
Seres Therapeutics Investor Presentation November 13, 2024 Exhibit 99.2

Disclaimers Forward Looking Statements This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this communication that do not relate to matters of historical fact should be considered forward-looking statements, including statements about the financial terms and future payments related to the VOWST sale; the timing and results of our clinical studies and data readouts; future product candidates, development plans and commercial opportunities; interactions with regulatory agencies; operating plans and our future cash runway; our ability to generate additional capital; our planned strategic focus; anticipated timing of any of the foregoing and other statements which are not historical fact. These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: (1) we have incurred significant losses, are not currently profitable and may never become profitable; (2) our need for additional funding; (3) our history of operating losses; (4) our novel approach to therapeutic intervention; (5) our reliance on third parties to conduct our clinical trials and manufacture our product candidates; (6) the competition we will face; (7) our ability to protect our intellectual property; (8) our ability to retain key personnel and to manage our growth; (9) the effect of the VOWST sale on our ability to retain and hire key personnel and maintain relationships with our customers, suppliers, advertisers, partners and others with whom we do business, or on our operating results and businesses generally; (10) the risks associated with the disruption of management’s attention from ongoing business operations due to the obligation to provide transition services; (11) our failure to receive the installment payments or the milestone payments in the future; (12) the uncertainty of impact of the 50/50 profit and loss sharing arrangement on our reported results and liquidity; and (13) we may not be able to realize the anticipated benefits of the VOWST sale. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC), on August 13, 2024, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this communication. Any such forward-looking statements represent management’s estimates as of the date of this communication. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this communication. Seres Therapeutics, Inc. © 2024
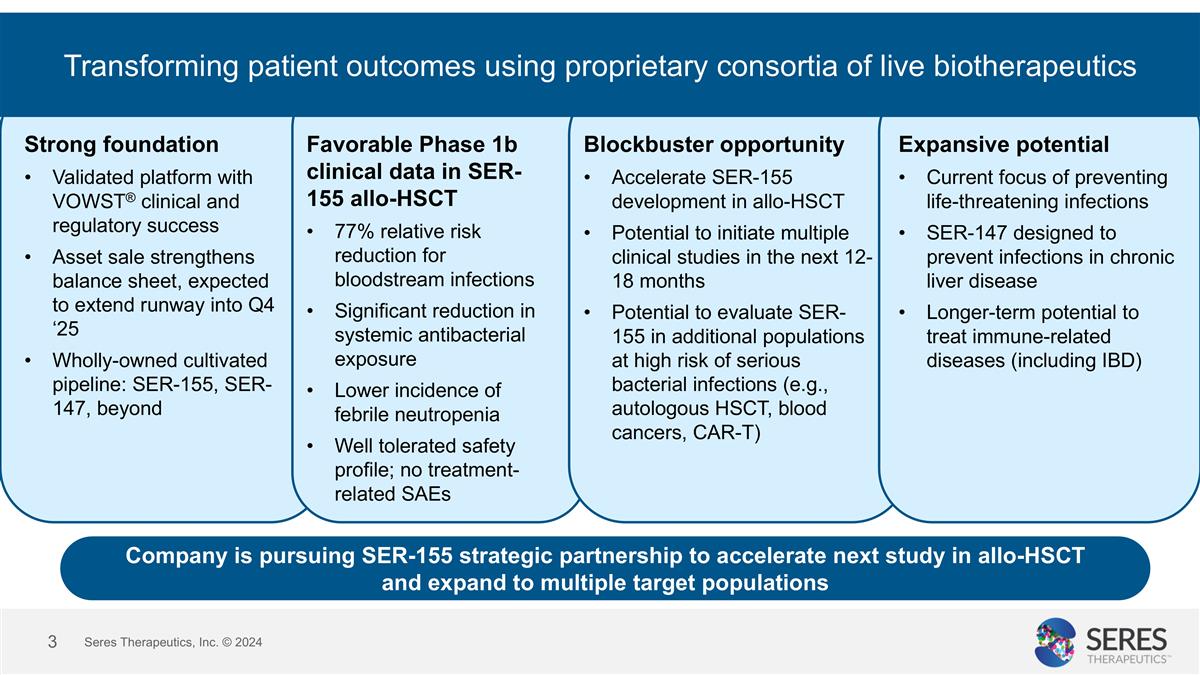
Transforming patient outcomes using proprietary consortia of live biotherapeutics Strong foundation Validated platform with VOWST® clinical and regulatory success Asset sale strengthens balance sheet, expected to extend runway into Q4 ‘25 Wholly-owned cultivated pipeline: SER-155, SER-147, beyond Favorable Phase 1b clinical data in SER-155 allo-HSCT 77% relative risk reduction for bloodstream infections Significant reduction in systemic antibacterial exposure Lower incidence of febrile neutropenia Well tolerated safety profile; no treatment-related SAEs Blockbuster opportunity Accelerate SER-155 development in allo-HSCT Potential to initiate multiple clinical studies in the next 12-18 months Potential to evaluate SER-155 in additional populations at high risk of serious bacterial infections (e.g., autologous HSCT, blood cancers, CAR-T) Expansive potential Current focus of preventing life-threatening infections SER-147 designed to prevent infections in chronic liver disease Longer-term potential to treat immune-related diseases (including IBD) Seres Therapeutics, Inc. © 2024 Company is pursuing SER-155 strategic partnership to accelerate next study in allo-HSCT and expand to multiple target populations
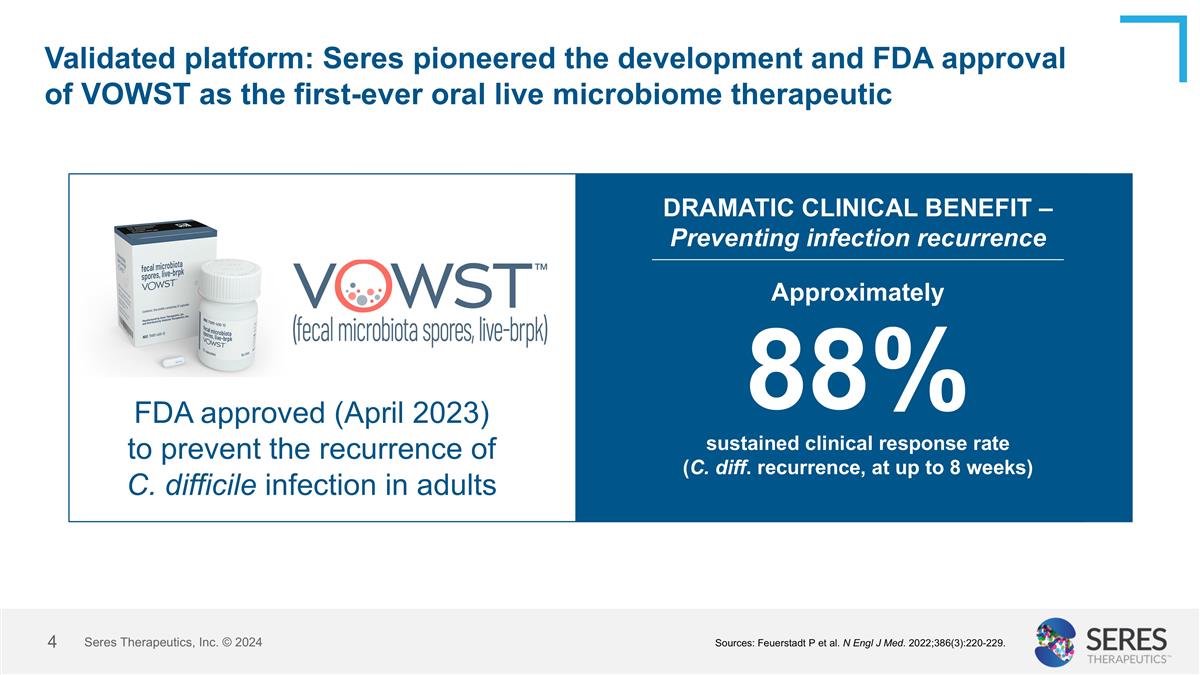
Validated platform: Seres pioneered the development and FDA approval of VOWST as the first-ever oral live microbiome therapeutic FDA approved (April 2023) to prevent the recurrence of C. difficile infection in adults Approximately 88% sustained clinical response rate (C. diff. recurrence, at up to 8 weeks) DRAMATIC CLINICAL BENEFIT – Preventing infection recurrence Seres Therapeutics, Inc. © 2024 Sources: Feuerstadt P et al. N Engl J Med. 2022;386(3):220-229.

VOWST asset sale completed September 30, 2024: transformational for Seres – provides resources to support SER-155 advancement KEY FINANCIAL TERMS VOWST asset purchase agreement provided infusion of capital and supports SER-155 development Asset sale extends operational runway into Q4 2025 Retires debt and other obligations $100M upfront payment to Seres, less ~$20M in net obligations due to an affiliate of SPN* $15M equity investment by SPN at closing $60M prepaid sales-based milestone at closing $75M in deferred payments due in 2025 (less ~$1.5M in employment-related payments) $275M in potential future sales-based milestone payments (subject to reductions for interest on prepaid milestone payment) Seres Therapeutics, Inc. © 2024 Transaction results in a more streamlined, focused Seres organization and lower cash burn rate *SPN: Société des Produits Nestlé S.A.
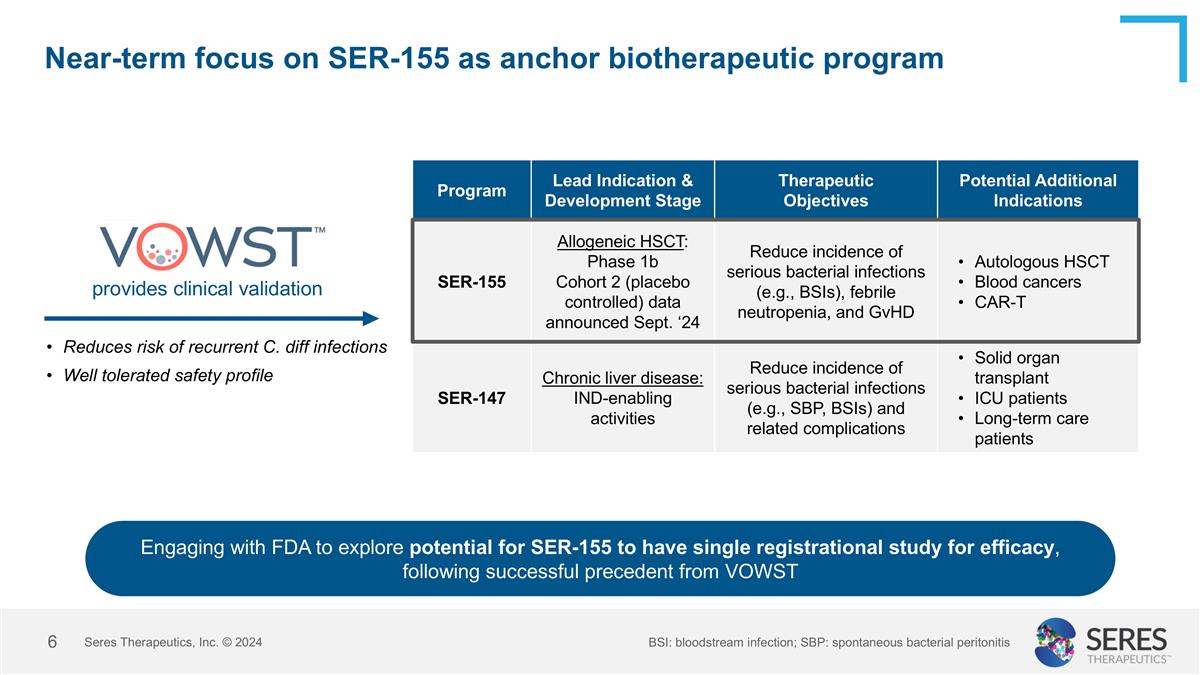
Near-term focus on SER-155 as anchor biotherapeutic program Program Lead Indication & Development Stage Therapeutic Objectives Potential Additional Indications SER-155 Allogeneic HSCT: Phase 1b Cohort 2 (placebo controlled) data announced Sept. ‘24 Reduce incidence of serious bacterial infections (e.g., BSIs), febrile neutropenia, and GvHD Autologous HSCT Blood cancers CAR-T SER-147 Chronic liver disease: IND-enabling activities Reduce incidence of serious bacterial infections (e.g., SBP, BSIs) and related complications Solid organ transplant ICU patients Long-term care patients provides clinical validation Reduces risk of recurrent C. diff infections Well tolerated safety profile Seres Therapeutics, Inc. © 2024 Engaging with FDA to explore potential for SER-155 to have single registrational study for efficacy, following successful precedent from VOWST BSI: bloodstream infection; SBP: spontaneous bacterial peritonitis
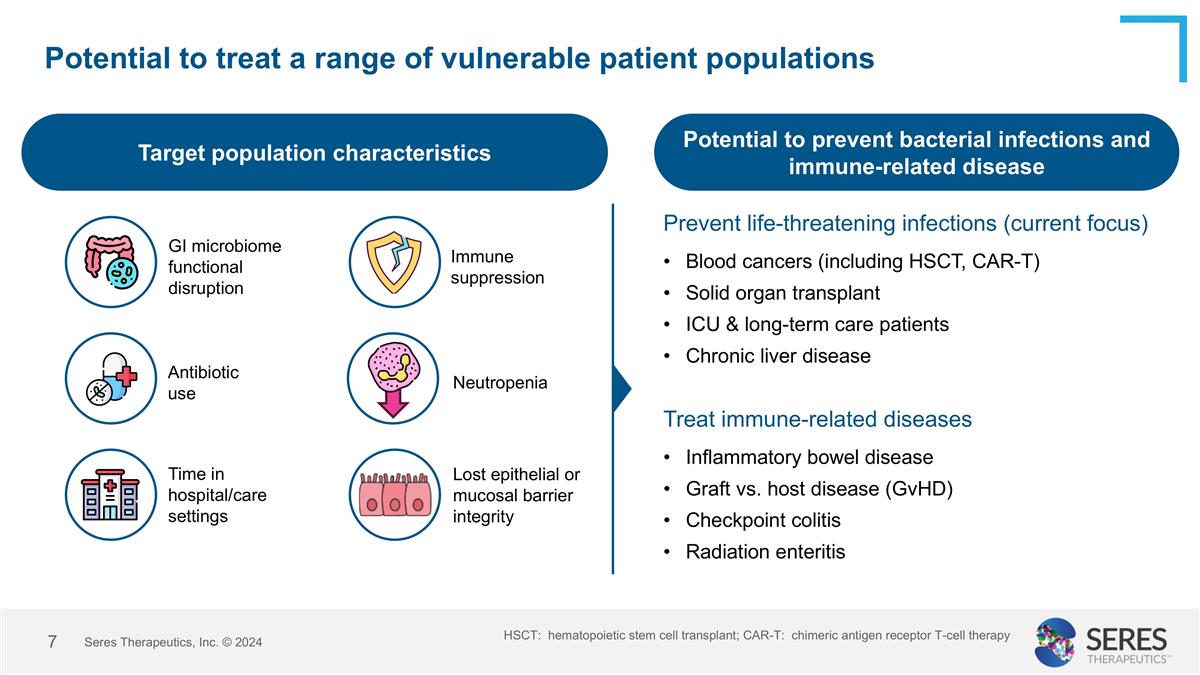
Potential to treat a range of vulnerable patient populations Seres Therapeutics, Inc. © 2024 Blood cancers (including HSCT, CAR-T) Solid organ transplant ICU & long-term care patients Chronic liver disease Target population characteristics Potential to prevent bacterial infections and immune-related disease Immune suppression GI microbiome functional disruption Antibiotic use Neutropenia Time in hospital/care settings Lost epithelial or mucosal barrier integrity Prevent life-threatening infections (current focus) Treat immune-related diseases Inflammatory bowel disease Graft vs. host disease (GvHD) Checkpoint colitis Radiation enteritis HSCT: hematopoietic stem cell transplant; CAR-T: chimeric antigen receptor T-cell therapy
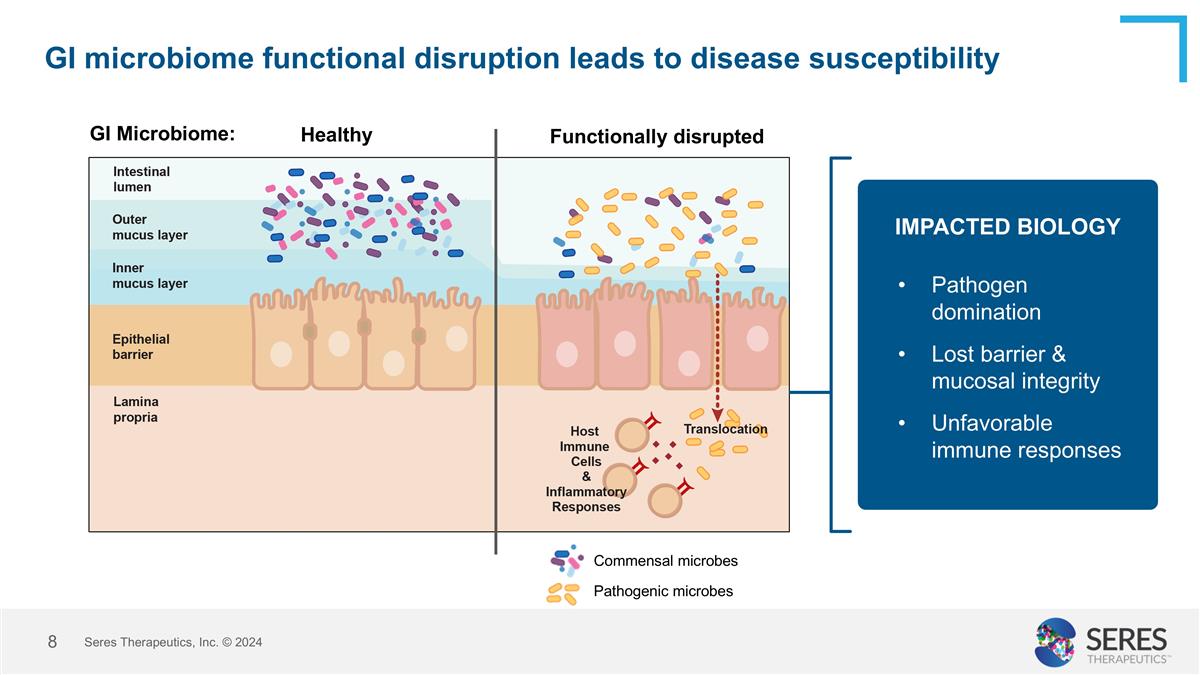
GI microbiome functional disruption leads to disease susceptibility Functionally disrupted Healthy GI Microbiome: Commensal microbes Pathogenic microbes Seres Therapeutics, Inc. © 2024 Pathogen domination Lost barrier & mucosal integrity Unfavorable immune responses IMPACTED BIOLOGY
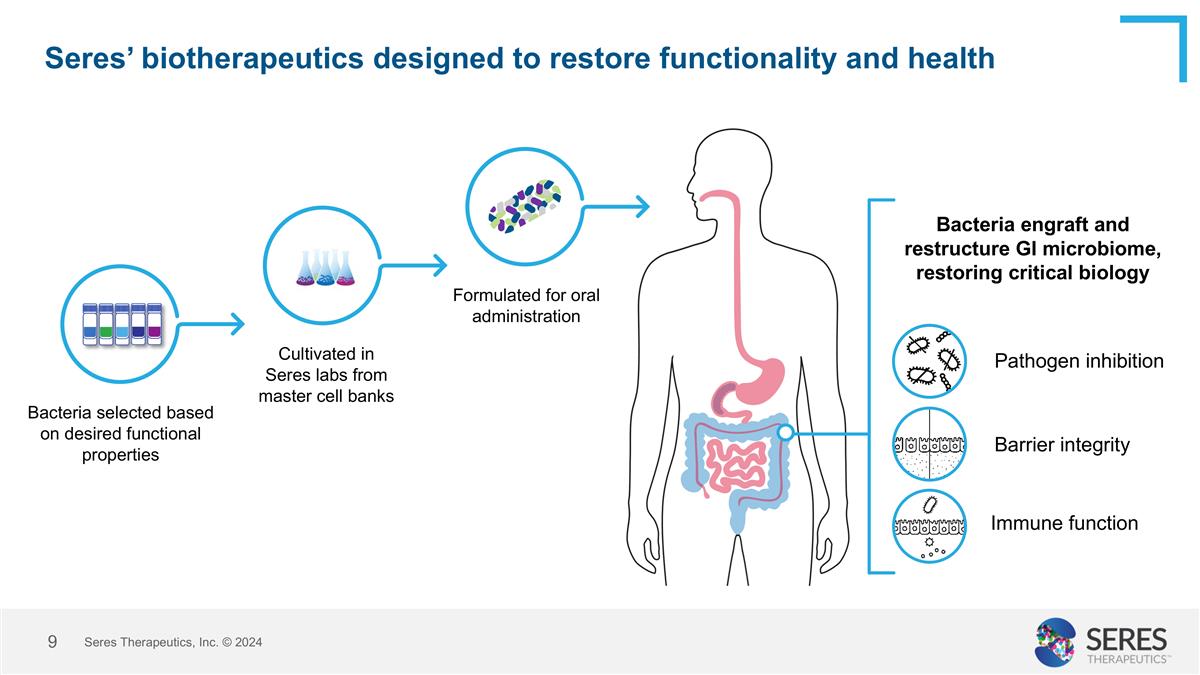
Seres’ biotherapeutics designed to restore functionality and health Formulated for oral administration Bacteria selected based on desired functional properties Cultivated in Seres labs from master cell banks Bacteria engraft and restructure GI microbiome, restoring critical biology Pathogen inhibition Barrier integrity Immune function Seres Therapeutics, Inc. © 2024
Seres’ biotherapeutics and pipeline candidates are expected to have well tolerated safety profile, reducing development risk Sources: Nanayakkara et al, CA Cancer J Clin 2021; Penack et al, Blood Adv 2020; Zheng et al, Infect Dis Ther 2021 Seres Therapeutics, Inc. © 2024 Based on GI bacteria naturally found in healthy humans, and not associated with disease VOWST product profile includes well tolerated safety without drug-related serious adverse events Well tolerated safety profile in multiple clinical trials and patient populations, including medically vulnerable allo-HSCT recipients Safety profile has potential to mitigate a primary cause of drug development failure
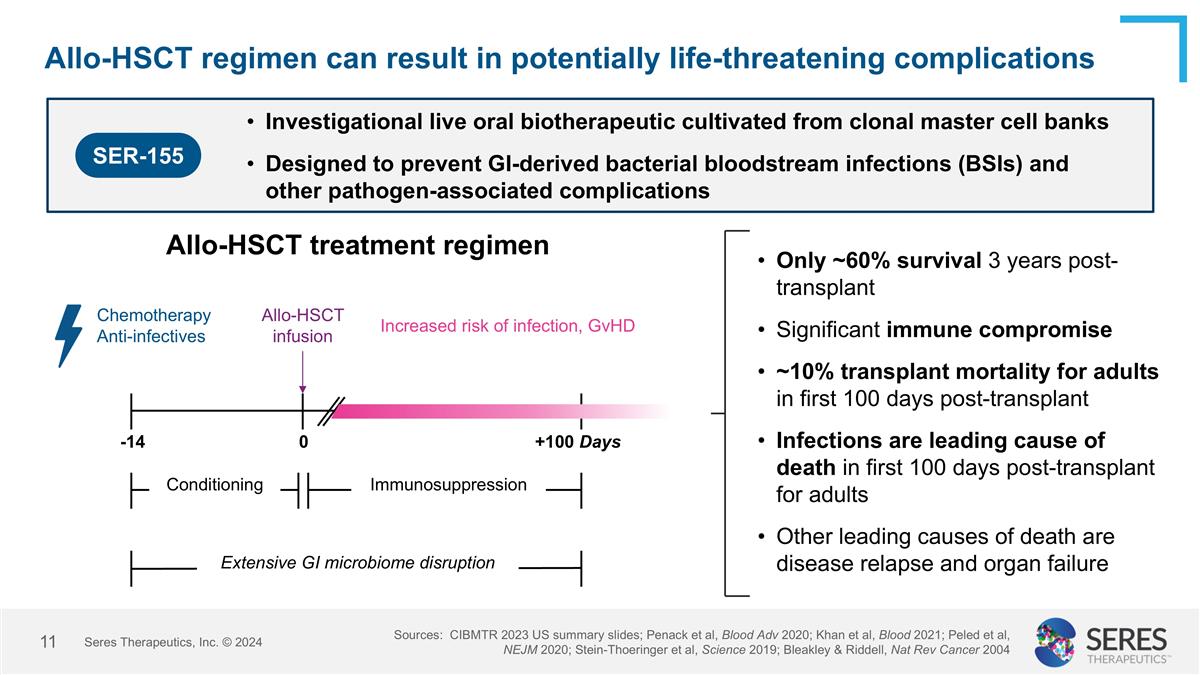
Allo-HSCT regimen can result in potentially life-threatening complications Only ~60% survival 3 years post-transplant Significant immune compromise ~10% transplant mortality for adults in first 100 days post-transplant Infections are leading cause of death in first 100 days post-transplant for adults Other leading causes of death are disease relapse and organ failure Allo-HSCT treatment regimen -14 0 +100 Days Conditioning Immunosuppression Chemotherapy Anti-infectives Increased risk of infection, GvHD Allo-HSCT infusion Extensive GI microbiome disruption Sources: CIBMTR 2023 US summary slides; Penack et al, Blood Adv 2020; Khan et al, Blood 2021; Peled et al, NEJM 2020; Stein-Thoeringer et al, Science 2019; Bleakley & Riddell, Nat Rev Cancer 2004 SER-155 Investigational live oral biotherapeutic cultivated from clonal master cell banks Designed to prevent GI-derived bacterial bloodstream infections (BSIs) and other pathogen-associated complications Seres Therapeutics, Inc. © 2024
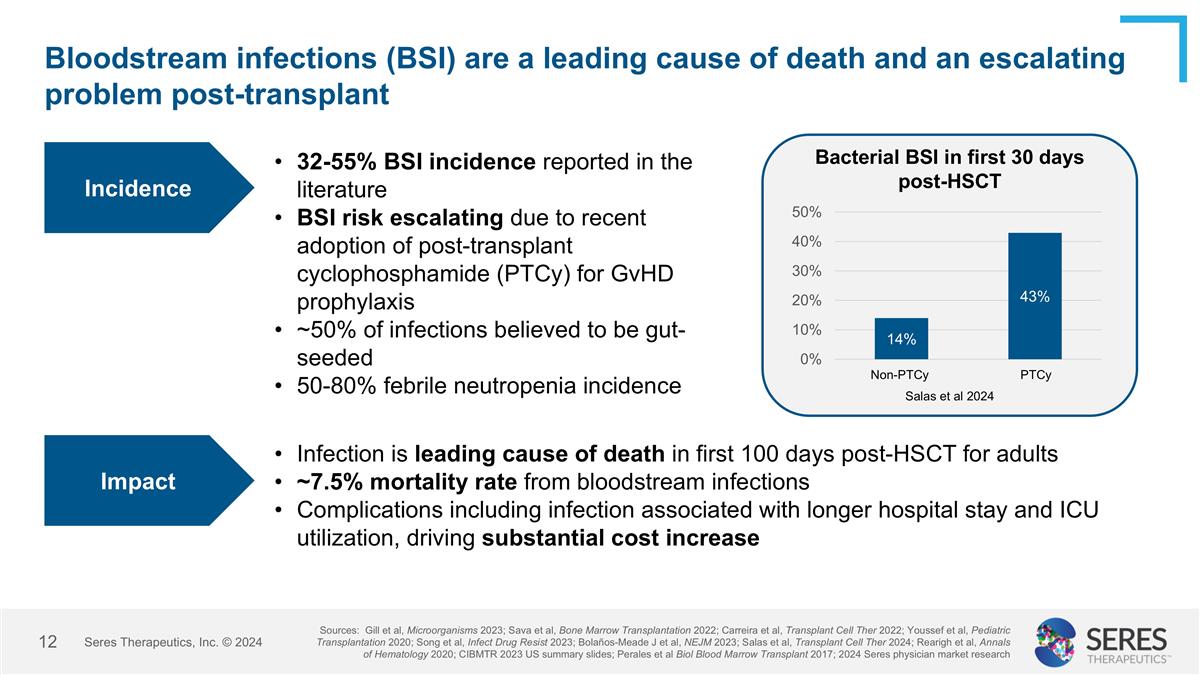
Bloodstream infections (BSI) are a leading cause of death and an escalating problem post-transplant 32-55% BSI incidence reported in the literature BSI risk escalating due to recent adoption of post-transplant cyclophosphamide (PTCy) for GvHD prophylaxis ~50% of infections believed to be gut-seeded 50-80% febrile neutropenia incidence Incidence Sources: Gill et al, Microorganisms 2023; Sava et al, Bone Marrow Transplantation 2022; Carreira et al, Transplant Cell Ther 2022; Youssef et al, Pediatric Transplantation 2020; Song et al, Infect Drug Resist 2023; Bolaños-Meade J et al, NEJM 2023; Salas et al, Transplant Cell Ther 2024; Rearigh et al, Annals of Hematology 2020; CIBMTR 2023 US summary slides; Perales et al Biol Blood Marrow Transplant 2017; 2024 Seres physician market research Bacterial BSI in first 30 days post-HSCT Salas et al 2024 Impact Infection is leading cause of death in first 100 days post-HSCT for adults ~7.5% mortality rate from bloodstream infections Complications including infection associated with longer hospital stay and ICU utilization, driving substantial cost increase Seres Therapeutics, Inc. © 2024 PTCy Non-PTCy
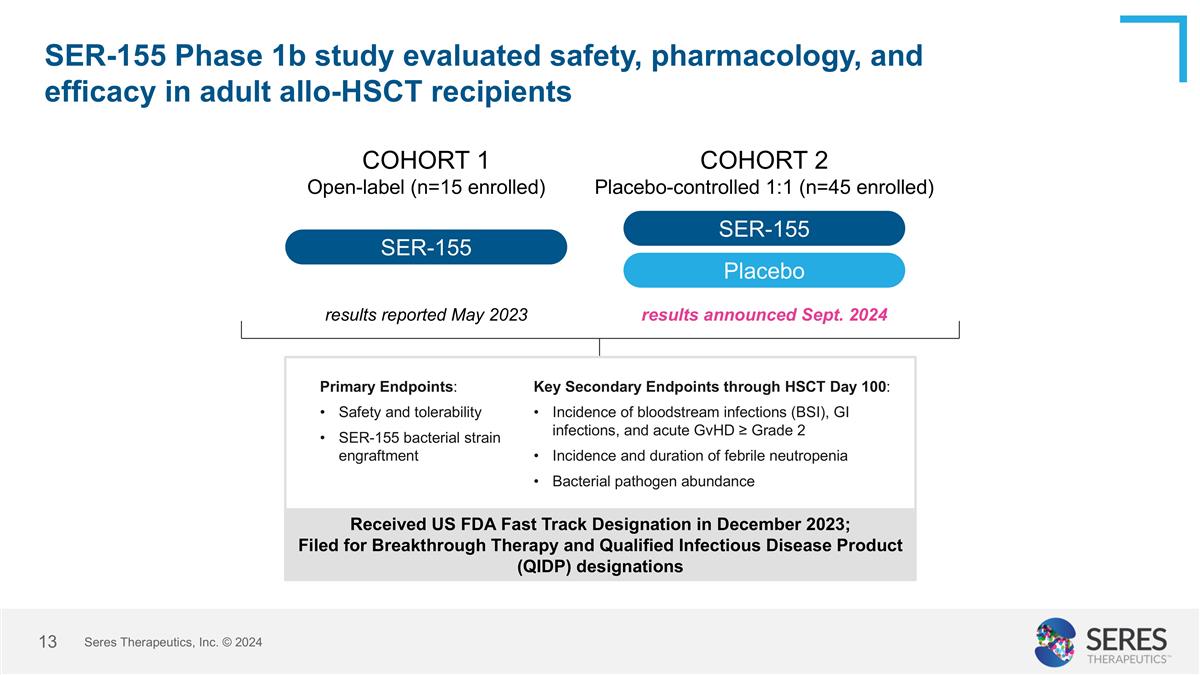
SER-155 Phase 1b study evaluated safety, pharmacology, and efficacy in adult allo-HSCT recipients SER-155 COHORT 1 Open-label (n=15 enrolled) COHORT 2 Placebo-controlled 1:1 (n=45 enrolled) SER-155 Placebo results reported May 2023 results announced Sept. 2024 Primary Endpoints: Safety and tolerability SER-155 bacterial strain engraftment Received US FDA Fast Track Designation in December 2023; Filed for Breakthrough Therapy and Qualified Infectious Disease Product (QIDP) designations Key Secondary Endpoints through HSCT Day 100: Incidence of bloodstream infections (BSI), GI infections, and acute GvHD ≥ Grade 2 Incidence and duration of febrile neutropenia Bacterial pathogen abundance Seres Therapeutics, Inc. © 2024
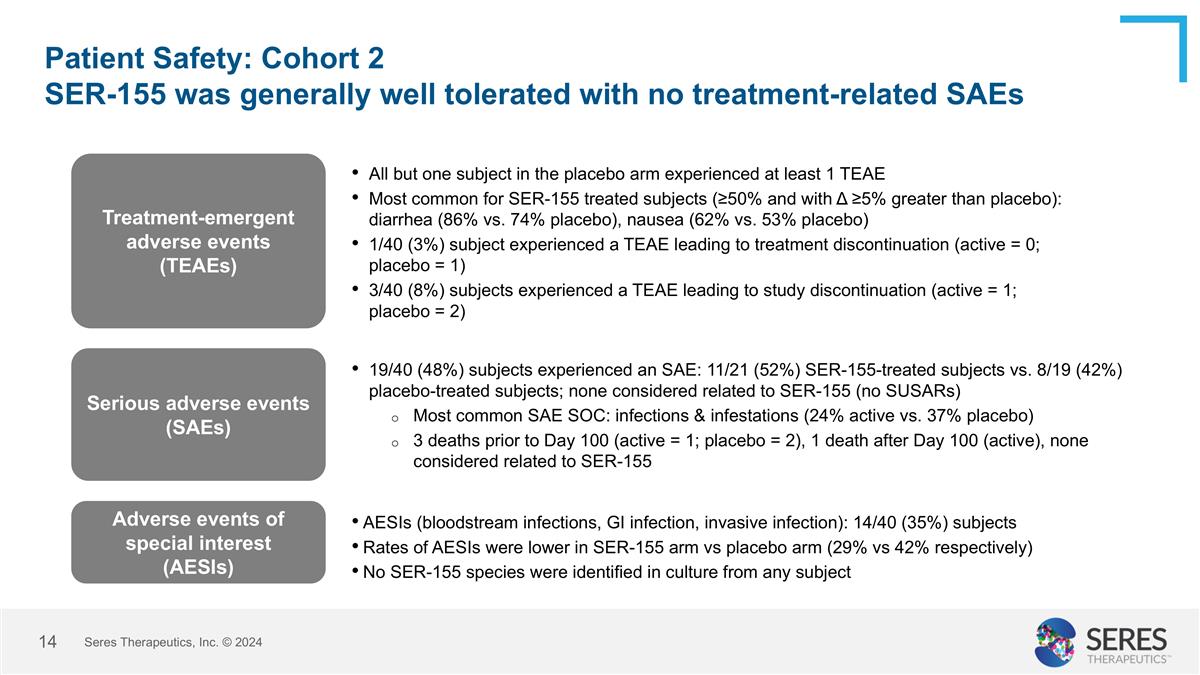
Patient Safety: Cohort 2 SER-155 was generally well tolerated with no treatment-related SAEs Seres Therapeutics, Inc. © 2024 Treatment-emergent adverse events (TEAEs) All but one subject in the placebo arm experienced at least 1 TEAE Most common for SER-155 treated subjects (≥50% and with Δ ≥5% greater than placebo): diarrhea (86% vs. 74% placebo), nausea (62% vs. 53% placebo) 1/40 (3%) subject experienced a TEAE leading to treatment discontinuation (active = 0; placebo = 1) 3/40 (8%) subjects experienced a TEAE leading to study discontinuation (active = 1; placebo = 2) Serious adverse events (SAEs) 19/40 (48%) subjects experienced an SAE: 11/21 (52%) SER-155-treated subjects vs. 8/19 (42%) placebo-treated subjects; none considered related to SER-155 (no SUSARs) Most common SAE SOC: infections & infestations (24% active vs. 37% placebo) 3 deaths prior to Day 100 (active = 1; placebo = 2), 1 death after Day 100 (active), none considered related to SER-155 Adverse events of special interest (AESIs) AESIs (bloodstream infections, GI infection, invasive infection): 14/40 (35%) subjects Rates of AESIs were lower in SER-155 arm vs placebo arm (29% vs 42% respectively) No SER-155 species were identified in culture from any subject
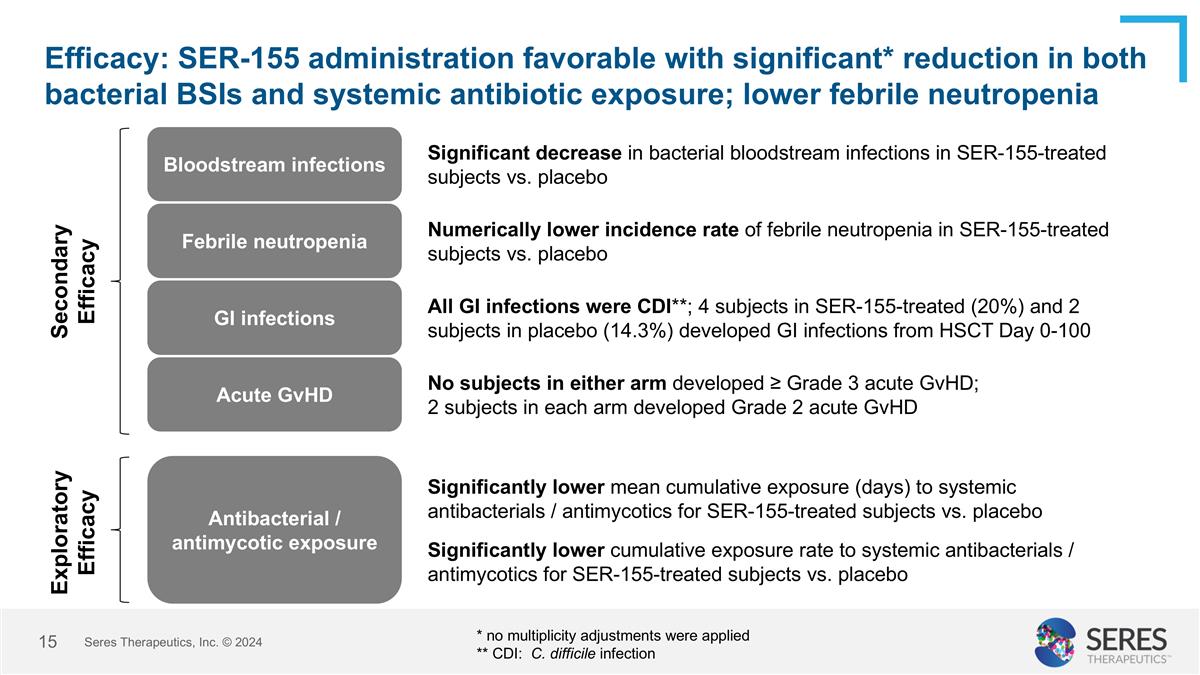
Efficacy: SER-155 administration favorable with significant* reduction in both bacterial BSIs and systemic antibiotic exposure; lower febrile neutropenia Seres Therapeutics, Inc. © 2024 Bloodstream infections Significant decrease in bacterial bloodstream infections in SER-155-treated subjects vs. placebo Acute GvHD No subjects in either arm developed ≥ Grade 3 acute GvHD; 2 subjects in each arm developed Grade 2 acute GvHD Numerically lower incidence rate of febrile neutropenia in SER-155-treated subjects vs. placebo Febrile neutropenia Antibacterial / antimycotic exposure Significantly lower mean cumulative exposure (days) to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo Significantly lower cumulative exposure rate to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo * no multiplicity adjustments were applied ** CDI: C. difficile infection Secondary Efficacy Exploratory Efficacy GI infections All GI infections were CDI**; 4 subjects in SER-155-treated (20%) and 2 subjects in placebo (14.3%) developed GI infections from HSCT Day 0-100
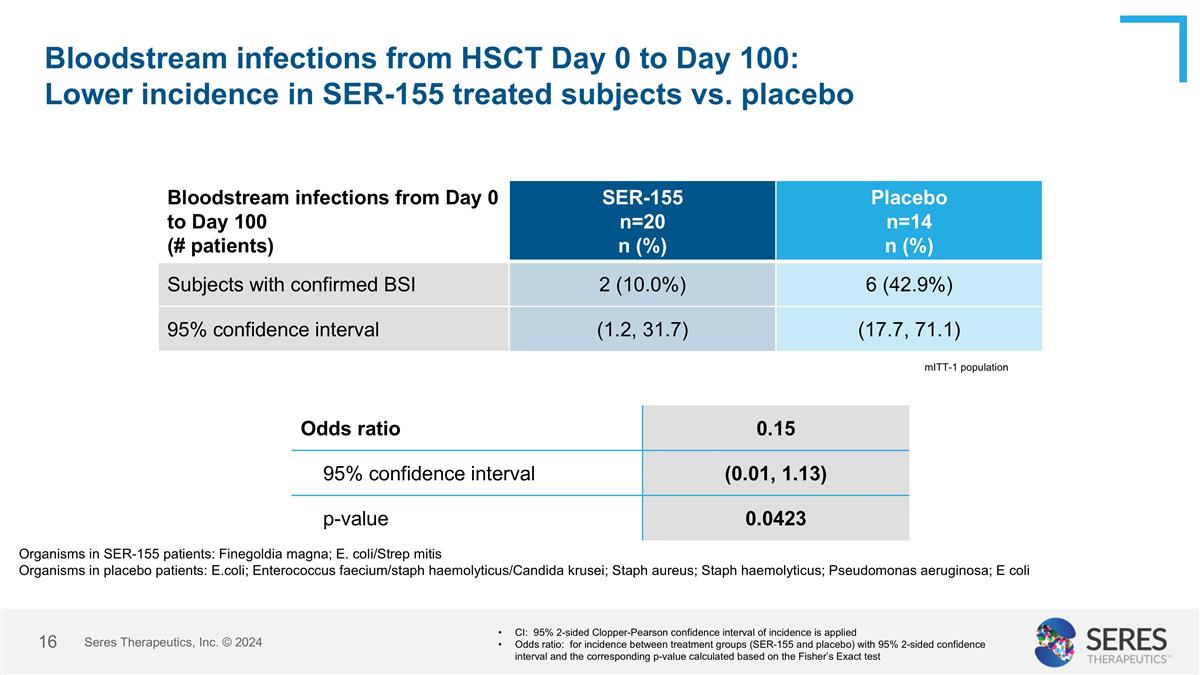
Bloodstream infections from HSCT Day 0 to Day 100: Lower incidence in SER-155 treated subjects vs. placebo Seres Therapeutics, Inc. © 2024 Bloodstream infections from Day 0 to Day 100 (# patients) SER-155 n=20 n (%) Placebo n=14 n (%) Subjects with confirmed BSI 2 (10.0%) 6 (42.9%) 95% confidence interval (1.2, 31.7) (17.7, 71.1) CI: 95% 2-sided Clopper-Pearson confidence interval of incidence is applied Odds ratio: for incidence between treatment groups (SER-155 and placebo) with 95% 2-sided confidence interval and the corresponding p-value calculated based on the Fisher’s Exact test Odds ratio 0.15 95% confidence interval (0.01, 1.13) p-value 0.0423 mITT-1 population Organisms in SER-155 patients: Finegoldia magna; E. coli/Strep mitis Organisms in placebo patients: E.coli; Enterococcus faecium/staph haemolyticus/Candida krusei; Staph aureus; Staph haemolyticus; Pseudomonas aeruginosa; E coli

Cumulative exposure to systemic antibacterials / antimycotics through HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Seres Therapeutics, Inc. © 2024 Cumulative Antibacterial or Antimycotic Exposure (HSCT Days) SER-155 n=20 n (SD) Placebo n=14 n (SD) Mean (SD) 9.2 (5.44) 21.1 (20.31) Median 9.0 14.0 Min, Max 0, 19 0, 74 Cumulative exposure is the sum of all days a subject received systemic antibacterials and/or antimycotics between HSCT Day 0 through Day 100; counting once per day regardless of number of agents taken 95% confidence interval and p-value based on independent samples t-test of the difference in mean days between SER-155 and placebo Mean Difference (95% CI) -11.9 (-23.85, -0.04) p-value 0.0494 mITT-1 population
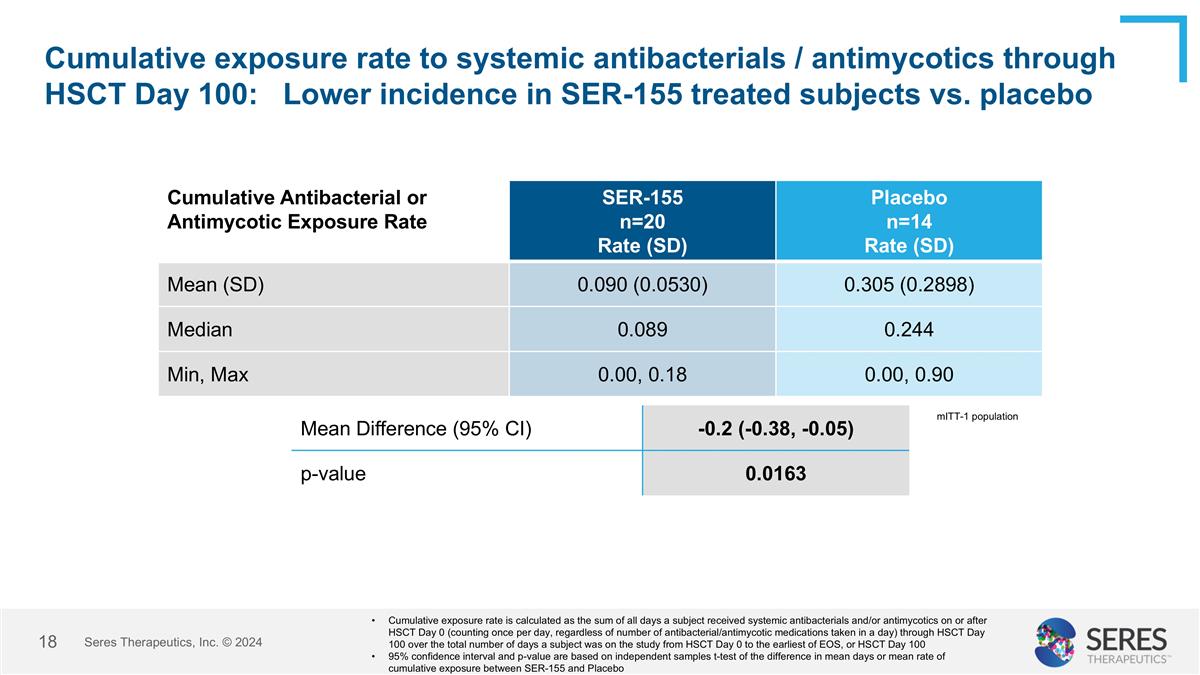
Cumulative exposure rate to systemic antibacterials / antimycotics through HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Seres Therapeutics, Inc. © 2024 Cumulative Antibacterial or Antimycotic Exposure Rate SER-155 n=20 Rate (SD) Placebo n=14 Rate (SD) Mean (SD) 0.090 (0.0530) 0.305 (0.2898) Median 0.089 0.244 Min, Max 0.00, 0.18 0.00, 0.90 Cumulative exposure rate is calculated as the sum of all days a subject received systemic antibacterials and/or antimycotics on or after HSCT Day 0 (counting once per day, regardless of number of antibacterial/antimycotic medications taken in a day) through HSCT Day 100 over the total number of days a subject was on the study from HSCT Day 0 to the earliest of EOS, or HSCT Day 100 95% confidence interval and p-value are based on independent samples t-test of the difference in mean days or mean rate of cumulative exposure between SER-155 and Placebo Mean Difference (95% CI) -0.2 (-0.38, -0.05) p-value 0.0163 mITT-1 population
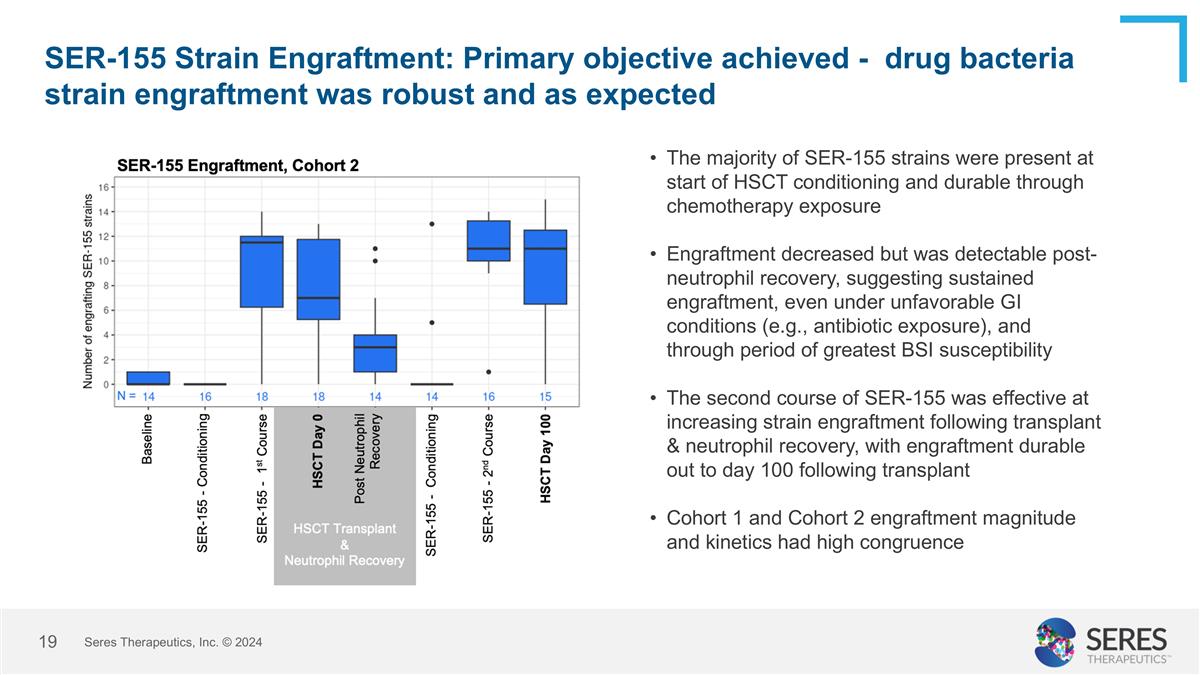
SER-155 Strain Engraftment: Primary objective achieved - drug bacteria strain engraftment was robust and as expected Seres Therapeutics, Inc. © 2024 The majority of SER-155 strains were present at start of HSCT conditioning and durable through chemotherapy exposure Engraftment decreased but was detectable post-neutrophil recovery, suggesting sustained engraftment, even under unfavorable GI conditions (e.g., antibiotic exposure), and through period of greatest BSI susceptibility The second course of SER-155 was effective at increasing strain engraftment following transplant & neutrophil recovery, with engraftment durable out to day 100 following transplant Cohort 1 and Cohort 2 engraftment magnitude and kinetics had high congruence
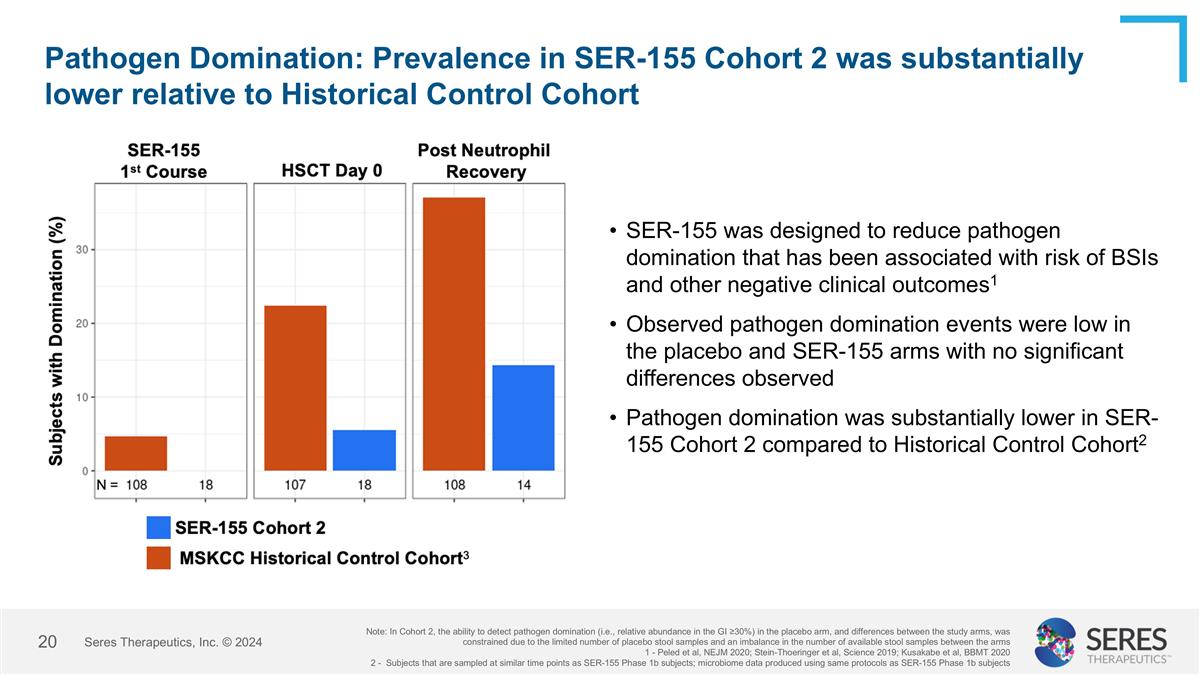
Pathogen Domination: Prevalence in SER-155 Cohort 2 was substantially lower relative to Historical Control Cohort Seres Therapeutics, Inc. © 2024 SER-155 was designed to reduce pathogen domination that has been associated with risk of BSIs and other negative clinical outcomes1 Observed pathogen domination events were low in the placebo and SER-155 arms with no significant differences observed Pathogen domination was substantially lower in SER-155 Cohort 2 compared to Historical Control Cohort2 Note: In Cohort 2, the ability to detect pathogen domination (i.e., relative abundance in the GI ≥30%) in the placebo arm, and differences between the study arms, was constrained due to the limited number of placebo stool samples and an imbalance in the number of available stool samples between the arms 1 - Peled et al, NEJM 2020; Stein-Thoeringer et al, Science 2019; Kusakabe et al, BBMT 2020 2 - Subjects that are sampled at similar time points as SER-155 Phase 1b subjects; microbiome data produced using same protocols as SER-155 Phase 1b subjects
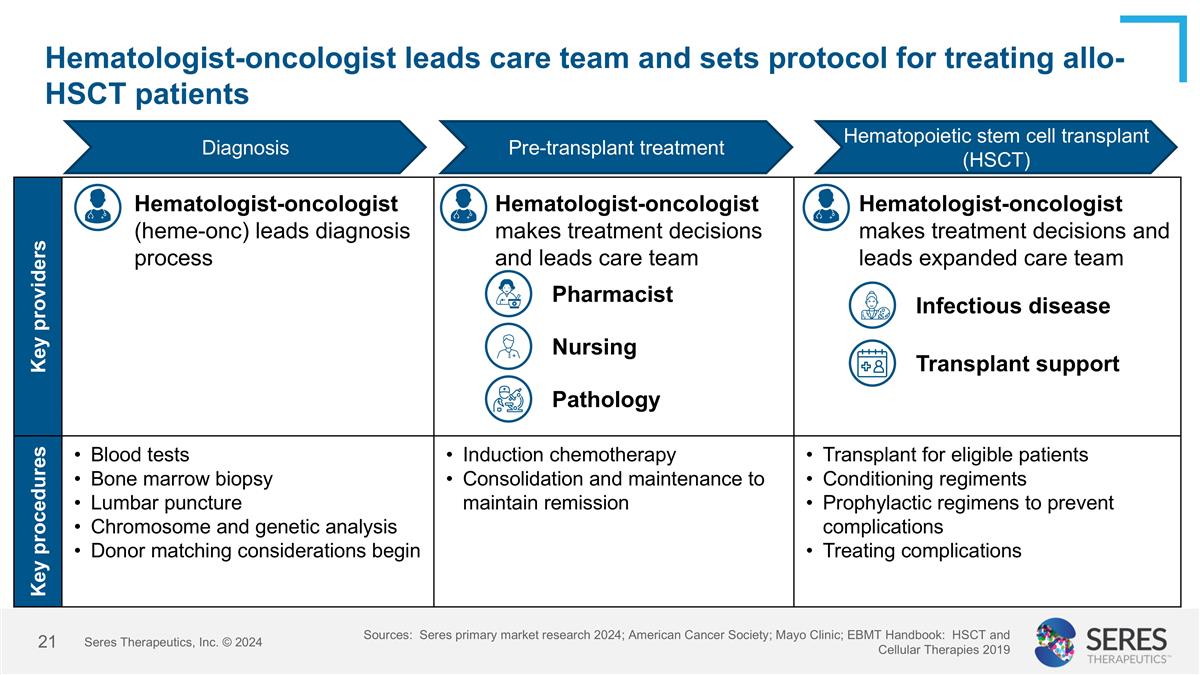
Key providers Key procedures Blood tests Bone marrow biopsy Lumbar puncture Chromosome and genetic analysis Donor matching considerations begin Induction chemotherapy Consolidation and maintenance to maintain remission Transplant for eligible patients Conditioning regiments Prophylactic regimens to prevent complications Treating complications Hematologist-oncologist leads care team and sets protocol for treating allo-HSCT patients Sources: Seres primary market research 2024; American Cancer Society; Mayo Clinic; EBMT Handbook: HSCT and Cellular Therapies 2019 Diagnosis Pre-transplant treatment Hematopoietic stem cell transplant (HSCT) Seres Therapeutics, Inc. © 2024 Hematologist-oncologist (heme-onc) leads diagnosis process Hematologist-oncologist makes treatment decisions and leads care team Hematologist-oncologist makes treatment decisions and leads expanded care team Pharmacist Nursing Infectious disease Pathology Transplant support
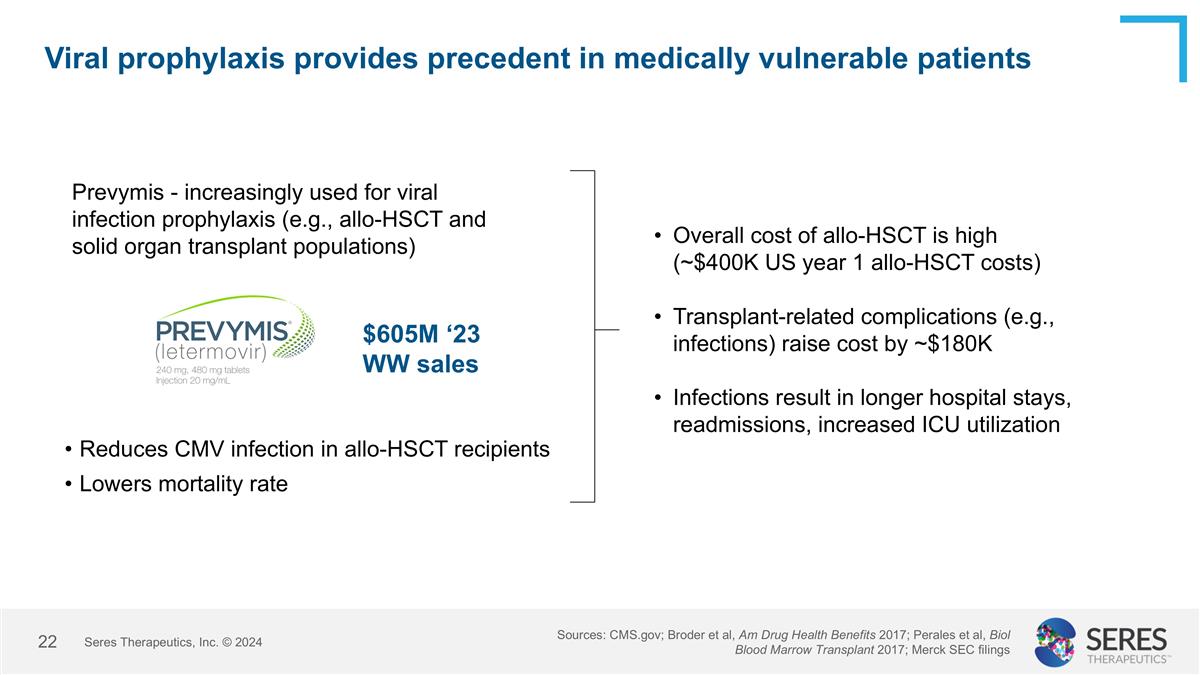
Viral prophylaxis provides precedent in medically vulnerable patients Prevymis - increasingly used for viral infection prophylaxis (e.g., allo-HSCT and solid organ transplant populations) $605M ‘23 WW sales Reduces CMV infection in allo-HSCT recipients Lowers mortality rate Overall cost of allo-HSCT is high (~$400K US year 1 allo-HSCT costs) Transplant-related complications (e.g., infections) raise cost by ~$180K Infections result in longer hospital stays, readmissions, increased ICU utilization Seres Therapeutics, Inc. © 2024 Sources: CMS.gov; Broder et al, Am Drug Health Benefits 2017; Perales et al, Biol Blood Marrow Transplant 2017; Merck SEC filings
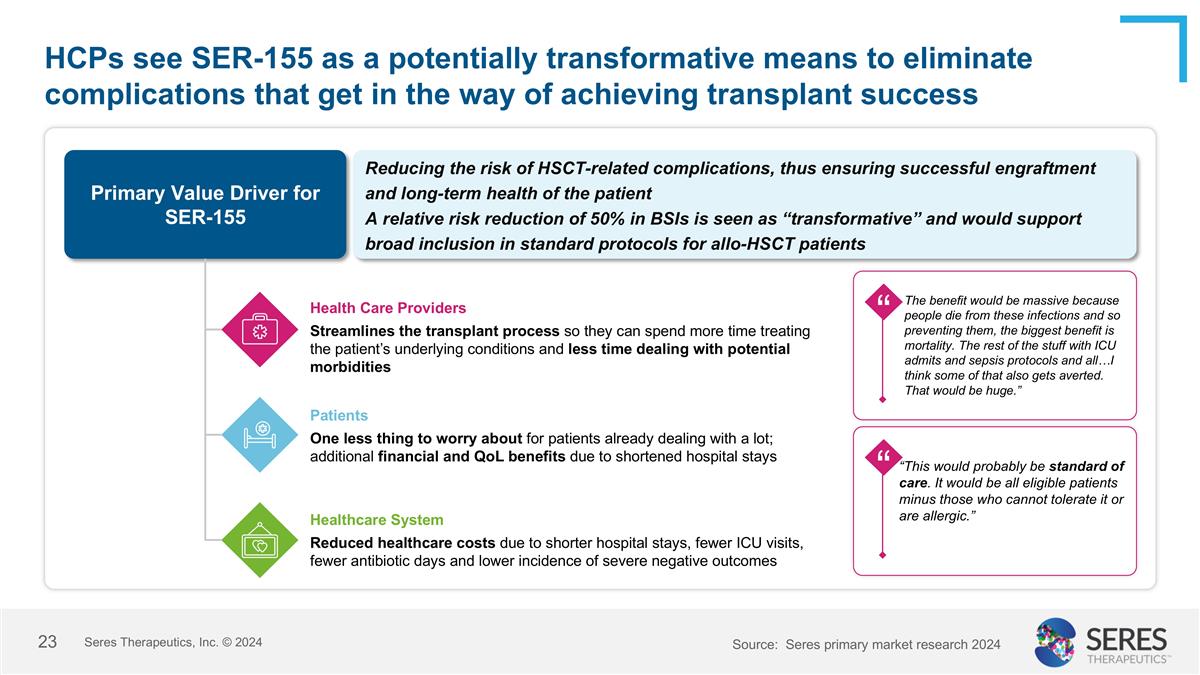
HCPs see SER-155 as a potentially transformative means to eliminate complications that get in the way of achieving transplant success Primary Value Driver for SER-155 Reducing the risk of HSCT-related complications, thus ensuring successful engraftment and long-term health of the patient A relative risk reduction of 50% in BSIs is seen as “transformative” and would support broad inclusion in standard protocols for allo-HSCT patients Health Care Providers Streamlines the transplant process so they can spend more time treating the patient’s underlying conditions and less time dealing with potential morbidities Patients One less thing to worry about for patients already dealing with a lot; additional financial and QoL benefits due to shortened hospital stays Healthcare System Reduced healthcare costs due to shorter hospital stays, fewer ICU visits, fewer antibiotic days and lower incidence of severe negative outcomes “This would probably be standard of care. It would be all eligible patients minus those who cannot tolerate it or are allergic.” “ “ “ The benefit would be massive because people die from these infections and so preventing them, the biggest benefit is mortality. The rest of the stuff with ICU admits and sepsis protocols and all…I think some of that also gets averted. That would be huge.” Source: Seres primary market research 2024 Seres Therapeutics, Inc. © 2024
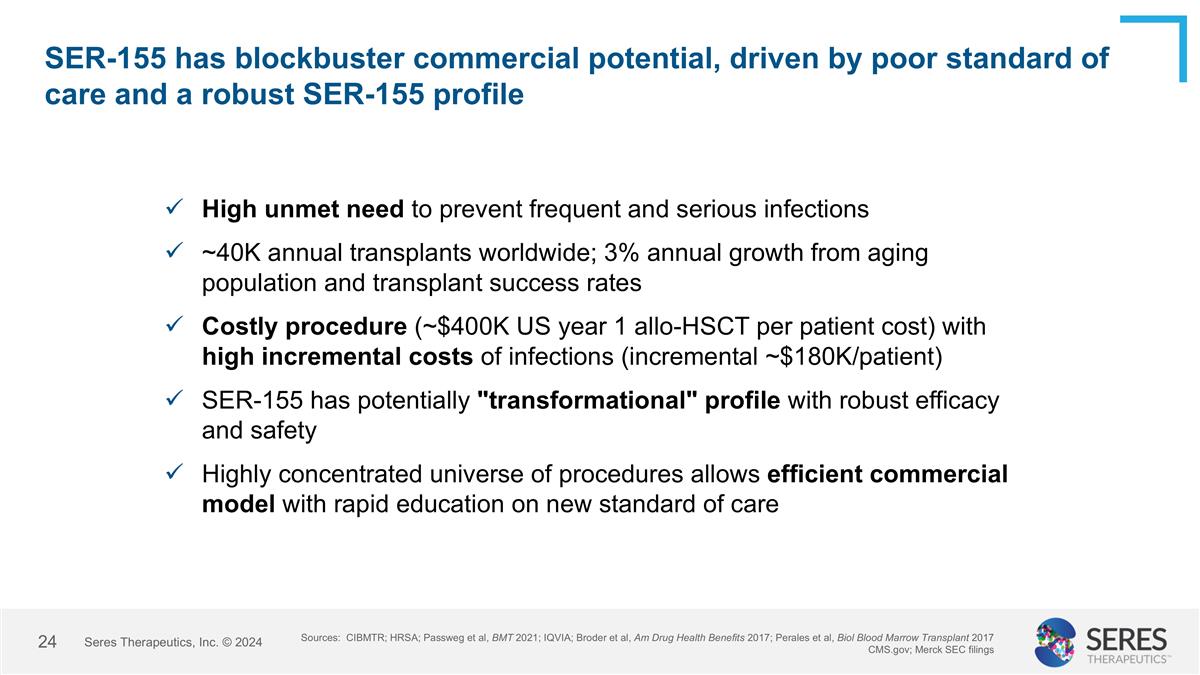
SER-155 has blockbuster commercial potential, driven by poor standard of care and a robust SER-155 profile Sources: CIBMTR; HRSA; Passweg et al, BMT 2021; IQVIA; Broder et al, Am Drug Health Benefits 2017; Perales et al, Biol Blood Marrow Transplant 2017 CMS.gov; Merck SEC filings High unmet need to prevent frequent and serious infections ~40K annual transplants worldwide; 3% annual growth from aging population and transplant success rates Costly procedure (~$400K US year 1 allo-HSCT per patient cost) with high incremental costs of infections (incremental ~$180K/patient) SER-155 has potentially "transformational" profile with robust efficacy and safety Highly concentrated universe of procedures allows efficient commercial model with rapid education on new standard of care Seres Therapeutics, Inc. © 2024
Accelerating SER-155 clinical development with positive Ph1b outcomes Seeking SER-155 strategic partnership to accelerate next study in allo-HSCT and expand to multiple target populations Aim to accelerate SER-155 development in allo-HSCT Potential to follow successful precedent from VOWST development with single registrational study for efficacy Engage with FDA on advancement of SER-155 allo-HSCT program Filed for Breakthrough Therapy and Qualified Infections Disease Product designations Expect to receive feedback by end of 2024 Intend to evaluate SER-155 in additional patient populations with high risk of serious bacterial infections Seres Therapeutics, Inc. © 2024
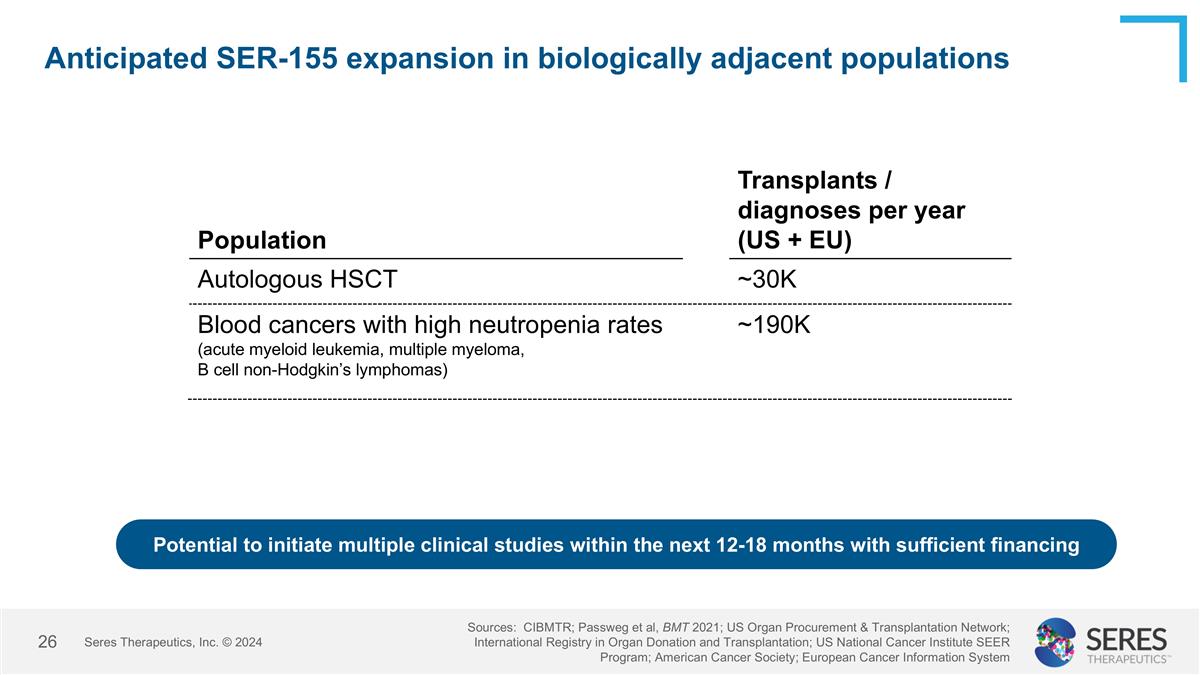
Anticipated SER-155 expansion in biologically adjacent populations Seres Therapeutics, Inc. © 2024 Sources: CIBMTR; Passweg et al, BMT 2021; US Organ Procurement & Transplantation Network; International Registry in Organ Donation and Transplantation; US National Cancer Institute SEER Program; American Cancer Society; European Cancer Information System Population Transplants / diagnoses per year (US + EU) Autologous HSCT ~30K Blood cancers with high neutropenia rates (acute myeloid leukemia, multiple myeloma, B cell non-Hodgkin’s lymphomas) ~190K Potential to initiate multiple clinical studies within the next 12-18 months with sufficient financing
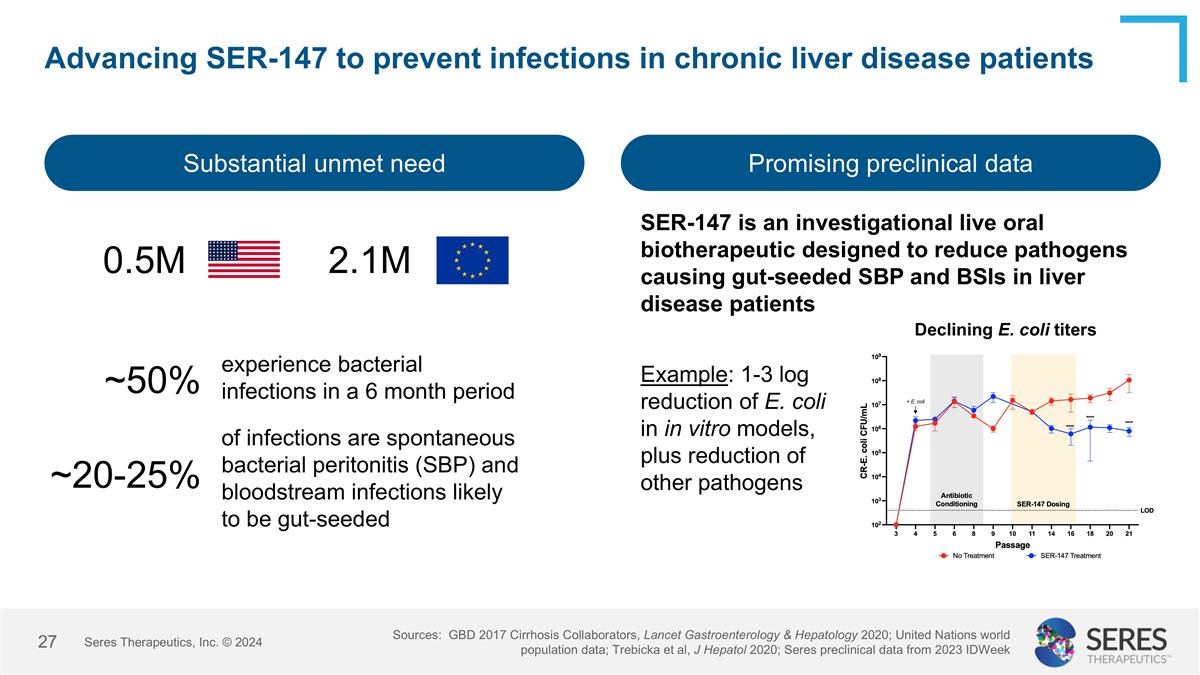
Advancing SER-147 to prevent infections in chronic liver disease patients Substantial unmet need Promising preclinical data Sources: GBD 2017 Cirrhosis Collaborators, Lancet Gastroenterology & Hepatology 2020; United Nations world population data; Trebicka et al, J Hepatol 2020; Seres preclinical data from 2023 IDWeek 0.5M 2.1M ~50% SER-147 is an investigational live oral biotherapeutic designed to reduce pathogens causing gut-seeded SBP and BSIs in liver disease patients Declining E. coli titers experience bacterial infections in a 6 month period ~20-25% of infections are spontaneous bacterial peritonitis (SBP) and bloodstream infections likely to be gut-seeded Seres Therapeutics, Inc. © 2024 Example: 1-3 log reduction of E. coli in in vitro models, plus reduction of other pathogens
Manufacturing platform delivers defined consortia in oral formulation using cost-effective production Novel formulations enabling consistent drug product composition, drug stability for distribution, and targeted drug delivery Quality systems to ensure product quality and stability, extending prior regulatory successes, including developing product release specifications with the FDA Highly intensive strain bioprocessing leveraging flexible, single-use manufacturing technology for cost-effective production Strain isolation and characterization pipeline to rapidly identify cGMP-suitable medium components Seres Therapeutics, Inc. © 2024
Maximizing opportunity going forward SER-155 Allo-HSCT: Engaging with FDA to accelerate; filed for Breakthrough Therapy and Qualified Infectious Disease Product designations Evaluate in additional populations with high risk of serious bacterial infections Prevent life-threatening infections in additional populations Treat immune-related diseases (e.g., IBD, GvHD, checkpoint colitis, radiation enteritis) Additional Opportunities SER-147 Chronic liver disease: Progressing towards IND readiness Indication expansion (e.g., ICU and long-term care patients, organ transplant) rCDI: Proven clinical and regulatory success; asset sale to Nestlé; Seres to participate in future milestones VOWST Seres Therapeutics, Inc. © 2024
Summary and path forward SER-155 Phase 1b placebo-controlled clinical results promising SER-155 administration associated with 77% relative risk reduction for bloodstream infections SER-155 administration associated with significant reduction in systemic antibiotic exposure and lower incidence of febrile neutropenia as compared to placebo through day 100 post HSCT SER-155 demonstrated generally well tolerated safety profile and confirmed drug bacteria strain engraftment Company is pursuing SER-155 strategic partnership to accelerate next study in allo-HSCT and expand to multiple target populations VOWST asset sale strengthens financial position $66.8M in cash at end Q3 2024; cash runway projected into Q4 2025 Fully retired outstanding debt VOWST asset sale closed in September; received $175M at closing less an ~$20M settlement of net obligations, and $75M (less ~$1.5M in employment-related payments) in installment payments due in 2025 + $275M potential future milestones Developing a pipeline of novel live biotherapeutics in areas of high unmet need SER-155 Phase 1b placebo-controlled clinical efficacy data further support Seres’ strategy Pipeline aims to bring transformative medicines to a wider set of patients, led by SER-155 while advancing SER-147 VOWST approval validates using live biotherapeutics to prevent life-threatening infections Seres Therapeutics, Inc. © 2024





























