
1 ReSTORE PHASE 3 TRIAL RESULTS FOR REZAFUNGIN L E A D I N G T H E S C I E N C E O F P R O T E C T I O N D e c 2 0 2 1

2 F O R WA R D - LO O K I N G S TAT E M E N T S T h e s e s l i d e s c o n t a i n f o r w a r d - l o o k i n g s t a t e m e n t s w i t h i n t h e m e a n i n g o f t h e P r i v a t e S e c u r i t i e s L i t i g a t i o n R e f o r m A c t o f 1 9 9 5 . The words “believe,” “may,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward- looking statements. Such statements include, but are not limited to, statements regarding Cidara’s expectations that the ReSTORE trial data will support an NDA submission in the U.S. and similar marketing authorization submissions in other countries; the potential timing of such submissions; and the likelihood that rezafungin, if approved, will be prescribed by physicians or included in formularies or treatment guidelines. Additional risks and uncertainties may emerge from time to time, and it is not possible for Cidara’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Cidara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. These slides are not intended to and do not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities in any jurisdiction, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. This presentation also contains estimates and other statistical data made by independent parties and by Cidara relating to market size and growth and other data about Cidara's industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of the future performance of the markets in which Cidara operates are necessarily subject to a high degree of uncertainty and risk, including, Cidara's ability to obtain additional financing; the success and timing of Cidara’s clinical trials and other research and development activities; receipt of necessary regulatory approvals for development and commercialization, as well as changes to applicable regulatory laws in the United States Securities and Exchange Commission, under the heading “Risk Factors.”

3 R E Z A F U N G I N O V E R A L L P H A S E 3 D E V E LO P M E N T P L A N P H A S E 3 T R E AT M E N T T R I A L POTENTIAL INDICATION OVERALL OBJECTIVE Treatment of candidemia & invasive candidiasis FDA: Day 30 All-Cause Mortality vs SOC P H A S E 3 P R O P H Y L A X I S T R I A L Prophylaxis against Aspergillus, Candida & Pneumocystis in allogeneic blood and marrow transplant patients Day 90 Fungal free survival vs standard of care PHASE 3 SIZE 187 patients1 (20% NI margin) 462 patients (12.5% NI margin) 1. mITT population.

4 Re S TO R E P H A S E 3 T R I A L D E S I G N 2 3 4 5 6 7 8 9 5 14 30 N= 93 Caspofungin Rezafungin N= 94 Week Day Week 1 2 3 4 5 6 7 8 9 Global Cure Global Cure (1° ENDPOINT – EMA) All-Cause Mortality (1° ENDPOINT – FDA) End of Follow Up Period 400/200mg Weekly 70/50mg Daily Dose Optional dose Dose Optional dose 1 Global Cure is defined as Clinical Cure (as assessed by the Primary Investigator), Mycological Eradication and Radiological Cure (for qualifying invasive candidiasis patients only). • A Phase 3, prospective, double-blind, randomized, international, multicenter trial • Evaluate the efficacy and safety of once-weekly IV rezafungin vs once-daily caspofungin followed by optional oral fluconazole step-down in the treatment of documented candidemia and/or IC • mITT population: All subjects in safety population who had documented Candida infection

5 Re S TO R E P H A S E 3 T R I A L R E S U LT S S U M M A RY P r i m a r y E f f i c a c y E n d p o i n t s S e c o n d a r y E f f i c a c y E n d p o i n t s E x p l o r a t o r y E f f i c a c y E n d p o i n t s S a f e t y • Both the FDA All-Cause Mortality at Day 30 as well as the EMA Global Cure at Day 14 endpoints were achieved • Early efficacy outcomes (Day 5 Global Cure, Day 5 Mycological Eradication) were either similar or trended higher in the rezafungin arm • Blood cultures were cleared more quickly in the rezafungin arm though the difference was not significant • Duration of ICU stay was lower in the rezafungin group compared to caspofungin • Rates of Adverse Events and Serious Adverse Events were similar between the two study arms

6 K E Y B A S E L I N E D E M O G R A P H I C S S I M I L A R A C R O S S A R M S DEMOGRAPHIC OR CHARACTERISTIC REZAFUNGIN n (%) CASPOFUNGIN n (%) Mean Age: ≥65 years 38 (40.9) 38 (40.4) Sex: Female 31 (33.3) 38 (40.4) Race Asian 23 (24.7) 31 (33.0) Black or African American 5 (5.4) 4 (4.3) White 59 (63.4) 55 (58.5) Other 1 (1.1) 2 (2.1) Not reported 4 (4.3) 1 (1.1) Final Diagnosis: Candidemia 64 (68.8) 67 (71.3) Final Diagnosis: Invasive Candidiasis 29 (31.2) 27 (28.7) Modified APACHE II score ≥20 12 (12.9) 17 (18.1) 10–19 42 (45.2) 40 (42.6) <10 38 (40.9) 37 (39.4) Absolute neutrophil count <500/μL 7 (7.5) 5 (5.3)

7 D AY 3 0 A L L - C A U S E M O R TA L I T Y ( P r i m a r y E n d p o i n t fo r F D A w a s A c h i e v e d ) A LL -C A U SE M O R TA LI TY D A Y 3 0 The upper limit of the 95% confidence interval for ACM is 14.4%, which is within the noninferiority margin of 20% established with the FDA. Rezafungin 400 mg/200 mg weekly (N=93) Caspofungin 70 mg/50 mg daily (N=94) 21.3% 23.7% 0% 10% 20% 30% 40% 22/93 20/94 95% CI –9.7 to 14.4

8 60.6% 59.1% 0% 10% 20% 30% 40% 50% 60% 70% D AY 1 4 G LO B A L C U R E ( P r i m a r y E n d p o i n t fo r E M A w a s A c h i e v e d ) G LO B A L C U R E D A Y 1 4 The lower limit of the 95% confidence interval for Day 14 Global Cure is –14.9%, which is within the noninferiority margin of –20% established with the EMA. 57/94 55/93 Rezafungin 400 mg/200 mg weekly (N=93) Caspofungin 70 mg/50 mg daily (N=94) 95% CI –14.9 to 12.7 Global Cure is defined as Clinical Cure (as assessed by the Primary Investigator), Mycological Eradication and Radiological Cure (for qualifying invasive candidiasis patients only).
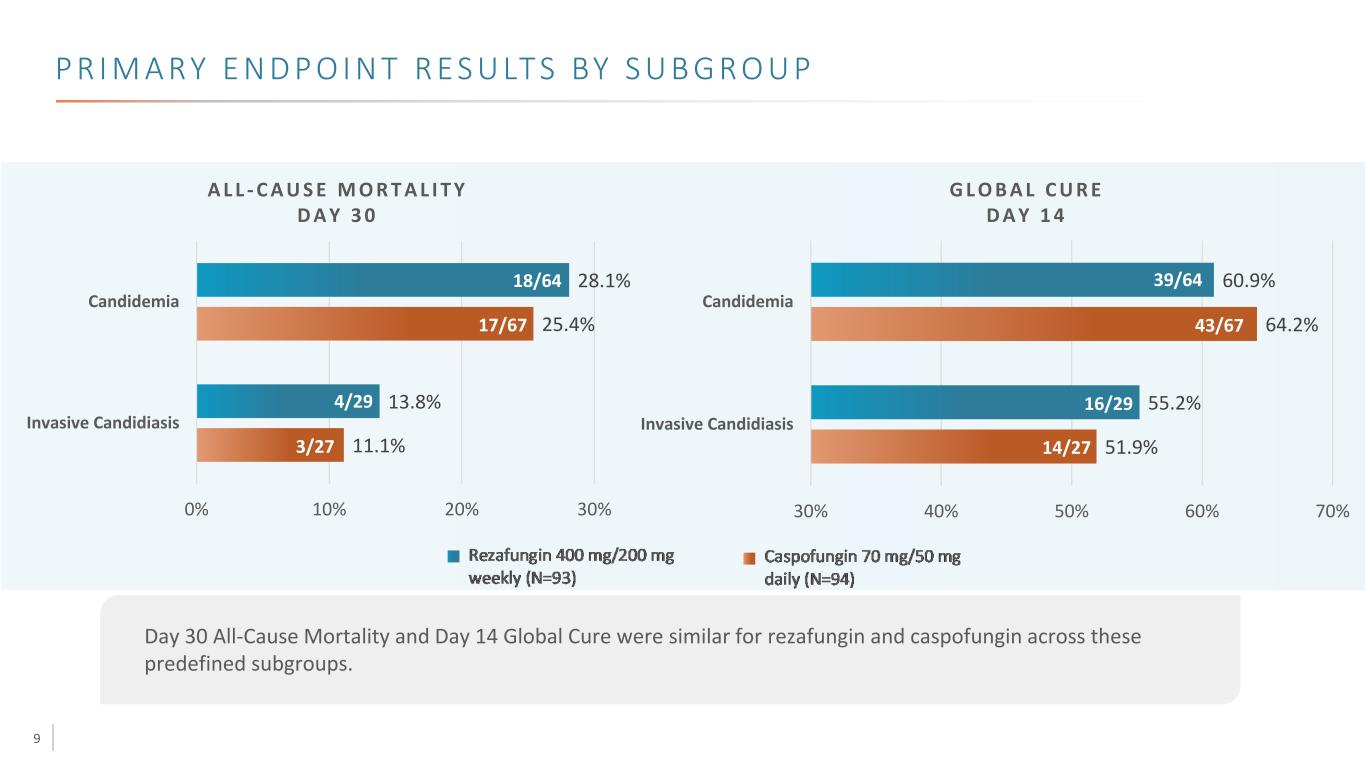
9 P R I M A RY E N D P O I N T R E S U LT S BY S U B G R O U P A L L - C A U S E M O R T A L I T Y D A Y 3 0 G L O B A L C U R E D A Y 1 4 Day 30 All-Cause Mortality and Day 14 Global Cure were similar for rezafungin and caspofungin across these predefined subgroups. 51.9% 64.2% 55.2% 60.9% 30% 40% 50% 60% 70% Invasive Candidiasis Candidemia 11.1% 25.4% 13.8% 28.1% 0% 10% 20% 30% Invasive Candidiasis Candidemia 4/29 3/27 18/64 17/67 39/64 43/67 16/29 14/27

10 G LO B A L C U R E AT D AY 5 , D AY 1 4 , A N D D AY 3 0 48.9% 60.6% 52.1% 49.5% 59.1% 55.9% 30% 40% 50% 60% 70% Global Cure Day 30 Global Cure Day 14 Global Cure Day 5 52/93 49/94 55/93 57/94 Rezafungin 400 mg/200 mg weekly (N=93) Caspofungin 70 mg/50 mg daily (N=94) Global Cures were similar between study arms across multiple timepoints. D a y 5 a n d D a y 3 0 a r e S e c o n d a r y E n d p o i n t s 46/93 46/94

11 M YC O LO G I C A L E R A D I C AT I O N AT D AY 5 , D AY 1 4 , A N D D AY 3 0 D a y 5 , D a y 1 4 , a n d D a y 3 0 a r e S e c o n d a r y E n d p o i n t s , i n C a n d i d e m i a O n l y 59.7% 70.1% 68.7% 62.5% 71.9% 78.1% 50% 60% 70% 80% Mycological Eradication Day 30 Mycological Eradication Day 14 Mycological Eradication Day 5 50/64 46/67 46/64 47/67 Rezafungin 400 mg/200 mg weekly (N=93) Caspofungin 70 mg/50 mg daily (N=94) Mycological Eradication was numerically higher for rezafungin at Day 5 and similar across arms at later timepoints. 40/64 40/67

12 P H A S E 3 E A R LY E F F I C A C Y A N D M E D I A N I C U S TAY 0 7 14Day % Neg. Blood Culture1,2 % Mycological Eradication1 Rz Cf2 Rz CfRz Cf 36/67 1 DAY 5 DAYS2 DAYS 30/65 49/66 41/64 50/64 46/67 % Neg. Blood Culture1,2 53.7 46.2 78.1 68.7 74.2 64.1 n/N n/Nn/N 1. Patients with candidemia only 2. Not powered for statistical comparison 3. All patients in the ICU on day 1 included except for those who died prior to ICU discharge S E C O N D A R Y A N D E X P L O R AT O R Y A N A LY S E S
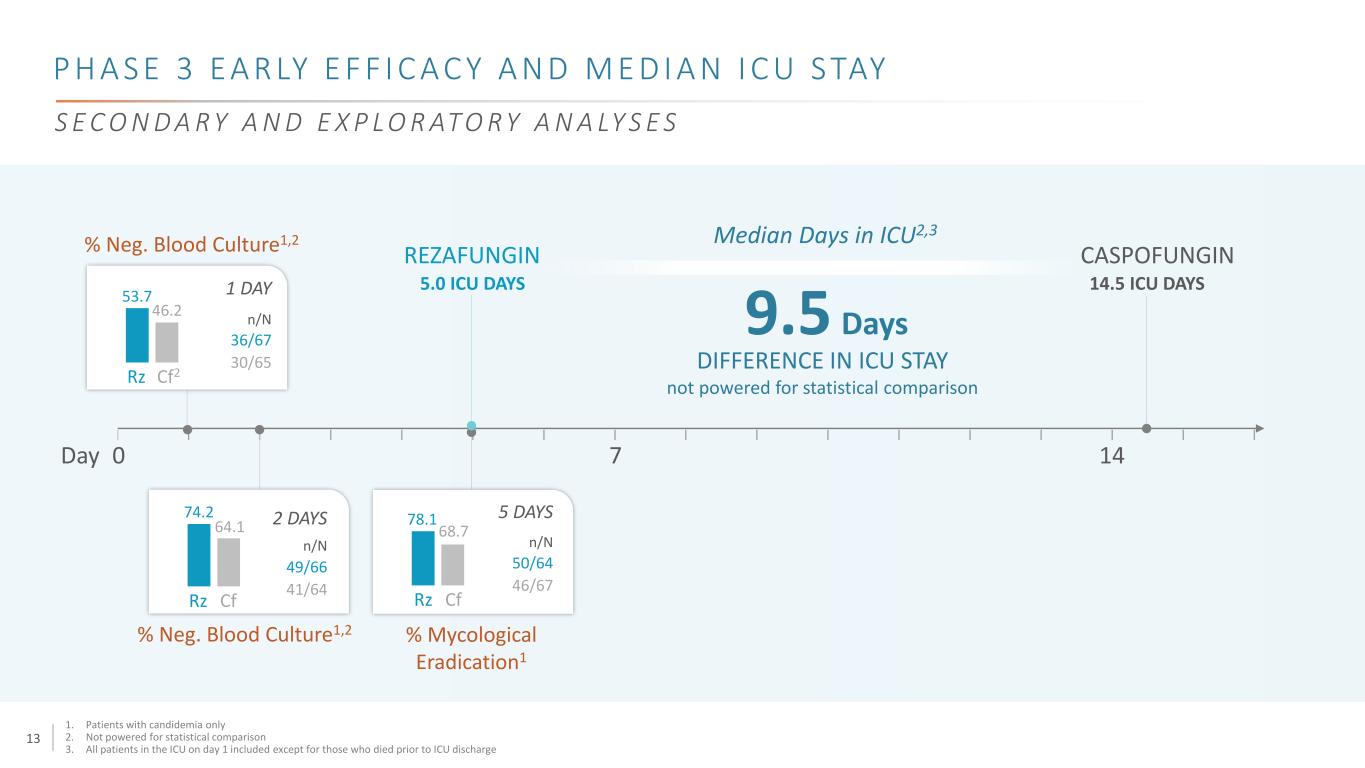
13 P H A S E 3 E A R LY E F F I C A C Y A N D M E D I A N I C U S TAY 0 7 14Day % Neg. Blood Culture1,2 % Mycological Eradication1 Rz Cf2 Rz CfRz Cf 36/67 1 DAY 5 DAYS2 DAYS 30/65 49/66 41/64 50/64 46/67 % Neg. Blood Culture1,2 53.7 46.2 78.1 68.7 74.2 64.1 n/N n/Nn/N 1. Patients with candidemia only 2. Not powered for statistical comparison 3. All patients in the ICU on day 1 included except for those who died prior to ICU discharge S E C O N D A R Y A N D E X P L O R AT O R Y A N A LY S E S 9.5 Days DIFFERENCE IN ICU STAY not powered for statistical comparison Median Days in ICU2,3 CASPOFUNGIN 14.5 ICU DAYS REZAFUNGIN 5.0 ICU DAYS

14 R E Z A F U N G I N P H A S E 2 A N D P H A S E 3 A L L - C AU S E M O R TA L I T Y R E S U LT S 21.3% 13.1% 23.7% 4.4% 15.8% 0% 10% 20% 30% All-Cause Mortality Day 30 All-Cause Mortality Day 30 12/76 8/61 2/46 22/93 20/94 P H A S E 2 P H A S E 3 Rezafungin 400 mg/200 mg weekly Caspofungin 70 mg/50 mg daily Rezafungin 400 mg/400 mg weekly Differences in Trials • Overall mortality higher in Ph3 • Ph3 enrolled during COVID (75% of Ph3 subjects were enrolled after March 2020) • Ph2 run in NA and Europe • Ph3 added Australia, South America, East Asia including China Impact of the above on study outcomes is unknown

15 R E Z A F U N G I N P H A S E 2 A N D P H A S E 3 D AY 1 4 C U R E R E S U LT S 60.6% 67.2% 59.1% 76.1% 60.5% 50% 60% 70% 80% 90% Global Cure Day 14 Overall Cure Day 14 46/76 41/61 35/46 55/93 57/94 P H A S E 2 1 P H A S E 3 2 Rezafungin 400 mg/200 mg weekly Caspofungin 70 mg/50 mg daily Rezafungin 400 mg/400 mg weekly 1. Overall Cure (Phase 2): resolution of systemic signs attributable to candidemia or invasive candidiasis AND mycological eradication as demonstrated by a single tissue/fluid culture or 2 negative blood cultures at least 12 hours apart 2. Global Cure (Phase 3): investigator assessment of clinical cure AND mycological eradication as demonstrated by a single negative blood or tissue/fluid culture AND (if pertinent) improvement or resolution of evidence of invasive candidiasis on radiographic imaging Differences in Trials • Overall rates of D14 global cure trended lower in Ph3 • Ph 3 enrolled during COVID • Expanded regions of enrollment • Increased rates of IC Impact of the above on study outcomes is unknown

16 Number of Subjects REZAFUNGIN 400 mg/200 mg Weekly N=98 n (%) CASPOFUNGIN 70 mg/50 mg Daily N=98 n (%) ≥1 TEAE 89 (90.8) 83 (84.7) Study drug-related* 16 (16.3) 9 (9.2) Serious AE 55 (56.1) 52 (53.1) Study drug-related* 2 (2.0) 3 (3.1) AE leading to study drug discontinuation 13 (13.3) 11 (11.2) Rezafungin was generally well tolerated and had a similar safety profile to caspofungin. S A F E T Y: S U M M A RY O F A D V E RS E E V E N T S * Study drug-related AEs may be considered related to study drug or placebo due to investigator blinding. 5 AEs in the rezafungin arm were considered related to placebo administration. 0 AEs in the caspofungin arm were considered related to placebo administration.

17 S A F E T Y: R E L AT E D S E R I O U S A D V E R S E E V E N T S A S D E T E R M I N E D BY T H E P I s • Rezafungin arm o Infusion-related reaction (Day 3) - Hypersensitivity reaction during the Day 3 infusion of saline placebo o Urticaria (Day 15) - Urticarial rash following oral placebo administration • Caspofungin arm o Hypertransaminasaemia (Day 14) - High liver function tests o Liver injury (Day 8) - High liver function tests o Anaphylactic shock (Day 3) - Anaphylactic reaction to Day 3 study drug infusion B o t h S A E s i n t h e r e z a f u n g i n a r m w e r e a s s o c i a t e d w i t h p l a c e b o a d m i n i s t r a t i o n

18 Re S TO R E P H A S E 3 T R I A L R E S U LT S S U M M A RY P r i m a r y E f f i c a c y E n d p o i n t s S e c o n d a r y E f f i c a c y E n d p o i n t s E x p l o r a t o r y E f f i c a c y E n d p o i n t s S a f e t y • Both the FDA All-Cause Mortality at Day 30 as well as the EMA Global Cure at Day 14 endpoints were achieved • Early efficacy outcomes (Day 5 Global Cure, Day 5 Mycological Eradication) were either similar or trended higher in the rezafungin arm • Blood cultures were cleared more quickly in the rezafungin arm though the difference was not significant • Duration of ICU stay was lower in the rezafungin group compared to caspofungin • Rates of Adverse Events and Serious Adverse Events were similar between the two study arms

19 REZAFUNGIN • No new treatments for IC in 15 years • NDA and EMA Filing expected mid-2022 • Go-to-market strategy optionality while preparing • Highly efficient market • Supply chain in place and launch supplies on hand • Fast Track, QIDP, Orphan designation for C/IC • Validated by Mundipharma partnership R e S TO R E P R O V I D E S E XC E L L E N T F O U N D AT I O N F O R C O M M E R C I A L I Z AT I O N

20 R e S TO R E P R O V I D E S E XC E L L E N T F O U N D AT I O N F O R C O M M E R C I A L I Z AT I O N Only drug in development successfully compared head-to-head in Ph3 with standard of care echinocandin in 1st line Candida treatment 1st and only once-weekly antifungal candidate High front-loaded dosing for rapid Candida clearance Substantial tissue and organ penetration No DDIs across two studies with relevant drugs May enable early discharge (ICU and Hospital) Active against tough to treat Candida strains including C. auris and azole-resistant Candida ADVANTAGES • No new treatments for IC in 15 years • NDA and EMA Filing expected mid-2022 • Go-to-market strategy optionality while preparing • Highly efficient market • Supply chain in place and launch supplies on hand • Fast Track, QIDP, Orphan designation for C/IC • Validated by Mundipharma partnership REZAFUNGIN

21 R e S TO R E D ATA A N D M A R K E T I N P U T D E F I N E C L E A R PAT I E N T O P P O R T U N I T I E S 1. 30% of patients with Candida receive therapy for documented disease whereas 70% receive empiric therapy only (Internal estimate). 2. 30% of patients who start on an echinocandin are still on an echinocandin on the last day of hospitalization because they could not be stepped down. Sofjan, Garey et al. Journal of Antimicrobial Resistance. Vol14, Sept 2018 INPATIENT REZAFUNGIN TARGETS 1. Documented Candida (not empiric) 2. Higher front-loaded dosing may be of benefit in critically ill (ICU, etc.) 3. Cannot step down (azole issues)2 4. Potential for early discharge (ICU and Hospital), increasing importance with COVID pandemic ~30% of Candida patients Targets for Rezafungin (Cidara R&D Day Presentation Sept 2021) 1 7 14 21 ICU General Ward Outpatient Suspected Confirmed IFI OUTPATIENT REZAFUNGIN TARGETS 1. Remain on echinocandin due to azole resistance 2. Remain on echinocandin due to azole tox/DDI 3. Unknown Candida pathogen 4. Once-weekly facilitates administration and adherence

22 Rezafungin is the only antifungal in clinical stage development for both 1st-line treatment and prophylaxis... …Fluconazole and voriconazole, which sold $1B and $800M at peak1,2 respectively, had a similar approach R E Z A F U N G I N : T W O P H A S E 3 P R O G R A M S V S S TA N D A R D S O F C A R E 10 – Infectious Disease 20 – Hem/Onc Antifungal Prophylaxis Focus:Candida Treatment Focus: + 1. Source: IQVIA 2. Peak year annual sales estimates for fluconazole and voriconazole are presented for illustrative purposes only and are not a forecast of potential rezafungin sales or revenue. Complete NDA Mid-2022 Ongoing 10 – Hem/Onc 20 – Infectious Disease

23 QUESTIONS L E A D I N G T H E S C I E N C E O F P R O T E C T I O N D e c 2 0 2 1

24 THANK YOU L E A D I N G T H E S C I E N C E O F P R O T E C T I O N D e c 2 0 2 1























