UNITED STATESSECURITIES AND EXCHANGE COMMISSION
| o | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| o | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 001-36575
MACROCURE LTD.
(Exact name of Registrant as specified in its charter)
ISRAEL
(Jurisdiction of incorporation or organization)
25 Hasivim Street
Petach Tikva 4959383, Israel
(Address of principal executive offices)
Mark Page
Chief Financial Officer
Telephone: +972-3-923-5556
E-mail: mark.page@macrocure.com
Macrocure Ltd.
25 Hasivim Street
Petach Tikva 4959383, Israel
(Name, telephone, e-mail and/or facsimile number and address of company contact person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | Name of each exchange on which registered |
| Ordinary shares, par value NIS 0.01 per share | | NASDAQ Global Market |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None.
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None.
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report: As of December 31, 2014, the registrant had outstanding 16,262,465 ordinary shares, par value NIS 0.01 per share.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated file, or a non-accelerated filer. See the definitions of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer o | Accelerated filer o | Non-accelerated filer x |
Indicate by check mark which basis for accounting the registrant has used to prepare the financing statements included in this filing:
U.S. GAAP o | International Financial Reporting Standards as issued by the International Accounting Standards Board x | Other o |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
o Item 17 o Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
MACROCURE LTD.
FORM 20-F
ANNUAL REPORT FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014
TABLE OF CONTENTS
| i |
| ii |
| ii |
| PART I |
| 1 |
| 1 |
| 1 |
| 34 |
| 64 |
| 64 |
| 78 |
| 99 |
| 103 |
| 105 |
| 105 |
| 119 |
| 120 |
| PART II |
| 120 |
| 120 |
| 121 |
| 121 |
| 121 |
| |
| |
| |
| 122 |
| 122 |
| 122 |
| PART III |
| 122 |
| 122 |
| 123 |
| 124 |
| Index to Consolidated Financial Statements | F-1 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain information included or incorporated by reference in this annual report on Form 20-F may be deemed to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often characterized by the use of forward-looking terminology such as “may,” “will,” “expect,” “anticipate,” “estimate,” “continue,” “believe,” “should,” “intend,” “project” or other similar words, but are not the only way these statements are identified.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future. The statements that we make regarding the following matters are forward-looking by their nature:
| | · | the timing and conduct of our trials of CureXcell, including statements regarding the timing, progress and results of current clinical trials, and our research and development programs; |
| | · | the clinical utility, potential advantages and timing or likelihood of regulatory filings and approvals of CureXcell; |
| | · | our estimates regarding the market opportunity for CureXcell; |
| | · | our plans for laying the groundwork for commercialization of CureXcell in the United States and elsewhere; |
| | · | our plans to develop a platform for development and commercialization of a broader range of regenerative medicine products; |
| | · | our strategy for expanding our geographical coverage and penetrating additional global markets; |
| | · | our estimates regarding expenses, future revenue, capital requirements and the need for additional financing; |
| | · | the impact of our research and development expenses as we continue developing CureXcell and additional regenerative medicine products; |
| | · | our ability to obtain and maintain adequate intellectual property rights and adequately protect and enforce such rights; |
| | · | our expectations regarding the time during that we will be an emerging growth company under the Jumpstart Our Business Startups, or JOBS, Act; |
| | · | the impact of government laws and regulations; and |
| | · | our expectations regarding the use of proceeds from our IPO. |
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate.
Readers are urged to carefully review and consider the various disclosures made throughout this annual report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects. In particular, please see the factors described in Item 3.D “Key Information - Risk Factors”, Item 4 “Information on the Company”, and Item 5 “Operating and Financial Review and Prospects”.
Any forward-looking statements in this annual report are made as of the date hereof, and we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
USE OF DATA AND TRADEMARKS
This annual report includes statistical data, market data and other industry data and forecasts, which we obtained from market research, publicly available information and independent industry publications and reports that we believe to be reliable sources. We have proprietary rights to our “CureXcell” trademark, which is registered under applicable intellectual property laws. Solely for convenience, our “CureXcell” trademark may appear without the “®” or “™” symbols in this annual report, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights to this trademark. We do not intend our use or display of other companies’ tradenames, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other company. Each trademark, tradename or service mark of any other company appearing in this annual report is the property of its respective holder.
| | CERTAIN ADDITIONAL TERMS AND CONVENTIONS |
In this annual report, unless the context otherwise requires:
| | • | references to “Macrocure,” “our company,” “the Company,” “the registrant,” “we,” “us,” and “our” refer to Macrocure Ltd.; |
| | • | references to “ordinary shares”, “our shares” and similar expressions refer to the Company’s Ordinary Shares, par value NIS 0.01 per share; |
| | • | references to “dollars”, “U.S. dollars”, “U.S. $” and “$” are to United States Dollars; |
| | • | references to “Euro” or “€”are to the Euro, the official currency of the Eurozone in the European Union; |
| | • | references to “shekels” and “NIS” are to New Israeli Shekels, the Israeli currency; |
| | • | references to “IFRS” are to the International Financial Reporting Standards, as issued by the International Accounting Standards Board, or “IASB”; |
| | • | references to the “articles” are to our amended and restated articles of association, as currently in effect; |
| | • | references to the “Companies Law” are to the Israeli Companies Law, 5759-1999, as amended; |
| | • | references to the “Securities Act” are to the Securities Act of 1933, as amended; |
| | • | references to the “Exchange Act” are to the Securities Exchange Act of 1934, as amended; |
| | • | references to “NASDAQ” are to the NASDAQ Stock Market; |
| | • | references to the “SEC” are to the United States Securities and Exchange Commission; |
| | • | references to the “IPO” are to the initial public offering of our ordinary shares in the United States, which was consummated on August 5, 2014; and |
| | • | references to the “IPO Prospectus” are to the final prospectus for the IPO, dated July 30, 2014, that we filed with the SEC pursuant to Securities Act Rule 424(b)(4) on July 31, 2014. |
Item 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Item 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
| | A. | Selected Financial Data |
Our historical consolidated financial statements are prepared in accordance with IFRS as issued by the IASB and are presented in U.S. dollars. The selected historical consolidated financial information as of December 31, 2014 and 2013 and for each of the three years ended December 31, 2014, 2013 and 2012 have been derived from, and should be read in conjunction with, our audited financial statements and the notes thereto appearing elsewhere in this annual report. The selected financial data as of December 31, 2012 has been derived from our audited financial statements not included in this annual report. Selected consolidated financial information as of, and for the years ended, December 31, 2011 and 2010 have been omitted from this annual report because of our status as an emerging growth company under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and as per related guidance provided by the SEC.
The information presented below is qualified by the more detailed historical consolidated financial statements set forth in this annual report, and should be read in conjunction with those consolidated financial statements, the notes thereto and the discussion under “Item 5 - Operating and Financial Review and Prospects” included elsewhere in this annual report.
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | | | 2012 | |
| | | (in thousands except share and per share data) |
| Consolidated Statements of Loss Data: | | | | | | | | | |
| Operating expenses(1): | | | | | | | | | |
| Research and development expenses, net | | $ | 15,542 | | | $ | 9,303 | | | $ | 7,168 | |
| General and administrative expenses | | | 5,374 | | | | 4,567 | | | | 1,631 | |
| Operating loss | | | (20,916 | ) | | | (13,870 | ) | | | (8,799 | ) |
| Financing income (expenses), net | | | (4,504 | ) | | | (4,305 | ) | | | 1,043 | |
| Loss before income tax | | | (25,420 | ) | | | (18,175 | ) | | | (7,756 | ) |
| Taxes on income | | | (31 | ) | | | (149 | ) | | | -- | |
| Loss for the year | | $ | (25,451 | ) | | $ | (18,324 | ) | | $ | (7,756 | ) |
| Net loss per share(basic and diluted) | | $ | (2.15 | ) | | $ | (2.46 | ) | | $ | (1.05 | ) |
| Other comprehensive loss | | | (26 | ) | | | - | | | | - | |
| Total comprehensive loss | | $ | (25,477 | ) | | | - | | | | - | |
| Weighted average number of ordinary shares used in computing loss per share, basic and diluted | | | 11,863,372 | | | | 7,444,042 | | | | 7,421,088 | |
| | | As of December 31, | |
| | | 2014 | | | 2013 | | | 2012 | |
| | | (in thousands) | |
| Consolidated Statements of Financial Position Data: | | | | | | | | | |
| Cash and cash equivalents | | $ | 10,868 | | | $ | 18,995 | | | $ | 15,322 | |
| Working capital (2) | | | 44,229 | | | | 17,593 | | | | 14,510 | |
| Total assets | | | 48,699 | | | | 20,738 | | | | 17,709 | |
| Total current liabilities | | | 2,488 | | | | 1,971 | | | | 1,499 | |
| Total non-current liabilities | | | - | | | | - | | | | 3,114 | |
| Total shareholders’ equity | | | 46,211 | | | | 18,767 | | | | 13,096 | |
| (1) | Includes share-based compensation expense as follows: |
| | | Year Ended December 31, | |
| | | 2014 | | | 2013 | | | 2012 | |
| | | (in thousands) | |
| | | |
| Research and development expenses, net | | $ | 452 | | | $ | 0 | | | $ | 210 | |
| General and administrative expenses | | | 1,211 | | | | 2,648 | | | | 31 | |
| Total share-based compensation expenses | | $ | 1,663 | | | $ | 2,648 | | | $ | 241 | |
| (2) | We define working capital as total current assets minus total current liabilities. |
| | B. | Capitalization and Indebtedness |
| | C. | Reasons for the Offer and Use of Proceeds |
Investing in our ordinary shares involves a high degree of risk. You should carefully consider the risks and uncertainties described below, in addition to the other information set forth in this annual report, including the consolidated financial statements and the related notes included elsewhere in this annual report, before purchasing our ordinary shares. If any of the following risks actually occurs, our business, financial condition, cash flows and results of operations could be materially adversely affected. In that case, the trading price of our ordinary shares would likely decline and you might lose all or part of your investment. The risks described below are not the only risks we face. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially adversely affect our business operations.
Risks Relating to Our Business and Industry
We depend on the success of CureXcell, our only product candidate. We may be unable to obtain approval of CureXcell for treatment of diabetic foot ulcers or venous leg ulcers in the United States and other markets.
At the present time, our focus is on obtaining regulatory approval for CureXcell, our only current product candidate. Our success depends on our ability to obtain regulatory approvals for CureXcell in the United States and in other markets. In order to support a Biologics License Application, or BLA, submission to the U.S. Food and Drug Administration, or the FDA, we are conducting two pivotal Phase 3 trials. We cannot predict whether these studies will be successful and, even if successful, whether the FDA will require additional studies before we make a BLA submission, how long the FDA will take to review CureXcell following our BLA submission or whether the FDA will ever approve our BLA submission. Similarly, we cannot predict how long regulatory authorities outside of the United States will take to provide CureXcell with marketing authorization in their jurisdictions or whether they will ever provide such authorizations. The grant of regulatory approval in one or more countries does not assure the grant of regulatory approval by other countries. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier clinical trials. The failure to receive such regulatory approvals, especially in the United States, would have a materially adverse impact on our business, financial condition, cash flows and results of operations.
Clinical failure can occur at any stage of clinical development. Because the results of earlier clinical trials do not necessarily predict future results, any product candidate we advance through clinical trials may not have favorable results in later clinical trials or receive regulatory approval.
Clinical failure can occur at any stage of clinical development. Clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical or preclinical trials. In addition, data obtained from trials are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval. Success in prior clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. In addition, the design of a clinical trial can determine whether its results will support approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. Clinical trials of potential products often reveal that it is not practical or feasible to continue development efforts. For example, if the results of our Phase 3 trials of CureXcell do not demonstrate significant efficacy, the prospects for approval of CureXcell would be materially adversely affected. Furthermore, if these trials fail to meet their primary statistical and clinical end points they will be disqualified for FDA approval in which case we would need to replace the failed study with a new Phase 3 trial, which would require significant additional capital, cause substantial delays in commercialization and materially adversely affect our business, financial condition, cash flows and results of operations.
Delays in the completion of our clinical trials could result in increased costs to us and delay or limit our ability to obtain regulatory approval for CureXcell.
Our two pivotal Phase 3 trials are currently ongoing. These clinical trials may be delayed or not be completed. Clinical trials may be delayed or may proceed less quickly than intended due to delays and difficulty in recruiting, enrolling and maintaining subjects. This may occur for a variety of reasons, including the failure to identify candidates that meet applicable enrollment criteria, competition from other clinical trial programs for the same indication as our product candidates and the inability to retain subjects in clinical trials due to the treatment protocol, personal issues, side effects or lack of efficacy. Our clinical trials may also be delayed if we suffer an interruption in the supply of CureXcell. In addition, one of our Phase 3 trials is in its early stages, and could be delayed if we fail to establish test sites and recruit physicians, patients and coordinators in the time frame we expect.
In addition, our Phase 3 clinical trials or any of our other clinical trials may be suspended or terminated by us, the FDA or other regulatory authorities due to a number of factors, including:
• failure to conduct the clinical trial in accordance with Good Clinical Practices, or GCP, regulatory requirements or our clinical protocols;
• failure to pass inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities;
• failure to comply with current Good Manufacturing Practices, or cGMP, or other applicable requirements;
• unforeseen safety issues or any determination that a clinical trial presents unacceptable health risks;
• changes in regulatory requirements and guidance; or
• lack of adequate funding to continue the clinical trial due to unforeseen costs resulting from enrollment delays, requirements to conduct additional trials and studies, increased expenses associated with the services of our contract research organizations, or CROs, and other third parties or other reasons.
Delays in the completion of our trials would correspondingly delay our ability to apply for and receive the regulatory approvals necessary to commercialize our product. Delays could also increase the costs associated with a particular trial. There is no assurance that we will have or be able to access the capital necessary to fund our operations for a longer period of time prior to commercialization or to fund increased costs for clinical trials. Accordingly, delays in the completion of our clinical trials would have a material adverse effect on our business, financial condition, cash flows and results of operations.
We may be required to complete additional Phase 3 trials in order to receive regulatory approval in the United States or in other countries.
In order to provide BLA approval for a proposed indication, the FDA typically requires two well-controlled Phase 3 trials that demonstrate safety and effectiveness for that specific indication. The FDA has indicated to us that one robust, well-controlled Phase 3 trial that demonstrates the safety and efficacy of CureXcell for each of the proposed indications (diabetic foot ulcers, or DFU, and venous leg ulcers, or VLU) may be sufficient for approval of a BLA for both of those indications; however, the FDA is under no obligation to grant approval under these circumstances and may require us to complete additional Phase 3 trials for one or both indications. Completing additional trials would involve considerable additional expense and would delay our ability to begin the commercialization of CureXcell, each of which would require substantial additional capital that we may not have or be able to access. In addition, it would prevent us from implementing and executing our business plan on a timely basis, which could harm our growth and prospects. Accordingly, a requirement that we complete additional Phase 3 clinical trials would have a material adverse effect on our business, financial condition, cash flows and results of operations. Further, even if the FDA does provide approval on the basis of our two planned Phase 3 trials, it is possible that regulators in other jurisdictions, including Europe, may require additional trials in order to grant approval.
We are a clinical stage biotechnology company with a history of operating losses. We expect to incur additional losses in the future and may never be profitable.
Since our inception in 2008, we have been focused on research and development and have not recognized any revenue. Additionally, we have incurred losses since inception, largely reflecting research and development and general and administrative expenses, and experienced net losses of $25.5 million, $18.3 million and $7.8 million in the years ended December 31, 2014, 2013 and 2012, respectively. As of December 31, 2014, we had an accumulated deficit of approximately $68.2 million. In addition to our current clinical trials in the United States, we may be required to conduct and successfully complete significant additional clinical trials and other tests and audits before we apply for regulatory approval for CureXcell in the United States, the European Union and other markets in order to commercialize CureXcell and start generating revenue. As a result, we anticipate that we will continue to incur significant additional losses and we may never be profitable or achieve revenue.
Our limited history makes it difficult to evaluate our business and prospects.
We have a limited operating history. While we have obtained regulatory approval from the Israeli Ministry of Health for certain applications of CureXcell as a medical device, we have only conducted limited sales of CureXcell in Israel for clinical purposes as part of our research and development activities. We have not fully commercialized CureXcell in any jurisdiction, and we have not received regulatory approvals for CureXcell as a biologic product from the FDA, the European Medicines Agency, or EMA, or other foreign regulatory bodies for any applications. There remains substantial doubt regarding whether we will receive the regulatory approvals necessary to sell CureXcell in the United States, Europe or elsewhere and whether we will successfully commercialize this product. Consequently, any predictions about our future performance may not be as accurate.
We may need substantial additional capital in the future, which could cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights, and if additional capital is not available, we may have to delay, reduce or cease operations.
To date, we have funded our operations primarily through private and public offerings of our securities and have not recognized any revenue. We expect to generate revenue primarily through sales of CureXcell. However, we still have to receive the approvals necessary to market this product in the United States, Europe and other jurisdictions outside of Israel. We expect this process will take at least several years to complete and to require substantial additional investment. In addition, before being able to market CureXcell, we would need to establish additional facilities to produce the product and hire and train a sales and marketing team, each of which would also require additional investment.
There are many factors that could cause us to need additional funding prior to product launch. These include that our estimates of the capital requirements necessary to complete these activities may have been too low; that we run into difficulties with our clinical trials that delay their completion or otherwise require additional investment; that we are required to complete additional clinical trials in order to obtain regulatory approvals; and that our regulatory approvals become delayed, thereby delaying our product launch. In addition, we will need to raise capital in order to commercialize CureXcell, including to build manufacturing facilities and establish a sales and marketing team.
In the event that these or other factors require that we obtain additional funding, we may not be able to raise this additional capital on reasonable terms or at all. Any additional funding may come from equity offerings, debt financings, collaborations, licensing arrangements or any other means. Further, securing additional financing may divert our management from our day-to-day activities, which may adversely affect our ability to develop and commercialize CureXcell.
If we are unable to raise additional capital when required or on acceptable terms, we may be required to:
• delay, scale back or discontinue the development or commercialization of CureXcell;
• seek corporate partners for CureXcell on terms that are less favorable than might otherwise be available; or
• relinquish or license on unfavorable terms our rights to CureXcell, which we otherwise would seek to develop or commercialize ourselves.
Any such consequence may have a material adverse effect on our business, operating results and prospects and on our ability to develop our pipeline products.
If we raise additional capital through the sale of equity or convertible debt securities, your ownership interest in us will be diluted, and the terms of the new equity securities may include liquidation or other preferences that adversely affect your rights as a shareholder. The incurrence of indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also impose restrictive covenants, such as limitations on our ability to incur additional debt or to issue additional equity, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. In addition, the issuance of additional equity securities by us, or the possibility of such issuance, may cause the market price of our ordinary shares to decline. In the event that we enter into collaborations or licensing arrangements in order to raise capital, we may be required to accept unfavorable terms, including relinquishing or licensing to a third party on unfavorable terms our rights to product candidates or intellectual property that we otherwise would seek to develop or commercialize ourselves or reserve for future potential arrangements when we might be able to achieve more favorable terms.
Development and commercialization of CureXcell requires our successful completion of the regulatory approval process as well as ongoing regulatory compliance, and may suffer delays or fail.
In the United States, the European Union and other markets, we will be required to apply for and receive marketing authorization before we can commercialize CureXcell. This process can be time consuming and complicated and may result in unanticipated delays. To secure marketing authorization, an applicant generally is required to submit an application that includes the data supporting preclinical and clinical safety and efficacy as well as detailed information on the manufacturing and control of the product, proposed labeling and additional information.
Before marketing authorization is granted, regulatory authorities generally require the inspection of the facilities and quality systems (including those of third parties) at which the product candidate is manufactured and tested to assess compliance with strictly enforced cGMP, as well as potential audits of the non-clinical and clinical trial sites that generated the data cited in the marketing authorization application. In addition, if a product is approved, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and record-keeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submission of safety and other post-marketing information and reports, registration and continued compliance with cGMP for any clinical trials that we conduct post-approval. Although our contract manufacturer’s facility in Israel is cGMP-certified, as is the manufacturing facility of the American Red Cross, our outsourced manufacturer in the United States, we may face difficulties in obtaining regulatory approval for any new manufacturing and quality control facilities we construct in order to commercialize our product.
We cannot predict how long the applicable regulatory authority or agency in any given jurisdiction will take to grant marketing authorization or whether any such authorizations will ultimately be granted. Regulatory agencies, including the FDA and the EMA, have substantial discretion in the approval process, and the approval process and the requirements governing clinical trials vary from country to country. The policies of the FDA, EMA or other regulatory authorities may change or may not be explicit, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of CureXcell. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States, Europe or elsewhere. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
Any delays or failures in obtaining or maintaining regulatory and marketing approval for CureXcell would adversely affect our business, prospects, financial condition and results of operations.
We have no experience in marketing or distributing products and no internal capability to do so, and are therefore subject to certain risks in relation to the commercialization of CureXcell.
��
We have not yet established a commercial organization for the marketing, sales and distribution of our single product candidate, CureXcell. Therefore, even if we receive approval to market CureXcell in the United States or other markets, in order to successfully commercialize CureXcell, we will need to build our marketing, sales, distribution, managerial and other non-technical capabilities. This involves many challenges, such as recruiting and retaining talented personnel; training employees; setting the appropriate system of incentives; managing additional headcount; and integrating new business units into an existing corporate infrastructure. The development of our own sales infrastructure will involve substantial expense, much of which we will incur well in advance of any marketing or sales. Moreover, we do not have experience as a company in establishing a significant sales infrastructure, and we cannot be certain that we will successfully develop this capability. We will have to compete with other pharmaceutical, biotechnology and wound care companies to recruit, hire, train and retain personnel for medical affairs, marketing and sales. If we fail to establish an effective sales and marketing infrastructure, we will be unable to successfully commercialize CureXcell, which in turn would have a material adverse effect on our business, financial condition and results of operations.
The commercial success of CureXcell will depend upon its degree of market acceptance.
Even if it successfully obtains marketing approvals, CureXcell may not gain market acceptance by physicians and their teams, healthcare payers and others in the medical community. If CureXcell does not achieve an adequate level of acceptance, we may not generate sufficient revenue to achieve or sustain profitability. The degree of market acceptance of CureXcell, if we receive marketing approval, will depend on a number of factors, some of which are beyond our control, including:
• the strength of our clinical data;
• the willingness of physicians to administer our product and their acceptance of it as part of the medical department routine, which itself may be influenced by various factors including ease of use and the compensation that doctors receive for administering CureXcell relative to other alternative treatments;
• the perceived risks of using a product derived from human blood;
• our success in obtaining third-party coverage or reimbursement for CureXcell;
• our ability to offer CureXcell for sale at a competitive price;
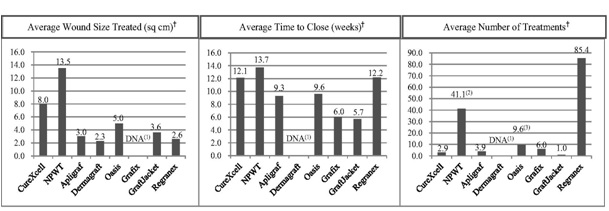
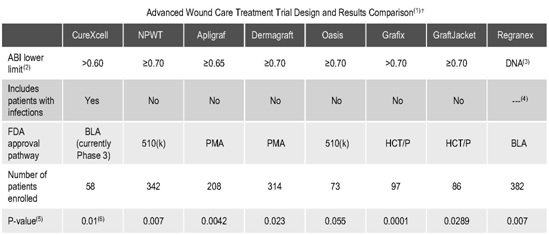
• the efficacy and potential advantages of CureXcell relative to other advanced wound care products;
• the prevalence and severity of side effects, if any;
• our reputation and the reputation of CureXcell;
• the efficacy, potential advantages and timing of introduction to the market of alternative treatments;
• the shelf life of CureXcell and our ability to manage the logistics of the end user supply chain; and
• our ability to reliably source sufficient raw materials and produce sufficient amounts of final product.
Failure to achieve market acceptance for CureXcell, if it is approved for commercial sale, would have a material adverse effect on our business, financial condition and results of operations.
We must receive adequate reimbursement policies for our product and the physicians that administer it in order to successfully commercialize CureXcell.
Should we receive the approvals necessary to market CureXcell, we will still need to apply to government and other third-party payers for them to reimburse physicians and patients to administer and use our product. Newly-approved healthcare products face significant uncertainty regarding both whether they are covered and their levels of reimbursement. Government and other healthcare payers, including Medicare, are increasingly attempting to contain healthcare costs by limiting both coverage and reimbursement levels. Even if CureXcell is approved by regulators, third-party payers may decline to cover it or may offer reimbursement rates that are insufficient to cover our cost to supply CureXcell or that otherwise fail to provide the revenue we expect to receive for the product. They may also set reimbursement rates for physicians who administer the product that are insufficient to cover the physicians’ costs or otherwise provide them with a disincentive to prescribe it. Further, once coverage and reimbursement rates are established, they may be changed or withdrawn in the future. The failure of government and other healthcare payers to cover or provide adequate reimbursement levels for CureXcell, could reduce its market acceptance, limit our growth and cause our revenue and results of operations to suffer. Further, delays in establishing coverage and reimbursement would delay the commercialization of our product, which would adversely affect our growth, operating results and financial position.
Prices in many countries, including many in Europe, are subject to local regulation and certain biological products, such as blood-derived products, are subject to price controls in several of the world’s principal markets, including many countries within the European Union. In these jurisdictions, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay or prevent our commercial launch of the product and negatively impact the revenue, if any, we are able to generate from the sale of the product in that country. The existence of direct and indirect price controls and pressures over our products could materially adversely affect our financial prospects and performance.
Future changes in government regulation or in the administration of government healthcare programs could adversely affect our commercialization of our products.
The United States and several other jurisdictions are considering, and have already enacted, a number of legislative and regulatory proposals to change the healthcare system in ways that may affect our current and future clinical trials and commercial sales, if and when CureXcell receives marketing approval. The continuing efforts of governments, insurance companies, managed care organizations and other payers of healthcare services to contain or reduce costs of healthcare may adversely affect:
• the market acceptance or demand for CureXcell;
• the ability to set a price that we believe is fair for CureXcell;
• our ability to generate revenue and achieve or maintain profitability;
• the level of taxes that we are required to pay; and
• the availability of capital.
We rely on a limited number of suppliers of whole blood in order to manufacture CureXcell.
Our primary raw material for producing CureXcell is whole blood. We purchase it from the American Red Cross and currently, in Israel, we purchase it from Magen David Adom, or MDA, Israel’s national blood bank. Our reliance on a limited number of suppliers involves risks. Specifically, our contract with the American Red Cross requires that we notify and seek pre-approval for significant changes to our manufacturing process, suppliers or operating procedures. This arrangement could delay our ability to make adjustments to our business, which could negatively impact our operations. Additionally, because of the short shelf life of our product, we do not have a banked inventory to use in the event of an interruption in supply. Accordingly, if our supplier in a given region becomes temporarily unable or unwilling to provide us with whole blood, we would be unable to produce CureXcell until this interruption ceases or we obtain an alternate supply. Further, if we were to permanently lose a supplier it may take a substantial amount of time and expense for us to secure other suppliers and we may be required to relocate our manufacturing to facilities closer to the new suppliers.
We must coordinate closely with our suppliers in order to obtain our raw materials.
Our need for a continuous supply of whole blood meeting certain specifications, often on short notice, involves risks. Our product has a short shelf life, which requires us to coordinate closely with each supplier in order to obtain the correct amount and type of raw materials on a day-to-day basis. Further, our regulatory approvals require using blood come from a donor meeting certain requirements, including for age and health. It may be difficult for our suppliers to provide us with the specific type of whole blood we need on short notice. If we are unable to obtain this supply on a regular basis it could make it difficult for us to meet our demand or could create logistical problems, either of which could adversely affect our current and future clinical trials and commercial sales if and when CureXcell receives marketing approval.
We currently rely on third parties to manufacture our product.
In the United States, the American Red Cross currently manufactures CureXcell for use in our clinical trials pursuant to a manufacturing agreement between the American Red Cross and us, which we refer to as the American Red Cross Manufacturing Agreement. Under this agreement, the American Red Cross performs the entire manufacturing process, including testing and quality assurance at its facility pursuant to the technical specifications provided by us, and then packages and ships the product to the various sites at which our clinical trials take place. Only American Red Cross personnel that have been trained by us may perform the services that the American Red Cross is required to provide under the American Red Cross Manufacturing Agreement. In Israel, the manufacturing of CureXcell is carried out by MDA technicians supervised by our employees at the MDA’s central facility where we have our own clean room pursuant to an agreement between us and MDA, which we refer to as the MDA Agreement. Accordingly, we do not directly control all aspects of the manufacturing of CureXcell. A failure by the American Red Cross or MDA to follow cGMP in the manufacturing of CureXcell may require that we recall and destroy the CureXcell that they produce and suspend their manufacturing.
The American Red Cross or MDA may delay or cease altogether manufacturing CureXcell for us for many reasons, including because their facility is affected by a war, terrorist attack, earthquake, fire, explosion, equipment or power failure, or because they have failed to adhere to cGMP. In any of these situations, our manufacturing capacity could be shut down for an extended period, we could experience a loss of raw materials, work in process or finished goods inventory and our ability to operate our business would be harmed. In addition, in any such event, the construction or contracting of new manufacturing and storage facilities, and obtaining regulatory approval for the new facilities could be expensive and time-consuming. During this period, we may be unable to manufacture CureXcell, which could delay our clinical trials and, in the future, materially adversely affect our revenue.
The ability of our third-party manufacturers to continue manufacturing and supplying CureXcell depends on their continued adherence to current good manufacturing practices regulations.
The manufacturing process for CureXcell is governed by detailed cGMP regulations. It is subject to the risk of product loss due to contamination, equipment failure or improper operation of equipment, validation activities or operator error. Failure by third-party manufacturers and quality operations units to adhere to established regulations or to meet a specification or procedure set forth in cGMP requirements could require that a CureXcell batch or materials be rejected and destroyed. Even minor deviations from normal manufacturing processes or quality requirements for our products could result in reduced production yields, defects and other supply disruptions.
Adherence to cGMP regulations and the effectiveness of our quality control systems are periodically assessed through inspections of manufacturing facilities by regulatory authorities. Such inspections could result in deficiency citations, which would require action to correct those deficiencies to the satisfaction of the applicable regulatory authorities. If critical deficiencies are noted or if recurrences are not prevented, we may have to recall CureXcell batches or suspend operations until appropriate measures are implemented. If microbial, viral or other contaminations are discovered in CureXcell or in the manufacturing facilities in which CureXcell is or will be made, such manufacturing facilities may need to be closed to investigate and remedy the contamination. Our manufacturing partners may be unable or unwilling to meet applicable regulatory and quality requirements. Any adverse developments affecting manufacturing operations for CureXcell may result in delays, inventory shortages, batch failures, withdrawals or recalls, or other interruptions in the supply of CureXcell, which, in turn, may have a material adverse effect on our current and future clinical trials and commercial sales if and when CureXcell receives marketing approval.
Since cGMP reflects ever-evolving standards, manufacturing processes and procedures must be regularly updated to comply with cGMP. These changes may cause us to incur additional costs and may adversely impact our results of operations. For example, more sensitive testing assays (if and when they become available) may be required or existing procedures or processes may require revalidation, all of which may be costly and time-consuming and could delay or prevent the manufacturing of CureXcell.
The short shelf life of our product exposes us to certain risks.
The FDA protocol for our clinical trials currently restricts the shelf life of a dose of CureXcell to seven days from completion of production, provided it is kept refrigerated between two to eight degrees Celsius. This exposes us to risks associated with production, storage and delivery of products with short shelf lives. These include the need to develop systems that can manage the complex logistics of obtaining the raw materials for CureXcell, manufacturing it and delivering each unit for administration to the patient, all in a very short period of time and within the prescribed temperature range. We will also need to anticipate the date CureXcell will be administered, coordinate with our suppliers, ensure timely delivery and coordinate the transfer of product between facilities to match the then current patient and clinical trial needs. If we fail to properly manage these logistics, we could gain a reputation for unreliability, which could harm our ability to complete our clinical trials and market acceptance of our product. We could also have to dispose of units that have expired at unacceptably high rates.
We depend on a sole supplier to obtain the transfusion and infusion bags in which our products are processed and packaged.
As CureXcell is a biologic product with living cells, it must be processed and packaged in kits consisting of sterile plastic transfusion and infusion bags that are designed to maintain the proper environment for CureXcell. We procure these bags from Maco Pharma, our sole supplier, which manufactures the bags on the basis of technological specifications that we provide pursuant to a manufacturing agreement. This agreement provides that Maco Pharma must manufacture the bags exclusively for us and that we must purchase the bags exclusively from it. The agreement also requires us to purchase a minimum amount of bags during each year. The agreement has an initial term of six years and is terminable by either party upon nine months’ notice. In the event that this agreement were to be terminated, identifying and qualifying an alternative source would require time and effort which may interfere with our ability to package CureXcell both for purposes of current and future clinical trials and commercial sales if and when CureXcell receives marketing approval. In addition, regulatory authorities could require that we conduct additional studies in support of a new supplier, which could result in significant additional costs or delays. Furthermore, we may not be able to procure an alternative source for CureXcell packages at all or at comparable quality or competitive prices or upon fair and reasonable contractual terms and conditions. Any interruption of our supply of bags in which to package CureXcell in a timely manner, or at all, would adversely affect our business, financial condition, cash flows and results of operations. Additionally, under our agreement with Maco Pharma, we are obligated to provide Maco Pharma with a right of first refusal for the manufacturing of any products containing specifications similar to those used in the manufacture of bags under the agreement.
Our products are derived from human blood, and there is an inherent risk of biological contamination and virus transmission in blood-derived products.
We derive CureXcell from donated human whole blood. Many disease-causing viruses, bacteria and other pathogens are present in the blood of infected individuals and if these infected individuals donate blood, the blood and possibly its byproducts would contain those pathogens. As a result, the FDA, EMA and other regulatory agencies extensively regulate the sourcing and screening of blood products and the production of products derived from whole blood. We rely on our suppliers to maintain compliance with these regulations. If any of our suppliers fail to adhere to regulations regarding the sourcing and screening of their blood or if the blood supply of any of our suppliers becomes contaminated, we could lose our ability to obtain blood from that supplier. If contaminated blood leads to our product becoming contaminated, we could become subject to substantial liability. If any of these were to occur, our reputation could be substantially harmed.
The safety concerns associated with blood-derived products in general may affect our ability to market our products. A significant contamination of the blood supply or studies that raise or substantiate concerns about the safety of our or other similar products could negatively impact public perception of all blood-derived products, which would adversely affect market acceptance of our product. Further, any failure in screening, whether by our manufacturers or manufacturers of other products, could adversely affect our reputation, the support we receive from the medical community and overall demand for our products.
New infectious diseases emerge in the human population from time to time. If a new infectious disease has a period during which the causative agent is present in the bloodstream but symptoms are not present, it is possible that blood donations could be contaminated by that infectious agent and remain undetected. Typically, early in an outbreak of a new disease, tests for the causative agent do not exist. During this early phase, our suppliers will need to rely on screening of donors (e.g., for behavioral risk factors or physical symptoms) to reduce the risk of blood contamination. Screening methods are generally less sensitive and specific than a direct test as a means of identifying potentially contaminated whole blood units. During the early phase of an outbreak of a new infectious disease, our ability to manufacture safe products would depend on the manufacturing process’ capacity to inactivate or remove the infectious agent. To the extent that a product’s manufacturing process is inadequate to inactivate or remove an infectious agent, our ability to manufacture and distribute that product would be impaired. If a new infectious disease were to emerge in the human population, the regulatory and public health authorities could impose precautions to limit the transmission of the disease that would impair our ability to procure whole blood, manufacture our products or both. Such precautionary measures could be taken before there is conclusive medical or scientific evidence that a disease poses a risk for blood-derived products.
We rely on arrangements and third parties for the conduct of our clinical trials and for the provision of other services.
We rely on third parties, such as contract research organizations, medical institutions and clinical investigators to conduct our trials, which limits our control over these activities. The third-party contractors may not assign as great of a priority to our clinical development programs or pursue them as diligently as we would if we were undertaking such programs directly and, accordingly, may not complete activities on schedule, or may not conduct the studies or our clinical trials in accordance with regulatory requirements or with our trial design. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, or if their performance is substandard or fails to meet regulatory requirements, our clinical trials may be negatively affected and we may be required to replace them. The replacement of these third parties would result in increased costs and delays, primarily due to the training and familiarization that their replacements would need to undergo. Consequently, our efforts to obtain regulatory approvals for, and to commercialize, CureXcell would be delayed. The third-party contractors may also have relationships with other commercial entities, some of whom may compete with us. If the third-party contractors work with our competitors, our competitive position may be harmed.
Our commercialization plans for CureXcell will require that we establish substantial manufacturing capacity, an undertaking that will involve a substantial amount of risk.
Should we receive the approvals necessary to market CureXcell, we will need to establish and obtain approval for the manufacturing facilities necessary to produce the quantities of product we hope to market. This effort will involve many risks. We will need to find space for these facilities that is close to our suppliers, meets our production needs and has ready access to the companies that ship our products. We may be unable to obtain production space that meets these requirements at reasonable cost or at all. We will also incur substantial expenses in order to build and outfit these new facilities, and the final costs to build them may be more than we anticipate. In addition, we may be asked by the FDA to conduct additional studies to obtain approval for the new facilities, which may be expensive and delay approval. We may fail to complete new manufacturing facilities on time or may not be able to obtain the regulatory and quality approvals, which would delay our ability to market CureXcell. We may also fail to properly construct the facilities, which could lead to problems, inefficiencies or delays in manufacturing. Any of the foregoing could materially adversely affect our business, financial condition, cash flows and results of operations.
CureXcell may cause unanticipated and undesirable side effects or have other properties that are currently unknown to us.
Should we receive the approvals necessary to market CureXcell, we expect that, like with most pharmaceutical products, our approval label for CureXcell will list certain side effects. If we or others identify problems with CureXcell or its underlying technology, including adverse events of unanticipated severity or frequency, problems with our manufacturers or manufacturing processes, or failure to comply with regulatory requirements, the following consequences, among others, may occur:
• restrictions on the clinical trials, marketing or manufacturing of the product, withdrawal of the product from clinical trials or the market, or voluntary or mandatory product recalls;
• fines, warning letters or holds on clinical trials;
• harm to our reputation, reduced demand for our products and loss of market acceptance;
• refusal by the regulatory authority to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals;
• product seizure or detention, or refusal to permit the import or export of products; and
• injunctions or the imposition of civil or criminal penalties.
Any of these events could adversely affect our current and future clinical trials and commercial sales if and when CureXcell receives marketing approval, which may materially adversely affect our business, financial condition, cash flows and results of operations.
We could be subject to product liability lawsuits, which could result in costly and time-consuming litigation and significant liabilities.
The development of biologic products involves an inherent risk of product liability claims and associated adverse publicity. CureXcell may be alleged or found to be harmful or to contain harmful substances. This would expose us to substantial risk of litigation and liability or may force us to discontinue production of CureXcell. Although we have product liability insurance covering up to $10 million in claims, the coverage may not insure us against all claims made and in some instances we may be required to pay substantial deductibles. Product liability insurance is costly and often limited in scope. We may not be able to obtain or maintain insurance on reasonable terms or to otherwise protect ourselves against potential product liability claims that could impede or prevent commercialization of CureXcell. Furthermore, a product liability claim could damage our reputation, whether or not such a claim has merit or is covered by insurance. A product liability claim against us or the withdrawal of a product from the market could have a material adverse effect on our business or financial condition. Additionally, product liability lawsuits, regardless of their success, would likely be time consuming and expensive to resolve and would divert management’s time and attention, which could materially adversely affect our business, financial condition, cash flows and results of operations.
We face competition from potential changes in medical practice and technology and the possibility that our competitors may develop products, treatments or procedures that are similar, more advanced, safer or more effective than ours.
The medical, biotechnology and pharmaceutical industries are intensely competitive and subject to significant technological and practice changes. We may face competition from many difference sources. Possible competitors include medical practitioners, pharmaceutical and wound care companies, academic and medical institutions, governmental agencies and public and private research institutions, among others. Should any competitor’s product candidates receive regulatory or marketing approval prior to ours, they may establish a strong market position and be difficult to displace, or will diminish the need for our products.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products, treatments or procedures that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any product that we may develop. Currently, there are a number of commercially available AWC products for the treatment of chronic and other hard-to-heal wounds, including, but not limited to, negative pressure wound therapy, or NPWT, skin substitutes, amniotic allografts, hyperbaric oxygen therapy and other treatment approaches. In addition, we licensed a patent related to the production of macrophages using osmotic shock, which we refer to as the Danon patent. Our current, more effective processes and products are covered by different patents. When the Danon patent expires in June 2015, MDA, which currently manufactures CureXcell in Israel, or any other party, can develop, manufacture and commercialize products that could compete against our products using the manufacturing process described in the Danon patent or design another process that circumvents the intellectual property directed to our current manufacturing processes. If any competing products are cheaper, more effective, safer or more marketable than CureXcell, our business could be materially adversely affected.
Many of our competitors may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we may have. Mergers and acquisitions in the pharmaceutical, biotechnology or wound care industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
If we fail to manage our growth effectively, our business could be disrupted.
Our future financial performance and ability to successfully commercialize CureXcell, and to compete effectively will depend, in part, on our ability to manage any future growth effectively. We expect to expand our work force and train, motivate and manage additional employees. Even following expansion, our facilities, personnel, systems, procedures and controls may not be adequate to support our future operations. Any failure to manage future growth effectively could have a material adverse effect on our business, financial condition, cash flows and results of operations.
We may not be successful in using our proprietary cell activation technology to establish a platform for the development and commercialization of a broad range of regenerative medicine products.
A key element of our long-term strategy is to use our proprietary cell activation technology to establish a platform for the development and commercialization of a broad range of regenerative medicine products, including product candidates for non-wound indications. Although we plan to expand our pipeline of product candidates, we may not be able to identify or develop product candidates that are safe and effective. Even if we are successful in building our pipeline, the potential product candidates that we identify may not be suitable for clinical development, including as a result of being shown to have harmful side effects or other characteristics that indicate that they are unlikely to receive marketing approval and achieve market acceptance. Research and development to identify new product candidates requires substantial technical, financial and human resources, and we may expend these resources on product candidates that ultimately prove to be unsuccessful. If we do not establish a platform for the development and commercialization of a broad range of regenerative medicine products, we may face difficulty in obtaining revenues in future periods, which could have a materially adverse impact on our business, financial condition, cash flows and results of operations.
Our business could suffer if we are unable to attract and retain key employees.
Our success depends upon the continued service and performance of our senior management and other key personnel, which currently include Nissim Mashiach (our President and Chief Executive Officer), Michael Molyneaux, MD (our Chief Medical Officer) and Mark Page (our Chief Financial Officer). The loss of the services of these personnel could delay or prevent the successful completion of our clinical trials, the commercialization of CureXcell or otherwise affect our ability to manage our company effectively and to carry out our business plan. Members of our senior management team may resign at any time. A high demand exists for senior management and other key personnel in the biotechnology and medical technology industries and we may not be able to continue to retain and attract such personnel.
Our growth and success also depend on our ability to attract and retain additional highly qualified and skilled sales and marketing, research and development, operational, managerial and finance personnel. Competition for skilled personnel is intense and the unexpected loss of an employee with a particular skill could materially adversely affect our operations until a replacement can be found and trained. While we have not experienced issues retaining our personnel, if we cannot retain our existing skilled scientific and operational personnel and attract and retain sufficiently-skilled additional scientific and operational personnel as required for our operations on acceptable terms, we may not be able to carry out our business plan.
Exchange rate fluctuations between the U.S. dollar and the Israeli shekel, as well as other non-U.S. currencies, may negatively affect our earnings.
The dollar is our functional and reporting currency. However, a portion of our operating expenses is incurred in shekels. As a result, we are exposed to the risks that the shekel may appreciate relative to the dollar, or, if the shekel instead devalues relative to the dollar, that the inflation rate in Israel may exceed the rate of devaluation of the shekel, or that the timing of that devaluation may lag behind inflation in Israel. In any such event, the dollar cost of our operations in Israel would increase and our dollar-denominated results of operations would be adversely affected. We cannot predict any future trends in the rate of inflation in Israel or the rate of appreciation (if any) of the shekel against the dollar. For example, the rate of devaluation of the dollar against the shekel was 7.5% and 2.3% in 2013 and 2012, respectively, which was compounded by inflation in Israel at a rate of 1.8% and 1.6%, respectively. This had the effect of increasing the dollar cost of our operations in Israel by 9.3% and 3.9% respectively, in such years. This trend was reversed in 2014, during which the dollar appreciated relative to the shekel by 10.7%, and the rate of inflation in Israel was negative (0.2%). If the trend of 2013 and 2012 returns, however, and the dollar cost of our operations in Israel increases, our dollar-measured results of operations will be adversely affected. Our operations also could be adversely affected if we are unable to effectively hedge against currency fluctuations in the future.
In addition, we intend to commercialize CureXcell in Europe and other geographical markets, such as Japan. Accordingly we may in the future generate revenue in currencies other than the dollar and the shekel, such as the Euro or the Yen. In that case, our operating results and cash flows may also subject to fluctuations due to changes in the relative values of the dollar and these foreign currencies. These fluctuations could negatively affect our operating results and could cause them to vary from quarter to quarter.
Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing.
Many of our research and development activities with respect to CureXcell involve the use of animal testing. This type of activity has been the subject of controversy and adverse publicity. Animal rights groups and numerous other organizations and individuals have attempted to stop animal testing by, amongst other things, lobbying for legislation and regulation in these areas. If the use of animal testing is restricted by legislation or regulation it may adversely affect our business.
Potential future acquisitions of or investments in companies or technologies may distract our management, may disrupt our business, negatively impact our financial condition and may not yield the returns expected.
We may acquire or make investments in businesses, technologies or products, whether complementary or otherwise, as a means to expand our business, if appropriate opportunities arise. We may be unable to identify future suitable acquisition or investment candidates, or, if we do identify suitable candidates, we may be unable to make the acquisitions or investments on reasonable terms or at all. In addition, we have no prior experience in integrating acquisitions and we could experience difficulties incorporating an acquired company’s personnel, operations, technology or product offerings into our own or in retaining and motivating key personnel from these businesses. We may also incur unanticipated liabilities. The financing of any such acquisition or investment, or of a significant general expansion of our business, may not be readily available on favorable terms. Any significant acquisition or investment, or major expansion of our business, may require us to explore external financing sources, such as an offering of our equity or debt securities. We cannot be certain that these financing sources will be available to us or that we will be able to negotiate commercially reasonable terms for any such financing, or that our actual cash requirements for an acquisition, investment or expansion will not be greater than anticipated. In addition, any indebtedness that we may incur in such a financing may inhibit our operational flexibility, while any equity securities that we may issue in connection with such a financing would dilute our shareholders. Any such difficulties could disrupt our ongoing business, distract our management and employees, increase our expenses and adversely affect our results of operations. Furthermore, we may not realize the anticipated benefits or synergies of any such acquisition or investment.
Regulatory approval for CureXcell may be limited to specific indications and conditions for which clinical safety and efficacy have been demonstrated, and the prescription or promotion of off-label uses could adversely affect our business.
Any regulatory approval that we receive for CureXcell would be limited to those specific indications for which CureXcell has been deemed safe and effective by the FDA, the EMA or other regulatory authority. Additionally, labeling restrictions may also limit the manner in which a product may be used. For example, we anticipate that CureXcell’s approval, if any, will be limited to wounds without any exposed bone or tendons and that it will not be indicated for pressure and post-surgical wounds. Physicians may, however prescribe a product for an unapproved, or “off-label,” use or may use a product in a manner that is inconsistent with the manufacturer’s labeling. To the extent such off-label use is pervasive and results in reduced efficacy or other adverse effects, the reputation of CureXcell in the marketplace may suffer.
Furthermore, while physicians may choose to prescribe treatments for uses that are not described in the product’s labeling and for uses that differ from those approved by regulatory authorities, our ability to promote the products is limited to those indications that are specifically approved by the FDA, the EMA or other regulatory authorities. Although regulatory authorities generally do not regulate the behavior of physicians, they do restrict communications by companies on the subject of off-label use. If our promotional activities fail to comply with these regulations or guidelines, we may be subject to warnings from, or enforcement action by, these authorities. In the United States, off-label promotion by pharmaceutical companies has resulted in significant litigation under the U.S. False Claims Act, violations of which may result in substantial civil penalties and fines. More generally, failure to follow the rules and guidelines of regulatory agencies relating to promotion and advertising, such as that promotional materials not be false or misleading, can result in refusal to approve a product, the suspension or withdrawal of an approved product from the market, product recalls, fines, disgorgement of money, operating restrictions, injunctions or criminal prosecution.
The implementation of healthcare reform in the United States may adversely affect our business.
Pursuant to the March 2010 adoption of the healthcare reform law in the United States, substantial changes are being made to the current system for paying for healthcare in the United States, including programs to extend medical benefits to millions of individuals who currently lack insurance coverage. The changes contemplated by the healthcare reform law are subject to rule-making and implementation timelines that extend for several years, and this uncertainty limits our ability to forecast changes that may occur in the future. However, implementation has already begun with respect to certain significant cost-saving measures under the healthcare reform law, for example with respect to several government healthcare programs that may cover the cost of our future products, including Medicaid, Medicare Parts B and D, and these efforts could have a materially adverse impact on our future financial prospects and performance.
For example, in order for a manufacturer’s products to be reimbursed by federal funding under Medicaid, the manufacturer must enter into a Medicaid rebate agreement with the Secretary of the U.S. Department of Health and Human Services, and pay certain rebates to the states based on utilization data provided by each state to the manufacturer and to the Centers for Medicare and Medicaid Services, or CMS, and pricing data provided by the manufacturer to the federal government. The states share this savings with the federal government, and sometimes implement their own additional supplemental rebate programs. Under the Medicaid drug rebate program, the rebate amount for most branded drug products was previously equal to a minimum of 15.1% of the Average Manufacturer Price, or AMP, or the AMP less Best Price, whichever is greater. Effective January 1, 2010, the healthcare reform law generally increases the size of the Medicaid rebates paid by manufacturers for single source and innovator multiple source (brand name) drug product from a minimum of 15.1% to a minimum of 23.1% of the AMP, subject to certain exceptions, for example, for certain clotting factors, the increase is limited to a minimum of 17.1% of the AMP. For non-innovator multiple source (generic) products, the rebate percentage is increased from a minimum of 11.0% to a minimum of 13.0% of AMP. In 2010, the healthcare reform law also newly extended this rebate obligation to prescription drugs covered by Medicaid managed care organizations. These increases in required rebates may adversely affect our future financial prospects and performance.
The healthcare reform law also creates new rebate obligations for our products under Medicare Part D, a partial, voluntary prescription drug benefit created by the United States federal government primarily for persons 65 years old and over. The Part D drug program is administered through private insurers that contract with CMS. Beginning in 2011, the healthcare reform law generally requires that in order for a drug manufacturer’s products to be reimbursed under Medicare Part D, the manufacturer must enter into a Medicare Coverage Gap Discount Program agreement with the Secretary of the U.S. Department of Health and Human Services, and reimburse each Medicare Part D plan sponsor an amount equal to 50% savings for the manufacturer’s brand name drugs and biologics which the Part D plan sponsor has provided to its Medicare Part D beneficiaries who are in the “donut hole” (or a gap in Medicare Part D coverage for beneficiaries who have expended certain amounts for drugs). The Part D plan sponsor is responsible for calculating and providing the discount directly to its beneficiaries and for reporting these amounts paid to CMS’s contractor, which notifies drug manufacturers of the rebate amounts it must pay to each Part D plan sponsor. The rebate requirement could adversely affect our future financial performance, particularly if contracts with Part D plans cannot be favorably renegotiated or the Part D plan sponsors fail to accurately calculate payments due in a manner that overstates our rebate obligation.
The healthcare reform law also introduced a biosimilar pathway that will permit companies to obtain FDA approval of generic versions of existing biologics based upon reduced documentation and data requirements deemed sufficient to demonstrate safety and efficacy than are required for the pioneer biologics. The new law provides that a biosimilar application may be submitted as soon as four years after the reference product is first licensed, and that the FDA may not make approval of an application effective until 12 years after the reference product was first licensed. With the likely introduction of biosimilars in the United States, we expect in the future to face greater competition from biosimilar products, including a possible increase in patent challenges. The FDA has reported meeting with sponsors who are interested in developing biosimilar products, and is developing regulations to implement the abbreviated regulatory review pathway.
Regarding access to our products, the healthcare reform law established and provided significant funding for a Patient-Centered Outcomes Research Institute to coordinate and fund Comparative Effectiveness Research, or CER. While the stated intent of CER is to develop information to guide providers to the most efficacious therapies, outcomes of CER could influence the reimbursement or coverage for therapies that are determined to be less cost-effective than others. Should any of our products be determined to be less cost-effective than alternative therapies, the levels of reimbursement for these products, or the willingness to reimburse at all, could be impacted, which could materially impact our future financial prospects and results.
We may be limited in the promotional claims we can make and may not be able to use information about our competitors to promote or market CureXcell without incurring significant regulatory or enforcement risks.
Various U.S. governmental agencies, including the FDA and the Federal Trade Commission, or the FTC, regulate the promotion and advertising of FDA approved medical products. Promotional materials and statements must not be false or misleading. Among other things, the FDA requires that promotional claims be supported by “substantial evidence,” which requires adequate, well-controlled clinical trials. Promotional claims must also reflect “fair balance” between the risks and benefits of a medical product. The FDA also has found comparative claims to be “false and misleading” when they are not supported by adequate, well-controlled, head-to-head comparison trials.
Disclaimers that the comparative claims are not based on head-to-head trials may not be sufficient to insulate the responsible party from an FDA or FTC enforcement action. False and misleading advertising and promotion is a violation of the Federal Food, Drug, and Cosmetic Act, or the FDCA, and subjects the responsible party to sanctions including, but not limited to, warning letters, injunctions, civil penalties and criminal prosecution. Additionally, a product is misbranded under the regulations if, in an effort to promote the product, a responsible party makes a false or misleading representation with respect to a competing drug, device or biologic. We have not conducted head-to-head trials with any other AWC therapy. As a result, we will be limited in our ability to market CureXcell using available data from separate trials of competing therapies. If we decide to market CureXcell’s benefits as compared to other AWC therapies, we will need to conduct head-to-head clinical trials which may be time consuming and expensive and may not be successful.
Certain of our business practices could become subject to scrutiny by regulatory authorities, as well as to lawsuits brought by private citizens. Failure to comply with applicable law or an adverse decision in lawsuits may result in adverse consequences to us.
The laws governing our conduct in the United States are enforceable by criminal, civil and administrative penalties. Violations of laws such as the FDCA the Public Health Service Act, the U.S. False Claims Act, provisions of the U.S. Social Security Act, including the provision known as the “Anti-Kickback Law,” or any regulations promulgated under their authority, may result in various administrative, civil and criminal sanctions, jail sentences, fines or exclusion from federal and state programs, as may be determined by Medicare, Medicaid, other regulatory authorities and the courts. We may come under the scrutiny of regulators and other government authorities or our practices may be found to violate applicable laws, rules and regulations or prompt lawsuits by private citizen “relators” under federal or state false claims laws.
For example, under the Anti-Kickback Law, and similar state laws and regulations, even common business arrangements, such as discounted terms and volume incentives for customers in a position to recommend or choose drugs and devices for patients, such as physicians and hospitals, can result in substantial legal penalties, including, among others, exclusion from Medicare and Medicaid programs. As a result, arrangements with potential referral sources must be structured with care to comply with applicable requirements. Also, certain business practices, such as payment of consulting fees to healthcare providers, sponsorship of educational or research grants, charitable donations, interactions with healthcare providers and financial support for continuing medical education programs, must be conducted within narrowly prescribed and controlled limits to avoid any possibility of wrongfully influencing healthcare providers to prescribe or purchase particular products or of rewarding past prescribing.
In addition, significant enforcement activity has taken place under federal and state false claims act statutes and violations of the U.S. False Claims Act can result in treble damages, and penalty of up to $11,000 for each false claim submitted for payment. The U.S. False Claims Act, as well as certain state false claims acts, permit relators to file complaints in the name of the United States (and if applicable, particular states). These relators may be entitled to receive up to 30% of total recoveries and have been active in pursuing cases against pharmaceutical companies. Where practices have been found to involve improper incentives to use products, the submission of false claims, or other improper conduct, government investigations and assessments of penalties against manufacturers have resulted in substantial damages and fines. In addition, to avoid exclusion from participation in federal healthcare programs, many manufacturers have been required to enter into corporate integrity agreements that prescribe allowable corporate conduct. Failure to satisfy requirements under the FDCA can also result in a variety of administrative, civil and criminal penalties, including injunctions or consent decrees that prescribe allowable corporate conduct.
To enhance compliance with applicable healthcare laws, and mitigate potential liability in the event of noncompliance, regulatory authorities, such as the Office of Inspector General of the U.S. Department of Health and Human Services, or OIG, have recommended the adoption and implementation of a comprehensive health care compliance program that generally contains the elements of an effective compliance and ethics program described in Section 8B2.1 of the U.S. Sentencing Commission Guidelines Manual. Increasing numbers of U.S.-based pharmaceutical companies have such programs. As CureXcell is not yet approved for marketing in the United States, we have not adopted U.S. healthcare compliance and ethics programs that generally incorporate the OIG’s recommendations, but even if we do, we may still be unable to avoid any compliance issues.
In addition, we are subject to analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances. Many of these laws differ from each other in significant ways and often are not preempted by the U.S. Health Insurance Portability and Accountability Act of 1996, thus complicating compliance efforts.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, it may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
As a public company with securities registered under the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, we are subject to the U.S. Foreign Corrupt Practices Act, or FCPA. The FCPA and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. We have implemented policies mandating compliance with these anti-bribery laws; however, we may operate in parts of the world that have experienced governmental corruption to some degree and in certain circumstances, strict compliance with anti-bribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different than in the United States. Our internal control policies and procedures may not be sufficient to effectively protect us against reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our financial condition, results of operations and cash flows.
We are subject to environmental, health and safety and other laws and regulations.
We are subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures. Although we maintain workers’ compensation insurance to cover the costs and expenses that may be incurred because of injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. Additional or more stringent laws and regulations affecting our operations may be adopted in the future. We may incur substantial capital costs and operating expenses and may be required to obtain consents to comply with any of these or certain other laws or regulations and the terms and conditions of any permits required pursuant to such laws and regulations, including costs to modify our operations or perform other corrective actions at our respective facilities. In addition, fines and penalties may be imposed for noncompliance with environmental, health and safety and other laws and regulations or for the failure to have, or comply with the terms and conditions of, required environmental or other permits or consents.
Risks Related to Our Intellectual Property
Our success depends in part on our ability to obtain and maintain protection for the intellectual property relating to or incorporated into our technology and products.
Our commercial success depends in part on our ability to obtain and maintain trade secret protection for our intellectual property and proprietary technologies, our products and their uses, as well as our ability to operate without infringing upon the proprietary rights of others. We rely on proprietary information (such as trade secrets, know-how and confidential information) to protect our intellectual property, including intellectual property that may not be patentable, or that we believe is best protected by means that do not require public disclosure. We generally seek to protect this proprietary information by entering into confidentiality agreements, or consulting, services or employment agreements that contain non-disclosure and non-use provisions with our employees, consultants, contractors, scientific advisors and third parties. However, we may fail to enter into the necessary agreements, and even if entered into, these agreements may be breached or otherwise fail to prevent disclosure, third-party infringement or misappropriation of our proprietary information, may be limited as to their term and may not provide an adequate remedy in the event of unauthorized disclosure or use of proprietary information. We have limited control over the protection of trade secrets used by our suppliers and service providers and could lose future trade secret protection if any unauthorized disclosure of such information occurs. In addition, our proprietary information may otherwise become known or be independently developed by our competitors or other third parties. To the extent that our employees, consultants, contractors, scientific advisors and other third parties use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our and relevant third parties’ proprietary rights, failure to obtain or maintain protection for our proprietary information could adversely affect our competitive business position and if third parties are able to establish that we are using their proprietary information without their permission, we may be required to obtain a license to that information, or if such a license is not available, re-design our products to avoid any such unauthorized use or temporarily delay or permanently stop manufacturing or sales of the affected products. Furthermore, laws regarding trade secret rights in certain markets where we operate may afford little or no protection to our trade secrets. Any of the foregoing could deteriorate our competitive advantages, undermine the trade secret and contractual protections afforded to our confidential information and have material adverse effects on our business.
We also rely on physical and electronic security measures to protect our proprietary information, but these security measures may be breached or may not provide adequate protection for our property. There is a risk that third parties may obtain and improperly utilize our proprietary information to our competitive disadvantage. We may not be able to detect or prevent the unauthorized use of such information or take appropriate and timely steps to enforce our intellectual property rights.
Some of our employees were previously employed at blood banks, universities or other biotechnology or pharmaceutical companies, including potential competitors. While we take steps to prevent our employees from using the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have inadvertently or otherwise used or disclosed intellectual property, trade secrets or other proprietary information of any such employee’s former employer. Litigation may be necessary to defend against these claims and, even if we are successful in defending ourselves, could result in substantial costs to us or be distracting to our management. If we fail to defend any such claims successfully, in addition to paying monetary damages and possible ongoing royalties, we may lose valuable intellectual property rights or personnel.
We may receive only limited protection, or no protection, from our issued patents and patent applications.
We have four patent families on file covering processes and resulting activated white blood cell compositions that we developed, and their use. From one of those families we have been granted three patents. One patent in each of the United States and the European Union has claims covering our process for producing activated white blood cell compositions, and one patent in Australia has claims covering our process of producing activated white blood cell compositions, the compositions themselves, and their use in treating wounds. However, the patent applications relating to our products, processes or technologies may not result in patents being issued, the claims that issue may have limited or no coverage of our products and technologies, and any patents that have been issued may not be adequate to protect our intellectual property or afford us patent protection for any significant period of time. Additionally, the enforceability or ownership of any issued patents may be challenged by third parties, and patents that we hold may be found by a judicial authority or the United States Patent and Trademark Office, or USPTO, or other relevant patent office or governmental authority, to be invalid or unenforceable. Other parties may independently develop similar or competing technology or design around any patents that may be issued to or held by us. In addition, we licensed the Danon patent, which relates to the production of macrophages using osmotic shock. Our current, more effective processes and products are covered by different patents. When the Danon patent expires in June 2015, MDA, which currently manufactures CureXcell in Israel, or any other party can develop, manufacture and commercialize products using the manufacturing process described in the Danon patent or design another process that circumvents the intellectual property directed to our current manufacturing processes. Further, the patents directed to the processes and products we developed will expire or they may otherwise cease to provide meaningful competitive advantage, and we may be unable to adequately develop new technologies and obtain future patent protection to preserve our competitive advantage or avoid adverse effects on our business.
At present, we consider the intellectual property relating to our use of hypo-osmotic shock to activate white blood cells, the technology that underlies CureXcell, to be material to the operation of our business as a whole.
Because our issued patents cover the process of manufacturing CureXcell, they offer protection against competition that is, to some extent, more limited than the protection provided by patents that claim products or chemical structures that were previously unknown. To the extent our issued patents remain limited to process claims, they may be less protective and it may be difficult for us to detect infringing products and enforce our patents against them. If a competitor were able to successfully design around our patents, we may not be able to block competition, and furthermore the competitor’s products may be more effective or commercially successful than our products. Further, the exclusivity of our ownership of our issued United States patent may be subject to our receipt of additional assignments from certain inventors. These inventors have previously executed assignments to us in connection with certain of the provisional applications related to the issued United States patent and we are beginning the process of obtaining confirmatory assignments from them. If patents covering CureXcell in various jurisdictions were subject to a successful challenge, our business and competitive advantage could be significantly affected.
In addition, the patent landscape in the biotechnology field is highly uncertain and involves complex legal, factual and scientific questions, and changes in either patent laws or in the interpretation of patent laws in the United States and other countries may diminish the value and strength of our intellectual property or narrow the scope of our patent protection. Particularly in recent years in the United States, there have been several major legislative developments and court decisions that have affected patent laws in significant ways. In addition, we may fail to apply for or be unable to obtain patents necessary to protect our technology, for example, due to prior uses of or claims to similar processes, or products or enforce our patents due to lack of information about the exact use of our process by third parties. Even if patents are issued to us, they may be challenged, narrowed, invalidated, held to be unenforceable or circumvented, which could limit our ability to prevent competitors from using similar technology or marketing similar products, or limit the length of time our technologies and products have patent protection.
Our material patents also may not afford us protection against competitors with similar technology. Because patent applications in the United States and many other jurisdictions are typically not published until 18 months after their filing, if at all, and because publications of discoveries in scientific literature often lag behind actual discoveries, neither we nor our licensors can be certain that we or they were the first to make the inventions claimed in our or their issued patents or pending patent applications, or that we or they were the first to file for protection of the inventions set forth in such patent applications (depending on the laws applicable in the relevant country at the time of filing). As a result, the patents we own and license may be invalidated in the future, and the patent applications we own and license may not be granted. For example, if a third party has also filed a patent application covering an invention similar to one covered in one of our patent applications, we may be required to participate in an adversarial proceeding known as an “interference proceeding,” declared by the USPTO or its foreign counterparts, to determine priority of invention. Also, as of March 16, 2013, the U.S. transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. The costs of these proceedings could be substantial and our efforts in them could be unsuccessful, resulting in a loss of our anticipated patent position. In addition, if a third party prevails in such a proceeding and obtains an issued patent, we may be prevented from practicing technology or marketing products covered by that patent. Additionally, patents and patent applications owned by third parties may prevent us from pursuing certain opportunities such as entering into specific markets or developing certain products. Finally, we may choose to enter into markets where certain competitors have patents or patent protection over technology that may impede our ability to compete effectively.
Our currently issued patents directed to the processes and products we developed are nominally due to expire in 2030, and the Danon patent, which we licensed before we developed our current processes and products, expires in June 2015. However, because of the extensive time required for development, testing and regulatory review of a potential product, and although such delays may entitle us to patent term extensions, it is possible that, before CureXcell can be commercialized in additional jurisdictions and/or before any of our future products can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantages of the patent. Our pending and future patent applications may not lead to the issuance of patents or, if issued, the patents may not be issued in a form that will provide us with any competitive advantage. We also cannot guarantee that:
• any of our present or future patents or patent claims or other intellectual property rights will not lapse or be invalidated, circumvented, challenged or abandoned;
• our intellectual property rights will provide competitive advantages or prevent competitors from making or selling competing products;
• our ability to assert our intellectual property rights against potential competitors or to settle current or future disputes will not be limited by our agreements with third parties, financial constraints, market realities, competitive concerns or other factors;
• any of our pending or future patent applications will be issued or have the coverage originally sought;
• our intellectual property rights will be enforced in jurisdictions where competition may be intense or where legal protection may be weak; or
• we will not lose the ability to assert our intellectual property rights against, or to license our technology to, others and collect royalties or other payments.
In addition, our competitors or others may design around our patents or protected technologies. Effective protection of our intellectual property rights may also be unavailable or limited in some countries, and even if available, we may fail to pursue or obtain necessary intellectual property protection in such countries, including because filing, prosecuting, maintaining and defending patents on product candidates in all countries throughout the world would be prohibitively expensive. In addition, the legal systems of certain countries, such as China, may not favor the aggressive enforcement of patents and other intellectual property rights, and the laws of certain foreign countries do not protect our rights to the same extent as the laws of the United States, especially with respect to method claims. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and we may be unable to prevent such competitors from importing those infringing products into territories where we have patent protection but enforcement is not as strong as in the United States or into jurisdictions in which we do not have patent protection. These products may compete with our product candidates and our patents and other intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions.
In order to preserve and enforce our patent and other intellectual property rights, we may need to make claims or file lawsuits against third parties. Such lawsuits could entail significant costs to us and divert our management’s attention from developing and commercializing our products. Lawsuits may ultimately be unsuccessful and may also subject us to counterclaims and cause our intellectual property rights to be challenged, narrowed, invalidated or held to be unenforceable.
Additionally, unauthorized use of our intellectual property may have occurred or may occur in the future. Any failure to detect or identify unauthorized use of, and otherwise adequately protect, our intellectual property could adversely affect our business, including by reducing the demand for our products. Any reported adverse events involving counterfeit products that purport to be our products could harm our reputation and the sale of our products. Moreover, if we are required to commence litigation related to unauthorized use, whether as a plaintiff or defendant, such litigation would be time-consuming, force us to incur significant costs and divert our attention and the efforts of our management and other employees, which could, in turn, result in lower revenue and higher expenses.
If we are unable to protect our CureXcell trademark from infringement or other violation, our business prospects may be harmed.
We own the trademark CureXcell, and have registered this trademark in the United States and Israel. Although we take steps to monitor the possible infringement or misuse of this trademark, it is possible that third parties may infringe, dilute or otherwise violate our trademark rights. Any unauthorized use of our trademark could harm our reputation or commercial interests. In addition, our enforcement against third-party infringers or violators may be unduly expensive and time-consuming, and the outcome may be an inadequate remedy.
We may be subject to claims that we infringe, misappropriate or otherwise violate the intellectual property rights of third parties.
Our development, marketing or sale of CureXcell may infringe or be accused of infringing one or more claims of an issued patent or may fall within the scope of one or more claims in a published patent application that may be subsequently issued and to which we do not hold a license or other rights. There may also be issued patents held by third parties that may be infringed or otherwise violated by our products, technologies and other activities, and we do not know whether or to what extent we are infringing or otherwise violating third party patents. We may also be subject to claims that we are infringing, misappropriating or otherwise violating other intellectual property rights, such as trademarks, copyrights or trade secrets. Third parties could therefore bring claims against us or our potential strategic partners that would cause us to incur substantial expenses, including litigation costs or costs associated with settlement, and, if successful against us, could cause us to pay substantial damages. Further, if such a claim were brought against us, we could be temporarily or permanently enjoined or otherwise forced to temporarily delay or permanently stop manufacturing or trials and sales of CureXcell.
If we are found to be infringing, misappropriating or otherwise violating the patent or other intellectual property rights of a third party, or in order to avoid or settle claims, we may choose or be required to seek a license from a third party and be required to pay license fees or royalties or both, which could be substantial. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property, or such rights might be restrictive and limit our present and future activities. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened claims, we or our potential strategic partners are unable to enter into licenses on acceptable terms.
There have been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. For example, in May 2014, Cognate Bioservices, Inc. filed a motion in an ongoing litigation matter involving one of our former employees seeking leave to file an amended complaint to add us as a defendant. The proposed amended complaint includes allegations of violations of the Computer Fraud and Abuse Act, misappropriation of products and misappropriation of trade secrets. In addition, to the extent that we gain greater visibility and market exposure as a public company in the United States, we face a greater risk of being involved in such litigation. In addition to possible infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference, opposition, re-examination and similar proceedings before the USPTO and its foreign counterparts, regarding intellectual property rights with respect to CureXcell. Additionally, recent legislative changes to U.S. patent laws under the Leahy-Smith America Invents Act (signed into law on September 16, 2011) include new procedures for third parties to challenge issued patents in the USPTO, and lower evidentiary standards or other advantages to the challenger may apply in certain USPTO proceedings compared to litigation in courts. Third parties could attempt to use these or other procedures to invalidate our patents or prevent us from enforcing them. Furthermore, the cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. A negative outcome could result in liability for monetary damages, including treble damages and attorneys’ fees if, for example, we are found to have willfully infringed a patent. A finding of infringement could prevent us from developing, marketing or selling a product or force us to cease some or all of our business operations. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace, and patent litigation and other proceedings may also absorb significant management time.
Under applicable employment laws, we may not be able to enforce covenants not to compete.
We generally enter into non-competition agreements with our employees. These agreements prohibit our employees, if they cease working for us, from competing directly with us or working for our competitors or clients for a limited period. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants developed while working for us. For example, Israeli labor courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer, which have been recognized by the courts, such as the protection of a company’s trade secrets or other intellectual property.
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
We have invested and expect to continue to invest a significant amount of resources in the development of intellectual property by our employees in the course of their employment for us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived by an employee during the scope of his or her employment with a company are regarded as ‘‘service inventions,’’ which belong to the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patent Law also provides that if there is no such agreement between an employer and an employee, the Israeli Compensation and Royalties Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for his or her inventions. Recent decisions by the Committee have created uncertainty in this area, as it held that employees may be entitled to remuneration for their service inventions despite having specifically waived any such rights. Further, the Committee has not yet determined the method for calculating this Committee-enforced remuneration or the criteria or circumstances under which an employee’s assignment of all rights and/or waiver of his or her right to remuneration will be disregarded. Although our employees have agreed to assign to us service invention rights, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could negatively affect our business.
Risks Related to an Investment in Our Ordinary Shares
The trading market for our ordinary shares is not always active, liquid and orderly, which may inhibit the ability of our shareholders to sell ordinary shares.
Prior to our IPO in August 2014, there was no public market for our ordinary shares. Since that time, the trading market for our ordinary shares has not always been active, liquid or orderly. The lack of an active market at times may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. An inactive market may also impair our ability to raise capital by selling shares and may impair our ability to acquire other companies by using our shares as consideration.
The market price of our ordinary shares may be subject to fluctuation and you could lose all or part of your investment.
The market price of our ordinary shares has been subject to considerable fluctuation since our IPO in August 2014, with the closing price per share having varied from a low of $7.01 to a high of $11.84. The stock market in general has been, and the market price of our ordinary shares in particular will likely continue to be, subject to fluctuation, whether due to, or irrespective of, our operating results and financial condition. The market price of our ordinary shares on the NASDAQ Global Market may fluctuate as a result of a number of factors, some of which are beyond our control, including, but not limited to:
• actual or anticipated variations in our and our competitors’ results of operations and financial condition;
• market acceptance of our products;
• the mix of products that we sell;
• changes in earnings estimates or recommendations by securities analysts, if our ordinary shares are covered by analysts;
• development of technological innovations or new competitive products by others;
• announcements of technological innovations or new products by us;
• publication of the results of clinical trials for CureXcell or other products;
• failure by us to achieve a publicly announced milestone;
• delays between our expenditures to develop and market products and the generation of sales from those products;
• developments concerning intellectual property rights, including our involvement in litigation;
• regulatory developments and the decisions of regulatory authorities as to the approval or rejection of new or modified products;
• changes in the amounts that we spend to develop, acquire or license new products, technologies or businesses;
• our sale or proposed sale, or the sale by our significant shareholders, of our ordinary shares or other securities in the future;
• changes in key personnel;
• success or failure of our research and development projects or those of our competitors;
• the trading volume of our ordinary shares; and
• general economic and market conditions and other factors, including factors unrelated to our operating performance.
These factors and any corresponding price fluctuations may materially adversely affect the market price of our ordinary shares and result in substantial losses being incurred by our investors. In the past, following periods of market volatility, public company shareholders have often instituted securities class action litigation. If we were involved in securities litigation, it could impose a substantial cost upon us and divert the resources and attention of our management from our business.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our ordinary shares, the price of our ordinary shares could decline.
The trading market for our ordinary shares relies in part on the research and reports that equity research analysts publish about us and our business. We do not have control over these analysts and we do not have commitments from them to continue to write research reports about us. The price of our ordinary shares could decline if one or more equity research analysts downgrades our ordinary shares or if those analysts issue other unfavorable commentary or cease publishing reports about us or our business.
Future sales of our ordinary shares could reduce the market price of our ordinary shares.
If our existing shareholders, particularly our directors, their affiliates, or our executive officers, sell a substantial number of our ordinary shares in the public market, the market price of our ordinary shares could decrease significantly. The perception in the public market that our shareholders might sell our ordinary shares could also depress the market price of our ordinary shares and could impair our ability to obtain capital, especially through an offering of equity securities. The 180 day restriction on resale of ordinary shares by our pre-IPO shareholders pursuant to our IPO has lapsed, and these shareholders now have the ability to sell our ordinary shares into the market. We have furthermore filed a registration statement on Form S-8 with the SEC covering all of the ordinary shares issuable under our stock option and share incentive plans, and such shares are available for resale currently. In addition, our sale of additional ordinary shares or similar securities in order to raise capital might have a similar negative impact on the share price of our ordinary shares. A decline in the price of our ordinary shares might impede our ability to raise capital through the issuance of additional ordinary shares or other equity securities, and may cause you to lose part or all of your investment in our ordinary shares.
Certain of our pre-IPO shareholders, including some of our directors, continue to own a majority of our ordinary shares and, as a result, are able to exercise significant control over us, and your interests may conflict with their interests, and your ability to influence corporate matters may be limited.
As of the start of February 2015, following the expiration of the 180 day lock-up period after our IPO, our most significant shareholders, who have held our ordinary shares since prior to our IPO (which include certain of our executive officers and directors, and/or their affiliates) continued to own nearly 70% of our ordinary shares. Accordingly, if they vote the shares that they own together, they may be able to significantly influence the outcome of matters required to be submitted to our shareholders for approval, including decisions relating to the election of our board of directors and the outcome of any proposed merger or consolidation of our company. These individuals’ interests may not be consistent with those of our other shareholders. In addition, these parties’ significant interest in us may discourage third parties from seeking to acquire control of us, which may adversely affect the market price of our ordinary shares.
We have broad discretion as to the use of the net proceeds from our IPO and may not use them effectively.
Our management has broad discretion in the application of the net proceeds from our IPO. Our shareholders may not agree with the manner in which our management chooses to allocate the net proceeds from our IPO. The failure by our management to apply these funds effectively could have a material adverse effect on our business, financial condition, cash flows and results of operations. Pending their use, we may invest the net proceeds from our IPO in a manner that produces insignificant or no income.
We have been incurring, and will continue to incur, increased costs as a result of operating as a public company, and our management has been, and will continue to be, required to devote substantial time to new compliance initiatives.
As a public company whose ordinary shares are listed in the United States, we incur accounting, legal, regulatory and other expenses that we did not incur as a private company, including costs associated with our reporting requirements under the Exchange Act. We also incur costs associated with corporate governance requirements, including requirements under Section 404 and other provisions of Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules implemented by the SEC and the NASDAQ Stock Market. These rules and regulations have increased our legal and financial compliance costs, introduced new costs such as investor relations and stock exchange listing fees, and have made some activities more time-consuming and costly. We are currently evaluating and monitoring developments with respect to the implementation of these rules, and we cannot predict or estimate the amount of additional costs that we may incur or the timing of such costs.
Changes in the laws and regulations affecting public companies will likely result in increased costs to us as we respond to their requirements. These laws and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We cannot predict or estimate the amount or timing of additional costs we may incur in order to comply with such requirements.
We have never paid cash dividends on our share capital, and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends on our share capital, nor do we anticipate paying any cash dividends on our share capital in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our ordinary shares will be investors’ sole source of gain for the foreseeable future. In addition, Israeli law limits our ability to declare and pay dividends, and may subject our dividends to Israeli withholding taxes.
As a foreign private issuer, we follow certain home country corporate governance practices instead of otherwise applicable SEC and NASDAQ requirements.
As a foreign private issuer, we follow certain home country corporate governance practices instead of those otherwise required under the NASDAQ Stock Market rules for domestic U.S. issuers. For instance, we follow home country practice in Israel with regard to the (i) quorum requirement for shareholder meetings, (ii) the required composition of, and authorities delegated to, the compensation committee of our board of directors and (iii) independent director oversight of director nominations requirement. See “Item 6.C. Directors, Senior Management and Employees—Board Practices.” We may in the future elect to follow home country practices in Israel (and consequently avoid the requirements that would otherwise apply to a U.S. company listed on the NASDAQ Global Market) with regard to other matters, as well, such as separate executive sessions of independent directors and non-management directors and the requirement to obtain shareholder approval for certain dilutive events (such as for the establishment or amendment of certain share-based compensation plans, issuances that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or more interest in the company and certain acquisitions of the stock or assets of another company). Following our home country governance practices as opposed to the requirements that otherwise apply to a U.S. company listed on the NASDAQ Global Market may provide less protection to you than what is accorded to investors under the NASDAQ Stock Market rules applicable to domestic U.S. issuers.
As a foreign private issuer, we are not subject to U.S. proxy rules and are exempt from filing certain Exchange Act reports.
As a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, including the requirement for an emerging growth company to disclose the compensation of the chief executive officer and other two highest compensated executive officers on an individual, rather than aggregate, basis. A recent amendment to regulations under the Israeli Companies Law, 5759-1999, or the Companies Law, requires us to disclose the annual compensation of our five most highly compensated officers on an individual, rather than aggregate, basis. However, this disclosure is not as extensive as that required of a U.S. domestic issuer. Besides our executive compensation disclosure leniencies, our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual and current reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act, and we are generally be exempt from filing quarterly reports with the SEC under the Exchange Act. Moreover, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information. These exemptions and leniencies reduce the frequency and scope of information and protections to which you may otherwise have been eligible in relation to a U.S. domestic issuer.
We will lose our foreign private issuer status if a majority of our directors or executive officers are U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
We are an “emerging growth company” and the reduced disclosure requirements applicable to emerging growth companies may make our ordinary shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements that are applicable to other public companies that are not “emerging growth companies.” Most of such requirements relate to disclosures that we would only be required to make if we cease to be a foreign private issuer in the future. Nevertheless, as a foreign private issuer that is an emerging growth company, we are not required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act for up to five fiscal years after the date of our IPO. We will remain an emerging growth company until the earliest of: (a) the last day of our fiscal year during which the fifth anniversary of the completion of our IPO occurs; (b) the last day of our fiscal year in which we have annual gross revenue of $1.0 billion or more; (c) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt securities; or (d) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act. When we are no longer deemed to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act. Some investors may find our ordinary shares less attractive as a result of our reliance on exemptions under the JOBS Act, which may cause there to be a less active trading market for our ordinary shares and a more volatile price for our shares.
We have not yet determined whether our existing internal control over financial reporting systems are compliant with Section 404 of the Sarbanes-Oxley Act.
Pursuant to Section 404 of the Sarbanes-Oxley Act and the related rules adopted by the SEC, starting with the annual report that we file with the SEC covering the 2015 fiscal year, our management will be required to report on the effectiveness of our internal control over financial reporting. In addition, once we no longer qualify as an “emerging growth company” under the JOBS Act and lose the ability to rely on the exemptions applicable to emerging growth companies discussed above, our independent registered public accounting firm will also need to attest to management’s assessment of the effectiveness of our internal control over financial reporting under Section 404 if we are an “accelerated filer” or “large accelerated filer” under the Exchange Act. We have not yet determined whether our existing internal controls over financial reporting systems are compliant with Section 404 and whether there are any material weaknesses or significant deficiencies in our existing internal controls. This determination will require the investment of substantial time and resources, including by our chief financial officer and other members of our senior management. As a result, the process related to this determination may divert internal resources and take a significant amount of time and effort to complete. In addition, we cannot predict the outcome of this determination and whether we will need to implement remedial actions in order to implement effective controls over financial reporting. The determination and any remedial actions required could result in us incurring additional costs that we did not anticipate, including the hiring of outside consultants. Irrespective of compliance with Section 404, any failure of our internal controls could have a material adverse effect on our stated results of operations and harm our reputation. As a result, we may experience higher than anticipated operating expenses, as well as higher independent auditor fees during and after the implementation of these changes. If we are unable to implement any of the required changes to our internal control over financial reporting effectively or efficiently or are required to do so earlier than anticipated, it could adversely affect our operations, financial reporting or results of operations and could result in an adverse opinion on internal controls from our independent auditors.
Our U.S. shareholders may suffer adverse tax consequences if we are characterized as a passive foreign investment company.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Our status as a PFIC may also depend on how quickly we use the cash proceeds from our IPO in our business. Based on estimates of our gross income and gross assets (including tangible assets and intangible assets based on the anticipated market value of our ordinary shares), our use of proceeds of our IPO, and the nature of our business, we do not believe that we were classified as a PFIC for the taxable year ending December 31, 2014. There can be no assurance, however, regarding our PFIC status for 2014, 2015 or any other taxable year. If we are characterized as a PFIC, our U.S. shareholders may suffer adverse tax consequences, including having gains realized on the sale of our ordinary shares treated as ordinary income, rather than as capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares by individuals who are U.S. Holders (as defined in “Item 10.E. Taxation—U.S. Federal Income Tax Consequences” of this annual report), and having interest charges apply to distributions by us and the proceeds of share sales. Certain elections exist that may alleviate some of the adverse consequences of PFIC status and would result in an alternative treatment (such as mark-to-market treatment) of our ordinary shares; however, we do not intend to provide the information necessary for U.S. Holders to make qualified electing fund elections if we are classified as a PFIC.
Risks Primarily Related to Our Operations in Israel
Part of our research and development facilities and other significant operations are located in Israel and, therefore, our results may be adversely affected by political, economic and military instability in Israel.
Part of our research and development facilities is located in Petach Tikva, Israel. In addition, certain of our key employees and officers, as well as the majority of our directors are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries.
In recent years, these have included hostilities between Israel and Hezbollah in Lebanon and Hamas in the Gaza strip, both of which resulted in rockets being fired into Israel causing casualties and disruption of economic activities. Most recently, in July 2014, an armed conflict took place between Israel and Hamas. In addition, Israel faces threats from more distant neighbors, in particular, Iran.
Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflict involving Israel could adversely affect our operations and results of operations.
Further, our operations could be disrupted by the obligations of personnel to perform military service. As of December 31, 2014, we had 19 employees based in Israel, certain of whom may be called upon to perform up to 54 days in each three year period (and in the case of non-officer commanders or officers, up to 70 or 84 days, respectively, in each three year period) of military reserve duty until they reach the age of 40 (and in some cases, depending on their specific military profession up to 45 or even 49 years of age) and, in certain emergency circumstances, may be called to immediate and unlimited active duty. Our operations could be disrupted by the absence of a significant number of employees related to military service, which could materially adversely affect our business and results of operations.
Several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies whether as a result of hostilities in the region or otherwise. In addition, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods based on Israeli government policies. Such actions, particularly if they become more widespread, may adversely impact our ability to sell our products.
We received an Israeli government grant for certain research and development activities. The terms of the grant require us to satisfy specified conditions and to pay penalties in addition to repayment of the grant upon certain events.
Our research and development efforts were financed in part through a grant from the Israeli Office of the Chief Scientist, or OCS. The total gross amount of the grant actually received by us from the OCS, including accrued LIBOR interest as of December 31, 2014, totaled approximately $0.8 million. As of December 31, 2014, we had not paid any royalties to the OCS.
Even following full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Israeli Law for the Encouragement of Industrial Research and Development, 5744-1984, and related regulations, or collectively, the R&D Law. When a company develops know-how, technology or products using OCS grants, the terms of these grants and the R&D Law restrict the transfer outside of Israel of such know-how, and the manufacturing or manufacturing rights of such products, technologies or know-how, without the prior approval of the OCS. Therefore, if aspects of our technologies are deemed to have been developed with OCS funding, the discretionary approval of an OCS committee would be required for any transfer to third parties outside of Israel of know-how or manufacturing or manufacturing rights related to those aspects of such technologies. We may not receive those approvals. Furthermore, the OCS may impose certain conditions on any arrangement under which it permits us to transfer technology or development out of Israel.
The transfer of OCS-supported technology or know-how or manufacturing or manufacturing rights related to aspects of such technologies outside of Israel may involve the payment of significant penalties and other amounts, depending upon the value of the transferred technology or know-how, the amount of OCS support, the time of completion of the OCS-supported research project and other factors. These restrictions and requirements for payment may impair our ability to sell our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction involving the transfer outside of Israel of technology or know-how developed with OCS funding (such as a merger or similar transaction) may be reduced by any amounts that we are required to pay to the OCS.
Provisions of Israeli law may delay, prevent or otherwise impede a merger with, or an acquisition of, us, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless, following consummation of the tender offer, the acquirer would hold at least 98% of the company’s outstanding shares. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition, unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek such appraisal rights.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred.
It may be difficult to enforce a judgment of a U.S. court against us or our officers and directors in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our officers and directors.
We are incorporated in Israel. Some of our executive officers and almost all of our directors reside outside of the United States, and most of our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
Your rights and responsibilities as a shareholder will be governed by Israeli law, which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our ordinary shares are governed by our amended articles of association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in U.S.-based companies. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain from abusing its power in the company, including, among other things, in voting at a general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a vote at a meeting of the shareholders or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company with regard to such vote or appointment. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders of U.S. companies.
Item 4. INFORMATION ON THE COMPANY
| | A. | History and Development of the Company |
Our History
Our legal and commercial name is Macrocure Ltd. We were formed as a company in Israel on January 14, 2008. We are a biotechnology company focused on developing, manufacturing and commercializing novel cell therapy products to address unmet needs in the treatment of chronic and other hard-to-heal wounds.
In August 2014, we completed our IPO, pursuant to which we sold 5.35 million ordinary shares for aggregate gross proceeds (before underwriting discounts, commissions and expenses) of $53.5 million. Upon the consummation of our IPO, our ordinary shares began trading on the NASDAQ Global Market, under the symbol “MCUR”.
We are subject to the provisions of the Israeli Companies Law, 5759-1999. Our corporate headquarters are located at 25 Hasivim Street, Petach Tikva 4959383, Israel. Our telephone number is +972-3-923-5556 and our web site is located at www.macrocure.com (the information contained therein or linked thereto shall not be considered incorporated by reference in this annual report). Our U.S. agent is Puglisi & Associates, located at 850 Library Avenue, Suite 204, Newark, Delaware 19711.
Principal Capital Expenditures
Our capital expenditures for fiscal years 2014, 2013 and 2012 amounted to $0.2 million, $0.1 million and $0.1 million, respectively. Capital expenditures consist primarily of investments in Leasehold improvement and the design of our new plant. We anticipate our capital expenditures in fiscal year 2015 to be approximately $4.0 million. We anticipate our capital expenditures in 2015 to be financed from the proceeds of our IPO in August 2014.
We are a biotechnology company focused on developing, manufacturing and commercializing novel cell therapy products to address unmet needs in the treatment of chronic and other hard-to-heal wounds. Our product candidate, CureXcell, is an AWC therapy to treat such wounds by injecting living human white blood cells that have been activated to facilitate the healing process. CureXcell is currently in two pivotal, Phase 3, double-blind clinical trials targeting a broad indication for the treatment of DFUs and VLUs. We anticipate results from our DFU clinical trial and interim data from our VLU clinical trial in the second half of 2015. We expect to submit our BLA to the FDA in late 2016. We also intend to pursue marketing authorization in Europe with the EMA. We already hold product approval for CureXcell as a medical device in Israel for the treatment of chronic and other hard-to-heal wounds, and have effectively and safely treated more than 5,000 patients in commercial or clinical study settings in Israel. To support our development and manufacturing initiatives for CureXcell, we have established operations in Philadelphia, Pennsylvania in the United States and the Tel Aviv region in Israel.
In a 131-patient post-marketing trial conducted in Israel and completed in 2011, CureXcell achieved full closure of hard-to-heal wounds in approximately 71% of patients in a study with a challenging patient population. In total, CureXcell has completed nine clinical trials to date. In addition to clinical data, our technologies have been presented in several peer-reviewed publications, and CureXcell has support from key opinion leaders, or KOLs, in the wound care field. Based on nearly 15 years of clinical data and experience, we believe CureXcell provides patients and physicians significant clinical benefits. While CureXcell’s completed clinical trials support this belief, our two ongoing Phase 3 clinical trials will be critical in obtaining regulatory approval and demonstrating CureXcell’s safety and efficacy. See “—CureXcell and its Clinical History” for a discussion of CureXcell’s completed and ongoing clinical trials.
CureXcell is an injectable suspension of living human white blood cells, including macrophages, neutrophils and lymphocytes that are crucial to initiating, promoting and completing the process of cellular regeneration and wound healing. We use our proprietary cell activation technology to trigger these cells to release growth factors and other biochemical factors that improve the healing environment in the wound bed and stimulate wound closure. We source white blood cells from fully-screened, healthy volunteer blood donors through established relationships with blood banks, including the American Red Cross. CureXcell is an easy to administer, ready-to-use, monthly therapy. A physician draws CureXcell from its package using a standard syringe for superficial injection into a patient’s wound. Based on our experience in Israel and clinical trials, a typical course of CureXcell treatment involves injection applications administered once per month for three months to achieve complete wound closure.
Through CureXcell and our proprietary cell based technology, we intend to target a significant unmet market opportunity with a large patient population that imposes a financial burden on healthcare systems. Chronic and other hard-to-heal wounds represent a $25 billion burden on the U.S. healthcare system alone. With our initial focus on the treatment of patients with hard-to-heal DFUs and VLUs, we expect to target a market of approximately $5.3 billion worldwide with a patient population of more than 1.7 million in the United States and Europe. We believe CureXcell will offer patients and physicians a highly efficacious, safe and easy-to-use therapy to treat these wounds.
Our Market Opportunity
Chronic and other hard-to-heal wounds, which are wounds that do not close within four weeks following the use of conventional medical treatment, represent a significant global market opportunity and a substantial unmet medical need. In many cases, these wounds take several months to heal and, in some cases, never heal. Patients with hard-to-heal DFUs and VLUs are initially treated in either a primary care setting or an emergency room and, if the wound cannot be treated at these locations, patients are then referred to a wound care center, vascular surgeon, orthopedic surgeon or podiatrist. According to the research firm The Advisory Board Company, there are currently over 1,500 wound care centers in the United States treating such patients.
Diabetic Foot Ulcers
Diabetes is a chronic, life-threatening disease with no known cure. According to the International Diabetes Foundation, or IDF, the worldwide prevalence of diabetes was approximately 380 million in 2013 and is expected to grow to approximately 590 million by 2035, due in part to an aging population and the rising incidence of obesity. In the United States alone, the IDF estimated that approximately 25 million people, or approximately 11% of the adult population, had diabetes in 2013.
If left untreated or mismanaged, diabetes can cause an increase in blood glucose levels, which in turn can lead to a reduction in blood flow to the feet and nerve damage that can cause patients to lose sensation in their feet. This loss of sensation may prevent these patients from noticing injuries to the feet that can develop into very difficult to heal open sores or ulcers. In the United States, 3.5% of the diabetic patients, or approximately 0.9 million people, develop DFUs every year, making DFUs one of the most significant, prevalent and recurring co-morbidities of diabetes. Unhealed DFUs can lead to amputation, significant disability or potentially death.
Venous Leg Ulcers
Chronic venous insufficiency, or the inability of blood vessels in the leg to properly return blood to the heart, varicose veins and chronic venous hypertension can result in reduced blood flow and pooling of blood in the legs. This can lead to fluid leakage into surrounding tissue, swelling, increased pressure on the veins and eventually the formation of VLUs. The risk of VLUs can be increased by heart disease and other vascular diseases, blood clots, obesity, smoking, lack of physical activity or work that requires many hours of standing. These slow-to-heal ulcers usually form on the sides of the lower leg, above the ankle and below the knee, and often recur if preventative steps are not taken. In the United States and Europe, VLUs affect more than 2.2 million people annually. VLUs can have a dramatic impact on a patient’s quality of life due to pain, odor and reduced mobility.
The Wound Healing Process
The normal wound healing process is a well-orchestrated, complex and interlinked series of phases in which cell interactions, growth factors and other biochemical factors mediated by white blood cells coordinate to restore function and rebuild damaged tissue. White blood cells are a major, widely dispersed cell population and the presence of activated white blood cells, such as macrophages, neutrophils and lymphocytes, at the wound surface is critical to maximizing a wound’s healing potential. Although wound healing occurs in a continuous, integrated manner, the overall process can be divided into three phases.
Inflammation (days 1-4) – After a wound develops, inflammatory mediators are released and cause blood vessels adjacent to the injured area to become more permeable leading to localized heat, swelling and discomfort. The wound produces straw colored liquid that bathes the wound with nutrients, actively cleanses the wound surface and facilitates healing by neutrophils (which clean the wound) and macrophages (which produce a variety of substances that regulate and promote healing). An individual’s ability to heal a wound is influenced by a number of systemic factors, such as age, co-morbidities and lifestyle as well as conditions at the wound site, such the quality of blood circulation.
Proliferation (days 4-21) – A decrease in wound size is achieved by a combination of the physiological processes of filling of the wound (granulation), reduction in wound size (contraction), and covering of the wound with skin (epithelialization). During granulation, new tissue forms while macrophages and lymphocytes produce a variety of substances that stimulate new blood vessel formation and recruit fibroblasts to the site. Fibroblasts are a type of cell that synthesizes the extracellular matrix and collagen fibers that form a scaffold for new tissue. After tissue production, fibroblasts congregate around the edge of the wound and work to pull the wound’s edges together which plays a significant part in the healing of large, open wounds. The regeneration of skin cells across the wound surface occurs during the final stage of proliferation.
Remodeling (day 21-year 2) – Remodeling of scar tissue is stimulated by macrophages and results in the reorganization of collagen fibers to maximize the strength of the wound area over time.
In healthy individuals with no underlying conditions, an acute wound should heal within three weeks with remodeling occurring over the next year or two. Conditions that impair blood circulation and suppress the immune response, such as diabetes or venous insufficiency, can disrupt the wound healing process leaving the wound in the inflammatory stage. CureXcell provides activated living human white blood cells that are crucial to restarting and completing the natural wound healing process in these patients.
Current Standard of Care and Other Advanced Wound Care Products
Initial treatment for DFUs and VLUs focuses on good ulcer care, or GUC. The key components of GUC include:
| | • | the optimal control of the underlying disease (for example, medical management of diabetes, vascular insufficiency or elevated venous pressure); |
| | • | offloading of DFUs using shoe modification, casts, walkers or other means; |
| | • | compression therapy for VLUs where the affected limb is wrapped with elastic bandages or support stockings to reduce swelling; |
| | • | local wound care, including the cleaning and removal of non-viable tissue (referred to as debridement) and application of wound dressings or bandages; |
| | • | the prevention and control of wound infection, including through the use of antibiotics; and |
| | • | ensuring the limb has adequate blood circulation. |
After failing to respond to GUC for four weeks, DFUs or VLUs are considered hard-to-heal, at which point AWC therapies are often administered.
Currently, there are a number of commercially available AWC products for the treatment of chronic and other hard-to-heal wounds, including, but not limited to, negative pressure wound therapy, or NPWT, skin substitutes, amniotic allografts, hyperbaric oxygen therapy and other treatment approaches.
| | Negative pressure wound therapy |
NPWT (such as products marketed by Kinetic Concepts, Inc. and Smith & Nephew plc), which is used to treat DFUs, is a topical treatment that promotes wound healing by applying a vacuum, continuously or intermittently (often daily or a number of times each week), through a special sealed dressing to draw fluid out from the wound and increase blood flow to the wound area.
This therapy can be very demanding for patients, often requiring frequent use over two to three months to achieve wound closure, which is a significant burden in both therapy time and physician costs.
Skin substitutes (such as Apligraf and Dermagraft, both marketed by Organogenesis Inc.) are swatches of tissue derived from human or animal materials that are typically applied to, or draped over, a wound.
These products generally require weekly treatment over the course of one to two months. Moreover, because many of these products utilize non-human biological material, such as animal collagen, these products are not broadly approved for use in Europe due to concerns there regarding the use of non-human biologic materials. In addition, depending on the size of the wound, there is costly product wastage due to the fixed size of the tissue swatches.
Amniotic allografts (such as EpiFix, marketed by MiMedx Group, Inc., and Grafix, marketed by Osiris Therapeutics, Inc.) are products made from human placenta, but they do not contain living cells, other than Grafix, which contains living stem cells.
Many of these products obtained FDA approval under the human cell and tissue products, or HCT/P, regulatory pathway, which does not require rigorous clinical data and FDA supervised clinical trials. As a result, many of these products have not demonstrated meaningful clinical benefits, which often reduces their adoption by providers and reimbursement by payers.
| | Hyperbaric oxygen therapy |
Hyperbaric oxygen therapy, which is used to treat DFUs, involves exposing the wound to an oxygen-rich environment under air pressure.
We are not aware of any randomized placebo controlled trials that support the clinical efficacy of expensive therapy.
| | Other treatment approaches |
Other treatment approaches include acellular non-human skin substitutes and growth factors (such as Oasis and Regranex, respectively, both of which are marketed by Smith & Nephew plc).
Our Solution
Our novel approach is to treat and close chronic and other hard-to-heal wounds by injecting the human body’s own wound healing and regenerative components directly into the wound itself. CureXcell is a unique combination of living human white blood cells that have been activated to facilitate the healing process and stimulate wound closure. CureXcell addresses each phase of healing in the impaired wound, including the production of growth factors and other biochemical factors involved in fibroblast activation, cell migration and extracellular matrix production, stimulating the body’s natural healing process. Our delivery method of direct superficial injection into the chronic wound allows for precise delivery of the cells into the defective wound tissue where they can be most effective. This is in contrast to other AWC products that are applied to the surface of the chronic wound and thus do not come into direct contact with the impaired wound cells below the surface layer. We believe the clinically differentiated profile of CureXcell will be attractive to patients and healthcare providers to treat hard-to-heal DFUs and VLUs without the drawbacks of currently available AWC products.
In order to produce CureXcell, we source white blood cells from fully-screened, healthy volunteer blood donors through established relationships with blood banks. We then activate the white blood cells through our proprietary hypo-osmotic shock cell activation technology, a process in which we change the concentration and pH of the suspension surrounding the cells. Once activated, these cells undergo an increase in gene expression that results in an increase in the cells’ secretion of numerous growth factors and other biochemical factors. The activated suspension is then placed into sterile packaging, akin to a blood bag. At the wound care clinic or other treatment site, a physician draws CureXcell from its package using a standard syringe for superficial injection into a patient’s wound. The biochemical factors found in CureXcell stimulate the normal wound healing process to begin and recruit other necessary cells already found in the wound bed, to facilitate the healing process. Based on our experience in Israel and clinical trials, a typical course of CureXcell treatment involves injection applications administered once per month for three months to achieve complete wound closure.
CureXcell has been approved as a medical device in Israel and has been utilized in more than 5,000 patients in commercial or clinical study settings with consistent results. Notably, in a 131-patient post-marketing trial we conducted in Israel and completed in 2011, CureXcell achieved complete wound closure in approximately 71% of hard-to-heal wounds. Patient inclusion criteria in this study were very broad, and permitted patients with large ulcers, poor circulation and co-morbidities, such as infection and amputation, to be included, while similar patients were excluded from many of the trials of other AWC products.
Comparison of Published Clinical Results of Hard-To-Heal and Chronic AWC Products
The first chart below compares the wound closure rate CureXcell achieved in a post-marketing clinical trial to the published wound closure rates reported for certain AWC products in the largest of their respective published clinical trials for DFUs. While these comparisons are not based on head-to-head studies of these AWC therapies and as a result the wound closure rates derived from these separate clinical trials may not be comparable and would not form a basis for marketing CureXcell, if approved, we believe these data suggest that CureXcell has the potential to achieve a high wound closure rate even though our trial had the broadest inclusion criteria among the studies presented. Specifically, our CureXcell trial included patients that would be expected to have the slowest healing rates, such as those with large ulcers, poor circulation and co-morbidities such as infection and amputation, while the trials of other AWC products excluded such patients. CureXcell closed approximately 68% of the wounds in this more challenging patient population over an average of 12 weeks and 2.9 treatments. CureXcell’s clinical trial did not include a sham treatment arm because CureXcell was already approved in Israel, which was the site of the trial. To assess the statistical significance of the trial data, we used an assumed sham closure rate, or AR, of 36%. We chose what we believe is a higher AR than would have been observed based on the patient inclusion criteria in our trial because it results in a more rigorous test of statistical significance. Despite using this more demanding AR, we still achieved statistically significant results as shown in the table below. We believe that had this trial included a sham treatment arm, the actual sham rate would have been lower than the AR. Based on our review of various published clinical trial reports for AWC therapies, we believe our actual sham rate would have been closer to that of three trials conducted by others that had patient inclusion criteria most comparable to those used in our trial; those trials observed sham closure rates between 15% and 20%.

This comparison does not reflect a head-to-head trial. Please see “Item 3.D. Risk Factors—Risks Relating to Our Business and Industry—We may be limited in the promotional claims we can make and may not be able to use information about our competitors to promote or market CureXcell without incurring significant regulatory or enforcement risks” for additional information.
AR* This bar represents an assumed sham closure rate, or AR, of 36%. This trial did not include a sham treatment arm because CureXcell was already approved in Israel, which was the site of the trial. We chose what we believe is a higher AR than what would be expected based on the patient inclusion criteria in our trial because the higher AR results in a more rigorous test of statistical significance.
RE* The Oasis trial’s control patients were treated with Regranex. This bar represents the percentage of Regranex-treated patients who had complete wound closure after 12 weeks of treatment in this trial.
† This comparison does not reflect a head-to-head trial. Please see “Item 3.D. Risk Factors—Risks Relating to Our Business and Industry—We may be limited in the promotional claims we can make and may not be able to use information about our competitors to promote or market CureXcell without incurring significant regulatory or enforcement risks” for additional information.
(1) Data Not Available: This portion of the trial’s complete results and protocol has not been formally published.
(2) As NPWT is continuously applied and the trial patient’s dressings were changed no fewer than three times per week, the chart assumes three treatments per week.
(3) As the clinician had discretion whether to reapply Oasis at weekly clinic visits, the chart assumes one treatment per week.
† This comparison does not reflect a head-to-head trial. Please see “Item 3.D. Risk Factors—Risks Relating to Our Business and Industry—We may be limited in the promotional claims we can make and may not be able to use information about our competitors to promote or market CureXcell without incurring significant regulatory or enforcement risks” for additional information.
(1) The CureXcell study was a post-marketing, observational, open-label single-arm trial conducted in Israel in 2010 and 2011; 131 patients were enrolled in the study, 58 of whom were treated for DFUs. The NPWT study was a post-marketing, multicenter, randomized, controlled trial studying DFUs with results published in 2008. The Apligraf study was a pivotal, Phase 3 randomized, controlled, prospective, multicenter trial studying noninfected neuropathic DFUs with results published in 2001. The Dermagraft study was a pivotal, Phase 3 randomized, controlled, prospective, single blind, multicenter trial studying chronic DFUs with results published in 2003. The Oasis study was a randomized, controlled, prospective, multicenter trial comparing Oasis wound matrix to Regranex gel for diabetic ulcers with results published in 2005. The Grafix study was a randomized, controlled, single blind multicenter trial for the treatment of chronic DFUs with an overview of results published by Osiris Therapeutics, Inc. in 2013. The GraftJacket study was a randomized, controlled, prospective, multicenter study comparing the proportion of healed DFUs and mean healing time between patients receiving acellular matrix and standard of care therapies with results published in 2009. The Regranex study was a Phase 3 randomized, placebo controlled, double-blind, multicenter trial for patients with chronic neuropathic diabetic ulcers with results published in 1998.
(2) ABI, or ankle-brachial index, is the ratio of the systolic blood pressure in a patient’s ankle to the systolic blood pressure in the patient’s arm. Lower measurements of ABI generally indicate poorer blood circulation in a patient’s leg, with corresponding adverse implications for wound healing.
(3) Data Not Available: The published results did not include an ABI lower limit; however, to ensure that arterial circulation was adequate, other measurements were taken.
(4) Any infection or cellulitis present before debridement had to be well-controlled before randomization and patients were excluded if osteomyelitis affecting the area of the target ulcer was present.
(5) “P-value” (relative to placebo) means the probability that no true difference exists between the rates for wound closure for the relevant patient group and the control group based on the observed results. For example, a “P-value” of less than 0.0001 indicates that there is a less than 1 in 10,000 chance that the rates in the treatment group and the observed result in the control group are the same. A “P-value” equal or less than 0.05 is generally accepted as meaning a given difference is statistically significant.
(6) To assess the statistical significance of the trial data, we used an assumed sham closure rate, or AR, of 36%. This trial did not include a sham treatment arm because CureXcell was already approved in Israel, which was the site of the trial. We chose what we believe is a higher AR than what would be expected based on the patient inclusion criteria in our trial because it results in a more rigorous statistical analysis. Using this more demanding AR, we still achieved statistically significant results as shown in the table above.
We believe the clinical efficacy of CureXcell and its ease-of-therapy and lower cost to achieve wound closure will drive adoption if CureXcell is approved in the United States and Europe. CureXcell is a once per month injection application and requires limited product preparation. This is in contrast to other AWC therapies that require daily or weekly applications and often significant product preparation. Accordingly, we believe CureXcell is easier for physicians to deliver and supports patient compliance. In addition, we can customize the CureXcell dosage size to different wound sizes and avoid the significant product waste that can be associated with other AWC products, especially skin substitutes. We believe that these features will position CureXcell to become a frontline AWC treatment for hard-to-heal DFUs and VLUs while also enjoying favorable reimbursement policies from payers. Accordingly, we believe the product will bring advantages to all primary stakeholders in the wound care space, namely patients, physicians and payers.
CureXcell and its Clinical History
Since its initial development, CureXcell has been tested in a total of 11 clinical studies designed to investigate its safety and efficacy, of which nine have been completed and two are still ongoing. CureXcell has been approved for treatment of chronic wounds by the Israeli Ministry of Health as a medical device and has been included in the Israeli health basket of reimbursable medications since 2011, enabling use of CureXcell for treatment of various types of chronic wounds throughout the country. Since 2000, more than 5,000 patients suffering from chronic and other hard-to-heal wounds were treated with CureXcell either in commercial or clinical study settings in Israel.
Our company was founded in 2008 with the goal of further developing and commercializing CureXcell and its underlying technology. After in-licensing the intellectual property underlying the development and manufacturing of CureXcell, in 2011, we submitted an investigational new drug application, or IND, to the FDA based on the conducted clinical studies of CureXcell’s safety and efficacy. Additionally, the FDA considered the results from our completed clinical trials and as these studies showed a good safety and efficacy profile for CureXcell when considered together, the FDA allowed us to proceed directly to Phase 3 trials without completing Phase 1 and 2 trials. We have not sought, and do not intend to seek, a Special Protocol Assessment from the FDA.
CureXcell is currently in two pivotal Phase 3, double-blind clinical trials targeting a broad indication for the treatment of DFUs and VLUs. We anticipate results from our DFU clinical trial and interim data from our VLU clinical trial in the second half of 2015. We expect to submit our BLA to the FDA in late 2016. We also intend to pursue marketing authorization in Europe with the EMA. In August 2013, we retained a CRO to carry out our clinical trials and implement the trial process planned by our clinical trials team.
CureXcell Current and Planned Phase 3 Clinical Trials
Pivotal Phase 3 Clinical Trial for Treatment of DFUs
We began this study in September 2011 and expect it to conclude in the second half of 2015. It is composed of two main phases: a core double-blind treatment phase and a follow-up phase. To ensure the blinding of the core double-blind phase, the study treatment is administered by an un-blinded treatment administrator, who is not involved with any other aspects of the study, including patient assessments (except those related to treatment administration); both patient and evaluator remain blinded.
The study include a total of 285 enrolled patients who will be allocated to either treatment with CureXcell or a control in combination with GUC based on a 2:1 assignment ratio, respectively. Up to 30 wound centers located primarily in the United States, with additional centers in Canada and Israel, are enrolled as sites in the study.
The primary efficacy endpoint is the proportion of patients with complete closure of their target ulcer at any time during the core double-blind treatment period, with sustained complete closure for four additional weeks. The target ulcer is the largest lower extremity ulcer at baseline that meets all entry criteria, provided it has not decreased in area more than 25% subsequent to patient screening. For purposes of this clinical trial, complete closure is defined as the achievement of 100% ulcer re-epithelialization with no drainage present and no dressing required.
The secondary efficacy endpoints include the following:
• proportion of patients with at least 50% closure of target ulcer during the core double-blind treatment phase;
• time to complete closure of the target ulcer during the core double-blind treatment phase with sustained complete closure for four additional weeks;
• proportion of patients whose target ulcer completely closed during the core double-blind treatment phase and remained closed after twelve weeks;
• proportion of patients with certain negative ulcer-related outcomes; and
• proportion of patients whose target ulcer recurred during the follow-up period after achieving closure during the core double-blind treatment phase.
As of December 31, 2014, we have completed the enrollment in our pivotal, double-blind, Phase III clinical trial of CureXcell in DFU. A total of 280 patients at 25 participating sites have been enrolled and randomized. An independent data monitoring committee last met in January 2015 to review the patient data and the committee recommended that the study continue unmodified.
Phase 3 Clinical Trial for Treatment of VLUs
We began this study in April 2014, expect to complete an interim analysis in the second half of 2015, and expect the study to conclude in the second half of 2016. This study has two main phases: a core double-blind treatment phase and a follow-up phase. To ensure the blinding of the core double-blind phase, the study treatment will be administered by an un-blinded treatment administrator, who will not be involved with any other aspects of the study, including patient assessments (except those related to treatment administration); both patient and evaluator will remain blinded.
The study will include a total of 252 enrolled patients who will be allocated to either treatment with CureXcell or a control in combination with GUC. We plan to enroll up to 30 wound centers, located in the United States, in the study.
The primary efficacy endpoint is time to complete closure of the target ulcer. For purposes of this clinical trial, complete closure of target ulcer is defined as 100% re-epithelialization without drainage or dressing requirements. Complete closure is confirmed at consecutive study visits two weeks apart. The time of complete closure will be the visit when 100% re-epithelialization without drainage or dressing requirements was first confirmed. The final confirmation of complete closure will occur two weeks after the first confirmation of complete closure.
The secondary efficacy endpoints include the following:
• proportion of complete closure of target ulcer within the treatment phase;
• percentage change from baseline in target ulcer surface area at the end of the treatment phase;
• proportion of complete closure of target ulcer at each visit within the treatment phase;
• percentage surface area reduction from baseline, at each visit within the treatment phase;
• proportion of target ulcer recurrence, during the follow-up phase;
• change in quality of life assessments from baseline; and
• reduction in pain from baseline.
Other Ongoing Studies
Mechanism of Action Study
The MOA study was conducted to evaluate the wound healing effects of CureXcell in an athymic rat splinted full-thickness dermal wound model. The study was intended to provide scientific evidence to clinical and regulatory teams supporting the biological activity of CureXcell and demonstrate the effect of CureXcell on multiple aspects of wound repair, including wound closure, angiogenesis, granulation tissue formation, collagen deposition and organization. The results demonstrated that treatment with CureXcell significantly accelerated wound closure and percent re-epithelialization as compared to sham wounds in this model and this treatment effect for the CureXcell wound cohort over the sham wound cohort did not diminish 14 days post treatment. It was also reported that CureXcell-treatment enhanced the granulation tissue formation response that was significantly greater, more robust and sustained than the sham control wounds. In regard to angiogenesis, immunohistochemistry analysis of von Willebrand Factor (vWF) and alpha-smooth muscle action was conducted. The data indicated that CureXcell-treatment enhances blood vessel formation and maturation as compared to the sham control wounds. To measure collagen deposition and organization, immunohistochemistry analysis of collagen I and trichrome staining was conducted. The data indicated that CureXcell-treatment enhances collagen deposition and organization. This data also suggests that CureXcell induces tissue remodeling and maturation.
In addition to in-vivo, an in-vitro product characterization study was also conducted to evaluate CureXcell’s composition. The analysis reported that fifty-five (55) different cytokines and growth factors were present in CureXcell. Also, the results, demonstrated that leukocytes activation resulted in alteration in cell subpopulations and increased production of biochemical factors that promote tissue repair and remodeling.
CureXcell Completed Clinical Studies
| Study | | Year | | Design | | Endpoint | | Number Treated (CureXcell /control) | | CureXcell Results(a) | | Control Results(a) | | P-value(b) |
| Post-marketing multi-center study to define procedures for the use in a community setting | | 2010-11 | | Observational, open-label, Phase 4 | | Full Wound Closure(c) | | 131/0 | | 70.9% | | N/A | | 0.01(d) |
| Post-marketing multi-center observational study | | 2009-12 | | Observational, Phase 4 | | Adverse Events(c) | | 70/0 | | 5.7%(e) | | N/A | | N/A(f) |
| Proof-of-concept study for the treatment of anal fissures | | 2008-09 | | Open-label, proof of concept | | Full Wound Closure | | 5/0 | | 40.0% | | N/A | | N/A(f) |
| Prophylaxis of post-sternotomy incision site infection study | | 2007 | | Prospective, single-blind, randomized | | Infection Prevention(c) | | 33/31 | | 97.0%(g) | | 83.9%(g) | | N/A(f) |
| Treatment of wounds with exposed bone study | | 2006-07 | | Observational, open-label, Phase 4 | | Full Wound Closure | | 6/0 | | 83.3% | | N/A | | N/A(f) |
| Hard-to-heal pressure ulcers study | | 2004-06 | | Prospective, open-label, non-randomized, Phase 4 | | Full Wound Closure(c) | | 66/38 | | 59.1% | | 5.3% | | 0.001 |
| Prophylaxis of saphenous vein harvesting incision site infection study | | 2004-06 | | Prospective, single-blind, randomized | | Infection Prevention(c) | | 22/22 | | 100%(g) | | 72.7%(g) | | N/A(f) |
| Infected leg wounds following saphenous vein harvesting study | | 2000-04 | | Retrospective, historically controlled, non-randomized, Phase 4 | | Full Wound Closure | | 95/113 | | 97.9% | | 85.8% | | 0.0001 |
| Deep sternal wound infections study | | 2000-03 | | Historically controlled, retrospective, non-randomized, Phase 4 | | Mortality(c) | | 66/64 | | 3.0%(h) | | 29.7%(h) | | 0.001 |
(a) Results reported as percent of healed patients unless otherwise noted.
(b) “P-value” (relative to placebo) means the probability that no true difference exists between the rates for wound closure for the relevant patient group and the control group based on the observed results. For example, a “P-value” of less than 0.0001 indicates that there is a less than 1 in 10,000 chance that the rates in the treatment group and the observed result in the control group are the same. A “P-value” equal or less than 0.05 is generally accepted as meaning a given difference is statistically significant.
(c) Included safety as a measured outcome.
(d) To assess the statistical significance of the trial data, we used an assumed sham closure rate, or AR, of 36%. This trial did not include a sham treatment arm because CureXcell was already approved in Israel, which was the site of the trial. We chose what we believe is a higher AR than what would be expected based on the patient inclusion criteria in our trial because it results in a more rigorous statistical analysis. Using this more demanding AR, we still achieved statistically significant results as shown in the table above.
(e) Results reported as percent of patients experiencing product-related Serious Adverse Events, or SAEs.
(f) P-values were not reported in these studies.
(g) Results reported as percent of uninfected patients.
(h) Results reported as percent of patient mortality; none of these were associated with the treatment.
Post-Marketing Multi-Center Study to Define Procedures for Use in a Community Setting. This study was a post-marketing, observational, open-label single-arm study conducted in Israel in 2010 and 2011. In this study, 131 patients with chronic wounds, including DFUs, pressure ulcers, VLUs and post-operative wounds, with no improvement for at least three weeks were treated with CureXcell. The patients enrolled into this study had significant co-morbid medical conditions and long-standing histories of diabetes or vascular disease. These patients were studied for complete wound closure or for a period of 24 weeks.
Complete wound closure was observed in 68.5% and 70.9% of treated patients at 24 weeks and at study completion, respectively (p<0.01). The wound type with the highest rate of wound closure at 24 weeks was VLUs, with a closure rate of 81.4%, followed by post-operative wounds, with a closure rate of 70.0%, and diabetic wounds, with a closure rate of 68.4%. Pressure wounds demonstrated a smaller wound closure rate at 24 weeks of 35.3%. Additionally, a total of 38 SAEs were reported in 29 patients, out of which seven were considered product-related. These events included four cases of wound infections and three cases of cellulitis.
Post-Marketing Multi-Center Observational Study. This study was a post-marketing, observational, open-label, single-arm study conducted in Israel from 2009 through 2012, and the primary endpoint was safety, as measured by incidence of adverse events. In this study, 70 patients with chronic and/or refractory wounds, including DFUs, sinus ulcers, pressure ulcers, post-operative ulcers and burn ulcers, with no improvement for at least four weeks were treated with CureXcell. The patients enrolled into this study had significant co-morbid medical conditions and long-standing histories of diabetes and vascular disease leading to limb amputations. The secondary endpoint was complete wound closure.
Complete wound closure was achieved in 51.4% of the treated patients. A total of 38 SAEs were reported in 22 patients, four of which were considered to be product-related. These events included one case of each of cellulitis, wound infection, osteopenia and necrosis of the toe with subsequent amputation. Wound infection, cellulitis and amputation are common clinical findings in patients with hard-to-heal wounds and complex underlying disease such as diabetes mellitus.
Proof-of-Concept Study on the Treatment of Anal Fissures. This study was a post-marketing, observational, open-label, proof-of-concept study conducted in Israel in 2008 and 2009. Patients were followed for fissure closure for four weeks. In 40%, or two out of five patients, the fissures were completely closed within four weeks from treatment. Two other patients experienced pain relief and a decrease in size of the wound. No adverse events were reported.
Prophylaxis of Post-Sternotomy Incision Site Infection Study. This study was a post-marketing, prospective, controlled, randomized, single-blind study conducted in Israel in 2007. In this study, 64 patients undergoing cardiac surgery were randomized to either CureXcell with standard of care treatment or standard of care treatment alone; 33 patients were in the CureXcell treated group and 31 patients were in the control group. Patients were followed for infection at the incision site throughout the study period of three months after hospital discharge. In the CureXcell treated group, 3.0%, or one of 33, of the patients experienced a deep sternal wound infection, compared to 16.1%, or five of 31, of the patients in the control group. No product-related SAEs were observed in this study.
Treatment of Wounds with Exposed Bone Study. This study was an observational, open-label, Phase 4 study conducted in Israel in 2006 and 2007. Six patients suffering from chronic wounds with exposed bone and refractory to other treatments were treated with CureXcell. Patients were followed until full wound closure was obtained. Five out of six, or 83.3%, of the wounds were closed within four to ten weeks. Additionally, no product-related adverse events were observed in the treated patients.
Hard-to-Heal Pressure Ulcers Study. This study was a historically prospective, Phase 4, open-label, non-parallel study conducted in Israel from 2004 through 2006. In this study, patients were treated with a standard of care treatment during the first year of the study and, during the second year of the study, patients were treated with CureXcell. The investigators designed the study in this manner to reduce the seasonal effect of wound-healing, as patients of each group were treated throughout the entire year. A total of 100 patients with stage III-IV pressure ulcers were enrolled into the study, out of which 66 patients were treated with CureXcell and four patients were included in both groups at different time periods. Patients were eligible if they suffered from at least one pressure ulcer at stage III-IV, and all patients were hospitalized, with most suffering from multiple co-morbidities and generally poor health. Patients were followed for complete wound closure and time to wound closure.
Of the ulcers treated with CureXcell in this study, 69.5%, or 98 out of 141, achieved wound closure compared to 13.3%, or 10 out of 75, of the ulcers in the control group (p<0.001). In addition, 59.1%, or 39 out of 66, of the patients treated with CureXcell achieved complete wound closure in comparison to 5.3%, or two out of 38, of the control patients (p<0.001). Also, a faster median healing time of 87.0 days was noted in patients treated with CureXcell when compared to a median healing time of 117.7 days in the control group, though this difference was not found to be statistically different.
For the different population subsets studies (patients with diabetes mellitus, patients with lower leg pressure ulcers, and patients with diabetes mellitus with baseline wounds greater than or equal to 15 cm2), similar results were observed. No adverse or SAEs related to CureXcell were reported.
Prophylaxis of Saphenous Vein Harvesting Incision Site Infection Study. This study was a post-marketing, prospective, randomized, single-blind study conducted in Israel from 2004 through 2006. In this study, 44 patients undergoing coronary artery bypass, or CABG, surgery were randomized to either CureXcell with standard of care treatment or standard of care treatment alone; 22 patients were placed in each group. Patients were followed for infection at the incision site throughout the study period of three months after hospital discharge. None of the patients in the CureXcell group experienced an infected saphenous vein incision site. This is compared to 27%, or six of 22, of the patients experiencing infection in the control group. No product-related SAEs were observed in this study.
Infected Leg Wounds following Saphenous Vein Harvesting Study. This study was a retrospective, historically controlled, non-randomized, Phase 4 study conducted in Israel from 2000 through 2004. A total of 208 patients suffering from infected leg wounds post-CABG surgery were enrolled into the study, out of which 95 patients were treated with CureXcell. Patients were followed for complete wound closure and time to wound closure.
Of the patients treated with CureXcell, 97.9%, or 93 out of 95, achieved wound closure compared to 85.8%, or 97 out of 113, of the patients in the control group (p<0.001). Additionally, the average time to wound closure was 47 days in the CureXcell group compared to 66.5 days in the control group.
Deep Sternal Wound Infections Study. This study was a historically controlled, retrospective, non-randomized, Phase 4 study conducted in Israel from 2000 through 2003. A total of 130 patients with deep sternal wound infections were included in the study, of which 66 patients were treated with CureXcell and data from 64 patients were retrospectively collected. All patients had previously undergone open heart surgery, with most of them suffering from multiple co-morbidities. Patients were followed for mortality and percent of wound closure.
Two out of 66, or 3.0%, the treatment group patients suffered late deaths, compared to 19 late deaths, or 29.7%, in the control group (p<0.001). Both deaths in the treatment group were considered unrelated to CureXcell. Sixty patients, or 90.9%, in the CureXcell group achieved complete wound closure. No CureXcell-related adverse events were observed. Furthermore, no deterioration or worsening of wound condition was observed in the treated patients.
In parallel to the groups above, an additional group of 50 patients with superficial sternal wound infection were treated by CureXcell and were monitored for complete wound closure. All of these patients achieved full closure in 22.6 days after a single CureXcell treatment.
Research and Development
A significant portion of our historical research and development efforts have focused on the CureXcell production process. Specifically, we have invested significantly in order to enable production in closed systems of kits containing transfusion bags. Similarly, our research and development strategy is centered on further developing the CureXcell production process so as to extend the shelf life of the product and to enable its packaging in containers other than transfusion bags, which will enable us to produce a greater range of dosages so as to further maximize product utilization. We are also researching the mode of action of our cell activation technology in order to leverage the technology for the development of a regenerative medicine product platform for non-wound indications. Our research and development team is located at our facilities in Israel and the United States, and consisted of 20 employees as of December 31, 2014.
In the past, we received government grants that were subject to the payment of royalties as part of our research and development programs approved by the OCS. The total gross amount of grants actually received by us from the OCS, including accrued LIBOR interest, totaled approximately $0.8 million as of December 31, 2014. According to the terms of the grants, the OCS is entitled to royalties equal to 3.0% to 4.5% of our sales, up until the amount of the grants is repaid in full. As of December 31, 2014, we had not paid any royalties to the OCS.
We incurred approximately $15.5 million, $9.3 million and $7.2 million in research and development expenses, net in the years ended December 31, 2014, 2013 and 2012, respectively.
Supply and Production
The production of CureXcell begins with whole blood units from healthy donors. The whole blood is separated into three components, red blood cells, plasma and white blood cells, through centrifugation. The white blood cells and plasma are the raw materials we use for production. These raw materials are processed in clean rooms in a process that currently takes approximately 20 hours from production initiation to product release and that involves transformation of plasma into serum (plasma without any blood cells and clotting proteins), activation of white blood cells through controlled hypo-osmotic shock achieved by the introduction of water and certain salt solutions, further centrifugation to separate out the activated white blood cells and suspension of the white blood cells in serum. The entire manufacturing process subsequent to processing of the raw materials occurs in a closed system of six plastic transfusion and infusion bags. We test for transfusion transmitted diseases and sterility at various points in the process.
In the United States, the American Red Cross currently supplies our raw material and manufactures CureXcell under agreements that we entered in March 2013 and July 2010, which we refer as the American Red Cross Supply Agreement and the American Red Cross Manufacturing Agreement, respectively. Under the American Red Cross Supply Agreement, the American Red Cross procures, pursuant to our request, white blood cells and plasma from human donors. Under the American Red Cross Manufacturing Agreement, after sourcing those materials, the American Red Cross performs the entire manufacturing process, including testing and quality assurance at its facility pursuant to the technical specifications provided by us, and then packages and ships the product to the sites at which our clinical trials take place. Only American Red Cross personnel that have been trained by us may perform the services that the American Red Cross is required to provide under the American Red Cross Manufacturing Agreement, and we are entitled to have a representative at the manufacturing plant for purposes of real time observation of production, testing, packaging, quality assurance, shipment and related activities.
The terms of each of the American Red Cross Supply Agreement and American Red Cross Manufacturing Agreement, each as amended in April 2014, will extend through April 25, 2017. If either party desires to extend either agreement further, it must notify the other party at least nine months prior to the end of the then-current term, and if the parties mutually agree to such an extension, they may negotiate an amendment. Each such agreement may be terminated by either party in the event of an uncured material breach by, or bankruptcy event (or, in the case of the American Red Cross Supply Agreement, the cessation of operations) of, the other party. Under our agreements with the American Red Cross, we pay the American Red Cross a fixed monthly payment, as well as additional fees that are tied to the amount of raw materials it supplies to, and the amount of CureXcell that it produces for, us.
In Israel, the source of our raw material is the whole blood inventory of MDA. In addition, the manufacturing of CureXcell is carried out by MDA technicians supervised by our employees at the MDA’s central blood bank facility where we have our own clean room. Pursuant to the MDA Agreement, we are obligated to pay MDA fixed per unit prices (subject to adjustment for the Israeli consumer price index and for significant changes in the costs of production). The MDA Agreement terminates upon the expiration of the Danon patent in June 2015, a material breach of the MDA Agreement or upon certain bankruptcy events. When the Danon patent expires in June 2015, MDA, or any other party can develop, manufacture and commercialize products using the manufacturing process described in the Danon patent or design another process that circumvents the intellectual property directed to our current manufacturing processes. In addition, we expect to pay MDA royalties of 1% of CureXcell net sales outside of Israel, defined as amounts we receive in connection with the marketing, distribution and sales of CureXcell, if and when CureXcell is offered for sale in such countries.
While we believe that our current production capacity is sufficient to meet the needs for our current and planned clinical studies as well as the expected initial demand for CureXcell if it receives FDA approval, in anticipation of approval and commercialization of CureXcell we intend to invest in the establishment of our production facilities equipped to receive raw material from blood banks and to produce commercial quantities of CureXcell in clean rooms.
As CureXcell is a biologic product with living cells, it must be processed and packaged in kits consisting of sterile plastic transfusion and infusion bags that are designed to maintain the proper environment for CureXcell. We procure these bags from a supplier located in France, which manufactures the bags on the basis of technological specifications that we provide.
The FDA protocol for our clinical trials currently restricts the shelf life of CureXcell to seven days from production completion. Further, our regulatory approvals require that we manufacture our product from blood of the same type as the patient who will be using it and that the blood come from a donor meeting certain requirements, including for age and health.
Marketing and Sales
We intend to commercialize CureXcell in the United States and Europe by building our commercial organization, establishing pricing and reimbursement and implementing a comprehensive marketing campaign and branding and training programs. In anticipation of (and subject to) regulatory approval of CureXcell, we will establish a focused commercial organization in the United States and certain targeted markets in Europe. We also intend to initiate processes with insurance companies and health maintenance organizations for reimbursement coverage in our target markets in the United States and to file for reimbursement in our target markets in the European Union. In the United States, we expect to establish reimbursement through an existing procedure code for therapeutic injections and to file for a new product code rather than be subject to the codes applicable to skin substitutes. We will also commence marketing CureXcell to physicians. Furthermore, we intend to build an internal sales force to target hospitals and U.S. Veterans Administration health centers that treat wounds and private wound care centers.
We also plan to enter into other international markets through collaboration with local distributors and leverage our approved registration files in the United States and Europe to obtain regional marketing authorizations. At that point, we anticipate entering into local distribution agreements for distribution in individual countries, subject to country-by-country regulatory approval, which may take additional time.
We have commissioned ongoing third-party preliminary pricing studies in the United States and in various European countries in order to suggest an approximate sales price per CureXcell treatment sample. We expect that the price point for CureXcell will reflect CureXcell’s benefits and potential cost savings relative to alternative treatments.
Intellectual Property
Our intellectual property and proprietary technology are important to the development and production of CureXcell and in developing and maintaining our competitive position. We seek to protect our intellectual property, core technologies and other know-how, through a combination of trade secrets, know how, confidential information, non-disclosure and confidentiality agreements, licenses, assignments of invention and other contractual arrangements with our employees, consultants, partners, suppliers, customers and others, as well as patents and trademarks. Additionally, we rely on our research and development program, clinical trials and know-how to advance CureXcell. We also rely on protection available under trademark laws, and we currently hold a registered trademark for the mark “CureXcell” in the United States and Israel.
We have five patent families on file covering processes and resulting activated white blood cell compositions that we developed, and their use. From one of those families we have been granted five patents. One patent in each of the United States and the European Union has claims covering our process for producing activated white blood cell compositions, and one patent in each of Australia, China and South Africa has claims covering our process of producing activated white blood cell compositions, the compositions themselves, and their use in treating wounds. From another of those families we have been granted one patent in Australia, which has claims covering producing activated white blood cell conditioned supernatant, the supernatants themselves, and their use in treating wounds. We also have three recently allowed applications and 41 additional pending applications in various jurisdictions, the most important of which separately cover the (i) method for activating white blood cells through hypo-osmotic shock and (ii) the composition of CureXcell. We submit applications under the Patent Cooperation Treaty, or PCT, which is an international patent law treaty that provides a unified procedure for filing a single initial patent application to seek patent protection for an invention simultaneously in each of the member states. Although a PCT application is not itself examined and cannot issue as a patent, it allows the applicant to seek protection in any of the member states through national-phase applications.
Because our issued patents cover the process of manufacturing CureXcell, they offer protection against competition that is, to some extent, more limited than the protection provided by patents which claim products or chemical structures which were previously unknown. To the extent our issued patents remain limited to process claims, it may be difficult for us to detect infringing products and enforce our patents against them. Absent patent-term extensions, our key patents are nominally due to expire in 2030.
While our policy is to obtain patents by application, license or otherwise, to maintain trade secrets and to seek to operate without infringing on the intellectual property rights of third parties, technologies related to our business have been rapidly developing in recent years. Additionally, patent applications that we may file or license from third parties may not result in the issuance of patents, the claims that issue may have limited or no coverage of our products and technologies, and our issued patents and any issued patents that we may receive in the future may be challenged, invalidated or circumvented. For example, we cannot predict the extent of claims that may be allowed or enforced in our patents nor be certain of the priority of inventions covered by pending third-party patent applications. If third parties prepare and file patent applications that also claim technology or therapeutics to which we have rights, we may have to partake in proceedings to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable to us. Moreover, because of the extensive time required for clinical development and regulatory review of a product we may develop, it is possible that, before CureXcell can be commercialized, whether for its currently anticipated applications or otherwise, related patents will have expired or will expire a short period following commercialization, thereby reducing the advantage of such patent. Further, the exclusivity of our ownership of our issued United States patent may be subject to our receipt of additional assignments from certain inventors. These inventors have previously executed assignments to us in connection with certain of the provisional applications related to the issued United States patent and we are beginning the process of obtaining confirmatory assignments from them. Loss or invalidation of certain of our patents, or a finding of unenforceability or limited scope of certain of our intellectual property, could have a material adverse effect on us. See “Item 3.D. Risk Factors—Risks Related to Our Intellectual Property—Our success depends in part on our ability to obtain and maintain protection for the intellectual property relating to or incorporated into our technology and products.”
In addition to patent protection, we also rely on trade secrets, including unpatented know-how, technology innovation, drawings, technical specifications and other proprietary information in attempting to develop and maintain our competitive position.
Danon License Agreement
In January 2008, we entered into an exclusive license agreement with Professor David Danon, pursuant to which we acquired use of certain technology that relates to culturing macrophages from blood and related technologies, which we refer to as the licensed technology. We did not use the specific technology licensed under this agreement to manufacture the CureXcell used in the clinical trials we describe herein and will not use it in the future because we developed our current, more effective processes and products that are covered by the four patent families described above. Our current technology utilizes a different method for preparing and activating the blood cells used to produce CureXcell than the licensed technology, resulting in a higher concentration of viable and activated cells and, as a result, a more effective product for advanced wound care. Under this license agreement, we received an exclusive worldwide right and license to certain technology, including patents and patent applications relating to the licensed technology, which expire in June 2015. We agreed to pay Professor Danon milestone payments upon the achievement of clinical milestones and royalties from sales derived from any products developed under the licensed technology. In January 2011, we entered into an amendment to the license agreement with Professor Danon pursuant to which we were granted an option to make a one-time payment of $1.2 million, plus Israeli value added tax, in consideration of his waiving any and all rights to future royalties under the original agreement. We exercised that right in April 2011 and paid the sum to Professor Danon. We are obligated to pay Professor Danon milestone payments not to exceed an aggregate of $2.0 million upon our receipt of certain regulatory approvals or upon certain other events. As of December 31, 2014, we have paid Professor Danon $0.2 million for consulting services.
Competition
The medical, biotechnology and pharmaceutical industries are intensely competitive and subject to significant technological and practice changes. While we believe that our innovative technology, knowledge, experience and scientific resources provide us with competitive advantages, we may face competition from many different sources with respect to CureXcell. Possible competitors include medical practitioners, pharmaceutical and wound care companies, academic and medical institutions, governmental agencies and public and private research institutions, among others. Any product that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
If we obtain regulatory approval for CureXcell, we would compete with the current methods and products for treatment of hard-to-heal wounds. Currently, there are a number of commercially available AWC products for the treatment of chronic and other hard-to-heal wounds, including, but not limited to, NPWT, skin substitutes, amniotic allografts, hyperbaric oxygen therapy and other treatment approaches. See “—Current Standard of Care and Other Advance Wound Care Products” above in this Item 4.B.
Government Regulation
Our business is subject to extensive government regulation. Regulation by governmental authorities in the United States, the European Union and other jurisdictions is a significant factor in the development, manufacture and marketing of CureXcell and in our ongoing research and development activities.
United States
Review and Approval of Biologics
CureXcell is an investigational drug in the United States and subject to various regulations. In the United States, the FDA regulates drugs and biologics under the Federal Food, Drug, and Cosmetic Act and implementing regulations and other laws, including the Public Health Service Act. The FDA has classified CureXcell as a biological product. Biologics require the submission of a BLA and approval by the FDA prior to being marketed in the United States. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to a variety of administrative or judicial sanctions, and enforcement actions brought by the FDA, the U.S. Department of Justice or other governmental entities. Possible sanctions may include the FDA’s refusal to approve pending BLAs, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties.
The process required by the FDA prior to marketing and distributing a biologic in the United States generally involves the following:
• completion of laboratory tests, animal studies and formulation studies in compliance with the FDA’s Good Laboratory Practices, or GLP, or cGMP regulations, as applicable;
• submission to the FDA of an IND application which must become effective before clinical trials may begin;
• approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated;
• performance of adequate and well-controlled clinical trials in accordance with GCP to establish the safety and efficacy of the product for each indication;
• preparation and submission to the FDA of a BLA or supplemental BLA;
• satisfactory completion of an FDA advisory committee review, if applicable;
• satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMP requirements, and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; and
• payment of user fees and FDA review and approval of the BLA.
Preclinical Studies
Preclinical studies include laboratory evaluation, as well as animal studies to assess the potential safety and efficacy of the product candidate. Preclinical safety tests must be conducted in compliance with FDA regulations regarding GLP. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND, which must become effective before clinical trials may be commenced.
Clinical Trials in Support of a BLA
Clinical trials involve the administration of an investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written study protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. An IND automatically becomes effective thirty days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin.
In addition, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health for public dissemination on their ClinicalTrials.gov website.
Clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Phase 1: The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
Phase 2: The drug is administered to a limited patient population to identify possible short-term adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
Phase 3: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Submission of a BLA to the FDA
In the United States, we are conducting our pivotal Phase 3 studies for CureXcell for the treatment of DFUs and VLUs to support a BLA submission to the FDA, which we currently anticipate will occur in late 2016. The results of the preclinical studies and clinical trials, together with other detailed information, including information on the manufacture, control and composition of the product, are submitted to the FDA as part of a BLA requesting approval to market the product candidate for a proposed indication. Under the Prescription Drug User Fee Act, as amended, applicants are required to pay fees to the FDA for reviewing a BLA. These user fees, as well as the annual fees required for commercial manufacturing establishments and for approved products, can be substantial. The BLA review fee alone can exceed $2,100,000, subject to certain limited deferrals, waivers and reductions that may be available. Each BLA submitted to the FDA for approval is typically reviewed for administrative completeness and reviewability within forty-five to sixty days following submission of the application. If found complete, the FDA will “file” the BLA, thus triggering a full review of the application. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission. The FDA’s established goal is to review 90% of BLA applications and original efficacy supplements given priority status within six months and 90% of applications and original efficacy supplements given standard status within ten months, whereupon a review decision is to be made. The FDA, however, may not approve a biologic within these established goals, and its review goals are subject to change from time to time. Further, the outcome of the review, even if generally favorable, may not be an actual approval but rather an “action letter” that describes additional work that must be completed before the application can be approved.
Before approving a BLA, the FDA inspects the facilities at which the product is manufactured or facilities that are significantly involved in the product development and distribution process, and will not approve the product unless cGMP compliance is satisfactory. The FDA may deny approval of a BLA if applicable statutory or regulatory criteria are not satisfied, or may require additional testing or information, which can delay the approval process. FDA approval of any application may include many delays or may never be granted. If a product is approved, the approval will impose limitations on the indicated uses for which the product may be marketed, may require that warning statements be included in the product labeling, and may require that additional studies be conducted following approval as a condition of the approval, may impose restrictions and conditions on product distribution, prescribing or dispensing in the form of a risk management plan, or impose other limitations.
Once a product is approved, marketing the product for other indicated uses or making certain manufacturing or other changes requires FDA review and approval of a supplement BLA or a new BLA, which may require additional clinical data and review fees. In addition, further post-marketing testing and surveillance to monitor the safety or efficacy of a product may be required. Also, product approvals may be withdrawn if compliance with regulatory standards is not maintained or if safety or manufacturing problems occur following initial marketing. In addition, new government requirements may be established that could delay or prevent regulatory approval of our product candidate under development.
Post-Approval Requirements
Any drug or biologic products for which we receive FDA approvals are subject to continuing regulation by the FDA. Certain requirements include, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information on an annual basis or more frequently for specific events, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements. These promotion and advertising requirements include, among others, standards for direct-to-consumer advertising, prohibitions against promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling, known as “off-label use,” and other promotional activities, such as those considered to be false or misleading. Failure to comply with FDA requirements can have negative consequences, including the immediate discontinuation of noncomplying materials, adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Such enforcement may also lead to scrutiny and enforcement by other government and regulatory bodies. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not encourage, market or promote such off-label uses. As a result, off-label promotion has formed the basis for litigation under the U.S. False Claims Act, violations of which are subject to significant civil fines and penalties. In addition, manufacturers of prescription products are required to disclose annually to the Center for Medicaid and Medicare any payments made to physicians in the United States under the Sunshine Act of 2012. These payments could be in cash or kind, could be for any reason, and are required to be disclosed even if the payments are not related to the approved product. Failure to fully disclose or not in time reporting could lead to penalties up to $1 million per year.
The manufacturing of CureXcell and any subsequent products is and will be required to comply with applicable FDA manufacturing requirements contained in the FDA’s cGMP regulations. CureXcell is currently manufactured on our behalf by the American Red Cross at its production facility in Pennsylvania, which is cGMP certified. The FDA’s cGMP regulations require, among other things, quality control and quality assurance, as well as the corresponding maintenance of comprehensive records and documentation. Drug and biologic manufacturers and other entities involved in the manufacture and distribution of approved drugs and biologics are also required to register their establishments and list any products they make with the FDA and to comply with related requirements in certain states. These entities are further subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in serious and extensive restrictions on a product, manufacturer or holder of an approved BLA, as well as lead to potential market disruptions. These restrictions may include recalls, suspension of a product until the FDA is assured that quality standards can be met, and continuing oversight of manufacturing by the FDA under a consent decree, which frequently includes the imposition of costs and continuing inspections over a period of many years, as well as possible withdrawal of the product from the market. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented. Other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
The FDA also may require post-marketing testing, or Phase 4 testing, as well as risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions on an approval that could otherwise restrict the distribution or use of CureXcell.
Pediatric Studies and Exclusivity
CureXcell is currently in Phase 3 clinical trials to investigate its safety and efficacy for the treatment of diabetic foot ulcers in adult diabetic patients. However, even when not pursuing a pediatric indication, under the Pediatric Research Equity Act of 2003, a BLA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With enactment of the Food and Drug Administration Safety and Innovation Act, or the FDASIA, in 2012, sponsors must also submit pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, the FDA, and the FDA’s internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in the FDASIA.
Separately, in the event the FDA makes a written request for pediatric data relating to a biologic product, a BLA sponsor who submits such data may be entitled to pediatric exclusivity. Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent and orphan exclusivity.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the “Hatch-Waxman Act,” which permits a patent restoration of up to five years for patent term lost during product development and the FDA regulatory review. The restoration period granted is typically one-half the time between the effective date of an IND and the submission date of a BLA, plus the time between the submission date of a BLA and the ultimate approval date. Patent term restoration cannot be used to extend the remaining term of a patent past a total of fourteen years from the product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple drugs for which approval is sought can only be extended in connection with one of the approvals. The USPTO reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
Biosimilar products
As part of the Patient Protection and Affordable Care Act of 2010, Public Law No. 111-148, under the subtitle of Biologics Price Competition and Innovation Act of 2009, or BPCI, a statutory pathway has been created for licensure, or approval, of biological products that are biosimilar to, and possibly interchangeable with, earlier biological products licensed under the Public Health Service Act. Also under the BPCI, innovator manufacturers of original reference biological products are granted twelve years of exclusive use before biosimilars can be approved for marketing in the United States. There are current legislative proposals to shorten this period from 12 years to seven years. The objectives of the BPCI are conceptually similar to those of the Hatch-Waxman Act, which established abbreviated pathways for the approval of drug products. The implementation of an abbreviated approval pathway for biological products is under the direction of the FDA and is currently being developed. In February 2012, the FDA published draft guidance documents on biosimilar product development. A biosimilar is defined in these documents as a biological product that is highly similar to an already approved biological product, notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biosimilar and the approved biological product in terms of safety, purity and potency. Under this proposed approval pathway, biological products are approved based on demonstrating they are biosimilar to, or interchangeable with, a biological product that is already approved by the FDA, which is called a reference product. The approval of a biologic product biosimilar to CureXcell could have a materially adverse impact on our business, may be significantly less costly to bring to the market and may be priced significantly lower than CureXcell, but such approval may only occur after our twelve-year exclusivity period.
European Union
The approval process of medicinal products in the European Union generally involves satisfactorily completing each of the following:
• laboratory tests, animal studies and formulation studies all performed in accordance with the applicable EU GLP or cGMP regulations;
• submission to the relevant national authorities and ethics committees of a clinical trial application, or CTA, which must be approved before human clinical trials may begin;
• performance of adequate, well-designed and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication;
• submission to the relevant competent authorities (for example, the EMA in case of an advanced therapy medicinal product) of a marketing authorization application, or MAA, which includes the data supporting preclinical and clinical safety and efficacy as well as detailed information on the product characteristics, composition and manufacture and composition and the control of the product development and proposed labeling as well as other information;
• inspection by the relevant national authorities of the manufacturing facility or facilities and quality systems (including those of third parties) at which the product is produced, to assess compliance with strictly enforced cGMP;
• potential audits of the preclinical and clinical trial sites that generated the data in support of the MAA; and
• review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product.
Quality/Preclinical studies
In order to assess the potential safety and efficacy of a product, tests include laboratory evaluations of product characterization, analytical tests and controls, as well as studies to evaluate pharmacological, pharmacokinetic and toxicity effects in animals. The conduct of the preclinical tests and formulation of the compounds for testing must comply with the relevant European Union regulations and requirements. In particular, GLP and cGMP compliance are required for toxicity testing. The results of such tests, together with relevant manufacturing control information and analytical data, are submitted as part of the CTA. For cell based medicinal products, the key focus is on product characterization and mode of action clarification. Conventional administration, distribution, metabolism, excretion studies, are not expected, however proof of concept in animals, as well as biodistribution, persistence of the cells in situ and toxicity (in particular, tumorigenicity and allosensitization) are requested.
Clinical trial approval
Pursuant to the Clinical Trials Directive 2001/20/EC, as amended, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, approval must be obtained from the competent national authority of a European Union member state in which a study is planned to be conducted. To this end, a CTA is submitted, which must be supported by an investigational medicinal product dossier and further supporting information prescribed by the Clinical Trials Directive and other applicable guidance documents. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the clinical trial application in that country.
Clinical drug development is often described as consisting of four temporal phases (Phase 1- 4), see for example EMA’s note for guidance on general considerations for clinical trials (CPMP/ICH/291/95).
• Phase 1 (Most typical kind of study: Human Pharmacology);
• Phase 2 (Most typical kind of study: Therapeutic Exploratory);
• Phase 3 (Most typical kind of study: Therapeutic Confirmatory); and
• Phase 4 (Variety of Studies: Therapeutic Use).
Studies in Phase 4 are all studies (other than routine surveillance) performed after drug approval and are related to the approved indication.
The phase of development provides an inadequate basis for classification of clinical trials because one type of trial may occur in several phases. The phase concept is a description, not a set of requirements. The temporal phases do not imply a fixed order of studies since for some drugs in a development plan the typical sequence will not be appropriate or necessary.
Pediatric Investigation Plans
On January 26, 2007, Regulation (EC) 1901/2006 came into force with its primary purpose being the improvement of the health of children without subjecting children to unnecessary trials, or delaying the authorization of medicinal products for use in adults. The regulation established the Pediatric Committee, or PDCO, which is responsible for coordinating the EMA’s activities regarding pharmaceutical drugs for children. The PDCO’s main role is to determine which studies the applicant needs to perform in the pediatric population as part of the PIP.
All applications for marketing authorization for new pharmaceutical products that were not authorized in the European Union prior to January 26, 2007 have to include the results of studies carried out in children of different ages. The PDCO determines the requirements and procedures of such studies, describing them in a PIP. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized. The PDCO can grant deferrals for some medicines, allowing a company to delay development of the medicine in children until there is enough information to demonstrate its effectiveness and safety in adults. The PDCO can also grant waivers when development of a medicine in children is not needed or is not appropriate, such as for diseases that only affect the elderly population.
Before a marketing authorization application can be filed, or an existing marketing authorization can be amended, the EMA confirms that the applicant complied with the studies’ requirements and measures listed in the PIP. Since the regulation became effective, several incentives for the development of medicines for children become available in the European Union, including:
• medicines that have been authorized for marketing in the European Union with the results of PIP studies included in the product information are eligible for an extension of their patent protection by six months. This is the case even when the studies’ results are negative;
• scientific advice and protocol assistance at the EMA are free of charge for questions relating to the development of medicines for children; and
• medicines developed specifically for children that are already authorized but are not protected by a patent or supplementary protection certificate, can apply for a pediatric use marketing authorization, or PUMA. If a PUMA is granted, the product will benefit from 10 years of market protection as an incentive.
Marketing authorization
Authorization to market a product in the European Union member states proceeds under one of four procedures: a centralized authorization procedure, a mutual recognition procedure, a decentralized procedure or a national procedure. A marketing authorization may be granted only to an applicant established in the European Union.
The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The centralized procedure is compulsory for medicines produced by certain biotechnological processes, products designated as orphan medicinal products and products with a new active substance indicated for the treatment of certain diseases (such as diabetes or cancer), and is optional for those products that are highly innovative or for which a centralized process is in the interest of patients. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. Under the centralized procedure in the European Union, the maximum time frame for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the Scientific Advice Working Party of the Committee of Medicinal Products for Human Use).
In general, if the centralized procedure is not followed, there are three alternative procedures.
• Mutual recognition procedure. If an authorization has been granted by one member state, or the Reference Member State, an application may be made for mutual recognition in one or more other member states, or the Concerned Member State(s).
• Decentralized procedure. The decentralized procedure may be used to obtain a marketing authorization in several European member states when the applicant does not yet have a marketing authorization in any country.
• National procedure. Applicants following the national procedure will be granted a marketing authorization that is valid only in a single member state. Furthermore, this marketing authorization is not based on recognition of another marketing authorization for the same product awarded by an assessment authority of another member state. If marketing authorization in only one member state is preferred, an application can be filed with the national competent authority of a member state. The national procedure can also serve as the first phase of a mutual recognition procedure.
It is not always possible for applicants to follow the national procedure. In the case of medicinal products in the category for which the centralized authorization procedure is compulsory, that procedure must be followed. In addition, the national procedure is not available in the case of medicinal product dossiers where the same applicant has already obtained marketing authorization in one of the other European Union member states or has already submitted an application for marketing authorization in one of the other member states and the application is under consideration. In the latter case, applicants must follow a mutual recognition procedure.
After a drug has been authorized, it must be launched within three years in the targeted markets in order to keep a valid MAA. Also it is a condition of maintaining the marketing authorization that all aspects relating to its quality, safety and efficacy must be kept under review. For cell-based medicinal products, a long term safety and efficacy post-marketing plan must be completed. Sanctions may be imposed for failure to adhere to the conditions of the marketing authorization. In extreme cases, the authorization may be revoked, resulting in withdrawal of the product from sale.
Period of authorization and renewals
Marketing authorization shall be valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder shall provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least nine months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization shall be valid for an unlimited period, unless the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the European Union market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization shall cease to be valid.
Regulatory data protection
Without prejudice to the law on the protection of industrial and commercial property, all applications for marketing authorization receive an 8+2+1 protection regime. This regime consists of a regulatory data protection period of eight years plus a concurrent market exclusivity of ten years plus an additional market exclusivity of one further year if, during the first eight years of those ten years, the marketing approval holder obtains an approval for one or more new therapeutic indications which, during the scientific evaluation prior to their approval, are determined to bring a significant clinical benefit in comparison with existing therapies. Under the current rules, a third party may reference the preclinical and clinical data of the original sponsor beginning eight years after first approval, but the third party may market a generic version only after ten (or eleven) years have lapsed.
Additional data protection can be applied for when an applicant has complied with all requirements as set forth in an approved PIP.
Manufacturing
The manufacturing of authorized drugs, for which a separate manufacturer’s license is mandatory, must be conducted in strict compliance with the EMA’s cGMP requirements and comparable requirements of other regulatory bodies, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. The EMA enforces its cGMP requirements through mandatory registration of facilities and inspections of those facilities. The EMA may have a coordinating role for these inspections while the responsibility for carrying them out rests with the member states competent authority under whose responsibility the manufacturer falls. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenue, and could subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil and criminal penalties.
Marketing and promotion
The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Community, notably under Directive 2001/83 in the European Community code relating to medicinal products for human use, as amended by Directive 2004/27. The applicable regulation aims to ensure that information provided by holders of marketing authorizations regarding their products is truthful, balanced and accurately reflects the safety and efficacy claims authorized by the EMA or by the competent authority of the authorizing member state. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
Review and Approval of Drug Products Outside the United States and the European Union
In addition to the above regulations, we must obtain approval of a product by the comparable regulatory authorities of foreign countries outside of the United States and the European Union before we can commence clinical trials or marketing of CureXcell in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA or EMA approval. In addition, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. In all cases, clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we obtain regulatory approval. In the United States and other markets, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payers. Third-party payers include government health administrative authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payer will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payer will pay for the drug product. Third-party payers may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drug products for a particular indication. Third-party payers are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of CureXcell, in addition to the costs required to obtain the FDA approvals. Additionally, CureXcell may not be considered medically necessary or cost-effective. A payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In March 2010, the President of the United States signed one of the most significant healthcare reform measures in decades. The healthcare reform law substantially changes the way healthcare will be financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The comprehensive $940 billion dollar overhaul is expected to extend coverage to approximately 32 million previously uninsured Americans. The healthcare reform law contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse, which will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
Additionally, the healthcare reform law, as limited by the U.S. Supreme Court’s decision in June 2012:
• increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%;
• requires collection of rebates for drugs paid by Medicaid managed care organizations; and
• imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs.
There have been proposed in Congress a number of legislative initiatives regarding healthcare, including possible repeal of the healthcare reform law. At this time, it remains unclear whether there will be any changes made to the healthcare reform law, whether to certain provisions or its entirety.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
Other U.S. Federal Healthcare Laws and Regulations
Healthcare providers, physicians and third-party payers play a primary role in the recommendation and prescription of drug products and biologics that are granted marketing approval. Arrangements with healthcare providers, third-party payers and other customers are subject to broadly applicable fraud and abuse and other healthcare laws and regulations. Such restrictions under applicable federal and state healthcare laws and regulations, include the following:
• the federal healthcare Anti-Kickback Law prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid;
• the U.S. False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
• the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
• HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
• the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
• the federal transparency requirements under the Health Care Reform Law require manufacturers of drugs, devices and medical supplies to report to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests; and
• analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers.
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Environmental, Health and Safety Matters
We are subject to extensive environmental, health and safety laws and regulations in a number of jurisdictions, primarily Israel, governing, among other things: the use, storage, registration, handling, emission and disposal of chemicals, waste materials and sewage; chemicals, air, water and ground contamination; air emissions and the cleanup of contaminated sites, including any contamination that results from spills due to our failure to properly dispose of chemicals, waste materials and sewage. Our operations use chemicals and produce waste materials and sewage and require permits from various governmental authorities including, local municipal authorities, the Ministry of Environmental Protection and the Ministry of Health. The Ministry of Environmental Protection and the Ministry of Health, local authorities and the municipal water and sewage company conduct periodic inspections in order to review and ensure our compliance with the various regulations.
These laws, regulations and permits could potentially require the expenditure by us of significant amounts for compliance or remediation. If we fail to comply with such laws, regulations or permits, we may be subject to fines and other civil, administrative or criminal sanctions, including the revocation of permits and licenses necessary to continue our business activities. In addition, we may be required to pay damages or civil judgments in respect of third-party claims, including those relating to personal injury (including exposure to hazardous substances we use, store, handle, transport, manufacture or dispose of), property damage or contribution claims. Some environmental, health and safety laws allow for strict, joint and several liability for remediation costs, regardless of comparative fault. We may be identified as a responsible party under such laws. Such developments could have a material adverse effect on our business, financial condition and results of operations.
In addition, laws and regulations relating to environmental, health and safety matters are often subject to change. In the event of any changes or new laws or regulations, we could be subject to new compliance measures or to penalties for activities, which were previously permitted. For instance, new Israeli regulations were promulgated in 2012 relating to the discharge of industrial sewage into the sewer system. These regulations establish new and potentially significant fines for discharging forbidden or irregular sewage into the sewage system.
Legal Proceedings
See “Item 8. Financial Information—Consolidated Financial Statements and Other Financial Information—Legal proceedings.”
| | C. | Organizational Structure |
Our corporate structure consists of Macrocure Ltd., our Israeli parent company, and Macrocure, Inc., its wholly-owned subsidiary, which was incorporated on November 15, 2012 under the laws of the State of Delaware.
| | | Property, Plants and Equipment |
Our principal executive offices are located at 25 Hasivim Street, Petach Tikva 4959383, Israel. We lease these facilities from Amot Investments Ltd. and Clal Insurance Company Ltd., pursuant to a lease agreement that expires on January 31, 2019, with an option to extend the term for two one-year periods. The facilities consist of approximately 1,460 square feet of space, and lease payments are approximately $10,000 per month. These facilities house part of our administrative functions and our research and development laboratories. Our Israeli manufacturing facilities are housed in MDA’s central blood bank facility located at the Tel Hashomer Hospital outside Tel Aviv, where MDA produces CureXcell under our supervision. Our United States manufacturing facilities are housed by in an American Red Cross facility in the United States. The facilities consist of approximately 255 square feet of space in Philadelphia, Pennsylvania.
Item 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
Item 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following discussion of our financial condition and results of operations in conjunction with the financial statements and the notes thereto included elsewhere in this annual report. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this annual report, particularly those in “Item 3.D. Risk Factors.”
Overview
We are a biotechnology company focused on developing, manufacturing and commercializing novel cell therapy products to address unmet needs in the treatment of chronic and other hard-to-heal wounds. Our product candidate, CureXcell, is an AWC therapy to treat such wounds by injecting living human white blood cells that have been activated to facilitate the healing process. CureXcell is currently in two pivotal, Phase 3, double-blind clinical trials targeting a broad indication for the treatment of DFUs and VLUs. We anticipate results from our DFU clinical trial and interim data from our VLU clinical trial in the second half of 2015. We expect to submit our BLA to the FDA in late 2016. We also intend to pursue marketing authorization in Europe with the EMA. We already hold product approval for CureXcell as a medical device in Israel for the treatment of chronic and other hard-to-heal wounds, and have effectively and safely treated more than 5,000 patients in commercial or clinical study settings in Israel. To support our development and manufacturing initiatives for CureXcell, we have established operations in Philadelphia, Pennsylvania in the United States and the Tel Aviv region in Israel.
To date, we have financed our operations primarily with the net proceeds from the IPO and private placements of our ordinary and preferred shares and warrants, and to a significantly lesser extent, through a government grant. Since our inception, we have incurred significant operating losses. Our net losses were $25.5 million, $18.3 million and $7.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. As of December 31, 2014, we had an accumulated deficit of $68.2 million. We have not recognized any revenue to date.
We expect to continue to incur significant expenses and operating losses for the foreseeable future. The net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially if and as we:
| | • | continue our Phase 3 clinical trials for CureXcell to support a BLA submission to the FDA; |
| | • | establish independent production facilities and our sales, marketing and distribution infrastructure to commercialize CureXcell in the United States; |
| | • | seek marketing approvals for CureXcell in territories other than the United States; |
| | • | maintain, expand and protect our intellectual property portfolio; |
| | • | hire additional operational, clinical, quality control and scientific personnel; |
| | • | add operational, financial and management information systems and personnel, including personnel to support our product development, any future commercialization efforts and our transition to a public company; and |
| | • | invest in research and development and regulatory approval efforts in order to utilize our technology as a platform to develop regenerative medicine products for other indications. |
Financial Operations Overview
Revenue
To date, we have not recognized any revenue, and we do not expect to recognize any revenue for the foreseeable future, if ever. We view the sale of CureXcell in Israel to be part of our research and development activities, rather than commercial in nature; for example, the price of the products sold in Israel is below their cost with no marketing efforts. Moreover, we view our operations in Israel as a beta site for testing and evaluating our products as part of our research and development activities aimed at obtaining product approval in the United States and the European Union, our primary future commercial markets. Our business strategy does not include the Israeli market as a targeted commercial market. Accordingly, we recognize the amounts that we receive from sales of CureXcell to health care professionals in Israel as an offset to our research and development expense. Our ability to generate recognizable revenue in the future will depend on the successful commercialization of CureXcell.
Operating expenses
Research and development expenses, net
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect research and development costs to increase significantly for the foreseeable future as we progress with our Phase 3 clinical trials. We do not believe that it is possible at this time to accurately project total program-specific expenses to reach regulatory approval and commercialization. There are numerous factors associated with the successful regulatory approval and commercialization of CureXcell, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time. Additionally, future regulatory and commercial factors beyond our control will affect our clinical development programs and plans.
Research and development expenses are reduced by amounts that we receive from sales of CureXcell to health care professionals in Israel. We view these sales and use of CureXcell to be part of our research and development activities, rather than commercial in nature. Accordingly, we view and characterize these sales as an extension of our research and development activities, rather than as standalone revenues.
From 2012 through 2014, our cumulative research and development expenses for CureXcell were $32.0 million, which is net of $1.4 million that we received from sales of CureXcell to health care professionals in Israel. Our net research and development expenses in the years ended December 31, 2014 and 2013 were $15.5 million and $9.3 million, respectively, which primarily related to the development of CureXcell. We charge all research and development expenses to operations as they are incurred. We expect research and development expenses to increase in the near term.
Research and development expenses consist primarily of costs incurred for our research activities, including:
| | • | employee-related expenses for research and development staff, including salaries, benefits and related expenses, including share-based compensation and travel expenses; |
| | • | expenses incurred under agreements with third parties, including the American Red Cross, MDA, contract research organizations and consultants that conduct quality assurance and regulatory activities and clinical trials; |
| | • | expenses incurred to design, develop and assess clinical trials; |
| | • | facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other operating costs; |
| | • | costs associated with development, clinical and preclinical activities and regulatory operations; |
| | • | costs associated with obtaining and maintaining patents and other intellectual property; and |
| | • | depreciation of tangible and intangible fixed assets used to develop CureXcell. |
The successful development of CureXcell and future product candidates, if any, is highly uncertain. At this time we cannot reasonably estimate the nature, timing and estimated costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from, CureXcell and future product candidates. This uncertainty is due to numerous risks and uncertainties associated with developing products, including the uncertainty of:
| | • | the scope, rate of progress and expense of our research and development activities; |
| | • | preclinical and clinical trial results and the duration of the trials; |
| | • | the terms and timing of regulatory approvals and the ability to obtain reimbursement for our product candidates; |
| | • | our ability to build the manufacturing capacity and have the raw materials necessary to meet the future market demands; |
| | • | the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; and |
| | • | the ability to commercialize, market and achieve market acceptance. |
A change in the outcome of any of these variables with respect to the development of CureXcell or other products that we may develop could result in a significant change in the costs and timing associated with their development. For example, if the FDA, EMA or other regulatory authority were to require us to conduct additional preclinical or clinical studies beyond those which we currently anticipate for the completion of clinical development of CureXcell or if we experience significant delays in enrollment in any clinical trials, we could be required to expend significant additional financial resources and time on the completion of the clinical development.
General and administrative expenses
Our general and administrative expenses consist principally of:
| | • | employee-related expenses for employees other than research and development staff, including salaries, benefits and related expenses, including share-based compensation and travel expenses; |
| | • | legal and professional fees for auditors and other consulting expenses not related to research and development activities; |
| | • | costs of office leases, communication and office expenses; |
| | • | information technology expenses; and |
| | • | depreciation of tangible fixed assets related to our general and administrative activities. |
We expect that our general and administrative expenses will increase in the future as our business expands and we incur additional general and administrative costs associated with being a public company in the United States, including compliance with the Sarbanes-Oxley Act and rules promulgated by the SEC. These public company-related increases will likely include, among other things, costs of additional personnel, additional legal fees, accounting and audit fees, directors’ liability insurance premiums and costs related to investor relations. During the year ended December 31, 2014, we paid up to $0.65 million in the aggregate to certain of our executive officers for their contribution to the completion of our IPO.
Financing income (expenses), net
Financing income (expenses), net, is obtained by subtracting our financing expense from our financing income and adding or subtracting the gain or loss, as applicable, that we have realized due to the revaluation of warrants at fair value, reclassification of outstanding warrants from a liability into equity on our balance sheet and recognizing expenses for a warrants granted to the lender of the convertible credit line. Financing income includes interest income and exchange rate differences and change in fair value of warrants held by investors. Financing expense consists primarily of change in fair value of warrants held by investors, a convertible loan credit line from a significant shareholder and exchange rate differences.
Taxes on income
The standard corporate tax rate in Israel is 26.5% (beginning in 2014), and was 25.0% for each of the 2013 and 2012 tax years. We do not generate taxable income in Israel, as we have historically incurred operating losses resulting in carry-forward losses for tax purposes totaling $38.0 million as of December 31, 2014. We anticipate that we will be able to carry forward these tax losses indefinitely to future tax years. Accordingly, we do not expect to pay taxes in Israel until we have taxable income after the full utilization of our carry forward tax losses. In 2013 and 2014, taxes on income included taxes on income of our U.S. subsidiary, which operates on a cost-plus basis.
Comparison of the years ended December 31, 2014 and 2013
The following table summarizes our results of operations for the years ended December 31, 2014 and 2013.
| | | Year ended December 31, | |
| | | 2014 | | | 2013 | |
| | | (in thousands) | |
| Operating expenses: | | | | | | |
Research and development expenses, net | | $ | 15,542 | | | $ | 9,303 | |
General and administrative expenses | | | 5,374 | | | | 4,567 | |
Operating loss | | | (20,916 | ) | | | (13,870 | ) |
Financing expense, net | | | (4,504 | ) | | | (4,305 | ) |
Taxes on income | | | (31 | ) | | | (149 | ) |
Loss for the year | | $ | (25,451 | ) | | $ | (18,324 | ) |
| Other comprehensive loss | | | (26 | ) | | | - | |
| Total comprehensive loss | | $ | (25,477 | ) | | | - | |
Operating expenses
Research and development expenses, net
Research and development expenses, net increased by $6.2 million, or 67%, to $15.5 million in the year ended December 31, 2014 from $9.3 million in the year ended December 31, 2013. The increase was primarily due to progress in the development of CureXcell, including increased expenditures due to clinical trial costs associated with the increased recruitment for the DFU trial and the opening and operating new clinical sites in support of the VLU.
General and administrative expenses
General and administrative expenses increased by $0.8 million, or 18%, to $5.4 million in the year ended December 31, 2014 from $4.6 million in the year ended December 31, 2013. The increase primarily related to expenses incurred during the IPO process and other public company costs.
Financing expense, net
Financing expenses, net increased by $0.2 million to $4.5 of expenses in the year ended December 31, 2014 from $4.3 million of expense in the year ended December 31, 2013, primarily due to one-time non-cash expenses associated with a convertible credit line, made available to the Company, and for the year ended December 31,2013, revaluation of warrants as part of the 2013 financing round.
Loss for the year
Due to the cumulative effect of the factors described above, the most significant of which were the increase in our operating expenses, particularly due to increased research and development expenses, our net loss increased by $7.1 million, or 39%, to $25.5 million in the year ended December 31, 2014 from $18.3 million in the year ended December 31, 2013.
Taxes on income
In the year ended December 31, 2014, we incurred less than $0.1 million of income tax expenses due to the implementation of transfer pricing guidelines related to our U.S. subsidiary.
Comparison of the years ended December 31, 2013 and 2012
The following table summarizes our results of operations for the years ended December 31, 2013 and 2012.
| | Year ended December 31, | |
| | 2013 | | 2012 | |
| | (in thousands) | |
| Operating expenses: | | | | | | |
Research and development expenses, net | | $ | 9,303 | | | $ | 7,168 | |
General and administrative expenses | | | 4,567 | | | | 1,631 | |
Operating loss | | | (13,870 | ) | | | (8,799 | ) |
Financing income (expense), net | | | (4,305 | ) | | | 1,043 | |
Taxes on income | | | (149 | ) | | | - | |
Loss for the year | | $ | (18,324 | ) | | $ | (7,756 | ) |
Operating expenses
Research and development expenses, net
Research and development expenses, net increased by $2.1 million, or 30%, to $9.3 million in the year ended December 31, 2013 from $7.2 million in the year ended December 31, 2012. The increase was primarily due to progress in the development of CureXcell, including increased expenditures due to the higher recruitment rate for our first Phase 3 trial, clinical trial protocol design changes and increased headcount of employees focused on clinical operations.
General and administrative expenses
General and administrative expenses increased by $2.9 million, or 180%, to $4.6 million in the year ended December 31, 2013 from $1.6 million in the year ended December 31, 2012. The increase primarily related to higher stock-based compensation expenses and changes in headcount of employees due to the progress in the development of CureXcell.
Financing income (expense), net
Financing income (expense), net decreased by $5.3 million to $4.3 million of expense in the year ended December 31, 2013 from $1.0 million of income in the year ended December 31, 2012. For the year ended December 31, 2013, financing expense, net primarily represented the revaluation of warrants as part the 2013 financing round and for the year ended December 31, 2012, financing income, net primarily represented revaluation of warrants to fair value.
Loss for the year
Due to the cumulative effect of the factors described above, most significant of which were the increase in our operating expenses, particularly due to increased research and development expenses, as well as the other expenses that we recognized, our net loss increased by 136% to $18.3 million in the year ended December 31, 2013 from $7.8 million in the year ended December 31, 2012.
Taxes on income
In the year ended December 31, 2013, we incurred income tax expense of $0.1 million due to the implementation of transfer pricing guidelines related to our U.S. subsidiary. We did not incur any taxes on income in the year ended December 31, 2012.
Effective Corporate Tax Rate
We are subject to corporate taxes in various countries in which we operate. Generally, Israeli companies are subject to corporate tax at a rate of 25% of a company’s taxable income for 2013, increasing to 26.5% as of 2014 and thereafter. However, our effective corporate tax rate in Israel could be significantly lower, due to tax benefits for which we may become eligible, as described below.
Israeli Tax Structure and Tax Programs That May Become Applicable to Our Company
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, provides several tax benefits for “Industrial Companies.” We may in the future qualify as an Industrial Company within the meaning of the Industry Encouragement Law.
The Industry Encouragement Law defines an “Industrial Company” as a company resident in Israel, of which 90% or more of its income in any tax year, other than income from defense loans, is derived from an “Industrial Enterprise” owned by it. An “Industrial Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
• amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which are used for the development or advancement of the Industrial Enterprise;
• under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and
• expenses related to a public offering are deductible in equal amounts over three years.
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
Tax Benefits
The Investment Law provides certain benefits for income generated by a “Preferred Company” through its “Preferred Enterprise” (as such terms are defined in the Investment Law) provided certain conditions are met. The definition of a Preferred Company includes a company incorporated in Israel that is not wholly-owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled and managed from Israel. As of January 1, 2014, a Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to its income derived by its Preferred Enterprise, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 9%.
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at source at the rate of 20% with respect to dividends to be distributed on or after January 1, 2014, subject to certain conditions, or such lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required to be withheld. Although, if subsequently distributed to individuals or a non-Israeli company, such dividends would be subject to withholding tax at source at a rate of 20% with respect to dividends to be distributed on or after January 1, 2014, subject to certain conditions, or such lower rate as may be provided in an applicable tax treaty.
We have examined the possible effect, if any, of the applicable provisions of the Investment Law on our financial statements and have decided, at this time, not to opt to apply the benefits under the Investment Law.
From time to time, the Israeli Government has discussed reducing the benefits available to companies under the Investment Law. The termination or substantial reduction of any of the benefits available under the Investment Law could materially increase our tax liabilities.
Liquidity and Capital Resources
To date, we have financed our operations primarily with the IPO and the net proceeds from private placements of our ordinary and preferred shares and warrants, and to a significantly lesser extent, through a government grant.
We believe that based on our current business plan, our existing cash, cash equivalents and the net proceeds from our IPO will be sufficient to meet our currently anticipated cash requirements through at least the next 12 months.
Cash flows
The following table summarizes our consolidated statement of cash flows for the years ended December 31, 2014, 2013 and 2012.
| | | Year ended December 31, | |
| | | 2014 | | 2013 | | | 2012 | |
| | | (in thousands) | |
| Net cash provided by (used in): | | | | | | | | | |
| Operating activities | | $ | (18,017 | ) | | $ | (9,939 | ) | | $ | (7,550 | ) |
| Investing activities | | | (36,804 | ) | | | (114 | ) | | | (136 | ) |
| Financing activities | | | 46,887 | | | | 13,750 | | | | 12,294 | |
| Net increase in cash and cash equivalents | | $ | (7,934 | ) | | $ | 3,697 | | | $ | 4,608 | |
Net cash used in operating activities
The use of cash in all periods reflected primarily our net losses from operations, as adjusted for non-cash charges and measurements and changes in components of working capital. Adjustments to net income for non-cash items include depreciation and amortization, gain or loss due to revaluation of our outstanding warrants, non- cash expenses due to convertible credit line, income tax expenses, non-cash interest expenses and share-based compensation.
Net cash used in operating activities was $18.0 million in the year ended December 31, 2014 compared to $9.9 million in the year ended December 31, 2013 and $7.6 million in the year ended December 31, 2012. The year-over-year increases were attributable primarily to an increase in our net loss to $25.5 million in the year ended December 31, 2014 from $18.3 million in the year ended December 31, 2013 and $7.8 million in 2012. The increases were offset, in part, in the case of the year ended December 31, 2013, by an adjustment due to the non-cash loss that we recorded in 2013 for the revaluation of our outstanding warrants and due to changes in our receivables and payables.
Net cash used in investing activities
The use of cash in investing activities has historically been primarily related to the purchases of property and equipment. Net cash used in investing activities was $36.8 million during the year ended December 31, 2014, and $0.1 million during each of the years ended December 31, 2013 and December 31, 2012. The increase was attributable primarily to investment in short-term bank deposits and available for sale financial assets, and for the years ended December 31, 2013 and 2012, reflecting non-material changes in amounts incurred in purchases of property and equipment.
Net cash provided by financing activities
Net cash provided by financing activities was $46.9 million during the year ended December 31, 2014 compared to $13.8 million during the year ended December 31, 2013 and $12.3 million during the year ended December 31, 2012. The increases year-over-year were attributable primarily to increases in the amount of cash proceeds that we raised pursuant to our IPO in 2014 relative to the preferred shares and warrant private placement financing that we consummated mainly with our existing shareholders in the year ended December 31, 2013, which itself reflected an increase in financing amount relative to the corresponding financing that we consummated in the year ended December 31, 2012. See “—Cash and funding sources.”
Cash and funding sources
Our primary source of financing in the year ended December 31, 2014 was the $46.7 million of proceeds that we raised in our IPO and $0.2 million in proceeds from the exercise of warrants. Our primary source of financing in the year ended December 31, 2013 primarily consisted of $14.1 million in proceeds that we raised from the issuance of preferred A shares to certain existing investors. Our primary sources of financing in the year ended December 31, 2012 were comprised of the $11.9 million in proceeds that we raised from the issuance of preferred A shares to certain existing investors and $0.4 million in proceeds from exercise of warrants. Other than lease guarantees, we have no ongoing material financial commitments, such as lines of credit, that we expect will affect our liquidity over the next five years.
Funding requirements
We believe that our existing cash, cash equivalents and the net proceeds from our IPO will be sufficient to meet our currently anticipated cash requirements through at least the next 12 months. We have based this estimate on assumptions that may prove to be wrong and we could exhaust our capital resources sooner than we currently expect.
Our present and future funding requirements will depend on many factors, including, among other things:
| | • | the progress, timing and completion of our two Phase 3 clinical trials for CureXcell; |
| | • | the time and costs involved in obtaining regulatory approval for CureXcell and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to CureXcell in our clinical studies; |
| | • | in connection with the anticipated commercialization of CureXcell, costs and time involved in the establishment of our own manufacturing facilities in the United States, Europe and rest of the world; |
| | • | the amounts we invest in research and development and regulatory approval efforts in order to utilize our technology as a platform to develop regenerative medicine products for other indications; and |
| | • | the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims or infringements raised by third parties. |
For more information as to the risks associated with our future funding needs, see “Item 3.D. Risk Factors—Risks Relating to Our Business and Industry—We may need substantial additional capital in the future, which could cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights, and if additional capital is not available, we may have to delay, reduce or cease operations.”
Royalties
Magen David Adom
We expect to pay MDA royalties of 1% of CureXcell net sales outside of Israel, defined as amounts we receive in connection with the marketing, distribution and sales of CureXcell, if and when CureXcell is offered for sale in such countries. For additional discussion of our relationship with MDA, see “Business—Supply and Production.”
Office of the Chief Scientist
We have received a grant as part of our research and development programs approved by the OCS. The requirements and restrictions for such grant are found in the R&D Law. Under the R&D Law, royalties of 3% to 4.5% of the revenues derived from sales of products or services developed in whole or in part using this OCS grant are payable to the Israeli government. The maximum aggregate royalties paid generally cannot exceed 100% of the grant made to us, plus annual interest generally equal to the 12-month LIBOR applicable to dollar deposits, as published on the first business day of each calendar year. The total gross amount of the grant actually received by us from the OCS, including accrued LIBOR interest as of December 31, 2014, totaled $0.8 million. As of December 31, 2014, we had not paid any royalties to the OCS.
In addition to paying any royalty due, we must abide by other restrictions associated with receiving such grant under the R&D Law that continue to apply following repayment to the OCS. These restrictions may impair our ability to outsource manufacturing, engage in change of control transactions or otherwise transfer our know-how outside of Israel by requiring us to obtain the approval of the OCS for certain actions and transactions and pay additional royalties and other amounts to the OCS. In addition, any change of control and any change of ownership of our ordinary shares that would make a non-Israeli citizen or resident an “interested party,” as defined in the R&D Law, requires prior written notice to the OCS. If we fail to comply with the R&D Law, we may be subject to criminal charges.
Application of Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with IFRS as issued by the IASB. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses during the reporting periods. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our consolidated financial statements appearing elsewhere in this annual report, we believe that the accounting policies discussed below are critical to our financial results and to the understanding of our past and future performance, as these policies relate to the more significant areas involving management’s estimates and assumptions. We consider an accounting estimate to be critical if: (a) it requires us to make assumptions because information was not available at the time or it included matters that were highly uncertain at the time we were making our estimate; and (b) changes in the estimate could have a material impact on our financial condition or results of operations.
Research and development expenses
Research expenses are recognized as expenses when incurred. Costs incurred on development projects are recognized as intangible assets as of the date as of which it can be established that it is probable that future economic benefits attributable to the asset will flow to us considering its commercial feasibility. This is generally the case when regulatory approval for commercialization is achieved and costs can be measured reliably. Given the current stage of the development of our product candidate, no development expenditures have yet been capitalized. Intellectual property-related costs for patents are part of the expenditure for the research and development projects. Therefore, registration costs for patents are expensed when incurred as long as the research and development project concerned does not meet the criteria for capitalization.
Share-based compensation
We account for our share-based compensation for employees in accordance with the provisions of IFRS 2 “Share-based Payment,” which requires us to measure the cost of share-based compensation based on the fair value of the award on the grant date.
We selected the Black-Scholes model as the most appropriate method for determining the estimated fair value of our share-based awards. The resulting cost of an equity incentive award is recognized as an expense over the requisite service period of the award, which is usually the vesting period. We recognize compensation expense over the vesting period using the accelerated method pursuant to which each vesting tranche is treated as a separate amortization period from grant date to vest date, and classify these amounts in the consolidated financial statements based on the department to which the related employee reports.
Option Valuations
The determination of the grant date fair value of options using an option pricing model is affected by estimates and assumptions regarding a number of complex and subjective variables. These variables include the expected volatility of our share price over the expected term of the options, share option exercise and cancellation behaviors, risk-free interest rates and expected dividends, which are estimated as follows:
| | • | Fair Value of our Ordinary Shares. Because our ordinary shares are publicly traded, we derive the fair value of the shares from the sales price (generally, the closing sales price) of the shares on the trading market on which our shares are listed, as discussed below in “—Valuation of our ordinary shares.” |
| | • | Volatility. The expected share price volatility was based on the historical average equity volatility of the ordinary shares of comparable drug companies that are publicly traded. |
| | • | Expected Term. The expected term of options granted represents the period of time that options granted are expected to be outstanding. Since adequate historical experience is not available to provide a reasonable estimate, the expected term is determined based on the midpoint between the available exercise dates (the end of the vesting periods) and the last available exercise date (the contracted expiry date). |
| | • | Risk-Free Rate. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the expected life of the options. |
| | • | Expected Dividend Yield. We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero. |
If any of the assumptions used in the Black-Scholes model change significantly, share-based compensation for future awards may differ materially compared with the awards granted previously.
The following table presents the weighted-average assumptions used to estimate the fair value of options granted to employees on the dates indicated.
| | | August 2013 | | | October 2013 | | | December 2013 | | | April 2014 | | | December and August 2014 | |
Expected volatility | | | 129% – 131 | % | | | 128% – 131 | % | | | 126% – 128 | % | | | 126% - 127 | % | | | 103%-104 | % |
| Expected term (in years) | | | 5.2 – 6.7 | | | | 4.9 – 6.8 | | | | 5.5 – 7.0 | | | | 5.5 - 7.0 | | | | 5.5 - 7.0 | |
Risk-free rate | | | 1.83 – 2.36 | % | | | 1.71 – 2.37 | % | | | 1.88 – 2.41 | % | | | 1.85% - 2.28 | % | | | 1.97% - 2.14 | % |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
The following table presents the grant dates, number of underlying shares and related exercise prices of awards granted to employees and non-employees since January 1, 2012 as well as the estimated fair value of the underlying ordinary shares on the grant date.
| Month of grant | | Number of shares subject to awards granted | | | Average exercise price per share | | | Estimated fair value per ordinary share at grant date | |
June 2012 | | | 8,740 | | | $ | 9.59 | | | $ | 3.78 | |
August 2013 | | | 573,068 | | | $ | 3.26 | | | $ | 4.80 | |
August 2013 | | | 286,534 | | | $ | 16.04 | | | $ | 4.80 | |
October 2013 | | | 25,760 | | | $ | 3.26 | | | $ | 3.63 | |
December 2013 | | | 55,982 | | | $ | 3.26 | | | $ | 5.37 | |
April 2014 | | | 370,116 | | | $ | 10.20 | | | $ | 9.80 | |
| August 2014 | | | 219,972 | | | | 10.00 | | | | 7.25 | |
| December 2014 | | | 50,000 | | | | 7.32 | | | | 7.37 | |
Based on the initial public offering price of per share in our IPO, the intrinsic value of the awards outstanding as of December 31, 2014 was $6.9 million, of which $5.6 million related to vested options and $1.3 million related to unvested options.
Ordinary Share Valuations
Please see the discussion regarding the fair value of our ordinary shares in “Management’s Discussion and Analysis of Financial Condition and Results of Operations— Share-based compensation—Ordinary Share Valuations” in our IPO Prospectus, which discussion is incorporated by reference herein.
Recent Accounting Pronouncements
Standards and Amendments Adopted by Us
We have adopted the following new amendments to International Accounting Standards, or IASs, and IFRS standards, with a date of initial application of January 1, 2014:
| | · | Amendment to IAS 32, Financial Instruments: Presentation |
| | · | Amendment to IAS 36, Impairment of Assets: Recoverable Amount Disclosures for Non-Financial Assets |
| | · | Amendment to IFRS 2, Share-Based Payment, definition of “vesting condition”. |
The implementation of these new standards and amendments has not had a material effect on our consolidated financial statements.
New Standards and Interpretations Not Yet Adopted by Us
| | · | IFRS 9, Financial Instruments |
A final version of this accounting standard, which includes revised guidance on the classification and measurement of financial instruments, and a new model for measuring impairment of financial assets, has been adopted by the IASB. This guidance has been added to the International IFRS chapter dealing with general hedge accounting requirements issued in 2013. IFRS 9 (2014) is effective for annual periods beginning on or after January 1, 2018 with early adoption being permitted. We have not yet commenced examining the effects of adopting IFRS 9 (2014) on our consolidated financial statements.
| | · | Amendment to IAS 24, “Related Party Disclosures” |
The definition of “Related Party Disclosures” was expanded to include entities that provide key management personnel, or KMP, services to a reporting entity, directly or through an affiliated entity of the company. We have not yet commenced examining the effects of adopting this amendment on our consolidated financial statements.
JOBS Act Exemptions
The JOBS Act permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards. We have not elected to avail ourselves of an exemption. This election is irrevocable.
| | C. | Research and development, patents and licenses, etc. |
For a description of our research and development programs and the amounts that we have incurred over the last three years pursuant to those programs, please see Item 4 “Information on the Company—Business Overview—Research and Development.”
Our results of operations and financial condition may be affected by various trends and factors discussed in “Item 3 - Key Information - Risk Factors”, including pricing regulations, third-party coverage and reimbursement policies, healthcare reform initiatives, the degree of market acceptance of our products in the healthcare field due, in part, to trends in that field, changes in political, military or economic conditions in Israel and in the Middle East, general slowing of local or global economies and decreased economic activity in one or more of our target markets.
| | | Off-Balance Sheet Arrangements |
We do not currently engage in off-balance sheet financing arrangements. In addition, we do not have any interest in entities referred to as variable interest entities, which includes special purposes entities and other structured finance entities.
| | F. | Tabular Disclosure of Contractual Obligations |
Our significant contractual obligations as of December 31, 2014 are summarized in the following table.
| | | Payments due by period | |
| | | Less than 1 year | | | Between 1 and 2 years | | | Between 2 and 5 years | | | More than 5 years | | | Total | |
| | | (in thousands) | |
Operating lease obligations(1) | | $ | 122 | | | $ | 107 | | | $ | 233 | | | $ | - | | | $ | 462 | |
| (1) | Operating lease obligations consist of payments pursuant to lease agreements for office and laboratory facilities and vehicle lease obligations. |
The contractual obligations listed in the above table do not include royalties that we may pay to MDA or the OCS based upon future sales of our products as we are unable at this time to estimate the actual amount or timing of these costs that we will incur in the future to these parties. The obligations listed in the table also exclude amounts that we are obligated to pay to our supplier of sterile plastic transfusion and infusion bags, under the supply agreement to which we are party with it. While that agreement requires us to purchase minimum amounts during each year of the six year term of the agreement ending in February 2020, the agreement is terminable by either party upon nine months’ prior written notice without further obligation, and it is therefore not deemed a firm contractual commitment. Based on our past order volume and current expectations for order volume in the foreseeable future, we believe that the minimum annual purchase amount under the agreement is immaterial.
Item 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
| | A. | Directors and Senior Management |
The following table sets forth information relating to our executive officers and directors as of the date of this annual report. Unless otherwise stated, the address for our directors and executive officers is c/o Macrocure Ltd., 25 Hasivim Street, Petach Tikva 4959383, Israel.
| | | | | |
| Name | | Age | | Position |
| Executive Officers | | | | |
| Nissim Mashiach | | 54 | | President and Chief Executive Officer |
| Michael Molyneaux, MD | | 44 | | Chief Medical Officer |
| Mark Page | | 42 | | Chief Financial Officer |
| Directors | | | | |
| David Ben Ami | | 54 | | Chairman of the Board of Directors |
Ze’ev Bronfeld(1) | | 64 | | Director |
Ranan Grobman(1)(2)(3) | | 37 | | Director |
Tomer Kariv(1) | | 53 | | Director |
Jonathan Kolber(1)(3) | | 52 | | Director |
Katherine Wolf(1)(2)(3) | | 48 | | External director |
Yuval Yanai(1)(2)(3) | | 62 | | External director |
(1) Independent director under the rules of the NASDAQ Stock Market.
(2) Member of our audit committee.
(3) Member of our compensation committee.
Executive Officers
Nissim Mashiach has served as our President and Chief Executive Officer since June 2012. Before joining our company, he served as General Manager at Ethicon, a Johnson & Johnson company, from 2009 to 2012. Prior to then, he served as President and Chief Operating Officer at Omrix Biopharmaceuticals, Inc., a company acquired by Johnson & Johnson in 2008. Prior to Omrix, Mr. Mashiach held leadership positions at several pharmaceutical companies. He holds an MBA from the University of Manchester, England, an MPharmSc from the Hebrew University, Jerusalem, Israel, and a BSc, Chemical Engineering from the Technion-Israel Institute of Technology, Haifa, Israel.
Michael Molyneaux joined our company in March 2013 as our Chief Medical Officer. Dr. Molyneaux served as the Medical Director at the Center for Advanced Wound Healing and Hyperbaric Oxygen Therapy at Passavant Area Hospital in Jacksonville, Illinois from August 2008 until August 2013, and currently remains a part-time physician at that healing center. He has also been actively engaged in clinical trial research since June 2005, acting as a principal investigator for the following wound care companies: Healthpoint Biotherapeutics, US Biotest, CoDa Therapeutics, Inc. and Derma Sciences, Inc. He completed his medical school and residency training at Dalhousie University in Nova Scotia, Canada and is board certified in both Canada and the United States. He also completed an MBA at Washington University in St. Louis, and a BSc, Biology at University Prince Edward Island in Prince Edward Island, Canada.
Mark Page has served as our Chief Financial Officer since February 2015. Prior to joining our company, Mr. Page served at Credit Suisse’s Global Healthcare Investment Banking Group where he led the firm’s medical device franchise since 2010. Mr. Page served as the lead banker on our IPO in August 2014 among other transactions. Prior to serving at Credit Suisse, he spent the majority of his healthcare career at Merrill Lynch & Co. (which subsequently became Bank of America Merrill Lynch). Since 1994, he has also held positions at Lehman Brothers Inc., PAREXEL International and Leerink Swann (now Leerink Partners). Mark earned his MBA from The Wharton School at the University of Pennsylvania and his BA in Economics from Haverford College.
Directors
David Ben-Ami, our Chairman of the Board and a co-founder of our company, has served on our board of directors since our founding in early 2008. Mr. Ben-Ami has more than 20 years of experience with activities in management, business development and corporate strategy in the life sciences industry. He served as Chief Executive Officer of NVR Labs from June 2005 to April 2010, Country Director of Boston Scientific Israel from June 2003 to May 2005, and Director of Business Development of Teva Israel from January 1999 to June 2003. Mr. Ben-Ami serves as a board member in BioCell Ltd. and Degania Silicone Ltd. and Entera Bio Ltd., among other medical device and pharmaceutical companies. He received his MBA and BA in Economics & Management from Tel-Aviv University.
Ze’ev Bronfeld has served on our board of directors since January 2008. Mr. Bronfeld has significant experience in the management and building of biotechnology companies. He is a co-founder of Biocell Ltd., an Israeli publicly traded holding company specializing in biotechnology companies and has served as its Chief Executive Officer since 1986. Mr. Bronfeld has been a director of Protalix Ltd. since 1996, and also currently serves as a director of Biocell Ltd. and D.N.A. Biomedical Solutions Ltd., which are publicly traded on the Tel Aviv Stock Exchange, and Protalix Inc., which is publicly traded on the American Stock Exchange. Mr. Bronfeld is also a director of a number of private companies. Mr. Bronfeld holds a BA in Economics from the Hebrew University of Jerusalem.
Ranan Grobman has served on our board of directors since May 2012. Mr. Grobman has been an active investor in Israeli high-tech companies for the last 14 years. Mr. Grobman has served as a general partner of Jerusalem Global Ventures, or JGV, since 2008 and has served in different capacities since 1998. Mr. Grobman, through JGV, serves as an advisor to Vaizra Ventures, a shareholder of our company since 2010. Mr. Grobman served as Vice President of Business Development at Certagon from 2006 to 2008 and Vice President Investment Banking at Yazam from 1999 to 2001.
Tomer Kariv joined our board of directors in March 2008. He is the co-founder and Chief Executive Officer of Pontifax, a group of Israeli-based life sciences venture funds focusing on investments in development stage bio-pharmaceutical and med-tech technologies and a shareholder of our company. Mr. Kariv serves as an active board member of many of the fund’s portfolio companies. Among other companies, Mr. Kariv serves as the Chairman of Check-Cap Ltd. and is a board member of Arno Therapeutics Inc. During the 10 years prior to establishing Pontifax, Mr. Kariv played a key role in investing, managing and nurturing technology driven companies and startups and has held senior management positions at top Israeli financial institutions. Mr. Kariv practiced law with Sullivan & Cromwell in New York, and holds a BA in Economics from Harvard University and a JD from Harvard Law School.
Jonathan Kolber joined our board of directors in March 2011. Mr. Kolber is a general partner of Viola Private Equity, a technology buyout and growth capital fund that is an affiliate of the Viola Group and is a shareholder of our company. From 1986 to 1997, Mr. Kolber was a founder and manager of Claridge Israel, which invested in Teva Pharmaceuticals, ECI Telecom, Osem and Optrotech. In 1998, Mr. Kolber became the Chief Executive Officer of Koor Industries, one of Israel’s largest conglomerates, which he sold to the IDB Group in 2006. He has served as Chairman, Chief Executive Officer and Director in over 40 public and private companies in Israel and North America. He is a director of the Peres Center for Peace and the Chairman of the Friends of the Tel Aviv Medical Center. He holds a Bachelor’s degree in Near Eastern Language and Literature from Harvard University and a Certificate of Advanced Arabic Language from the American University of Cairo.
Katherine Wolf joined our board of directors effective upon the listing of our ordinary shares on NASDAQ. Since January 2014, Ms. Wolf has served as Chief Executive Officer, Co-Founder and Partner of Rocket Science Healthcare LLC. From September 2012 to January 2014, Ms. Wolf provided independent consulting services. From September 2008 to August 2012, Ms. Wolf was the Chief Financial Officer and Executive Vice President, Corporate Development at Vision-Sciences, Inc., a NASDAQ-listed medical device company in the flexible endoscopy space. From June 2005 to April 2008, Ms. Wolf was a Managing Director at HSBC Securities (USA) Inc. in its investment banking division, where she was a member of the firm’s Health Care Group and ran the investment banking MedTech effort. Prior to HSBC, she worked at Bear, Stearns & Co. from 2000 to 2005 in the Health Care Group, rising to the level of Managing Director. Ms. Wolf holds an M.B.A. from Harvard Business School and a B.A. from Williams College.
Yuval Yanai joined our board of directors effective upon the listing of our ordinary shares on NASDAQ. Mr. Yanai served, from September 2005 through March 2014, as Senior Vice President and Chief Financial Officer of Given Imaging Ltd. From October 2000 through August 2005, he served as Senior Vice President and Chief Financial Officer of Koor Industries Ltd., one of Israel’s largest holding companies. Prior to that, from April 1998 to September 2000, he served as Vice President and Chief Financial Officer of NICE Systems Ltd., an Israeli global provider of Insight from Interactions, and, from 1991 to April 1998, he was the Vice President, Finance and Chief Financial Officer of Elscint Ltd., a former Israeli company engaged in the manufacturing of medical imaging devices that was acquired by larger companies in this field. He joined Elscint in 1985 and served as Corporate Controller and Corporate Treasurer through 1991. Mr. Yanai serves as a director in Check-Cup Ltd., Medical Compression Systems Ltd., Compulab Ltd. And Efranat Ltd. Mr. Yanai is also the Chairman of The Israeli Fund for UNICEF. Previously, Mr. Yanai served as a director of Citycon Oj, Starplast Industries Ltd., Adama Ltd. (formerly Makteshim-Agan Industries Ltd.), ECI Telecom Ltd., Equity One, Inc., BVR Systems Ltd., Tadiran Communication Ltd., The Elisra Group and Telrad Networks Ltd. Mr. Yanai holds a B.Sc. in Accounting and Economics from Tel-Aviv University.
Arrangements Concerning Election of Directors; Family Relationships
Pursuant to our articles of association in effect prior to our IPO, certain of our shareholders had rights to appoint members of our board of directors. All rights to appoint directors terminated upon the closing of our IPO, although currently-serving directors (other than external directors) who were elected prior to the IPO will continue to serve pursuant to their election until our 2015 annual meeting of shareholders, at which their current term as directors will expire. MDA has a right to appoint a non-voting observer to our board of directors, but has not exercised this right. We are not a party to, and are not aware of, any voting agreements among our shareholders pursuant to which any of our directors was elected. Two of our directors, Tomer Kariv and Jonathan Kolber, are brothers-in-law. Other than that relationship, there are no family relationships among our executive officers and directors.
Compensation of Directors and Executive Officers
The aggregate compensation expensed and share-based compensation and other payments expensed by us and our subsidiaries to our directors and executive officers with respect to the year ended December 31, 2014 was $3.6 million. This amount includes approximately $0.3 million set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include business travel, relocation, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in our industry.
Compensation of Office Holders
The table below outlines the compensation paid to our five most highly compensated senior office holders (as defined in the Companies Law and described under “Board Practices—External Directors” below) during or with respect to the year ended December 31, 2014, in the disclosure format of Regulation 21 of the Israeli Securities Regulations (Periodic and Immediate Reports), 1970. We refer to the five individuals for whom disclosure is provided herein as our Covered Executives.
For purposes of the table and the summary below, and in accordance with the above mentioned securities regulations, “compensation” includes base salary, bonuses, equity-based compensation, retirement or termination payments, benefits and perquisites such as car, phone and social benefits and any undertaking to provide such compensation.
Summary Compensation Table
Information Regarding the Covered Executive(1) | |
Name and Principal Position(2) | | Base Salary ($) | | | Benefits and Perquisites ($)(3) | | | Variable compensation ($)(4) | | | Equity-Based Compensation ($)(5) | | | Total ($) | |
| Nissim Mashiach, CEO | | | 350,000 | | | | 59,440 | | | | 850,000 | | | | 783,692 | | | | 2,043,132 | |
| Michael Molyneaux, CMO | | | 220,000 | | | | 46,465 | | | | 155,000 | | | | 167,915 | | | | 589,380 | |
| Shai Lankry, VP Finance | | | 134,176 | | | | 52,891 | | | | 85,000 | | | | 190,240 | | | | 462,307 | |
| David Ben Ami, Chairman of the BOD | | | 192,000 | | | | 65,232 | | | | - | | | | - | | | | 257,232 | |
Each Other Director of the Company (6) | | | 15,000 | | | | | | | | | | | | 50,497 | | | | 65,497 | |
| (1) | All amounts reported in the table are in terms of cost to our company, as recorded in our financial statements. |
| | |
| (2) | All current executive officers listed in the table are full-time employees or consultants of our company. Cash compensation amounts denominated in currencies other than the U.S. dollar were converted into U.S. dollars at the average conversion rate for 2014. |
| | |
| (3) | Amounts reported in this column include benefits and perquisites, including those mandated by applicable law. Such benefits and perquisites may include, to the extent applicable to the Covered Executive, payments, contributions and/or allocations for savings funds, pension, severance, vacation, car or car allowance, medical insurances and benefits, risk insurances (e.g., life, disability, accident), convalescence pay, payments for social security, tax gross-up payments and other benefits and perquisites consistent with our guidelines. |
| | |
| (4) | Amounts reported in this column refer to bonus payments that were paid with respect to 2014. |
| | |
| (5) | Amounts reported in this column represent the expense recorded in our financial statements for the year ended December 31, 2014 with respect to equity-based compensation. Assumptions and key variables used in the calculation of such amounts are described in paragraph (__) of Note __ to our audited consolidated financial statements, which are included in this annual report. |
| | |
| (6) | Each of our directors, other than Mr. Ben Ami (who serves as a consultant to our company), received identical compensation in 2014, consisting of an option grant and a prorated portion of an annual fee of $30,000, which was payable for the remaining portion of the year following the consummation of the IPO on August 5, 2014. |
Agreements with Executive Officers; Consulting and Directorship Services Provided by Directors
We have entered into written confidentiality, non-competition/solicitation and inventions assignment agreements with all of our executive officers. These agreements contain standard provisions for a company in our industry regarding non-competition, confidentiality of information and assignment of inventions. See “Item 3.D. Risk Factors—Risks Relating to Our Intellectual Property—Under applicable employment laws, we may not be able to enforce covenants not to compete” for a further description of the enforceability of non-competition clauses. Our executive officers will not receive benefits upon the termination of their respective employment with us, other than payment of salary and benefits (and limited accrual of vacation days) during the required notice period for termination of their employment, which varies for each individual. See “Item 7.B. Related Party Transactions—Agreements and Arrangements with, and Compensation of, Directors and Executive Officers” for additional information.
We receive consulting and directorship services from one of our directors. The amount payable pursuant to this arrangement has been approved by our board of directors and shareholders. Other than with respect to our directors that are also executive officers, there are no arrangements or understandings between us, on the one hand, and any of our directors, on the other hand, providing for benefits upon termination of their service as directors of our company.
Board of Directors
Under the Companies Law, the management of our business is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are also appointed by our board of directors, and are subject to the terms of any applicable employment agreements that we may enter into with them.
Under our articles, our board of directors must consist of at least four and not more than eleven directors, including at least two external directors required to be appointed under the Companies Law. We elected two external directors prior to the consummation of our IPO, and our shareholders ratified such election at a special general meeting of shareholders held in November 2014. Our external directors are serving a three-year term pursuant to the requirements of the Companies Law. Under our articles, all of our other directors are elected on an annual basis, to serve until the next annual meeting of shareholders and until their successors are elected and duly qualified. External directors must be elected by a special majority of shareholders, while other directors may be elected by an ordinary majority of the voting power present and voting, in person or by proxy, at each annual meeting.
All directors (other than external directors) may be removed by a vote of 65% of the voting power of our shareholders at a general meeting of our shareholders or upon the occurrence of certain events, in accordance with the Companies Law and our articles. External directors may be removed from office only under the limited circumstances set forth in the Companies Law. See “—External Directors” in this Item 6.C. below.
In addition to election by our shareholders, our articles allow our board of directors to appoint directors to fill vacancies on our board, for a term of office equal to the remaining term of office of the director(s) whose office(s) was vacated.
Our board of directors has determined that a majority of our directors (six, including our two external directors) qualify as “independent directors” under the NASDAQ Listing Rules. The definitions of “independent director” under the NASDAQ Listing Rules and “external director” under the Companies Law overlap to a certain extent such that we would generally expect the two directors who will serve as external directors to satisfy the independence requirements under the NASDAQ Listing Rules. The definition of external director under the Companies Law includes a set of statutory criteria that must be satisfied, including criteria whose aim is to ensure that there is no factor that would impair the ability of the external director to exercise independent judgment, which is similar to the corresponding standard for an independent director under the NASDAQ Listing Rules.
In accordance with the exemption available to foreign private issuers under the NASDAQ Listing Rules, we do not follow the requirements of the NASDAQ Listing Rules with regard to the process of nominating directors, and instead follow Israeli law and practice, in accordance with which our board of directors (or a committee thereof) is authorized to recommend to our shareholders director nominees for election.
Under the Companies Law and our articles, nominees for directors may also be proposed by any shareholder holding at least 1% of our outstanding voting power. However, any such shareholder may make such a nomination only if a written notice of such shareholder’s intent to make such nomination has been delivered to our registered Israeli office within seven days after we publish notice of our upcoming annual general meeting (or within 14 days after we publish a preliminary notification of an upcoming annual general meeting). Any such notice must include certain information, including, among other things, a description of all arrangements between the nominating shareholder and the proposed director nominee(s) and any other person pursuant to which the nomination(s) are to be made by the nominating shareholder, the consent of the proposed director nominee(s) to serve as our director(s) if elected and a declaration signed by the nominee(s) declaring that there is no limitation under the Companies Law preventing their election, and that all of the information that is required under the Companies Law to be provided to us in connection with such election has been provided.
Under the Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial expertise. See “—External Directors” below. In determining the number of directors required to have such expertise, our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the minimum number of directors of our company who are required to have accounting and financial expertise is one.
External Directors
Under the Companies Law, our board of directors must include at least two members who qualify as external directors. We elected two external directors—Katherine Wolf and Yuval Yanai—prior to the consummation of our IPO, whose election was ratified at a special general meeting of shareholders held in November 2014. Both of our external directors serve on our audit committee and compensation committee.
The provisions of the Companies Law set forth special approval requirements for the election of external directors. External directors must be elected by a majority vote of the shares present and voting at a meeting of shareholders, provided that either:
| | • | such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding abstentions, to which we refer as a disinterested majority; or |
| | • | the total number of shares voted by non-controlling shareholders and by shareholders who do not have a personal interest in the election of the external director against the election of the external director does not exceed 2% of the aggregate voting rights in the company. |
The term “controlling shareholder” is defined in the Companies Law as a shareholder with the ability to direct the activities of the company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder holds (within the meaning of the Companies Law) 50% or more of the voting rights in a company or has the right to appoint the majority of the directors of the company or its general manager. With respect to certain matters, a controlling shareholder is deemed to include a shareholder that holds 25% or more of the voting rights in a public company if no other shareholder holds more than 50% of the voting rights in the company, but excludes a shareholder whose power derives solely from his or her position as a director of the company or from any other position with the company.
The initial term of an external director is three years. Thereafter, an external director may be re-elected by shareholders to serve in that capacity for additional three-year terms, provided that certain conditions are satisfied and that either:
| | (i) | his or her service for each such additional term is recommended by one or more shareholders holding at least 1% of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate voting rights in the company; or |
| | (ii) | his or her service for each such additional term is recommended by the board of directors and is approved at a meeting of shareholders by the same majority required for the initial election of an external director (as described above). |
The term of office for external directors for Israeli companies traded on certain foreign stock exchanges, including the NASDAQ Global Market, may be extended indefinitely in increments of additional three-year terms, in each case provided that the audit committee and the board of directors of the company confirm that, in light of the external director’s expertise and special contribution to the work of the board of directors and its committees, the reelection for such additional period(s) is beneficial to the company, and provided that the external director is reelected subject to the same shareholder vote requirements as if elected for the first time (as described above). Prior to the reelection of the external director at a general meeting of shareholders, the company’s shareholders must be informed of the term previously served by him or her and of the reasons why the board of directors and audit committee recommended the extension of his or her term.
External directors may be removed from office by a special general meeting of shareholders called by the board of directors, which approves such dismissal by the same shareholder vote percentage required for their election or by a court, in each case, only under limited circumstances, including ceasing to meet the statutory qualifications for appointment, or violating their duty of loyalty to the company.
If an external directorship becomes vacant and there are fewer than two external directors on the board of directors at the time, then the board of directors is required under the Companies Law to call a shareholders’ meeting as soon as practicable to appoint a replacement external director.
Each committee of the board of directors that exercises powers of the board of directors must include at least one external director, except that the audit committee and the compensation committee must include all external directors then serving on the board of directors. Under the Companies Law, external directors of a company are prohibited from receiving, directly or indirectly, any compensation from the company other than for their services as external directors pursuant to the Companies Law and the regulations promulgated thereunder. Compensation of an external director is determined prior to his or her appointment and may not be changed during his or her term subject to certain exceptions.
The Companies Law provides that a person is not qualified to serve as an external director if (i) the person is a relative of a controlling shareholder of the company, or (ii) if that person or his or her relative, partner, employer, another person to whom he or she was directly or indirectly subordinate, or any entity under the person’s control, has or had, during the two years preceding the date of appointment as an external director: (a) any affiliation or other disqualifying relationship with the company, with any person or entity controlling the company or a relative of such person, or with any entity controlled by or under common control with the company; or (b) in the case of a company with no shareholder holding 25% or more of its voting rights, had at the date of appointment as an external director, any affiliation or other disqualifying relationship with a person then serving as chairman of the board or chief executive officer, a holder of 5% or more of the issued share capital or voting power in the company or the most senior financial officer.
The term “relative” is defined under the Companies Law as a spouse, sibling, parent, grandparent or descendant; spouse’s sibling, parent or descendant; and the spouse of each of the foregoing persons.
Under the Companies Law, the term “affiliation” and the similar types of disqualifying relationships include (subject to certain exceptions):
| | • | an employment relationship; |
| | • | a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships); |
| | • | service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director was elected as a director of the private company in order to serve as an external director following the initial public offering. |
The term “office holder” is defined under the Companies Law as the chief executive officer (referred to in the law as a general manager), chief business manager, deputy general manager, vice general manager, any other person assuming the responsibilities of any of these positions regardless of that person’s title, a director and any other manager directly subordinate to the general manager.
In addition, a person may not serve as an external director if that person’s position or professional or other activities create, or may create, a conflict of interest with that person’s responsibilities as a director or otherwise interfere with that person’s ability to serve as a director or if the person is an employee of the Israel Securities Authority or an Israeli stock exchange. A person may furthermore not continue to serve as an external director if he or she received direct or indirect compensation from the company, including amounts paid pursuant to indemnification or exculpation contracts or commitments and insurance coverage for his or her service as an external director, other than as permitted by the Companies Law and the regulations promulgated thereunder.
Following the termination of an external director’s service on a board of directors, such former external director and his or her spouse and children may not be provided a direct or indirect benefit by the company, its controlling shareholder or any entity under its controlling shareholder’s control. This includes engagement as an office holder or director of the company or a company controlled by its controlling shareholder or employment by, or provision of services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by the former external director. This restriction extends for a period of two years with regard to the former external director and his or her spouse or child and for one year with respect to other relatives of the former external director.
If at the time at which an external director is appointed all members of the board of directors who are not controlling shareholders or relatives of controlling shareholders of the company are of the same gender, the external director to be appointed must be of the other gender. A director of one company may not be appointed as an external director of another company if a director of the other company is acting as an external director of the first company at such time.
According to regulations promulgated under the Companies Law, a person may be appointed as an external director only if he or she has professional qualifications or if he or she has accounting and financial expertise (each, as defined below). In addition, at least one of the external directors must be determined by our board of directors to have accounting and financial expertise. However, if at least one of our other directors (i) meets the independence requirements under the Exchange Act, (ii) meets the standards of the NASDAQ Listing Rules for membership on the audit committee, and (iii) has accounting and financial expertise as defined under the Companies Law, then neither of our external directors is required to possess accounting and financial expertise as long as each possesses the requisite professional qualifications.
A director with accounting and financial expertise is a director who, due to his or her education, experience and skills, possesses an expertise in, and an understanding of, financial and accounting matters and financial statements, such that he or she is able to understand the financial statements of the company and initiate a discussion about the presentation of financial data. A director is deemed to have professional qualifications if he or she has any of (i) an academic degree in economics, business management, accounting, law or public administration, (ii) an academic degree or has completed another form of higher education in the primary field of business of the company or in a field which is relevant to his/her position in the company, or (iii) at least five years of experience serving in one of the following capacities, or at least five years of cumulative experience serving in two or more of the following capacities: (a) a senior business management position in a company with a significant volume of business; (b) a senior position in the company’s primary field of business; or (c) a senior position in public administration or service. The board of directors is charged with determining whether a director possesses financial and accounting expertise or professional qualifications.
Our board of directors has determined that of our two external directors, Yuval Yanai possesses accounting and financial expertise, while Katherine Wolf possesses professional qualifications.
Board Committees
Audit Committee
Companies Law Requirements
Pursuant to the requirements of the Companies Law, we appointed an audit committee upon the closing of our IPO. An audit committee must be comprised of at least three directors, including all of the external directors, one of whom must serve as chairman of the committee. The audit committee may not include the chairman of the board, a controlling shareholder of the company, a relative of a controlling shareholder, a director employed by or providing services on a regular basis to the company, to a controlling shareholder or to an entity controlled by a controlling shareholder, or a director who derives most of his or her income from a controlling shareholder. In addition, under the Companies Law, the majority of the directors serving on the audit committee of a publicly traded company must be unaffiliated directors. In general, an “unaffiliated director” under the Companies Law is defined as either an external director or as a director who meets the following criteria:
| | • | he or she meets the qualifications for being appointed as an external director, except for the requirements that the director (i) be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed for trading outside of Israel) and (ii) possess accounting and financial expertise or professional qualifications; and |
| | • | he or she has not served as a director of the company for a period exceeding nine consecutive years. For this purpose, a break of less than two years in the service shall not be deemed to interrupt the continuation of the service. |
NASDAQ Listing Requirements
Under the NASDAQ Listing Rules, we are required to maintain an audit committee consisting of at least three independent directors, each of whom is financially literate and one of whom has accounting or related financial management expertise.
Our audit committee consists of Mr. Yanai (chair), Mr. Grobman and Ms. Wolf. All of the members of our audit committee are independent directors in accordance with Rule 10A-3(b)(1) under the Exchange Act and satisfy the independent director requirements under the NASDAQ Listing Rules.
All members of our audit committee meet the requirements for financial literacy under the NASDAQ Listing Rules. Mr. Yanai is an audit committee financial expert as such term is defined in the rules of the SEC.
Audit Committee Role
Our board of directors has adopted an audit committee charter that sets forth the responsibilities of the audit committee consistent with the rules and regulations of the SEC and the NASDAQ Listing Rules, as well as the requirements for such committee under the Companies Law, including the following:
| | • | oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors in accordance with Israeli law; |
| | • | recommending the engagement or termination of the person filling the office of our internal auditor; and |
| | • | recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors. |
Our audit committee provides assistance to our board of directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent of management.
Under the Companies Law, our audit committee will be responsible for:
| | (i) | determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices; |
| | (ii) | determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and whether such transaction is extraordinary or material under the Companies Law) (see “—Approval of Related Party Transactions under Israeli Law”); |
| | (iii) | establishing the approval process for certain transactions with a controlling shareholder or in which a controlling shareholder has a personal interest; |
| | (iv) | where the board of directors approves the work plan of the internal auditor, examining such work plan before its submission to the board of directors and proposing amendments thereto; |
| | (v) | examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to fulfill his responsibilities; |
| | (vi) | examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment of our auditor; and |
| | (vii) | establishing procedures for the handling of employees’ complaints as to deficiencies in the management of our business and the protection to be provided to such employees. |
Our audit committee may not approve any actions requiring its approval (see “—Approval of Related Party Transactions under Israeli Law” in this Item 6.C. below), unless at the time of the approval a majority of the committee’s members are present, which majority consists entirely of unaffiliated directors, including at least one external director.
Compensation Committee and Compensation Policy
The members of the compensation committee of our board of directors are Ms. Wolf (chair), Mr. Grobman, Mr. Kolber and Mr. Yanai. All of the members of our compensation committee are independent under the NASDAQ Listing Rules.
Under the Companies Law, the board of directors of a public company must appoint a compensation committee. The compensation committee must be comprised of at least three directors, including all of the external directors, who must constitute a majority of the members of the compensation committee. However, subject to certain exceptions, Israeli companies whose securities are traded on stock exchanges such as the NASDAQ Global Market, and who do not have a controlling shareholder, do not have to meet this majority requirement; provided, however, that the compensation committee meets other Companies Law composition requirements, as well as the requirements of the jurisdiction where the company’s securities are traded. Each compensation committee member who is not an external director must be a director whose compensation does not exceed an amount that may be paid to an external director. The compensation committee is subject to the same limitations as the audit committee under the Companies Law as to who may not be a member of the committee.
The duties of the compensation committee include recommending to the company’s board of directors a policy regarding the terms of engagement of office holders, to which we refer as a compensation policy. Such policy must be adopted by the company’s board of directors, after considering the recommendations of the compensation committee, and must be approved by the company’s shareholders, which approval requires what we refer to as a Special Majority Approval for Compensation. A Special Majority Approval for Compensation requires shareholder approval by a majority vote of the shares present and voting at a meeting of shareholders called for such purpose, provided that either: (a) such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in such compensation arrangement; or (b) the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in the compensation arrangement and who vote against the arrangement does not exceed 2% of the company’s aggregate voting rights. Under the Companies Law, subject to certain conditions, the board of directors may adopt the compensation policy even if it is not approved by the shareholders. Under the Companies Law, a company must adopt a compensation policy within nine months following its becoming a public company. Pursuant to those requirements, our compensation committee and board of directors adopted our compensation policy, and our shareholders approved it at a special general meeting of shareholders held on December 30, 2014.
The compensation policy must serve as the basis for decisions concerning the financial terms of employment or engagement of office holders, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The compensation policy must relate to certain factors, including the advancement of the company’s objectives, the company’s business plan and its long-term strategy, and the creation of appropriate incentives for office holders. It must also consider, among other things, the company’s risk management, size and the nature of its operations. The compensation policy must furthermore consider additional factors, including:
| | • | the education, skills, expertise and accomplishments of the relevant office holder; |
| | • | the office holder’s roles and responsibilities and prior compensation agreements with him or her; |
| | • | the relationship between the cost of the terms offered and the cost of the compensation of the other employees of the company, including those employed through manpower companies; |
| | • | the impact of disparities in salary upon work relationships in the company; |
| | • | the possibility of reducing variable compensation at the discretion of the board of directors; |
| | • | the possibility of setting a limit on the exercise value of non-cash variable share-based compensation; and |
| | • | as to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company. |
The compensation policy must also include the following principles:
| | • | the link between variable compensation and long-term performance and measurable criteria; |
| | • | the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation; |
| | • | the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements; |
| | • | the minimum holding or vesting period for variable, share-based compensation; and |
| | • | maximum limits for severance compensation. |
The compensation committee is responsible for (a) recommending the compensation policy to a company’s board of directors for its approval (and subsequent approval by its shareholders) and (b) duties related to the compensation policy and to the compensation of a company’s office holders, including:
| | • | recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years); |
| | • | recommending to the board of directors periodic updates to the compensation policy; |
| | • | assessing implementation of the compensation policy; and |
| | • | determining whether the compensation terms of the chief executive officer of the company need not be brought to approval of the shareholders. |
Compensation Committee Role
Our board of directors has adopted a compensation committee charter setting forth the responsibilities of the compensation committee, which include:
| | • | the responsibilities set forth in the compensation policy; |
| | • | reviewing and approving the granting of options and other incentive awards to the extent such authority is delegated by our board of directors; and |
| | • | reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors. |
Internal Auditor
Under the Companies Law, the board of directors of an Israeli public company must appoint an internal auditor recommended by the audit committee. An internal auditor may not be:
| | • | a person (or a relative of a person) who holds more than 5% of the company’s outstanding shares or voting rights; |
| | • | a person (or a relative of a person) who has the power to appoint a director or the general manager of the company; |
| | • | an office holder (including a director) of the company (or a relative thereof); or |
| | • | a member of the company’s independent accounting firm, or anyone on its behalf. |
The role of the internal auditor is to examine, among other things, our compliance with applicable law and orderly business procedures. The audit committee is required to oversee the activities and to assess the performance of the internal auditor as well as to review the internal auditor’s work plan. Pursuant to the foregoing requirements, we have appointed Hila Barr-Hoisman from Deloitte Brightman Almagor Zohar Israel, as our internal auditor.
Approval of Related Party Transactions under Israeli Law
Fiduciary Duties of Directors and Executive Officers
The Companies Law codifies the fiduciary duties that office holders owe to a company. Each person listed in the table under “—Executive Officers and Directors” is an office holder under the Companies Law.
An office holder’s fiduciary duties consist of a duty of care and a duty of loyalty. The duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of loyalty requires that an office holder act in good faith and in the best interests of the company.
The duty of care includes a duty to use reasonable means to obtain:
| | • | information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and |
| | • | all other important information pertaining to any such action. |
The duty of loyalty includes a duty to:
| | • | refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or personal affairs; |
| | • | refrain from any activity that is competitive with the company; |
| | • | refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and |
| | • | disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder. |
Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions
The Companies Law requires that an office holder promptly disclose to the company any personal interest that he or she may be aware of and all related material information or documents concerning any existing or proposed transaction with the company. An interested office holder’s disclosure must be made promptly and in any event no later than the first meeting of the board of directors at which the transaction is considered. A personal interest includes an interest of any person in an action or transaction of a company, including a personal interest of such person’s relative or of a corporate body in which such person or a relative of such person is a 5% or greater shareholder, director or general manager or in which he or she has the right to appoint at least one director or the general manager, but excluding a personal interest stemming from one’s ownership of shares in the company.
A personal interest furthermore includes the personal interest of a person for whom the office holder holds a voting proxy or the personal interest of the office holder with respect to his or her vote on behalf of a person for whom he or she holds a proxy even if such person has no personal interest in the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction. Under the Companies Law, an extraordinary transaction is defined as any of the following:
| | • | a transaction other than in the ordinary course of business; |
| | • | a transaction that is not on market terms; or |
| | • | a transaction that may have a material impact on a company’s profitability, assets or liabilities. |
If it is determined that an office holder has a personal interest in a transaction, approval by the board of directors is required for the transaction, unless the company’s articles of association provide for a different method of approval. Further, so long as an office holder has disclosed his or her personal interest in a transaction, the board of directors may approve an action by the office holder that would otherwise be deemed a breach of his or her duty of loyalty. However, generally, a company may only approve a transaction or action in which an office holder has a personal interest that is in the best interests of the company. An extraordinary transaction in which an office holder has a personal interest requires approval first by the company’s audit committee and subsequently by the board of directors. The compensation of, or an undertaking to indemnify or insure, an office holder who is not a director requires approval first by the company’s compensation committee, then by the company’s board of directors. If such compensation arrangement or an undertaking to indemnify or insure is inconsistent with the company’s stated compensation policy, or if the office holder is the chief executive officer (apart from a number of specific exceptions), then such arrangement is further subject to a Special Majority Approval for Compensation. Arrangements regarding the compensation, indemnification or insurance of a director require the approval of the compensation committee, board of directors and, subject to certain exceptions, shareholders by ordinary majority, in that order, and under certain circumstances, a Special Majority Approval for Compensation. If shareholders of a company do not approve the compensation terms of office holders, other than directors, the compensation committee and board of directors may override the shareholders’ decision, subject to certain conditions.
Generally, a person who has a personal interest in a matter that is considered at a meeting of the board of directors or the audit committee may not be present at such a meeting or vote on that matter unless the chairman of the board of directors or audit committee (as applicable) determines that he or she should be present in order to present the transaction that is subject to approval. If a majority of the members of the audit committee or the board of directors (as applicable) has a personal interest in the approval of a transaction, then all directors may participate in discussions of the audit committee or the board of directors (as applicable) on such transaction and the voting on approval thereof, but shareholder approval is also required for such transaction.
Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions
Pursuant to the Companies Law, the disclosure requirements regarding personal interests that apply to directors and executive officers also apply to a controlling shareholder of a public company. In the context of a transaction involving a shareholder of the company, a controlling shareholder also includes a shareholder who holds 25% or more of the voting rights in the company if no other shareholder holds more than 50% of the voting rights in the company. For this purpose, the holdings of all shareholders who have a personal interest in the same transaction will be aggregated. The approval of the audit committee, the board of directors and the shareholders of the company, in that order, is required for (a) extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, (b) the engagement of a controlling shareholder or his or her relative, directly or indirectly, for the provision of services to the company, (c) the terms of engagement and compensation of a controlling shareholder or his or her relative who is not an office holder or (d) the employment of a controlling shareholder or his or her relative by the company, other than as an office holder. In addition, the shareholder approval requires one of the following, which we refer to as a Special Majority:
| | • | at least a majority of the shares held by all shareholders who do not have a personal interest in the transaction and who are present and voting at the meeting are voted in favor of the transaction, excluding abstentions; or |
| | • | the shares voted against the transaction by shareholders who have no personal interest in the transaction and who are present and voting at the meeting do not exceed 2% of the voting rights in the company. |
To the extent that any such transaction with a controlling shareholder is for a period extending beyond three years, approval is required once every three years, unless, with respect to certain transactions, the audit committee determines that the duration of the transaction is reasonable given the circumstances related thereto.
Arrangements regarding the compensation, indemnification or insurance of a controlling shareholder in his or her capacity as an office holder require the approval of the compensation committee, board of directors and shareholders by a Special Majority and the terms thereof may not be inconsistent with the company’s stated compensation policy.
Pursuant to regulations promulgated under the Companies Law, certain transactions with a controlling shareholder or his or her relative, or with directors, that would otherwise require approval of a company’s shareholders may be exempt from shareholder approval upon certain determinations of the audit committee or the compensation committee, as the case may be, and board of directors. Under these regulations, a shareholder holding at least 1% of the issued share capital of the company may require, within 14 days of the publication of such determinations, that despite such determinations by the relevant committee and the board of directors, such transaction will require shareholder approval under the same majority requirements that would otherwise apply to such transactions.
Shareholder Duties
Pursuant to the Companies Law, a shareholder has a duty to act in good faith and in a customary manner toward the company and other shareholders and to refrain from abusing his or her power in the company, including, among other things, in voting at a general meeting and at shareholder class meetings with respect to the following matters:
| | • | an amendment to the company’s articles of association; |
| | • | an increase of the company’s authorized share capital; |
| | • | the approval of related party transactions and acts of office holders that require shareholder approval. |
A shareholder also has a general duty to refrain from discriminating against other shareholders.
In addition, certain shareholders have a duty of fairness toward the company. These shareholders include a controlling shareholder, a shareholder who knows that he or she has the power to determine the outcome of a shareholder vote and a shareholder who has the power to appoint or to prevent the appointment of an office holder of the company or other power towards the company. The Companies Law does not define the substance of the duty of fairness, except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty of fairness.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our amended and restated articles of association which are effective prior to the closing of our IPO include such a provision. A company may not exculpate in advance a director from liability arising out of a prohibited dividend or distribution to shareholders.
Under the Companies Law, a company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking made in advance of an event or following an event, provided its articles of association include a provision authorizing such indemnification:
| | • | financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court; however, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria; |
| | • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding, and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction; and |
| | • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent. |
Under the Companies Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder, if and to the extent provided in the company’s articles of association:
| | • | a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company; |
| | • | a breach of duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder; and |
| | • | a financial liability imposed on the office holder in favor of a third party. |
Under the Companies Law, a company may not indemnify, exculpate or insure an office holder against any of the following:
| | • | a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company; |
| | • | a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| | • | an act or omission committed with intent to derive illegal personal benefit; or |
| | • | a fine or forfeit levied against the office holder. |
Under the Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to certain office holders or under certain circumstances, also by the shareholders. See “—Approval of Related Party Transactions under Israeli Law.”
Our articles permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted or to be permitted by the Companies Law.
We have obtained directors and officers liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by the Companies Law. In addition, we have entered into agreements with each of our directors and executive officers exculpating them from liability to us for damages caused to us as a result of a breach of duty of care and undertaking to indemnify them, in each case, to the fullest extent permitted by our amended and restated articles of association and the Companies Law, including with respect to liabilities resulting from our IPO to the extent that these liabilities are not covered by insurance. In the opinion of the SEC, however, indemnification of directors and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics applicable to all of our directors and employees, including our Chief Executive Officer, Chief Financial Officer, Vice President Finance, controller or principal accounting officer, or other persons performing similar functions, which is a “code of ethics” as defined in Item 16B of Form 20-F promulgated by the SEC. We have posted the full text of the Code of Business Conduct and Ethics on our website at www.macrocure.com. Information contained on, or that can be accessed through, our website does not constitute a part of this annual report and is not incorporated by reference herein. If we make any amendment to the Code of Business Conduct and Ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the SEC. Under Item 16B of Form 20-F, if a waiver or amendment of the Code of Business Conduct and Ethics applies to our principal executive officer, principal financial officer, principal accounting officer or controller and relates to standards promoting any of the values described in Item 16B(b) of Form 20-F, we are required to disclose such waiver or amendment on our website in accordance with the requirements of Instruction 4 to such Item 16B.
As of December 31, 2014, we had 28 employees, 19 based in Israel and 9 based in the United States. The total number of our full-time employees and the distribution of our employees according to main areas of activity, as of the end of each of the last three fiscal years, are set forth in the following table.
| | | Number of full-time employees by area of activity as of | |
| | | December 31, | |
| | | 2014 | | | 2013 | | | 2012 | |
| Area of Activity | | | | | | | | | |
| General and administrative | | | 8 | | | | 9 | | | | 10 | |
| Research and development | | | 20 | | | | 20 | | | | 14 | |
| Total | | | 28 | | | | 29 | | | | 24 | |
During the periods covered by the above tables, we did not employ a significant number of temporary employees, although we did utilize a CRO for overseeing and implementing our clinical trials in the United States, which itself had many employees.
Israeli labor laws govern the length of the workday and workweek, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination, payments to the National Insurance Institute, and other conditions of employment and include equal opportunity and anti-discrimination laws. While none of our employees is party to any collective bargaining agreements, certain provisions of the collective bargaining agreements between the Histadrut (General Federation of Labor in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Associations) are applicable to our employees in Israel by order of the Israeli Ministry of the Economy. These provisions primarily concern pension fund benefits for all employees, insurance for work-related accidents, recuperation pay and travel expenses. We generally provide our employees with benefits and working conditions beyond the required minimums.
We have never experienced any employment-related work stoppages and believe our relationships with our employees are good.
Equity Incentive Plans
2008 Stock Option Plan
In November 2008, we adopted our 2008 Stock Option Plan, or the 2008 Plan. The 2008 Plan permits the grant of options to our directors, employees, officers, consultants and service providers, among others.
The initial reserved pool under the 2008 Plan was 690,000 ordinary shares, which, following subsequent increases and decreases, and option exercises, now consists of a total of 1,127,322 ordinary shares (that are issuable under outstanding options). The 2008 Plan expires in November 2018. The 2008 Plan is administered by our board of directors or a committee designated by our board of directors, which determines, subject to Israeli law, the grantees of options, the terms of the options, including exercise prices, vesting schedules, acceleration of vesting, the type of option and the other matters necessary or desirable for, or incidental to the administration of the 2008 Plan. The 2008 Plan provides for the issuance of options under various Israeli tax regimes including, without limitation, pursuant to Sections 102 and 3(i) of the Israeli Income Tax Ordinance (New Version) 1961, or the Ordinance.
Section 102 of the Ordinance allows employees, directors and officers, who are not controlling shareholders and who are Israeli residents, to receive favorable tax treatment for compensation in the form of shares or options. Section 102 of the Ordinance includes two alternatives for tax treatment involving the issuance of options or shares to a trustee for the benefit of the grantees and also includes an additional alternative for the issuance of options or shares directly to the grantee. Section 102(b)(2) of the Ordinance, which provides the most favorable tax treatment for grantees, permits the issuance to a trustee under the “capital gains track.” In order to comply with the terms of the capital gains track, all options granted under a specific plan and subject to the provisions of Section 102 of the Ordinance, as well as the shares issued upon exercise of such options and other shares received following any realization of rights with respect to such options, such as share dividends and share splits, must be registered in the name of a trustee selected by the board of directors and held in trust for the benefit of the relevant employee, director or officer. The trustee may not release these options or shares to the relevant grantee before the second anniversary of the registration of the options in the name of the trustee. However, under this track, we are not allowed to deduct an expense with respect to the issuance of the options or shares.
The 2008 Plan provides that options granted to our employees, directors and officers who are not controlling shareholders and who are considered Israeli residents are intended to qualify for special tax treatment under the “capital gains track” provisions of Section 102(b)(2) of the Ordinance. Under the 2008 Plan, our Israeli non-employee service providers and controlling shareholders may only be granted options under Section 3(i) of the Ordinance, which does not provide for similar tax benefits.
Options granted under the 2008 Plan are subject to vesting schedules and generally expire ten years from approval of the options and generally vest over four years commencing on the date of grant, such that 25% vests on the first anniversary of the date of grant and an additional 6.25% vests at the end of each subsequent three-month period thereafter for 36 months. Under the 2008 Plan, in the event of termination of employment or services for reasons of disability or death, the grantee, or in the case of death, his or her legal successor, may exercise options that have vested prior to termination within a period of twelve months after the date of termination. If a grantee’s employment or service is terminated for cause, all of the grantee’s vested and unvested options expire on the date of termination. If a grantee’s employment or service is terminated without cause, the grantee may exercise his or her vested options within 180 days after the date of termination. Any expired or unvested options are returned to the pool for reissuance.
The exercise price and the number and/or type of shares issuable upon exercise of options under the 2008 Plan shall be adjusted due to a stock split (forward or reverse), stock dividend, recapitalization or similar adjustment affecting our outstanding share capital. The exercise price per share of any outstanding option that has not been exercised yet and has not expired yet shall be reduced by the net amount payable on each share of our outstanding share capital due to any cash dividend distributed to our shareholders.
The 2008 Plan provides that in the event of a merger or consolidation of our company, or a sale of all, or substantially all, of our shares or assets or other transaction having a similar effect on us, then without the consent of the option holder, our board of directors or its designated committee, as applicable, may but is not required to (i) cause any outstanding award to be assumed or an equivalent award to be substituted by such successor corporation, or (ii) in case the successor corporation refuses to assume or substitute the award (a) provide the grantee with the option to exercise the award as to all or part of the shares (even a portion not then otherwise vested) or (b) cancel the options against payment in cash in an amount determined by the board of directors or the committee as fair in the circumstances. Notwithstanding the foregoing, our board of directors or its designated committee may upon such event amend or terminate the terms of any award, including conferring the right to purchase any other security or asset that the board of directors shall deem, in good faith, appropriate. Pursuant to the foregoing provisions of the 2008 Plan, our board of directors has determined that upon the occurrence of any such merger or similar event, the vesting of options granted to certain of our executive officers will accelerate, thereby enabling such officers to exercise those options (even to the extent not otherwise exercisable).
Following the adoption of our 2013 Plan (as described below), all ordinary shares underlying awards under the 2008 Plan that expire or that are cancelled, terminated or forfeited for any reason are automatically added to, and become available for grant under, the 2013 Plan.
2013 Share Incentive Plan
In October 2013, we adopted our 2013 Share Incentive Plan, or the 2013 Plan. The 2013 Plan permits the grant of options, restricted shares and other share-based awards to our directors, employees, officers, consultants, advisors and service providers, among others.
The reserved pool under the 2013 Plan was 1,108,324 ordinary shares which following subsequent events now consists of a total of 1,152,484 ordinary shares (that are either (i) issuable under outstanding options or (ii) available for issuance under future share-based grants). Pursuant to a recent amendment to the 2013 Plan, the number of shares issuable under the 2013 Plan will automatically increase on an annual basis, commencing in January 2015, by an amount equal to the lesser of (i) 2% of the number of outstanding shares as of the prior year-end and (ii) an amount determined by our board of directors. No grants may be made under the 2013 Plan after October 2023, although the 2013 Plan will remain in effect until the expiration of all outstanding awards granted prior to that time. The 2013 Plan is administered by our board of directors or a committee designated by our board of directors, which determines, subject to Israeli law, the grantees of options, the terms of the options, including exercise prices, vesting schedules, acceleration of vesting, the type of option and the other matters necessary or desirable for, or incidental to the administration of the 2013 Plan. The 2013 Plan provides for the issuance of options under various tax regimes including, without limitation, pursuant to Sections 102 and 3(i) of the Ordinance.
The 2013 Plan provides that options granted to our employees, directors and officers who are not controlling shareholders and who are considered Israeli residents are intended to qualify for special tax treatment under the “capital gains track” provisions of Section 102(b)(2) of the Ordinance. Our Israeli non-employee service providers and controlling shareholders may only be granted options under Section 3(i) of the Ordinance, which does not provide for similar tax benefits.
Options granted under the 2013 Plan generally vest over four years commencing on the date of grant, such that 25% vests on the first anniversary of the date of grant and an additional 6.25% vests at the end of each subsequent three-month period thereafter for 36 months. Under the 2013 Plan, in the event of termination of employment or services for reasons of disability or death, the grantee, or in the case of death, his or her legal successor, may exercise options that have vested prior to termination within a period of one year after the date of termination. If a grantee’s employment or service is terminated for cause, all of the grantee’s vested and unvested options expire on the date of termination. If a grantee’s employment or service is terminated for any other reason, the grantee may exercise his or her vested options within 90 days after the date of termination. Any expired or unvested options are returned to the pool for reissuance.
The 2013 Plan provides that in the event of a merger or consolidation of our company, or a sale of all, or substantially all, of our shares or assets or other transaction having a similar effect on us, then without the consent of the option holder, our board of directors or its designated committee, as applicable, may but is not required to (i) cause any outstanding award to be assumed or an equivalent award to be substituted by such successor corporation, or (ii) in case the successor corporation refuses to assume or substitute the award (a) provide the grantee with the option to exercise the award as to all or part of the shares or (b) cancel the options against payment in cash in an amount determined by the board of directors or the committee as fair in the circumstances. Notwithstanding the foregoing, our board of directors or its designated committee may upon such event amend or terminate the terms of any award, including conferring the right to purchase any other security or asset that the board of directors shall deem, in good faith, appropriate. In the case of a stock split (forward or revenue), stock dividend, recapitalization or similar adjustment affecting our outstanding share capital, the exercise price and the number and/or type of shares issuable upon exercise of options under the 2013 Plan shall be adjusted accordingly.
Options granted under the 2013 Plan to U.S. residents may qualify as incentive stock options within the meaning of Section 422 of the Code, or may be non-qualified. The exercise price for “incentive stock options” must not be less than the fair market value on the date on which an option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital.
The following table presents certain data for our 2008 and 2013 Plans as of December 31, 2014.
| Plan | | Total ordinary shares reserved under plan | | | Shares available for future grants under plan | | | Aggregate number of options exercised | | | Aggregate number of options outstanding | | | Weighted average exercise price of options outstanding | |
2008 Stock Option Plan | | | 1,379,172 | | | | — | | | | 251,850 | | | | 1,127,322 | | | $ | 6.00 | |
2013 Share Incentive Plan | | | 1,152,467 | | | | 430,683 | | | | — | | | | 721,784 | | | $ | 9.15 | |
Share Ownership of Executive Officers and Directors
For information concerning the overall beneficial ownership of our ordinary shares by our executive officers and directors, please see the table in Item 7. “Major Shareholders and Related Party Transactions—Major Shareholders” below. As of December 31, 2014, options to purchase 1,228,934 ordinary shares granted to our directors and executive officers were outstanding under our share option plans at a weighted average exercise price of $8.05 per share. The ordinary shares held by our directors and executive officers do not have voting rights that differ from those enjoyed by all holders of our ordinary shares.
Item 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
The following table sets forth information with respect to the beneficial ownership of our shares as of February 28, 2015 by:
| | · | each person or entity known by us to own beneficially more than 5% of our outstanding shares; |
| | · | each of our directors and executive officers individually; and |
| | · | all of our executive officers and directors as a group. |
The beneficial ownership of ordinary shares is determined in accordance with the rules of the SEC and generally includes any ordinary shares over which a person exercises sole or shared voting or investment power, or the right to receive the economic benefit of ownership. For purposes of the table below, we deem shares subject to options or warrants that are currently exercisable or exercisable within 60 days of February 28, 2015 to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person. The percentage of shares beneficially owned is based on 16,702,225 ordinary shares outstanding as of February 28, 2015, which excluded an additional 1,339,244 ordinary shares issuable upon exercise of warrants at an exercise price of NIS 0.01 per share that were outstanding as of that date.
As of February 28, 2015, we had three holders of record of our ordinary shares in the United States, including Cede & Co., the nominee of The Depository Trust Company. These shareholders held in the aggregate 6,248,104 ordinary shares, or 38.6% of our outstanding ordinary shares as of February 28, 2015. The number of record holders in the United States is not representative of the number of beneficial holders nor is it representative of where such beneficial holders are resident since many of these ordinary shares were held by brokers or other nominees.
All of our shareholders, including the shareholders listed below, have the same voting rights attached to their ordinary shares. See “Item 10.B. Articles of Association.” None of our principal shareholders or our directors and executive officers has different or special voting rights with respect to his, her or its ordinary shares. Unless otherwise noted below, each shareholder’s address is c/o Macrocure Ltd., 25 Hasivim Street, Petach Tikva 4959383, Israel.
A description of any material relationship that our principal shareholders have had with us or any of our predecessors or affiliates within the past three years is included below under “Item 7.B. Related Party Transactions.”
| | | Number and Percentage of Ordinary Shares Beneficially Owned | |
| Name | | Number | | | Percent | |
5% or Greater Shareholders (other than directors and executive officers) | | | | | | |
Pontifax (Israel) and affiliated venture funds(1) | | | 1,861,134 | (2) | | | 11.2 | % |
Viola Private Equity I, L.P.(3) | | | 1,822,006 | (4) | | | 10.8 | % |
Viatcheslav Mirilashvili(5) | | | 3,333,032 | (6) | | | 20.5 | % |
| Directors and Executive Officers | | | | | | | | |
| Nissim Mashiach | | | 859,602 | (7) | | | 5.0 | % |
| Michael Molyneaux, MD | | | 20,236 | | | | 0.1 | % |
| Mark Page | | | — | | | | — | |
| David Ben Ami | | | 1,840,000 | | | | 11.3 | % |
| Ze’ev Bronfeld | | | 2,688,746 | | | | 16.5 | % |
| Ranan Grobman | | | 383,303 | (8) | | | 2.4 | % |
| Tomer Kariv | | | 1,861,134 | (9) | | | 11.2 | % |
| Jonathan Kolber | | | — | (10) | | | — | |
| Yuval Yanai | | | — | | | | — | |
| Katherine Wolf | | | — | | | | — | |
| All Directors and Executive Officers as a Group (10 persons) | | | 7,653,021 | (11) | | | 46.6 | % |
| | (1) | The address of Pontifax (Israel) and its affiliated venture funds, to which we refer collectively as Pontifax, is 14 Shenkar St., Herzliya Pituach, PO Box 4093, Herzliya, 46140, Israel. Each of Mr. Tomer Kariv, who is the chief executive officer of Pontifax, and Mr. Ron Nussbaum, shares voting and dispositive power with respect to the shares held by Pontifax. |
| | | |
| | (2) | Includes an aggregate of 320,344 ordinary shares issuable upon the exercise of warrants at an exercise price per share of NIS 0.01, all of which are currently exercisable. |
| | | |
| | (3) | The address of Viola Private Equity I, L.P., or Viola, is Ackerstein Towers, Building D, 12 Abba Eban Avenue, 46120 Herzliya Pituach, Israel. Mr. Harel Beit-On, Mr. Shlomo Dovrat, Mr. Avi Zeevi and Mr. Eylon Penchas hold indirect interests in, and are directors in, and/or shareholders of, various entities that are the general partners of Viola and may be deemed to be the beneficial owners of the shares held by Viola. |
| | | |
| | (4) | Includes an aggregate of 669,898 ordinary shares issuable upon the exercise of warrants at the weighted average exercise price of NIS 0.01, all of which are currently exercisable. |
| | | |
| | (5) | The address of Viatcheslav Mirilashvili is Hamanofim St., Herzliya Pituach, Herzliya, 46725, Israel. |
| | | |
| | (6) | Consists of (i) 2,693,770 ordinary shares held by Viatcheslav Mirilashvili, and (ii) (a) 391,184 ordinary shares and (b) 248,078 ordinary shares issuable upon the exercise of warrants that are currently exercisable at the weighted average exercise price per share of NIS 0.01, held by Vaizra Ventures Ltd., or Vaizra Ventures, an entity in which Mr. Mirilashvili indirectly holds 100% of the equity. As described in footnote (8) below, an entity for which Ranan Grobman, a director of our company, serves as a director and in which he holds a 40% equity interest, holds a currently exercisable option to purchase 10% of the ordinary shares of our company beneficially owned by Mr. Mirilashvili (including shares held or beneficially owned by Vaizra Ventures), in the aggregate. |
| | | |
| | (7) | Consists entirely of options to purchase ordinary shares, all of which are currently exercisable. |
| | | |
| | (8) | Includes 333,303 ordinary shares that constitute 10% of the 3,333,032 ordinary shares beneficially owned by Mr. Mirilashvili (including via Vaizra Ventures), in the aggregate, which are subject to a currently exercisable option to purchase that is held by an entity in which Mr. Grobman holds a 40% equity interest and for which he serves as a director. Mr. Grobman disclaims beneficial ownership of those 333,303 ordinary shares except to the extent of his pecuniary interest therein. |
| | | |
| | (9) | Consists of the 1,861,134 ordinary shares beneficially owned by Pontifax, for which Mr. Kariv serves as chief executive officer. Mr. Kariv and Ran Nussbaum share voting and dispositive power with respect to the shares held by Pontifax. |
| | | |
| | (10) | Excludes the 1,794,506 ordinary shares beneficially owned by Viola, for which Mr. Kolber serves as a general partner, as Mr. Kolber does not possess voting or dispositive power with respect to those shares. |
| | | |
| | (11) | Please see footnotes 7 through 10 above for information concerning the beneficial ownership of our directors and executive officers. |
| | B. | Related Party Transactions |
The following is a description of material transactions, or series of related material transactions, since January 1, 2014, to which we were or will be a party and in which the other parties included or will include our directors, executive officers, holders of more than 10% of our voting securities or any member of the immediate family of any of the foregoing persons.
Registration Rights
We are party to a Second Amended and Restated Registration Rights Agreement, dated July 22, 2014 (which became effective upon the closing of our IPO) with certain of our security holders, to which we refer as the registration rights agreement. Under the registration rights agreement, holders of a total of 3,746,010 of our ordinary shares (which excludes ordinary shares issuable upon exercise of certain of our outstanding warrants, whose holders also possess registration rights) have the right to require us to register their ordinary shares under the Securities Act under specified circumstances and will have incidental registration rights as described below.
Demand Registration Rights
At any time, the holders of at least 50% of the registrable securities then outstanding may request that we file a registration statement (including, once we are eligible to use Form F-3, which we anticipate will occur twelve months following the consummation of our IPO, a registration of the sale of their shares on a delayed or continuous basis under Form F-3) with respect to the registrable securities held by them. This demand right is subject to an anticipated aggregate offering price, net of selling expenses, of at least $5.0 million in an ordinary demand registration and $1.0 million for a registration on Form F-3. Upon receipt of such registration request, we are obligated to use our best efforts to effect, as soon as practicable, the registration under the Securities Act of all registrable securities that the Holders request to be registered. Our shareholders have the right to utilize their demand rights up to three times for an ordinary demand and up to two times in every 12 month period in the case of a demand for registration on Form F-3.
We will not be obligated to file a registration statement at any such time if in the good faith judgment of our board of directors (as reflected in a certificate delivered by our chief executive officer or the chairman of our board of directors), such registration would be seriously detrimental to our company, provided that we do not use that exemption more than once in any 12 month period. We also have the right not to effect or take any action to effect a registration statement during the period of 180 days following the effective date of a previous registration.
Piggyback Registration Rights
In addition, if we register any of our ordinary shares in connection with the public offering of such securities solely for cash, the holders of all registrable securities are entitled to notice of the registration and to include all or a portion of their registrable securities in the registration. If the public offering that we are effecting is underwritten, the right of any shareholder to include shares in the registration related thereto is conditioned upon the shareholder accepting the terms of the underwriting as agreed between us and the underwriters. Each shareholder may furthermore only include such quantity of registrable securities as the underwriters in their sole discretion determine will not jeopardize the success of our offering.
Other Provisions
We will pay all registration expenses (other than underwriting discounts and selling commissions) and the reasonable fees and expenses of a single counsel for the selling shareholders, related to any demand or piggyback registration. The demand and piggyback registration rights described above will expire five years after our initial public offering, or, in respect of any individual shareholder, when such shareholder (together with any affiliates of the shareholder with whom such shareholder must aggregate its sales under Rule 144 promulgated under the Securities Act) is able to sell all of its registrable securities within any three month period.
Agreements and Arrangements with, and Compensation of, Directors and Executive Officers
We have entered into written confidentiality, non-competition/solicitation and inventions assignment agreements with each of our executive officers. However, the enforceability of the non-competition provisions may be limited under applicable law. Our executive officers will not receive benefits upon the termination of their employment with us, other than payment of salary and benefits (and limited accrual of vacation days) during the required notice period for termination of their employment, which varies for each individual.
Indemnification agreements
Our amended and restated articles of association permit us to exculpate, indemnify and insure each of our directors and office holders to the fullest extent permitted by the Companies Law. Prior to the closing of this offering, we will enter into indemnification agreements with each of our directors and executive officers, undertaking to indemnify them to the fullest extent permitted by the Companies Law, including with respect to liabilities resulting from a public offering of our shares, to the extent that these liabilities are not covered by insurance. We have also obtained directors and officers insurance for each of our executive officers and directors. For further information, see “Item 6.C. Board Practices—Exculpation, Insurance and Indemnification of Directors and Officers.”
Convertible Loan Credit Line from Significant Shareholder
On July 10, 2014, we entered into a convertible loan agreement with Viatcheslav Mirilashvili, who together with Vaizra Ventures, of which he indirectly owns 100% of the equity, constitutes a significant shareholder of ours, pursuant to which Mr. Mirilashvili, to whom we refer as the lender, made available to us a line of credit in an amount of up to $10.0 million that we were permitted to draw upon in one or more installments, at our sole discretion. Any amounts outstanding under the line of credit as of the consummation of the IPO were to automatically convert into ordinary shares of our company to be issued to the lender at the price per share being paid by the public in the IPO, although the underwriters would not have received any discounts or commissions in respect of that conversion. We did not draw from the line of credit at all, and the line of credit expired pursuant to its terms upon the consummation of our IPO.
In consideration of the lender’s having provided the line of credit to us, we issued to the lender a warrant to purchase 439,760 of our ordinary shares at a price per share equal to NIS 0.01. The warrant was to expire 10 years following the date of the convertible loan agreement, or earlier if we were to consummate a merger, sale of all or substantially all of our assets, license of all or substantially all of our intellectual property or similar transaction.
On February 12, 2015 the lender exercised the warrants to purchase 439,760 of our ordinary shares at a price per share equal to NIS 0.01.
Participation in Our IPO
Certain of our shareholders who held our ordinary shares since prior to our IPO, including affiliates of certain of our directors, purchased (or their affiliates purchased) an aggregate of 1,170,500 ordinary shares in our IPO at the price paid by the public ($10 per share), in the following amounts: Viatcheslav Mirilashvili: 900,000 ordinary shares; Pontifax (Israel) and affiliated venture funds: 75,000 ordinary shares; Ranan Grobman: 40,000 ordinary shares; Viola Private Equity I, L.P.: 27,500 ordinary shares; and other pre-IPO shareholders: 128,000 ordinary shares. The underwriters received the same underwriting discount on the shares purchased by these persons as they did on the other shares sold to the public in our IPO. The shares purchased by these persons were subject to the 180 day lock-up agreements entered into by our pre-IPO shareholders with the underwriters for our IPO (which lock-ups have subsequently expired).
| | C. | Interests of Experts and Counsel |
Item 8. FINANCIAL INFORMATION
| | A. | Consolidated Statements and Other Financial Information |
Consolidated Financial Statements
We have appended our consolidated financial statements at the end of this annual report, starting at page F-2, as part of this annual report.
Legal Proceedings
On July 2, 2014, the Directorate of Courts of Israel initiated a legal proceeding in the Israeli Magistrate Court of Rishon Lezion in which the court was requested to collect certain evidence and documents, including a laptop computer, which was used by a former employee. This proceeding was initiated in Israel at the request of the United States District Court for the District of Maryland, or the U.S. District Court, based on the Hague Convention on the Taking of Evidence Abroad in Civil or Commercial Matters (March 18, 1970). The purpose of the proceeding in Israel is to assist Cognate Bioservices, Inc., or Cognate, and additional plaintiffs to collect evidence that can be used in a legal action that they initiated against the former employee and that is pending in the U.S. District Court for, among other things, misappropriation of trade secrets. In August 2014, the Israeli court ordered our company to submit to it by September 17, 2014 all documents and evidence requested by the U.S. District Court, accompanied by a discovery affidavit. We objected to this order, based on various grounds, including that it jeopardizes our confidential information and trade secrets and subjects us to undue burden. A court hearing was held on September 30, 2014, followed by a decision rendered by the Israeli court. The Israeli court responded to the request of the U.S. District Court by noting our argument that all documents sought by the U.S. District Court are confidential and constitute trade secrets. The Israeli court further indicated that if the U.S. District Court is not satisfied with this response or requests the Israeli court to take additional action, it should provide its opinion on some questions that need to be clarified before we are required to provide the documents sought, including, inter alia, the degree of relevance of the documents to the case and the extent to which the requested documents are essential or crucial to the proceeding. On October 19, 2014, the Israeli court decided that the transcript of the hearing and all the decisions of the Israeli court shall be delivered to the judicial authority in Maryland by the unit of Legal Aid between Countries in the Directorate of Courts of Israel.
Due to the early stage of the above proceedings, we are not in a position to determine the effect of this demand and order on our company.
From time to time, we may become party to additional litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. Except as described above, we are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations.
Dividend Policy
We have never declared or paid cash dividends to our shareholders, and we do not intend to pay cash dividends in the foreseeable future. We intend to reinvest any earnings in developing and expanding our business. Any future determination relating to our dividend policy will be at the discretion of our board of directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, our strategic goals and plans to expand our business, applicable law and other factors that our board of directors may deem relevant.
See “Item 3.D. Risk Factors—Risks Related to an Investment in Our Ordinary Shares—We have never paid cash dividends on our share capital, and we do not anticipate paying any cash dividends in the foreseeable future” and “Item 10.B. Articles of Association—Description of Share Capital—Dividend and Liquidation Rights.”
No significant changes have occurred since December 31, 2014, except as otherwise disclosed in this annual report.
Item 9. THE OFFER AND LISTING
Our ordinary shares have been quoted on the NASDAQ Global Market under the symbol “MCUR” since July 31, 2014. Prior to that date, there was no public trading market for our ordinary shares. Our IPO was priced at $10.00 per share on July 30, 2014. The following table sets forth for the periods indicated the high and low closing sales prices per ordinary share as reported on NASDAQ:
| | | | | | | |
| | | (in U.S. dollars) | |
| Annual: | | | | | | |
| 2014 (beginning July 31, 2014) | | $ | 7.01 | | | $ | 9.71 | |
| Quarterly: | | | | | | | | |
| First Quarter 2015 (through February 28, 2015) | | | 7.53 | | | | 11.84 | |
| Fourth Quarter 2014 | | | 7.01 | | | | 8.30 | |
| Third Quarter 2014 (beginning July 31, 2014) | | | 7.10 | | | | 9.71 | |
| Most Recent Six Months: | | | | | | | | |
| February 2015 | | | 9.35 | | | | 11.84 | |
| January 2015 | | | 7.53 | | | | 9.88 | |
| December 2014 | | | 7.30 | | | | 8.30 | |
| November 2014 | | | 7.22 | | | | 8.00 | |
| October 2014 | | | 7.01 | | | | 7.68 | |
| September 2014 | | | 7.10 | | | | 9.60 | |
Not applicable.
See “—Listing Details” above.
Not applicable.
Not applicable.
Not applicable.
Item 10. ADDITIONAL INFORMATION
Not applicable.
| | B. | Articles of Association |
Registration Number and Purposes of the Company. Our registration number with the Israeli Registrar of Companies is 51-408376-5. Our purpose as set forth in our articles is to engage in any lawful activity or business
Voting Rights and Conversion. All ordinary shares have identical voting and other rights in all respects.
Transfer of Shares. Our fully paid ordinary shares are issued in registered form and may be freely transferred under our articles of association, unless the transfer is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trade. The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our articles of association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
Election of Directors. Our ordinary shares do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect all of our directors, subject to the special approval requirements for external directors described under “Item 6.C. Board Practices—External Directors.”
Under our articles, our board of directors must consist of at least four and not more than eleven directors, including at least two external directors required to be appointed under the Companies Law. The amendment of the minimum and maximum number of directors of our company requires the approval of at least 65% of the voting power present and voting (excluding abstentions) at a general meeting of our shareholders.
Pursuant to our articles, each of our directors, other than the external directors, for whom special election requirements apply under the Companies Law, is elected by a simple majority vote of holders of our voting shares, participating and voting at each annual general meeting of our shareholders. Each director, other than the external directors, serves on our board of directors until he or she is removed by a vote of not less than 65% of the voting power of our shareholders present and voting (excluding abstentions) at a general or special meeting of our shareholders or upon the occurrence of certain events, in accordance with the Companies Law and our articles, including the election of his or her successor or his or her earlier death or resignation. In addition, our articles allow our board of directors to appoint directors to fill vacancies on the board of directors to serve for a term of office equal to the remaining period of the term of office of the directors(s) whose office(s) have been vacated. External directors are elected for an initial term of three years, may be elected for additional terms of three years each under certain circumstances, and may be removed from office pursuant to the terms of the Companies Law. Please see “Item 6.C. Board Practices—Board of Directors—External Directors.”
Dividend and Liquidation Rights. We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Companies Law, dividend distributions are determined by the board of directors and do not require the approval of the shareholders of a company unless the company’s articles of association provide otherwise. Our articles do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the Companies Law, the distribution amount is limited to the greater of retained earnings or earnings generated over the previous two years, according to our then last reviewed or audited financial statements, provided that the end of the period to which the financial statements relate is not more than six months prior to the date of the distribution. If we do not meet such criteria, then we may distribute dividends only with court approval. In each case, we are only permitted to distribute a dividend if our board of directors and the court, if applicable, determines that there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Exchange Controls. There are currently no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares or interest or other payments to non-residents of Israel, except for shareholders who are subjects of countries that are, or have been, in a state of war with Israel.
Shareholder Meetings. Under Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year and no later than 15 months after the date of the previous annual general meeting. All shareholder meetings other than the annual general meeting of shareholders are referred to in our amended and restated articles of association as special general meetings. Our board of directors may call special general meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides that our board of directors is required to convene a special general meeting upon the written request of (i) any two or more of our directors or one-quarter or more of the members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of our outstanding issued shares and 1% or more of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Subject to the provisions of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date of the meeting. Furthermore, the Companies Law requires that resolutions regarding the following matters must be passed at a general meeting of our shareholders:
| • | amendments to our articles of association; |
| | |
| • | appointment or termination of our auditors; |
| | |
| • | appointment of external directors; |
| | |
| • | approval of certain related party transactions; |
| | |
| • | increases or reductions of our authorized share capital; |
| | |
| • | a merger; and |
| | |
| • | the exercise of our board of directors’ powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management. |
The Companies Law requires that a notice of any annual general meeting or special general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes, among others, the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, or an approval of a merger, notice must be provided at least 35 days prior to the meeting.
Under the Companies Law and under our articles, shareholders are not permitted to take action by way of written consent in lieu of a meeting.
Voting Rights
Quorum Requirements
Pursuant to our articles, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting. As permitted under the Companies Law and the NASDAQ Listing Rules, due to our status as a foreign private issuer, the quorum required for our general meetings of shareholders consists of at least two shareholders present in person, by proxy or written ballot who hold or represent between them at least 25% of the total outstanding voting rights. A meeting adjourned for lack of a quorum is generally adjourned to the same day in the following week at the same time and place or to a later time or date if so specified in the notice of the meeting. At the reconvened meeting, subject to certain exceptions, any two or more shareholders present in person or by proxy shall constitute a lawful quorum.
Vote Requirements
Our articles provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Companies Law or by our articles. Under the Companies Law, each of (i) the approval of an extraordinary transaction with a controlling shareholder, (ii) the terms of employment or other engagement of the controlling shareholder of the company or such controlling shareholder's relative (even if such terms are not extraordinary), (iii) the compensation of a company's chief executive officer, (iv) the approval of a compensation policy with respect to a company's office holders, (v) service of the same person as chief executive officer and chairman of the board and (vi) certain other matters, requires the approval of special majorities by shareholders, including, where applicable, a Special Majority and a Special Majority Approval for Compensation, as described above under “Item 6.C. Board Parctices—Approval of Related Party Transactions under Israeli Law.” Under our articles, the alteration of the rights, privileges, preferences or obligations of any class of our shares requires a simple majority of the class so affected (or such other percentage of the relevant class that may be set forth in the governing documents relevant to such class), in addition to the ordinary majority vote of all classes of shares voting together as a single class at a shareholder meeting.
Further exceptions to the simple majority vote requirement are a resolution for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350 of the Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting and voting on the resolution. Our articles also impose certain supermajority voting requirements, which provide that approval by at least 65% of the voting power of our shareholders present and voting (excluding abstentions) at a general meeting is required for any of (i) the amendment of the minimum and maximum number of our directors under our articles, or (ii) removal of a director from office.
Access to Corporate Records
Under the Companies Law, shareholders are provided access to: minutes of our general meetings; our shareholders register and principal shareholders register, articles of association and annual audited financial statements; and any document that we are required by law to file publicly with the Israeli Registrar of Companies or the Israel Securities Authority. In addition, shareholders may request to be provided with any document related to an action or transaction requiring shareholder approval under the related party transaction provisions of the Companies Law. We may deny this request if we believe it has not been made in good faith or if such denial is necessary to protect our interest or protect a trade secret or patent.
Modification of Class Rights
Under the Companies Law and our articles, the rights attached to any class of share, such as voting, liquidation and dividend rights, may be amended by adoption of a resolution by the holders of a majority of the shares of that class present at a separate class meeting, or otherwise in accordance with the rights attached to such class of shares, as set forth in our articles.
Registration Rights
For a description of the registration rights that we have granted to certain of our shareholders and warrant holders, please see “Item 7.B. Related Party Transactions—Registration Rights” above.
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of an Israeli public company and who would as a result hold over 90% of the target company’s issued and outstanding share capital is required by the Companies Law to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a public Israeli company and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of shares.
Upon a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli court as described above.
If (a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company or of the applicable class or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares from shareholders who accepted the tender offer that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class.
Special Tender Offer
The Companies Law provides that, subject to certain exceptions, an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company. This requirement does not apply if there is already another holder of at least 25% of the voting rights in the company. Similarly, the Companies Law provides that an acquisition of shares in a public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the company who holds more than 45% of the voting rights in the company, subject to certain exceptions.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) the offeror acquired shares representing at least 5% of the voting power in the company and (ii) the number of shares tendered by shareholders who accept the offer exceeds the number of shares held by shareholders who object to the offer (excluding the purchaser, controlling shareholders, holders of 25% or more of the voting rights in the company or any person having a personal interest in the acceptance of the tender offer). If a special tender offer is accepted, the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger
The Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described under the Companies Law are met, by a majority vote of each party’s shareholders. In the case of the target company, approval of the merger further requires a majority vote of each class of its shares.
For purposes of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of shares represented at the meeting of shareholders that are held by parties other than the other party to the merger, or by any person (or group of persons acting in concert) who holds (or hold, as the case may be) 25% or more of the voting rights or the right to appoint 25% or more of the directors of the other party, vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same Special Majority approval that governs all extraordinary transactions with controlling shareholders (as described under “Item 6.C. Board Practices—Approval of Related Party Transactions under Israeli Law—Fiduciary Duties of Directors and Executive Officers—Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”).
If the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the petition of holders of at least 25% of the voting rights of a company. For such petition to be granted, the court must find that the merger is fair and reasonable, taking into account the respective values assigned to each of the parties to the merger and the consideration offered to the shareholders of the target company.
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger is filed with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
Anti-Takeover Measures under Israeli Law
The Companies Law allow us to create and issue shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights with respect to voting, distributions or other matters and shares having preemptive rights. As of the closing of this offering, no preferred shares will be authorized under our amended and restated articles of association. In the future, if we do authorize, create and issue a specific class of preferred shares, such class of shares, depending on the specific rights that may be attached to it, may have the ability to frustrate or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization and designation of a class of preferred shares will require an amendment to our amended and restated articles of association, which requires the prior approval of the holders of a majority of the voting power attaching to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled to participate and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Companies Law as described above in this Item 10.B. under “—Voting Rights.”
Borrowing Powers
Pursuant to the Companies Law and our articles, our board of directors may exercise all powers and take all actions that are not required under law or under our amended and restated articles of association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
Changes in Capital
Our articles enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Companies Law and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits, require the approval of both our board of directors and an Israeli court.
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is Continental Stock Transfer & Trust Company.
For a description of the registration rights that we granted under our registration rights agreement, please refer to “Item 7.B. Related Party Transactions—Registration Rights.”
We entered into an underwriting agreement by and among our company and Credit Suisse Securities (USA) LLC and Jefferies LLC, as representatives of the underwriters, on July 30, 2014, with respect to the ordinary shares sold in our IPO. We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect of such liabilities.
In 1998, Israeli currency control regulations were liberalized significantly, so that Israeli residents generally may freely deal in foreign currency and foreign assets, and non-residents may freely deal in Israeli currency and Israeli assets. There are currently no Israeli currency control restrictions on remittances of dividends on the ordinary shares or the proceeds from the sale of the shares provided that all taxes were paid or withheld; however, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
Non-residents of Israel may freely hold and trade our securities. Neither our articles nor the laws of the State of Israel restrict in any way the ownership or voting of ordinary shares by non-residents, except that such restrictions may exist with respect to citizens of countries which are in a state of war with Israel. Israeli residents are allowed to purchase our ordinary shares.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our ordinary shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction. This summary does not discuss all of the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of such investors include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion.
Certain Israeli Tax Consequences
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. Israeli capital gains tax is imposed on the disposal of capital assets by a non-Israeli resident if such assets are either (i) located in Israel; (ii) shares or rights to shares in an Israeli resident company, or (iii) represent, directly or indirectly, rights to assets located in Israel, unless a specific exemption is available or unless a tax treaty between Israel and the seller's country of residence provides otherwise.
Capital gain is generally subject to tax at the corporate tax rate (26.5% in 2014 and thereafter), if generated by a company, or if generated by an individual (from the sale of an asset purchased on or after January 1, 2003) at the rate of 25% or at a rate of 30% in the case of sale of shares by a Substantial Shareholder (i.e., a person who holds, directly or indirectly, alone or together with another, 10% or more of any of the company's “means of control” (including, among other things, the right to receive profits of the company, voting rights, the right to receive proceeds upon liquidation and the right to appoint a director)) at the time of sale or at any time during the preceding 12-month period. Individual and corporate shareholders dealing in securities in Israel are taxed at the tax rates applicable to business income (a corporate tax rate for a corporation and a marginal tax rate of up to 48% for an individual in 2014).
Notwithstanding the foregoing, a non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a recognized stock exchange in Israel or outside of Israel will generally be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of 25% or more in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly. In addition, such exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares are deemed to be business income.
Additionally, a sale of shares by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, under the United States-Israel Tax Treaty, the disposition of shares by a shareholder who (i) is a U.S. resident (for purposes of the treaty), (ii) holds the shares as a capital asset, and (iii) is entitled to claim the benefits afforded to such person by the treaty, is generally exempt from Israeli capital gains tax. Such exemption will not apply if (i) the capital gain arising from the disposition can be attributed to a permanent establishment in Israel; (ii) the shareholder holds, directly or indirectly, shares representing 10% or more of the voting rights during any part of the 12-month period preceding the disposition, subject to certain conditions; or (iii) such U.S. resident is an individual and was present in Israel for a period or periods aggregating to 183 days or more during the relevant taxable year. In such case, the sale, exchange or disposition of our ordinary shares would be subject to Israeli tax, to the extent applicable; however, under the United States-Israel Tax Treaty, the taxpayer would be permitted to claim a credit for such taxes against the U.S. federal income tax imposed with respect to such sale, exchange or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The United States-Israel Tax Treaty does not relate to U.S. state or local taxes.
In some instances, whether or not our shareholders are liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale. Specifically, in transactions involving a sale of all of the shares of an Israeli resident company, in the form of a merger or otherwise, the Israel Tax Authority may require from shareholders who are not liable for Israeli tax to sign declarations in forms specified by this authority or obtain a specific exemption from the Israel Tax Authority to confirm their status as a non-Israeli resident, and, in the absence of such declarations or exemptions, may require the purchaser of the shares to withhold taxes at source.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents are generally subject to Israeli withholding tax on the receipt of dividends paid on our ordinary shares at the rate of 25%. With respect to a person who is a Substantial Shareholder at the time of receiving the dividend or at any time during the preceding 12 months, the applicable withholding tax rate is 30%, unless such Substantial Shareholder holds such shares through a nominee company, in which case the rate is 25%.
For example, under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the United States-Israel Tax Treaty) is 25%. However, for dividends (not generated by an Approved Enterprise, a Benefited Enterprise or a Preferred Enterprise as defined under the Israeli Law for the Encouragement of Capital Investments, 5719-1959) paid to a U.S. corporation holding 10% or more of the outstanding voting rights throughout the tax year in which the dividend is distributed as well as during the previous tax year, the maximum rate of withholding tax is generally 12.5%, provided that not more than 25% of the gross income for such preceding year consists of certain types of dividends and interest. U.S. residents who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction for U.S. federal income tax purposes in the amount of the taxes withheld, subject to detailed limitations under U.S. laws applicable to foreign tax credits.
A non-Israeli resident who receives dividends from which tax was withheld is generally exempt from the obligation to file tax returns in Israel with respect to such income, provided that (i) such income was not generated from business conducted in Israel by the taxpayer for more than 180 days during the tax year and (ii) the taxpayer has no other taxable sources of income in Israel with respect to the period for which a tax return is required to be filed.
We cannot assure you that in the event we declare a dividend we will designate the income that we may distribute in a way that will reduce shareholders' tax liability.
Excess Tax
Beginning on January 1, 2013, an additional tax liability at the rate of 2% was added to the applicable tax rate on the annual taxable income of individuals who are subject to tax in Israel (whether any such individual is an Israeli resident or non-Israeli resident) exceeding NIS 811,560 (in 2014) which amount is linked to the annual change in the Israeli consumer price index, including, but not limited to, dividends, interest and capital gain, subject to the provisions of an applicable tax treaty.
Certain United States Federal Income Tax Consequences
The following is a description of certain United States federal income tax consequences relating to the acquisition, ownership and disposition of our ordinary shares by a U.S. Holder (as defined below). This description addresses only the United States federal income tax consequences to U.S. Holders that hold our ordinary shares as capital assets. This description does not address tax considerations applicable to U.S. Holders that may be subject to special tax rules, including, without limitation:
| | · | banks, financial institutions or insurance companies; |
| | · | real estate investment trusts, regulated investment companies or grantor trusts; |
| | · | dealers or traders in securities, commodities or currencies; |
| | · | tax-exempt entities or organizations, including an “individual retirement account” or “Roth IRA” as defined in Section 408 or 408A of the Code, respectively; |
| | · | certain former citizens or long-term residents of the United States; |
| | · | persons that received our shares as compensation for the performance of services; |
| | · | persons that will hold our shares as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for United States federal income tax purposes; |
| | · | partnerships (including entities classified as partnerships for United States federal income tax purposes) or other pass-through entities, or holders that will hold our shares through such an entity; |
| | · | holders that acquire ordinary shares as a result of holding or owning our preferred shares; |
| | · | holders whose “functional currency” is not the U.S. Dollar; or |
| | · | holders that own directly, indirectly or through attribution 10.0% or more of the voting power or value of our shares. |
Moreover, this description does not address the United States federal estate, gift or alternative minimum tax consequences, or any state, local or foreign tax consequences, of the acquisition, ownership and disposition of our ordinary shares.
This description is based on the Code, existing, proposed and temporary United States Treasury Regulations and judicial and administrative interpretations thereof, in each case as in effect and available on the date hereof. All of the foregoing is subject to change, which change could apply retroactively and could affect the tax consequences described below. There can be no assurances that the U.S. Internal Revenue Service, or IRS, will not take a different position concerning the tax consequences of the acquisition, ownership and disposition of our ordinary shares or that such a position would not be sustained. Holders should consult their own tax advisors concerning the U.S. federal, state, local and foreign tax consequences of purchasing, owning and disposing of our ordinary shares in their particular circumstances.
For purposes of this description, a “U.S. Holder” is a beneficial owner of our ordinary shares that, for United States federal income tax purposes, is:
| | · | a citizen or resident of the United States; |
| | · | a corporation (or other entity treated as a corporation for United States federal income tax purposes) created or organized in or under the laws of the United States or any state thereof, including the District of Columbia; |
| | · | an estate the income of which is subject to United States federal income taxation regardless of its source; or |
| | · | a trust if such trust has validly elected to be treated as a United States person for United States federal income tax purposes or if (1) a court within the United States is able to exercise primary supervision over its administration and (2) one or more United States persons have the authority to control all of the substantial decisions of such trust. |
If a partnership (or any other entity treated as a partnership for United States federal income tax purposes) holds our ordinary shares, the tax treatment of a partner in such partnership will generally depend on the status of the partner and the activities of the partnership. Such a partner or partnership should consult its tax advisor as to the particular United States federal income tax consequences of acquiring, owning and disposing of our ordinary shares in its particular circumstance
You should consult your tax advisor with respect to the United States federal, state, local and foreign tax consequences of acquiring, owning and disposing of our ordinary shares.
Distributions
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” if you are a U.S. Holder, the gross amount of any distribution made to you with respect to our ordinary shares before reduction for any Israeli taxes withheld therefrom, other than certain distributions, if any, of our ordinary shares distributed pro rata to all our shareholders, generally will be includible in your income as dividend income to the extent such distribution is paid out of our current or accumulated earnings and profits as determined under United States federal income tax principles. We do not expect to maintain calculations of our earnings and profits under United States federal income tax principles. Therefore, if you are a U.S. Holder you should expect that the entire amount of any distribution generally will be reported as dividend income to you. Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may qualify for the preferential rates of taxation with respect to dividends on ordinary shares if certain requirements, including stock holding period requirements, are satisfied by the recipient and the company is eligible for the benefits of the United States-Israel Tax Treaty.
However, such dividends will not be eligible for the dividends received deduction generally allowed to corporate U.S. Holders. To the extent that the amount of any distribution by us exceeds our current and accumulated earnings and profits as determined under United States federal income tax principles, it will be treated first as a return of your adjusted tax basis in our ordinary shares and thereafter as either long-term or short-term capital gain depending upon whether the U.S. Holder has held our ordinary shares for more than one year as of the time such distribution is received.
Subject to certain conditions and limitations, Israeli tax withheld on dividends may be deducted from your taxable income or credited against your United States federal income tax liability. If you are a U.S. Holder, dividends paid to you with respect to our ordinary shares will generally be treated as foreign source income, which may be relevant in calculating your foreign tax credit limitation. However, for periods in which we are a “United Stated-owned foreign corporation,” a portion of dividends paid by us may be treated as U.S. source solely for purposes of the foreign tax credit. We would be treated as a United States-owned foreign corporation if 50% or more of the total value or total voting power of our stock is owned, directly, indirectly or by attribution, by United States persons. To the extent any portion of our dividends is treated as U.S. source income pursuant to this rule, the ability of a U.S. Holder to claim a foreign tax credit for any Israeli withholding taxes payable in respect of our dividends may be limited. A U.S. Holder entitled to benefits under the United States-Israel Tax Treaty may, however, elect to treat any dividends as foreign source income for foreign tax credit purposes if the dividend income is separated from other income items for purposes of calculating the U.S. Holder’s foreign tax credit. U.S. Holders should consult their own tax advisors about the impact of, and any exception available to, the special sourcing rule described in this paragraph, and the desirability of making, and the method of making, such an election.
The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if you do not satisfy certain minimum holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and you should consult your tax advisor to determine whether and to what extent you will be entitled to this credit.
Sale, Exchange or Other Disposition of Ordinary Shares
Subject to the discussion below under “Passive Foreign Investment Company Considerations,” if you are a U.S. Holder, you generally will recognize gain or loss on the sale, exchange or other taxable disposition of our ordinary shares equal to the difference between the amount realized on such sale, exchange or other taxable disposition and your adjusted tax basis in our ordinary shares, and such gain or loss will be capital gain or loss. The adjusted tax basis in an ordinary share generally will be equal to the cost of such ordinary share. Except as discussed below with respect to foreign currency gain or loss, if you are a non-corporate U.S. Holder, capital gain from the sale, exchange or other taxable disposition of ordinary shares is generally eligible for a preferential rate of taxation applicable to capital gains, if your holding period for such ordinary shares exceeds one year (i.e., such gain is long-term capital gain). The deductibility of capital losses for United States federal income tax purposes is subject to limitations under the Code. Any such gain or loss that a U.S. Holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
A U.S. Holder’s initial tax basis in the ordinary shares will generally be the U.S. dollar value of the purchase price of our ordinary shares on the date of purchase. If our ordinary shares are treated as traded on an ‘‘established securities market,’’ a cash basis U.S. Holder or, if it elects, an accrual basis U.S. Holder, will determine the U.S. dollar value of the cost of such ordinary shares by translating the amount paid at the spot rate of exchange on the settlement date of the purchase. Such an election by an accrual basis U.S. Holder must be applied consistently from year to year and cannot be revoked without the consent of the IRS. The amount realized generally will be the U.S. dollar value of the payment received determined on the date of disposition. If our ordinary shares are treated as traded on an established securities market, a cash basis taxpayer, or, if it elects, an accrual basis taxpayer, will determine the U.S. dollar value of the amount realized by translating the amount realized (as determined on the trade date) at the spot rate of exchange on the settlement date of the sale.
On the settlement date, the U.S. Holder will recognize U.S. source foreign currency gain or loss (taxable as ordinary income or loss) equal to the difference (if any) between the U.S. dollar value of the amount received based on the exchange rates in effect on the date of sale or other disposition and the settlement date. However, in the case of ordinary shares traded on an established securities market that are sold by a cash basis U.S. Holder (or an accrual basis U.S. Holder that so elects), the amount realized will be based on the exchange rate in effect on the settlement date for the sale, and no exchange gain or loss will be recognized at that time.
Passive Foreign Investment Company Considerations
PFIC Status of the Company
If we were to be classified as a “passive foreign investment company,” or PFIC, in any taxable year, a U.S. Holder would be subject to special rules generally intended to reduce or eliminate any benefits from the deferral of U.S. federal income tax that a U.S. Holder could derive from investing in a non-U.S. company that does not distribute all of its earnings on a current basis.
A non-U.S. corporation will be classified as a PFIC for federal income tax purposes in any taxable year in which, after applying certain look-through rules with respect to the income and assets of subsidiaries, either:
| | · | at least 75% of its gross income is “passive income”; or |
| | · | at least 50% of the average quarterly value of its total gross assets (which, assuming we were a non-publicly traded CFC for the year being tested may be measured by the adjusted tax basis of our assets or, if we were a publicly traded CFC or not a CFC, the total value of our assets may be measured in part by the market value of our ordinary shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production of passive income. |
Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income, and includes amounts derived by reason of the temporary investment of funds raised in offerings of our ordinary shares. If a non-U.S. corporation owns directly or indirectly at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation’s income. If we are classified as a PFIC in any year with respect to which a U.S. Holder owns our ordinary shares, we will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding years during which the U.S. Holder owns our ordinary shares, regardless of whether we continue to meet the tests described above.
Based on estimates of our gross income and gross assets (including tangible assets and intangible assets based on the anticipated market value of our ordinary shares), our intended use of the proceeds of this offering, and the nature of our business, we do not believe that we were classified as a PFIC for the taxable year ended December 31, 2014 or that we will be classified as a PFIC for the taxable year ending December 31, 2015. However, because PFIC status is based on our income, assets, and activities for the entire taxable year, including how quickly we utilize the cash proceeds from our IPO in our business, it is not possible to finally determine whether we will be characterized as a PFIC for the 2015 taxable year until after the close of such year. Moreover, we must determine our PFIC status annually based on tests that are factual in nature; thus our status in future years will depend on our income, assets, and activities in those years as well as fluctuations in the market price of our ordinary shares. Accordingly, there can be no assurance regarding our PFIC status for the current or any future any taxable year.
If we were a PFIC, and you are a U.S. Holder, then unless you make the “mark-to-market” election described below, a special tax regime will apply to both (a) any “excess distribution” by us to you (generally, your ratable portion of distributions in any year which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding period for our ordinary shares) and (b) any gain realized on the sale or other disposition of the ordinary shares. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (i) the excess distribution or gain had been realized ratably over your holding period, (ii) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax, at the U.S. Holder’s regular ordinary income rate for the current year and would not be subject to the interest change discussed below), and (iii) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years. In addition, dividend distributions made to you will not qualify for the lower rates of taxation applicable to long-term capital gains discussed above under “—Distributions.”
If a U.S. Holder makes the mark-to-market election, then, in lieu of being subject to the tax and interest charge rules discussed above, the U.S. Holder generally will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. Holder makes the election, the U.S. Holder’s tax basis in the ordinary shares will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election).
The mark-to-market election is available only if we are a PFIC and our ordinary shares are “regularly traded” on a “qualified exchange.” Our ordinary shares will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter. The NASDAQ Global Market is a qualified exchange for this purpose. Because a mark-to-market election cannot be made for any lower-tier PFICs that we may own, a U.S. Holder may continue to be subject to the tax and interest charge rules discussed above with respect to such holder’s indirect interest in any investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes, including stock in any of the company’s subsidiaries that are treated as PFICs. If a U.S. Holder makes a mark-to-market election, it will be effective for the taxable year for which the election is made and all subsequent taxable years unless our ordinary shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election.
In certain circumstances, the adverse tax consequences of the special tax regime described above may be mitigated if a U.S. Holder makes a timely “qualified electing fund” election (a “QEF election”) (rather than a mark-to-market election) with respect to its interest in a PFIC; however, we do not intend to provide U.S. Holders with the information required to implement a QEF election with respect to our ordinary shares.
If we are determined to be a PFIC, the general tax treatment for U.S. Holders described in this section would apply to indirect distributions and gains deemed to be realized by U.S. Holders in respect of any of our subsidiaries that also may be determined to be PFICs.
If a U.S. Holder owns ordinary shares during any year in which we are a PFIC, the U.S. Holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to the company, generally with the U.S. Holder’s federal income tax return for that year.
U.S. Holders should consult their tax advisors regarding whether we are a PFIC and the potential application of the PFIC rules.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gains from the disposition of ordinary shares. Each U.S. Holder that is an individual, estate or trust is urged to consult its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in our ordinary shares.
Backup Withholding Tax and Information Reporting Requirements
United States backup withholding tax and information reporting requirements may apply to certain payments to certain holders of stock. Information reporting generally will apply to payments of dividends on, and to proceeds from the sale or redemption of, our ordinary shares made within the United States, or by a United States payer or United States middleman, to a holder of our ordinary shares, other than an exempt recipient (including a payee that is not a United States person that provides an appropriate certification and certain other persons). A payer will be required to withhold backup withholding tax from any payments of dividends on, or the proceeds from the sale or redemption of, ordinary shares within the United States, or by a United States payer or United States middleman, to a holder, other than an exempt recipient, if such holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with, or establish an exemption from, such backup withholding tax requirements. Any amounts withheld under the backup withholding rules will be allowed as a credit against the beneficial owner’s United States federal income tax liability, if any, and any excess amounts withheld under the backup withholding rules may be refunded, provided that the required information is timely furnished to the IRS.
Foreign Asset Reporting
Certain U.S. Holders who are individuals are required to report information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for shares held in accounts maintained by financial institutions) by filing IRS Form 8938 (Statement of Specified Foreign Financial Assets) with their federal income tax return. U.S. Holders are urged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of our ordinary shares.
The above description is not intended to constitute a complete analysis of all tax consequences relating to acquisition, ownership and disposition of our ordinary shares. You should consult your tax advisor concerning the tax consequences of your particular situation.
| | F. | Dividends and Paying Agents |
Not applicable.
Not applicable.
We are currently subject to the informational requirements of the Exchange Act applicable to foreign private issuers and fulfill the obligations of these requirements by filing reports with the SEC. As a foreign private issuer, we are exempt from the rules under the Exchange Act relating to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we intend to file with the SEC, within 120 days after the end of each subsequent fiscal year, an annual report on Form 20-F containing financial statements which will be examined and reported on, with an opinion expressed, by an independent public accounting firm. We also intend to file with the SEC reports of foreign private issuer on Form 6-K containing unaudited financial information for the first three quarters of each fiscal year, within 60 days after the end of each quarter.
You may read and copy any document we file with the SEC without charge at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
Item 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market risk is the risk of loss related to changes in market prices, including interest rates and foreign exchange rates, of financial instruments that may adversely impact our consolidated financial position, results of operations or cash flows.
Foreign currency exchange risk
The U.S. dollar is our functional and reporting currency. A portion of our expenses are denominated in shekels, accounting for 17%, 20% and 26% of our expenses in the years ended December 31, 2014, 2013 and 2012, respectively. This exposes us to risk associated with exchange rate fluctuations vis-à-vis the U.S. dollar. See “Item 3.D. Risk Factors—Risks Relating to Our Business and Industry—Exchange rate fluctuations between the U.S. dollar and the Israeli shekel, as well as other non-U.S. currencies, may negatively affect our earnings.” Furthermore, we anticipate that a portion of our expenses, principally of salaries and related personnel expenses, will continue to be denominated in shekels.
To the extent the U.S. dollar weakens against the shekel, we will experience a negative impact on our profit margins. A devaluation of the shekel in relation to the U.S. dollar has the effect of reducing the U.S. dollar amount of our expenses or payables that are payable in shekels, unless those expenses or payables are linked to the U.S. dollar. Conversely, any increase in the value of the shekel in relation to the U.S. dollar has the effect of increasing the U.S. dollar value of our unlinked shekel expenses, which would have a negative impact on our profit margins. In 2014, the value of the shekel depreciated relative to the dollar by 10.7%, and the rate of inflation in Israel was negative 0.2%. In 2013, the value of the shekel appreciated in relation to the U.S. dollar by 7.5%, the effect of which was compounded by inflation in Israel, at a rate of 1.8%. In 2012, the value of the shekel appreciated in relation to the U.S. dollar by 2.3%, the effect of which was compounded by inflation in Israel, at the rate of 1.6%.
Because exchange rates between the U.S. dollar and the shekel (as well as between the U.S. dollar and other currencies) fluctuate continuously, such fluctuations have an impact on our results and period-to-period comparisons of our results. The effects of foreign currency re-measurements are reported in our consolidated financial statements of loss.
We do not currently engage in currency hedging activities in order to reduce this currency exposure, but we may begin to do so in the future. Instruments that may be used to hedge future risks may include foreign currency forward and swap contracts. These instruments may be used to selectively manage risks, but there can be no assurance that we will be fully protected against material foreign currency fluctuations.
Inflation-related risks
We do not believe that the rate of inflation in Israel has had a material impact on our business to date, however, our costs in Israel will increase if inflation in Israel exceeds the devaluation of the shekel against the U.S. dollar or if the timing of such devaluation lags behind inflation in Israel.
Item 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
Item 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
| MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
Initial Public Offering
The effective date of the registration statement (File no. 333- 196961) for our IPO of ordinary shares, par value NIS 0.01, was July 30, 2014. The offering commenced on July 18, 2014 and was closed on August 5, 2014. J.P. Credit Suisse Securities (USA) LLC and Jefferies LLC acted as joint book runners and representatives of the underwriters. Oppenheimer & Co. Inc. and Nomura Securities International, Inc. acted as additional underwriters for the offering. We registered 5,350,000 ordinary shares in the offering and granted the underwriters a 30-day over-allotment option to purchase up to 802,500 additional shares from us to cover over-allotments. The over-allotment was not exercised.
As a result, we issued and sold a total of 5,350,000 ordinary shares at a price per share of $10.00 with aggregate gross proceeds of $53.5 million. Under the terms of the offering, we incurred aggregate underwriting discounts of approximately $3.7 million and expenses of approximately $3.1 million in connection with the offering, resulting in net proceeds to us of approximately $46.7 million.
From the effective date of the registration statement and until December 31, 2014, we had not used the net proceeds of the IPO. We intend to use the net proceeds of the IPO as follows: (i) between $25 million and $35 million for the clinical and regulatory development of CureXcell, which includes between $10 million and $15 million for our ongoing Phase 3 DFU clinical trial and between $15 million and $20 million for our ongoing Phase 3 VLU clinical trial; (ii) between $15 million and $20 million to establish our commercial manufacturing capabilities in the United States; and (iii) the balance, if any, for other general corporate purposes, which may include the development of our platform for regenerative medicine products
None of the net proceeds of the offering was paid directly or indirectly to any director or officer, of ours or to their associates, persons owning ten percent or more of any class of our equity securities, or to any of our affiliates.
Item 15. CONTROLS AND PROCEDURES
| | (a) | Disclosure controls and procedures |
Our Chief Executive Officer and Chief Financial Officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act) as of December 31, 2014, have concluded that, as of such date, our disclosure controls and procedures were effective and ensured that information required to be disclosed by us in reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure and is recorded, processed, summarized and reported within the time periods specified by the SEC’s rules and forms.
| | (b)-(c) Management annual report on internal control over financial reporting and attestation report of the registered public accounting firm |
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the company’s registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
| | (d) | Changes in internal control over financial reporting |
Based on the evaluation conducted by our management, with the participation of our principal executive and principal financial officers, pursuant to Rules 13a-15(d) and 15d-15(d) promulgated under the Exchange Act, our management (including such officers) has concluded that the below-described changes have occurred with respect to our internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) during 2014, which have materially affected, and are reasonably likely to materially affect, our internal control over financial reporting. Over the course of 2014, in connection with our IPO and our becoming a reporting company under the Exchange Act, we began, and continued, to implement, and to evaluate the effectiveness of, our internal control over financial reporting in an effort to ensure that we can provide reasonable assurance concerning the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles.
Item 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that Yuval Yanai is an audit committee financial expert as defined by the SEC rules and is an independent director under the NASDAQ Listing Rules.
We have adopted a Corporate Code of Ethics and Conduct applicable to our executive officers, directors and all other employees. A copy of the code is delivered to every employee of Macrocure Ltd. and its subsidiary, and is available to investors and others on our website at http:// http://investor.macrocure.com/corporate-governance.cfm or by contacting our investor relations department. Any waivers of this code for executive officers or directors will be disclosed through the filing of a report of foreign private issuer on Form 6-K or on our website.
Item 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Principal Accountant Fees and Services
We paid the following fees for professional services rendered by Somekh Chaikin, Certified Public Accountants (Isr.), a Member Firm of KPMG International, an independent registered public accounting firm, for the years ended December 31, 2014 and 2013:
| | | | | | | |
| Audit Fees (1) | | $ | 296 | | | $ | 75 | |
| Tax Fees (2) | | | 11 | | | | 8 | |
| Total | | $ | 307 | | | $ | 83 | |
| (1) | “Audit fees” are aggregate fees for audit services for each of the years shown in this table, including fees associated with the annual audit, reviews of our quarterly financial results submitted in Reports of Foreign Private Issuer on Form 6-K, the Registration Statement on Form F-1 related to our initial public offering, consultation on various accounting issues and audit services provided in connection with other statuary or regulatory filings. |
| (2) | “Tax fees” are fees for tax services rendered by our auditors for tax compliance and for tax consulting associated with international transfer pricing. |
Audit Committee Pre-Approval Policies and Procedures
Our audit committee has adopted a pre-approval policy for the engagement of our independent accountant to perform certain audit and non-audit services. Pursuant to this policy, which is designed to assure that such engagements do not impair the independence of our auditors, the audit committee pre-approves annually a catalog of specific audit and non-audit services in the categories of audit services, audit-related services and tax services that may be performed by our independent accountants.
Item 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Item 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Item 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Item 16G. CORPORATE GOVERNANCE
As a foreign private issuer, we are permitted to comply with Israeli corporate governance practices instead of the NASDAQ Listing Rules, provided that we disclose those NASDAQ Listing Rule requirements with which we do not comply and the equivalent Israeli requirements that we follow instead. We currently rely on this “foreign private issuer exemption” with respect to the following matters:
| | (i) | Quorum Requirement for Meetings of Our Shareholders: As permitted under the Companies Law, pursuant to our articles of association, the quorum required for an ordinary meeting of shareholders consists of at least two shareholders present in person or by proxy, who hold or represent between them at least 25% of the voting power of our shares (and, with respect to an adjourned meeting, generally one or more shareholders who hold or represent any number of shares), instead of 33 1/3% of the issued share capital provided under the NASDAQ Listing Rules. |
| | (ii) | Independent Director Oversight of Executive Officer Compensation: As described under Item 6.C (“Board Practices”— “Board of Directors”— “Board Committees”— “Compensation Committee and Compensation Policy”), under the Companies Law, the compensation of our executives and other office holders is subject to a compensation policy and to the recommendations of a compensation committee of our board of directors that we have adopted and appointed, respectively. The required composition of that committee and the procedure for approval of compensation under that Companies Law amendment differ slightly from those under NASDAQ Listing Rule 5605(d), which requires that the compensation of executive officers be recommended or determined solely by independent directors or by a compensation committee of the board consisting solely of independent directors (as defined under the NASDAQ Listing Rules). While there is significant practical overlap as to who qualifies to serve on the compensation committee under the Companies Law and the NASDAQ Listing Rules, the requirements are not identical, and we comply with the Companies Law requirement. |
| | (iii) | Independent Director Oversight of Nominations: Under Israeli law, there is no requirement to have an independent nominating committee or the independent directors of a company select (or recommend for selection) director nominees, as is required under NASDAQ Listing Rule 5605(e) for a U.S. domestic issuer. Our board of directors handles this process, as is permitted under our amended articles and the Companies Law. We also need not adopt a formal board resolution or charter addressing the director nominations process and such related matters as may be required under the U.S. federal securities laws, as NASDAQ requires for a U.S. issuer. |
Item 16H. MINE SAFETY DISCLOSURE
Not applicable.
Item 17. FINANCIAL STATEMENTS
See pages F-2 through F-38 of this annual report.
Item 18. FINANCIAL STATEMENTS
Not applicable.
See exhibit index incorporated herein by reference.
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | MACROCURE LTD. | |
| | By: | /s/ Nissim Mashiach | |
| Date: March 18, 2015 | | Nissim Mashiach President and Chief Executive Officer | |
| | | ANNUAL REPORT ON FORM 20-F INDEX OF EXHIBITS | |
Exhibit No. | | Description | |
| 1.1 | | Articles of Association of Macrocure Ltd., as amended | |
| 2.1 | | Specimen ordinary share certificate of Macrocure Ltd. (incorporated by reference to Exhibit 4.1 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196991)) | |
| 4.1 | | Second Amended and Restated Registration Rights Agreement, dated July 22, 2014 (but effective upon the closing of Macrocure Ltd.’s IPO), by and among Macrocure Ltd. and certain security holders of Macrocure Ltd. (incorporated by reference to Exhibit 4.3 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196991)) | |
| 4.2 | | Form of Indemnification Agreement (incorporated by reference to Exhibit 10.8 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 4.3 | | Summary English Translation of Macrocure Ltd. 2008 Stock Option Plan (incorporated by reference to Exhibit 10.1 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 4.4.1 | | Macrocure Ltd. 2013 Share Incentive Plan (incorporated by reference to Exhibit 10.2 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 4.4.2 | | Amendment No. 1 to Macrocure Ltd. 2013 Share Incentive Plan (incorporated by reference to Exhibit 10.2.1 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 4.5 | | Summary English Translation of License Agreement, dated January 31, 2008, by and between Macrocure Ltd. and Professor David Danon, as amended by an Addendum, dated January 16, 2011 (incorporated by reference to Exhibit 10.3 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 4.6.1 | | Non Clinical and Clinical Manufacturing Agreement, dated July 11, 2010, by and between Macrocure Ltd. and the American National Red Cross, Penn-Jersey Region (incorporated by reference to Exhibit 10.4.1 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961))# | |
| 4.6.2 | | Amendment No. 1, entered into as of April 23, 2014, to Non Clinical and Clinical Manufacturing Agreement, by and between Macrocure Ltd. and the American National Red Cross, Penn-Jersey Region (incorporated by reference to Exhibit 10.4.2 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961))# | |
| 4.7.1 | | Source Leukocytes and Plasma Services Agreement, entered into effective as of March 26, 2013, by and between Macrocure Ltd. and the American National Red Cross, Penn-Jersey Region (incorporated by reference to Exhibit 10.5.1 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961))# | |
| 4.7.2 | | Amendment No. 1, entered into as of April 23, 2014, to Source Leukocytes and Plasma Services Agreement, by and between Macrocure Ltd. and the American National Red Cross, Penn-Jersey Region (incorporated by reference to Exhibit 10.5.2 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961))# | |
| 4.8 | | Summary English Translation of Sales, Marketing and Research & Development Agreement, dated January 23, 2008, by and between Macrocure Ltd. and Magen David Adom (incorporated by reference to Exhibit 10.6 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 4.9 | | Manufacturing Agreement, dated February 26, 2014, between Maco Productions, Maco Pharma and Macrocure Ltd. (incorporated by reference to Exhibit 10.7 to the registrant’s Registration Statement on Form F-1, as amended (SEC File No. 333-196961)) | |
| 8.1 | | List of subsidiaries of the Registrant | |
| 12.1 | | Certificate of Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002 | |
| 12.2 | | Certificate of Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002 | |
| 13.1 | | Certificate of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002 | |
| 15.1 | | Consent of Somekh Chaikin, Independent Registered Public Accounting Firm, a Member Firm of KPMG International | |
| Confidential treatment was received for portions of this document. The omitted portions of this document were filed separately with the SEC. |
Macrocure Ltd. Consolidated Financial Statements As of December 31, 2014 |
Macrocure Ltd.
Consolidated Financial Statements as of December 31, 2014
Contents
| | Page |
| | |
| F-2 |
| | |
| F-3 |
| | |
| F-4 |
| | |
| F-5 |
| | |
| F-6 |
| | |
| F-7 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Macrocure Ltd.
We have audited the accompanying consolidated statements of financial position of Macrocure Ltd. and its subsidiary (hereafter – the “Company”) as of December 31, 2014 and 2013, and the related consolidated statements of loss and other comprehensive loss, changes in equity and cash flows for each of the years in the three-year period ended December 31, 2014. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the Standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2014, in conformity with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”).
/s/ Somekh Chaikin
Somekh Chaikin
Certified Public Accountants (Isr.)
Member Firm of KPMG International
Tel Aviv, Israel
Macrocure Ltd.
Consolidated Statements of Financial Position as at
U.S. dollars in thousands
| | | | | | December 31 | | | December 31 | |
| | | | | | | | | | |
| Assets | | | | | | | | | |
| Current assets | | | | | | | | | |
| Cash and cash equivalents | | | 4 | | | | 10,868 | | | | 18,995 | |
| Short-term investments | | | 5 | | | | 35,313 | | | | - | |
| Other receivable | | | 6 | | | | 536 | | | | 569 | |
| Total current assets | | | | | | | 46,717 | | | | 19,564 | |
| | | | | | | | | | | | | |
| Non-current assets | | | | | | | | | | | | |
| Property and equipment, net | | | 7 | | | | 451 | | | | 330 | |
| Intangible assets, net | | | 8 | | | | 276 | | | | 827 | |
| Deposits | | | 5 | | | | 1,255 | | | | 17 | |
| | | | | | | | | | | | | |
| Total non-current assets | | | | | | | 1,982 | | | | 1,174 | |
| | | | | | | | | | | | | |
| Total assets | | | | | | | 48,699 | | | | 20,738 | |
| | | | | | | | | | | | | |
| Liabilities and Shareholders’ Equity | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Current liabilities | | | | | | | | | | | | |
| Trade and other payables | | | 9 | | | | 2,488 | | | | 1,971 | |
| Total current liabilities | | | | | | | 2,488 | | | | 1,971 | |
| | | | | | | | | | | | | |
| Total liabilities | | | | | | | 2,488 | | | | 1,971 | |
| | | | | | | | | | | | | |
| Shareholders’ equity | | | 11 | | | | | | | | | |
| Ordinary shares of NIS 0.01 par value | | | | | | | 45 | | | | 20 | |
| Series A preferred shares of NIS 0.01 par value | | | | | | | - | | | | * | |
| Share premium | | | | | | | 95,941 | | | | 48,158 | |
| Capital reserves | | | | | | | 6,167 | | | | 5,117 | |
| Warrants held by shareholders | | | | | | | 12,256 | | | | 8,219 | |
| Accumulated deficit | | | | | | | (68,198 | ) | | | (42,747 | ) |
Total shareholders’ equity | | | | | | | 46,211 | | | | 18,767 | |
| | | | | | | | | | | | | |
| Total liabilities and shareholders’ equity | | | | | | | 48,699 | | | | 20,738 | |
* Represents an amount lower than $1.
The accompanying notes are an integral part of these consolidated financial statements.
Macrocure Ltd.
U.S. dollars in thousands
| | | | | | | |
| | | | | | | | | | | | | |
| Research and development expenses, net | | | 14 | | | | 15,542 | | | | 9,303 | | | | 7,168 | |
| General and administrative expenses | | | 15 | | | | 5,374 | | | | 4,567 | | | | 1,631 | |
| | | | | | | | | | | | | | | | | |
| Operating Loss | | | | | | | (20,916 | ) | | | (13,870 | ) | | | (8,799 | ) |
| | | | | | | | | | | | | | | | | |
| Financing income | | | 16A | | | | 40 | | | | 193 | | | | 1,051 | |
| Financing expense | | | 16B | | | | (4,544 | ) | | | (4,498 | ) | | | (8 | ) |
| Financing income (expense), net | | | | | | | (4,504 | ) | | | (4,305 | ) | | | 1,043 | |
| | | | | | | | | | | | | | | | | |
| Loss before income tax | | | | | | | (25,420 | ) | | | (18,175 | ) | | | (7,756 | ) |
| Taxes on income | | | 17 | | | | (31 | ) | | | (149 | ) | | | - | |
| | | | | | | | | | | | | | | | | |
| Loss for the year | | | | | | | (25,451 | ) | | | (18,324 | ) | | | (7,756 | ) |
| | | | | | | | | | | | | | | | | |
| Other Comprehensive Loss that will be transferred to profit or loss: | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net change in fair value of available for sale financial assets | | | | | (26 | ) | | | - | | | | - | |
| | | | | | | | | | | | | | | |
| Total comprehensive loss for the year | | | | | (25,477 | ) | | | - | | | | - | |
| | | | | | | | | | | | | | | |
| Loss per share - basic and diluted (in U.S. dollars) | 12 | | | | (2.15 | ) | | | (2.46 | ) | | | (1.05 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
Macrocure Ltd.
U.S. dollars in thousands
| | | | | | | | | | | | | | | Warrants | | | | | | | |
| | | Ordinary | | | Preferred | | | | | | Capital | | | held by | | | Accumulated | | | | |
| | | | | | | | | | | | Reserve | | | | | | | | | | |
| For the year ended December 31, 2014: | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance as of January 1, 2014 | | | 20 | | | | * | | | | 48,158 | | | | 5,117 | | | | 8,219 | | | | (42,747 | ) | | | 18,767 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss for the year: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Other comprehensive loss for the year | | | - | | | | - | | | | - | | | | (26 | ) | | | - | | | | - | | | | (26 | ) |
| Loss for the year | | | - | | | | - | | | | - | | | | - | | | | - | | | | (25,451 | ) | | | (25,451 | ) |
| | | | | | | | | | | | | | | | (26 | ) | | | | | | | (25,451 | ) | | | (25,477 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of ordinary shares, net of issuance costs | | | 25 | | | | (*)- | | | | 46,664 | | | | - | | | | - | | | | - | | | | 46,689 | |
| Expiration of warrants and options | | | - | | | | - | | | | 308 | | | | (308 | ) | | | - | | | | - | | | | - | |
| Exercise of options and warrants | | | *- | | | | - | | | | 811 | | | | (279 | ) | | | (334 | ) | | | - | | | | 198 | |
Share-based compensation | | | - | | | | - | | | | - | | | | 1,663 | | | | - | | | | - | | | | 1,663 | |
| Grant of warrants | | | - | | | | - | | | | - | | | | | | | | 4,371 | | | | - | | | | 4,371 | |
| Balance as of December 31 ,2014 | | | 45 | | | | - | | | | 95,941 | | | | 6,167 | | | | 12,256 | | | | (68,198 | ) | | | 46,211 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the year ended December 31, 2013: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of January 1, 2013 | | | 20 | | | | * | | | | 34,394 | | | | 2,483 | | | | 622 | | | | (24,423 | ) | | | 13,096 | |
| Issuance of Preferred shares | | | - | | | | * | | | | 13,750 | | | | - | | | | - | | | | - | | | | 13,750 | |
| Reclassification of warrants | | | - | | | | - | | | | - | | | | - | | | | 7,597 | | | | - | | | | 7,597 | |
| Expiration of warrants and options | | | - | | | | - | | | | 14 | | | | (14 | ) | | | - | | | | - | | | | - | |
| Share-based compensation | | | - | | | | - | | | | - | | | | 2,648 | | | | - | | | | - | | | | 2,648 | |
| Loss for the year | | | - | | | | - | | | | - | | | | - | | | | - | | | | (18,324 | ) | | | (18,324 | ) |
| Balance as of December 31 ,2013 | | | 20 | | | | * | | | | 48,158 | | | | 5,117 | | | | 8,219 | | | | (42,747 | ) | | | 18,767 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the year ended December 31, 2012: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of January 1, 2012 | | | 19 | | | | * | | | | 21,958 | | | | 4,004 | | | | 1,027 | | | | (16,667 | ) | | | 10,341 | |
| Issuance of Preferred shares and warrants | | | - | | | | * | | | | 9,883 | | | | - | | | | - | | | | - | | | | 9,883 | |
| Exercise of warrants and options | | | 1 | | | | * | | | | 2,154 | | | | (1,686 | ) | | | (81 | ) | | | - | | | | 387 | |
| Expiration of warrants and options | | | - | | | | - | | | | 400 | | | | (76 | ) | | | (324 | ) | | | - | | | | - | |
| Share based compensation | | | - | | | | - | | | | - | | | | 241 | | | | - | | | | - | | | | 241 | |
| Loss for the year | | | - | | | | - | | | | - | | | | - | | | | - | | | | (7,756 | ) | | | (7,756 | ) |
| Balance as of December 31 ,2012 | | | 20 | | | | * | | | | 34,394 | | | | 2,483 | | | | 622 | | | | (24,423 | ) | | | 13,096 | |
* Represents an amount lower than $1.
The accompanying notes are an integral part of these consolidated financial statements.
Macrocure Ltd.
U.S. dollars in thousands
| | | | | |
| | | | | | | | | | | |
| Cash flows from operating activities: | | | | | | | | | | |
| Loss for the year | | | | (25,451 | ) | | | (18,324 | ) | | | (7,756 | ) |
| | | | | | | | | | | | | | |
Adjustments: | | | | | | | | | | | | | |
| Depreciation | | | | 106 | | | | 89 | | | | 98 | |
| Amortization | | | | 551 | | | | 551 | | | | 551 | |
| Finance expense (income), net | | | | 4,504 | | | | 4,305 | | | | (1,043 | ) |
| Taxes on income | | | | 31 | | | | 149 | | | | - | |
| Share based compensation | | | | 1,663 | | | | 2,648 | | | | 241 | |
| | | | | 6,855 | | | | 7,742 | | | | (153 | ) |
Changes in operating assets and liability items: | | | | | | | | | | | | | |
| Decrease (increase) in other receivable | | | | 158 | | | | 118 | | | | (65 | ) |
| Increase in trade and other payables | | | | 692 | | | | 440 | | | | 331 | |
| | | | | 850 | | | | 558 | | | | 266 | |
| | | | | | | | | | | | | | |
| Income tax paid | | | | (303 | ) | | | - | | | | - | |
| Interest received | | | | 32 | | | | 85 | | | | 93 | |
| Net cash used in operating activities | | | | (18,017 | ) | | | (9,939 | ) | | | (7,550 | ) |
| | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | |
| Purchase of property and equipment | | | | (227 | ) | | | (116 | ) | | | (130 | ) |
| Decrease (increase) in long terms deposits | | | | (1,238 | ) | | | 2 | | | | (6 | ) |
| Short-term investments | | | | (17,829 | ) | | | - | | | | - | |
| Investment in available for sale financial assets | | | | (17,563 | ) | | | - | | | | - | |
| Repayment of available for sale financial assets | | | | 53 | | | | - | | | | - | |
| Net cash used in investing activities | | | | (36,804 | ) | | | (114 | ) | | | (136 | ) |
| | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | |
| Proceeds from issuance of shares and warrants, net | | | | | | | | | | | | | |
| of issuance costs | | | | 46,689 | | | | 13,750 | | | | 9,883 | |
| Proceeds from issuance of warrants at fair value | | | | | | | | | | | | | |
| through profit or loss | | | | - | | | | - | | | | 2,024 | |
| Exercise of warrants | | | | 198 | | | | - | | | | 387 | |
| Net cash provided by financing activities | | | | 46,887 | | | | 13,750 | | | | 12,294 | |
| | | | | | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | | (7,934 | ) | | | 3,697 | | | | 4,608 | |
| Effect of exchange rate changes on cash and | | | | | | | | | | | | | |
| cash equivalents | | | | (193 | ) | | | (24 | ) | | | (4 | ) |
| Cash and cash equivalents at beginning | | | | | | | | | | | | | |
| of the year | | | | 18,995 | | | | 15,322 | | | | 10,718 | |
| Cash and cash equivalents at end of the year | | | | 10,868 | | | | 18,995 | | | | 15,322 | |
The accompanying notes are an integral part of these consolidated financial statements.
Macrocure Ltd.
| U.S. dollars in thousands (except share and per share data) |
| Note 1 - The Reporting Entity |
| | 1. | 1.Macrocure Ltd. was incorporated in Israel on January 14, 2008. The registered address of the Company's office is 25 Hasivim St. Petach Tikva, Israel. Since its inception, the Company has been engaged in the biotechnology field and focused on developing, manufacturing and commercializing novel cell therapy products to address unmet needs in the treatment of chronic and other hard-to-heal wounds as well as other potential regenerative medicine applications. |
The Company’s lead cell-based biological product, CureXcell, is currently in a Phase 3 pivotal clinical trial targeting a broad indication for the treatment of hard-to-heal diabetic foot ulcers (“DFU”). The Company also commenced a phase 3 trial for the treatment of hard-to-heal venous leg ulcers (“VLU”). The Company’s main goal is to submit a Biologics License Application (“BLA”) with the U.S. Food and Drug Administration (“FDA”), and an application for marketing authorization with the European Medicines Agency (“EMA”). CureXcell was approved for treatment of DFUs and VLUs by the Israeli Ministry of Health in 2000.
| | 2. | The Company has incurred operational losses in each year since its inception and does not expect to generate significant revenue unless and until it obtains marketing approval for CureXcell. |
| | 3. | On August 5, 2014 the Company closed an Initial Public Offering (“IPO”) of its ordinary shares, which resulted in the sale of 5,350,000 ordinary shares at a public offering price of $10 per share, before underwriting discounts and offering expenses, and from that date the Company’s shares are traded on the NASDAQ Global Market under the symbol of “MCUR”. The underwriters had a 30-day option to purchase up to 802,500 additional shares at a public offering price of $10 per share, no option was exercised by the underwriters. The Company received net proceeds from the IPO of approximately $46.7 million (net of issuance costs and underwriting discounts of approximately $6.8 million). |
In addition, 81,435 preferred A shares and 27,241 warrants to purchase preferred A shares were automatically converted into 3,746,010 ordinary shares and 1,253,086 warrants to purchase ordinary shares, respectively. In addition, the vesting of the remaining, unvested portion of the options to purchase 859,602 ordinary shares held by an officer of the Company was accelerated.
| | 4. | The consolidated financial statements of the Company as of and for the year ended December 31, 2014 comprise the Company and its wholly owned U.S. subsidiary (together referred to as the “Company”). |
| Note 2 - Basis of Preparation |
A. Statement of Compliance
The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards ("IFRS"), as issued by the international accounting standards board (“IASB”).
The consolidated financial statements have been prepared on the historical cost basis, except for financial instruments, which are measured at fair value as available-for-sale.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 2 - Basis of Preparation (cont'd) |
C. Functional and presentation currency
These consolidated financial statements are presented in U.S. dollars, which is the Company's functional currency. The U.S dollar is the currency of the primary economic environment in which the Company operates and expects to operate in the foreseeable future.
D. Use of estimates and judgment
In preparing these consolidated financial statements, management has made judgments, estimates and assumptions that affect the application of the Company’s accounting policies and the reported amounts recognized in the financial statements. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to estimates are recognized prospectively.
Critical estimates computed by the Company that may cause a material adjustment to the carrying amounts of liabilities in the future periods are discussed below:
Fair value of warrants held by shareholders: The fair value of warrants held by shareholders is determined using the Option-Pricing Model ("OPM"). The Company exercises discretion for the purpose of determining assumptions, based principally on the existing market conditions at each reporting date. See Note 11B(8) for the parameters used to calculate the fair value of the warrants and see Note 13 for fair value hierarchy.
Fair value of share-based compensation: The Company grants share-based compensation to employees and consultants. The fair value of the share options is measured at the grant date using the Black-Scholes OPM and assumptions regarding unobservable inputs used in the valuation models.
The value of the transactions, measured as described above, is recognized as an expense over the vesting period. Concurrently with the periodic recognition of an expense, an increase is recognized in a capital reserve, within the Company’s equity. See Note 11C for the assumptions used to calculate the fair value of options.
| Note 3 - Significant Accounting Policies |
The accounting policies set out below have been applied consistently for all periods presented in these consolidated financial statements, except as explained in Note 3N, and have been applied consistently by the Company entities.
A. Basis of consolidation
(1) Subsidiary
A subsidiary is an entity controlled by the Company. The Company controls an entity when it is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. The financial statements of the subsidiary are included in the consolidated financial statements from the date that control commences until the date that control is lost.
(2) Transactions eliminated on consolidation
Intercompany balances and transactions, and any unrealized income and expenses arising from Intercompany transactions, are eliminated in preparing the consolidated financial statements.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 3 - Significant Accounting Policies (cont’d) |
B. Foreign currency transactions
Transactions in foreign currencies are translated to the functional currency of the Company entities at exchange rates at the dates of the transactions. Monetary assets and liabilities denominated in foreign currencies at the reporting date are translated to the functional currency at the exchange rate at the reporting date. The foreign currency gain or loss on monetary items is the difference between amortized cost in the functional currency at the beginning of the year, adjusted for effective interest and payments during the year, and the amortized cost in foreign currency translated at the exchange rate at the end of the year.
Non-monetary assets and liabilities denominated in foreign currencies that are measured at fair value are translated to the functional currency at the exchange rate at the date that the fair value was determined. Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using the exchange rate at the date of the transaction.
Foreign currency differences arising on translation are recognized in profit or loss.
C. Financial instruments
(1) Non-derivative financial assets
Initial recognition of financial assets
The Company initially recognizes loans and receivables and deposits on the date that they are created. All other financial assets acquired in a regular way purchase, including assets designated at fair value through profit or loss, are recognized initially on the trade date at which the Company becomes a party to the contractual provisions of the instrument, meaning on the date the Company undertook to purchase or sell the asset. Non-derivative financial instruments comprise deposits, investments in debt securities, accounts receivable and cash and cash equivalents.
Cash and cash equivalents include cash balances available for immediate use and short-term highly liquid investments (with original maturities of three months or less).
Derecognition of financial assets
Financial assets are derecognized when the contractual rights of the Company to the cash flows from the asset expire, or the Company transfers the rights to receive the contractual cash flows on the financial asset in a transaction in which substantially all the risks and rewards of ownership of the financial asset are transferred.
Regular way sales of financial assets are recognized on the trade date, meaning on the date the Company undertook to sell the asset.
Available-for-sale financial assets
The Company’s investments in debt securities classified as available-for-sale financial assets. Available-for-sale financial assets are recognized initially at fair value plus any directly attributable transaction costs. Subsequent to initial recognition, they are measured at fair value and changes therein, other than impairment losses, foreign currency differences and the accrual of effective interest on available-for-sale debt instruments, are recognized directly in other comprehensive income and presented within equity in a reserve for financial assets classified as available-for-sale. When an investment is derecognized, the cumulative gain or loss in the reserve for available-for-sale financial assets is transferred to profit or loss.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 3 - Significant Accounting Policies (cont’d) |
C. Financial instruments (cont’d)
(2) Non-derivative financial liabilities
Non-derivative financial liabilities include trade and other payables.
Initial recognition of financial liabilities
Financial liabilities are recognized initially on the trade date at which the Company becomes a party to the contract creating the obligation.
Financial liabilities are recognized initially at fair value plus any directly attributable transaction costs. Subsequent to initial recognition, financial liabilities are measured at amortized cost using the effective interest method.
Derecognition of financial liabilities
Financial liabilities are derecognized when the obligation of the Company, as specified in the agreement, expires or when the obligation is discharged or cancelled.
(3) Share capital
Ordinary and Preferred shares are classified as equity. Incremental costs directly attributable to the issuance of the shares are recognized as a deduction from equity, net of any tax effects.
(4) Issuance of Warrants
| | (1) | Consideration received in respect of warrants issued by the Company as part of capital raises, under which, upon exercise, the Company would issue a fixed amount of its own equity instruments in exchange for a fixed amount of cash is recognized and classified as equity in the statements of financial position. |
| | (2) | Consideration received in respect of warrants issued by the Company as part of capital raises, under which, upon exercise, the Company would issue variable number of its own equity instruments (e.g. due to net settlement feature) are recognized and classified as derivatives. Accordingly, the warrants are measured at fair value through profit or loss. |
D. Property and equipment
Property and equipment are measured at cost less accumulated depreciation and accumulated impairment losses. Cost includes expenditures that are directly attributable to the acquisition of the asset. Purchased software that is integral to the functionality of the related equipment is capitalized as part of that equipment.
Gains and losses on disposal of a fixed asset item are determined by comparing the net proceeds from disposal with the carrying amount of the asset, and are recognized in profit or loss.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 3 - Significant Accounting Policies (cont’d) |
D. Property and equipment (cont’d)
An asset is depreciated from the date it is ready for use, meaning the date it reaches the location and condition required for it to operate in the manner intended by management. Depreciation is recognized in profit or loss on a straight-line basis over the estimated useful life of each part of the property and equipment, since this most closely reflects the expected pattern of consumption of the future economic benefits embodied in the asset.
The estimated useful lives for the current and comparative periods are as follows:
| | · | Furniture and office equipment | 3-12 years |
| | · | | The shorter of the lease term and the useful life |
Depreciation methods and useful lives are reviewed at the end of each reporting year and adjusted if appropriate.
E. Intangible assets
Separately acquired intangible assets are shown at historical cost. The cost of separately acquired intangible asset comprises its purchase price, and any acquisition related costs.
Licensed Technology (as defined in Note 10A) has a finite useful life and is carried at cost less accumulated amortization. Amortization is calculated using the straight-line method to allocate the cost of licenses over their useful life, see also Note 8B.
F. Research and development expenses, net
Research and development expenses are recognized in profit or loss when incurred. An intangible asset arising from a development project or from the development phase of an internal project is recognized if the Company can demonstrate the technical feasibility of completing the intangible asset so that it will be available for use or sale; the Company’s intention to complete the intangible asset; and the Company’s ability to measure reliably the expenditure attributable to the intangible asset during its development. Since the Company’s research and development projects are often subject to regulatory approval procedures and other uncertainties, the conditions for the capitalization of costs incurred before receipt of approvals are not normally satisfied, and, therefore, research and development expenses are recognized in profit or loss when incurred.
The Company’s research and development expenses are presented net of any incremental income that is generated as part of its research and development activities.
As of December 31, 2014, no development expenditures have met the recognition criteria and thus the Company expensed all of its development expenditures as incurred.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 3 - Significant Accounting Policies (cont’d) |
| | G. | Impairment of non-financial assets |
Assets that are subject to depreciation are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs to sell and its value in use.
Income tax includes current and deferred tax. Current tax is the expected tax payable (or receivable) on the taxable income for the year, using tax rates enacted or substantively enacted at the reporting date.
A provision for uncertain tax positions, or reduction in deferred tax asset, is recognized when it is more probable than not that the Company will have to use its economic resources to pay the obligation.
Deferred tax is recognized for temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes.
Deferred tax is measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date. A deferred tax asset is recognized for unused tax losses, tax benefits and deductible temporary differences, to the extent that it is probable that there will be future taxable profits against which such tax benefits can be utilized. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realized.
As of December 31, 2014, no deferred tax assets have been recorded since it is not probable that the Company will have future taxable income against which any tax losses, benefits or deductible temporary differences can be utilized.
(1) Post-employment benefits
The Company's liability for severance pay is pursuant to Section 14 of the Israeli Severance Compensation Act, 1963 ("Section 14"). The majority of the Company’s employees are included under this section and are entitled only to monthly deposits made in the employee’s name with insurance companies at a rate of 8.33% of an employee’s monthly salary. Payments in accordance with Section 14 release the Company from any future severance payments in respect of those employees. The funds deposited are made available to the employee at the time the employer-employee relationship is terminated, regardless of cause of termination. The severance pay liabilities and deposits under Section 14 are accounted for as defined contribution benefits and accordingly are not reflected in the balance sheet, as the severance pay risks have been irrevocably transferred to the severance funds.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 3 - Significant Accounting Policies (cont’d) |
| | I. | Employee benefits (cont’d) |
(2) Short-term benefits
Short-term employee benefit obligations are measured on an undiscounted basis and are expensed as the related service is provided or upon the actual absence of the employee when the benefit is not accumulated. A liability is recognized for the amount expected to be paid under short-term cash bonus or profit-sharing plans if the Company has a present legal or constructive obligation to pay this amount as a result of past service provided by the employee and the obligation can be estimated reliably. The employee benefits are classified, for measurement purposes, as short-term benefits or as other long-term benefits depending on when the Company expects the benefits to be wholly settled.
(3) Termination benefits
Termination benefits are recognized as an expense when the Company is committed demonstrably, without realistic possibility of withdrawal, to a formal detailed plan to terminate employment before the normal retirement date.
(4) Share based compensation
The grant date fair value of share-based compensation awards granted to employees is recognized as a salary expense, with a corresponding increase in equity, over the period that the employees become unconditionally entitled to the awards. The amount recognized as an expense in respect of share-based compensation awards that are conditional upon meeting service and non-market performance conditions, is adjusted to reflect the number of awards that are expected to vest. In respect of other service providers, where the fair value of the goods or services received as consideration of equity instruments cannot be measured reliably, they are measured by reference to the fair value of the equity instruments granted.
Government grants are recognized initially at fair value when there is reasonable assurance that they will be received and the Company will comply with the conditions associated with the grant. Unconditional government grants are recognized when the Company is entitled to receive them. Grants that compensate the Company for expenses incurred are presented as a deduction from the corresponding expense.
Grants from the Israel Office of the Chief Scientist (“OCS”) with respect to research and development projects are accounted for as forgivable loans according to IAS 20. Grants received from the OCS are recognized as a liability according to their fair value on the date of their receipt, unless on that date it is reasonably certain that the amount received will not be refunded. The amount of the liability is reexamined each period, and any changes in the present value of the cash flows discounted at the original interest rate of the grant are recognized in profit or loss. The difference between the amount received and the fair value of the liability on the date of the receipt of the grant is recognized as a deduction from research and development expenses.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 3 - Significant Accounting Policies (cont’d)
| | J. | Government grants (cont’d) |
As of December 31, 2014, the Company’s management estimates that the Company will not be required to refund grants received from the OCS which relate to an inactive research and development project, and accordingly, no provision was included in the financial statements.
K. Financing income and expenses
Financing income includes interest income, foreign currency gains and change in fair value of warrants held by shareholders which have been recorded as financial liabilities. Interest income is recognized as it accrues using the effective interest method.
Financing expense includes bank charges, changes in foreign currency losses and change in fair value of warrants held by shareholders.
In the statements of cash flows, interest received is presented as part of cash flows from operating activities.
Foreign currency gains and losses on financial assets and financial liabilities are reported on a net basis as either financing income or financing expenses, depending on whether foreign currency movements are in a net gain or net loss position.
L. Loss per share
The Company presents basic and diluted earnings or loss per share (“EPS”) data for its ordinary shares. Basic EPS is calculated by dividing the loss attributable to ordinary shareholders of the Company by the weighted average number of ordinary shares outstanding during the year, which includes, inter alia, ordinary shares issuable for little or no consideration. There is no difference between basic and diluted EPS since there are no dilutive potential ordinary shares.
M. Segment reporting
The Company does not present segment information as the Company currently operates in a single segment.
N. Changes in accounting policies
The Company has adopted the following new standards and amendments to standards, with a date of initial application of January 1, 2014.
(1) Amendment to IAS 32, Financial Instruments: Presentation
| | (2) | Amendment to IAS 36, Impairment of Assets: Recoverable Amount Disclosures for Non-Financial Assets |
(3) Amendment to IFRS 2, Share-Based Payment, definition of “vesting condition”.
The implementation of the new standards and amendments did not have a material effect on the consolidated financial statements.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| Note 3 - Significant Accounting Policies (cont’d) |
O. New standards and interpretations not yet adopted
· IFRS 9, Financial Instruments
A final version of the standard, which includes revised guidance on the classification and measurement of financial instruments, and a new model for measuring impairment of financial assets. This guidance has been added to the chapter dealing with general hedge accounting requirements issued in 2013.
IFRS 9 (2014) is effective for annual periods beginning on or after January 1, 2018 with early adoption being permitted.
· Amendment to IAS 24, “Related Party Disclosures”
The definition of the term was expanded to include entities that provide key management personnel (KMP) services to the reporting entity, directly or through another entity of the Company.
The Company has not yet commenced examining the effects of adopting IFRS 9 (2014) andAmendment to IAS 24 on the financial statements.
Note 4 - Cash and Cash Equivalents
| | | | |
| | | | | | | |
| Cash for immediate withdrawal | | | 2,531 | | | | 3,130 | |
| Bank deposits (*) | | | 8,337 | | | | 15,865 | |
| | | | | | | | | |
| | | | 10,868 | | | | 18,995 | |
| | (*) Bank deposits bear interest ranging from 0.1% to 0.5%. |
Due to a contractual requirement to provide a bank guaranty to the lessor of the Company’s offices, the Company was required by the bank to maintain a minimum level of cash and cash equivalents as security for the bank guaranty in the amount of $60, linked to the Consumer Price Index (“CPI”), however, no specific deposit is required to be designated for that specific purpose (see also Note 10H).
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 5 - Investments
| | | | |
| | | | | | | |
| Available for sale debt securities | | | 17,484 | | | | - | |
| Bank deposits (*) | | | 17,829 | | | | - | |
| | | | | | | | | |
| | | | 35,313 | | | | - | |
| Long-term investments: | | | | | | | | |
| Deposits (*) | | | 1,255 | | | | 17 | |
| | (*) Bank deposits bear interest ranging from 0.25% to 1.4%. |
Note 6 – Other Receivable
| | | | |
| | | | | | | |
| Government authorities | | | 149 | | | | 19 | |
| Prepaid expenses | | | 157 | | | | 348 | |
| Healthcare professionals – see Note 10B | | | 171 | | | | 196 | |
| Other | | | 59 | | | | 6 | |
| | | | | | | | | |
| | | | 536 | | | | 569 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 7 - Property and Equipment, Net
Composition of property and equipment and the depreciation thereof, grouped by major classifications and the changes during the year ended at December 31, 2014 is as follows:
| | | | | | | | | | |
| | | Balance at the beginning of the year | | | | | | | | | Balance at the beginning of the year | | | | | | | | | Property and equipment, net | |
| Buildings under construction | | | - | | | | 100 | | | | 100 | | | | - | | | | - | | | | - | | | | 100 | |
| Furniture and Office equipment | | | 31 | | | | - | | | | 31 | | | | 9 | | | | 3 | | | | 12 | | | | 19 | |
| Computers | | | 194 | | | | (3 | ) | | | 191 | | | | 91 | | | | 35 | | | | 126 | | | | 65 | |
| Leasehold improvements | | | 103 | | | | 118 | | | | 221 | | | | 91 | | | | 24 | | | | 115 | | | | 106 | |
| Laboratory equipment | | | 429 | | | | 12 | | | | 441 | | | | 236 | | | | 44 | | | | 280 | | | | 161 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | 757 | | | | 227 | | | | 984 | | | | 427 | | | | 106 | | | | 533 | | | | 451 | |
Composition of property and equipment and the depreciation thereof, grouped by major classifications and the changes during the year ended at
December 31, 2013 is as follows:
| | | | | | | | | | |
| | | Balance at the beginning of the year | | | | | | | | | Balance at the beginning of the year | | | | | | | | | Property and equipment, net | |
| Furniture and Office equipment | | | 28 | | | | 3 | | | | 31 | | | | 7 | | | | 2 | | | | 9 | | | | 22 | |
| Computers | | | 101 | | | | 93 | | | | 194 | | | | 68 | | | | 23 | | | | 91 | | | | 103 | |
| Leasehold improvements | | | 98 | | | | 5 | | | | 103 | | | | 71 | | | | 20 | | | | 91 | | | | 12 | |
| Laboratory equipment | | | 414 | | | | 15 | | | | 429 | | | | 192 | | | | 44 | | | | 236 | | | | 193 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | 641 | | | | 116 | | | | 757 | | | | 338 | | | | 89 | | | | 427 | | | | 330 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 8 - Intangible Assets
| | A. | Composition of Intangible assets and the amortization thereof and changes during the years ending at December 31, 2014 and 2013 are as follows: |
| | | | |
| | | | | | | |
| | | | | | | |
Cost: | | | | | | |
| Balance at the beginning of the year | | | 2,541 | | | | 2,541 | |
| Additions | | | - | | | | - | |
| | | | | | | | | |
| Balance at the end of the year | | | 2,541 | | | | 2,541 | |
| | | | | | | | | |
Accumulated depreciation: | | | | | | | | |
| Balance at the beginning of the year | | | 1,714 | | | | 1,163 | |
| Additions | | | 551 | | | | 551 | |
| | | | | | | | | |
| Balance at the end of the year | | | 2,265 | | | | 1,714 | |
| | | | | | | | | |
| Depreciated cost | | | 276 | | | | 827 | |
| | B. | Intangible assets represent acquired Licensed Technology including additional acquisition related costs (see Note 10A). |
The Company estimates the useful life of the Licensed Technology in accordance with the duration of the patent, which will expire in June 2015. Amortization of the intangible asset is included in research and development expenses.
Note 9 - Trade and Other Payables
| | | | |
| | | | | | | |
| Trade payables | | | 61 | | | | 1,095 | |
| Payroll and related accruals | | | 845 | | | | 243 | |
| Government authorities | | | 59 | | | | 244 | |
| Accrued expenses | | | 1,509 | | | | 203 | |
| Other payables | | | 14 | | | | 186 | |
| | | | | | | | | |
| | | | 2,488 | | | | 1,971 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 10 - Contingent Liabilities and Commitments
| | A. | In January 2008, the Company entered into an agreement with Professor David Danon (the “Researcher”) to acquire a license to use technology relating to culturing macrophages from blood (the “Licensed Technology”). Pursuant to the agreement, the Company received an exclusive worldwide license to certain technology, including patents and additional patent applications relating to the Licensed Technology. In consideration for the Licensed Technology, the Company is required to pay certain milestone payments upon achievement of clinical milestones (FDA, EMA, CE MARK) and royalties from sales derived from any products developed under the Licensed Technology. The royalties are calculated based on a fixed percentage of the Company’s sales subject to certain terms and conditions. The total amount of the clinical milestones payment shall not exceed $1,000. The agreement is for an unlimited period and subject to certain terms and conditions set forth therein. During 2008 the Company paid the Researcher an amount of $206 as part of the payments relating to the clinical milestones and capitalized the amount as an intangible asset. |
In January 2011, the parties signed an amendment to the agreement according to which, the Company was granted an option to pay one-time payment of $1,156 in consideration of waiving future royalty payments under the original agreement. In April 2011 the Company exercised the option and paid the Researcher $1,156. This amount was recorded as an intangible asset.
In addition, in January 2008, the Company entered into a consulting agreement with the Researcher for consulting services pursuant to which the Researcher is entitled to a fixed monthly fee of $11. Consultancy payments will be paid to the Researcher until the later of reaching one of the regulatory milestones (FDA/CE MARK) or the end of the research.
In September 2010, the Company’s Board of Directors approved the grant of options to purchase 183,218 ordinary shares of the Company to the intermediary in the Licensed Technology acquisition transaction for his professional services and arranging the transaction.
The options, which vested immediately, are exercisable at an exercise price of NIS 0.01 per share until the earlier of: (i) approximately eight years following the date of grant; or (ii) a Merger or Acquisition event (“M&A event”) as defined in the agreement.
The Company estimated the fair value of the options on the date of grant to be approximately $1,179. This amount was capitalized in the Company's financial statements to the Intangible assets which represents the Licensed Technology.
| | B. | In January 2008, the Company entered into an agreement with Magan David Adom (“MDA”), Israel’s national blood bank, under which MDA supplies the blood that serves as the raw material for, and manufactures, the Company's CureXcell product in Israel. Pursuant to the agreement, the manufacturing of the Company's CureXcell product is carried out by MDA and is supervised by the Company's employees at the MDA’s central blood bank facility. Under the agreement, MDA produces CureXcell based on the Licensed Technology and based on the Company’s needs. The CureXcell product is sold to health care professionals in Israel for clinical purposes as part of the Company’s research and development activities. |
| | Research and development expenses are presented net of proceeds received from sales of CureXcell to healthcare professionals in Israel since these sales are an integral part of the Company’s research and development activities rather than standalone revenues in the ordinary course of business (see Note 14). |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 10 - Contingent Liabilities and Commitments (cont’d)
B. (cont’d)
Under the agreement, the Company is obligated to pay MDA fixed per unit charges (subject to adjustment for the Israeli CPI and for significant changes in the costs of production). In addition, the Company is also obligated to pay MDA royalties determined as a percentage of net sales, derived outside of Israel if based on the technology covered by the U.S. patent or any changes, updates or future developments on this technology developed by the Company together with MDA. The royalty rate is 2% of net sales in countries in which the regulatory approval is obtained primarily on the basis of the Israeli Ministry of Health approval and at a cost of less than $100, which currently includes only Israel. The royalty rate is 1% in all other countries. The MDA agreement terminates upon the loss of the patent protection for the Licensed Technology, a material breach of the MDA agreement or upon certain bankruptcy events.
| | C. | In July 2010, the Company entered into an agreement with the American Red Cross (“ARC”) to provide raw materials and produce the clinical trial supplies of the Company’s CureXcell for North America. According to the agreement, the ARC produces CureXcell based on the Company’s needs, and manufactures it in the ARC’s facility, but only by personnel that have been trained by the Company and under the Company’s observation and supervision. |
In March 2013, the Company entered into an additional agreement with the ARC according to which, in addition to the manufacturing and supply of CureXcell, the ARC will provide the Company with leukocytes and plasma products for the Company’s clinical trials.
Under the agreements, the Company is obligated to pay the ARC a fixed monthly payment in respect to the space used by the Company in the ARC’s facility and additional fixed fee per unit charges for raw materials that the ARC supplies and CureXcell batches that are produced by the ARC on the Company’s behalf.
In April 2014, the Company’s agreements with the ARC were extended until April 2017.
| | D. | In December 2009, the Company entered into a Master Services Agreement with a contract service organization (the “Initial CRO”) regarding the Company’s clinical trials in North America. Pursuant to the agreement, the Initial CRO will provide the Company full-service support for the clinical trial towards achieving the regulatory approval in the United States. |
During 2014 and 2013, the services provided by the Initial CRO were in the amount of $0 and $1,064, respectively.
| | E. | In September 2013, the Company entered into a Master Services Agreement with a contract service organization (the “Second CRO”), pursuant to which it retained the Second CRO to carry out the Company's clinical trials and implement the trial process planned by the Company's clinical trials team. Work is carried out by the Second CRO on a project by project basis, in accordance with project work orders submitted by the Company. |
During 2014 and 2013, the services provided by the Second CRO were in the amount of $2,962 and $385, respectively.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 10 - Contingent Liabilities and Commitments (cont’d)
| | F. | In February 2014, the Company entered into a manufacturing agreement with a third-party (the “Manufacturer”), for the manufacture of the kits of sterile plastic transfusion and infusion bags. Under the agreement, the Manufacturer exclusively manufactures and sells the kits to the Company and the Company is similarly required to purchase the kits exclusively from the Manufacturer during the term of the agreement, subject to the Manufacturer’s compliance with certain terms. The Manufacturer is responsible under the agreement for submitting documentation for obtaining regulatory approval for the kits following receipt from the Company of such documentation. |
| | The agreement can be terminated by either party upon nine month formal notice. |
G. Office of Chief Scientist
The Company partially financed its research and development expenditures under programs sponsored by the OCS for the support of certain research and development activities conducted in Israel. In return for the OCS’s participation, the Company is committed to pay royalties at a rate of 3% - 4.5% of sales of the developed products linked to U.S. dollars, until repayment of 100% of the amount of grants received, plus annual interest at the LIBOR rate. As of December 31, 2014 the Company’s total commitment for royalties payable with respect to future sales, based on OCS participation received, totaled approximately $800 (including accrued LIBOR interest). In addition, the OCS may impose certain conditions on any arrangement under which it permits the Company to transfer know-how or development and manufacturing out of Israel.
As of December 31, 2014, the Company’s management estimates that the Company will not be required to refund grants received from the OCS which relate to inactive research and development projects, and accordingly, no provision was included in the financial statements.
| | H. | In December 2013, the Company entered into a new lease agreement for its facilities and relocated its offices and laboratory. The term of the new lease agreement is five years, commencing February 1, 2014, with two options for additional two-year periods. Monthly lease fees are $10, linked to CPI. The Company issued a guarantee letter of $60 thousands, in respect of the facilities. |
Rental expenses (including management fee) for the years ended December 31, 2014 and 2013 were $135 and $129, respectively.
On July 10, 2014, the Company entered into a convertible loan agreement with a related party (the ‘‘Lender’’), pursuant to which the Lender has made available a line of credit to the Company in an amount of up to $10 million that the Company may draw upon in one or more installments, at its sole discretion. The line of credit would expire upon the earlier of (i) the consummation of an IPO or (ii) 12 months following the execution of the convertible loan agreement. Any amounts outstanding under the line of credit as of the consummation of an IPO will automatically convert into ordinary shares of the Company to be issued to the Lender at the price per share being paid by the public in an offering.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 10 - Contingent Liabilities and Commitments (cont’d)
I. (cont’d)
In consideration of this line of credit, the Company issued to the Lender warrants to purchase 439,760 ordinary shares of the Company at a price per share equal to NIS 0.01. The warrants will expire 10 years following the date of the convertible loan agreement, or earlier if the Company consummate a merger, sale of all or substantially all of its assets, license of all or substantially all of its intellectual property or similar transaction.
Further to that mentioned in Note 1(3), on August 5, 2014, the Company closed an IPO of its ordinary shares and the Company’s right to drew funds under the convertible credit line was terminated without any amount was drawn.
As a result of the aforesaid, the Company recognized a financing expense in the amount of $4,371 that reflects the line of credit provided, and on the other hand an increase in warrants held by shareholders based on the fair value of the warrants which was determined according to the Company’s equity value.
Regarding the exercise of the warrants, see Note 20(B).
J. Litigation
On July 2, 2014, following a request of the United States District Court for the District of Maryland (the "U.S. Court"), the Directorate of Courts of Israel (the "Court of Israel") initiated a legal proceeding in which the Court of Israel was requested to collect certain evidence and documents, including a laptop computer, which were used by a former employee of the Company. The purpose of the proceeding in Israel is to assist Cognate Bioservices, Inc.("Cognate"), and additional plaintiffs to collect evidence that can be used in a legal action that they initiated against the former employee and that is pending in the U.S. Court for, among other things, misappropriation of trade secrets. The Company objected to this order, based on various grounds, including that it jeopardizes the Company's confidential information and trade secrets and subjects the Company to undue burden.
Due to the early stage of the above proceedings, the effect of this legal proceeding on the Company cannot be determined.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 11 - Equity
A. Share capital
Composed as of December 31, 2014 and 2013 of shares of NIS 0.01 par value, as follows:
| | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | | | | | | | |
| Ordinary share | | | 100,000,000 | | | | 99,875,141 | | | | 16,262,465 | | | | 7,056,630 | |
| Series A preferred shares | | | - | | | | 124,859 | | | | - | | | | 81,435 | |
| | | | | | | | | | | | | | | | | |
| | | | 100,000,000 | | | | 100,000,000 | | | | 16,262,465 | | | | 7,138,065 | |
1. Rights attached to share
Each ordinary share is entitled to one vote at meetings of the Company’s shareholders, to appoint, dismiss and replace directors, to receive bonus shares, profits and dividends as declared by the Board of Directors and to participate in distribution of surplus assets of the Company upon its liquidation, subject to preferred shares preference.
2. Share split
On July 18, 2014, the Company effected a bonus share distribution of 46-to-1 bonus shares (equivalent to a 46-for-1 stock split). For accounting purposes, this transaction was recorded as a share split and accordingly, all ordinary shares, options to purchase ordinary shares, warrant to purchase ordinary shares and loss per share amounts have been adjusted retroactively for all periods presented in these financial statements.
3. Conversion of series A preferred shares
See Note 1(3) regarding the automatic conversion of series A preferred shares and warrants to purchase preferred A shares into ordinary shares and warrants to purchase ordinary shares, respectively, as part of the Company’s IPO.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 11 - Equity (Cont’d)
B. Financing rounds
| | 1. | In February 2008, the Company entered into a share purchase agreement (the “2008 Share Purchase Agreement”) with several third-party investors. Pursuant to the 2008 Share Purchase Agreement, the investors purchased 1,068,580 ordinary shares of the Company and were granted warrants to purchase 156,584 ordinary shares of the Company at an exercise price equal to the par value, for total consideration of $5,194 (net of issuance costs of $56). |
In August 2009, the Company entered into a share purchase agreement (the “2009 Share Purchase Agreement”) with several third-party investors. Pursuant to the 2009 Share Purchase Agreement, the investors purchased 1,099,492 ordinary shares of the Company and were granted warrants to purchase 183,218 ordinary shares of the Company at an exercise price of $10.54 per share, for total consideration of $7,371 (net of issuance costs of $372).
The proceeds from the 2008 Share Purchase Agreement and 2009 Share Purchase Agreement were allocated to warrants classified as equity in the statement of financial position, based on their fair value as of the grant date, and to the ordinary shares and share premium.
As of December 31, 2014, none of the warrants granted under the 2008 Share Purchase Agreement have been exercised and 36,708 warrants under the 2009 Share Purchase Agreement were exercised for total consideration of $388, and the remaining 146,510 warrants expired.
| | 2. | In September 2010, the Company entered into a share purchase agreement (the “2010 Share Purchase Agreement”) with several investors. Pursuant to the 2010 Share Purchase Agreement, the investors agreed to purchase 22,111 Preferred A shares from the Company and the Company agreed to issue to each investor warrants to purchase a number of Preferred A shares equal to 50% of its original number of Preferred A shares (i.e., at a 2 share to 1 warrant ratio); 50% of the warrants had an exercise price of $882 per share, and the remaining 50% had an exercise price of $1,176 per share. The warrants were fully vested upon the closing of 2010 Share Purchase Agreement and are exercisable until the earlier of: (i) 5 years after the closing date of the 2010 Share Purchase Agreement; or (ii) certain merger or acquisition events. The investors may exercise the warrants either for cash or in a cashless manner. |
The closing of the 2010 Share Purchase Agreement was subject to the fulfillment of the Investigation New Drug (“IND”) Event, as defined under the 2010 Share Purchase Agreement.
In March 2011, the conditions for closing were met and the Company issued 22,111 Preferred A shares and warrants for total consideration of $12,919 (net of issuance costs of $81).
The total consideration was allocated to warrants classified as a derivative liability due to their net share settlement feature, based on their fair value at the grant date. The remaining amount was allocated to the Preferred A shares and share premium. The warrants are measured at fair value through profit or loss.
In April 2012, following the approval of a share purchase agreement of 2012 (the “2012 Share Purchase Agreement”), the Company amended the 2010 Share Purchase Agreement. Pursuant to the amendment, the exercise prices of all of the warrants that were granted to the investors in accordance with the 2010 Share Purchase Agreement was modified to be $708 per share. This reduction in the exercise price was reflected in the fair value of the warrants as of December 31, 2012.
As of December 31, 2014, 492 warrants granted under 2010 Share Purchase Agreement have been exercised.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 11 - Equity (Cont’d)
B. Financing rounds (cont’d)
| | 3. | In April 2012, the Company entered into the 2012 Share Purchase Agreement with several investors. Pursuant to the 2012 Share Purchase Agreement, the investors purchased 20,287 Preferred A shares from the Company and the Company issued to each investor warrants to purchase a number of Preferred A shares equal to 50% of its original number of Preferred A shares (i.e., at a two share to one warrant ratio), at an exercise price of $708 per share. The warrants were fully vested and are exercisable until the earlier of: (i) 5 years after the closing date of the 2012 Share Purchase Agreement; or (ii) certain M&A events. The investors may exercise the warrants either for cash or in a cashless manner. |
The total consideration as determined in the 2012 Share Purchase Agreement was $11,907 (net of issuance costs of $21) and was allocated to warrants classified as a derivative liability due to their net share settlement feature, based on their fair value at the grant date. The remaining amount was allocated to the Preferred A shares and share premium. The warrants are measured at fair value through profit or loss.
The Company estimated the fair value of the warrants as of the date of grant, using the Black-Scholes option-pricing model to be approximately $44 (see Note 11B(8) for the parameters used to calculate the fair value of the warrants).
As of December 31, 2014, 442 warrants granted under the 2012 Share Purchase Agreement have been exercised.
| | 4. | In July 2013, the warrants to purchase the Company’s Preferred A shares that were granted to the investors pursuant to the 2010 Share Purchase Agreement and the 2012 Share Purchase Agreement were amended such that (i) the exercise price per each Preferred A share issuable upon the exercise of each such warrant was reduced to NIS 0.01; (ii) the number of Preferred A warrants was increased by an additional 5,691 Preferred A warrants (approximately 2.5% of the Company’s fully diluted share capital following the Recapitalization as defined below) and were granted to the holders of such warrants on a pro-rata basis; and (iii) all Preferred A warrants were fully vested and are exercisable until certain M&A events ("Recapitalization"). |
| | As of December 31, 2014, 250 warrants from the additional warrants granted have been exercised. |
Following the above mentioned reduction in the exercise price of the warrants granted under the 2010 Share Purchase Agreement and the 2012 Share Purchase Agreement, the Company changed the warrants classification from derivative instruments measured at fair value through profit and loss to equity, since upon the change in exercise price the warrants became “in substance” shares and no longer met the definition of derivatives in accordance with IAS 32. See Note 11B(8) for the inputs that were used in the calculation of the fair value of the warrants on the Recapitalization day.
| | 5. | In July 2013, following the Recapitalization, the Company entered into a share purchase agreement (the "2013 Share Purchase Agreement") with some of the Company's then current shareholders as well as new investors, pursuant to which they purchased 39,037 Preferred A shares of the Company for an aggregate purchase price of $13,750 (net of issuance costs of $300). |
The proceeds from the 2013 Share Purchase Agreement were allocated to Preferred A shares and share premium.
| | 6. | During 2014, 1,184 warrants to preferred A shares were exercised for 54,464 ordinary shares (based on the adjusted conversion rate upon the share split described in Note 11A(2)). During 2013, no warrants were exercised. |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 11 - Equity (Cont’d)
B. Financing rounds (cont’d)
| | 7. | See Note 1(3) regarding the Company’s IPO. |
| | 8. | The assumptions used to calculate the fair value of the warrants held by shareholders are as follows: |
| | | July 1, | |
| | | | 2013(*) | |
Company’s equity value (1) | | | 70,240 | |
Expected term (in years) (2) | | | 2.25 | |
Expected volatility (3) | | | 120 | % |
Risk-free interest rate (4) | | | 0.38 | % |
| Dividend yield | | | 0 | % |
| | (*) | In July 2013, following the Recapitalization event described in Note 11B(4), the warrants were classified from derivatives instruments measured at fair value through profit and loss to equity. |
| | (1) | The Company’s equity value as of July 1, 2013 was estimated based on July 2013 financing round. |
| | (2) | Based on the period until liquidation event (September 30, 2015). |
| | (3) | Based on the average equity volatility of comparable drug companies that are publicly traded. |
| | (4) | Based on zero coupon U.S. treasury bonds fixed with maturity equal to expected terms. |
| | C. | Share-based compensation |
| | (1) | Expense recognized in the statement of loss and other comprehensive loss is as follows: |
The expense that was recognized for services received from employees and service providers is as follows:
| | | | |
| | | | | | | |
| Research and development | | | 452 | | | | - | |
| General and administrative | | | 1,211 | | | | 2,648 | |
| | | | | | | | | |
| Total share-based compensation | | | 1,663 | | | | 2,648 | |
(2) Share based compensation plans for employees and consultants:
The Company has two option plans for employees and consultants under which up to 2,531,639 ordinary shares are reserved for issuance. As of December 31, 2014, 430,683 ordinary shares of the Company were still available for future grant. Any options that are forfeited or not exercised before expiration become available for future grants.
Options granted under the Company’s 2008 and 2013 Israeli Share Option Plans (hereinafter, the “Plans”) are exercisable in accordance with the terms of the Plans, within 10 years from the date of grant, against payment of an exercise price. The options for employees vest over a period of two to four years.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 11 – Equity (cont’d)
| | C. | Share-based compensation (cont’d) |
(3) Option grants during 2014 and 2013:
| | 1. | On August 22, 2013, the Company granted options to purchase 573,068 and 286,534 ordinary shares under the Plans at exercise prices of $3.26 and $16.04 per share, respectively, to its chief executive officer. In accordance with the executive’s employment agreement, upon the consummation of an IPO or M&A event during the employment period or during the notice period upon termination, the unvested options will become fully vested and exercisable. |
| | 2. | On October 29, 2013, the Company granted options to purchase 25,760 ordinary shares under the Plans at an exercise price of $3.26 per share to its employee and consultant. |
| | 3. | On December 26, 2013, the Company granted options to purchase 55,982 ordinary shares under the Plans at an exercise price of $3.26 per share to its employees. |
| | 4. | On April 13, 2014, the Company granted options to purchase 370,070 ordinary shares under the Plans at an exercise price of $10.20 per share to its employees and consultant. |
| | 5. | On July 22, 2014, the Company granted options to purchase 219,972 ordinary shares under the Plans at an exercise price of $10.00 per share to its Board of Directors members. |
| | 6. | On December 31, 2014, the Company granted options to purchase 50,000 ordinary shares under the Plans at an exercise price of $7.32 per share to its employee. |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 11 – Equity (cont’d)
| | C. | Share-based compensation (cont’d) |
| | (4) | The number and weighted average exercise prices of options are as follows: |
| | | Weighted average exercise price | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Outstanding at January 1 | | | 5.78 | | | | 2.93 | | | | 1,341,544 | | | | 440,128 | |
| Expired and forfeited | | | 4.33 | | | | 6.52 | | | | (80,592 | ) | | | (39,928 | ) |
| Exercised | | | 0.39 | | | | - | | | | (67,850 | ) | | | - | |
| Granted | | | 9.85 | | | | 7.13 | | | | 656,004 | | | | 941,344 | |
| Outstanding at December 31 | | | 7.23 | | | | 5.78 | | | | 1,849,106 | | | | 1,341,544 | |
| | | Options outstanding as of December 31, 2014 | |
| | | | | | Weighted average remaining contractual life | | | Weighted average exercise price | |
| Range of exercise prices (U.S. Dollar) | | | | | | | | |
| 0.01 – 3.26 | | | 860,982 | | | | 7.97 | | | | 2.48 | |
| 4.91 – 7.32 | | | 111,548 | | | | 7.28 | | | | 5.99 | |
| 10.00 – 16.04 | | | 876,576 | | | | 9.14 | | | | 12.06 | |
| Total | | | 1,849,106 | | | | 8.49 | | | | 7 | |
| | | | | | | |
| Weighted average share prices (in U.S. dollar) | | | 7.25-9.80 | | | | 3.65-5.37 | |
| Expected life of share options (in years)(*) | | | 5.5-7.0 | | | | 4.9-7 | |
| Expected volatility | | | 104%-127 | % | | | 126%-131 | % |
| Risk-free interest rate | | | 1.85%-2.28 | % | | | 1.71%-2.41 | % |
| Dividend yield | | | 0 | % | | | 0 | % |
| | (*) | The expected life of the share options is based on the midpoints between the available exercise dates (the end of the vesting periods) and the last available exercise date (the contractually expiry date), as adequate historical experience is not available to provide a reasonable estimate. |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 12 - Basic and Diluted Loss Per Share
Basic and Diluted
Basic and Diluted loss per share is calculated by dividing the loss of the Company by the weighted average number of the issued and outstanding ordinary shares during the year (2014 - 362,756, 2013 - 387,412), which includes warrants and options to purchase ordinary shares that granted with an exercise price of par value.
| | | | |
| | | | | | | |
| Loss for the year | | | (25,451 | ) | | | (18,324 | ) |
| Weighted average number of Ordinary shares outstanding | | | 11,863,372 | | | | 7,444,042 | |
| | | | | | | | | |
| Basic and diluted loss per share (in U.S. dollars) | | | (2.15 | ) | | | (2.46 | ) |
| Number of options excluded from the diluted loss per share | | | | | | | | |
| calculation due to their anti-dilutive effect | | | 1,826,152 | | | | 4,254,402 | |
Note 13 - Financial Instruments
The Company has exposure to the following risks from its use of financial instruments:
This Note presents quantitative and qualitative information about the Company’s exposure to each of the above risks, and the Company’s objectives, policies and processes for measuring and managing risk.
| | B. | Risk management framework |
The Company’s board of directors has overall responsibility for carrying out risk management activities. In this regard, the finance department identifies, defines and assesses financial risks. Risk management policies are reviewed regularly to reflect changes in market conditions and the Company’s activities. The Company, through its training and management of standards and procedures, aims to develop a disciplined and constructive control environment in which all employees understand their roles and obligations.
Credit risk is the risk of financial loss to the Company if a counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Company’s cash and cash equivalents and accounts receivable.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
| | Cash and cash equivalent and short-term deposits |
On December 31, 2014 and 2013, the Company held cash and cash equivalents of $10,868 and $18,995, respectively. In addition on December 31, 2014 and 2013, the Company held short-term deposits of $17,829 and $0, respectively. The Company’s cash and cash equivalents and deposits are deposited with financial institutions having a high credit rating.
| | Available for sale financial assets |
On December 31, 2014 and 2013, the Company held Available for sale financial Assets of $17,484 and $0, respectively. The Company’s available for sale financial assets comprised of debt securities issued by corporations that have a credit rating of at least A1/A+ (from three different rating agencies).
The maximum exposure to credit risk for investments in debt instruments by type of counterparty was as follows:
| | | | |
| | | | | | | |
| | | | | | | |
| Available-for-sale financial assets: | | | | | | |
| Debentures issued by entities: | | | | | | |
| Rated AA | | | 2,784 | | | | - | |
| Rated AA- | | | 5,473 | | | | - | |
| Rated A+ | | | 7,009 | | | | - | |
| Rated A | | | 2,218 | | | | - | |
| | | | 17,484 | | | | - | |
Liquidity risk is the risk that the Company will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Company’s approach to managing liquidity is to ensure, as far as possible, that it has sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Company’s reputation.
Company management monitors rolling forecasts of the Company’s liquidity reserves on the basis of anticipated cash flows and maintains the liquidity balances at a level that it believes is sufficient to meet its needs.
As of December 31, 2014 and 2013 the Company’s contractual obligation of financial liability is in respect of Trade and other payables in the amount of $2,488 and $1,971, respectively. The contractual maturity of this financial liability is less than one year and in its carrying amount.
The contractual obligations do not include royalties that the Company may be obligated to pay to MDA or the OCS, as detailed under Note 10G based upon future sales of its products, as the Company is unable to estimate the actual amount or timing of these costs that will incur in the future to these parties.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Market risk is the risk that changes in market prices, such as foreign exchange rates, the consumer price index, or CPI, interest rates and equity prices will affect the Company’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimizing the return.
| | (1) | Foreign currency risks |
The Company’s activities are partly denominated in foreign currencies, which exposes the Company to risks resulting from changes in exchange rates. The Company does not use derivatives to hedge currency risk.
The Company’s exposure to foreign currency risk was as follows:
| | (a) | The exposure to foreign currency risk |
The Company’s exposure to foreign currency risk was as follows:
| | | | |
| | | | | | | | | | |
| Financial assets and | | | | | | | | | |
| financial liabilities: | | | | | | | | | |
| Current assets: | | | | | | | | | |
| Cash and cash equivalents | | | 9,802 | | | | 1,066 | | | | 10,868 | |
| Short term investments | | | 35,313 | | | | | | | | 35,313 | |
| Accounts receivable | | | 184 | | | | 339 | | | | 523 | |
| Non-current assets | | | | | | | | | | | | |
| Deposits | | | 1,246 | | | | 9 | | | | 1,255 | |
| Current liabilities: | | | | | | | | | | | | |
| Trade and other payable | | | (2,132 | ) | | | (366 | ) | | | (2,498 | ) |
| | | | | | | | | | | | | |
| | | | 44,413 | | | | 1,048 | | | | 45,461 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 13 - Financial Instruments (cont’d)
| | (1) | Foreign currency risks (cont’d) |
(a) The exposure to foreign currency risk (cont’d)
| | | | |
| | | | | | | | | | | | | |
| Financial assets and | | | | | | | | | | | | |
| financial liabilities: | | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | | |
| Cash and cash | | | | | | | | | | | | |
| equivalents | | | 17,988 | | | | 1,007 | | | | - | | | | 18,995 | |
| Accounts receivable | | | 300 | | | | 269 | | | | - | | | | 569 | |
| Non-current assets | | | | | | | | | | | | | | | | |
| Deposits | | | - | | | | 17 | | | | - | | | | 17 | |
| Current liabilities: | | | | | | | | | | | | | | | | |
| Trade and other payable | | | (882 | ) | | | (1,019 | ) | | | (70 | ) | | | (1,971 | ) |
| | | | | | | | | | | | | | | | | |
| | | | 17,406 | | | | 274 | | | | (70 | ) | | | 17,610 | |
A change as of December 31 in the exchange rates of the following currencies against the U.S. dollar, as indicated below would have affected the measurement of financial instruments denominated in other currencies and would have increased (decreased) profit or loss and equity by the amounts shown below. This analysis is based on currency exchange rate that the Company considered to be reasonably possible at the end of the reporting period. The analysis assumes that all other variables remain constant.
| | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Change in the exchange rate of: | | | | | | | | | | | | |
| 5% in the NIS | | | 45 | | | | (45 | ) | | | 14 | | | | (14 | ) |
| 10% in the NIS | | | 90 | | | | (90 | ) | | | 27 | | | | (27 | ) |
| 5% in the Euro | | | - | | | | - | | | | (4 | ) | | | 4 | |
| 10% in the Euro | | | - | | | | - | | | | (7 | ) | | | 7 | |
* The effect of the change on equity is the same as on profit or loss.
As of December 31, 2014, the Company has debt securities classified as available for sale financial investments, for which the Company is exposed to risk of fluctuations in the security price that is determined by reference to the quoted market price.
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 13 - Financial Instruments (cont’d)
| | (2) | Security price risk (cont’d) |
Security price risk – sensitivity analysis
The Company’s investments in securities include investments in corporate debt securities. The sensitivity analysis below presents the effect of a change in debt security prices (or the underlying assets) on the fair value of securities held by the Company, assuming that all other variables remain constant.
A change in debt security prices would have increased (decreased) equity by the amounts shown below (including tax effects):
| | | | |
| | | | | | | |
| Increase of 5% | | | 223 | | | | 868 | |
| Increase of 10% | | | 453 | | | | 1,736 | |
| Decrease of 5% | | | - | | | | (868 | ) |
| Decrease of 10% | | | - | | | | (1,736 | ) |
F. Fair value
| | (1) | Financial instruments which their fair value approximates their carrying amounts |
The carrying amounts of certain financial assets and liabilities, including cash and cash equivalents, receivables, deposits, trade and other payables, are the same as or approximate to their fair value.
The table below analyzes financial instruments carried at fair value, using a valuation method in accordance with the fair value hierarchy level. The different levels have been defined as follows:
| | — | Level 1: quoted prices (unadjusted) in active markets for identical instruments |
| | — | Level 2: inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly |
| | — | Level 3: inputs that are not based on observable market data (unobservable inputs). |
| | | December 31, 2014 | |
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| Available-for-sale financial | | | | | | | | | | | | |
| assets | | | 17,484 | | | | - | | | | - | | | | 17,484 | |
| | | | | | | | | | | | | | | | | |
| | | | 17,484 | | | | - | | | | - | | | | 17,484 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 14 - Research and Development Expenses, Net
| | | | |
| | | | | | | | | | |
| Payroll and related expenses | | | 2,618 | | | | 2,065 | | | | 1,380 | |
| Materials, subcontractors and consultants | | | 12,387 | | | | 6,498 | | | | 5,116 | |
| Depreciation and amortization | | | 573 | | | | 631 | | | | 641 | |
| Rent, insurance and maintenance | | | 198 | | | | 182 | | | | 148 | |
| Other | | | 209 | | | | 379 | | | | 369 | |
| | | | 15,985 | | | | 9,755 | | | | 7,654 | |
| Less – income from healthcare professionals (see Note 10B) | | | 443 | | | | 452 | | | | 486 | |
| | | | | | | | | | | | | |
| | | | 15,542 | | | | 9,303 | | | | 7,168 | |
Note 15 - General and Administrative Expenses
| | | | |
| | | 2014 | | | 2013 | | | 2012 | |
| Payroll and related expenses | | | 2,575 | | | | 3,927 | | | | 1,102 | |
| Professional expenses | | | 1,895 | | | | 431 | | | | 290 | |
| Rent, insurance and maintenance | | | 241 | | | | 141 | | | | 115 | |
| Other | | | 663 | | | | 68 | | | | 124 | |
| | | | | | | | | | | | | |
| | | | 5,374 | | | | 4,567 | | | | 1,631 | |
Note 16 - Financing Income (Expense), Net
| | | | |
| | | | | | | | | | |
| Interest income | | | 40 | | | | 94 | | | | 102 | |
| Foreign exchange income,net | | | - | | | | 99 | | | | 68 | |
| Change in fair value of warrants held by shareholders | | | - | | | | - | | | | 881 | |
| | | | | | | | | | | | | |
| | | | 40 | | | | 193 | | | | 1,051 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 16 - Financing Income (Expense), Net (Cont’d)
| | | | |
| | | 2014 | | | 2013 | | | 2012 | |
| Credit line (See Note 10I) | | | 4,371 | | | | - | | | | - | |
| Bank charges | | | 15 | | | | 15 | | | | 8 | |
| Foreign exchange loss, net | | | 158 | | | | - | | | | - | |
| Change in fair value of warrants held by shareholders | | | - | | | | 4,483 | | | | - | |
| | | | | | | | | | | | | |
| | | | 4,544 | | | | 4,498 | | | | 8 | |
| | | | | | | | | | | | | |
| Financing income (expenses), net | | | (4,504 | ) | | | (4,305 | ) | | | (1,043 | ) |
Note 17 - Taxes on Income
| | A. | Details regarding the tax environment of the Company |
(1) Corporate tax rate
| | (a) | Presented below are the tax rates relevant to the Company in the years 2011-2014: |
2012 - 25%
2013 - 25%
2014 - 26.5%
On August 5, 2013, the Knesset passed the Law for Changes in National Priorities (Legislative Amendments for Achieving Budget Objectives for 2013 and 2014) - 2013, by which, inter alia, the corporate tax rate would be raised by 1.5% to a rate of 26.5% commencing January 1, 2014.
| | (b) | On February 4, 2010, Amendment 174 to the Income Tax Ordinance (New Version) – 1961 (hereinafter – the “Ordinance”) was published in the Official Gazette. The amendment added Section 87A to the Ordinance, which provides a temporary order whereby Accounting Standard No. 29 “Adoption of International Financial Reporting Standards (IFRS)” that was issued by the Israel Accounting Standards Board shall not apply when determining the taxable income for the 2007, 2008 and 2009 tax years even if this standard was applied when preparing the financial statements (hereinafter – the “Temporary Order”). On January 12, 2012, Amendment 188 to the Ordinance was issued, according by which the Temporary Order was amended so that Standard 29 shall not apply when determining the taxable income for 2010 and 2011. On July 31, 2014 Amendment 202 to the Ordinance was issued, by which the Temporary Order was extended to the 2012 and 2013 tax years, effective retroactively as of January 1, 2012. |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 17 - Taxes on Income (cont’d)
| | A. | Details regarding the tax environment of the Company (cont’d) |
| | (2) | Benefits under the Law for the Encouragement of Capital Investments |
(a) Beneficiary enterprise
The Company has elected the year of 2012 as the year of election for the “Beneficiary Enterprise”. The income generated by the “Beneficiary Enterprise” is exempt from tax over a period of 2 years and is subject to a reduced rate of company tax for a period of up to 5 years beginning with the year in which the Company first had taxable income (limited to a maximum period of 12 years from the year of election). The tax benefit period of the beneficiary enterprise that commenced operations in 2012 has not yet commenced. The benefits are contingent upon compliance with the terms of the Encouragement Law.
A company having a beneficiary enterprise that distributes a dividend from exempt income, will be required in the tax year of the dividend distribution to pay corporate tax on the amount of the dividend distributed at the tax rate that would have been applicable to it in the year the income was produced if it had not been exempt from tax.
| | (3) | Benefits under the Law for the Encouragement of Industry (Taxes) - 1969 |
If the Company qualifies as an “Industrial Company” as defined in the Law for the Encouragement of Industry (Taxes) – 1969, it is entitled to benefits of which the most significant ones are as follows:
(a) Higher rates of depreciation.
| | (b) | Amortization in three equal annual portions of issuance expenses when registering shares for trading as from the date the shares of the company were registered. |
| | (c) | An 8-year period of amortization for patents and know-how serving in the development of the enterprise. |
| | (d) | The possibility of submitting consolidated tax returns by companies in the same line of business. |
| | (4) | Macrocure Inc., the U.S. subsidiary, is taxed based on U.S. tax laws. The tax rates applicable to the subsidiary is approximately 35% (Federal and State). Taxes on income recorded in profit or loss are current tax expenses of this subsidiary. |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
B. Unrecognized deferred tax assets
As of December 31, 2014 and 2013, the Company had carry forward losses for tax purposes in the amount of $34,927 and $27,303, respectively. The tax losses may be carried forward and offset against taxable income in the future for an indefinite period.
As of December 31, 2014 and 2013, the Company had deductible temporary differences in the net amount of $13,009 and $8,359, respectively.
The Company has not recorded deferred tax assets with respect to carry forward losses and deductible temporary differences because it is not probable that future taxable profit will be available against which the Company can use the benefits therefrom.
C. Tax assessments
The Company and its subsidiary have not been assessed since incorporation. In accordance with the Israeli Income Tax Ordinance, tax assessments of the Company through tax year 2010 are considered final.
The main reconciling item between the statutory tax rate of the Company and the effective tax rate is current year tax losses and benefits for which no deferred tax assets were created.
Note 18 - Geographical Information
The Company’s non-current assets based on their geographical locations are as follows:
| | | | |
| | | | | | | |
| Israel | | | 511 | | | | 994 | |
| United States | | | 216 | | | | 163 | |
| | | | | | | | | |
| | | | 727 | | | | 1,157 | |
Macrocure Ltd.
Notes to the Consolidated Financial Statements as of December 31, 2014
| U.S. dollars in thousands (except share and per share data) |
Note 19 - Related Parties
Compensation and benefits to key management personal
In addition to their salaries, the Company provides non-cash benefits to a director and executive officers (such as a car, medical insurance, etc.), and contributes to a post-employment defined benefit plan on their behalf. The Company’s executive officers are subject to mutual term of notice of 3-9 months.
Executive officers also participate in the Company’s share option programs. For further information see Note 11C regarding share-based compensation.
Compensation and benefits to key management personnel (including one director) are as follows:
| | | | |
| | | | | | | |
| | | Number of | | | Amount in | | | Number of | | | Amount in | |
| | | | | | | | | | | | | |
| Short-term benefits | | | 10 | | | | 2,298 | | | | 6 | | | | 1,149 | |
| Share-based compensation | | | 9 | | | | 1,445 | | | | 4 | | | | 2,648 | |
| Total | | | | | | | 3,743 | | | | | | | | 3,797 | |
For further information regarding convertible loan agreement with a related party see Note 10 I.
Note 20 - Subsequent Events
| | A. | On February 5, 2015 the Board of directors resolved to increase the number of ordinary shares of the Company reserved for issuance under the 2013 Plan by 400,000 options. |
| | B. | Further to what is described in Note 10I, on February 12, 2015 the lender of the convertible loan exercised the warrants to purchase 439,760 ordinary shares of the Company at a price per share equal to NIS 0.01. |
F - 38