
Corporate Overview March 16, 2017 Exhibit 99.2

Forward looking statements Some of the statements in this presentation constitute “forward looking statements” under the Private Securities Litigation Reform Act of 1995. Such statements are subject to factors, risks and uncertainties (such as those detailed in the Company’s periodic filings with the SEC) that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any forward looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so.
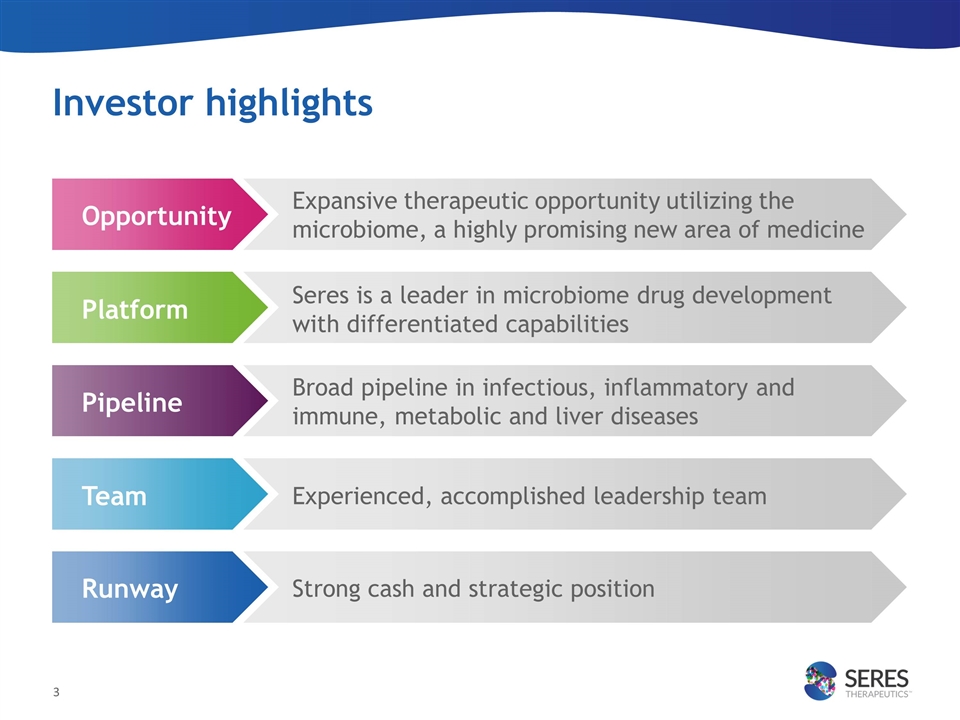
Investor highlights Expansive therapeutic opportunity utilizing the microbiome, a highly promising new area of medicine Seres is a leader in microbiome drug development with differentiated capabilities Broad pipeline in infectious, inflammatory and immune, metabolic and liver diseases Experienced, accomplished leadership team Strong cash and strategic position Platform Opportunity Pipeline Team Runway
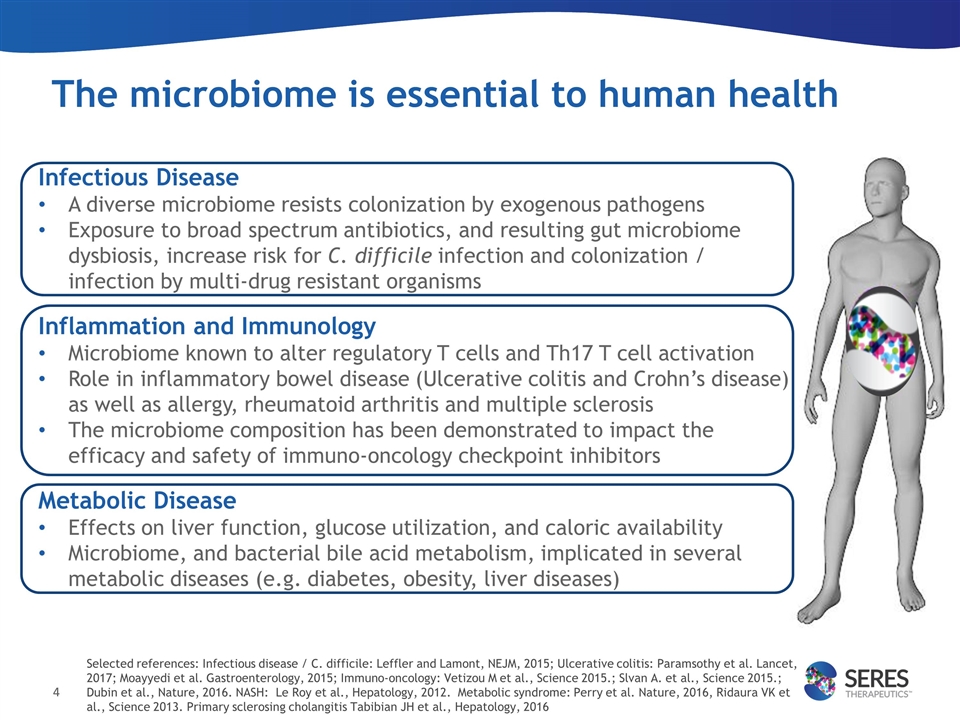
The microbiome is essential to human health Infectious Disease A diverse microbiome resists colonization by exogenous pathogens Exposure to broad spectrum antibiotics, and resulting gut microbiome dysbiosis, increase risk for C. difficile infection and colonization / infection by multi-drug resistant organisms Inflammation and Immunology Microbiome known to alter regulatory T cells and Th17 T cell activation Role in inflammatory bowel disease (Ulcerative colitis and Crohn’s disease) as well as allergy, rheumatoid arthritis and multiple sclerosis The microbiome composition has been demonstrated to impact the efficacy and safety of immuno-oncology checkpoint inhibitors Metabolic Disease Effects on liver function, glucose utilization, and caloric availability Microbiome, and bacterial bile acid metabolism, implicated in several metabolic diseases (e.g. diabetes, obesity, liver diseases) Selected references: Infectious disease / C. difficile: Leffler and Lamont, NEJM, 2015; Ulcerative colitis: Paramsothy et al. Lancet, 2017; Moayyedi et al. Gastroenterology, 2015; Immuno-oncology: Vetizou M et al., Science 2015.; Slvan A. et al., Science 2015.; Dubin et al., Nature, 2016. NASH: Le Roy et al., Hepatology, 2012. Metabolic syndrome: Perry et al. Nature, 2016, Ridaura VK et al., Science 2013. Primary sclerosing cholangitis Tabibian JH et al., Hepatology, 2016
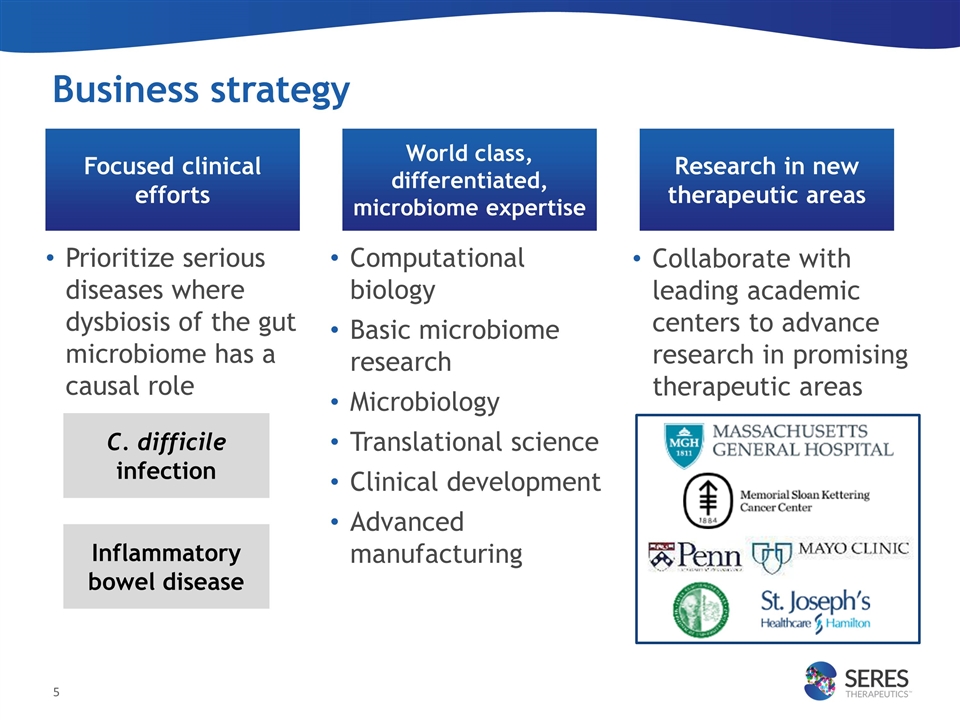
Prioritize serious diseases where dysbiosis of the gut microbiome has a causal role Focused clinical efforts Computational biology Basic microbiome research Microbiology Translational science Clinical development Advanced manufacturing World class, differentiated, microbiome expertise Collaborate with leading academic centers to advance research in promising therapeutic areas Research in new therapeutic areas Business strategy Inflammatory bowel disease C. difficile infection
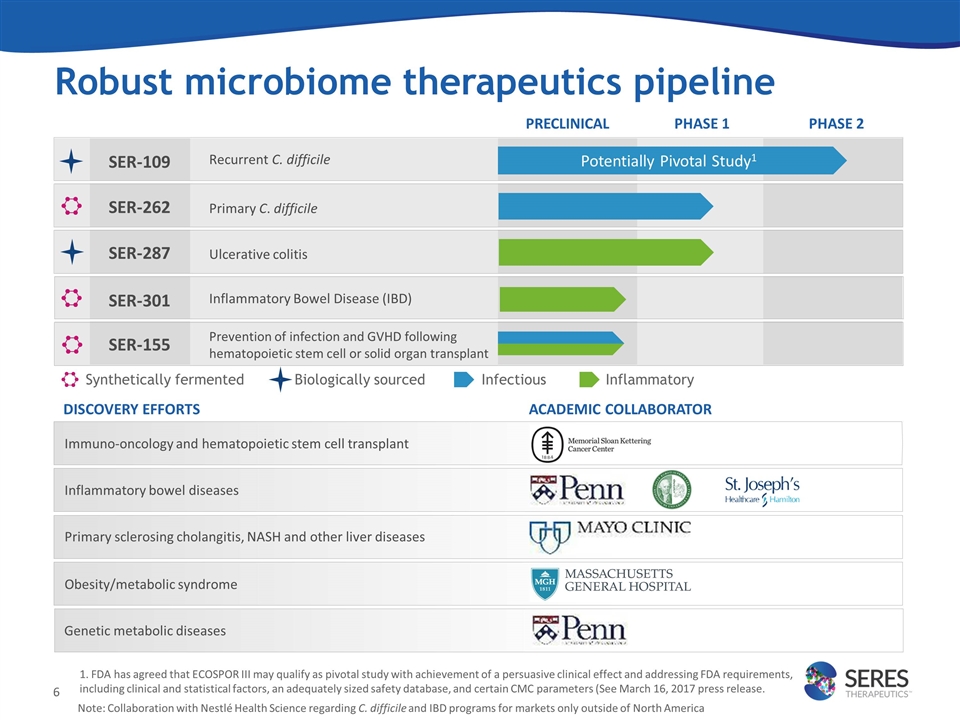
Robust microbiome therapeutics pipeline SER-109 DISCOVERY EFFORTS PRECLINICAL PHASE 1 PHASE 2 SER-262 SER-287 Inflammatory bowel diseases Primary sclerosing cholangitis, NASH and other liver diseases Immuno-oncology and hematopoietic stem cell transplant SER-301 ACADEMIC COLLABORATOR SER-155 Potentially Pivotal Study1 Recurrent C. difficile Primary C. difficile Ulcerative colitis Inflammatory Bowel Disease (IBD) Prevention of infection and GVHD following hematopoietic stem cell or solid organ transplant Synthetically fermented Infectious Inflammatory Note: Collaboration with Nestlé Health Science regarding C. difficile and IBD programs for markets only outside of North America Obesity/metabolic syndrome Biologically sourced Genetic metabolic diseases 1. FDA has agreed that ECOSPOR III may qualify as pivotal study with achievement of a persuasive clinical effect and addressing FDA requirements, including clinical and statistical factors, an adequately sized safety database, and certain CMC parameters (See March 16, 2017 press release.
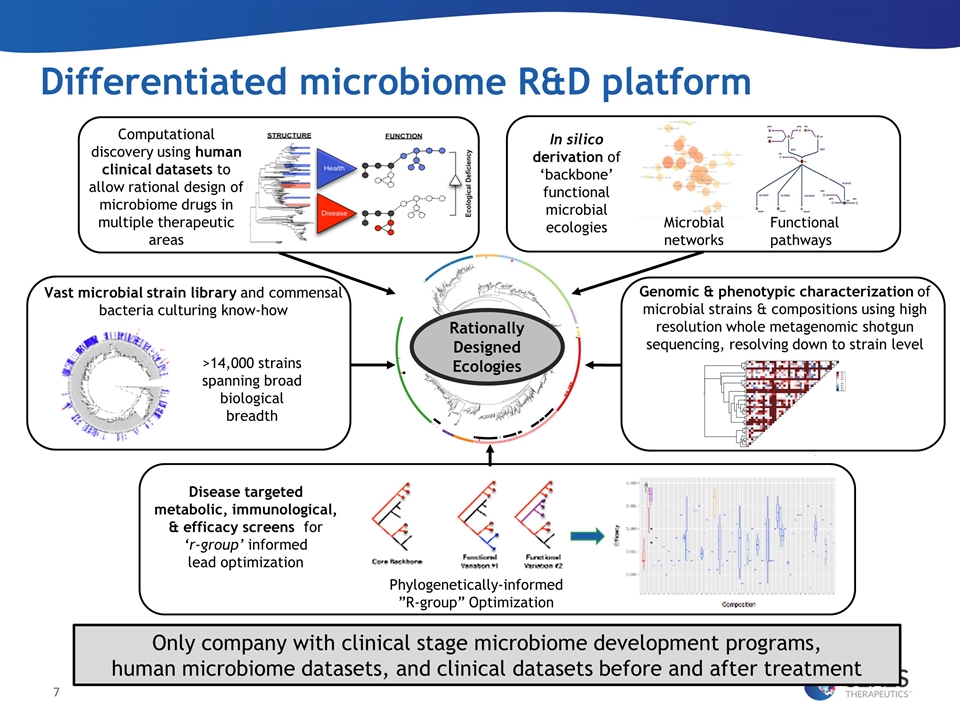
Differentiated microbiome R&D platform Computational discovery using human clinical datasets to allow rational design of microbiome drugs in multiple therapeutic areas Vast microbial strain library and commensal bacteria culturing know-how Disease targeted metabolic, immunological, & efficacy screens for ‘r-group’ informed lead optimization Rationally Designed Ecologies Functional pathways Microbial networks In silico derivation of ‘backbone’ functional microbial ecologies Genomic & phenotypic characterization of microbial strains & compositions using high resolution whole metagenomic shotgun sequencing, resolving down to strain level >14,000 strains spanning broad biological breadth Compositional Variants Efficacy Metric Phylogenetically-informed ”R-group” Optimization Only company with clinical stage microbiome development programs, human microbiome datasets, and clinical datasets before and after treatment
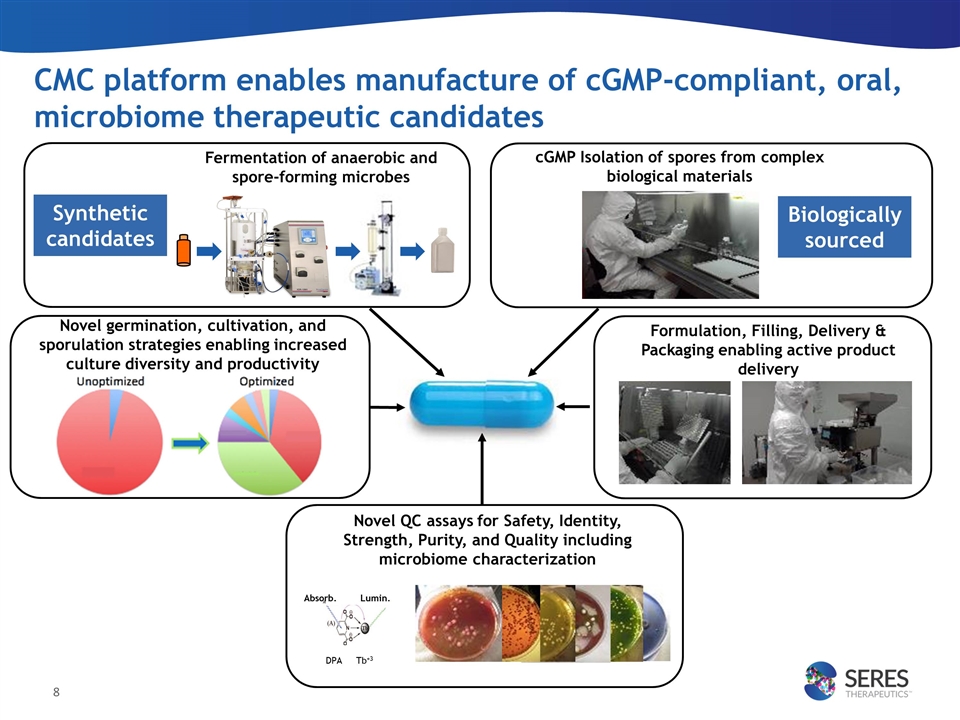
CMC platform enables manufacture of cGMP-compliant, oral, microbiome therapeutic candidates Fermentation of anaerobic and spore-forming microbes Formulation, Filling, Delivery & Packaging enabling active product delivery cGMP Isolation of spores from complex biological materials Synthetic candidates Biologically sourced Novel QC assays for Safety, Identity, Strength, Purity, and Quality including microbiome characterization Absorb. Lumin. DPA Tb+3 Novel germination, cultivation, and sporulation strategies enabling increased culture diversity and productivity aa

Clostridium difficile Infection Overview and R&D Programs Leading the Microbiome Revolution
C. difficile infection overview Infectious disease caused by toxin producing anaerobic, spore-forming bacteria, resulting in diarrhea, abdominal pain, fever, and nausea Leading cause of hospital-acquired infection in the US; approximately 29,000 deaths/year Infection caused by two-hit process: Disruption of gut microbiome and exposure to pathogenic spores ~25% of patients with primary CDI recur Risk of relapse increases with each recurrence Leffler and Lamont, New England Journal of Medicine, 2015
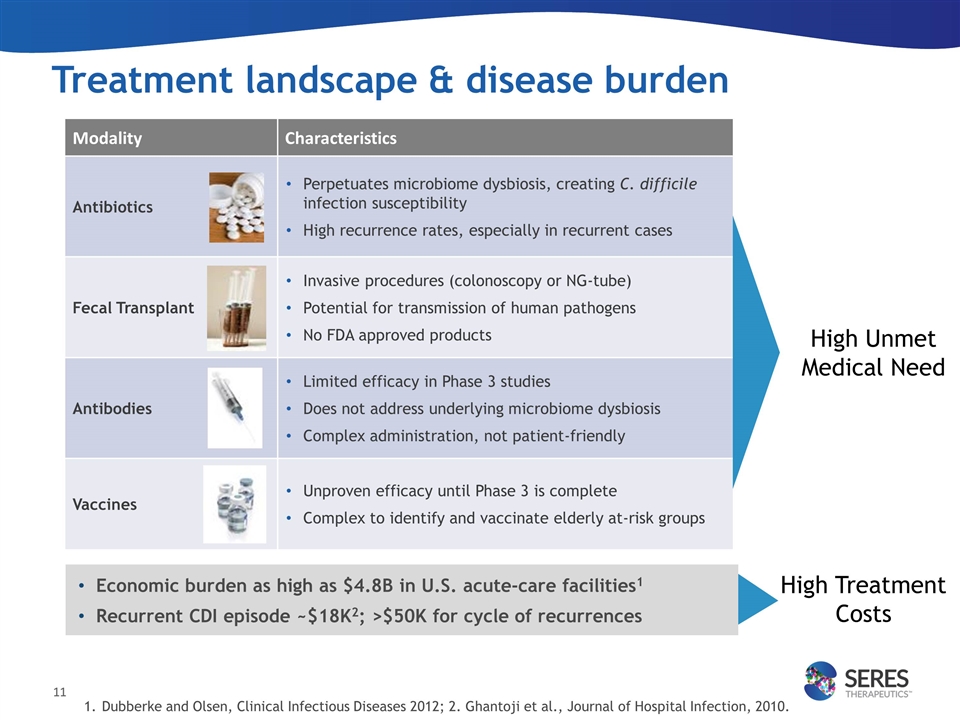
Treatment landscape & disease burden Economic burden as high as $4.8B in U.S. acute-care facilities1 Recurrent CDI episode ~$18K2; >$50K for cycle of recurrences Modality Characteristics Antibiotics Perpetuates microbiome dysbiosis, creating C. difficile infection susceptibility High recurrence rates, especially in recurrent cases Fecal Transplant Invasive procedures (colonoscopy or NG-tube) Potential for transmission of human pathogens No FDA approved products Antibodies Limited efficacy in Phase 3 studies Does not address underlying microbiome dysbiosis Complex administration, not patient-friendly Vaccines Unproven efficacy until Phase 3 is complete Complex to identify and vaccinate elderly at-risk groups High Unmet Medical Need Dubberke and Olsen, Clinical Infectious Diseases 2012; 2. Ghantoji et al., Journal of Hospital Infection, 2010. High Treatment Costs
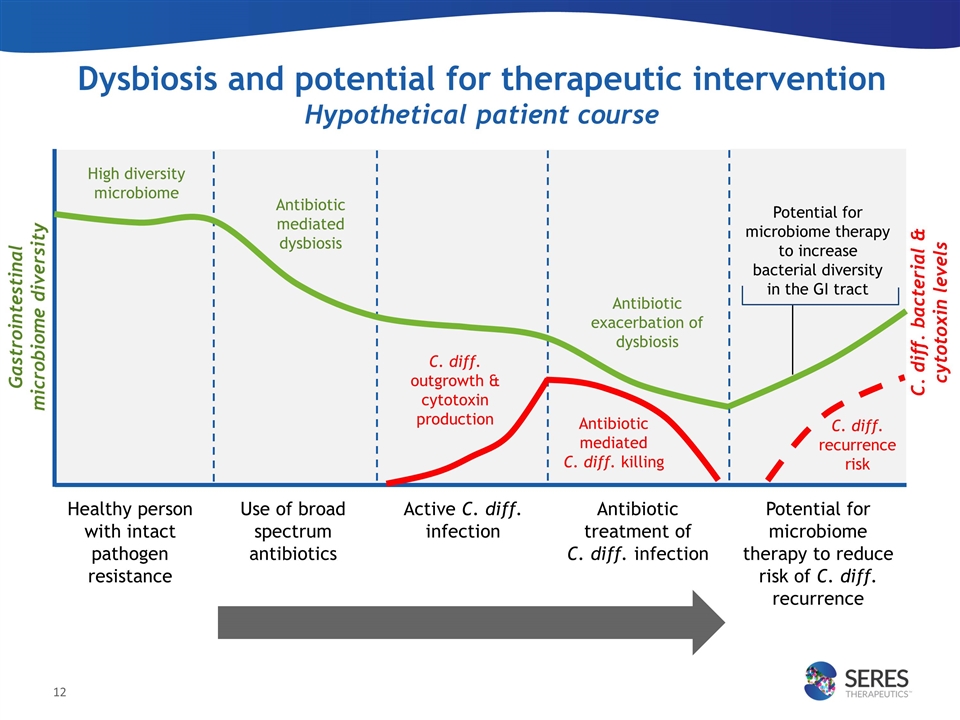
Dysbiosis and potential for therapeutic intervention Hypothetical patient course Healthy person with intact pathogen resistance High diversity microbiome Use of broad spectrum antibiotics Active C. diff. infection Antibiotic treatment of C. diff. infection Potential for microbiome therapy to reduce risk of C. diff. recurrence Antibiotic mediated C. diff. killing Antibiotic exacerbation of dysbiosis Gastrointestinal microbiome diversity C. diff. bacterial & cytotoxin levels C. diff. recurrence risk C. diff. outgrowth & cytotoxin production Potential for microbiome therapy to increase bacterial diversity in the GI tract Antibiotic mediated dysbiosis
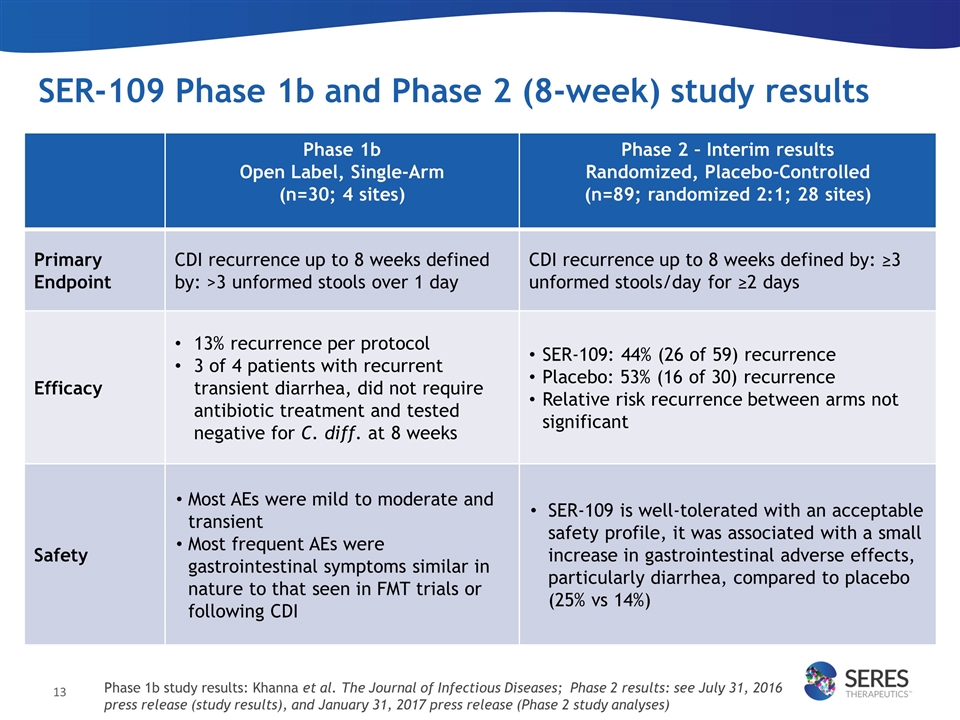
SER-109 Phase 1b and Phase 2 (8-week) study results n=59 n=30 n=28 n=15 n=31 n=15 Phase 1b Open Label, Single-Arm (n=30; 4 sites) Phase 2 – Interim results Randomized, Placebo-Controlled (n=89; randomized 2:1; 28 sites) Primary Endpoint CDI recurrence up to 8 weeks defined by: >3 unformed stools over 1 day CDI recurrence up to 8 weeks defined by: ≥3 unformed stools/day for ≥2 days Efficacy 13% recurrence per protocol 3 of 4 patients with recurrent transient diarrhea, did not require antibiotic treatment and tested negative for C. diff. at 8 weeks SER-109: 44% (26 of 59) recurrence Placebo: 53% (16 of 30) recurrence Relative risk recurrence between arms not significant Safety Most AEs were mild to moderate and transient Most frequent AEs were gastrointestinal symptoms similar in nature to that seen in FMT trials or following CDI SER-109 is well-tolerated with an acceptable safety profile, it was associated with a small increase in gastrointestinal adverse effects, particularly diarrhea, compared to placebo (25% vs 14%) Phase 1b study results: Khanna et al. The Journal of Infectious Diseases; Phase 2 results: see July 31, 2016 press release (study results), and January 31, 2017 press release (Phase 2 study analyses)
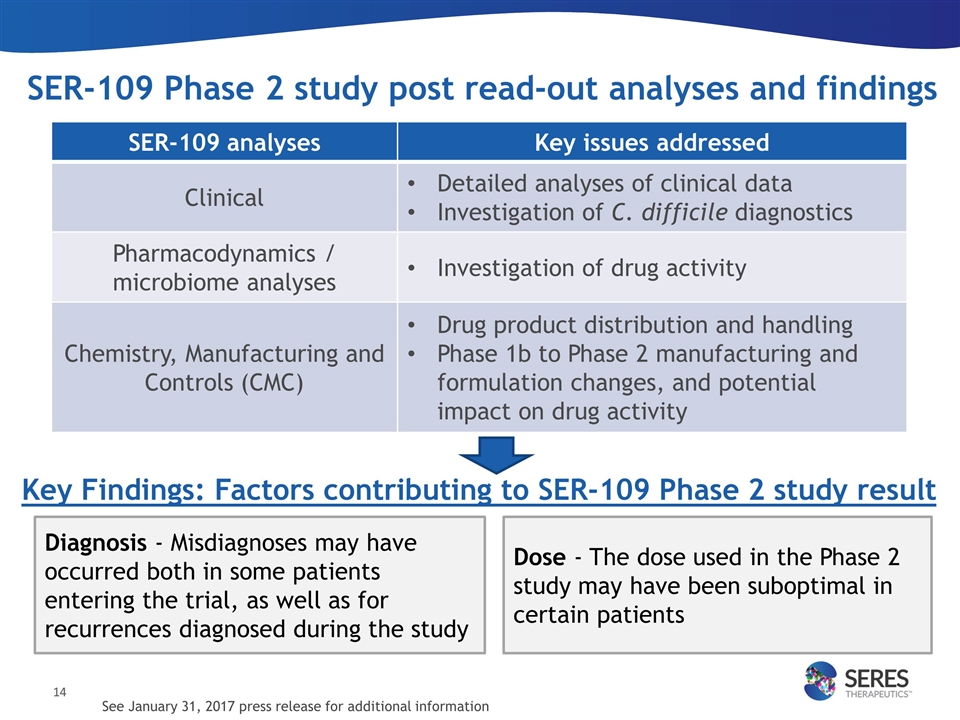
SER-109 Phase 2 study post read-out analyses and findings SER-109 analyses Key issues addressed Clinical Detailed analyses of clinical data Investigation of C. difficile diagnostics Pharmacodynamics / microbiome analyses Investigation of drug activity Chemistry, Manufacturing and Controls (CMC) Drug product distribution and handling Phase 1b to Phase 2 manufacturing and formulation changes, and potential impact on drug activity Diagnosis - Misdiagnoses may have occurred both in some patients entering the trial, as well as for recurrences diagnosed during the study Key Findings: Factors contributing to SER-109 Phase 2 study result Dose - The dose used in the Phase 2 study may have been suboptimal in certain patients See January 31, 2017 press release for additional information
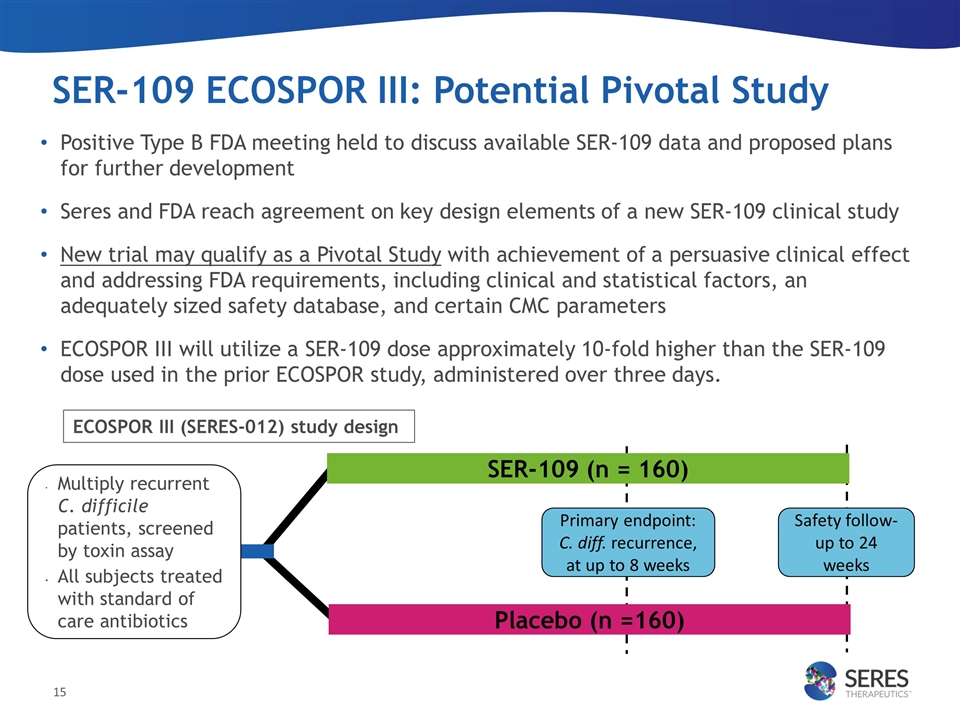
SER-109 ECOSPOR III: Potential Pivotal Study Positive Type B FDA meeting held to discuss available SER-109 data and proposed plans for further development Seres and FDA reach agreement on key design elements of a new SER-109 clinical study New trial may qualify as a Pivotal Study with achievement of a persuasive clinical effect and addressing FDA requirements, including clinical and statistical factors, an adequately sized safety database, and certain CMC parameters ECOSPOR III will utilize a SER-109 dose approximately 10-fold higher than the SER-109 dose used in the prior ECOSPOR study, administered over three days. Multiply recurrent C. difficile patients, screened by toxin assay All subjects treated with standard of care antibiotics Primary endpoint: C. diff. recurrence, at up to 8 weeks Safety follow-up to 24 weeks ECOSPOR III (SERES-012) study design SER-109 (n = 160) Placebo (n =160)
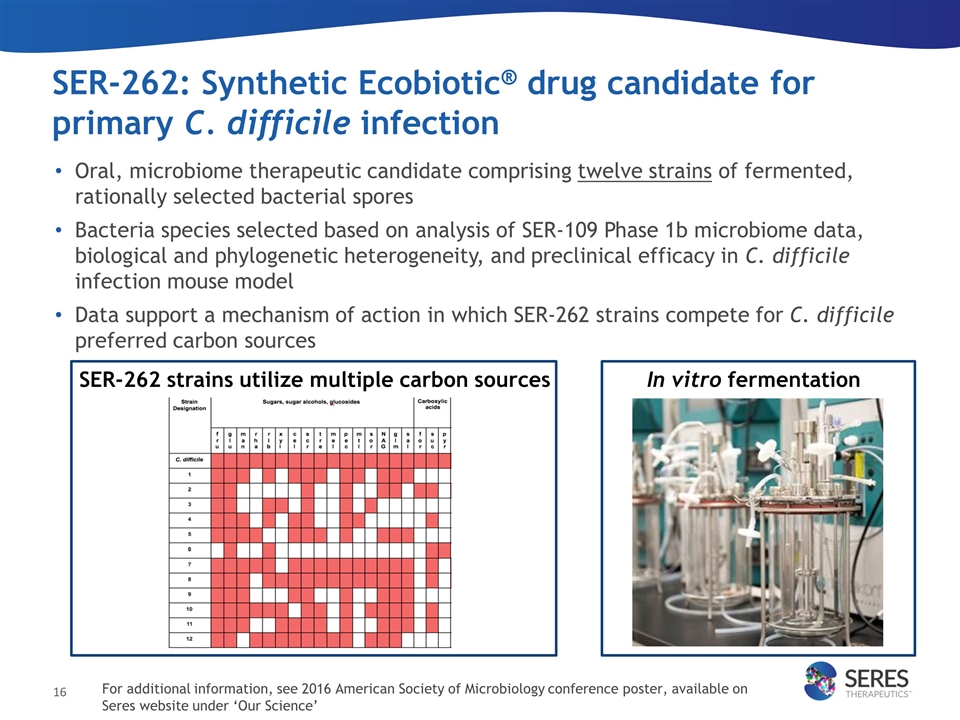
SER-262: Synthetic Ecobiotic® drug candidate for primary C. difficile infection SER-262 strains utilize multiple carbon sources In vitro fermentation Oral, microbiome therapeutic candidate comprising twelve strains of fermented, rationally selected bacterial spores Bacteria species selected based on analysis of SER-109 Phase 1b microbiome data, biological and phylogenetic heterogeneity, and preclinical efficacy in C. difficile infection mouse model Data support a mechanism of action in which SER-262 strains compete for C. difficile preferred carbon sources For additional information, see 2016 American Society of Microbiology conference poster, available on Seres website under ‘Our Science’
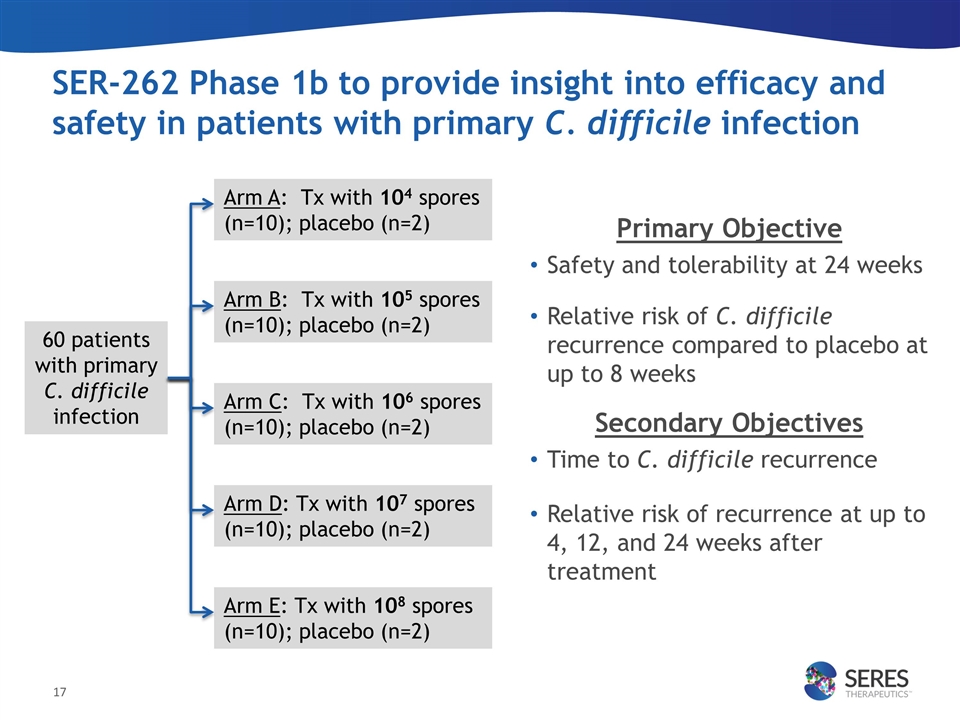
SER-262 Phase 1b to provide insight into efficacy and safety in patients with primary C. difficile infection Primary Objective Safety and tolerability at 24 weeks Relative risk of C. difficile recurrence compared to placebo at up to 8 weeks Secondary Objectives Time to C. difficile recurrence Relative risk of recurrence at up to 4, 12, and 24 weeks after treatment Arm A: Tx with 104 spores (n=10); placebo (n=2) Arm B: Tx with 105 spores (n=10); placebo (n=2) Arm C: Tx with 106 spores (n=10); placebo (n=2) Arm D: Tx with 107 spores (n=10); placebo (n=2) 60 patients with primary C. difficile infection Arm E: Tx with 108 spores (n=10); placebo (n=2)

Inflammatory Bowel Disease Overview and R&D Programs Leading the Microbiome Revolution
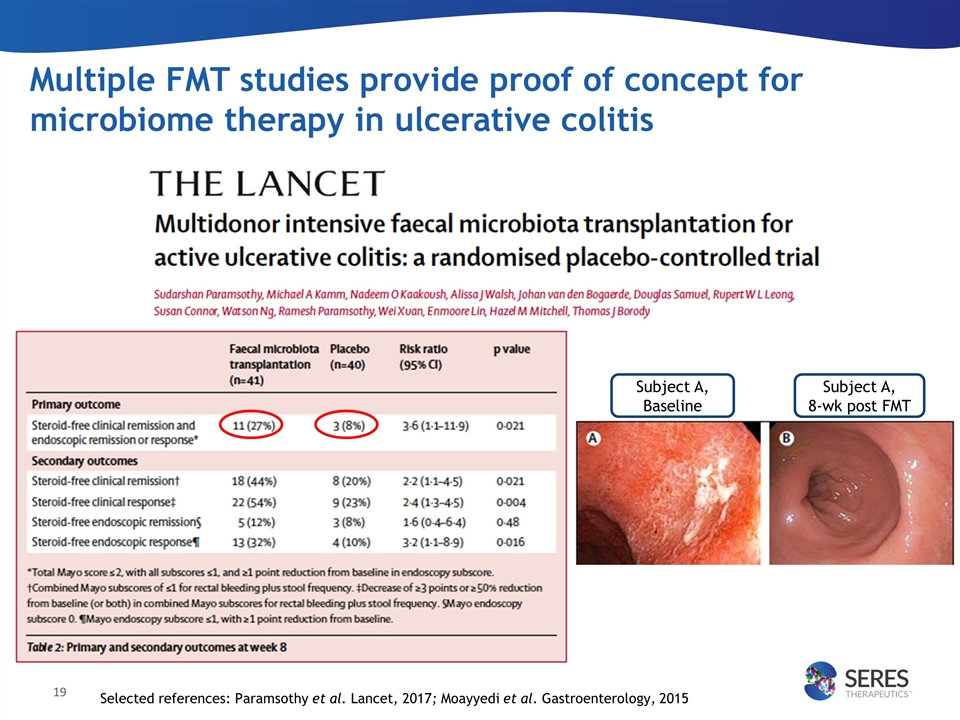
Selected references: Paramsothy et al. Lancet, 2017; Moayyedi et al. Gastroenterology, 2015 Multiple FMT studies provide proof of concept for microbiome therapy in ulcerative colitis Subject A, Baseline Subject A, 8-wk post FMT

SER-287 Inflammatory Bowel Disease (IBD) opportunity Significant unmet need for improved therapies for IBD Large US population: ~700K ulcerative colitis, ~700K Crohn’s Only~30% of patients respond to currently approved therapies Many therapies are immunosuppressive, limiting widespread use SER-287 target profile: Oral Alternative mechanistic approach, potential mono or combo therapy Not expected to be immunosuppressive SER-287 development opportunity: Initial development as induction therapy for ulcerative colitis Potential development as UC maintenance therapy, Crohn’s disease
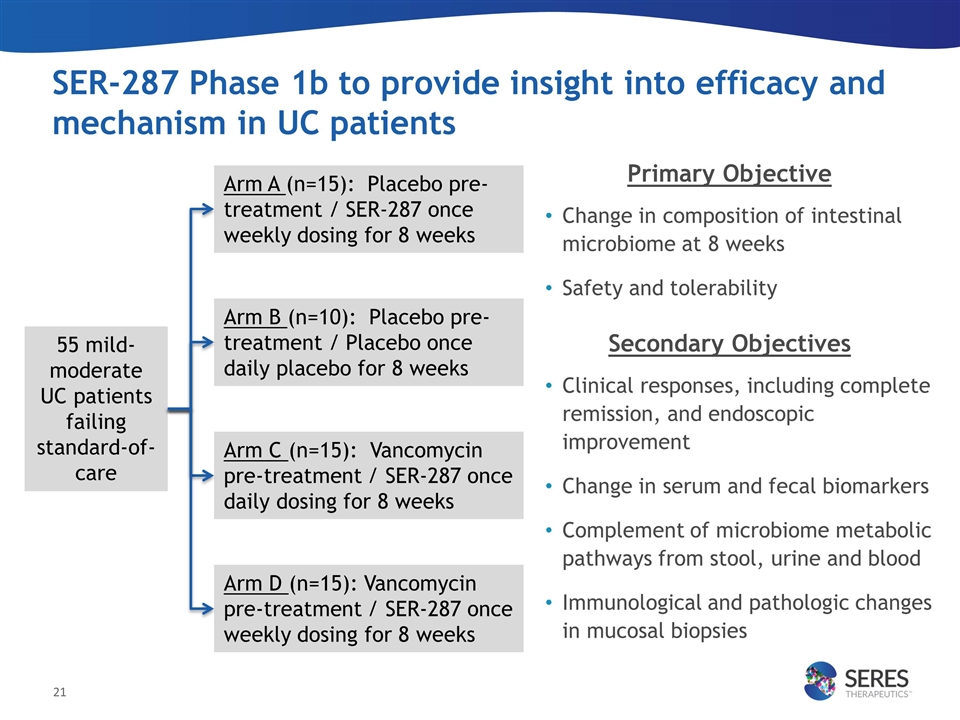
SER-287 Phase 1b to provide insight into efficacy and mechanism in UC patients Primary Objective Change in composition of intestinal microbiome at 8 weeks Safety and tolerability Secondary Objectives Clinical responses, including complete remission, and endoscopic improvement Change in serum and fecal biomarkers Complement of microbiome metabolic pathways from stool, urine and blood Immunological and pathologic changes in mucosal biopsies Arm A (n=15): Placebo pre-treatment / SER-287 once weekly dosing for 8 weeks Arm B (n=10): Placebo pre-treatment / Placebo once daily placebo for 8 weeks Arm C (n=15): Vancomycin pre-treatment / SER-287 once daily dosing for 8 weeks Arm D (n=15): Vancomycin pre-treatment / SER-287 once weekly dosing for 8 weeks 55 mild-moderate UC patients failing standard-of-care
SER-301: Synthetic Ecobiotic® therapeutic candidate for inflammatory bowel disease Follow-on therapeutic candidate to SER-287 in preclinical development for inflammatory bowel disease Oral, microbiome therapeutic candidate comprising fermented, rationally selected bacteria Selection of SER-301 bacterial composition to be based on: SER-287 study data (clinical and microbiome analysis) Existing collaborations evaluating analysis of FMT ulcerative colitis clinical study data Preclinical screening for microbial function, immunological assay, and animal models

Additional R&D Opportunities Leading the Microbiome Revolution
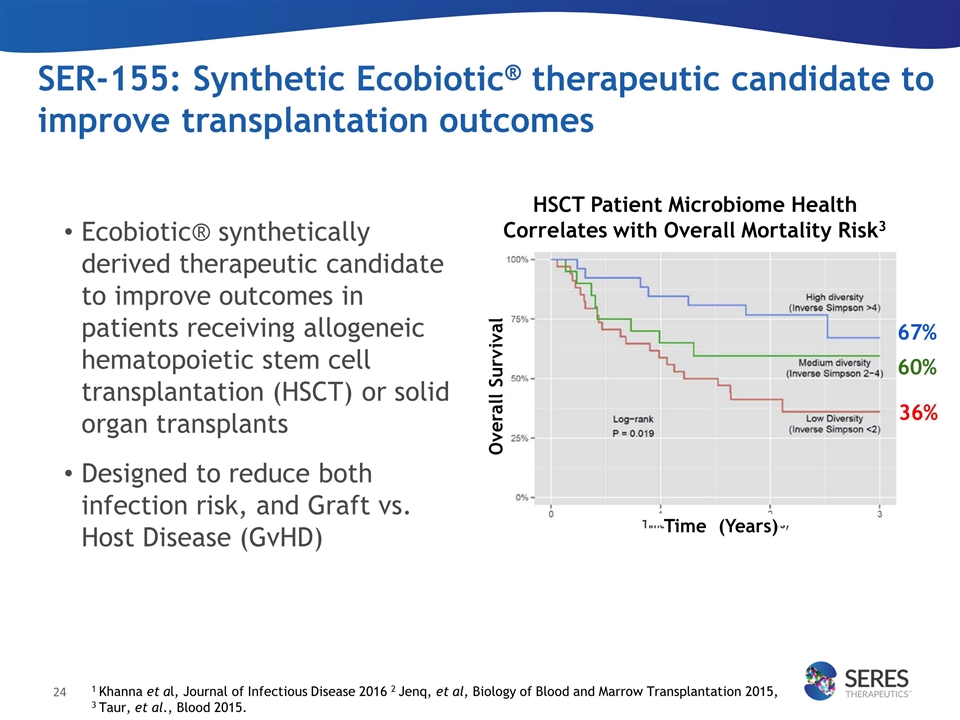
SER-155: Synthetic Ecobiotic® therapeutic candidate to improve transplantation outcomes 1 Khanna et al, Journal of Infectious Disease 2016 2 Jenq, et al, Biology of Blood and Marrow Transplantation 2015, 3 Taur, et al., Blood 2015. 36% 60% 67% Overall Survival HSCT Patient Microbiome Health Correlates with Overall Mortality Risk3 Time (Years) Ecobiotic® synthetically derived therapeutic candidate to improve outcomes in patients receiving allogeneic hematopoietic stem cell transplantation (HSCT) or solid organ transplants Designed to reduce both infection risk, and Graft vs. Host Disease (GvHD)
Immuno-oncology microbiome therapeutic opportunity Therapeutic Objectives To improve efficacy: Modulate immune response, improve clinical response to therapeutic checkpoint inhibitors To improve safety: Reduce anti-CTLA4 induced colitis by providing microbial ecologies correlated with improved patient outcomes
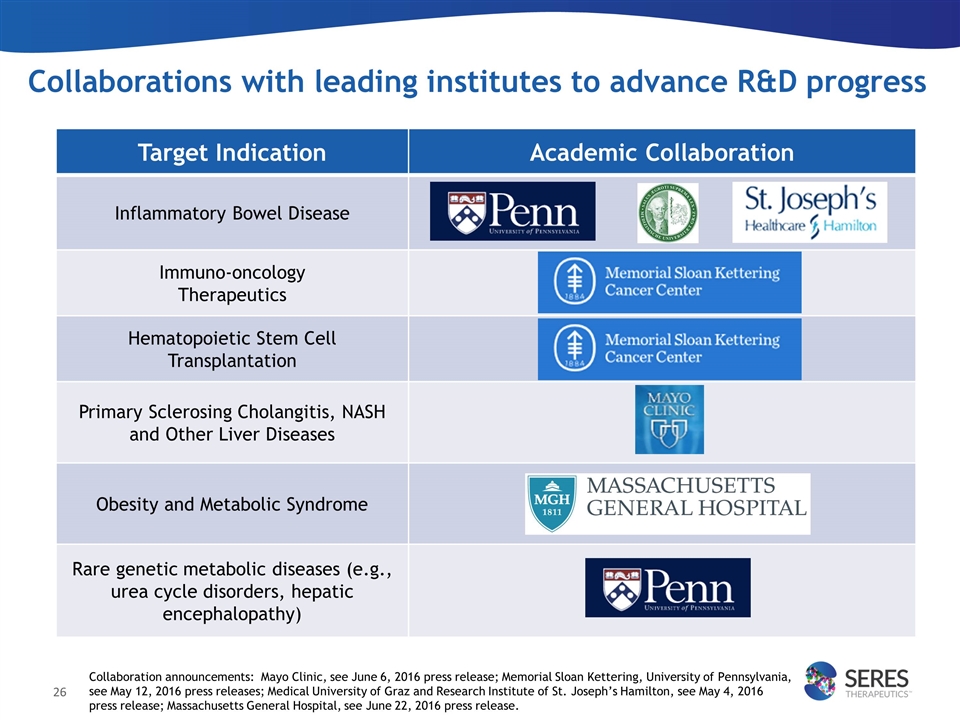
Collaborations with leading institutes to advance R&D progress Target Indication Academic Collaboration Inflammatory Bowel Disease Immuno-oncology Therapeutics Hematopoietic Stem Cell Transplantation Primary Sclerosing Cholangitis, NASH and Other Liver Diseases Obesity and Metabolic Syndrome Rare genetic metabolic diseases (e.g., urea cycle disorders, hepatic encephalopathy) Collaboration announcements: Mayo Clinic, see June 6, 2016 press release; Memorial Sloan Kettering, University of Pennsylvania, see May 12, 2016 press releases; Medical University of Graz and Research Institute of St. Joseph’s Hamilton, see May 4, 2016 press release; Massachusetts General Hospital, see June 22, 2016 press release.
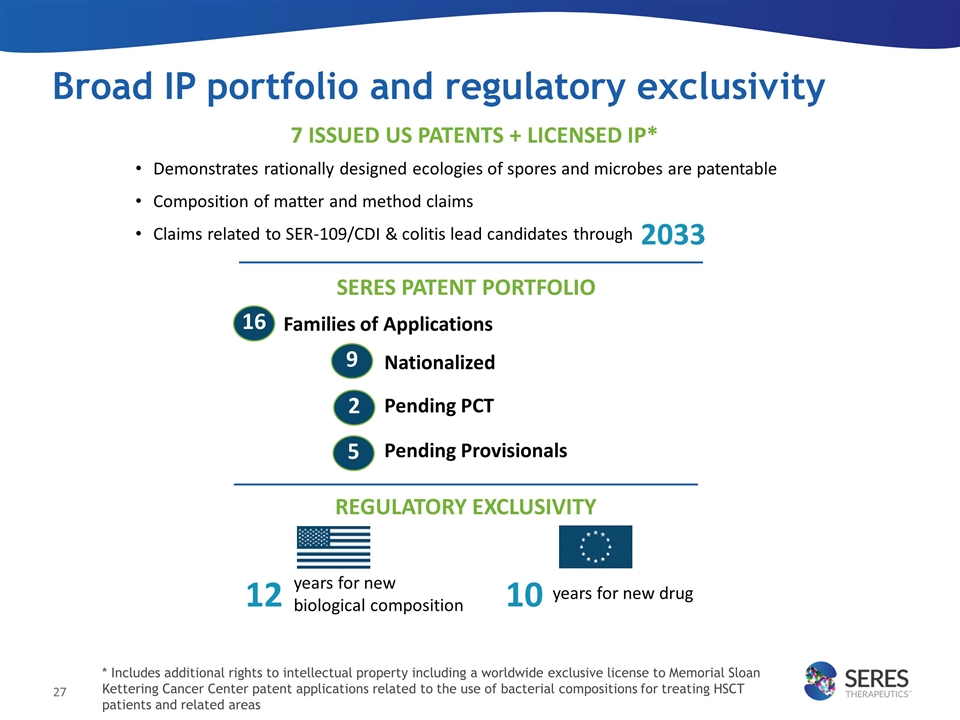
Broad IP portfolio and regulatory exclusivity Families of Applications Nationalized Pending PCT Pending Provisionals SERES PATENT PORTFOLIO 16 9 2 REGULATORY EXCLUSIVITY years for new biological composition 12 years for new drug 10 7 ISSUED US PATENTS + LICENSED IP* Demonstrates rationally designed ecologies of spores and microbes are patentable Composition of matter and method claims Claims related to SER-109/CDI & colitis lead candidates through 2033 5 * Includes additional rights to intellectual property including a worldwide exclusive license to Memorial Sloan Kettering Cancer Center patent applications related to the use of bacterial compositions for treating HSCT patients and related areas
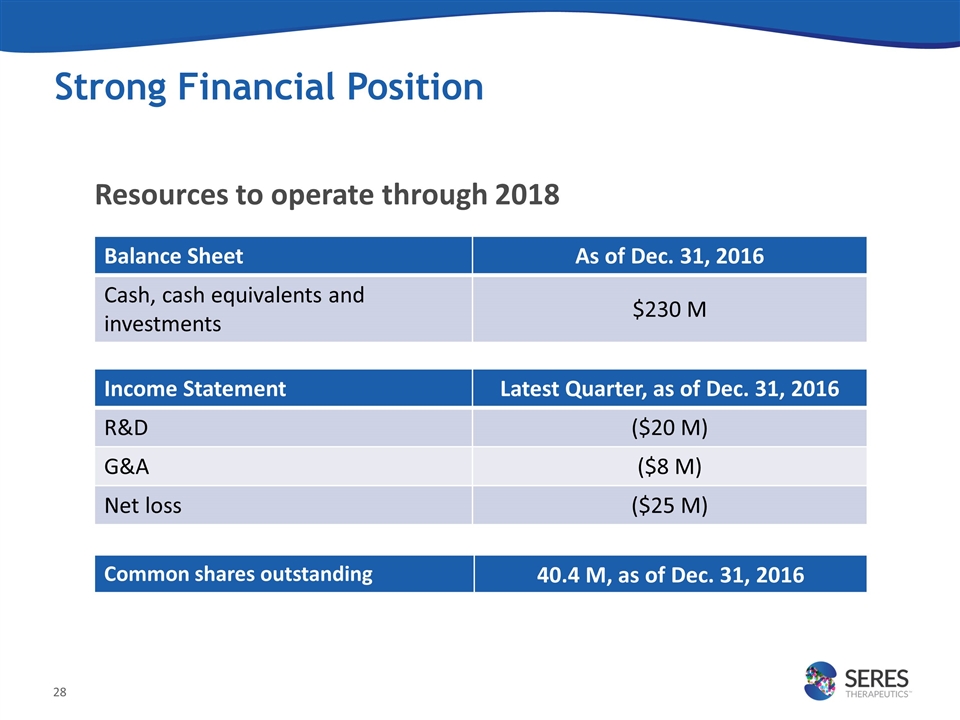
Resources to operate through 2018 Strong Financial Position Balance Sheet As of Dec. 31, 2016 Cash, cash equivalents and investments $230 M Income Statement Latest Quarter, as of Dec. 31, 2016 R&D ($20 M) G&A ($8 M) Net loss ($25 M) Common shares outstanding 40.4 M, as of Dec. 31, 2016
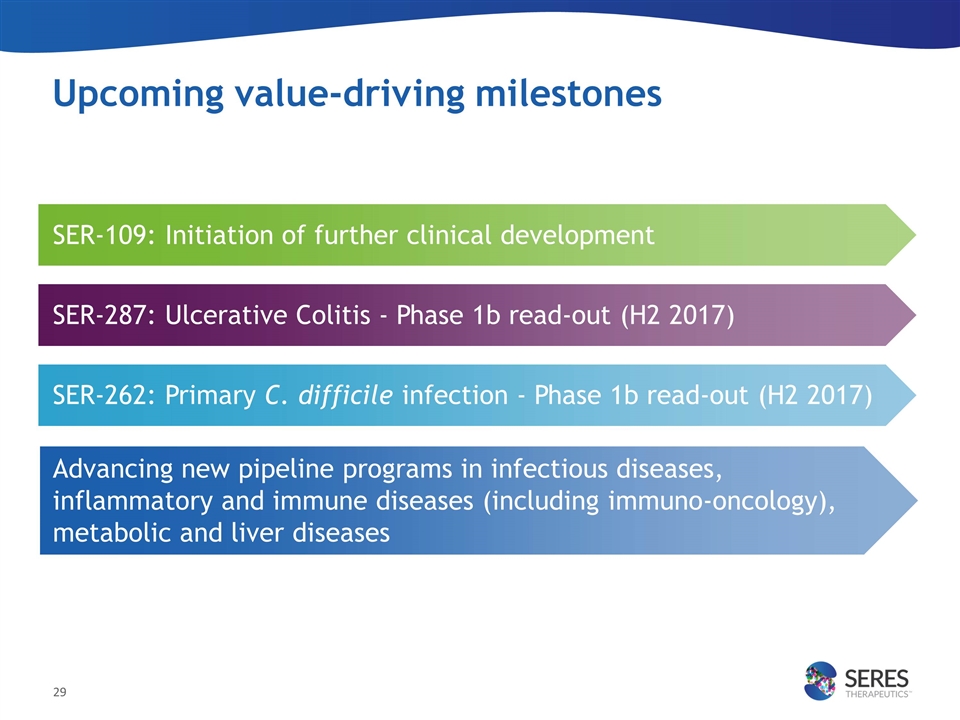
SER-109: Completion of FDA dialogue regarding future development SER-109: Initiation of further clinical development SER-287: Ulcerative Colitis - Phase 1b read-out (H2 2017) SER-262: Primary C. difficile infection - Phase 1b read-out (H2 2017) Advancing new pipeline programs in infectious diseases, inflammatory and immune diseases (including immuno-oncology), metabolic and liver diseases Upcoming value-driving milestones




























