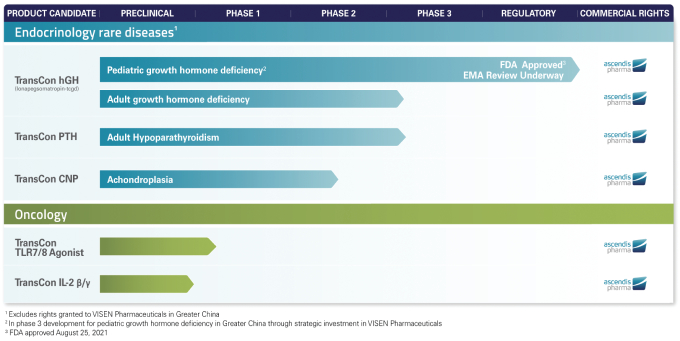
Our phase 3 pediatric program for lonapegsomatropin-tcgd consists of the heiGHt, fliGHt and enliGHten Trials. The heiGHt Trial was a randomized, open label, active-controlled phase 3 registrational trial that enrolled 161 children with GHD who had not previously been treated. The fliGHt Trial was designed to evaluate lonapegsomatropin-tcgd in subjects who were primarily treatment experienced with daily somatropin, although a subgroup of younger subjects were treatment-naïve. Nearly all subjects who completed the heiGHt or fliGHt Trials have enrolled in the open-label extension study, or the enliGHten Trial, which is designed to provide long-term safety data to support the regulatory submissions for lonapegsomatropin-tcgd. We initiated the enliGHten Trial in 2017 as the first subjects began to roll over from the heiGHt Trial, and we have enrolled approximately 300 pediatric subjects. Data from enliGHten formed the long-term safety database supporting our Biologics License Application submission to the FDA for lonapegsomatropin-tcgd for the treatment of pediatric GHD which was approved in August 2021, as well as submission of an MAA to the European Medicines Agency, or EMA, which occurred in September 2020.
Additionally, in January 2021, we announced 104-week analysis of data from the ongoing enliGHten Trial, including follow-up on subjects from height who continued into enliGHten. The data showed maintenance of a treatment advantage in subjects initially treated with lonapegsomatropin-tcgd beyond the first year of therapy. The safety results, which were comparable to Genotropin in the phase 3 heiGHt Trial, were consistent across the phase 3 clinical trials.
In September 2020, we filed a Clinical Trial Notification, or CTN, with the Pharmaceuticals and Medical Devices Agency, or PMDA in Japan, to initiate our phase 3 riGHt Trial of lonapegsomatropin-tcgd for the treatment for pediatric GHD. The primary objective of the riGHt Trial is to evaluate and compare the AHV of 40 Japanese prepubertal treatment-naïve children with GHD treated with weekly lonapegsomatropin-tcgd to that of a commercially available daily somatropin formulation at 52 weeks.
In July 2020, the EMA adopted a decision agreeing with the positive opinion from the Paediatric Development Committee, or PDCO, which approved the proposed Paediatric Investigation Plan, or PIP, for lonapegsomatropin-tcgd. The PDCO endorsed the lonapegsomatropin-tcgd program as acceptable for assessment of safety and efficacy for the use of lonapegsomatropin-tcgd as a treatment for GHD in children from six months to less than 18 years of age, mirroring the population covered by the studies conducted in the program.
In April 2020, we received orphan drug designation from the FDA for lonapegsomatropin-tcgd in the United States for the treatment of GHD. The FDA grants orphan designation to drugs that are intended for the treatment, diagnosis, or prevention of rare diseases or disorders that affect fewer than 200,000 people in the United States, and potentially may be safer or more effective than already approved products.
In October 2019, we received orphan designation from the European Commission, or EC, for lonapegsomatropin-tcgd for GHD. Orphan designation is granted to therapies aimed at the treatment, prevention or diagnosis of a disease that is life-threatening or chronically debilitating, affects no more than five in 10,000 persons in the European Union and for which no satisfactory method of diagnosis, prevention, or treatment has been authorized (or the product would provide significant additional benefit over existing therapies).
Additionally, we have initiated the foresiGHt Trial, a global phase 3 study with the aim to demonstrate the metabolic benefits of lonapegsomatropin-tcgd in adults with GHD, and in Greater China, VISEN Pharmaceuticals completed the patient enrollment of 154 treatment-naïve, prepubertal children for the ongoing phase 3 pivotal trial of lonapegsomatropin-tcgd in patients with pediatric GHD in March 2021. We intend to pursue other indications for lonapegsomatropin-tcgd consistent with our strategy to create sustainable growth.
Palopegteriparatide
We are using our TransCon technology platform to develop palopegteriparatide, an investigational once-daily long-acting prodrug of parathyroid hormone, or PTH, as a potential treatment for adult hypoparathyroidism, or HP, a rare endocrine disorder of calcium and phosphate metabolism. Palopegteriparatide is designed to replace PTH at physiologic levels for 24 hours each day to address both the short-term symptoms and long-term complications of HP.
Current standard of care (SoC) for HP patients primarily consists of active vitamin D and oral calcium supplementation. However, since PTH is not present at the kidney to facilitate calcium reabsorption from the urine, the goal of SoC is to maintain serum calcium (sCa) levels just below or within the lower part of the normal range and thereby limit as much as possible the damage from excess urinary calcium. Nonetheless, SoC frequently leads to significant sCa fluctuations accompanied by symptomatic hyper- or hypocalcemia. SoC with active vitamin D and calcium have been shown to contribute to the risk of renal disease.
HP also poses a high burden on the healthcare system despite current SoC. For example, one survey of 374 patients showed that 72% experienced more than ten symptoms in the preceding twelve months, with symptoms experienced for a mean of 13 ± 9 hours a day. Other studies showed that 79% of HP cases require hospitalizations and that patients with the disease have a four-fold increase in the risk of renal disease compared to healthy controls. Patients often experience decreased quality of life. We conducted a survey of 42 patients which found that 100 percent of subjects reported negative psychological impacts, interference with daily life and impact on physical functioning from HP, and that 76 percent were either no longer able to work or experienced interference with work productivity.
Palopegteriparatide is currently in phase 3 development as a treatment for adult HP. In September 2020, we submitted an amendment to our IND to initiate PaTHway, our North American and European phase 3 double-blinded, placebo controlled clinical trial evaluating the safety, tolerability and efficacy of palopegteriparatide in adults with HP following discussions with FDA and European regulatory authorities. On July 6, 2021, we announced that the PaTHway Trial reached the target enrollment.
In addition, on July 6, 2021, we announced the receipt of orphan drug designation for palopegteriparatide from the Japanese Ministry of Health, Labor, and Welfare. Furthermore, we announced the acceptance of the CTN for the PaTHway Japan Trial, a single-arm, phase 3 trial of palopegteriparatide in a minimum of 12 Japanese subjects with HP.
On June 2, 2021, VISEN announced IND approval from the Center for Drug Evaluation on the National Medical Products Administration for the phase 3 clinical trial of palopegteriparatide in adult subjects with HP, the PaTHway China Trial.
On May 10, 2021, we announced preliminary 58-week results from the continuing open-label extension, or OLE, portion of the PaTH Forward Trial, a global phase 2 trial evaluating the safety, tolerability, and efficacy of its investigational product candidate, palopegteriparatide, in adult subjects with HP.
3