Exhibit 99.4

Transforming Immuno - Oncology Using Next - Generation Immune Cell Engagers Corporate Presentation November 2017

2 2 Forward - looking statements / safe harbor This presentation and the accompanying oral commentary contain “forward - looking” statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation and the accompanying o ral commentary, including statements regarding our future financial condition, business strategy and plans and objectives of management for future operations, are forward - looking statements. In some cases, you can identify forward - looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “might,” “app rox imately,” “expect,” “predict,” “could,” “potentially” or the negative of these terms or other similar expressions. Forward - looking stateme nts appear in a number of places throughout this presentation and the accompanying oral commentary and include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, ou r ongoing and planned preclinical development and clinical trials, our collaborations and development of our products in combin ati on with other therapies, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals f or our product candidates our intellectual property position, our collaboration activities, our ability to develop commercial functi ons , expectations regarding clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, fu ture transactions, growth and strategies, the industry in which we operate, the trends that may affect the industry or us and the ris ks uncertainties and other factors described under the heading “Risk Factors” in Affimed’s filings with the Securities and Excha nge Commission. Forward - looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. Forward - looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these forw ard - looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in th e f orward - looking statements, even if new information becomes available in the future.
3 Affimed Pioneering immune cell - based cancer immunotherapies Approach ▪ Eliminating tumor cells by engaging NK cells or T cells Pipeline ▪ Clinical and preclinical assets based on tetravalent bispecific antibody formats Leader in NK cell engagement ▪ AFM13 is the most advanced NK cell engager in clinic with solid Phase 1 data ▪ Suitable for combinations with checkpoint inhibitors (CPIs), adoptive NK cell transfer or cytokines D ifferentiated T cell - based approach ▪ Two molecules in clinical development based on AFMD platform Partnerships with industry, academia, and advocacy groups ▪ Merck (MSD), MD Anderson Cancer Center (MDACC), Leukemia & Lymphoma Society (LLS )

4 Affimed’s pipeline
5 5 Q3/17 updates (1) Corporate Update ▪ Dr. Wolfgang Fischer, former Global Head of Program and Project Management of Sandoz Biopharmaceuticals (Novartis Group) joined Affimed as COO in Sep 2017. He will also assume responsibility as interim CMO in close collaboration with Affimed’s clinical team. T cell engager programs ▪ Phase 1 dose - escalation trials of AFM11 (CD19/CD3) in ALL and in NHL • Ongoing with patients currently being recruited to the 4th (ALL) and 3rd dose cohorts (NHL), respectively ▪ Amphivena’s Phase 1 study of AMV564 (CD33/CD3) in AML ongoing

6 6 Q3/17 updates (2) NK cell engager programs ▪ AFM13 (CD30/CD16A) Phase 1b combination study with Merck’s Keytruda in r/r HL • Dose escalation completed, dose expansion cohort open and recruiting • Data from highest dose cohort of escalation phase show 3 partial metabolic responses of 3 analyzed patients at first tumor assessment • Data from the escalation phase are planned to be presented as poster at ASH 2017 ▪ AFM13 translational Phase 1b/2a study in CD30+ lymphoma (IST led by Columbia University) • Enrollment completed into first cohort, ongoing into further cohorts • Complete response of cutaneous lesions in first patient, suffering from anaplastic large - cell lymphoma (ALCL) with cutaneous manifestation, systemic evaluation ongoing ▪ AFM13 Phase 2 monotherapy in r/r HL (IST led by the German Hodgkin Study Group) • Open to recruit under new study design ▪ MD Anderson Cancer Center collaboration to evaluate AFM13 in combination with MDACC’s NK cell product • Preclinical research ongoing ▪ Preclinical programs • Novel tetravalent, bispecific antibody formats aimed at tailoring PK profiles developed in addition to TandAbs • Final candidates selected for AFM24 (EGFR/CD16A) and AFM26 (BCMA/CD16A)

7 Affimed’s tetravalent bispecific antibody formats Most competitors Bivalent, T cell focus Avidity, high specificity, tailored PK Antibody binding domains AFMD Tetravalent, NK and T cells Tumor cell T cell Tumor cell NK cell Tumor cell T cell Tumor antigen CD16A CD3 Tumor cell NK cell CD3 Tumor antigen Versatile immune cell engager formats designed for avidity, high specificity and tailored PK

8 NK cells ▪ Crucial in the body’s defense against pathogens and malignantly transformed cells ▪ There is a positive correlation between NK cell infiltration and clinical outcome in patients 1 , but the need for specific NK cell engagement remains ▪ NK cell engagers can address immune evasion, which compromises immune cell activation ▪ CD16A as an attractive target : • Key activating receptor capable of “arming” the NK cell • High affinity targeting of a specific epitope on CD16A enables activation of ADCC Targeting NK cells Therapeutic use of NK cell function is a novel and promising approach to kill tumor cells POC for NK cell - mediated tumor killing ▪ NK cells showed efficacy in Phase 1 clinical trial in r/r AML ( n=9) 2 • 4/9 patients with CR in relapsed/refractory AML ▪ Demonstrated safety and ability to induce remissions in leukemia patients 3 • NK cells critical to graft - vs. - leukemia, but no severe GvHD • Consistent observations across studies, >100 patients + Cytokines 1 Sconocchia et al, 2011; Imai et al, 2000 2 Romee et al., Sci. Transl. Med., 2016; 3 Koehl et al., Oncoimmunology, 2015; Bachanova et al., Blood, 2014; Curti et al., Blood, 2014; Rubnitz et al., J. Clin. Oncol. , 2 010; Miller et al., Blood, 2005
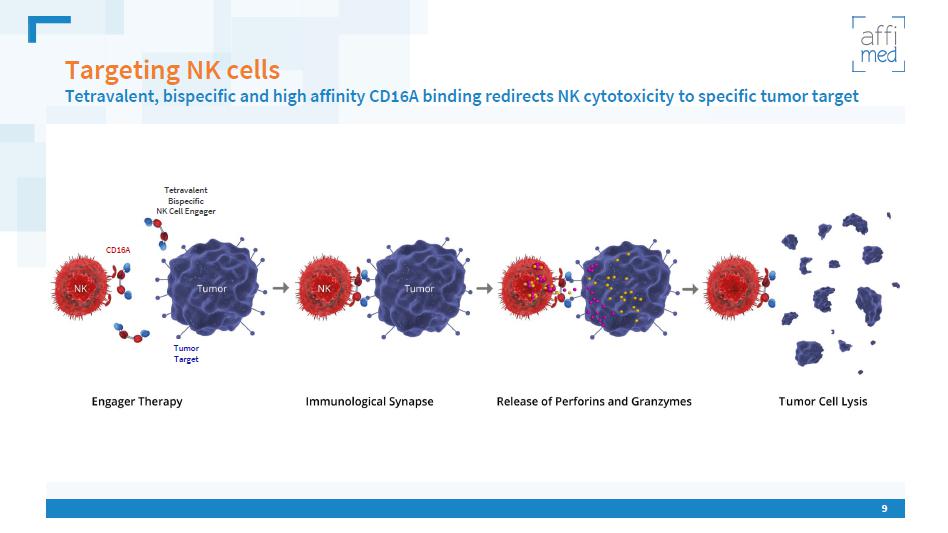
9 Targeting NK cells Tetravalent , bispecific and high affinity CD16A binding redirects NK cytotoxicity to specific tumor target Tetravalent Bispecific NK Cell Engager CD16 A Tumor Target
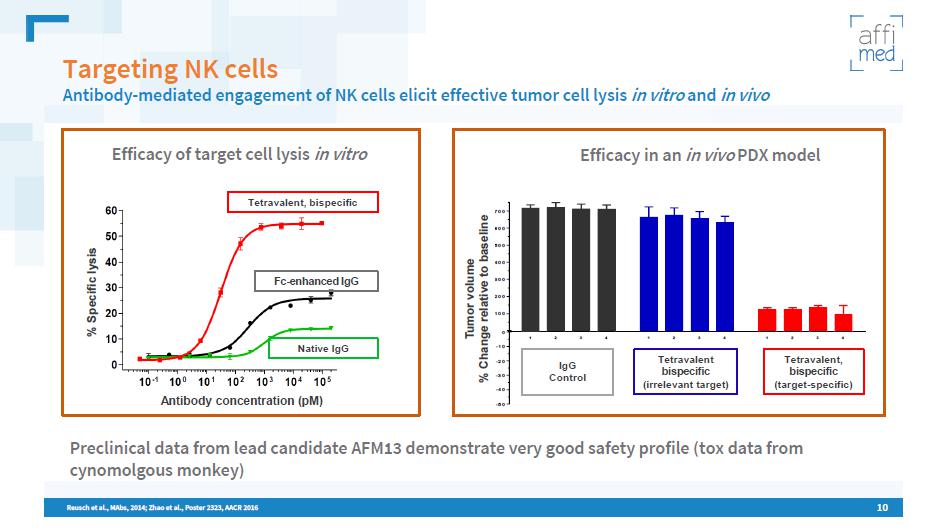
10 Targeting NK cells Antibody - mediated engagement of NK cells elicit effective tumor cell lysis in vitro and in vivo Efficacy of target cell lysis in vitro Tetravalent, bispecific Fc - enhanced IgG Native IgG Reusch et al., MAbs , 2014; Zhao et al., Poster 2323, AACR 2016 1 2 3 4 1 2 3 4 1 2 3 4 -50 -40 -30 -20 -10 0 100 200 300 400 500 600 700 Efficacy in an in vivo PDX model Tumor volume % Change relative to baseline Tetravalent, bispecific (target - specific) Tetravalent bispecific (irrelevant target) IgG Control Preclinical data from lead candidate AFM13 demonstrate very good safety profile ( tox data from cynomolgous monkey) % S pecific lysis Antibody concentration ( pM )

11 Unique target CD16A ▪ Only receptor triggering ADCC and not requiring additional co - stimulatory signals ▪ Constitutively expressed on ~95% of NK cells Tetravalent bispecific NK cells engagers ▪ Up to 1000x higher affinity for CD16A than monoclonal antibodies ▪ Binding largely unaffected by competing IgG ▪ High potency independent of whether NK cells express high or low affinity CD16A (V/F) ▪ No binding to CD16B Targeting NK cells through high affinity binding to CD16A Addressing need of targeting malignant cells that escape elimination by current therapeutics Current IgG - based approache s AFMD approach Adapted from Koch & Tesar, Transfus . Med. Chemother ., 2017

12 Targeting NK cells Tetravalent, bispecific NK cell engagers can kill cells with very low target expression Target expression as low as a few thousand receptors per cell (CD30) In vitro potency of AFM13; EC50 [ pM ] a CD30 Target expression as low as a hundred receptors per cell (HLA - A2/MMP1 003 ) Lead Candidate cell line HLA - A2 MMP1 Mean EC 50 [ pM ] B - CPAP + + 1690 KMS - 27 + - no JVM - 2 + - no ES - 2 - + no BXPC - 3 - + no MM.1S - - no KARPAS - 299 - - no NK cell

13 13 Phase 1: Safety and clinical activity demonstrated in heavily pre - treated HL patients ▪ Favorable safety profile determined ▪ Tumor shrinkage in 62 % ( 8/13) and PRs in 23% (3/13) of patients at doses of at least 1.5 mg/kg Phase 2a monotherapy in r/r HL (IST by GHSG, ongoing) confirms single agent activity ▪ Evidence of AFM13 single agent activity in patients which failed standard treatments including B.V. and were anti - PD1 naïve (2/7 evaluable patients with PR) Phase 1b in r/r HL in combination with Merck’s Keytruda® (ongoing) with first data readout ▪ Dose escalation (3 cohorts) completed; dose expansion cohort initiated at highest dose ▪ Data analysis of 3 months - response rates is ongoing with 9/12 patients completed: • Cohort 1: 2 metabolic PRs, 1 PD (3/3 analyzed) • Cohort 2: 1 metabolic CR, 1 metabolic PR, 1 PD (3/3 analyzed ) • Cohort 3: 3 metabolic PRs (3/6 analyzed ) • Presentation of dose escalation data planned for ASH 2017 Annual Meeting in December AFM13 (CD30/CD16A ) (1) Clinical activity observed in mono - and combination - therapeutic setting

14 AFM13 (CD30/CD16A ) (2) Clinical activity observed in mono - and combination - therapeutic setting » First evidence that NK cell engagers are able to induce tumor regression in this indication Phase 1b/2a study in r/r CD30+ lymphoma (IST by Columbia University, ongoing) with first efficacy data ▪ Translational study in patients with cutaneous manifestation enabling serial biopsies ▪ First cohort fully enrolled (1/3 analyzed), recruitment into further cohorts ongoing ▪ Patient suffering from anaplastic large - cell lymphoma (ALCL) with cutaneous manifestation ▪ Complete response of cutaneous lesions (systemic evaluation ongoing ) Baseline Day 3 Day 21
15 MDACC Collaboration Combination of NK cell - engaging bispecifics with adoptive NK cell transfer Development collaboration ▪ Investigation of Affimed’s NK cell engagers in combination with MDACC’s cord blood - derived NK cells ▪ Initially focused on AFM13 in HL ▪ Approach independent of a patient‘s endogenous NK cell count with potential applicability at the time of/shortly after ASCT ▪ May pave way for combinations in further indications, e.g. multiple myeloma

16 16 AFM24 Targeting EGFR: Development of NK cell engagers to treat solid tumors Medical need for a novel approach to treat EGFR+ solid tumors ▪ Widen therapeutic window and address resistant patient population Targeting EGFR ▪ EGFR as validated target in solid tumors, however side effects negatively impact market potential ( skin toxicity ) ▪ Receptor blocking (e.g. cetuximab) cannot address activating mutations (K - RAS) AFM24 ▪ Differentiated MOA (NK cell activation vs. solely receptor inhibition): • Increased potency compared to cetuximab enabling NK cell - mediated killing of EGFR low cells • Broad target population including patients resistant to standard of care such as mAbs (e.g. cetuximab) ▪ Safety : • First evidence of beneficial profile in single and repeated - dose toxicity pilot studies in cynomolgus monkey (TandAb format )

17 AFM24 Efficacy against Ras - mutated, cetuximab - resistant HCT - 116 cells in a humanized mouse model Tumor growth (T/C ratios) Time ( days ) AFM24_001 cetuximab w/o antibody antibody concentration [pM] % s p e c i f i c l y s i s 10 -2 10 -1 10 0 10 1 10 2 10 3 10 4 10 5 0 20 40 60 80 100 120 w/o AFM24 w/o antibody cetuximab HCT-116 % S pecific lysis Antibody concentration ( pM ) AFM24_001 cetuximab w/o antibody Efficacy in vitro Efficacy in vivo
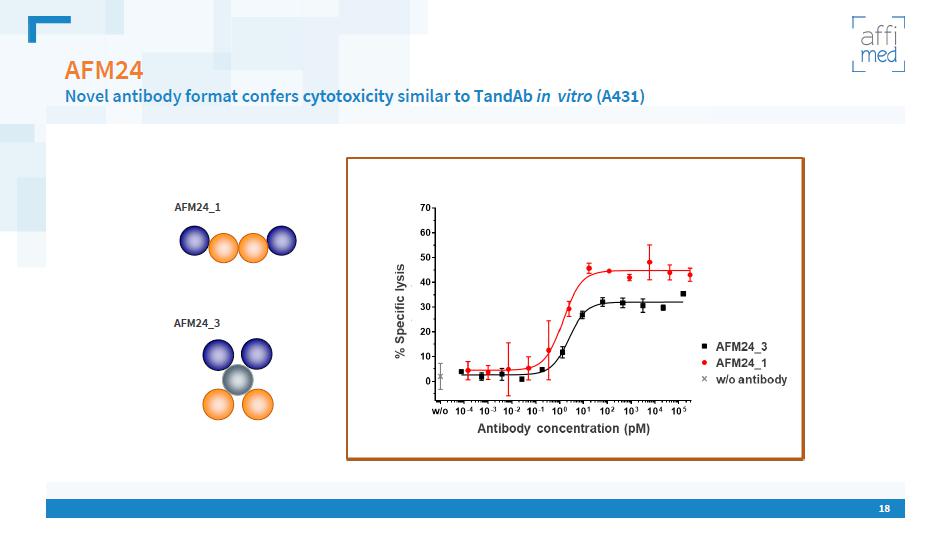
18 AFM24 Novel antibody format confers cytotoxicity similar to TandAb in vitro (A431) AFM24_1 AFM24_3
19 19 AFM26 Leveraging BCMA as target in autologous stem cell transplant (ASCT) - eligible patients Medical need for a novel approach to treat multiple myeloma ▪ Treatment at or shortly after ASCT to eliminate minimal residual disease (MRD ), avoiding relapse Targeting BCMA ▪ BCMA is a highly promising target for therapeutic intervention based on early clinical data (CAR - T and ADCs) ▪ Low expression of BCMA is a significant hurdle to eliminate malignant cells ▪ NKs are the first population of lymphocytes to recover post transplant; opportunity to exploit AFM26 in ASCT setting ▪ Opportunity for combination of AFM26 with adoptive NK cell transfer AFM26 ▪ Differentiated MOA: High affinity engagement of NK cells • Distinguished from other approaches (e.g. daratumumab , elotuzumab ) • Allows targeting cells expressing very low levels of BCMA ▪ Safety : Lower cytokine release vs BiTE ▪ Convenience: Potential benefit as novel format is designed to confer significantly better PK profile compared to TandAb

20 AFM26 Killing of tumor cells expressing only a few hundred receptors per cell (BCMA ) Cell line a BCMA NCI - H929 51480 RPMI - 8226 14700 MM.1S 13180 HuNS1 1440 ARH - 77 1180 MC/CAR 450 0 5 10 15 20 25 B C M A M F I N C I - H 9 2 9 R P M I - 8 2 2 6 M M . 1 S H u N S 1 A R H 7 7 M C / C A R N A L M - 6 -50 0 50 100 150 200 E C 5 0 [ p M ] n/a BCMA(+) BCMA(-)

21 Q3 2017 Cash flow statement ▪ Raised € 16.4m net proceeds in follow - on financing in Q1/2017 and from second loan tranche of € 2.5m in Q2/2017 ▪ Runway at least until YE/2018 * short - term deposits In thousands of € 9M 2017 Cash and cash equivalents and financial assets* end of period 41,813 Cash and cash equivalents and financial assets* beginning of period 44,894 Cash and Cash equivalents at the beginning of the period 35,407 FX related changes to Cash and Cash equivalents (1,366) Net cash used in operating activities (20,679) Cash Flow from investing activities (225) Cash Flow from financing activities 20,206 Cash and Cash equivalents at the end of the period 33,343

22 22 Path forward Maximize value from pipeline and technologies Expand NK cell engagement leadership • Develop AFM13 (CD30/CD16A) in combination with Keytruda® and as monotherapy in r/r HL and in CD30+ lymphoma • Advance AFM24 (EGFRwt/CD16A) in solid tumors (incl. lung, head and neck, and colon cancer) • Advance AFM26 (BCMA/CD16A) in multiple myeloma • Continue to explore NK cell engager combination potential with CPIs, adoptive NK cell therapy (MDACC) or immune activating agents Focus on DLBCL, MCL and ALL in T cell engagement ▪ Generate POC for T cell engagers with AFM11 (CD19/CD3) ▪ Additional POC through AMV564 (CD33/CD3) in AML Expand platforms (multiple formats, targeting MHC - peptide complexes) Use pipeline and technologies to create value through both next - generation products and partnership opportunities























