
Ascendis Pharma A/S ENDO Data Review April 3, 2017 Exhibit 99.1

Cautionary Note On Forward-Looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, such as statements regarding our future results of operations and financial position, including our business strategy, prospective products, availability of funding, clinical trial results, product approvals and regulatory pathways, collaborations, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products, are forward-looking statements. These forward-looking statements are based on our current expectations and beliefs, as well as assumptions concerning future events. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the results discussed in the forward-looking statements. These risks, uncertainties and other factors are more fully described in our reports filed with or submitted to the Securities and Exchange Commission, including, without limitation, our most recent Annual Report on Form 20-F filed with the SEC on March 22, 2017, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In light of the significant uncertainties in our forward-looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. Any forward-looking statement made by us in this presentation speaks only as of the date of this presentation and represents our estimates and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these statements publicly, whether as a result of new information, future events or otherwise after the date of this presentation. This presentation concerns product candidates that are or have been under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency or other foreign regulatory authorities. These product candidates are currently limited by U.S. Federal law to investigational use, and no representations are made as to their safety or effectiveness for the purposes for which they are being investigated. Ascendis is a trademark that we use in this presentation. Any other trademarks appearing in this presentation are the property of their respective holders.

Agenda: ENDO 2017 Data Review Introduction – Jan Mikkelsen, Chief Executive Officer Presentation Highlights TransCon Growth Hormone (4 posters) Jonathan Leff, M.D. Chief Medical Officer TransCon PTH (2 posters; one late-breaker) David B. Karpf, M.D. VP, Clinical Development TransCon CNP (2 posters; one late-breaker) Kennett Sprogøe, PhD, Senior Vice President, Product Innovation Q&A
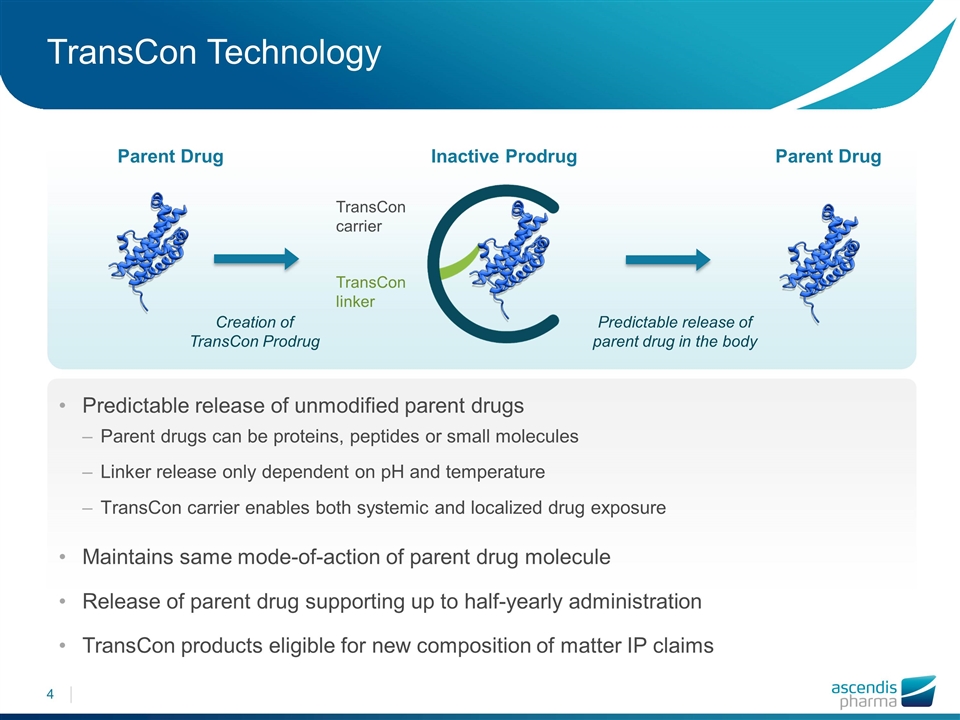
TransCon Technology Predictable release of parent drug in the body Parent Drug Parent Drug TransCon carrier TransCon linker Inactive Prodrug Predictable release of unmodified parent drugs Parent drugs can be proteins, peptides or small molecules Linker release only dependent on pH and temperature TransCon carrier enables both systemic and localized drug exposure Maintains same mode-of-action of parent drug molecule Release of parent drug supporting up to half-yearly administration TransCon products eligible for new composition of matter IP claims Creation of TransCon Prodrug
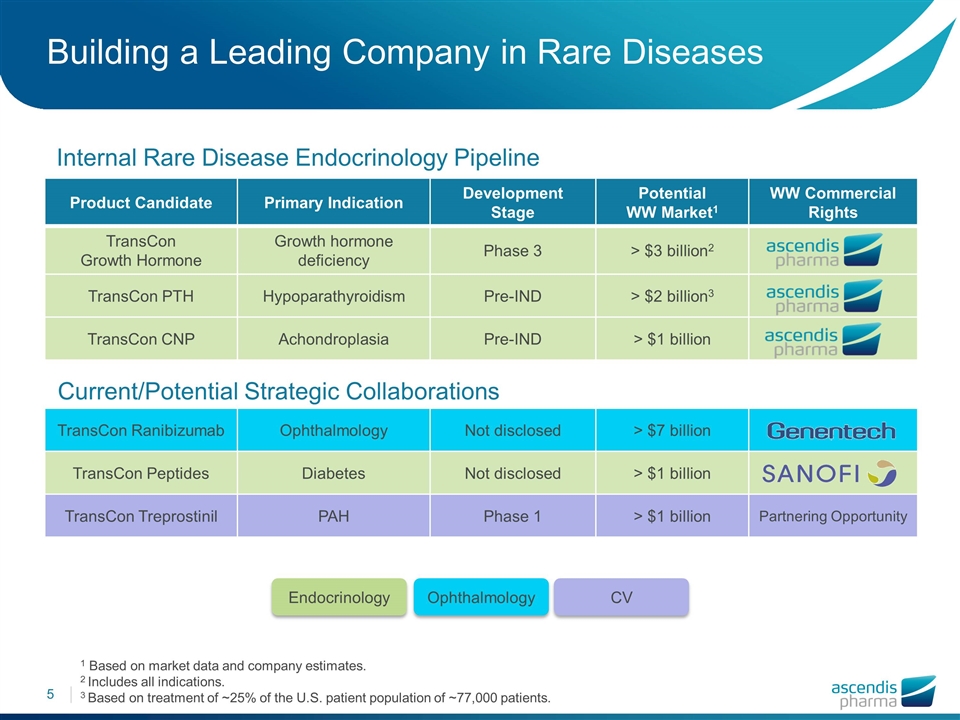
Product Candidate Primary Indication Development Stage Potential WW Market1 WW Commercial Rights TransCon Growth Hormone Growth hormone deficiency Phase 3 > $3 billion2 TransCon PTH Hypoparathyroidism Pre-IND > $2 billion3 TransCon CNP Achondroplasia Pre-IND > $1 billion TransCon Ranibizumab Ophthalmology Not disclosed > $7 billion TransCon Peptides Diabetes Not disclosed > $1 billion TransCon Treprostinil PAH Phase 1 > $1 billion Partnering Opportunity Endocrinology Ophthalmology CV Internal Rare Disease Endocrinology Pipeline Current/Potential Strategic Collaborations Building a Leading Company in Rare Diseases 1 Based on market data and company estimates. 2 Includes all indications. 3 Based on treatment of ~25% of the U.S. patient population of ~77,000 patients.

TransCon Growth Hormone: Once-Weekly Replacement Therapy Jonathan Leff, M.D. Chief Medical Officer

Efficacy Safety (including immunogenicity) Tolerability TransCon Growth Hormone Target Product Profile Comparable to Daily Growth Hormone = Weekly subcutaneous administration Single injection/dose Convenience 31G needle Room temperature storage Device Easy to use Empty-all design (controlled substance)
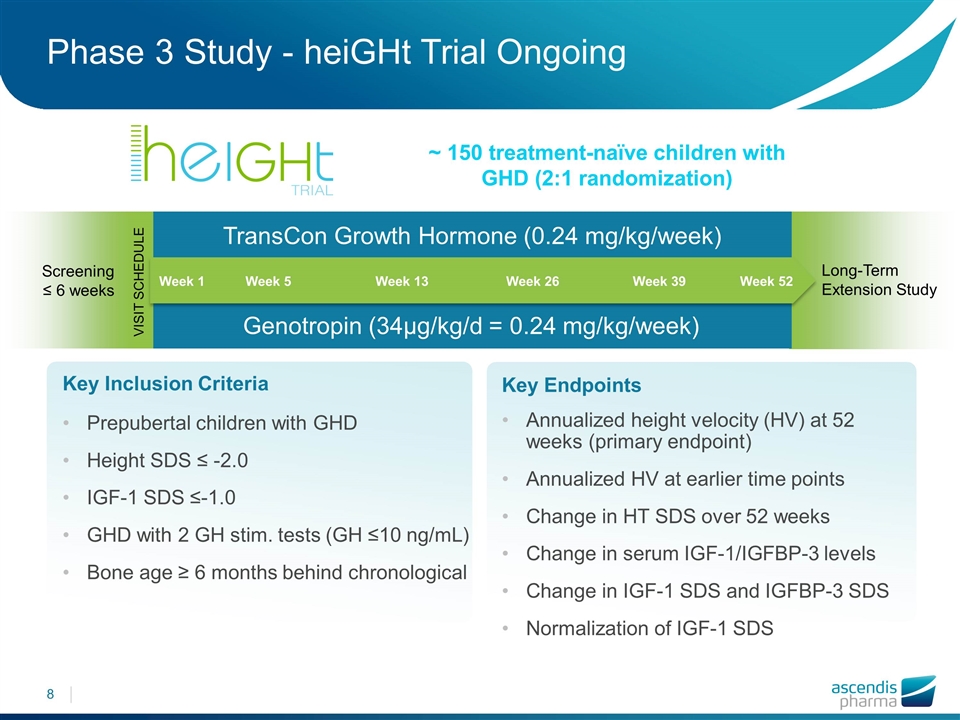
Phase 3 Study - heiGHt Trial Ongoing Key Inclusion Criteria Prepubertal children with GHD Height SDS ≤ -2.0 IGF-1 SDS ≤-1.0 GHD with 2 GH stim. tests (GH ≤10 ng/mL) Bone age ≥ 6 months behind chronological TransCon Growth Hormone (0.24 mg/kg/week) Genotropin (34µg/kg/d = 0.24 mg/kg/week) Screening ≤ 6 weeks Key Endpoints Annualized height velocity (HV) at 52 weeks (primary endpoint) Annualized HV at earlier time points Change in HT SDS over 52 weeks Change in serum IGF-1/IGFBP-3 levels Change in IGF-1 SDS and IGFBP‑3 SDS Normalization of IGF-1 SDS Long-Term Extension Study Week 1 Week 5 Week 13 Week 52 Week 26 Week 39 ~ 150 treatment-naïve children with GHD (2:1 randomization) VISIT SCHEDULE
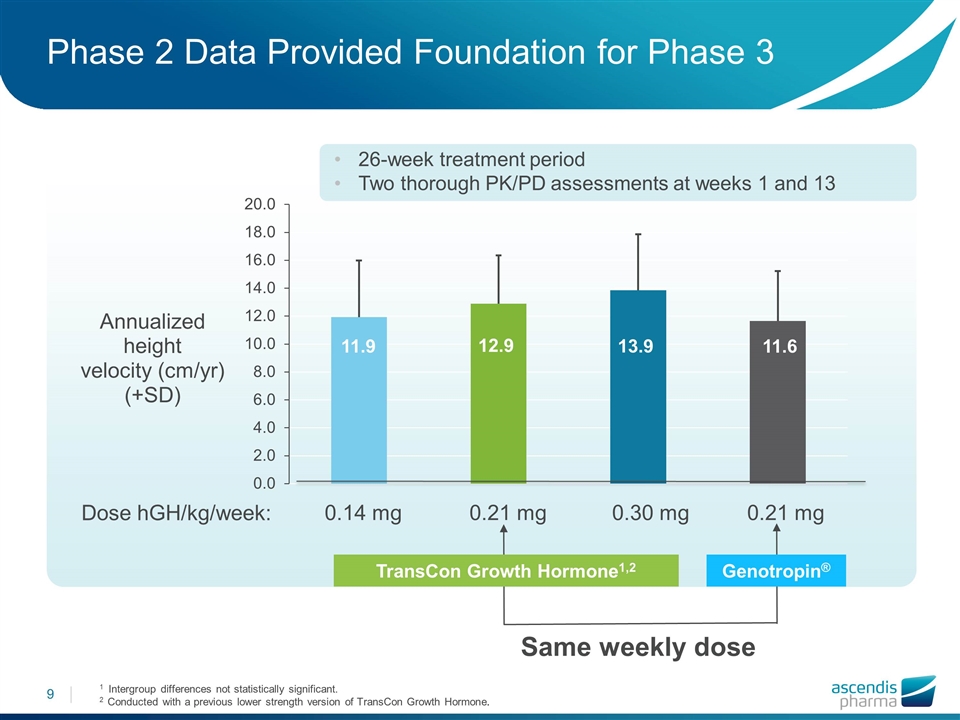
Phase 2 Data Provided Foundation for Phase 3 Same weekly dose 0.14 mg 0.21 mg 0.30 mg 0.21 mg Dose hGH/kg/week: 1 Intergroup differences not statistically significant. 2 Conducted with a previous lower strength version of TransCon Growth Hormone. 26-week treatment period Two thorough PK/PD assessments at weeks 1 and 13 TransCon Growth Hormone1,2 Genotropin®
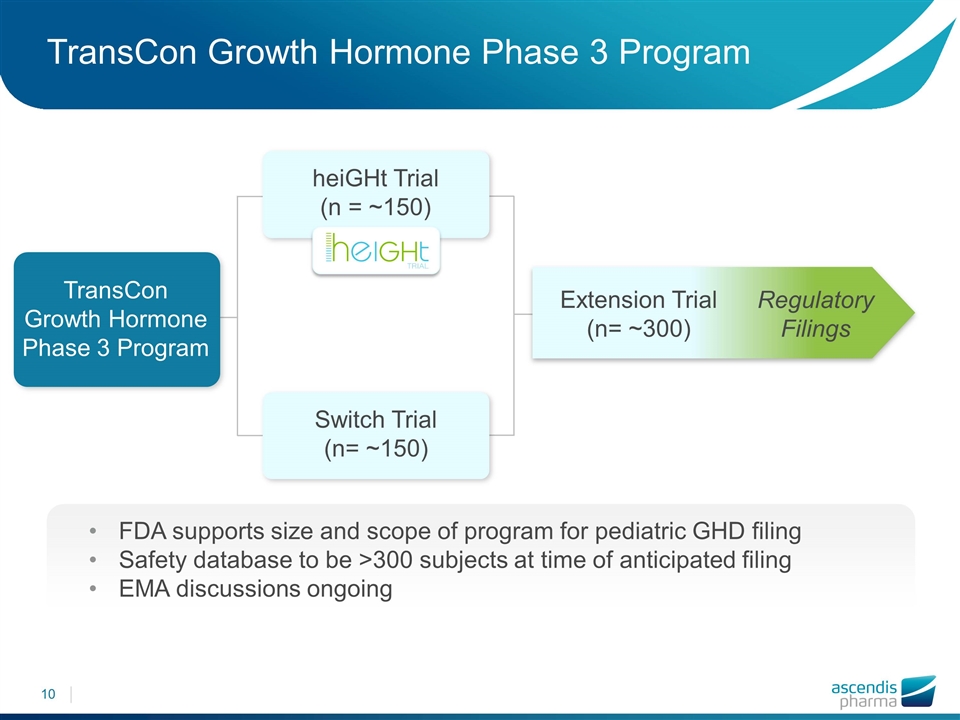
TransCon Growth Hormone Phase 3 Program heiGHt Trial (n = ~150) Switch Trial (n= ~150) Extension Trial (n= ~300) TransCon Growth Hormone Phase 3 Program FDA supports size and scope of program for pediatric GHD filing Safety database to be >300 subjects at time of anticipated filing EMA discussions ongoing Regulatory Filings

Auto-Injector Designed to Optimize Adherence and Compliance Auto-injector planned during extension study and for commercial launch Simple operation with few user steps A single low-volume (<0.60 mL) injection for patients less than 60kg Small needle comparable to daily hGH (31G, 4mm) Room temperature storage No waste due to empty-all design Enabled for Bluetooth® connectivity to IT health care solutions Device lifetime at least 4 years Key Features
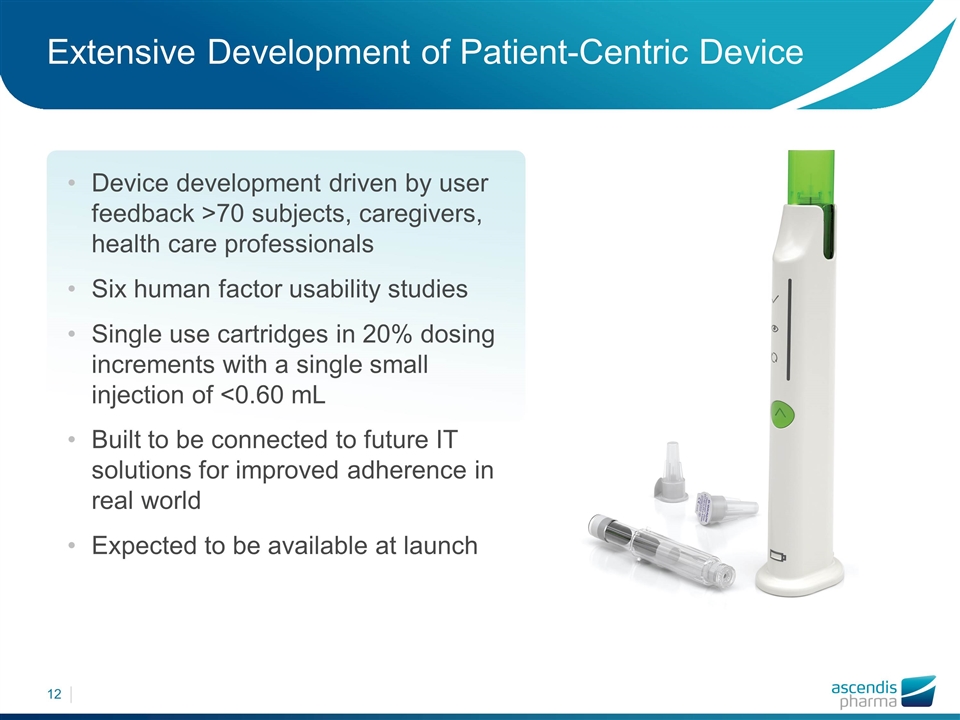
Device development driven by user feedback >70 subjects, caregivers, health care professionals Six human factor usability studies Single use cartridges in 20% dosing increments with a single small injection of <0.60 mL Built to be connected to future IT solutions for improved adherence in real world Expected to be available at launch Extensive Development of Patient-Centric Device

TransCon PTH: Once-daily for Hypoparathyroidism David B. Karpf, M.D. VP, Clinical Development
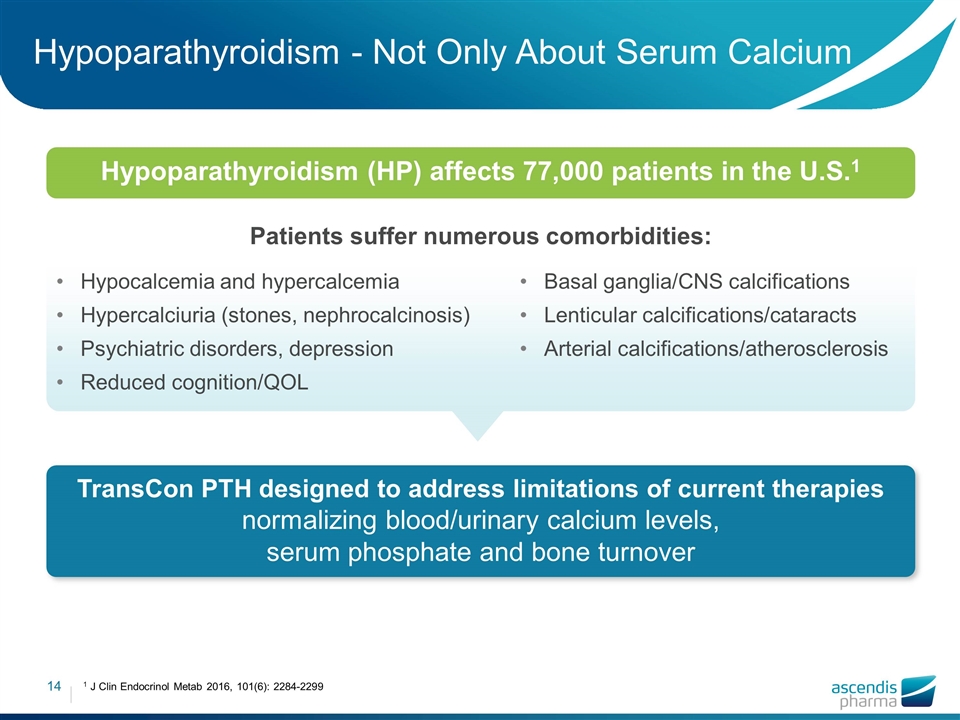
Hypoparathyroidism - Not Only About Serum Calcium Patients suffer numerous comorbidities: TransCon PTH designed to address limitations of current therapies normalizing blood/urinary calcium levels, serum phosphate and bone turnover Basal ganglia/CNS calcifications Lenticular calcifications/cataracts Arterial calcifications/atherosclerosis 1 J Clin Endocrinol Metab 2016, 101(6): 2284-2299 Hypoparathyroidism (HP) affects 77,000 patients in the U.S.1 Hypocalcemia and hypercalcemia Hypercalciuria (stones, nephrocalcinosis) Psychiatric disorders, depression Reduced cognition/QOL
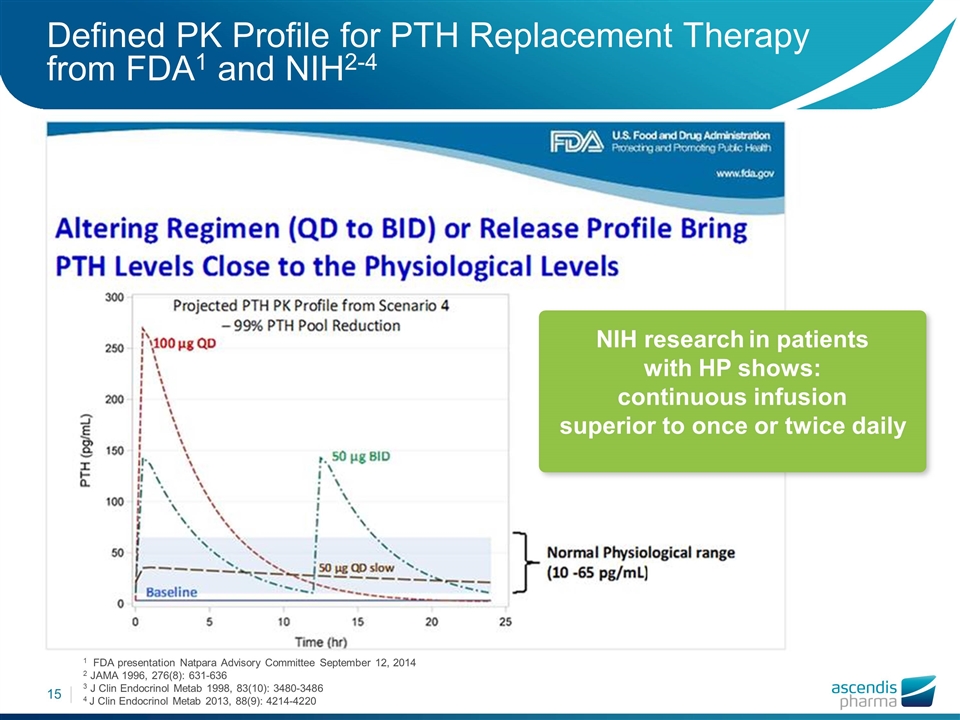
Defined PK Profile for PTH Replacement Therapy from FDA1 and NIH2-4 1 FDA presentation Natpara Advisory Committee September 12, 2014 2 JAMA 1996, 276(8): 631-636 3 J Clin Endocrinol Metab 1998, 83(10): 3480-3486 4 J Clin Endocrinol Metab 2013, 88(9): 4214-4220 NIH research in patients with HP shows: continuous infusion superior to once or twice daily
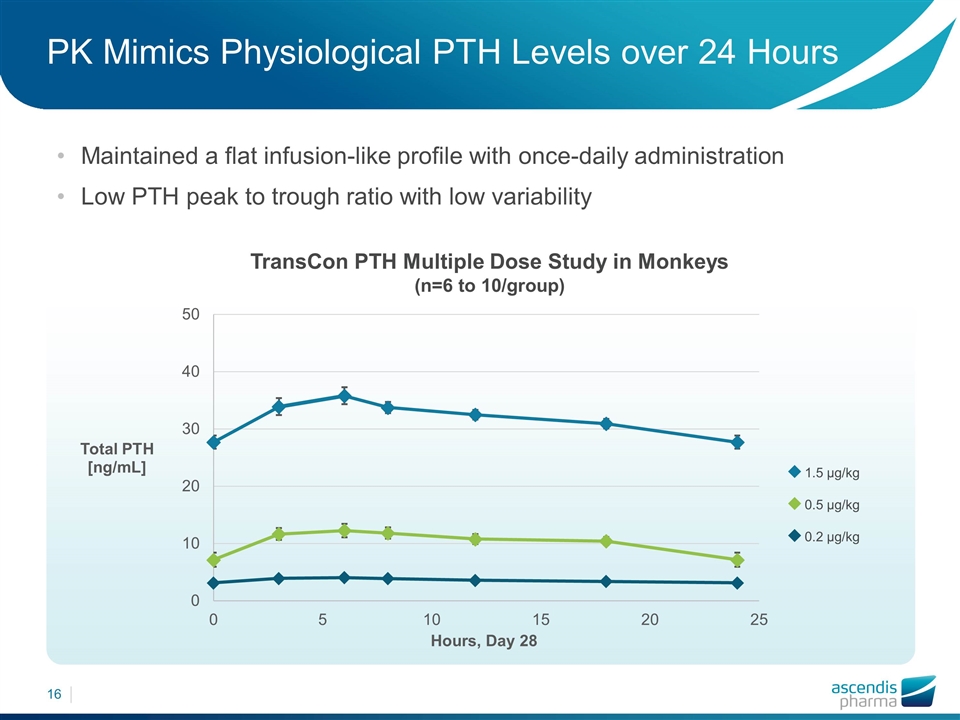
PK Mimics Physiological PTH Levels over 24 Hours Maintained a flat infusion-like profile with once-daily administration Low PTH peak to trough ratio with low variability TransCon PTH Multiple Dose Study in Monkeys (n=6 to 10/group) 1.5 µg/kg 0.5 µg/kg 0.2 µg/kg
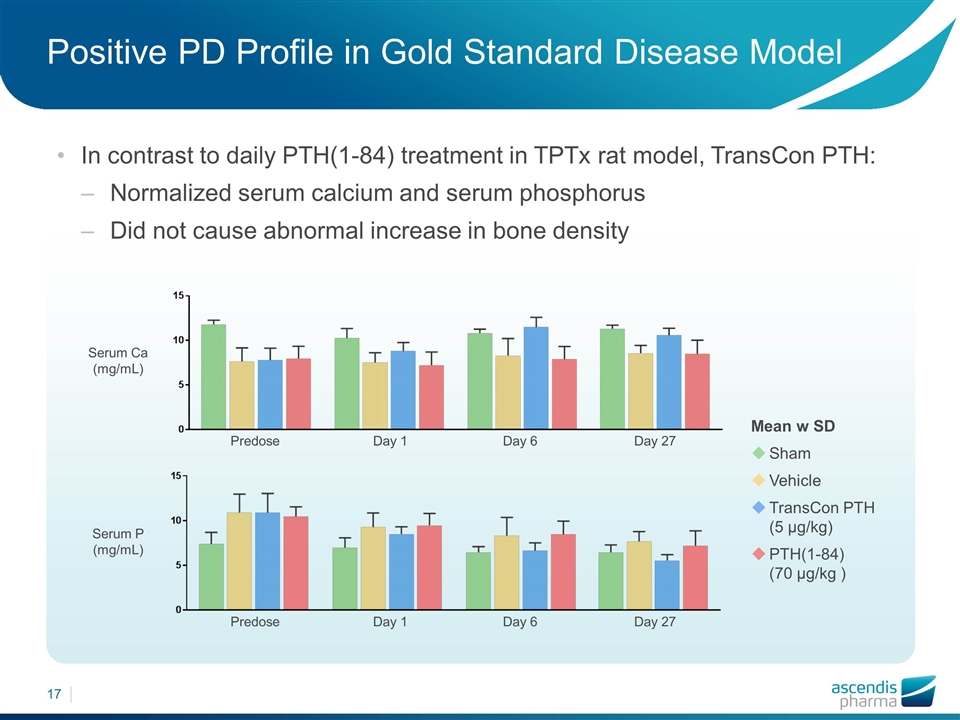
Positive PD Profile in Gold Standard Disease Model In contrast to daily PTH(1-84) treatment in TPTx rat model, TransCon PTH: Normalized serum calcium and serum phosphorus Did not cause abnormal increase in bone density Mean w SD Sham Vehicle TransCon PTH (5 µg/kg) PTH(1-84) (70 µg/kg ) Serum Ca (mg/mL) Serum P (mg/mL) Predose Day 1 Day 6 Day 27 Predose Day 1 Day 6 Day 27
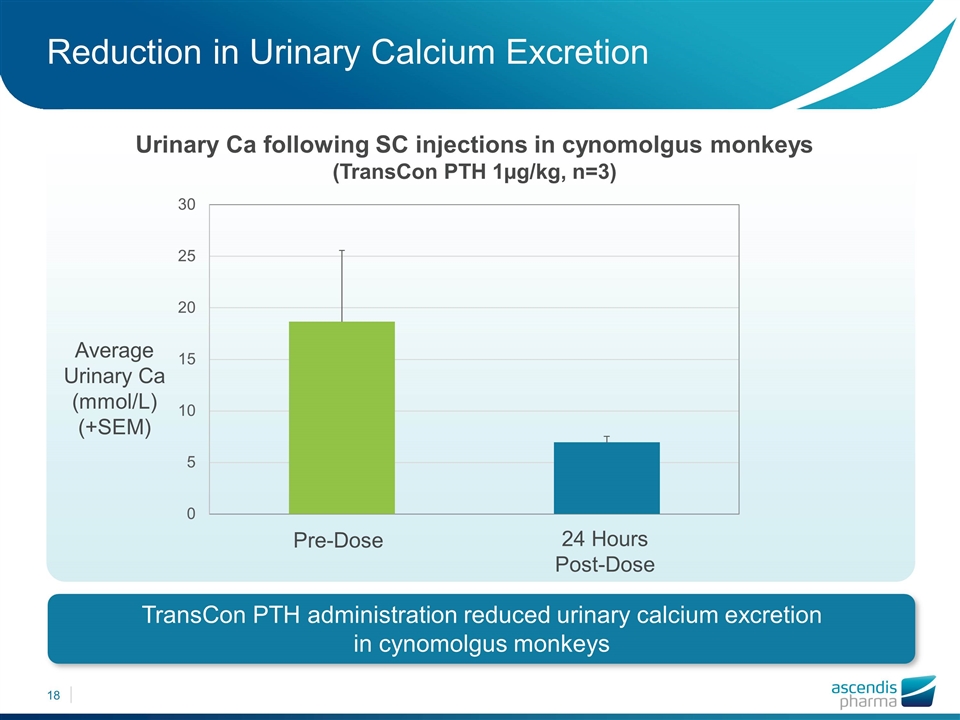
Reduction in Urinary Calcium Excretion TransCon PTH administration reduced urinary calcium excretion in cynomolgus monkeys Urinary Ca following SC injections in cynomolgus monkeys (TransCon PTH 1µg/kg, n=3) Average Urinary Ca (mmol/L) (+SEM) 24 Hours Post-Dose Pre-Dose

TransCon CNP: Once-Weekly CNP for Achondroplasia Kennett Sprogøe, PhD Senior Vice President, Product Innovation
Achondroplasia – A Serious and Disabling Disease Autosomal dominant genetic disorder Patients suffer numerous comorbidities No FDA-approved therapy Only option to improve height is surgical limb lengthening Most common form of human dwarfism Approximately 250,000 patients worldwide1 80% born to average-sized parents Ear infections/sleep apnea Obesity Bowed legs 1 Lancet 2007, 370: 162-172 Back/spine/cord compression Cardiovascular complications Dental complications
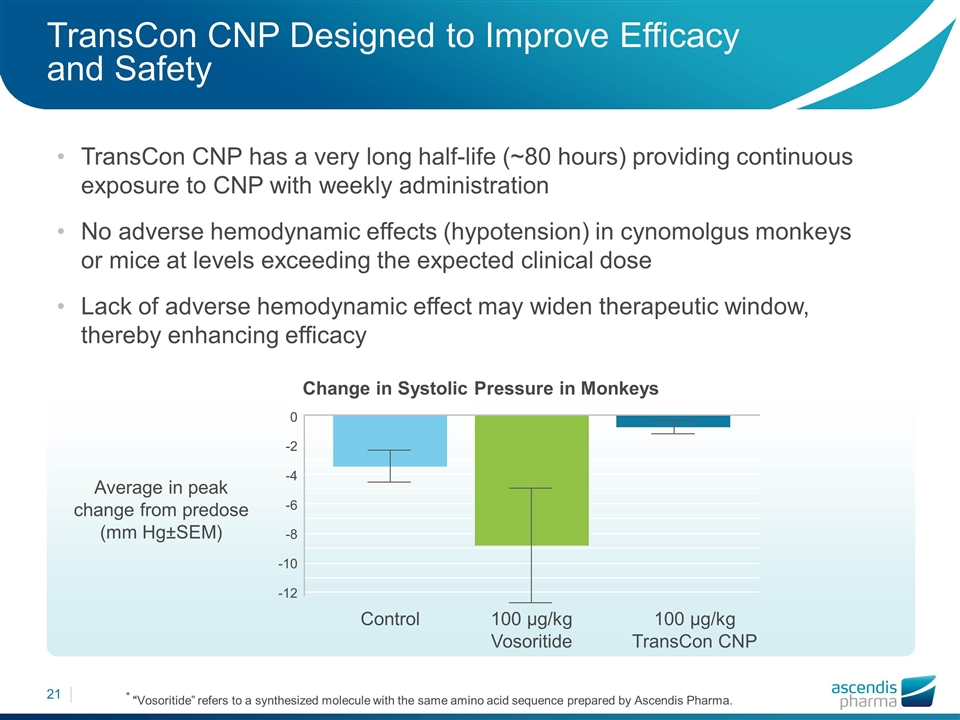
TransCon CNP Designed to Improve Efficacy and Safety TransCon CNP has a very long half-life (~80 hours) providing continuous exposure to CNP with weekly administration No adverse hemodynamic effects (hypotension) in cynomolgus monkeys or mice at levels exceeding the expected clinical dose Lack of adverse hemodynamic effect may widen therapeutic window, thereby enhancing efficacy * “Vosoritide” refers to a synthesized molecule with the same amino acid sequence prepared by Ascendis Pharma. Control Average in peak change from predose (mm Hg±SEM) 100 µg/kg Vosoritide 100 µg/kg TransCon CNP 0 -2 -4 -6 -8 -10 -12 Change in Systolic Pressure in Monkeys
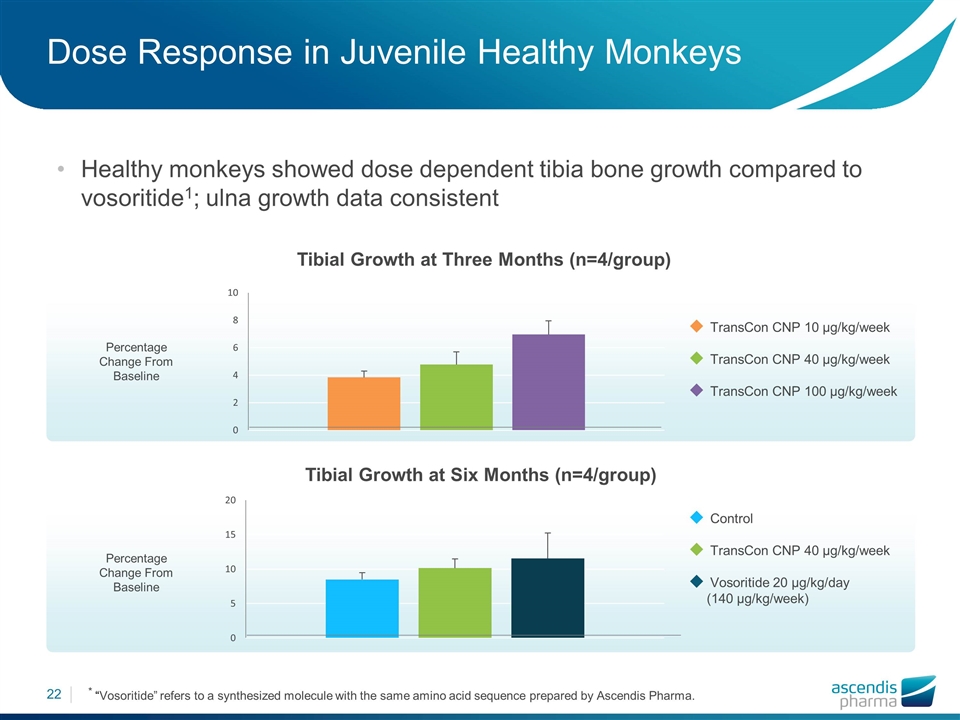
Control TransCon CNP 40 µg/kg/week Vosoritide 20 µg/kg/day (140 µg/kg/week) Percentage Change From Baseline Dose Response in Juvenile Healthy Monkeys Healthy monkeys showed dose dependent tibia bone growth compared to vosoritide1; ulna growth data consistent * “Vosoritide” refers to a synthesized molecule with the same amino acid sequence prepared by Ascendis Pharma. Percentage Change From Baseline Tibial Growth at Three Months (n=4/group) Tibial Growth at Six Months (n=4/group) TransCon CNP 10 µg/kg/week TransCon CNP 40 µg/kg/week TransCon CNP 100 µg/kg/week
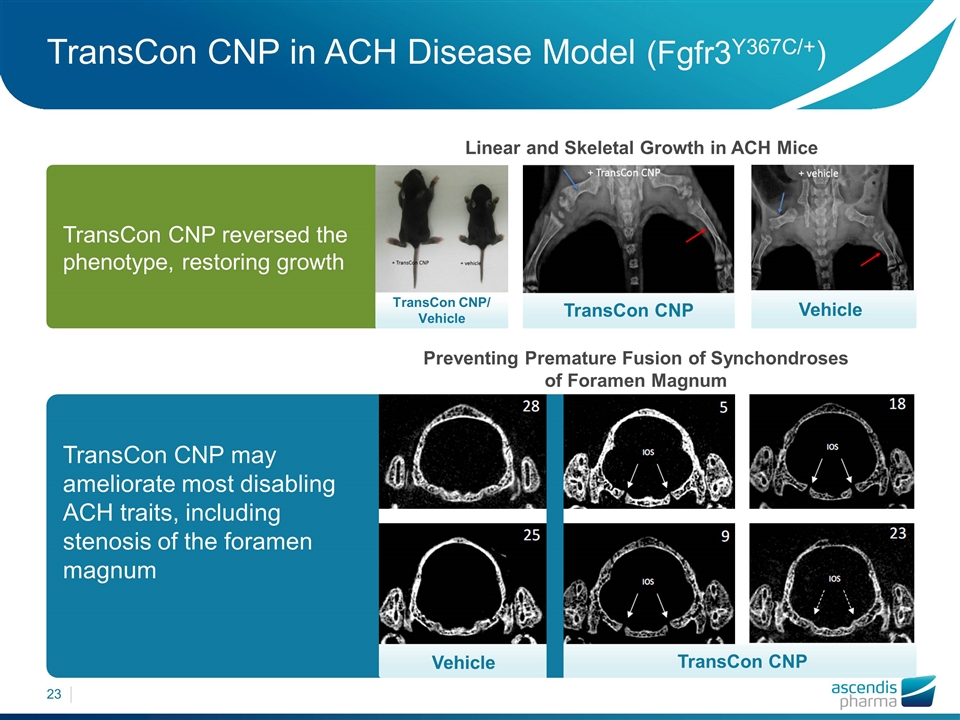
TransCon CNP/ Vehicle Vehicle TransCon CNP may ameliorate most disabling ACH traits, including stenosis of the foramen magnum TransCon CNP in ACH Disease Model (Fgfr3Y367C/+) Preventing Premature Fusion of Synchondroses of Foramen Magnum Linear and Skeletal Growth in ACH Mice Vehicle TransCon CNP TransCon CNP reversed the phenotype, restoring growth TransCon CNP
ENDO Conference Highlights Efficacy without potential dose-limiting cardiovascular adverse effects; once-weekly dosing in ACH IND or equivalent filing expected 4Q 2017 TransCon Growth Hormone TransCon PTH TransCon CNP Infusion-like profile potentially addresses all aspects of hypoparathyroidism Regulatory filing planned in Australia during 2Q 2017; Phase 1 to begin 3Q 2017 Only long-acting GH in clinical development that delivers unmodified GH, achieving same distribution and effects as hGH Phase 3 heiGHt Trial on track to complete enrollment 4Q 2017

Q&A
























