MEDIFOCUS INC.
FORM 51-102FI
Management Discussion and Analysis
for the three months ended June 30, 2019
August 29, 2019
The following sets out the Management’s Discussion and Analysis (“MD&A”) of the financial position and results of operations for the three months ended June 30, 2019 of Medifocus Inc. (the “Company”, “Medifocus” or“we”). The MD&Ais dated August 29, 2019 andshould be read in conjunction with the Company’sunauditedcondensed interim consolidated financial statements for the three months ended June 30, 2019 and 2018. All dollar amounts are presented in United States dollars unless otherwise noted. The functional currency of the Company and its subsidiary is the United States dollar, and the presentation currency is the United States dollar. Additional information relating to the Company is available on SEDAR atwww.sedar.com.
Forward-Looking Statements
This management’s discussion and analysis may contain statements that are “Forward-looking Statements”. These include statements about the Company’s expectations, beliefs, plans, objectives and assumptions about future events or performance. These statements are often, but not always, made through the use of words or phrases such as “will likely result”, “are expected to”, “will continue”. “anticipate”, “believes”, “estimate”, “intend”, “plan”, “would”, and “outlook” or statements to the effect that actions, events or results “will”, “may”, “should” or “would” be taken, occur or be achieved. Forward-looking statements are not historical facts, and are subject to a number of risks and uncertainties beyond the Company’s control. Accordingly, the Company’s actual results could differ materially from those suggested by these forward-looking statements for various reasons discussed throughout this analysis. Forward-looking statements are made on the basis of the beliefs, opinions, and estimates of the Company’s management on the date the statements are made and, other than in compliance with applicable securities laws, the Company does not undertake any obligation to update forward-looking statements if the circumstances or management’s beliefs, opinions or estimates should change. Readers should not place undue reliance on forward-looking statements.
Reporting currency
Effective April 1, 2014, the Company changed is reporting and functional currency from the Canadian dollar to the U.S. dollar in anticipation of filing its financial statements with the U.S. Securities and Exchange Commission;
| 2. | Overview of Financial Performance |
The Company recognized revenues of $562,068 for the three months ended June 30, 2019, a decrease of $175,322, or 24%, compared to the same three-month period of 2018. The decrease of total sales is primarily due to the $144,783 decrease in direct product sales. The Prolieve disposal kit supply delayed caused by parts shortage in June 2019 had contributed to the reduction of certain sales during the quarter. Additionally, the insurance reimbursement rates were reduced during the end of FY 2019 and several of our service sales prices were reduced.Our sales had declined in general since the initial cost cutting measures were implemented in August 2014 when the Company started to gradually eliminate sales positions and mobile services in areas where there was low demand and ultimately not profitable. The emerging of other BPH treatment options have also affected our business negatively. As the result of the cost reduction measures implemented,our operating expenses were reduced significantly over the last five years, from $4,651,973 for the fiscal year ended March 31, 2015 to $4,494,867 for the fiscal year ended March 31, 2016 and from $1,996,188 for the fiscal year ended March 31, 2017 to $1,593,789 for the fiscal year ended March 31, 2018. Our operating expenses for the year ended March 31, 2019 were further reduced to $1,358,208 from $1,593,789 for the year ended March 31, 2018.
The Company realized a$116,587 increaseinloss from operation for the three months ended June 30, 2019 from the same period a year ago, largely caused by lower gross profit due to lower sales and reduced sales prices for our product and higher general and administration expenses which were caused by an increase in legal expenses.
The cost of sales for the three months ended June 30, 2019 was $387,390. Gross margin was 31%, changed from 32% a year ago.
| 3. | Company History and Business |
Our business was started by Dr. Augustine Cheung, our former Chief Executive Officer, as an outgrowth of his academic interest and work in the field of microwave technology and the thermotherapy treatment of disease while he was a professor at the University of Maryland and George Washington University. In 1982, he founded A.Y. Cheung Associates Inc. to pursue this work. A.Y. Cheung Associates Inc. changed its name to Cheung Laboratories, Inc. in 1984, and Cheung Laboratories Inc. subsequently changed its name to Celsion Corporation (“Celsion”) in 1998.
At Celsion, Dr. Cheung and his team began developing technologies for the treatment of BPH and breast cancer using thermotherapy technology, leading to the development and commercialization of the Prolieve® system for the treatment of BPH. In 2007, Celsion sold the Prolieve® system and technology to Boston Scientific Corporation (“Boston Scientific”) for $60 million. Dr. Cheung also began developing the APA 1000 system for the treatment of breast cancer. The rights to key elements of APA 1000 were licensed from MIT pursuant to an Exclusive Patent License Agreement (“Patent License Agreement”) dated October 24, 1997.
In 2005 Celsion transferred all its interest in this license and other rights to APA 1000 to its wholly-owned subsidiary, Celsion (Canada) Limited (“Celsion Canada”). On January 16, 2006, Dr. Cheung resigned from Celsion’s board of directors and his position as Celsion’s Chief Scientific Officer, and purchased Celsion Canada for $20,000,000 (Canadian dollars). The purchase price was paid by issuing: (a) a personal $1.5 million promissory note; and (b) an $ 18.5 million royalty payable at the rate of 5% of the net sales on sales of products developed using APA technology, once such products become commercialized. The $1.5 million promissory note was secured by 1,508,050 shares of Celsion’s common stock. After Dr. Cheung’s default on payment of the promissory note, Celsion agreed in 2009 with Dr. Cheung to retain the 1,508,000 shares of Celsion’s common stock that it held as security in full satisfaction of the $1.5 million promissory note.
Medifocus Inc. was incorporated on April 25, 2005 under the Business Corporations Act (Ontario) as a CPC. Under Canadian law, a CPC is a newly created Canadian company having no assets, other than cash, which is permitted to conduct an initial public offering of its securities (“IPO”) and obtain a listing of its shares on the TSXV. A CPC may then uses the funds raised in the IPO to identify and evaluate assets or businesses which, when acquired, qualify the CPC for listing as a regular issuer on the TSXV.
On June 29, 2006 Medifocus Inc., completed its IPO on the TSXV of 4,600,000 shares at a price of $0.20 (Canadian dollars) per share receiving gross proceeds of $920,000 (Canadian dollars). In order to gain improved access to funding, Medifocus Inc. engaged in a share exchange offer with Celsion Canada in 2008 pursuant to which Celsion Canada became a wholly-owned subsidiary of Medifocus. Concurrently with the exchange offer, Medifocus completed a private placement of units, receiving gross proceeds of $2 million (Canadian dollars). In addition, Medifocus issued 903,112 shares to Celsion at a deemed value of $0.50 (Canadian dollars) per share, in partial satisfaction of an approximate $600,000 (Canadian dollars) liability that was owed to Celsion. After the completion of the share exchange transaction, we continued our development of the APA 1000 technology for the treatment of breast cancer. Phase I and Phase II clinical trials were originally completed by Celsion. Subsequently, the Company received approvals from both the FDA and the Canadian Bureau of Medical Devices to conduct a pivotal Phase III breast cancer treatment study. We have begun the pivotal Phase III clinical trials but, such trials have been proceeding at a slow pace due to lack of funding. We plan to complete the pivotal Phase III trial when funding is available.
The Patent License Agreement with MIT was amended on June 16, 2007. The amended agreement requires us to pay MIT a 5% royalty on the net sales of any products derived from APA 1000, and an annual maintenance fee of $50,000. MIT is entitled to receive royalties for so long as the patents relating to the APA technology are valid or the Patent License Agreement is terminated.
On July 24, 2012, we acquired the Prolieve® technology and related assets from Boston Scientific pursuant to an Asset Purchase Agreement dated June 25, 2012, amended on July 24, 2012 (the “Asset Purchase Agreement”). The purchase price was $3,662,115, of which $2,535,610 was paid on the closing of the transaction. Additionally, we entered into a contingent consideration arrangement under which we will pay Boston Scientific up to $2,500,000, to be paid in quarterly installments at a rate of 10% of the sales of Prolieve® products which is estimated to have contingent balance through September 30, 2018. Sales are defined as the gross amount invoiced for sales, distributions, licenses, leases, transfers, and other dispositions. As of June 30, 2018, $1,977,967 is due to Boston Scientific under the contingent consideration arrangement, of which $1,902,387 is past due.
Our Products
A. Prolieve® Thermodilatation™ System
Our first commercial heat-based therapy system, Prolieve®, is used to treat benign prostatic hyperplasia or “BPH.” BPH is a condition in which the prostate gland becomes enlarged and restricts the flow of urine through the urethra. Our clinical studies have shown that the treatment of this condition with the Prolieve® system improves urine flow by decreasing the enlarged prostate’s pressure on the urethra through the heating, dilation and shrinking of the prostate tissue surrounding it. The BPH drug therapy market is estimated to be about $4 billion in major developed countries according to Decision Resources Group. This number does not include non-drug treatments and the patients who are on “Watchful Waiting” due to the side effects of some of the treatment options. While the market for minimally invasive BPH treatment is approximately $150 million according to Medtech Insight, we believe that Prolieve®® can be a viable alternative to drug therapy due to its safety and efficacy profiles and thus has the potential to increase the market for minimally invasive BPH treatment.
What Is Benign Prostatic Hyperplasia?
Millions of aging men experience symptoms resulting from BPH, a non-cancerous urological disease in which the prostate enlarges and constricts the urethra. The prostate is a walnut-sized gland surrounding the male urethra that produces seminal fluid and plays a key role in sperm preservation and transportation. The prostate frequently enlarges with age. As the prostate expands, it compresses or constricts the urethra, thereby restricting the normal passage of urine. This restriction may require a patient to exert excessive bladder pressure to urinate. Because urination is one of the body’s primary means of cleansing impurities, the inability to urinate adequately increases the possibility of infection and bladder and kidney damage.
BPH Symptoms
The symptoms of BPH usually involve problems with emptying the bladder or storing urine in the bladder. However, the severity of the symptoms can vary widely, from mild and barely noticeable to serious and disruptive. Common BPH symptoms include:
| ● | Pushing or straining to begin urination; |
| ● | Dribbling after urination; |
| ● | A frequent need to urinate, sometimes every 2 hours or less; |
| ● | A recurrent, sudden, or uncontrollable urge to urinate; |
| ● | Feeling the bladder has not completely emptied after urination; |
| ● | Pain during urination; and |
| ● | Waking at night to urinate. |
In extreme cases, a man may be completely unable to urinate. In such situations, emergency medical attention is required.
An enlarged prostate does not cause prostate cancer or directly affect sexual function. However, many men experience sexual dysfunction and BPH symptoms at the same time. This is due to aging and the common medical conditions older men often encounter, including vascular disease and diabetes. Because these conditions take place with aging, sexual dysfunction tends to be more pronounced in men with BPH.
BPH Complications
BPH is not a form of prostate cancer and does not lead to prostate cancer. Accordingly, BPH is not life-threatening. However, as many men know, BPH may be lifestyle-threatening and can cause great discomfort, inconvenience, and awkwardness and complications such as:
| ● | Acute urinary retention, which is a condition that results in a complete inability to urinate. A tube called a catheter may be needed to drain urine from the bladder. |
| ● | Chronic urinary retention, which is a partial blockage of urine flow that causes urine to remain in the bladder. In rare cases, this may lead to kidney damage if it goes undiagnosed for too long. |
| ● | Urinary tract infection, which can cause pain or burning during urination, foul-smelling urine, or fever and chills. |
| ● | Other complications from BPH may include bladder stones or bladder infections. |
| ● | Having BPH does not directly affect one’s sexual function. However, it is common for the symptoms of BPH and sexual dysfunction to occur at the same time. |
Prevalence of BPH and Market Opportunity
BPH is an age-related disorder the incidence of which increases with maturation of the population. According to urologyhealth.org, by age 60, more than half of men have BPH. By age 85, about 90 percent of men have BPH. As the population continues to age and life expectancy increases, the prevalence of BPH can be expected to continue to increase.
Treatment Alternatives for BPH
Several types of treatments are available for enlarged prostate. They include medications, surgery and minimally invasive surgery. The best treatment choice for patients depends on several factors, including how much the symptoms bother them, the size of their prostate, other health conditions the patients may have, their age and preference. If symptoms are not severe, a patient may decide not to have treatment and wait to see whether their symptoms become more bothersome over time.
Watchful Waiting
When a patient first develops symptoms caused by BPH, physicians generally prescribe drugs as the first treatment option, but usually leave the decision to their patients. Due to the low success rate, high costs, side effects and complications associated with BPH drug therapies, some patients diagnosed with BPH prefer to be regularly monitored by their doctors, but choose not to begin a drug therapy. The patients who opt out of therapy fall into a group referred to as “watchful waiting.” Often, BPH symptom persistence and worsening or an acute urinary event may force the patient to move on to some other form of therapy.
Drug Therapy
Medications are the most common treatment for moderate symptoms of prostate enlargement but if a patient stops taking medicine, the symptoms will usually return. Medications used to relieve symptoms of enlarged prostate include several types of drugs, such as Alpha-Blockers (such as Flomax®) and Alpha Reductase Inhibitors (such as Proscar®). Drug therapy costs approximately $1,000 per year or more in the United States, must be maintained for life, and does not offer consistent relief to many BPH patients. Many of the currently available BPH drugs also have appreciable side effects, such as: headache, fatigue, impotence, dizziness, and low blood pressure.
Surgical Intervention
Two of the primary surgical procedures to treat BPH are transurethral resection of the prostate (“TURP”) and laser procedures. TURP has traditionally been a common procedure for enlarged prostate for many years. It is a procedure in which the prostatic urethra and surrounding diseased tissue in the prostate are trimmed with a telescopic knife, thereby widening the urethral channel for urine flow. While the TURP procedure generally has been considered the most effective treatment available for the relief of BPH symptoms, the procedure has its shortcomings. In the first instance, TURP generally requires from one to three days of post-operative hospitalization. In addition, a substantial percentage, approximately 5-10%, of patients who undergo TURP encounter significant complications, which can include painful urination, infection, impotence, incontinence, and excessive bleeding. Further, retrograde ejaculation, a condition in which semen released during ejaculation enters the bladder rather than exiting the penis, occurs in up to 90% of patients who undergo a TURP procedure, with a long-term side effect in up to 75% of such patients.
Laser surgeries (also called laser therapies) use high-energy lasers to destroy or remove overgrown prostate tissue. Options for laser therapy depend on prostate size, the location of the overgrown areas. During prostate laser surgery, a combined visual scope and laser is inserted through the tip of the patient’s penis into the urethra, which is surrounded by the prostate. Using the laser, doctors remove prostate tissue that are squeezing the urethra and blocking urine flow, thus making a new larger tube for urine to pass through. Lasers use concentrated light to generate precise and intense heat. Risks of laser surgery include: temporary difficulty urinating and post treatment catheterization, urinary tract infection, narrowing of the urethra as scars form, retrograde ejaculation, and erection problems.
Accordingly, neither drug therapies nor the surgical alternatives appear to provide fully satisfactory, cost-effective treatment solutions for BPH sufferers.
Our Approach: The Prolieve® Thermodilatation™ System
The Prolieve® Thermodilatation™ System was originally and primarily developed and commercialized by our current management, product development, clinical and regulatory teams. Such development occurred while such teams were employed at Celsion Corporation from 1997 to 2004, at an estimated cost of $20,000,000. As discussed above, Celsion sold the Prolieve® system, technology and related assets to Boston Scientific Corporation in 2007 for $60 million. In June 2012, Medifocus reached an agreement with Boston Scientific for the purchase of all of the assets of its Prolieve® business, including all Prolieve® inventory, the mobile service distribution assets, as well as the intellectual property associated with the Prolieve® technology.
Employing a patented 46 Fr. dilating balloon that enhances the efficiency of thermotherapy via a small microwave antenna embedded within a disposable 18 Fr. treatment catheter, Prolieve® Transurethral Thermodilatation™ (TUTD™) is the only FDA-approved Thermodilatation™ device on the market for treating BPH. Prolieve® TUTD™ is a fast in-office procedure performed under local anesthesia, with more than 100,000 cases thus far successfully performed in the U.S. since the initial FDA’s PMA approval for the device. Nearly 90% of all treated patients do not require a post treatment urinary catheter, in contrast to the vast majority of patients treated with other minimally-invasive BPH therapies. Thus, in addition to providing immediate symptomatic relief for BPH patients, Prolieve® has demonstrated long-term durable clinical benefits in the completed study accepted by the FDA.
In a randomized one-year clinical trial, conducted at 14 centers across the United States, patients undergoing treatment with Prolieve® achieved measurably greater improvement in symptoms after three months compared to a control group using a drug, Proscar, which is commonly prescribed to treat BPH condition.
Based upon a study conducted by Boston Scientific (the “Prolieve® Study”), patients treated with the Prolieve® system experienced a symptom reduction of 22% three days following treatment. Furthermore, most patients that undergo the Prolieve® treatment do not require post-treatment catheterization. Based upon the Prolieve® Study, 94% of patients that underwent the Prolieve® treatment were catheter free immediately following the treatment, and 100% of such patients were catheter free after three days. Accordingly, we believe that patients that undergo the Prolieve® treatment should be able to resume their normal activities shortly after the treatment.
Five Year Post Study Approved
In May 2018, the United States Food and Drug Administration (FDA) completed the review of the Company’s rigorous FDA mandated Post Approval Study (PAS). The 5-year follow-up study has satisfactorily fulfilled the PAS requirements. The PAS was conducted on a cohort of 225 symptomatic BPH patients treated with the Company’s Prolieve® Thermodilatation™ System. The 12-year PAS with 5-year follow-up data confirms long-term safety, efficacy and durability with improved lower urinary tract symptoms, urinary flow rate, quality of life, and minimal sexual side effects when compared to an untreated age-matched male population. In addition, the PAS has demonstrated stabilization of serum Prostate-Specific Antigen (PSA) level and prostate size during the 5-year follow-up period. The table below summarizes the key findings of the PAS:
| 85%Post-Treatment Catheter-Free Rate |
Minimal/No SexualSideEffects: ErectileDysfunction: 0.3 per 100person-years Retrograde Ejaculation: 0.3 per 100person-years |
Improvementof Mean AUASymptomScore: Baseline =20.1 vs.Year 5 =12.8 |
ImprovementofPeakFlow Rate (Qmax):Baseline =8.6mL/secvs.Year 5 =12.8 mL/sec |
Improvementof Quality of Life (QoL) Score:Baseline =22.0 vs.Year 5 =16.5 |
Stabilizationof BPHSymptoms:83%reported No Progression atYear5 |
Stabilizationof SerumPSAandProstate Size |
The Prolieve® system is comprised of two components. The first component is a freestanding module that contains a microwave generator and computerized controls that regulates and monitors the delivery of heat to the enlarged prostate tissue. The second component is our proprietary disposable catheter that is attached to the module. This component contains an internal balloon that is inflated after it is inserted through the urethra to the point of constriction. Upon inflation of the balloon, the tissue is heated by microwaves delivered via the catheter, resulting in dilation of the urethra. Our computer system in the module monitors and regulates the heat being applied to ensure maximum safety and efficiency. The Prolieve® system is covered by 45 core patents, which were acquired as part of the acquisition of the Prolieve®® assets from Boston Scientific Corporation in 2012.
The combined effect of this “heat plus compression” therapy is twofold: first, the heat denatures the proteins in the wall of the urethra, causing a stiffening of the opening created by the inflated balloon, forming a biological stent. Second, the heat serves effectively to kill off prostate cells outside the wall of the urethra, thereby creating sufficient space for the enlarged natural opening. In addition, the Prolieve® system’s temperature (46º C to 54º C) is sufficient to kill prostatic cells surrounding the urethra wall, thereby creating space for the enlargement of the urethra opening. However, the relatively low temperature is not sufficient to cause swelling in the urethra.
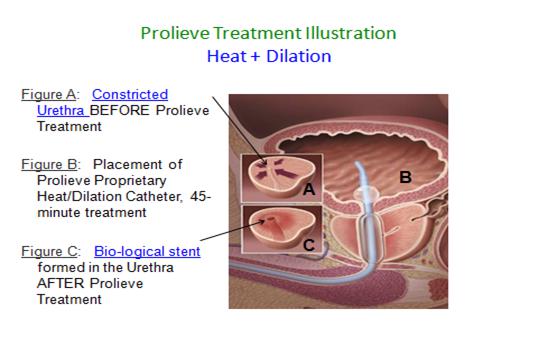
The Prolieve® system is designed with patients’ needs and comfort in mind. In general, it does not require sedation or post-operative catheterization and provides rapid symptomatic relief from BPH. BPH patients can be treated using Prolieve® in urologic offices throughout the United States. In addition, the Prolieve® treatment is also made available to physicians utilizing our mobile service.
Since acquiring the Prolieve® assets from Boston Scientific Corporation in July 2012, we have been concentrating our corporate development efforts on developing these assets into a business. We are focusing on increasing sales from our installed base of systems and from our mobile service. In addition, the Company entered into distribution agreement with seven independent distributors in certain territories in the U.S. and Puerto Rico. Boston Scientific Corporation had sold approximately 250 Prolieve® systems and approximately 80,000 disposable catheter kits in the United States prior to Boston Scientific Corporation’s sale of the Prolieve®® assets to us in 2012. Our current business strategy is to utilize social media, key opinion leaders, centers of excellence and independent mobile service providers to increase the utility and market presence of Prolieve®. In the U.S. market, we do not intend to actively market the Prolieve® system itself but, rather, our strategy is to grow revenue through the direct sale of disposable catheter kits to physicians with Prolieve® systems installed and, increasingly, through independent distributors and our mobile service, which eliminates physicians’ need to purchase, and learn how to operate, the Prolieve® system. However, if U.S. or international customers choose to purchase the Prolieve® system itself, we will accommodate their needs to the best of our ability.
We currently have approximately 165 systems that were acquired as part of the Prolieve®® asset purchase from Boston Scientific Corporation. We do not currently have an agreement with a manufacturer to produce additional Prolieve® systems, although we believe that there are several qualified medical device contract manufacturers, including Sanmina, that are capable of manufacturing the system if our current inventory is depleted. For the year ended March 31, 2019, 100% of our revenues came from the sales of our disposable catheters used in each treatment or the provision of mobile services that provide therapy using our disposable catheters. The disposable catheters are manufactured in Mexico by Lake Region Medical Center, formerly known as Accelent Corporation. We currently have an agreement with Lake Region Medical Center to supply these catheters, pursuant to which we order the number of catheters we estimate we will need for a 12-month period. We have no other source of catheters at the present time. Due to the complicated nature of these kits, as well as FDA manufacturing standards imposed on suppliers, the Company does not believe that an alternate supplier of catheters is readily available.
In addition to the Prolieve® technology, the installed base of Prolieve® systems and related patents acquired from Boston Scientific Corporation, we also acquired a fleet of 15 vans, each equipped with two Prolieve® systems. Currently we own nine vans. This mobile fleet allows us to provide Prolieve® therapy to patients in certain geographical areas whose health care providers do not have access to one of our permanently installed systems. The mobile Prolieve® system is identical to the permanently installed systems.
Our mobile Prolieve® systems are deployed by our scheduler upon the request of a physician. Our scheduler then coordinates the timing of the requested appointment with one of our medical technicians. On the day of the appointment, our medical technician arrives at the physician’s office and the Prolieve® module is brought into the physician’s office. Under the physician’s supervision, a catheter is inserted into the urethra to the point of constriction, and the Prolieve® treatment is administered by our medical technician under the physician’s supervision.
Competition
There are several treatment options for BPH. The first is traditional surgery, known as trans-urethral resection procedure, or “TURP.” This surgery requires a hospital stay, sedation, and a post-operative recovery period. Other newer BPH treatment technologies include Urolift and Rezum. We are aware that Urologix LLC offers microwave-based treatment with which we compete. Unlike the Urologix’ treatment, which solely utilizes heat, our Prolieve®® therapy combines heat and compression (via the inflated balloon). According to Medtech Insight, the surgical and minimally invasive treatment market for BPH is approximately $150 million in the U.S.
However, the majority of BPH patients undergoing treatment today choose medical therapy instead of surgery. Pursuant to such medical therapy, patients take daily doses of medicine to shrink the prostate in order to improve function. These medicines are known to cause side effects, and must be taken daily to be effective. We believe our Prolieve® treatment can be a viable alternative to drug therapy due the demonstrated efficacy and side effect profile.
Prescribed medicines for BPH treatment in major industrialized countries is currently believed to be approximately $4 billion annually. These medicines are manufactured and sold by some of the world’s largest pharmaceutical companies, including Eli Lilly, Glaxo Smith Kline and Merck & Co. These companies market their drugs to physicians and directly to the public through television, radio, the internet and conventional print media. With the substantial investment made by these companies in developing, commercializing and marketing these drugs, and the size of the BPH treatment market, these companies represent a significant competitive threat to our Prolieve® therapy, and to our company. We are also aware that non-prescription herbal supplements promoted to relieve BPH symptoms are being aggressively marketed to the public; these products also compete with Prolieve®.
B. Adaptive Phased Array Technology (APA 1000)
Our second product, APA 1000, which is a minimally invasive breast cancer treatment, is developed, but has not been cleared by the FDA for commercial use. Both Phase I and Phase II clinical trials were completed by Celsion, establishing the system’s safety and efficacy on a limited scale. We have begun pivotal Phase III clinical trials, but have proceeded slowly in such trials because of insufficient funds. The Phase III clinical trial is designed to demonstrate that the combination of focused heat and neo-adjuvant chemotherapy could shrink the size of the tumor 40% more over using chemotherapy alone. In the Phase II clinical trial, a 50% increase in tumor size reduction using focused heat and neo-adjuvant chemotherapy was observed over using chemotherapy alone. In the Phase II trial, two heat treatments were applied while in the Phase III trial, three heat treatments are applied. We believe that, if the Phase III trial is successful, it will show that the combination of focused heat and neo-adjuvant chemotherapy could downsize a cancer tumor enough to allow a surgeon to perform a lumpectomy rather than a mastectomy, thereby preserving the affected breast.
The APA 1000 system delivers heat precisely to breast tumors. While using heat to kill cancerous tumors has been considered effective for many years, heat therapy has not become a part of standard treatment for cancer because of the inability to safely apply it to tumors without damaging healthy tissue. When treating cancer, physicians seek to minimize damage to healthy tissue. It is our belief that the APA 1000 system precisely focuses microwave heat on diseased tissue, sparing adjacent tissue. Precision is achieved through the utilization of “Star Wars” technology that we have exclusively licensed from MIT and have adapted for medical use in our APA 1000 system.
Adaptive Phased Array Technology Illustration
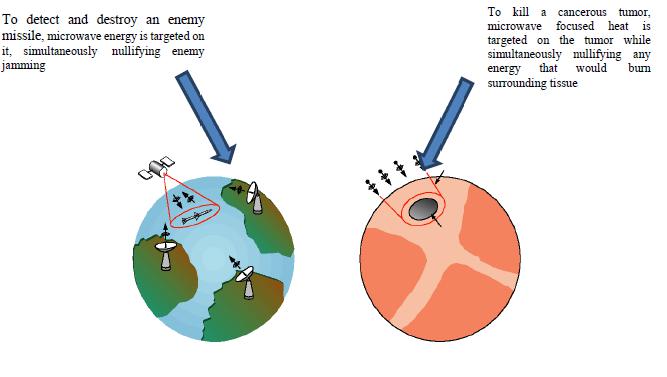
Our microwave control technology known as “Adaptive Phased Array,” or “APA.”, was originally developed at Massachusetts Institute of Technology (“MIT”) for the U.S. Department of Defense. This technology permits properly designed microwave devices to focus and concentrate energy targeted at diseased tissue areas deep within the body and to heat them selectively, without adverse impact on surrounding healthy tissue. In the treatment of breast cancer, the APA technology applies the same principal used in MIT’s “Star Wars” program of detecting missiles.
In the treatment of breast cancer, the APA technology applies the same principal used in MIT’s “Star Wars” program of detecting missiles.
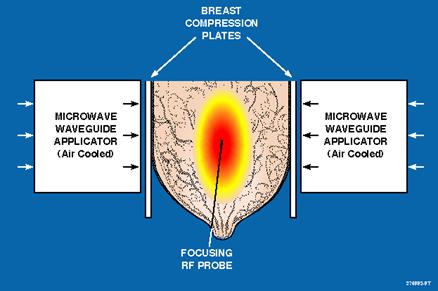
APA 1000 Breast Cancer Treatment Illustration
| ● | An RF needle probe inserted at tumor center provides feedback signal to focus microwave energy at tumor center to induce shrinkage without harming surrounding tissue. |
| ● | Focused microwave energy (43-44°C) combined with chemotherapy achieves an average of 88% tumor size reduction in Phase II clinical trials. |
Treatment with APA 1000 may accomplish several objectives. First, we believe that it destroys many cancer cells, and substantially shrinks cancerous cells that are not destroyed. If tumors are shrunk small enough, a patient may not need to have the entire breast removed. Second, we believe that the application of APA 1000 heat therapy boosts the effectiveness of subsequent chemotherapy and radiation therapy.
There can be no assurance that we will complete the pivotal Phase III clinical trial, or that the FDA will approve of the APA 1000 for sale in the United States. Even if the APA 1000 successfully completes the pivotal Phase III clinical trial and the FDA permits us to sell this system, there can be no assurance that it will be adopted by health care industry.
As stated earlier, we are progressing at a very slow pace through Phase III clinical trials due to lack of funding, and are currently focusing our corporate activities and resources on expanding our Prolieve® operations. We estimate that the cost of completing Phase III clinical trials will be approximately $7,500,000. Subject to obtaining financing, we may resume of the pivotal Phase III trial in the future. We previously negotiated arrangements with physicians and medical centers in the United States and Canada to conduct this trial. Because the pace of the trial has been slow, we have closed the clinical site in Canada. There can be no assurance that the remaining site in the U.S. will be interested in continuing the study trials. If it is not, we would then need to make alternative arrangements, of which there can be no assurance.
Effective April 1, 2016, the Company adopted ASU 2014-15,Presentation of Financial Statements-Going Concern (Subtopic 205-40), which requires management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date the financial statements are issued. Management’s evaluations are based on relevant conditions and events that are known and reasonably to be knowable as of August 29, 2019. Based on the following, management believes that it is probable that management will be unable to meet its obligations as they come due within one year that the financial statements are issued.
The Company’s operations are subject to certain risks and uncertainties including, among others, current and potential competitors with greater resources, lack of operating history and uncertainty of future profitability and possible fluctuations in financial results. Since inception, the Company has incurred substantial operating losses, principally from expenses associated with the Company’s Prolieve® operation, research and development and financing activities. The Company believes these expenditures are essential for the commercialization of its technologies. The Company expects its operating losses to continue in the near future as it continues its Prolieve® sales and marketing activities. Due to continued operating losses, there is substantial doubt regarding the Company’s ability to continue as a going concern. The Company’s ability to achieve profitability is dependent upon its ability to operate its Prolieve® business profitably and to obtain governmental approvals, produce, and market and sell its new product candidates. There can be no assurance that the Company will be able to commercialize its technology successfully or that profitability will ever be achieved. The operating results of the Company have fluctuated significantly in the past. The Company expects that its operating results will fluctuate significantly in the future and will depend on a number of factors, many of which are outside the Company’s control.
The Company will need substantial additional funding in order to sustain its operation, to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from the sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Additionally, the Company is not in compliance with the provisions of outstanding debt agreements, and it has not remitted quarterly royalty payments to Boston Scientific Corporation pursuant to the terms of its purchase agreement for Prolieve. The Company has not paid interest owing to certain holders of the convertible debentures, and is in default of the terms of the debentures.
Management is continuing its efforts to obtain additional funds through equity financing and through the negotiation of debt agreements to ensure that the Company can meet its obligations and sustain operations. Additionally, the Company is reducing costs of operations, as the Company is eliminating certain positions that do not hold value to the Company.
The consolidated financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue in existence
Comparison of Three Months Ended June 30, 2019 and 2018
The table below summarizes our results of operations for the three months ended June 30, 2019 and 2018:
| | | Three months ended June 30, | |
| | | 2019 | | | 2018 | |
| Sales | | | | | | |
| Products | | $ | 183,359 | | | $ | 328,142 | |
| Services | | | 378,709 | | | | 409,248 | |
| Total Sales | | | 562,068 | | | | 737,390 | |
| Costs of Sales | | | | | | | | |
| Products | | | 109,402 | | | | 197,985 | |
| Services | | | 277,988 | | | | 288,348 | |
| Total Costs of Sales | | | 387,390 | | | | 486,333 | |
| Gross Profit | | | 174,678 | | | | 251,057 | |
| Operating Expenses | | | | | | | | |
| Research and development | | | 27,733 | | | | 27,409 | |
| Sales and marketing | | | 16,175 | | | | 7,310 | |
| General and administrative | | | 312,313 | | | | 288,348 | |
| Total Operating Expenses | | | 356,221 | | | | 316,013 | |
| Loss from Operations | | | (181,543 | ) | | | (64,956 | ) |
| Other Income (Expense) | | | | | | | | |
| Interest and discount accretion | | | (245,070 | ) | | | (230,955 | ) |
| Loss from change in fair value of contingent consideration (Note 2) | | | (667 | ) | | | (14,793 | ) |
| Gain on recovery of HST receivable | | | — | | | | — | |
| Other income (expense) | | | (22,104 | ) | | | 29,244 | |
| Total Other Income (Expense) | | | (267,841 | ) | | | (216,504 | ) |
| Net Loss | | $ | (449,384 | ) | | $ | (281,460 | ) |
| Net Loss per share basic and diluted | | $ | (0.00 | ) | | $ | (0.00 | ) |
| Weighted average common shares outstanding—basic and diluted | | | 184,984,215 | | | | 184,984,215 | |
Sales
The Company’s revenue from the sale of its Prolieve® products and services decreased 24% from $737,390 for the three months ended June 30, 2018 to $562,068 for the three months ended June 30, 2019. Product sales during the quarters ended June 30, 2019 and June 30, 2018 consisted solely of single-use catheters. The decrease of total sales for the quarter ended June 30, 2019 is due to the decrease in direct product sales caused by the lack of supply for our Prolieve® disposable kits. Additionally, a reduction in insurance reimbursement rates has caused a decrease in our sales revenue.
Costs of Goods Sold, Costs of Services and Gross Profit
The costs of sales for products primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our Prolieve® intellectual property, warranty costs, warehousing costs, freight and handling charges. Costs of sales for services consist primarily of the costs to provide mobile services to our patients, including depreciation of our mobile consoles and vehicle fleet, and payroll and benefit costs.
Costs of goods sold as a percentage of product sales was 60% for the three months ended June 30, 2019 as compared to 67% for the three months ended June 30, 2018. The decrease is primarily due to the reduction of the fixed amortization expense for the three months in 2019. Costs of services as a percentage of services sales was 73% for the three months ended June 30, 2019, as compared to 68% for the three months ended June 30, 2018. The increase is due to lower weighted average selling price and higher express shipping cost at the end of the quarter caused by the Prolieve® disposable kit supply issues, offset by the reduction of the fixed amortization costs. The total gross profit decreased to $174,678 for the three months ended June 30, 2019 from $238,790 for the three months ended June 30, 2018, as a result of decreased revenue.
Net Loss
Our loss from operation for the quarter ended June 30, 2019 was $181,543, an increase of $116,587, or 179%, from the same quarter in 2018, primarily due to the decrease in gross profit from lower sales and the increase in operating expenses. Our net loss of $449,382 for the quarter ended June 30, 2019, compared to $281,460 for the same period in 2018.
Research and Development Expenses
For the three months ended June 30, 2019, the Company incurred research and development expenses of $27,733, slight increase from the $27,409 for the same period in 2018.
Sales and Marketing Expenses
Sales and marketing expenses primarily include Prolieve® promotion costs, such as trade shows, costs of travel, and other direct marketing expenses for Prolieve® and other business promotion costs. Sales and marketing expenses for the three months ended June 30, 2019 were $16,175, compared to $7,310 for three months ended June 30, 2018. The increase is primarily caused by the higher travel expenses for trade shows and increased in-house marketing. The Company plans to utilize social media, direct mailing, and independent distributors to sustain and grow its Prolieve® business.
General and Administrative Expenses
General and administrative expenses for the three months ended June 30, 2019 increased $23,965, or 8% to $312,313, from $288,348 for three month ended June 30, 2018. The increase is primarily due to the legal fee expenses incurred for a lawsuit filed against the Company.
Other Income (Expense)
Other income (expense) primarily consists of interest and accretion expense, losses from change in the fair value of the contingent consideration. Total other income (expense) of ($267,839) for the three months ended June 30, 2019 reflects an increase from our total other income (expense) of ($216,504) for three months ended June 30, 2018, largely due to the increase in interest expenses and a loss in the change in foreign exchange rates for the 3 months ending June 30, 2019 as compared to a gain in foreign exchange rates for the 3 months ending June 30, 2018
On July 24, 2012 the Company purchased from Boston Scientific Corporation all of the assets, and assumed certain liabilities, relating to the Prolieve® Thermodilatation System (“Prolieve®”), a FDA approved device for the treatment of Benign Prostatic Hyperplasia (“BPH”). The total purchase consideration consisted of the following:
| Cash | | $ | 2,535,610 | |
| Fair value of contingent consideration | | | 1,126,505 | |
| Total consideration | | $ | 3,662,115 | |
The maximum amount payable pursuant to the terms of the contingent consideration is $2.5 million. The fair value was determined by calculating its present value based on its payment terms using an interest rate of 24% (the Company’s estimated unsecured borrowing rate). The contingent consideration is paid quarterly at a rate of 10% of sales of Prolieve® products. The fair value of the contingent consideration is adjusted for changes in the estimated future payments with the adjustment being reflected in profit or loss.
The Company accounted for its acquisition of Prolieve® by recording all tangible assets and intangible assets acquired, and liabilities assumed, at their respective fair values on the acquisition date. The fair value assigned to identifiable intangible assets acquired was determined using a cost approach and was based on the Company’s best estimates; this intangible asset is being amortized on a straight-line basis over its estimated useful life of ten years.
The following summarizes the fair value of the assets acquired assumed in the transaction:
| Inventory | | $ | 463,338 | |
| Equipment | | | 736,662 | |
| Intangible assets | | | 2,462,115 | |
| Total consideration | | $ | 3,662,115 | |
| | 6. | Medifocus Holding Joint Venture |
On November 8, 2013, we entered into an agreement with Ideal Concept Group Limited (“Ideal Concept”) to develop our Prolieve® business and APA technology in a geographic area referred to as “Asia Pacific” in the agreement (the “JV Agreement”). The countries comprising of Asia Pacific are not specified in the JV Agreement. Pursuant to the JV Agreement, Medifocus and Ideal Concept agreed to capitalize a company, Medifocus Holding Limited (“Medifocus Holding”), to develop this business. Medifocus Holding was incorporated in the British Virgin Islands on June 28, 2012.
The JV Agreement states that, at the outset, Ideal Concept will own 60% of Medifocus Holding and we will own 40%. Through March 31, 2015, Medifocus Inc. has made total contributions to Medifocus Holding of approximately $214,735 in cash and Prolieve® equipment. In addition to capital contributions, the shareholders are obligated to provide loans to the JV of up to HKD 4,000,000 (or approximately $520,000). Ideal Concept previously agreed, through November 8, 2014, to loan us the funds necessary to satisfy our portion of the required shareholder contributions to Medifocus Holding. Such loan would bear interest at 6% per year and be secured by our ownership interest in Medifocus Holding. No such loans were made to us by Ideal Concept and we did not make any further investments or loans in the joint venture. Pursuant to the terms of our joint venture, our equity ownership in Medifocus Holdings LLC had been reduced over the last two years and was eventually bought out by Ideal Concept in March 2016.
Pursuant to the terms of the JV Agreement and a License and Distribution Agreement dated as of November 8, 2013, Medifocus Holding will engage in clinical testing, and obtaining approval from China Food and Drug Administration of the People’s Republic of China (“CFDA”) for all products relating to Prolieve® and the APA technology. Medifocus Holding has been in communication with the CFDA and continues to evaluate the regulatory requirements for commercialization of Prolieve® in China. There is no assurance that the CFDA will approve Prolieve® for commercialization in China. Additionally, Medifocus Holding has been in discussions with several hospitals in China regarding conducting clinical testing. As of the date of this annual report, no clinical testing has begun in China. During fiscal 2016, Medifocus Holding entered into a distribution agreement with a South Korea-based distributor to market Prolieve® in South Korea, subject to regulatory approvals from the South Korean government.
Medifocus Holding is required to pay us a royalty of 5% of the first $10,000,000 in sales of the catheter kits and control units utilized in the Prolieve® business. After $10,000,000 in sales has been reached, the royalty decreases to 3%. For all other products we develop, Medifocus Holding is required to pay us a royalty of 7.5% on net sales of such products.
| | 7. | Liquidity and capital resources |
The Company’s primary cash requirements are to fund operations, including research and development programs and collaborations, and to support general and administrative activities. The Company’s future capital requirements will depend on many factors, including, but not limited to:
| ● | sales of the Company’s Prolieve® products and services; |
| ● | pricing and payment terms with customers; |
| ● | costs of the disposable catheter kits and payment terms with suppliers; and |
| ● | capital expenditures and equipment purchases to support product launches |
In December 2013, the Company raised gross proceeds of $ 3,540,000 from the sale of convertible redeemable promissory notes and warrants (the “Units”). Each Unit consists of (i) a $10,000 face value convertible redeemable promissory note, bearing 8% annual interest and due in three years (“Note”), which is convertible into shares of common stock beginning six months after the Closing Date of the offering at a conversion price of $0.25 per share, and (ii) three-year warrants to purchase 20,000 shares of common stock at a price of $0.30 per Share. The net proceeds from the offering was used for Prolieve® operations including research and development activities, capital expenditures, repayment of debt and working capital.
In a second closing in March 2014, the Company issued 200 additional Units to the investors, receiving gross proceeds of $2,000,000. The additional notes are convertible into 8,000,000 shares of common stock. Each warrant entitles the holder to acquire 20,000 common shares (for a total of 4,000,000 common shares) at an exercise price of $0.30 per share and expire on December 18, 2016. The warrants were classified as equity and were recorded as additional paid in capital at their estimated fair value of $572,999.
Our $0.43 million unsecured promissory note made to a lender in July 2012 (included in our contractual obligations table on page 38) and the accrued but unpaid interest of $CAD 0.2 million as of December 31, 2013, was originally due October 23, 2013. The lender has extended the due date of this promissory note to June 30, 2014 and we are currently in discussion with the lender to negotiate a payment plan for this note. Subsequent to March 31, 2015 we have made no principal and interest payments to the lender and are currently in negotiations with the lender regarding the extension of the due date. If such negotiations fail, the lender may declare all amounts due and payable immediately. The lender would not have a right to seize any of the Company’s assets because the promissory note is unsecured. Further, if the lender were to retain counsel or initiate litigation to enforce its rights and interests under the promissory note, the Company would be required to pay all reasonable costs and expenses of the lender.
In the fiscal year ending March 31, 2015, the Company raised approximately $1.6 million from the sale of Units. Each Unit was priced at $0.16 and consists of one Share, and a detachable stock purchase warrant to purchase one Share at $0.25 per share.
The company also received funds prior to March 31, 2015 for common shares in the amount of $1,705,000 for future shares to be issued. On May 12, 2015, the company issued 38,750,000 common shares at a price of $0.044 per common share for gross proceeds of $ 1,705,000 as part of this transaction.
For the fiscal year ending March 31, 2016, the Company received gross proceeds of $775,000 from the sale of common stock and warrants (the “Units”). Each Unit was priced at $10,000 and consists of 200,000 Shares, and a detachable stock purchase warrant to purchase 100,000 Shares at $0.10 per share.
In the quarter ended June 30, 2016, the Company obtained a loan of $200,000 from a lender at a monthly interest rate of 1.25%. The loan was secured with all the intellectual property and proprietary rights related to the Prolieve® system. Subsequently, in August 2016, the Company obtained an additional $200,000 in loan from the same lender, at the same terms, and secured with the same intellectual property and proprietary rights related to the Prolieve® system. In October 2016, the Company obtained an additional $100,000 in loan from the same lender, at the same terms, and secured with the same intellectual property and proprietary rights related to the Prolieve® system.
The Company extends credit to customers on an unsecured basis and payment terms are typical 30 to 60 days from delivery or service. Management assesses the collectability of its receivables based on a periodic customer-by-customer analysis, considering historical collection experience as well as customer-specific conditions; when a specific customer account is determined to be uncollectible the Company provides an allowance equal to the estimated uncollectible amounts. Receivables are written off when it is determined that amounts are uncollectible. The Company established an allowance for doubtful accounts of approximately $39,556 as of June 30, 2018,
Our cash and cash equivalents of approximately $79,990 on hand at June 30, 2018 are not sufficient to fund operations through June 30, 2019. We estimate that the external funding requirement for the next 12 months will be at least $500,000 to maintain and grow the Prolieve® business in the U.S. and the essential corporate activities. The Company is currently in default with certain lenders and creditors, and owed Boston Scientific Corporation $1,977,967 in accrued but unpaid sales royalties. We have suspended the APA 1000’s Phase III clinical trials and the research and development activities in the heat-activated immunotherapy business due to the lack of funding. If we are not able to raise additional capital, we will need to take certain measures to further reduce our operating costs, including reducing our staff, curtailing our research and development efforts and our clinical trials, and reducing the costs we plan to spend to operate our Prolieve® business. As such, we would not be able to achieve the growth of the Prolieve® business, complete the development, testing and commercialization of our product candidates. If adequate funding is not available, the Company will delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. The Management is continuing its efforts to obtain additional funds so that the Company can meet its operating obligations and sustain operations.
We do not have any committed sources of financing and cannot give assurance that alternate funding will be available in a timely manner, on acceptable terms or at all. We may need to pursue dilutive equity financings, such as the issuance of shares of common stock, convertible debt or other equity-linked securities, which financings could dilute the percentage ownership of our current common stockholders and could significantly lower the market value of our common stock.
Net Cash Provided by Operations
Net cash used in operating activities was $69,824 for the three months ended June 30, 2019 compared to the $6,268 net cash used in operating activities during the three months ended June 30, 2018. The increase in the use of cash of approximately $63,556 is primarily due to an increase in net loss n offset by a decrease in cash used for accounts payable.
Net Cash Provided by/Used in Investing Activities
There were no investing transactions for the three months ending 2019 or 2018.
Net Cash Used in Financing Activities
There were no financing activities for the three months ended June 30, 2019 and 2018.
An investment in shares of our common stock (which we refer to as the “Shares”) involves a high degree of risk. You should carefully consider the risks described below and the risks described elsewhere in this annual report under the sections entitled “Item 4. Information on the Company” before deciding whether to invest in our shares. The following is a summary of the risk factors that we believe are most relevant to our business. These are factors that, individually or in the aggregate, could cause our actual results to differ significantly from anticipated or historical results. The occurrence of any of the risks could harm our business and cause the price of our common stock to decline, and investors may lose all or part of their investment. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. The risks and uncertainties described below and in the incorporated documents are not the only risks and uncertainties that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of these risks actually occurs, our business, financial condition and results of operations would suffer. The risks discussed below also include forward-looking statements, and our actual results may differ substantially from those discussed in these forward-looking statements. See “Special Note Regarding Forward-Looking Statements” at the beginning of Part I of this annual report. Except as required by law, we undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events, or otherwise.
We have a history of significant losses and expect to continue such losses for the foreseeable future.
Since our inception in 2005, our expenses have substantially exceeded our revenues, resulting in continuing losses and an accumulated deficit of $36,443,359 at March 31, 2019. In addition, our net loss for the year ended March 31, 2019 was $1,470,532. Such operating losses are the result of limited revenues from our Prolieve® sales not being sufficient to offset the expenses associated with the Prolieve® operation and other corporate expenses. We may continue to experience operating losses unless and until we generate significant revenue from Prolieve®, as well as the development of other new products and these products have been clinically tested, approved by the FDA or other regulatory authorities, and successfully commercialized.
Litigation.
In June 2018, W.L. Pate, JR and Charles C. Shelton filed a lawsuit in the District Court of Harris County, Texas to seek monetary relief of over $200,000 but not more than $1,000,000 from Medifocus Inc. for a transaction that did not materialize. Although the Company does not believe the suit has any merits and has not accrued for any amount in its financial statements as of March 31, 2019, any judgement unfavorable to the Company can potentially cause significant financial hardship and other damages to the Company. In addition, certain note holders of the Company had threatened to take legal actions against the Company due to the default on the notes. Such legal actions will also cause significant financial hardship and other damages to the Company.
We may not be able to generate significant revenue for the foreseeable future.
Prior to July 2012, we devoted our resources to maintaining and developing the APA 1000. We will not be able to market the APA 1000 until we have completed clinical testing and obtained all necessary governmental approvals. On July 26, 2012, we acquired from Boston Scientific Corporation the Prolieve® business for the treatment of BPH and, since that time, we had assembled a sales and service team to market the Prolieve® system. Due to our cost reduction measures implemented since March 2016, we currently do not have a dedicated sales team to market Prolieve®. Our current revenue is primarily derived from sales of our single-use treatment catheters, treatments delivered through our mobile service and limited sales of Prolieve® consoles. Our lack of product diversification means that we may be negatively affected by changes in market conditions and in regulation (including regulation affecting reimbursement for our products). In addition, at the present time our APA 1000 system is still in clinical testing stage and cannot be marketed until we have completed clinical testing and obtained necessary governmental approval. Accordingly, our revenue sources are, and will remain extremely limited until and unless our Prolieve® system is marketed successfully and/or until our other new products are clinically tested, approved by the FDA or other regulatory authorities, and successfully commercialized. We cannot guarantee that our products will be successfully tested, approved by the FDA or other regulatory authorities, or commercialized, successfully or otherwise, at any time in the foreseeable future, if at all.
Our future may depend on our ability to obtain additional financing. If we do not obtain such financing, we may have to cease our operations and investors could lose their entire investment.
We have yet to operate profitably or generate positive cash flows from operations on annual basis, and there is no assurance that we will operate profitably or will generate positive cash flow in the future. As a result, we have very limited funds, and such funds may not be adequate to take advantage of current, planned and unanticipated business opportunities. Even if our funds prove to be sufficient to pursue current, planned and unanticipated business opportunities, we may not have enough capital to fully develop such opportunities. As of March 31, 2019, our total liabilities exceeded our tangible assets by $11,177,420.
We are dependent on the proceeds of future financing in order to grow the Prolieve® business and to develop and commercialize other products. There can be no assurance that we will be able to raise the additional capital resources necessary to permit us to pursue our business plan. Finally, the continued growth of our business may require additional funding from time to time to be used by us for general corporate purposes, such as acquisitions, investments, repayment of debt, capital expenditures, repurchase of capital stock and additional purposes identified by the Company.
Accordingly, our ultimate success may depend upon our ability to raise additional capital. There can be no assurance that any additional financing will be available to us. As additional capital is needed, we may not be able to obtain additional equity or debt financing. Even if financing is available, it may not be available on terms that are favorable or acceptable to us, or in sufficient amounts to satisfy our requirements. Any inability to obtain additional financing will likely have a material adverse effect on our business operations and could result in the loss of your entire investment.
Our independent registered public accountants have expressed substantial doubt regarding our ability to continue as a going concern.
Our auditors have expressed their opinion that there is substantial doubt about the Company’s ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of these uncertainties. Our ability to continue as a going concern is dependent upon our ability to successfully raise adequate additional financing and our ability to successfully develop our sales and marketing programs and commence our planned operations. We cannot assure you that we will be able to obtain additional financing or achieve profitability in our operations. Our failure to obtain additional financing or achieve profitability in our operations could require the Company to liquidate our business interests, and could result in the loss of your entire investment.
The loss of certain of our key personnel, or any inability to attract and retain additional personnel, could negatively affect our business.
Our future success depends to a significant extent on the continued service of certain key employees who have been intimately involved with, and primarily responsible for, the invention, development and commercialization efforts for our technology and products. The loss of services of those key employees could adversely affect our business and our ability to implement our business plan.
Our future success will also depend on our ability to attract, retain and motivate highly skilled personnel to assist us with product development, commercialization and other facets of our business plan. If we fail to hire and retain a sufficient number of qualified individuals to fully meet the needs of the business of the Company, it may have an adverse effect on our business and results of operations.
One of our shareholders owns a significant percentage of our Shares and could exert significant influence over matters requiring shareholder approval.
Mr. Tak Cheung Yam, a former director of the Company, through Integrated Assets Management (Asia) Ltd, currently owns 25,386,742 Shares, or 13.72% of the Company’s outstanding common stock. In addition, Mr. Yam, through Integrated Assets Management (Asia) Ltd, owns convertible notes in the amount of $2,811,737, including accrued and unpaid interest. As a result, Mr. Yam may have considerable influence over our management, our decision-making process, our business strategy and affairs and matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as mergers, consolidations or the sale of substantially all of our assets. Mr. Yam’s interests may differ from those of other shareholders of the Company, and, Mr. Yam will have the ability to exercise influence over our business and may take actions that are not in our or our public shareholders’ best interests. Furthermore, this concentration of ownership may have the effect of delaying or preventing a change in control, including a merger, consolidation or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control, even if such a change in control would benefit our other stockholders.
Our internal sales and marketing capability is limited and we may need to enter into alliances with others possessing such capabilities to commercialize our products internationally.
Currently our primary source of revenue is through the sales of disposable catheter treatment kits and mobile services in the U.S., as well as limited number of Prolieve® consoles to our distributor in Asia. We are dependent upon our limited sales and marketing capability for the successful marketing of our Prolieve® system. There can be no assurance that we will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our Prolieve® system.
We intend to market our other products, either directly or through other strategic alliances and distribution arrangements with third parties. There can be no assurance that we will be able to enter into third-party marketing or distribution arrangements on advantageous terms or at all. To the extent that we do enter into such arrangements, we will be dependent on our marketing and distribution partners. In entering into third-party marketing or distribution arrangements, we expect to incur significant additional expense. There can be no assurance that, to the extent that we sell products directly or we enter into any commercialization arrangements with third parties, such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our products and services.
We do not manufacture the Prolieve® system ourselves, and rely on a third-party supplier to supply us with the proprietary disposable catheters used with our Prolieve® system.
The Prolieve® systems we currently have in inventory were manufactured by Sanmina Corporation for Boston Scientific Corporation prior to our acquisition of the Prolieve® assets, and we do not currently have an agreement with Sanmina for the production of additional Prolieve® systems. Accordingly, if our current inventory becomes insufficient to meet the business growth in both the U.S. and international markets, we will have to engage Sanmina Corporation, or another manufacturer, to produce such additional systems. Further, the proprietary disposable catheter kits used with the Prolieve® system are manufactured by Lake Regional Medical Center (formerly Accellent Inc.) in its facility in Mexico. Due to the complexity of these catheter kits, as well as FDA standards applicable to manufacturers of such kits, the Company has not identified an alternative supplier for these catheter kits. If, for any reason, we are unable to obtain new Prolieve® systems manufactured by Sanmina Corporation, or we are no longer able to purchase the catheter kits from Lake Regional Medical Center in sufficient amounts, on an as-needed basis and on acceptable terms, or if either manufacturer becomes unable or unwilling to continue to supply us with new Prolieve® systems and disposable catheter kits, it would have a material adverse effect on our business and operations. There can be no assurance that we could find new manufacturers to fulfill our needs, that any such manufacturer would be FDA approved, or that such manufacturers would be willing to provide us with the required products under commercially acceptable terms. If we are unable to find additional manufacturers and suppliers and it results in a disruption to our business, there would be a material adverse effect on our business and results of operations.
The slow pace of our APA 1000 Breast Cancer System’s Phase III clinical trials could result in additional delays and increased costs of completing the trials in the future.
Our focus now is attaining profitability for our Prolieve® business. Accordingly, we have allocated most of our resources to this goal, compounding this with the lack of funding, the progress of the pivotal Phase III clinical trials of our APA 1000 breast cancer treatment system has been very slow. We estimate that the Phase III clinical trials will cost approximately $7,500,000. We currently do not have the financing in place to complete these trials. There can be no assurance that such financings will be available at all, or on terms favorable to us. Further, there can be no assurance as to when, or even if, we will succeed in making Prolieve® profitable. Our inability to do so may make it more difficult for us to raise funds for the pivotal Phase III clinical trial of the APA 1000. If we are able achieve profitable Prolieve® operations, there can be no assurance that we will be able to generate enough funds from the Prolieve® business to finance the pivotal Phase III clinical trial. Furthermore, we cannot predict the effect of the slow pace of the pivotal Phase III trial could have on the costs and other critical aspects of the Phase III clinical trial. There is the risk that this uncertainty could negatively impact our business plans, and our ability to raise additional funds for further development of our APA 1000 business.
We may not receive regulatory approval from the U.S. Food and Drug Administration (“FDA”) to market the APA 1000.
Drugs and medical devices in the United States are regulated by the FDA, which requires that new medicines and medical devices be demonstrated to be both safe and effective. This is accomplished by conducting staged clinical trials that are subject to the FDA’s review, analysis and approval. While the Phase I and Phase II clinical trials for APA 1000 have been completed, and we received approval from the FDA and Health Canada to begin the pivotal Phase III clinical trials, as of today, a very limited number of patients out of a planned 238-person trial in the pivotal Phase III clinical trial, have been treated with APA 1000. There can be no assurance that our Phase III clinical trial will be completed, and if it is completed, that it will demonstrate APA 1000’s safety and efficacy, and that we will subsequently receive the FDA’s approval for us to commence marketing. If we complete the pivotal Phase III clinical trial and receive FDA approval to market APA 1000, there can be no assurance that APA 1000 will be adopted for use by the healthcare industry, and that this business will be profitable.
We may not succeed in developing a meaningful market share of the benign prostatic hyperplasia (“BPH”) treatment markets with Prolieve®, and our Prolieve® business may not become profitable.
The BPH market is highly competitive, and is presently dominated by large, international pharmaceutical companies that promote the use of proprietary drugs to treat this condition. These companies, which include, Eli Lilly, Glaxo Smith Kline, Merck & Co., and others, aggressively market their drugs to primary care physicians, and to consumers through television, print, digital and other media. Because the market for BPH treatment is large and growing, and the manufacturers of these medications have made substantial investments in their development and marketing, we expect them to vigorously defend their market positions. In addition, we face intense competition from surgical and other minimally invasive treatment modalities. Because our financial, marketing and sales resources are much smaller than those of the pharmaceutical companies, we are at significant competitive disadvantage, which will make it difficult for us to substantially expand our Prolieve® business.
Recent or future health care reform laws in the U.S. could have a negative impact on our business.
Our business, financial condition, results of operations and cash flows could be significantly and adversely affected by recent or future healthcare reform legislation. We cannot predict what healthcare programs and regulations will ultimately be implemented at the federal or state level, or the effect of any future legislation or regulation. However, any changes that lower reimbursement for our products or reduce medical procedure volumes could have a material adverse effect our business and results of operations.
Our APA 1000 system and future products utilizing the adaptive phased array technology depend on the license agreement with MIT, and our immunotherapy and gene therapy development and commercialization efforts utilizing the heat-activated gene technology depend on the license agreement with Duke University to permit us to use patented technologies.
Our success depends, in substantial part, on our ability to maintain our rights under license agreements that grant us the rights to use patented technologies. We have entered into a license agreement with MIT under which we have exclusive rights to commercialize medical treatment products and procedures based on MIT’s Adaptive Phased Array technology. The MIT license agreement contains license fee, royalty and/or research support provisions, testing and regulatory milestones, and other performance requirements that we must meet by certain deadlines. If we breach these or other provisions of the license agreements, we could lose our ability to use the subject technologies and it could have a material adverse effect on our business. Further, we cannot guarantee that any patent or other technology rights licensed to us by others will not be challenged or circumvented successfully by third parties, or that the rights granted will provide adequate protection. We are aware of published patent applications and issued patents belonging to others, and it is not clear whether any of these patents or applications, or other patent applications of which we may not have any knowledge, will require us to alter any of our potential products or processes, pay licensing fees to others or cease certain activities. Litigation, which could result in substantial costs, may also be necessary to enforce any patents issued to or licensed by us or to determine the scope and validity of others’ claimed proprietary rights. We also rely on trade secrets and confidential information that we seek to protect, in part, by confidentiality agreements with our corporate partners, collaborators, employees, and consultants. We cannot guarantee that these agreements will not be breached, that, even if not breached, that they are adequate to protect our trade secrets, that we will have adequate remedies for any breach or that our trade secrets will not otherwise become known to, or will not be discovered independently by, competitors.
We may not be able to protect the intellectual property that is integral to our business, or we may be subject to claims of intellectual property infringement by third parties, either of which could have a material adverse effect on our business.
Much of our potential success and value lies in our ownership and use of intellectual property. Our inability or failure to protect our intellectual property may negatively affect our business and value. Our ability to compete effectively is dependent in large part upon the maintenance and protection of the intellectual property we own and licenses from MIT. We will rely on patents, trademarks, trade secret and copyright law, as well as confidentiality procedures to establish and protect our intellectual property rights. It may be possible for a third party to copy or otherwise obtain and use the proprietary technology presently owned by or licensed to us without authorization. Policing unauthorized use of our intellectual property is difficult. The steps we take may not prevent misappropriation of our intellectual property, and the agreements we enter may not be enforceable. In addition, effective intellectual property protection may be unavailable or limited in some jurisdictions outside the United States. Litigation may be necessary in the future to enforce or protect our intellectual property rights or to determine the validity and scope of the proprietary rights of others. Such litigation could cause us to incur substantial costs and divert resources away from our business, which in turn could have a material adverse effect on our business, results of operations, financial condition and profitability.
We may be subject to damaging and disruptive intellectual property litigation.
Although we are not currently aware that our products or services infringe any published patents or registered trademarks, we may be subject to infringement claims in the future. Because patent applications are kept confidential for a period of time after filing, applications may have been filed that, if issued as patents, could relate to our business. Parties making claims of infringement may be able to obtain injunctive or other equitable relief that could effectively block us from providing its products and services in the United States and other jurisdictions and could cause us to pay substantial damages. In the event of a successful claim of infringement, we may need to obtain one or more licenses from third parties, which may not be available at a reasonable cost, if at all. The defense of any lawsuit could result in time-consuming and expensive litigation, regardless of the merits of such claims, as well as resulting damages, license fees, royalty payments and restrictions on our ability to provide products or services, any of which could harm our business.
Our business is subject to numerous and evolving state, federal and foreign regulations and we may not be able to secure the government approvals needed to develop and market our products.
Our research and development activities, pre-clinical tests and clinical trials, and ultimately the manufacturing, marketing and labeling of our products, are all subject to extensive regulation by the FDA and foreign regulatory agencies. Pre-clinical testing and clinical trial requirements and the regulatory approval process typically take years and require the expenditure of substantial resources. Further, additional government regulation may be established that could prevent or delay regulatory approval of our product candidates. Delays or rejections in obtaining regulatory approvals would adversely affect our ability to commercialize any product candidates and our ability to generate product revenues or royalties.
The FDA and foreign regulatory agencies require that the safety and efficacy of product candidates be supported through adequate and well-controlled clinical trials. If the results of pivotal clinical trials do not establish the safety and efficacy of our product candidates to the satisfaction of the FDA and other foreign regulatory agencies, we will not receive the approvals necessary to market such product candidates.
Even if regulatory approval of a product candidate is granted, the approval may include significant limitations on the indicated uses for which the product may be marketed. In addition, we are subject to inspections and regulations by the FDA. Medical devices must also continue to comply with the FDA’s Quality System Regulation, or QSR. Compliance with such regulations requires significant expenditures of time and effort to ensure full technical compliance. The FDA stringently applies regulatory standards for manufacturing.
We are subject to the periodic inspection of our clinical trials, facilities, procedures and operations and/or the testing of our products by the FDA to determine whether our systems and processes are in compliance with FDA regulations. Following such inspections, the FDA may issue notices on Form 483 and warning letters that could cause us to modify certain activities identified during the inspection. A Form 483 notice is generally issued after an FDA inspection and lists conditions the FDA inspectors believe may violate FDA regulations. FDA guidelines specify that a warning letter is issued only for violations of “regulatory significance” for which the failure to adequately and promptly achieve correction may be expected to result in an enforcement action.
Failure to comply with FDA and other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production and/or distribution, suspension of the FDA’s review of product applications, enforcement actions, injunctions and criminal prosecution. Under certain circumstances, the FDA also has the authority to revoke previously granted product approvals. Although we have internal compliance programs, if these programs do not meet regulatory agency standards or if our compliance is deemed deficient in any significant way, it could have a material adverse effect on the Company.
We are also subject to record keeping and reporting regulations, including FDA’s mandatory Medical Device Reporting, or MDR, regulation. Labeling and promotional activities are regulated by the FDA and, in certain instances, by the Federal Trade Commission.
Many states in which we do or in the future may do business or in which our products may be sold impose licensing, labeling or certification requirements that are in addition to those imposed by the FDA. There can be no assurance that one or more states will not impose regulations or requirements that have a material adverse effect on our ability to sell our products.
In many of the foreign countries in which we may do business or in which our products may be sold, we will be subject to regulation by national governments and supranational agencies as well as by local agencies affecting, among other things, product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. There can be no assurance that one or more countries or agencies will not impose regulations or requirements that could have a material adverse effect on our ability to sell our products.
Failure to comply with applicable regulatory requirements, can result in, among other things, warning letters, fines, injunctions and other equitable remedies, civil penalties, recall or seizure of products, total or partial suspension of production, refusal of the government to grant approvals, pre-market clearance or pre-market approval, withdrawal of approvals and criminal prosecution of the Company and its employees, all of which would have a material adverse effect on our business.
The success of our products may be harmed if the government, private health insurers and other third-party payors do not provide sufficient coverage or reimbursement.
Our current and future revenues are subject to uncertainties regarding health care reimbursement and reform. Our ability to commercialize our new cancer treatment system successfully will depend in part on the extent to which reimbursement for the costs of such products and related treatments will be available from government health administration authorities, private health insurers and other third-party payors. The reimbursement status of newly approved medical products is subject to significant uncertainty. We cannot guarantee that adequate third-party insurance coverage will be available for us to establish and maintain price levels sufficient for us to realize an appropriate return on our investment in developing new therapies. Government, private health insurers, and other third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products approved for marketing by the FDA. Accordingly, even if coverage and reimbursement are provided by government, private health insurers, and third-party payors for uses of our products, market acceptance of these products would be adversely affected if the reimbursement available proves to be unprofitable for health care providers. We may be unable to sell our products on a profitable basis if third-party payers deny coverage, or provide low reimbursement rates.
Our products may not achieve sufficient acceptance by the medical community to sustain our business.
Although we have received a PMA from the FDA for our Prolieve® system for the treatment of BPH, we can offer no assurance that the Prolieve® system will be accepted by the medical community widely. Our breast cancer treatment development project using the APA technology is currently in Phase III clinical trials. It may prove not to be effective in practice. If testing and clinical practice do not confirm the safety and efficacy of our systems or, even if further testing and practice produce positive results but the medical community does not view these new forms of treatment as effective and desirable, our efforts to market our new products may fail, with material adverse consequences to our business.
We face intense competition and the failure to compete effectively could adversely affect our ability to develop and market our products.
There are many companies and other institutions engaged in research and development of various technologies, both for prostate disease and cancer treatment products that seek treatment outcomes similar to those that we are pursuing. We believe that the level of interest by others in investigating the potential of possible competitive treatments and alternative technologies will continue and may increase. Potential competitors engaged in all areas of BPH and cancer treatment research in the United States and other countries include, among others, major pharmaceutical, specialized technology companies, and universities and other research institutions. Most of our competitors and potential competitors have substantially greater financial, technical, human and other resources, and may also have far greater experience, than do we, both in pre-clinical testing and human clinical trials of new products and in obtaining FDA and other regulatory approvals. One or more of these companies or institutions could succeed in developing products or other technologies that are more effective than the products and technologies that we have been or are developing, or which would render our technology and products obsolete and non-competitive. Furthermore, if we are permitted to commence commercial sales of any of our products, we will also be competing, with respect to manufacturing efficiency and marketing, with companies having substantially greater resources and experience in these areas.
If we become subject to product liability claims, we may be required to pay damages that exceed our insurance coverage and the value of our assets.
We currently carry product liability insurance in the amount of $5,000,000 per occurrence, which may be inadequate to satisfy liabilities we may incur. Any claim brought against us, regardless of its merit, could result in the increase of our product liability insurance rates or our inability to obtain future coverage on acceptable terms, or at all. In addition, if our product liability coverage is inadequate to pay a damage award, we would have to pay any shortfall out of our assets, which may be insufficient, or by securing additional funds, of which there can be no assurance. Even a meritless or unsuccessful product liability claim made against us could harm our reputation, cause us to incur significant legal fees and result in the diversion of management’s attention from managing our business. Any of these occurrences or events would have a material adverse effect on our business.
Our Relationship with Medifocus Holdings Ltd. could cause us to effectively transfer rights to our technology in major markets in Asia, and to lose rights to sell and market our products in Asia.
In 2013 we entered into a License and Distribution Agreement with Medifocus Holdings Ltd. for the distribution of Prolieve® and other future products in Asia. Medifocus Holdings Ltd. is subject to a variety of risks including, without limitation, obtaining adequate financing to operate the business, recruiting management with expertise to market, promote, and produce products and having the capability of obtaining required regulatory approvals from various foreign governments in order sell products. Our right to receive royalties from the sale of products by Medifocus Holdings Ltd. will prove to be worthless if there are no sales.
Damage to our reputation, for whatever reason, could have a material adverse effect on our business.
Our ability to market and sell Prolieve®, APA 1000 and new products in major world markets, including the United States, could be adversely affected in the future by negative publicity resulting from, among others, the joint venture, adverse regulatory decisions by international bodies related to our products, controversy surrounding our products and the businesses activities of the joint venture, litigation arising from the joint venture and use of products, over which we will have very little, if any, control.
We have elected to use the extended transition period for complying with new or revised accounting standards.
Pursuant to Section 107(b) of the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), we have elected to use the extended transition period for complying with new or revised accounting standards for an “emerging growth company.” This election will permit, but not require, us to delay the adoption of new or revised accounting standards that will have different effective dates for public and private companies until those standards apply to private companies. Consequently, our financial statements may not be comparable to companies that comply with public company effective dates.
Our Shares are deemed to be “Penny Stocks,” which means that there are significant restrictions on stockbrokers and dealers recommending our Shares for purchase.
Our common stock is considered to be a “penny stock” pursuant to the rules promulgated under Section 15(g) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As a result, our securities are subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in the sale of shares of penny stock to persons other than established customers or “accredited investors” (as such term is defined in Rule 501 of Regulation D promulgated under the Securities Act of 1933, as amended (the “Securities Act”)). Under such rules, a broker-dealer must, prior to a transaction in a penny stock not otherwise exempt from those rules, deliver a standardized risk disclosure document prepared by the SEC, which specifies information about penny stocks and the nature and significance of risks of the penny stock market. A broker-dealer must also provide the customer with bid and offer quotations for the penny stock, the compensation of the broker-dealer, and sales person in the transaction, and monthly account statements indicating the market value of each penny stock held in the customer’s account. In addition, the penny stock rules require that, prior to a transaction in a penny stock not otherwise exempt from the penny stock rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for stock that is subject to the penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules may discourage investor interest in and limit the marketability of our securities, and limit the current investors’ ability to sell their shares of our common stock.
We may never pay dividends.
We have never declared or paid any dividends on our Shares since our inception. We do not intend to pay cash dividends on our Shares for the foreseeable future, and currently intend to retain any future earnings to fund the development and growth of our business. The payment of cash dividends, if any, on the Shares will rest solely within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors. We currently intend to use any revenues, as well as proceeds from any financings, to assist us in obtaining our business objectives, and not for the payment of any dividends upon our Shares.
Shareholders may suffer dilution of the value of their Shares by our issuance of additional Shares in the future.
As of June 30, 2019, we have stock options that are convertible or exchangeable into 10,700,000 Shares. Additionally, we may sell and issue additional Shares, or other securities that are convertible into Shares, in the future, to raise funds and for other purposes. The Company may continue to discuss with the holders of the 8% Convertible Notes (the “Notes”) on converting the matured Notes at a price that is significantly lower than the original Conversion Price in the Notes. The issuance of additional Shares, whether through the conversion of convertible notes, the exercise of warrants or options, or an issuance of Shares in connection with a financing, will dilute our current shareholders’ ownership in the Company, and will reduce shareholders’ voting power proportionally.
Future sales of Shares, securities convertible into Shares, and other securities may negatively affect our stock price.
Future sales of Shares and/or other securities that are convertible into Shares could have a significant negative effect on the market price of our Shares, and the number of Shares outstanding could increase substantially. This increase, in turn, could dilute future earnings per share. Dilution and the availability of a large amount of securities for sale, and the possibility of additional issuances and sales of Shares or other classes of securities may negatively affect both the trading price and liquidity of our Shares.
The market for our Shares is, and may continue to be, limited and highly volatile, which may generally affect any future price of our Shares.
The lack of an orderly market for our common stock may negatively affect the volume of trading and market price for our common stock.
Historically, the volume of trades for our Shares has been limited. Moreover, the prices at which our Shares have traded have fluctuated widely on a percentage basis. There can be no assurance as to the prices at which our Shares will trade in the future, although they may continue to fluctuate significantly. Prices for our Shares will be determined in the marketplace and may be influenced by many factors, including, without limitation, the following:
| | ● | the depth and liquidity of the markets for our Shares; |
| | ● | investor perception of the Company and the industry in which we participate; |
| | ● | general economic and market conditions; |
| | ● | statements or changes in opinions, ratings or earnings estimates made by brokerage firms or industry analysts relating to the market in which we do business or relating to us specifically, as has occurred in the past; |
| | ● | quarterly variations in our results of operations; |
| | ● | general market conditions or market conditions specific to technology industries; and |
| | ● | domestic and international macroeconomic factors. |
An active trading market for the Shares may not exist in the future. Even if a market for our Shares continues to exist, investors may not be able to resell their Shares at or above the purchase price for which such investors purchased such Shares. In addition, the stock market has recently experienced extreme price and volume fluctuations. These fluctuations are often unrelated to the operating performance of the specific companies. As a result of the factors identified above, a stockholder (due to personal circumstances) may be required to sell its Shares at a time when our stock price is depressed due to random fluctuations, possibly based on factors beyond our control
| | 9. | Critical Accounting Policies |
A “critical accounting policy” is one that is both important to the portrayal of our financial condition and results of operations and that requires management’s most difficult, subjective or complex judgments. Such judgments are often the result of a need to make estimates about the effect of matters that are inherently uncertain. The preparation of our financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
A summary of our critical accounting policies, including those that require the use of significant estimates and judgment, follows. A more comprehensive description of all of our significant accounting policies is contained in Note 1 to our consolidated financial statements.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. In the accompanying consolidated financial statements, estimates are used for, but not limited to, the expected economic life and value of our licensed technology, allowance for doubtful accounts, value of contingent consideration, value of our debt issuances, accruals for estimated product returns, warrant relative fair value calculation, allowance for inventory obsolescence, allowance for our net operating loss carry forward and related valuation allowance for tax purposes and our stock-based compensation related to employees and directors, consultants and advisors.. Actual results could differ from those estimates.
Revenue Recognition
The Company sells products that are used in the treatment of Benign Prostate Hyperplasia. The Company recognizes product revenue, net of sales taxes, from the sale of catheters upon shipment delivery to the customer. Revenue from the sale of products is measured at the fair value of the consideration received or receivable, net of estimated returns. Revenue from the mobile service is recognized upon completion of the services by our mobile technicians or independent contractors, which is generally upon treatment of the patient.
The Company does not have a return policy that allows customers to return product, however the company has allowed returns on a limited customer by customer basis. The Company’s estimate for returns is based upon its historical experience with actual returns. While such experience has allowed for reasonable estimation in the past, history may not always be an accurate indicator of future returns. The Company continually monitors its estimates for returns and makes adjustments when it believes that actual product returns may differ from the established accruals. We record a provision for estimated returns in the same period as the related revenue is recorded. The provision for estimated sales returns is based on historical sales returns, analysis of credit memo data and specific customer-based circumstances.
Inventory
Inventory is valued at the lower of cost or market and consists primarily of console units and single-use treatment catheters. Current inventory of catheters consists of the direct costs of acquiring the inventory from vendors. Non-current inventory of console units, which were originally held for sale, were classified as property & equipment during the year ended March 31, 2016 as the Company began using the console units in operations. The carrying amount was adjusted prior to the transfer of the asset for any depreciation expense that would have been recognized had the asset been classified as held for sale. The Company recognized a loss on impairment of long-lived assets in other income (expense) of the statement of operations and comprehensive loss in the amount of $99,020, during the year ended March 31, 2016, related to transaction.
Inventory is relieved using the first-in, first-out method.
Stock-Based Compensation
ASC 718 “Compensation - Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period), on a graded vesting basis.
The Company accounts for stock-based compensation issued to non-employees and consultants in accordance with the provisions of ASC 505-50 “Equity - Based Payments to Non-Employees.” Measurement of share-based payment transactions with non-employees is based on the fair value of whichever is more reliably measurable: (a) the goods or services received; or (b) the equity instruments issued. The fair value of the share-based payment transaction is determined at the earlier of performance commitment date or performance completion date.
The estimated fair value of the options and warrants that are ultimately expected to vest based on performance related conditions, as well as the options and warrants that are expected to vest based on future service, is recorded over the instrument’s requisite service period and charged to stock-based compensation. In determining the amount of options and warrants that are expected to vest, the Company takes into account, voluntary termination behavior as well as trends of actual option and warrant forfeitures.
Research and Development Expenses
Research and development costs are expensed as incurred.
Contingent Consideration
In accordance with ASC 805, upon the purchase of Prolieve®, the Company recognized a contingent consideration obligation as part of the consideration transferred in exchange for the acquired business. The initial measurement of the contingent consideration obligation was based on its estimated fair value. The contingent consideration obligation has been remeasured to fair value at each reporting date and will continue to be remeasured until the contingency is resolved. The contingent consideration obligation has been re-measured to fair value at each reporting date and will continue to be re-measured until the contingency is resolved, which was during the three-month period ended June 30, 2019. The contingent consideration is $0 and $11,139 as of June 30, 2019 and March 31, 2019, respectively.
Intangible Assets
Intangible assets consist of intellectual property and customer relationships for our Prolieve® business acquired in July 2012. These intangible assets were originally recorded at fair value and are amortized on a straight-line basis over their estimated useful lives of 10 years. The Company reviews its intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, in a manner similar to that for property and equipment.
As of March 31, 2019, management determined, during the review of intangible assets, that the life of their intellectual property and customer relationships should be extended until the year March 31, 2029.
Recent Accounting Pronouncements
None of the accounting pronouncements we were required to or otherwise adopted in any of the periods contained in this report had a material impact on our results of operations, financial condition or cash flows. Additionally, we are evaluating all issued and unadopted Accounting Standards Updates and believe the adoption of these standards also will not have a material impact on our results of operations, financial position, or cash flows.
Fair Value Measurements
The Company’s unaudited condensed interim consolidated statements of financial position include various financial instruments (primarily cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, and notes payable) recorded at cost, which approximates their fair value. Fair value is the price that would be received from the sale of an asset or paid to transfer a liability assuming an orderly transaction in the most advantageous market at the measurement date. U.S. GAAP establishes a hierarchical disclosure framework which prioritizes and ranks the level of observability of inputs used in measuring fair value. These tiers include:
| | ● | Level 1—Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs. |
| | | |
| | ● | Level 2—Observable market-based inputs other than quoted prices in active markets for identical assets or liabilities. |
| | | |
| | ● | Level 3—Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs. |
In connection with the acquisition of Prolieve®, the Company owes additional purchase consideration of up to $2.5 million (contingent consideration) based on the sales of Prolieve® products after their acquisition. The contingent consideration is measured at fair value on a recurring basis using level 3 inputs, and the fair value is determined using unobservable inputs such as the discount rate.
The Company has no financial assets and liabilities measured at fair value on a non-recurring basis. The Company’s long-lived assets are measured at fair value on a non-recurring basis only when an impairment is deemed to occur.
Interest Rate Risk
Because of the short-term maturities of our cash and cash equivalents, we do not believe that an increase in market interest rates would have a significant impact on their realized value. The interest rates on our various outstanding debt instruments, including promissory and convertible notes, are fixed. Because of the fixed rates, a change in market interest rates would not have a material impact on interest expense associated with the debt.
Exchange Rate Risk
The Company’s reporting currency is the U.S. dollar and, accordingly, the Company reports its financial results in U.S. dollars. The Company’s functional currency and that of its wholly owned subsidiary is the U.S. dollar. Therefore, because certain financial transactions are denominated in Canadian dollars, we report those transactions in U.S. dollars using prevailing exchange rates at the time of the transaction. Transaction gains and losses on the settlement of these transactions, and on outstanding receivables and payables that are denominated in Canadian dollars, are included in the determination of our net loss.
Equity Price Risk
Historically, the Company has issued equity securities, and equity-linked securities such convertible debt, stock purchase warrants and stock options, to investors, employees and vendors. Equity and equity-linked securities are initially recorded in our financial statements at their fair values, and depending on the nature of the security may require periodic re-measurement at fair value. Changes in the market price of our common stock can have an impact on the value of the securities issued which could have a direct impact on those fair values, earnings, and cash flow.
| | 11. | Summary of Quarterly Results |
The following table sets forth, for the quarters indicated, information relating to the Company’s revenue, net loss and loss per common shares.
| | | Revenues | | | Net Loss | | | Basic and
Diluted EPS | |
| September 30, 2017 | | | 557,600 | | | | (476,277 | ) | | | (0.00 | ) |
| December 31, 2017 | | | 722,810 | | | | (312,396 | ) | | | (0.00 | ) |
| March 31, 2018 | | | 677,440 | | | | (429,793 | ) | | | (0.00 | ) |
| June 30, 2018 | | | 737,390 | | | | (281,460 | ) | | | (0.00 | ) |
| September 30, 2018 | | | 704,195 | | | | (388,521 | ) | | | (0.00 | ) |
| December 31, 2018 | | | 714,510 | | | | (279,938 | ) | | | (0.00 | ) |
| March 31, 2019 | | | 610,645 | | | | (520,613 | ) | | | (0.00 | ) |
| June 30, 2019 | | | 562,068 | | | | (449,384 | ) | | | (0.00 | ) |
For further quarterly financial information, please refer to the Company’s unaudited condensed interim consolidated financial statements that have been filed on SEDAR.com
| | 12. | Transactions with Related Parties |
The management team and directors, along with their remuneration for the three months ended June 30, 2019 is presented below:
| Individual | | Position | | Cash | | Options | | Shares | | Total |
| Grant B. Walsh | | | Director | | | $ | 6,000 | | | | | | | | | | | $ | 6,000 | |
| William Jow, MD | | | CEO | | | $ | 49,500 | | | | | | | | | | | $ | 49,500 | |
| Joseph S. C. Chan | | | Director | | | $ | 6,000 | | | | | | | | | | | $ | 6,000 | |
| Dr. Augustine P. Y. Chow | | | Director | | | $ | 6,000 | | | | | | | | | | | $ | 6,000 | |
| Raymond Tong | | | Director | | | $ | 6,000 | | | | | | | | | | | $ | 6,000 | |
| Mirsad Jakubovic | | | CFO | | | $ | 6,000 | | | | | | | | | | | $ | 6,000 | |
On January 16, 2006, the Company’s wholly owned subsidiary, Celsion (Canada) Inc. purchased from Celsion Corporation(USA) [“Celsion”]all of the assets relating to breast cancer Microfocus APA 1000 System (“System”), consisting of the microwave machine technology, the APA technology licensed from MIT, and all related intellectual and regulatory property (collectively, the “Business”). The Company has a commitment to pay a 5% royalty to Celsion on the net sales of products sold by and patent royalties received by the Company and its successors and assignees. Total royalties paid are not to exceed $18,500,000. Royalties will not be payable until the System can be placed in the market following successful completion of the pivotal clinical trial and receipt of approval to market the System in the US and Canada from the FDA and Health Canada. The Company has an additional commitment to pay a 5% royalty to MIT on the net sales of products, upon commercialization. If the Company does not apply for or does not receive FDA approval to enter at least one phase III clinical trials of a licensed product prior to the earlier of the termination of the agreement or June 25, 2018, the Company shall pay $10,000 to MIT. If the Company receives approval for sale of at least one licensed product or discovery product then the Company shall pay MIT $100,000. As of June 30, 2019, this requirement has not been met and no payment is due.
On October 1, 2017, the Company entered into an amended 5-year operating lease agreement. Future minimum payments under the operating lease for office space as of June 30, 2019 are as follows:
| 2020 | | $ | 86,801 | |
| 2021 | | $ | 89,416 | |
| 2022 | | $ | 92,093 | |
| 2023 | | $ | 62,604 | |
The Company has a purchase order commitment with its primary vendor for a total amount of $613,418 of the Prolieve kits through December 31, 2019.
During the year ended March 31, 2018, the Company entered into a research and development project agreement with Urobois Limited. The Company has paid $5,000 since the signing of the agreement and will make milestone payments to Urobois Limited in the amount of $20,000 through the completion of the agreement. The project is currently on hold.
The Company has agreed to indemnify its directors and officers and certain of its employees in accordance with the Company’s by-laws. As of June 30, 2019, the Company has not secured insurance policies that may provide coverage against certain claims.
In June 2018, W.L. Pate, JR and Charles C. Shelton filed a lawsuit in the District Court of Harris County, Texas to seek monetary relief of over $200,000 but not more than $1,000,000 from Medifocus Inc. for a transaction that did not materialize. Although the Company does not believe the suit has any merits and has not accrued for any amount in its financial statements as of June 30, 2019, any judgement unfavorable to the Company can potentially cause significant financial hardship and other damages to the Company. In addition, certain note holders of the Company had threatened to take legal actions against the Company due to the default on the notes. Such legal actions will also cause significant financial hardship and other damages to the Company.
Outstanding Share Data as of August 29, 2019
| | | Number or Principal Amount Outstanding | | Maximum Number of
Common Shares
Issuable, if Convertible,
Exercisable or
Exchangeable |
| Common Shares | | | 184,984,215 | | | | N/A | |
| Stock Options | | | 10,700,000 | | | | 10,700,000 | |
| Convertible debenture | | | - | | | | - | |
| Warrants outstanding | | | - | | | | - | |
| Maximum common shares outstanding | | | | | | | 195,684.215 | |
| | 16. | Off-Balance Sheet Arrangements |
As of the date of this filing, the Company does not have any off-balance sheet arrangements that have, or reasonably likely to have, a current or future effect upon the financial performance or financial condition of the Company, including, and without limitation, such considerations as liquidity and capital resources.
The Company has not entered into any significant transaction, nor is it currently reviewing any such transaction, which requires board approval, shareholder approval or regulatory approval that has not been discussed within this MD&A.
| | 18. | Current Accounting Pronouncements Not Effective |
ASU 2014-09,Revenue from Contracts with Customers (Topic 606),which provides guidance for revenue recognition for contracts. This guidance requires an entity to review contracts in five steps and will result in enhanced disclosures regarding the nature, amount, timing and uncertainty of revenue arising from contracts with customers. This standard is effective for fiscal years beginning after December 15, 2017 and early adoption is permitted only as of annual reporting periods for fiscal years beginning after December 15, 2016. We are currently evaluating the impact, if any, that this new guidance will have on the Company’s Condensed Interim Consolidated Financial Statements.
ASU 2015-11,Inventory (Topic 330): Simplifying the Measurement of Inventory, changes the measurement principle for certain inventory methods from the lower of cost or market to the lower of cost and net realizable value. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. This ASU does not apply to inventory that is measured using Last-in First-out (“LIFO”) or the retail inventory method. The provisions of ASU 2015-11 are effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The Company is currently evaluating this guidance to determine the impact it may have the Company’s Condensed Interim Consolidated Financial Statements.
ASU 2015-17,Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes, was issued to simplify the classification of deferred taxes on the balance sheet. The new guidance would require that deferred taxes be classified as non-current assets and liabilities based on the taxpaying jurisdiction. Application of the standard, which can be applied prospectively or retrospectively, is required for fiscal years beginning on or after December 15, 2016 and for interim periods within that year. The adoption of the amended guidance is not expected to have a material impact on the Company’s Condensed Interim Consolidated Financial Statements.
| | 19. | Disclosure Controls and Procedures |
Under the supervision and with the participation of the Company’s management, including its Chief Executive Officer and Chief Financial Officer, the Company evaluated the effectiveness of the design and operation of its disclosure controls and procedures (as defined in Rule 13a-15(e) or 15d-15(e) under the U.S. Exchange Act) for the quarter ended June 30, 2019. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of the end of the period covered by this report, the Company’s disclosure controls and procedures were effective to ensure that information required to be disclosed by the Company in the reports it files or submits under the U.S. Exchange Act is recorded, processed, summarized and reported within the time periods specified in applicable rules and forms.
| | 20. | Internal controls over Financial Reporting |
Our management has evaluated, with the participation of our Chief Executive Officer, changes in our internal controls over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) during the three months ended June 30, 2019. In connection with such evaluation, there have been no changes to our internal control over financial reporting that occurred during the three months ended June 30, 2019 that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
The Directors of the Company have approved the disclosure contained in this MD&A and a copy will be provided to anyone who requests it.
Condensed Interim Consolidated Financial Statements
Medifocus Inc.
June 30, 2019 and 2018
Management’s Responsibility for the Condensed Interim Consolidated Financial Statements
The accompanying unaudited condensed interim consolidated financial statements of Medifocus Inc. (the “Company”) are the responsibility of management and have been approved by the Board of Directors.
The unaudited condensed interim consolidated financial statements have been prepared by management, on behalf of the Board of Directors, in accordance with the accounting policies disclosed in the notes to the unaudited condensed interim consolidated financial statements. Where necessary, management has made informed judgments and estimates in accounting for transactions which were not complete at the date of the reporting period. In the opinion of management, the unaudited condensed interim consolidated financial statements have been prepared within acceptable limits of materiality and are in accordance with International Financial Reporting Standards.
Management has established systems of internal control over the financial reporting process, which are designed to provide reasonable assurance that relevant and reliable financial information is produced.
The Board of Directors is responsible for reviewing and approving the unaudited condensed interim consolidated financial statements together with other financial information of the Company and for ensuring that management fulfills its financial reporting responsibilities. An Audit Committee assists the Board of Directors in fulfilling this responsibility. The Audit Committee meets with management to review the financial reporting process and the unaudited condensed interim consolidated financial statements together with other financial information of the Company. The Audit Committee reports its findings to the Board of Directors for its consideration in approving the unaudited condensed interim consolidated financial statements together with other financial information of the Company for issuance to the shareholders.
Management recognizes its responsibility for conducting the Company’s affairs in compliance with established financial standards, and applicable laws and regulations, and for maintaining proper standards of conduct for its activities.
| “William Jow” | “Mirsad Jakubovic” |
| Dr. William Jow | Mirsad Jakubovic |
| Chief Executive Officer | Chief Executive Officer |
Notice of no Auditor Review of Interim Financial Statements
The accompanying unaudited condensed interim consolidated financial statements of the Company have been prepared by and are the responsibility of management.
The Company’s independent auditor has not performed a review of these financial statements in accordance with standards established by the Canadian Institute of Chartered Accountants for a review of interim financial statements by an entity’s auditor.
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
(in U.S. dollars)
| | | June 30, 2019 | | | March 31, 2019 | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 137,598 | | | $ | 183,104 | |
| Accounts receivable, net (Note 1) | | | 480,746 | | | | 499,383 | |
| Inventory, net (Note 1) | | | 84,411 | | | | 109,706 | |
| Other assets | | | 57,159 | | | | 44,790 | |
| Total Current Assets | | | 759,914 | | | | 836,983 | |
| Property and equipment, net (Note 3) | | | 66,004 | | | | 97,919 | |
| Deposits (Note 1) | | | 29,726 | | | | 29,725 | |
| Intangible assets, net (Note 4) | | | 781,099 | | | | 801,127 | |
| Total Assets | | $ | 1,636,743 | | | $ | 1,765,754 | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 148,073 | | | $ | 89,175 | |
| Accrued expenses | | | 622,801 | | | | 631,382 | |
| Accrued interest payable | | | 3,814,092 | | | | 3,550,337 | |
| Promissory notes payable (Note 5) | | | 772,790 | | | | 767,156 | |
| Payable to Boston Scientific Corporation (Note 2) | | | 2,190,867 | | | | 2,179,061 | |
| Contingent consideration, current portion (Note 2) | | | — | | | | 11,139 | |
| Convertible notes payable (net of discount), current portion (Note 5) | | | 5,410,000 | | | | 5,410,000 | |
| Total Current Liabilities | | | 12,958,623 | | | | 12,638,250 | |
| Contingent consideration | | | — | | | | — | |
| Total liabilities | | | 12,958,623 | | | | 12,638,250 | |
| Commitments and contingencies (Note 5 and Note 8) | | | | | | | | |
| Stockholders’ deficit: | | | | | | | | |
| Common stock (no par value, unlimited shares authorized, 184,984,215 and 184,984,215 shares issued and outstanding as of June 30, 2019 and March 31, 2019, respectively. | | | 14,295,388 | | | | 14,295,388 | |
| Common stock issuable (Note 6) | | | 445,400 | | | | 445,400 | |
| Additional paid-in capital | | | 10,830,075 | | | | 10,830,075 | |
| Accumulated deficit | | | (36,892,743 | ) | | | (36,443,359 | ) |
| Total Stockholders’ Deficit | | | (11,321,880 | ) | | | (10,872,496 | ) |
| Total Liabilities and Stockholders’ Deficit | | $ | 1,636,743 | | | $ | 1,765,754 | |
| Going Concern (Note 1) | | | | | | | | |
See accompanying notes to unaudited condensed interim consolidated financial statements.
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF OPERATIONS
(in U.S. dollars)
| | | Three months ended June 30, | |
| | | 2019 | | | 2018 | |
| Sales | | | | | | |
| Products | | $ | 183,359 | | | $ | 328,142 | |
| Services | | | 378,709 | | | | 409,248 | |
| Total Sales | | | 562,068 | | | | 737,390 | |
| Costs of Sales | | | | | | | | |
| Products | | | 109,402 | | | | 197,985 | |
| Services | | | 277,988 | | | | 288,348 | |
| Total Costs of Sales | | | 387,390 | | | | 486,333 | |
| Gross Profit | | | 174,678 | | | | 251,057 | |
| | | | | | | | | |
| Operating Expenses | | | | | | | | |
| Research and development | | | 27,733 | | | | 27,409 | |
| Sales and marketing | | | 16,175 | | | | 7,310 | |
| General and administrative | | | 312,313 | | | | 281,294 | |
| Total Operating Expenses | | | 356,221 | | | | 316,013 | |
| Loss from Operations | | | (181,543 | ) | | | (64,956 | ) |
| Other Income (Expense) | | | | | | | | |
| Interest and discount accretion | | | (245,070 | ) | | | (230,955 | ) |
| Loss from change in fair value of contingent consideration (Note 2) | | | (667 | ) | | | (14,793 | ) |
| Gain on recovery of HST receivable | | | — | | | | — | |
| Other income (expense) | | | (22,104 | ) | | | 29,244 | |
| Total Other Income (Expense) | | | (267,841 | ) | | | (216,504 | ) |
| Net Loss | | $ | (449,384 | ) | | $ | (281,460 | ) |
| Net Loss per share basic and diluted | | $ | (0.00 | ) | | $ | (0.00 | ) |
| Weighted average common shares outstanding—basic and diluted | | | 184,984,215 | | | | 184,984,215 | |
See accompanying notes to unaudited condensed interim consolidated financial statements.
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF CASH FLOWS
(in U.S. dollars)
| | | Three months ended June 30, | |
| | | 2019 | | | 2018 | |
| Net Loss | | $ | (449,384 | ) | | $ | (281,460 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 51,943 | | | | 93,839 | |
| Loss on change in fair value of contingent consideration | | | 667 | | | | 14,793 | |
| Provision (recovery) for bad debts and warranties | | | 5,795 | | | | (6,807 | ) |
| Changes in operating assets and liabilities | | | | | | | | |
| Decrease (increase) in accounts receivable | | | 12,842 | | | | (32,960 | ) |
| Decrease in inventory | | | 25,295 | | | | 63,609 | |
| (Increase) in other current assets | | | (12,370 | ) | | | (19,634 | ) |
| Increase (decrease) in accounts payable | | | 58,898 | | | | (63,901 | ) |
| (Decrease) in accrued expenses | | | (8,581 | ) | | | (4,513 | ) |
| Increase in accrued interest | | | 245,071 | | | | 230,766 | |
| Net cash used in operating activities | | | (69,824 | ) | | | (6,268 | ) |
| | | | | | | | | |
| FINANCING ACTIVITIES: | | | | | | | | |
| Net cash provided by financing activities | | | — | | | | — | |
| Effect of exchange rate changes on cash and cash equivalents | | | 24,318 | | | | (29,244 | ) |
| (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | | | (45,506 | ) | | | (35,512 | ) |
| CASH AND CASH EQUIVALENTS, BEGINNING OF THE PERIOD | | | 183,104 | | | | 115,502 | |
| CASH AND CASH EQUIVALENTS, END OF THE PERIOD | | $ | 137,598 | | | $ | 79,990 | |
| | | | | | | | | |
| Cash paid for interest | | $ | — | | | $ | — | |
See accompanying notes to unaudited condensed interim consolidated financial statements.
MEDIFOCUS, INC.
UNAUDITED CONDENSED INTERIM CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
(in U.S. dollars)
| | | | | | | | | | | | Additional | | | | | | Total | |
| | | Common Stock | | | Common Stock | | | Common Stock | | | Paid-in | | | Accumulated | | | Stockholders’ | |
| | | Shares | | | Amount | | | Issuable | | | Capital | | | Deficit | | | Deficit | |
| Balance at April 1, 2018 | | | 184,984,215 | | | $ | 14,295,388 | | | $ | 123,809 | | | $ | 10,830,075 | | | $ | (34,972,827 | ) | | $ | (9,723,555 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | (281,460 | ) | | | (281,460 | ) |
| Balance at June 30, 2018 | | | 184,984,215 | | | $ | 14,295,388 | | | $ | 123,809 | | | $ | 10,830,075 | | | $ | (35,254,287 | ) | | $ | (10,005,015 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at April 1, 2018 | | | 184,984,215 | | | $ | 14,295,388 | | | $ | 445,400 | | | $ | 10,830,075 | | | $ | (36,443,359 | ) | | $ | (10,872,496 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | (449,384 | ) | | | (449,384 | ) |
| Balance at June 30, 2018 | | | 184,984,215 | | | $ | 14,295,388 | | | $ | 445,400 | | | $ | 10,830,075 | | | $ | (36,892,743 | ) | | $ | (11,321,880 | ) |
See accompanying notes to unaudited condensed interim consolidated financial statements.
MEDIFOCUS, INC.
NOTES TO UNAUDITED CONDENSED INTERIM
CONSOLIDATED FINANCIAL STATEMENTS
AS OF THE THREE MONTHS ENDED JUNE 30, 2019
1. BUSINESS, GOING CONCERN, LIQUIDITY AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Description of Business and Current Financial Condition
Medifocus Inc. (the “Company” or “Medifocus”) was incorporated under the Business Corporations Act (Ontario) on April 25, 2005. Medifocus develops and commercializes minimally invasive focused heat systems for the treatment of cancerous and benign tumors, and enlarged prostate, medically known as Benign Prostate Hyperplasia (“BPH”).
The Company owns two focused heat technology platforms with comprehensive US and international patent protection:
| | ● | The Endo-thermotherapy Platform-from which Prolieve Thermodilatation System (“Prolieve”) was developed, can potentially be used to treat cancers in prostate, rectal, cervical and esophageal, and |
| | | |
| | ● | The Adaptive Phased Array (“APA”) Microwave Focusing Platform-invented by MIT, licensed to Medifocus, directs precisely focused microwave energy at tumor center to induce shrinkage or eradication of tumors without undue harm to surrounding tissue. The Company’s APA 1000 Breast Cancer Treatment System, developed from the APA technology platform is currently in pivotal Phase-III clinical trials. |
Going Concern Consideration
Effective April 1, 2016, the Company adopted ASU 2014-15,Presentation of Financial Statements-Going Concern (Subtopic 205-40), which requires management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date the financial statements are issued. Management’s evaluations are based on relevant conditions and events that are known and reasonably to be knowable as of August 29, 2019. Based on the following, management believes that it is probable that management will be unable to meet its obligations as they come due within one year that the financial statements are issued.
The Company’s operations are subject to certain risks and uncertainties including, among others, current and potential competitors with greater resources, lack of operating history and uncertainty of future profitability and possible fluctuations in financial results. Since inception, the Company has incurred substantial operating losses, principally from expenses associated with the Company’s Prolieve operation, research and development and financing activities. The Company believes these expenditures are essential for the commercialization of its technologies. The Company expects its operating losses to continue in the near future as it continues its Prolieve sales and marketing activities. Due to continued operating losses, there is substantial doubt regarding the Company’s ability to continue as a going concern. The Company’s ability to achieve profitability is dependent upon its ability to operate its Prolieve business profitably and to obtain governmental approvals, produce, and market and sell its new product candidates. There can be no assurance that the Company will be able to commercialize its technology successfully or that profitability will ever be achieved. The operating results of the Company have fluctuated significantly in the past. The Company expects that its operating results will fluctuate significantly in the future and will depend on a number of factors, many of which are outside the Company’s control.
The Company will need substantial additional funding in order to sustain its operation, to complete the development, testing and commercialization of its product candidates. The commitment to these projects will require additional external funding, at least until the Company is able to generate sufficient cash flow from the sale of one or more of its products to support its continued operations. If adequate funding is not available, the Company may be required to delay, scale back or eliminate certain aspects of its operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force it to relinquish rights to certain of its technologies, products or potential markets or that could impose onerous financial or other terms. Furthermore, if the Company cannot fund its ongoing development and other operating requirements, particularly those associated with its obligations to conduct clinical trials under its licensing agreements, it will be in breach of these licensing agreements and could therefore lose its license rights, which could have material adverse effects on its business. Additionally, the Company is not in compliance with the provisions of outstanding debt agreements, and it has not remitted quarterly royalty payments to Boston Scientific Corporation pursuant to the terms of its purchase agreement for Prolieve. The Company has not paid interest owing to certain debt holders of the convertible debentures, and is in default of the terms of the debentures. As of June 30, 2019, certain debt holders have threatened to launch legal action against the Company related to the outstanding debt.
Management is continuing its efforts to obtain additional funds through equity financing and through the negotiation of debt agreements to ensure that the Company can meet its obligations and sustain operations. Additionally, the Company has reduced costs of operations, as the Company has eliminated certain positions that do not hold value to the Company. Management has also reduced debt by settling the debt with common shares. Although the Company’s positive cash flows from operating activities is a positive sign for the Company, it is not enough to eliminate the risk of uncertainty in the Company’s future.
These factors raise substantial doubt about the ability of the Company to continue as a going concern. The consolidated financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue in existence. Such adjustments could be material
The Company’s going concern
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements of Medifocus, Inc. have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”) and include the accounts of the Company and its wholly-owned subsidiary Celsion (Canada) Inc. All intercompany transactions have been eliminated. There were no transactions for Celsion (Canada) Inc. for the three month periods ended June 30, 2019 and 2018.
Unless otherwise noted, all references to “$” or “dollar” refer to the United States dollar. The Company operates in a single business segment, focused heat systems for targeted thermotherapy of surface, subsurface and deep seated localized and regional cancers. Substantially all of the Company’s revenue is generated, and assets are located, in the United States.
Foreign Currency
Effective April 1, 2013, the Company changed its reporting currency from the Canadian dollar (“CAD”) to the U.S. dollar in anticipation of filing its financial statements with the U.S. Securities and Exchange Commission. Effective April 1, 2014, the Company changed its functional currency and that of its wholly owned subsidiary to the U.S. dollar. As a result, all translation adjustments prior to April 1, 2014 were recognized into other income (expense) in the year ending March 31, 2015.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. The consolidated financial statements include significant estimates for the expected economic life and value of our licensed technology, allowance for doubtful accounts, value of contingent consideration, value of our debt issuances,accruals for estimated product returns, allowance for inventory obsolescence,allowance for our net operating loss carry forward, contingencies and related valuation allowance for tax purposes, stock-based compensation related to employees and directors, consultants and advisors. Because of the use of estimates inherent in the financial reporting process, actual results could differ significantly from those estimates.
Currency Risk
The Company held its cash balances within banks in Canada in Canadian dollars and with banks in United States in United States dollars. The Company’s operations are mainly conducted in United States of America however the Company has transactions in Canada which are affected by the fluctuation of the currency rates. The value of the United States dollar against the Canadian dollar may fluctuate with the changes in economic conditions.
During the three months ended June 30, 2019, in comparison to the prior year, the Canadian dollar strengthened in relation to the US dollar and upon the translation of the Company’s debt and accrued expenses held in Canadian dollars, the Company recorded a currency loss of $24,318 (2018-Gain of $29,244), in other income (expense) on the consolidated statements of operations and comprehensive loss.
Credit risk and economic dependence
Credit risk is the risk that one party to a financial instrument will cause a financial loss for the other party by failing to discharge an obligation. The financial instruments that potentially subject the Company to credit risk consist of cash and accounts receivable. The Company maintains cash with high credit quality financial institutions located in the United States and Canada.
The Company provides credit to its customers in the normal course of its operations. It carries out, on a continuing basis, credit checks on its customers. The Company’s operations rely significantly on one supplier and Company can not easily source alternative suppliers.
Credit Concentration
One customer represented a concentration of approximately 15% of total trade receivables for the years ended March 31, 2019 and 2018. No individual customer represented more than 10% of revenues for the years ended March 31, 2019, 2018 and 2017. The Company’s sales are primarily in the United States.
Vendor Concentration and Vendor Deposits
The Company currently purchases 100% of its Prolieve catheter inventory from one supplier. Alternative suppliers and alternative catheters are not currently available. As of June 30, 2019 and 2018, the Company maintained a deposit of $0 and $221,330, respectively, with its vendor.
Fair Value Measurements
The Company’s consolidated statements of financial position include various financial instruments (primarily cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, payable to Boston Scientific Corporation, accrued interest payable, and notes payable) recorded at cost, which approximates their fair value. Fair value is the price that would be received from the sale of an asset or paid to transfer a liability assuming an orderly transaction in the most advantageous market at the measurement date. U.S. GAAP establishes a hierarchical disclosure framework which prioritizes and ranks the level of observability of inputs used in measuring fair value. These tiers include:
| | ● | Level 1—Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs. |
| | | |
| | ● | Level 2—Observable market-based inputs other than quoted prices in active markets for identical assets or liabilities. |
| | | |
| | ● | Level 3—Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs. |
In connection with the acquisition of Prolieve, the Company owes additional purchase consideration of up to $2.5 million (contingent consideration) based on the sales of Prolieve products after their acquisition. The contingent consideration is measured at fair value on a recurring basis using Level 3 inputs, and the fair value is determined using unobservable inputs such as the discount rate. The change in the fair value of the contingent consideration of $35,878, $54,185 and $83,189 for the years ended March 31, 2019, 2018 and 2017, respectively, is reflected as “loss from change in fair value of contingent consideration” in the accompanying consolidated statements of operations and comprehensive loss.See note 2.
The Company has no financial assets and liabilities measured at fair value on a non-recurring basis. The Company’s long-lived assets are measured at fair value on a non-recurring basis only when an impairment is deemed to occur.
Fair Value of Financial Instruments
The carrying amounts of financial instruments classified as current assets or liabilities, including accounts receivable, accounts payable and accrued expenses approximate fair value because of the short maturity of these instruments.
Other Comprehensive (Loss) Income and Accumulated Other Comprehensive (Loss) Income
Other comprehensive (loss) income includes the total of the Company’s net loss and all other changes in equity other than transactions with owners, including changes in equity for cumulative translation adjustments resulting from the consolidation of foreign subsidiary as the financial statements of the subsidiary was previously accounted for using the local currency as the functional currency. The Company did not recognize any foreign currency translation losses during the three-month periods ending June 30, 2019 and 2018.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents. All interest bearing and non-interest-bearing accounts are guaranteed by the FDIC up to $250,000. The Company may maintain cash balances in excess of FDIC coverage. Management considers this to be a normal business risk.
Accounts Receivable – Trade and Harmonized Sales Tax
Trade
The Company extends credit to customers on an unsecured basis and payment terms are typically 30 days from delivery or service. The Company’s receivables are primarily related to Prolieve products and services. Management uses the aging account method to assess the company’s allowance for doubtful accounts. The aging account method uses the number of days outstanding for the underlying invoices that have been past due. Receivables are written off when it is determined that the underlying invoices are uncollectible.
The Company maintained an allowance for doubtful accounts of $42,318 and $36,523 as of June 30, 2019 and March 31, 2019, respectively.
Harmonized Sales Tax
During the year ended March 31, 2016 the Company had a receivable from a Canadian tax agency for a harmonized sales tax, however, management was uncertain as to the collectability of the asset. During the year ended March 31, 2016, the Company decided to write-off the entire balance until the receivable is collected. During the year ended March 31, 2017, the collectability was ensured and the Company recovered a significant portion of the receivable. As the recovery (write-off) is considered by the Company as an infrequent occurrence and it is not part of the trade receivable balance, the transaction is included as a gain (loss) in other income (expense) in the statements of operations and comprehensive loss for the years ended March 31, 2017.
Accounts Receivable consisted of the following as of June 30, 2019 and March 31, 2019.
| | | June 30, 2019 | | | March 31, 2019 | |
| Accounts receivable trade | | $ | 516,195 | | | $ | 529,542 | |
| Accounts receivable - Harmonized sales tax | | | 6,871 | | | | 6,364 | |
| Allowance for doubtful accounts | | | (42,318 | ) | | | (36,523 | ) |
| | | $ | 480,746 | | | $ | 499,383 | |
Inventory
Inventory is valued at the lower of cost or market and consists primarily of console units and single-use treatment catheters. Current inventory of catheters and consoles consist of the direct costs of acquiring the inventory from vendors. Certain non-current inventory of console units, which were originally held for sale, were classified as property and equipment during the year ended March 31, 2016 as the Company began using the console units in operations. The carrying amount was adjusted prior to the transfer of the assets for any depreciation expense that would have been recognized since acquisition had the asset been classified as held for sale. The Company recognized a loss on impairment of long-lived assets in other income (expense) of the statement of operations and comprehensive loss in the amount of $99,020, during the year ended March 31, 2016, related to this transaction.
Inventory is relieved using the first-in, first-out method and consists of the following at June 30, 2019 and March 31, 2019.
| | | June 30, 2019 | | | March 31, 2019 | |
| | | | | | | |
| Finished Goods – Catheters | | $ | 79,698 | | | $ | 104,994 | |
| Finished Goods – Consoles | | | 4,712 | | | | 4,712 | |
| | | | | | | | | |
| Total Inventory | | $ | 84,410 | | | $ | 109,706 | |
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is provided over the estimated useful lives of the related assets, ranging from three to seven years, using the straight-line method. Major renewals and improvements are capitalized and ordinary repairs and maintenance are expensed as incurred.
The Company reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An asset is considered impaired if its carrying amount exceeds the future net undiscounted cash flows that the asset is expected to generate. If such asset is considered to be impaired, the impairment recognized is the amount by which the carrying amount of the asset, if any, exceeds its fair value determined using a discounted cash flow model.
Equity Method Investments
During the year ended March 31, 2018, the Company entered into a sale agreement of their rights to the Gene Therapy platform along with a $50,000 deposit for consideration of $100,000 to ThermeGene Corporation which was offset by approximately $64,000 in payables leaving a remaining receivable balance of approximately $36,000. The Company recorded a $50,000 gain in connection with the sale of the platform. The Company will receive 10% of the purchaser’s shares, ThermeGene, which are valued at $0, which will remain anti-dilutive until ThermeGene Corporation raises $2,000,000. During the year ended March 31, 2019, the receivable in the amount of approximately $36,000 was deemed uncollectable and written off as a loss and the write off is included in other income (expense) in the accompanying consolidated statements of operations and comprehensive loss. There was no activity for the three-month period ending June 30, 2019.
Contingent Consideration
In accordance with ASC 805, upon the purchase of Prolieve, the Company recognized a contingent consideration obligation as part of the consideration transferred in exchange for the acquired business. The initial measurement of the contingent consideration obligation was based on its estimated fair value. The contingent consideration obligation has been re-measured to fair value at each reporting date and will continue to be re-measured until the contingency is resolved, which was during the three-month period ended June 30, 2019. The contingent consideration is $0 and $11,139 as of June 30, 2019 and March 31, 2019, respectively.
Convertible Debt Instruments
When the Company has determined that the embedded conversion options should not be bifurcated from their host instruments the Company accounts for convertible debt instruments in accordance with ASC 470-20Debt with Conversion and Other Options. The Company records, when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. The Company amortizes any debt discount over the term of the notes, using the straight-line method, which approximates the effective interest method. The Company records, when necessary, any induced conversion expense, at the time of conversion for the difference between the reduced conversion price per share and the original conversion price per share.
Intangible Assets
Intangible assets consist of intellectual property and customer relationships for our Prolieve business acquired in July 2012. These intangible assets were originally recorded at fair value and are amortized on a straight-line basis over their estimated useful lives of 10 years. The Company reviews its intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, in a manner similar to that for property and equipment.
As of March 31, 2019, management determined, during the review of intangible assets, that the life of their intellectual property and customer relationships should be extended until the year March 31, 2029.
Revenue Recognition
The Company sells products and provides services which are used in the treatment of BPH. The Company recognizes revenue, net of sales taxes, from the sale of Prolieve consoles and catheters upon shipment to the customer. Revenue from the sale of products is measured at the fair value of the consideration received or receivable, net of any estimated returns. Revenue from the mobile service is recognized upon completion of the services, which is generally upon treatment of the patient.
The Company does not have a return policy that allows customers to return product, however the Company has allowed returns on a limited customer by customer basis. The Company’s estimate for returns is based upon its historical experience with actual returns, however such returns have historically been limited. While such experience has allowed for reasonable estimation in the past, history may not always be an accurate indicator of future returns. The Company continually monitors its estimates for returns and makes adjustments when it believes that actual product returns may differ from the established accruals, if any. We record a provision for estimated returns in the same period as the related revenue is recorded.
Costs of Sales—Products
Costs of goods sold primarily include the cost of products sold to customers on a first-in first-out basis, along with amortization expense of our intellectual property, warranty costs, warehousing costs, freight and handling charges. Warehousing costs include payroll and benefit costs.
Costs of Sales—Services
Costs of services consist primarily of the costs to provide mobile services to our patients, including catheter cost, amortization expense of our intellectual property, depreciation of our mobile consoles and vehicle fleet, and payroll and benefit costs.
Product Warranty Liabilities
Prolieve products are covered by warranties against defects in material and workmanship for periods of up to 12 months. We record a liability for warranty claims at the time of sale based on the trend in the historical ratio of product failure rates, material usage and service delivery costs to sales, the historical length of time between the sale and resulting warranty claim and other factors. The accrued liability for warranty provisions was approximately $4,000 and $4,800 as of June 30, 2019 and March 31, 2019, respectively.
Research and Development Expenses
Research and development costs are expensed as incurred.
Income Taxes
Income taxes are accounted for under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in results of operations in the period that the tax rate change occurs. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
A tax position is recognized as a benefit only if it is “more likely than not” that the tax position taken would be sustained in a tax examination, presuming that a tax examination will occur. The Company recognizes interest and/or penalties related to income tax matters in the income tax expense category.
Stock-Based Compensation
ASC 718 “Compensation - Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period), on a graded vesting basis.
The Company accounts for stock-based compensation issued to non-employees and consultants in accordance with the provisions of ASC 505-50 “Equity - Based Payments to Non-Employees.” Measurement of share-based payment transactions with non-employees is based on the fair value of whichever is more reliably measurable: (a) the goods or services received; or (b) the equity instruments issued. The fair value of the share-based payment transaction is determined at the earlier of performance commitment date or performance completion date.
The estimated fair value of the options and warrants that are ultimately expected to vest based on performance related conditions, as well as the options and warrants that are expected to vest based on future service, is recorded over the instrument’s requisite service period and charged to stock-based compensation. In determining the amount of options and warrants that are expected to vest, the Company takes into account, voluntary termination behavior as well as trends of actual option and warrant forfeitures.
Profit Sharing Plan
The Company sponsors a defined contribution retirement plan through a Section 401(k) profit sharing plan. Employees may contribute up to 15% of their pre-tax compensation. Participants are eligible for matching Company contributions up to 3% of eligible compensation dependent on the level of voluntary contributions. Company matching contributions totaled approximately $4,060 and $3,908 for the three months ended June 30, 2019 and 2018, respectively.
Shipping and Handling
The Company incurs shipping and handling costs related to their Prolieve operations. The Company includes these expenses into the costs of sales on the Company’s unaudited consolidated statement of operations and comprehensive loss. Shipping and handling costs totaled approximately $18,100 and $12,600 for the three months ended June 30, 2019 and 2018, respectively.
Net Loss Per Share
Basic net loss per share is computed by dividing net loss available to common shareholders by the weighted-average number of shares of common shares outstanding during the period.
For periods of net loss, diluted loss per share is calculated similarly to basic loss per share because the impact of all dilutive potential common shares is anti-dilutive due to the net losses. Outstanding stock options of 10,700,000, 10,700,000 and 6,500,000 and outstanding stock purchase warrants of 0, 0 and 18,013,250 to purchase commons shares for the three month period ending June 30, 2019 and the year ended March 31, 2019, were considered anti-dilutive and therefore were not included in the calculation of diluted shares. Additionally, for the three months ended June 30, 2019 and the year ended March 31, 2019, convertible promissory notes convertible into 21,640,000, 21,640,000 and 22,160,000, respectively, shares of common stock were also considered anti-dilutive and therefore were not included in the calculation of diluted shares.
Newly Adopted Accounting Pronouncements
ASU No. 2014-09,Revenue from Contracts with Customers (Topic 606),which provides guidance for revenue recognition for contracts. This guidance requires an entity to review contracts in five steps and will result in enhanced disclosures regarding the nature, amount, timing and uncertainty of revenue arising from contracts with customers. This standard is effective for fiscal years beginning after December 15, 2017 and early adoption is permitted only as of annual reporting periods for fiscal years beginning after December 15, 2016. The adoption of this guidance did not have a material impact on the company’s unaudited condensed interim consolidated financial statements.
ASU No. 2016-01, Financial Instruments-Overall (Subtopic 825-10) - Recognition and Measurement of Financial Assets and Financial Liabilities, which addresses certain aspects of recognition, measurement, presentation, and disclosure of financial instruments. Most notably, this new guidance requires equity investments (except those accounted for under the equity method of accounting or those that result in consolidation of the investee) to be measured at fair value with changes in fair value recognized in net income. This new guidance is effective for annual reporting periods beginning after December 15, 2017. The adoption of this guidance did not have a material impact on the company’s unaudited condensed interim consolidated financial statements.
ASU No. 2016-15,Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments.The ASU provides clarity to preparers on the treatment of eight specific items within an entity’s statement of cash flows. The guidance becomes effective for all public entities in fiscal years beginning after December 15, 2017, including interim periods therein. Early adoption of the guidance, including within an interim period, is permitted. The adoption of this guidance did not have a material impact on the company’s unaudited condensed interim consolidated financial statements.
ASU No. 2017-09,Compensation-Stock Compensation (Topic 718): “Scope of Modification Accounting”.The ASU amends the scope of modification accounting for share-based payment arrangements and provides guidance on the types of changes to the terms or conditions of share-based payment awards to which an entity would be required to apply modification accounting under ASC 718. The guidance becomes effective for annual reporting periods, including interim periods within those annual reporting periods, beginning after December 15, 2017. The adoption of this guidance did not have a material impact on the company’s unaudited condensed interim consolidated financial statements.
Recent Accounting Pronouncements
ASU No. 2018-13,Fair Value Measurement (Topic 820).The ASU eliminates certain disclosures requirements for fair value measurements for all entities, requires public entities to disclose certain new information and modifies some disclosures requirements. The guidance becomes effective for annual reporting periods, including interim periods within those annual reporting periods, beginning after December 15, 2019. This guidance is not expected to have a material impact on the company’s unaudited condensed interim consolidated financial statements.
ASU No. 2018-11 and 2018-10,Leases (Topic 842).The ASU discusses the effective date and transition requirements for the amendments related to separating components of a contract are the same as the effective date and transition requirements in ASU No. 2016-02. This guidance is not expected to have a material impact on the company’s unaudited condensed interim consolidated financial statements.
ASU No. 2018-07,Compensation-Stock Compensation (Topic 718): These amendments expand the scope of Topic 718, Compensation—Stock Compensation (which currently only includes share-based payments to employees) to include share-based payments issued to nonemployees for goods or services. Consequently, the accounting for share-based payments to nonemployees and employees will be substantially aligned. The ASU supersedes Subtopic 505-50, Equity—Equity-Based Payments to Non-Employees. The guidance becomes effective for annual reporting periods, including interim periods within those annual reporting periods, beginning after December 15, 2018. This guidance is not expected to have a material impact on the company’s unaudited condensed interim consolidated financial statements.
Emerging Growth Company Status
The Company is an “emerging growth company” as defined in section 3(a) of the Exchange Act, as amended by the United States Jumpstart Our Business Startups Act, enacted on April 5, 2012 (the “JOBS Act”), and will continue to qualify as an “emerging growth company” until the earliest to occur of: (a) the last day of the fiscal year during which the Company has total annual gross revenues of $1,000,000,000 (as such amount is indexed for inflation every 5 years by the SEC) or more; (b) the last day of the Company’s fiscal year following the fifth anniversary of the date of the first sale of the Company’s common equity securities pursuant to an effective registration statement under the Securities Act; (c) the date on which the Company has, during the previous 3-year period, issued more than $1,000,000,000 in non-convertible debt; or (d) the date on which we are deemed to be a ‘large accelerated filer’, as defined in Exchange Act Rule 12b–2.
Generally, a company that registers any class of its securities under section 12 of the Exchange Act is required to include in the second and all subsequent annual reports filed by it under the Exchange Act, a management report on internal controls over financial reporting and, subject to an exemption available to companies that meet the definition of a “smaller reporting company” in Exchange Act Rule 12b-2, an auditor attestation report on management’s assessment of internal controls over financial reporting. However, for so long as the Company will continue to qualify as an emerging growth company, the Company will be exempt from the requirement to include an auditor attestation report in the Company’s annual reports filed under the Exchange Act, even if the Company does not qualify as a “smaller reporting company”. In addition, section 103(a)(3) of the Sarbanes-Oxley Act of 2002 has been amended by the JOBS Act to provide that, among other things, auditors of an emerging growth company are exempt from any rules of the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the Company.
Any U.S. domestic issuer that is an emerging growth company is able to avail itself to the reduced disclosure obligations regarding executive compensation in periodic reports and proxy statements, and to not present to its shareholders a nonbinding advisory vote on executive compensation, obtain approval of any golden parachute payments not previously approved, or present the relationship between executive compensation actually paid and the Company’s financial performance. As a foreign private issuer, the Company is not subject to such requirements, and will not become subject to such requirements even if the Company was to cease to be an emerging growth company.
As a reporting issuer under the securities legislation of the Canadian provinces of Ontario, British Columbia, and Alberta, the Company is required to comply with all new or revised accounting standards that apply to Canadian public companies. Pursuant to Section 107(b) of the JOBS Act, an emerging growth company may elect to utilize an extended transition period for complying with new or revised accounting standards for public companies until such standards apply to private companies. The Company elected to utilize this extended transition period. However, while the Company elected to utilize this extended transition period, our unaudited condensed interim consolidated financial statements as of June 30, 2019 reflect the adoption of all required accounting standards for public companies.
2. BUSINESS ACQUISITION AND CONTINGENT CONSIDERATION
On July 24, 2012 the Company purchased from Boston Scientific Corporation (“BSC”), in a taxable transaction, all of the assets, relating to the Prolieve, an FDA approved device for the treatment of BPH. The total purchase consideration consisted of the following:
| Cash | | $ | 2,535,610 | |
| Fair value of contingent consideration | | | 1,126,505 | |
| Total consideration | | $ | 3,662,115 | |
The maximum amount of $2,500,000 is payable as contingent consideration pursuant to the terms of the agreement. The fair value of the contingent consideration was determined by calculating its present value based on its payment terms using an interest rate of 24% (our estimated unsecured borrowing rate). The contingent consideration is payable quarterly at a rate of 10% of sales of Prolieve products. As of March 31, 2019, $2,179,061 of royalties are due to BSC of which $2,117,097 is past due.
The activity of the non-contingent and contingent consideration obligation for the years ending March 31, 2019, 2018 and 2017 and the allocation is as follows:
| Activity is as follows: | | Non-Contingent | | | Contingent | | | Total | |
| Balance at April 1, 2018 | | $ | 1,902,387 | | | $ | 251,935 | | | $ | 2,154,322 | |
| Less: payments | | | — | | | | — | | | | — | |
| Change in non-contingent/contingent | | | 75,580 | | | | (60,787 | ) | | | 14,793 | |
| Balance at June 30, 2018 | | $ | 1,977,967 | | | | 191,148 | | | | 2,169,115 | |
| | | | | | | | | | | | | |
| Balance at April 1, 2019 | | | 2,179,061 | | | | 11,139 | | | | 2,190,200 | |
| Less: payments | | | — | | | | — | | | | — | |
| Change in non-contingent/contingent | | | 11,806 | | | | (11,139 | ) | | | 667 | |
| Balance at June 30, 2019 | | $ | 2,190,867 | | | $ | — | | | $ | 2,190,867 | |
| | | | | | | | | | | | | |
| Allocated as follows as of June 30, 2019: | | | | | | | | | | | | |
| Payable to Boston Scientific Corp. | | $ | 2,190,867 | | | $ | — | | | $ | 2,190,867 | |
| Contingent consideration – current | | $ | — | | | $ | — | | | $ | — | |
| Contingent consideration – non current | | $ | — | | | $ | — | | | $ | — | |
3. PROPERTY AND EQUIPMENT
Property and equipment consists of the following as of June 30, 2019 and March 31, 2019:
| | | June 30, 2019 | | | March 31, 2019 | |
| Machinery and equipment (5-7 year life) | | $ | 35,937 | | | $ | 35,937 | |
| Mobile consoles (7 year life) | | | 798,667 | | | | 798,667 | |
| Automobiles (3 year life) | | | 18,376 | | | | 18,376 | |
| Furniture and fixtures (3-5 year life) | | | 20,000 | | | | 20,000 | |
| | | | 872,980 | | | | 872,980 | |
| Accumulated depreciation | | | (806,976 | ) | | | (775,061 | ) |
| Total | | $ | 66,004 | | | $ | 97,919 | |
Depreciation expense was approximately $32,000 and $32,000 for the three-month period ended June 30, 2019 and 2018, respectively.
4. INTANGIBLE ASSETS
Intangible assets include intellectual properties and customer relationships relating to the Prolieve technology, acquired at a cost of $2.5 million. The intellectual properties expire over several years commencing September 2021 and continuing until February 2029. These assets are being amortized on a straight-line basis over ten years; amortization expense was $20,028 and $61,553 for the three months ended June 30, 2019 and 2018, respectively.
Future amortization expense related to the net carrying amount of intangible assets is estimated to be as follows:
| 2020 | | $ | 80,113 | |
| 2021 | | | 80,113 | |
| 2022 | | | 80,113 | |
| 2022 | | | 80,113 | |
| 2022 | | | 80,113 | |
| Thereafter | | | 380,534 | |
| Total | | $ | 781,099 | |
5. PROMISSORY NOTES PAYABLE, CONVERTIBLE NOTES PAYABLE AND ACCURED INTEREST PAYABLE
In fiscal year 2013, the Company raised bridge financing of approximately $435,000. The bridge financing lender received a promissory note, with interest payable at 2% per month after October 23, 2012. The original maturity date of the promissory note was October 23, 2013 and was subsequently extended until June 30, 2014 at which time the Company began paying additional interest of 2% per month on accrued interest with an additional interest charge of .09% per month on current interest expense. As of June 30, 2019, the note remains in default and is due in full. The Company has a total principal and accrued interest balance of approximately $1,174,655 and $1,092,000 as of June 30, 2019 and March 31, 2019, respectively. Interest expense of approximately $58,000 and $55,000 was recognized on the promissory note and accrued interest for the three-month period ended June 30, 2019 and March 31, 2019, respectively.
In fiscal year 2014, the Company issued, in two separate tranches, 554 units of 8% redeemable promissory convertible notes (the “Notes”) together with Series C stock purchase warrants (the “Warrants”) to various accredited investors in an offering exempt from registration in the U.S pursuant to regulations D and S under the U.S. Federal Securities rules and regulations, receiving gross proceeds of $5,540,000. The notes are convertible into 22,160,000 shares of common stock. Each warrant entitled the holder to acquire 20,000 common shares (for a total of 11,080,000 common shares) at an exercise price of $0.30 per share and expired on December 18, 2016 and March 7, 2017. The warrants were classified as equity, were recorded as additional paid in capital at their estimated fair value of $1,532,877, and were considered a non-cash financing activity. The Company recognized a beneficial conversion feature of $195,938 and deferred financing fees (consisting of both cash payments and the fair value of stock purchase warrants classified as equity) of $558,552 which were fully amortized using the effective interest method through the fiscal year ended March 31, 2017. The Company has accrued interest of $2,582,408 owing to holders of the convertible debentures as of June 30, 2019, of which $2,425,694 is past due.
During the year ending March 31, 2018, the company settled $130,000 of the convertible notes and $24,514 of accrued interest at a settlement price of CAD $0.05 ($0.04) per share. The fair value of the stock price was $0.032 per share on the date of the settlement; therefore, per the guidance ASC 470-60, “Trouble Debt Restructuring by Debtors”, the Company recognized a gain of $30,705 for the year ending March 31, 2018. Additionally, the Company recorded a common stock issuable in the amount of $123,809 for 3,862,850 shares. During the year March 31, 2019, additional interest in the amount of $25,551 was granted to the note holders as their shares were not issued during the year ended March 31, 2019. The additional interest was settled for 890,884 shares issuable which had a fair value of $16,667, based on the fair value of $0.019. A gain of $8,885 was recorded related to the transaction. The remaining shareholders have not settled their debt as of June 30, 2019.
In connection with the convertible notes, the Company recognized interest expense of $156,714 and 144,779 for the three-month periods ended June 30, 2019 and 2018, respectively.
On May 13, 2016, the Company entered into a loan agreement in the amount of $200,000. The financing lender will receive interest payable of 1.25% per month. The maturity date for the outstanding amount was November 30, 2016 and default interest of 2% per month began accruing effective that date as the loan is in default. The Company recognized $12,000 and $12,000 in interest expense for the three-month periods ended June 30, 2019 and 2018, respectively. Accrued interest related to the loan balance is $139,442 and $127,442 as of June 30, 2019 and March 31, 2019, respectively. The loan is secured by the Company’s assets.
On August 1, 2016, the Company entered into a loan agreement in the amount of $200,000. The financing lender will receive interest payable of 1.25% per month. The maturity date for the outstanding amount was January 31, 2017 and default interest of 2% per month began accruing effective of that date as the loan is in default. The Company recognized $12,000 and $12,000 in interest expense for the three-month periods ended June 30, 2019 and 2018, respectively. Accrued interest related to the loan balance is $130,678 and $118,678 as of June 30, 2019 and March 31, 2019, respectively. The loan is secured by the Company’s assets.
On October 30, 2016, the Company entered into a loan agreement in the amount of $100,000. The financing lender will receive interest payable of 1.25% per month. The maturity date for the outstanding amount is April 30, 2017 and default interest of 2% per month began accruing effective of that date as the loan is in default. The Company recognized $6,000 and $6,000 in interest expense for the three-month periods ended June 30, 2019 and 2018, respectively. Accrued interest related to the loan balance is $59,698 and $53,698 as of June 30, 2019 and March 31, 2019, respectively. The loan is secured by the Company’s assets.
6. EQUITY AND STOCK-BASED COMPENSATION
Common Stock
Authorized share capital consists of unlimited common shares with no par value.
On December 14, 2015, the Company completed a private placement of 15,500,000 units at a price of $0.05 per unit raising gross proceeds of $775,000 (net proceeds of $713,000). Each unit consisted of one common share and 0.5 common share purchase warrant. Each whole warrant entitled the holder to acquire one common share at an exercise price of $0.10 until December 14, 2017. As of March 31, 2018, these warrants are expired.
Management determined the warrants to have a fair value of $0.015 per warrant and accordingly, $176,169 of the proceeds from the issuance was allocated to additional paid in capital, and the balance of the proceeds was allocated to common shares.
A relative fair value calculation was used to determine the carrying value of the warrants. The fair value of the warrants issued was estimated using a Black-Scholes pricing model with the following assumptions:
| Risk free interest rate | | | 0.58% to 0.97 | % |
| Expected life in years | | | 2 - 3 years | |
| Expected volatility | | | 98.96 to 146.6 | % |
The Company uses the contract life as its expected life. Volatility is calculated based on actual weekly trading history of the Company’s common stock. The risk-free rate is based on the daily yield curve of U.S. treasury bills.
Common stock issuable
Prior to March 31, 2015, the Company received funds for common shares in the amount of $1,561,000 (net of fees) as part of a future private placement occurring on May 12, 2015. All shares were issued during the year ended March 31, 2016. During the year ended March 31, 2016 the Company resolved to pay accrued expenses with common stock in the amount of $140,452.
As of March 31, 2018, the Company has a total of shares issuable of 3,862,850 in the amount of $123,809 at a settlement price of CAD $0.05 ($0.04), as a result of an agreement to settle convertible debt of $130,000 plus accrued interest. As of March 31, 2019, additional 890,884 shares issuable, in the amount of $16,667 at a settlement price of CAD $0.05 ($0.04) were granted to the debt holders. As of June 30, 2019, the shares have not been issued.
As of March 31, 2019, the Company agreed to settle CA$400,000 ($304,923) of debt with the directors and the CFO for has a total of shares issuable of 8,000,000 which is included as common stock issuable in the Company’s unaudited condensed interim consolidated statements of changes in stockholders deficit. As of June 30, 2019, the shares have not been issued.
Stock Purchase Warrants
The Company did not have any stock purchase warrants outstanding as of June 30, 2019 and March 31, 2019:
Stock Options and Stock Option Plan
The purpose of the stock option plan (“the Plan”) is to attract, retain and motivate persons as key service providers to the Company and to advance the interests of the Company by providing such persons with the opportunity, through share options, to acquire a proprietary interest in the Company and benefit from its growth. The options are non-assignable and may be granted for a term not exceeding five years.
The Company may grant stock options to directors, senior officers and service providers by resolution of the Board of Directors. The exercise price will reflect the market price of the Company’s stock on the date of the grant. The maximum number of stock options outstanding under the Plan is limited 10% of issued shares.
During the year ending March 31, 2016, the Company issued 10,100,000 options to directors and officers of the Company which vested immediately upon issuance. The Company recorded $183,410 in stock compensation expense related to these options.
During the year ended March 31, 2018, the Company issued 4,200,000 shares of stock options to the Company’s CEO with an exercise price of CAD$0.05 ($0.04) per share. The options vested immediately and have an expected life of 5 years. The Company recorded $85,294 in stock compensation expense related to these options.
No stock-based compensation cost was recorded for the three months ending June 30, 2019 and 2018.
The Company measures the cost of stock option awards based on the grant-date fair value of the award. The cost is recognized over the period during which an employee is required to provide service in exchange for the award or the requisite service period. The Company recognizes stock-based compensation expense over the vesting periods of the awards, adjusted for estimated forfeitures. A summary of the stock option activity for the three month periods ended June 30, 2019 and 2018 is presented below:
| | | | | | Weighted average | | | Weighted Average Remaining | | | Aggregate | |
| | | Option Shares | | | exercise price | | | Contractual Life | | | Intrinsic Value | |
| | | # | | | $ | | | (years) | | | $ | |
| Outstanding April 1, 2018 | | | 10,700,000 | | | | 0.051 | | | | 3.43 | | | $ | — | |
| Granted | | | — | | | | — | | | | — | | | | | |
| Exercised | | | — | | | | — | | | | — | | | | | |
| Cancelled/Expired | | | — | | | | — | | | | — | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding, March, 31, 2019 | | | 10,700,000 | | | $ | 0.051 | | | | 3.18 | | | $ | — | |
| | | | | | | | | | | | | | | | | |
| Outstanding April 1, 2018 | | | 10,700,000 | | | $ | 0.051 | | | | 2.43 | | | | | |
| Granted | | | — | | | | — | | | | — | | | $ | — | |
| Exercised | | | — | | | | — | | | | — | | | | | |
| Cancelled/Expired | | | — | | | | — | | | | — | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding, March, 31, 2019 | | | 10,700,000 | | | $ | 0.051 | | | | 2.18 | | | $ | — | |
| | | | | | | | | | | | | | | | | |
| Exercisable, March 31, 2019 | | | 10,700,000 | | | | | | | | | | | | | |
The weighted average fair value of the option grants issued in fiscal year 2016 was $0.018 per share. The option grants were estimated on the date of the grant using the Black-Scholes option pricing model and using the following assumptions:
| Expected option life (years) | | | 2.5 years | |
| Risk free interest rate | | | 1.12 | % |
| Expected volatility | | | 106 | % |
The weighted average fair value of the option grants issued in fiscal year 2018 was $0.020 per share. The option grants were estimated on the date of the grant using the Black-Scholes option pricing model and using the following assumptions:
| Expected option life (years) | | | 5 years | |
| Risk free interest rate | | | 1.56 | % |
| Expected volatility | | | 122 | % |
7. INCOME TAXES
The Company is domiciled in Canada and files Canadian federal and certain provincial tax returns. The Company had no provision (benefit) for income taxes for the years ending March 31, 2019, 2018 and 2017 as a result of its net losses and full valuation allowance against its deferred assets. There are no significant changes as of June 30, 2019.
The following is the statutory rates which apply to the Company
| | | For the years ending March 31, | |
| | | 2019 | | | 2018 | | | 2017 | |
| Income Tax Statutory Income Tax Rates | | | | | | | | | | | | |
| United States | | | 21.00 | % | | | 21.00 | % | | | 35.00 | % |
| Canada | | | 26.50 | % | | | 26.50 | % | | | 26.50 | % |
The reconciliation of income taxes at statutory income tax rates (Canada - 26.5%) to the income tax expense is as follows:
| | | For the years ending March 31, | |
| | | 2019 | | | 2018 | | | 2017 | |
| Loss before income taxes | | $ | (1,471,000 | ) | | $ | (1,541,000 | ) | | $ | (1,570,000 | ) |
| Expected income recovery based on statutory rate | | | (390,000 | ) | | | (408,000 | ) | | | (416,000 | ) |
| Adjustment to expected income tax benefit | | | | | | | | | | | | |
| Stock based compensation | | | - | | | | 23,000 | | | | - | |
| Permanent differences | | | 2,000 | | | | 2,000 | | | | 3,000 | |
| Change in benefit of tax assets not recognized | | | 388,000 | | | | 383,000 | | | | 413,000 | |
| Deferred income tax provision (recovery) | | | - | | | | - | | | | - | |
Deferred Income Tax
Deferred tax asset components as of March 31, 2019 and 2018 is as follows:
| | | As of March 31, | |
| | | 2019 | | | 2018 | |
| Non-capital loss carry-forwards | | $ | 21,730,000 | | | $ | 22,586,000 | |
| Equipment | | | 1,888,000 | | | | 1,769,000 | |
| Net deferred tax asset | | $ | 23,618,000 | | | $ | 24,355,000 | |
As the Company has recognized substantial cumulative losses from operations and has not earned significant revenues, it has provided a 100% valuation allowance on the net deferred tax asset as of March 31, 2019 and 2018. There are no significant changes as of June 30, 2019
The Company has non-capital losses which may, under certain circumstances, be applied against future taxable income and which expire as follows:
| 2026 | | $ | 110,000 | |
| 2027 | | | 11,000 | |
| 2028 | | | 33,000 | |
| 2029 | | | 5,000 | |
| 2030 | | | 1,209,000 | |
| 2031 | | | 1,537,000 | |
| 2032 | | | 1,073,000 | |
| 2033 | | | 3,047,000 | |
| 2034 | | | 3,800,000 | |
| 2035 | | | 4,556,000 | |
| 2036 | | | 3,969,000 | |
| 2037 | | | 685,000 | |
| 2038 | | | 629,000 | |
| 2038 | | | 1,086,000 | |
| | | $ | 21,730,000 | |
The Company is subject to income taxes in numerous jurisdictions. Significant judgment is required in evaluating the Company’s tax positions and determining its provision for income taxes. The Company establishes liabilities for tax-related uncertainties based on estimates of whether, and the extent to which, additional taxes will be due. These liabilities are established when the Company believes that certain positions might be challenged despite its belief that its tax return positions are fully supportable. The Company adjusts these liabilities in light of changing facts and circumstances, such as the outcome of a tax audit. The provision for income taxes includes the impact of changes to the liability that is considered appropriate. The Company has identified no material uncertain tax positions as of March 31, 2019 and 2018. There are no significant changes as of June 30, 2019.
The Company is subject to income tax audits in all jurisdictions for which it is required to file tax returns. Tax audits by their nature are often complex and can require several years to complete. Neither the Company nor any of its subsidiaries is currently under audit in any jurisdiction. All of the Company’s income tax returns remain subject to examination by tax authorities.
During the year ended March 31, 2017, the Company recorded $20,000 in tax penalties to the IRS for failure to timely file income tax returns for the prior years and is included in other income (expense) on the consolidated statements of operations and comprehensive loss. No penalties were recorded for either fiscal 2019 or 2018. No penalties were recorded for the 3 months ended June 30, 2019.
8. COMMITMENTS AND CONTINGENCIES
On January 16, 2006, the Company’s wholly owned subsidiary, Celsion (Canada) Inc. purchased from Celsion Corporation(USA) [“Celsion”]all of the assets relating to breast cancer Microfocus APA 1000 System (“System”), consisting of the microwave machine technology, the APA technology licensed from MIT, and all related intellectual and regulatory property (collectively, the “Business”). The Company has a commitment to pay a 5% royalty to Celsion on the net sales of products sold by and patent royalties received by the Company and its successors and assignees. Total royalties paid are not to exceed $18,500,000. Royalties will not be payable until the System can be placed in the market following successful completion of the pivotal clinical trial and receipt of approval to market the System in the US and Canada from the FDA and Health Canada. The Company has an additional commitment to pay a 5% royalty to MIT on the net sales of products, upon commercialization. If the Company does not apply for or does not receive FDA approval to enter at least one phase III clinical trials of a licensed product prior to the earlier of the termination of the agreement or June 25, 2018, the Company shall pay $10,000 to MIT. If the Company receives approval for sale of at least one licensed product or discovery product then the Company shall pay MIT $100,000. As of March 31, 2019, this requirement has not been met and no payment is due.
On October 1, 2017, the Company entered into an amended 5-year operating lease agreement. All vehicle leases expired during year ending March 31, 2018. Future minimum payments under the operating lease for office space as of June 30, 2019 are as follows:
| 2020 | | $ | 86,801 | |
| 2021 | | $ | 89,416 | |
| 2022 | | $ | 92,093 | |
| 2023 | | $ | 62,604 | |
The Company recognized total rent expense of $30,567 and $27,373 for the three-month periods ended June 30, 2019 and 2018, respectively.
The Company has a purchase commitment with its primary vendor in which to purchase 2,160 Prolieve Kits at $286 for a total amount of $617,716 through December 31, 2019.
During the year ended March 31, 2019, the Company entered into a research and development project agreement with Urobois Limited. The Company has paid $5.000 to Urobois Limited will make milestone payments to Urobois Limited in the amount of $20,000 through the completion of the agreement. The project is currently put on hold.
In June 2018, W.L. Pate, JR and Charles C. Shelton filed a lawsuit in the District Court of Harris County, Texas to seek monetary relief of over $200,000 but not more than $1,000,000 from Medifocus Inc. for a transaction that did not materialize. Although the Company does not believe the suit has any merits and has not accrued for any amount in its financial statements as of June 30, 2019, any judgement unfavorable to the Company can potentially cause significant financial hardship and other damages to the Company.
9. RELATED PARTY TRANSACTIONS
The Company has entered into several transactions with a director and an officer. Descriptions of the related party transactions are as follows:
| | ● | The Company made direct revenue sales to Dr. William Jow, the Company’s CEO effective October 1, 2016, in the amount of $0 and $7,000 during the three-month periods ending June 30, 2019 and 2018, respectively. There was a trade receivable balance of $0 as of June 30, 2019 and March 31, 2019, respectively, related to these transactions. As of June 30, 2019, and March 31, 2019, respectively, the Company has an outstanding liability to Dr. William Jow in the amount of $33,000 and $16,500 for compensation and expense reimbursements. See also Note 6 for stock options issued to the CEO during the year ending March 31, 2018. |
| | | |
| | ● | The Company made no sales to Medifocus Asia, Ltd., during the three month periods ended June 30, 2019 and 2018, respectively. There was a trade receivable balance of approximately $0 as of June 30, 2019 and March 31, 2019. Mr. Augustine Chow and Mr. Raymond Tong, both directors of Medifocus, Inc, are also directors of Medifocus Asia, Ltd. Additionally, Mr. Augustine Chow and Mr. Raymond Tong have significant investments in Medifocus Asia, Ltd. |
| | | |
| | ● | The Company has accrued compensation expenses owed to the CFO and the board of directors as of June 30, 2019 and 2018 as follows: The amounts are unsecured, due on demand and bear no interest. |
| | | CFO | | | Directors | |
| March 31, 2019 | | $ | 66,651 | | | $ | 235,636 | |
| June 30, 2019 | | $ | 71,688 | | | $ | 247,729 | |
| | ● | During the year ended March 31, 2018, the Company settled a $50,000 convertible debt and $16,304 in related accrued interest charges due to Douglas Liu, Vice President of Finance, for 1,657,595 shares issuable. During the year ended March 31, 2019 an additional 325,157 shares issuable were granted for additional interest costs. There was no activity during the period ending June 30, 2019. |
| | | |
| | ● | Augustine Chow, a director of Medifocus Inc, is also a director of Gwyneth Gold Limited which has a substantial investment in the Company. Additionally, Gwyneth Gold Limited is the holder of a $790,000 convertible note with the company and is owed $434,702 and $410,687 in accrued interest as of June 30, 2019 and March 31, 2019. |
| | | |
| | ● | During the year ended March 31, 2019, the directors and CFO settled CA$400,000 ($304,923) of debt for 8 million shares issuable. |
10. RECLASSIFICATIONS
Certain reclassifications were made to the three-month period ending June 30, 2018, to make them comparable to the June 30, 2019 financial statements.
Medifocus, Inc. Announces Increase in Revenue and Positive Cash Flow from
Operation in Fiscal Year Ended March 31, 2019 and Global Market Expansion
with Prolieve® Procedures Performed in Singapore
COLUMBIA, MD and Toronto, ON- August 29, 2019 – Medifocus, Inc. (OTCQB: MDFZF and TSXV: MFS) (“Medifocus” or the “Company”), a biotechnology company with a portfolio of medical products encompassing thermotherapy systems for the treatment of Benign Prostatic Hyperplasia (BPH) and Breast Cancer, reported a 4% increase in revenue and a 15% decrease in operating expenses for the fiscal year ended March 31, 2019, compared to the same period in 2018.
Prolieve®sales were $2,766,740 for the year ended March 31, 2019, compared to $2,666,800 for the previous fiscal year, while the operating expenses for the recent fiscal year were reduced by 15% to $1,358,208, compared to $1,593,789 for the previous year. During the fiscal year ended March 31, 2019, the Company also recorded a $133,041 positive cash flow from operating activities, compared to $21,544 for the year ended March 31, 2018.
Dr. William Jow, President and Chief Executive Officer of Medifocus, commented, “Since I became CEO less than three years ago, we have substantially reduced operating losses by improving operational effectiveness. Despite costly sales and marketing campaigns launched by our competitors, we have managed to convince the urologists that Prolieve is proven safe and effective confirmed by our recently completed and approved FDA 5-year Follow-up Study. We are very pleased to see a small but meaningful uptick in revenue from Prolieve®operation in the last fiscal year and to continue achieving positive cash flow from operating activities.”
Dr. Jow continued, “With Prolieve®procedures recently performed in Singapore after Hong Kong, we anticipate a boost in our future Prolieve® sales. Thanks to collaborations with our Asian partners, we continue to look forward to seeing more Asian and Global markets open up for Prolieve® in the near future.”
“We will immediately rebrand Prolieve®as the only ThermoDilatation™ (TUTD™) that can treat and prevent the progression of BPH with stabilization of Prostatic-specific Antigen (PSA) and prostate volume as evidenced by our FDA Post Approval Study.” He added, “Furthermore, we can now expand other utilizations of Prolieve®, especially by offering a proven-safe treatment option for men who elect to undergo Active Surveillance for early prostate cancer, which has become a huge marketplace with great potential in recent years.”
About the Prolieve® Thermodilatation™ System
The Prolieve® Thermodilatation™ System offers potential relief to the millions of men who suffer from Benign Prostatic Hyperplasia (BPH), a condition that becomes common as men age. Approximately 50% of men over 50 have significant enlargement of the prostate gland, and this rises to about 90% among those over 70 years old. As the prostate enlarges, it constricts the urethra, thereby restricting the passage of urine. Persistent restriction and further progression of BPH, if left untreated, often results in worsening of lower urinary tract symptoms (LUTS). Further complications of this process may result in urinary retention, bladder stones and infection, as well as compromised bladder and kidney function.
The Prolieve® System is a novel focused heat therapy which utilizes a unique combination of focused heat energy directed at the prostate in combination with a patented, water cooled and pressurized dilatation balloon to achieve immediate and long-term relief of BPH symptoms with very minimal treatment related side effects after a brief in-office procedure performed under local anesthesia. The unique advantage of this combined “heat plus compression” therapy is twofold: first, the heat denatures local tissue proteins of the (balloon) dilated urethra, resulting in an expanded and stiffened urethral lumen functioning as a biological stent. Second, the compression reduces local blood flow thus increasing the thermal efficiency of the microwave energy to achieve apoptosis and tissue ablation in the targeted prostate gland. As the transurethral microwave energy is applied to the entire prostate, a computerized feedback system involving the rectal temperature probe renders the rectal temperature not to exceed 41-42◦C, thus ensuring safety of the rectal wall and neurovascular bundles essential to preservation of erectile function.
About Medifocus, Inc.:
Medifocus, Inc. (TSXV-MFS, OTC-MDFZF) is a Biotechnology Company with a portfolio of medical technologies that utilize patented Focal Thermal Technology to treat conditions ranging from Prostate Diseases to Breast Cancer. Its Prolieve® Thermodilatation™ System offers symptomatic relief to men with Benign Prostatic Hyperplasia (BPH) through a simple, fast, in-office treatment. Prolieve® is both FDA and Medicare approved for treating symptomatic BPH with over 100,000 cases performed in the U.S. alone, and with proven long-term safety, efficacy and durability. Its APA 1000 Breast Cancer Treatment System was licensed from Massachusetts Institute of Technology and developed by the Medifocus team. The Targeted Focal Thermotherapy has been demonstrated in Phase 2 clinical trials to offer significantly better tumor shrinkage in patients treated with the combined Chemothermal therapy compared those treated with Chemotherapy alone. APA 1000 was also shown to be effective in reducing margin positivity among patients treated with such thermotherapy prior to lumpectomy.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
For more details, please visit:
www.medifocusinc.com
www.prolieve.com
www.facebook.com/pages/Medifocus-Inc-Company-Page/546315028715627



