Exhibit 1

July 2015 Macrocure Ltd. NASDAQ: MCUR Corporate Presentation

Safe harbor statement This presentation contains forward-looking statements within the meaning of Section 27A of the U.S. Securities Act of 1933, as amended, Section 21E of the U.S. Securities and Exchange Act of 1934, as amended, and the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Any and all statements concerning our business and financial performance and condition, as well as our plans, objectives and expectations for our business, operations and financial performance and condition. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms including ‘‘anticipates,’’ ‘‘believes,’’ ‘‘could,’’ ‘‘estimates,’’ ‘‘expects,’’ ‘‘intends,’’ ‘‘may,’’ ‘‘plans,’’ ‘‘potential,’’ ‘‘predicts,’’ ‘‘projects,’’ ‘‘should,’’ ‘‘will,’’ ‘‘ would,’’ and similar expressions intended to identify forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. You should not unduly rely on any forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation, to conform these statements to actual results or to changes in our expectations. These forward-looking statements speak only as of the date of this presentation, and we assume no obligation to update or revise these forward-looking statements for any reason . The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company.
Macrocure - overview ▪ Late-stage Phase 3 biotechnology company with novel, cell therapy platform ▪ Lead BLA product candidate, CureXcell, is a monthly injectable therapy, with potential to transform conventional AWC category ▪ Initial focus on $5 billion DFU and VLU market opportunity in U.S. and EU ▪ Significant near-term milestones: - August 2015: VLU Futility Analysis - October 2015: DFU Phase 3 data readout - 1H 2016: VLU Phase 3 data readout ▪ Commercial-scale manufacturing and reimbursement initiatives underway supporting attractive 80 %+ gross margin ▪ Proven management team experienced in developing and commercializing biologics

Reshaping chronic wound care therapy 4 CureXcell’s offers meaningful benefits to patients, physicians and payers compared with existing medical device therapies CureXcell Advantages Ready-to-use and easy to administer in less than 3 mins Direct contact with wound to heal from the inside out Once a month injection Estimated per patient treatment cost ~$6k Challenge for existing products Complex and challenging product preparation and application Limited by topical application of wound surface Multi-monthly treatments Average per patient treatment cost ~$10k

CureXcell’s multi-billion dollar market opportunity 5 We believe the sizeable AWC market is underpenetrated due to lack of effective products ▪ Targeting chronic hard-to-heal wounds that have not closed after four weeks of standard of care treatment ▪ ~ 15 % of hard-to-heal wounds result in amputations ▪ 80 % of all amputations in the US are a result of non-healing DFUs Total market size, # of p atients (in thousands) Parameter DFU VLU Total Hard-to-heal incidence in U.S. 443 562 1,005 Hard-to-heal incidence in EU5 233 505 738 Total hard-to-heal Incidence 676 1,067 1,743 Hard-to-heal market size, in dollars (in $millions) Parameter DFU VLU Total U.S. $1,180 $2,303 $3,483 EU5 $757 $1,662 $2,419 Total $1,937 $3,965 $5,902 Data Source: 2014 The Frankel Group Associates LLC - AWC market assessment report
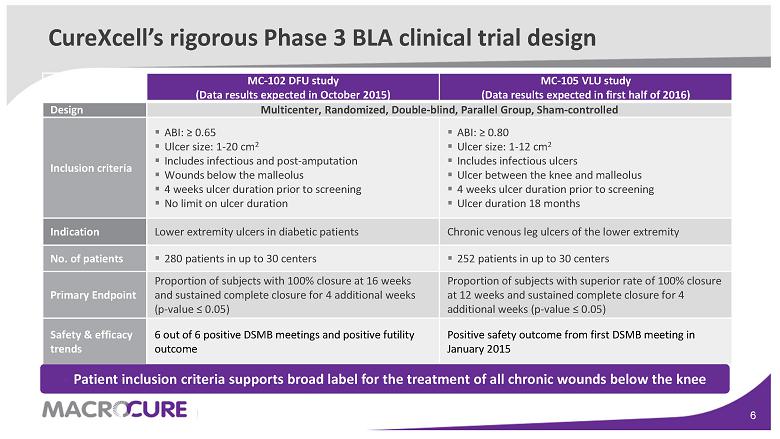
CureXcell’s rigorous Phase 3 BLA clinical trial design
MC-102 DFU study (Data results expected in October 2015) MC-105 VLU study (Data results expected in first half of 2016) Design Multicenter, Randomized, Double-blind, Parallel Group, Sham-controlled Inclusion criteria ▪ ABI: ≥ 0.65 ▪ Ulcer size: 1-20 cm 2 ▪ Includes infectious and post-amputation ▪ Wounds below the malleolus ▪ 4 weeks ulcer duration prior to screening ▪ No limit on ulcer duration ▪ ABI: ≥ 0.80 ▪ Ulcer size: 1-12 cm 2 ▪ Includes infectious ulcers ▪ Ulcer between the knee and malleolus ▪ 4 weeks ulcer duration prior to screening ▪ Ulcer duration 18 months Indication Lower extremity ulcers in diabetic patients Chronic venous leg ulcers of the lower extremity No. of patients ▪ 280 patients in up to 30 centers ▪ 252 patients in up to 30 centers Primary Endpoint Proportion of subjects with 100% closure at 16 weeks and sustained complete closure for 4 additional weeks (p-value ≤ 0.05) Proportion of subjects with superior rate of 100% closure at 12 weeks and sustained complete closure for 4 additional weeks (p-value ≤ 0.05) Safety & efficacy trends 6 out of 6 positive DSMB meetings and positive futility outcome Positive safety outcome from first DSMB meeting in January 2015 ▪ Patient inclusion criteria supports broad label for the treatment of all chronic wounds below the knee

Proven track record supports our current clinical plan ▪ CureXcell’s 15+ year clinical history is compelling - Over 5,000 patients treated - 9 completed clinical trials across a variety of chronic and acute/surgical wound types - Existing MC-103 efficacy data is key precedent for our current US Phase 3 BLA trials ▪ C urrent Phase 3 FDA trials are highly consistent in design with our largest precedent study, MC-103
Key Study Inclusion Criteria MC-103 DFU & VLU Israeli open-label study MC-102 DFU US BLA Study MC-105 VLU US BLA Study Infection yes yes yes Post amputation yes yes N/A Large wounds yes yes yes Low ABI (blood flow) yes yes N/A Good Ulcer Care yes yes yes Source: 8 abstracts reviewed by a medical conference scientific committee and 11 peer reviewed publications
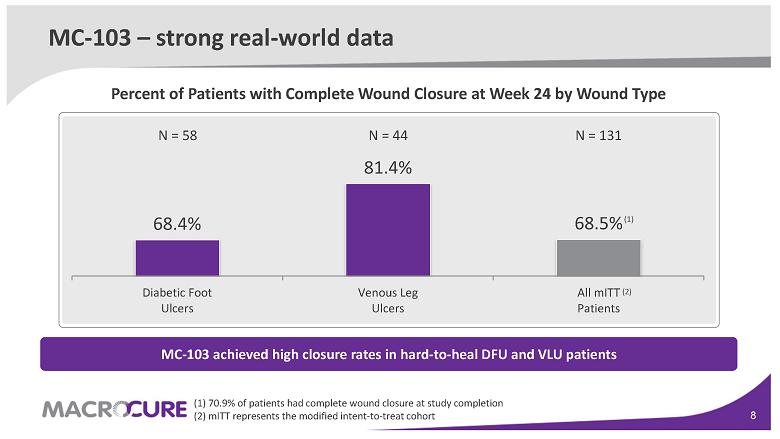
MC-103 – strong real-world data
68.4% 81.4% 68.5% Diabetic Foot Ulcers Venous Leg Ulcers All mITT Patients N = 58 N = 44 N = 131 MC-103 achieved high closure rates in hard-to-heal DFU and VLU patients Percent of Patients with Complete Wound Closure at Week 24 by Wound Type (1) (1) 70.9 % of patients had complete wound closure at study completion (2) mITT represents the modified intent-to-treat cohort (2)

CureXcell case studies
▪ 53 yr. old male; multiple co-morbidities ▪ DFU; No improvement with SOC after 3 mos. ▪ Closed after 2 CureXcell injections In certain instances, CureXcell was effective in wounds for which an alternative treatment did not work ▪ 56 yr. old male; multiple co-morbidities ▪ DFU; post toe amputation ▪ Closed after 2 CureXcell injections ▪ 64 yr. old male; multiple co-morbidities ▪ DFU for 8 years without closure ▪ Closed after 3 CureXcell injections
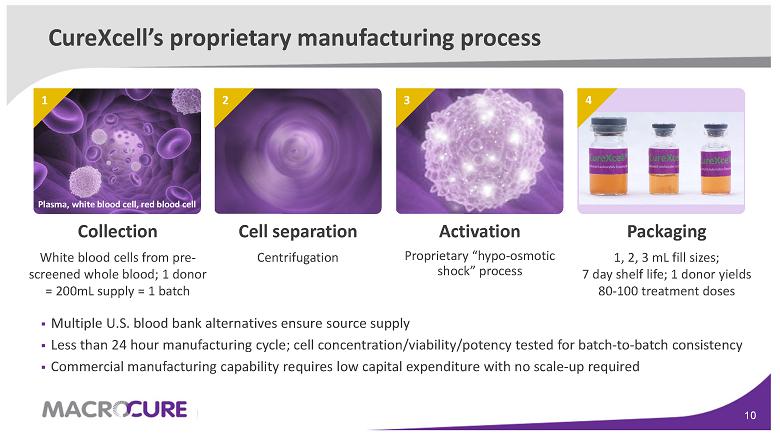
Activation Proprietary “hypo-osmotic shock” process Packaging 1, 2, 3 mL fill sizes; 7 day shelf life; 1 donor yields 80-100 treatment doses Cell separation Centrifugation Collection White blood cells from pre-screened whole blood; 1 donor = 200mL supply = 1 batch CureXcell’s proprietary manufacturing process
3 1 2 Plasma, white blood cell, red blood cell ▪ Multiple U.S. blood bank alternatives ensure source supply ▪ Less than 24 hour manufacturing cycle; cell concentration/viability/potency tested for batch-to-batch consistency ▪ Commercial manufacturing capability requires low capital expenditure with no scale-up required 4

Our proprietary activation process is key 11 The rapid movement of water across the cell membrane results in the opening of ion channels Our proprietary activation process uses white blood cells as a biological factory for growth factor and cytokine production ▪ Step 1 . Hypo-osmotic shock: Precise timing enables WBC activation that allows the release of cytokines and growth factors ▪ Step 2 . Incubation Period: Achieves consistent and stable cytokines and growth factor concentration necessary for wound healing and tissue regeneration

MOA: 55 growth factors & cytokines differentiate CureXcell 12 ▪ Comprehensive Mechanism of Action data provides unique approach to address the common deficient pathways that exist in both DFU and VLU ▪ Demonstrated statistically significant amplification of growth factors and cytokines ▪ Measurable activation and potency throughout CureXcell’s shelf life Selected Human Factors Angiogenesis Cell Proliferation Collagen I production Re-epithelialization Anti-catabolic Changes Post-Activation IL-1RA x 100-fold IL-8 x x 100-fold VEGF-A x 55-fold EGF x x x 27-fold Angiopoietin-1 x 8-fold PDGF-AA & -BB x x x 5-fold FGF-2 (bFGF) x x x 2-fold TIMPs 1, 2 & 3 x 2-fold

Executing our commercial manufacturing strategy ▪ Commercial manufacturing site anticipated in greater New Jersey/Philadelphia area - Extends existing clinical US manufacturing capability with the American Red Cross in Philadelphia, PA - Final site location and lease agreement to be finalized in Q3 2015 - Facility completion and validation for BLA submission in 2H 2016 ▪ Low capital expenditure requirement - Closed system, disposable bag technology - No scale-up required - Expansion capacity to accommodate future growth
Over the longer-term, we believe 1 to 2 US facilities and 1 European facility sufficient to address the market

Executing our reimbursement strategy ▪ Our formative reimbursement initiatives are already underway ▪ Post BLA approval, we intend to file for new procedure (CPT) and product (HCPCS) codes for CureXcell ▪ CureXcell is a biologic drug and therefore we are pursuing drug-based reimbursement - Approval process typically takes 9 to 12 months post FDA approval - Current HCPCS code J 3590 – ‘unclassified biologics’ ▪ Currently, there is no expectation that CureXcell will be placed under the existing high or low cost skin substitute bundle
We believe CureXcell’s product profile, efficacy, ease-of-use and patient convenience will enhance our reimbursement strategy
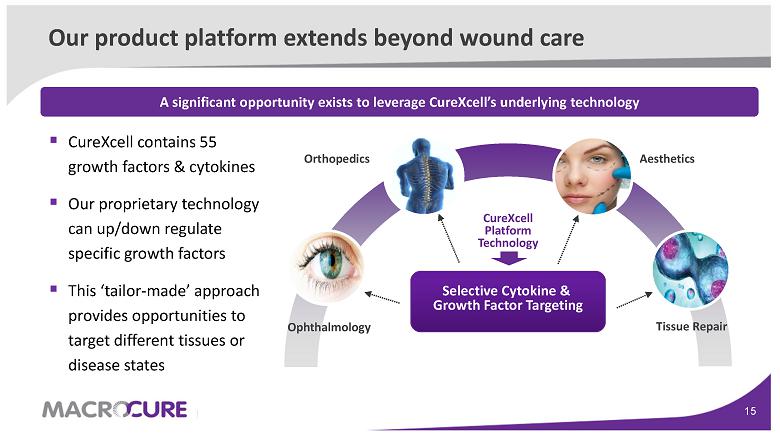
Our product platform extends beyond wound care
Ophthalmology Aesthetics Orthopedics CureXcell Platform Technology ▪ CureXcell contains 55 growth factors & cytokines ▪ Our proprietary technology can up/down regulate specific growth factors ▪ This ‘tailor-made’ approach provides opportunities to target different tissues or disease states Selective Cytokine & Growth Factor Targeting A significant opportunity exists to leverage CureXcell’s underlying technology Tissue Repair
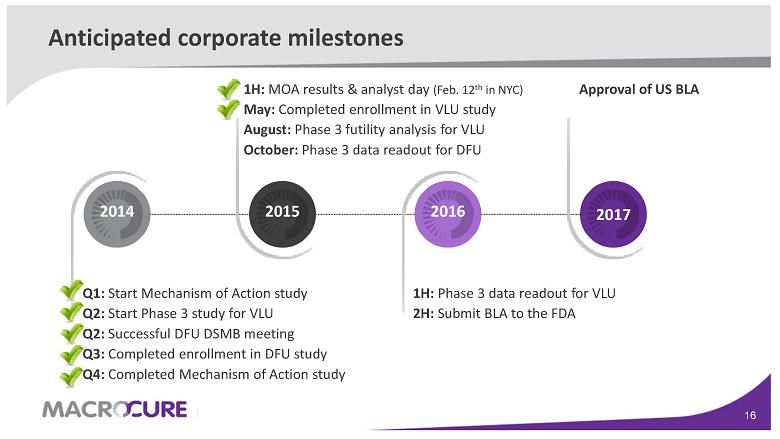
Anticipated corporate milestones
Q1: Start Mechanism of Action study Q2: Start Phase 3 study for VLU Q2: Successful DFU DSMB meeting Q3: Completed enrollment in DFU study Q4: Completed Mechanism of Action study 2014 1H: MOA results & analyst day (Feb. 12th in NYC) May: Completed enrollment in VLU study August: Phase 3 futility analysis for VLU October: Phase 3 data readout for DFU 2015 1H: Phase 3 data readout for VLU 2H: Submit BLA to the FDA 2016 A pproval of US BLA 2017
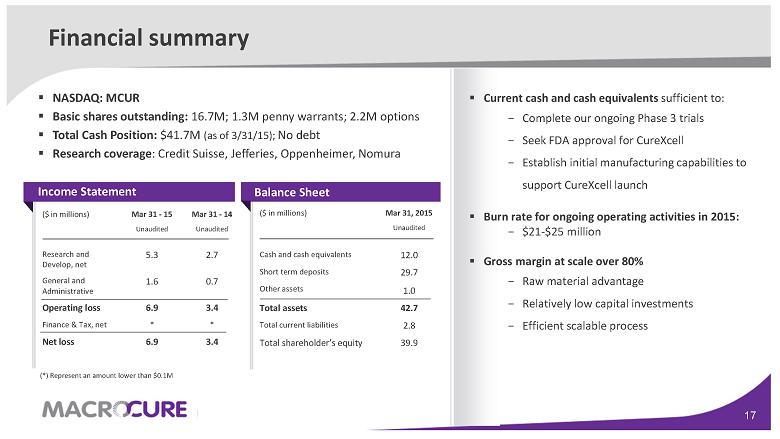
Mar 31, 2015 Unaudited ($ in millions) 12.0 Cash and cash equivalents 29.7 1.0 Short term deposits Other assets 42.7 Total assets 2.8 Total current liabilities 39.9 Total shareholder’s equity Financial summary
Mar 31 - 14 Unaudited Mar 31 - 15 Unaudited ($ in millions) 2.7 5.3 Research and Develop, net 0.7 1.6 General and Administrative 3.4 6.9 Operating loss * * Finance & Tax, net 3.4 6.9 Net loss Balance Sheet ▪ NASDAQ: MCUR ▪ Basic shares outstanding: 16.7M; 1.3M penny warrants; 2.2M options ▪ Total Cash Position: $41.7M (as of 3/31/15); No debt ▪ Research coverage: Credit Suisse, Jefferies, Oppenheimer, Nomura Income Statement ▪ Current cash and cash equivalents sufficient to: − Complete our ongoing Phase 3 trials − Seek FDA approval for CureXcell − Establish initial manufacturing capabilities to support CureXcell launch ▪ Burn rate for ongoing operating activities in 2015: − $21-$25 million ▪ Gross margin at scale over 80 % − Raw material advantage − Relatively low capital investments − Efficient scalable process (*) Represent an amount lower than $0.1 M

Macrocure - summary ▪ Late - stage Phase 3 biotechnology company with novel, cell therapy platform ▪ Lead BLA product candidate, CureXcell, is a monthly injectable therapy, with potential to transform conventional AWC category ▪ Initial focus on $5 billion DFU and VLU market opportunity in U.S. and EU ▪ Significant near - term milestones: - August 2015: VLU Futility Analysis - October 2015: DFU Phase 3 data readout - 1H 2016: VLU Phase 3 data readout ▪ Commercial - scale manufacturing and reimbursement initiatives underway supporting attractive 80%+ gross margin ▪ Proven management team experienced in developing and commercializing biologics



















