Exhibit 10.4
SCHEDULE 1
WORK ORDER 2
ThisWORK ORDER dated as of the date of last signature below
BETWEEN
(1) Kitov Pharmaceuticals Holdings Ltd., One Azrieli Center, Round Tower, 23rd Floor, Tel Aviv 670110 (hereinafter referred to as “KITOV”) and
(2)JAVA CLINICAL RESEARCH LIMITED, a company incorporated in Ireland, registered number 311398, with registered office address 88 Harcourt Street , Dublin 2, and located at Denshaw House 21 Lower Baggot Street , Ireland (hereinafter referred to as “JAVA”).
WHEREASKitov and JAVA are bound by the terms of the Master Services Agreement dated 04 February 2014 between Kitov and JAVA (the "Master Services Agreement"), Kitov and JAVA wish to set out the specific details of services to be provided by JAVA in respect of study
Title of trial: A Prospective Randomized Placebo Controlled Study to Evaluate the Effect of Celecoxib on the Efficacy and Safety of Amlodipine on Renal and Vascular Function in Subjects with Existing Hypertension Requiring Antihypertensive Therapy
THE PARTIES NOW HEREBY AGREE AS FOLLOWS:
| 1. | JAVA shall provide the services as outlined in Schedule 2 hereto (the "Services") in accordance with the timelines set out in Schedule 3 hereto and the terms and conditions of the Master Services Agreement together with the additional provisions as set out in the following Schedules: |
| | Schedule 1 | Timelines |
| | | |
| | Schedule 2 | Services Budget and Payment Schedule Java Clinical Research |
| | | |
| | Schedule 3 | Services Budget and Payment Schedule CROSNT |
| | | |
| | Schedule 4 | Payment Plan |
| | | |
| | Schedule 5 | Personnel / Contacts for Notices and Payments |
This Work Order has an effective date as of the last date of signature and will remain valid until completion of Services described herein and when Kitov has paid the final invoice in accordance with the terms of the Master Services Agreement, unless otherwise terminated in accordance with the provisions of Clause 12 of the Master Services Agreement.
SIGNED BY
KITOV PHARMACEUTICAL HOLDINGS LTD JAVA CLINICAL RESEARCH LTD
| Signature:__________________________ | | Signature:__________________________ |
| | | |
| By:________________________________ | | By:________________________________ |
| (PrintName) | | |
| | | |
| Title: ______________________________ | | Title: ______________________________ |
| | | |
| Date: ______________________________ | | Date: ______________________________ |
SCHEDULE 1: TIMELINES
The Work Order is based on the following estimated timelines:
| Milestone | | Target Date |
| MHRA Approval | | September 2016 |
| October | | October 2016 |
| First Site Initiation Visit | | October 2016 |
| First Clinical Trial Subject Recruited | | October 2016 |
| Last Clinical Trial Subject Recruited | | 17 February 2017 |
| LPLV | | 31 March 2017 |
| DB Lock | | May 2017 |
| Final Study Report | | June/ July 2017 |
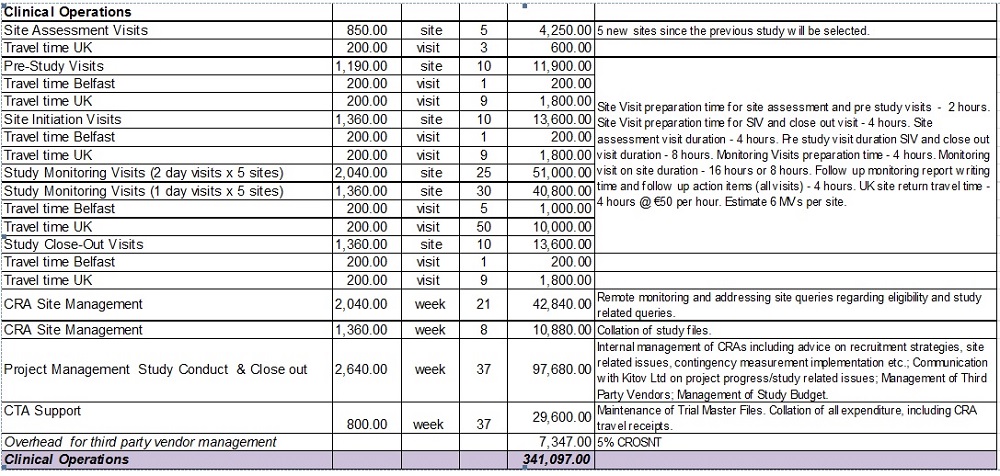
SCHEDULE 2: BUDGET JAVA CLINICAL RESEARCH
| Study Reference KIT-302-03-92 | | | | |
| | | | | |
| Projected Timelines: | | | | |
| Project Preparation | | 15 weeks | | 04 July 2016 - 17 October 2016 |
| Study Recruitment and Conduct Period | | 24 weeks | | 17 October 2016 - 31 March 2017 |
| | | | | Last patient randomised 17/02/2017 |
| | | | | Last Patient Last Visit 31/03/2017 |
| Close out period | | 13 weeks | | 13 March 2017 - 30 June 2017 |
| | | | | |
| Indication: | | Hypertension | | |
| | | | | |
| Key Assumptions: | | | | |
| Number of patients to be screened | | 150 | | |
| Number of patients to be randomised | | 105 | | |
| | | | | |
| Sites | | 10 sites | | |
| Number of monitoring visits (150 patients) | | Estimated pool of monitoring visits 55 (5/6) | | |
| | | | | |
| Total CRF pages per patient | | TBC | | |

SCHEDULE 3: BUDGET CROSNT
| Biometrics Management | | Total Cost (EUR) | |
| TOTAL | | | 15,423.00 | |
| | | | | |
| Data Management | | Total Cost (EUR) | |
| CRF Design | | | 3,365.20 | |
| Data Management Plan | | | 3,830.30 | |
| Programming and Validation of Database | | | 10,108.80 | |
| Programming and Validation of Consistency Checks | | | 8,095.20 | |
| Data Load set-up | | | 2,706.00 | |
| User acceptance testing | | | 1,971.20 | |
| Data Cleaning | | | 17,577.20 | |
| SAE Reconciliation | | | 1,033.80 | |
| Production of Listing for Medical Review | | | 873.90 | |
| Coding | | | 3,158.40 | |
| Metrics/Tracking Report | | | 789.60 | |
| SAS External Data Load | | | 1,071.00 | |
| Database Lock | | | 958.80 | |
| Database Transfer | | | 119.00 | |
| TMF Management | | | 714.00 | |
| Documentation Archiving | | | 2,380.00 | |
| DM Teleconferences | | | 1,353.60 | |
| TOTAL | | | 60,106.00 | |
| | | | | |
| eCRF management and hosting | | Total Cost (EUR) | |
| EDC System setup, documentation and maintenance & Help Desk (2nd level) | | | 1,249.50 | |
| eCRF License fee, hosting, e-Learning and 1st Level Help Desk | | | 49,461.00 | |
| Patient Data Reports | | | 1,725.50 | |
| Implementation & Maintenance of Access Authorisation | | | 8,131.20 | |
| e-Learning User Management | | | 2,310.00 | |
| CD Archives | | | 163.35 | |
| TOTAL | | | 63,040.55 | |
| | | | | |
| Pharmacovigilance | | Total Cost (EUR) | |
| Initial Set Up Costs (to include registration with EMA and all competent authorities, Development of Safety Management Plan | | | 9,270.00 | |
| Monthly costs for duration of study for provision of responsible person for PV Qualified medic, PV Governance & Project Quality, Safety database hosting, generation of SAE monthly tracking sheets | | | 7,416.00 | |
| SAE Handling Costs | | | 2,472.00 | |
| Generation and submission of SUSARS listings to Investigators | | | 103.00 | |
| TOTAL | | | 19,261.00 | |
| | | | | |
| TOTAL | | | 157,830.55 | |
| | | | | |
| DISCOUNT | | | 10,881.42 | |
| | | | | |
| TOTAL INCLUDING DISCOUNT | | | 146,949.13 | |
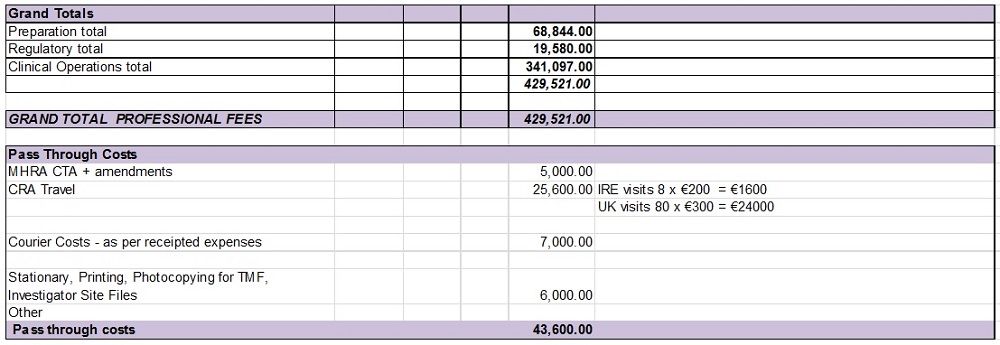
SCHEDULE 4: PAYMENT SCHEDULES
Payment Schedules Java
Total Budget €429,521
| Step of Payment | | Percentage | | | Due Amount (EUR) | |
| Upfront fee at exchange of contracts | | | 30 | % | | | 128, 856 | |
| Less Upfront fee at LOI contracts | | | | | | € | 50,000.00 | |
| Balance due on exchange of contracts | | | | | | € | 78,856. | |
| Study Conduct period Invoiced monthly during randomisation | | | 50 | % | | € | 214,760 | |
| LPLV | | | 10 | % | | € | 42,952 | |
| DB Lock | | | 5 | % | | € | 21,476 | |
| Final study report | | | 5 | % | | € | 21,477 | |
Payment Schedules CROSNT
Total Budget€146,949.13
| Step of Payment | | Percentage | | | Due Amount (EUR) | |
| Upfront fee at exchange of contracts | | | 30 | % | | € | 44,084.74 | |
| Database Go live | | | 10 | % | | € | 14,694.91 | |
| Following 25 Subjects randomised | | | 10 | % | | € | 14,694.91 | |
| Following 50 Subjects randomised | | | 10 | % | | € | 14,694.91 | |
| Following 75 Subjects randomised | | | 10 | % | | € | 14,694.91 | |
| Following 100 Subjects randomised | | | 10 | % | | € | 14,694.91 | |
| LPLV | | | 10 | % | | € | 14,694.91 | |
| DB Lock | | | 10 | % | | € | 14,694.92 | |
SCHEDULE 5: Personnel / Contacts for Notices and Payments
For; Java CLINICAL RESEARCH Ltd
Ruth Nallen
Managing Director
Java Clinical Research
Denshaw House
121 Lower baggot street
Dublin 2
Emial; rnallen@javacr.com
For : KITOV PHARMACEUTICAL HOLDINGS LTD
Mr Simcha Rock
Chief Financial Officer
One Azrieli Center, Round Tower,
23rdFloor,
Tel Aviv
Israel 670110
Email; simcha@kitovpharma.com
Page 8 of 8


