
SER-109 ECOSPOR IV Study Results June 7, 2022 Exhibit 99.1

Some of the statements in this presentation constitute “forward looking statements” under the Private Securities Litigation Reform Act of 1995, including, but not limited to, the timing and potential approval of SER-109 and its potential to be a first-in-class therapeutic; the market for SER-109; our capacity for commercial supply of SER-109; the anticipated indication of SER-109; the potential impact of Infection Protection microbiome therapeutics; our development opportunities and plans; the ultimate safety and efficacy data for our products; the potential of microbiome therapeutics to treat and prevent disease; and other statements which are not historical fact. Such statements are subject to important factors, risks and uncertainties, such as those discussed under the caption "Risk Factors" in the Company’s Quarterly Report on Form 10-Q filed on May 4, 2022, and its other filings with the SEC, that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any forward-looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so. Forward Looking Statements
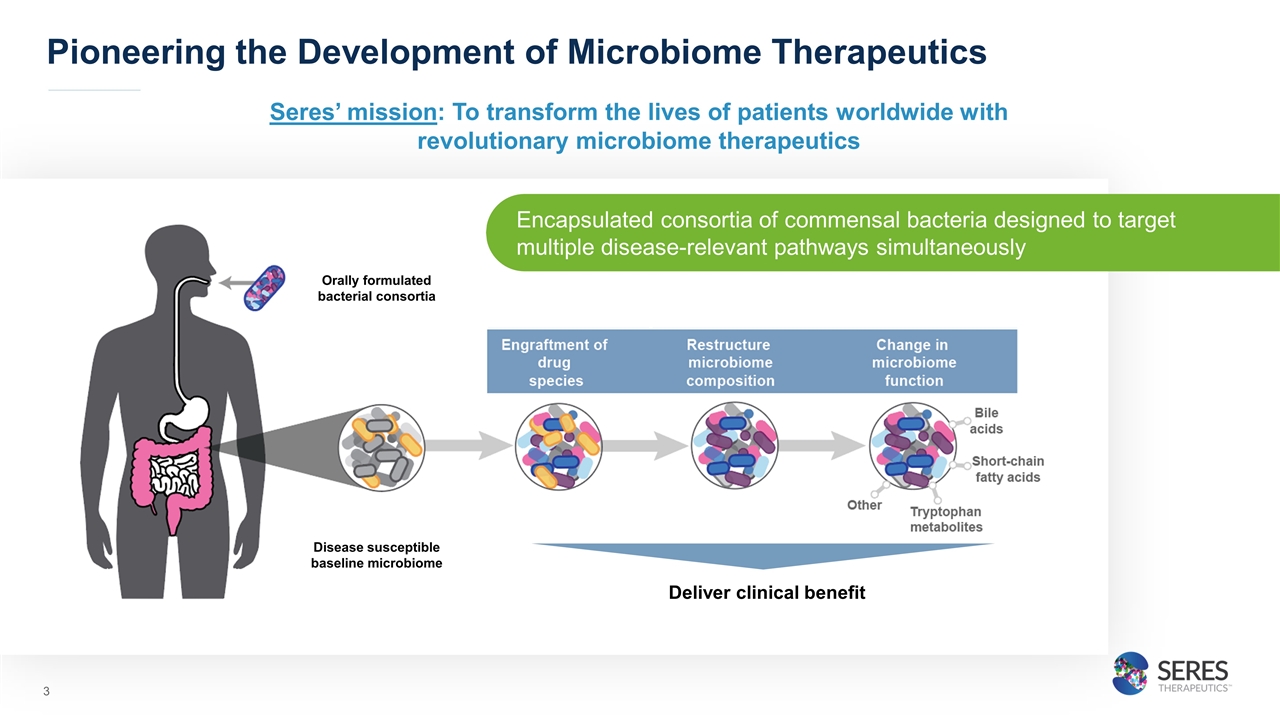
Pioneering the Development of Microbiome Therapeutics Deliver clinical benefit Disease susceptible baseline microbiome Orally formulated bacterial consortia Encapsulated consortia of commensal bacteria designed to target multiple disease-relevant pathways simultaneously Seres’ mission: To transform the lives of patients worldwide with revolutionary microbiome therapeutics
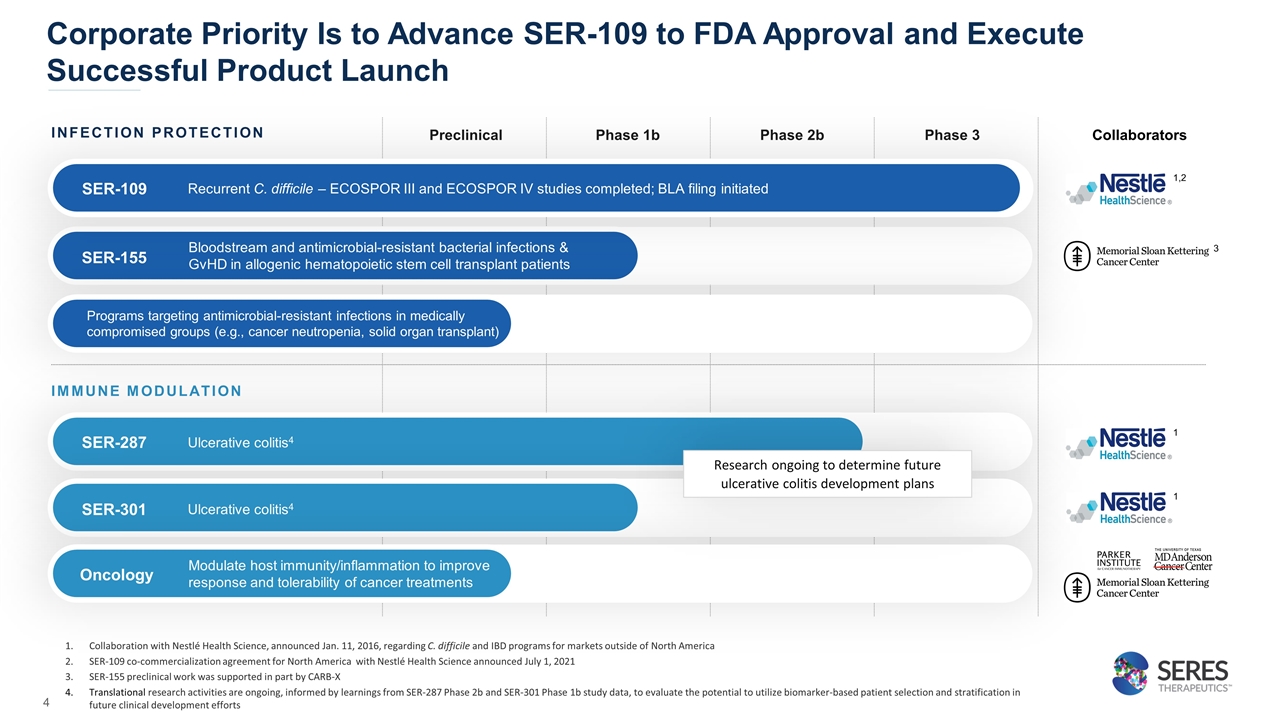
Corporate Priority Is to Advance SER-109 to FDA Approval and Execute Successful Product Launch Collaboration with Nestlé Health Science, announced Jan. 11, 2016, regarding C. difficile and IBD programs for markets outside of North America SER-109 co-commercialization agreement for North America with Nestlé Health Science announced July 1, 2021 SER-155 preclinical work was supported in part by CARB-X Translational research activities are ongoing, informed by learnings from SER-287 Phase 2b and SER-301 Phase 1b study data, to evaluate the potential to utilize biomarker-based patient selection and stratification in future clinical development efforts Immunotherapy Collaborators INFECTION PROTECTION Preclinical Phase 1b Phase 2b Phase 3 SER-155 Bloodstream and antimicrobial-resistant bacterial infections & GvHD in allogenic hematopoietic stem cell transplant patients IMMUNE MODULATION SER-287 Ulcerative colitis4 SER-301 Ulcerative colitis4 Modulate host immunity/inflammation to improve response and tolerability of cancer treatments Programs targeting antimicrobial-resistant infections in medically compromised groups (e.g., cancer neutropenia, solid organ transplant) SER-109 Recurrent C. difficile – ECOSPOR III and ECOSPOR IV studies completed; BLA filing initiated 1,2 3 Oncology Research ongoing to determine future ulcerative colitis development plans 1 1
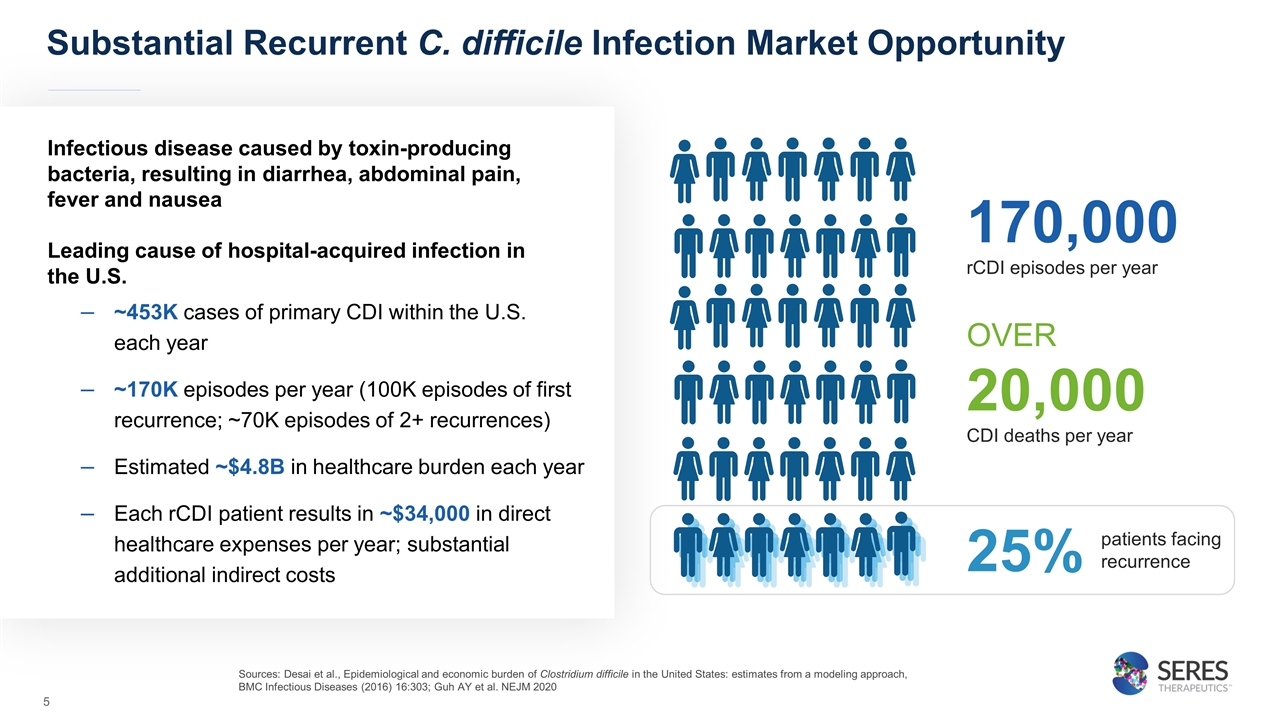
Substantial Recurrent C. difficile Infection Market Opportunity Infectious disease caused by toxin-producing bacteria, resulting in diarrhea, abdominal pain, fever and nausea Leading cause of hospital-acquired infection in the U.S. ~453K cases of primary CDI within the U.S. each year ~170K episodes per year (100K episodes of first recurrence; ~70K episodes of 2+ recurrences) Estimated ~$4.8B in healthcare burden each year Each rCDI patient results in ~$34,000 in direct healthcare expenses per year; substantial additional indirect costs Sources: Desai et al., Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach, BMC Infectious Diseases (2016) 16:303; Guh AY et al. NEJM 2020 OVER 20,000 CDI deaths per year 170,000 rCDI episodes per year patients facing recurrence 25%
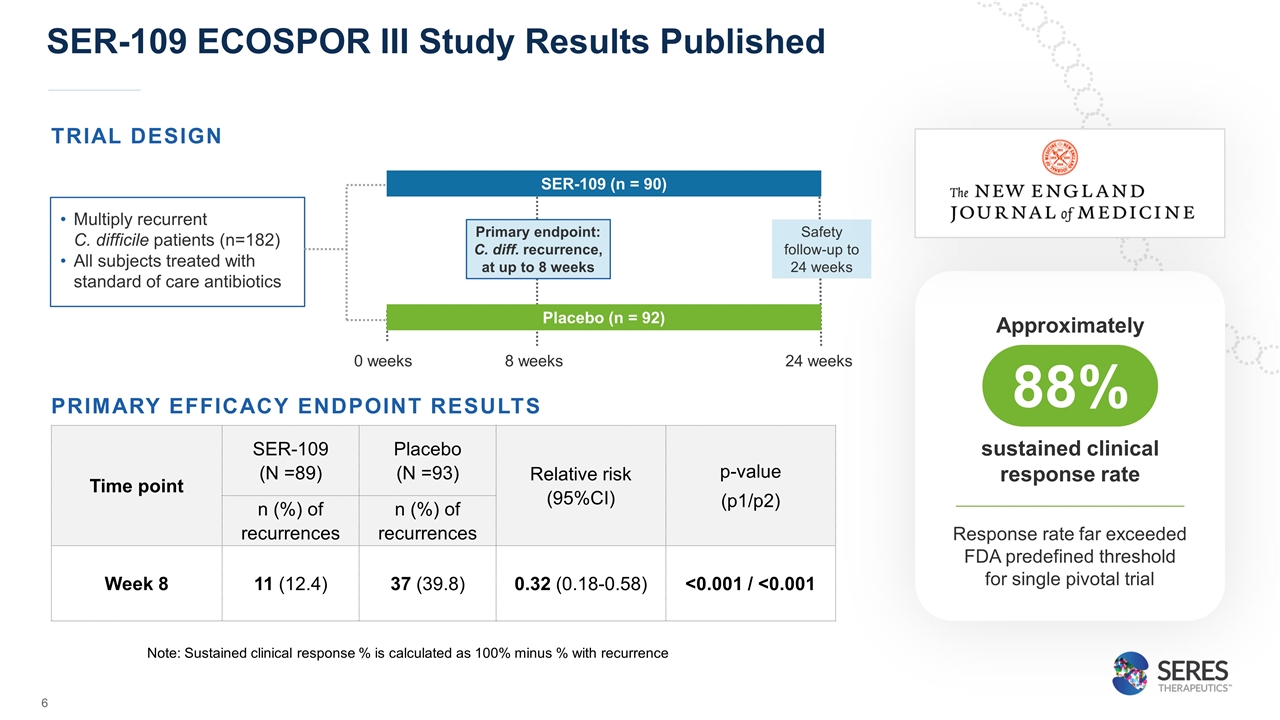
Time point SER-109 (N =89) Placebo (N =93) Relative risk (95%CI) p-value (p1/p2) n (%) of recurrences n (%) of recurrences Week 8 11 (12.4) 37 (39.8) 0.32 (0.18-0.58) <0.001 / <0.001 TRIAL DESIGN SER-109 ECOSPOR III Study Results Published Primary endpoint: C. diff. recurrence, at up to 8 weeks SER-109 (n = 90) Multiply recurrent C. difficile patients (n=182) All subjects treated with standard of care antibiotics Placebo (n = 92) 0 weeks 8 weeks 24 weeks Safety follow-up to 24 weeks PRIMARY EFFICACY ENDPOINT RESULTS Note: Sustained clinical response % is calculated as 100% minus % with recurrence Approximately 88% sustained clinical response rate Response rate far exceeded FDA predefined threshold for single pivotal trial

Favorable Safety Profile Observed in ECOSPOR III SER-109 was well tolerated, with no treatment-related serious adverse events (SAEs) and an adverse event profile comparable to placebo Overall incidence of patients who experienced AEs was similar between SER-109 and placebo arms Following ECOSPOR III study results, FDA requested that a BLA filing include a safety database with at least 300 subjects administered SER-109 at the commercial dose and followed for 24 weeks
SER-109 ECOSPOR IV Study Results
SER-109 ECOSPOR IV Study Overview Provides 24-week data on additional patients administered SER-109 at commercial dose to fulfill FDA request Incorporated patients similar to those commonly treated in clinical practice Includes 1st recurrence patients (29% of total enrollment) Diagnostic criteria at study entry included both PCR and toxin Study had two open label cohorts receiving SER-109, with each having an 8-week primary efficacy period and a subsequent 16-week follow-up period Cohort 1: Subjects previously in ECOSPOR III (n=29) with a CDI recurrence within 8 weeks after SER-109 or placebo Cohort 2: Safety and tolerability in subjects receiving SER-109 at the dose used in ECOSPOR III (n=234). All had at least one CDI recurrence and had responded to CDI antibiotic therapy. Allowed PCR and toxin diagnostic testing for entry.
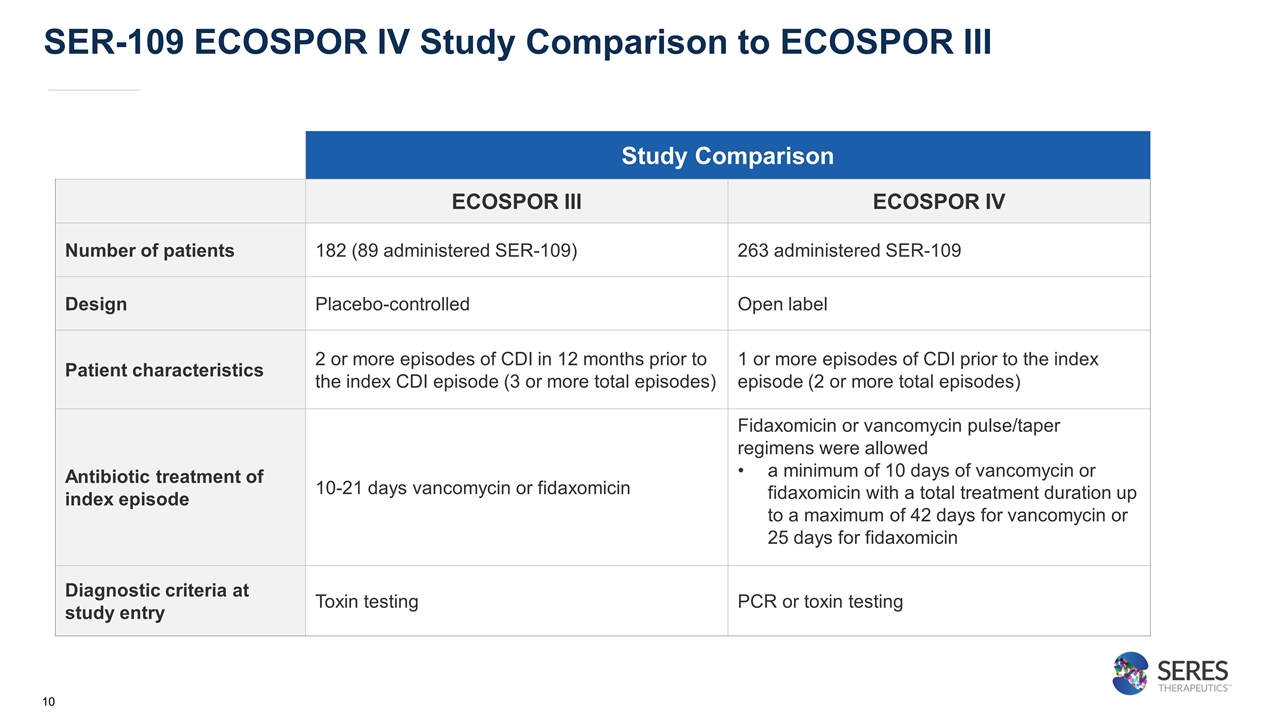
SER-109 ECOSPOR IV Study Comparison to ECOSPOR III Study Comparison ECOSPOR III ECOSPOR IV Number of patients 182 (89 administered SER-109) 263 administered SER-109 Design Placebo-controlled Open label Patient characteristics 2 or more episodes of CDI in 12 months prior to the index CDI episode (3 or more total episodes) 1 or more episodes of CDI prior to the index episode (2 or more total episodes) Antibiotic treatment of index episode 10-21 days vancomycin or fidaxomicin Fidaxomicin or vancomycin pulse/taper regimens were allowed a minimum of 10 days of vancomycin or fidaxomicin with a total treatment duration up to a maximum of 42 days for vancomycin or 25 days for fidaxomicin Diagnostic criteria at study entry Toxin testing PCR or toxin testing
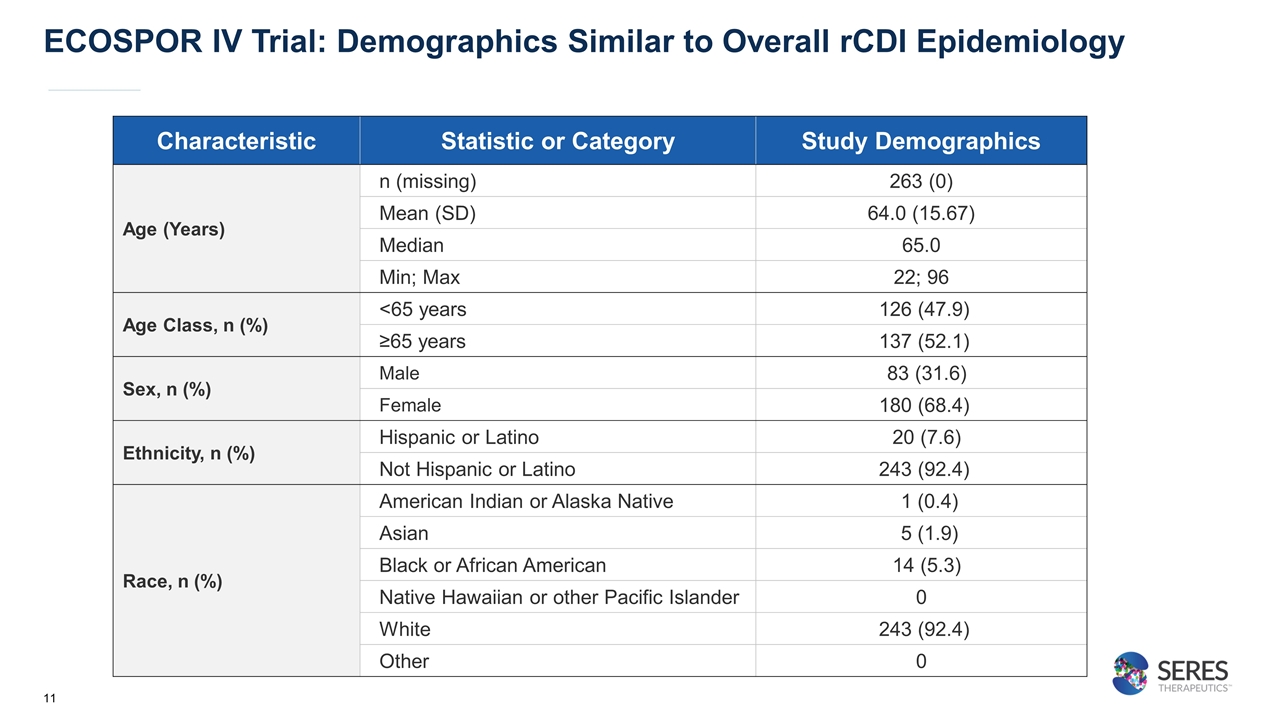
ECOSPOR IV Trial: Demographics Similar to Overall rCDI Epidemiology Characteristic Statistic or Category Study Demographics Age (Years) n (missing) 263 (0) Mean (SD) 64.0 (15.67) Median 65.0 Min; Max 22; 96 Age Class, n (%) <65 years 126 (47.9) ≥65 years 137 (52.1) Sex, n (%) Male 83 (31.6) Female 180 (68.4) Ethnicity, n (%) Hispanic or Latino 20 (7.6) Not Hispanic or Latino 243 (92.4) Race, n (%) American Indian or Alaska Native 1 (0.4) Asian 5 (1.9) Black or African American 14 (5.3) Native Hawaiian or other Pacific Islander 0 White 243 (92.4) Other 0
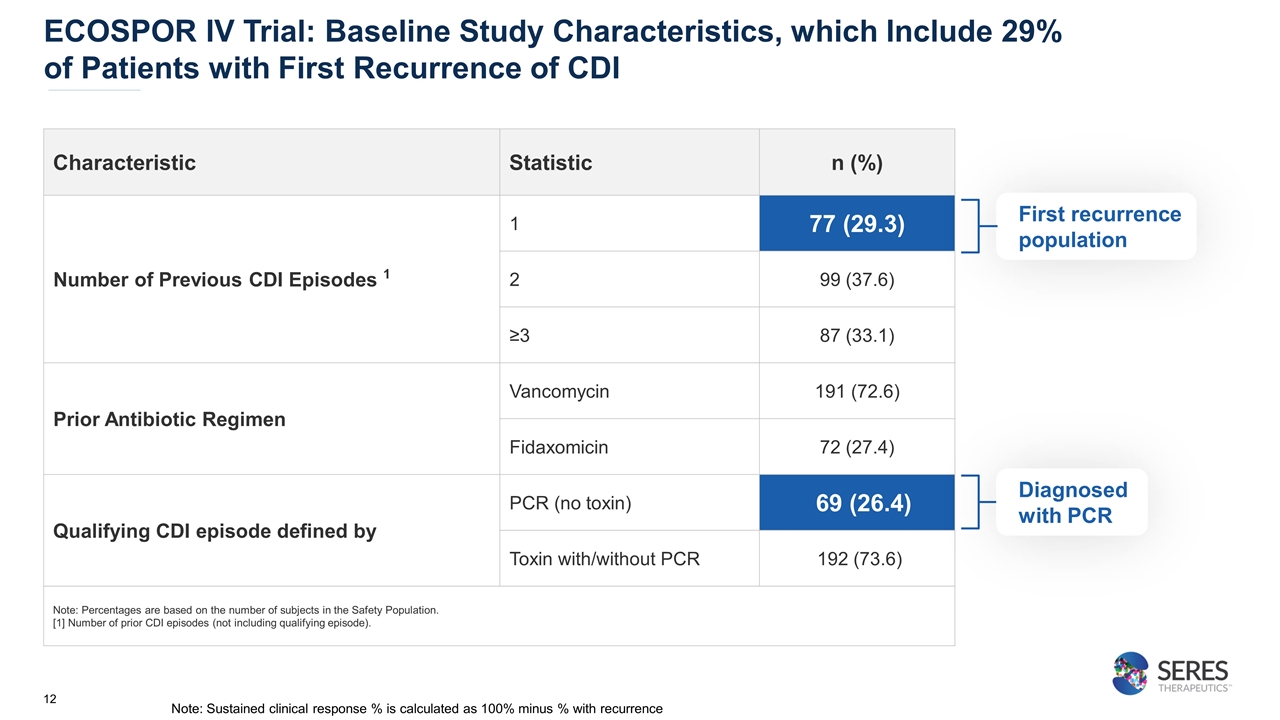
ECOSPOR IV Trial: Baseline Study Characteristics, which Include 29% of Patients with First Recurrence of CDI Characteristic Statistic n (%) Number of Previous CDI Episodes 1 1 77 (29.3) 2 99 (37.6) ≥3 87 (33.1) Prior Antibiotic Regimen Vancomycin 191 (72.6) Fidaxomicin 72 (27.4) Qualifying CDI episode defined by PCR (no toxin) 69 (26.4) Toxin with/without PCR 192 (73.6) Note: Percentages are based on the number of subjects in the Safety Population. [1] Number of prior CDI episodes (not including qualifying episode). First recurrence population Diagnosed with PCR Note: Sustained clinical response % is calculated as 100% minus % with recurrence
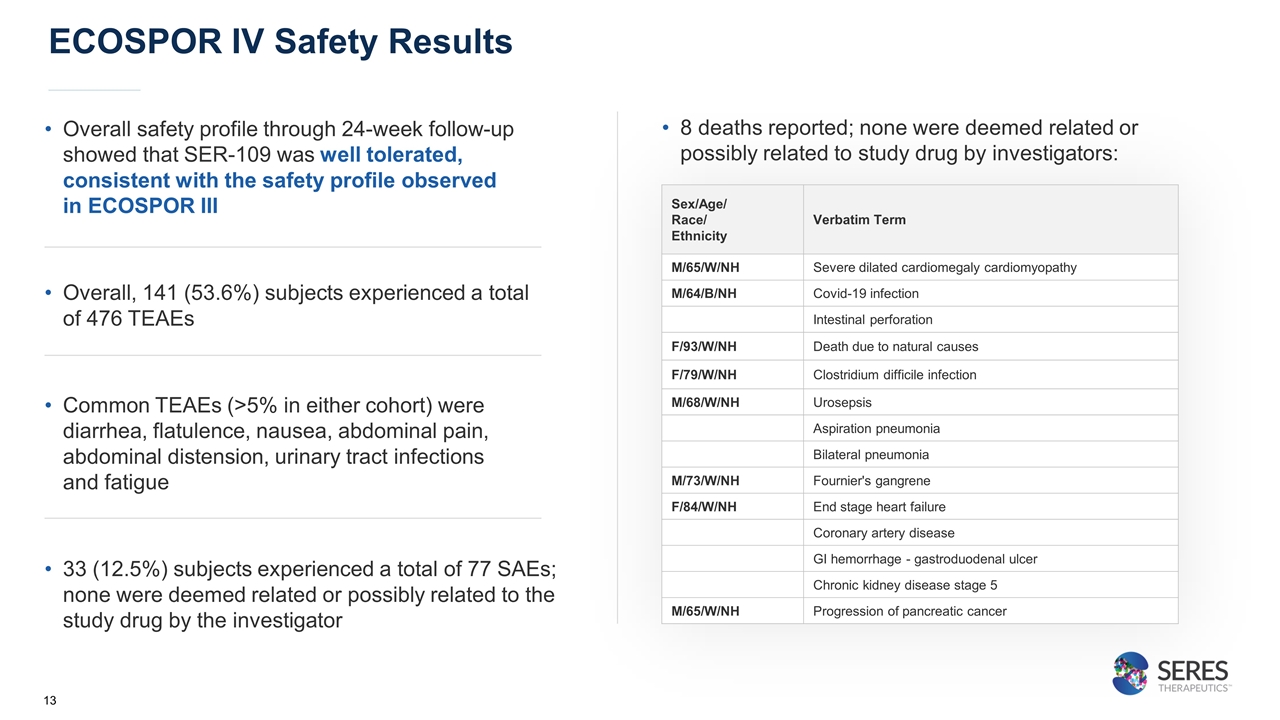
ECOSPOR IV Safety Results Sex/Age/ Race/ Ethnicity Verbatim Term M/65/W/NH Severe dilated cardiomegaly cardiomyopathy M/64/B/NH Covid-19 infection Intestinal perforation F/93/W/NH Death due to natural causes F/79/W/NH Clostridium difficile infection M/68/W/NH Urosepsis Aspiration pneumonia Bilateral pneumonia M/73/W/NH Fournier's gangrene F/84/W/NH End stage heart failure Coronary artery disease GI hemorrhage - gastroduodenal ulcer Chronic kidney disease stage 5 M/65/W/NH Progression of pancreatic cancer 8 deaths reported; none were deemed related or possibly related to study drug by investigators: Overall safety profile through 24-week follow-up showed that SER-109 was well tolerated, consistent with the safety profile observed in ECOSPOR III Overall, 141 (53.6%) subjects experienced a total of 476 TEAEs Common TEAEs (>5% in either cohort) were diarrhea, flatulence, nausea, abdominal pain, abdominal distension, urinary tract infections and fatigue 33 (12.5%) subjects experienced a total of 77 SAEs; none were deemed related or possibly related to the study drug by the investigator
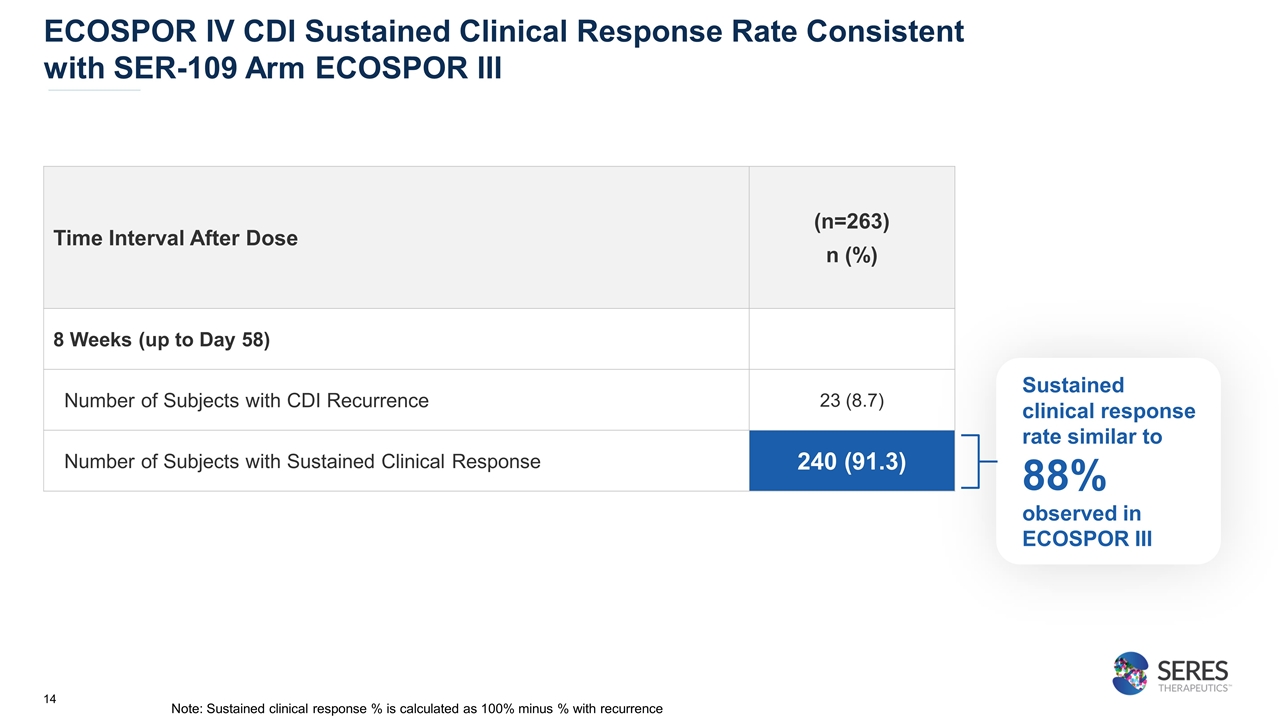
ECOSPOR IV CDI Sustained Clinical Response Rate Consistent with SER-109 Arm ECOSPOR III Sustained clinical response rate similar to 88% observed in ECOSPOR III Time Interval After Dose (n=263) n (%) 8 Weeks (up to Day 58) Number of Subjects with CDI Recurrence 23 (8.7) Number of Subjects with Sustained Clinical Response 240 (91.3) Note: Sustained clinical response % is calculated as 100% minus % with recurrence
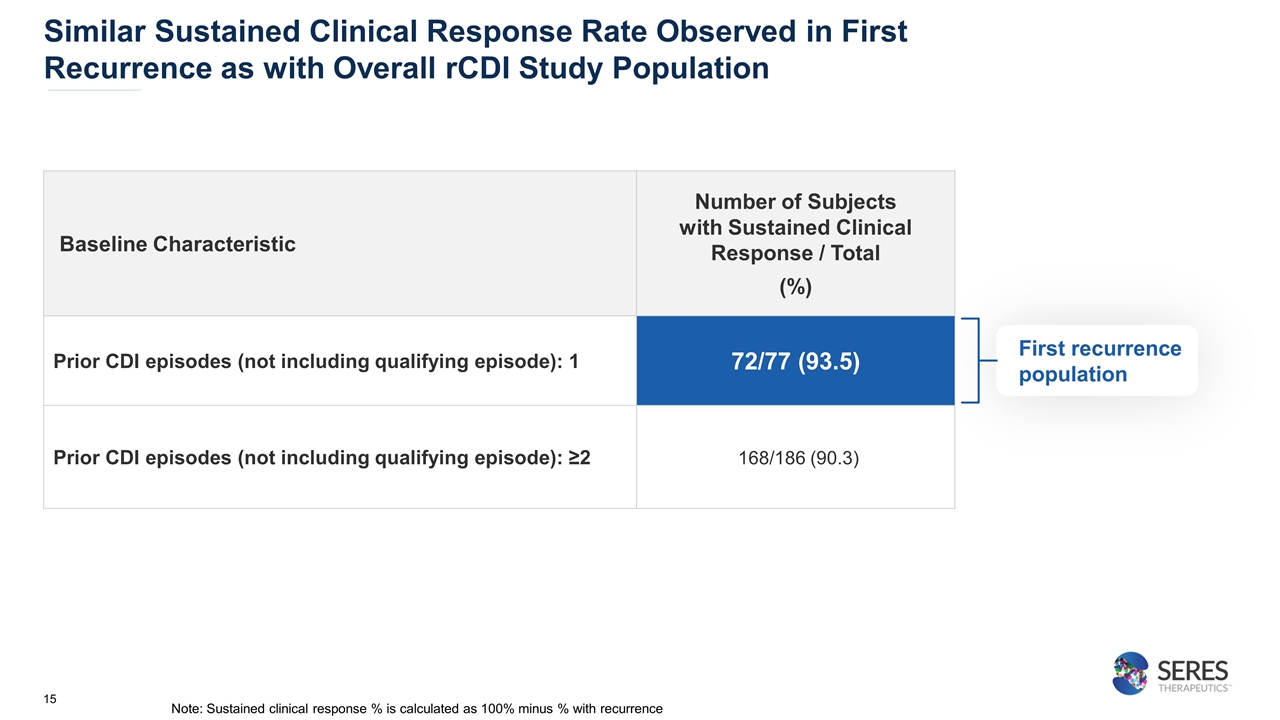
Similar Sustained Clinical Response Rate Observed in First Recurrence as with Overall rCDI Study Population First recurrence population Baseline Characteristic Number of Subjects with Sustained Clinical Response / Total (%) Prior CDI episodes (not including qualifying episode): 1 72/77 (93.5) Prior CDI episodes (not including qualifying episode): ≥2 168/186 (90.3) Note: Sustained clinical response % is calculated as 100% minus % with recurrence
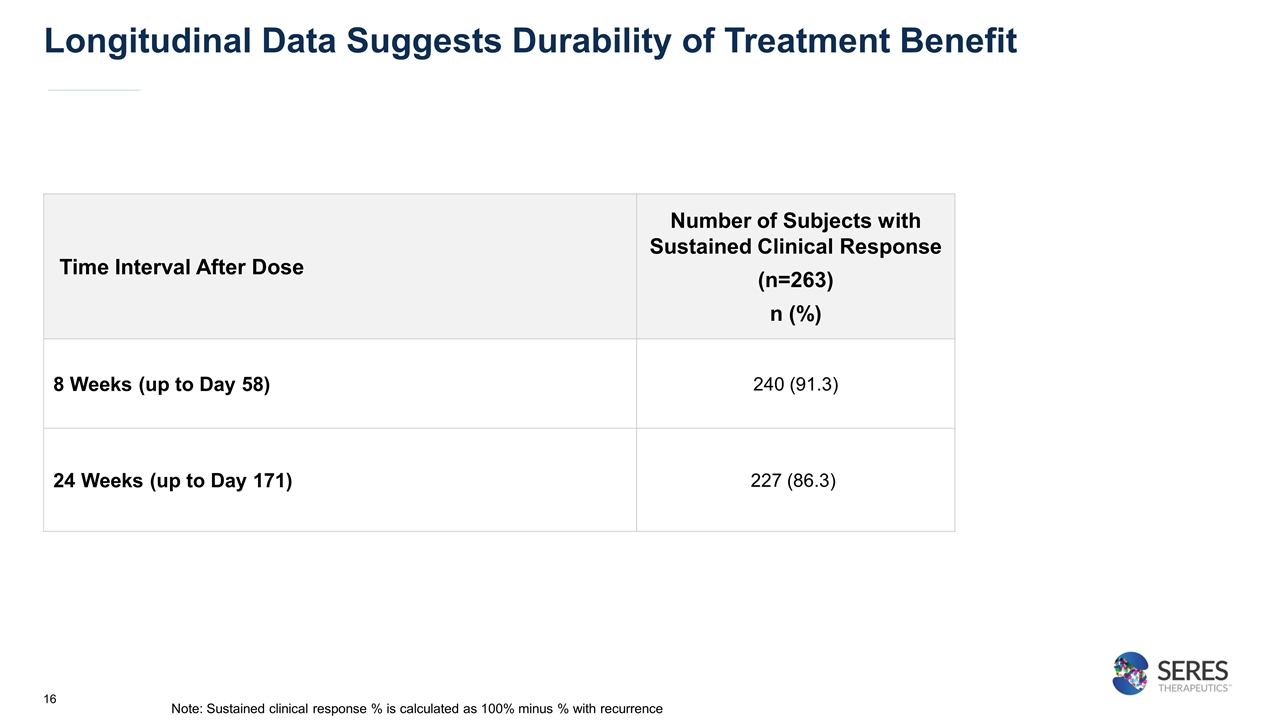
Longitudinal Data Suggests Durability of Treatment Benefit Time Interval After Dose Number of Subjects with Sustained Clinical Response (n=263) n (%) 8 Weeks (up to Day 58) 240 (91.3) 24 Weeks (up to Day 171) 227 (86.3) Note: Sustained clinical response % is calculated as 100% minus % with recurrence
Overall ECOSPOR IV Study Conclusions Reaffirms and extends ECOSPOR III efficacy results ECOSPOR IV CDI sustained clinical response rate provides additional evidence of substantial efficacy, consistent with the results obtained in SER-109 arm of ECOSPOR III ECOSPOR IV study demonstrates similar sustained clinical response rate in patients with first or later recurrences and regardless of CDI diagnostic method. First recurrence data are consistent with similar pathology of microbiome disruption underlying all recurrent CDI events. Favorable SER-109 safety results Safety profile shows that SER-109 was well tolerated, consistent with SER-109 ECOSPOR III study where SER-109 safety profile was similar to placebo arm ECOSPOR IV study results support: SER-109 clinical benefit across entire recurrent CDI patient population SER-109 BLA filing for recurrent CDI; potential first approved microbiome therapeutic
BLA Filing Now Initiated; Anticipate SER-109 Launch H1 2023 BLA submission FDA review Assume Priority Review based on Breakthrough Therapy Designation BLA submission initiated; on track for completion in mid-2022 Expanded access program ongoing across multiple US sites Anticipated approval in H1 2023 Potential SER-109 approval and launch

Well Positioned to Meet Commercial Demand at Launch and Beyond SER-109 commercial supply See Seres and Bacthera collaboration press release issued Nov. 10, 2021 + In-house GMP manufacturing and quality control, supported by CMOs Joint venture between Chr. Hansen and Lonza with offices in Switzerland and Denmark Bacthera collaboration provides redundancy and expands upon existing commercial supply capacity
Seres, Nestlé Health Science SER-109 Co-Commercialization License Agreement for North America – Maximizing Commercial Opportunity Broadly engaging KOLs leveraging Seres and Aimmune, Medical Affairs teams (e.g., DDW 2022) Deploying Aimmune payer field team with robust value proposition and rCDI education Continuing Market Education Efforts Key Market Research Activities in Progress Conducting customer segmentation Progressing pricing analysis Leveraging Efficient Infrastructure for Launch Integrating existing Aimmune capabilities and expertise across commercial and G&A for launch Note: Aimmune Therapeutics, a Nestle Health Science company, is leading SER-109 commercialization prep activities

Maximizing the Opportunity in Infection Protection and AMR SER-109 rCDI SER-155 BSI & GvHD in allogeneic HSCT recipients Broad preclinical portfolio Driving to an additional clinical development program in 2023 Potentially up to 3 additional programs within 3 years Rich set of therapeutic adjacencies to C. difficile SER-155 clinical development ongoing Additional Opportunities Initiated BLA Filing Active Clinical Development Pre-clinical Portfolio Autologous HSCT Cancer Neutropenia Solid Organ Transplant Cirrhosis Broadly Target Antimicrobial Resistant Infections




















