
New Hope For Serious Infections Corporate Presentation January 2017 Exhibit 99.1

Forward-Looking Statements These slides and the accompanying oral presentation (the “Presentation”) contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding the effectiveness, safety, long-acting nature, anticipated human dosing, potential to treat infections and other attributes of CD101 IV and CD101 topical, as well as the incidence of fungal infections (and related potential market sizes) and the effectiveness and treatment protocols for competitive therapies. Statements regarding the effectiveness, safety, potential to treat infections and other attributes of and plans for CD201, as well as the intended design of current and future Cloudbreak compounds, are also forward-looking. Risks that contribute to the uncertain nature of the forward-looking statements include: the success and timing of Cidara’s preclinical studies, clinical trials and other research and development activities; regulatory developments in the United States and foreign countries; changes in Cidara’s plans to develop and commercialize its product candidates; Cidara’s ability to obtain additional financing; Cidara’s ability to obtain and maintain intellectual property protection for its product candidates; and the loss of key scientific or management personnel. These and other risks and uncertainties are described more fully in Cidara’s Form 10-K and Form 10-Q, each as most recently filed with the United States Securities and Exchange Commission (SEC), under the heading “Risk Factors.” All forward-looking statements contained in the Presentation speak only as of the date on which they were made. Cidara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Two Platforms, Multiple Programs CD101 topical for VVC, Phase 2 CD101 IV for candidemia, Phase 2 CD101 Echinocandin Antifungal In-vivo proof of concept: antifungal Development candidate: antibacterial Volker Brinkmann, Wikimedia.org Cloudbreak™ Immunotherapy
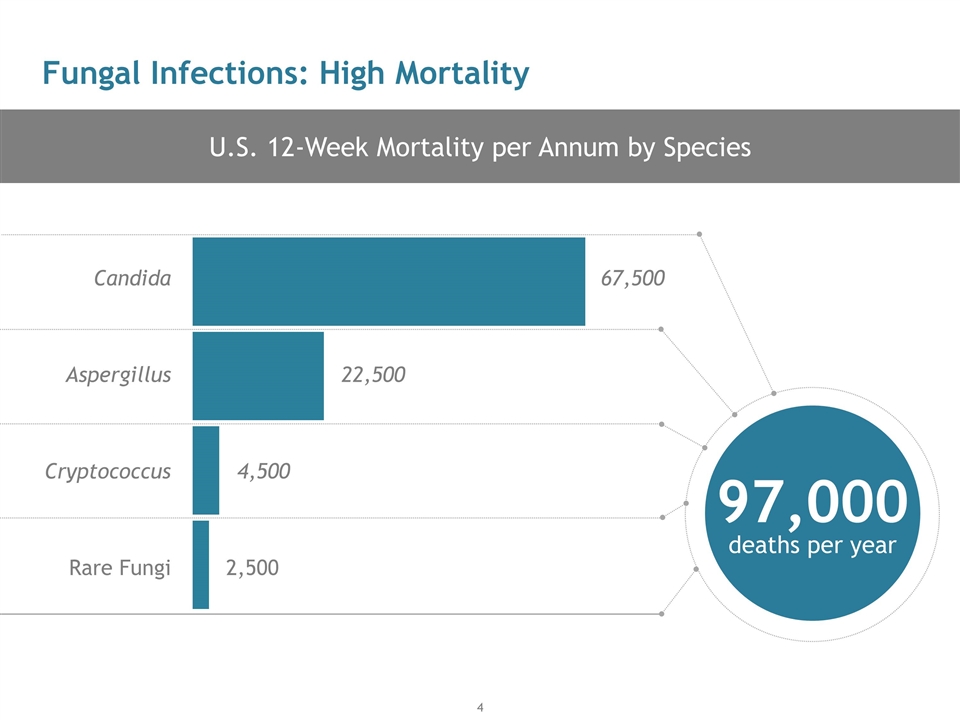
U.S. 12-Week Mortality per Annum by Species Fungal Infections: High Mortality Rare Fungi Cryptococcus Aspergillus Candida 2,500 4,500 22,500 67,500 97,000 deaths per year
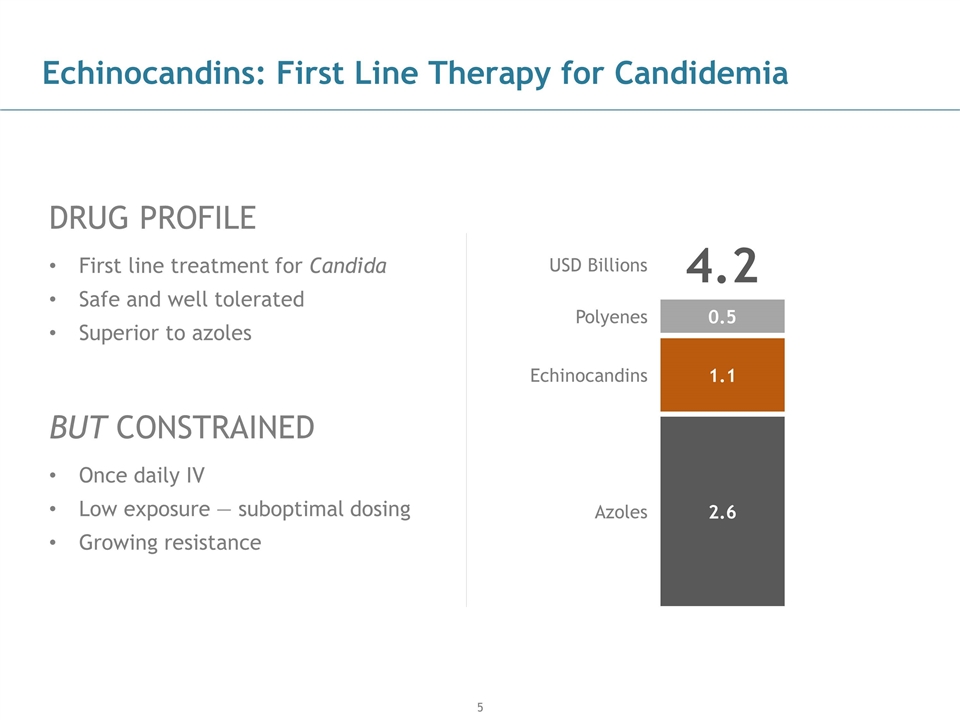
Echinocandins: First Line Therapy for Candidemia DRUG PROFILE First line treatment for Candida Safe and well tolerated Superior to azoles BUT CONSTRAINED Once daily IV Low exposure — suboptimal dosing Growing resistance USD Billions 4.2 0.5 Polyenes 1.1 Echinocandins 2.6 Azoles
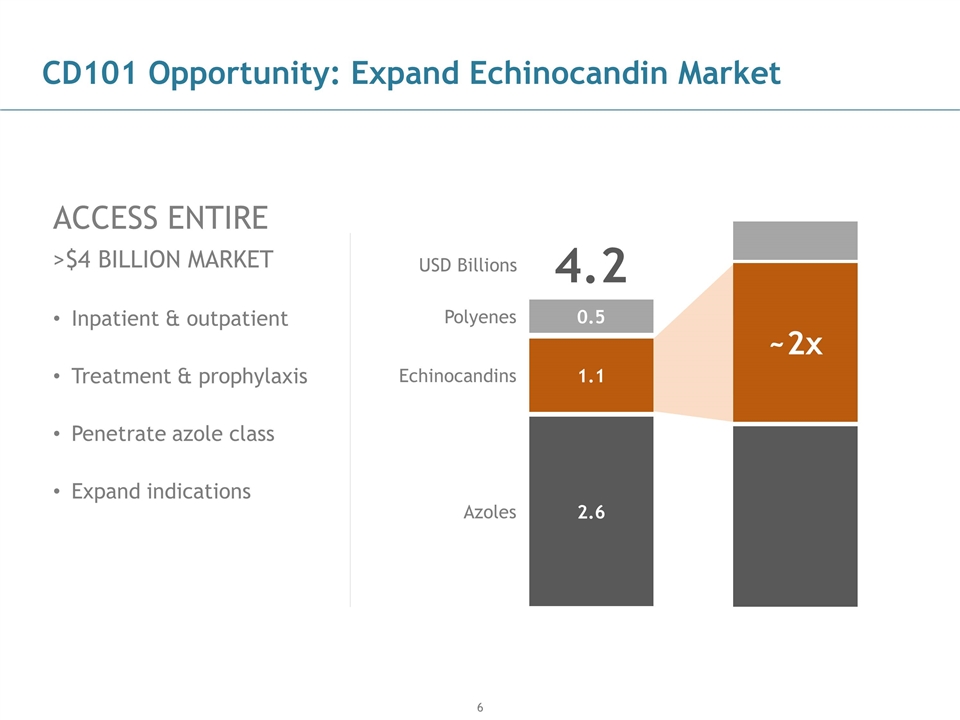
CD101 Opportunity: Expand Echinocandin Market ACCESS ENTIRE >$4 BILLION MARKET Inpatient & outpatient Treatment & prophylaxis Penetrate azole class Expand indications 1.1 2.6 0.5 4.2 Polyenes Echinocandins Azoles ~2x USD Billions
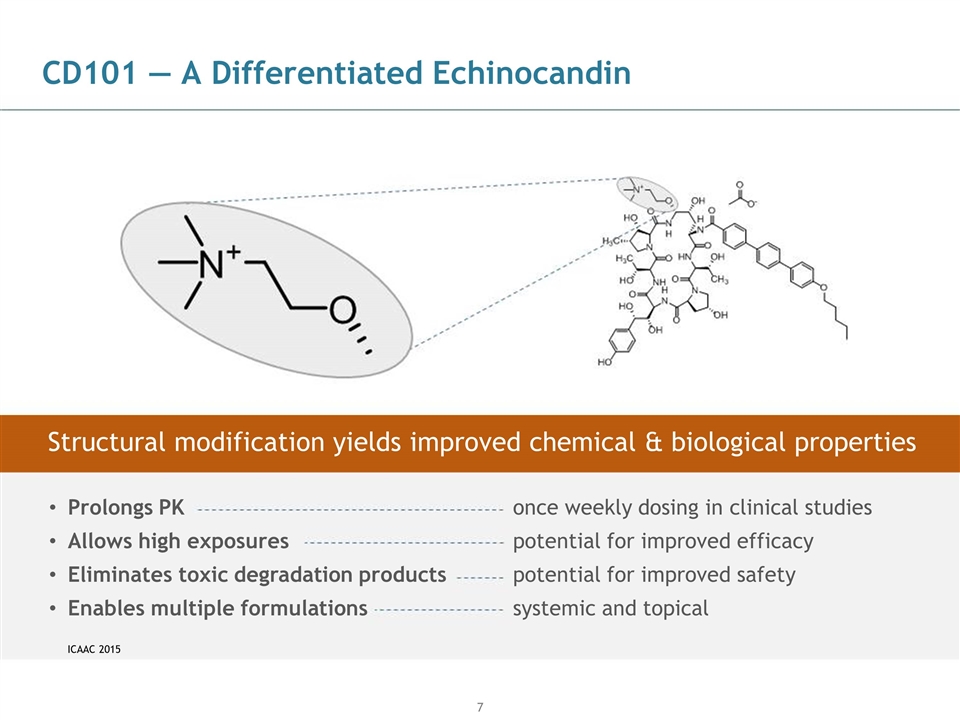
CD101 — A Differentiated Echinocandin ICAAC 2015 Prolongs PK Allows high exposures Eliminates toxic degradation products Enables multiple formulations once weekly dosing in clinical studies potential for improved efficacy potential for improved safety systemic and topical Structural modification yields improved chemical & biological properties
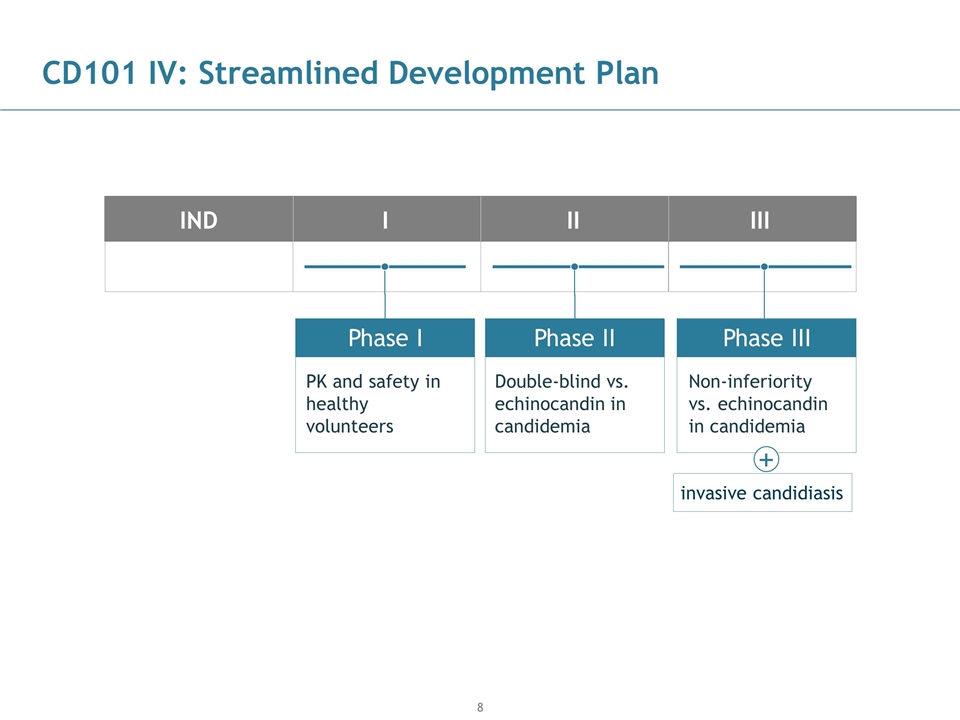
CD101 IV: Streamlined Development Plan PK and safety in healthy volunteers Double-blind vs. echinocandin in candidemia Phase I Phase II Non-inferiority vs. echinocandin in candidemia IND I II III Phase III + invasive candidiasis
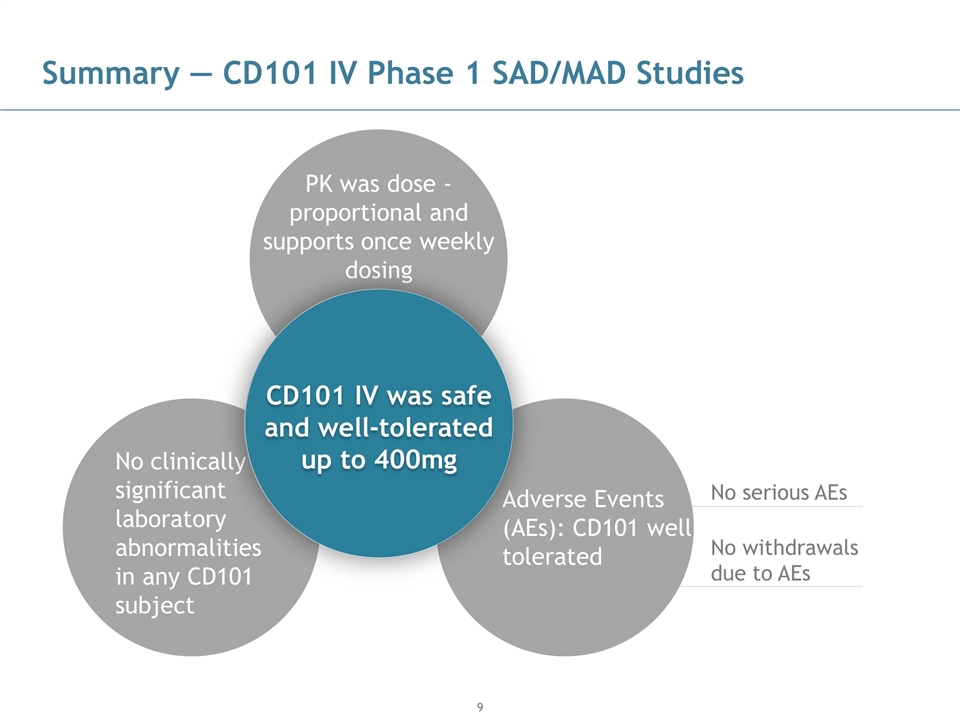
Summary — CD101 IV Phase 1 SAD/MAD Studies No serious AEs No withdrawals due to AEs PK was dose -proportional and supports once weekly dosing No clinically significant laboratory abnormalities in any CD101 subject Adverse Events (AEs): CD101 well tolerated CD101 IV was safe and well-tolerated up to 400mg
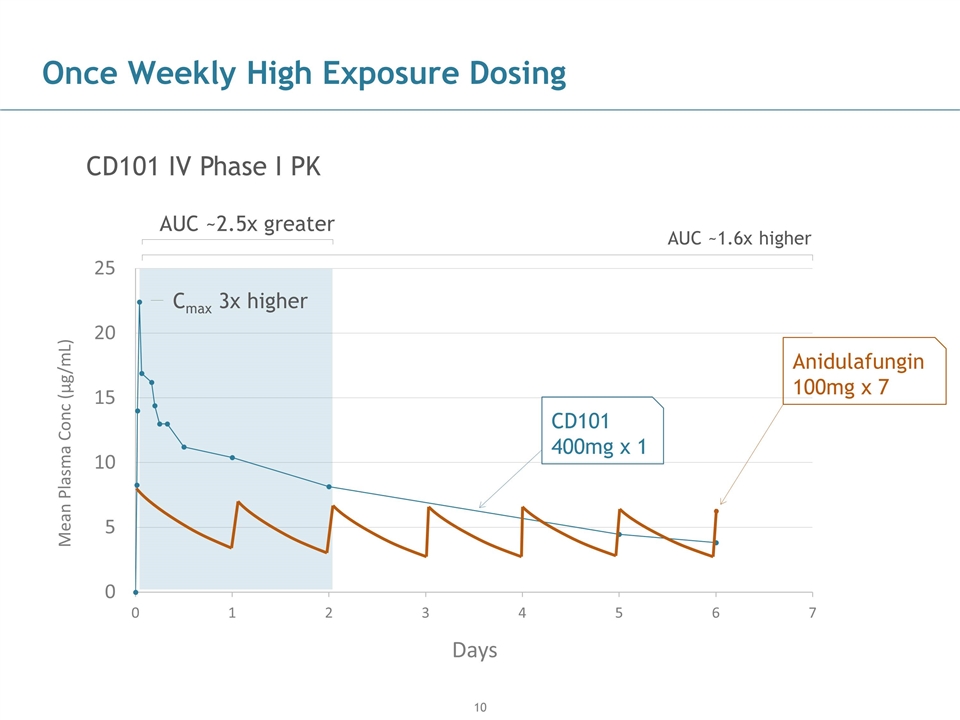
AUC ~2.5x greater AUC ~1.6x higher Once Weekly High Exposure Dosing CD101 IV Phase I PK Cmax 3x higher Anidulafungin 100mg x 7 CD101 400mg x 1
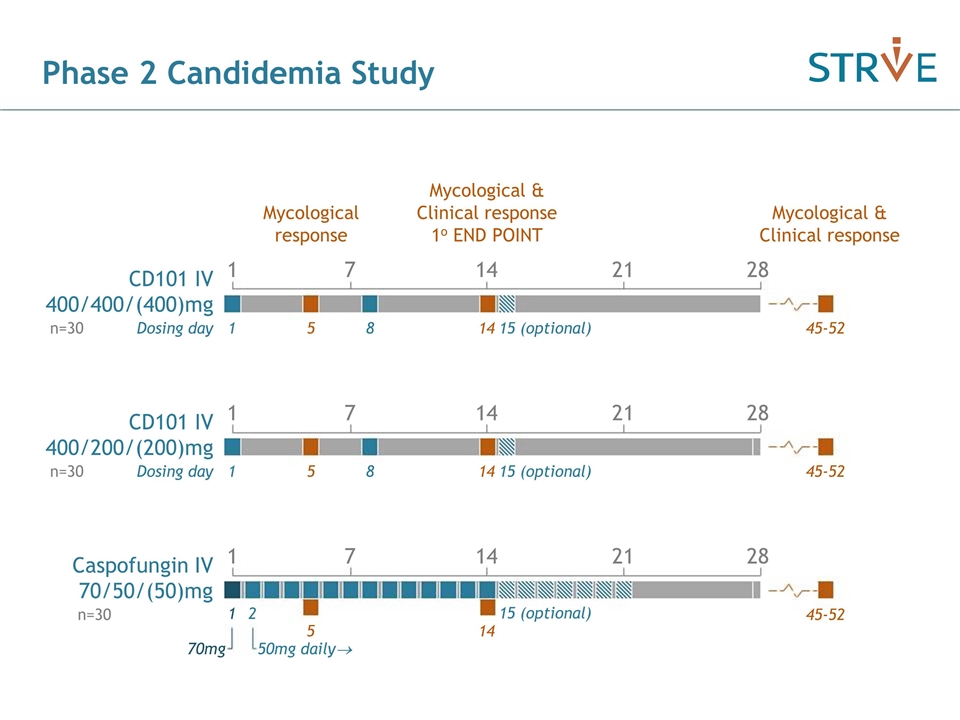
Phase 2 Candidemia Study CD101 IV 400/400/(400)mg 1 8 Dosing day 15 (optional) 5 Mycological response 14 Mycological & Clinical response 1o END POINT Mycological & Clinical response 45-52 n=30 CD101 IV 400/200/(200)mg 1 8 Dosing day 15 (optional) 5 14 45-52 n=30 1 7 14 21 28 1 7 14 21 28 Caspofungin IV 70/50/(50)mg 1 7 14 21 28 5 14 45-52 n=30 70mg 50mg daily® 15 (optional) 1 2
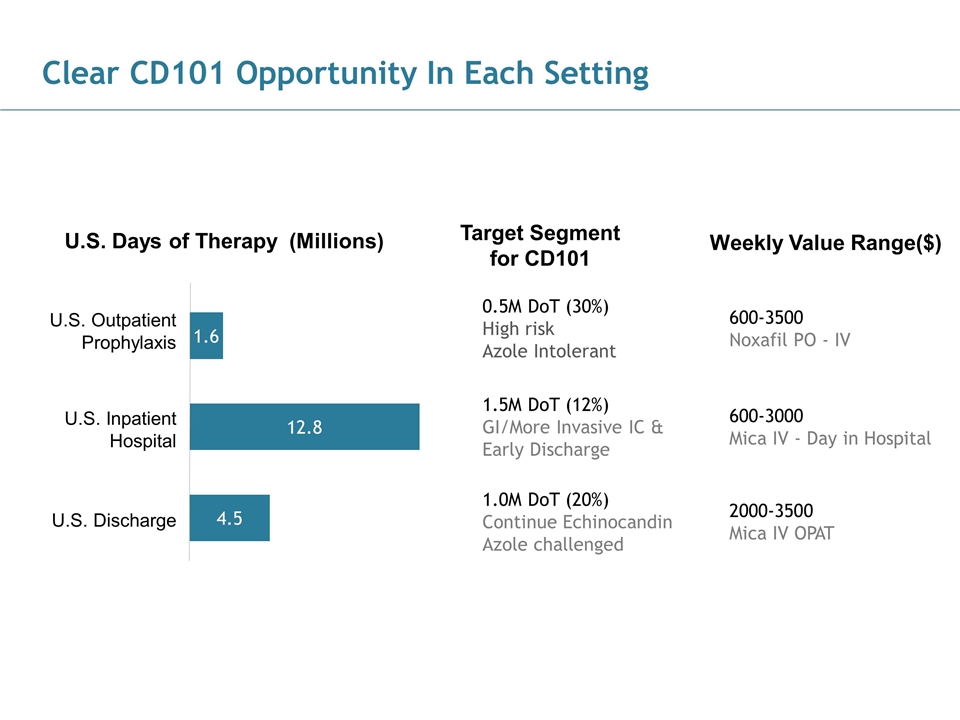
186, 89, 21 43, 124, 154 28, 83, 102 102, 102, 102 170, 170, 170 U.S. Days of Therapy (Millions) U.S. Outpatient Prophylaxis U.S. Inpatient Hospital U.S. Discharge Clear CD101 Opportunity In Each Setting 1.5M DoT (12%) GI/More Invasive IC & Early Discharge 1.0M DoT (20%) Continue Echinocandin Azole challenged 0.5M DoT (30%) High risk Azole Intolerant Target Segment for CD101 Weekly Value Range($) 600-3000 Mica IV - Day in Hospital 2000-3500 Mica IV OPAT 600-3500 Noxafil PO - IV
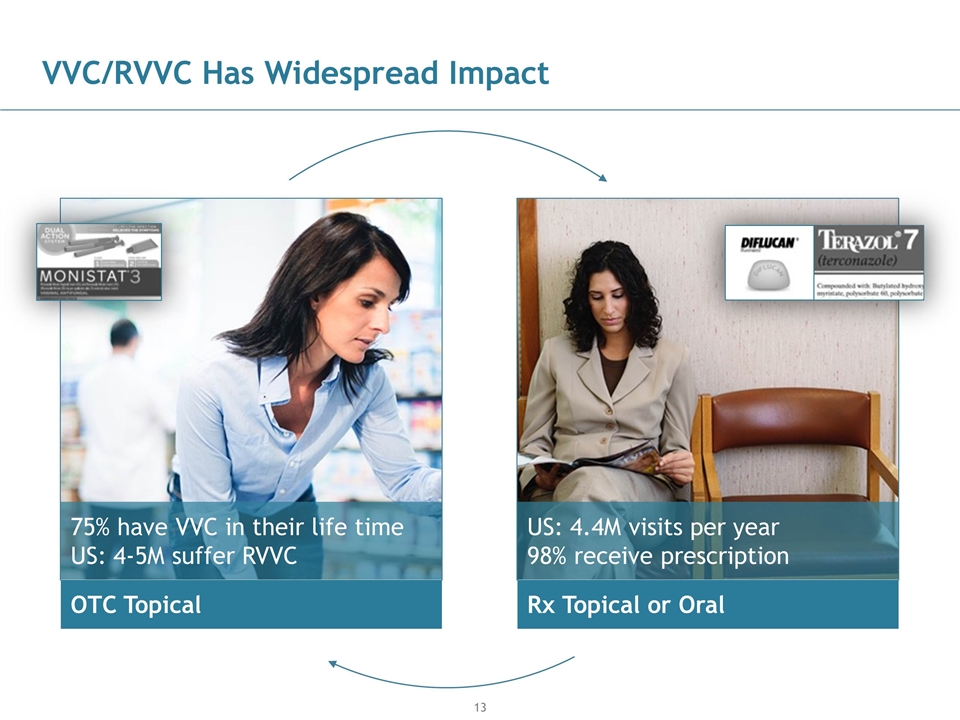
VVC/RVVC Has Widespread Impact OTC Topical 75% have VVC in their life time US: 4-5M suffer RVVC Rx Topical or Oral US: 4.4M visits per year 98% receive prescription

Existing Therapies Have Significant Limitations 30-40% women fail acute treatment 50% relapse in RVVC No coverage of non-albicans Candida Increased risk of miscarriage (flucon) CDC guidelines recommend only using topical antifungals to treat pregnant women with VVC, including for longer periods than usual if these infections persist or recur
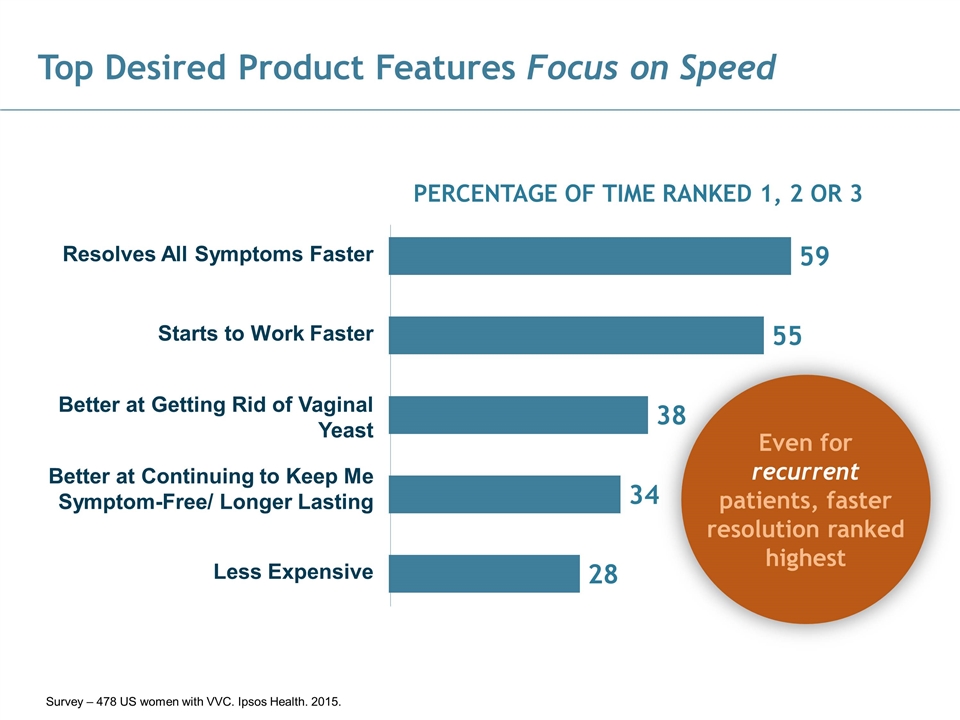
Top Desired Product Features Focus on Speed Resolves All Symptoms Faster Better at Getting Rid of Vaginal Yeast Better at Continuing to Keep Me Symptom-Free/ Longer Lasting Less Expensive Starts to Work Faster PERCENTAGE OF TIME RANKED 1, 2 OR 3 Even for recurrent patients, faster resolution ranked highest Survey – 478 US women with VVC. Ipsos Health. 2015.
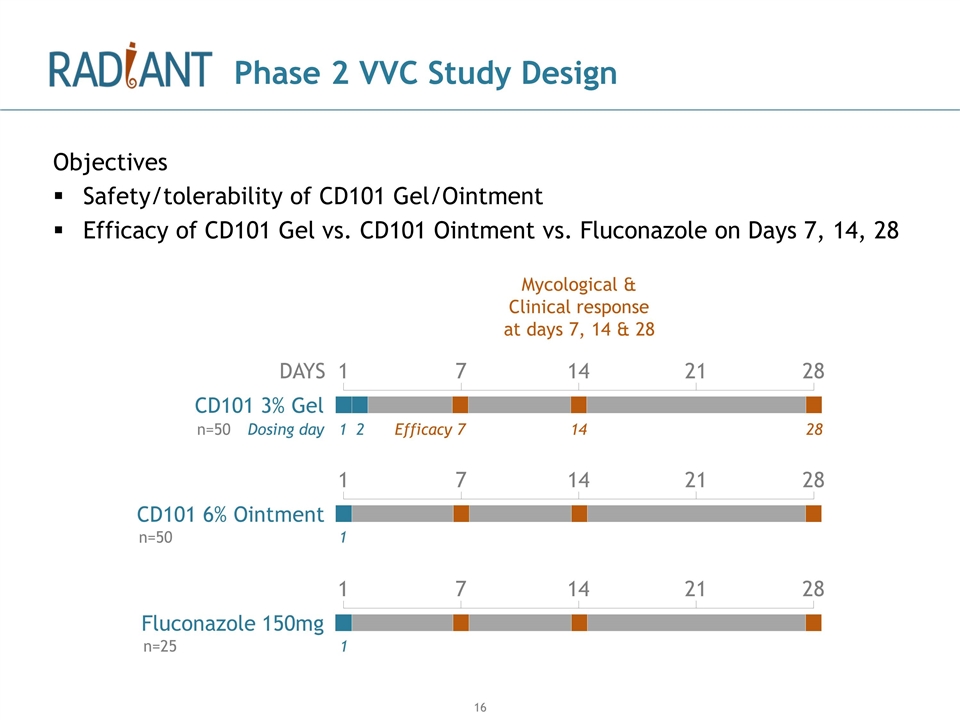
Phase 2 VVC Study Design Objectives Safety/tolerability of CD101 Gel/Ointment Efficacy of CD101 Gel vs. CD101 Ointment vs. Fluconazole on Days 7, 14, 28 DAYS CD101 3% Gel CD101 6% Ointment Fluconazole 150mg 1 2 Dosing day 1 1 1 7 14 21 28 1 7 14 21 28 1 7 14 21 28 Efficacy 7 14 28 n=50 n=50 n=25 Mycological & Clinical response at days 7, 14 & 28
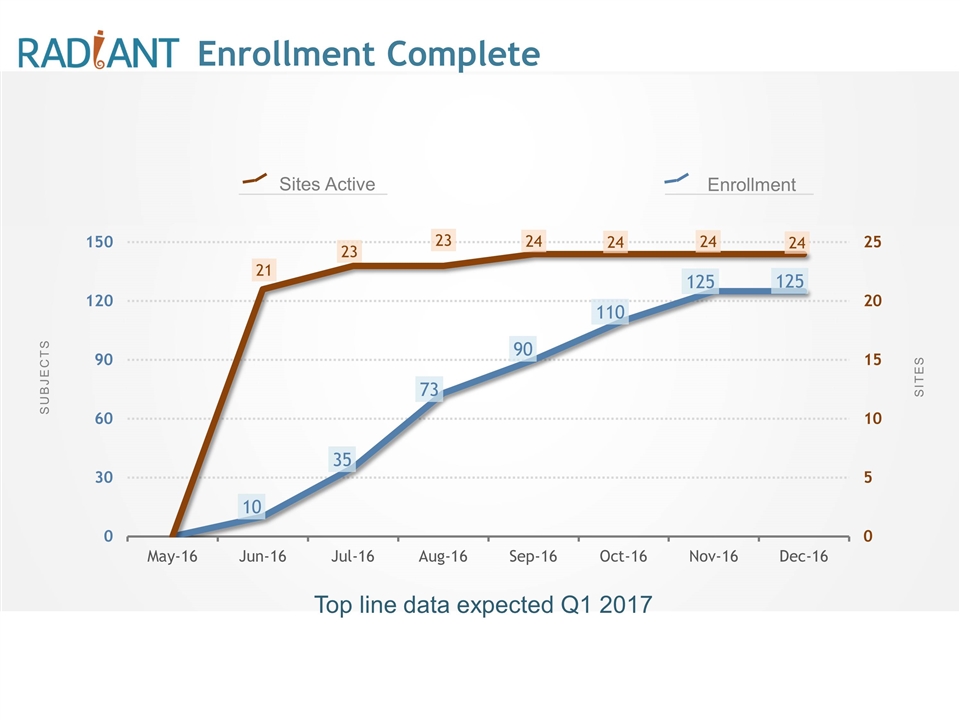
SUBJECTS SITES Sites Active Enrollment Top line data expected Q1 2017 Enrollment Complete
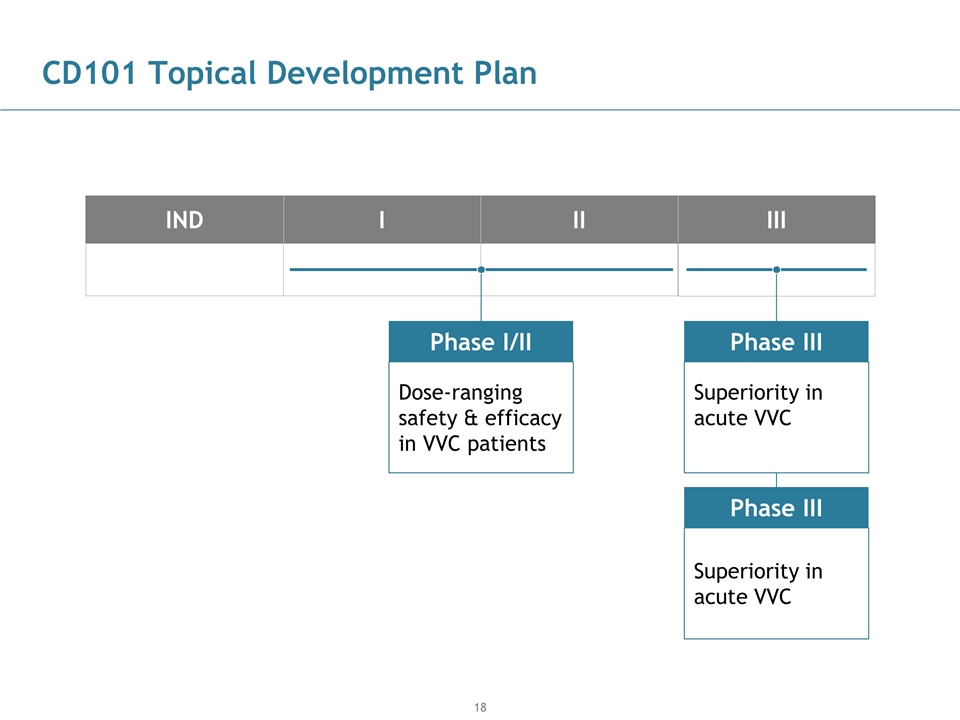
CD101 Topical Development Plan Superiority in acute VVC Superiority in acute VVC Phase I/II Phase III IND I II III Phase III Dose-ranging safety & efficacy in VVC patients
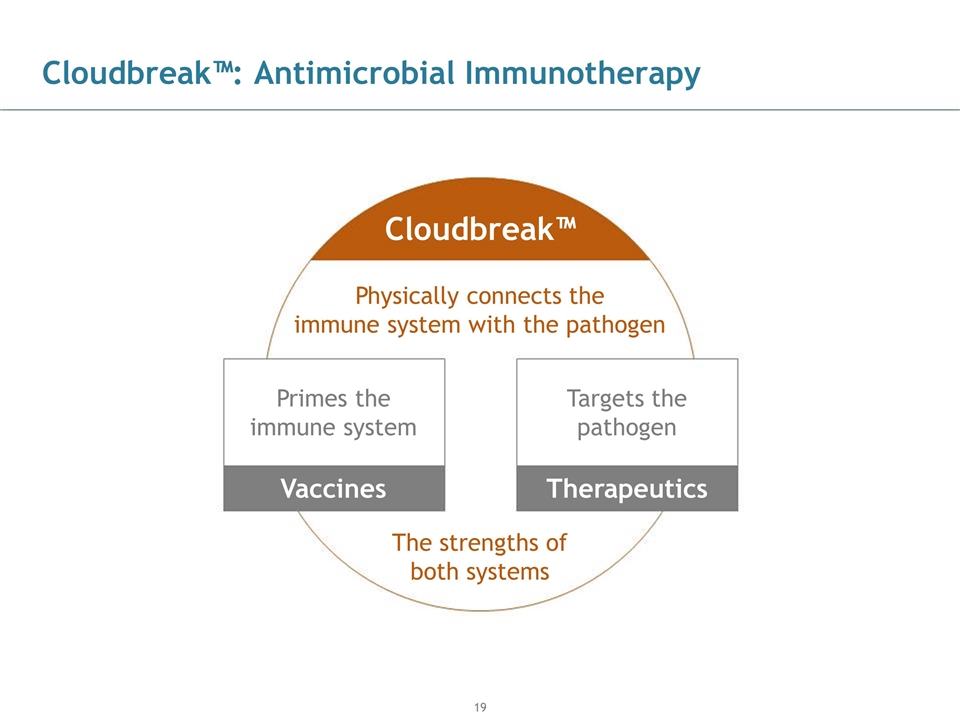
Cloudbreak™: Antimicrobial Immunotherapy Physically connects the immune system with the pathogen Vaccines Primes the immune system Therapeutics Targets the pathogen The strengths of both systems Cloudbreak™
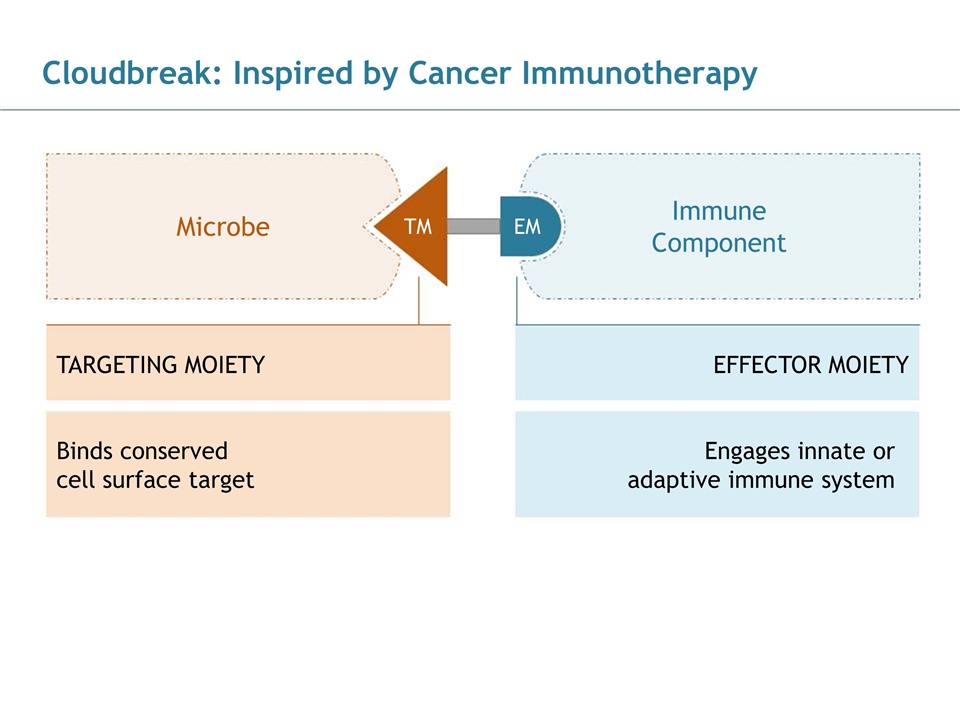
Cloudbreak: Inspired by Cancer Immunotherapy 186, 89, 21 43, 124, 154 28, 83, 102 102, 102, 102 170, 170, 170 Binds conserved cell surface target Engages innate or adaptive immune system TARGETING MOIETY Microbe Immune Component TM EM EFFECTOR MOIETY
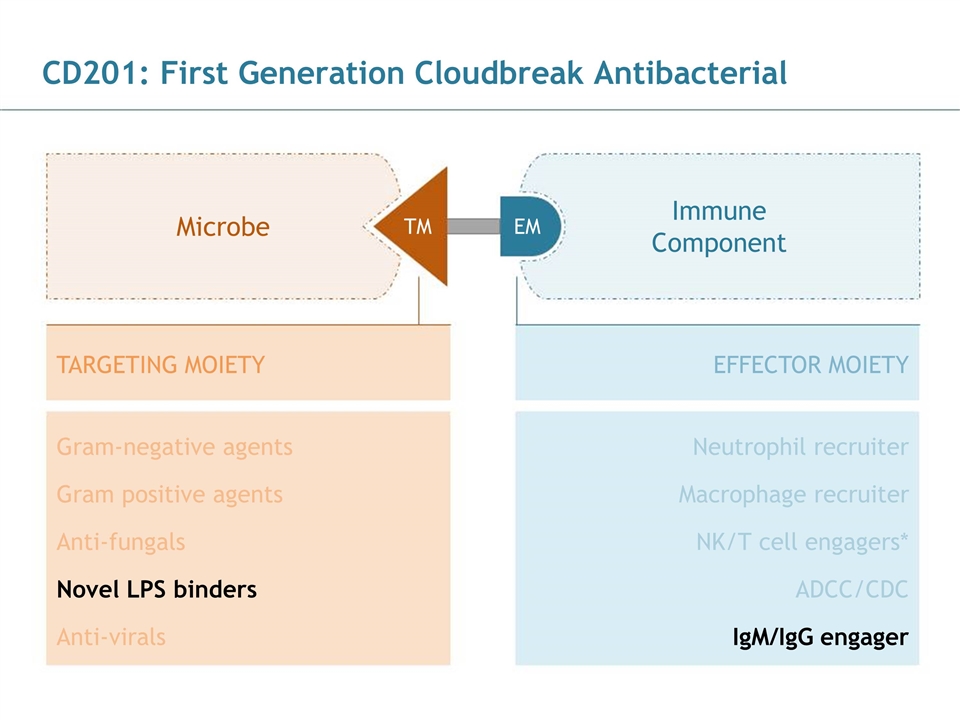
CD201: First Generation Cloudbreak Antibacterial EFFECTOR MOIETY TARGETING MOIETY Microbe Immune Component TM EM Gram-negative agents Gram positive agents Neutrophil recruiter Novel LPS binders Anti-virals IgM/IgG engager Macrophage recruiter NK/T cell engagers* ADCC/CDC Anti-fungals
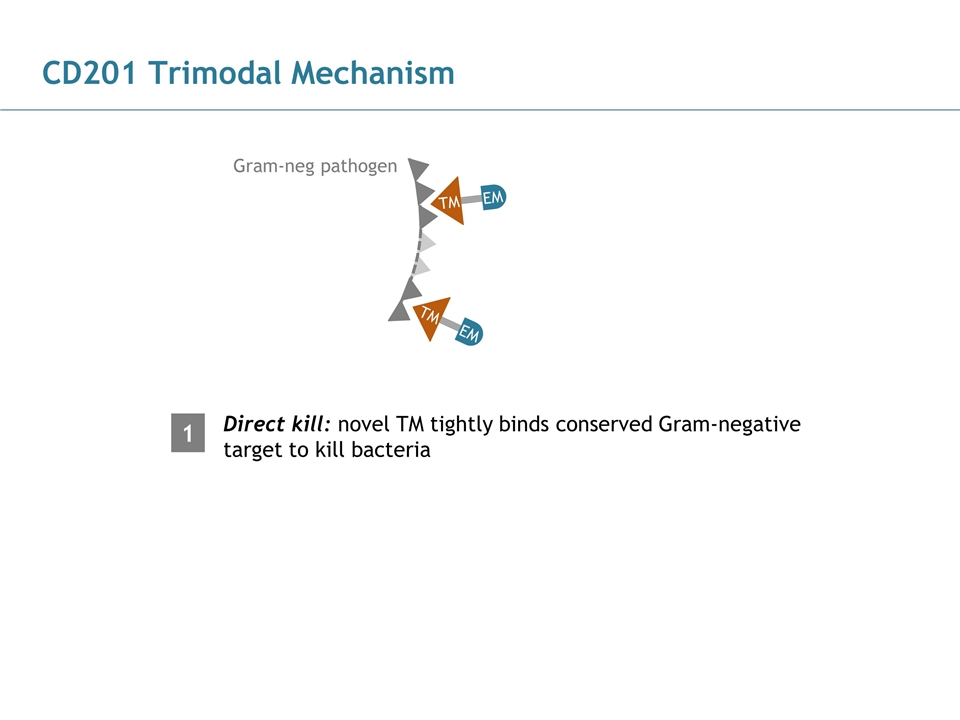
CD201 Trimodal Mechanism Direct kill: novel TM tightly binds conserved Gram-negative target to kill bacteria Gram-neg pathogen TM EM TM EM 1
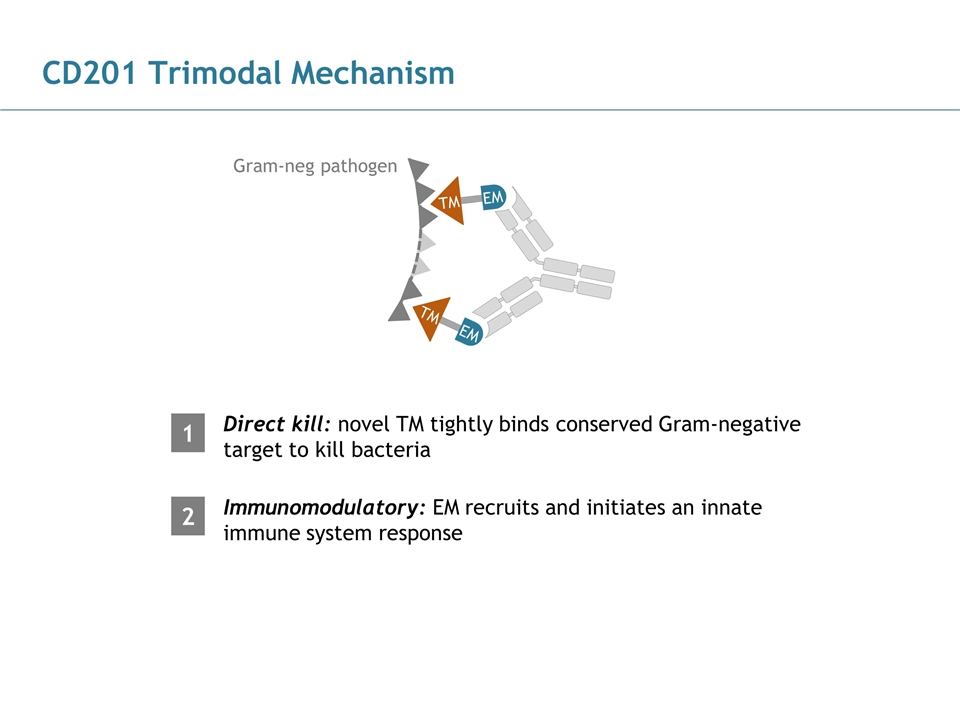
CD201 Trimodal Mechanism Direct kill: novel TM tightly binds conserved Gram-negative target to kill bacteria Gram-neg pathogen Immunomodulatory: EM recruits and initiates an innate immune system response 1 2 TM EM TM EM
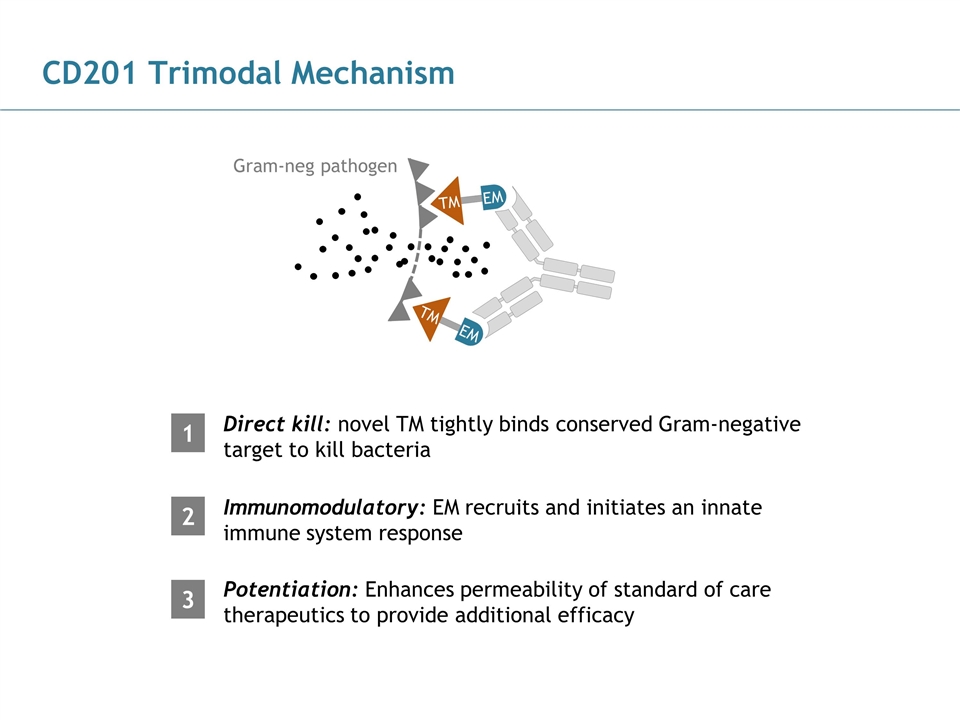
CD201 Trimodal Mechanism Direct kill: novel TM tightly binds conserved Gram-negative target to kill bacteria Gram-neg pathogen Immunomodulatory: EM recruits and initiates an innate immune system response 1 2 Potentiation: Enhances permeability of standard of care therapeutics to provide additional efficacy 3 TM EM TM EM
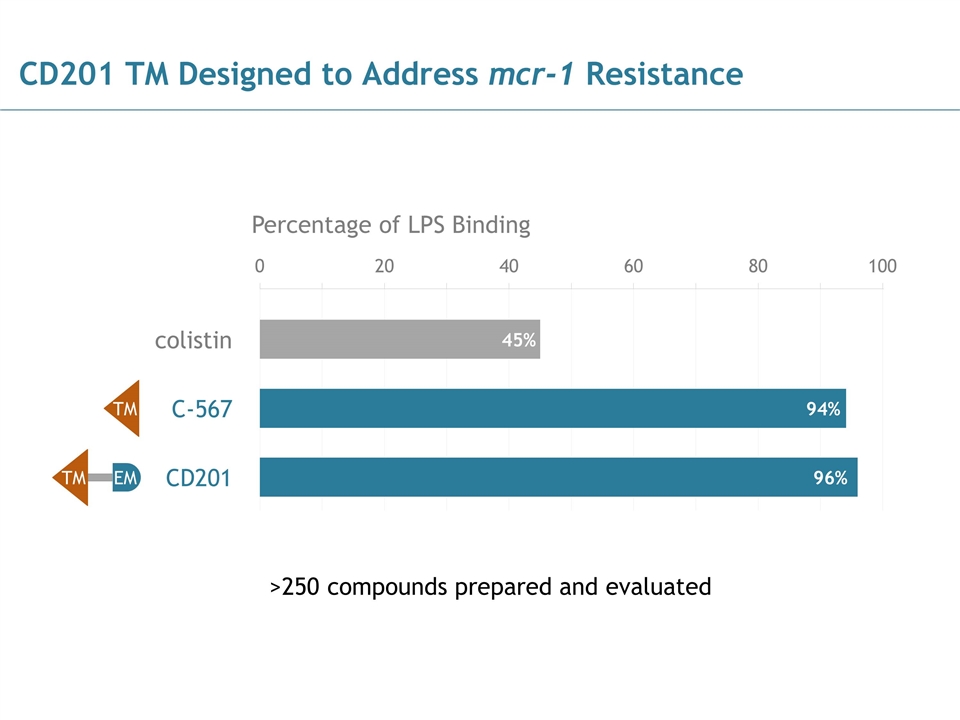
CD201 TM Designed to Address mcr-1 Resistance colistin C-567 CD201 Percentage of LPS Binding >250 compounds prepared and evaluated 0 20 40 60 80 100 45% 94% 96% TM EM TM
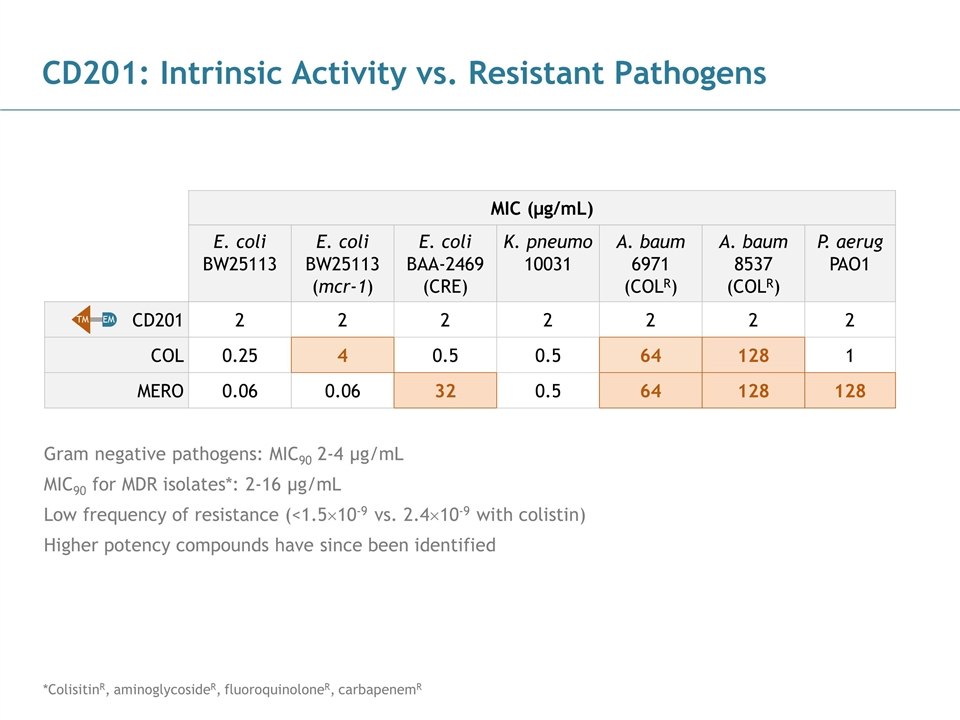
CD201: Intrinsic Activity vs. Resistant Pathogens Gram negative pathogens: MIC90 2-4 µg/mL MIC90 for MDR isolates*: 2-16 µg/mL Low frequency of resistance (<1.5´10-9 vs. 2.4´10-9 with colistin) Higher potency compounds have since been identified MIC (µg/mL) E. coli BW25113 E. coli BW25113 (mcr-1) E. coli BAA-2469 (CRE) K. pneumo 10031 A. baum 6971 (COLR) A. baum 8537 (COLR) P. aerug PAO1 CD201 2 2 2 2 2 2 2 COL 0.25 4 0.5 0.5 64 128 1 MERO 0.06 0.06 32 0.5 64 128 128 TM EM *ColisitinR, aminoglycosideR, fluoroquinoloneR, carbapenemR
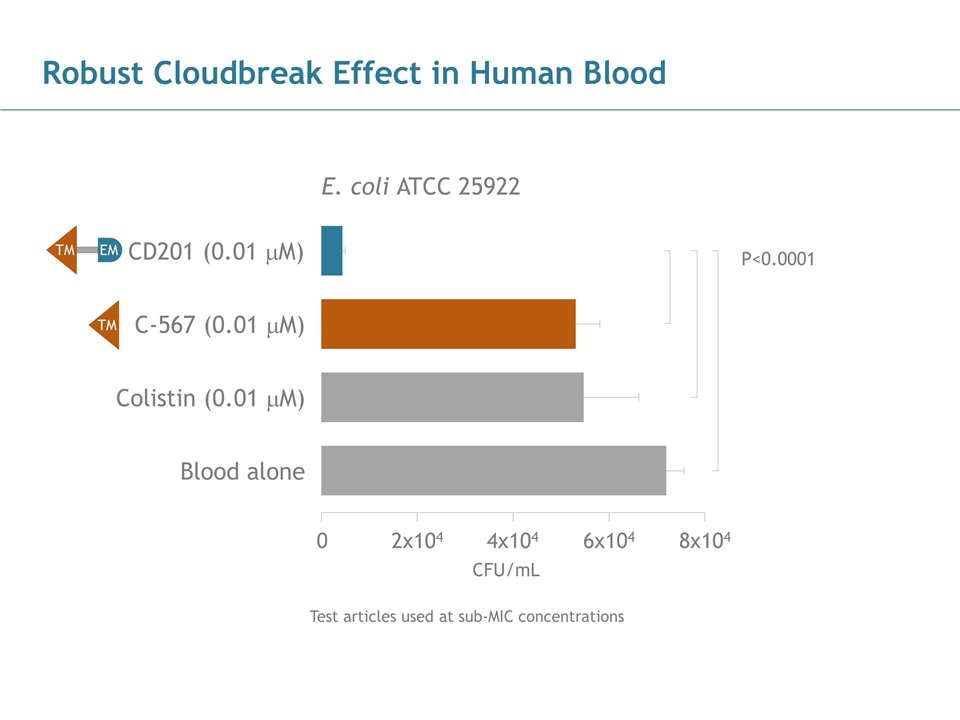
Robust Cloudbreak Effect in Human Blood Test articles used at sub-MIC concentrations E. coli ATCC 25922 0 2x104 4x104 6x104 8x104 CD201 (0.01 mM) C-567 (0.01 mM) Colistin (0.01 mM) Blood alone TM EM TM P<0.0001 CFU/mL
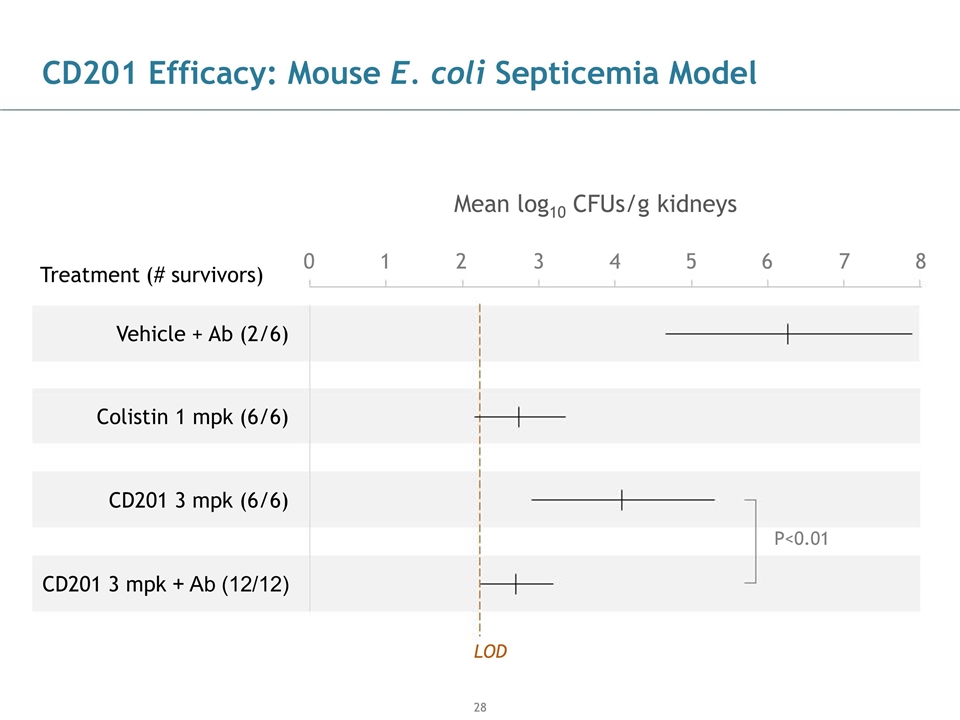
CD201 Efficacy: Mouse E. coli Septicemia Model Mean log10 CFUs/g kidneys P<0.01 LOD 0 1 2 3 4 5 6 7 8 Vehicle + Ab (2/6) Colistin 1 mpk (6/6) CD201 3 mpk (6/6) CD201 3 mpk + Ab (12/12) Treatment (# survivors)
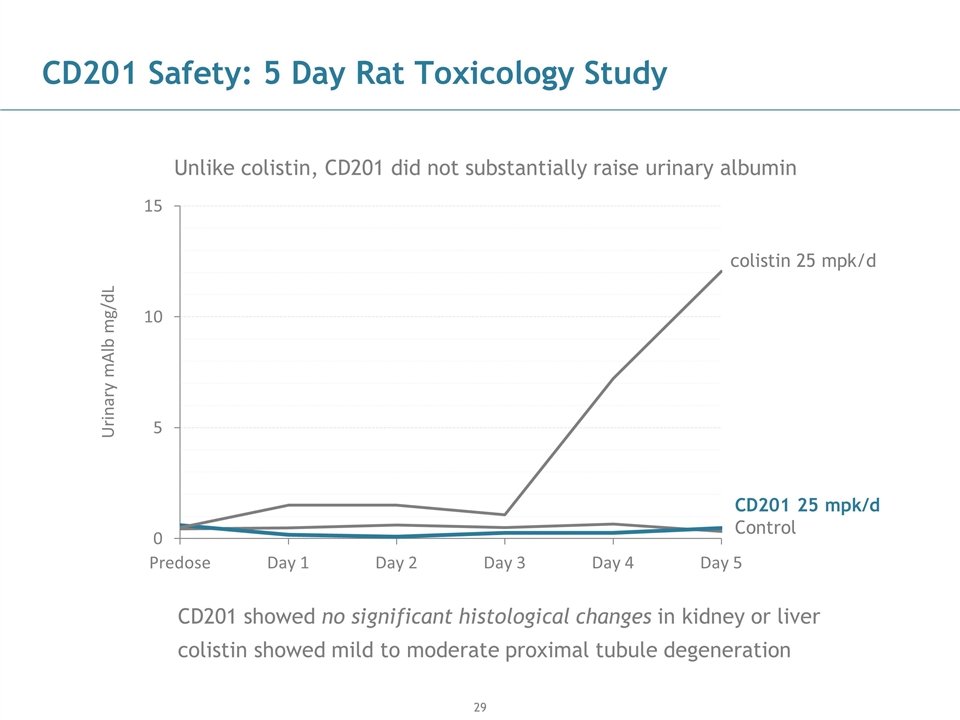
CD201 Safety: 5 Day Rat Toxicology Study CD201 showed no significant histological changes in kidney or liver colistin showed mild to moderate proximal tubule degeneration Unlike colistin, CD201 did not substantially raise urinary albumin Control CD201 25 mpk/d colistin 25 mpk/d

Cidara’s Development Pipeline CD101 Cloudbreak™ IV Topical Antibacterial Antifungal Antiviral Discovery in vitro in vivo IND Enabling Phase I Phase II
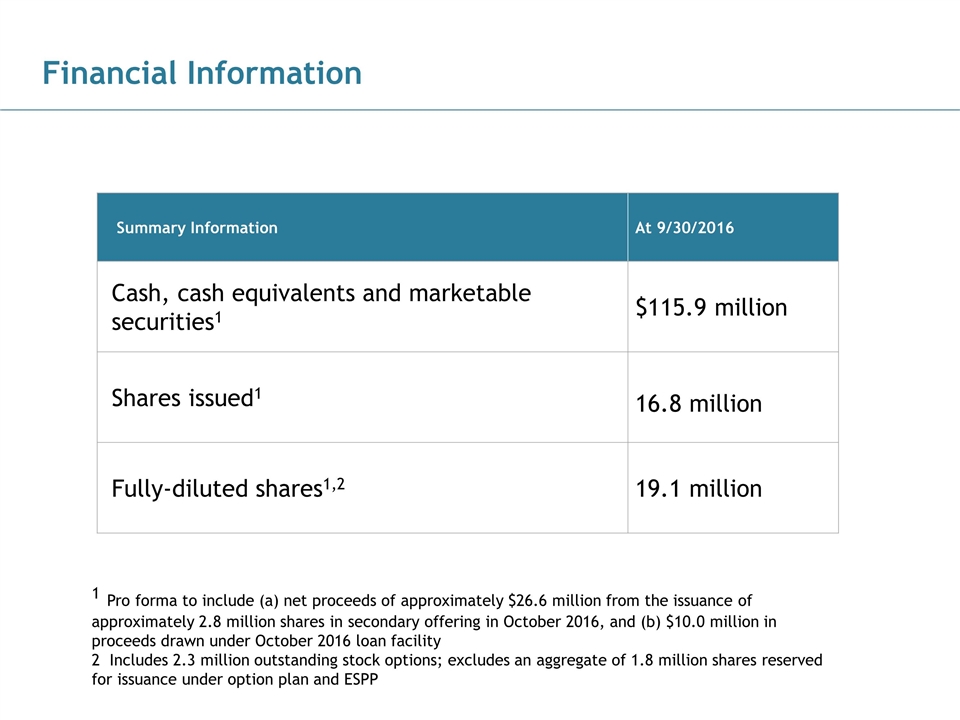
Financial Information 186, 89, 21 43, 124, 154 28, 83, 102 102, 102, 102 170, 170, 170 Summary Information At 9/30/2016 Cash, cash equivalents and marketable securities1 $115.9 million Shares issued1 16.8 million Fully-diluted shares1,2 19.1 million 1 Pro forma to include (a) net proceeds of approximately $26.6 million from the issuance of approximately 2.8 million shares in secondary offering in October 2016, and (b) $10.0 million in proceeds drawn under October 2016 loan facility 2 Includes 2.3 million outstanding stock options; excludes an aggregate of 1.8 million shares reserved for issuance under option plan and ESPP
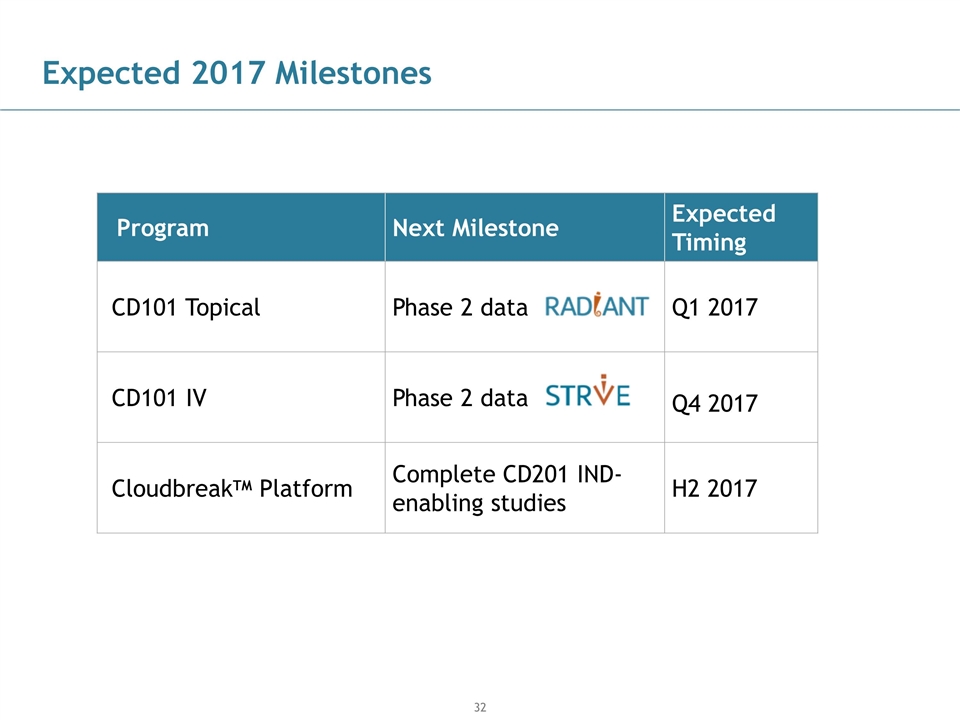
Expected 2017 Milestones Program Next Milestone Expected Timing CD101 Topical Phase 2 data Q1 2017 CD101 IV Phase 2 data Q4 2017 CloudbreakTM Platform Complete CD201 IND-enabling studies H2 2017

New Hope For Serious Infections Corporate Presentation January 2017
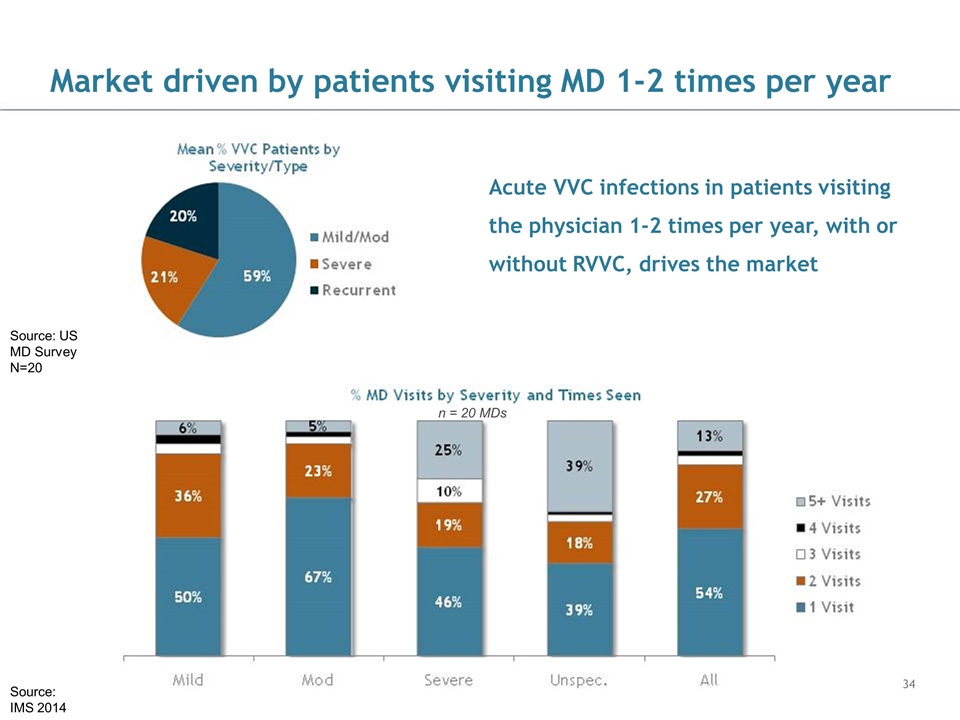
Market driven by patients visiting MD 1-2 times per year Source: IMS 2014 n = 20 MDs Source: US MD Survey N=20 Acute VVC infections in patients visiting the physician 1-2 times per year, with or without RVVC, drives the market

































