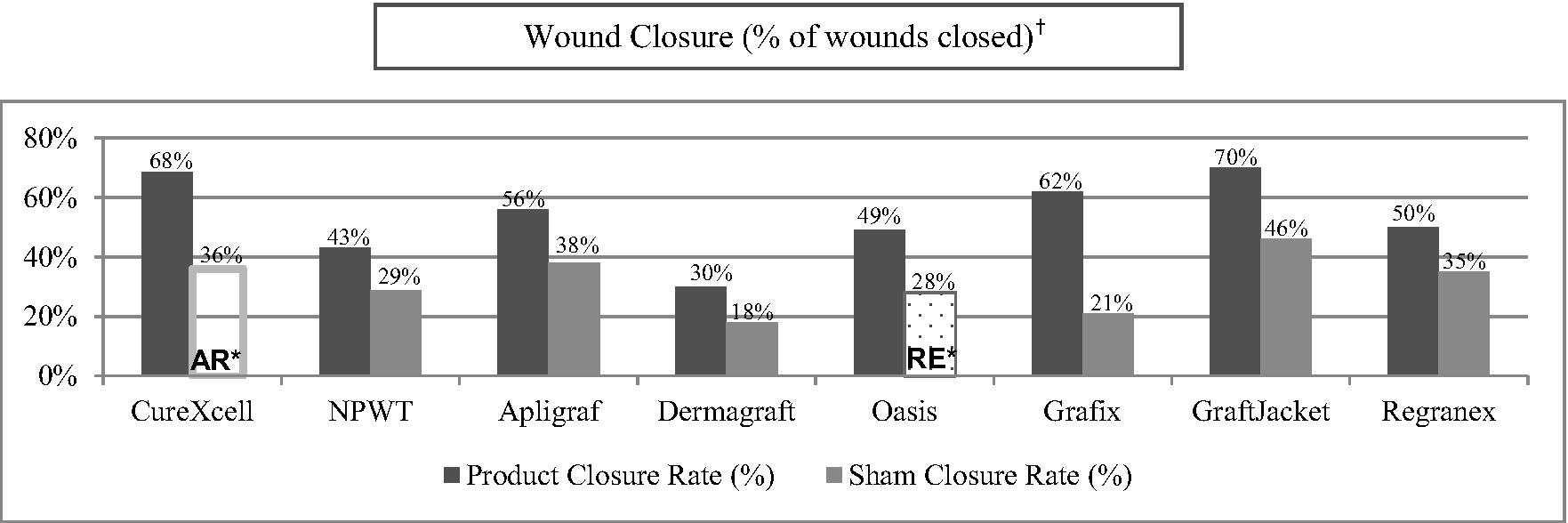
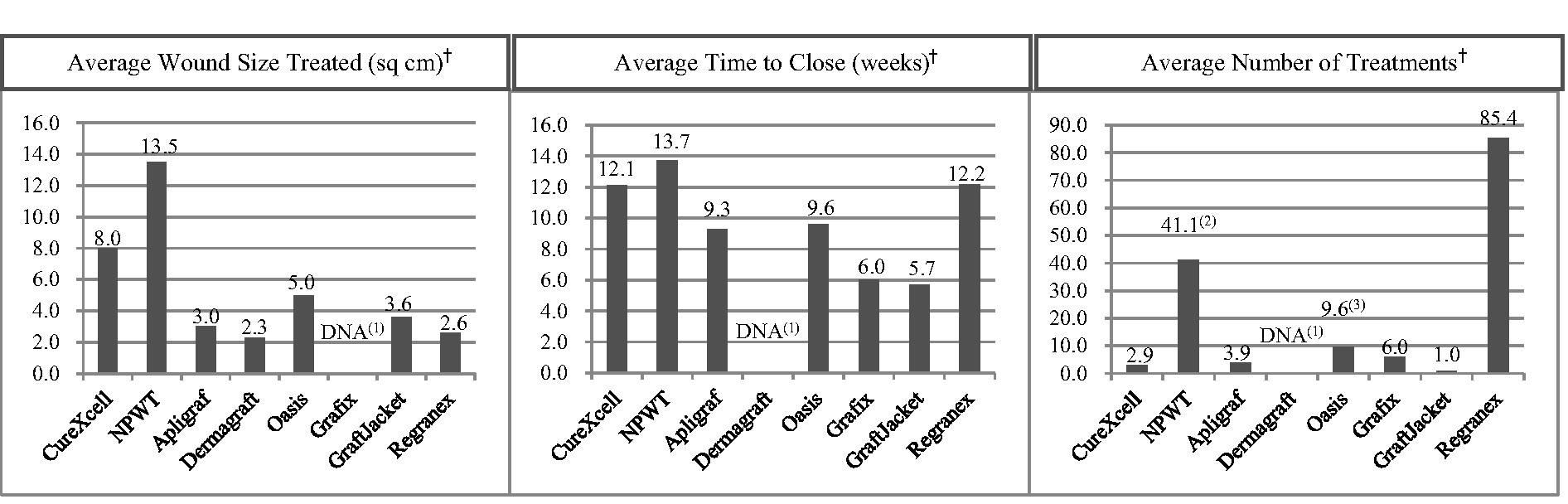
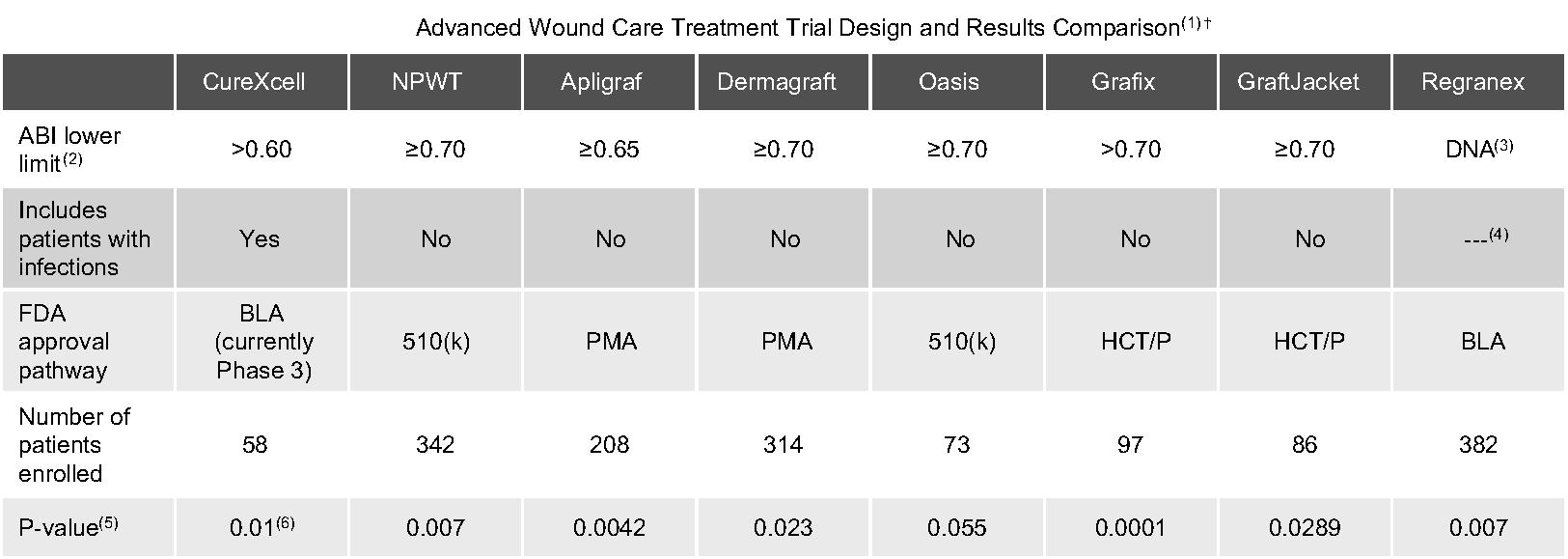
Of the ulcers treated with CureXcell in this study, 69.5%, or 98 out of 141, achieved wound closure compared to 13.3%, or 10 out of 75, of the ulcers in the control group (p<0.001). In addition, 59.1%, or 39 out of 66, of the patients treated with CureXcell achieved complete wound closure in comparison to 5.3%, or two out of 38, of the control patients (p<0.001). Also, a faster median healing time of 87.0 days was noted in patients treated with CureXcell when compared to a median healing time of 117.7 days in the control group, though this difference was not found to be statistically different.
For the different population subsets studies (patients with diabetes mellitus, patients with lower leg pressure ulcers, and patients with diabetes mellitus with baseline wounds greater than or equal to 15 cm2), similar results were observed. No adverse or SAEs related to CureXcell were reported.
Prophylaxis of Saphenous Vein Harvesting Incision Site Infection Study. This study was a post-marketing, prospective, randomized, single-blind study conducted in Israel from 2004 through 2006. In this study, 44 patients undergoing coronary artery bypass, or CABG, surgery were randomized to either CureXcell with standard of care treatment or standard of care treatment alone; 22 patients were placed in each group. Patients were followed for infection at the incision site throughout the study period of three months after hospital discharge. None of the patients in the CureXcell group experienced an infected saphenous vein incision site. This is compared to 27%, or six of 22, of the patients experiencing infection in the control group. No product-related SAEs were observed in this study.
Infected Leg Wounds following Saphenous Vein Harvesting Study. This study was a retrospective, historically controlled, non-randomized, Phase 4 study conducted in Israel from 2000 through 2004. A total of 208 patients suffering from infected leg wounds post-CABG surgery were enrolled into the study, out of which 95 patients were treated with CureXcell. Patients were followed for complete wound closure and time to wound closure.
Of the patients treated with CureXcell, 97.9%, or 93 out of 95, achieved wound closure compared to 85.8%, or 97 out of 113, of the patients in the control group (p<0.001). Additionally, the average time to wound closure was 47 days in the CureXcell group compared to 66.5 days in the control group.
Deep Sternal Wound Infections Study. This study was a historically controlled, retrospective, non-randomized, Phase 4 study conducted in Israel from 2000 through 2003. A total of 130 patients with deep sternal wound infections were included in the study, of which 66 patients were treated with CureXcell and data from 64 patients were retrospectively collected. All patients had previously undergone open heart surgery, with most of them suffering from multiple co-morbidities. Patients were followed for mortality and percent of wound closure.
Two out of 66, or 3.0%, the treatment group patients suffered late deaths, compared to 19 late deaths, or 29.7%, in the control group (p<0.001). Both deaths in the treatment group were considered unrelated to CureXcell. Sixty patients, or 90.9%, in the CureXcell group achieved complete wound closure. No CureXcell-related adverse events were observed. Furthermore, no deterioration or worsening of wound condition was observed in the treated patients.
In parallel to the groups above, an additional group of 50 patients with superficial sternal wound infection were treated by CureXcell and were monitored for complete wound closure. All of these patients achieved full closure in 22.6 days after a single CureXcell treatment.
Research and Development
A significant portion of our historical research and development efforts have focused on the CureXcell production process. Specifically, we have invested significantly in order to enable production in closed systems of kits containing transfusion bags. Similarly, our research and development strategy is centered on further developing the CureXcell production process so as to extend the shelf life of the product and to enable its packaging in containers other than transfusion bags, which will enable us to produce a greater range of dosages so as to further maximize product utilization. We are also researching the mode of action of our cell activation technology in order to leverage the technology for the development of a regenerative medicine product platform for non-wound indications. In addition, we intend to commence a multinational study to test the safety and efficacy of a product using our technology for anti-reabsorbtive medication induced ONJ. Our research and development team is located at our facilities in Israel and the United States, and consisted of 20 employees as of March 31, 2014.
In the past, we received government grants that were subject to the payment of royalties as part of our research and development programs approved by the OCS. The total gross amount of grants actually received by us from the OCS, including accrued LIBOR interest, totaled approximately $0.8 million as of March 31, 2014. According to the terms of the grants, the OCS is entitled to royalties equal to 3.0% to 4.5% of our sales, up until the amount of the grants is repaid in full. As of March 31, 2014, we had not paid any royalties to the OCS.