- DBVT Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
6-K Filing
DBV Technologies (DBVT) 6-KCurrent report (foreign)
Filed: 7 Jan 19, 4:04pm

The Epicutaneous Immunotherapy Company January 2019 ©Genoskin Exhibit 99.1

Safe Harbor This presentation contains forward looking statements including, but not limited to, statements concerning the outcome or success of DBV’s clinical trials; its ability to successfully gain regulatory approvals and commercialize products; its ability to successfully advance its pipeline of product candidates; the rate and degree of market acceptance of its products; and its ability to develop sales and marketing capabilities. Forward looking statements are subject to a number of risks, uncertainties and assumptions. Moreover, DBV operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for DBV’s management to predict all risks, nor can DBV assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward looking statements it may make. In light of these risks, uncertainties and assumptions, the forward looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. You should not rely upon forward looking statements as predictions of future events. Although DBV believes that the expectations reflected in the forward looking statements are reasonable, it cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward looking statements will be achieved or occur. Moreover, except as required by law, neither DBV nor any other person assumes responsibility for the accuracy and completeness of the forward looking statements. Forward looking statements in this presentation represent DBV’s views only as of the date of this presentation. DBV undertakes no obligation to update or review any forward looking statement, whether as a result of new information, future developments or otherwise, except as required by law.
Advancing novel skin immunotherapies for patients with food allergies and other immunological diseases Limited innovation in the field of food allergies has left millions of patients underserved today We are focused on discovering, developing and commercializing our novel skin immunotherapy product candidates using our proprietary Viaskin Technology Platform Potent activation of the immune system with the Viaskin patch No active passage of antigen into the bloodstream Proprietary manufacturing equipment designed, engineered and developed by DBV Pioneering a New Class of Immunotherapy
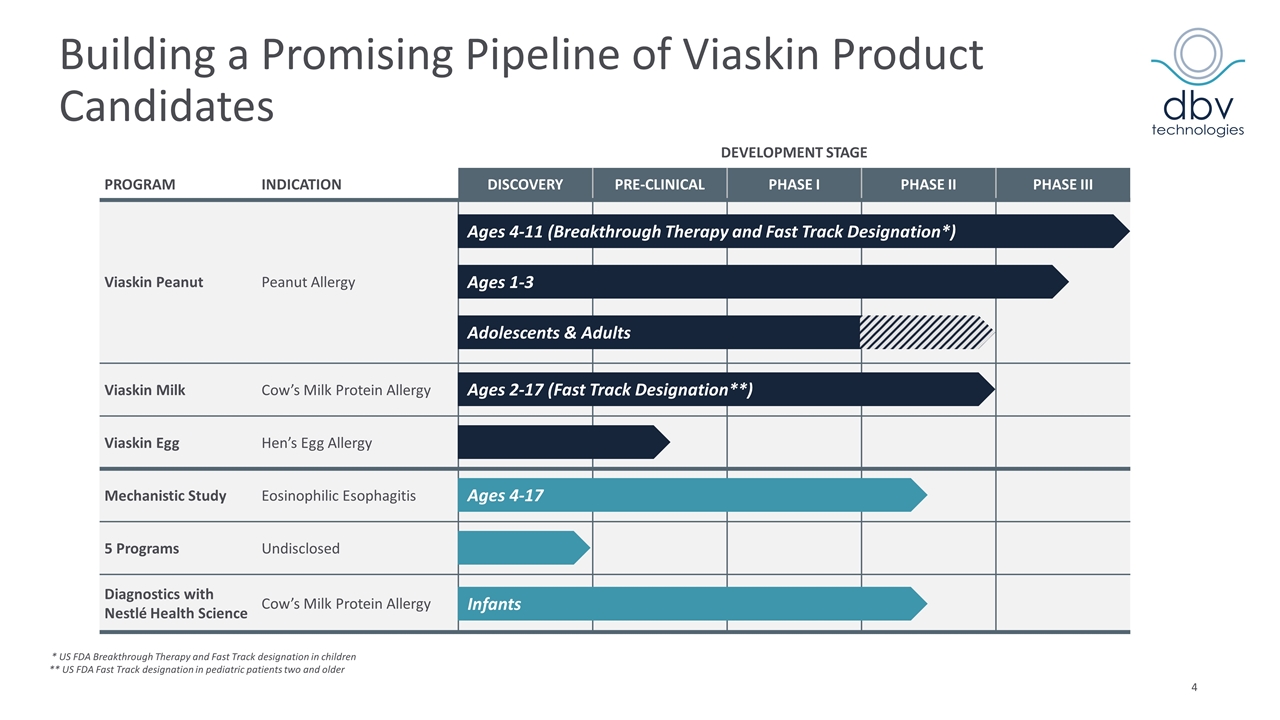
Building a Promising Pipeline of Viaskin Product Candidates DEVELOPMENT STAGE PROGRAM INDICATION DISCOVERY PRE-CLINICAL PHASE I PHASE II PHASE III Viaskin Peanut Peanut Allergy Viaskin Milk Cow’s Milk Protein Allergy Viaskin Egg Hen’s Egg Allergy Mechanistic Study Eosinophilic Esophagitis 5 Programs Undisclosed Diagnostics with Nestlé Health Science Cow’s Milk Protein Allergy Ages 4-11 (Breakthrough Therapy and Fast Track Designation*) Ages 1-3 Ages 2-17 (Fast Track Designation**) Ages 4-17 Adolescents & Adults Infants * US FDA Breakthrough Therapy and Fast Track designation in children ** US FDA Fast Track designation in pediatric patients two and older
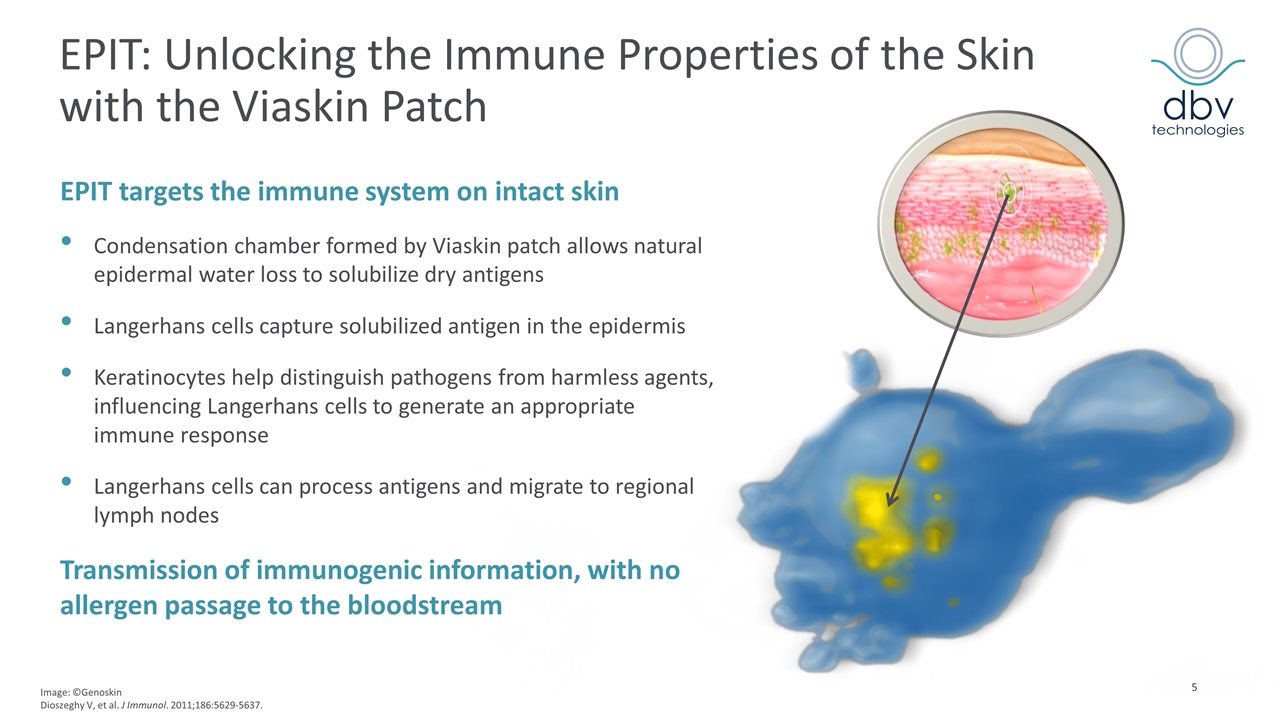
EPIT: Unlocking the Immune Properties of the Skin with the Viaskin Patch Image: ©Genoskin Dioszeghy V, et al. J Immunol. 2011;186:5629-5637. EPIT targets the immune system on intact skin Condensation chamber formed by Viaskin patch allows natural epidermal water loss to solubilize dry antigens Langerhans cells capture solubilized antigen in the epidermis Keratinocytes help distinguish pathogens from harmless agents, influencing Langerhans cells to generate an appropriate immune response Langerhans cells can process antigens and migrate to regional lymph nodes Transmission of immunogenic information, with no allergen passage to the bloodstream
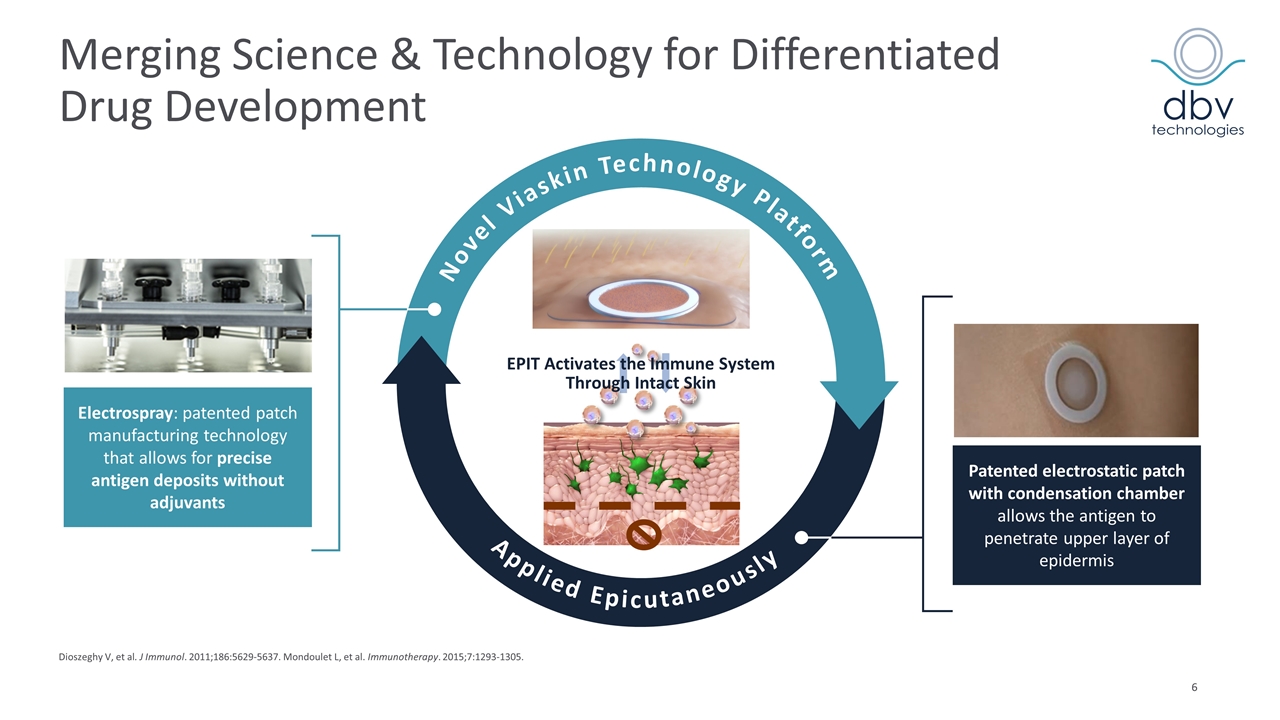
Merging Science & Technology for Differentiated Drug Development Applied Epicutaneously Electrospray: patented patch manufacturing technology that allows for precise antigen deposits without adjuvants Patented electrostatic patch with condensation chamber allows the antigen to penetrate upper layer of epidermis EPIT Activates the Immune System Through Intact Skin Dioszeghy V, et al. J Immunol. 2011;186:5629-5637. Mondoulet L, et al. Immunotherapy. 2015;7:1293-1305. Novel Viaskin Technology Platform

Where We Are Today: Preparing to Resubmit BLA for Viaskin Peanut Leadership team expanded and strengthened in January 2019 Key medical, manufacturing and regulatory changes implemented ahead of anticipated BLA resubmission for Viaskin Peanut Dr. Hugh Sampson to oversee scientific and medical strategy globally as CSO and interim CMO Julie O’Neill to direct all manufacturing operations and oversee resubmission of BLA Launch preparation ongoing with experienced commercial team in place Accomplished pharma marketer Kevin Trapp joined as Chief Commercial Officer in August 2018 North American headquarters established in Summit, New Jersey Search for a US-based Chief Medical Officer ongoing Company to carefully allocate resources throughout BLA resubmission process Over €150 million cash/cash equivalents and no debt (as of September 30, 2018)
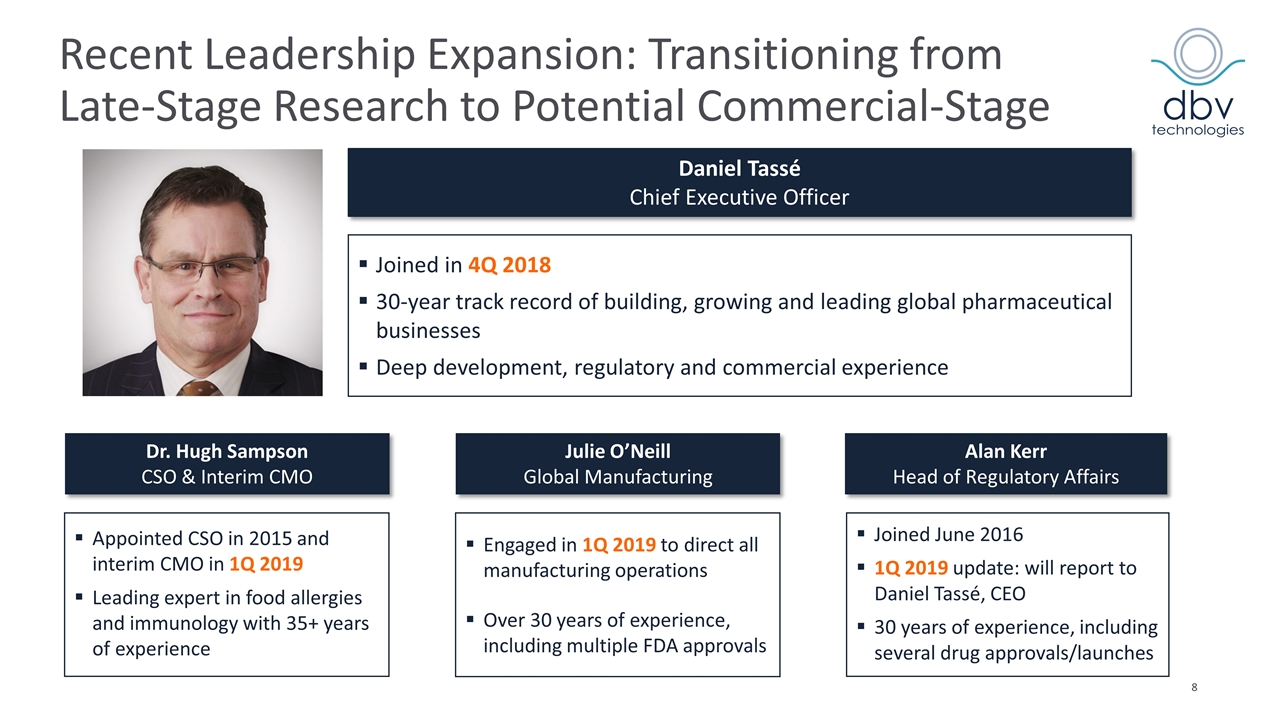
Recent Leadership Expansion: Transitioning from Late-Stage Research to Potential Commercial-Stage Julie O’Neill Global Manufacturing Dr. Hugh Sampson CSO & Interim CMO Alan Kerr Head of Regulatory Affairs Appointed CSO in 2015 and interim CMO in 1Q 2019 Leading expert in food allergies and immunology with 35+ years of experience Engaged in 1Q 2019 to direct all manufacturing operations Over 30 years of experience, including multiple FDA approvals Joined June 2016 1Q 2019 update: will report to Daniel Tassé, CEO 30 years of experience, including several drug approvals/launches Joined in 4Q 2018 30-year track record of building, growing and leading global pharmaceutical businesses Deep development, regulatory and commercial experience Daniel Tassé Chief Executive Officer
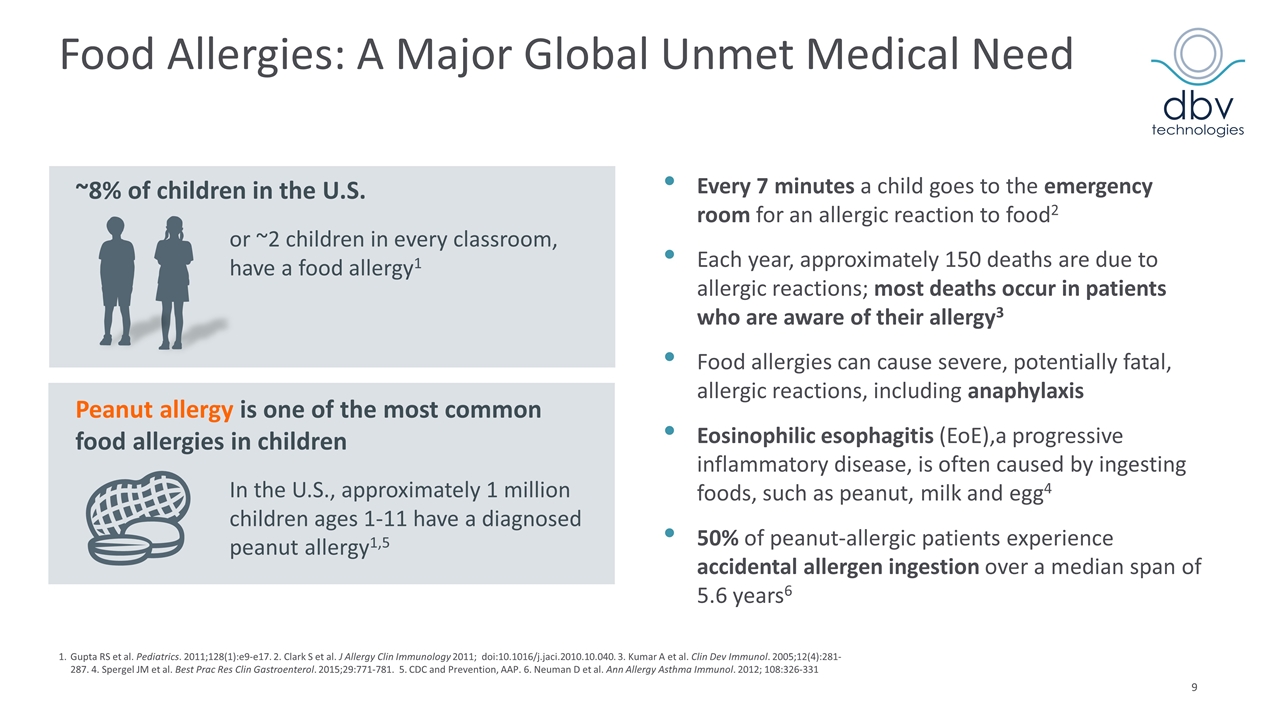
Food Allergies: A Major Global Unmet Medical Need Every 7 minutes a child goes to the emergency room for an allergic reaction to food2 Each year, approximately 150 deaths are due to allergic reactions; most deaths occur in patients who are aware of their allergy3 Food allergies can cause severe, potentially fatal, allergic reactions, including anaphylaxis Eosinophilic esophagitis (EoE),a progressive inflammatory disease, is often caused by ingesting foods, such as peanut, milk and egg4 50% of peanut-allergic patients experience accidental allergen ingestion over a median span of 5.6 years6 ~8% of children in the U.S. In the U.S., approximately 1 million children ages 1-11 have a diagnosed peanut allergy1,5 or ~2 children in every classroom, have a food allergy1 Peanut allergy is one of the most common food allergies in children 1.Gupta RS et al. Pediatrics. 2011;128(1):e9-e17. 2. Clark S et al. J Allergy Clin Immunology 2011; doi:10.1016/j.jaci.2010.10.040. 3. Kumar A et al. Clin Dev Immunol. 2005;12(4):281-287. 4. Spergel JM et al. Best Prac Res Clin Gastroenterol. 2015;29:771-781. 5. CDC and Prevention, AAP. 6. Neuman D et al. Ann Allergy Asthma Immunol. 2012; 108:326-331
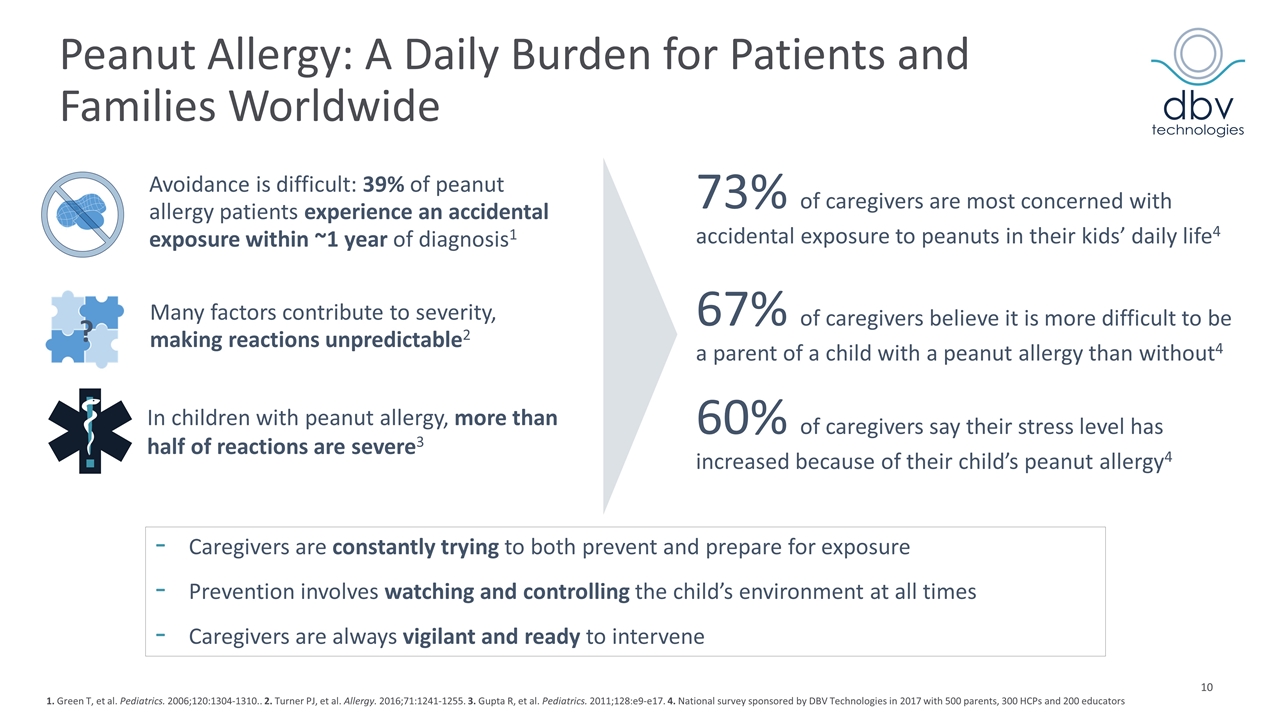
Peanut Allergy: A Daily Burden for Patients and Families Worldwide 10 Avoidance is difficult: 39% of peanut allergy patients experience an accidental exposure within ~1 year of diagnosis1 Many factors contribute to severity, making reactions unpredictable2 ? 1. Green T, et al. Pediatrics. 2006;120:1304-1310.. 2. Turner PJ, et al. Allergy. 2016;71:1241-1255. 3. Gupta R, et al. Pediatrics. 2011;128:e9-e17. 4. National survey sponsored by DBV Technologies in 2017 with 500 parents, 300 HCPs and 200 educators 73% of caregivers are most concerned with accidental exposure to peanuts in their kids’ daily life4 67% of caregivers believe it is more difficult to be a parent of a child with a peanut allergy than without4 60% of caregivers say their stress level has increased because of their child’s peanut allergy4 Caregivers are constantly trying to both prevent and prepare for exposure Prevention involves watching and controlling the child’s environment at all times Caregivers are always vigilant and ready to intervene In children with peanut allergy, more than half of reactions are severe3
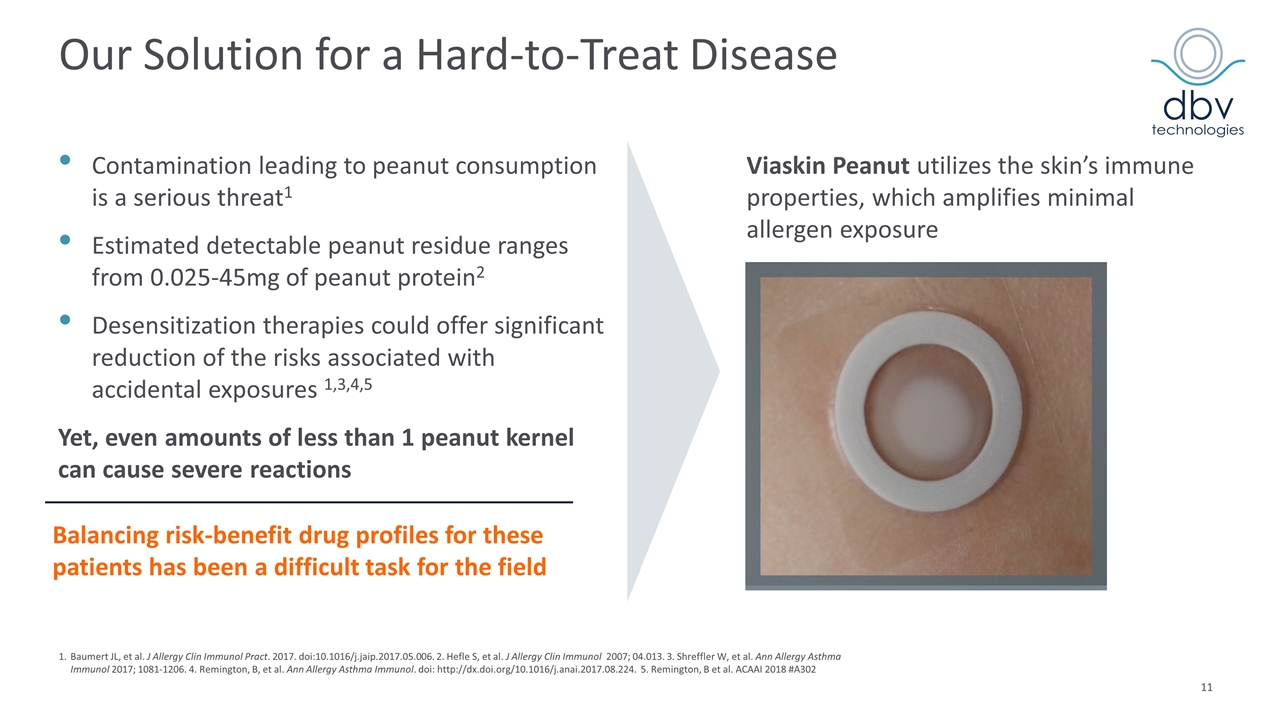
Our Solution for a Hard-to-Treat Disease Contamination leading to peanut consumption is a serious threat1 Estimated detectable peanut residue ranges from 0.025-45mg of peanut protein2 Desensitization therapies could offer significant reduction of the risks associated with accidental exposures 1,3,4,5 Yet, even amounts of less than 1 peanut kernel can cause severe reactions Viaskin Peanut utilizes the skin’s immune properties, which amplifies minimal allergen exposure Balancing risk-benefit drug profiles for these patients has been a difficult task for the field 1.Baumert JL, et al. J Allergy Clin Immunol Pract. 2017. doi:10.1016/j.jaip.2017.05.006. 2. Hefle S, et al. J Allergy Clin Immunol 2007; 04.013. 3. Shreffler W, et al. Ann Allergy Asthma Immunol 2017; 1081-1206. 4. Remington, B, et al. Ann Allergy Asthma Immunol. doi: http://dx.doi.org/10.1016/j.anai.2017.08.224. 5. Remington, B et al. ACAAI 2018 #A302
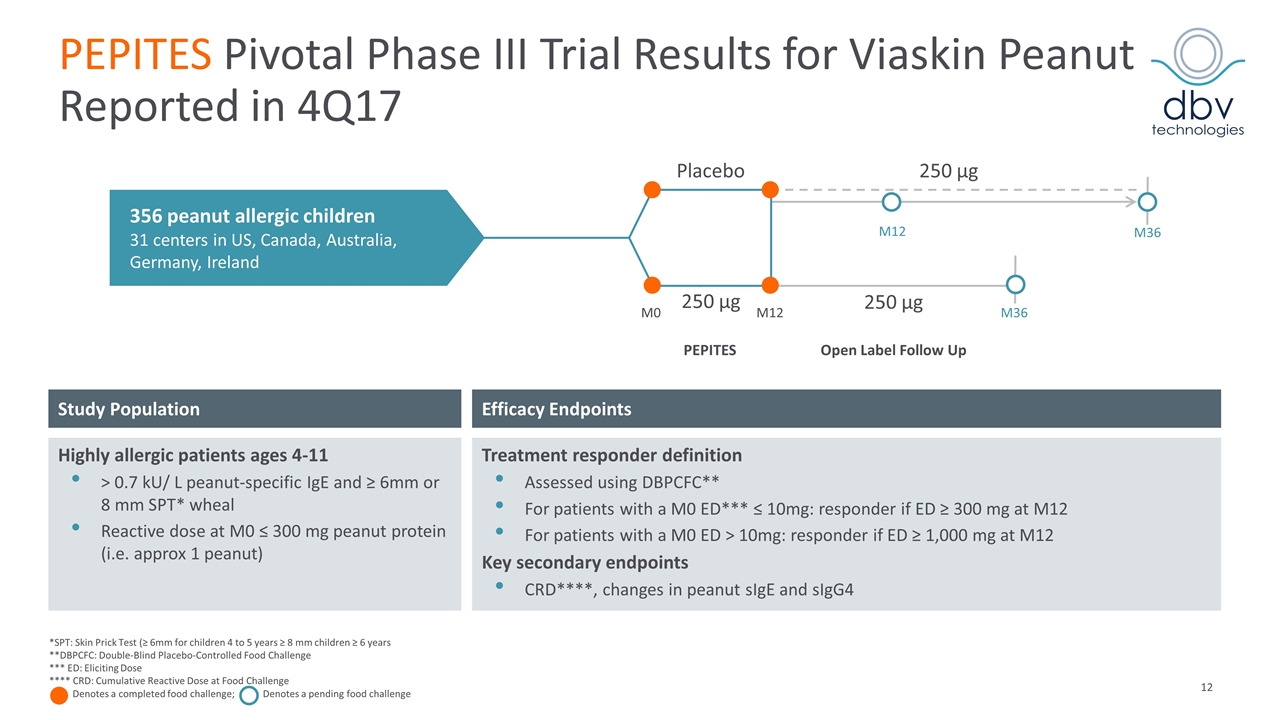
PEPITES Pivotal Phase III Trial Results for Viaskin Peanut Reported in 4Q17 Efficacy Endpoints Study Population Highly allergic patients ages 4-11 > 0.7 kU/ L peanut-specific IgE and ≥ 6mm or 8 mm SPT* wheal Reactive dose at M0 ≤ 300 mg peanut protein (i.e. approx 1 peanut) Treatment responder definition Assessed using DBPCFC** For patients with a M0 ED*** ≤ 10mg: responder if ED ≥ 300 mg at M12 For patients with a M0 ED > 10mg: responder if ED ≥ 1,000 mg at M12 Key secondary endpoints CRD****, changes in peanut sIgE and sIgG4 *SPT: Skin Prick Test (≥ 6mm for children 4 to 5 years ≥ 8 mm children ≥ 6 years **DBPCFC: Double-Blind Placebo-Controlled Food Challenge *** ED: Eliciting Dose **** CRD: Cumulative Reactive Dose at Food Challenge Denotes a completed food challenge; Denotes a pending food challenge 356 peanut allergic children 31 centers in US, Canada, Australia, Germany, Ireland 250 µg 250 µg M0 M12 M36 PEPITES Open Label Follow Up Placebo 250 µg M36 M12
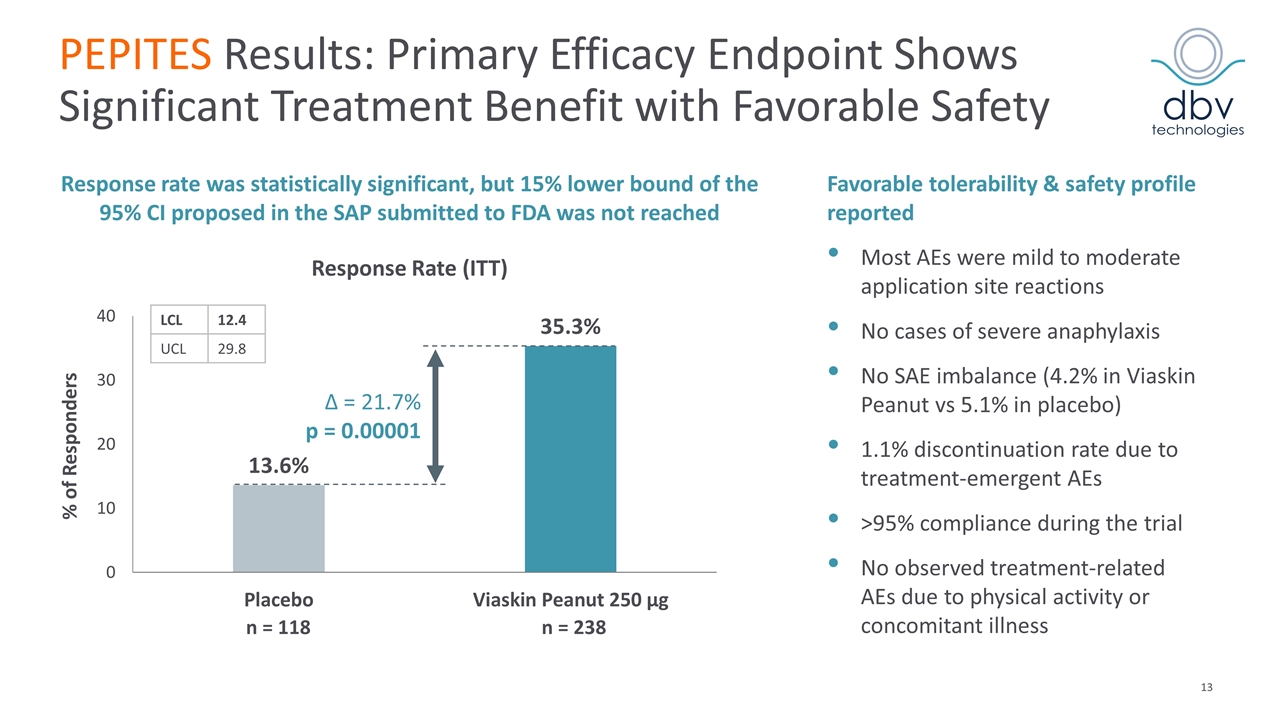
PEPITES Results: Primary Efficacy Endpoint Shows Significant Treatment Benefit with Favorable Safety Favorable tolerability & safety profile reported Most AEs were mild to moderate application site reactions No cases of severe anaphylaxis No SAE imbalance (4.2% in Viaskin Peanut vs 5.1% in placebo) 1.1% discontinuation rate due to treatment-emergent AEs >95% compliance during the trial No observed treatment-related AEs due to physical activity or concomitant illness Δ = 21.7% p = 0.00001 n = 118 n = 238 Response Rate (ITT) % of Responders Response rate was statistically significant, but 15% lower bound of the 95% CI proposed in the SAP submitted to FDA was not reached LCL 12.4 UCL 29.8
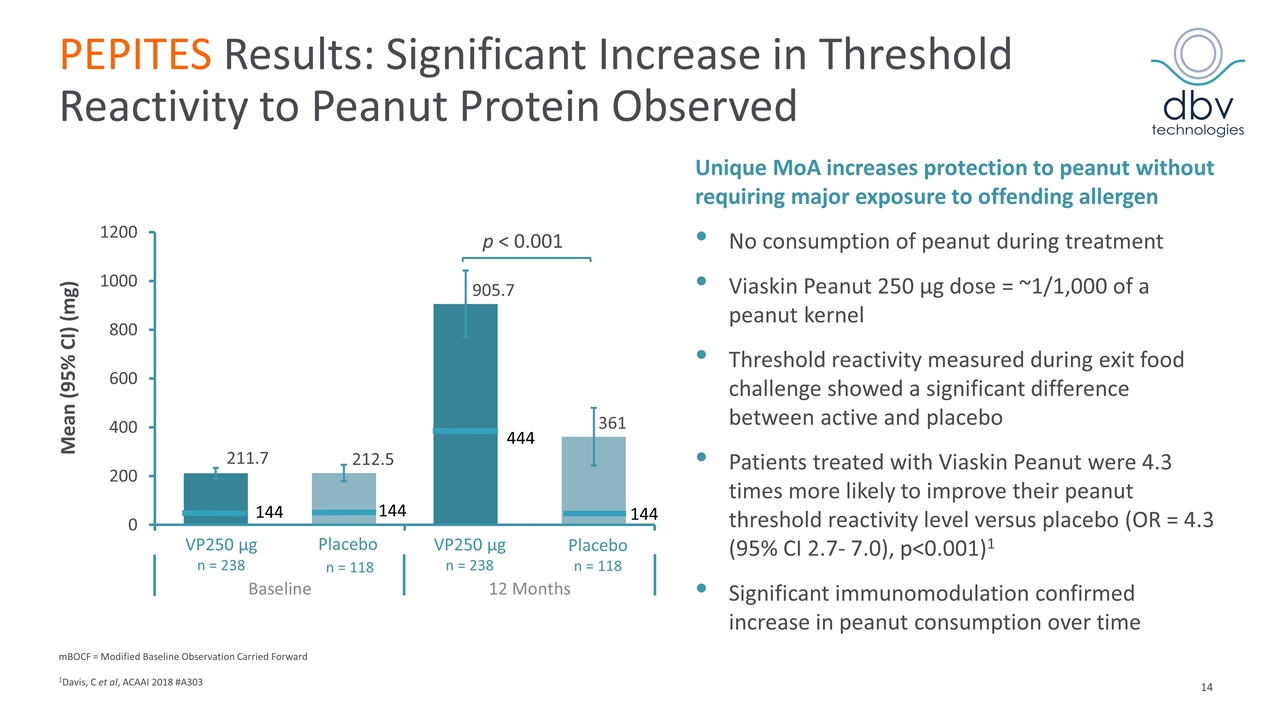
PEPITES Results: Significant Increase in Threshold Reactivity to Peanut Protein Observed mBOCF = Modified Baseline Observation Carried Forward 1Davis, C et al, ACAAI 2018 #A303 Unique MoA increases protection to peanut without requiring major exposure to offending allergen No consumption of peanut during treatment Viaskin Peanut 250 μg dose = ~1/1,000 of a peanut kernel Threshold reactivity measured during exit food challenge showed a significant difference between active and placebo Patients treated with Viaskin Peanut were 4.3 times more likely to improve their peanut threshold reactivity level versus placebo (OR = 4.3 (95% CI 2.7- 7.0), p<0.001)1 Significant immunomodulation confirmed increase in peanut consumption over time Mean (95% CI) (mg)
Viaskin Peanut 250 µg Clinical Profile Patients treated with Viaskin Peanut 250 µg were only exposed to ~1 peanut via the skin after 3 years1,2 Favorable safety and tolerability observed to date No cases of severe anaphylaxis due to treatment reported Drop-out rate due to AEs < 2% after 12 months (Phase I, Phase II and Phase III trials) Over 95% compliance observed for up to three years of treatment Most commonly reported AEs are mild to moderate application site reactions Consistent treatment benefit observed in multiple clinical trials Statistically significant higher rate of responders compared to placebo after 12 months of treatment in Phase II and Phase III trials Significant increase in threshold reactivity (CRD) to peanut protein observed after the first year of treatment Consistent biomarker trends in peanut-specific IgE and IgG4 across Phase II and Phase III studies Viaskin Peanut 250 µg has shown progressive and long-lasting desensitization after 12, 241,2 and 361,2 months of treatment Fleischer et al. AAAAI 2019, #L32. 2. Sampson et al. JAMA. 2017;318(18):1798-1809. doi:10.1001/jama.2017.16591; Shreffler et al. AAAAI 2017, #L7
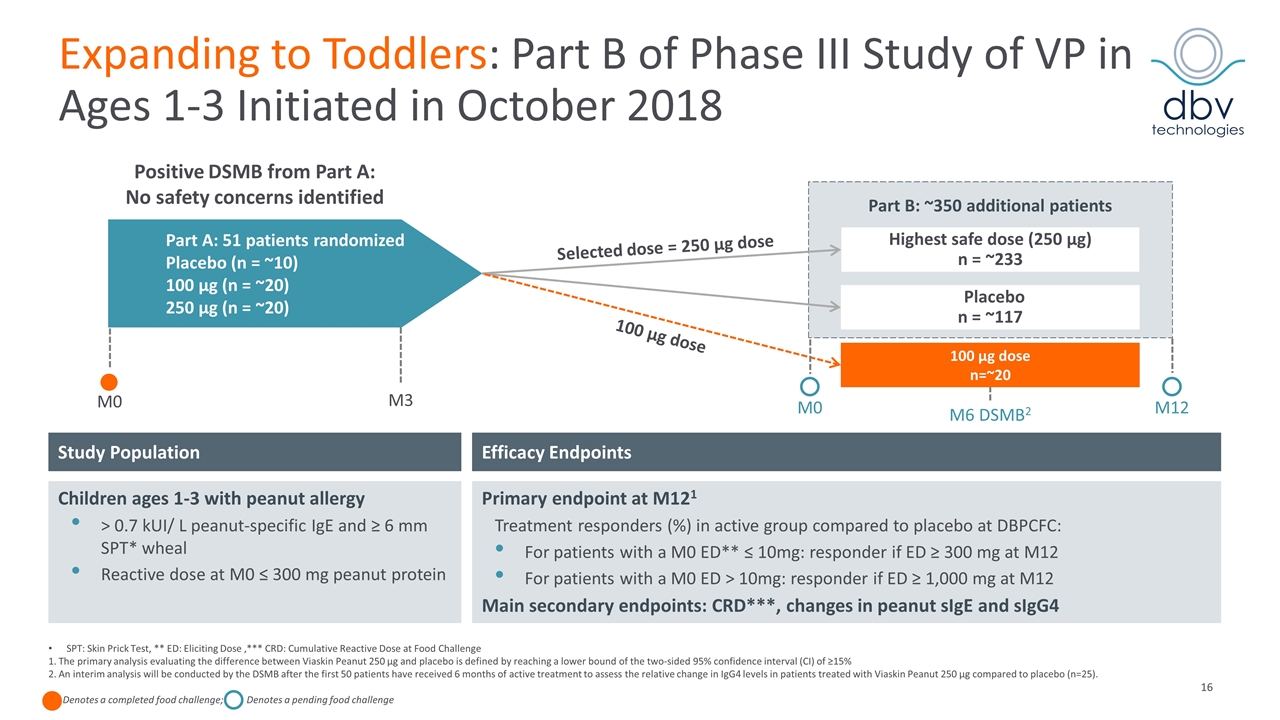
SPT: Skin Prick Test, ** ED: Eliciting Dose ,*** CRD: Cumulative Reactive Dose at Food Challenge 1. The primary analysis evaluating the difference between Viaskin Peanut 250 μg and placebo is defined by reaching a lower bound of the two-sided 95% confidence interval (CI) of ≥15% 2. An interim analysis will be conducted by the DSMB after the first 50 patients have received 6 months of active treatment to assess the relative change in IgG4 levels in patients treated with Viaskin Peanut 250 µg compared to placebo (n=25). Denotes a completed food challenge; Denotes a pending food challenge Expanding to Toddlers: Part B of Phase III Study of VP in Ages 1-3 Initiated in October 2018 M12 M3 Part A: 51 patients randomized Placebo (n = ~10) 100 µg (n = ~20) 250 µg (n = ~20) Highest safe dose (250 µg) n = ~233 Placebo n = ~117 100 µg dose n=~20 Part B: ~350 additional patients 100 µg dose Positive DSMB from Part A: No safety concerns identified Efficacy Endpoints Study Population Children ages 1-3 with peanut allergy > 0.7 kUI/ L peanut-specific IgE and ≥ 6 mm SPT* wheal Reactive dose at M0 ≤ 300 mg peanut protein Primary endpoint at M121 Treatment responders (%) in active group compared to placebo at DBPCFC: For patients with a M0 ED** ≤ 10mg: responder if ED ≥ 300 mg at M12 For patients with a M0 ED > 10mg: responder if ED ≥ 1,000 mg at M12 Main secondary endpoints: CRD***, changes in peanut sIgE and sIgG4 M0 M0 Selected dose = 250 µg dose M6 DSMB2
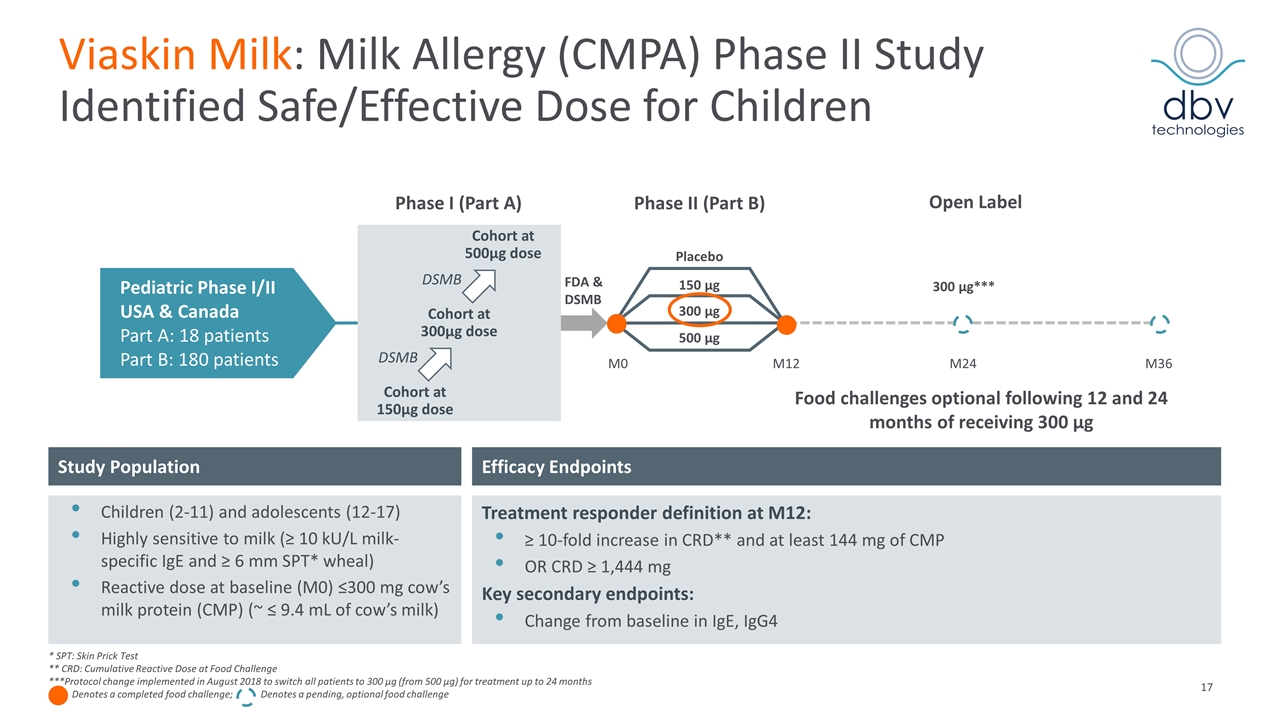
Viaskin Milk: Milk Allergy (CMPA) Phase II Study Identified Safe/Effective Dose for Children Pediatric Phase I/II USA & Canada Part A: 18 patients Part B: 180 patients M0 Phase I (Part A) Cohort at 150µg dose Cohort at 300µg dose Cohort at 500µg dose DSMB DSMB FDA & DSMB Placebo 300 µg 150 µg 500 µg Phase II (Part B) M12 M24 M36 Efficacy Endpoints Study Population Children (2-11) and adolescents (12-17) Highly sensitive to milk (≥ 10 kU/L milk-specific IgE and ≥ 6 mm SPT* wheal) Reactive dose at baseline (M0) ≤300 mg cow’s milk protein (CMP) (~ ≤ 9.4 mL of cow’s milk) Treatment responder definition at M12: ≥ 10-fold increase in CRD** and at least 144 mg of CMP OR CRD ≥ 1,444 mg Key secondary endpoints: Change from baseline in IgE, IgG4 * SPT: Skin Prick Test ** CRD: Cumulative Reactive Dose at Food Challenge ***Protocol change implemented in August 2018 to switch all patients to 300 µg (from 500 µg) for treatment up to 24 months Denotes a completed food challenge; Denotes a pending, optional food challenge Open Label Food challenges optional following 12 and 24 months of receiving 300 µg 300 µg***
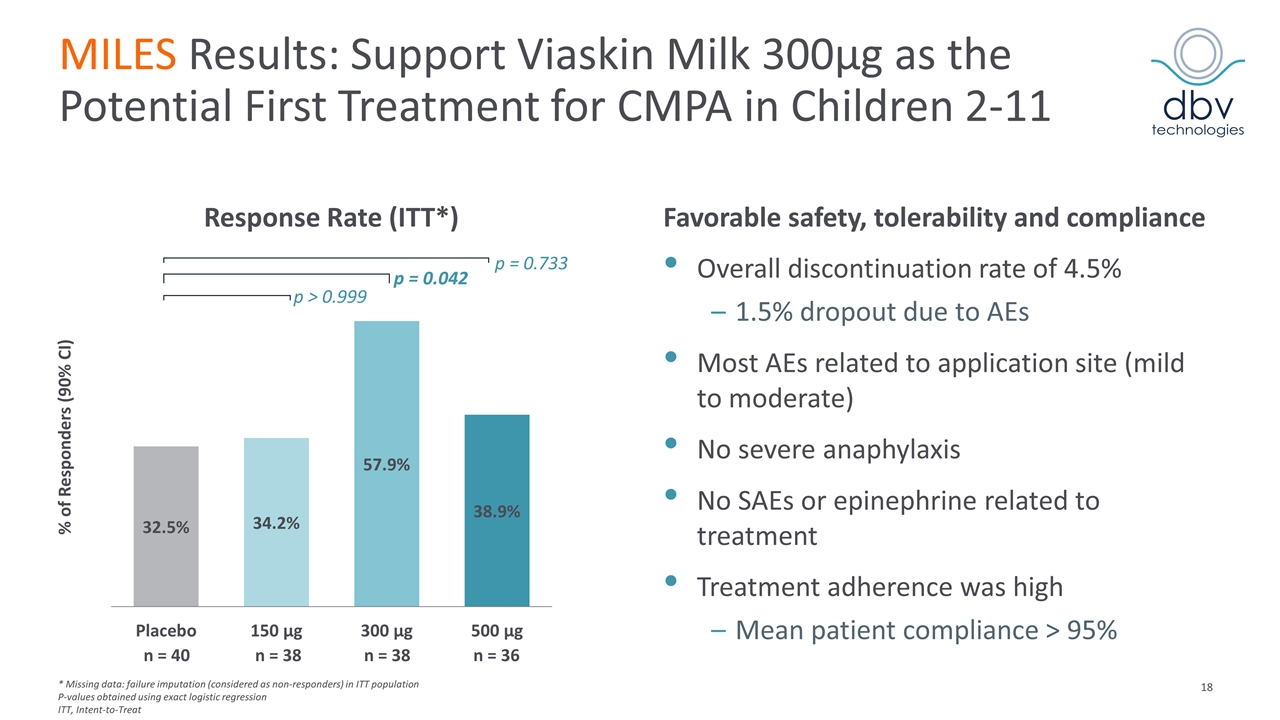
MILES Results: Support Viaskin Milk 300µg as the Potential First Treatment for CMPA in Children 2-11 Response Rate (ITT*) Favorable safety, tolerability and compliance Overall discontinuation rate of 4.5% 1.5% dropout due to AEs Most AEs related to application site (mild to moderate) No severe anaphylaxis No SAEs or epinephrine related to treatment Treatment adherence was high Mean patient compliance > 95% % of Responders (90% CI) p = 0.733 p = 0.042 p > 0.999 n = 40 n = 38 n = 38 n = 36 * Missing data: failure imputation (considered as non-responders) in ITT population P-values obtained using exact logistic regression ITT, Intent-to-Treat

DBV Technologies: Pioneering a New Class of Immunotherapy CEO Daniel Tassé joined DBV in November 2018 Key medical, manufacturing and regulatory leadership changes announced ahead of anticipated resubmission of BLA for Viaskin Peanut Phase III clinical program for Viaskin Peanut in children ages 4-11 completed (PEPITES & REALISE trials) in 2018 Positive preliminary Phase I/II data in second food allergy candidate, Viaskin Milk Key commercial roles, including Kevin Trapp, Chief Commercial Officer, recruited and onboarded in Summit, New Jersey office Continuing to build a talented team with global headquarters in US and France 3Q 2018 cash position of €153.9mn

Viaskin Platform
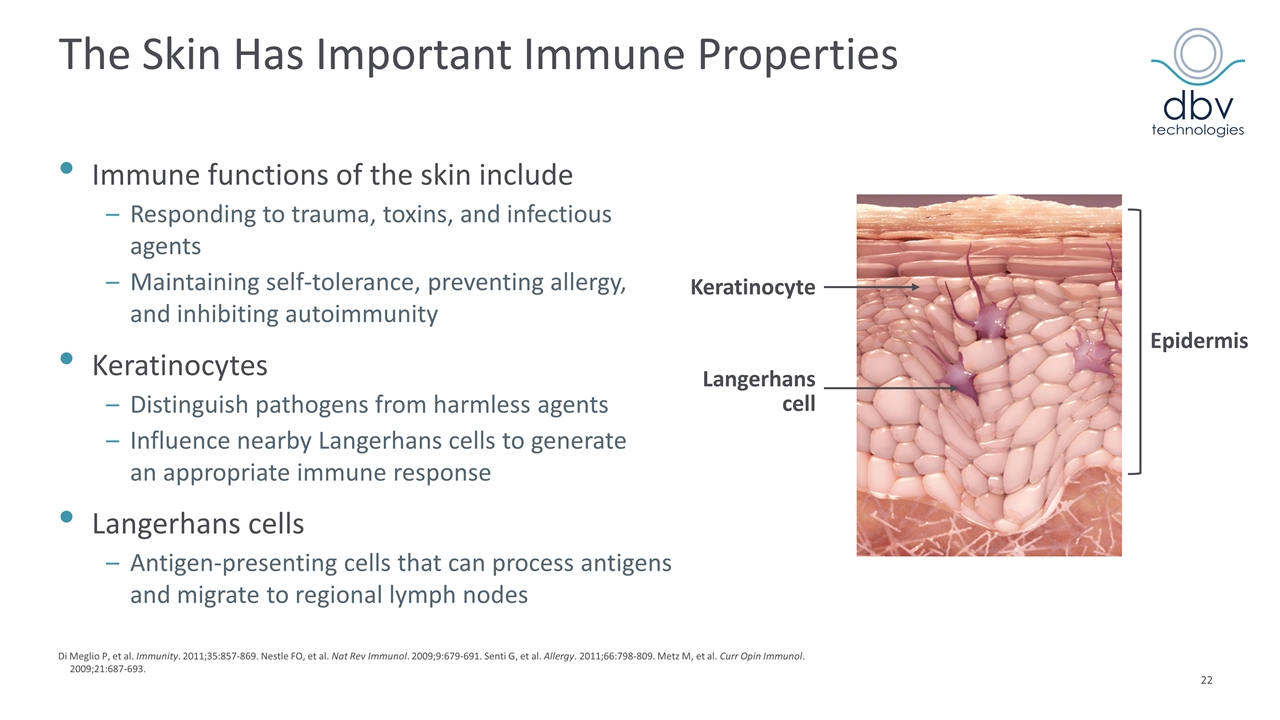
Di Meglio P, et al. Immunity. 2011;35:857-869. Nestle FO, et al. Nat Rev Immunol. 2009;9:679-691. Senti G, et al. Allergy. 2011;66:798-809. Metz M, et al. Curr Opin Immunol. 2009;21:687-693. Langerhans cell Keratinocyte Epidermis The Skin Has Important Immune Properties Immune functions of the skin include Responding to trauma, toxins, and infectious agents Maintaining self-tolerance, preventing allergy, and inhibiting autoimmunity Keratinocytes Distinguish pathogens from harmless agents Influence nearby Langerhans cells to generate an appropriate immune response Langerhans cells Antigen-presenting cells that can process antigens and migrate to regional lymph nodes
Modular components à technology versatility Highly scalable Broadly applicable platform Manufacturing capabilities In-house development and engineering of electrospray machines Development and engineering expertise at DBV [ES GEN4.0 machine] Proprietary & Patented Manufacturing Capabilities Developed by DBV
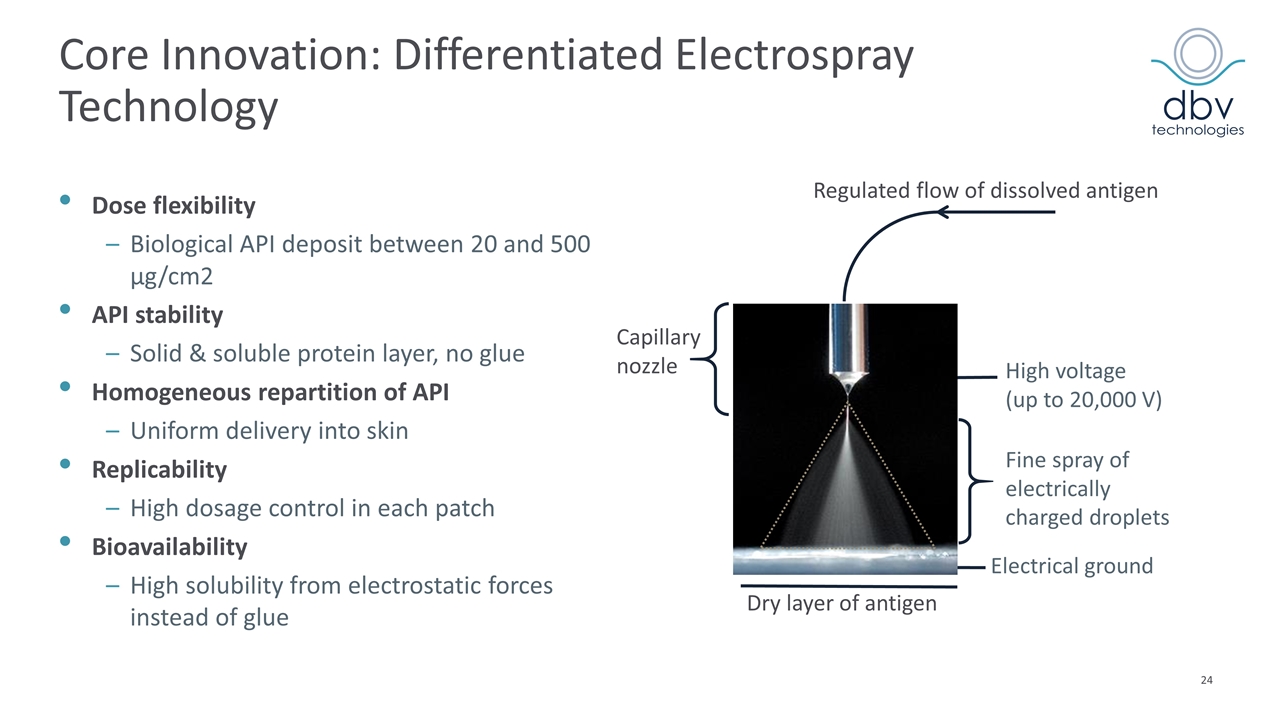
Dose flexibility Biological API deposit between 20 and 500 µg/cm2 API stability Solid & soluble protein layer, no glue Homogeneous repartition of API Uniform delivery into skin Replicability High dosage control in each patch Bioavailability High solubility from electrostatic forces instead of glue Electrical ground High voltage (up to 20,000 V) Regulated flow of dissolved antigen Fine spray of electrically charged droplets Dry layer of antigen Capillary nozzle Core Innovation: Differentiated Electrospray Technology
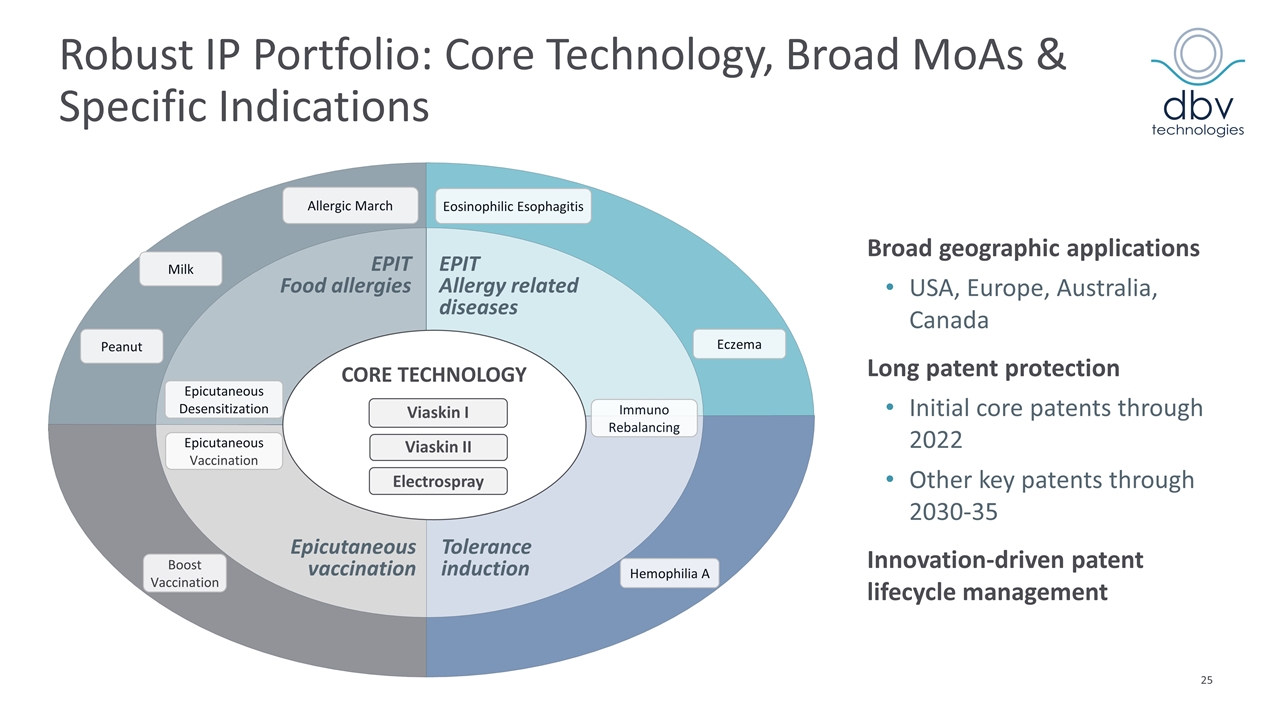
Boost Vaccination Peanut Eczema Eosinophilic Esophagitis Hemophilia A Allergic March Electrospray Viaskin II Viaskin I CORE TECHNOLOGY EPIT Food allergies EPIT Allergy related diseases Epicutaneous vaccination Immuno Rebalancing Tolerance induction Epicutaneous Desensitization Epicutaneous Vaccination Broad geographic applications USA, Europe, Australia, Canada Long patent protection Initial core patents through 2022 Other key patents through 2030-35 Innovation-driven patent lifecycle management Robust IP Portfolio: Core Technology, Broad MoAs & Specific Indications Milk

Viaskin Peanut Phase III Program: PEPITES & REALISE
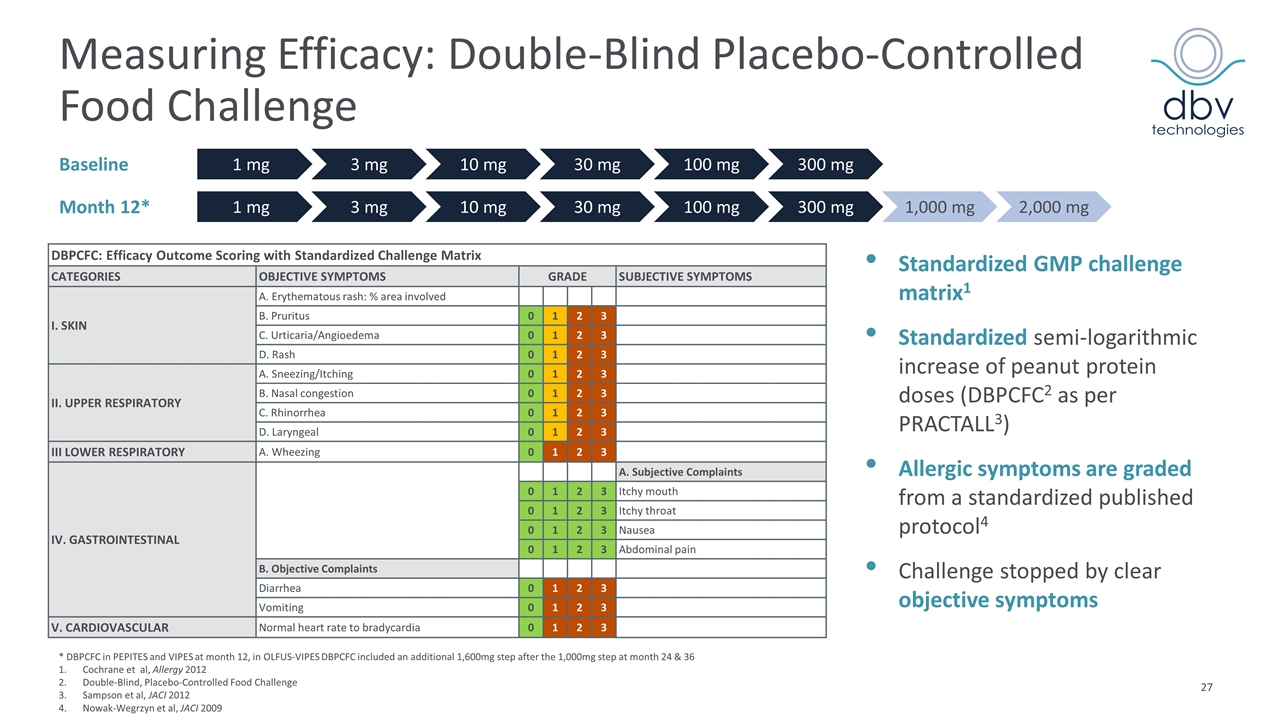
Measuring Efficacy: Double-Blind Placebo-Controlled Food Challenge Standardized GMP challenge matrix1 Standardized semi-logarithmic increase of peanut protein doses (DBPCFC2 as per PRACTALL3) Allergic symptoms are graded from a standardized published protocol4 Challenge stopped by clear objective symptoms * DBPCFC in PEPITES and VIPES at month 12, in OLFUS-VIPES DBPCFC included an additional 1,600mg step after the 1,000mg step at month 24 & 36 Cochrane et al, Allergy 2012 Double-Blind, Placebo-Controlled Food Challenge Sampson et al, JACI 2012 Nowak-Wegrzyn et al, JACI 2009 Month 12* 1 mg 3 mg 10 mg 30 mg 100 mg 300 mg 1,000 mg 2,000 mg Baseline 1 mg 3 mg 10 mg 30 mg 100 mg 300 mg DBPCFC: Efficacy Outcome Scoring with Standardized Challenge Matrix CATEGORIES OBJECTIVE SYMPTOMS GRADE SUBJECTIVE SYMPTOMS I. SKIN A. Erythematous rash: % area involved B. Pruritus 0 1 2 3 C. Urticaria/Angioedema 0 1 2 3 D. Rash 0 1 2 3 II. UPPER RESPIRATORY A. Sneezing/Itching 0 1 2 3 B. Nasal congestion 0 1 2 3 C. Rhinorrhea 0 1 2 3 D. Laryngeal 0 1 2 3 III LOWER RESPIRATORY A. Wheezing 0 1 2 3 IV. GASTROINTESTINAL A. Subjective Complaints 0 1 2 3 Itchy mouth 0 1 2 3 Itchy throat 0 1 2 3 Nausea 0 1 2 3 Abdominal pain B. Objective Complaints Diarrhea 0 1 2 3 Vomiting 0 1 2 3 V. CARDIOVASCULAR Normal heart rate to bradycardia 0 1 2 3
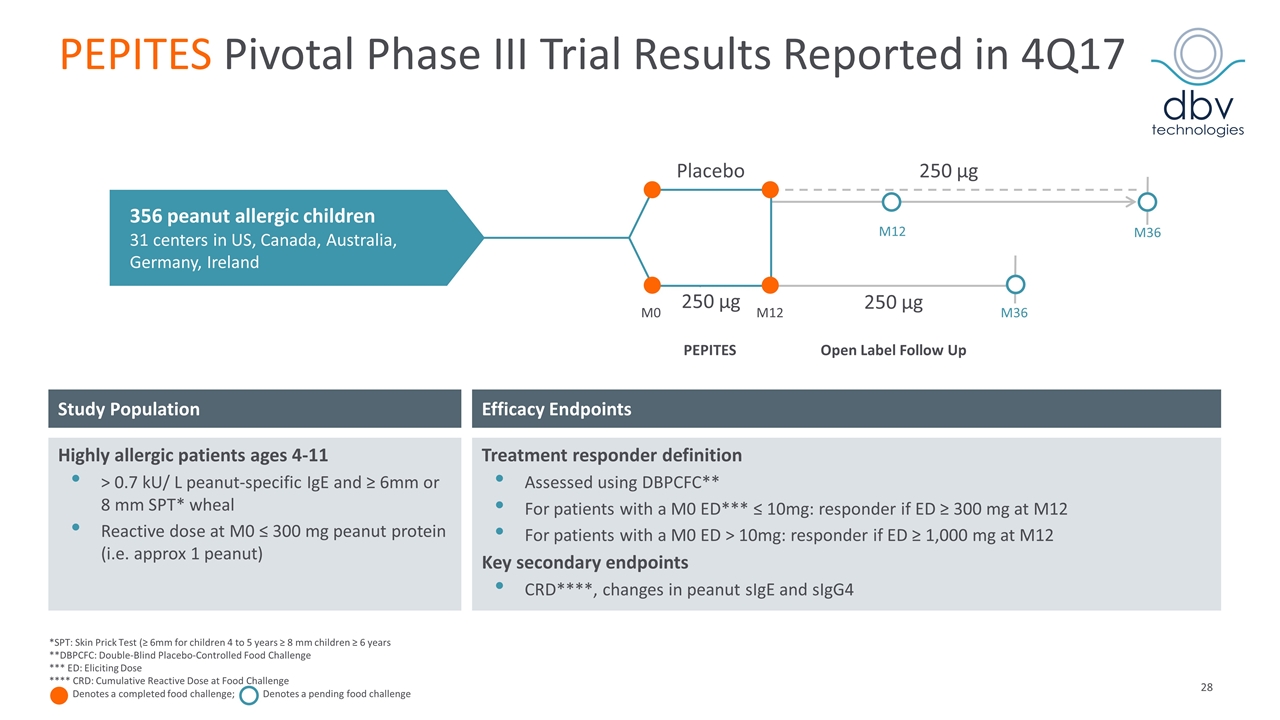
PEPITES Pivotal Phase III Trial Results Reported in 4Q17 Efficacy Endpoints Study Population Highly allergic patients ages 4-11 > 0.7 kU/ L peanut-specific IgE and ≥ 6mm or 8 mm SPT* wheal Reactive dose at M0 ≤ 300 mg peanut protein (i.e. approx 1 peanut) Treatment responder definition Assessed using DBPCFC** For patients with a M0 ED*** ≤ 10mg: responder if ED ≥ 300 mg at M12 For patients with a M0 ED > 10mg: responder if ED ≥ 1,000 mg at M12 Key secondary endpoints CRD****, changes in peanut sIgE and sIgG4 *SPT: Skin Prick Test (≥ 6mm for children 4 to 5 years ≥ 8 mm children ≥ 6 years **DBPCFC: Double-Blind Placebo-Controlled Food Challenge *** ED: Eliciting Dose **** CRD: Cumulative Reactive Dose at Food Challenge Denotes a completed food challenge; Denotes a pending food challenge 356 peanut allergic children 31 centers in US, Canada, Australia, Germany, Ireland 250 µg 250 µg M0 M12 M36 PEPITES Open Label Follow Up Placebo 250 µg M36 M12
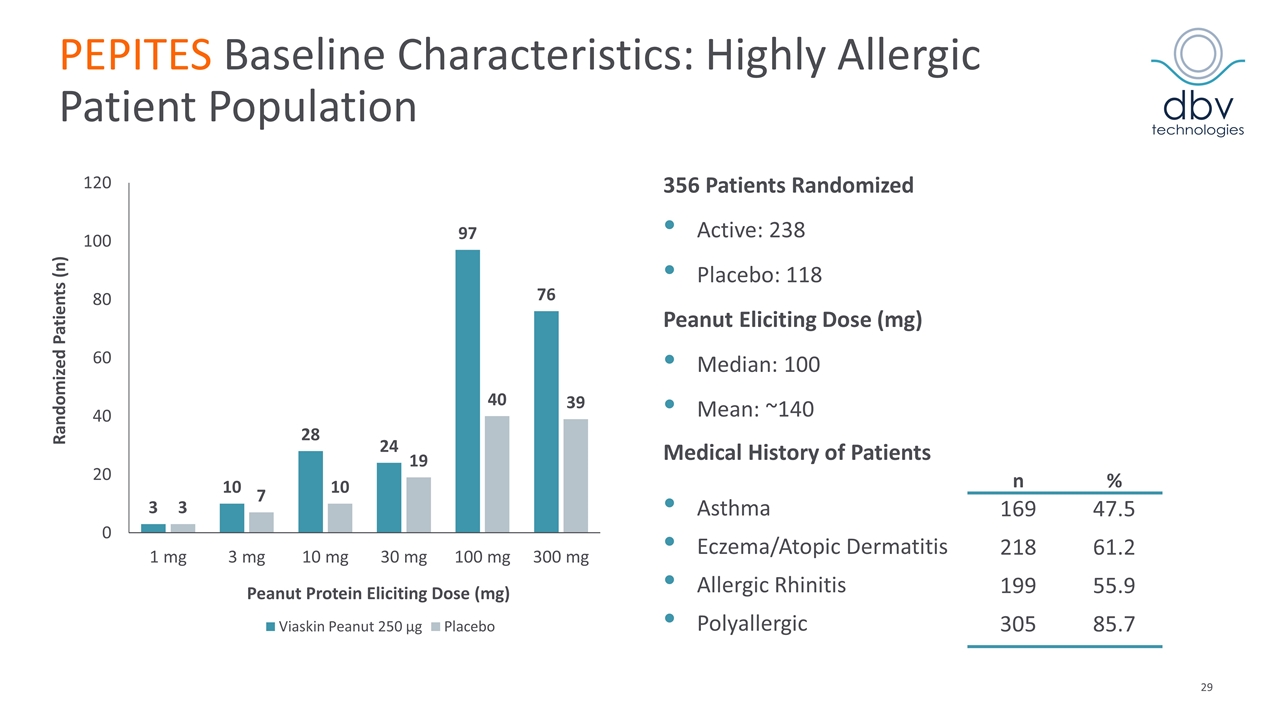
PEPITES Baseline Characteristics: Highly Allergic Patient Population 356 Patients Randomized Active: 238 Placebo: 118 Peanut Eliciting Dose (mg) Median: 100 Mean: ~140 Medical History of Patients n % Asthma 169 47.5 Eczema/Atopic Dermatitis 218 61.2 Allergic Rhinitis 199 55.9 Polyallergic 305 85.7
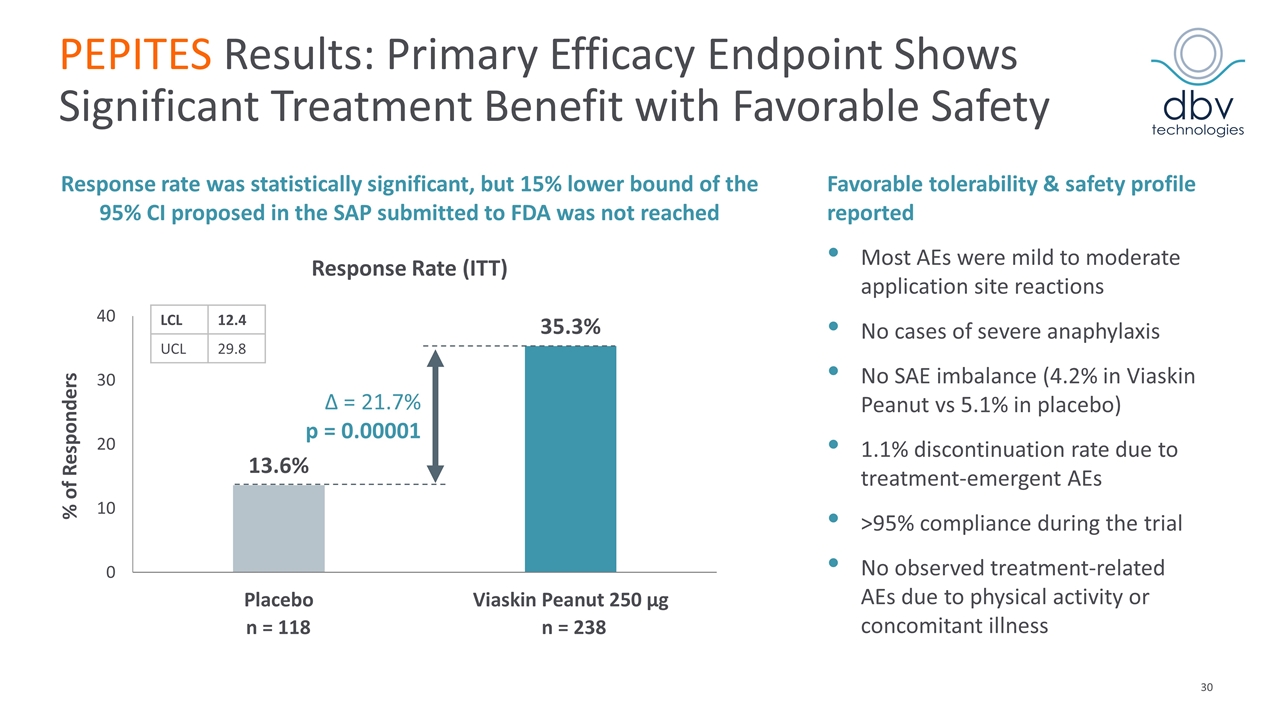
PEPITES Results: Primary Efficacy Endpoint Shows Significant Treatment Benefit with Favorable Safety Favorable tolerability & safety profile reported Most AEs were mild to moderate application site reactions No cases of severe anaphylaxis No SAE imbalance (4.2% in Viaskin Peanut vs 5.1% in placebo) 1.1% discontinuation rate due to treatment-emergent AEs >95% compliance during the trial No observed treatment-related AEs due to physical activity or concomitant illness Δ = 21.7% p = 0.00001 n = 118 n = 238 Response Rate (ITT) % of Responders Response rate was statistically significant, but 15% lower bound of the 95% CI proposed in the SAP submitted to FDA was not reached LCL 12.4 UCL 29.8
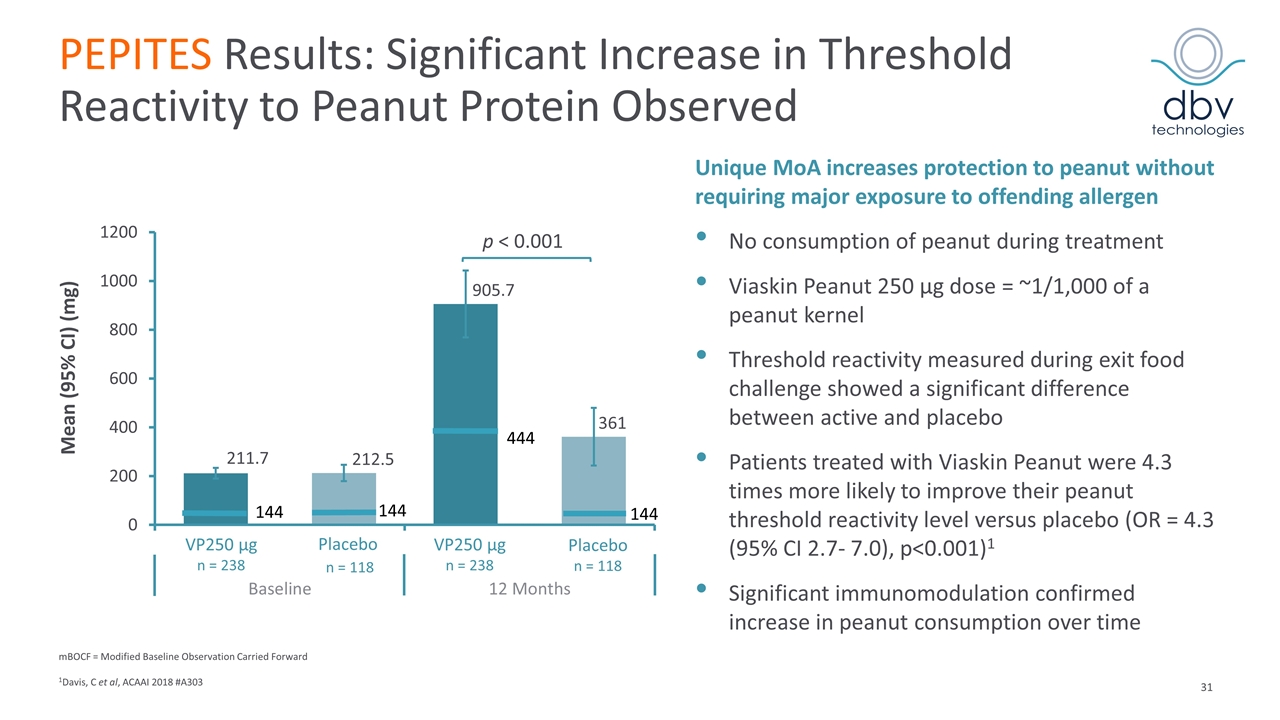
PEPITES Results: Significant Increase in Threshold Reactivity to Peanut Protein Observed mBOCF = Modified Baseline Observation Carried Forward 1Davis, C et al, ACAAI 2018 #A303 Unique MoA increases protection to peanut without requiring major exposure to offending allergen No consumption of peanut during treatment Viaskin Peanut 250 μg dose = ~1/1,000 of a peanut kernel Threshold reactivity measured during exit food challenge showed a significant difference between active and placebo Patients treated with Viaskin Peanut were 4.3 times more likely to improve their peanut threshold reactivity level versus placebo (OR = 4.3 (95% CI 2.7- 7.0), p<0.001)1 Significant immunomodulation confirmed increase in peanut consumption over time Mean (95% CI) (mg)
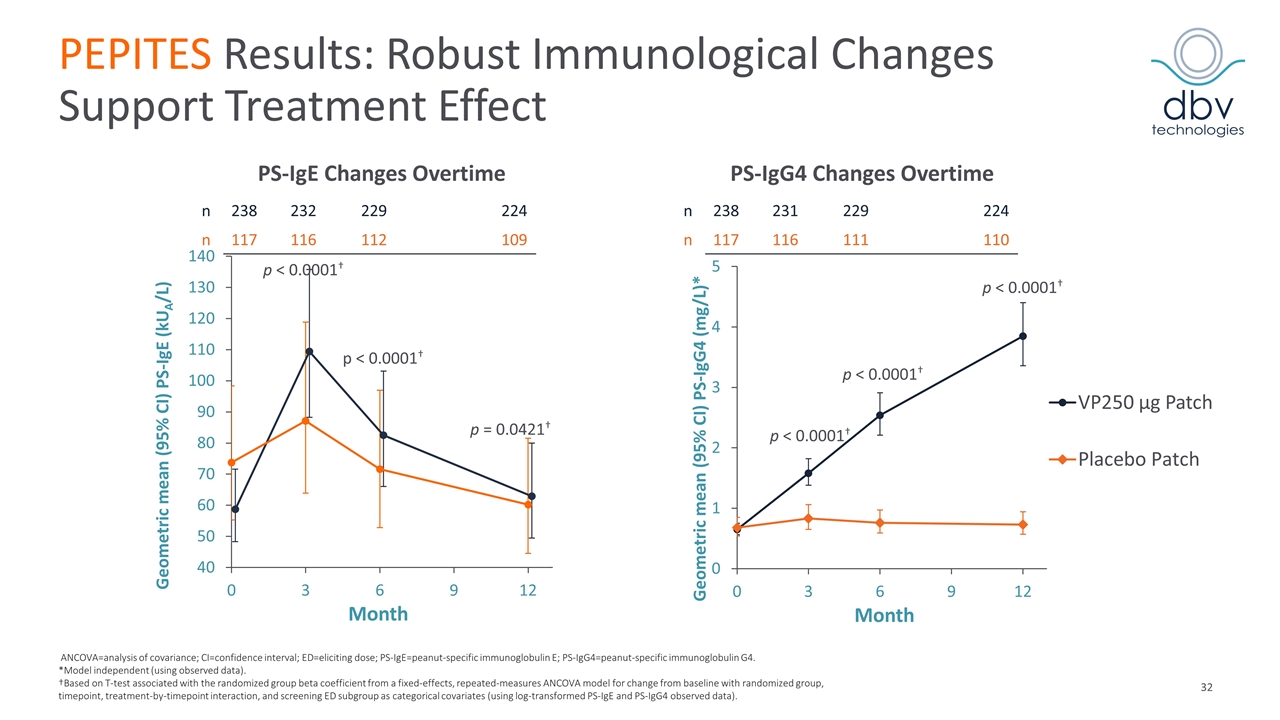
PEPITES Results: Robust Immunological Changes Support Treatment Effect p < 0.0001† p < 0.0001† p < 0.0001† n 238 232 229 224 n 117 116 112 109 n 238 231 229 224 n 117 116 111 110 p < 0.0001† p < 0.0001† p = 0.0421† ANCOVA=analysis of covariance; CI=confidence interval; ED=eliciting dose; PS-IgE=peanut-specific immunoglobulin E; PS-IgG4=peanut-specific immunoglobulin G4. *Model independent (using observed data). †Based on T-test associated with the randomized group beta coefficient from a fixed-effects, repeated-measures ANCOVA model for change from baseline with randomized group, timepoint, treatment-by-timepoint interaction, and screening ED subgroup as categorical covariates (using log-transformed PS-IgE and PS-IgG4 observed data). PS-IgE Changes Overtime PS-IgG4 Changes Overtime
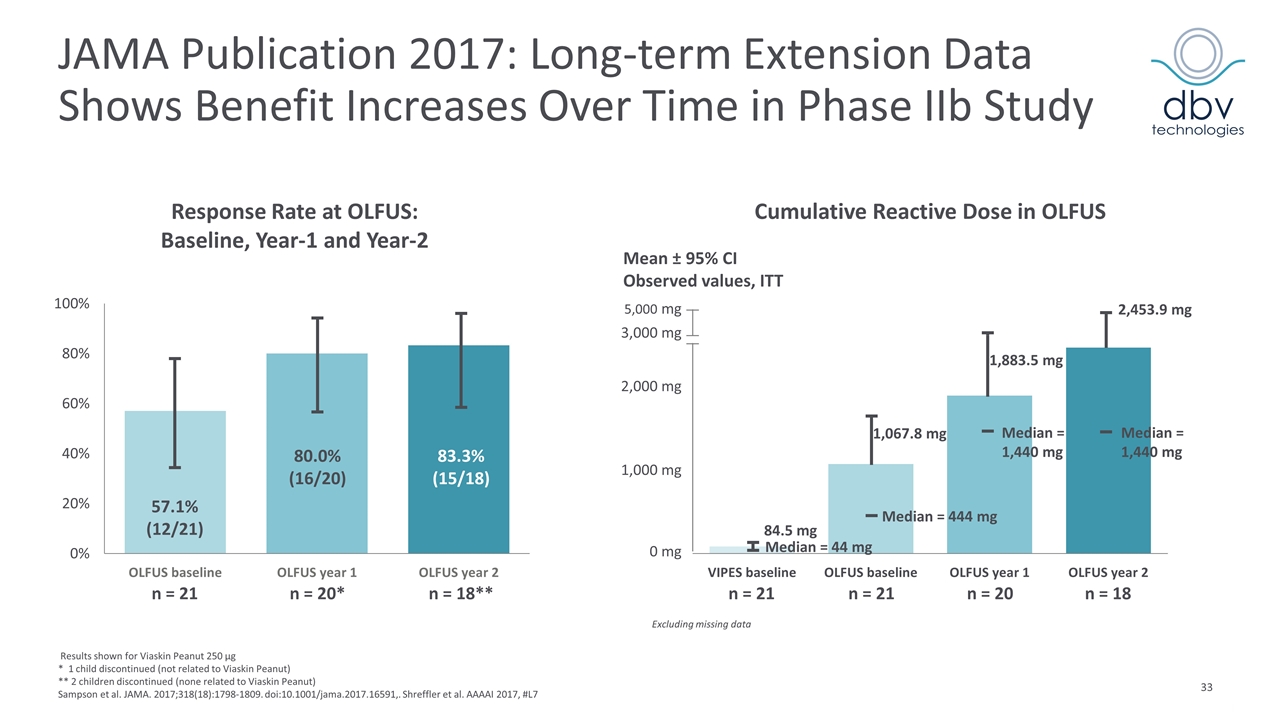
JAMA Publication 2017: Long-term Extension Data Shows Benefit Increases Over Time in Phase IIb Study Results shown for Viaskin Peanut 250 µg * 1 child discontinued (not related to Viaskin Peanut) ** 2 children discontinued (none related to Viaskin Peanut) Sampson et al. JAMA. 2017;318(18):1798-1809. doi:10.1001/jama.2017.16591,. Shreffler et al. AAAAI 2017, #L7 Response Rate at OLFUS: Baseline, Year-1 and Year-2 Cumulative Reactive Dose in OLFUS Excluding missing data n = 21 n = 21 n = 20 n = 18 0 mg 1,000 mg 2,000 mg 3,000 mg 5,000 mg Median = 1,440 mg Median = 1,440 mg Median = 444 mg Median = 44 mg Mean ± 95% CI Observed values, ITT n = 21 n = 20* n = 18** 57.1% (12/21) 80.0% (16/20) 83.3% (15/18) 2,453.9 mg 1,883.5 mg 1,067.8 mg 84.5 mg
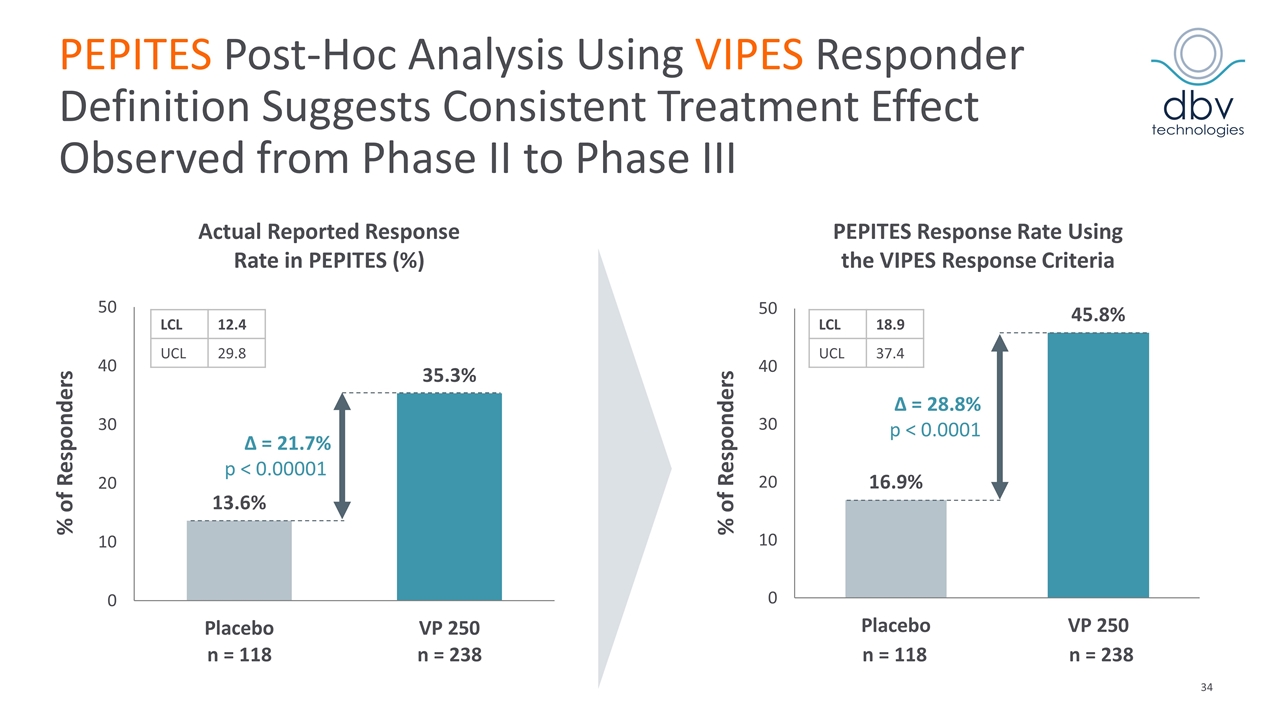
PEPITES Post-Hoc Analysis Using VIPES Responder Definition Suggests Consistent Treatment Effect Observed from Phase II to Phase III Δ = 28.8% p < 0.0001 Δ = 21.7% p < 0.00001 % of Responders % of Responders n = 238 n = 118 Actual Reported Response Rate in PEPITES (%) PEPITES Response Rate Using the VIPES Response Criteria n = 238 n = 118 LCL 12.4 UCL 29.8 LCL 18.9 UCL 37.4
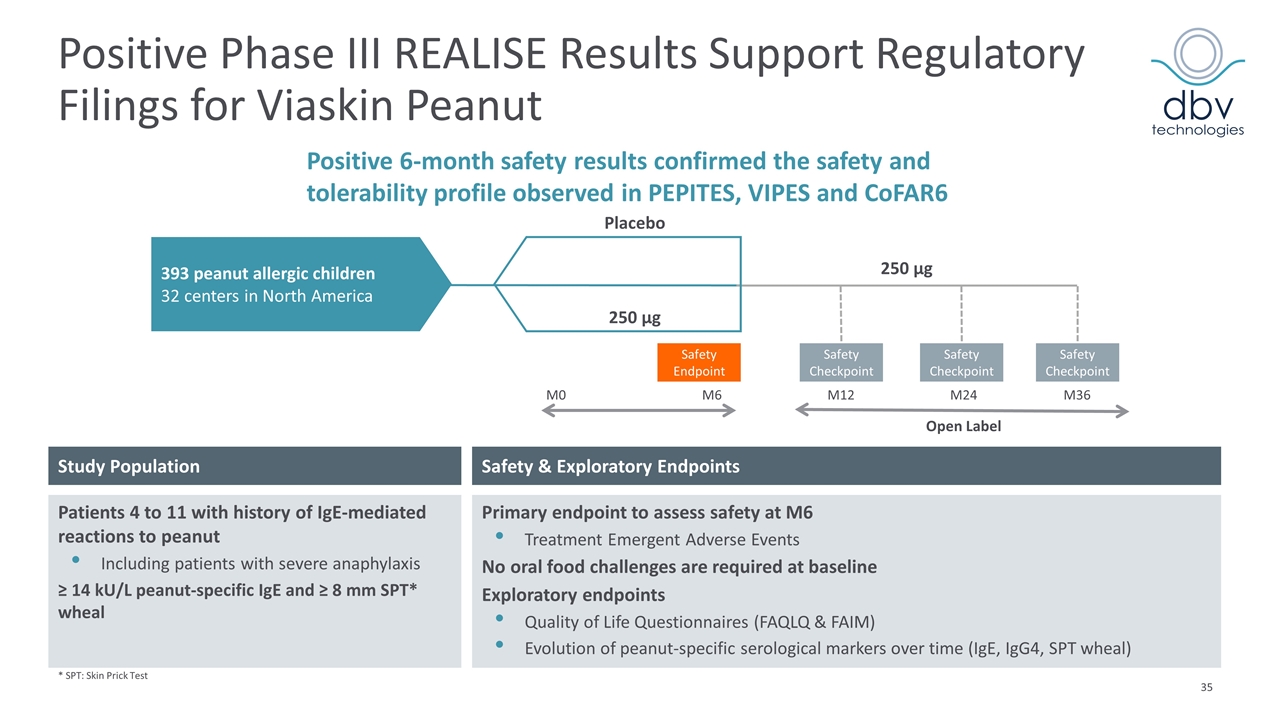
Positive Phase III REALISE Results Support Regulatory Filings for Viaskin Peanut * SPT: Skin Prick Test Patients 4 to 11 with history of IgE-mediated reactions to peanut Including patients with severe anaphylaxis ≥ 14 kU/L peanut-specific IgE and ≥ 8 mm SPT* wheal Safety & Exploratory Endpoints Primary endpoint to assess safety at M6 Treatment Emergent Adverse Events No oral food challenges are required at baseline Exploratory endpoints Quality of Life Questionnaires (FAQLQ & FAIM) Evolution of peanut-specific serological markers over time (IgE, IgG4, SPT wheal) Study Population Placebo 393 peanut allergic children 32 centers in North America 250 µg 250 µg Safety Checkpoint Safety Checkpoint Safety Checkpoint M36 M24 M12 M0 M6 Open Label Safety Endpoint Positive 6-month safety results confirmed the safety and tolerability profile observed in PEPITES, VIPES and CoFAR6

Viaskin Peanut Phase II Program: VIPES, OLFUS-VIPES & COFAR6
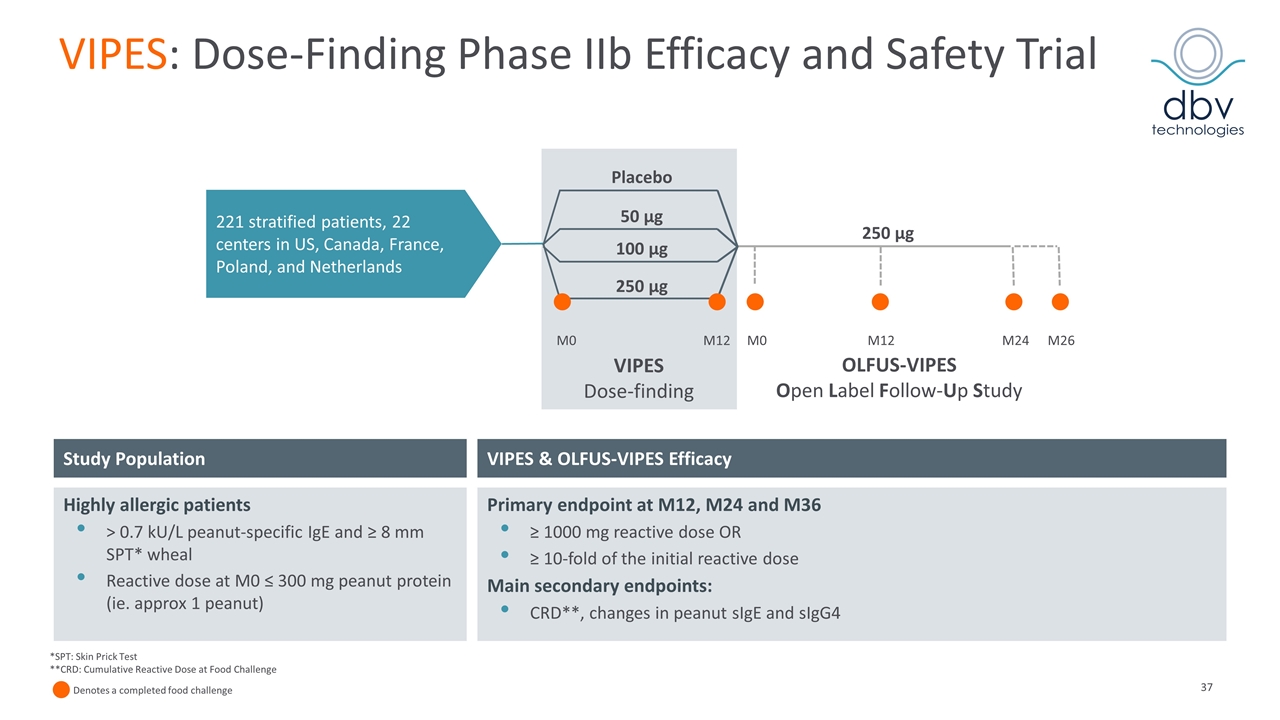
VIPES: Dose-Finding Phase IIb Efficacy and Safety Trial M0 M12 M12 M24 M26 Placebo 50 µg 100 µg 250 µg VIPES Dose-finding 250 µg 221 stratified patients, 22 centers in US, Canada, France, Poland, and Netherlands OLFUS-VIPES Open Label Follow-Up Study M0 VIPES & OLFUS-VIPES Efficacy Study Population Highly allergic patients > 0.7 kU/L peanut-specific IgE and ≥ 8 mm SPT* wheal Reactive dose at M0 ≤ 300 mg peanut protein (ie. approx 1 peanut) Primary endpoint at M12, M24 and M36 ≥ 1000 mg reactive dose OR ≥ 10-fold of the initial reactive dose Main secondary endpoints: CRD**, changes in peanut sIgE and sIgG4 *SPT: Skin Prick Test **CRD: Cumulative Reactive Dose at Food Challenge Denotes a completed food challenge
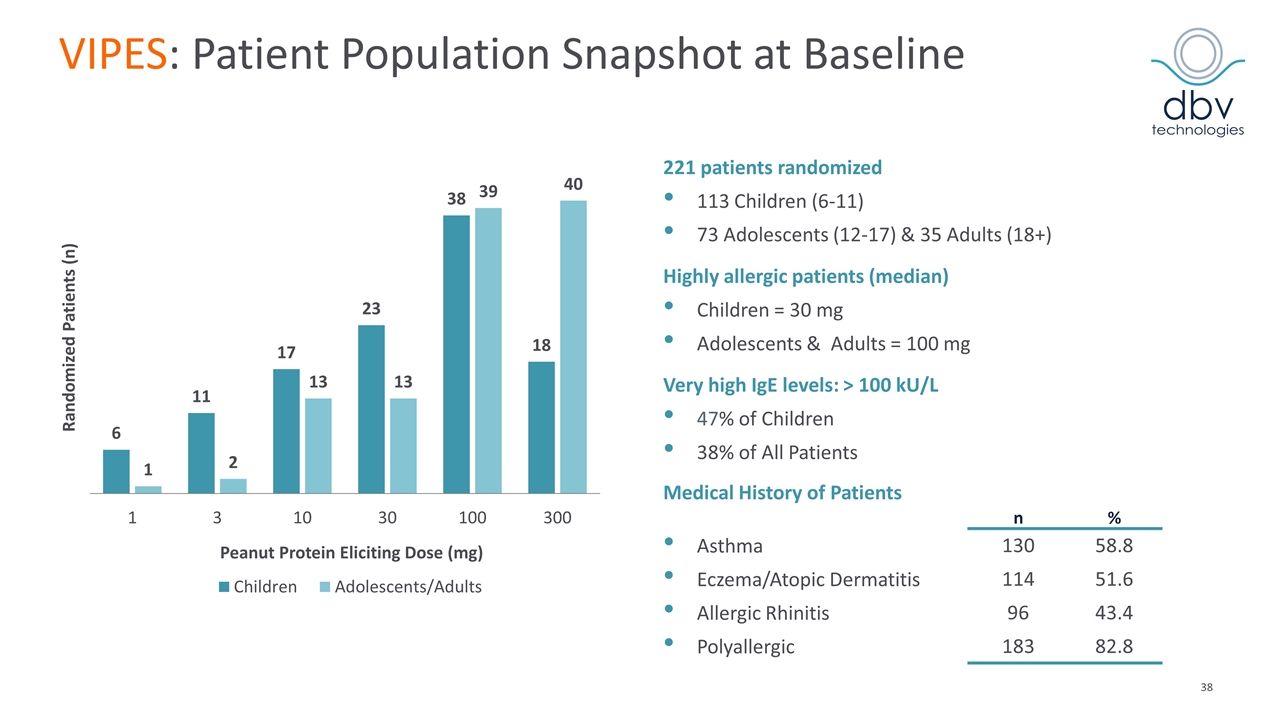
VIPES: Patient Population Snapshot at Baseline 221 patients randomized 113 Children (6-11) 73 Adolescents (12-17) & 35 Adults (18+) Highly allergic patients (median) Children = 30 mg Adolescents & Adults = 100 mg Very high IgE levels: > 100 kU/L 47% of Children 38% of All Patients Medical History of Patients n % Asthma 130 58.8 Eczema/Atopic Dermatitis 114 51.6 Allergic Rhinitis 96 43.4 Polyallergic 183 82.8
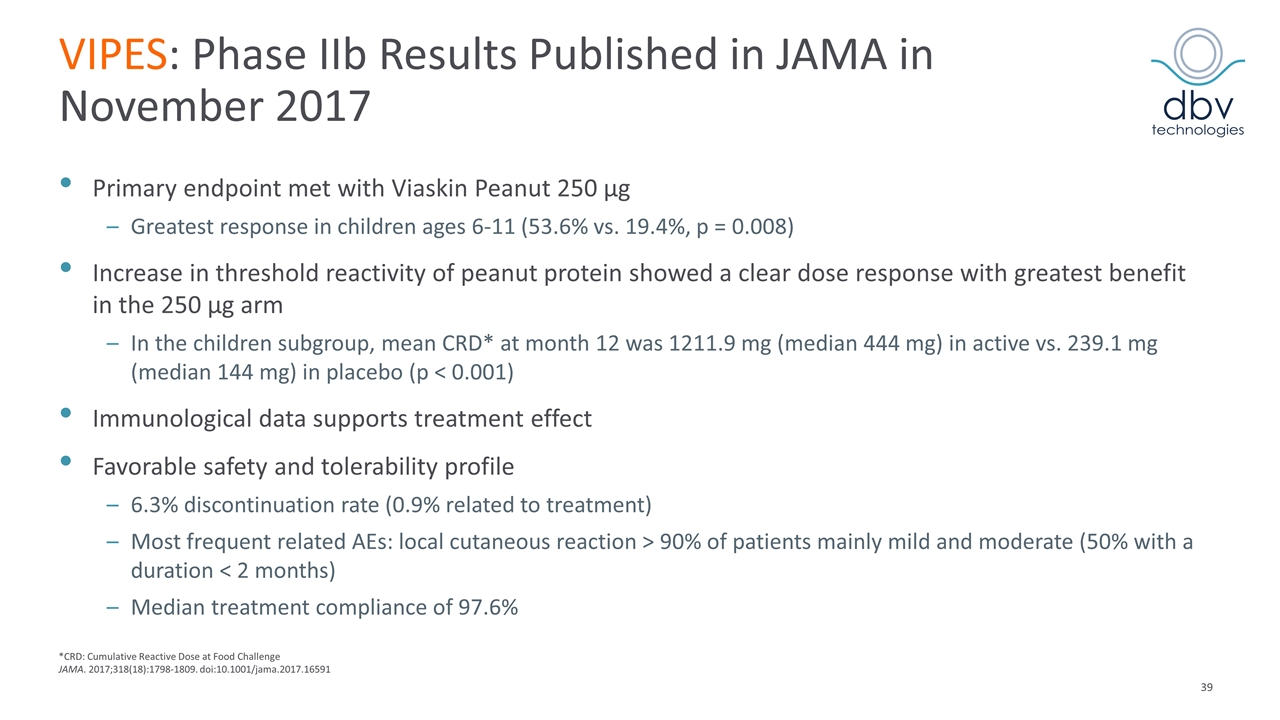
VIPES: Phase IIb Results Published in JAMA in November 2017 Primary endpoint met with Viaskin Peanut 250 µg Greatest response in children ages 6-11 (53.6% vs. 19.4%, p = 0.008) Increase in threshold reactivity of peanut protein showed a clear dose response with greatest benefit in the 250 µg arm In the children subgroup, mean CRD* at month 12 was 1211.9 mg (median 444 mg) in active vs. 239.1 mg (median 144 mg) in placebo (p < 0.001) Immunological data supports treatment effect Favorable safety and tolerability profile 6.3% discontinuation rate (0.9% related to treatment) Most frequent related AEs: local cutaneous reaction > 90% of patients mainly mild and moderate (50% with a duration < 2 months) Median treatment compliance of 97.6% *CRD: Cumulative Reactive Dose at Food Challenge JAMA. 2017;318(18):1798-1809. doi:10.1001/jama.2017.16591
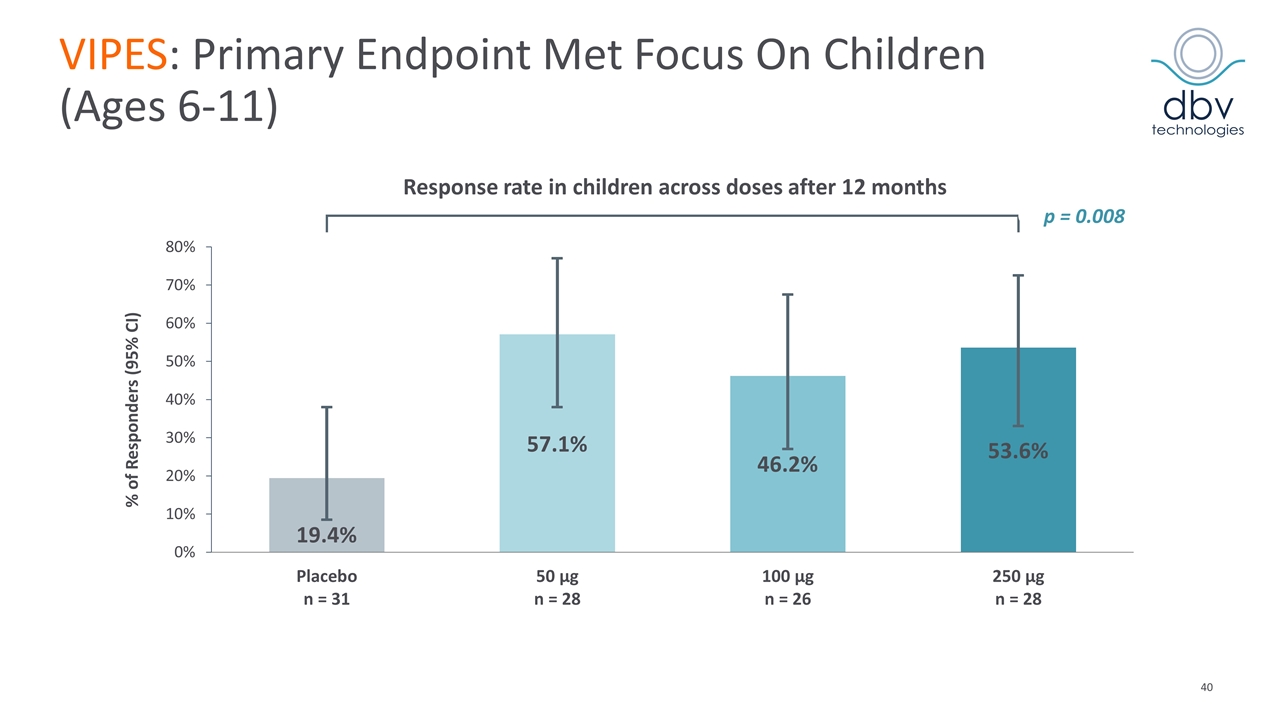
VIPES: Primary Endpoint Met Focus On Children (Ages 6-11) Response rate in children across doses after 12 months % of Responders (95% CI) p = 0.008
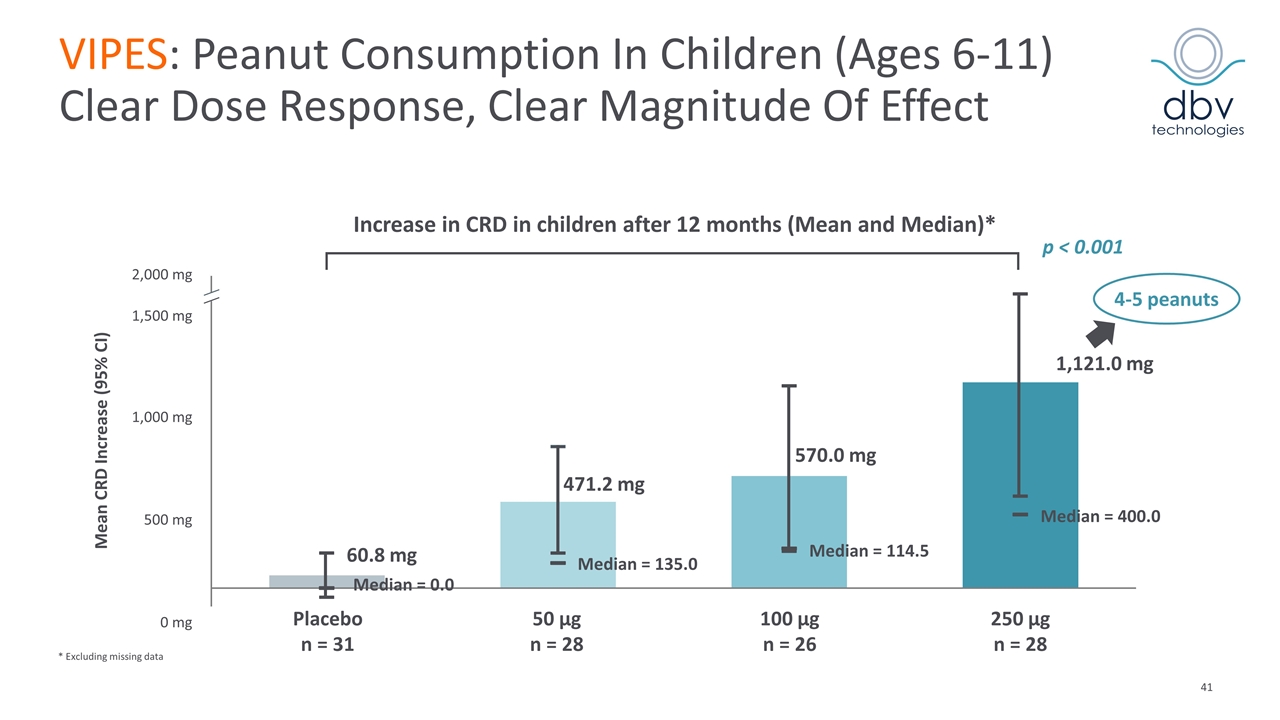
1,000 mg 1,500 mg 2,000 mg 1,121.0 mg Median = 135.0 Median = 0.0 Median = 114.5 Median = 400.0 570.0 mg 471.2 mg 60.8 mg Mean CRD Increase (95% CI) 4-5 peanuts p < 0.001 Placebo n = 31 100 µg n = 26 VIPES: Peanut Consumption In Children (Ages 6-11) Clear Dose Response, Clear Magnitude Of Effect * Excluding missing data Increase in CRD in children after 12 months (Mean and Median)* 250 µg n = 28 50 µg n = 28
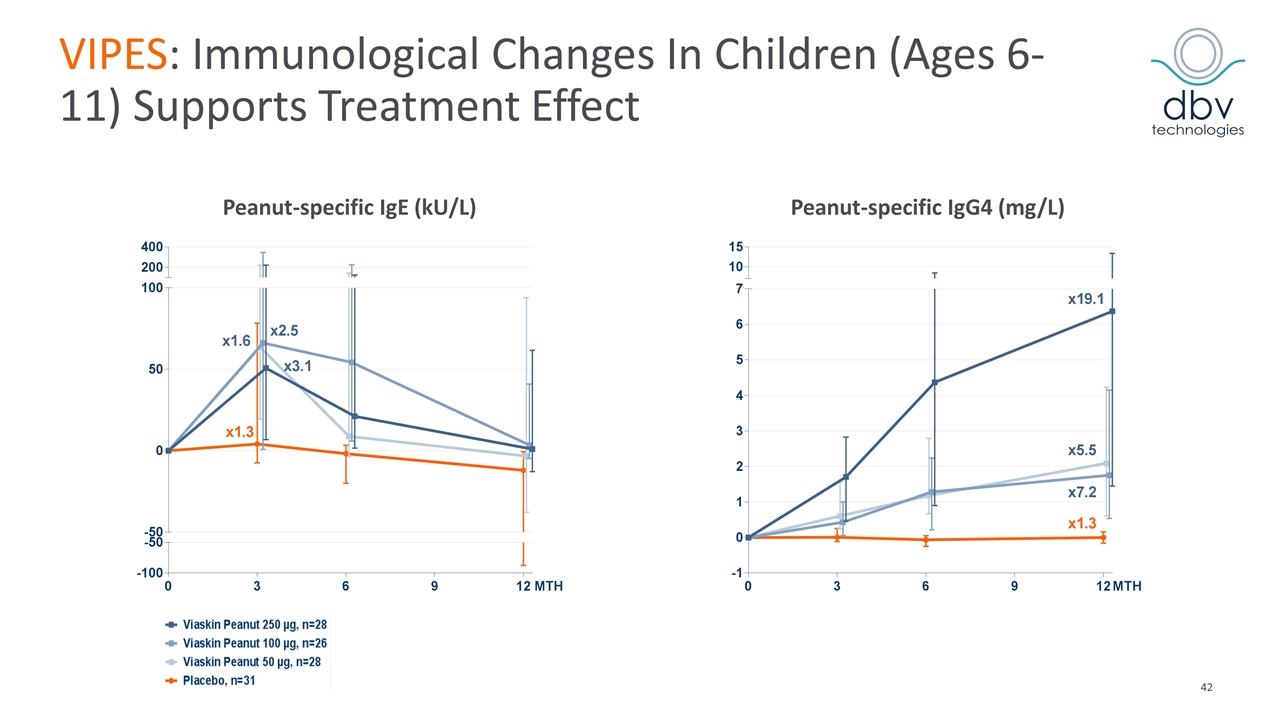
VIPES: Immunological Changes In Children (Ages 6-11) Supports Treatment Effect Peanut-specific IgE (kU/L) Peanut-specific IgG4 (mg/L)
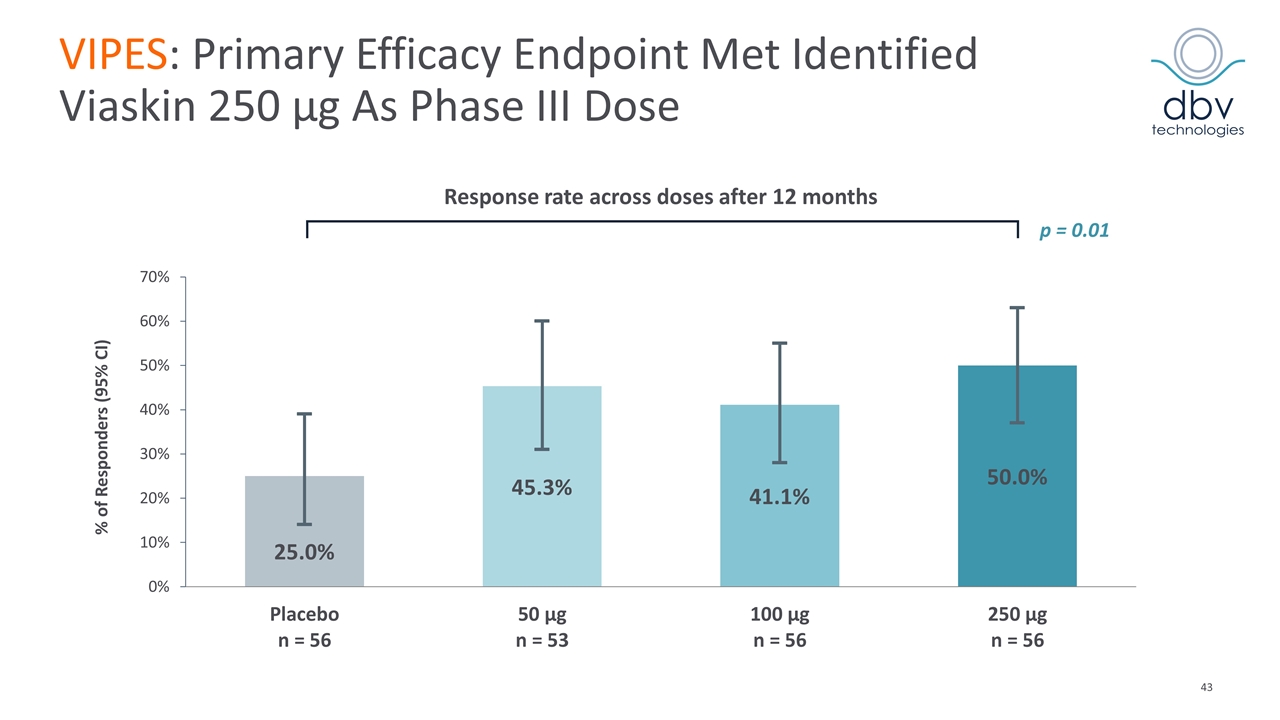
VIPES: Primary Efficacy Endpoint Met Identified Viaskin 250 µg As Phase III Dose Response rate across doses after 12 months % of Responders (95% CI) p = 0.01
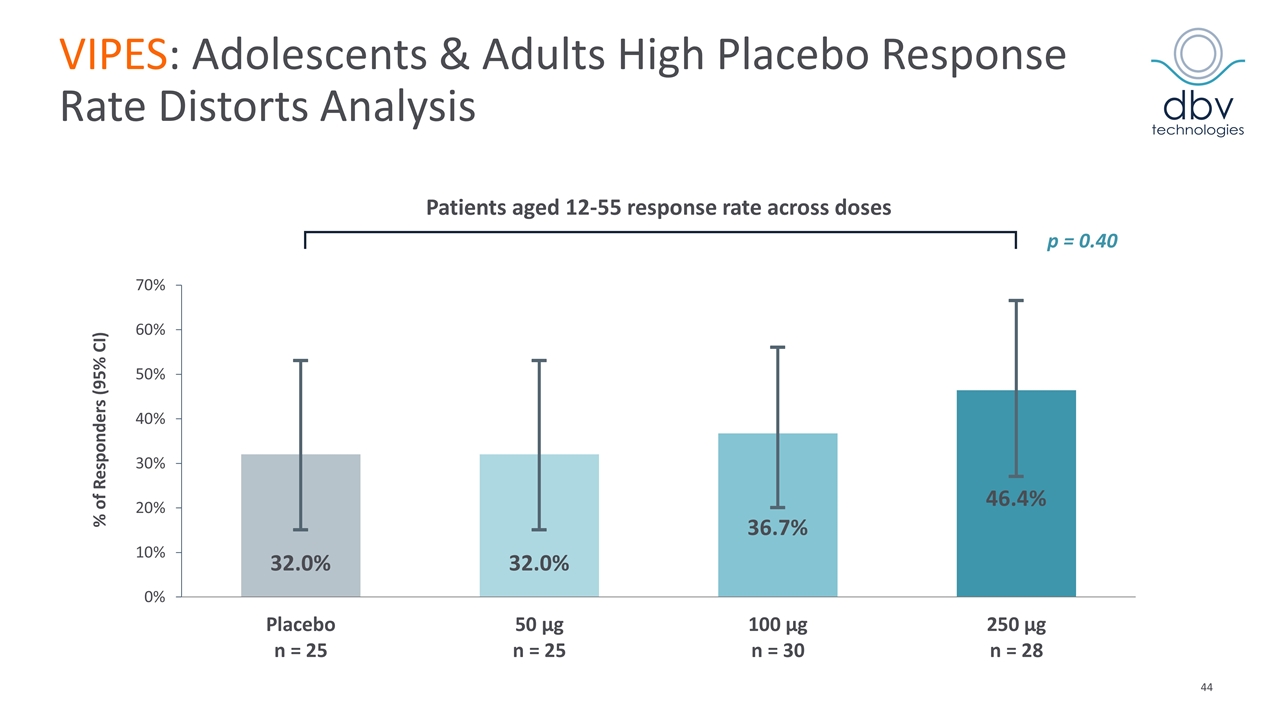
VIPES: Adolescents & Adults High Placebo Response Rate Distorts Analysis Patients aged 12-55 response rate across doses % of Responders (95% CI) p = 0.40
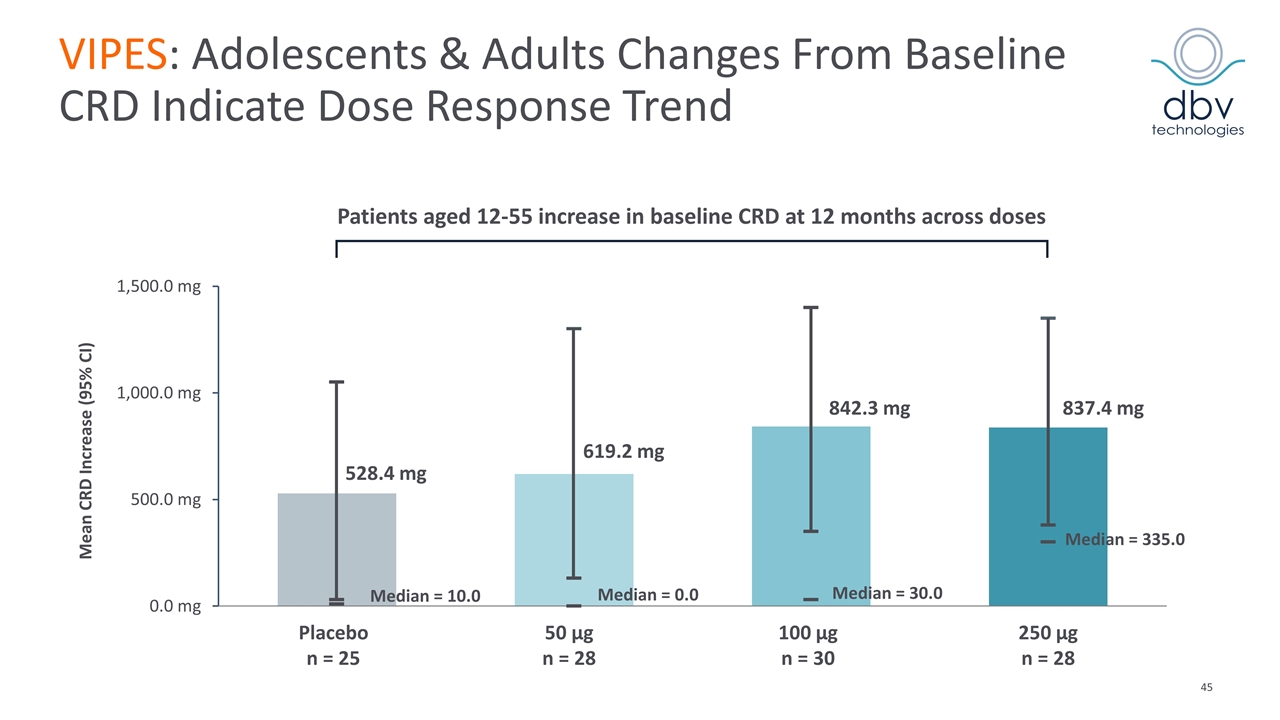
Patients aged 12-55 increase in baseline CRD at 12 months across doses 837.4 mg Median = 0.0 Median = 10.0 Median = 30.0 Median = 335.0 842.3 mg 619.2 mg 528.4 mg VIPES: Adolescents & Adults Changes From Baseline CRD Indicate Dose Response Trend Mean CRD Increase (95% CI) Placebo n = 25 100 µg n = 30
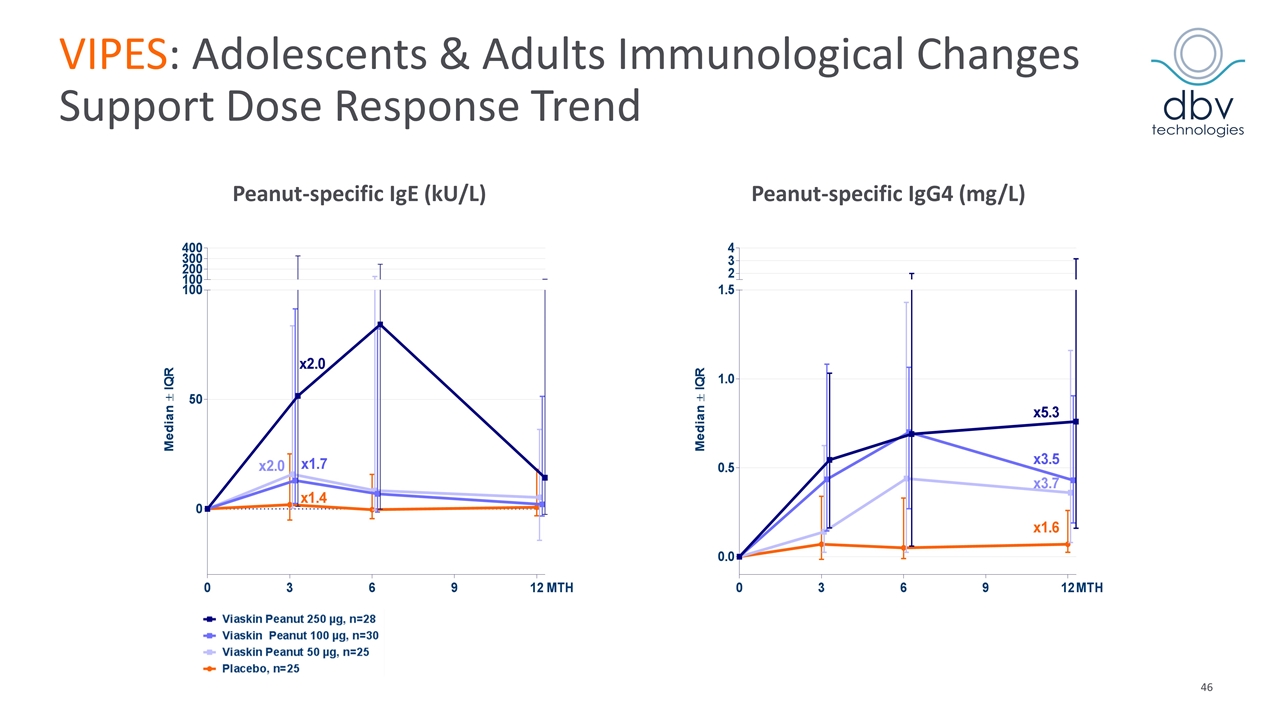
VIPES: Adolescents & Adults Immunological Changes Support Dose Response Trend Peanut-specific IgE (kU/L) Peanut-specific IgG4 (mg/L)
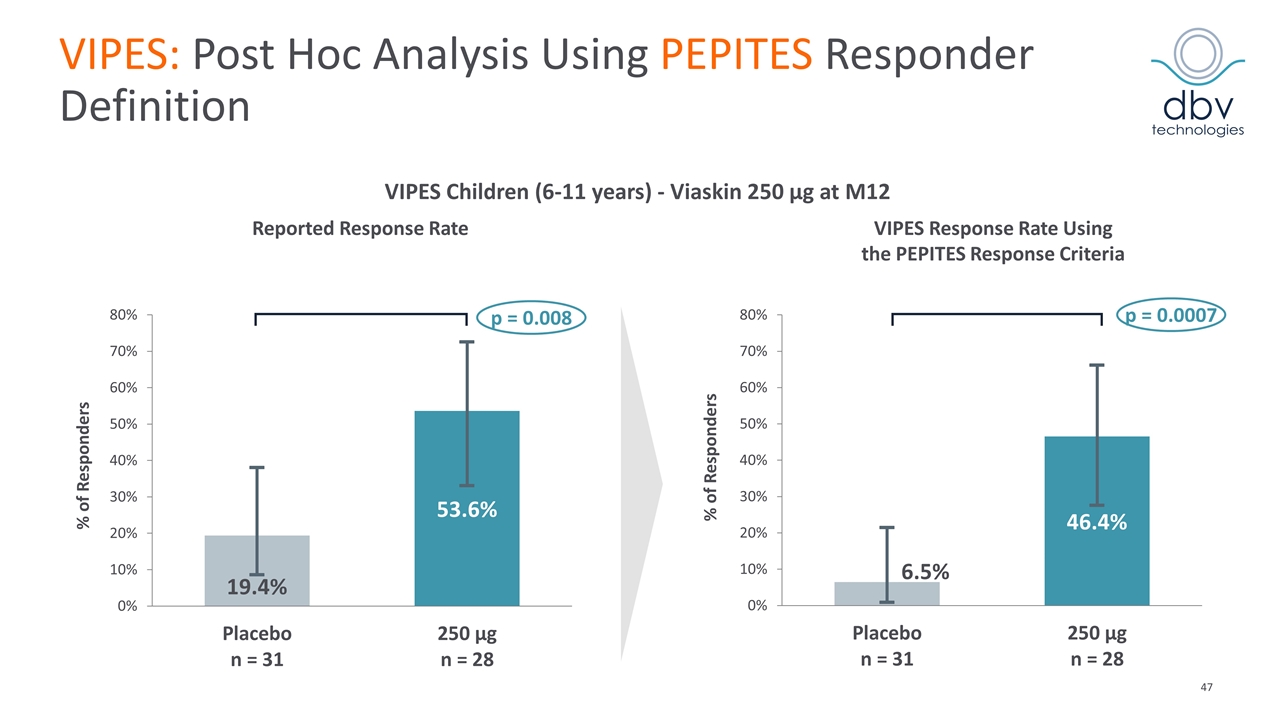
VIPES: Post Hoc Analysis Using PEPITES Responder Definition % of Responders Reported Response Rate VIPES Response Rate Using the PEPITES Response Criteria p = 0.008 p = 0.0007 VIPES Children (6-11 years) - Viaskin 250 μg at M12 % of Responders
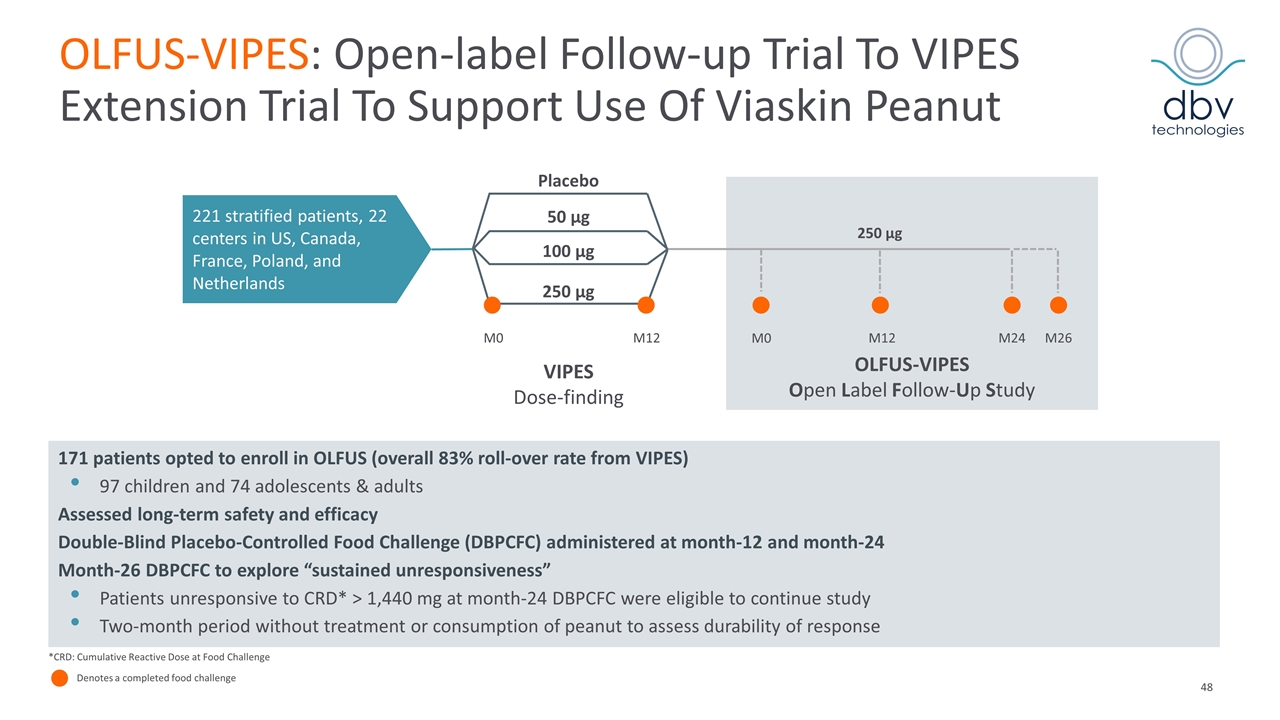
OLFUS-VIPES: Open-label Follow-up Trial To VIPES Extension Trial To Support Use Of Viaskin Peanut M0 M12 M12 M24 M26 Placebo 50 µg 100 µg 250 µg VIPES Dose-finding 250 µg 221 stratified patients, 22 centers in US, Canada, France, Poland, and Netherlands OLFUS-VIPES Open Label Follow-Up Study M0 *CRD: Cumulative Reactive Dose at Food Challenge Denotes a completed food challenge 171 patients opted to enroll in OLFUS (overall 83% roll-over rate from VIPES) 97 children and 74 adolescents & adults Assessed long-term safety and efficacy Double-Blind Placebo-Controlled Food Challenge (DBPCFC) administered at month-12 and month-24 Month-26 DBPCFC to explore “sustained unresponsiveness” Patients unresponsive to CRD* > 1,440 mg at month-24 DBPCFC were eligible to continue study Two-month period without treatment or consumption of peanut to assess durability of response
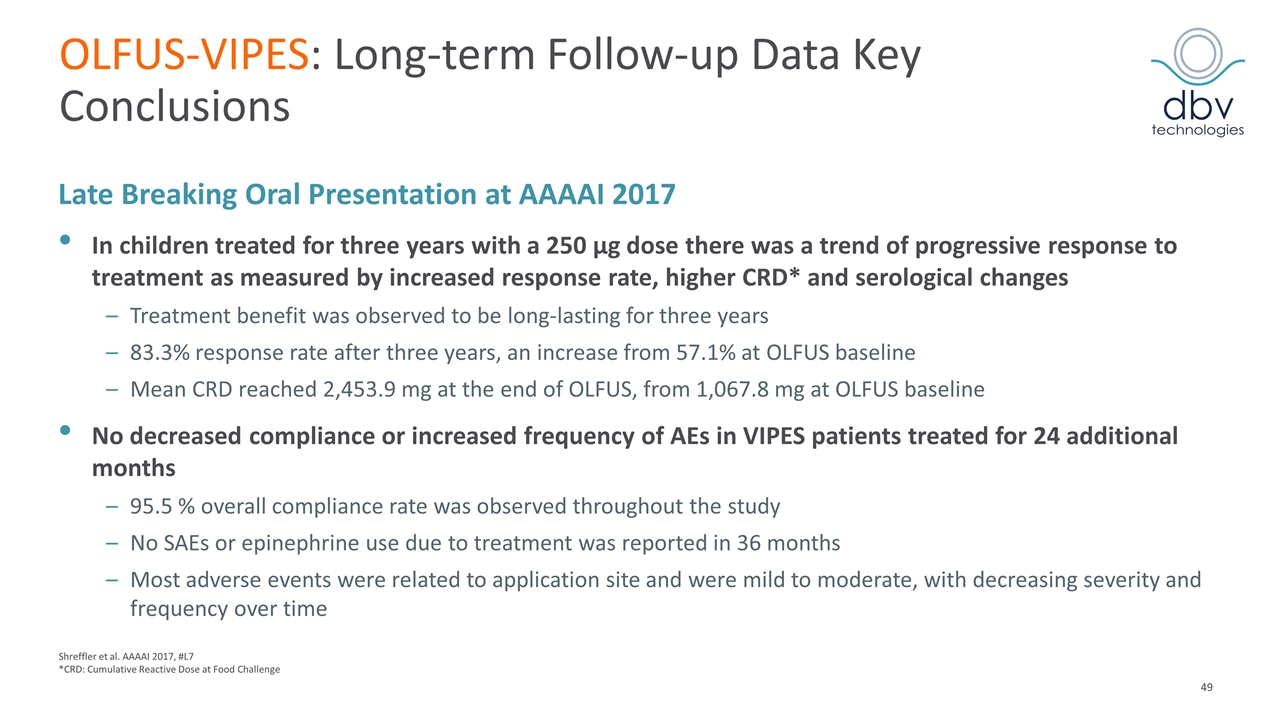
OLFUS-VIPES: Long-term Follow-up Data Key Conclusions Late Breaking Oral Presentation at AAAAI 2017 In children treated for three years with a 250 µg dose there was a trend of progressive response to treatment as measured by increased response rate, higher CRD* and serological changes Treatment benefit was observed to be long-lasting for three years 83.3% response rate after three years, an increase from 57.1% at OLFUS baseline Mean CRD reached 2,453.9 mg at the end of OLFUS, from 1,067.8 mg at OLFUS baseline No decreased compliance or increased frequency of AEs in VIPES patients treated for 24 additional months 95.5 % overall compliance rate was observed throughout the study No SAEs or epinephrine use due to treatment was reported in 36 months Most adverse events were related to application site and were mild to moderate, with decreasing severity and frequency over time Shreffler et al. AAAAI 2017, #L7 *CRD: Cumulative Reactive Dose at Food Challenge
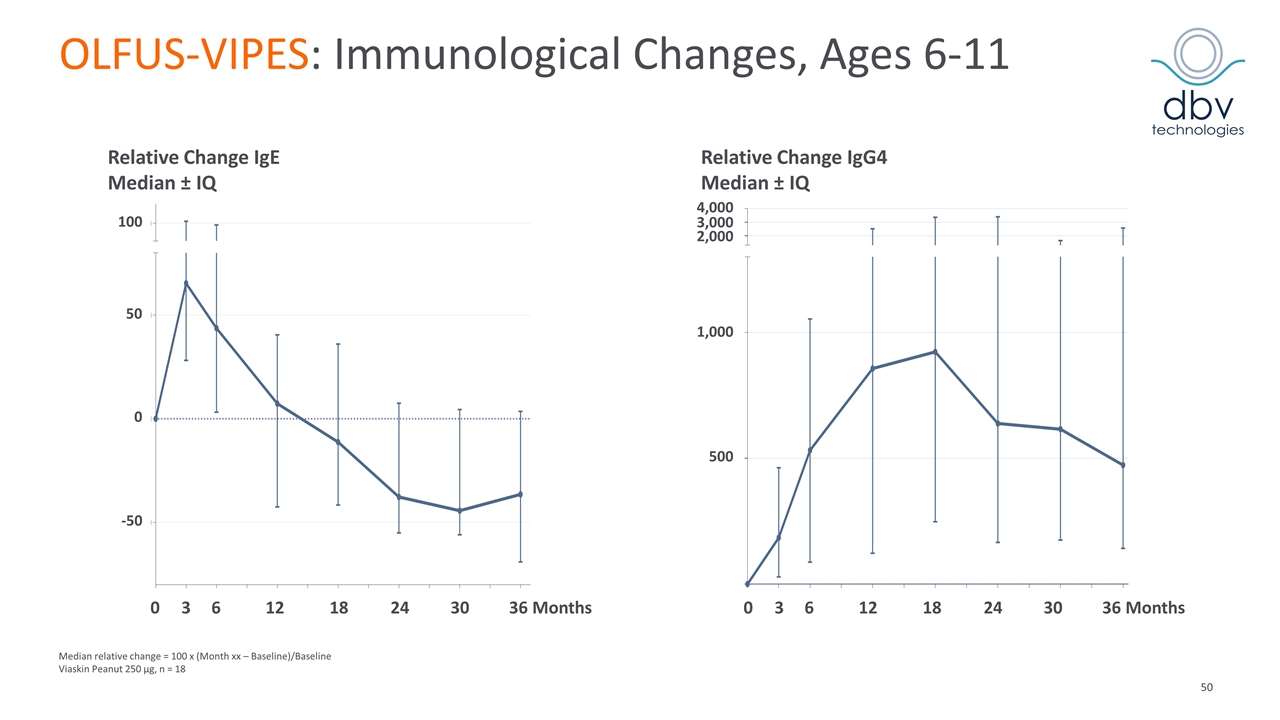
OLFUS-VIPES: Immunological Changes, Ages 6-11 Median relative change = 100 x (Month xx – Baseline)/Baseline Viaskin Peanut 250 µg, n = 18 0 3 6 12 18 24 30 36 Months 100 50 0 -50 0 3 6 12 18 24 30 36 Months 4,000 3,000 2,000 1,000 500 Relative Change IgE Median ± IQ Relative Change IgG4 Median ± IQ
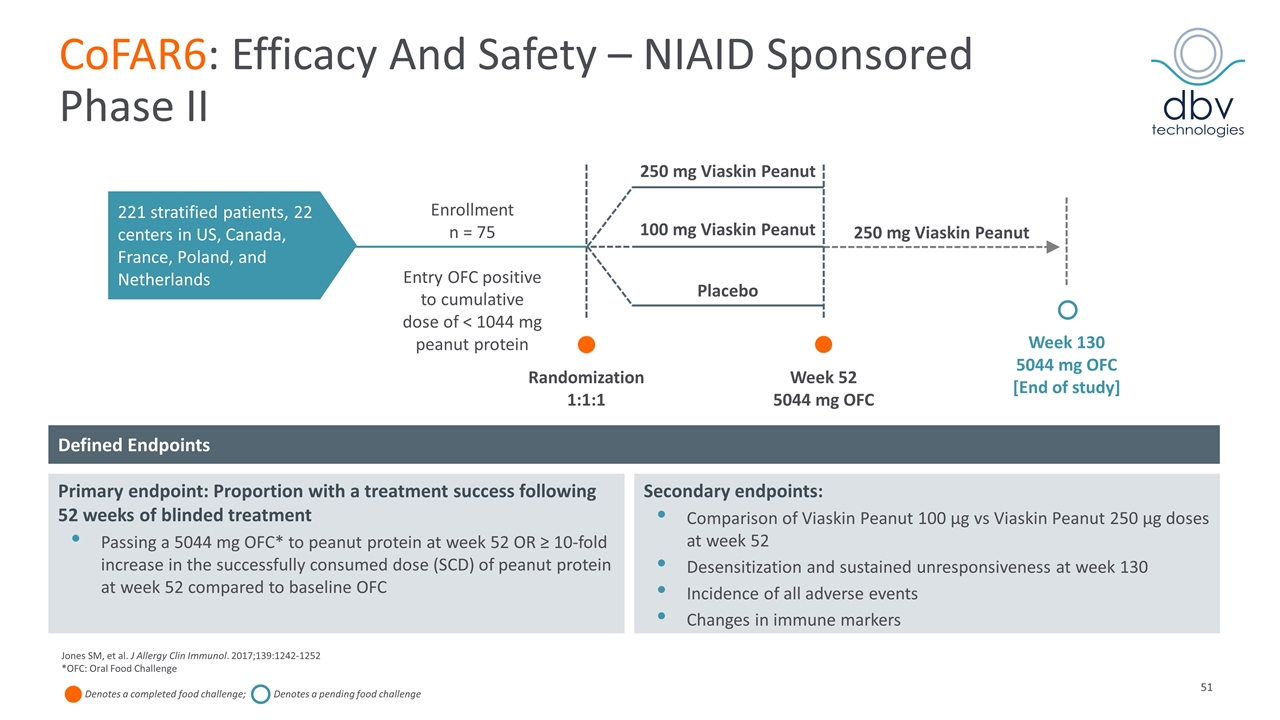
CoFAR6: Efficacy And Safety – NIAID Sponsored Phase II 221 stratified patients, 22 centers in US, Canada, France, Poland, and Netherlands Enrollment n = 75 Entry OFC positive to cumulative dose of < 1044 mg peanut protein Randomization 1:1:1 Week 52 5044 mg OFC 250 mg Viaskin Peanut Week 130 5044 mg OFC [End of study] Placebo 100 mg Viaskin Peanut 250 mg Viaskin Peanut Defined Endpoints Primary endpoint: Proportion with a treatment success following 52 weeks of blinded treatment Passing a 5044 mg OFC* to peanut protein at week 52 OR ≥ 10-fold increase in the successfully consumed dose (SCD) of peanut protein at week 52 compared to baseline OFC Secondary endpoints: Comparison of Viaskin Peanut 100 µg vs Viaskin Peanut 250 µg doses at week 52 Desensitization and sustained unresponsiveness at week 130 Incidence of all adverse events Changes in immune markers Jones SM, et al. J Allergy Clin Immunol. 2017;139:1242-1252 *OFC: Oral Food Challenge Denotes a completed food challenge; Denotes a pending food challenge
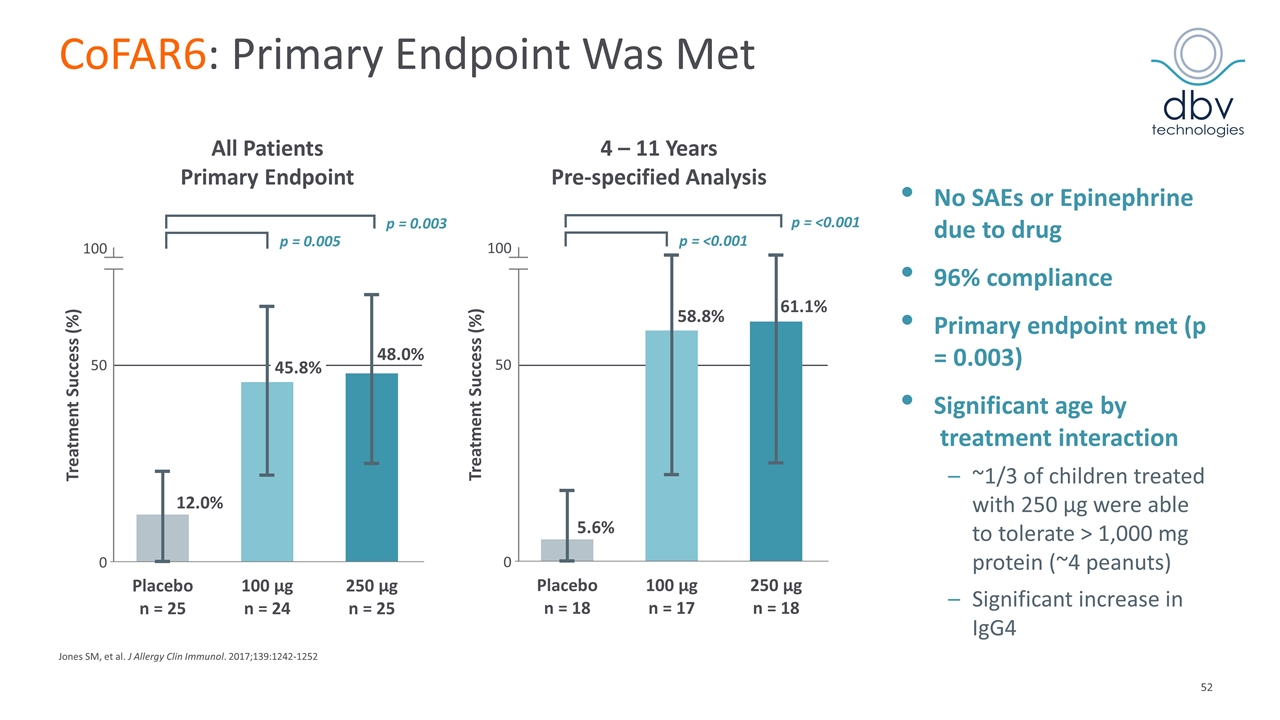
CoFAR6: Primary Endpoint Was Met No SAEs or Epinephrine due to drug 96% compliance Primary endpoint met (p = 0.003) Significant age by treatment interaction ~1/3 of children treated with 250 µg were able to tolerate > 1,000 mg protein (~4 peanuts) Significant increase in IgG4 Jones SM, et al. J Allergy Clin Immunol. 2017;139:1242-1252 All Patients Primary Endpoint 4 – 11 Years Pre-specified Analysis Treatment Success (%) 100 50 0 p = 0.005 p = 0.003 Treatment Success (%) 100 50 0 p = <0.001 p = <0.001

Viaskin Milk Phase II Program: MILES
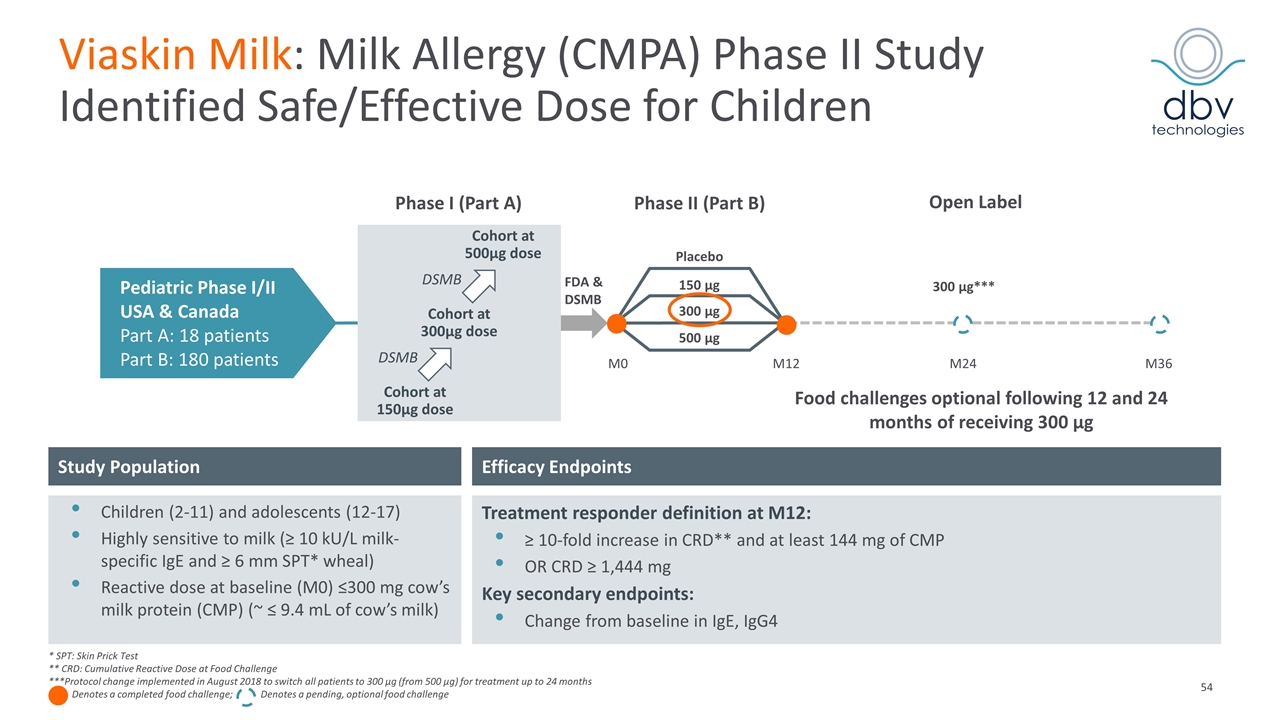
Viaskin Milk: Milk Allergy (CMPA) Phase II Study Identified Safe/Effective Dose for Children Pediatric Phase I/II USA & Canada Part A: 18 patients Part B: 180 patients M0 Phase I (Part A) Cohort at 150µg dose Cohort at 300µg dose Cohort at 500µg dose DSMB DSMB FDA & DSMB Placebo 300 µg 150 µg 500 µg Phase II (Part B) M12 M24 M36 Efficacy Endpoints Study Population Children (2-11) and adolescents (12-17) Highly sensitive to milk (≥ 10 kU/L milk-specific IgE and ≥ 6 mm SPT* wheal) Reactive dose at baseline (M0) ≤300 mg cow’s milk protein (CMP) (~ ≤ 9.4 mL of cow’s milk) Treatment responder definition at M12: ≥ 10-fold increase in CRD** and at least 144 mg of CMP OR CRD ≥ 1,444 mg Key secondary endpoints: Change from baseline in IgE, IgG4 * SPT: Skin Prick Test ** CRD: Cumulative Reactive Dose at Food Challenge ***Protocol change implemented in August 2018 to switch all patients to 300 µg (from 500 µg) for treatment up to 24 months Denotes a completed food challenge; Denotes a pending, optional food challenge Open Label Food challenges optional following 12 and 24 months of receiving 300 µg 300 µg***
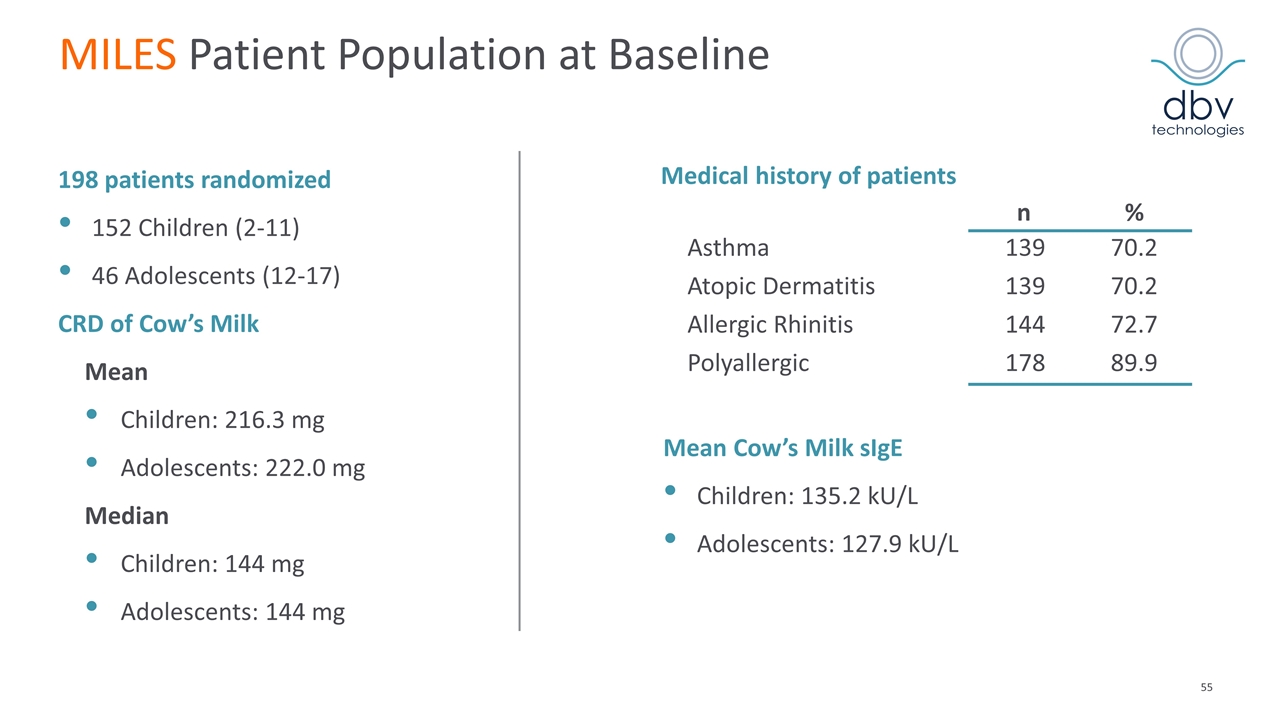
MILES Patient Population at Baseline 198 patients randomized 152 Children (2-11) 46 Adolescents (12-17) CRD of Cow’s Milk Mean Children: 216.3 mg Adolescents: 222.0 mg Median Children: 144 mg Adolescents: 144 mg Medical history of patients n % Asthma 139 70.2 Atopic Dermatitis 139 70.2 Allergic Rhinitis 144 72.7 Polyallergic 178 89.9 Mean Cow’s Milk sIgE Children: 135.2 kU/L Adolescents: 127.9 kU/L
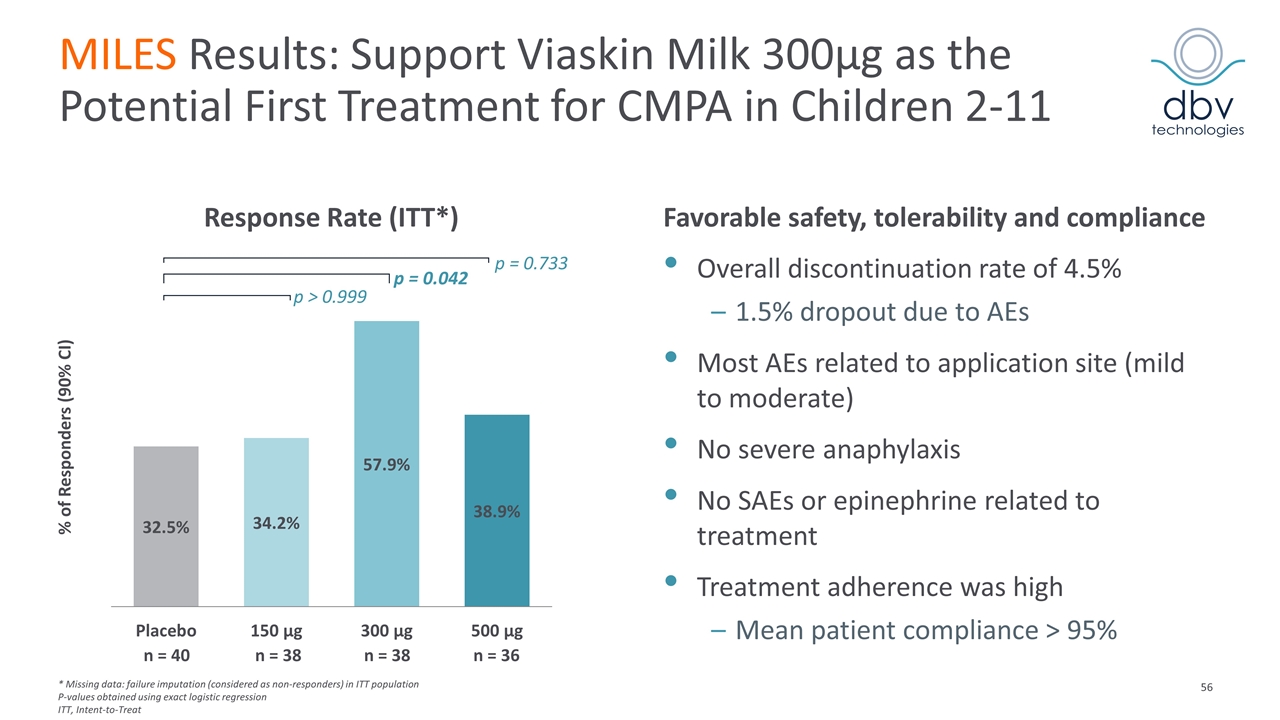
MILES Results: Support Viaskin Milk 300µg as the Potential First Treatment for CMPA in Children 2-11 Response Rate (ITT*) Favorable safety, tolerability and compliance Overall discontinuation rate of 4.5% 1.5% dropout due to AEs Most AEs related to application site (mild to moderate) No severe anaphylaxis No SAEs or epinephrine related to treatment Treatment adherence was high Mean patient compliance > 95% % of Responders (90% CI) p = 0.733 p = 0.042 p > 0.999 n = 40 n = 38 n = 38 n = 36 * Missing data: failure imputation (considered as non-responders) in ITT population P-values obtained using exact logistic regression ITT, Intent-to-Treat
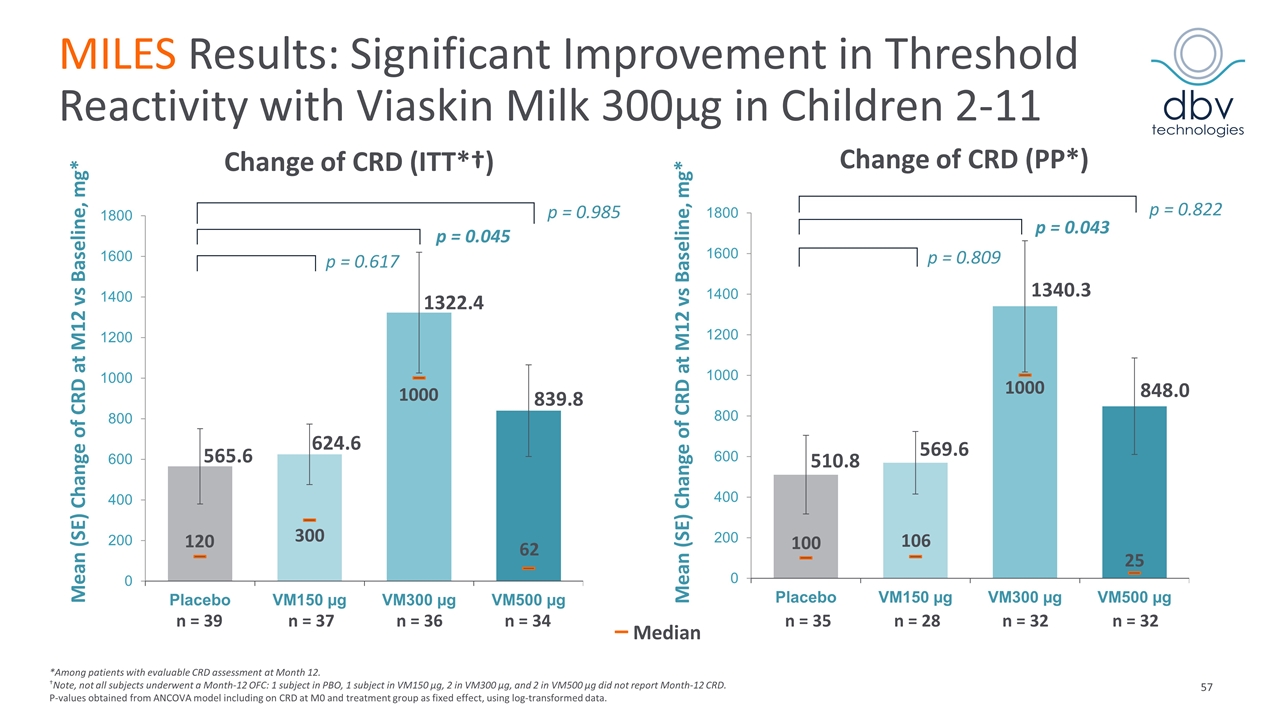
MILES Results: Significant Improvement in Threshold Reactivity with Viaskin Milk 300µg in Children 2-11 *Among patients with evaluable CRD assessment at Month 12. †Note, not all subjects underwent a Month-12 OFC: 1 subject in PBO, 1 subject in VM150 µg, 2 in VM300 µg, and 2 in VM500 µg did not report Month-12 CRD. P-values obtained from ANCOVA model including on CRD at M0 and treatment group as fixed effect, using log-transformed data. Mean (SE) Change of CRD at M12 vs Baseline, mg* Change of CRD (PP*) p = 0.822 p = 0.043 p = 0.809 510.8 569.6 1340.3 848.0 100 106 1000 25 n = 35 n = 28 n = 32 n = 32 Mean (SE) Change of CRD at M12 vs Baseline, mg* Change of CRD (ITT*†) p = 0.985 p = 0.045 p = 0.617 565.6 624.6 1322.4 839.8 120 300 1000 n = 39 n = 37 n = 36 n = 34 62 – Median
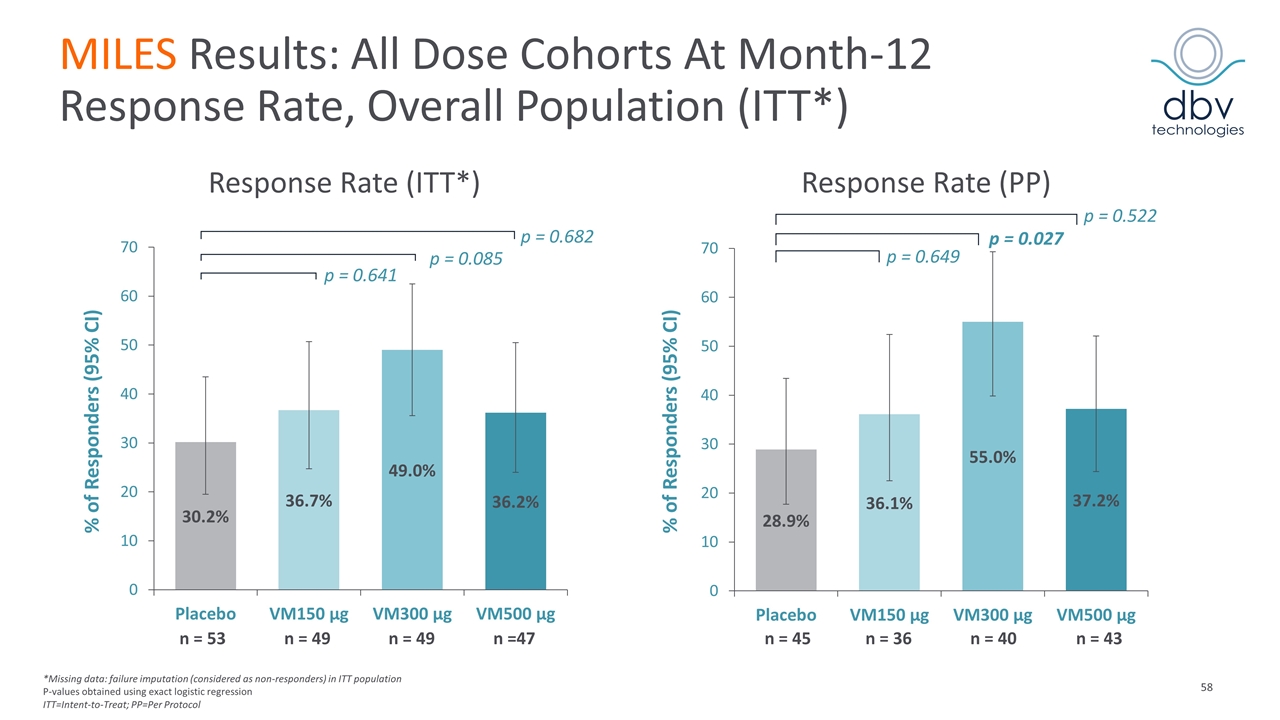
MILES Results: All Dose Cohorts At Month-12 Response Rate, Overall Population (ITT*) Response Rate (PP) % of Responders (95% CI) p = 0.682 p = 0.085 p = 0.641 % of Responders (95% CI) p = 0.522 p = 0.027 p = 0.649 Response Rate (ITT*) *Missing data: failure imputation (considered as non-responders) in ITT population P-values obtained using exact logistic regression ITT=Intent-to-Treat; PP=Per Protocol n = 53 n = 49 n = 49 n =47 n = 45 n = 36 n = 40 n = 43
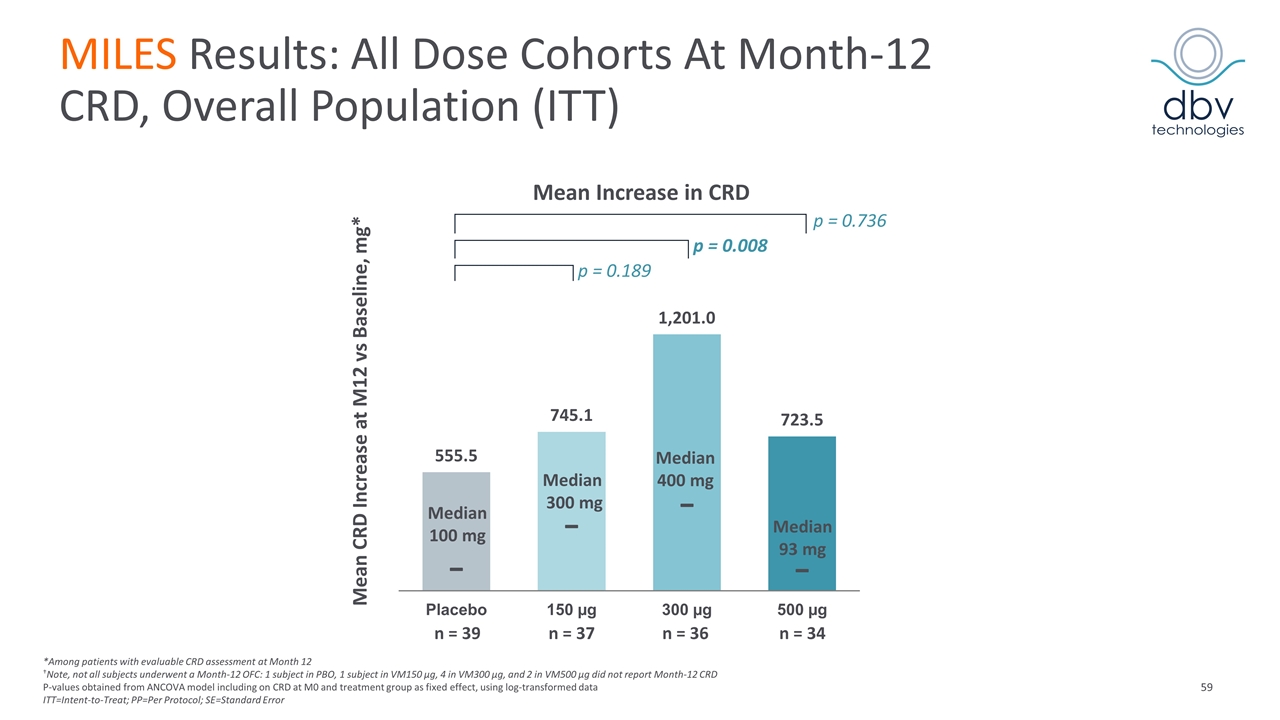
MILES Results: All Dose Cohorts At Month-12 CRD, Overall Population (ITT) Mean CRD Increase at M12 vs Baseline, mg* Mean Increase in CRD p = 0.736 p = 0.008 p = 0.189 Median 100 mg Median 300 mg Median 400 mg Median 93 mg n = 39 n = 37 n = 36 n = 34 *Among patients with evaluable CRD assessment at Month 12 †Note, not all subjects underwent a Month-12 OFC: 1 subject in PBO, 1 subject in VM150 µg, 4 in VM300 µg, and 2 in VM500 µg did not report Month-12 CRD P-values obtained from ANCOVA model including on CRD at M0 and treatment group as fixed effect, using log-transformed data ITT=Intent-to-Treat; PP=Per Protocol; SE=Standard Error
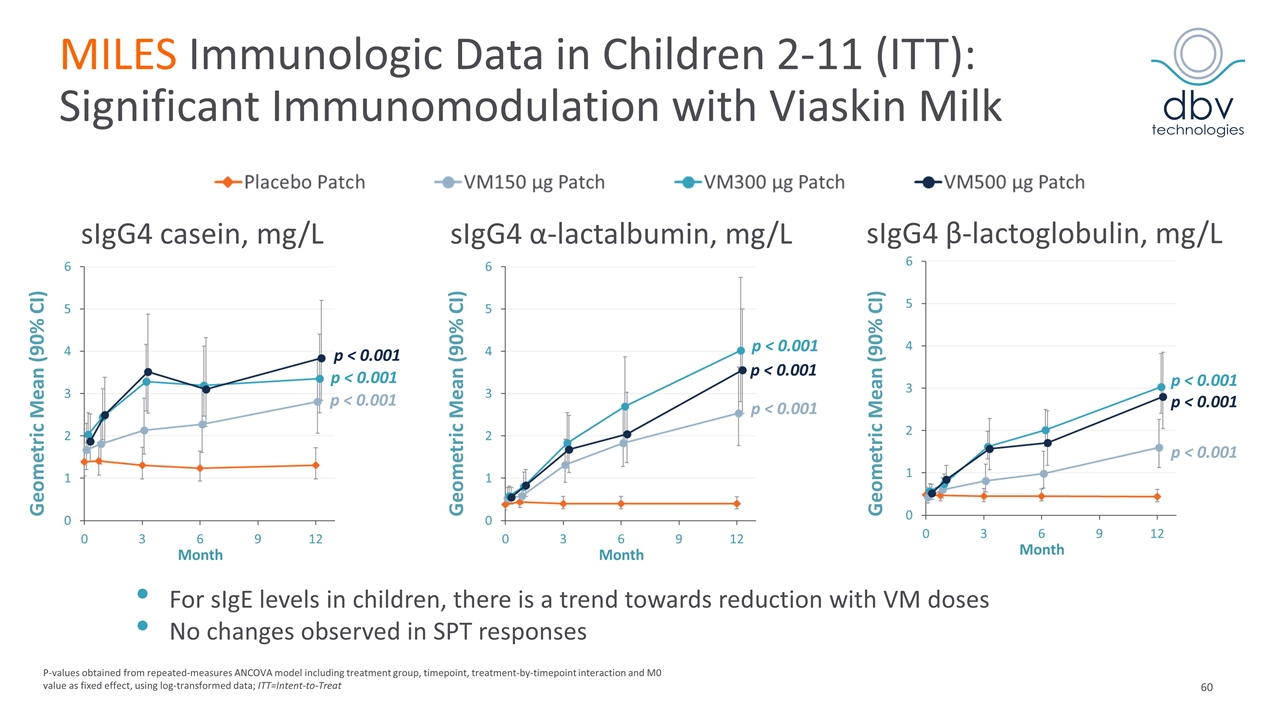
MILES Immunologic Data in Children 2-11 (ITT): Significant Immunomodulation with Viaskin Milk sIgG4 casein, mg/L sIgG4 α-lactalbumin, mg/L sIgG4 β-lactoglobulin, mg/L p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 p ˂ 0.001 Geometric Mean (90% CI) Geometric Mean (90% CI) Geometric Mean (90% CI) P-values obtained from repeated-measures ANCOVA model including treatment group, timepoint, treatment-by-timepoint interaction and M0 value as fixed effect, using log-transformed data; ITT=Intent-to-Treat For sIgE levels in children, there is a trend towards reduction with VM doses No changes observed in SPT responses
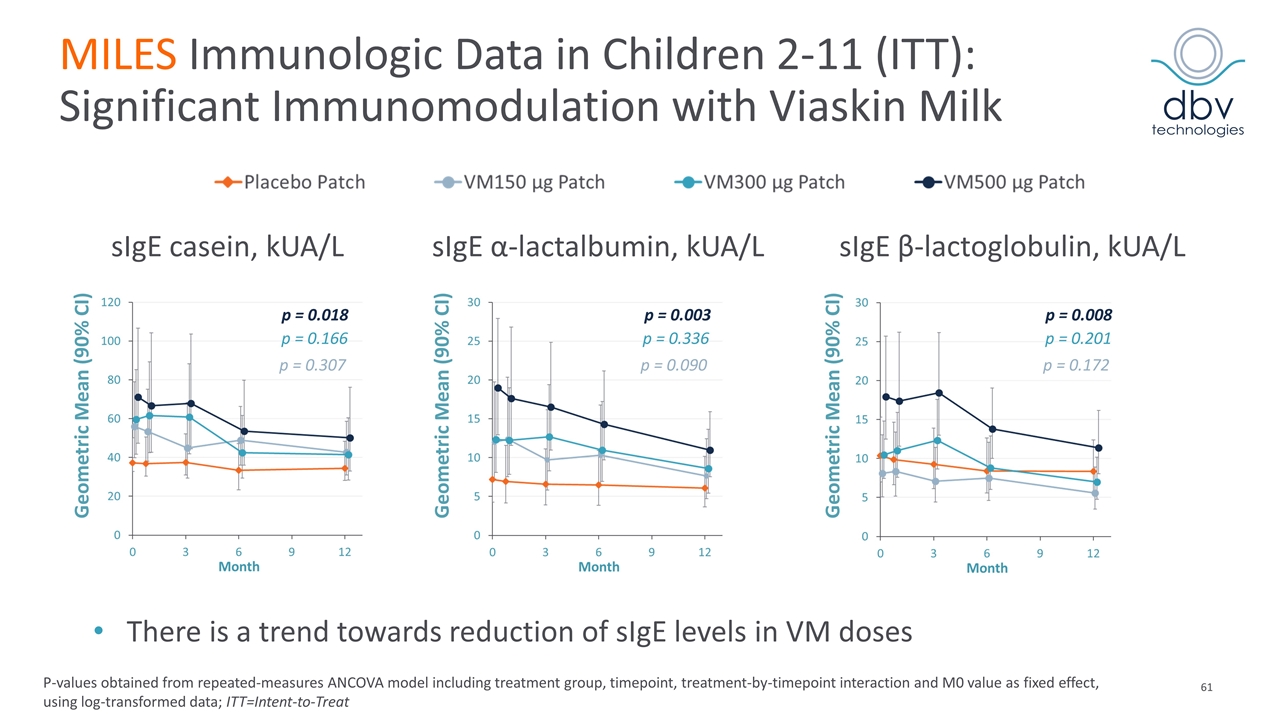
MILES Immunologic Data in Children 2-11 (ITT): Significant Immunomodulation with Viaskin Milk P-values obtained from repeated-measures ANCOVA model including treatment group, timepoint, treatment-by-timepoint interaction and M0 value as fixed effect, using log-transformed data; ITT=Intent-to-Treat p = 0.018 sIgE casein, kUA/L sIgE α-lactalbumin, kUA/L sIgE β-lactoglobulin, kUA/L p = 0.307 p = 0.166 p = 0.090 p = 0.336 p = 0.003 p = 0.172 p = 0.201 p = 0.008 Geometric Mean (90% CI) Geometric Mean (90% CI) Geometric Mean (90% CI) There is a trend towards reduction of sIgE levels in VM doses
