Started |
| Dr. Augustine Cheung | | Chief Executive Officer President, Director | | August 28, 2008 |
| | |
| John Mon | | Chief Operating Officer | | August 28, 2008 |
| | |
| Mirsad Jakubovic | | Chief Financial Officer, Director | | November 25, 2008, January 6, 2014 |
| | |
| Douglas Liu | | Director of Business Development | | January 1, 2013 |
| | |
| Grant B. Walsh | | Chairman of Board of Directors | | August 28, 2008 |
| | |
| Joseph S. C. Chan | | Director | | August 28, 2008 |
| | |
| Dr. Augustine P.Y. Chow | | Director | | August 28, 2008 |
| | |
| Raymond Tong | | Director | | January 27, 2015 |
Dr. Augustine Y, Cheung, Ph.D., 68, was a Director of Celsion (Canada) Limited from January 2006 to November 2008 and previously President, Chief Executive Officer and a Director of Celsion Corporation (formerly Cheung Laboratories, Inc.) from February 1985 to March 2006. Dr. Cheung was assistant and associate Professor at the University of Maryland, School of Medicine (1974-1980) and Associate
42
Professor of Engineering at the George Washington University (1980-1985). Dr. Cheung received the degrees of B.S., M.S. and Ph.D, all from the University of Maryland in 1969, 1971 and 1973, respectively. Dr. Cheung is a full-time member of the Company’s management team. Dr. Cheung is the brother-in-law of John Mon, the Company’s Chief Operating Officer.
John Mon, 63, has over 20 years’ experience in the medical device industry. From the late 1980s through 2008, Mr. Mon held various positions at Celsion, including VP Product Development, VP New Technology, General Manager, Corporate Secretary, and he has served as a director of Celsion. Additionally, he was responsible for global sales and marketing. During his 20 years with Celsion, Mr. Mon worked with the FDA to gain approval for IDE/Pre-market Approval/510K submissions. He also worked with surgical and oncology clinicians, electromagnetic (microwave) engineers and patent attorneys to develop various thermotherapy and breast cancer related devices. He has authored and co- authored a number of granted and pending patents in the field of microwave technology. Mr. Mon received his B.S. in Economics from the University of Maryland. He is the brother-in-law of Dr. Cheung, the Company’s Chief Executive Officer and President.
Mirsad Jakubovic, 52, is a chartered accountant. His experience includes working as the Director of Finance and Administration for Havana House Cigar and Tobacco Merchants Ltd., and as Director of Finance and Administration for Swatch Group Canada Ltd. Mr. Jakubovic received his MBA from the Richard Ivey School of Business and his B.Comm. from the University of Toronto.
Mr. Douglas Liu, 51, was a financial analyst with HII Enterprises Inc. in New York from 1994 to 1996, performing budget and cash flow preparation and analysis, as well as cross border joint venture investment evaluation. From 1996 to 2005, he was the assistant to the Chief Financial Officer and shareholders administrator at Celsion Corporation, assisting the management in budget and business plan development and analysis, in the structuring, negotiation and completion of multiple rounds of PIPE offerings. He also worked closely with auditors and securities attorneys in the preparation of Celsion Corp’s filings with the U.S. SEC. From 2005 to 2012, he was a private investor in various public and startup companies and occasionally worked as independent consultant in corporate finance matters. Mr. Liu graduated magna cum laude from the Ohio State University with a bachelor’ s degree in finance and international business.
Mr. Joseph S.C. Chan, MBA, 69, is a director of Harmony Asset Limited since December 2006, a director of Champion Minerals Inc. since November 2009 and a director of MBMI Resources Inc. since April 2011. Mr. Chan has over 30 years of accounting and management experience. He obtained an MBA from Edinburgh Business School, Heriot Watt University, Scotland, U.K. He is a member of The Institute of Chartered Accountants of England and Wales, the Hong Kong Institute of Certified Public Accountants, the Chartered Institute of Management Accountants as well as a member of the Certified General Accountants’ Association of Canada.
Dr. Augustine P.Y. Chow, M.Sc., Ph.D, 62, until May 2015, had served as the Chief Executive Officer of Harmony Asset Limited, a publicly listed investment company in Hong Kong and Toronto, since 1996. He is currently a director of Celsion Corporation (AMEX) and Kaisun Energy Group Ltd., and is a former director of Augyva Mining Resources Inc. (TSXV) and Jian ePayment Systems Limited (HKEX). From 1990 to 1998, Dr. Chow was the Chief Executive Officer of Allied Group of Companies. Dr. Chow received the degrees of M.Sc. from London Business School, Ph.D. from the University of South Australia, a DBA from Southern Cross University, and an Engineering Doctorate from the City University of Hong Kong. Dr. Chow also is a director of Gwynneth Gold Limited, a significant investor in our Company.
43
Grant B. Walsh, MBA, C.Dir., 66, is the Chairman of the Board of Directors of the Company. He also serves as Chairman and/ or Director of Canada Lands Company Limited (a Canadian Crown Corporation), Downsview Park Inc., Old Port of Montreal, Montreal Science Centre and Algoma University. He is also the Chairman and CEO of Walsh Delta Group Inc., a firm specializing in governance, strategy, leadership, and performance improvement. Mr. Walsh has served as a director and/or senior executive of various public, private, for-profit and not-for-profit healthcare and service organizations in both the United States and Canada. In addition to CEO roles, Mr. Walsh was Executive Vice President of the ServiceMaster Company in Chicago, Illinois, where he was accountable for $550 million in revenue, 30,000 employees, and 10,000 properties in 44 states and Canada. Assets under his leadership exceeded $30 billion. Mr. Walsh has been Executive-in-Residence and Adjunct Professor at the DeGroote School of Business of McMaster University. Mr. Walsh holds a Master of Business Administration degree from Southern Illinois University and a designation as a Chartered Director from McMaster University and the Conference Board of Canada. His undergraduate degree in English and Philosophy is from Roberts Wesleyan College.
Dr Raymond C. F. Tong, M.D., 56, is the Chief Executive Officer of Harmony Medical Inc, an Asian investment group active in the introduction and distribution of medical and healthcare products and services in China and the Asia. Dr. Tong obtained his medical degree from the University of Toronto. He is also an independent Director of Shanghai CP Guojian Pharmaceutical, the largest bio-pharmaceutical manufacturer in China, and is also Chairman of Shanghai Kedu Healthcare Group, one of the largest medical equipment distributor and third-party service provider in China, representing products from GE, Philips, Siemens and Kodak and other multi-nationals. Dr Tong’s earlier career included senior management positions in China with Pfizer and Ball Corporation. He was also responsible for the Healthcare Investment Division of CITIC Pacific in Hong Kong.
Exchange Rate Table
The following table sets forth the average exchange rate for one Canadian dollar expressed in terms of one U.S. dollar for each of the last five fiscal years. The average rate was calculated using the average of the exchange rates, as calculated on each day in the period.
| | | | |
Year | | Average | |
| |
2015 (through March 31) | | | 0.8057 | |
2014 | | | 0.9054 | |
2013 | | | 0.9710 | |
2012 | | | 1.0004 | |
2011 | | | 1.0110 | |
2010 | | | 0.9709 | |
The following table sets forth the high and low exchange rates for one Canadian dollar expressed in terms of one U.S. dollar for each month during the previous six months.
| | | | | | | | |
Month | | Low | | | High | |
| | |
March 2015 | | | 0.7791 | | | | 0.8060 | |
February 2015 | | | 0.7876 | | | | 0.8095 | |
January 2015 | | | 0.7813 | | | | 0.8562 | |
December 2014 | | | 0.8568 | | | | 0.8839 | |
November 2014 | | | 0.8732 | | | | 0.8936 | |
October 2014 | | | 0.8783 | | | | 0.9017 | |
44
The exchange rates are based upon the noon buying rate as quoted by the Bank of Canada. At March 31, 2015, the exchange rate for one Canadian dollar expressed in terms of one U.S. dollar, as quoted by The Bank of Canada at 4 p.m. Eastern Time, equaled $0.7895.
Summary Compensation Table
The following table sets out all annual andlong-term compensation for services in all capacities to the Company for the fiscal years ended March 31, 2015, 2014, and 2013 in respect of the individuals who served as the Chief Executive Officer, the Chief Financial Officer, and Chief Operating Officer of the Company (collectively, the “Named Executive Officers”). Other than as disclosed below, no other executive officer received in excess of $150,000 in total salary, bonus and other compensation for the fiscal year ended March 31, 2014. Please note that, aside from salary, all amounts in the following compensation tables are in Canadian dollars.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Non-equity incentive
plan compensation
($) | | | | | | | | | | |
Name and Principal Position | | Year | | | Salary
(USD$) | | | Share-Based
Awards
(Can.$)(2) | | | Option-Based
Awards
(Can.$) | | | Annual
Incentive
Plans | | | Long-term
Incentive
Plans | | | Pension
Value
(Can.$) | | | All
Other
Compensation
(Can.$) | | | Total
Compensation
($) | |
Dr. Augustine Y. Cheung President and CEO | |
| 2015
2014
2013 |
| | $
| 215,262
243,600
240,000
|
| |
| —
—
— |
| |
| —
—
380,000 |
(3) | |
| —
— — |
| |
| —
— — |
| |
| —
— — |
| |
| —
— — |
| |
| 215,262
243,600 620,000 |
|
| | | | | | | | | |
Mirsad Jakubovic (1) | | | 2015 | | | | 75,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 75,000 | |
Chief Financial Officer | | | 2014 | | | | 75,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 75,000 | |
| | | 2013 | | | | 75,000 | | | | — | | | | 123,500 | (3) | | | — | | | | — | | | | — | | | | — | | | | 198,500 | |
| | | | | | | | | |
John Mon | | | 2015 | | | $ | 203,846 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 203,846 | |
Chief Operating Officer | | | 2014 | | | | 203,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 203,000 | |
| | | 2013 | | | | 200,000 | | | | — | | | | 237,500 | (3)
| | | —
| | | | —
| | | | —
| | | | —
| | | | 437,500
| |
45
Notes:
| (1) | Mirsad Jakubovic was appointed Chief Financial Officer on November 25, 2008. Mr. Jakubovic was paid compensation of CAD $75,000 for each of the last three fiscal years. |
| (2) | The share grants were all recognized at a price of $0.18 per share. |
| (3) | Options were granted December 17, 2012 and vested over 12 months from the date of the grant. Each option has a term of three (3) years and an exercise price of $0.19. The fair value of the stock option grants were estimated at the date of the grant using a Black-Scholes option-pricing model in accordance with the standard methodology applicable to time vested stock option grants. The following assumptions were used in the model (being the same assumptions used for financial reporting purposes): risk-free interest rate of 1.1%, expected volatility in the market price of the Corporation’s shares of 156.0%, expected life of 3 years and a dividend yield of nil% |
Option Grants
No stock options were granted to our executive officers and directors during the fiscal year ended March 31, 2015.
46
Value Vested or Earned During the Year
No stock options were exercised by any Named Executive Officer, or vested during the fiscal year ended March 31, 2015.
Compensation of Directors
Our directors of each receive an annual fee of CAD $20,000 as compensation for their services as directors. In addition, each committee chairman receives an additional CAD $15,000 annually, and the Chairman of the Board of Directors receives an additional CAD $30,000 annually.
Directors are also eligible to participate in the Company’s Stock Option Plan (the “Option Plan”) on an on-going basis. As of March 31, 2015, an aggregate of 8,025,000 stock options had been granted under the Option Plan, of which an aggregate of 5,375,000 stock options had been granted to directors or former directors.
The following table sets out all annual andlong-term compensation for services in all capacities to the Company for the fiscal year ended March 31, 2015 of each of the directors (other than named Executive Officers).
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Name | | Fees
earned | | | Share-
based
awards | | | Option-
based
awards | | Non-equity
incentive plan
compensation | | | Pension
value | | | All other
compensation | | | Total | |
Joseph S. C. Chan | | | 31,250 | | | | — | | | | | | — | | | | — | | | | — | | | | 31,250 | |
Dr. Augustine P.Y. Chow | | | 20,000 | | | | — | | | | | | — | | | | — | | | | — | | | | 20,000 | |
Tak Cheung Yam | | | 15,000 | | | | — | | | | | | — | | | | — | | | | — | | | | 15,000 | |
Grant Walsh | | | 53,750 | | | | — | | | | | | — | | | | — | | | | 18,000 | (1) | | | 71,750 | |
Raymond Tong | | | 5,000 | | | | — | | | | | | — | | | | — | | | | — | | | | 5,000 | |
| (1) | Reimbursable travel and administrative expenses |
Incentive Plan Awards
OutstandingShare-Based Awards andOption-Based Awards
As of March 31, 2015, the following table sets forth information in respect of all stock options granted to our directors. No options were granted as a result of repricing.
| | | | | | | | | | | | | | | | | | | | | | |
| | | Option-based Awards | | Share-based Awards | |
Name | | Number of
securities
underlying
unexercised
options
(#) | | | Option
exercise price
(Can.$) | | | Option expiration
date | | Number of
shares or units
of shares that
have not
vested
(#) | | | Market or
payout value of
share-based
awards that
have not vested
(Can.$) | | | Market or
payout value of
vested share-
based awards
not paid out or
distributed
(CanT$) | |
Joseph S. C. Chan | | | 300,000 | | | | 0.20 | | | March 17, 2016 | | | — | | | | — | | | | — | |
| | | 150,000 | | | | 0.19 | | | December 17, 2015 | | | | | | | | | | | | |
Dr. Augustine P.Y. Chow | | | 300,000 | | | | 0.20 | | | March 17, 2016 | | | — | | | | — | | | | — | |
| | | 150,000 | | | | 0.19 | | | December 17, 2015 | | | | | | | | | | | | |
Grant Walsh | | | 500,000 | | | | 0.20 | | | March 17, 2016 | | | — | | | | — | | | | — | |
| | | 250,000 | | | | 0.19 | | | December 17, 2015 | | | | | | | | | | | | |
47
Value Vested or Earned During the Year
No stock options were exercised by any director, or vested during the fiscal year ended March 31, 2015. Following his resignation from the Board, 225,000 options owing to Mr. Tak Cheung Yam expired without exercise.
Outstanding Options
Set forth below is a summary of the outstanding options under the Option Plan to purchase Shares as of March 31, 2015:
All executive officers, past executive officers, directors, past directors of the Company, and key employees, as a group:
| | | | | | | | | | | | | | | | |
Holder | | Number of
Shares Under
Option | | | Date of Grant | | | Expiry Date | | | Exercise | |
| | | | |
Grant Walsh | | | 500,000 | | | | March 17, 2011 | | | | March 17, 2016 | | | $ | 0.20 | |
| | | | |
Joseph S. C. Chan | | | 300,000 | | | | March 17, 2011 | | | | March 17, 2016 | | | $ | 0.20 | |
| | | | |
Dr. Augustine P.Y. Chow | | | 300,000 | | | | March 17, 2011 | | | | March 17, 2016 | | | $ | 0.20 | |
| | | | |
Dr. Augustine Cheung | | | 500,000 | | | | March 17, 2011 | | | | March 17, 2016 | | | $ | 0.20 | |
| | | | |
John Mon | | | 400,000 | | | | March 17, 2011 | | | | March 17, 2016 | | | $ | 0.20 | |
| | | | |
Mirsad Jakubovic | | | 200,000 | | | | March 17, 2011 | | | | March 17, 2016 | | | $ | 0.20 | |
| | | | |
Grant B. Walsh | | | 250,000 | | | | December 17, 2012 | | | | December 17, 2015 | | | $ | 0.19 | |
| | | | |
Dr. Augustine Y. Cheung | | | 2,000,000 | | | | December 17, 2012 | | | | December 17, 2015 | | | $ | 0.19 | |
| | | | |
Joseph S. C. Chan | | | 150,000 | | | | December 17, 2012 | | | | December 17, 2015 | | | $ | 0.19 | |
| | | | |
Dr. Augustine P.Y. Chow | | | 150,000 | | | | December 17, 2012 | | | | December 17, 2015 | | | $ | 0.19 | |
| | | | |
Ernie Eves | | | 150,000 | | | | December 17, 2012 | | | | January 6, 2016 | | | $ | 0.19 | |
| | | | |
John Mon | | | 1,250,000 | | | | December 17, 2012 | | | | December 17, 2015 | | | $ | 0.19 | |
| | | | |
Mirsad Jakubovic | | | 650,000 | | | | December 17, 2012 | | | | December 17, 2015 | | | $ | 0.19 | |
| | | | |
Kurt O’Neill | | | 500,000 | | | | January 9, 2013 | | | | January 9, 2016 | | | $ | 0.24 | |
| | | | | | | | | | | | | | | | |
| | | 7,300,000 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
48
Options Granted during Fiscal Year Ended March 31, 2013
No stock options were granted to our executive officers and directors, past executive officers and directors, and key employees in the fiscal years ended March 31, 2014 or March 31, 2015. During the year ended March 31, 2013, 5,325,000 stock options were granted to our executive officers and directors, past executive officers and directors, and key employees. The following table sets out the options granted to such individuals during the year ended March 31, 2013.
| | | | | | | | | | | | | | | | |
| Name | | Securities Under
Options Granted
(#) | | | % of Total
Options granted
to Employees in
the Financial
Year | | | Exercise or Base
Price
(Can.$/Security) | | | Market Value of Securities Underlying Options on the
Date of Grant (Can.$/Security) | |
Grant B. Walsh | | | 250,000 | | | | 4 | % | | $ | 0.19 | | | | 20,000 | |
Dr. Augustine Y. Cheung | | | 2,000,000 | | | | 34 | % | | $ | 0.19 | | | | 160,000 | |
Joseph S. C. Chan | | | 150,000 | | | | 3 | % | | $ | 0.19 | | | | 12,000 | |
Dr. Augustine P.Y. Chow | | | 150,000 | | | | 3 | % | | $ | 0.19 | | | | 12,000 | |
Ernie Eves(1) | | | 150,000 | | | | 3 | % | | $ | 0.19 | | | | 12,000 | |
Tak Cheung Yam(3) | | | 225,000 | | | | 4 | % | | $ | 0.19 | | | | 18,000 | |
John Mon | | | 1,250,000 | | | | 21 | % | | $ | 0.19 | | | | 100,000 | |
Mirsad Jakubovic | | | 650,000 | | | | 11 | % | | $ | 0.19 | | | | 52,000 | |
| | | | |
Kurt O’Neill(2) | | | 500,000 | | | | 9 | % | | $ | 0.24 | | | | 40,000 | |
| | | | | | | | | | | | | | | | |
| | | | |
| | | 5,325,000 | | | | | | | | | | | | | |
| (1) | Mr. Eves resigned did not stand for re-election at our 2014 annual meeting. |
| (2) | Mr. O’Neill resigned from his position at Medifocus Inc. in July 2014. |
| (3) | Mr. Tak Cheung Yam resigned from his position at Medifocus Inc. in January 2015. |
Upon exercise in accordance with the terms thereof, each of these options entitles the holder thereof to acquire one Share.
Stock Awards
At our Annual and Special Meeting of Shareholders held on November 28, 2012 our shareholders approved a resolution authorizing the issuance of up to 3,000,000 Shares to directors and officers in lieu of a portion of the remuneration to which such persons were entitled. The goal of such stock awards is to provide our officers and directors with an increased proprietary stake in the Company, while allowing us to deploy more of its cash on hand on execution of its business plan. All stock awards will be done at a deemed price of at least the “Discounted Market Price,” as such term is defined under applicable TSXV regulations.
49
At March 31, 2015 a total of 1,755,095 Shares have been issued to the following persons and in the following amounts.
| | | | | | |
Name | | Title | | Number of Shares Awarded | |
Dr. Augustine Cheung | | President, CEO, & Director | | | 792,058 | |
Ernie Eves(1) | | Director | | | 500,000 | |
John Mon | | Chief Operating Officer | | | 363,037 | |
Mirsad Jakubovic | | Chief Financial Officer | | | 100,000 | |
| (1) | Mr. Eves is no longer a director of the Company. |
Employment Contracts
We have no written employment contracts with any members of senior management.
Termination Agreements for Executive Officers and Directors
There are no termination agreements in effect for the Company’s executive officers or directors. Although our Compensation Committee has indicated a desire to grant senior executive officers a severance package that could result in up to two years of salary, plus a bonus, in the event of termination of employment, no employment agreements have been entered into with senior management. In the event we do enter into employment agreements with our senior executive officers, we are unable to state at this time what the terms of such employment agreements would be.
Stock Option Plan
Our shareholders approved the Option Plan on November 28, 2012. The number of Shares reserved for issuance under the Option Plan may not exceed 10% of the total number of Shares issued and outstanding from time to time. At March 31, 2015, the Company had 127,542,120 Shares outstanding. An aggregate of 8,825,000 options have been granted by the Company under the Option Plan to date and none of these options have been exercised. Accordingly, 3,929,212 options currently remain available for future grant under the Option Plan (based upon 10% of the aggregate number of issued and outstanding Shares at March 31, 2015).
The purpose of the Option Plan is to attract, retain and motivate persons as key service providers to the Company and to advance the interests of the Company by providing such persons with the opportunity, through share options, to acquire a proprietary interest in the Company and benefit from its growth. The options are non-assignable and may be granted for a term not exceeding five years.
Options may be granted under the Option Plan only to directors, officers, employees and other service providers subject to the rules and regulations of applicable regulatory authorities and any Canadian stock exchange upon which the Shares may be listed or may trade from time to time. The number of Shares reserved for issue to any one person pursuant to the Option Plan within any one year period may not exceed 5% of the issued and outstanding Shares. The maximum number of Shares which may be reserved for issuance to insiders under the Option Plan, any other employer stock option plans or options for services is 10% of the aggregate number of issued and outstanding Shares at the date of grant (on a non-diluted basis). The maximum number of Shares which may be issued to insiders under the Option Plan, together with any previously established or proposed share compensation arrangements, within any one year period, is 10% of the aggregate number of issued and outstanding Shares. The maximum number of Shares which may be issued to any insider and his or her associates under the Option Plan, together with any previously established or proposed share compensation arrangements, within any one year period, is 5% of the aggregate number of issued and outstanding Shares at the date of grant (on a non-diluted basis). The maximum number of Shares which may be granted to any consultant under the Option Plan, any other employer stock option plans or options for services, within any one year
50
period, is 2% of the aggregate number of issued and outstanding Shares at the date of grant (on a non-diluted basis). The maximum number of Shares which may be granted to any “investor relations person” under the Option Plan, any other employer stock option plans or options for services, within any one year period, is 2% of the aggregate number of issued and outstanding Shares at the date of grant (on a non-diluted basis).
The exercise price of options issued may not be less than the market value of the Shares at the time the option is granted, subject to any discounts permitted by applicable legislative and regulatory requirements.
Pension, Retirement and other Similar Benefits
No amount has been set aside by the Company during the last fiscal year to provide pension, retirement or similar benefits for our directors and officers pursuant to any existing plan provided or contributed to by us or our subsidiary company, or otherwise.
Warrants
At March 31, 2015 we had the following warrants outstanding.
| | | | | | | | | | | | | | | | | | | | |
| | | Number
# | | | Exercise
Price
$ | | | Black-Scholes
Values
$ | | | Expiry
Date | | | Year
of
Issue | |
Share purchase warrants | | | 2,449,997 | | | | 0.50 | | | | 583,702 | | | | 4/24/2015 | (1) | | | 2011 | |
Share purchase warrants | | | 3,745,000 | | | | 0.30 | | | | 192,180 | | | | 2/24/2016 | | | | 2011 | |
Share purchase warrants | | | 18,367,263 | | | | 0.20 | | | | 1,024,758 | | | | 6/8/2015 | (2) | | | 2012 | |
Share purchase warrants | | | 22,200,000 | | | | 0.20 | | | | 1,238,597 | | | | 6/8/2015 | (2) | | | 2012 | |
Share purchase warrants | | | 22,196,795 | | | | 0.20 | | | | 1,284,137 | | | | 9/21/2015 | | | | 2012 | |
Share purchase warrants | | | 13,056,997 | | | | 0.20 | | | | 743,924 | | | | 1/14//2016 | | | | 2013 | |
Share purchase warrants | | | 8,336,400 | | | | 0.30 | | | | 745,316 | | | | 12/18/2016 | | | | 2013 | |
Share purchase warrants | | | 4,644,400 | | | | 0.30 | | | | 419,274 | | | | 3/7/2017 | | | | 2014 | |
Share purchase warrants | | | 10,281,250 | | | | 0.25 | | | | 1,231,934 | | | | 9/15/2017 | | | | 2015 | |
| | | | | | | | | | | | | | | | | | | | |
Outstanding, end of year | | | 105,278,102 | | | | | | | | 7,463,822 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| 1. | Subsequent to the end of the year, 2,449,997 outstanding common share purchase warrants expired without exercise. |
| 2. | Subsequent to the end of the year, 40,567,263 outstanding common share purchase warrants were extended to June 8, 2016. |
51
The following table sets forth information regarding our current directors. Our directors serve one-year terms or until their successors are elected and accept their positions.
| | |
| Name | | Year Appointed Director |
Joseph Shuen Chuen Chan | | 2010 |
| |
Dr. Augustine Cheung | | 2008 |
| |
Dr. Augustine P.Y. Chow | | 2010 |
| |
Raymond Tong | | 2015 |
| |
Grant B. Walsh | | 2008 |
Committees of the Board of Directors
The Board of Directors of the Company (the “Board”) currently has the following standing committees: (i) an Audit Committee; (ii) a Compensation Committee; and (iii) a Governance Committee.
Composition of the Audit Committee
The members of the audit committee are Joseph S.C. Chan (Chairman), Grant B. Walsh, and Raymond Tong. Each of the Audit Committee members is considered independent and are financially literate.
The principal responsibilities of the Audit Committee include: (i) appointing, and overseeing the work of, any public accounting firm that the Company employs for the purpose of preparing or issuing an audit report or related work; (ii) approving the compensation of any such public accounting firm; (iii) approving all auditing services and non-audit services that the Company’s auditors provide to the Company; (iv) resolving any disagreements between the Company’s management and the auditor regarding financial reporting; (v) establishing procedures for the receipt, retention, and treatment of complaints that the Company receives regarding accounting, internal accounting controls or auditing matters and for the confidential, anonymous submission by employees of the Company of concerns regarding questionable accounting or auditing matters; (vi) assisting the Board in the oversight of (1) the integrity of the Company’s financial statements, (2) the Company’s compliance with legal and regulatory requirements, (3) the auditor’s qualifications and independence, and (4) the performance of the Company’s external audit functions; and (vii) determining appropriate funding for (x) compensation to any public accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit, review or attest services for the Company, (y) compensation to any advisors employed by the Company to assist the Committee in the conduct of its duties, and (z) ordinary administrative expenses of the Committee that are necessary or appropriate in carrying out its duties.
The Audit Committee must be comprised of at least 3, and no more than 5, independent directors, all of whom must be financially literate, and have knowledge of the Company’s industry, and the ability to understand business and financial risks and related controls and control processes. The Audit Committee is required to meet at least four times each year.
Compensation Committee
The members of Compensation Committee are Dr. Augustine P.Y. Chow, Grant B. Walsh, Raymond Tong and Joseph S. C. Chan, all of whom are considered independent.
The Compensation Committees principal responsibilities include: (i) reviewing, and recommending to the Board, the compensation and benefit plans of the Chief Executive Officer, key executives, and the Directors of the Company; (ii) reviewing, and recommending to the Board, the Company’s compensation, benefit and compensation-related policies; (iii) advising the Board on the appointment of health and retirement benefit plan administrators, trustees and other similarly required positions; and (iv) reviewing, and recommending to the Board, equity plans and other long-term compensation programs.
52
The Compensation Committee receives recommendations from management and reviews and makes recommendations to the Board regarding the granting of stock options or common shares to directors, executive officers or employees, as well as compensation for executive officers and directors’ fees, if any, from time to time. Executive officers and directors may be compensated in cash and/or common shares for their expert advice and contribution towards our success.
The form and amount of such compensation will be evaluated by the Compensation Committee, which will be guided by the following goals:
| | • | | compensation should be commensurate with the time spent by the executive officers and directors in meeting their obligations and reflective of the compensation paid by companies similar in size and business to the Company; and |
| | • | | the structure of the compensation should be simple, transparent and easy for shareholders to understand. |
Generally, the Compensation Committee and the Board strive to balance the Company’s structure as aCanadian-listed Company whose operations and employees are primarily in the United States. Due to the Company’s size and development stage, the Compensation Committee has not yet considered it necessary to consult with any third party advisors in determining the directors’ and officers’ compensation. Outside advisors may be engaged by the Compensation Committee in the future for that purpose.
In reaching compensation decisions, the Compensation Committee considered the individual performance of each executive officer as well as the overall performance of the Company, taking consideration its size and stage of development.
The Audit Committee must be comprised of 3 or more members of the Board, all of whom must be considered independent. Our Chief Executive Officer is also considered an ex officio member of the Compensation Committee, and participates in all matters except the final recommendations regarding CEO compensation arrangements. The Compensation Committee meets at such times as the committee shall determine, which is typically at least two times per fiscal year.
Governance Committee
The members of the Governance Committee are Grant Walsh, Raymond Tong, and Dr. Augustine P.Y. Chow and Joseph S. C. Chan.
The Governance Committees primary responsibilities include: (i) assisting the Board with the recruitment, retention and evaluation of our Chief Executive Officer, and to ensure that a succession plan is in place; (ii) approving the strategic direction, major strategies, plans and actions of the Company; (iii) providing high level operational oversight including quality, fiscal responsibility and implementation of strategic direction; and (iv) ensuring that the Company operates in a manner which is consistent with legal, ethical and moral principles.
Additionally, the Governance Committee is responsible for considering and making recommendations to the Board concerning the appropriate size, functions and needs of the Board. The Corporate Governance Committee may, at its sole discretion, engage director search firms and has the sole authority to approve the fees and other retention terms with respect to any such firms. When considering a candidate for the Board, the Governance Committee is guided by the following principles:
| | • | | Each Director should be an individual of the highest character and integrity, have an inquiring mind, experience at a strategy / policy setting level, or otherwise at a senior executive level of experience, and the ability to work well with others. |
53
| | • | | Each Director shall have sufficient time available to devote to the affairs of the Company to carry out the responsibilities of a Director. Directors are expected to make a commitment to prepare for, and attend, meetings of the Board and its Committees on a reasonably regular basis. Each Director shall strive to attend at least 75% of the meetings each year for which the Director is expected to participate. |
| | • | | Each independent Director should be free of any conflict of interest that would interfere with the independence and proper performance of the responsibilities of a Director. |
| | • | | Directors should not be chosen as representatives of a constituent group or organization and shall act in the best interests of the Corporation as mandated by Canadian corporate law (Canada Business Act). |
| | • | | Directors should have an equity interest in the Company. Toward that end, each Director may change all or a part of Director’s fees to Company Common Shares. |
The Corporate Governance Committee also has the authority, as necessary and appropriate, to consult with other counsel and outside advisors to assist in its duties to the Company.
At March 31, 2015, we had 26 employees, a decrease from 31 employees at March 31, 2014. This decrease is a result of our efforts to restructure the Prolieve business and operate our business more efficiently.
Of our full time employees, 16 are regional Prolieve sales representatives and Prolieve mobile service technicians. The rest are corporate management, engineering, regulatory compliance and support staff.
None of our current employees are members of a labor union and, consequently, there is no relationship between our management and any labor union.
54
The following table sets forth the shareholdings of the Company’s directors and senior management at March 31, 2015.
| | | | | | | | | | | | | | | | |
NAME | | SHARES OWNED | | | SHARE
CONVERTIBLE
FROM
PROMISSORY
NOTESAND
OPTIONSOR
WARRANTS
VESTEDOR
VESTING WITHIN
60DAYS | | | BENEFICIAL
OWNERSHIP | | | PERCENTAGE
OF
OUTSTANDING
SHARES* | |
| | | | |
Dr. Augustine Cheung | | | 6,727,370 | | | | 2,500,000 | | | | 9,227,370 | | | | 7.10 | % |
| | | | |
John Mon | | | 1,141,667 | | | | 2,336,667 | | | | 3,478,334 | | | | 2.68 | % |
| | | | |
Mirsad Jakubovic | | | 1,814,667 | | | | 2,371,667 | | | | 4,186,334 | | | | 3.22 | % |
| | | | |
Douglas Liu | | | 250,000 | | | | 500,000 | | | | 750,000 | | | | 0.59 | % |
| | | | |
Joseph S. C. Chan | | | 250,000 | | | | 450,000 | | | | 700,000 | | | | 0.55 | % |
| | | | |
Dr. Augustine P.Y. Chow | | | 850,000 | | | | 450,000 | | | | 1,300,000 | | | | 1.02 | % |
| | | | |
Grant B. Walsh | | | 450,000 | | | | 750,000 | | | | 1,200,000 | | | | 0.94 | % |
| | | | |
Raymond C. Tong | | | 90,909 | | | | | | | | | | | | 0.07 | % |
| | | | |
Officers & Directors, as a group | | | 11,574,613 | | | | 9,358,334 | | | | 20,932,947 | | | | 15.29 | % |
| * | At March 31, 2015, the Company had 127,542,120 Shares outstanding. This number excludes the 38,750,000 shares issuable for private placement as of March 31, 2015, and the 7,571,959 shares issuable for debt conversion as of March 31, 2015. |
55
| Item 7. | Major Shareholders and Related Party Transactions. |
At June 30, 2015, we had 166,292,120 Shares outstanding. To the knowledge of our directors and senior officers, the following are the only persons or companies who beneficially own, directly or indirectly, or exercise control or direction over Shares carrying more than 5% of our outstanding Shares:
| | | | | | | | | | |
Name | | No. of Shares | | | Percentage | | | Natural Persons(6) |
Integrated Asset Management (ASIA) Limit | | | 51,382,575 | (1) | | | 26.72 | % | | Tak Cheung Yam |
Gwynneth Gold Limited | | | 35,528,114 | (2) | | | 19.20 | % | | Vincent Cheung |
Augustine Y. Cheung | | | 9,227,370 | (3) | | | 5.47 | % | | N/A |
Chatwin Management Limited | | | 17,300,000 | (4) | | | 9.83 | % | | Temmy Wong |
Star Example Limited | | | 13,333,334 | (5) | | | 7.71 | % | | Kwong Chi Shing Savio |
Notes:
| (1). | Includes warrants to purchase 13,333,333 Shares at $0.20 per Share, warrants to purchase 3,700,000 Shares at $0.30 per Share and warrants to purchase 1,562,500 Shares at $0.25 per Share, and the 7,400,000 Shares that could be issued after June 18 2014 pursuant to the terms of a convertible promissory note. |
| (2). | Includes warrants to purchase 12,400,001 Shares at $0.20 per Share, warrants to purchase 1,580,000 Shares at $0.30 per Share and warrants to purchase 1,562,500 Shares at $0.25 per Share, and 3,160,000 Shares that could be issued after June 18. 2014 pursuant to the terms of a convertible promissory note. |
| (3). | Includes stock options to purchase 2,000,000 Shares at $0.19 per Share and stock options to purchase 500,000 Shares at $0.20 per Share. |
| (4). | Includes warrants to purchase 7,600,000 Shares at $0.20 per Share, warrants to purchase 700,000 Shares at $0.30 per Share, and the 1,400,000 Shares that could be issued after June 18. 2014 pursuant to the terms of a convertible promissory note. |
| (5). | Includes warrants to purchase 6,666,667 Shares at $0.20 per Share. |
| (6). | The individuals listed in this column represent the natural persons who have or share the power to direct the voting or disposition of the securities listed, or to receive the economic benefit of ownership of such securities. |
At June 29, 2015, we had 56 U.S. shareholders of record, holding 31,145,852 Shares, which represented approximately 19% of our outstanding Shares. At such date, there were no arrangements, the operation of which could result in a change of control. All shareholders have the same voting rights with respect to the Shares.
| B. | Related party transactions. |
No executive officer, director, or person owning at least 5% of our Shares, or any affiliate thereof, has or any has had any material interest, directly or indirectly, in any transaction involving our company since April 1, 2010, or in any proposed transaction involving our company.
In February 2015, Mr. Tak Cheung Yam, a former director of the Company, through Integrated Assets Management (Asia) Ltd, purchased 9,090,909 Shares in a private offering. Mr. Yam paid $0.044 per Share, for an aggregate purchase price of $400,000. Further, Mr. Yam, through Integrated Assets Management (Asia) Ltd, currently owns 25,386,742 Shares, or 15.27% of the Company’s outstanding common stock. Integrated Assets Management (Asia) Ltd also owns exercisable warrants to purchase an additional 18,595,833 Shares. If Mr. Yam chooses to exercise all these warrants, he will control 23.18% of our Common Stock. In addition, Mr. Yam, through Integrated Assets Management (Asia) Ltd, owns a convertible note that can be converted into 7,400,00 Shares. If Mr. Yam chooses to convert the notes to Shares, and to exercise all his warrants, he will effectively control 26.03% of our outstanding shares. In February 2015, Mr. Yam purchased an additional 9,090,909 shares through Integrated Assets Management (Asia) Ltd. at $0.044 per share.
56
| C. | Interests of Experts and Counsel |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
| Item 8. | Financial Information. |
| | A. | Consolidated statements and other financial information. |
See “Item 18. Financial Statements.”
Litigation
We are not currently involved in any legal proceedings of a material nature, nor are we aware of any legal proceedings known to be contemplated by any governmental authorities.
Dividend Policy
We have never paid a dividend and it is unlikely that we will declare or pay a dividend in the foreseeable future. The Board, in its sole discretion, may declare dividends in the future, and determine the amount and payment date of such dividends. In making such determinations, the Board will consider our financial requirements and other relevant conditions prevailing at the time. We currently intend to use any revenues, as well as proceeds from any financings, to assist us in obtaining our business objectives, and not for the payment of any dividends upon our Shares.
Except as disclosed in the notes to the audited financial statements, there have been no significant changes since the date of the Company’s audited financial statements at March 31, 2015.
| Item 9. | The Offer and Listing. |
| | A. | Offer and listing details. |
Our Shares have traded in Canada on the TSXV since July 31, 2006, under the symbol “MFS”. In the United States, our Shares traded on the OTC Pink Sheets from December 2009 through June 29, 2012, under the symbol “MDFZ”. Our shares have traded in the United States on the OTCQX since June 30, 2011, under the symbol “MDFZF”.
The TSXV
Our Shares did not trade in Canada between August 6, 2011 and May 16, 2012 because of cease trade orders imposed by the Ontario Securities Commission and the British Columbia Securities Commission in September 2011. The cease trade orders were imposed because of our failure to file audited financial statements for the fiscal year ended March 31, 2011 and related filings. After we filed these financial statements, the cease trade orders were lifted by the Ontario Securities Commission and the British Columbia Securities Commission in December 2011, and our Shares resumed trading on the Toronto Venture Exchange on May 16, 2012.
57
The annual high and low market prices in Canadian dollars for the common shares of the Company for the five most recent fiscal years as traded on the TSXV were as follows:
| | | | | | | | |
| Fiscal Year Ended March 31, | | Low (Cdn$) | | | High (Cdn$) | |
| | |
2015 | | | 0.04 | | | | 0.22 | |
2014 | | | 0.12 | | | | 0.25 | |
2013 | | | 0.10 | | | | 0.31 | |
2012 | | | 0.12 | | | | 0.33 | |
2011 | | | 0.08 | | | | 0.35 | |
The quarterly high and low market prices in Canadian dollars for the common shares of the Company for each quarterly period within the two most recent fiscal years as traded on the TSXV were as follows:
| | | | | | | | |
| Quarter Ended | | Low (Cdn$) | | | High (Cdn$) | |
| | |
March 31, 2015 | | | 0.04 | | | | 0.11 | |
December 31, 2014 | | | 0.08 | | | | 0.12 | |
September 30, 2014 | | | 0.10 | | | | 0.16 | |
June 30, 2014 | | | 0.12 | | | | 0.22 | |
March 31, 2014 | | | 0.18 | | | | 0.25 | |
December 31, 2013 | | | 0.15 | | | | 0.25 | |
September 30, 2013 | | | 0.13 | | | | 0.21 | |
June 30, 2013 | | | 0.12 | | | | 0.25 | |
The monthly high and low market prices in Canadian dollars for the common shares of the Company for the most recent six months as traded on the TSXV were as follows:
| | | | | | | | |
| Month | | Low (Cdn$) | | | High (Cdn$) | |
| | |
May 2015 | | | 0.05 | | | | 0.16 | |
April 2015 | | | 0.04 | | | | 0.06 | |
March 2015 | | | 0.04 | | | | 0.05 | |
February 2015 | | | 0.05 | | | | 0.09 | |
January 2015 | | | 0.05 | | | | 0.11 | |
December 2014 | | | 0.09 | | | | 0.11 | |
The closing price of our Shares on the TSXV on March 31, 2015 was $0.04 (Canadian dollars).
58
The OTCQX
The annual high and low market prices in U.S. dollars for the common shares of the Company for the five most recent fiscal years as traded on the OTCQX were as follows:
| | | | | | | | |
| Fiscal Year Ended March 31, | | Low ($) | | | High ($) | |
| | |
2015 | | | 0.03 | | | | 0.19 | |
2014 | | | 0.11 | | | | 0.25 | |
2013 | | | 0.11 | | | | 0.35 | |
2012 | | | 0.08 | | | | 0.35 | |
2011 | | | 0.10 | | | | 0.40 | |
The quarterly high and low market prices in U.S. dollars for the common shares of the Company for each quarterly period within the two most recent fiscal years as traded on the OTCQX were as follows:
| | | | | | | | |
| Quarter Ended | | Low ($) | | | High ($) | |
| | |
March 31, 2015 | | | 0.03 | | | | 0.09 | |
December 31, 2014 | | | 0.07 | | | | 0.11 | |
September 30, 2014 | | | 0.09 | | | | 0.14 | |
June 30, 2014 | | | 0.12 | | | | 0.19 | |
March 31, 2014 | | | 0.15 | | | | 0.23 | |
December 31, 2013 | | | 0.16 | | | | 0.25 | |
September 30, 2013 | | | 0.14 | | | | 0.21 | |
June 30, 2013 | | | 0.11 | | | | 0.25 | |
The monthly high and low market prices in U.S. dollars for the common shares of the Company for the most recent six months as traded on the OTCQX were as follows:
| | | | | | | | |
| Month | | Low ($) | | | High ($) | |
| | |
May 2015 | | | 0.04 | | | | 0.13 | |
April 2015 | | | 0.03 | | | | 0.05 | |
March 2015 | | | 0.03 | | | | 0.05 | |
February 2015 | | | 0.03 | | | | 0.07 | |
January 2015 | | | 0.05 | | | | 0.09 | |
December 2014 | | | 0.08 | | | | 0.09 | |
The closing price of our Shares on the OTCQX on March 31, 2015 was $0.04.
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
Our Shares have traded in Canada on the TSXV since July 31, 2006, under the symbol “MFS”. In the United States, our Shares traded on the OTC Pink Sheets from December 2009 through June 29, 2012, under the symbol “MDFZ”. Our shares have traded in the United States on the OTCQX since June 30, 2011, under the symbol “MDFZF”.
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
59
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
| Item 10. | Additional Information. |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
| B. | Memorandum and articles of association. |
I. General
The Company is a corporation governed by the Business Corporations Act (Ontario) and the regulations promulgated thereunder (collectively referred to as the “Act”). The Company was incorporated in the Province of Ontario, Canada on April 25, 2005. The Company’s corporate objectives and purpose are unrestricted. The Company is authorized to issue an unlimited number of Shares.
II. Shares
Holders of the Shares are entitled to one vote per share upon all matters presented to the holders of the Shares at a meeting of such shareholders. The Shares have no rights regarding preference, conversion, exchange, preemptive rights or cumulative voting rights. Further, there are no provisions for redemption, purchase for cancellation, surrender or sinking or purchase funds for the Shares, and our shareholders have no liability for further capital calls.
The holders of Shares are entitled to the payment of any dividend declared by our Board of Directors, if at all, and, upon liquidation, to receive such of our assets that are distributable to holders of the Shares. All Shares rank equally as to dividends and as to the distribution of the Company’s assets in the event of a liquidation, dissolution or winding up of the Company.
The Act contains provisions that require a “special resolution” for effecting certain corporate actions. Such a “special resolution” requires the approval of two-thirds of the votes cast on a resolution submitted to the shareholders. The principle corporate actions for which the Company would require a “special resolution” include: (i) an amendment to the provisions relating to the outstanding capital of the Company; (ii) a sale of all or substantially all of the assets of the Company; (iii) an amalgamation of the Company with another company, other than a subsidiary; (iv) a winding-up of the Company; (v) a continuance of the Company into another jurisdiction; (vi) a statutory court approved arrangement under the Act (essentially a corporate reorganization such as an amalgamation, sale of assets, winding-up, etc.); and (vii) a change of name.
60
III. Directors
Pursuant to Section 132 of the Act, a director who is a party to, or who is a director or officer of or has a material interest in any person who is a party to, a material contract or transaction or proposed material contract or transaction with us shall disclose to us the nature and extent of that interest and shall not vote on any resolution to approve such contract or transaction.
Section 137 of the Act provides that the directors shall be paid such remuneration for their services as the board of directors may from time to time determine.
Section 184 of the Act provides that the board may from time to time on our behalf, without authorization of shareholders:
| | • | | borrow money upon Company credit; |
| | • | | issue, reissue, sell or pledge debt obligations of the Company; |
| | • | | guarantee on our behalf to secure performance of any obligation of any person; and |
| | • | | mortgage, hypothecate, pledge or otherwise create a security interest in all or any of our currently owned or subsequently acquired property of the Company, to secure any obligations of the Company. |
There are no provisions in the Company’s by-laws relating to retirement or non-retirement of directors under an age limit requirement. A director need not be a shareholder. At least 25% of directors must be resident Canadians and at least two of the directors must be considered independent.
IV. Annual and Special Meetings
The annual meeting and special meetings of shareholders are held at such time and place as the board of directors, the chairman of the board, the managing director or the president shall determine. Notice of meetings are sent out to shareholders not less than 21 nor more than 50 days before the date of such meeting. All shareholders at the record date are entitled to notice of the meeting and have the right to attend the meeting. Shareholders entitled to vote at an annual or special meeting may do so in person or by proxy. Our directors do not stand for reelection at staggered intervals.
Shareholders may submit to the Company a notice of a proposal to be discussed at the Company’s annual meeting. A proposal for the nomination of the election of directors must be signed by one or more holders of Shares representing, in the aggregate, not less than five per cent of the Shares. The Board may call a special meeting of shareholders at any time. Holders of not less than 5 percent of the Company’s issued Shares that carry the right to vote at a meeting may require the directors to call a special meeting of shareholders for a specified purpose.
IV. Miscellaneous
There are no provisions in either the Company’s Articles of Incorporation or By-laws that would have the effect of delaying, deferring or preventing a change in control of the Company and that would operate only with respect to a merger, acquisition or corporate restructuring involving the Company or its subsidiary. There are no by-law provisions governing the ownership threshold above which shareholder ownership must be disclosed. With respect to the matters discussed in this Item 10B, the law applicable to the Company is not significantly different from United States law.
61
The following is a summary of the material contracts of the Company. The descriptions of the agreements below are qualified, in their entirety, by the agreements, themselves, as set forth in “Item 19. Exhibits.”
Agreements with Maxim Group LLC
On June 27, 2013 we entered into two agreements with Maxim Group LLC (“Maxim”). In the first agreement, Maxim agreed to provide us with (i) general financial advisory services, and (ii) investment banking services (the “Financial Advisory Agreement”). In the second agreement, Maxim agreed to act as lead underwriter in a $20,000,000 follow-up offering to our $6,000,000 Unit Offering, which commenced in August 2013 (the “Follow-Up Offering Agreement”). As of the date of this annual report, no offering has commenced in connection with the Follow-Up Offering Agreement.
Further, on February 25, 2014 we entered into an additional agreement with Maxim appointing Maxim as our exclusive agent in connection with a proposed $10 million private placement of our securities (the “February 25, 2014 Agreement”). This offering was subsequently re-negotiated as a $6 million private placement of our securities, which commenced on May 12, 2014 and closed on September 12, 2014 with a gross proceed of $1,645,000. We have not registered this offering with the SEC as it is exempt from registration.
| (1) | Financial Advisory Agreement |
Under the Financial Advisory Agreement, Maxim is to provide the us with advice related to: (i) our valuation, (ii) our capitalization, (iii) our strategic partnerships, (iv) United States trading and fund-raising, and (v) the performance of various other duties.
The initial term of this agreement is six months, after which time either party may terminate the agreement upon 30 days written notice. Pursuant to the Financial Advisory Agreement, Maxim will received compensation of $10,000 per month. In addition, in the event of a financing, Maxim is entitled to receive compensation of: (i) a cash fee of 8% of the capital raised; (ii) a cash fee of 1% in unallocated expenses; and (ii) warrants to purchase shares up to 9% of the amount of shares underlying the securities sold in the offering. The exercise price of the warrants would be 110% of the price of the securities (or exercise price of warrants issued in the offering), and the warrants will expire five years after they are issued. Further, in the event we engage in a transaction such as a merger, acquisition, joint venture, strategic alliance, sale of assets, or similar transaction, Maxim will be entitled to receive a fee of 3% of the value of such transaction. In addition to the above compensation, we agreed to issue Maxim warrants to purchase 4 million shares, at an exercise price of $0.20 per Share, exercisable for five years. Through March 31, 2014 Maxim has earned a total of $80,000 in compensation pursuant to the Financial Advisory Agreement.
In addition, during the course of this agreement and for twelve month thereafter, in the event of either a public or private offering of our securities Maxim will have the right to be the lead book running manager or exclusive agent for such financing. This agreement is still in full force and effect.
| (2) | Follow-Up Offering Agreement |
Pursuant to this agreement, which expired on August 31, 2014, the Company appointed Maxim to be its exclusive lead managing underwriter and book runner in connection with a proposed public offering of the Company’s securities of up to $20,000,000 of our securities, to commence at some point after the conclusion of our $6,000,000 Unit Offering.
62
The actual size of the offering, the type and number of securities to be offered, and the offering price will be determined at a later time by the Company and Maxim. The agreement provides customary outs for Maxim in case it decides, for any number of reasons, not to proceed with an offering.
Under the Follow-Up Offering Agreement, Maxim would receive an underwriting discount of 9% of the public offering price of the securities in the offering. The Company paid Maxim a $15,000 advance upon execution of the Follow-Up Offering Agreement, to be applied to the underwriting discount. Maxim is entitled to receive an additional $45,000 if and when the Company files a registration statement on either Form S-1 or F-1 with the SEC covering the securities to be offered.
In the event that the Company and Maxim agree to proceed with such an offering of our securities, it is anticipated that we would enter into an underwriting agreement setting forth the terms of the offering and each party’s obligations and responsibilities. Pursuant to the Follow-Up Offering Agreement, we have agreed that any underwriting agreement will provide Maxim an option to acquire up to an additional 15% of the securities offered in such offering, as an over-allotment option.
When such potential offering closes, Maxim will be entitled to receive warrants to purchase shares equal to 5% of the total amount of securities sold in the offering. The warrants will not be exercisable for the first six months after the potential offering closes, and will expire five years after the closing. The exercise price of the warrants will be 110% of the public offering price of the securities sold in the offering.
As of March 31, 2014, we have not initiated an offering that would result in the payment of any amounts to Maxim, or the performance of any services by Maxim, under the Follow-Up Offering Agreement. The Follow-Up Offering Agreement terminated upon its terms on April 30, 2015. We anticipate entering into a similar agreement, upon similar terms, in the near future.
| (3) | February 25, 2014 Agreement |
On February 25, 2014, we entered into a new agreement appointing Maxim as our exclusive agent in connection with a proposed $10 million private placement of our securities. Under the February 25, 2014 Agreement, Maxim is entitled to receive: (i) a cash commission of 9% of the gross proceeds; and (ii) warrants, upon closing of such private placement, covering the number of securities equal to 9% of the total number of securities sold in the offering, exercisable for two years at a price equal to 115% of the price paid by investors in the offering. Upon signing the agreement, we paid Maxim a $15,000 as an advance against its placement fee. The agreement expires on October 31, 2014, after which it can be terminated by either party upon 30 days written notice.
Under the terms of the February 25, 2014 Agreement, we have granted Maxim, for a period of eighteen months from the date of the closing, the right to act as lead managing underwriter and book runner, or minimally, as a co-lead manager and book runner with at least 50% of the economics, or in the case of a three-handed deal, 33% of the economics for all future public and private equity and debt offerings we engage in. Such right, however, is contingent upon the closing of the offering contemplated by this agreement.
Agreement with Healthios Capital Markets, LLC
On September 19, 2013 we entered into an agreement with Healthios Capital Markets, LLC (“Healthios”) pursuant to which Healthios agreed to assist us in raising capital in our $6,000,000 Unit Offering (the “2013 Unit Offering”). Under the terms of the agreement, after a minimum of $3,000,000 was raised in the 2013 Unit Offering, Healthios was granted the non-exclusive right to assist us in any
63
subsequent private offerings of its securities, or transaction such as a merger, acquisition, joint venture, strategic alliance, sale of assets, or similar transaction. The 2013 Unit Offering closed on March 8, 2014. We did not register this offering with the SEC.
The initial term of this agreement was six months, but the agreement could be extended on a month-to-month basis after six months. Under this agreement, Healthios was paid a monthly retainer of $15,000 for the first four months of the agreement, and $5,000 for subsequent months. This agreement was terminated, effective February 27, 2014.
Under this agreement, Healthios was entitled to a cash commission of 6% of the aggregate amount of the funds actually raised. Healthios was also entitled to receive warrants entitling it to purchase securities equal to 5% of the aggregate consideration in the same security and at the same price as the offering. Such warrants would have a term of five years and would be exercisable on a non-cash basis in the event of a change in control of the Company.
In the event we engage in a transaction such as a merger, acquisition, joint venture, strategic alliance, sale of assets, or similar transaction, during the period of the agreement until February 27, 2015, with any entity Healthios introduced to it, or where Healthios rendered any services, Healthios will be entitled to the same fee paid to Maxim for such transaction. If no fee is paid to Maxim, Healthios will be entitled to a fee equal to 3% of the value of such transaction.
Through March 31, 2014, Healthios received a total of $70,000 in compensation from us pursuant to its monthly retainer under this agreement.
Agreements in Connection with Medifocus Holding Joint Ventures
On November 8, 2013 we entered into an agreement with Ideal Concept Group Limited to develop our Prolieve business and products based upon APA technology in a geographic area referred to as Asia Pacific. Medifocus Holding, our joint venture with Ideal Concept Group Limited, was formed as a result of this agreement. Reference is made to “Item 4. Information on the Company” for a description of the terms of this agreement.
License and Distribution Agreement between Medifocus Inc. and Medifocus Holding Limited (BVI). Reference is made to “Item 4. Information on the Company” for a description of the terms of this agreement.
Option Agreements in Connection with Duke University
On May 1st, 2015, the Company entered into an option agreement with Duke University regarding heat-activated and tumor targeted Immunotherapy. The two parties are actively negotiating an exclusive license to the patent rights of a Duke invention for the development of heat-activated and tumor targeted Immunotherapy for treatment of cancer and other diseases. This option to license and develop Duke’s technology runs through December 30, 2015. The technology, described in the agreement as a “method for selective expression of therapeutic genes in cancer cells by hyperthermia,” provides the design basis for an adenoviral gene delivery construct that releases IL-12 upon activation by the temperature rise caused by focused thermotherapy. Temperature activation provides the ability to excise spatial and temporal control over the release of the IL-12 therapeutics, leading to enhanced efficacy and reduced treatment induced toxicity. The patent also provides for the possibility of adding other Cytokines and/or Biological Modifiers in combination with IL-12 within the construct to further enhance efficacy. The technology is expected to provide Medifocus a foundation on which it can build a pipeline of heat activated and tumor targeted genetic and molecular therapeutics for treatment of cancer and other diseases.
64
The above descriptions of our agreements are summaries only. The full agreements are set forth at “Item 19. Exhibits.”
There are no laws, governmental decrees or regulations in Canada that restrict the export or import of capital or which affect the remittance of dividends, interest or other payments to non-resident holders of our shares, other than the withholding tax requirements (Reference is made to “Item 10E.”) and the Proceeds of Crime (Money Laundering) and Terrorist Financing Act. The Proceeds of Crime (Money Laundering) and Terrorist Financing Act requires that persons and entities report the importation or exportation of currency or monetary instruments of a value equal to or greater than $10,000 to Canadian customers officers in the prescribed form and manner.
There are no limitations under the laws of Canada or the Province of Ontario, or in our constituting documents, with respect to the right of non-resident or foreign owners to hold or vote common shares other than those imposed by the Investment Canada Act.
The Investment Canada Act is a federal Canadian statute which regulates the acquisition of control of existing Canadian businesses and the establishment of new Canadian businesses by an individual, government or entity that is a “non-Canadian” as defined in the Investment Canada Act. Such investments are generally reviewable under the Investment Canada Act by the Minister, designated as being responsible for the administration of the Investment Canada Act. Reviewable investments, generally, may not be implemented prior to the Minister’s determining that the investment is likely to be of “net benefit to Canada” based on the criteria set out in the Investment Canada Act. Generally, investments by non-Canadians consisting of the acquisition of control of Canadian businesses which are otherwise non-reviewable and the establishment of new Canadian businesses are subject to certain notification requirements under the Investment Canada Act in the prescribed form and manner.
Management of the Company believes that it is not currently a “non-Canadian” for purposes of the Investment Canada Act and therefore it is not subject to the Act. However, if the Company were to become a “non-Canadian” in the future, acquisitions of control of Canadian businesses by the Company would become subject to the Investment Canada Act. Generally, the direct acquisition by a “non-Canadian” of an existing Canadian business with gross assets of $5 million or more is reviewable under the Investment Canada Act, unless the business is acquired by a WTO investor in which the thresholds for transactions are expected to be CAD $354 million in 2014 (the 2014 threshold is expected to be published in the Canada Gazette in early 2014). Generally, indirect acquisitions of existing Canadian businesses (with gross assets over $50 million) are reviewable under the Investment Canada Act, except in situations involving “WTO investors” where indirect acquisitions are generally not reviewable but are nonetheless subject to notification.
Under the Investment Canada Act, the Minister may order a review of any investment by a non-Canadian, regardless of the size of the interest acquired or the value of the assets involved, where the Minister has reasonable grounds to believe that such an investment could be injurious to national security. No guidelines or other explanatory statements have been issued to provide guidance on the scope of the national security review power.
65
Acquisitions of businesses related to Canada’s cultural heritage or national identity (regardless of the value of assets involved) may also be reviewable under the Investment Canada Act. In addition, investments to establish new, unrelated businesses are not generally reviewable but are nonetheless subject to notification. An investment to establish a new business that is related to the non-Canadian’s existing business in Canada is not subject to notification under the Investment Canada Act unless such investment relates to Canada’s cultural heritage or national identity.
Any proposed take-over of the Company by a “non-Canadian” would likely only be subject to the simple notification requirements of the Investment Canada Act, as in all likelihood that non-Canadian would be a “WTO investor” for purposes of the Investment Canada Act and the Company would not likely exceed the applicable review threshold for a “WTO Investor.” Generally, a “WTO investor” is an individual, other than a Canadian, who is a national of a country that is a member of the World Trade Organization or a business entity controlled by such an individual. Virtually all countries of the Western world are members of the World Trade Organization. A take-over offer from a non-WTO Investor would be reviewable if the net book value of the Company’s assets exceeded $5 million for a direct acquisition, or exceeded $50 million for an indirect acquisition.
10.E.1. Certain Canadian Federal Income Tax Consequences - General
The following is a brief summary of the material Canadian federal income tax consequences to a holder of the Shares (a “Holder”). This summary is applicable only to Holders who are residents of the United States, have never been resident in Canada, deal at arm’s length with the Company, hold their Shares as capital property, and who will not use or hold the Shares in carrying on business in Canada. Special rules, which are not discussed in this summary, may apply to a United States Holder that is an issuer that carries on business in Canada and elsewhere.
This summary is based upon the provisions of the Income Tax Act of Canada and the regulations thereunder (collectively, the “Act”) and that Canada-United States Tax Convention (the “Treaty”) as of the date of this annual report, and the current administrative practices of Canada Customs and Revenue Agency. This summary does not take into account provincial income tax consequences.
This summary is of a general nature only and is not intended to be, and should not be construed to be, legal, business or tax advice to any particular Holder or prospective Holder of Shares, and no opinion or representation with respect to the tax consequences to any Holder or prospective Holder of Shares is made. Accordingly, Holders and prospective Holders of Shares are strongly urged to consult their own tax advisors with respect to the income tax consequences of purchasing, owning and disposing of our common shares in their particular circumstances.
10.E.2. Dividends
Dividends paid on shares of a corporation to a non-resident Holder will be subject under the Act to withholding tax at a standard rate of 25%, subject to a reduction under the provisions of the Treaty, which withholding tax is deducted at source by the Company. Pursuant to the Treaty, the standard withholding tax rate is reduced to 15% on dividends paid on shares of a corporation resident in Canada (such as the Company) to residents of the United States, and also provides for a further reduction of the withholding tax rate to 5% where the beneficial owner of the dividends is a corporation resident in the United States that owns at least 10% of the voting shares of the corporation paying the dividend.
66
10.E.3. Disposition of Common Shares
A Holder who disposes of shares of a corporation, including by deemed disposition on death, will not normally be subject to Canadian tax on any capital gain (or capital loss) thereby realized unless the common share constituted “taxable Canadian property” as defined by the Act. Generally, a common share of a public corporation will not constitute taxable Canadian property of a Holder if the share is listed on a designated stock exchange unless the Holder or persons with whom the Holder did not deal at arm’s length, alone or together, held or held options to acquire, at any time within the five years preceding the disposition, 25% or more of the shares of any class of the capital stock of the Company. The TSXV is a designated stock exchange under the Act.
A Holder who is a resident of the United States and realizes a capital gain on a disposition of a common share that was taxable Canadian property will nevertheless, by virtue of the Treaty, generally be exempt from Canadian tax thereon unless (a) more than 50% of the value of the common shares is derived from, or from an interest inreal or immovable property situated in Canada including Canadian real estate, Canadian timber resource properties, Canadian mineral resource properties, and options in respect of property of the aforementioned (b) the common share formed part of the Business property of a permanent establishment that the Holder has or had in Canada within the 12 month period preceding the disposition, or (c) the Holder is an individual who (i) was a resident of Canada at any time during the 10 years immediately preceding the disposition, and for a total of 120 months during any period of 20 consecutive years, preceding the disposition, and (ii) owned the common share when he ceased to be resident in Canada.
A Holder who is subject to Canadian tax in respect of a capital gain realized on a disposition of a common share must include one-half of the capital gain (taxable capital gain) in computing the Holder’s taxable income earned in Canada. The Holder may, subject to certain limitations, deduct one-half of any capital loss (allowable capital loss) arising on a disposition of taxable Canadian property from taxable capital gains realized in the year of disposition in respect of taxable Canadian property and, to the extent not so deductible, from such taxable capital gains realized in any of the three preceding years or any subsequent year.
10.E.4. United States Taxation
Material U.S. Federal Income Tax Considerations
The following is a discussion of certain material U.S. federal income tax considerations that may be relevant to our shareholders. This discussion is based upon the provisions of the Internal Revenue Code of 1986 (the “Code”), legislative history, applicable U.S. Treasury Regulations promulgated thereunder, judicial authority and administrative interpretations, as of the date of this annual report, all of which are subject to change, possibly with retroactive effect, or are subject to different interpretations. Changes in these authorities may cause the U.S. federal income tax considerations to vary substantially from those described below.
This discussion applies only to beneficial owners of our Shares that own the Shares as “capital assets” (generally, for investment purposes) and does not comment on all aspects of U.S. federal income taxation that may be important to certain shareholders in light of their particular circumstances, such as shareholders subject to special tax rules (e.g., financial institutions, regulated investment companies, real estate investment trusts, insurance companies, traders in securities that have elected the mark-to-market method of accounting for their securities, persons liable for alternative minimum tax, broker-dealers, tax-exempt organizations, or former citizens or long-term residents of the United States) or shareholders that hold our Shares as part of a straddle, hedge, conversion, constructive sale or other integrated transaction for U.S. federal income tax purposes, all of whom may be subject to U.S. federal income tax rules that
67
differ significantly from those summarized below. If a partnership or other entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our Shares, the tax treatment of its partners generally will depend upon the status of the partner and the activities of the partnership. Partners in partnerships holding our Shares should consult their own tax advisors to determine the appropriate tax treatment of the partnership’s ownership of our Shares.
No ruling has been requested from the IRS regarding any matter affecting the Company or its shareholders. Accordingly, statements made herein may not be sustained by a court if contested by the IRS.
This discussion does not address any U.S. estate, gift or alternative minimum tax consideration or tax considerations arising under the laws of any state, local or non-U.S. jurisdiction. Shareholders are urged to consult their own tax advisors regarding the U.S. federal, state, local, non-U.S. and other tax consequences of owning and disposing of our Shares.
U.S. Federal Income Taxation of U.S. Holders
As used herein, the term “U.S. Holder” means a beneficial owner of our Shares that is for U.S. federal income tax purposes: (a) a U.S. citizen or U.S. resident alien (a U.S. Individual Holder); (b) a corporation, or other entity taxable as a corporation that was created or organized under the laws of the United States, any state thereof, or the District of Columbia; (c) an estate whose income is subject to U.S. federal income taxation regardless of its source; or (d) a trust that either is subject to the supervision of a court within the United States and has one or more U.S. persons with authority to control all of its substantial decisions or has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
Distributions
Any distributions made by us to a U.S. Holder generally will constitute dividends, which may be taxable as ordinary income or “qualified dividend income” as described in more detail in the paragraph below, to the extent of our current and accumulated earnings and profits allocated to the U.S. Holder’s shares, as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits allocated to the U.S. Holder’s Shares will be treated first as a nontaxable return of capital to the extent of the U.S. Holder’s tax basis in our Shares and thereafter as capital gain, which will be either long-term or short-term capital gain depending upon whether the U.S. Holder has held the Shares for more than one year. U.S. Holders that are corporations generally will not be entitled to claim a “dividends received” deduction with respect to any distributions they receive from us. For purposes of computing allowable foreign tax credits for U.S. federal income tax purposes, dividends received with respect to our Shares will be treated as foreign source income and, generally, will be treated as “passive category income,” or in the case of certain types of U.S. Holders, “general category income.”
Under current law, subject to holding-period requirements and certain other limitations, dividends received with respect to our publicly traded shares by a U.S. Holder who is an individual, trust or estate (a Non-Corporate U.S. Holder generally will be treated as qualified dividend income that is taxable to such Non-Corporate U.S. Holder at preferential capital gain tax rates.
Sale, Exchange or Other Disposition of Our Shares
A U.S. Holder generally will recognize capital gain or loss upon a sale, exchange or other disposition of our Shares in an amount equal to the difference between the amount realized by the U.S. Holder from such sale, exchange or other disposition and the U.S. Holder’s tax basis in such Shares.
68
Gain or loss recognized upon a sale, exchange or other disposition of our Shares generally will be (a) treated as long-term capital gain or loss if the U.S. Holder’s holding period is greater than one year at the time of the sale, exchange or other disposition, or short-term capital gain or loss otherwise, and (b) treated as U.S. source income or loss, as applicable, for foreign tax credit purposes. Non-Corporate U.S. Holders may be eligible for preferential rates of U.S. federal income tax in respect of long-term capital gains. A U.S. Holder’s ability to deduct capital losses is subject to certain limitations.
Consequences of Possible CFC Classification
If CFC Shareholders (generally, U.S. Holders who each own, directly, indirectly or constructively, 10% or more of the total combined voting power of all classes of our outstanding Shares entitled to vote) own directly, indirectly or constructively more than 50% of either the total combined voting power of all classes of our outstanding Shares entitled to vote or the total value of all of our outstanding Shares, we generally would be treated as a controlled foreign corporation, or a CFC. Certain disclosure requirement apply to CFC Shareholders, whether or not we are a CFC. Investors are urged to consult with their own tax advisors regarding the possible application of these disclosure requirements to their investment in our Shares.
CFC Shareholders are treated as receiving current distributions of their respective share of certain income of the CFC and earnings invested in U.S. property during the year without regard to any actual distributions. In addition, CFC Shareholders are subject to certain burdensome U.S. federal income tax and administrative requirements. In addition, a person who is or has been a CFC Shareholder may be taxed at ordinary rates on all or a portion of the Shareholders income from disposition of shares of the CFC. U.S. persons who may, individually or together with a statutorily related person, obtain a substantial interest in us should consider the potential implications of being treated as a CFC Shareholder.
The U.S. federal income tax consequences to U.S. Holders who are not CFC Shareholders would not change in the event we become a CFC in the future.
PFIC Status and Significant Tax Consequences
Special and adverse U.S. federal income tax rules apply to a U.S. Holder that holds stock in a non-U.S. entity treated as a corporation and classified as a PFIC for U.S. federal income tax purposes. In general, we will be treated as a PFIC for any taxable year in which either (a) at least 75% of our gross income consists of passive income and (b) at least 50% of the average value of our assets is attributable to assets that produce passive income, or are held for the production of passive income. For purpose of these tests, passive income includes dividends, interest, gains from the sale or exchange of investment property and rents and royalties (subject to certain exclusions including the exclusion for rents and royalties derived in connection with the active conduct of a trade or business) but does not include income derived from the performance of services. Based on the current composition of our assets and operations (and that of our subsidiaries), we intend to take the position that we are not now and have never been a PFIC. Further, although we intend to conduct our affairs in a manner to avoid being classified as a PFIC with respect to any taxable year, there can be no assurance that the nature of our operations, and therefore the composition of our income and assets, will remain the same in the future. Moreover, the market value of our stock may be treated as reflecting the value of our assets at any given time. Therefore, a decline in the market value of our stock (which is not within our control) may impact the determination of whether we are a PFIC. Because our status as a PFIC for any taxable year will not be determinable until after the end of the taxable year, there can be no assurance that we will not be considered a PFIC for any future taxable year.
69
If we were to be treated as a PFIC for any taxable year a U.S. Holder may be subject to special rules resulting in increased tax liability and may also be subject to certain filing requirements.
U.S. Holders are strongly urged to consult their own tax advisors regarding the PFIC rules, including the PFIC annual reporting requirements, as well as applicability, availability and advisability of, and procedure for, making available elections with respect to us, and the U.S. federal income tax consequences of making such elections.
U.S. Return Disclosure Requirements for U.S. Individual Holders
U.S. Individual Holders that hold certain specified foreign financial assets, including stock in a foreign corporation that is not held in an account maintained by a financial institution, with an aggregate value in excess of certain thresholds that vary depending on the individual’s tax status and residency may be required to report such assets on IRS Form 8938 with their tax return for that taxable year. Penalties apply for failure to properly complete and file Form 8938. Investors are encouraged to consult with their own tax advisors regarding the possible application of this disclosure requirement to their investment in our Shares. The IRS anticipates issuing regulations that will require certain domestic entities to file Form 8958. Investors are urged to consult their own tax advisors regarding possible application of these requirements to their investment in our Shares in the future.
U.S. Federal Income Taxation of Non-U.S. Holders
A beneficial owner of our Shares (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that is not a U.S. Holder is referred to herein as a non-U.S. Holder.
Distributions
In general, a non-U.S. Holder is not subject to U.S. federal income tax on distributions received from us with respect to our Shares unless the distributions are effectively connected with the non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment that the non- U.S. Holder maintains in the United States). If a non-U.S. Holder is engaged in a U.S. trade or business and the distribution is deemed to be effectively connected to that trade or business, the non-U.S. Holder generally will be subject to U.S. federal income tax on that distribution in the same manner as if it were a U.S. Holder.
Sale, Exchange or Other Disposition of Our Shares
In general, a non-U.S. Holder is not subject to U.S. federal income tax on any gain resulting from the disposition of our Shares unless (a) such gain is effectively connected with the non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment that the non-U.S. Holder maintains in the United States) or (b) the non-U.S. Holder is an individual who is present in the United States for 183 days or more during the taxable year in which those Shares are disposed of (and certain other requirements are met). If a non-U.S. Holder is engaged in a U.S. trade or business and the disposition of Shares is deemed to be effectively connected to that trade or business, the non-U.S. Holder generally will be subject to U.S. federal income tax on the resulting gain in the same manner as if it were a U.S. Holder.
70
Medicare Tax on Unearned Income
Certain Non-Corporate U.S. Holders, including certain beneficiaries of foreign estates and trusts, are subject to a 3.8% tax on certain investment income, including dividends and gain from the sale or other disposition of our Shares.
Information Reporting and Backup Withholding
In general, payments of distributions or the proceeds of a disposition of our Shares to a Non-Corporate U.S. Holder will be subject to information reporting requirements. These payments to a Non-Corporate U.S. Holder also may be subject to backup withholding if the U.S. Holder:
| | • | | fails to timely provide an accurate taxpayer identification number; |
| | • | | is notified by the IRS that it has failed to report all interest or distributions required to be shown on its U.S. federal income tax returns; or |
| | • | | in certain circumstances, fails to comply with applicable certification requirements |
Non-U.S. Holders may be required to establish their exemption from information reporting and backup withholding on payments made to them within the United States by certifying their status on an IRS Form W-8BEN, W-8ECI, or W-8IMY, as applicable.
Backup withholding is not an additional tax. Rather, a holder generally may obtain a credit for any amount withheld against its liability for U.S. federal income tax (and obtain a refund of any amounts withheld in excess of such liability) by accurately completing and timely filing a U.S. federal income tax return with the IRS.
| F. | Dividends and paying agents. |
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
We are filing this Form as an annual report under the Exchange Act and, as such, there is no requirement to provide any information under this item.
Any statement in this annual report about any of our contracts or other documents is not necessarily complete. If the contract or document is filed as an exhibit to this annual report or is incorporated by reference, the contract or document is deemed to modify our description. You must review the exhibits themselves for a complete description of any contract or document.
You may request a copy free of charge by mail to 10240 Old Columbia Road, Suite G, Columbia, Maryland 21046, or by telephone at 410-290-5734.
You may also review a copy of our filings with the SEC, including exhibits and schedules filed with or incorporated by reference in this annual report and future filings with the SEC, at the SEC’s public reference facilities in Room 1580, 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of such materials from the Public Reference Section of the SEC, Room 1580, 100 F Street, N.E., Washington, D.C. 20549, at prescribed rates. You may call the SEC at 1-800-SEC-0330 for further information on the public reference rooms. The SEC maintains a website (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
71
You may read and copy any reports, statements or other information that we file with the SEC at the addresses indicated above and you may also access some of them electronically at the website set forth above. These SEC filings are also available to the public from commercial document retrieval services.
| I. | Subsidiary information. |
Not applicable.
| Item 11. | Quantitative and Qualitative Disclosures About Market Risk. |
We are exposed to various market risks including interest rate risks and equity price risks, which may affect our results of operations and financial condition and, consequently, the value of our company. We currently do not hedge interest rate exposure or foreign currency exchange exposure. We have not used derivative financial instruments for speculation or trading purposes.
Interest Rate Risk
Because of the short-term maturities of our cash and cash equivalents, we do not believe that an increase in market interest rates would have a significant impact on their realized value. The interest rates on our various outstanding debt instruments, including promissory and convertible notes, are fixed. Because of the fixed rates, a change in market interest rates would not have a material impact on interest expense associated with the debt.
72
Equity Price Risk
Historically, the Company has issued equity securities, and equity-linked securities such convertible debt, stock purchase warrants and stock options, to investors, employees and vendors. Equity and equity-linked securities are initially recorded in our financial statements at their fair values, and depending on the nature of the security may require periodic remeasurement at fair value. Changes in the market price of our common stock can have an impact on the value of the securities issued which could have a direct impact on those fair values, earnings, and cash flow.
| Item 12. | Description of Securities Other than Equity Securities. |
Not applicable.
PART II
| Item 13. | Defaults, Dividend Arrearages and Delinquencies. |
As noted below, the Company is not in compliance with the provisions of outstanding debt agreements, and it has not remitted quarterly royalty payments to Boston Scientific Corporation pursuant to the terms of its purchase agreement for Prolieve. The Company has not paid interest owing to certain holders of the convertible debentures, and is in a technical default of the terms of the debentures. Management is continuing its efforts to obtain additional funds through equity financing and through the negotiation of debt agreements to ensure that the Company can meet its obligations and sustain operations.
Convertible Notes
In fiscal 2014, the Company issued 554 Units of 8% Redeemable Promissory Convertible Notes (the “Notes”) together with Series C Stock Purchase Warrants (the “Warrants”) to various accredited investors in an offering exempt from registration in the U.S pursuant to regulations D and S under the U.S. Federal Securities rules and regulations, receiving gross proceeds of $5,540,000. The Company has not paid $378,291 interest owing to certain holders of the convertible debentures, of which $307,491 is past due, and is in a technical default of the terms of the debentures. The investors have not accelerated the terms of the debenture.
Note Payable
In fiscal 2013, the Company raised bridge financing of CAD550,000. The bridge financing lender received a promissory note, with interest is payable at 2% per month after October 23, 2012. The original maturity date of the promissory notes was October 23, 2013 and was subsequently extended until June 30, 2014. As at March 31, 2015, the note remains matured and the Company is currently in discussions with the lender on a further extension of the maturity date. The company made principle payments of approximately $178,000 during the year ended March 31, 2015.
Boston Scientific Corporation
On July 24, 2012 the Company purchased from Boston Scientific Corporation (“BSC”), in a taxable transaction, all of the assets, relating to the Prolieve Thermodilatation System (“Prolieve”), a FDA approved device for the treatment of Benign Prostatic Hyperplasia (“BPH”). The maximum amount of $2,500,000 is payable as contingent consideration pursuant to the terms of the agreement. The contingent consideration is payable quarterly at a rate of 10% of sales of Prolieve products. As of March 31, 2015, $831,632 of royalties is due to Boston Scientific Corporation. Of this amount, approximately $728,632 is past due.
73
| Item 14. | Material Modifications to the Rights of Security Holders and Use of Proceeds. |
None.
| Item 15. | Controls and Procedures. |
| A. | Disclosure Controls and Procedures. |
Disclosure controls and procedures are defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act to mean controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and includes, without limitation, controls and procedures designed to ensure that such information is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
As required under the Exchange Act, we have carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer, of the effectiveness of our disclosure controls and procedures as of March 31, 2015. Based on such evaluation, we have concluded that, as of such date, our disclosure controls and procedures were not effective to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in applicable SEC rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer, as appropriate, to allow timely discussions regarding required disclosure.
| B. | Management’s Annual Report on Internal Control Over Financial Reporting. |
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the company’s registered public accounting firm due to a transition period established by the rules of the Securities and Exchange Commission for newly public companies.
| C. | Attestation Report of the Registered Public Accounting Firm. |
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the company’s registered public accounting firm due to a transition period established by the rules of the Securities and Exchange Commission for newly public companies.
| D. | Changes in Internal Control Over Financial Reporting. |
Our management has evaluated, with the participation of our Chief Executive Officer, changes in our internal controls over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) during the year ended March 31, 2015. In connection with such evaluation, there have been no changes to our internal control over financial reporting that occurred during the year ended March 31, 2015 that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting. While there have been no significant changes, we have assessed our internal controls as being deficient and will be taking steps during fiscal 2016 to remedy such deficiencies.
74
| Item 16A. | Audit Committee Financial Expert. |
The Board determined that Mr. Joseph S. C. Chan is an “audit committee financial expert” as defined in Item 16A of Form 20-F under the Exchange Act, and that Mr. Chan is independent.
The Company has not yet adopted a code of ethics but is evaluating its internal procedures to determine the necessity and contents of such a code of ethics. In the event that it is determined that a code of ethics is necessary, an appropriate code will be implemented.
| Item 16C. | Principal Accountant Fees and Services. |
The following summarizes the total fees billed by our external auditors for each of the years ended March 31, 2015 and March 31, 2014. All dollar amounts are exclusive of applicable taxes.
| | | | | | | | |
| | | 2015 | | | 2014 | |
Audit Fees | | $ | 52,500 | | | $ | 42,500 | |
Audit-Related Fees | | $ | 3,750 | | | $ | 2,500 | |
Tax Fees | | $ | 7,500 | | | | — | |
All Other Fees | | | — | | | | — | |
Audit Fees
This category includes the aggregate fees billed by our independent auditor for the audit of our consolidated annual financial statements, reviews of interim financial statements and attestation services that are provided in connection with statutory and regulatory filings or engagements.
Audit Related Fees
This category includes the aggregate fees billed in each of the last two fiscal years for assurance and related services by the independent auditors that are reasonably related to the performance of the audits or reviews of the financial statements and are not reported above under “Audit Fees,” and generally consist of fees for other engagements under professional auditing standards, accounting and reporting consultations,
Tax Fees
This category includes the aggregate fees billed in each of the last two fiscal years for professional services rendered by the independent auditors for tax compliance, tax planning and tax advice.
All Other Fees
This category includes the aggregate fees billed in each of the last two fiscal years for products and services rendered by the independent auditors, other than the services reported above.
75
Policy on Pre-Approval by Audit Committee of Services Performed by Independent Auditors
We do not currently have a pre-approval policy regarding the services performed by our independent auditors. Rather, our shareholders appoint our independent auditors at our Annual Meeting of Shareholders, and authorize our directors to fix the auditor’s remuneration. At our Annual Meeting of Shareholders held in January 2015, our shareholders appointed Stegman & Company (“Stegman”) as our independent auditor, and authorized our directors to fix Stegman’s remuneration. At our Annual Meeting of Shareholders held in January 2014, our shareholders appointed Sievert & Sawrantschuk, LLP as our independent auditors.
We intend on developing and implementing a pre-approval policy with respect to the services performed by independent auditors in the near future.
| Item 16D. | Exemptions from the Listing Standards for Audit Committees. |
Not applicable.
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers. |
The Company did not purchase any of its common shares during the financial year ended March 31, 2015.
| Item 16F. | Change in Registrant’s Certifying Accountant. |
The Company previously engaged the accounting firm of S&W LLP (formerly Sievert & Sawrantschuk, LLP, Chartered Accountants) to audit the Company’s financial statements for purposes of filing such financial statements in Canada. S&W LLP is not registered with the Public Company Accounting Oversight Board (“PCAOB”) and, as a result, we would not be able to include financial statements audited by S&W LLP in our Registration Statement on Form 20-F, filed. Accordingly, on May 31, 2013, the Company appointed a PCAOB registered auditing firm as its auditing firm in connection with the audit of financial statements filed in our Registration Statement on Form 20-F filed on September 17, 2014.
Concurrently, we engaged Stegman & Company (“Stegman”) in connection with the preparation of our audited financial statements for the years ended March 31, 2014, March 31, 2013, and March 31 2012, to be included in the Registration Statement. The change in auditors was discussed between the Company’s senior management and Board of Directors, was approved by the Company’s senior management and ratified by the Audit Committee of the Board of Directors.
During the past three fiscal years and through the present time, there have been no disagreements with either S&W LLP or Stegman on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure. There has been no adverse opinion or a disclaimer of opinion, or qualification or modification as to uncertainty, audit scope, or accounting principles in our principal accountant’s report on the Company’s financial statements for either of the past three years, except as described in the audit report dated July 29, 2014 in the March 31, 2014 audited financial statements and a possible change to the accounting of the convertible debt instrument to conform with the IFRS treatment of a convertible instrument denominated in a currency other than its functional currency. The direct impact of this potential restatement for the March 31, 2014 financial statements has not been determined at this time.
76
We furnished S&W LLP with a copy of the disclosures made in this Item 16F, and we have requested that S&W LLP furnish us with a letter addressed to the SEC stating whether it agrees with the above statements and, if not, to state in the letter the respects in which it does not agree. A copy of this letter is included herewith as Exhibit 15.2.
| Item 16G. | Corporate Governance. |
Not applicable.
| Item 16H. | Mine Safety Disclosure. |
Not applicable.
PART III
| Item 17. | Financial Statements. |
See “Item 18. Financial Statements.”
| Item 18. | Financial Statements. |
The following financial statements have been filed as part of this annual report.
| | • | | Report of Independent Registered Public Accounting Firm |
| | • | | Consolidated Balance Sheets of the Company for the years ended March 31, 2015 and 2014 |
| | • | | Consolidated Statements of Operations and Comprehensive Loss for the years ended March 31, 2015, 2014 and 2013 |
| | • | | Consolidated Statements of Changes in Stockholders’ Equity for the years ended March 31, 2015, 2014 and 2013 |
| | • | | Consolidated Statements of Cash Flows for the years ended March 31, 2015, 2014, and 2013 |
| | • | | Notes to the Consolidated Financial Statements |
77
| (a) | The following documents are filed as part of this annual report. |
| | |
Exhibit
No. | | Exhibit |
| |
| 1.1* | | Certificate of Incorporation of Medifocus Inc. |
| |
| 1.2* | | Articles of Amendment to Certificate of Incorporation of Medifocus Inc. |
| |
| 1.3* | | By-Law No. One of Medifocus Inc. |
| |
| 1.4* | | By-Law No. Two of Medifocus Inc. |
| |
| 2.1* | | Form of Common Stock Certificate |
| |
| 4.1* | | Asset Purchase Agreement dated as of June 25, 2012 between Boston Scientific Corporation, Affiliates of Boston Scientific Corporation, and Medifocus Inc. |
| |
| 4.2* | | Amendment No. 1, dated July 24, 2012, to Asset Purchase Agreement between Boston Scientific Corporation and Medifocus Inc |
| |
| 4.3* | | Bill of Sale and Assignment, dated July 24, 2012, between Boston Scientific Corporation and Medifocus Inc. |
| |
| 4.4* | | Transition Services Agreement, dated July 24, 2012, between Boston Scientific Corporation and Medifocus Inc. |
| |
| 4.5* | | Non-Exclusive License Agreement, dated July 24, 2012, between Medifocus Inc. and Boston Scientific Corporation (Buyer Out-License Agreement) |
| |
| 4.6* | | Non-Exclusive License Agreement, dated July 24, 2012, between Medifocus Inc. and Boston Scientific Corporation (Seller Out-License Agreement) |
| |
| 4.7* | | Patent Assignment dated July 24, 2012 between Boston Scientific Corporation, Boston Scientific Scimed, Inc., and Boston Scientific Limited (collectively, the “Assignors”) and Medifocus Inc. (“Assignee”) |
| |
| 4.8* | | Trademark Assignment dated July 24, 2012 between Boston Scientific Scimed, Inc. (“Assignor”) and Medifocus Inc. (“Assignee”) |
| |
| 4.9* | | Assumption Agreement dated July 24, 2012 between Boston Scientific Corporation and Medifocus Inc. |
| |
| 4.10* | | Patent License Agreement dated October 24, 1997 between Massachusetts Institute of Technology and Cheung Laboratories, Inc. |
| |
| 4.11* | | First Amendment to Patent License Agreement, effective May 23, 2002, between Massachusetts Institute of Technology and Celsion Corporation |
| |
| 4.12* | | Second Amendment to Patent License Agreement, effective March 7, 2005, between Massachusetts Institute of Technology and Celsion Corporation |
| |
| 4.13* | | Third Amendment to Patent License Agreement, effective June 16, 2007, between Massachusetts Institute of Technology and Celsion (Canada) Limited |
| |
| 4.14* | | Fourth Amendment to Patent License Agreement, effective June 1, 2009, between Massachusetts Institute of Technology and Medifocus Inc. |
| |
| 4.15* | | Fifth Amendment to Patent License Agreement, effective March 29, 2013, between Massachusetts Institute of Technology and Medifocus Inc. |
78
| | |
| |
| 4.16* | | Sixth Amendment to Patent License Agreement, effective July 15, 2013, between Massachusetts Institute of Technology and Medifocus Inc. |
| |
| 4.17* | | Agreement dated January 16, 2006 between Celsion USA, Celsion Canada, and Dr. Augustine Cheung |
| |
| 4.18* | | Agreement dated November 8, 2013 between Medifocus Inc. and Ideal Concept Group Limited |
| |
| 4.19* | | License and Distribution Agreement dated as of November 8, 2013, between Medifocus Inc. and Medifocus Holding Limited (BVI) |
| |
| 4.20* | | Letter Agreement dated June 27, 2013 between Medifocus Inc. and Maxim Group LLC |
| |
| 4.21* | | Engagement Letter dated June 27, 2013 between Medifocus Inc. and Maxim Group LLC |
| |
| 4.22* | | Advisory Services Agreement, dated September 19, 2013, between Healthios Capital Markets LLC and Medifocus Inc. |
| |
| 4.23* | | Letter Agreement dated January 5, 2014 between Medifocus Inc. and Ernie Eves (regarding Mr. Eves’ resignation from the Medifocus Inc. Board of Directors) |
| |
| 4.24* | | Letter Agreement dated February 25, 2014, between Medifocus, Inc. and Maxim Group LLC |
| |
| 4.25* | | Stock Option Plan (Approved by the Company’s shareholders on January 16, 2014) |
| |
| 4.26** | | Medical Product Manufacturing Services Agreement, dated March 11, 2004, between Celsion Corporation and VENUSA Corporation, as subsequently assigned by Celsion Corporation to the Company. |
| |
| 4.27# | | Option Agreement, effective May 1, 2015, between Medifocus Inc. and Duke University |
| |
| 8.1* | | List of wholly-owned subsidiaries |
| |
| 12.1# | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 12.2# | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.1# | | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.2# | | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 15.1# | | Consent of Stegman & Company, Certified Public Accountants |
| |
| 15.2# | | Concurrence Letter from Sievert & Sawrantschuk LLP to the Securities and Exchange Commission, dated July 8, 2015 |
| * | Previously filed with the Company’s Registration Statement on Form 20-F, filed with the Securities and Exchange Commission on September 17, 2014 |
| ** | Previously filed with Amendment No. 2 to the Company’s Registration Statement on Form 20-F/A, filed with the Securities and Exchange Commission on November 14, 2014. |
| (b) | Financial Statement Schedules |
None.
79
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | |
| MEDIFOCUS INC. |
| |
| | /s/ Augustine Cheung, Ph.D. |
| | Augustine Cheung, Ph.D. |
| | President and Chief Executive Officer |
Date: July 16, 2015
80
MEDIFOCUS, INC.
CONSOLIDATED BALANCE SHEETS
(in U.S. dollars)
| | | | | | | | |
| | | March 31, | |
| | | 2015 | | | 2014 | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 1,312,479 | | | $ | 1,292,509 | |
Accounts receivable, net | | | 1,057,705 | | | | 2,029,396 | |
Inventory, net | | | 79,604 | | | | 518,763 | |
Deferred financing costs | | | 191,228 | | | | 184,430 | |
Other assets | | | 38,800 | | | | 31,550 | |
| | | | | | | | |
Total Current Assets | | | 2,679,816 | | | | 4,056,648 | |
| | | | | | | | |
Inventory, net | | | 190,276 | | | | 190,276 | |
Property and equipment, net | | | 469,035 | | | | 543,429 | |
Deferred financing costs, long term | | | 91,698 | | | | 282,926 | |
Deposits | | | 221,330 | | | | 242,307 | |
Intangible assets, net | | | 1,766,332 | | | | 2,012,544 | |
| | | | | | | | |
Total Assets | | $ | 5,418,487 | | | $ | 7,328,130 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 606,527 | | | $ | 1,016,255 | |
Accrued expenses | | | 568,919 | | | | 375,869 | |
Accrued interest payable | | | 567,955 | | | | 187,572 | |
Promissory notes payable | | | 282,303 | | | | 401,714 | |
Payable to Boston Scientific Corporation | | | 831,632 | | | | 409,861 | |
Contingent consideration, current portion | | | 440,663 | | | | 408,531 | |
Convertible notes payable (net of discount), current portion | | | 4,426,984 | | | | — | |
| | | | | | | | |
Total Current Liabilities | | | 7,724,983 | | | | 2,799,802 | |
| | | | | | | | |
Convertible notes payable (net of discount) | | | — | | | | 3,898,574 | |
Contingent consideration | | | 590,516 | | | | 764,123 | |
| | | | | | | | |
Total liabilities | | | 8,315,499 | | | | 7,462,499 | |
| | | | | | | | |
Commitments and contingencies (Note 8) | | | | | | | | |
Stockholders’ deficit: | | | | | | | | |
Common stock (no par value, unlimited shares authorized, 127,542,120 and 117,260,870 shares issued and outstanding in 2015 and 2014) | | | 12,782,563 | | | | 12,372,498 | |
Common stock issuable | | | 1,561,000 | | | | — | |
Additional paid-in capital | | | 9,659,740 | | | | 8,625,531 | |
Accumulated other comprehensive loss | | | — | | | | (203,553 | ) |
Accumulated deficit | | | (26,900,315 | ) | | | (20,928,845 | ) |
| | | | | | | | |
Total Stockholders’ Deficit | | | (2,897,012 | ) | | | (134,369 | ) |
| | | | | | | | |
Total Liabilities and Stockholders’ Deficit | | $ | 5,418,487 | | | $ | 7,328,130 | |
| | | | | | | | |
See accompanying notes to consolidated financial statements.
F-1
MEDIFOCUS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in U.S. dollars)
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2015 | | | 2014 | | | 2013 | |
Sales | | | | | | | | | | | | |
Products | | $ | 1,212,224 | | | $ | 2,704,593 | | | $ | 1,188,288 | |
Services | | | 3,007,235 | | | | 2,411,913 | | | | 612,083 | |
| | | | | | | | | | | | |
Total Sales | | | 4,219,459 | | | | 5,116,506 | | | | 1,800,371 | |
| | | | | | | | | | | | |
Costs of Sales | | | | | | | | | | | | |
Products | | | 665,382 | | | | 1,167,493 | | | | 1,057,171 | |
Services | | | 2,990,089 | | | | 2,381,605 | | | | 855,737 | |
| | | | | | | | | | | | |
Total Costs of Sales | | | 3,655,471 | | | | 3,549,098 | | | | 1,912,908 | |
| | | | | | | | | | | | |
Gross Profit (Deficit) | | | 563,988 | | | | 1,567,408 | | | | (112,537 | ) |
| | | | | | | | | | | | |
Operating Expenses | | | | | | | | | | | | |
Research and development | | | 399,212 | | | | 472,810 | | | | 411,045 | |
Sales and marketing | | | 1,549,460 | | | | 2,122,203 | | | | 2,407,723 | |
General and administrative | | | 2,703,301 | | | | 3,859,202 | | | | 2,402,210 | |
| | | | | | | | | | | | |
Total Operating Expenses | | | 4,651,973 | | | | 6,454,215 | | | | 5,220,978 | |
| | | | | | | | | | | | |
Loss from Operations | | | (4,087,985 | ) | | | (4,886,807 | ) | | | (5,333,515 | ) |
| | | | | | | | | | | | |
Other Income (Expense) | | | | | | | | | | | | |
Net loss from equity method investment | | | (55,735 | ) | | | (159,000 | ) | | | — | |
Loss on elimination of translation adjustment | | | (203,553 | ) | | | — | | | | — | |
Foreign exchange gain (loss) | | | 21,366 | | | | (24,398 | ) | | | (135,044 | ) |
Loss from change in fair value of contingent consideration | | | (280,296 | ) | | | (705,355 | ) | | | — | |
Interest and discount accretion | | | (1,365,267 | ) | | | (217,337 | ) | | | (377,964 | ) |
| | | | | | | | | | | | |
Total Other Income (Expense) | | | (1,883,485 | ) | | | (1,106,090 | ) | | | (513,008 | ) |
| | | | | | | | | | | | |
Net Loss | | | (5,971,470 | ) | | | (5,992,897 | ) | | | (5,846,523 | ) |
Other Comprehensive Income (Loss) | | | 203,553 | | | | (116,524 | ) | | | (66,366 | ) |
| | | | | | | | | | | | |
Net Comprehensive Loss | | $ | (5,767,917 | ) | | $ | (6,109,421 | ) | | $ | (5,912,889 | ) |
| | | | | | | | | | | | |
Net Loss per share basic and diluted | | $ | (0.05 | ) | | $ | (0.05 | ) | | $ | (0.07 | ) |
| | | | | | | | | | | | |
Weighted average common shares outstanding—basic and diluted | | | 122,809,928 | | | | 117,260,870 | | | | 84,042,487 | |
| | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
F-2
MEDIFOCUS, INC.
CONSOLIDATED STATEMENTS CASH FLOWS
(in U.S. dollars)
| | | | | | | | | | | | |
| | | Year ended March 31, | |
| | | 2015 | | | 2014 | | | 2013 | |
Net Loss | | $ | (5,971,470 | ) | | $ | (5,992,897 | ) | | $ | (5,846,523 | ) |
Adjustments to reconcile net loss to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 360,886 | | | | 357,697 | | | | 261,716 | |
Loss on fixed asset disposal | | | 4,374 | | | | — | | | | — | |
Loss on elimination of translation adjustment | | | 203,553 | | | | — | | | | — | |
Stock based compensation | | | — | | | | 94,801 | | | | 859,614 | |
Accretion of deferred financing costs and debt discount | | | 712,841 | | | | 122,251 | | | | 67,380 | |
Loss on change in fair value of contingent consideration | | | 280,296 | | | | 705,355 | | | | — | |
Future shares issuance for compensation | | | — | | | | — | | | | 271,655 | |
Losses from equity method investment | | | 55,735 | | | | 159,000 | | | | — | |
Provisions for bad debts and warranties | | | 71,367 | | | | 46,000 | | | | 20,000 | |
Changes in operating assets and liabilities, net of amounts acquired | | | | | | | | | | | | |
Decrease/(Increase) in accounts receivable | | | 844,589 | | | | (1,334,881 | ) | | | (510,798 | ) |
Decrease/(Increase) in inventory | | | 439,159 | | | | (146,124 | ) | | | (109,090 | ) |
(Increase)/Decrease in other current assets | | | (7,250 | ) | | | (6,824 | ) | | | 107,461 | |
Decrease/(Increase) in deposits | | | 20,977 | | | | 57,693 | | | | (300,000 | ) |
Decrease/(Increase) in deferred financing costs | | | — | | | | (467,357 | ) | | | — | |
(Decrease)/Increase in accounts payable | | | (409,730 | ) | | | 489,371 | | | | (1,260,236 | ) |
(Decrease)/Increase in other accrued expenses | | | 193,050 | | | | 374,516 | | | | (1,094,141 | ) |
(Decrease)/Increase in other accrued interest | | | 380,383 | | | | 79,408 | | | | 51,126 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (2,821,240 | ) | | | (5,461,991 | ) | | | (7,481,836 | ) |
| | | | | | | | | | | | |
INVESTING ACTIVITIES: | | | | | | | | | | | | |
Cash paid for business acquisition | | | — | | | | — | | | | (2,535,610 | ) |
Payment of contingent consideration | | | — | | | | (309,133 | ) | | | — | |
Investment in joint venture | | | — | | | | (159,000 | ) | | | — | |
Purchases of property and equipment | | | (44,653 | ) | | | (16,059 | ) | | | (30,486 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (44,653 | ) | | | (484,192 | ) | | | (2,566,096 | ) |
| | | | | | | | | | | | |
FINANCING ACTIVITIES: | | | | | | | | | | | | |
Proceeds from sale of common stock, net of issuance costs | | | 1,444,274 | | | | — | | | | 11,337,902 | |
Private placement for common stock issuable | | | 1,561,000 | | | | — | | | | — | |
Proceeds from note payable | | | — | | | | 5,540,000 | | | | 548,295 | |
Principal repayments on note payable | | | (178,488 | ) | | | — | | | | (259,345 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 2,826,786 | | | | 5,540,000 | | | | 11,626,852 | |
Effect of exchange rate changes on cash and cash equivalents | | | 59,077 | | | | (27,330 | ) | | | 86,213 | |
| | | | | | | | | | | | |
INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | | | 19,970 | | | | (433,513 | ) | | | 1,665,133 | |
CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR | | | 1,292,509 | | | | 1,726,022 | | | | 60,889 | |
| | | | | | | | | | | | |
CASH AND CASH EQUIVALENTS, END OF YEAR | | $ | 1,312,479 | | | $ | 1,292,509 | | | $ | 1,726,022 | |
| | | | | | | | | | | | |
Cash paid for: | | | | | | | | | | | | |
Interest | | $ | 185,964 | | | $ | 132,751 | | | $ | 245,816 | |
Income taxes | | | — | | | | — | | | | — | |
See accompanying notes to consolidated financial statements.
F-3
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(in U.S. dollars)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock Shares | | | Common Stock Amount | | | Common Stock Shares Issuable | | | Common Stock Issuable | | | Additional Paid-in Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income (Loss) | | | Total Stockholders’ Equity (Deficit) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Balance at April 1, 2012 | | | 34,218,512 | | | $ | 4,774,837 | | | | — | | | $ | 769,231 | | | $ | 1,012,248 | | | $ | (9,089,425 | ) | | $ | (20,663 | ) | | $ | (2,553,772 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 859,614 | | | | — | | | | — | | | | 859,614 | |
Issuance of common shares in private placement | | | 75,821,055 | | | | 6,501,023 | | | | — | | | | — | | | | 4,678,302 | | | | — | | | | — | | | | 11,179,325 | |
Shares issued as part of debt extinguishment | | | 4,755,545 | | | | 837,229 | | | | — | | | | (719,387 | ) | | | — | | | | — | | | | — | | | | 117,842 | |
Extension of warrants | | | — | | | | (26,162 | ) | | | — | | | | — | | | | 26,162 | | | | — | | | | — | | | | — | |
Cancellation of shares to be issued | | | — | | | | — | | | | — | | | | (49,844 | ) | | | — | | | | — | | | | — | | | | (49,844 | ) |
Shares issued in satisfaction of debt | | | 1,090,000 | | | | 271,655 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 271,655 | |
Shares issued as part of debt conversion | | | 1,409,091 | | | | 166,153 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 166,153 | |
Foreign currency translation | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (66,366 | ) | | | (66,366 | ) |
Cancellation of shares | | | (33,333 | ) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Net loss | | | | | | | — | | | | — | | | | — | | | | — | | | | (5,846,523 | ) | | | — | | | | (5,846,523 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at March 31, 2013 | | | 117,260,870 | | | $ | 12,524,735 | | | | — | | | | — | | | $ | 6,576,326 | | | $ | (14,935,948 | ) | | $ | (87,029 | ) | | $ | 4,078,084 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 94,801 | | | | — | | | | — | | | | 94,801 | |
Warrants and beneficial conversion feature associated with convertible notes | | | — | | | | — | | | | — | | | | — | | | | 1,782,167 | | | | — | | | | — | | | | 1,782,167 | |
Extension of warrants and adjustments to common stock | | | — | | | | (152,237 | ) | | | — | | | | — | | | | 172,237 | | | | — | | | | — | | | | 20,000 | |
Foreign currency translation | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (116,524 | ) | | | (116,524 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (5,992,897 | ) | | | — | | | | (5,992,897 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at March 31, 2014 | | | 117,260,870 | | | $ | 12,372,498 | | | | — | | | | — | | | $ | 8,625,531 | | | $ | (20,928,845 | ) | | $ | (203,553 | ) | | $ | (134,369 | ) |
| | | | | | | �� | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common shares in private placement | | | 10,281,250 | | | | 410,065 | | | | — | | | | — | | | | 1,034,209 | | | | — | | | | — | | | | 1,444,274 | |
Cash received for future private placement | | | — | | | | — | | | | 38,750,000 | | | | 1,561,000 | | | | — | | | | — | | | | — | | | | 1,561,000 | |
Foreign currency translation | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 203,553 | | | | 203,553 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (5,971,470 | ) | | | — | | | | (5,971,470 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at March 31, 2015 | | | 127,542,120 | | | $ | 12,782,563 | | | | 38,750,000 | | | $ | 1,561,000 | | | $ | 9,659,740 | | | $ | (26,900,315 | ) | | $ | — | | | $ | (2,897,012 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
F-4
MEDIFOCUS, INC.
NOTES TO AUDITED CONSOLIDATED FINANCIAL STATEMENTS
AS OF MARCH 31, 2015
1. BUSINESS, LIQUIDITY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Description of Business and Current Financial Condition
Medifocus Inc. (the “Company” or “Medifocus”) was incorporated under the Business Corporations Act (Ontario) on April 25, 2005. Medifocus develops and commercializes minimally invasive focused heat systems for the treatment of cancerous and benign tumors, and enlarged prostate, medically known as Benign Prostate Hyperplasia (“BPH”). After the acquisition of Prolieve® from the Boston Scientific Corporation, Medifocus now owns a revenue-generating commercial BPH treatment product targeting the BPH drug therapy market and generating cash flows to support the development and commercialization of other heat systems for targeted thermotherapy of surface, subsurface and deep seated localized and regional cancers.
The Company owns two technology platforms with comprehensive US and international patent protection:
| | • | | The Endo-thermotherapy Platform-from which Prolieve was developed, can potentially be used to treat cancers in prostate, rectal, cervical and esophageal, and |
| | • | | The Adaptive Phased Array (“APA”) Microwave Focusing Platform-invented by MIT, licensed to Medifocus, directs precisely focused microwave energy at tumor center to induce shrinkage or eradication of tumors without undue harm to surrounding tissue. The Company’s APA 1000 Breast Cancer Treatment System, developed from the APA technology platform is currently in pivotal Phase-III clinical trials. |
Going Concern Consideration
The Company’s operations are subject to certain risks and uncertainties including, among others, current and potential competitors with greater resources, dependence on significant customers, lack of operating history and uncertainty of future profitability and possible fluctuations in financial results. Since inception, the Company has incurred substantial operating losses, principally from expenses associated with the Company’s research and development, financing activities, and development of new technologies. The Company believes these expenditures are essential for the commercialization of its technologies. The Company expects its operating losses to continue for the foreseeable future as it continues its product development efforts and undertakes its sales and marketing activities. Due to continued substantial operating losses, there is substantial doubt regarding the Company’s ability to continue as a going concern. The Company’s ability to achieve profitability is dependent upon its ability to obtain governmental approvals, produce, and market and sell its new product candidates. There can be no assurance that the Company will be able to commercialize its technology successfully or that profitability will ever be achieved. The operating results of the Company have fluctuated significantly in the past. The Company expects that its operating results will fluctuate significantly in the future and will depend on a number of factors, many of which are outside the Company’s control.
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from the sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Additionally, the Company is not in compliance with the provisions of outstanding debt agreements, and it has not remitted quarterly royalty payments to Boston Scientific Corporation pursuant to the terms of its purchase agreement for Prolieve. The Company has not paid interest owing to certain holders of the convertible debentures, and is in a technical default of the terms of the debentures. The investors have not accelerated the terms of the debenture. Management is continuing its efforts to obtain additional funds through equity financing and through the negotiation of debt agreements to ensure that the Company can meet its obligations and sustain operations.
F-5
The financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue in existence.
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements of Medifocus Inc. have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”) and include the accounts of the Company and its wholly-owned subsidiary Celsion (Canada) Inc. All intercompany transactions have been eliminated. There were no transactions for Celsion (Canada) Inc. for the year ending March 31, 2015.
Unless otherwise noted, all references to “$” or “dollar” refer to the United States dollar. Certain items in the prior period financial statements have been reclassified to conform to the current period presentation. The Company operates in a single business segment, focused heat systems for targeted thermotherapy of surface, subsurface and deep seated localized and regional cancers. Substantially all of the Company’s revenue is generated, and assets are located, in the United States.
Foreign Currency
Effective April 1, 2013, the Company changed its reporting currency from the Canadian dollar (“CAD”) to the U.S. dollar in anticipation of filing its financial statements with the U.S. Securities and Exchange Commission. Effective April 1, 2014, the Company changed its functional currency and that of its wholly owned subsidiary to the U.S. dollar. As a result, all prior year translation adjustments were recognized into income.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. The consolidated financial statements include significant estimates for the expected economic life and value of our licensed technology, allowance for doubtful accounts, value of contingent consideration, value of our debt issuances, accruals for estimated product returns, allowance for inventory obsolescence, allowance for our net operating loss carry forward and related valuation allowance for tax purposes and our stock-based compensation related to employees and directors, consultants and advisors. Because of the use of estimates inherent in the financial reporting process, actual results could differ significantly from those estimates.
Credit Concentration
The Company’s customers are primarily physicians and physician organizations in the U.S.For the year ended March 31, 2015, one customer represented approximately 12% of total revenues. No individual customer represented more than 10% of revenues for the years ended March 31, 2014 and 2013.
Vendor Concentration
The Company currently purchases 100% of its Prolieve catheter inventory from one supplier. Alternative suppliers and alternative catheters are not currently available. The company maintains a deposit of $221,330 with its vendor.
Fair Value Measurements
The Company’s consolidated balance sheets include various financial instruments (primarily cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, and notes payable) recorded at cost, which approximates their fair value. Fair value is the price that would be received from the sale of an asset or paid to transfer a liability assuming an orderly transaction in the most advantageous market at the measurement date. U.S. GAAP establishes a hierarchical disclosure framework which prioritizes and ranks the level of observability of inputs used in measuring fair value. These tiers include:
| | • | | Level 1—Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs. |
| | • | | Level 2—Observable market-based inputs other than quoted prices in active markets for identical assets or liabilities. |
| | • | | Level 3—Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs. |
F-6
In connection with the acquisition of Prolieve, the Company owes additional purchase consideration of up to $2.5 million (contingent consideration) based on the sales of Prolieve products after their acquisition. The contingent consideration is measured at fair value on a recurring basis using level 3 inputs, and the fair value is determined using unobservable inputs such as the discount rate. The change in the fair value of the contingent consideration of $280,296 and $705,355 and nil for the years ended March 31, 2015, 2014 and 2013, respectively, is reflected as “loss from change in fair value of contingent consideration” in the accompanying consolidated statements of operations.See note 2.
The Company has no financial assets and liabilities measured at fair value on a non-recurring basis. The Company’s long-lived assets are measured at fair value on a non-recurring basis only when an impairment is deemed to occur.See notes 3 and 4.
Fair Value of Financial Instruments
The carrying amounts of financial instruments classified as current assets or liabilities, including accounts receivable, accounts payable and accrued expenses approximate fair value because of the short maturity of these instruments.
Comprehensive Income (Loss) and Accumulated Other Comprehensive Income (Loss)
Comprehensive income (loss) includes the total of the Company’s net income (loss) and all other changes in equity other than transactions with owners, including changes in equity for cumulative translation adjustments resulting from the consolidation of foreign subsidiaries as the financial statements of the subsidiaries were previously accounted for using the local currency as the functional currency.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents. All interest bearing and non-interest bearing accounts are guaranteed by the FDIC up to $250,000. The Company may maintain cash balances in excess of FDIC coverage. Management considers this to be a normal business risk.
Accounts Receivable
The Company extends credit to customers on an unsecured basis and payment terms are typically 30 days from delivery or service. The Company’s receivables are primarily related to Prolieve products and services. Management assesses the collectability of its receivables based on a periodic customer-by-customer analysis, considering historical collection experience as well as customer-specific conditions; when a specific customer account is determined to be uncollectible, the Company provides an allowance equal to the estimated uncollectible amounts. Receivables are written off when it is determined that amounts are uncollectible. The Company maintained an allowance for doubtful accounts of $74,289 and $34,289 as of March 31, 2015 and 2014, respectively.
| | | | | | | | |
| | | 2015 | | | 2014 | |
Accounts receivable trade | | $ | 920,022 | | | $ | 1,821,704 | |
Accounts receivable - Harmonized sales tax | | | 211,972 | | | | 241,981 | |
Allowance for doubtful accounts | | | (74,289 | ) | | | (34,289 | ) |
| | | | | | | | |
| | $ | 1,057,705 | | | $ | 2,029,396 | |
| | | | | | | | |
Inventory
Inventory consists primarily of console units and single-use treatment catheters. Inventory consists of the direct costs of acquiring the inventory from vendors. Inventory of console units are considered non-current since the sales period is usually in excess of one year.
Inventory is valued at the lower of cost or net realizable value. Net realizable value represents the estimated selling price for inventories less costs necessary to make the sale. In determining net realizable value, we consider, at a minimum, selling prices, reimbursements charges, and changes in demand for products due to competitive conditions or market acceptance. A provision is recognized to reduce the cost of inventories to the estimated net realizable values, if required, however no provision was recognized for the years ending March 31, 2015, 2014 and 2013. We also analyze the level of inventory on hand on a periodic basis, in relation to estimated customer requirements to determine whether write-downs for excess, obsolete, or slow-moving inventory are required. Any significant or unanticipated change in the factors noted above could have a significant impact on the value of inventories and on reported operating results.
F-7
Inventory is relieved using the first-in, first-out method and consists of the following at March 31, 2015 and 2014.
| | | | | | | | |
| | | 2015 | | | 2014 | |
Current inventory – Catheters | | $ | 79,604 | | | $ | 518,763 | |
Non-current inventory - Consoles | | | 190,276 | | | | 190,276 | |
| | | | | | | | |
| | $ | 269,880 | | | $ | 709,039 | |
| | | | | | | | |
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is provided over the estimated useful lives of the related assets, ranging from three to seven years, using the straight-line method. Major renewals and improvements are capitalized and ordinary repairs and maintenance are expensed as incurred.
The Company reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An asset is considered impaired if its carrying amount exceeds the future net undiscounted cash flows that the asset is expected to generate. If such asset is considered to be impaired, the impairment recognized is the amount by which the carrying amount of the asset, if any, exceeds its fair value determined using a discounted cash flow model.
Contingent Consideration
In accordance with ASC 805, upon the purchase of Prolieve, the Company recognized a contingent consideration obligation as part of the consideration transferred in exchange for the acquired business. The initial measurement of the contingent consideration obligation was based on its estimated fair value. The contingent consideration obligation has been remeasured to fair value at each reporting date and will continue to be remeasured until the contingency is resolved. The changes in fair value are recognized in earnings. The obligation outstanding totaled $1,031,179 and $1,172,654 as of March 31, 2015 and 2014.See Note 2.
Intangible Assets
Intangible assets consist of intellectual property and customer relationships for our Prolieve business acquired in July 2012. These intangible assets were originally recorded at fair value and are amortized on a straight line basis over their estimated useful lives of 10 years. The Company reviews its intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, in a manner similar to that for property and equipment.
Deferred Financing Fees and Other Assets
As part of the convertible debt transaction (see note 5), the Company has unamortized deferred financing fees in the amount of $282,926 and $467,357 as of March 31, 2015 and 2014, respectively. Other assets primarily include a vendor deposit and prepaid rent.
Revenue Recognition
The Company sells products and provides services which are used in the treatment of BPH. The Company recognizes revenue, net of sales taxes, from the sale of Prolieve consoles and catheters upon shipment to the customer. Revenue from the sale of products is measured at the fair value of the consideration received or receivable, net of any estimated returns. Revenue from the mobile service is recognized upon completion of the services, which is generally upon treatment of the patient.
The Company does not have a return policy that allows customers to return product, however the company has allowed returns on a limited customer by customer basis. The Company’s estimate for returns is based upon its historical experience with actual returns, however such returns have historically been limited. While such experience has allowed for reasonable estimation in the past, history may not always be an accurate indicator of future returns. The Company continually monitors its estimates for returns and makes adjustments when it believes that actual product returns may differ from the established accruals, if any. We record a provision for estimated returns in the same period as the related revenue is recorded.
F-8
Costs of Sales—Products
Costs of goods sold primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our intellectual property, warranty costs, warehousing costs, freight and handling charges. Warehousing costs include payroll and benefit costs, including related stock-based compensation expense.
Costs of Sales—Services
Costs of services consist primarily of the costs to provide mobile services to our patients, including catheter cost, depreciation of our mobile consoles and vehicle fleet, and payroll and benefit costs, including related stock-based compensation expense.
Warranty Liabilities
Prolieve products are covered by warranties against defects in material and workmanship for periods of up to 12 months. We record a liability for warranty claims at the time of sale based on the trend in the historical ratio of product failure rates, material usage and service delivery costs to sales, the historical length of time between the sale and resulting warranty claim and other factors. The accrued liability for warranty provisions was $37,960 and $31,650 for the years ended March 31, 2015 and 2014, respectively, and is included in accrued expenses in the accompanying consolidated balance sheets.
Research and Development Expenses
Research and development costs are expensed as incurred.
Income Taxes
Income taxes are accounted for under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in results of operations in the period that the tax rate change occurs. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
A tax position is recognized as a benefit only if it is “more likely than not” that the tax position taken would be sustained in a tax examination, presuming that a tax examination will occur. The Company recognizes interest and/or penalties related to income tax matters in the income tax expense category. The Company remains subject to examination for income tax returns for the years ending after 2010.
Stock-Based Compensation
Compensation costs for all stock-based awards is measured at fair value on the date of the grant using an option pricing model and is recognized over the service period for awards expected to vest. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the current estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Profit Sharing Plan
The Company sponsors a defined contribution retirement plan through a Section 401(k) profit sharing plan. Employees may contribute up to 15% of their pre-tax compensation. Participants are eligible for matching Company contributions up to 3% of eligible compensation dependent on the level of voluntary contributions. Company matching contributions totaled $75,000, $78,000 and $17,000, respectively, for the years ended March 31, 2015, 2014 and 2013.
Net Income (Loss) Per Share
Basic earnings (loss) per share is computed by dividing net income (loss) available to common shareholders by the weighted-average number of shares of common shares outstanding during the period.
F-9
For periods of net loss, diluted loss per share is calculated similarly to basic loss per share because the impact of all dilutive potential common shares is anti-dilutive due to the net losses. For the years ended March 31, 2015, 2014 and 2013, outstanding stock options of 8,525,000 for each of the years, and warrants outstanding to purchase 105,278,102, 94,996,882 and 86,106,777 commons shares, respectively were considered anti-dilutive and therefore were not included in the calculation of diluted shares. For the years ended March 31, 2015 and 2014, convertible promissory notes convertible into 22,160,000 shares of common stock were considered anti-dilutive and therefore were not included in the calculation of diluted shares.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (FASB) issued ASU 2014-09 – Revenue from Contracts with Customers providing guidance for revenue recognition for contracts. This guidance requires an entity to review contracts in five steps and will result in enhanced disclosures regarding the nature, amount, timing and uncertainty of revenue arising from contracts with customers. This standard is effective for fiscal years beginning after December 15, 2016 and early adoption is not permitted. We are currently evaluating the impact, if any, that this new guidance will have on our financial statements.
ASU No. 2014-12,Compensation-Stock Compensation (Topic 718): Accounting for Share Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Periodamendments require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. Guidance in Topic 718 as it relates to awards with performance conditions that affect vesting should be applied to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. As indicated in the definition of vest, the stated vesting period (which includes the period in which the performance target could be achieved) may differ from the requisite service period. The amendments of ASU 2014-12 are effective for interim and annual periods beginning after December 15, 2015. The adoption of the amended guidance is not expected to have a material on the Consolidated Financial Statements.
ASU No. 2014-16,Derivatives and Hedging (Topic 815): Determining Whether the Host Contract in a Hybrid Financial instrument in the Form of a ShareisMore Akin to Debt or Equity.In November 2014, the F ASB issued amended guidance that clarifies how current GAAP should be interpreted in evaluating the economic characteristics and risks of a host contract in a hybrid financial instrument that is issued in the form of a share. Specifically, the amendments clarify that an entity should consider all relevant terms and features -including the embedded derivative features being evaluated for bifurcation -in evaluating the nature of the host contract. Furthermore, the amendments clarify that no single term or feature would necessarily determine the economic characteristics and risks of the host contract. Rather, the nature of the host contract depends upon the economic characteristics and risks of the entire hybrid financial instrument. The amended guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015, with early adoption permitted. The effects of initially adopting the amended guidance should be applied on a modified retrospective basis to existing hybrid financial instruments issued in the form of a share as of the beginning of the fiscal year for which the amendments are effective and shall be reported as a cumulative-effect adjustment directly to retained earnings as of the beginning of the year of adoption. The adoption of the amended guidance is not expected to have a material impact on the Consolidated Financial Statements.
ASU No. 2015-03,Interest-Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs.In April 2015, the FASB issued amended guidance to address the different balance sheet presentation requirements for debt issuance costs and debt discounts and premiums. The amended guidance requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the amended guidance. The amended guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015, with early adoption permitted for financial statements that have not been previously issued. The amended guidance should be applied retrospectively, wherein the balance sheet of each individual period presented should be adjusted to reflect the period-specific effects of applying the amended guidance. The Company currently has approximately $283,000 of debt issuance costs recorded in the Consolidated Balance Sheets that will be required to be reclassified and presented as a direct deduction from the debt liability upon adoption of the amended guidance. The adoption of the amended guidance is not expected to have an impact on the Company’s Consolidated Statements of Income.
F-10
In August 2014, the FASB issued ASU 2014-15 – Presentation of Financial Statements – Going Concern. This guidance requires management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. This guidance is effective for fiscal years beginning after December 15, 2016 and early adoption is permitted. We do not expect the adoption of this guidance to have a material impact on our financial statements.
Emerging Growth Company Status
We are an “emerging growth company” as defined in section 3(a) of the Exchange Act, as amended by the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), and will continue to qualify as an “emerging growth company” until the earliest to occur of: (a) the last day of the fiscal year during which we have total annual gross revenues of $1,000,000,000 (as such amount is indexed for inflation every 5 years by the SEC) or more; (b) the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (c) the date on which we have, during the previous 3-year period, issued more than $1,000,000,000 in non-convertible debt; or (d) the date on which we are deemed to be a ‘large accelerated filer’, as defined in Exchange Act Rule 12b–2.
Generally, a company that registers any class of its securities under section 12 of the Exchange Act is required to include in the second and all subsequent annual reports filed by it under the Exchange Act, a management report on internal controls over financial reporting and, subject to an exemption available to companies that meet the definition of a “smaller reporting company” in Exchange Act Rule 12b-2, an auditor attestation report on management’s assessment of internal controls over financial reporting. However, for so long as we continue to qualify as an emerging growth company, we will be exempt from the requirement to include an auditor attestation report in our annual reports filed under the Exchange Act, even if we do not qualify as a “smaller reporting company”. In addition, section 103(a)(3) of the Sarbanes-Oxley Act of 2002 has been amended by the JOBS Act to provide that, among other things, auditors of an emerging growth company are exempt from any rules of the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the company.
Any U.S. domestic issuer that is an emerging growth company is able to avail itself to the reduced disclosure obligations regarding executive compensation in periodic reports and proxy statements, and to not present to its shareholders a nonbinding advisory vote on executive compensation, obtain approval of any golden parachute payments not previously approved, or present the relationship between executive compensation actually paid and our financial performance. As a foreign private issuer, we are not subject to such requirements, and will not become subject to such requirements even if we were to cease to be an emerging growth company.
As a reporting issuer under the securities legislation of the Canadian provinces of Ontario, British Columbia, and Alberta, we are required to comply with all new or revised accounting standards that apply to Canadian public companies. Pursuant to Section 107(b) of the JOBS Act, an emerging growth company may elect to utilize an extended transition period for complying with new or revised accounting standards for public companies until such standards apply to private companies. We have elected to utilize this extended transition period. However, while we have elected to utilize this extended transition period, our audited consolidated financial statements as of March 31, 2015 reflect the adoption of all required accounting standards for public companies.
F-11
2. BUSINESS ACQUISITION AND CONTINGENT CONSIDERATION
On July 24, 2012 the Company purchased from Boston Scientific Corporation [“BSC”], in a taxable transaction, all of the assets, relating to the Prolieve Thermodilatation System (“Prolieve”), a FDA approved device for the treatment of Benign Prostatic Hyperplasia (“BPH”). The total purchase consideration consisted of the following:
| | | | |
Cash | | $ | 2,535,610 | |
| Fair value of contingent consideration | | | 1,126,505 | |
| | | | |
Total consideration | | $ | 3,662,115 | |
| | | | |
The maximum amount of $2,500,000 is payable as contingent consideration pursuant to the terms of the agreement. The fair value of the contingent consideration was determined by calculating its present value based on its payment terms using an interest rate of 24% (our estimated unsecured borrowing rate). The contingent consideration is payable quarterly at a rate of 10% of sales of Prolieve products. As at March 31, 2015, $831,632 of royalties is due to BSC. Of this amount, approximately $728,632 is past due.
As of March 31, 2015, the balance due is allocated as follows:
| | | | | | | | | | | | |
| | | Non Contingent
portion | | | Contingent
portion | | | Total | |
Balance at April 1, 2012 | | $ | — | | | $ | — | | | $ | — | |
Additions | | | 180,597 | | | | 890,020 | | | | 1,070,617 | |
Change in fair market value | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Balance at March 31, 2013 | | | 180,597 | | | | 890,020 | | | | 1,070,617 | |
| | | | | | | | | | | | |
Less: payments | | | (309,133 | ) | | | | | | | (309,133 | ) |
Change in fair market value | | | 538,397 | | | | 282,634 | | | | 821,031 | |
| | | | | | | | | | | | |
Balance at March 31, 2014 | | | 409,861 | | | | 1,172,654 | | | | 1,582,515 | |
| | | | | | | | | | | | |
Less: payments | | | — | | | | — | | | | — | |
Change in fair market value | | | 421,771 | | | | (141,475 | ) | | | 280,296 | |
| | | | | | | | | | | | |
Balance at March 31, 2015 | | | 831,632 | | | | 1,031,179 | | | | 1,862,811 | |
| | | | | | | | | | | | |
Allocated as follows: | | | | | | | | | | | | |
| | | | | | | | | | | | |
Payable to BSC | | $ | 831,632 | | | $ | — | | | $ | 831,632 | |
| | | | | | | | | | | | |
Contingent Payable to BSC - current | | $ | — | | | $ | 440,663 | | | $ | 440,663 | |
| | | | | | | | | | | | |
Contingent Payable to BSC - long term | | $ | — | | | $ | 590,516 | | | $ | 590,516 | |
| | | | | | | | | | | | |
3. PROPERTY AND EQUIPMENT
Property and equipment consists of the following as of March 31, 2015 and 2014:
| | | | | | | | |
| | | March 31, 2015 | | | March 31, 2014 | |
Machinery and equipment (5-7 year life) | | $ | 75,982 | | | $ | 88,160 | |
Mobile consoles (7 year life) | | | 678,845 | | | | 666,174 | |
Furniture and fixtures (3-5 year life) | | | 20,000 | | | | 19,049 | |
| | | | | | | | |
| | | 774,827 | | | | 773,383 | |
Accumulated depreciation | | | (305,792 | ) | | | (229,954 | ) |
| | | | | | | | |
Total | | $ | 469,035 | | | $ | 543,429 | |
| | | | | | | | |
F-12
Depreciation expense was approximately $115,000, $111,000 and $78,000 for the years ended March 31, 2015, 2014 and 2013, respectively.
4. INTANGIBLE ASSETS
Intangible assets include intellectual properties relating to the Prolieve technology, acquired at a cost of $2.5 million. These assets are being amortized on a straight-line basis over ten years; amortization expense was $246,212, $246,212 and $225,730 for the years ended March 31, 2015, 2014 and 2013, respectively.
Future amortization expense related to the net carrying amount of intangible assets is estimated to be as follows:
| | | | |
2016 | | $ | 246,212 | |
2017 | | | 246,212 | |
2018 | | | 246,212 | |
2019 | | | 246,212 | |
2020 | | | 246,212 | |
| | | | |
Sub-total | | | 1,231,060 | |
| | | | |
2021 and thereafter | | | 535,272 | |
| | | | |
Total | | $ | 1,766,332 | |
| | | | |
5. DEBT
In fiscal 2013, the Company raised bridge financing of approximately U.S. $435,000. The bridge financing lender received a promissory note, with interest payable at 2% per month after October 23, 2012. The original maturity date of the promissory notes was October 23, 2013 and was subsequently extended until June 30, 2014. As of March 31, 2015, the note remains in default and due in full. The Company is currently in discussions with the lender on a further extension of the maturity date. Interest expense of approximately $117,000, $138,000 and $55,000 was recognized on the promissory note for 2015, 2014 and 2013 respectively.
In fiscal 2014, the Company issued 554 Units of 8% Redeemable Promissory Convertible Notes (the “Notes”) together with Series C stock Purchase Warrants (the “Warrants”) to various accredited investors in an offering exempt from registration in the U.S pursuant to regulations D and S under the U.S. Federal Securities rules and regulations, receiving gross proceeds of $5,540,000. The notes are convertible into 22,160,000 shares of common stock. Each warrant entitles the holder to acquire 20,000 common shares (for a total of 11,080,000 common shares) at an exercise price of $0.30 per share and expire on December 18, 2016. The warrants were classified as equity, were recorded as additional paid in capital at their estimated fair value of $1,532,877, and are considered a non-cash financing activity. The Company recognized a beneficial conversion feature of $195,938 and deferred financing fees (consisting of both cash payments and the fair value of stock purchase warrants classified as equity) of $558,552 which are amortized using the effective interest method through December 18, 2016. The Company has not paid $378,291 interest owing to certain holders of the convertible debentures, of which $307,491 is past due, and is in a technical default of the terms of the debentures. The investors have not accelerated the terms of the debenture.
In connection with the above transaction, the Company recognized interest expense of $464,010 and accretion expense of $712,841 for the year ended March 31, 2015, and interest expense of $94,000 and accretion expense of $161,002, for the year ended March 31, 2014.
6. EQUITY AND STOCK-BASED COMPENSATION
Authorized share capital consists of unlimited common shares with no par value.
F-13
On September 15, 2014, the Company completed a private placement of 10,281,250 units at a price of USD $0.16 per unit raising gross proceeds of $1,645,000 (net proceeds of $1,561,000). Each unit consisted of one common share and one common share purchase warrant. Each warrant entitled the holder to acquire one common share at an exercise price of USD $0.25 until September 15, 2017. Management determined the warrants to have a fair value of $0.12 per warrant and accordingly, $1,231,934 of the proceeds from the issuance was allocated to additional paid in capital, and the balance of the proceeds was allocated to common shares.
A relative fair value calculation was used to determine the carrying value of the warrants. The fair value of the warrants issued was estimated using a Black-Scholes pricing model with the following assumptions:
| | |
Risk free interest rate | | 0.94% to 0.99% |
| Expected life in years | | 3 year |
Expected volatility | | 146.6% |
Common stock issuable
Prior to March 31, 2015, the company received funds for common shares in the amount of $1,561,000 (net of fees) as part of a future private placement occurring subsequent to year end. The shares were issued on May 12, 2015 and further disclosed in Note 10.
Stock Purchase Warrants
The Company had stock purchase warrants outstanding as of March 31, 2015, 2014 and 2013 for the purchase of common shares, as follows:
| | | | | | | | | | | | | | | | |
| | | | March 31,
2015 | | | March 31,
2014 | |
Year of Issue | | Exercise
Price | | | Expiration | | | Underlying
Shares | | | Underlying
Shares | |
2011 | | $ | 0.50 | | | | 4/24/2015 | | | | 2,449,997 | | | | 2,449,997 | |
2011 | | $ | 0.30 | | | | 2/24/2016 | | | | 3,745,000 | | | | 3,745,000 | |
2013 | | $ | 0.20 | | | | 6/8/2015 | | | | 18,367,263 | | | | 18,367,263 | |
2013 | | $ | 0.20 | | | | 6/8/2015 | | | | 22,200,000 | | | | 22,200,000 | |
2013 | | $ | 0.20 | | | | 9/21/2015 | | | | 22,196,795 | | | | 22,196,765 | |
2013 | | $ | 0.20 | | | | 1/14/2016 | | | | 13,056,997 | | | | 13,056,997 | |
2014 | | $ | 0.30 | | | | 12/18/2016 | | | | 8,336,400 | | | | 8,336,400 | |
2014 | | $ | 0.30 | | | | 3/7/2017 | | | | 4,644,400 | | | | 4,644,400 | |
2015 | | $ | 0.25 | | | | 9/15/2017 | | | | 10,281,250 | | | | — | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | 105,278,102 | | | | 94,996,822 | |
| | | | | | | | | | | | | | | | |
Warrant Modifications
During the years ended March 31, 2015 and 2014 the Company extended the expiration date of certain outstanding stock warrants issued as part of private placements. Such modifications require an allocation between common stock and additional paid in capital and does not impact the net loss and total deficit of the Company.
Stock Options
The Company may grant stock options to directors, senior officers and service providers by resolution of the Board of Directors. The exercise price will reflect the market price of the Company’s stock on the date of the grant. The maximum number of stock options outstanding under the stock option plan is limited 10% of issued shares.
F-14
The Company measures the cost of stock option awards based on the grant-date fair value of the award. The cost is recognized over the period during which an employee is required to provide service in exchange for the award or the requisite service period. The Company recognizes stock-based compensation expense over the vesting periods of the awards, adjusted for estimated forfeitures. The stock-based compensation cost that was incurred by the Company was $0, $94,801 and $859,613 for the years ended March 31, 2015, 2014 and 2013, respectively. Stock-based compensation was included in the consolidate statement of operations as follows:
| | | | | | | | | | | | |
| | | 2015 | | | 2014 | | | 2013 | |
Cost of services | | $ | — | | | $ | 10,427 | | | $ | 11,497 | |
Sales and marketing expenses | | | — | | | | 32,231 | | | | 35,536 | |
General and administrative expenses | | | — | | | | 52,142 | | | | 812,580 | |
| | | | | | | | | | | | |
Total | | $ | — | | | $ | 94,801 | | | $ | 859,613 | |
| | | | | | | | | | | | |
A summary of Plan activity in 2015, 2014 and 2013 follows:
| | | | | | | | | | | | | | | | |
| | | Option
Shares | | | Weighted
Average
Exercise Price | | | Weighted
Average
Remaining
Contractual
Life (years) | | | Aggregate
Intrinsic Value | |
Outstanding, April 1, 2012 | | | 3,000,000 | | | $ | 0.20 | | | | 3.0 | | | $ | 75,000 | |
Granted to officers and directors | | | 4,825,000 | | | $ | 0.19 | | | | 3.0 | | | $ | 168,875 | |
Granted to employees | | | 1,000,000 | | | $ | 0.24 | | | | 3.0 | | | $ | — | |
Cancelled | | | (300,000 | ) | | $ | 0.20 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding, March 31, 2013 | | | 8,525,000 | | | $ | 0.20 | | | | 3.0 | | | $ | 243,875 | |
| | | | | | | | | | | | | | | | |
Cancelled | | | (270.000 | ) | | $ | 0.20 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding, March 31, 2014 and 2015 | | | 8,255,000 | | | | | | | | | | | | | |
Exercisable, March 31, 2015 | | | 8,255,000 | | | $ | 0.20 | | | | 1.0 | | | $ | — | |
| | | | | | | | | | | | | | | | |
The quoted market price for the Company’s common stock at March 31, 2015 was less than the weighted average exercise price of the outstanding stock options, resulting in no intrinsic value at that date. The fair value of each option grant in 2013 was estimated on the date of grant using a Black-Scholes option pricing model using the following assumptions:
| | |
Risk free interest rate | | 1.20% |
Expected life in years | | 2 year |
Expected volatility | | 158.0% |
7. INCOME TAXES
The Company is domiciled in Canada and files Canadian federal and certain provincial tax returns. The Company had no provision (benefit) for income taxes in 2015, 2014 and 2013 as a result of its net losses and full valuation allowance against its deferred tax assets.
The following table reconciles our losses before income taxes by jurisdiction:
| | | | | | | | | | | | |
| | | Years ended March 31, | |
| | | 2015 | | | 2014 | | | 2013 | |
U.S | | $ | — | | | $ | — | | | $ | 112,537 | |
Canada | | $ | 5,971,470 | | | $ | 5,992,897 | | | $ | 5,733,986 | |
| | | | | | | | | | | | |
| | $ | 5,971,470 | | | $ | 5,992,897 | | | $ | 5,846,523 | |
| | | | | | | | | | | | |
F-15
The difference between the Company’s expected income tax provision (benefit) from applying the Canadian federal statutory tax rates to the pre-tax loss and the actual income tax provision (benefit) relates primarily to the effect of the following:
| | | | | | | | | | | | |
| | | Year Ended March 31, | |
| | | 2015 | | | 2014 | | | 2013 | |
Canadian statutory rate | | | (26.50 | %) | | | (26.50 | %) | | | (28.50 | %) |
Non-deductible expenses | | | 3.80 | | | | 3.80 | | | | 4.50 | |
Valuation allowance | | | 22.70 | | | | 22.70 | | | | 24.00 | |
| | | | | | | | | | | | |
Income tax provision (benefit) | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % |
| | | | | | | | | | | | |
Deferred income taxes reflect the net tax effects of differences between the bases of assets and liabilities for financial reporting and income tax purposes. The Company’s deferred income tax assets and liabilities consisted of the following:
| | | | | | | | | | | | |
| | | Year Ended March 31, | |
| | | 2015 | | | 2014 | | | 2013 | |
Net operating carry forwards | | $ | 4,658,383 | | | $ | 4,025,503 | | | $ | 3,266,027 | |
Research and development expenses | | | — | | | | (976,078 | ) | | | 976,078 | |
Intangible assets | | | — | | | | — | | | | 38,000 | |
Non-deductible expenses | | | — | | | | — | | | | 57,927 | |
Valuation allowance | | | (4,658,383 | ) | | | (3,049,925 | ) | | | (4,338,032 | ) |
| | | | | | | | | | | | |
Income tax provision (benefit) | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
The Company is required to establish a valuation allowance for deferred tax assets if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The Company considers the projected future taxable income and tax planning strategies in making this assessment. Based upon the level of historical taxable income and projections for future taxable income in the periods which the deferred tax assets are deductible, the Company has determined that a full valuation allowance is required as of March 31, 2015, 2014 and 2013.
The Company is subject to income taxes in numerous jurisdictions. Significant judgment is required in evaluating the Company’s tax positions and determining its provision for income taxes. The Company establishes liabilities for tax-related uncertainties based on estimates of whether, and the extent to which, additional taxes will be due. These liabilities are established when the Company believes that certain positions might be challenged despite its belief that its tax return positions are fully supportable. The Company adjusts these liabilities in light of changing facts and circumstances, such as the outcome of a tax audit. The provision for income taxes includes the impact of changes to the liability that is considered appropriate. The Company has identified no material uncertain tax positions as of March 31, 2015.
The Company is subject to income tax audits in all jurisdictions for which it is required to file tax returns. Tax audits by their nature are often complex and can require several years to complete. Neither the Company nor any of its subsidiaries is currently under audit in any jurisdiction. All of the Company’s income tax returns remain subject to examination by tax authorities.
As a Canadian domiciled company, the Company has never filed a tax return in the United States. U.S. federal tax legislation was enacted in 2004 to address perceived U.S. tax concerns in “corporate inversion” transactions. A “corporate inversion” generally occurs when a non-U.S. Company acquires “substantially all” of the equity interests in, or the assets of, a U.S. Company or partnership, if, after the acquisition, former equity holders of the U.S. Company or partnership own a specified level of stock in the non-U.S. Company. The tax consequences of these rules depend upon the percentage identity of stock ownership that results. Generally, in “80 percent identity” transactions (i.e., former equity holders of the U.S. Company owns 80% or more of the equity of the non-U.S. acquiring entity, excluding certain equity interests), the tax benefits of the inversion are limited by treating the non-U.S. acquiring entity as a domestic entity for U.S. tax purposes. In “60-80 percent identity” transactions, the benefits of the inversion are limited by barring certain corporate-level “toll charges” from being offset by certain tax attributes of the U.S. Company (e.g., loss carry-forwards), and imposing excise taxes on certain stock-based compensation held by “insiders” of the U.S. Company. Management is of the view that a corporate inversion has resulted from the reverse takeover transaction it completed in fiscal 2009; however, it has not yet determined whether the Company is subject to the “80 percent” or the “60-80 percent” identity with respect to the transactions undertaken in the fiscal 2009 year since the interpretation of which categories of stock ownership are to be considered under the inversion rules is not yet settled. The Company has not filed income tax returns in Canada or United States since 2009. The Company has had losses in every jurisdiction in these years, income tax expenses and non-filing penalties are insignificant.
F-16
8. COMMITMENTS AND CONTINGENCIES
On January 16, 2006, the Company’s wholly owned subsidiary, Celsion (Canada) Inc. purchased from Celsion Corporation(USA) [“Celsion”]all of the assets relating to breast cancer Microfocus APA 1000 System (“System”), consisting of the microwave machine technology, the APA technology licensed from MIT, and all related intellectual and regulatory property (collectively, the “Business”). The Company has a commitment to pay a 5% royalty to Celsion on the net sales of products sold by and patent royalties received by the Company and its successors and assignees. Total royalties paid are not to exceed $18,500,000. Royalties will not be payable until the System can be placed in the market following successful completion of the pivotal clinical trial and receipt of approval to market the System in the US and Canada from the FDA and Health Canada. The Company has an additional commitment to pay a 5% royalty to MIT on the net sales of products, upon commercialization.
Future minimum payments under operating leases for office space and vehicles are as follows:
| | | | |
2016 | | $ | 195,492 | |
2017 | | $ | 201,545 | |
2018 | | $ | 169,419 | |
The Company recognized total rent expense of $192,919, $176,616 and $91,596 for the years ended March 31, 2015, 2014 and 2013, respectively.
The Company has agreed to indemnify its directors and officers and certain of its employees in accordance with the Company’s by-laws. The Company maintains insurance policies that may provide coverage against certain claims.
9. RECLASSIFICATIONS
Certain reclassifications to 2014 and 2013 financial presentation have been made to conform to the 2015 presentation. These reclassifications did not affect previous reported net loss or total stockholders’ deficit.
10. SUBSEQUENT EVENTS
On May 12, 2015, the company issued 38,750,000 common shares at a price of $0.044 per common share for gross proceeds of US$1,705,000 received prior to March 31, 2015, as part of a non-brokered private placement. These shares are recorded as common shares issuable as at March 31, 2015.
Subsequent to the end of the year, 2,449,997 outstanding common share purchase warrants expired without exercise.
Subsequent to the end of the year, 40,567,263 outstanding common share purchase warrants were extended to June 8, 2016.
F-17
EXHIBIT INDEX
| | |
Exhibit
No. | | Exhibit |
| |
| 4.27 | | Option Agreement, effective May 1, 2015, between Medifocus Inc. and Duke University |
| |
| 12.1 | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 12.2 | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.1 | | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.2 | | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 15.1 | | Consent of Stegman & Company, Certified Public Accountants |
| |
| 15.2 | | Concurrence Letter from Sievert & Sawrantschuk LLP to the Securities and Exchange Commission, dated July 8, 2015 |



