- DBVT Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
6-K Filing
DBV Technologies (DBVT) 6-KCurrent report (foreign)
Filed: 8 Jan 20, 9:42pm

Investigational Viaskin peanut in children with peanut allergy the Pepites open label extension (PEOPLE) trial: TOPLINE RESULTS Epicutaneous immunotherapy and Viaskin® Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority. Exhibit 99.2

Safe harbor This presentation contains forward looking statements including, but not limited to, statements concerning the outcome or success of DBV’s clinical trials; its ability to successfully gain regulatory approvals and commercialize products; its ability to successfully advance its pipeline of product candidates; the rate and degree of market acceptance of its products; and its ability to develop sales and marketing capabilities. Forward looking statements are subject to a number of risks, uncertainties and assumptions. Moreover, DBV operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for DBV’s management to predict all risks, nor can DBV assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward looking statements it may make. In light of these risks, uncertainties and assumptions, the forward looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. You should not rely upon forward looking statements as predictions of future events. Although DBV believes that the expectations reflected in the forward looking statements are reasonable, it cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward looking statements will be achieved or occur. Moreover, except as required by law, neither DBV nor any other person assumes responsibility for the accuracy and completeness of the forward looking statements. Forward looking statements in this presentation represent DBV’s views only as of the date of this presentation. DBV undertakes no obligation to update or review any forward looking statement, whether as a result of new information, future developments or otherwise, except as required by law. Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority.
Peanut allergy REPRESENTS a serious BURDEN AMID CONSTANT FEAR OF ACCIDENTAL EXPOSURE Peanut allergy is one of the most common, persistent and potentially life-threatening food allergies, affecting nearly one million children in the U.S.1 The majority of peanut-allergic children will react to 300 mg of peanut protein (approximately 1 peanut) or less, with some individuals reacting to as little as 1 mg2 A constant fear of accidental peanut exposure leads to reduced quality of life for patients and their families In clinical trials, the degree of desensitization is evaluated by the change in a patient's eliciting dose (ED)3 Based on risk modeling, immunotherapy could offer meaningful reduction of the risks associated with accidental exposures by increasing a patient’s eliciting dose4 1. Gupta R, et al. Pediatrics. 2011;128:e9-17. 2. Deschildre A, et al. Clin Exp Allergy. 2016;46(4):610-620. 3. Food and Drug Administration. Meeting of the Allergenic Products Advisory Committee. https://www.fda.gov/media/95543/download. Accessed January 7, 2020. 4. Remington BC, et al. Annals Allergy Asthma Immunol. 2019;123:488-493.e2. . Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority.
PEOPLE: an open-label extension of PEPItes assessing long-term effects of viaskin peanut Patients who completed the Phase III PEPITES trial were eligible to enroll in PEOPLE Safety and efficacy of long-term treatment with VP 250 μg, up to 5 years of treatment Assessment of clinical benefit after 36 months of treatment with VP 250 μg Double-blind, placebo-controlled food challenge (DBPCFC) assessing ED Sustained unresponsiveness following 2 months of treatment discontinuation (M37 & M38) Unique opportunity to assess long-term effects of epicutaneous immunotherapy (EPITTM) Maintenance and/or improvement of treatment effect Further confirmation of tolerability and safety profile observed in the clinical program to date Further explore the mechanism of action associated with EPIT, potential for differentiation relative to OIT or SLIT Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority.
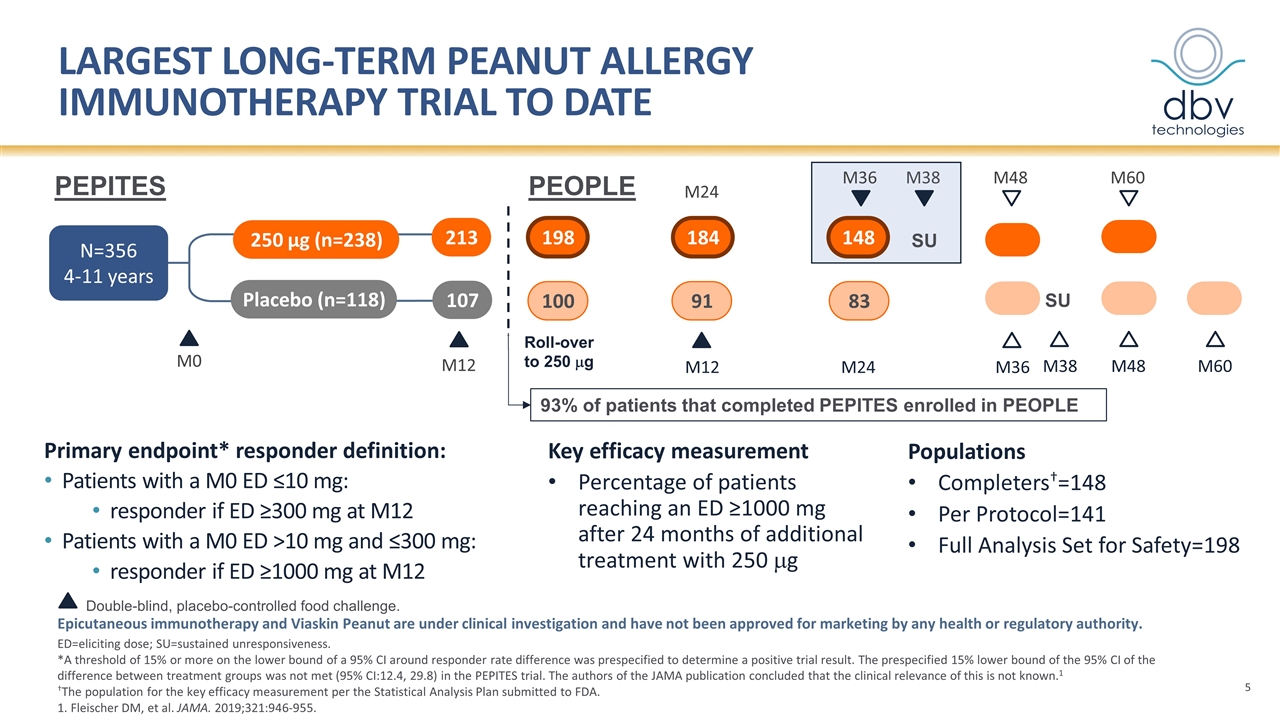
M12 Placebo (n=118) 250 µg (n=238) N=356 4-11 years 213 107 M0 largest long-term peanut allergy immunotherapy trial to date Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority. ED=eliciting dose; SU=sustained unresponsiveness. *A threshold of 15% or more on the lower bound of a 95% CI around responder rate difference was prespecified to determine a positive trial result. The prespecified 15% lower bound of the 95% CI of the difference between treatment groups was not met (95% CI:12.4, 29.8) in the PEPITES trial. The authors of the JAMA publication concluded that the clinical relevance of this is not known.1 †The population for the key efficacy measurement per the Statistical Analysis Plan submitted to FDA. 1. Fleischer DM, et al. JAMA. 2019;321:946-955. Primary endpoint* responder definition: Patients with a M0 ED ≤10 mg: responder if ED ≥300 mg at M12 Patients with a M0 ED >10 mg and ≤300 mg: responder if ED ≥1000 mg at M12 PEPITES PEOPLE 198 100 M12 M24 184 148 91 83 M24 M36 Roll-over to 250 mg M36 M48 M48 M60 M38 Populations Completers†=148 Per Protocol=141 Full Analysis Set for Safety=198 93% of patients that completed PEPITES enrolled in PEOPLE SU Double-blind, placebo-controlled food challenge. M38 Key efficacy measurement Percentage of patients reaching an ED ≥1000 mg after 24 months of additional treatment with 250 mg M60 SU
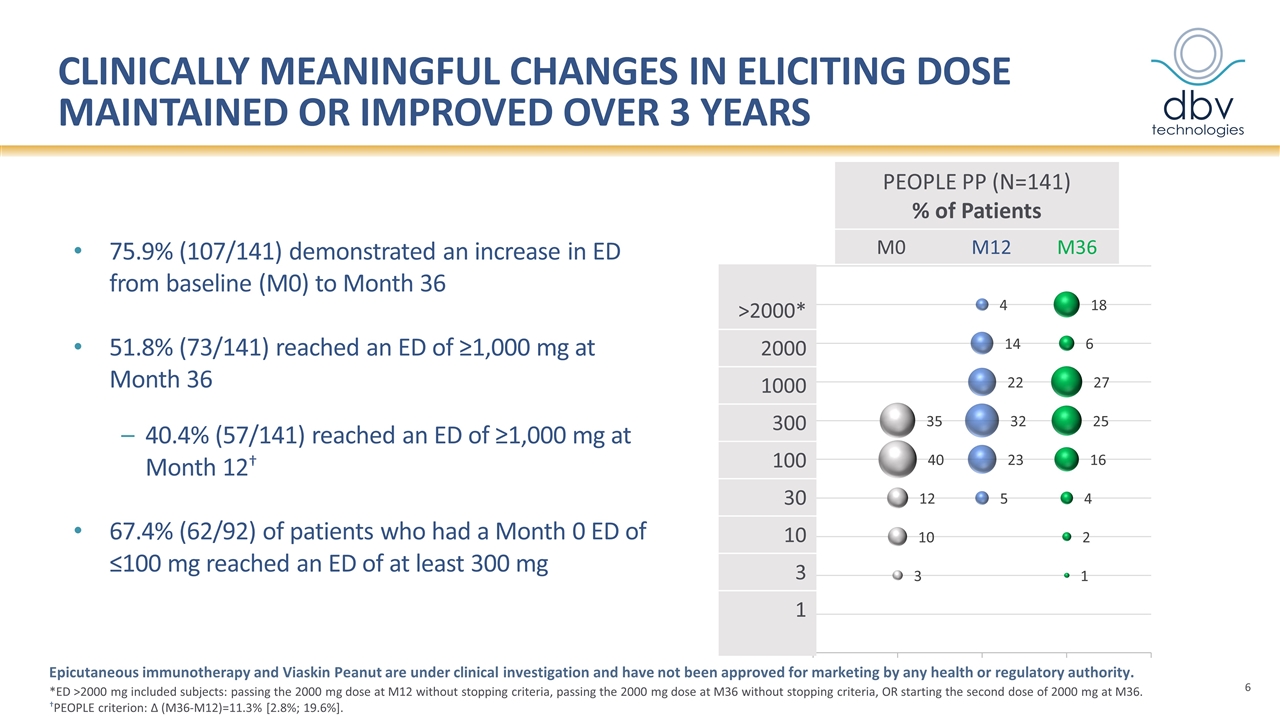
Clinically meaningful changes in Eliciting Dose maintained or improved over 3 years 75.9% (107/141) demonstrated an increase in ED from baseline (M0) to Month 36 51.8% (73/141) reached an ED of ≥1,000 mg at Month 36 40.4% (57/141) reached an ED of ≥1,000 mg at Month 12† 67.4% (62/92) of patients who had a Month 0 ED of ≤100 mg reached an ED of at least 300 mg Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority. PEOPLE PP (N=141) % of Patients M0 M12 M36 >2000* 2000 1000 300 100 30 10 3 1 *ED >2000 mg included subjects: passing the 2000 mg dose at M12 without stopping criteria, passing the 2000 mg dose at M36 without stopping criteria, OR starting the second dose of 2000 mg at M36. †PEOPLE criterion: ∆ (M36-M12)=11.3% [2.8%; 19.6%].
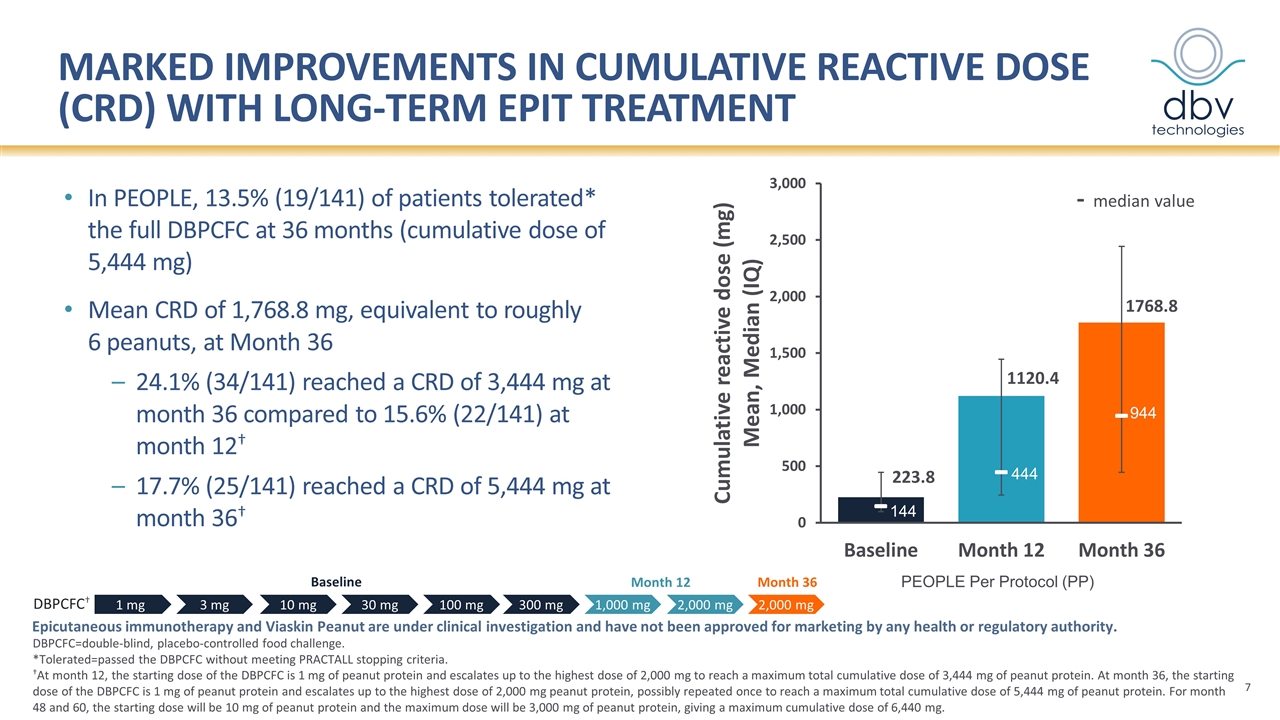
Marked improvements in cumulative reactive dose (CRD) with long-term EPIT treatment - median value In PEOPLE, 13.5% (19/141) of patients tolerated* the full DBPCFC at 36 months (cumulative dose of 5,444 mg) Mean CRD of 1,768.8 mg, equivalent to roughly 6 peanuts, at Month 36 24.1% (34/141) reached a CRD of 3,444 mg at month 36 compared to 15.6% (22/141) at month 12† 17.7% (25/141) reached a CRD of 5,444 mg at month 36† DBPCFC=double-blind, placebo-controlled food challenge. *Tolerated=passed the DBPCFC without meeting PRACTALL stopping criteria. †At month 12, the starting dose of the DBPCFC is 1 mg of peanut protein and escalates up to the highest dose of 2,000 mg to reach a maximum total cumulative dose of 3,444 mg of peanut protein. At month 36, the starting dose of the DBPCFC is 1 mg of peanut protein and escalates up to the highest dose of 2,000 mg peanut protein, possibly repeated once to reach a maximum total cumulative dose of 5,444 mg of peanut protein. For month 48 and 60, the starting dose will be 10 mg of peanut protein and the maximum dose will be 3,000 mg of peanut protein, giving a maximum cumulative dose of 6,440 mg. Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority. Month 12 Baseline 1 mg 3 mg 10 mg 30 mg 100 mg 300 mg 1,000 mg 2,000 mg 2,000 mg DBPCFC† Month 36 PEOPLE Per Protocol (PP) 144 444 944
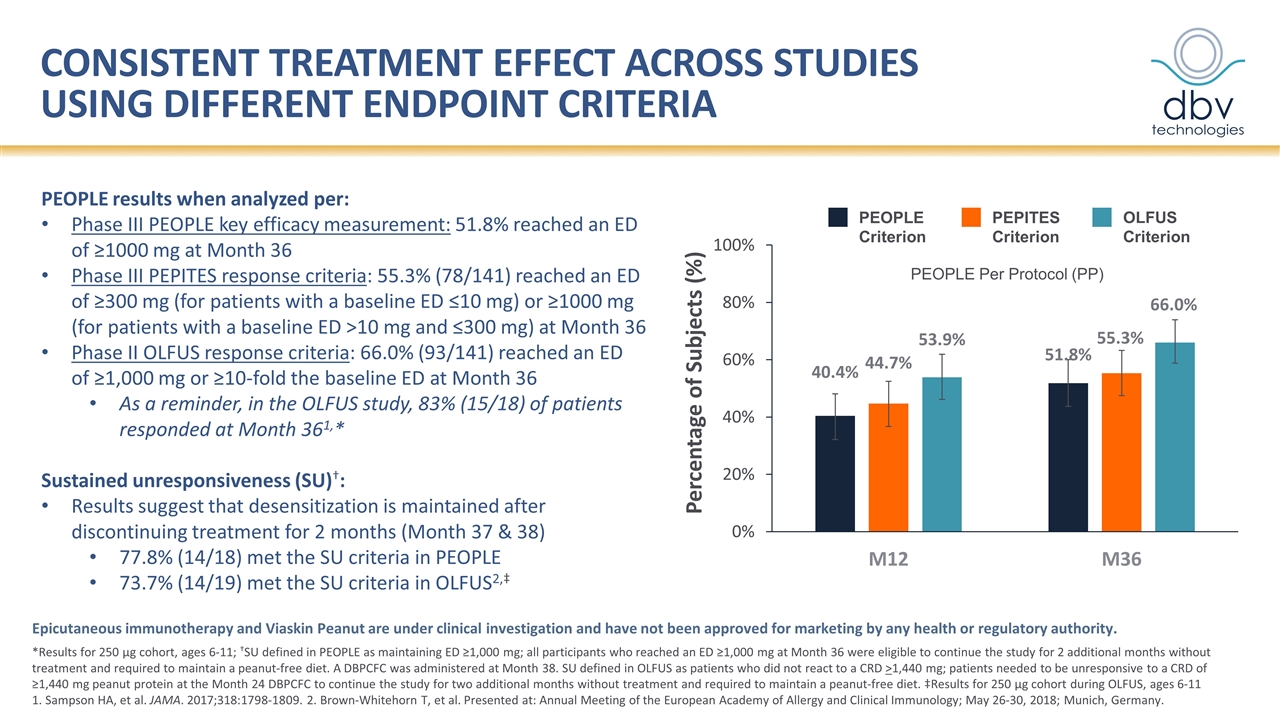
Consistent treatment effect across studies USING DIFFERENT endpoint criteria *Results for 250 µg cohort, ages 6-11; †SU defined in PEOPLE as maintaining ED ≥1,000 mg; all participants who reached an ED ≥1,000 mg at Month 36 were eligible to continue the study for 2 additional months without treatment and required to maintain a peanut-free diet. A DBPCFC was administered at Month 38. SU defined in OLFUS as patients who did not react to a CRD >1,440 mg; patients needed to be unresponsive to a CRD of ≥1,440 mg peanut protein at the Month 24 DBPCFC to continue the study for two additional months without treatment and required to maintain a peanut-free diet. ‡Results for 250 µg cohort during OLFUS, ages 6-11 1. Sampson HA, et al. JAMA. 2017;318:1798-1809. 2. Brown-Whitehorn T, et al. Presented at: Annual Meeting of the European Academy of Allergy and Clinical Immunology; May 26-30, 2018; Munich, Germany. PEOPLE results when analyzed per: Phase III PEOPLE key efficacy measurement: 51.8% reached an ED of ≥1000 mg at Month 36 Phase III PEPITES response criteria: 55.3% (78/141) reached an ED of ≥300 mg (for patients with a baseline ED ≤10 mg) or ≥1000 mg (for patients with a baseline ED >10 mg and ≤300 mg) at Month 36 Phase II OLFUS response criteria: 66.0% (93/141) reached an ED of ≥1,000 mg or ≥10-fold the baseline ED at Month 36 As a reminder, in the OLFUS study, 83% (15/18) of patients responded at Month 361,* Sustained unresponsiveness (SU)†: Results suggest that desensitization is maintained after discontinuing treatment for 2 months (Month 37 & 38) 77.8% (14/18) met the SU criteria in PEOPLE 73.7% (14/19) met the SU criteria in OLFUS2,‡ Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority. PEOPLE Criterion PEPITES Criterion OLFUS Criterion PEOPLE Per Protocol (PP) Percentage of Subjects (%)
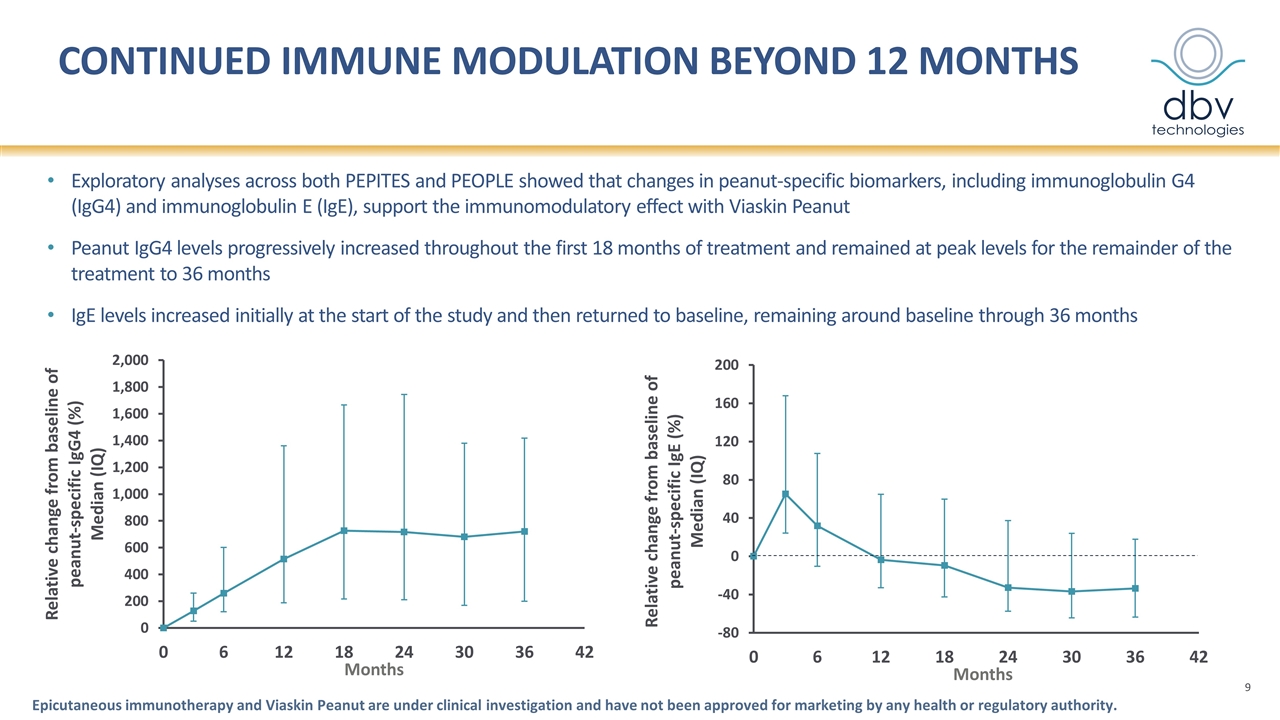
Continued Immune modulation beyond 12 months Exploratory analyses across both PEPITES and PEOPLE showed that changes in peanut-specific biomarkers, including immunoglobulin G4 (IgG4) and immunoglobulin E (IgE), support the immunomodulatory effect with Viaskin Peanut Peanut IgG4 levels progressively increased throughout the first 18 months of treatment and remained at peak levels for the remainder of the treatment to 36 months IgE levels increased initially at the start of the study and then returned to baseline, remaining around baseline through 36 months Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority.
safety profile consistent with that observed in the clinical program to date No treatment-related serious adverse events (SAEs) were reported Most commonly reported treatment-related AEs were mild to moderate skin reactions Local administration site reactions observed in 92.9% (184/198) of patients over 36 months In year 3 of treatment, local skin reactions observed in 29.9% (55/198) of patients 4 children discontinued due to treatment-emergent adverse events between the start of PEOPLE and Month 36 No epinephrine intake related to treatment observed in the PEOPLE study 1 patient experienced 1 possibly-related mild anaphylaxis* that resolved without treatment Treatment compliance remained high throughout the study Mean compliance of 98% observed over 3 years of treatment High adherence and low dropout rate 50 patients discontinued† between the start of PEOPLE and Month 36 27 patients discontinued between Month 30-36, with 21 of the 27 identifying food challenge fright/distaste as the reason for consent withdrawal *Anaphylaxis was defined according to NIAID, which has been shown to be highly sensitive but moderately specific, in an attempt to capture as many reactions as possible. Severe anaphylaxis was defined by presence of cyanosis, hypoxia, hypotension, confusion, loss of consciousness, or incontinence. †Includes those who discontinued prior to or during the Month 36 DBPCFC. Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority.

Summary Approximately 3 out of 4 patients experienced an increase in their ED from baseline, showing a majority of patients benefited from the investigational therapy 51.8% reached an eliciting dose (ED) of at least 1,000 mg peanut protein (about three peanuts) Robust treatment response with 13.5% of patients tolerating* a cumulative dose of 5,444 mg at Month 36 Median CRD increased approximately 7x from baseline (144 mg) to Month 36 (944 mg) 77.8% (14/18) demonstrated sustained unresponsiveness, defined by maintaining an ED ≥ 1,000 mg following a 2-month period without Viaskin Peanut treatment, suggesting that desensitization may be maintained after discontinuing treatment Safety profile was consistent with previously published controlled Phase 3 studies Viaskin Peanut was observed to be well tolerated over the three-year treatment period No treatment-related epinephrine use reported in PEOPLE Consistent results observed throughout Phase II and Phase III clinical studies EPIT has the potential to modify the immune response in a durable way, offering potential long-term protection against the risk of reaction from accidental exposure Epicutaneous immunotherapy and Viaskin Peanut are under clinical investigation and have not been approved for marketing by any health or regulatory authority. *Tolerated=passed the DBPCFC without meeting PRACTALL stopping criteria.