Exhibit 99.1
MEDIFOCUS INC.
FORM 51-102FI
Management Discussion and Analysis
for the three and six months ended
September 30, 2015
November 30, 2015
1
1. Introduction
The following sets out the Management’s Discussion and Analysis (“MD&A”) of the financial position and results of operations for the three and six months ended September 30, 2015 of Medifocus Inc. (the “Company”, “Medifocus” or we). The MD& should be read in conjunction with the Company’s condensed interim consolidated financial statements for the three and six months ended September 30, 2015 and 2014. All dollar amounts are presented in United States dollars unless otherwise noted. The functional currency of the Company and its subsidiary is the United States dollar, and the presentation currency is the United States dollar. Additional information relating to the Company is available on SEDAR atwww.sedar.com.
Forward-Looking Statements
This management’s discussion and analysis may contain statements that are “Forward-looking Statements”. These include statements about the Company’s expectations, beliefs, plans, objectives and assumptions about future events or performance. These statements are often, but not always, made through the use of words or phrases such as “will likely result”, “are expected to”, “will continue”. “anticipate”, “believes”, “estimate”, “intend”, “plan”, “would”, and “outlook” or statements to the effect that actions, events or results “will”, “may”, “should” or “would” be taken, occur or be achieved. Forward-looking statements are not historical facts, and are subject to a number of risks and uncertainties beyond the Company’s control. Accordingly, the Company’s actual results could differ materially from those suggested by these forward-looking statements for various reasons discussed throughout this analysis. Forward-looking statements are made on the basis of the beliefs, opinions, and estimates of the Company’s management on the date the statements are made and, other than in compliance with applicable securities laws, the Company does not undertake any obligation to update forward-looking statements if the circumstances or management’s beliefs, opinions or estimates should change. Readers should not place undue reliance on forward-looking statements.
2. Reporting currency
Effective April 1, 2014, the Company changed is reporting and functional currency from the Canadian dollar to the U.S. dollar in anticipation of filing its financial statements with the U.S. Securities and Exchange Commission.
3. Overview of Financial Performance
The Company recognized revenues of $2,261,370 for the six months ended September 30, 2015. This is a decrease of $29,796 compared to the same six month period of 2014, a decrease of 1.30%. During FY 2015, the Company re-examined its Prolieve sales and costs structure since acquiring the Prolieve business from Boston Scientific Corporation in July 2012. Sales had been developing steadily, but management found that profitability was marginal in some of the sales territories. Management concluded that the Company must refocus the allocation of resources and improve the Prolieve business’ operational efficiency by implementing the following:
| | • | | Create a more efficient sales organization by eliminating less productive sales positions, particularly in territories with high service costs. |
| | • | | Support our customer base in a more profit-oriented sales model with the goal to improve gross margin and profitability. |
| | • | | Grow new accounts by using hybrid mobile service technicians to supplement the sales team. |
| | • | | Focus on major metropolitan markets that generally provide higher margins and require less servicing costs |
| | • | | Re-evaluate uneconomical remote accounts to reduce travel and servicing costs. |
The restructuring involved a reduction of 65% of our sales force. As a result the Company experienced a significant decrease in revenue from Prolieve during the last three quarters of FY 2015 and the first two quarters of FY 2016. However, we expect revenues to stabilize in the remainder of FY 2016 and in FY 2017.
The cost of sales for the six months ended September 30, 2015 was $1,644,972 a decrease of $310,002 for the same period of 2014. Gross margin was 27.3% for the six month period, an increase of 12.6% for the same period ended September 30, 2014. The increase in the gross profit margin is due to the Company’s restructuring of the Prolieve business as the company continues to experience less costs related to mobile sales.
2
During the year ended March 31, 2014, Medifocus created Medifocus Holding Limited, together with Ideal Concept Group Limited to develop our Prolieve business and APA technology in Asia Pacific. Medifocus owns 40% of Medifocus Holdings Limited and Idea Concept Group holds 60%. Medifocus Holdings Limited will evaluate opportunities in Asia Pacific; engage in clinical testing and obtaining approval from China Food and Drug Administration of the People’s Republic of China (“CFDA”) for all products relating to Prolieve and the APA technology.
Medifocus Holdings Limited is required to pay Medifocus a royalty of 5% of the first $10,000,000 in sales of the catheter kits and control units utilized in the Prolieve business. After $10,000,000 in sales has been reached, the royalty decreases to 3%. For all other products developed by Medifocus, Holdings Ltd. is required to pay Medifocus royalty of 7.5% on net sales of such products.
4. Company History and Business
Our business was started by Dr. Augustine Cheung, our President and Chief Executive Officer, as an outgrowth of his academic interest and work in the field of microwave technology and the thermotherapy treatment of disease while he was a professor at the University of Maryland and George Washington University. In 1982, he founded A.Y. Cheung Associates Inc. to pursue this work. A.Y. Cheung Associates Inc. changed its name to Cheung Laboratories, Inc. in 1984, and Cheung Laboratories Inc. subsequently changed its name to Celsion Corporation (“Celsion”) in 1998.
At Celsion, Dr. Cheung began developing technologies for the treatment of BPH and breast cancer using thermotherapy technology, leading to the development and commercialization of the Prolieve system for the treatment of BPH. In 2007, Celsion sold the Prolieve system and technology to Boston Scientific Corporation (“Boston Scientific”) for $60 million. Dr. Cheung also began developing the APA 1000 system for the treatment of breast cancer. The rights to key elements of APA 1000 were licensed from MIT pursuant to an Exclusive Patent License Agreement (“Patent License Agreement”) dated October 24, 1997.
In 2005 Celsion transferred all its interest in this license and other rights to APA 1000 to its wholly-owned subsidiary, Celsion (Canada) Limited (“Celsion Canada”). On January 16, 2006, Dr. Cheung resigned from Celsion’s board of directors and his position as Celsion’s Chief Scientific Officer, and purchased Celsion Canada for $20,000,000 (Canadian dollars). The purchase price was paid by issuing: (a) a personal $1.5 million promissory note; and (b) an $ 18.5 million royalty payable at the rate of 5% of the net sales on sales of products developed using APA technology, once such products become commercialized. The $1.5 million promissory note was secured by 1,508,050 shares of Celsion’s common stock. After Dr. Cheung’s default on payment of the promissory note, Celsion agreed in 2009 with Dr. Cheung to retain the 1,508,000 shares of Celsion’s common stock that it held as security in full satisfaction of the $1.5 million promissory note.
Medifocus Inc. was incorporated on April 25, 2005 under the Business Corporations Act (Ontario) as a CPC. Under Canadian law, a CPC is a newly created Canadian company having no assets, other than cash, which is permitted to conduct an initial public offering of its securities (“IPO”) and obtain a listing of its shares on the TSXV. A CPC may then uses the funds raised in the IPO to identify and evaluate assets or businesses which, when acquired, qualify the CPC for listing as a regular issuer on the TSXV.
On June 29, 2006 Medifocus Inc., completed its IPO on the TSXV of 4,600,000 shares at a price of $0.20 (Canadian dollars) per share receiving gross proceeds of $920,000 (Canadian dollars). In order to gain improved access to funding, Medifocus Inc. engaged in a share exchange offer with Celsion Canada in 2008 pursuant to which Celsion Canada became a wholly-owned subsidiary of Medifocus. Concurrently with the exchange offer, Medifocus completed a private placement of units, receiving gross proceeds of $2 million (Canadian dollars). In addition, Medifocus issued 903,112 shares to Celsion at a deemed value of $0.50 (Canadian dollars) per share, in partial satisfaction of an approximate $600,000 (Canadian dollars) liability that was owed to Celsion. After the completion of the share exchange transaction, we continued our development of the APA 1000 technology for the treatment of breast cancer. Phase I and Phase II clinical trials were originally completed by Celsion. Subsequently, the Company received approvals from both the FDA and the Canadian Bureau of Medical Devices to conduct a pivotal Phase III breast cancer treatment study. We have begun the pivotal Phase III clinical trials but, such trials have been proceeding at a slow pace due to lack of funding. We plan to complete the pivotal Phase III trial when funding is available.
3
The Patent License Agreement with MIT was amended on June 16, 2007. The amended agreement requires us to pay MIT a 5% royalty on the net sales of any products derived from APA 1000, and an annual maintenance fee of $50,000. MIT is entitled to receive royalties for so long as the patents relating to the APA technology are valid or the Patent License Agreement is terminated.
On July 24, 2012 we acquired the Prolieve technology and related assets from Boston Scientific pursuant to an Asset Purchase Agreement dated June 25, 2012, amended on July 24, 2012 (the “Asset Purchase Agreement”). The purchase price was $3,662,115, of which $2,535,610 was paid on the closing of the transaction. Additionally, we entered into a contingent consideration arrangement under which we will pay Boston Scientific up to $2,500,000, to be paid in quarterly installments at a rate of 10% of the sales of Prolieve products. Sales are defined as the gross amount invoiced for sales, distributions, licenses, leases, transfers, and other dispositions. As of September 30, 2015, approximately $1,944,450 remains payable to Boston Scientific under the contingent consideration arrangement, $917,002 of which is past due.
Our Products
1. The Prolieve Thermodilatation System
Prolieve is used to treat benign prostatic hyperplasia or “BPH.” BPH is a condition in which the prostate gland becomes enlarged and restricts the flow of urine through the urethra. Our clinical studies have shown that the treatment of this condition with the Prolieve system improves urine flow by decreasing the enlarged prostate’s pressure on the urethra through the heating, dilation and shrinking of the prostate tissue surrounding it. The BPH drug therapy market is estimated to be about $4 billion in major developed countries according to Decision Resources Group. This number does not include non-drug treatments and the patients who are on “Watchful Waiting” due to the side effects of some of the treatment options. While the market for minimally invasive BPH treatment is approximately $150 million according to Medtech Insight, we believe that Prolieve can be a viable alternative to drug therapy due to its safety and efficacy profiles and thus has the potential to increase the market for minimally invasive BPH treatment.
What Is Benign Prostatic Hyperplasia?
Millions of aging men experience symptoms resulting from BPH, a non-cancerous urological disease in which the prostate enlarges and constricts the urethra. The prostate is a walnut-sized gland surrounding the male urethra that produces seminal fluid and plays a key role in sperm preservation and transportation. The prostate frequently enlarges with age. As the prostate expands, it compresses or constricts the urethra, thereby restricting the normal passage of urine. This restriction may require a patient to exert excessive bladder pressure to urinate. Because urination is one of the body’s primary means of cleansing impurities, the inability to urinate adequately increases the possibility of infection and bladder and kidney damage.
BPH Symptoms
The symptoms of BPH usually involve problems with emptying the bladder or storing urine in the bladder. However, the severity of the symptoms can vary widely, from mild and barely noticeable to serious and disruptive. Common BPH symptoms include:
| | • | | Pushing or straining to begin urination; |
| | • | | Dribbling after urination; |
| | • | | A frequent need to urinate, sometimes every 2 hours or less; |
| | • | | A recurrent, sudden, or uncontrollable urge to urinate; |
| | • | | Feeling the bladder has not completely emptied after urination; |
| | • | | Pain during urination; and |
| | • | | Waking at night to urinate. |
In extreme cases, a man may be completely unable to urinate. In such situations, emergency medical attention is required.
4
An enlarged prostate does not cause prostate cancer or directly affect sexual function. However, many men experience sexual dysfunction and BPH symptoms at the same time. This is due to aging and the common medical conditions older men often encounter, including vascular disease and diabetes. Because all of these conditions take place with aging, sexual dysfunction tends to be more pronounced in men with BPH.
BPH Complications
BPH is not a form of prostate cancer and does not lead to prostate cancer. Accordingly, BPH is not life-threatening. However, as many men know, BPH may be lifestyle-threatening and can cause great discomfort, inconvenience, and awkwardness and complications such as:
| | • | | Acute urinary retention, which is a condition that results in a complete inability to urinate. A tube called a catheter may be needed to drain urine from the bladder. |
| | • | | Chronic urinary retention, which is a partial blockage of urine flow that causes urine to remain in the bladder. In rare cases, this may lead to kidney damage if it goes undiagnosed for too long. |
| | • | | Urinary tract infection, which can cause pain or burning during urination, foul-smelling urine, or fever and chills. |
| | • | | Other complications from BPH may include bladder stones or bladder infections. |
| | • | | Having BPH does not directly affect one’s sexual function. However, it is common for the symptoms of BPH and sexual dysfunction to occur at the same time. |
Prevalence of BPH and Market Opportunity
BPH is an age-related disorder the incidence of which increases with maturation of the population. According to urologyhealth.org, by age 60, more than half of men have BPH. By age 85, about 90 percent of men have BPH. As the population continues to age and life expectancy increases, the prevalence of BPH can be expected to continue to increase.
Treatment Alternatives for BPH
Several types of treatments are available for enlarged prostate. They include medications, surgery and minimally invasive surgery. The best treatment choice for patients depends on several factors, including how much the symptoms bother them, the size of their prostate, other health conditions the patients may have, their age and preference. If symptoms are not severe, a patient may decide not to have treatment and wait to see whether their symptoms become more bothersome over time.
Watchful Waiting
When a patient first develops symptoms caused by BPH, physicians generally prescribe drugs as the first treatment option, but usually leave the decision to their patients. Due to the low success rate, high costs, side effects and complications associated with BPH drug therapies, some patients diagnosed with BPH prefer to be regularly monitored by their doctors, but choose not to begin a drug therapy. The patients who opt out of therapy fall into a group referred to as “watchful waiting.” Often, BPH symptom persistence and worsening or an acute urinary event may force the patient to move on to some other form of therapy.
Drug Therapy
Medications are the most common treatment for moderate symptoms of prostate enlargement but if a patient stops taking medicine, the symptoms will usually return. Medications used to relieve symptoms of enlarged prostate include several different types of drugs, such as Alpha-Blockers (such as Flomax®) and Alpha Reductase Inhibitors (such as Proscar®). Drug therapy costs approximately $1,000 per year or more in the United States, must be maintained for life, and does not offer consistent relief to a large number of BPH patients. Many of the currently available BPH drugs also have appreciable side effects, such as: headache, fatigue, impotence, dizziness, and low blood pressure.
Surgical Intervention
Two of the primary surgical procedures to treat BPH are transurethral resection of the prostate (“TURP”) and laser procedures. TURP has traditionally been a common procedure for enlarged prostate for many years. It is a procedure in which the prostatic urethra and surrounding diseased tissue in the prostate are trimmed with a telescopic knife, thereby widening the urethral channel for urine flow. While the TURP procedure generally has
5
been considered the most effective treatment available for the relief of BPH symptoms, the procedure has its shortcomings. In the first instance, TURP generally requires from one to three days of post-operative hospitalization. In addition, a substantial percentage, approximately 5-10%, of patients who undergo TURP encounters significant complications, which can include painful urination, infection, impotence, incontinence, and excessive bleeding. Further, retrograde ejaculation, a condition in which semen released during ejaculation enters the bladder rather than exiting the penis, occurs in up to 90% of patients who undergo a TURP procedure, with a long-term side effect in up to 75% of such patients.
Laser surgeries (also called laser therapies) use high-energy lasers to destroy or remove overgrown prostate tissue. Options for laser therapy depend on prostate size, the location of the overgrown areas. During prostate laser surgery, a combined visual scope and laser is inserted through the tip of the patient’s penis into the urethra, which is surrounded by the prostate. Using the laser, doctors remove prostate tissue that are squeezing the urethra and blocking urine flow, thus making a new larger tube for urine to pass through. Lasers use concentrated light to generate precise and intense heat. Risks of laser surgery include: temporary difficulty urinating and post treatment catheterization, urinary tract infection, narrowing of the urethra as scars form, retrograde ejaculation, and erection problems. Accordingly, neither drug therapies nor the surgical alternatives appear to provide fully satisfactory, cost-effective treatment solutions for BPH sufferers.
Our Approach: The Prolieve Thermodilatation System
The Prolieve Thermodilatation System was originally and primarily developed and commercialized by our current management, product development, clinical and regulatory teams. Such development occurred while such teams were employed at Celsion Corporation from 1997 to 2004, at an estimated cost of $20,000,000. Further, the development and commercialization occurred under the leadership of Dr. Augustine Cheung, who was Celsion’s president at the time. Dr. Cheung is currently our chief executive officer. As discussed above, Celsion sold the Prolieve system, technology and related assets to Boston Scientific Corporation in 2007 for $60 million. In June 2012, Medifocus reached an agreement with Boston Scientific for the purchase of all of the assets of its Prolieve business, including all Prolieve inventory, the mobile service distribution assets, as well as the intellectual property associated with the Prolieve technology.
Prolieve is an in-office procedure that minimizes patient discomfort and the need for post-treatment catheterization. In a randomized one-year clinical trial, conducted at 14 centers across the United States, patients undergoing treatment with Prolieve achieved measurably greater improvement in symptoms after three months compared to a control group using a drug, Proscar, which is commonly prescribed to treat BPH condition.
The purpose of the Prolieve system is to provide a relatively painless and effective alternative to drug therapy and certain types of surgical procedures to treat the symptoms of BPH. Prolieve is a minimally invasive treatment option for BPH. Unlike other microwave-based BPH treatments, Prolieve utilizes both microwave heat, delivered via a catheter, and proprietary balloon compression to both heat the prostate and dilate the prostatic urethra, and to shrink enlarged prostate tissue. The 45-minute Prolieve treatment is administered on an outpatient basis in a physician’s office and can be done with topical anesthesia only. We estimate that over 100,000 patients have been treated since the FDA PMA was granted. Many patients treated with Prolieve experience immediate symptom relief. Based upon a study conducted by Boston Scientific (the “Prolieve Study”), patients treated with the Prolieve system experienced a symptom reduction of 22% three days following treatment. Furthermore, most patients that undergo the Prolieve treatment do not require post-treatment catheterization. Based upon the Prolieve Study, 94% of patients that underwent the Prolieve treatment were catheter free immediately following the treatment, and 100% of such patients were catheter free after three days. Accordingly, we believe that patients that undergo the Prolieve treatment should be able to resume their normal activities shortly after the treatment.
The Prolieve system is comprised of two components. The first component is a freestanding module that contains a microwave generator and computerized controls that regulates and monitors the delivery of heat to the enlarged prostate tissue. The second component is our proprietary disposable catheter that is attached to the module. This component contains an internal balloon that is inflated after it is inserted through the urethra to the point of constriction. Upon inflation of the balloon, the tissue is heated by microwaves delivered via the catheter, resulting in dilation of the urethra. Our computer system in the module monitors and regulates the heat being applied to ensure maximum safety and efficiency. The Prolieve system is covered by 55 core patents, which were acquired as part of the acquisition of the Prolieve assets from Boston Scientific Corporation in 2012.
6
The combined effect of this “heat plus compression” therapy is twofold: first, the heat denatures the proteins in the wall of the urethra, causing a stiffening of the opening created by the inflated balloon, forming a biological stent. Second, the heat serves effectively to kill off prostate cells outside the wall of the urethra, thereby creating sufficient space for the enlarged natural opening. In addition, the Prolieve system’s temperature (46º C to 54º C) is sufficient to kill prostatic cells surrounding the urethra wall, thereby creating space for the enlargement of the urethra opening. However, the relatively low temperature is not sufficient to cause swelling in the urethra.
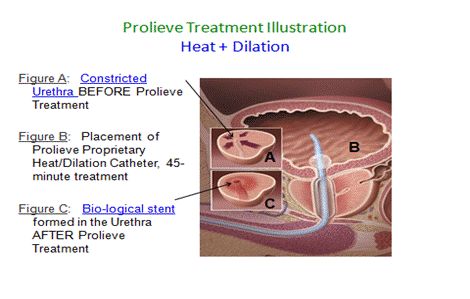
The Prolieve system is designed with patients’ needs and comfort in mind. In general, it does not require sedation or post-operative catheterization and provides rapid symptomatic relief from BPH. BPH patients can be treated using Prolieve in urologic offices throughout the United States. In addition, the Prolieve treatment is also made available to physicians utilizing our nationwide mobile service.
The Prolieve system is currently in use in the United States. Although it is generating revenue, (gross revenues of $2,261,370 for the six months ended September 30, 2015) our Prolieve operations are not, and have never been profitable, and there can be no assurance that they will ever become profitable.
Since acquiring the Prolieve assets from Boston Scientific Corporation in July 2012, we have been concentrating our corporate development efforts on developing these assets into a business. We are focusing on increasing sales from our installed base of systems, and from our mobile systems, described below. We have been increasing the number of persons directly supporting our Prolieve operations from eight in July 2012 to twenty-one at September 30, 2015. The Prolieve operations are currently supported by three sales professionals, eleven mobile technicians, one schedule coordinator, three persons responsible for regulatory compliance matters, and three engineering and support staff.
Boston Scientific Corporation had sold approximately 250 Prolieve systems and approximately 80,000 disposable catheter kits in the United States prior to Boston Scientific Corporation’s sale of the Prolieve assets to us in 2012. Our current business strategy is to increase revenues from these installed systems. In the U.S. market, we do not intend to actively market the Prolieve system itself but, rather, our strategy is to grow revenue through the direct sale of disposable catheter kits to physicians with Prolieve systems installed and, increasingly, through our mobile service, which eliminates physicians’ need to purchase, and learn how to operate, the Prolieve system. However, if U.S. or international customers choose to purchase the Prolieve system itself, we will accommodate such costumers’ needs to the best of our ability.
We currently have approximately 120 systems that were acquired as part of the Prolieve asset purchase from Boston Scientific Corporation. We do not currently have an agreement with a manufacturer for the production of additional Prolieve systems, although we believe that there are several qualified medical device contract manufacturers, including Sanmina, that are capable of manufacturing the system if our current inventory is depleted. At this time, 100% of our revenues come from the sales of our disposable catheters used in each treatment or the provision of mobile services that provide therapy using our disposable catheters. The disposable catheters are manufactured in Mexico by Lake Region Medical Center, formerly known as Accelent Corporation. We currently have an
7
agreement with Lake Region Medical Center to supply these catheters, pursuant to which we order the number of catheters we estimate we will need for a 12-month period. We have no other source of catheters at the present time. Due to the complicated nature of these kits, as well as FDA manufacturing standards imposed on suppliers, the Company does not believe that an alternate supplier of catheters is readily available.
In addition to the Prolieve technology, the installed base of Prolieve systems and related patents acquired from Boston Scientific Corporation, we also acquired a fleet of 15 vans, each equipped with two Prolieve systems. This mobile fleet allows us to provide Prolieve therapy to patients whose health care providers do not have access to one of our permanently installed systems. The mobile Prolieve system is identical to the permanently installed systems.
Our mobile Prolieve systems are deployed by our dispatcher and scheduler upon the request of a physician. Our scheduler then coordinates the timing of the requested appointment with one of our medical technicians. On the day of the appointment, our medical technician arrives at the physician’s office and the Prolieve module is brought into the physician’s office. Under the physician’s supervision, a catheter is inserted into the urethra to the point of constriction, and the Prolieve treatment is administered by our medical technician under the physician’s supervision. In most cases, the patient’s symptoms are eliminated immediately and normal urination bladder function is restored.
Competition
There are several treatment options for BPH. The first is traditional surgery, known as trans-urethral resection procedure, or “TURP.” This surgery requires a hospital stay, sedation, and a post-operative recovery period. Further, we are aware of two other minimally invasive, microwave-based, treatments with which we compete. The first such treatment is offered by Urologix. The second is offered by Thermatrix. Unlike these two treatments, which solely utilize heat, our Prolieve therapy combines heat and compression (via the inflated balloon).
According to Medtech Insight, the surgical and minimally invasive treatment market for BPH is approximately $150 million in the U.S. However, the majority of BPH patients undergoing treatment today choose medical therapy instead of surgery. Pursuant to such medical therapy, patients take daily doses of medicine to shrink the prostate in order to improve function. These medicines are known to cause side effects, and must be taken daily to be effective. We believe our Prolieve treatment can be a viable alternative to drug therapy due the demonstrated efficacy and side effect profile. We intend to explore the possibility of delivering the Prolieve treatment using our mobile service at general practitioners’ offices to provide a treatment option for BPH patients who are in drug therapy and patients on “Watchful Waiting”.
Prescribed medicines for BPH treatment in major industrialized countries is currently believed to be approximately $4 billion annually. These medicines are manufactured and sold by some of the world’s largest pharmaceutical companies, including Eli Lilly, Glaxo Smith Kline and Merck & Co. These companies market their drugs to physicians and directly to the public through television, radio, the internet and conventional print media. With the substantial investment made by these companies in developing, commercializing and marketing these drugs, and the size of the BPH treatment market, these companies represent a significant competitive threat to our Prolieve therapy, and to our company. We are also aware that non-prescription herbal supplements promoted to relieve BPH symptoms are being aggressively marketed to the public; these products also compete with Prolieve.
2.Adaptive Phased Array Technology (APA 1000)
The APA 1000, which is a minimally invasive breast cancer treatment, is developed, but has not been cleared by the FDA for commercial use. Both Phase I and Phase II clinical trials were completed by Celsion, establishing the system’s safety and efficacy on a limited scale. We have begun pivotal Phase III clinical trials, but have proceeded slowly in such trials because of insufficient funds. We are planning to complete the pivotal Phase III clinical trial of APA 1000 when we obtain adequate funding to do so. The Phase III clinical trial is designed to demonstrate that the combination of focused heat and neo-adjuvant chemotherapy could shrink the size of the tumor 40% more over using chemotherapy alone. In the Phase II clinical trial, a 50% increase in tumor size reduction using focused heat and neo-adjuvant chemotherapy was observed over using chemotherapy alone. In the Phase II trial, two heat treatments were applied while in the Phase III trial, three heat treatments are applied. We believe that, if the Phase III trial is successful, it will show that the combination of focused heat and neo-adjuvant chemotherapy could downsize a cancer tumor enough to allow a surgeon to perform a lumpectomy rather than a mastectomy, thereby preserving the affected breast.
The APA 1000 system delivers heat precisely to breast tumors. While using heat to kill cancerous tumors has been considered effective for many years, heat therapy has not become a part of standard treatment for cancer because of the inability to safely apply it to tumors without damaging healthy tissue. When treating cancer, physicians seek to
8
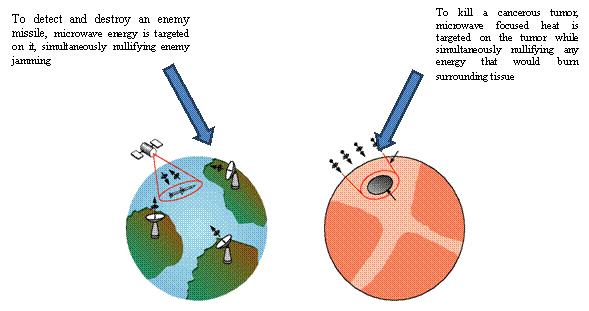
minimize damage to healthy tissue. It is our belief that the APA 1000 system precisely focuses microwave heat on diseased tissue, sparing adjacent tissue. Precision is achieved through the utilization of “Star Wars” technology that we have exclusively licensed from MIT and have adapted for medical use in our APA 1000 system.
Adaptive Phased Array Technology Illustration
Our current management team has been working with researchers at Massachusetts Institute of Technology (“MIT”) who had developed, originally for the U.S. Department of Defense, a microwave control technology known as “Adaptive Phased Array,” or “APA.” This technology permits properly designed microwave devices to focus and concentrate energy targeted at diseased tissue areas deep within the body and to heat them selectively, without adverse impact on surrounding healthy tissue. Since licensing the APA technology from MIT, our management team has been working together with Dr. Alan J. Fenn, the inventor of the patented technology. This collaboration has included technology transfer and technical/engineering assistance to develop and design our current APA Breast Cancer treatment device. In addition, Dr. Fenn has collaborated and advised the Company on the design of the clinical protocol, clinical study support, and device usage training of the current FDA breast cancer study as well as assisting the Company in developing new clinical protocol and new treatment devices using the APA technology licensed from MIT.
In the treatment of breast cancer, the APA technology applies the same principal used in MIT’s “Star Wars” program of detecting missiles.
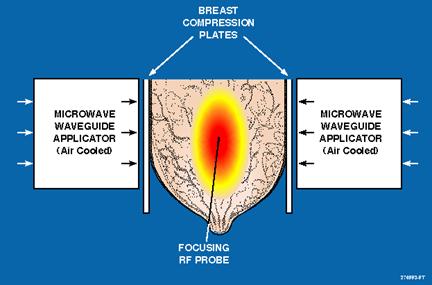
APA 1000 Breast Cancer Treatment Illustration
| | • | | An RF needle probe inserted at tumor center provides feedback signal to focus microwave energy at tumor center to induce shrinkage without harming surrounding tissue. |
| | • | | Focused microwave energy (43-44°C) combined with chemotherapy achieves an average of 88% tumor size reduction in Phase II clinical trials. |
9
Treatment with APA 1000 may accomplish several objectives. First, we believe that it destroys many cancer cells, and substantially shrinks cancerous cells that are not destroyed. If tumors are shrunk small enough, a patient may not need to have the entire breast removed. Second, we believe that the application of APA 1000 heat therapy boosts the effectiveness of subsequent chemotherapy and radiation therapy.
There can be no assurance that we will complete the pivotal Phase III clinical trial, or that the FDA will approve of the APA 1000 for sale in the United States. Even if the APA 1000 successfully completes the pivotal Phase III clinical trial and the FDA permits us to sell this system, there can be no assurance that it will be adopted by health care industry.
As stated earlier, we are progressing at a slow pace through Phase III clinical trials due to lack of funding, and are currently focusing our corporate activities and resources on expanding our Prolieve operations. We estimate that the cost of completing Phase III clinical trials will be approximately $7,500,000. We currently plan, subject to obtaining financing, to fully resume of the pivotal Phase III trial during the present calendar year, although there can be no assurance we will resume clinical trials.
10
Further, in anticipation of commencing the pivotal Phase III clinical trial, we previously negotiated arrangements with physicians and medical centers in the United States and Canada to conduct this trial. Because the pace of the trial has been slow, there can be no assurance that the persons and institutions with which we have previously made arrangements will be available to proceed on the same terms, or at all, when we are ready. In such event, we would then need to make alternative arrangements, of which there can be no assurance.
5. Going Concern
The Company’s operations are subject to certain risks and uncertainties including, among others, current and potential competitors with greater resources, dependence on significant customers, lack of operating history and uncertainty of future profitability and possible fluctuations in financial results. Since inception, the Company has incurred substantial operating losses, principally from expenses associated with the Company’s research and development, financing activities, and development of new technologies. The Company believes these expenditures are essential for the commercialization of its technologies. The Company expects its operating losses to continue for the foreseeable future as it continues its product development efforts and undertakes its sales and marketing activities. Due to continued substantial operating losses, there is substantial doubt regarding the Company’s ability to continue as a going concern. The Company’s ability to achieve profitability is dependent upon its ability to obtain governmental approvals, produce, and market and sell its new product candidates. There can be no assurance that the Company will be able to commercialize its technology successfully or that profitability will ever be achieved. The operating results of the Company have fluctuated significantly in the past. The Company expects that its operating results will fluctuate significantly in the future and will depend on a number of factors, many of which are outside the Company’s control.
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from the sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Additionally, the Company is not in compliance with the provisions of outstanding debt agreements, and it has not remitted quarterly royalty payments to Boston Scientific Corporation pursuant to the terms of its purchase agreement for Prolieve. The Company has not paid interest owing to certain holders of the convertible debentures, and is in a technical default of the terms of the debentures. The investors have not accelerated the terms of the debenture. Management is continuing its efforts to obtain additional funds through equity financing and through the negotiation of debt agreements to ensure that the Company can meet its obligations and sustain operations.
The financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue in existence.
11
6. Results of Operations
Comparison of Three Months Ended September 30, 2015 and 2014
The table below summarizes our results of operations for the three months ended September 30, 2015 and 2014:
| | | | | | | | |
| | | Three months ending
September 30, | |
| | | 2015 | | | 2014 | |
Sales | | | | | | | | |
Products | | $ | 357,006 | | | $ | 179,855 | |
Services | | | 779,354 | | | | 461,842 | |
| | | | | | | | |
Total Sales | | | 1,136,360 | | | | 641,697 | |
| | | | | | | | |
Costs of Sales | | | | | | | | |
Products | | | 172,977 | | | | 117,576 | |
Services | | | 641,284 | | | | 681,229 | |
| | | | | | | | |
Total Costs of Sales | | | 814,261 | | | | 798,805 | |
| | | | | | | | |
Gross Profit | | | 322,099 | | | | (157,108 | ) |
| | | | | | | | |
Operating Expenses | | | | | | | | |
Research and development | | | 161,903 | | | | 73,226 | |
Sales and marketing | | | 409,530 | | | | 440,962 | |
General and administrative | | | 708,473 | | | | 754,273 | |
| | | | | | | | |
Total Operating Expenses | | | 1,279,906 | | | | 1,268,461 | |
| | | | | | | | |
Loss from Operations | | | (957,807 | ) | | | (1,425,569 | ) |
| | | | | | | | |
Other Income (Expense) | | | | | | | | |
Interest and discount accretion | | | (343,758 | ) | | | (398,204 | ) |
Loss from change in fair value of contingent consideration | | | (44,492 | ) | | | (189,228 | ) |
Net loss from equity method investment | | | — | | | | (55,735 | ) |
Other income (expense) | | | 47,879 | | | | 24,522 | |
| | | | | | | | |
Total Other Income (Expense) | | | (340.371 | ) | | | (618,645 | ) |
| | | | | | | | |
Net Loss | | $ | (1,298,178 | ) | | $ | (2,044,214 | ) |
| | | | | | | | |
Sales
The Company’s revenue from the sale of its Prolieve system products and services increased from $641,697 for the three months ended September 30, 2014 to $1,136,360 for the three months ended September 30, 2015. Product sales in 2015 and 2014 consisted solely of single-use catheters. The increase of 77% was due to a significant decrease in product revenue in 2014 as a result of our restructuring of the Prolieve operation initiated in August 2014.
We anticipate that sales of Prolieve products and service will continue to increase in fiscal year 2016 as a result of our continuing efforts to sell our single-use catheters product and services across the U.S. While we expect single-use catheter product sales to increase in 2016 over the 2015 levels, we expect sales of our mobile services to continue to increase at a somewhat higher rate.
Costs of Good Sold, Costs of Services and Gross Profit
The costs of sales for products primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our Prolieve intellectual property, warranty costs, warehousing costs, freight and handling charges. Costs of sales for services consist primarily of the cost of products sold to customers on a first-in
12
first-out basis, along with amortization expense of our Prolieve intellectual property, warranty costs, warehousing costs, freight, handling charges, and additionally, costs to provide mobile services to our patients, including depreciation of our mobile consoles and vehicle fleet, payroll, benefit costs and travel expenses.
Costs of products sold as a percentage of product sales was 48% for the three months ended September 30, 2015 as compared to 65% for the three months ended September 30, 2014, and costs of services as a percentage of services sales was 82% for the three months ended September 30, 2015 as compared to 148% for the three months ended September 30, 2014. As a result, total gross profit increased from a negative gross profit of $157,108 for the three months ended September 30, 2014 to a gross profit of $322,099 for the three months ended September 30, 2015. The gross profit related to our product sales increased as fixed amortization expense of our intangible assets had a lesser impact. Gross profit related to our services sales increased due to the Company’s restructuring of the Prolieve business in August 2014. We anticipate that as our sales continue to grow and that our gross margins on both product sales and services will continue to increase as the effect of fixed charges have a relative lesser impact on total margin and the Company strives to be cost effective.
Net Loss
Our net loss of $1,298,178 decreased from our net loss of $2,044,214 for the three months ended September 30, 2015 and 2014, respectively. The decrease in our net loss is due to the increase in our gross profit of approximately $480,000 along with other expenses of approximately $280,000 for the three months ended September 30, 2015 as compared to the same period in 2014.
Research and Development Expenses
For the three months ended September 30, 2015, the Company incurred research and development expenses of $161,903 an increase from the $73,226 for the three months ended September 30, 2014. Research and development expenses for both periods consisted primarily of costs incurred with respect to the Phase III clinical study for our APA 1000 Breast Cancer System, as well as costs related to the Prolieve post-marketing study.
Sales and Marketing Expenses
Sales and marketing expenses include the costs of our sales force (including labor, travel, and other direct marketing expenses) for Prolieve and other business promotion costs.
Sales and marketing expenses for the three months ended September 30, 2015 were $409,530, a decrease of 7% from the three months ended September 30, 2014 expenses of $440,962. The decrease is primarily the result of the restructuring of the Prolieve business in the Company’s efforts to reduce costs by eliminating certain sales positions and scaling back marketing activities and travel expenses. The company continues to monitor sales and marketing costs related to our efforts to the Prolieve business more efficiently.
General and Administrative Expenses
General and administrative expenses for the three months ended September 30, 2015 decreased 6% to $708,473, from the three month ended September 30, 2014 expenses of $754,273, as there was a decrease in legal and accounting fees, corporate salaries and related benefits and consultant fees as the company focused on operating more efficiently. Further, our expenses associated with legal and accounting fees and consulting fees were higher during the three months ended September 30, 2014 because the company was going through the process of initial registration under the Exchange Act. The company will continue to find ways to decrease costs related to corporate expenses.
Other Income (Expense)
Other income (expense) primarily consists of interest and accretion expense, losses from change in the fair value of the contingent consideration. Total other income (expense) of ($340,371) in 2015 reflects a decrease from our total other income (expense) of ($618,645) in September 30, 2014 primarily due to changes in the fair value of the contingent consideration in 2015.
13
Comparison of Six Months Ended September 30, 2015 and 2014
The table below summarizes our results of operations for the six months ended September 30, 2015 and 2014:
| | | | | | | | |
| | | Six months ending
September 30, | |
| | | 2015 | | | 2014 | |
Sales | | | | | | | | |
Products | | $ | 684,694 | | | $ | 1,058,654 | |
Services | | | 1,577,276 | | | | 1,232,512 | |
| | | | | | | | |
Total Sales | | | 2,261,370 | | | | 2,291,166 | |
| | | | | | | | |
Costs of Sales | | | | | | | | |
Products | | | 348,870 | | | | 540,301 | |
Services | | | 1,296,102 | | | | 1,414,673 | |
| | | | | | | | |
Total Costs of Sales | | | 1,644,972 | | | | 1,954,974 | |
| | | | | | | | |
Gross Profit | | | 616,398 | | | | 336,192 | |
| | | | | | | | |
Operating Expenses | | | | | | | | |
Research and development | | | 244,546 | | | | 125,997 | |
Sales and marketing | | | 662,897 | | | | 1,090,337 | |
General and administrative | | | 1,303,470 | | | | 1,598,928 | |
| | | | | | | | |
Total Operating Expenses | | | 2,210,913 | | | | 2,815,262 | |
| | | | | | | | |
Loss from Operations | | | (1,594,515 | ) | | | (2,479,070 | ) |
| | | | | | | | |
Other Income (Expense) | | | | | | | | |
Interest and discount accretion | | | (685,013 | ) | | | (671,848 | ) |
Loss from change in fair value of contingent consideration | | | (81,640 | ) | | | (189,228 | ) |
Net loss from equity method investment | | | — | | | | (55,735 | ) |
Other income (expense) | | | 34,921 | | | | 12,049 | |
| | | | | | | | |
Total Other Income (Expense) | | | (731,732 | ) | | | (904,762 | ) |
| | | | | | | | |
Net Loss | | $ | (2,326,247 | ) | | $ | (3,383,832 | ) |
| | | | | | | | |
Sales
The Company’s revenue from the sale of its Prolieve system products and services decreased from $2,291,166 for the six months ended September 30, 2014 to $2,261,370 for the six months ended September 30, 2015. Product sales in 2015 and 2014 consisted solely of single-use catheters. The slight decrease of total sales for the six months ended September 30, 2015 is due to a significant decrease in product sales of 35.3% offset by increase in service revenue of 28.0% from 2014 as a result of our restructuring of the Prolieve operation initiated in August 2014.
We anticipate that sales of Prolieve products and service will continue to increase in fiscal year 2016 as a result of our continuing efforts to sell our single-use catheters product and services across the U.S. While we expect single-use catheter product sales to increase in 2016 over the 2015 levels, we expect sales of our mobile services to continue to increase at a somewhat higher rate.
Costs of Good Sold, Costs of Services and Gross Profit
The costs of sales for products primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our Prolieve intellectual property, warranty costs, warehousing costs, freight and handling charges. Costs of sales for services consist primarily of the cost of products sold to customers on a first-in
14
first-out basis, along with amortization expense of our Prolieve intellectual property, warranty costs, warehousing costs, freight, handling charges, and additionally, costs to provide mobile services to our patients, including depreciation of our mobile consoles and vehicle fleet, payroll, benefit costs and travel expenses.
Costs of products sold as a percentage of product sales was 51% for the six months ended September 30, 2015 and September 30, 2014, and costs of services as a percentage of services sales was 82% for the six months ended September 30, 2015 as compared to 115% for the three months ended September 30, 2014. As a result, total gross profit increased from $336,192 for the six months ended September 30, 2014 to $616,398 for the six months ended September 30, 2015. The gross profit percentage related to our product sales remained constant for the six month period ended September 30, 2015. Gross profit related to our services sales increased due to the Company’s restructuring of the Prolieve business in August 2014. We anticipate that as our sales continue to grow, margins on both product sales and services will increase as the effect of fixed charges continue to have a relative lesser impact on total margin.
Net Loss
Our net loss of $2,236,247 decreased from our net loss of $3,383,832 for the six months ended September 30, 2015 and 2014, respectively. The significant decrease in our net loss is due to several factors. The company had completed its restructuring of the Prolieve business noted above, lower general and administrative and sales and marketing expenses in the current year, and decreased losses on the fair value of the contingent consideration offset by increased research and development expenses.
Research and Development Expenses
For the six months ended September 30, 2015, the Company incurred research and development expenses of $244,546 an increase from the $125,997 for the six months ended September 30, 2014. Research and development expenses for both periods consisted primarily of costs incurred with respect to the Phase III clinical study for our APA 1000 Breast Cancer System, as well as costs related to the Prolieve post-marketing study.
Sales and Marketing Expenses
Sales and marketing expenses include the costs of our sales force (including labor, travel, and other direct marketing expenses) for Prolieve and other business promotion costs.
Sales and marketing expenses for the six months ended September 30, 2015 were $662,897, a decrease of 39% from the six months ended September 30, 2014 expenses of $1,090,337. The decrease is primarily the result of the restructuring of the Prolieve business in the Company’s efforts to reduce costs by eliminating certain sales positions and scaling back marketing activities and travel expenses. The company continues to monitor sales and marketing costs related to our efforts to the Prolieve business more efficiently.
General and Administrative Expenses
General and administrative expenses for the six months ended September 30, 2015 decreased 19% to $1,303,470, from the six month ended September 30, 2014 expenses of $1,598,928, as there was a decrease in legal and accounting fees, corporate salaries, payroll costs and consultant fees as the company focused on operating more efficiently. Further, our expenses associated with legal and accounting fees and consulting fees were higher during the six months ended September 30, 2014 because the company was going through the process of initial registration under the Exchange Act. The company will continue to find ways to decrease costs related to corporate expenses.
Other Income (Expense)
Other income (expense) primarily consists of interest and accretion expense, losses from our equity method investments and changes in the fair value of contingent consideration related to our Prolieve acquisition. Total other income (expense) of ($731,732) in 2015 reflects decreased expense from our total other income (expense) of ($904,762) in September 30, 2014 is primarily due to decreased losses in the fair value of the contingent consideration.
15
7. Business Acquisition
On July 24, 2012 the Company purchased from Boston Scientific Corporation all of the assets, and assumed certain liabilities, relating to the Prolieve Thermodilatation System (“Prolieve”), a FDA approved device for the treatment of Benign Prostatic Hyperplasia (“BPH”). The total purchase consideration consisted of the following:
| | | | |
Cash | | $ | 2,535,610 | |
Fair value of contingent consideration | | | 1,126,505 | |
| | | | |
Total consideration | | $ | 3,662,115 | |
| | | | |
The maximum amount payable pursuant to the terms of the contingent consideration is $2.5 million; its fair value was determined by calculating its present value based on its payment terms using an interest rate of 24% (the Company’s estimated unsecured borrowing rate). The contingent consideration is paid quarterly at a rate of 10% of sales of Prolieve products. The fair value of the contingent consideration is adjusted for changes in the estimated future payments with the amount of adjustment reflected in profit or loss.
The Company accounted for its acquisition of Prolieve by recording all tangible assets and intangible assets acquired, and liabilities assumed, at their respective fair values on the acquisition date. The fair value assigned to identifiable intangible assets acquired was determined using a cost approach and was based on the Company’s best estimates; this intangible asset is being amortized on a straight-line basis over its estimated useful life of ten years.
The following summarizes the fair value of the assets acquired assumed in the transaction:
| | | | |
Inventory | | $ | 463,338 | |
Equipment | | | 736,662 | |
Intangible assets | | | 2,462,115 | |
| | | | |
Total consideration | | $ | 3,662,115 | |
| | | | |
8. Medifocus Holding Joint Venture
On November 8, 2013, we entered into an agreement with Ideal Concept Group Limited (“Ideal Concept”) to develop our Prolieve business and APA technology in a geographic area referred to as “Asia Pacific” in the agreement (the “JV Agreement”). The countries comprising of Asia Pacific are not specified in the JV Agreement. Pursuant to the JV Agreement, Medifocus and Ideal Concept agreed to capitalize a company, Medifocus Holding Limited (“Medifocus Holding”), to develop this business. Medifocus Holding was incorporated in the British Virgin Islands on June 28, 2012.
The JV Agreement states that, at the outset, Ideal Concept will own 60% of Medifocus Holding and the Company will own 40%. Through September 30, 2015, Medifocus Inc. has made total contributions to Medifocus Holding of approximately $214,735 in cash and Prolieve equipment. In addition to capital contributions, the shareholders are obligated to provide loans to the JV of up to HKD 4,000,000 (or approximately $520,000). Ideal Concept previously agreed, through November 8, 2014, to loan the Company funds necessary to satisfy our portion of the required shareholder contributions to Medifocus Holding. Such loan would bear interest at 6% per year and be secured by our ownership interest in Medifocus Holding. No such loans were made to us by Ideal Concept and, based on Medifocus Holdings’ current business plan; we do not expect to make any further investments or loans in the joint venture.
Pursuant to the terms of the JV Agreement and a License and Distribution Agreement dated as of November 8, 2013, Medifocus Holding will engage in clinical testing, and obtaining approval from China Food and Drug Administration of the People’s Republic of China (“CFDA”) for all products relating to Prolieve and the APA technology. Medifocus Holding has been in communication with the CFDA and is currently in the process of preparing the required documentation with the CFDA for commercialization of Prolieve in China. There is no assurance that the CFDA will approve Prolieve for commercialization in China. Additionally, Medifocus Holding has been in discussions with several hospitals in China regarding conducting clinical testing. As of the date of this quarterly report, no clinical testing has begun in China.
16
The JV Agreement outlines the respective obligations of Ideal Concept and ourselves. The Company will be responsible for: (i) providing Medifocus Holding with an exclusive license to our rights in the Prolieve business and APA technology; (ii) applying for and maintaining the patents and other intellectual property rights throughout the world; (iii) directing and managing all research and development activities in Asia Pacific; and (iv) providing on-site and off-site technical support and training to Medifocus Holding’s personnel. Ideal Concept’s responsibilities will include: (i) formulating a business plan to evaluate opportunities in Asia Pacific; (ii) assisting in the performance of clinical trials relating to CFDA approval; (iii) providing assistance in establishing manufacturing arrangements for products; and (iv) managing the financial affairs of Medifocus Holding, including the cash flow, arranging funding, and assisting in developing markets in Asia Pacific.
Medifocus Holding is required to pay us a royalty of 5% of the first $10,000,000 in sales of the catheter kits and control units utilized in the Prolieve business. After $10,000,000 in sales has been reached, the royalty decreases to 3%. For all other products we develop, Medifocus Holding is required to pay us a royalty of 7.5% on net sales of such products.
9. Liquidity and capital resources
The Company’s primary cash requirements are to fund operations, including research and development programs and collaborations, to support general and administrative activities, and to fund acquisitions of products or businesses. The Company’s future capital requirements will depend on many factors, including, but not limited to:
| | • | | sales of the Company’s Prolieve products and services; |
| | • | | pricing and payment terms with customers; |
| | • | | costs of raw materials and payment terms with suppliers; |
| | • | | capital expenditures and equipment purchases to support product launches; and |
| | • | | business and product acquisitions. |
In December 2013, the Company raised gross proceeds of $3.6 million from the sale of convertible redeemable promissory notes and warrants (the “Units”). Each Unit consists of (i) a $10,000 face value convertible redeemable promissory note, bearing 8% annual interest and due in three years (“Note”), which is convertible into shares of common stock beginning six months after the Closing Date of the offering at a conversion price of $0.25 per share, and (ii) three-year warrants to purchase 20,000 shares of common stock at a price of $0.30 per Share. The net proceeds from the offering is to be used for Prolieve operations and for general corporate purposes, including research and development activities, capital expenditures, repayment of debt and working capital.
In a second closing in March 2014, the Company issued 200 additional Units to the investors, receiving gross proceeds of $2,000,000. The additional notes are convertible into 8,000,000 shares of common stock. Each warrant entitles the holder to acquire 20,000 common shares (for a total of 4,000,000 common shares) at an exercise price of $0.30 per share and expire on December 18, 2016. The warrants were classified as equity and were recorded as additional paid in capital at their estimated fair value of $572,999.
Our $0.6 million unsecured promissory note made to a lender in July 2012 and the accrued but unpaid interest of $0.2 million as of December 31, 2013, was originally due October 23, 2013. The lender extended the due date of this promissory note to June 30, 2014 and we are currently in discussion with the lender to negotiate a payment plan for this note. During FY 2015, the company made payments towards principal and interest. Subsequent to March 31, 2015 we have made no principal and interest payments to the lender and are currently in negotiations with the lender regarding the extension of the due date. If such negotiations fail, the lender may declare all amounts due and payable immediately. The lender would not have a right to seize any of the Company’s assets because the promissory note is unsecured. Further, if the lender were to retain counsel or initiate litigation to enforce its rights and interests under the promissory note, the Company would be required to pay all reasonable costs and expenses of the lender.
In the fiscal year ending March 31, 2015, the Company raised approximately $1.6 million from the sale of Units. Each Unit was priced at $0.16 and consists of one Share, and a detachable stock purchase warrant to purchase one Share at $0.25 per share.
The Company extends credit to customers on an unsecured basis and payment terms are typical 30 days from delivery or service. The Company’s receivables have increased significantly since its acquisition of Prolieve in July 2012 as a result of increasing sales of Prolieve products and services. Management uses the aging account method to assess the company’s allowance for doubtful accounts. The aging method uses the number of days outstanding the underlying invoices have been past due. Receivables are written off when it is determined that the underlying invoices are uncollectible. The Company maintained an allowance for doubtful accounts of $60,569 as of September 30, 2015.
17
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Management is continuing its efforts to obtain additional funds so that the Company can meet its obligations and sustain operations.
We do not have any committed sources of financing and cannot give assurance that alternate funding will be available in a timely manner, on acceptable terms or at all. We may need to pursue dilutive equity financings, such as the issuance of shares of common stock, convertible debt or other equity-linked securities, which financings could dilute the percentage ownership of our current common stockholders and could significantly lower the market value of our common stock.
Our cash and cash equivalents of approximately $374,799 on hand at September 30, 2015 are not sufficient to fund operations through September 30, 2016. We estimate that the external funding requirement for the next 12 months will be approximately $8 million to grow the Prolieve business in the U.S. and internationally, to accelerate the APA 1000’s Phase III clinical trials, and to start the research and development activities in the heat-activated immunotherapy business that the Company intends to pursue. We will need to raise substantial additional capital in the near future to fund our planned future operations beyond 2015, and we anticipate that such financing transactions will likely be dilutive to our current shareholders. If we are not able to raise additional capital, we will need to take certain measures to reduce our operating costs, including reducing our staff, curtailing our research and development efforts and our clinical trials, and reducing the costs we plan spend to grow our Prolieve business. As such, we would not be able to maintain the growth of the Prolieve business, complete the development, testing and commercialization of our product candidates.
Net Cash Used In Operations
Net cash used in operating activities was $905,925 for the six months ended September 30, 2015 compared to $1,952,429 during the six months ended September 30, 2014. The reduction in the use of cash in 2015 of approximately $1,046,000 is primarily due to the decrease in net loss, an increase in the Company’s accounts payable balances in 2015 compared to significant decreases in accounts payable in 2014 offset by an increases in our inventory levels.
Net Cash Provided by/Used in Investing Activities
Net cash used in investing activities for the six months ended September 30, 2015 and 2014 was $0 and $26,355, respectively, as there were limited investing transactions in both periods.
Net Cash Used in Financing Activities
Net cash provided by financing activities was $1,265,786 for the six months ended September 30, 2014. During the six months ended September 30, 2014, the company received financing of approximately $1,400,000 offset by principal payments of approximately $178,000 on notes payable. There was no financing activity for the six months ended September 30, 2015.
18
10. Risk Factors
An investment in shares of our common stock (which we refer to as the “Shares”) involves a high degree of risk. You should carefully consider the risks described below and the risks described elsewhere in this quarterly report under the sections entitled “Item 4. Information on the Company” before deciding whether to invest in our shares. The following is a summary of the risk factors that we believe are most relevant to our business. These are factors that, individually or in the aggregate, could cause our actual results to differ significantly from anticipated or historical results. The occurrence of any of the risks could harm our business and cause the price of our common stock to decline, and investors may lose all or part of their investment. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. The risks and uncertainties described below and in the incorporated documents are not the only risks and uncertainties that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of these risks actually occurs, our business, financial condition and results of operations would suffer. The risks discussed below also include forward-looking statements, and our actual results may differ substantially from those discussed in these forward-looking statements. See “Special Note Regarding Forward-Looking Statements” at the beginning of Part I of this interim report. Except as required by law, we undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events, or otherwise.
We have a history of significant losses and expect to continue such losses for the foreseeable future.
Since our inception in 2005, our expenses have substantially exceeded our revenues, resulting in continuing losses and an accumulated deficit of $29,226,562 at September 30, 2015. In addition, our net loss for the six month period ended September 30, 2015 was $2,326,247. Such operating losses are the result of our commitment to continuing our product research, development and commercialization programs, which is only partially offset by limited revenues from the sale of our Prolieve system and related disposables. We expect to continue to experience significant operating losses unless and until we generate significant revenue from Prolieve, as well as the development of other new products and these products have been clinically tested, approved by the FDA or other regulatory authorities, and successfully commercialized.
We may not be able to generate significant revenue for the foreseeable future.
Since 2005, we have devoted our resources to developing the APA 1000, but we will not be able to market the APA 1000 until we have completed clinical testing and obtained all necessary governmental approvals. On July 26, 2012, we acquired from Boston Scientific Corporation the Prolieve Thermodilatation system business for the treatment of BPH and, since that time, we have assembled a sales and service team to market the Prolieve system. All of our current revenue is derived from sales of our Prolieve control units and more importantly, our single-use treatment catheters and treatments delivered through our mobile service. There can be no assurance as to how much revenue will be generated by Prolieve sales. Our lack of product diversification means that we may be negatively affected by changes in market conditions and in regulation (including regulation affecting reimbursement for our products). In addition, at the present time our APA 1000 system is still in clinical testing stage and cannot be marketed until we have completed clinical testing and obtained necessary governmental approval. Accordingly, our revenue sources are, and will remain extremely limited until and unless our Prolieve system is marketed successfully and/or until our other new products are clinically tested, approved by the FDA or other regulatory authorities, and successfully commercialized. We cannot guarantee that our products will be successfully tested, approved by the FDA or other regulatory authorities, or commercialized, successfully or otherwise, at any time in the foreseeable future, if at all.
Our future is dependent upon our ability to obtain additional financing. If we do not obtain such financing, we may have to cease our operations and investors could lose their entire investment.
We have yet to operate profitably or generate positive cash flows from operations, and there is no assurance that we will operate profitably or will generate positive cash flow in the future. As a result, we have very limited funds, and such funds may not be adequate to take advantage of current, planned and unanticipated business opportunities. Even if our funds prove to be sufficient to pursue current, planned and unanticipated business opportunities, we may not have enough capital to fully develop such opportunities.
Further, our capital requirements relating to the manufacturing and marketing of our products have been, and will continue to be, significant. We are dependent on the proceeds of future financing in order to continue in business and to develop and commercialize proposed products. There can be no assurance that we will be able to raise the additional capital resources necessary to permit us to pursue our business plan. Finally, the continued growth of our business may require additional funding from time to time to be used by us for general corporate purposes, such as acquisitions, investments, repayment of debt, capital expenditures, repurchase of capital stock and additional purposes identified by the Company.
19
Accordingly, our ultimate success may depend upon our ability to raise additional capital. There can be no assurance that any additional financing will be available to us. As additional capital is needed, we may not be able to obtain additional equity or debt financing. Even if financing is available, it may not be available on terms that are favorable or acceptable to us, or in sufficient amounts to satisfy our requirements. Any inability to obtain additional financing will likely have a material adverse effect on our business operations, and could result in the loss of your entire investment.
Our independent registered public accountants have expressed substantial doubt regarding our ability to continue as a going concern.
Our auditors have expressed their opinion that there is substantial doubt about the Company’s ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of these uncertainties. Our ability to continue as a going concern is dependent upon our ability to successfully raise adequate additional financing and our ability to successfully develop our sales and marketing programs and commence our planned operations. We cannot assure you that we will be able to obtain additional financing or achieve profitability in our operations. Our failure to obtain additional financing or achieve profitability in our operations could require the Company to liquidate our business interests, and could result in the loss of your entire investment.
Our decision to not have a full-time Chief Financial Officer may negatively affect our business and operations.
Our Chief Financial Officer (or “CFO”) serves only on a part-time basis and may be subject to conflicts of interest. The CFO devotes a portion of his working time to other business endeavors, which may lead to conflicts of interest, including deciding how much time to devote to our affairs. The CFO position is critical to our operations, and our failure to fill this position on a full-time basis may negatively impact our business and operations. It may also lead to the late filing of financial reports and other required disclosures, or the filing of noncompliant financial reports and other required disclosures, which could have numerous consequences, including administrative proceedings by the Securities and Exchange Commission (the “SEC”), claims under Section 10 of the Exchange Act and, if our Shares become listed on a national exchange, cease trade orders or the de-listing of the Shares on such exchange. Further, having a part-time CFO has, and may continue to, negatively impact the effectiveness of our disclosure controls and our internal controls over financial reporting. No assurances can be given that our CFO will transition to a full-time basis, or that we will be able to identify or afford a full-time qualified candidate for this position.
We operate with de-centralized management, and may be unable to hire additional personnel to support, manage and control our operations.
Our CFO performs his functions for us on a part-time, non-exclusive basis, and resides in Toronto, Canada. Further, we believe that we are understaffed and need to hire additional personnel to support, manage and control our operations in order to operate optimally. In the past, the combination of not having our CFO at our headquarters and being understaffed have contributed to the late filing of financial reports in Canada. These late filings resulted in temporary cease trade orders being issued, and a multi-month suspension of trading of our shares on the Toronto Venture Exchange (the “TSXV”). Although the Company has recently hired a qualified outside accounting consultant to minimize this risk. Until we become profitable or obtain additional financing, these factors will persist, raising the risk in the future of us making late filings of financial reports in Canada and in the U.S., and having our Shares suspended from trading. Moreover, until we can afford to hire additional staff, we will continue to operate with less than optimal support, management and control of our operations. The Company recently hired a qualified outside accounting consultant to provide support to our management and CFO, but we cannot assure you that such efforts will effectively minimize such risks. If we continue to operate with de-centralized management and insufficient staffing, it may have a material adverse effect on our business.
We identified a material weakness in our internal control over financial reporting that could affect the reliability of our financial statements and have other adverse consequences.
We identified a material weakness in our internal control over financial reporting as of March 31, 2015. We determined that a material weakness existed because we did not employ a sufficient number of qualified accounting personnel to ensure all required adjustments are made to the Company’s books which would allow for the preparation and presentation of the Company’s financial statements in conformity with accounting principles generally accepted in the United States of America.
20
Failure to have effective internal control over financial reporting could impair our ability to produce accurate financial statements on a timely basis which could ultimately lead to a restatement of our financial statements. If, as a result of deficiencies in our internal control over financial reporting, we cannot provide reliable financial statements, our business decision processes may be adversely affected, our business and results of operations could be harmed, investors could lose confidence in our reported financial information and our ability to obtain additional financing, or additional financing on favorable terms, could be adversely affected. In addition, failure to maintain effective internal control over financial reporting could result in investigations or sanctions by regulatory authorities.
In an effort to remediate this material weakness, we retained and have continued to work closely with qualified consultants. Further, we continue to search for qualified personnel and consultants to help ensure proper and timely accounting disclosures. Thus far, retaining outside consultants has not yet remediated this material weakness. Further, we are in the process of updating all internal controls, including financial reporting, and properly documenting all such controls. There can be no assurance that the material weakness identified or that any additional material weaknesses will not arise in the future due to our failure to implement and maintain adequate internal control over financial reporting.
The loss of certain of our key personnel, or any inability to attract and retain additional personnel, could negatively affect our business.
Our future success depends to a significant extent on the continued service of Dr. Augustine Cheung, our President, and John Mon, our Chief Operating Officer. Both of these individuals have been intimately involved with, and primarily responsible for, the invention, development and commercialization efforts for both of our products. The loss of services of either individual would adversely affect our business and our ability to implement our business plan.
Our future success will also depend on our ability to attract, retain and motivate highly skilled personnel to assist us with product development, commercialization and other facets of our business plan. If we fail to hire and retain a sufficient number of qualified individuals to fully meet the needs of the business of the Company, it may have an adverse effect on our business and results of operations.
One of our shareholders owns a significant percentage of our Shares and could exert significant influence over matters requiring shareholder approval.
Mr. Tak Cheung Yam, a former director of the Company, through Integrated Assets Management (Asia) Ltd, currently owns 26,023,106 Shares, or 14.97% of the Company’s outstanding common stock. Together with Integrated Assets Management (Asia) Ltd, Mr. Yam also owns exercisable warrants and options to purchase an additional 18,595,833 Shares. If Mr. Yam chooses to exercise all these warrants and options, he will control 23.18% of our Common Stock. In addition, Mr. Yam, through Integrated Assets Management (Asia) Ltd, owns a convertible note that can be converted into 7,400,000 Shares. If Mr. Yam chooses to convert the note to Shares, and to exercise all his warrants and options, he will effectively control 26.03% of our outstanding shares. As a result, Mr. Yam may have significant influence over our management, our decision-making process, our business strategy and affairs and matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as mergers, consolidations or the sale of substantially all of our assets. Mr. Yam’s interests may differ from those of other shareholders of the Company, and, Mr. Yam will have the ability to exercise influence over our business and may take actions that are not in our or our public shareholders’ best interests. Furthermore, this concentration of ownership may have the effect of delaying or preventing a change in control, including a merger, consolidation or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control, even if such a change in control would benefit our other stockholders.
Our internal sales and marketing capability is limited and we may need to enter into alliances with others possessing such capabilities to commercialize our products internationally.
Currently our primary source of revenue is through the sales of disposable catheter treatment kits and mobile services in the U.S. Consequently, we are dependent upon our limited sales and marketing capability for the successful marketing of our Prolieve system. There can be no assurance that we will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our Prolieve system.
21
We intend to market our other products, if and when such products are approved for commercialization by the FDA or other regulatory authorities, either directly or through other strategic alliances and distribution arrangements with third parties. There can be no assurance that we will be able to enter into third-party marketing or distribution arrangements on advantageous terms or at all. To the extent that we do enter into such arrangements, we will be dependent on our marketing and distribution partners. In entering into third-party marketing or distribution arrangements, we expect to incur significant additional expense. There can be no assurance that, to the extent that we sell products directly or we enter into any commercialization arrangements with third parties, such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our products and services.
We do not manufacture the Prolieve system ourselves, and rely on a third-party supplier to supply us with the proprietary disposable catheters used with our Prolieve system.
The Prolieve systems we currently have in inventory were manufactured by Sanmina Corporation for Boston Scientific Corporation prior to our acquisition of the Prolieve assets, and we do not currently have an agreement with Sanmina for the production of additional Prolieve systems. Accordingly, if our current inventory becomes insufficient to meet the business growth in both the U.S. and international markets, we will have to engage Sanmina Corporation, or another manufacturer, to produce such additional systems. Further, the proprietary disposable catheter kits used with the Prolieve system are manufactured by Lake Regional Medical Center (formerly Accellent Inc.) in its facility in Mexico. Due to the complexity of these catheter kits, as well as FDA standards applicable to manufacturers of such kits, the Company has not identified an alternative supplier for these catheter kits. If, for any reason, we are unable to obtain new Prolieve systems manufactured by Sanmina Corporation, or we are no longer able to purchase the catheter kits from Lake Regional Medical Center in sufficient amounts, on an as-needed basis and on acceptable terms, or if either manufacturer becomes unable or unwilling to continue to supply us with new Prolieve systems and disposable catheter kits, it would have a material adverse effect on our business and operations. There can be no assurance that we could find new manufacturers to fulfill our needs, that any such manufacturer would be FDA approved, or that such manufacturers would be willing to provide us with the required products under commercially acceptable terms. If we are unable to find additional manufacturers and suppliers and it results in a disruption to our business, there would be a material adverse effect on our business and results of operations.
The slow pace of our APA 1000 Breast Cancer System’s Phase III clinical trials could result in additional delays and increased costs of completing the trials in the future.
Our main focus at this time is attaining profitability for our Prolieve business. Accordingly, we have allocated most of our resources to this goal, compounding this with the lack of funding the progress of the pivotal Phase III clinical trials of our APA 1000 breast cancer treatment system has been very slow. We estimate that the Phase III clinical trials will cost approximately $7,500,000. We currently do not have the financing in place to accelerate and complete these trials. There can be no assurance that such financings will be available at all, or on terms favorable to us. Further, there can be no assurance as to when, or even if, we will succeed in making Prolieve profitable. Our inability to do so may make it more difficult for us to raise funds for the pivotal Phase III clinical trial of the APA 1000. In the event that we are able achieve profitable Prolieve operations, there can be no assurance that we will be able to generate enough funds from the Prolieve business to finance the pivotal Phase III clinical trial. Furthermore, we cannot predict the effect of the slow pace of the pivotal Phase III trial could have on the costs and other critical aspects of the Phase III clinical trial. There is the risk that this uncertainty could negatively impact our business plans, and our ability to raise additional funds for further development of our APA 1000 business.
We may not receive regulatory approval from the U.S. Food and Drug Administration (“FDA”) to market the APA 1000.
Drugs and medical devices in the United States are regulated by the FDA, which requires that new medicines and medical devices be demonstrated to be both safe and effective. This is accomplished by conducting staged clinical trials that are subject to the FDA’s review, analysis and approval. While the Phase I and Phase II clinical trials for APA 1000 have been completed, and we received approval from the FDA and Health Canada to begin the pivotal Phase III clinical trials, as of today, a very limited number of patients out of a planned 238 person trial in the pivotal Phase III clinical trial, have been treated with APA 1000. There can be no assurance that our Phase III clinical trial will be completed, and if it is completed, that it will demonstrate APA 1000’s safety and efficacy, and that we will subsequently receive the FDA’s approval for us to commence marketing. In the event that we complete the pivotal Phase III clinical trial and receive FDA approval to market APA 1000, there can be no assurance that APA 1000 will be adopted for use by the healthcare industry, and that this business will be profitable. If the APA 1000 is not adopted for use by the healthcare industry, or we are not able to become profitable, it would have a material adverse effect on our business and results of operations.
22
We may not succeed in developing a meaningful market share of the benign prostatic hyperplasia (“BPH”) treatment markets with Prolieve, and our Prolieve business may not become profitable.
The BPH market is highly competitive, and is presently dominated by large, international pharmaceutical companies that promote the use of proprietary drugs to treat this condition. These companies, which include, Eli Lilly, Glaxo Smith Kline, Merck & Co., and others, aggressively market their drugs to primary care physicians, and to consumers through television, print, digital and other media. Because the market for BPH treatment is large and growing, and the manufacturers of these medications have made substantial investments in their development and marketing, we expect them to vigorously defend their market positions. In addition, we face strong competition from surgical and other minimally invasive treatment modalities. Although we have received a PMA from the FDA for our Prolieve system for the treatment of BPH, we can offer no assurance that the Prolieve system will be accepted by the medical community widely. Because our financial, marketing and sales resources are much smaller than those of the pharmaceutical companies, we are at significant competitive disadvantage, which will make it difficult for us to substantially expand our Prolieve business. Our inability to expand our Prolieve business, achieve profitability and capture significant market share of the BPH treatment market will adversely affect us.
Recent health care reform laws in the U.S. could have a negative impact on our business.
Our business, financial condition, results of operations and cash flows could be significantly and adversely affected by recent healthcare reform legislation, including, most immediately, by the medical device excise tax that became effective on January 1, 2013. The Patient Protection and Affordable Care Act and Health Care and Education Reconciliation Act of 2010 (the “Healthcare Reform Acts”) were enacted into law in March 2010. As a company that operated in the United States, the Healthcare Reform Acts may materially impact our business and operations. Certain provisions of the Healthcare Reform Acts will not be effective for a number of years and there are many programs and requirements for which the details have not yet been fully established. Accordingly, it is unclear what the full impact will be from the Healthcare Reform Acts.
However, beginning in January 2013, the Healthcare Reform Acts impose a 2.3% excise tax on sales of our Prolieve products in the United States. We expect the new tax will materially and adversely affect our business, cash flows and results of operations. The Healthcare Reform Acts also contain a number of Medicare provisions aimed at improving quality and decreasing costs. The Medicare provisions include value-based payment programs, increased funding of comparative effectiveness research, reduced hospital payments for avoidable readmissions and hospital acquired conditions, and pilot programs to evaluate alternative payment methodologies that promote care coordination (such as bundled physician and hospital payments). Additionally, the Healthcare Reform Acts include a reduction in the annual rate of inflation for Medicare payments to hospitals that began in 2011 and the establishment of an independent payment advisory board to recommend ways of reducing the rate of growth in Medicare spending beginning in 2014. We cannot predict what healthcare programs and regulations will ultimately be implemented at the federal or state level, or the effect of any future legislation or regulation. However, any changes that lower reimbursement for our products or reduce medical procedure volumes could have a material adverse effect our business and results of operations.
Our APA 1000 system and future products utilizing the adaptive phased array technology depend on license agreements with MIT to permit us to use patented technologies.
Our success depends, in substantial part, on our ability to maintain our rights under license agreements that grant us the rights to use patented technologies. We have entered into a license agreement with MIT under which we have exclusive rights to commercialize medical treatment products and procedures based on MIT’s Adaptive Phased Array technology. The MIT license agreement contains license fee, royalty and/or research support provisions, testing and regulatory milestones, and other performance requirements that we must meet by certain deadlines. If we were to breach these or other provisions of the license agreement, we could lose our ability to use the subject technology, as well as compensation for our efforts in developing or exploiting the technology. Any such loss of rights and access to technology could have a material adverse effect on our business.
Further, we cannot guarantee that any patent or other technology rights licensed to us by others will not be challenged or circumvented successfully by third parties, or that the rights granted will provide adequate protection. We are aware of published patent applications and issued patents belonging to others, and it is not clear whether any of these patents or applications, or other patent applications of which we may not have any knowledge, will require us to alter any of our potential products or processes, pay licensing fees to others or cease certain activities. Litigation, which could result in substantial costs, may also be necessary to enforce any patents issued to or licensed by us or to determine the scope and validity of others’ claimed proprietary rights. We also rely on trade secrets and confidential information that we seek to protect, in part, by confidentiality agreements with our corporate partners, collaborators, employees, and consultants. We cannot guarantee that these agreements will not be breached, that, even if not breached, that they are adequate to protect our trade secrets, that we will have adequate remedies for any breach or that our trade secrets will not otherwise become known to, or will not be discovered independently by, competitors.
23
We may not be able to protect the intellectual property that is integral to our business, or we may be subject to claims of intellectual property infringement by third parties, either of which could have a material adverse effect on our business.
Much of our potential success and value lies in our ownership and use of intellectual property. Our inability or failure to protect our intellectual property may negatively affect our business and value. Our ability to compete effectively is dependent in large part upon the maintenance and protection of the intellectual property we own and licenses from MIT. We will rely on patents, trademarks, trade secret and copyright law, as well as confidentiality procedures to establish and protect our intellectual property rights. It may be possible for a third party to copy or otherwise obtain and use the proprietary technology presently owned by or licensed to us without authorization. Policing unauthorized use of our intellectual property is difficult. The steps we take may not prevent misappropriation of our intellectual property, and the agreements we enter into may not be enforceable. In addition, effective intellectual property protection may be unavailable or limited in some jurisdictions outside the United States. Litigation may be necessary in the future to enforce or protect our intellectual property rights or to determine the validity and scope of the proprietary rights of others. Such litigation could cause us to incur substantial costs and divert resources away from our business, which in turn could have a material adverse effect on our business, results of operations, financial condition and profitability.
We may be subject to damaging and disruptive intellectual property litigation.
Although we are not currently aware that our products or services infringe any published patents or registered trademarks, we may be subject to infringement claims in the future. Because patent applications are kept confidential for a period of time after filing, applications may have been filed that, if issued as patents, could relate to our business.
Parties making claims of infringement may be able to obtain injunctive or other equitable relief that could effectively block us from providing its products and services in the United States and other jurisdictions and could cause us to pay substantial damages. In the event of a successful claim of infringement, we may need to obtain one or more licenses from third parties, which may not be available at a reasonable cost, if at all. The defense of any lawsuit could result in time-consuming and expensive litigation, regardless of the merits of such claims, as well as resulting damages, license fees, royalty payments and restrictions on our ability to provide products or services, any of which could harm our business.
Intellectual property rights are difficult to enforce in China, which could harm our business.
Chinese commercial law is relatively undeveloped compared with the commercial law in many of our other major markets and limited protection of intellectual property is available in China as a practical matter. We have formed a joint venture with Ideal Concepts Inc. to commercialize our products in the “Asia Pacific,” including China. Accordingly, any local design, manufacture, distribution or marketing of products that we undertake in China could subject us to an increased risk that unauthorized parties will be able to copy or otherwise obtain or use our intellectual property, which could harm our business. We may also have limited legal recourse in the event we encounter patent or trademark infringers, which could have a material adverse effect on our business and results of operations.
Our business is subject to numerous and evolving state, federal and foreign regulations and we may not be able to secure the government approvals needed to develop and market our products.
Our research and development activities, pre-clinical tests and clinical trials, and ultimately the manufacturing, marketing and labeling of our products, are all subject to extensive regulation by the FDA and foreign regulatory agencies. Pre-clinical testing and clinical trial requirements and the regulatory approval process typically take years and require the expenditure of substantial resources. Further, additional government regulation may be established that could prevent or delay regulatory approval of our product candidates. Delays or rejections in obtaining regulatory approvals would adversely affect our ability to commercialize any product candidates and our ability to generate product revenues or royalties.
The FDA and foreign regulatory agencies require that the safety and efficacy of product candidates be supported through adequate and well-controlled clinical trials. If the results of pivotal clinical trials do not establish the safety and efficacy of our product candidates to the satisfaction of the FDA and other foreign regulatory agencies, we will not receive the approvals necessary to market such product candidates.
24
Even if regulatory approval of a product candidate is granted, the approval may include significant limitations on the indicated uses for which the product may be marketed. In addition, we are subject to inspections and regulations by the FDA. Medical devices must also continue to comply with the FDA’s Quality System Regulation, or QSR. Compliance with such regulations requires significant expenditures of time and effort to ensure full technical compliance. The FDA stringently applies regulatory standards for manufacturing.
We are subject to the periodic inspection of our clinical trials, facilities, procedures and operations and/or the testing of our products by the FDA to determine whether our systems and processes are in compliance with FDA regulations. Following such inspections, the FDA may issue notices on Form 483 and warning letters that could cause us to modify certain activities identified during the inspection. A Form 483 notice is generally issued at the conclusion of an FDA inspection and lists conditions the FDA inspectors believe may violate FDA regulations. FDA guidelines specify that a warning letter is issued only for violations of “regulatory significance” for which the failure to adequately and promptly achieve correction may be expected to result in an enforcement action.
Failure to comply with FDA and other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production and/or distribution, suspension of the FDA’s review of product applications, enforcement actions, injunctions and criminal prosecution. Under certain circumstances, the FDA also has the authority to revoke previously granted product approvals. Although we have internal compliance programs, if these programs do not meet regulatory agency standards or if our compliance is deemed deficient in any significant way, it could have a material adverse effect on the Company.
We are also subject to record keeping and reporting regulations, including FDA’s mandatory Medical Device Reporting, or MDR, regulation. Labeling and promotional activities are regulated by the FDA and, in certain instances, by the Federal Trade Commission.
Many states in which we do or in the future may do business or in which our products may be sold impose licensing, labeling or certification requirements that are in addition to those imposed by the FDA. There can be no assurance that one or more states will not impose regulations or requirements that have a material adverse effect on our ability to sell our products.
In many of the foreign countries in which we may do business or in which our products may be sold, we will be subject to regulation by national governments and supranational agencies as well as by local agencies affecting, among other things, product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. There can be no assurance that one or more countries or agencies will not impose regulations or requirements that could have a material adverse effect on our ability to sell our products.
Failure to comply with applicable regulatory requirements, can result in, among other things, warning letters, fines, injunctions and other equitable remedies, civil penalties, recall or seizure of products, total or partial suspension of production, refusal of the government to grant approvals, pre-market clearance or pre-market approval, withdrawal of approvals and criminal prosecution of the Company and its employees, all of which would have a material adverse effect on our business.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act.
Our business operations in countries outside the United States, for example through our Chinese joint venture, may be subject to anti-corruption laws and regulations, including restrictions imposed by the Foreign Corrupt Practices Act (the “FCPA”). The FCPA and similar anti-corruption laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. We cannot provide assurance that our internal controls and procedures will always protect us from criminal acts committed by our employees or third parties with whom we work. If we are found to be liable for violations of the FCPA or similar anti-corruption laws in international jurisdictions, either due to our own acts or out of inadvertence, or due to the acts or inadvertence of others, we could suffer from criminal or civil penalties which could have a material and adverse effect on our results of operations, financial condition and cash flows.
25
The success of our products may be harmed if the government, private health insurers and other third-party payors do not provide sufficient coverage or reimbursement.
Our current and future revenues are subject to uncertainties regarding health care reimbursement and reform. Our ability to commercialize our new cancer treatment system successfully will depend in part on the extent to which reimbursement for the costs of such products and related treatments will be available from government health administration authorities, private health insurers and other third-party payors. The reimbursement status of newly approved medical products is subject to significant uncertainty. We cannot guarantee that adequate third-party insurance coverage will be available for us to establish and maintain price levels sufficient for us to realize an appropriate return on our investment in developing new therapies. Government, private health insurers, and other third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products approved for marketing by the FDA. Accordingly, even if coverage and reimbursement are provided by government, private health insurers, and third-party payors for uses of our products, market acceptance of these products would be adversely affected if the reimbursement available proves to be unprofitable for health care providers. We may be unable to sell our products on a profitable basis if third-party payers deny coverage, or provide low reimbursement rates.
Our products may not achieve sufficient acceptance by the medical community to sustain our business.
Although we have received a PMA from the FDA for our Prolieve system for the treatment of BPH, we can offer no assurance that the Prolieve system will be accepted by the medical community widely. Our breast cancer treatment development project using the APA technology is currently in Phase III clinical trials. It may prove not to be effective in practice. If testing and clinical practice do not confirm the safety and efficacy of our systems or, even if further testing and practice produce positive results but the medical community does not view these new forms of treatment as effective and desirable, our efforts to market our new products may fail, with material adverse consequences to our business.
We face intense competition and the failure to compete effectively could adversely affect our ability to develop and market our products.
There are many companies and other institutions engaged in research and development of various technologies, both for prostate disease and cancer treatment products that seek treatment outcomes similar to those that we are pursuing. We believe that the level of interest by others in investigating the potential of possible competitive treatments and alternative technologies will continue and may increase. Potential competitors engaged in all areas of BPH and cancer treatment research in the United States and other countries include, among others, major pharmaceutical, specialized technology companies, and universities and other research institutions. Most of our competitors and potential competitors have substantially greater financial, technical, human and other resources, and may also have far greater experience, than do we, both in pre-clinical testing and human clinical trials of new products and in obtaining FDA and other regulatory approvals. One or more of these companies or institutions could succeed in developing products or other technologies that are more effective than the products and technologies that we have been or are developing, or which would render our technology and products obsolete and non-competitive. Furthermore, if we are permitted to commence commercial sales of any of our products, we will also be competing, with respect to manufacturing efficiency and marketing, with companies having substantially greater resources and experience in these areas.
If we become subject to product liability claims, we may be required to pay damages that exceed our insurance coverage and the value of our assets.
We currently carry product liability insurance in the amount of $5,000,000 per occurrence, which may be inadequate to satisfy liabilities we may incur. Any claim brought against us, regardless of its merit, could result in the increase of our product liability insurance rates or our inability to obtain future coverage on acceptable terms, or at all. In addition, if our product liability coverage is inadequate to pay a damage award, we would have to pay any shortfall out of our assets, which may be insufficient, or by securing additional funds, of which there can be no assurance. Even a meritless or unsuccessful product liability claim made against us could harm our reputation, cause us to incur significant legal fees and result in the diversion of management’s attention from managing our business. Any of these occurrences or events would have a material adverse effect on our business.
26
Our joint venture with Ideal Concepts Inc. could cause us to effectively transfer rights to our technology in major markets in Asia, and to lose rights to sell and market our products in Asia.
We recently formed a joint venture with Ideal Concept Inc. As a new business, this joint venture is subject to a variety of risks including, without limitation, obtaining adequate financing to operate the business, recruiting management with expertise to market, promote, and produce products and having the capability of obtaining required regulatory approvals from various foreign governments in order sell products. Pursuant to the terms of our joint venture, our equity ownership in the joint venture can be reduced, and eventually eliminated, if we are unable to contribute financing to it in the future. This is a distinct possibility because of our current financial condition, and also because we will be borrowing funds from Ideal Concept Inc. to enable us to contribute a portion of the funds required to be invested by us in the joint venture. In addition, our right to receive royalties from the sale of products by the joint venture will prove to be worthless if there are no sales.
We could have disagreements with Ideal Concepts Inc. over the territory covered by the joint venture, and over other key aspects of the joint venture.
The territory covered by the joint venture is described as “Asia Pacific”, which is not defined in the agreement. In addition, other important aspects, terms and conditions of the joint venture are absent or unclear in the agreement establishing the joint venture. Accordingly, we could have disagreements with Ideal Concepts Inc. over rights and responsibilities of Ideal Concepts Inc. and us, as well as on other issues. If not resolved, these issues could have adverse consequences on the joint venture, make it difficult or impossible to sell products, result in litigation and cause us to incur substantial liabilities.
Damage to our reputation, for whatever reason, could have a material adverse effect on our business.
Our ability to market and sell Prolieve, APA 1000 and new products in major world markets, including the United States, could be adversely affected in the future by negative publicity resulting from, among others, the joint venture, adverse regulatory decisions by international bodies related to our products, controversy surrounding our products and the businesses activities of the joint venture, litigation arising from the joint venture and use of products, over which we will have very little, if any, control.
We have elected to use the extended transition period for complying with new accounting standards.
Pursuant to Section 107(b) of the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), we have elected to use the extended transition period for complying with new or revised accounting standards for an “emerging growth company.” This election will permit us to delay the adoption of new or revised accounting standards that will have different effective dates for public and private companies until those standards apply to private companies. Consequently, our financial statements may not be comparable to companies that comply with public company effective dates.
Our Shares are deemed to be “Penny Stocks,” which means that there are significant restrictions on stockbrokers and dealers recommending our Shares for purchase.
Our common stock is considered to be a “penny stock” pursuant to the rules promulgated under Section 15(g) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As a result, our securities are subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in the sale of shares of penny stock to persons other than established customers or “accredited investors” (as such term is defined in Rule 501 of Regulation D promulgated under the Securities Act of 1933, as amended (the “Securities Act”)). Under such rules, a broker-dealer must, prior to a transaction in a penny stock not otherwise exempt from those rules, deliver a standardized risk disclosure document prepared by the SEC, which specifies information about penny stocks and the nature and significance of risks of the penny stock market. A broker-dealer must also provide the customer with bid and offer quotations for the penny stock, the compensation of the broker-dealer, and sales person in the transaction, and monthly account statements indicating the market value of each penny stock held in the customer’s account. In addition, the penny stock rules require that, prior to a transaction in a penny stock not otherwise exempt from the penny stock rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for stock that is subject to the penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules may discourage investor interest in and limit the marketability of our securities, and limit the current investors’ ability to sell their shares of our common stock.
We may never pay dividends.
We have never declared or paid any dividends on our Shares since our inception. We do not intend to pay cash dividends on our Shares for the foreseeable future, and currently intend to retain any future earnings to fund the development and growth of our business. The payment of cash dividends, if any, on the Shares will rest solely within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors. We currently intend to use any revenues, as well as proceeds from any financings, to assist us in obtaining our business objectives, and not for the payment of any dividends upon our Shares.
27
Shareholders may suffer dilution of the value of their Shares by our issuance of additional Shares in the future.
As of September 30, 2015, we have outstanding debt, warrants and options that are convertible or exchangeable into 133,243,105 Shares. Additionally, we have plans to sell and issue additional Shares, or other securities that are convertible into Shares, in the future, in order to raise funds and for other purposes. The issuance of additional Shares, whether through the conversion of convertible notes, the exercise of warrants or options, or an issuance of Shares in connection with a financing, will dilute our current shareholders’ ownership in the Company, and will reduce shareholders’ voting power proportionally.
Future sales of Shares, securities convertible into Shares, and other securities may negatively affect our stock price.
Future sales of Shares and/or other securities that are convertible into Shares could have a significant negative effect on the market price of our Shares, and the number of Shares outstanding could increase substantially. This increase, in turn, could dilute future earnings per share. Dilution and the availability of a large amount of securities for sale, and the possibility of additional issuances and sales of Shares or other classes of securities may negatively affect both the trading price and liquidity of our Shares.
The market for our Shares is, and may continue to be, limited and highly volatile, which may generally affect any future price of our Shares.
The lack of an orderly market for our common stock may negatively affect the volume of trading and market price for our common stock. Historically, the volume of trades for our Shares has been limited. Moreover, the prices at which our Shares have traded have fluctuated widely on a percentage basis. There can be no assurance as to the prices at which our Shares will trade in the future, although they may continue to fluctuate significantly. Prices for our Shares will be determined in the marketplace and may be influenced by many factors, including, without limitation, the following:
| | • | | the depth and liquidity of the markets for our Shares; |
| | • | | investor perception of the Company and the industry in which we participate; |
| | • | | general economic and market conditions; |
| | • | | statements or changes in opinions, ratings or earnings estimates made by brokerage firms or industry analysts relating to the market in which we do business or relating to us specifically, as has occurred in the past; |
| | • | | quarterly variations in our results of operations; |
| | • | | general market conditions or market conditions specific to technology industries; and |
| | • | | domestic and international macroeconomic factors. |
An active trading market for the Shares may not exist in the future. Even if a market for our Shares continues to exist, investors may not be able to resell their Shares at or above the purchase price for which such investors purchased such Shares.
In addition, the stock market has recently experienced extreme price and volume fluctuations. These fluctuations are often unrelated to the operating performance of the specific companies. As a result of the factors identified above, a stockholder (due to personal circumstances) may be required to sell its Shares at a time when our stock price is depressed due to random fluctuations, possibly based on factors beyond our control.
11. Critical Accounting Policies
A “critical accounting policy” is one that is both important to the portrayal of our financial condition and results of operations and that requires management’s most difficult, subjective or complex judgments. Such judgments are often the result of a need to make estimates about the effect of matters that are inherently uncertain. The preparation of our financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
28
A summary of our critical accounting policies, including those that require the use of significant estimates and judgment, follows. A more comprehensive description of all of our significant accounting policies is contained in Note 1 to our unaudited consolidated interim financial statements.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. In the accompanying unaudited consolidated interim financial statements, estimates are used for, but not limited to, stock-based compensation, allowance for doubtful accounts, accruals for estimated product returns, allowance for inventory obsolescence, allowances for contingencies, value of contingent consideration, deferred taxes and valuation allowance, and the depreciable lives of tangible and intangible assets. Actual results could differ from those estimates.
Revenue Recognition
The Company sells products that are used in the treatment of Benign Prostate Hyperplasia. The Company recognizes revenue, net of sales taxes, from the sale of Prolieve consoles and catheters upon shipment delivery to the customer. Revenue from the sale of products is measured at the fair value of the consideration received or receivable, net of estimated returns. Revenue from the mobile service is recognized upon completion of the services, which is generally upon treatment of the patient.
The Company does not have a return policy that allows customers to return product, however the company has allowed returns on a limited customer by customer basis. The Company’s estimate for returns is based upon its historical experience with actual returns. While such experience has allowed for reasonable estimation in the past, history may not always be an accurate indicator of future returns. The Company continually monitors its estimates for returns and makes adjustments when it believes that actual product returns may differ from the established accruals. We record a provision for estimated returns in the same period as the related revenue is recorded. The provision for estimated sales returns is based on historical sales returns, analysis of credit memo data and specific customer-based circumstances.
Inventory
Inventory is valued at the lower of cost or market and consists primarily of console units and single-use treatment catheter. Inventory consists of the direct costs of acquiring the inventory from vendors. Inventory of console units are considered non-current since the sales period is usually in excess of one year.
Inventory is relieved using the first-in, first-out method.
Stock-Based Compensation
Compensation costs for all stock-based awards is measured at fair value on the date of the grant using an option pricing model and is recognized over the service period for awards expected to vest. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the current estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Research and Development Expenses
Research and development costs are expensed as incurred.
Contingent Consideration
In accordance with ASC 805, upon the purchase of Prolieve, the Company recognized a contingent consideration obligation as part of the consideration transferred in exchange for the acquired business. The initial measurement of the contingent consideration obligation was based on its estimated fair value. The contingent consideration obligation has been re-measured to fair value at each reporting date and will continue to be re-measured until the contingency is resolved. The changes in fair value are recognized in earnings.
29
Intangible Assets
Intangible assets consist of intellectual property and customer relationships for our Prolieve business acquired in July 2012. These intangible assets were originally recorded at fair value and are amortized on a straight line basis over their estimated useful lives of 10 years. The Company reviews its intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, in a manner similar to that for property and equipment.
Current Accounting Pronouncements
None of the recent accounting pronouncements in any of the periods contained in this report had a material impact on our results of operations, financial condition or cash flows.
12. Financial Instruments
Fair Value Measurements
The Company’s unaudited condensed interim consolidated statements of financial position include various financial instruments (primarily cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, and notes payable) recorded at cost, which approximates their fair value. Fair value is the price that would be received from the sale of an asset or paid to transfer a liability assuming an orderly transaction in the most advantageous market at the measurement date. U.S. GAAP establishes a hierarchical disclosure framework which prioritizes and ranks the level of observability of inputs used in measuring fair value. These tiers include:
| | • | | Level 1—Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs. |
| | • | | Level 2—Observable market-based inputs other than quoted prices in active markets for identical assets or liabilities. |
| | • | | Level 3—Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs. |
In connection with the acquisition of Prolieve, the Company owes additional purchase consideration of up to $2.5 million (contingent consideration) based on the sales of Prolieve products after their acquisition. The contingent consideration is measured at fair value on a recurring basis using level 3 inputs, and the fair value is determined using unobservable inputs such as the discount rate.
The Company has no financial assets and liabilities measured at fair value on a non-recurring basis. The Company’s long-lived assets are measured at fair value on a non-recurring basis only when an impairment is deemed to occur.
Interest Rate Risk
Because of the short-term maturities of our cash and cash equivalents, we do not believe that an increase in market interest rates would have a significant impact on their realized value. The interest rates on our various outstanding debt instruments, including promissory and convertible notes, are fixed. Because of the fixed rates, a change in market interest rates would not have a material impact on interest expense associated with the debt.
Exchange Rate Risk
The Company’s reporting currency is the U.S. dollar and, accordingly, the Company reports its financial results in U.S. dollars. The Company’s functional currency and that of its wholly owned subsidiary is the U.S. dollar. Therefore, because certain financial transactions are denominated in Canadian dollars, we report those transactions in U.S. dollars using prevailing exchange rates at the time of the transaction. Transaction gains and losses on the settlement of these transactions, and on outstanding receivables and payables that are denominated in Canadian dollars, are included in the determination of our net loss.
Equity Price Risk
Historically, the Company has issued equity securities, and equity-linked securities such convertible debt, stock purchase warrants and stock options, to investors, employees and vendors. Equity and equity-linked securities are initially recorded in our financial statements at their fair values, and depending on the nature of the security may require periodic re-measurement at fair value. Changes in the market price of our common stock can have an impact on the value of the securities issued which could have a direct impact on those fair values, earnings, and cash flow.
30
13. Summary of Quarterly Results
The following table sets forth, for the quarters indicated, information relating to the Company’s revenue, net loss and loss per common shares.
| | | | | | | | | | | | |
| | | Revenues | | | Net Loss | | | Basic and
Diluted EPS | |
December 31, 2013 | | $ | 1,282,837 | | | ($ | 1,464,500 | ) | | ($ | 0.01 | ) |
March 31, 2014 | | | 1,677,138 | | | | (2,096,551 | ) | | | (0.02 | ) |
June 30, 2014 | | | 1,649,469 | | | | (1,339,618 | ) | | | (0.01 | ) |
September 30, 2014 | | | 641,697 | | | | (2,044,214 | ) | | | (0.02 | ) |
December 31, 2014 | | | 895,973 | | | | (1,368,824 | ) | | | (0.01 | ) |
March 31, 2015 | | | 1,032,320 | | | | (1,015,261 | ) | | | (0.01 | ) |
June 30, 2015 | | | 1,125,010 | | | | (1,028,069 | ) | | | (0.01 | ) |
September 30, 2015 | | | 1,136,360 | | | | (1,298,178 | ) | | | (0.01 | ) |
For further quarterly financial information, please refer to the Company’s unaudited condensed interim consolidated financial statements that have been filed on SEDAR.com
14. Transactions with Related Parties
The management team and directors, along with their remuneration for the six months ended September 30, 2015 is presented below:
| | | | | | | | | | | | | | | | | | | | |
Individual | | Position | | | Cash | | | Options | | | Shares | | | Total | |
Grant B. Walsh | | | Director | | | $ | 25,660 | | | | — | | | | — | | | $ | 25,660 | |
Dr. Augustine Y. Cheung | | | CEO | | | $ | 58,708 | | | | — | | | | — | | | $ | 58,708 | |
Joseph S. C. Chan | | | Director | | | $ | 13,817 | | | | — | | | | — | | | $ | 13,817 | |
Dr. Augustine P. Y. Chow | | | Director | | | $ | 7,895 | | | | — | | | | — | | | $ | 7,895 | |
Raymond Tong | | | Director | | | $ | 7,895 | | | | — | | | | — | | | $ | 7,895 | |
John Mon | | | COO | | | $ | 106,000 | | | | — | | | | — | | | $ | 106,000 | |
Mirsad Jakubovic | | | CFO | | | $ | 29,608 | | | | — | | | | — | | | $ | 29,608 | |
15. Commitments
On January 16, 2006 Celsion purchased from Celsion Corporation (USA) all of the assets relating to breast cancer Microfocus APA 1000 System (“System”), consisting of the microwave machine technology, the APA technology licensed from MIT, and all related intellectual and regulatory property (collectively, the “Business”). The Company has a commitment to pay a 5% royalty to Celsion on the net sales of products sold by and patent royalties received by the Company and its successors and assignees. Total royalties paid are not to exceed US $18,500,000. Royalties will not be payable until the System can be placed in the market following successful completion of the pivotal clinical trial and receipt of approval to market the System in the US and Canada from the FDA and Health Canada. The Company has an additional commitment to pay a 5% royalty to MIT on the net sales of products, upon commercialization.
Future minimum payments under operating leases for office space and vehicles are as follows:
| | | | |
2016 | | $ | 198,519 | |
2017 | | $ | 201,545 | |
2018 | | $ | 112,946 | |
16. Contingencies
The Company has agreed to indemnify its directors and officers and certain of its employees in accordance with the Company’s by-laws. The Company maintains insurance policies that may provide coverage against certain claims.
31
17. Other MD&A Disclosure
Outstanding Share Data as of November 30, 2015
| | | | | | | | |
| | | Number or Principle
Amount Outstanding | | | Maximum Number of
Common Shares Issuable, if
Convertible, Exercisable or
Exchangeable | |
Common Shares | | | 166,292,120 | | | | N/A | |
Stock Options | | | 8,525,000 | | | | 8,525,000 | |
Convertible debenture | | | 22,160,000 | | | | 22,160,000 | |
Warrants outstanding | | | 102,828,105 | | | | 102,828,105 | |
Maximum common shares outstanding | | | | | | | 299,805,225 | |
18. Off-Balance Sheet Arrangements
As of the date of this filing, the Company does not have any off-balance sheet arrangements that have, or reasonably likely to have, a current or future effect upon the financial performance or financial condition of the Company, including, and without limitation, such considerations as liquidity and capital resources.
19. Proposed transactions
The Company has not entered into any significant transaction, nor is it currently reviewing any such transaction, which requires board approval, shareholder approval or regulatory approval that has not been discussed within this MD&A.
20. Current Accounting Pronouncements Not Effective
In May 2014, the Financial Accounting Standards Board (FASB) issued ASU 2014-09 – Revenue from Contracts with Customers providing guidance for revenue recognition for contracts. This guidance requires an entity to review contracts in five steps and will result in enhanced disclosures regarding the nature, amount, timing and uncertainty of revenue arising from contracts with customers. This standard is effective for fiscal years beginning after December 15, 2017 and early adoption is permitted for fiscal years beginning after December 15, 2016. We are currently evaluating the impact, if any, that this new guidance will have on the Company’s Condensed Interim Consolidated Financial Statements.
ASU No. 2014-12, Compensation-Stock Compensation (Topic 718): Accounting for Share Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period amendments require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. Guidance in Topic 718 as it relates to awards with performance conditions that affect vesting should be applied to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. As indicated in the definition of vest, the stated vesting period (which includes the period in which the performance target could be achieved) may differ from the requisite service period. The amendments of ASU 2014-12 are effective annual periods and interim periods within those annual periods beginning after December 15, 2015. The adoption of the amended guidance is not expected to have a material on the Company’s Condensed Interim Consolidated Financial Statements.
ASU No. 2014-16, Derivatives and Hedging (Topic 815): Determining Whether the Host Contract in a Hybrid Financial instrument in the Form of a Share is More Akin to Debt or Equity. In November 2014, the FASB issued amended guidance that clarifies how current GAAP should be interpreted in evaluating the economic characteristics and risks of a host contract in a hybrid financial instrument that is issued in the form of a share. Specifically, the
32
amendments clarify that an entity should consider all relevant terms and features -including the embedded derivative features being evaluated for bifurcation -in evaluating the nature of the host contract. Furthermore, the amendments clarify that no single term or feature would necessarily determine the economic characteristics and risks of the host contract. Rather, the nature of the host contract depends upon the economic characteristics and risks of the entire hybrid financial instrument. The amended guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015, with early adoption permitted. The effects of initially adopting the amended guidance should be applied on a modified retrospective basis to existing hybrid financial instruments issued in the form of a share as of the beginning of the fiscal year for which the amendments are effective and shall be reported as a cumulative-effect adjustment directly to retained earnings as of the beginning of the year of adoption. The adoption of the amended guidance is not expected to have a material impact on the Company’s Condensed Interim Consolidated Financial Statements.
ASU No. 2015-03, Interest-Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs. In April 2015, the FASB issued amended guidance to address the different balance sheet presentation requirements for debt issuance costs and debt discounts and premiums. The amended guidance requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the amended guidance. The amended guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015, with early adoption permitted for financial statements that have not been previously issued. The amended guidance should be applied retrospectively, wherein the balance sheet of each individual period presented should be adjusted to reflect the period-specific effects of applying the amended guidance. The Company currently has approximately $188,000 of debt issuance costs recorded in the Consolidated Balance Sheets that will be required to be reclassified and presented as a direct deduction from the debt liability upon adoption of the amended guidance. The adoption of the amended guidance is not expected to have an impact on the Company’s Condensed Interim Consolidated Statements of Loss and Comprehensive Loss.
In August 2014, the FASB issued ASU 2014-15 – Presentation of Financial Statements – Going Concern. This guidance requires management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. This guidance is effective for the annual period after December 15, 2016 and for the annual periods and interim periods thereafter. Early application is permitted. We do not expect the adoption of this guidance to have a material impact on the Company’s Condensed Interim Consolidated Financial Statements.
In July 2015, the FASB issued ASU 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” This ASU changes the measurement principle for certain inventory methods from the lower of cost or market to the lower of cost and net realizable value. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. This ASU does not apply to inventory that is measured using Last-in First-out (“LIFO”) or the retail inventory method. The provisions of ASU 2015-11 are effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The Company is currently evaluating this guidance to determine the impact it may have to its consolidated financial statements.
21. Disclosure Controls and Procedures
Disclosure controls and procedures are defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act to mean controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and includes, without limitation, controls and procedures designed to ensure that such information is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
As required under the Exchange Act, we have carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer, of the effectiveness of our disclosure controls and procedures as of September 30, 2015. Based on such evaluation, we have concluded that, as of such date, our disclosure controls and procedures were not effective to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in applicable SEC rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer, as appropriate, to allow timely discussions regarding required disclosure.
33
22. Internal controls over Financial Reporting
Our management has evaluated, with the participation of our Chief Executive Officer, changes in our internal controls over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) during the six months ended September 30, 2015. In connection with such evaluation, there have been no changes to our internal control over financial reporting that occurred during the six months ended September 30, 2015 that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting. While there have been no significant changes, we have assessed our internal controls as being ineffective and will be taking steps during fiscal 2016 to remedy such deficiencies.
23. Approvals
The Directors of the Company have approved the disclosure contained in this MD&A and a copy will be provided to anyone who requests it.
34
Condensed Interim Consolidated Financial Statements
Medifocus Inc.
For the three months ended September 30, 2015 and September 30, 2014
Management’s Responsibility for the Condensed Interim Consolidated
Financial Statements
The accompanying unaudited condensed interim consolidated financial statements of Medifocus Inc. (the “Company”) are the responsibility of management and have been approved by the Board of Directors.
The unaudited condensed interim consolidated financial statements have been prepared by management, on behalf of the Board of Directors, in accordance with the accounting policies disclosed in the notes to the unaudited condensed interim consolidated financial statements. Where necessary, management has made informed judgments and estimates in accounting for transactions which were not complete at the date of the reporting period. In the opinion of management, the unaudited condensed interim consolidated financial statements have been prepared within acceptable limits of materiality and are in accordance with U.S. GAAP.
Management has established systems of internal control over the financial reporting process, which are designed to provide reasonable assurance that relevant and reliable financial information is produced.
The Board of Directors is responsible for reviewing and approving the unaudited condensed interim consolidated financial statements together with other financial information of the Company and for ensuring that management fulfills its financial reporting responsibilities. An Audit Committee assists the Board of Directors in fulfilling this responsibility. The Audit Committee meets with management to review the financial reporting process and the unaudited condensed interim consolidated financial statements together with other financial information of the Company. The Audit Committee reports its findings to the Board of Directors for its consideration in approving the unaudited condensed interim consolidated financial statements together with other financial information of the Company for issuance to the shareholders.
Management recognizes its responsibility for conducting the Company’s affairs in compliance with established financial standards, and applicable laws and regulations, and for maintaining proper standards of conduct for its activities.
| | |
| “Augustine Cheung” | | “Mirsad Jakubovic” |
| Dr. Augustine Cheung | | Mirsad Jakubovic |
| Chief Executive Officer | | Chief Financial Officer |
Notice of no Auditor Review of Interim Financial Statements
The accompanying unaudited condensed interim consolidated financial statements of the Company have been prepared by and are the responsibility of management. The Company’s independent auditor has not performed a review of these financial statements.
Page F-1
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENT OF FINANCIAL POSITION
(in U.S. dollars)
| | | | | | | | |
| | | September 30,
2015 | | | March 31,
2015 | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 374,799 | | | $ | 1,312,479 | |
Accounts receivable, net | | | 883,820 | | | | 1,057,705 | |
Inventory, net | | | 145,488 | | | | 79,604 | |
Deferred financing costs | | | 188,177 | | | | 191,228 | |
Other assets | | | 37,479 | | | | 38,800 | |
| | | | | | | | |
Total Current Assets | | | 1,629,763 | | | | 2,679,816 | |
| | | | | | | | |
Inventory, net | | | 191,776 | | | | 190,276 | |
Property and equipment, net | | | 412,005 | | | | 469,035 | |
Deferred financing costs, long term | | | — | | | | 91,698 | |
Deposits | | | 271,330 | | | | 221,330 | |
Intangible assets, net | | | 1,643,226 | | | | 1,766,332 | |
| | | | | | | | |
Total Assets | | $ | 4,148,100 | | | $ | 5,418,487 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 908,398 | | | $ | 606,527 | |
Accrued expenses | | | 577,384 | | | | 568,919 | |
Accrued interest payable | | | 813,706 | | | | 567,955 | |
Promissory notes payable | | | 268,159 | | | | 282,303 | |
Payable to Boston Scientific Corporation | | | 1,030,638 | | | | 831,632 | |
Contingent consideration, current portion | | | 473,630 | | | | 440,663 | |
Convertible notes payable (net of discount), current portion | | | 4,718,810 | | | | 4,426,984 | |
| | | | | | | | |
Total Current Liabilities | | | 8,790,725 | | | | 7,724,983 | |
| | | | | | | | |
Contingent consideration | | | 440,182 | | | | 590,516 | |
| | | | | | | | |
Total liabilities | | | 9,230,907 | | | | 8,315,499 | |
| | | | | | | | |
Commitments and contingencies (Note 8) | | | | | | | | |
Stockholders’ deficit: | | | | | | | | |
Common stock (no par value, unlimited shares authorized, 166,292,120 and 127,542,120 shares issued and outstanding in September 30, 2015 and March 31, 2015, respectively. | | | 14,343,563 | | | | 12,782,563 | |
Common stock issuable | | | 140,452 | | | | 1,561,000 | |
Additional paid-in capital | | | 9,659,740 | | | | 9,659,740 | |
Accumulated deficit | | | (29,226,562 | ) | | | (26,900,315 | ) |
| | | | | | | | |
Total Stockholders’ Deficit | | | (5,082,807 | ) | | | (2,897,012 | ) |
| | | | | | | | |
Total Liabilities and Stockholders’ Deficit | | $ | 4,148,100 | | | $ | 5,418,487 | |
| | | | | | | | |
See accompanying notes to unaudited consolidated financial statements.
Page F-2
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF
LOSS AND COMPREHENSIVE LOSS
(in U.S. dollars)
| | | | | | | | | | | | | | | | |
| | | Three Months Ended
September 30, | | | Six Months Ended
September 30, | |
| | | 2015 | | | 2014 | | | 2015 | | | 2014 | |
Sales | | | | | | | | | | | | | | | | |
Services | | $ | 779,354 | | | $ | 461,842 | | | $ | 1,577,276 | | | $ | 1,232,512 | |
Products | | | 357,006 | | | | 179,855 | | | | 684,094 | | | | 1,058,654 | |
| | | | | | | | | | | | | | | | |
Total Sales | | | 1,136,360 | | | | 641,697 | | | | 2,261,370 | | | | 2,291,166 | |
| | | | | | | | | | | | | | | | |
Cost of Sales | | | | | | | | | | | | | | | | |
Services | | | 641,284 | | | | 681,229 | | | | 1,296,102 | | | | 1,414,673 | |
Products | | | 172,977 | | | | 117,576 | | | | 348,870 | | | | 540,301 | |
| | | | | | | | | | | | | | | | |
Total Cost of Sales | | | 814,261 | | | | 798,805 | | | | 1,644,972 | | | | 1,954,974 | |
| | | | | | | | | | | | | | | | |
Gross Profit (Loss) | | | 322,099 | | | | (157,108 | ) | | | 616,398 | | | | 336,192 | |
| | | | | | | | | | | | | | | | |
Operating Expenses | | | | | | | | | | | | | | | | |
Research and development | | | 161,903 | | | | 73,226 | | | | 244,546 | | | | 125,997 | |
Sales and marketing | | | 409,530 | | | | 440,962 | | | | 662,897 | | | | 1,090,337 | |
General and administrative | | | 708,473 | | | | 754,273 | | | | 1,303,470 | | | | 1,598,928 | |
| | | | | | | | | | | | | | | | |
Total Operating Expenses | | | 1,279,906 | | | | 1,268,461 | | | | 2,210,913 | | | | 2,815,262 | |
| | | | | | | | | | | | | | | | |
Loss from Operations | | | (957,807 | ) | | | (1,425,569 | ) | | | (1,594,515 | ) | | | (2,479,070 | ) |
| | | | | | | | | | | | | | | | |
Other Income (Expense) | | | | | | | | | | | | | | | | |
Interest and discount accretion | | | (343,758 | ) | | | (398,204 | ) | | | (685,013 | ) | | | (671,848 | ) |
Loss from change in fair value of contingent consideration | | | (44,492 | ) | | | (189,228 | ) | | | (81,639 | ) | | | (189,228 | ) |
Net loss from equity method investment | | | — | | | | (55,735 | ) | | | — | | | | (55,735 | ) |
Other income | | | 47,879 | | | | 24,522 | | | | 34,920 | | | | 12,049 | |
| | | | | | | | | | | | | | | | |
Total Other Income (Expense) | | | (340,371 | ) | | | (618,645 | ) | | | (731,732 | ) | | | (904,762 | ) |
| | | | | | | | | | | | | | | | |
Net Loss and Net Comprehensive Loss | | $ | (1,298,178 | ) | | $ | (2,044,214 | ) | | $ | (2,326,247 | ) | | $ | (3,383,832 | ) |
| | | | | | | | | | | | | | | | |
Net Loss per share basic and diluted | | $ | (0.01 | ) | | $ | (0.02 | ) | | $ | (0.01 | ) | | $ | (0.03 | ) |
| | | | | | | | | | | | | | | | |
Weighted average common shares outstanding—basic and diluted | | | 166,292,120 | | | | 118,937,161 | | | | 157,398,677 | | | | 118,103,596 | |
| | | | | | | | | | | | | | | | |
See accompanying notes to unaudited consolidated financial statements.
Page F-3
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF CASH FLOWS
(in U.S. dollars)
| | | | | | | | |
| | | Six months ended September 30, | |
| | | 2015 | | | 2014 | |
Net Loss | | $ | (2,326,247 | ) | | $ | (3,383,832 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization | | | 180,136 | | | | 180,595 | |
Accretion of deferred financing costs and debt discount | | | 386,575 | | | | 346,802 | |
Loss on change in fair value of contingent consideration | | | 81,639 | | | | 189,228 | |
Provisions for bad debts and warranties | | | 330 | | | | 44,700 | |
Product warranty expense | | | 74,909 | | | | 52,998 | |
Losses from equity method investment | | | — | | | | 55,735 | |
Changes in operating assets and liabilities | | | | | | | | |
Decrease in accounts receivable | | | 163,034 | | | | 247,820 | |
(Increase) decrease in inventory | | | (142,293 | ) | | | 445,767 | |
Decrease (increase) in other current assets | | | 1,321 | | | | (6,749 | ) |
(Increase) in deposits | | | (50,000 | ) | | | — | |
Increase (decrease) in accounts payable | | | 384,323 | | | | (466,167 | ) |
Increase in accrued expenses | | | 125,095 | | | | 52,724 | |
Increase in accrued interest | | | 215,253 | | | | 287,950 | |
| | | | | | | | |
Net cash used in operating activities | | | (905,925 | ) | | | (1,952,429 | ) |
| | | | | | | | |
INVESTING ACTIVITIES: | | | | | | | | |
Purchase of fixed assets | | | — | | | | (26,355 | ) |
| | | | | | | | |
Net cash used in investing activities | | | — | | | | (26,355 | ) |
FINANCING ACTIVITIES: | | | | | | | | |
Issuance of common shares | | | — | | | | 1,444,274 | |
Principal repayments on note payable | | | — | | | | (178,488 | ) |
| | | | | | | | |
Net cash provided by financing activities | | | — | | | | 1,265,786 | |
Effect of exchange rate changes on cash and cash equivalents | | | (31,755 | ) | | | 26,551 | |
| | | | | | | | |
DECREASE IN CASH AND CASH EQUIVALENTS | | | (937,680 | ) | | | (686,447 | ) |
CASH AND CASH EQUIVALENTS, BEGINNING OF THE PERIOD | | | 1,312,479 | | | | 1,292,509 | |
| | | | | | | | |
CASH AND CASH EQUIVALENTS, END OF THE PERIOD | | $ | 374,799 | | | $ | 606,062 | |
| | | | | | | | |
Cash paid for Interest | | $ | 40,000 | | | $ | 37,500 | |
NON CASH FINANCING ACTIVITIES | | | | | | | | |
Issuance of common shares issuable | | $ | 1,561,000 | | | $ | — | |
Accrued expense settled with common shares issuable | | $ | 140,452 | | | $ | — | |
See accompanying notes to unaudited consolidated financial statements.
Page F-4
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
(in U.S. dollars)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | Common | | | Common | | | Common | | | | | | | | | Other | | | Total | |
| | | Stock | | | Stock | | | Stock | | | Additional | | | Accumulated | | | Comprehensive | | | Stockholders’ | |
| | | Shares | | | Amount | | | Issuable | | | Paid-in Capital | | | Deficit | | | Income (Loss) | | | Deficit | |
Balance at April 1, 2014 | | | 117,260,870 | | | $ | 12,372,498 | | | $ | — | | | $ | 8,625,531 | | | $ | (20,928,845 | ) | | $ | (203,553 | ) | | $ | (134,369 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (1,339,618 | ) | | | — | | | | (1,339,618 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at June 30, 2014 | | | 117,260,870 | | | | 12,372,498 | | | | — | | | | 8,625,531 | | | | (22,268,463 | ) | | | (203,553 | ) | | | (1,473,987 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common shares in private placement | | | 10,281,250 | | | | 410,065 | | | | — | | | | 1,034,209 | | | | — | | | | — | | | | 1,444,274 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (2,044,214 | ) | | | — | | | | (2,044,214 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 30, 2014 | | | 127,542,120 | | | $ | 12,782,563 | | | $ | — | | | $ | 9,659,740 | | | $ | (24,312,677 | ) | | $ | (203,553 | ) | | $ | (2,073,927 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at April 1, 2015 | | | 127,542,120 | | | $ | 12,782,563 | | | $ | 1,561,000 | | | $ | 9,659,740 | | | $ | (26,900,315 | | | ) $ | — | | | $ | (2,897,012 | ) |
Issuance of common shares issuable | | | 38,750,000 | | | | 1,561,000 | | | | (1,561,000 | ) | | | — | | | | — | | | | — | | | | — | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (1,028,069 | ) | | | — | | | | (1,028,069 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at June 30, 2015 | | | 166,292,120 | | | | 14,343,563 | | | | — | | | | 9,659,740 | | | | (27,928,384 | ) | | | — | | | | (3,925,081 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common shares issuable | | | — | | | | — | | | | 140,452 | | | | — | | | | — | | | | — | | | | 140,452 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (1,298,178 | ) | | | — | | | | (1,298,178 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at September 30, 2015 | | | 166,292,120 | | | $ | 14,343,563 | | | $ | 140,452 | | | $ | 9,659,740 | | | $ | (29,226,562 | ) | | $ | — | | | $ | (5,082,807 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to unaudited consolidated financial statements.
Page F-5
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
1. BUSINESS, LIQUIDITY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Description of Business and Current Financial Condition
Medifocus Inc. (the “Company” or “Medifocus”) was incorporated under the Business Corporations Act (Ontario) on April 25, 2005. Medifocus develops and commercializes minimally invasive focused heat systems for the treatment of cancerous and benign tumors, and enlarged prostate, medically known as Benign Prostate Hyperplasia (“BPH”). After the acquisition of Prolieve® from the Boston Scientific Corporation, Medifocus now owns a revenue-generating commercial BPH treatment product targeting the BPH drug therapy market and generating cash flows to support the development and commercialization of other heat systems for targeted thermotherapy of surface, subsurface and deep seated localized and regional cancers.
The Company owns two technology platforms with comprehensive US and international patent protection:
| | • | | The Endo-thermotherapy Platform-from which Prolieve was developed, can potentially be used to treat cancers in prostate, rectal, cervical and esophageal, and |
| | • | | The Adaptive Phased Array (“APA”) Microwave Focusing Platform-invented by MIT, licensed to Medifocus, directs precisely focused microwave energy at tumor center to induce shrinkage or eradication of tumors without undue harm to surrounding tissue. The Company’s APA 1000 Breast Cancer Treatment System, developed from the APA technology platform is currently in pivotal Phase-III clinical trials. |
Going Concern Consideration
The Company’s operations are subject to certain risks and uncertainties including, among others, current and potential competitors with greater resources, dependence on significant customers, lack of operating history and uncertainty of future profitability and possible fluctuations in financial results. Since inception, the Company has incurred substantial operating losses, principally from expenses associated with the Company’s research and development, financing activities, and development of new technologies. The Company believes these expenditures are essential for the commercialization of its technologies. The Company expects its operating losses to continue for the foreseeable future as it continues its product development efforts and undertakes its sales and marketing activities. Due to continued substantial operating losses, there is substantial doubt regarding the Company’s ability to continue as a going concern. The Company’s ability to achieve profitability is dependent upon its ability to obtain governmental approvals, produce, and market and sell its new product candidates. There can be no assurance that the Company will be able to commercialize its technology successfully or that profitability will ever be achieved. The operating results of the Company have fluctuated significantly in the past. The Company expects that its operating results will fluctuate significantly in the future and will depend on a number of factors, many of which are outside the Company’s control.
The Company will need substantial additional funding in order to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from the sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Additionally, the Company is not in compliance with the provisions of outstanding debt agreements, and it has not remitted quarterly royalty payments to Boston Scientific Corporation pursuant to the terms of its purchase agreement for Prolieve. The Company has not paid interest owing to certain holders of the convertible debentures, and is in a technical default of the terms of the debentures. The investors have not accelerated the terms of the debenture. Management is continuing its efforts to obtain additional funds through equity financing and through the negotiation of debt agreements to ensure that the Company can meet its obligations and sustain operations.
Page F-6
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
The condensed interim consolidated financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue in existence.
Basis of Presentation and Principles of Consolidation
These condensed interim consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”). In the opinion of management the accompanying condensed interim consolidated financial statements include all adjustments, consisting of normal recurring adjustments, which are necessary to present fairly the Company’s financial position, results of operations and cash flows. The condensed interim results of operations are not necessarily indicative of the results that may occur for the full fiscal year. Certain information and footnote disclosure normally included in the financial statements prepared in accordance with U.S. GAAP have been omitted pursuant to instruction, rules and regulations provided by the United States Security and Exchange Commission. Management believes that the disclosures provided herein are adequate to make the information presented not misleading when these condensed interim consolidated financial statements are read in conjunction with the audited financial statements.
The accompanying condensed interim consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary Celsion (Canada) Inc. All intercompany transactions have been eliminated. There were no transactions for Celsion (Canada) Inc. for the year ended March 31, 2015 and the six month period ended September 30, 2015.
Unless otherwise noted, all references to “$” or “dollar” refer to the United States dollar. The Company operates in a single business segment, focused heat systems for targeted thermotherapy of surface, subsurface and deep seated localized and regional cancers. Substantially all of the Company’s revenue is generated, and assets are located, in the United States.
Foreign Currency
Effective April 1, 2014, the Company changed its functional currency and that of its wholly owned subsidiary to the U.S. dollar.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. The condensed interim consolidated financial statements include significant estimates for the expected economic life and value of our licensed technology, allowance for doubtful accounts, value of contingent consideration, value of our debt issuances, accruals for estimated product returns, allowance for inventory obsolescence, allowance for our net operating loss carry forward and related valuation allowance for tax purposes and our stock-based compensation related to employees and directors, consultants and advisors. Because of the use of estimates inherent in the financial reporting process, actual results could differ significantly from those estimates.
Credit Concentration
The Company’s customers are primarily physicians and physician organizations in the U.S.No individual customer represented more than 10% of revenues for the six month period ended September 30, 2015 and 2014.
Vendor Concentration
The Company currently purchases 100% of its Prolieve catheter inventory from one supplier. Alternative suppliers and alternative catheters are not currently available. The company maintains a deposit of $221,330 with its vendor.
Page F-7
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
Fair Value Measurements
The Company’s condensed interim consolidated statements of financial position include various financial instruments (primarily cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, and notes payable) recorded at cost, which approximates their fair value. Fair value is the price that would be received from the sale of an asset or paid to transfer a liability assuming an orderly transaction in the most advantageous market at the measurement date. U.S. GAAP establishes a hierarchical disclosure framework which prioritizes and ranks the level of observability of inputs used in measuring fair value. These tiers include:
| | • | | Level 1—Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs. |
| | • | | Level 2—Observable market-based inputs other than quoted prices in active markets for identical assets or liabilities. |
| | • | | Level 3—Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs. |
In connection with the acquisition of Prolieve, the Company owes additional purchase consideration of up to $2.5 million (contingent consideration) based on the sales of Prolieve products after their acquisition. The contingent consideration is measured at fair value on a recurring basis using level 3 inputs, and the fair value is determined using unobservable inputs such as the discount rate. The change in the fair value of the contingent consideration of $44,492 and $189,228 for the three month period ended September 30, 2015 and 2014 and $81,640 and $189,228 for the six month period ended September 30, 2015 and 2014, respectively, is reflected as “loss from change in fair value of contingent consideration” in the accompanying consolidated statements of operations.See note 2.
The Company has no financial assets and liabilities measured at fair value on a non-recurring basis. The Company’s long-lived assets are measured at fair value on a non-recurring basis only when an impairment is deemed to occur.
Fair Value of Financial Instruments
The carrying amounts of financial instruments classified as current assets or liabilities, including accounts receivable, accounts payable and accrued expenses approximate fair value because of the short maturity of these instruments.
Comprehensive Loss and Accumulated Other Comprehensive Loss
Comprehensive loss includes the total of the Company’s net loss and all other changes in equity other than transactions with owners, including changes in equity for cumulative translation adjustments resulting from the consolidation of foreign subsidiaries as the financial statements of the subsidiaries were previously accounted for using the local currency as the functional currency.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents. All interest bearing and non-interest bearing accounts are guaranteed by the FDIC up to $250,000. The Company may maintain cash balances in excess of FDIC coverage. Management considers this to be a normal business risk.
Accounts Receivable
The Company extends credit to customers on an unsecured basis and payment terms are typically 30 days from delivery or service. The Company’s receivables are primarily related to Prolieve products and services. Management uses the aging account method to assess the company’s allowance for doubtful accounts. The aging account method uses the number of days outstanding the underlying invoices have been past due. Receivables are written off when it is determined that the underlying invoices are uncollectible.
The Company maintained an allowance for doubtful accounts of $60,569 and $74,289 as of September 30, 2015 and March 31, 2015, respectively.
| | | | | | | | |
| | | September 30, | | | March 31, | |
Accounts receivable trade | | $ | 744,194 | | | $ | 920,022 | |
Accounts receivable—Harmonized sales tax | | | 200,195 | | | | 211,972 | |
Allowance for doubtful accounts | | | (60,569 | ) | | | (74,289 | ) |
| | | | | | | | |
| | $ | 883,820 | | | $ | 1,057,705 | |
| | | | | | | | |
Page F-8
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
Inventory
Inventory is valued at the lower of cost or market and consists primarily of console units and single-use treatment catheters. Inventory consists of the direct costs of acquiring the inventory from vendors. Inventory of console units are considered non-current since the sales period is usually in excess of one year.
Inventory is relieved using the first-in, first-out method and consists of the following at September 30, 2015 and March 31, 2015.
| | | | | | | | |
| | | September 30 | | | March 31 | |
Current inventory – Catheters | | $ | 145,488 | | | $ | 79,604 | |
Non-current inventory - Consoles | | | 191,776 | | | | 190,276 | |
| | | | | | | | |
| | $ | 337,264 | | | $ | 269,880 | |
| | | | | | | | |
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is provided over the estimated useful lives of the related assets, ranging from three to seven years, using the straight-line method. Major renewals and improvements are capitalized and ordinary repairs and maintenance are expensed as incurred.
The Company reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An asset is considered impaired if its carrying amount exceeds the future net undiscounted cash flows that the asset is expected to generate. If such asset is considered to be impaired, the impairment recognized is the amount by which the carrying amount of the asset, if any, exceeds its fair value determined using a discounted cash flow model.
Contingent Consideration
In accordance with ASC 805, upon the purchase of Prolieve, the Company recognized a contingent consideration obligation as part of the consideration transferred in exchange for the acquired business. The initial measurement of the contingent consideration obligation was based on its estimated fair value. The contingent consideration obligation has been re-measured to fair value at each reporting date and will continue to be re-measured until the contingency is resolved. The changes in fair value are recognized in earnings. The obligation outstanding totaled $913,812 and $1,031,179 as of September 30, 2015 and March 31, 2015, respectively.
Intangible Assets
Intangible assets consist of intellectual property and customer relationships for our Prolieve business acquired in July 2012. These intangible assets were originally recorded at fair value and are amortized on a straight line basis over their estimated useful lives of 10 years. The Company reviews its intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, in a manner similar to that for property and equipment.
Deferred Financing Fees and Other Assets
As part of the convertible debt transaction (see note 5), the Company has unamortized deferred financing fees in the amount of $188,177 and $282,926 as of September 30, 2015 and March 31, 2015, respectively. Other assets primarily includes prepaid rent.
Page F-9
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
Revenue Recognition
The Company sells products and provides services which are used in the treatment of BPH. The Company recognizes revenue, net of sales taxes, from the sale of Prolieve consoles and catheters upon shipment to the customer. Revenue from the sale of products is measured at the fair value of the consideration received or receivable, net of any estimated returns. Revenue from the mobile service is recognized upon completion of the services, which is generally upon treatment of the patient.
The Company does not have a return policy that allows customers to return product, however the company has allowed returns on a limited customer by customer basis. The Company’s estimate for returns is based upon its historical experience with actual returns, however such returns have historically been limited. While such experience has allowed for reasonable estimation in the past, history may not always be an accurate indicator of future returns. The Company continually monitors its estimates for returns and makes adjustments when it believes that actual product returns may differ from the established accruals, if any. We record a provision for estimated returns in the same period as the related revenue is recorded.
Costs of Sales—Products
Costs of goods sold primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our intellectual property, warranty costs, warehousing costs, freight and handling charges. Warehousing costs include payroll and benefit costs, including related stock-based compensation expense.
Costs of Sales—Services
Costs of services consist primarily of the costs to provide mobile services to our patients, including catheter cost, depreciation of our mobile consoles and vehicle fleet, and payroll and benefit costs, including related stock-based compensation expense.
Warranty Liabilities
Prolieve products are covered by warranties against defects in material and workmanship for periods of up to 12 months. We record a liability for warranty claims at the time of sale based on the trend in the historical ratio of product failure rates, material usage and service delivery costs to sales, the historical length of time between the sale and resulting warranty claim and other factors. The accrued liability for warranty provisions was approximately, $38,000 for each of the periods ended September 30, 2015 and March 31, 2015.
Research and Development Expenses
Research and development costs are expensed as incurred.
Income Taxes
Income taxes are accounted for under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in results of operations in the period that the tax rate change occurs. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
A tax position is recognized as a benefit only if it is “more likely than not” that the tax position taken would be sustained in a tax examination, presuming that a tax examination will occur. The Company recognizes interest and/or penalties related to income tax matters in the income tax expense category. The Company remains subject to examination for income tax returns for the years ending after 2010.
Page F-10
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
Stock-Based Compensation
Compensation costs for all stock-based awards is measured at fair value on the date of the grant using an option pricing model and is recognized over the service period for awards expected to vest. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the current estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Profit Sharing Plan
The Company sponsors a defined contribution retirement plan through a Section 401(k) profit sharing plan. Employees may contribute up to 15% of their pre-tax compensation. Participants are eligible for matching Company contributions up to 3% of eligible compensation dependent on the level of voluntary contributions. Company matching contributions totaled $14,966 and $27,033 for the three month period ended September 30, 2015 and 2014, respectively. For the six month period ended September 30, 2015 and 2014, matching contributions were $27,943 and $43,472, respectively.
Net Loss Per Share
Basic net loss per share is computed by dividing net loss available to common shareholders by the weighted-average number of shares of common shares outstanding during the period.
For periods of net loss, diluted loss per share is calculated similarly to basic loss per share because the impact of all dilutive potential common shares is anti-dilutive due to the net losses. For the three and six month periods ended September 30, 2015 and 2014, outstanding stock options of 8,525,000 for each of the periods, and warrants outstanding to purchase 80,631,310 and 105,278,102 commons shares, respectively, were considered anti-dilutive and therefore were not included in the calculation of diluted shares. For the three and six month periods ended September 30, 2015 and 2014, convertible promissory notes convertible into 22,160,000 shares of common stock were considered anti-dilutive and therefore were not included in the calculation of diluted shares.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (FASB) issued ASU 2014-09 – Revenue from Contracts with Customers providing guidance for revenue recognition for contracts. This guidance requires an entity to review contracts in five steps and will result in enhanced disclosures regarding the nature, amount, timing and uncertainty of revenue arising from contracts with customers. This standard is effective for fiscal years beginning after December 15, 2017 and early adoption is permitted for fiscal years beginning after December 15, 2016. We are currently evaluating the impact, if any, that this new guidance will have on the Company’s Condensed Interim Consolidated Financial Statements.
ASU No. 2014-12,Compensation-Stock Compensation (Topic 718): Accounting for Share Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Periodamendments require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. Guidance in Topic 718 as it relates to awards with performance conditions that affect vesting should be applied to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. As indicated in the definition of vest, the stated vesting period (which includes the period in which the performance target could be achieved) may differ from the requisite service period. The amendments of ASU 2014-12 are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. The adoption of the amended guidance is not expected to have a material impact on the Company’s Condensed Interim Consolidated Financial Statements.
Page F-11
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
ASU No. 2014-16,Derivatives and Hedging (Topic 815): Determining Whether the Host Contract in a Hybrid Financial instrument in the Form of a ShareisMore Akin to Debt or Equity.In November 2014, the FASB issued amended guidance that clarifies how current GAAP should be interpreted in evaluating the economic characteristics and risks of a host contract in a hybrid financial instrument that is issued in the form of a share. Specifically, the amendments clarify that an entity should consider all relevant terms and features -including the embedded derivative features being evaluated for bifurcation -in evaluating the nature of the host contract. Furthermore, the amendments clarify that no single term or feature would necessarily determine the economic characteristics and risks of the host contract. Rather, the nature of the host contract depends upon the economic characteristics and risks of the entire hybrid financial instrument. The amended guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015, with early adoption permitted. The effects of initially adopting the amended guidance should be applied on a modified retrospective basis to existing hybrid financial instruments issued in the form of a share as of the beginning of the fiscal year for which the amendments are effective and shall be reported as a cumulative-effect adjustment directly to retained earnings as of the beginning of the year of adoption. The adoption of the amended guidance is not expected to have a material impact on the Company’s Condensed Interim Consolidated Financial Statements.
ASU No. 2015-03,Interest-Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs.In April 2015, the FASB issued amended guidance to address the different balance sheet presentation requirements for debt issuance costs and debt discounts and premiums. The amended guidance requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the amended guidance. The amended guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015, with early adoption permitted for financial statements that have not been previously issued. The amended guidance should be applied retrospectively, wherein the balance sheet of each individual period presented should be adjusted to reflect the period-specific effects of applying the amended guidance. The Company currently has approximately $188,177 of debt issuance costs recorded in the Condensed Interim Consolidated Statements of Financial Position that will be required to be reclassified and presented as a direct deduction from the debt liability upon adoption of the amended guidance. The adoption of the amended guidance is not expected to have an impact on the Company’s Condensed Interim Consolidated Statements of Loss and Comprehensive Loss.
In August 2014, the FASB issued ASU 2014-15 – Presentation of Financial Statements – Going Concern. This guidance requires management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. This guidance is effective for the annual period after December 15, 2016 and for the annual periods and interim periods thereafter. Early application is permitted. We do not expect the adoption of this guidance to have a material impact on the Company’s Condensed Interim Consolidated Financial Statements.
In July 2015, the FASB issued ASU 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” This ASU changes the measurement principle for certain inventory methods from the lower of cost or market to the lower of cost and net realizable value. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. This ASU does not apply to inventory that is measured using Last-in First-out (“LIFO”) or the retail inventory method. The provisions of ASU 2015-11 are effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The Company is currently evaluating this guidance to determine the impact it may have to its consolidated financial statements.
Emerging Growth Company Status
We are an “emerging growth company” as defined in section 3(a) of the Exchange Act, as amended by the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), and will continue to qualify as an “emerging growth company” until the earliest to occur of: (a) the last day of the fiscal year during which we have total annual gross revenues of $1,000,000,000 (as such amount is indexed for inflation every 5 years by the SEC) or more; (b) the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (c) the date on which we have, during the previous 3-year period, issued more than $1,000,000,000 in non-convertible debt; or (d) the date on which we are deemed to be a ‘large accelerated filer’, as defined in Exchange Act Rule 12b–2.
Page F-12
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
Generally, a company that registers any class of its securities under section 12 of the Exchange Act is required to include in the second and all subsequent annual reports filed by it under the Exchange Act, a management report on internal controls over financial reporting and, subject to an exemption available to companies that meet the definition of a “smaller reporting company” in Exchange Act Rule 12b-2, an auditor attestation report on management’s assessment of internal controls over financial reporting. However, for so long as we continue to qualify as an emerging growth company, we will be exempt from the requirement to include an auditor attestation report in our annual reports filed under the Exchange Act, even if we do not qualify as a “smaller reporting company”. In addition, section 103(a)(3) of the Sarbanes-Oxley Act of 2002 has been amended by the JOBS Act to provide that, among other things, auditors of an emerging growth company are exempt from any rules of the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the company.
Any U.S. domestic issuer that is an emerging growth company is able to avail itself to the reduced disclosure obligations regarding executive compensation in periodic reports and proxy statements, and to not present to its shareholders a nonbinding advisory vote on executive compensation, obtain approval of any golden parachute payments not previously approved, or present the relationship between executive compensation actually paid and our financial performance. As a foreign private issuer, we are not subject to such requirements, and will not become subject to such requirements even if we were to cease to be an emerging growth company.
As a reporting issuer under the securities legislation of the Canadian provinces of Ontario, British Columbia, and Alberta, we are required to comply with all new or revised accounting standards that apply to Canadian public companies. Pursuant to Section 107(b) of the JOBS Act, an emerging growth company may elect to utilize an extended transition period for complying with new or revised accounting standards for public companies until such standards apply to private companies. We have elected to utilize this extended transition period. However, while we have elected to utilize this extended transition period, our condensed interim consolidated financial statements as of September 30, 2015 reflect the adoption of all required accounting standards for public companies.
2. BUSINESS ACQUISITION AND CONTINGENT CONSIDERATION
On July 24, 2012 the Company purchased from Boston Scientific Corporation (“BSC”), in a taxable transaction, all of the assets, relating to the Prolieve Thermodilatation System (“Prolieve”), a FDA approved device for the treatment of Benign Prostatic Hyperplasia (“BPH”). The total purchase consideration consisted of the following:
| | | | |
Cash | | $ | 2,535,610 | |
Fair value of contingent consideration | | | 1,126,505 | |
| | | | |
Total consideration | | $ | 3,662,115 | |
| | | | |
The maximum amount of $2,500,000 is payable as contingent consideration pursuant to the terms of the agreement. The fair value of the contingent consideration was determined by calculating its present value based on its payment terms using an interest rate of 24% (our estimated unsecured borrowing rate). The contingent consideration is payable quarterly at a rate of 10% of sales of Prolieve products. As of September 30, 2015, $1,030,638 of royalties is due to BSC of which $917,002 is past due.
As of September 30, 2015, the balance due is allocated as follows:
| | | | | | | | | | | | |
| | | Non Contingent
portion | | | Contingent
portion | | | Total | |
Balance at April 1, 2015 | | $ | 831,632 | | | $ | 1,031,179 | | | $ | 1,862,811 | |
Less: payments | | | — | | | | — | | | | — | |
Change in fair market value | | | 199,006 | | | | (117,367 | ) | | | 81,639 | |
| | | | | | | | | | | | |
Balance at September 30, 2015 | | $ | 1,030,638 | | | $ | 913,812 | | | $ | 1,944,450 | |
| | | | | | | | | | | | |
Allocated as follows: | | | | | | | | | | | | |
Payable to BSC | | $ | 1,030,638 | | | $ | — | | | $ | 1,030,638 | |
| | | | | | | | | | | | |
Contingent Payable to BSC—current | | $ | — | | | $ | 473,630 | | | $ | 473,630 | |
| | | | | | | | | | | | |
Contingent Payable to BSC—long term | | $ | — | | | $ | 440,182 | | | $ | 440,182 | |
| | | | | | | | | | | | |
Page F-13
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
3. PROPERTY AND EQUIPMENT
Property and equipment consists of the following as of September 30, 2015 and March 31, 2015:
| | | | | | | | |
| | | September 30, 2015 | | | March 31, 2015 | |
Machinery and equipment (5-7 year life) | | $ | 75,982 | | | $ | 75,982 | |
Mobile consoles (7 year life) | | | 678,845 | | | | 678,845 | |
Furniture and fixtures (3-5 year life) | | | 20,000 | | | | 20,000 | |
| | | | | | | | |
| | | 774,827 | | | | 774,827 | |
Accumulated depreciation | | | (362,822 | ) | | | (305,792 | ) |
| | | | | | | | |
Total | | $ | 412,005 | | | $ | 469,035 | |
| | | | | | | | |
Depreciation expense was approximately $28,000 and $27,000 for the three month period ended September 30, 2015 and 2014 and $57,000 and $57,000 for the six month period ended September 30, 2015 and 2014, respectively.
4. INTANGIBLE ASSETS
Intangible assets include intellectual properties and customer relationships relating to the Prolieve technology, acquired at a cost of $2.5 million. These assets are being amortized on a straight-line basis over ten years; amortization expense was $61,553 for the three month period ended September 30, 2015 and 2014, and $123,106 for the six month period ended September 30, 2015, and 2014, respectively.
Future amortization expense related to the net carrying amount of intangible assets is estimated to be as follows:
| | | | |
2016 | | $ | 246,212 | |
2017 | | | 246,212 | |
2018 | | | 246,212 | |
2019 | | | 246,212 | |
2020 | | | 246,212 | |
| | | | |
Sub-total | | | 1,231,060 | |
2021 and thereafter | | | 412,166 | |
| | | | |
Total | | $ | 1,643,226 | |
| | | | |
Page F-14
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
5. DEBT
In fiscal 2013, the Company raised bridge financing of approximately U.S. $435,000. The bridge financing lender received a promissory note, with interest payable at 2% per month after October 23, 2012. The original maturity date of the promissory notes was October 23, 2013 and was subsequently extended until June 30, 2014. As of September 30, 2015, the note remains in default and due in full. The Company is currently in discussions with the lender on a further extension of the maturity date. Interest expense of approximately $26,000 and $30,000 was recognized on the promissory note for the three month period ended September 30, 2015 and 2014, respectively. Interest expense of approximately $54,000 and $60,000 was recognized on the promissory note for the six month period ended September 30, 2015 and 2014, respectively.
In fiscal 2014, the Company issued 554 Units of 8% Redeemable Promissory Convertible Notes (the “Notes”) together with Series C stock Purchase Warrants (the “Warrants”) to various accredited investors in an offering exempt from registration in the U.S pursuant to regulations D and S under the U.S. Federal Securities rules and regulations, receiving gross proceeds of $5,540,000. The notes are convertible into 22,160,000 shares of common stock. Each warrant entitles the holder to acquire 20,000 common shares (for a total of 11,080,000 common shares) at an exercise price of $0.30 per share and expire on December 18, 2016. The warrants were classified as equity, were recorded as additional paid in capital at their estimated fair value of $1,532,877, and are considered a non-cash financing activity. The Company recognized a beneficial conversion feature of $195,938 and deferred financing fees (consisting of both cash payments and the fair value of stock purchase warrants classified as equity) of $558,552 which are amortized using the effective interest method through December 18, 2016. The Company has accrued interest of $576,590 owing to holders of the convertible debentures as of September 30, 2015, of which $465,790 is past due to certain holders, and is in a technical default of the terms of the debentures. The investors have not accelerated the terms of the debenture.
In connection with the above transaction, the Company recognized interest expense of $116,012 and 114,844 for the three month period ended September 30, 2015 and 2014; interest expense of $238,318 and $227,472 for the six month period ended September 30, 2015 and 2014; accretion expense of $195,939 and $175,728 for the three month period ended September 30, 2015 and 2014; and accretion expense of $386,574 and $346,803 for the six month period ended September 30, 2015 and 2014, respectively.
6. EQUITY AND STOCK-BASED COMPENSATION
Authorized share capital consists of unlimited common shares with no par value.
On September 15, 2014, the Company completed a private placement of 10,281,250 units at a price of USD $0.16 per unit raising gross proceeds of $1,645,000 (net proceeds of $1,444,274). Each unit consisted of one common share and one common share purchase warrant. Each warrant entitled the holder to acquire one common share at an exercise price of USD $0.25 until September 15, 2017. Management determined the warrants to have a fair value of $0.12 per warrant and accordingly, $1,231,934 of the proceeds from the issuance was allocated to additional paid in capital, and the balance of the proceeds was allocated to common shares.
A relative fair value calculation was used to determine the carrying value of the warrants. The fair value of the warrants issued was estimated using a Black-Scholes pricing model with the following assumptions:
| | |
| Risk free interest rate | | 0.94% to 0.99% |
| Expected life in years | | 3 year |
| Expected volatility | | 146.6% |
Common stock issuable
Prior to March 31, 2015, the company received funds for common shares in the amount of $1,561,000 (net of fees) as part of a future private placement occurring on May 12, 2015. As of September 30, 2015, the company has no common stock issuable.
Page F-15
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
Stock Purchase Warrants
The Company had stock purchase warrants outstanding as of September 30, 2015 and March 31, 2015 as follows:
| | | | | | | | | | | | | | | | |
| | | | | | | | | September 30,
2015 | | | March 31,
2015 | |
Year of issue | | Exercise
Price | | | Expiration | | | Underlying
Shares | | | Underlying
Shares | |
2011 | | $ | 0.50 | | | | 4/24/2015 | | | | — | | | | 2,449,997 | |
2011 | | $ | 0.30 | | | | 2/24/2016 | | | | 3,745,000 | | | | 3,745,000 | |
2013 | | $ | 0.20 | | | | 6/8/2016 | | | | 18,367,263 | | | | 18,367,263 | |
2013 | | $ | 0.20 | | | | 6/8/2016 | | | | 22,200,000 | | | | 22,200,000 | |
2013 | | $ | 0.20 | | | | 9/21/2015 | | | | — | | | | 22,196,765 | |
2013 | | $ | 0.20 | | | | 1/14/2016 | | | | 13,056,997 | | | | 13,056,997 | |
2014 | | $ | 0.30 | | | | 12/18/2016 | | | | 8,336,400 | | | | 8,336,400 | |
2014 | | $ | 0.30 | | | | 3/7/2017 | | | | 4,644,400 | | | | 4,644,400 | |
2015 | | $ | 0.25 | | | | 9/15/2017 | | | | 10,281,250 | | | | 10,281,250 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | 80,631,310 | | | | 105,278,072 | |
| | | | | | | | | | | | | | | | |
Warrant Modifications
During the six month period ended September 30, 2015 and 2014 the Company extended the expiration date of certain outstanding stock warrants issued as part of private placements. Such modifications require an allocation between common stock and additional paid in capital and does not impact the net loss and total deficit of the Company.
Stock Options
The Company may grant stock options to directors, senior officers and service providers by resolution of the Board of Directors. The exercise price will reflect the market price of the Company’s stock on the date of the grant. The maximum number of stock options outstanding under the stock option plan is limited 10% of issued shares.
The Company measures the cost of stock option awards based on the grant-date fair value of the award. The cost is recognized over the period during which an employee is required to provide service in exchange for the award or the requisite service period. The Company recognizes stock-based compensation expense over the vesting periods of the awards, adjusted for estimated forfeitures. There was no stock-based compensation cost that was incurred by the Company for the three and six month periods ended September 30, 2015 and 2014, respectively.
A summary of the Plan as of September 30, 2015 and March 31, 2015 and changes therein are presented below:
| | | | | | | | | | | | | | | | |
| | | September 30, 2015 | | | March 31, 2015 | |
| | | Number
# | | | Weighted
average
exercise
price
$ | | | Number
# | | | Weighted
average
exercise
price
$ | |
Outstanding, beginning of year | | | 8,525,000 | | | $ | 0.20 | | | | 8,525,000 | | | $ | 0.20 | |
Expired | | | — | | | | | | | | — | | | | | |
Granted | | | — | | | | | | | | — | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding, end of year | | | 8,525,000 | | | $ | 0.20 | | | | 8,525,000 | | | $ | 0.20 | |
| | | | | | | | | | | | | | | | |
Options exercisable, end of year | | | 8,525,000 | | | | | | | | 8,525,000 | | | | | |
| | | | | | | | | | | | | | | | |
Page F-16
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
7. INCOME TAXES
The Company is domiciled in Canada and files Canadian federal and certain provincial tax returns. The Company had no provision (benefit) for income taxes as of September 30, 2015 and March 31, 2014, as a result of its net losses and full valuation allowance against its deferred tax assets.
The Company is required to establish a valuation allowance for deferred tax assets if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The Company considers the projected future taxable income and tax planning strategies in making this assessment. Based upon the level of historical taxable income and projections for future taxable income in the periods which the deferred tax assets are deductible, the Company has determined that a full valuation allowance is required as of September 30, 2015 and March 31, 2015.
The Company is subject to income taxes in numerous jurisdictions. Significant judgment is required in evaluating the Company’s tax positions and determining its provision for income taxes. The Company establishes liabilities for tax-related uncertainties based on estimates of whether, and the extent to which, additional taxes will be due. These liabilities are established when the Company believes that certain positions might be challenged despite its belief that its tax return positions are fully supportable. The Company adjusts these liabilities in light of changing facts and circumstances, such as the outcome of a tax audit. The provision for income taxes includes the impact of changes to the liability that is considered appropriate. The Company has identified no material uncertain tax positions as of September 30, 2015 and March 31, 2015.
The Company is subject to income tax audits in all jurisdictions for which it is required to file tax returns. Tax audits by their nature are often complex and can require several years to complete. Neither the Company nor any of its subsidiaries is currently under audit in any jurisdiction. All of the Company’s income tax returns remain subject to examination by tax authorities.
As a Canadian domiciled company, the Company has never filed a tax return in the United States. U.S. federal tax legislation was enacted in 2004 to address perceived U.S. tax concerns in “corporate inversion” transactions. A “corporate inversion” generally occurs when a non-U.S. Company acquires “substantially all” of the equity interests in, or the assets of, a U.S. Company or partnership, if, after the acquisition, former equity holders of the U.S. Company or partnership own a specified level of stock in the non-U.S. Company. The tax consequences of these rules depend upon the percentage identity of stock ownership that results. Generally, in “80 percent identity” transactions (i.e., former equity holders of the U.S. Company owns 80% or more of the equity of the non-U.S. acquiring entity, excluding certain equity interests), the tax benefits of the inversion are limited by treating the non-U.S. acquiring entity as a domestic entity for U.S. tax purposes. In “60-80 percent identity” transactions, the benefits of the inversion are limited by barring certain corporate-level “toll charges” from being offset by certain tax attributes of the U.S. Company (e.g., loss carry-forwards), and imposing excise taxes on certain stock-based compensation held by “insiders” of the U.S. Company. Management is of the view that a corporate inversion has resulted from the reverse takeover transaction it completed in fiscal 2009; however, it has not yet determined whether the Company is subject to the “80 percent” or the “60-80 percent” identity with respect to the transactions undertaken in the fiscal 2009 year since the interpretation of which categories of stock ownership are to be considered under the inversion rules is not yet settled. The Company has not filed income tax returns in Canada or United States since 2009. The Company has had losses in every jurisdiction in these years, income tax expenses and non-filing penalties are insignificant.
8. COMMITMENTS AND CONTINGENCIES
On January 16, 2006, the Company’s wholly owned subsidiary, Celsion (Canada) Inc. purchased from Celsion Corporation(USA) [“Celsion”]all of the assets relating to breast cancer Microfocus APA 1000 System (“System”), consisting of the microwave machine technology, the APA technology licensed from MIT, and all related intellectual and regulatory property (collectively, the “Business”). The Company has a commitment to pay a 5% royalty to Celsion on the net sales of products sold by and patent royalties received by the Company and its successors and assignees. Total royalties paid are not to exceed $18,500,000. Royalties will not be payable until the
Page F-17
MEDIFOCUS, INC. NOTES TO UNAUDITED CONDENSED INTERIM CONSOLIDATED
FINANCIAL STATEMENTS
System can be placed in the market following successful completion of the pivotal clinical trial and receipt of approval to market the System in the US and Canada from the FDA and Health Canada. The Company has an additional commitment to pay a 5% royalty to MIT on the net sales of products, upon commercialization.
Future minimum payments under operating leases for office space and vehicles are as follows:
| | | | |
2016 | | $ | 198,519 | |
2017 | | $ | 201,545 | |
2018 | | $ | 112,946 | |
The Company recognized total rent expense of $48,132 and $50,443 for the three month periods ended September 30, 2015 and 2014 and $97,896 and $99,166 for the six month periods September 30, 2015 and 2014, respectively.
The Company has agreed to indemnify its directors and officers and certain of its employees in accordance with the Company’s by-laws. The Company maintains insurance policies that may provide coverage against certain claims.
Page F-18
FORM 52-109FV2
CERTIFICATION OF INTERIM FILINGS
VENTURE ISSUER BASIC CERTIFICATE
I, Dr. Augustine Y. Cheung, President and Chief Executive Officer of Medifocus Inc., certify the following:
| 1. | Review: I have reviewed the interim financial report and interim MD&A (together, the “interim filings”) of Medifocus Inc. (the “issuer”) for the interim period ended September 30, 2015. |
| 2. | No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, for the period covered by the interim filings. |
| 3. | Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings. |
Date: November 30, 2015.
| | |
| (signed) “Augustine Y. Cheung” |
| |
| Name: | | Dr. Augustine Y. Cheung |
| Title: | | President and Chief Executive Officer |
NOTE TO READER
In contrast to the certificate required for non-venture issuers under National Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings (NI 52-109), this Venture Issuer Basic Certificate does not include representations relating to the establishment and maintenance of disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as defined in NI 52-109. In particular, the certifying officers filing this certificate are not making any representations relating to the establishment and maintenance of
| | i) | controls and other procedures designed to provide reasonable assurance that information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation; and |
| | (ii) | a process to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the issuer’s GAAP. |
The issuer’s certifying officers are responsible for ensuring that processes are in place to provide them with sufficient knowledge to support the representations they are making in this certificate. Investors should be aware that inherent limitations on the ability of certifying officers of a venture issuer to design and implement on a cost effective basis DC&P and ICFR as defined in NI 52-109 may result in additional risks to the quality, reliability, transparency and timeliness of interim and annual filings and other reports provided under securities legislation.
FORM 52-109FV2
CERTIFICATION OF INTERIM FILINGS
VENTURE ISSUER BASIC CERTIFICATE
I, Mirsad Jakubovic, Chief Financial Officer of Medifocus Inc., certify the following:
| 1. | Review: I have reviewed the interim financial report and interim MD&A (together, the “interim filings”) of Medifocus Inc. (the “issuer”) for the interim period ended September 30, 2015. |
| 2. | No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, for the period covered by the interim filings. |
| 3. | Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings. |
Date: November 30, 2015.
| | |
| (signed) “Mirsad Jakubovic” |
| |
| Name: | | Mirsad Jakubovic |
| Title: | | Chief Financial Officer |
NOTE TO READER
In contrast to the certificate required for non-venture issuers under National Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings (NI 52-109), this Venture Issuer Basic Certificate does not include representations relating to the establishment and maintenance of disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as defined in NI 52-109. In particular, the certifying officers filing this certificate are not making any representations relating to the establishment and maintenance of
| | i) | controls and other procedures designed to provide reasonable assurance that information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation; and |
| | (ii) | a process to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the issuer’s GAAP. |
The issuer’s certifying officers are responsible for ensuring that processes are in place to provide them with sufficient knowledge to support the representations they are making in this certificate. Investors should be aware that inherent limitations on the ability of certifying officers of a venture issuer to design and implement on a cost effective basis DC&P and ICFR as defined in NI 52-109 may result in additional risks to the quality, reliability, transparency and timeliness of interim and annual filings and other reports provided under securities legislation.



